Abstract
Loss of repressor element 1 silencing transcription factor (REST) occurs in 20% of breast cancers and correlates with a poor patient prognosis. However, the molecular basis for enhanced malignancy in tumors lacking REST (RESTless) is only partially understood. We used multiplatform array data from the Cancer Genome Atlas to identify consistent changes in key signaling pathways. Of the proteins screened in the reverse-phase protein array, we found that insulin receptor substrate 1 (IRS1) is the most highly upregulated protein in RESTless breast tumors. Analysis of breast tumor cell lines showed that REST directly represses IRS1, and cells lacking REST have increased levels of IRS1 mRNA and protein. We find that the upregulation of IRS1 function is both necessary and sufficient for enhanced signaling and growth in breast cancer cells lacking REST. IRS1 overexpression is sufficient to phenocopy the enhanced activation of the signaling hubs AKT and mitogen-activated protein kinase (MAPK) of MCF7 cells lacking REST. Loss of REST renders MCF7 and MDA-MB-231 breast tumor cells dependent on IRS1 activity for colony formation in soft agar. Inhibition of the type 1 insulin-like growth factor receptor (IGF1R) reduces the enhanced signaling, growth, and migration in breast tumor cells that occur upon REST loss. We show that loss of REST induces a pathogenic program that works through the IGF1R/IRS1 pathway.
INTRODUCTION
We recently identified a novel subset of breast cancers that lack the repressor element 1 (RE-1) silencing transcription factor (REST). Loss of REST occurs in ∼20% of all human breast cancers (termed RESTless), regardless of hormone receptor status (1). Patients with tumors lacking REST function have decreased disease-free survival and an aggressive disease course compared to those of patients with tumors expressing REST (RESTfl) (1). MCF7 cells lacking REST give rise to significantly more tumors in mouse xenografts and correlate with enhanced soft-agar colony formation in vitro, illustrating that REST acts as a bona fide tumor suppressor for breast epithelia (2).
REST represses ∼2,000 target genes by recruiting chromatin-modifying complexes to RE-1 sites in the regulatory region of its target genes (3–10). REST was first identified as a breast tumor suppressor in a screen for factors whose loss conferred anchorage-independent growth in human mammary epithelial cells (11). Knockdown of REST in MCF7 cells increases clonogenicity, enhances cell proliferation, and suppresses apoptosis (2, 12). However, the mechanisms by which REST regulates these oncogenic pathways are only partially understood (1, 2).
An understanding of the mechanisms that promote tumor growth and metastasis is key to developing effective treatment programs for patients. We previously developed a biomarker assay that identifies a subgroup of aggressive tumors based on immunohistological staining and a gene signature comprised of 24 REST target genes (1). However, there is no targeted therapeutic approach to treat this set of tumors. To identify key effectors that distinguish RESTless from RESTfl tumors, we compared proteomic and genomic expression data from the Cancer Genome Atlas (TCGA) resource (13). This analysis identified insulin receptor substrate 1 (IRS1) as the most highly upregulated protein out of 171 screened signaling molecules in RESTless tumors.
IRS1 is the principal effector of the insulin and insulin-like growth factor (IGF) signaling pathways in breast epithelial cells, and these pathways are known to promote transformation, affect metabolism, and enhance metastasis for breast cancer cells (14–18). Increasing evidence suggests an important role for the type 1 IGF receptor (IGF1R) in modulating malignant phenotypes of breast cancer cells. Here we show that REST directly represses the IRS1 gene; loss of REST causes the upregulation of IRS1 mRNA and protein in multiple breast cancer cell lines. We find that enhanced IRS1 function is both necessary and sufficient to confer increased downstream signaling and growth in cells lacking REST.
MATERIALS AND METHODS
Cell lines and culture.
All cell lines were purchased from and authenticated by the American Type Culture Collection and grown at 37°C in 5% CO2. MCF7 and MDA-MB-231 cells were grown in Dulbecco's modified Eagle's medium (DMEM), while ZR751 cells were grown in RPMI with l-glutamine and 4.5 g/liter glucose (Mediatech). All cell lines were supplemented with 10% fetal bovine serum (FBS) (Gibco) and penicillin (100 IU/ml), streptomycin (100 μg/ml), and amphotericin B (250 ng/ml) (Mediatech). Phenol red-free DMEM (PRFM) was used in all growth and migration assays. Insulin-like growth factor (IGF; GroPep Bioreagents) was added to growth media at the concentrations indicated. For all signaling analyses, cells were seeded in phenol red-free medium for 2 days, deprived of serum for overnight, and treated with IGF for 10 min and 2 h for MCF7 and MDA-MB-231 cells, respectively, prior to harvesting of lysates for immunoblot analysis.
Retroviral knockdown and overexpression.
RESTnorm cells were transduced with nontargeting short hairpin RNA (shRNA), while RESTlow cells were transduced with two different sets of shRNA targeting REST. REST knockdown in MDA-MB-231 and ZR751 cells was conducted by using shRNA in the pLKO.1 vector system with The RNAi Consortium (TRC) lentiviral packaging from Open Biosystems according to the distributor's instructions. TRC REST shRNA (catalog number TRCN0000014784 or TRCN0000014785) and Addgene plasmid 1864, a gift from David Sabatini, were used as nontargeting scrambled shRNA (19). Viral particles were packaged with plasmids psPAX2 (Addgene plasmid 12260) and pMD2.G (Addgene plasmid 12259) according to the manufacturer's instructions. Viral infections were conducted according to standard Addgene protocols by using psPAX2 (Addgene plasmid 12260) and pMD2.G (Addgene plasmid 12259) viral packaging particles. Stable REST knockdown in MCF7 cells was accomplished with a Dharmacon Smart vector lentiviral shRNA delivery system, as previously described (1, 2). A second stable REST knockdown in MCF7 cells was achieved by using the above-mentioned methods with TRC REST shRNA from Open Biosystems (catalog number TRCN0000014784). shRNA set 1 for both MCF7 and MDA-MB-231 cells was used for all subsequent experiments in this study.
Retroviral expression vectors, pLNCX2-IRS1-HA (IRS1), pLNCX2-IRS1-HA Dominant Negative (dominant-negative form of IRS1 [IRS1-DN]), or pLNCX2-Control (control), were overexpressed in MCF7 cells by using the RetroPack PT67 retroviral packaging system (Clontech) according to the manufacturer's instructions, and virus was packaged as previously described (20).
Transient transfection of small interfering RNA (siRNA) targeted to IRS1 was achieved by using SMARTpool On-Target Plus IRS1 siRNA with the On-Target Plus nontargeting pool for control siRNA from Dharmacon. Cells were transfected with DharmaFECT transfection reagent (Dharmacon) according to the manufacturer's protocol. At 1 day posttransfection, cells received phenol red-free medium (10% charcoal-dextran-stripped fetal bovine serum [C/d FBS]) for 2 days prior to IGF treatment, as described above.
Assembly of expression constructs.
The pLNCX2-IRS1-HA overexpression plasmid was provided by the Laboratory of Adrian Lee (20). The IRS1-DN plasmid was constructed by amplifying the first 1,548 bp of IRS1 from the pLNCX2-IRS1-HA plasmid, which eliminates residues 517 to 1243 of IRS1. The IRS1-DN fragment with a hemagglutinin (HA) tag was then cloned into the empty pLNCX2 vector by using MluI and ClaI restriction enzymes at the multiple-cloning site, and inserts were verified by sequencing. Forward primer 5′-AGCTGGTTTAGTGAACCGTCAGATC-3′ and reverse primer 5′-CTTTCGGAACCGATTATCCAGAT-3′ were used for the amplification of IRS1-DN.
To construct the pGL3 Pro-IRS1RE1 plasmid, the RE-1 site ∼12.4 kb upstream of the transcription start site of IRS1 was amplified from MCF7 genomic DNA (http://genome.ucsc.edu/cgi-bin/hgTracks?db=hg19&position=chr2%3A227648999-227679713&hgsid=413898241_KmxMsJdP8OE4agxyPcmYqQ2GHnYU). Primers used for the amplification of the RE-1 site upstream of IRS1 from genomic DNA were as follows (5′ to 3′): forward primer GATCGTGCTACTGCACTCCA and reverse primer TGCTCGTGCATGTGGATATT. The amplified fragment encompassing the RE-1 site was cloned into pGL3-Promoter (Promega) by using Acc65I and MluI and was confirmed by sequencing.
