Abstract
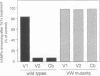
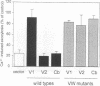
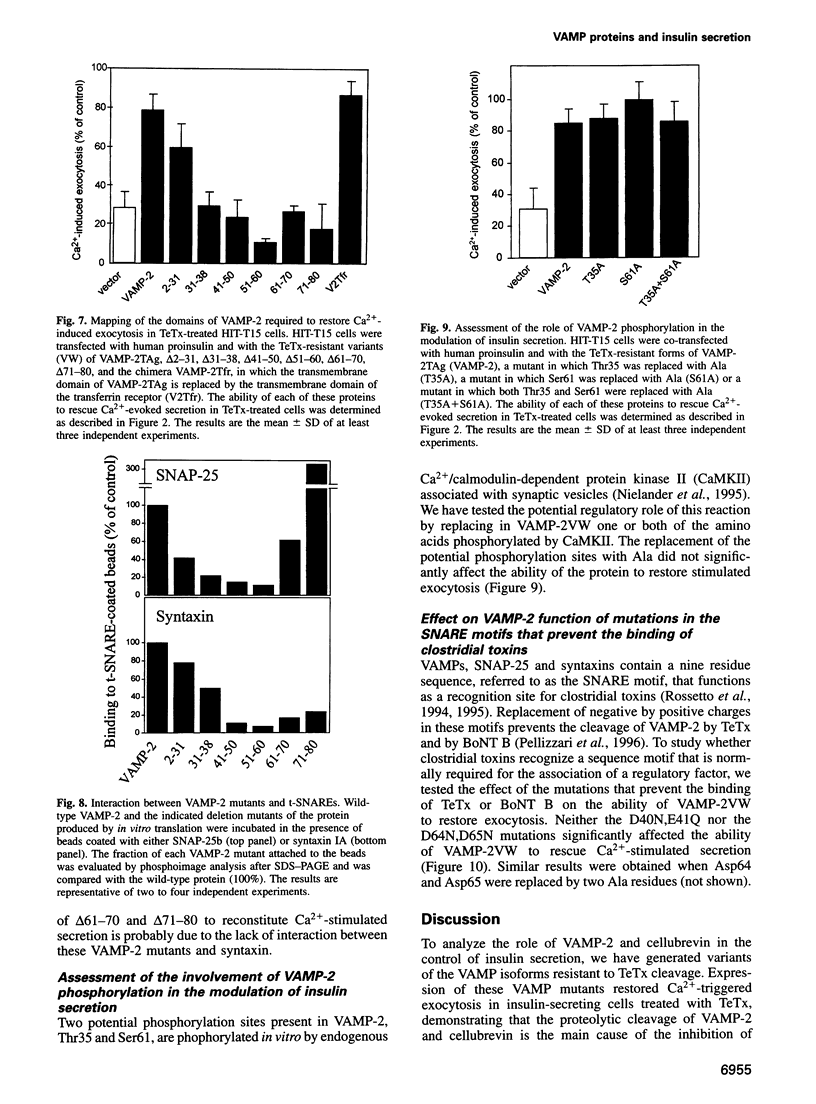
Vesicle-associated membrane protein-2 (VAMP-2) and cellubrevin are associated with the membrane of insulin-containing secretory granules and of gamma-aminobutyric acid (GABA)-containing synaptic-like vesicles of pancreatic beta-cells. We found that a point mutation in VAMP-2 preventing targeting to synaptic vesicles also impairs the localization on insulin-containing secretory granules, suggesting a similar requirement for vesicular targeting. Tetanus toxin (TeTx) treatment of permeabilized HIT-T15 cells leads to the proteolytic cleavage of VAMP-2 and cellubrevin and causes the inhibition of Ca2+-triggered insulin exocytosis. Transient transfection of HIT-T15 cells with VAMP-1, VAMP-2 or cellubrevin made resistant to the proteolytic action of TeTx by amino acid replacements in the cleavage site restored Ca2+-stimulated secretion. Wild-type VAMP-2, wild-type cellubrevin or a mutant of VAMP-2 resistant to TeTx but not targeted to secretory granules were unable to rescue Ca2+-evoked insulin release. The transmembrane domain and the N-terminal region of VAMP-2 were not essential for the recovery of stimulated exocytosis, but deletions preventing the binding to SNAP-25 and/or to syntaxin I rendered the protein inactive in the reconstitution assay. Mutations of putative phosphorylation sites or of negatively charged amino acids in the SNARE motif recognized by clostridial toxins had no effect on the ability of VAMP-2 to mediate Ca2+-triggered secretion. We conclude that: (i) both VAMP-2 and cellubrevin can participate in the exocytosis of insulin; (ii) the interaction of VAMP-2 with syntaxin and SNAP-25 is required for docking and/or fusion of secretory granules with the plasma membrane; and (iii) the phosphorylation of VAMP-2 is not essential for Ca2+-stimulated insulin exocytosis.