The REST expression system was generated using the tetracycline-on (Tet-on) system, where transcription of the REST gene is activated in the presence of doxycycline (Dox). PCR amplification of REST from the pMT-REST plasmid was used to construct the Tet-on REST plasmid (8), and enhanced green fluorescent protein (EGFP) was PCR amplified from the pAdTrack plasmid (Addgene plasmid 1604) for the Tet-on GFP plasmid (21). REST and GFP were cloned into the PLVX-TRE3G plasmid (Clontech), using the MluI and BamHI restrictions sites. Stable Tet-inducible constructs were generated according to the manufacturer's directions, using the Lenti-X Ten-On 3G inducible expression system (Clontech) in MDA-MB-231 cells. MCF7 cells were transiently transfected with plasmids by using Lipofectamine LTX (Life Technologies) prior to doxycycline (Dox) treatment. Treatment with 100 μg/ml doxycycline for 24 h overnight in DMEM was used to induce REST expression prior to harvesting of cells for RNA or protein analysis.
Quantitative real-time PCR.
Cells were seeded in triplicate into PRFM for 1 day, followed by serum deprivation for 2 days. RNA was extracted with TRIzol (Invitrogen) according to the manufacturer's protocol and reversed transcribed for quantitative real-time PCR (RT-PCR) as previously described (2). The primer set consisting of forward primer 5′-CAGAGGACCGTCAGTAGCTCAA-3′ and reverse primer 5′-GGAAGATATGAGGTCCTAGTTGTGAAT-3′ was used for the amplification of IRS1. The primer set consisting of forward primer 5′-GCCCCGCGAGCACAGAG-3′ and reverse primer 5′-CACGATGGAGGGGAAGAC-3′ was used to amplify actin.
Western immunoblotting and chromatin immunoprecipitation.
Cells were harvested in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) and quantified by using the DC protein assay (Bio-Rad, Hercules, CA). Protein lysates (30 μg) were separated via SDS-PAGE, transferred onto polyvinyl difluoride membranes, and blocked in 5% milk. Antibodies used for Western blotting were REST (catalog number 07-579; Millipore), IRS1 (catalog number 06-2648; Upstate), phospho-IRS1 (Tyr612) (catalog number 44816G; Invitrogen), actin (catalog number 691001; MP Biomedicals), and HA (catalog number sc-7392; Santa Cruz Biotechnology) antibodies. IGF1R (catalog number 9750), IRS2 (catalog number 4502), insulin receptor (IR) (catalog number 3025), IRS1 N terminus (catalog number 3407), AKT (catalog number 9272), phospho-AKT (Thr308) (catalog number 9275), extracellular signal-regulated kinase (ERK) (catalog number 4695), and phospho-ERK (Thr202/Tyr204) (catalog number 4370) antibodies were purchased from Cell Signaling Technology.
Chromatin immunoprecipitation (ChIP) was conducted as previously described (2, 8). ChIP was conducted by using 2 μg of REST antibody (Millipore) or rabbit IgG (Sigma) with 300 μg of protein. The primer set for IRS1 was forward primer 5′-GCTTTGGTAGTTCCCCTTCC-3′ and reverse primer 5′-TGCTCGTGCATGTGGATATT-3′. Primers for brain-derived neurotrophic factor (BDNF) were forward primer 5′-TTACAGCGCGGCCAAGAAGACTAC-3′ and reverse primer 5′-CCATCCGCACGTGACAAACC-3′. Primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were forward primer 5′-CTCCCTGAGGGTTCTTTG-3′ and reverse primer 5′-ATCTTACGGAGGCCTCGC-3′.
Luciferase reporter assay.
MCF7 cells were seeded in triplicate in 24-well plates at 5,000 cells/well for cotransfection of pGL3-Promoter or pGL3-ProIRS1RE1 with RL-TK renilla (Promega), using Lipofectamine LTX (Invitrogen). At 2 days posttransfection, cells were lysed and measured by using the Dual-Luciferase reporter assay system (Promega).
Anchorage-independent growth.
MDA-MB-231 and MCF7 cells were starved in phenol red-free medium with 10% C/d FBS 2 days prior to seeding in agar. For all soft-agar assays, phenol red-free medium agar with 10% C/d FBS consisting of equal parts of 2× DMEM from powder (Corning) and a 1.2% prewarmed agar solution (catalog number BP1423; Fisher Scientific) was prepared for the base (0.6%) at 2 ml per well in a 6-well plate. Cells at 10,000 cells per well were seeded in 0.3% medium agar over the base layer, and 2 ml of phenol red-free DMEM (10% C/d FBS) was added as a feeder layer, which contained the following drugs: dimethyl sulfoxide (DMSO), 10 μM LY294002 (AdipoGen), 10 μM U0126 (Tocris, Bristol, United Kingdom), 1 μM OSI-906 in MCF7 cells or 20 μM OSI-906 in MDA-MB-231 cells (Selleck Chemicals), or 10 μM AG1478 (Cayman Chemical Co). The top feeder layer was replenished every 3 days, and colonies were allowed to form for 2 to 3 weeks. Colonies were stained with 0.005% crystal violet, imaged, and quantified by using ImageQuant LAS 4000 and ImageQuantTL software (GE Healthcare Life Sciences). For quantification, one colony was scored as a group of 50 or more cells. Experimental replicates were analyzed for statistical significance, using raw values for each individual experiment. Data from each experiment were subsequently normalized to the control in order to combine experiments for analysis of variance (ANOVA).
Monolayer culture.
To measure cell growth, cells were seeded into phenol red-free DMEM with 10% C/d FBS at 10,000 cells per well in a 96-well plate. For IGF treatments, the medium was replaced the next day with phenol red-free/serum-free DMEM in the presence of IGF for 3 days. The cell number was quantified by using by a crystal violet or MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay as previously described (22, 23).
Cell migration.
Cell migration was tested in blind-well chemotaxis chambers containing 8-μm uncoated, polycarbonate membranes (Neuro Probe Inc.). Cells were starved overnight in phenol red-free/serum-free DMEM (PRFM). IGF (200 ng/ml) or 10% FBS in serum-free PRFM was added to the bottom chamber as a chemoattractant, and cells in serum-free PRFM were added to the upper chamber at 20,000 cells in 0.2 ml per chamber. Migration was assessed 24 h later by scraping cells that adhered to the top of the membrane, followed by fixation of the membrane in methanol for 15 min and staining of the cells on the bottom of the membrane with 1% crystal violet in 50% methanol for 15 min. After washing with water, the membranes were imaged, and cells were quantified in ImageJ. Although experimental replicates were first analyzed for statistical significance by using raw values for each individual experiment, data were subsequently relativized in order to combine experiments for ANOVA.
RESULTS
IRS1 protein is upregulated in RESTless tumors.
To identify the most functionally relevant growth mechanisms in patients with breast cancer lacking REST, we compared gene and protein lists using the Cancer Genome Atlas (TCGA) breast invasive carcinoma data set (https://tcga-data.nci.nih.gov/docs/publications/brca_2012/) (13). The data set of TCGA is a multiplatform analysis of 825 tumors that allowed us to compare the transcriptomes and proteomes in tumors with and those without REST. We previously developed a RESTless gene signature, which consists of a list of 24 REST target genes whose expression is significantly increased upon REST loss. This gene signature detects the loss of REST function, regardless of the mechanism by which REST itself is lost (1). To compare breast tumors that expressed REST with those that had lost REST function, we performed unsupervised hierarchical clustering of all estrogen receptor-positive (ER+) tumors using the RESTless gene signature (Fig. 1A). This is a conservative approach that underestimates the number of tumors that lack REST. This method also highlights only ER+ tumors, although immunohistochemical staining with a REST antibody highlights 50% of triple-negative tumors as being RESTless (1). Nevertheless, this method allows the identification, using the 24-gene signature, of the following subset of ER+ tumors that lack REST function from whole-genome expression data sets: TCGA-AO-A03T, TCGA-BH-A0DE, TCGA-AO-A0J3, TCGA-E2-A15R, TCGA-AO-A0J7, TCGA-A8-A08J, TCGA-D8-A13Y, TCGA-BH-A0B8, TCGA-BH-A18L, TCGA-A1-A0SK, TCGA-A8-A09R, TCGA-E2-A14T, TCGA-C8-A1HG, TCGA-B6-A0X4, TCGA-AR-A0U2, TCGA-E2-A105, TCGA-A8-A08I, TCGA-A8-A082, TCGA-BH-A0HN, and TCGA-AO-A12B. These tumors were used for reverse-phase protein array (RPPA) analysis.
FIG 1.
Protein expression in tumors lacking REST. (A) ER+ tumors were subjected to unsupervised hierarchical clustering using Euclidean distance with the 24-gene RESTless gene signature. The heat map shows a group of tumors clustered together that exhibited high expression levels of the majority of RESTless signature genes (labeled). (B) GSEA on the above-mentioned tumors in TCGA demonstrates a robust enrichment of REST target genes in those samples labeled “RESTless” (P < 10−3; false discovery rate [q] < 10−3; enrichment score [ES] = 0.466; normalized ES [NES] = 2.258). (C) Interaction network of upregulated proteins (displayed by EMBL STRING as gene symbols). The confidence view (left) shows stronger associations by thicker lines. Interactions in the interaction view (right) are indicated as follows: red lines, inhibitory; blue lines, binding; yellow lines, coexpressed; yellow dots, directionality of interaction with unknown action. (D) Schematic of MEK/ERK and PI3K/AKT pathways downstream of IRS1.
To further characterize this putative group of RESTless tumors, we performed a gene set enrichment analysis (GSEA) of the two tumor groups using a list of REST target genes (24). The gene list is derived from a ChIP sequencing (ChIP-seq) experiment described previously by Johnson et al., which we previously demonstrated could parse RESTless from RESTfl tumors (1, 4). After removing the genes in the REST gene signature and restricting genes in the data set of TCGA, GSEA with the restricted list yielded a robust GSEA signal (for 615 genes) (Fig. 1B). This result confirms that there is a significant enrichment of REST target genes in the group of tumors highlighted by the RESTless gene signature in the database of TCGA.
The data set of TCGA is associated with a RPPA platform that allowed us, for the first time, to evaluate changes in protein and signal transduction in RESTless tumors. We performed rank product analysis on the RPPA to uncover those signal transduction components most differentially expressed between RESTless and RESTfl tumors, which are listed in Table 1. Of the 171 proteins and phosphoproteins on the RPPA platform, IRS1 was the most robustly upregulated protein in RESTless tumors (2.21-fold at a P value of 6.4 × 10−4). STRING analysis (http://string-db.org/), which highlights known functional or physical interactions between genes, shows robust associations of 8 proteins from Table 1, suggesting that the IGF1R/IRS1 pathway is systematically changed in RESTless breast cancer (Fig. 1C). Importantly, IRS1 and IGF1R are upstream of signaling cascades involved in cell growth, metabolism, metastasis, and survival (Fig. 1D) (14, 18, 20, 25–32). IRS1 (total protein) was the only member of the IRS family spotted on the protein array, precluding conclusions regarding the other IRS proteins.
TABLE 1.
Upregulated proteins in RESTless tumors as determined by RPPA analysis
| Gene symbol | Protein | RPPA antibody | Fold changea |
|---|---|---|---|
| IRS1 | Insulin receptor substrate 1 | IRS1 | 2.21** |
| IGFBP2 | Insulin-like growth factor binding protein 2 | IGFBP2 | 2.01** |
| EIF4EBP1 | Eukaryotic translation initiation factor 4E binding protein 1 | 4EBP1 | 1.91** |
| CLDN7 | Claudin 7 | Claudin 7 | 1.85* |
| CCND1 | Cyclin D1 | Cyclin D1 | 1.84** |
| TAZ | Tafazzin | pTAZS89 | 1.75* |
| MSH2 | MutS protein homolog 2 | MSH2 | 1.68* |
| EIF4EBP1 | Eukaryotic translation initiation factor 4E binding protein 1 | p4EBP1T70 | 1.66* |
| RB1 | Retinoblastoma | pRbS807/S811 | 1.65* |
| BRAF | Rapidly accelerated fibrosarcoma | B-Raf | 1.63* |
| XRCC1 | X-ray repair cross-complementing protein 1 | XRCC1 | 1.62* |
| RPS6KB1 | Ribosomal protein S6 kinase, 70 kDa, polypeptide 1 | p70S6K | 1.61* |
| IGF1R | Insulin-like growth factor 1 receptor | IGF1Rbeta | 1.57* |
| MAPK9 | Mitogen-activated protein kinase 9 | JNK2 | 1.48* |
| EIF4EBP1 | Eukaryotic translation initiation factor 4E binding protein 1 | p4EBP1T37 | 1.45* |
*, P value of <0.05; **, P value of <0.005.
Five of the upregulated proteins from Table 1 were not connected to the IRS1 hub in STRING (i.e., designated “orphan nodes”), including CLDN7, TAZ, MSH2, XRCC1, and BRAF. The orphan nodes were not studied extensively here but may correlate with a variety of processes important for tumor advantage. For example, both MSH2 and XRCC1 are involved in DNA repair and stability (33–35). CLDN7 regulates tight-junction formation to hamper lipid and membrane protein diffusion (36). TAZ is involved in cardiolipin metabolism, and its expression has been observed to correlate with tumor invasiveness (37). Interestingly, TAZ overexpression in MCF10A cells causes cell migration and invasion, and knockdown of TAZ in MCF7 cells reduces anchorage-independent growth and tumorigenesis in nude mice (37). Finally, BRAF is a proto-oncogene involved in directing cell growth (38). Although these orphan nodes did not connect in the STRING analysis, they are still significantly upregulated in RESTless tumors and could potentially play a key role in mediating tumor aggression in this cohort. In this study, however, we focused on those nodes that formed a network, which was clustered around the IGF1R/IRS1 pathway.
REST directly represses IRS1.
Given that REST is a transcriptional repressor, we hypothesized that one or more genes encoding the proteins in Table 1 are directly repressed by REST and become upregulated upon the loss of REST. We compared the list of genes in Table 1 to genes containing functional RE-1 sites as judged by ChIP-seq performed previously by Johnson et al. (4). The only gene with a RE-1 site encoding a differentially expressed protein in the RPPA analysis was the IRS1 gene. Inspection of the IRS1 locus by using the UCSC Genome Browser (version GRCh37/hg19; February 2009) showed a very strong ChIP signal (at the maximal capped-score value of 1,000) 12,425 bp upstream of the annotated IRS1 promoter in all 10 cell lines assayed (A549, GM12878, hESC, HeLa, HepG2, K562, PANC-1, PFSK-1, SK-N-SH, and U87) (http://genome.ucsc.edu/cgi-bin/hgTracks?db=hg19&position=chr2%3A227675540-227676345&hgsid=427546677_d3c7eaUSydyvUT4nEPfiwGaXg2KP).
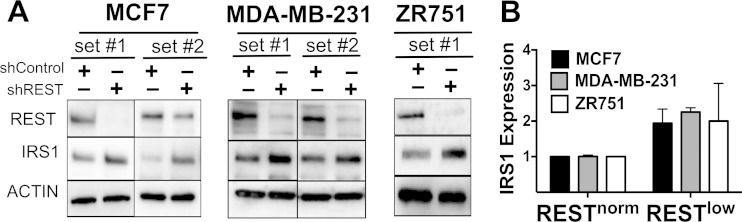
Because IRS1 was the most significantly upregulated protein in Table 1, we tested the hypothesis that the loss of REST is sufficient to derepress IRS1 expression. We knocked down REST in cell lines using lentiviral delivery of REST shRNA. Anti-REST shRNA (to generate RESTlow cells) showed increased levels of IRS1 (∼2×) in MCF7 (ER+), MDA-MB-231 (estrogen receptor-negative [ER−]), and ZR751 (ER+) cells (Fig. 2). These results are consistent with the hypothesis that IRS1 is upregulated in RESTless breast tumors due to a loss of REST-mediated repression.
FIG 2.
Loss of REST leads to increased IRS1 protein levels. (A) Western blot images showing REST and IRS1 expression in MCF7 (ER+), MDA-MB-231 (ER−), and ZR751 (ER+) cells. (B) Quantifications of relative protein expression depicted in a graph (for shRNA set 1), expressed as fold changes compared to levels in RESTnorm cells (n = 3).
Since IRS1 protein became upregulated upon REST loss and since IRS1 has a RE-1 site 12.4 kb upstream of the promoter (Fig. 3A), we predicted that REST directly represses IRS1 transcription. We confirmed REST binding to the IRS1 RE-1 site in both the MCF7 and MDA-MB-231 cell lines by ChIP and observed significant enrichment of the IRS1 RE-1 binding site in both cell types (Fig. 3B and C). Indeed, the ChIP signal at IRS1 was similar to that found at BDNF, a well-characterized REST target gene (2, 39).