Full text
PDF



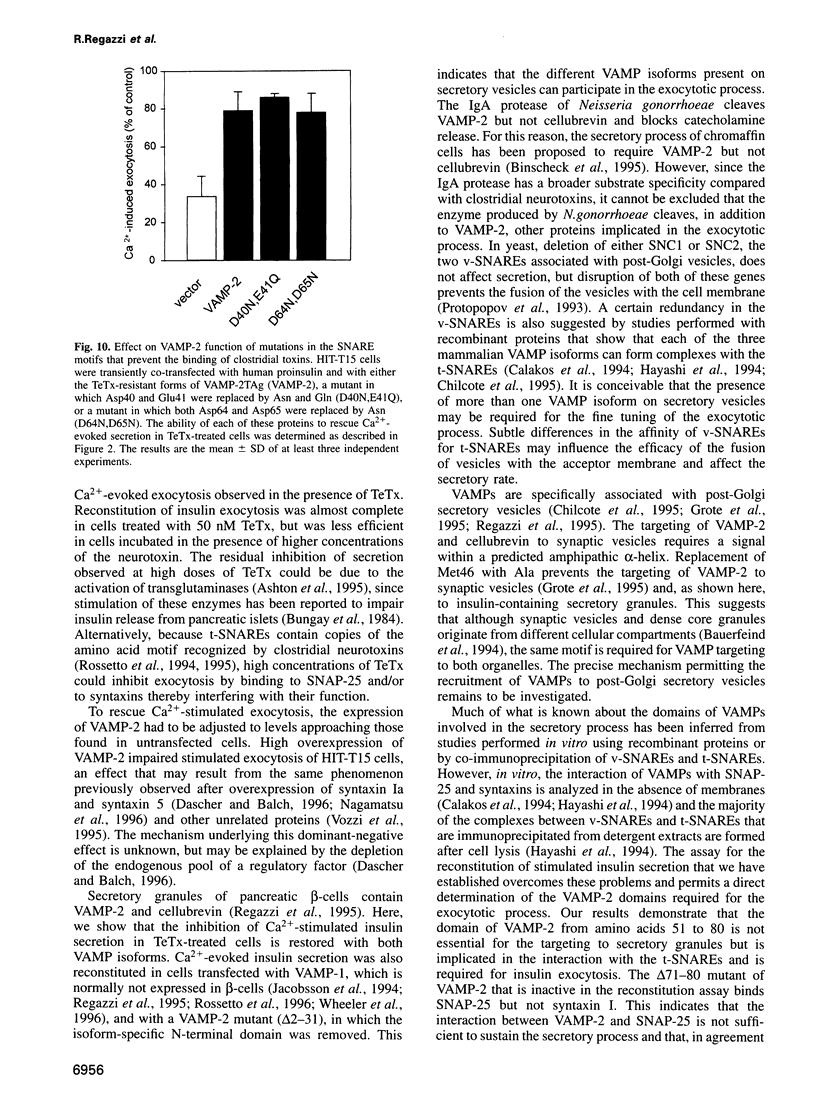



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asfari M., Janjic D., Meda P., Li G., Halban P. A., Wollheim C. B. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992 Jan;130(1):167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- Ashton A. C., Li Y., Doussau F., Weller U., Dougan G., Poulain B., Dolly J. O. Tetanus toxin inhibits neuroexocytosis even when its Zn(2+)-dependent protease activity is removed. J Biol Chem. 1995 Dec 29;270(52):31386–31390. doi: 10.1074/jbc.270.52.31386. [DOI] [PubMed] [Google Scholar]
- Bauerfeind R., Ohashi M., Huttner W. B. Biogenesis of secretory granules and synaptic vesicles. Facts and hypotheses. Ann N Y Acad Sci. 1994 Sep 15;733:233–244. doi: 10.1111/j.1749-6632.1994.tb17273.x. [DOI] [PubMed] [Google Scholar]
- Binscheck T., Bartels F., Bergel H., Bigalke H., Yamasaki S., Hayashi T., Niemann H., Pohlner J. IgA protease from Neisseria gonorrhoeae inhibits exocytosis in bovine chromaffin cells like tetanus toxin. J Biol Chem. 1995 Jan 27;270(4):1770–1774. doi: 10.1074/jbc.270.4.1770. [DOI] [PubMed] [Google Scholar]
- Bungay P. J., Potter J. M., Griffin M. The inhibition of glucose-stimulated insulin secretion by primary amines. A role for transglutaminase in the secretory mechanism. Biochem J. 1984 May 1;219(3):819–827. doi: 10.1042/bj2190819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calakos N., Bennett M. K., Peterson K. E., Scheller R. H. Protein-protein interactions contributing to the specificity of intracellular vesicular trafficking. Science. 1994 Feb 25;263(5150):1146–1149. doi: 10.1126/science.8108733. [DOI] [PubMed] [Google Scholar]
- Chapman E. R., An S., Barton N., Jahn R. SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils. J Biol Chem. 1994 Nov 4;269(44):27427–27432. [PubMed] [Google Scholar]
- Chilcote T. J., Galli T., Mundigl O., Edelmann L., McPherson P. S., Takei K., De Camilli P. Cellubrevin and synaptobrevins: similar subcellular localization and biochemical properties in PC12 cells. J Cell Biol. 1995 Apr;129(1):219–231. doi: 10.1083/jcb.129.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher C., Balch W. E. Mammalian Sly1 regulates syntaxin 5 function in endoplasmic reticulum to Golgi transport. J Biol Chem. 1996 Jul 5;271(27):15866–15869. doi: 10.1074/jbc.271.27.15866. [DOI] [PubMed] [Google Scholar]
- Dascher C., Ossig R., Gallwitz D., Schmitt H. D. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol Cell Biol. 1991 Feb;11(2):872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink L. A., Trimble W. S., Scheller R. H. Two vesicle-associated membrane protein genes are differentially expressed in the rat central nervous system. J Biol Chem. 1989 Jul 5;264(19):11061–11064. [PubMed] [Google Scholar]
- Ferro-Novick S., Jahn R. Vesicle fusion from yeast to man. Nature. 1994 Jul 21;370(6486):191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Galli T., Chilcote T., Mundigl O., Binz T., Niemann H., De Camilli P. Tetanus toxin-mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor-containing vesicles in CHO cells. J Cell Biol. 1994 Jun;125(5):1015–1024. doi: 10.1083/jcb.125.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E., Hao J. C., Bennett M. K., Kelly R. B. A targeting signal in VAMP regulating transport to synaptic vesicles. Cell. 1995 May 19;81(4):581–589. doi: 10.1016/0092-8674(95)90079-9. [DOI] [PubMed] [Google Scholar]
- Hayashi T., McMahon H., Yamasaki S., Binz T., Hata Y., Südhof T. C., Niemann H. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 1994 Nov 1;13(21):5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson G., Bean A. J., Scheller R. H., Juntti-Berggren L., Deeney J. T., Berggren P. O., Meister B. Identification of synaptic proteins and their isoform mRNAs in compartments of pancreatic endocrine cells. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12487–12491. doi: 10.1073/pnas.91.26.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lang J., Nishimoto I., Okamoto T., Regazzi R., Kiraly C., Weller U., Wollheim C. B. Direct control of exocytosis by receptor-mediated activation of the heterotrimeric GTPases Gi and G(o) or by the expression of their active G alpha subunits. EMBO J. 1995 Aug 1;14(15):3635–3644. doi: 10.1002/j.1460-2075.1995.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A., Van Dyke M., Stock J. Predicting coiled coils from protein sequences. Science. 1991 May 24;252(5009):1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- McMahon H. T., Ushkaryov Y. A., Edelmann L., Link E., Binz T., Niemann H., Jahn R., Südhof T. C. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993 Jul 22;364(6435):346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- Nagamatsu S., Fujiwara T., Nakamichi Y., Watanabe T., Katahira H., Sawa H., Akagawa K. Expression and functional role of syntaxin 1/HPC-1 in pancreatic beta cells. Syntaxin 1A, but not 1B, plays a negative role in regulatory insulin release pathway. J Biol Chem. 1996 Jan 12;271(2):1160–1165. doi: 10.1074/jbc.271.2.1160. [DOI] [PubMed] [Google Scholar]
- Nielander H. B., Onofri F., Valtorta F., Schiavo G., Montecucco C., Greengard P., Benfenati F. Phosphorylation of VAMP/synaptobrevin in synaptic vesicles by endogenous protein kinases. J Neurochem. 1995 Oct;65(4):1712–1720. doi: 10.1046/j.1471-4159.1995.65041712.x. [DOI] [PubMed] [Google Scholar]
- Niemann H., Blasi J., Jahn R. Clostridial neurotoxins: new tools for dissecting exocytosis. Trends Cell Biol. 1994 May;4(5):179–185. doi: 10.1016/0962-8924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Pellizzari R., Rossetto O., Lozzi L., Giovedi' S., Johnson E., Shone C. C., Montecucco C. Structural determinants of the specificity for synaptic vesicle-associated membrane protein/synaptobrevin of tetanus and botulinum type B and G neurotoxins. J Biol Chem. 1996 Aug 23;271(34):20353–20358. doi: 10.1074/jbc.271.34.20353. [DOI] [PubMed] [Google Scholar]
- Protopopov V., Govindan B., Novick P., Gerst J. E. Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae. Cell. 1993 Sep 10;74(5):855–861. doi: 10.1016/0092-8674(93)90465-3. [DOI] [PubMed] [Google Scholar]