FIG 3.
REST directly represses IRS1. (A) Primer sites flanking the RE-1 site positioned 12.4 kb upstream of the IRS1 promoter were used for ChIP and RE-1 reporter assays. (B and C) ChIP of REST at GAPDH, BDNF, and IRS1 in MCF7 (B) and MDA-MB-231 (C) cells (n = 3). (D) Luciferase reporter assay with pGL3Pro-IRS1RE1 (RE-1 site) or pGL3Pro (empty) transfected in MCF7 cells. A two-way ANOVA was used for statistical analysis (n = 3) (***, P = 0.0008; n.s., not significant). (E to G) IRS1 mRNA levels are higher in RESTlow MCF7 (n = 15) (E), MDA-MB-231 (n = 3) (F), and ZR751 (n = 6) (G) breast cancer cells. Data were analyzed with an unpaired, two-sided t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To test whether the IRS1 RE-1 site is functional at a heterologous promoter in our cell lines, we conducted a luciferase reporter assay. A luciferase reporter with the RE-1 site upstream of the simian virus 40 (SV40) promoter (pGL3Pro-IRS1RE1) or the parent luciferase reporter (pGL3-Pro) was transfected into RESTnorm or RESTlow MCF7 cells. Lower luciferase activity was observed in RESTnorm cells that were transfected with pGL3Pro-IRS1RE1 than in cells that were transfected with pGL3Pro. Cells lacking REST generated similar reporter signals with both plasmids (Fig. 3D). These results show that the IRS1 RE-1 site confers repression on a heterologous promoter in a REST-dependent manner. We hypothesized that if IRS1 is a direct REST target, the transcript levels of IRS1 would be higher in cells lacking REST. Indeed, MCF7, MDA-MB-231, and ZR751 cells had significantly higher IRS1 transcript levels upon the loss of REST (Fig. 3E to G).
The above-described results suggest that REST loss is sufficient to induce IRS1 expression. To test whether REST overexpression is sufficient to repress IRS1, we used a Tet-on REST expression system to increase REST protein levels in cells. In MDA-MB-231 cells harboring a Dox-inducible REST construct, the addition of doxycycline increased REST mRNA levels 2.5-fold and reduced IRS1 mRNA levels to less than half of control levels (Fig. 4A). However, despite having reduced IRS1 mRNA levels with REST overexpression, IRS1 protein levels were only marginally reduced (Fig. 4B). These results suggest that a loss of REST is sufficient to increase IRS1 mRNA and protein levels, whereas a gain of REST (while suppressing IRS1 mRNA) fails to reduce protein levels. This implies that there are other mechanisms that prevent a reduction of IRS1 protein levels in response to decreased mRNA levels. Induction of REST expression increased REST occupancy at the IRS1 RE-1 site (Fig. 4C). These data show that endogenous IRS1 transcript levels are dependent on REST levels in MDA-MB-231 cells.
FIG 4.
Induced REST expression represses IRS1 mRNA in MDA-MB-231 cells but not in MCF7 cells. (A) REST and IRS1 mRNAs extracted from MDA-MB-231 cells expressing Tet-inducible REST. Significance was determined by using a t test (n = 3). (B) Western blotting of MDA-MB-231 cells expressing Tet-REST treated with 100 μg/ml doxycycline for 24 h. (C) ChIP of REST or IgG to determine REST occupancy at IRS1 in Tet-induced REST MDA-MB-231 cells treated with doxycycline (n = 3). (D) Same as panel A but with MCF7 cells (n = 3). (E) Western blot of MCF7 cells expressing Tet-REST treated with 100 μg/ml doxycycline for 24 h. (F) Same as panel C but with MCF7 cells (n = 3).
Using the same Tet-on REST expression system, we also evaluated whether REST overexpression reduced IRS1 mRNA and protein levels in MCF7 cells. Upon REST induction, neither IRS1 transcript nor protein levels showed a significant reduction (Fig. 4D and E). We hypothesized that REST occupancy at the IRS1 promoter may be “saturated,” such that overexpression of REST does not cause any further loading at RE-1, thereby resisting significant reductions in mRNA or protein levels. Overexpression of REST with doxycycline treatment did not increase the ChIP signal at IRS1 compared to that of untreated cells (Fig. 4F). We likely did not observe reduced IRS1 transcript and protein levels upon REST overexpression in MCF7 cells because REST is already maximally bound at the IRS1 RE-1 site. Therefore, REST loss in MCF7 cells is sufficient to induce IRS1 mRNA and protein, but overexpression is not sufficient to reduce IRS1 expression. Together, these results suggest that IRS1 is a direct REST target gene, and IRS1 becomes derepressed upon REST loss. Importantly, these data suggest that a loss of REST in human breast tumors is directly linked to the upregulation of IRS1, a potent oncoprotein.
Cells lacking REST have increased IGF1R/IRS1 signaling.
IRS adaptor proteins are important for modulating the cellular response to IGF (40, 41), and increased levels of IRS1 have been observed to sensitize cells to lower levels of IGF for signaling and growth (42). To study the functional activity of increased IRS1 levels in the absence of REST, we monitored downstream signaling by examining AKT and ERK phosphorylation (26, 43–47). Both MCF7 and MDA-MB-231 cells were used to examine signaling in response to IGF. We employed multiple time points and doses to observe differences in the activation of downstream signaling in RESTnorm and RESTlow cells. MCF7 cells required treatment with 1 or 10 ng/ml IGF to observe increased levels of pIRS1Y612, pAKTT308, and pERKT202/T204 in RESTlow cells compared to RESTnorm cells (Fig. 5A, compare lanes 3 and 6). We also tested RESTlow MDA-MB-231 cells, which showed increased levels of phosphorylation at pIRS1Y612 and pAKTT308 in response to 20 ng/ml IGF compared to those in RESTnorm cells (Fig. 5B, compare lanes 2 and 5). However, pERKT202/Y204 was constitutively activated regardless of IGF addition (these cells contain mutant K-Ras [48]) and did not respond to the loss of REST. Together, these results suggest that REST loss promotes enhanced signaling in response to IGF in breast cancer cells, independent of hormone receptor status.
FIG 5.
Cells lacking REST have enhanced signaling in response to IGF. Shown are Western blot images from lysates of RESTnorm and RESTlow cells treated with IGF for 10 min for MCF7 cells (A) or for 2 h for MDA-MB-231 cells (B) (n = 3).
IGF1R/IRS1 signaling is required for enhanced growth in cells lacking REST.