- Reetz A., Solimena M., Matteoli M., Folli F., Takei K., De Camilli P. GABA and pancreatic beta-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretion. EMBO J. 1991 May;10(5):1275–1284. doi: 10.1002/j.1460-2075.1991.tb08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzi R., Li G. D., Deshusses J., Wollheim C. B. Stimulus-response coupling in insulin-secreting HIT cells. Effects of secretagogues on cytosolic Ca2+, diacylglycerol, and protein kinase C activity. J Biol Chem. 1990 Sep 5;265(25):15003–15009. [PubMed] [Google Scholar]
- Regazzi R., Ravazzola M., Iezzi M., Lang J., Zahraoui A., Andereggen E., Morel P., Takai Y., Wollheim C. B. Expression, localization and functional role of small GTPases of the Rab3 family in insulin-secreting cells. J Cell Sci. 1996 Sep;109(Pt 9):2265–2273. doi: 10.1242/jcs.109.9.2265. [DOI] [PubMed] [Google Scholar]
- Regazzi R., Wollheim C. B., Lang J., Theler J. M., Rossetto O., Montecucco C., Sadoul K., Weller U., Palmer M., Thorens B. VAMP-2 and cellubrevin are expressed in pancreatic beta-cells and are essential for Ca(2+)-but not for GTP gamma S-induced insulin secretion. EMBO J. 1995 Jun 15;14(12):2723–2730. doi: 10.1002/j.1460-2075.1995.tb07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetto O., Deloye F., Poulain B., Pellizzari R., Schiavo G., Montecucco C. The metallo-proteinase activity of tetanus and botulism neurotoxins. J Physiol Paris. 1995;89(1):43–50. doi: 10.1016/0928-4257(96)80550-X. [DOI] [PubMed] [Google Scholar]
- Rossetto O., Gorza L., Schiavo G., Schiavo N., Scheller R. H., Montecucco C. VAMP/synaptobrevin isoforms 1 and 2 are widely and differentially expressed in nonneuronal tissues. J Cell Biol. 1996 Jan;132(1-2):167–179. doi: 10.1083/jcb.132.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetto O., Schiavo G., Montecucco C., Poulain B., Deloye F., Lozzi L., Shone C. C. SNARE motif and neurotoxins. Nature. 1994 Dec 1;372(6505):415–416. doi: 10.1038/372415a0. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Mechanisms of intracellular protein transport. Nature. 1994 Nov 3;372(6501):55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994 Mar 1;4(3):220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Sadoul K., Lang J., Montecucco C., Weller U., Regazzi R., Catsicas S., Wollheim C. B., Halban P. A. SNAP-25 is expressed in islets of Langerhans and is involved in insulin release. J Cell Biol. 1995 Mar;128(6):1019–1028. doi: 10.1083/jcb.128.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B. R., Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992 Oct 29;359(6398):832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Söllner T., Bennett M. K., Whiteheart S. W., Scheller R. H., Rothman J. E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993 Nov 5;75(3):409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Vozzi C., Ullrich S., Charollais A., Philippe J., Orci L., Meda P. Adequate connexin-mediated coupling is required for proper insulin production. J Cell Biol. 1995 Dec;131(6 Pt 1):1561–1572. doi: 10.1083/jcb.131.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M. B., Sheu L., Ghai M., Bouquillon A., Grondin G., Weller U., Beaudoin A. R., Bennett M. K., Trimble W. S., Gaisano H. Y. Characterization of SNARE protein expression in beta cell lines and pancreatic islets. Endocrinology. 1996 Apr;137(4):1340–1348. doi: 10.1210/endo.137.4.8625909. [DOI] [PubMed] [Google Scholar]