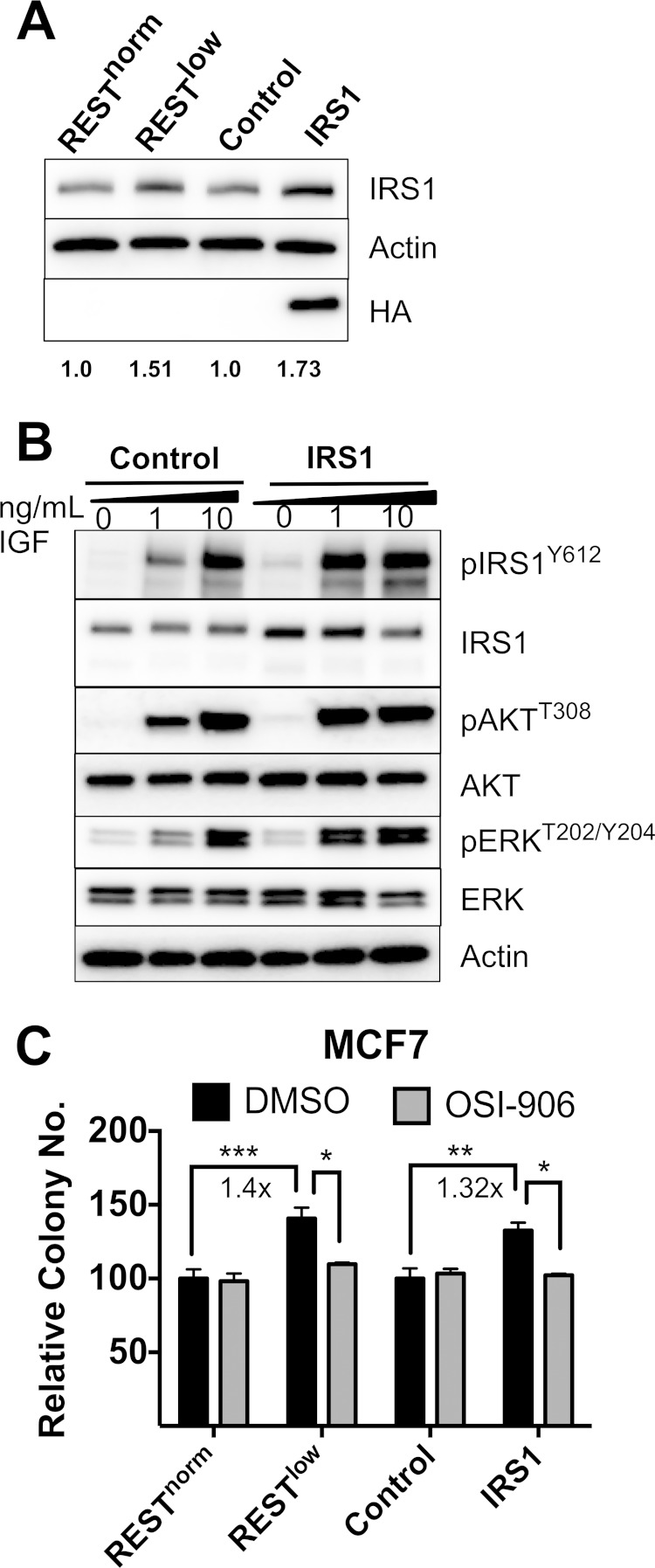
We previously showed that a loss of REST in breast cancer cells conferred an increased plating efficiency in focus formation assays and enhanced anchorage-independent growth in multiple breast cancer cell lines (2). We used this assay to analyze whether increased signaling downstream of IGF1R contributes to RESTlow cell growth (Fig. 6A). In soft agar, RESTlow MCF7 cells gave rise to >2-fold more colonies than did RESTnorm cells (Fig. 6B and D). Treatment with the IGF1R tyrosine kinase inhibitor OSI-906 and the epidermal growth factor receptor (EGFR) inhibitor AG1478 inhibited this enhanced growth. Neither antagonist impacted the colony formation of RESTnorm MCF7 cells, demonstrating specific requirements for both IGFR and EGFR signaling in cells lacking REST.
FIG 6.
Heightened signaling of PI3K/AKT and MAPK/ERK is required for enhanced growth of RESTlow cells. (A) Inhibitors of the IGF1R/IRS1 pathway. (B and C) Soft-agar growth with inhibitor treatment in RESTnorm and RESTlow MCF7 (n = 27) (B) or MDA-MB-231 (n = 18) (C) cells. A two-way ANOVA was used to determine significance. Relative colony numbers are expressed as the proportion of colonies compared to that of DMSO-treated RESTnorm cells. Significance is annotated relative to DMSO treatment. *, P < 0.05; **, P < 0.005; ***, P < 0.001; †, P < 0.0001. (D) Representative pictures of colonies from panels B and C. (E and F) Western blotting of MCF7 cells (n = 2) (E) or MDA-MB-231 cells (n = 3) (F) treated with OSI-906 for 30 min prior to stimulation with IGF. (G) Quantitation of pERK levels from OSI-906 and IGF treatments of MDA-MB-231 cells (n = 3).
To test the hypothesis that enhanced signaling downstream of IRS1 is necessary for RESTlow colony formation, the experiment was repeated in the presence of the phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 or the MEK inhibitor U0126 (Fig. 6B). Both inhibitors eliminated enhanced colony formation in RESTlow cells, with little effect on RESTnorm cells. Pretreatment with OSI-906 also diminished downstream IGF-mediated activation of pIRS1Y612, pAKTT308, and pERKT202/Y204 in MCF7 cells (Fig. 6E). Together, these results are consistent with the hypothesis that IGF1R signaling through IRS1 and subsequent PI3K/AKT and MAPK/ERK activation are necessary for the growth advantage observed in cells lacking REST.
We also performed a soft-agar colony formation assay with MDA-MB-231 cells. RESTlow MBA-MD-231 cells had a nearly 2-fold increase in the number of colonies compared to RESTnorm cells (Fig. 6C and D). As with MCF7 cells, treatment with OSI-906 eliminated the differential colony formation efficiency of RESTlow cells. However, EGFR inhibition failed to impact RESTlow colony formation, suggesting that IGF1R but not EGFR signaling is necessary for enhanced RESTlow colony formation. Both LY294002 and U0126 eliminated the RESTlow colony formation advantage over RESTnorm cells.
Figure 6F shows that IGF1R inhibition with OSI-906 blocks the enhanced activation of pIRS1Y612 and pAKTT308 in RESTlow MDA-MB-231 cells in response to IGF. OSI-906 in the absence of IGF reduced pERKT202/Y204 levels in both RESTnorm and RESTlow cells, suggesting that background or basal levels of ERK activation are not dependent on REST. However, OSI-906 in the presence of IGF resulted in a greater reduction of pERKT202/Y204 levels in RESTlow cells than in RESTnorm cells (Fig. 6F and G). This result suggests that ERK activation becomes more dependent on ligand-bound IGF1R activity in the absence of REST.
IRS1 function is necessary and sufficient for enhanced growth of RESTlow cells.
We next tested whether the high IRS1 levels observed in RESTlow cells were sufficient to drive increased levels of signaling downstream of IRS1. Others have previously reported enhanced PI3K/AKT and MEK/ERK signaling, as well as increased soft-agar colony formation, in the presence of exogenous IRS1 overexpression (41, 42). We stably overexpressed IRS1 in MCF7 cells using a vector containing HA-tagged IRS1 (MCF7-IRS1) at levels comparable to those found in RESTlow MCF7 cells (Fig. 7A). MCF7-IRS1 cells showed increased levels of pIRS1Y612, pAKTT308, and pERKT202/Y204 (Fig. 7B) when treated with 1 ng/ml of IGF, while MCF7 control cells required 10 ng/ml of IGF to induce similar levels of phosphorylation. This result supports the hypothesis that overexpression of IRS1 is sufficient to explain the enhanced IGF response, as observed from increased levels of IRS1, AKT, and ERK phosphorylation. Moreover, when MCF7-IRS1 cells were seeded into soft agar, they gave rise to a similar number of colonies as RESTlow MCF7 cells (Fig. 7C). Therefore, IRS1 overexpression is sufficient to enhance anchorage-independent growth to levels similar to those observed in cells lacking REST.
FIG 7.
Overexpression of IRS1 is sufficient to enhance signaling and growth in soft agar. (A) Western blot showing overexpression of IRS1-HA in MCF7 cells (pLNCX-IRS1-HA) alongside an empty vector control (pLNCX2-control). (B) Western blot of MCF7-IRS1 cells treated with IGF. (C) Soft-agar assay with MCF7-IRS1 cells and RESTlow MCF7 cells with OSI-906 treatment. Significance was determined by two-way ANOVA (n = 3). Relative colony numbers are expressed as the proportion of colonies compared to that of DMSO-treated RESTnorm cells (***, P < 0.001; **, P < 0.01; *, P < 0.05).
Upon receptor phosphorylation, IRS1 is subsequently phosphorylated at the C terminus and becomes the docking site for SH-2-containing proteins, such as p85 and Grb2, which activate PI3K/AKT and MAPK/ERK signaling, respectively (reviewed in reference 28). To determine whether elevated IRS1 levels are necessary for increased growth and signaling in RESTlow cells, we expressed a dominant negative form of IRS1 (IRS1-DN) (Fig. 8A). This IRS1-DN construct lacks C-terminal amino acids 517 to 1243 and can no longer act as a docking site for proteins to initiate downstream signaling to PI3K/AKT or MAPK/ERK. Exogenous expression of truncated IRS1 acts as a signaling sink by binding to receptors but preventing tyrosyl phosphorylation of endogenous IRS1, such that subsequent downstream activation of molecules is inhibited (49). Both MCF7 and MDA-MB-231 cells were transduced with the IRS1-DN expression vector containing a HA tag (Fig. 8B). Full-length IRS1 migrated as a molecular mass of ∼185 kDa by SDS-PAGE, and IRS1-DN migrated at ∼70 kDa (as observed by HA tag Western blotting). IRS1-DN levels in cells were high relative to endogenous levels, as observed via Western blot analysis with an IRS1 antibody raised to the N terminus (data not shown). Although endogenous levels of IRS1 in MCF7 cells were reduced upon the introduction of IRS1-DN, there was little to no reduction in IRS1 levels in MDA-MB-231 cells.
FIG 8.
IRS1 activity is necessary for enhanced signaling and growth in MCF7 and MDA-MB-231 cells. (A) Schematic of protein domains in the IRS1-DN construct compared to full-length IRS1. The domains conserved in the IRS1-DN are the pleckstrin homology (PH) domain and the phosphotyrosine binding (PTB) domain. (B) Western blot showing IRS1-DN with a HA tag or the empty vector (control) stably expressed in MCF7 and MDA-MB-231 cells with or without REST. (C and D) Western blot showing lysates from control or IRS1-DN MCF7 (C) and MDA-MB-231 (D) cells in the presence (+) or absence (−) of REST that were treated with IGF (n = 3). (E and F) Relative colony numbers of RESTnorm or RESTlow MCF7 (n = 6) (E) or MDA-MB-231 (n = 9) (F) cells expressing IRS1-DN with OSI-906 treatment, expressed as the proportion of colonies compared to that of DMSO-treated RESTnorm control cells. Significance was determined by two-way ANOVA. (G and H) siRNA directed to IRS1 or nontargeting siRNA was transfected into cells prior to stimulation with IGF to measure downstream signaling in MCF7 (G) or MDA-MB-231 (H) cells.
RESTnorm and RESTlow cells expressing IRS1-DN were evaluated for IRS1 activity in the presence of IGF. The expression of IRS1-DN abrogated the enhanced levels of pIRS1Y612 in both MCF7 and MDA-MB-231 cells (Fig. 8C and D; see also below). Using MCF7 and MDA-MB-231 cells expressing IRS1-DN, we tested whether IRS1 activity was necessary for the anchorage-independent growth advantage in RESTlow cells. IRS1-DN eliminated the enhanced colony formation of RESTlow cells without impacting colony formation in RESTnorm cells (Fig. 8E). RESTlow MCF7 cells were specifically sensitive to OSI-906, and cells expressing IRS1-DN demonstrated no further decrease in growth with the IGF1R antagonist. The requirement for IRS1 was also tested in RESTlow MDA-MB-231 cells by introducing IRS1-DN (Fig. 8F). The preferential colony formation of RESTlow MDA-MB-231 cells was abolished in the presence of IRS1-DN.
To test the importance of IRS1 in downstream signaling, we knocked down IRS1 via siRNA in both MCF7 and MDA-MB-231 cells and examined signaling downstream of IGF1R/IRS1 in RESTnorm and RESTlow cells. Anti-IRS1 siRNA (siIRS1) reduced total and phospho-IRS1 levels, whereas scrambled siRNA did not (Fig. 8G). In scrambled-siRNA-treated cells, IGF elicited higher levels of pIRS1, pAKT, and pERK in RESTlow than in RESTnorm MCF7 cells, as observed in Fig. 5A (Fig. 8G). However, siIRS1 abolished this differential activation of AKT and ERK. We also examined the effects of siIRS1 on signaling in MDA-MB-231 cells (Fig. 8H). Similar to the data shown in Fig. 5B, we observed increased levels of pIRS1 and pAKT in RESTlow cells in response to IGF treatment, but these signals were reduced upon knockdown of IRS1 (Fig. 8H). These results show that increased IRS1 levels are necessary for the increased signaling observed in the IGF1R pathway upon REST loss.
REST loss causes cell-type-specific phenotypes.
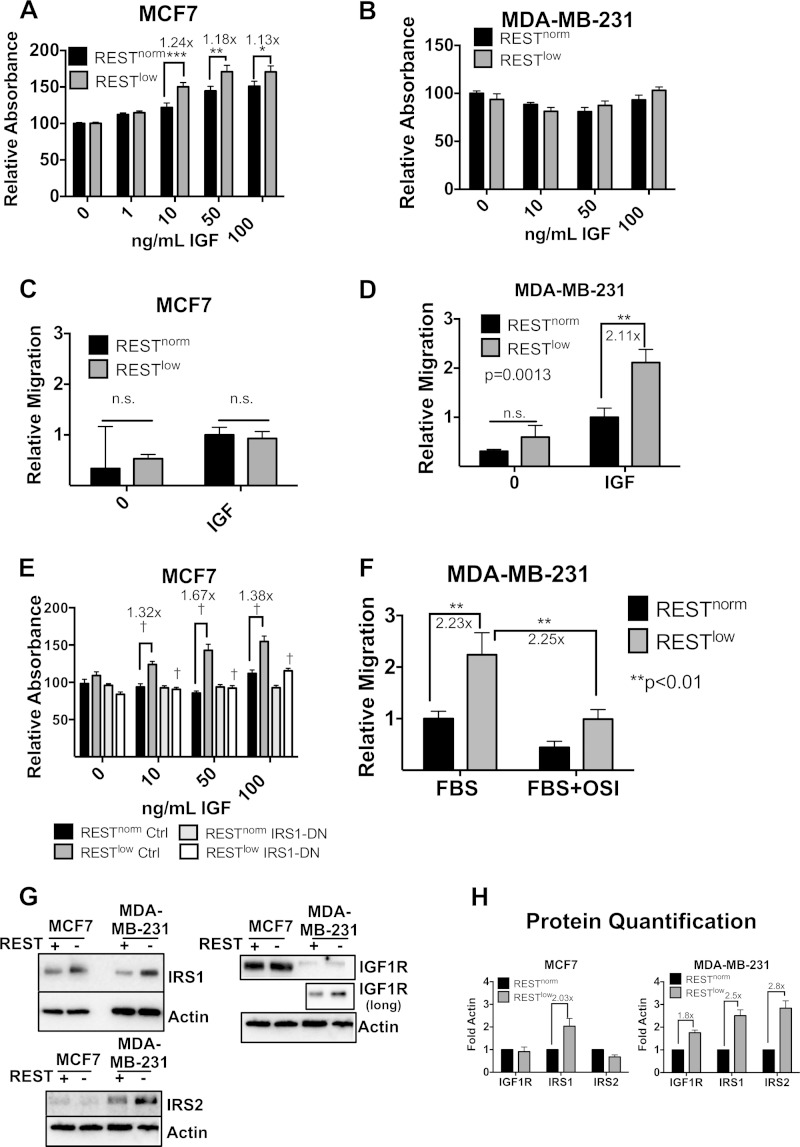
Signaling downstream of IGF1R/IRS1 is important for mediating a number of cellular phenotypes. We tested whether cells lacking REST were sensitized to IGF in monolayer cell growth. In response to a range of IGF concentrations, RESTlow MCF7 cells demonstrated a marginal, but significant (20%), increase in cell number compared to the number of RESTnorm cells in the presence of 10 and 50 ng/ml IGF after 3 days of growth (Fig. 9A). However, MDA-MB-231 cells displayed no significant difference in cell number between RESTnorm and RESTlow cells in response to IGF (Fig. 9B).
FIG 9.
Loss of REST confers cell-type-specific phenotypes. (A and B) Relative absorbance of crystal violet stain representing cell numbers for MCF7 (n = 24) (A) and MDA-MB-231 (n = 18) (B) cells, expressed as a signal proportional to the RESTnorm, untreated signal. Significance was determined by two-way ANOVA. ***, P < 0.001; **, P < 0.01; *, P < 0.05. (C and D) Migration of MCF7 (n = 17) (C) and MDA-MB-231 (n = 12) (D) cells with IGF treatment in chemotaxis chambers. A two-way ANOVA was used for statistical analysis. (E) MTT signal in the presence of IGF in RESTnorm or RESTlow cells expressing IRS1-DN (n = 12). Relative absorbance is presented as the signal relative to that with zero treatment of RESTnorm control cells. A two-way ANOVA was used to determine significance (†, P < 0.0001). (F) Migration of MDA-MB-231 cells using 10% FBS as a chemoattractant with or without 20 μM OSI-906 treatment in the upper chamber (n = 6). A two-way ANOVA was used to determine significance. (G) Western blot showing expression of receptors in MCF7 and MDA-MB-231 cells. The IGF1R (long) blot for MDA-MB-231 cells was imaged separately with a longer exposure to observe expression differences. (H) Data from blots from 4 different experiments were compiled and quantified.
We then evaluated IGF-mediated cell migration in RESTnorm versus RESTlow cells. While very little cell migration was observed in MCF7 cells (Fig. 9C), we found that MDA-MB-231 cells lacking REST manifested a significantly higher migration rate in response to IGF than did control cells (Fig. 9D). Thus, utilization of the IGF1R pathway upon REST loss plays an important role in both ER+ and ER− cells but likely mediates cell-type-specific effects. Whereas REST loss enhances IGF-mediated monolayer growth/survival in MCF7 cells, REST loss confers enhanced migration in MDA-MB-231 cells.
We further evaluated the requirement for IGF1R/IRS1 in the respective cell-specific phenotypes. Figure 9E shows that the number of RESTlow MCF7 cells is increased with IGF treatment, which is eliminated in cells expressing IRS1-DN. Thus, MCF7 cells require IRS1 activity for enhanced IGF-mediated growth or survival in monolayer culture upon the loss of REST. The requirement for the IGF1R pathway in mediating enhanced migration of RESTlow MDA-MB-231 cells was also evaluated. We used 10% FBS as a chemoattractant and evaluated whether IGF1R signaling was necessary for migration, regardless of the growth factors used to attract migratory cells. We observed increased migration in cells lacking REST using 10% FBS as a chemoattractant (Fig. 8F). However, treatment with OSI-906 significantly reduced cell migration in RESTlow cells in the presence of 10% FBS. This suggests that the activation of the IGF1R pathway is required for MDA-MB-231 cell migration upon REST loss.
Given the complex network of interactions involved in regulating IGF1R pathway signaling, we also examined the relative expression levels of the receptors IGF1R and insulin receptor (IR) as well as IRS2. IGF1R can heterodimerize with IR to activate downstream signaling via IRS1 or IRS2 (reviewed in reference 32). We observed no difference in IR protein levels in RESTlow over RESTnorm MCF7 and MDA-MB-231 cells (data not shown). Interestingly, whereas IGF1R displayed a nearly 2-fold-increased level in RESTlow MDA-MB-231 cells, it was invariant in MCF7 cells (Fig. 9G and H). IRS2 manifests a robust induction (2.8×) in protein levels upon REST loss in MDA-MB-231 cells but not in MCF7 cells. Thus, there are cell-specific alterations in IR, IGF1R, and IRS2 upon REST loss, whereas IRS1 is universally induced across the cell types examined here.
DISCUSSION
In this study, we find that IRS1, a REST target gene, is upregulated in human breast cancer as well as multiple breast cancer cell lines. Although there are cell type variations in the expressions of other proteins and receptors in the IGF1R pathway, we have identified IRS1 function as being necessary and sufficient for the enhanced signaling and growth observed in both MCF7 and MDA-MB-231 cells upon REST loss. Using patient data, we validated several genes and proteins that were differentially expressed between RESTless and RESTfl breast cancers and provided a molecular mechanism that, in part, explains how the loss of REST contributes to enhanced cell growth.
IRS1 upregulation is necessary and sufficient for the enhanced growth and signaling pathways in breast cancer cells lacking REST. Overexpression of IRS1 leads to the transformation of mouse embryonic fibroblasts (50) and is required for the full transformation activity of other oncogenes, such as SV40 T antigen in mouse embryonic fibroblasts (51) and Ras in 32D cells, a mouse myeloid progenitor line (52). High expression levels of IRS1 in ER+ breast tumors also correlate with poorer disease-free survival, which is consistent with the prognosis for RESTless breast cancer patients (53). Our results show that IRS1 is upregulated in RESTless human tumors (Table 1) and in multiple breast cancer cell lines upon knockdown of REST (Fig. 2). We find that REST directly represses the IRS1 gene, and the loss of REST leads to increased IRS1 transcript and protein levels.
REST directly represses the IRS1 gene.
IRS1 is the most highly upregulated protein in RESTless tumors observed in the RPPA analysis (see the list of tumors in “IRS1 protein is upregulated in RESTless tumors” above) and is the only protein in the list of differentially expressed proteins whose gene possesses an RE-1 site. IRS1 is also the only IRS isoform with a REST binding site. Upon REST loss, we find that IRS1 transcripts are significantly upregulated in multiple cell lines (Fig. 3E to G) and show that REST directly represses IRS1. Although we observed reduced IRS1 mRNA levels upon overexpression of REST in MDA-MB-231 cells, this reduction does not lead to a concomitant reduction in IRS1 protein levels in MCF7 or MDA-MB-231 cells. This implies that there are other mechanisms that maintain a minimal IRS1 protein complement in cells independent of mRNA abundance.
Although few studies have examined the role of REST in human breast tumors, we previously observed that Lin28A expression is significantly enhanced in human tumors lacking REST (2) and was found to be necessary for enhanced RESTlow cell growth. Interestingly, an important link for the Lin28/let-7 axis in regulating components of the IGF1R/IRS pathway has been reported (54). Lin28, a REST target that becomes upregulated in human breast tumors, regulates let-7 microRNA (miRNA) processing (2, 55, 56). Several genes in the IGF1R pathway have conserved let-7 target sequences, including IGF1, IGF1R, IRS2, PIK3IP1, AKT2, and IGFBP2. The observation of increased IGF1R and IRS2 protein levels in MDA-MB-231 cells supports a role for REST regulation of key components of the IGF1R pathway beyond directly binding to RE-1 sites.
Cells lacking REST acquire increased signaling downstream of IGF1R/IRS1.
In this report, we identify a requirement for signaling downstream of IGF1R for soft-agar growth of both MCF7 and MDA-MB-231 cells lacking REST (Fig. 6). Previous studies have shown that a loss of REST activates AKT (11, 12). In addition, our data show that MCF7 cells lacking REST have increased levels of pIRS1Y612, pAKTT308, and pERKT202/Y204, and MDA-MB-231 cells lacking REST have increased levels of pIRS1Y612 and pAKTT308 (Fig. 5). We further provide a mechanism for the enhanced signaling that we observed upon REST loss. Heightened signaling in the IGF1R pathway, which is important for regulating cell growth, also translates to increased soft-agar colony formation of RESTlow cells (Fig. 6).
IRS1 function is necessary and sufficient for altered signaling and growth in cells lacking REST.
We confirmed that IRS1 was both necessary and sufficient to recapitulate enhanced growth in soft agar and increased signaling downstream of IGF1R in cells lacking REST (Fig. 7 and 8). IGF1R requires insulin receptor substrates to activate biological effects (41), so IRS1 activity in RESTlow cells was reduced through IRS1-DN expression or treatment with OSI-906 to determine the requirement for the IGF1R pathway in mediating signaling and growth. Previous studies found that the introduction of dominant-negative IRS1 into hepatocellular carcinoma cells ablated anchorage-independent growth and tumor formation in nude mice (49). Both OSI-906 treatment and expression of IRS1-DN not only eliminated the heightened phosphorylation of IRS1 in RESTlow cells but also reduced anchorage-independent growth in MCF7 and MDA-MB-231 cells (Fig. 8C to F). This suggests that functional IRS1 is necessary for the growth advantage of RESTlow cells.
We also find that increased levels of IRS1 are necessary for the enhanced signaling found in RESTlow cells. IRS1 knockdown using siRNA reduced pIRS1, pAKT, and pERK signals in MCF7 cells (Fig. 8G and H). In MDA-MB-231 cells, pIRS1 and pAKT signals were reduced with siIRS1, but ERK activity remained the same between RESTnorm and RESTlow cells. However, ERK activity was reduced with OSI-906 and IGF cotreatment to a greater extent in RESTlow cells (Fig. 6F), suggesting that IGF1R may still contribute to ERK activity in MDA-MB-231 cells lacking REST.
REST loss confers cell-specific phenotypes to breast cancer cell lines.
Although we observed increased soft-agar colony formation for both MCF7 and MDA-MB-231 cells lacking REST, upregulation of the IGF1R pathway confers other cell-type-specific phenotypes. Thus, REST loss confers increased monolayer growth only to MCF7 cells and increased migration only to MDA-MB-231 cells. Upon the loss of REST, MDA-MB-231 cells have increased levels of both IRS1 and IRS2 (Fig. 9). While IRS1 activation has typically been associated with the proliferation of breast cancer cells, IRS2 has typically been associated with the motility of breast cancer cells (40, 41, 57, 58). Overexpression of either IRS1 or IRS2 in transgenic mice was recently shown to cause breast tumor metastasis (20). Furthermore, overexpression of either IRS1 or IRS2 has been observed to sensitize breast cancer cells to the promigratory activity of IGF (59), which we have observed in MDA-MB-231 cells lacking REST. Because IRS2 is required for cell migration and has been shown to regulate cell motility and tumor metastasis (41, 58–61), IRS2, in addition to IRS1, likely plays a key role in RESTlow MDA-MB-231 cells. Furthermore, MDA-MB-231 cells lacking REST have enhanced IGF1R expression, which has also been shown to contribute to cell migration (18, 62). Although the cooperation between IRS1 and IRS2 in mediating cell migration is beyond the scope of this study, IGF1R, IRS1, and IRS2 could be involved in mediating cell migration in cells lacking REST, as IGF1R inhibition reduced cell migration.
Clinical significance of REST loss in breast cancer.
Loss of REST function in breast cancer occurs through alternative splicing and may also occur through increased ubiquitination (1, 63). REST loss in human mammary epithelial cells causes cell transformation, and we have identified a key role for REST in regulating tumor aggression (2, 11). Patients with RESTless breast cancer have a poor prognosis, and loss of REST in breast cancer cells leads to enhanced tumor growth in mouse xenografts (2). Our results suggest that the IGF1R/IRS1 pathway in RESTless breast cancer may be a critical pathway to target in breast cancer therapy. Given that inhibition with OSI-906 reduces RESTlow cell growth and signaling across multiple cell types, the IGF1R/IRS1 pathway may be a candidate for targeted therapy of RESTless breast cancer. Mice with estrogen-independent MCF7 xenografts that were cotreated with OSI-906 and fulvestrant showed superior tumor regression compared to mice treated with either drug alone (64). However, IGF1R inhibitors in clinical trials with combination hormonal therapies in second-line breast cancer treatment have not yet shown a clear advantage and have an undefined rationale for use (reviewed in reference 65). Perhaps, specifically targeting tumors lacking REST that exhibit a heightened reliance on IGFR/IRS1 signaling may be of benefit for improving these therapeutic outcomes.
The IGF1R inhibitor ganitumab, alone or in combination with exemestane or fulvestrant, did not increase progression-free survival (66). Effective IGF1R inhibition is clearly contingent on the cell's dependence on IGF1R and important adaptor molecules, such as IRS1. For example, treatment with αIR-3, an IGF1R inhibitor, inhibited cell growth and motility only when IRS1 or IRS2 was expressed, suggesting that adaptor molecules are required to elicit a cellular response to IGF1R inhibition and should be evaluated when selecting patient cohorts for clinical trials (41).
IRS1 expression has been proposed as a functional biomarker for the biological responsiveness of IGF1R (41). Given that we can identify RESTless tumors using a REST biomarker stain, we propose that this stratification may be important for future selection of patients who will respond best to anti-IGF1R therapeutics.
ACKNOWLEDGMENTS
This work was supported by NINDS grant R01 NS065067, NIH training grant 5T32GM008688-14, and postdoctoral grant T32 CA157322, Molecular and Cellular Mechanisms of Tumor Development.
We thank John Newton and the Wisconsin Dual Sports Riders for funding and support. We thank Adrian Lee for the pLNCX2-IRS1 plasmid.
Footnotes
This work is dedicated to Cindy Stegemann and Norma Kalk, who are remembered for their battle against breast cancer.
REFERENCES
- 1.Wagoner MP, Gunsalus KT, Schoenike B, Richardson AL, Friedl A, Roopra A. 2010. The transcription factor REST is lost in aggressive breast cancer. PLoS Genet 6:e1000979. doi: 10.1371/journal.pgen.1000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunsalus KTW, Wagoner MP, Meyer K, Potter WB, Schoenike B, Kim S, Alexander CM, Friedl A, Roopra A. 2012. Induction of the RNA regulator LIN28A is required for the growth and pathogenesis of RESTless breast tumors. Cancer Res 72:3207–3216. doi: 10.1158/0008-5472.CAN-11-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoenherr CJ, Anderson DJ. 1995. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science 267:1360–1363. doi: 10.1126/science.7871435. [DOI] [PubMed] [Google Scholar]
- 4.Johnson DS, Mortazavi A, Myers RM, Wold B. 2007. Genome-wide mapping of in vivo protein-DNA interactions. Science 316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 5.Bruce AW, Donaldson IJ, Wood IC, Yerbury SA, Sadowski MI, Chapman M, Göttgens B, Buckley NJ. 2004. Genome-wide analysis of repressor element 1 silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) target genes. Proc Natl Acad Sci U S A 101:10458–10463. doi: 10.1073/pnas.0401827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tapia-Ramírez J, Eggen BJ, Peral-Rubio MJ, Toledo-Aral JJ, Mandel G. 1997. A single zinc finger motif in the silencing factor REST represses the neural-specific type II sodium channel promoter. Proc Natl Acad Sci U S A 94:1177–1182. doi: 10.1073/pnas.94.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Myers SJ, Dingledine R. 1999. Transcriptional repression by REST: recruitment of Sin3A and histone deacetylase to neuronal genes. Nat Neurosci 2:867–872. doi: 10.1038/13165. [DOI] [PubMed] [Google Scholar]
- 8.Roopra A, Qazi R, Schoenike B, Daley TJ, Morrison JF. 2004. Localized domains of G9a-mediated histone methylation are required for silencing of neuronal genes. Mol Cell 14:727–738. doi: 10.1016/j.molcel.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Roopra A, Sharling L, Wood IC, Briggs T, Bachfischer U, Paquette AJ, Buckley NJ. 2000. Transcriptional repression by neuron-restrictive silencer factor is mediated via the Sin3-histone deacetylase complex. Mol Cell Biol 20:2147–2157. doi: 10.1128/MCB.20.6.2147-2157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, Roopra A. 2006. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci 9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- 11.Westbrook TF, Martin ES, Schlabach MR, Leng Y, Liang AC, Feng B, Zhao JJ, Roberts TM, Mandel G, Hannon GJ. 2005. A genetic screen for candidate tumor suppressors identifies REST. Cell 121:837–848. doi: 10.1016/j.cell.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Lv H, Pan G, Zheng G, Wu X, Ren H, Liu Y, Wen J. 2010. Expression and functions of the repressor element 1 (RE-1)-silencing transcription factor (REST) in breast cancer. J Cell Biochem 110:968–974. doi: 10.1002/jcb.22610. [DOI] [PubMed] [Google Scholar]
- 13.Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, Fulton LL, Dooling DJ, Ding L, Mardis ER, Wilson RK, Ally A, Balasundaram M, Butterfield YSN, Carlsen R, Carter C, Chu A, Chuah E, Chun H-JE, Coope RJN, Dhalla N, Guin R, Hirst C, Hirst M, Holt RA, Lee D, Li HI, Mayo M, Moore RA, Mungall AJ, Pleasance E, Gordon Robertson A, Schein JE, Shafiei A, Sipahimalani P, Slobodan JR, Stoll D, Tam A, Thiessen N, Varhol RJ, Wye N, Zeng T, Zhao Y, Birol I, Jones SJM, Marra MA, Cherniack AD, Saksena G, Onofrio RC, Pho NH, et al. 2012. Comprehensive molecular portraits of human breast tumours. Nature 490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollak M. 2008. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Litzenburger BC, Cui X, Delgado DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM, Carboni JM, Lee AV. 2007. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and Snail. Mol Cell Biol 27:3165–3175. doi: 10.1128/MCB.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arteaga CL, Kitten LJ, Coronado EB, Jacobs S, Kull FC, Allred DC, Osborne CK. 1989. Blockade of the type I somatomedin receptor inhibits growth of human breast cancer cells in athymic mice. J Clin Invest 84:1418–1423. doi: 10.1172/JCI114315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaleko M, Rutter WJ, Miller AD. 1990. Overexpression of the human insulinlike growth factor I receptor promotes ligand-dependent neoplastic transformation. Mol Cell Biol 10:464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachdev D. 2008. Regulation of breast cancer metastasis by IGF signaling. J Mammary Gland Biol Neoplasia 13:431–441. doi: 10.1007/s10911-008-9105-5. [DOI] [PubMed] [Google Scholar]
- 19.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 20.Dearth RK, Cui X, Kim HJ, Kuiatse I, Lawrence NA, Zhang X, Divisova J, Britton OL, Mohsin S, Allred DC, Hadsell DL, Lee AV. 2006. Mammary tumorigenesis and metastasis caused by overexpression of insulin receptor substrate 1 (IRS-1) or IRS-2. Mol Cell Biol 26:9302–9314. doi: 10.1128/MCB.00260-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. 1998. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillies RJ, Didier N, Denton M. 1986. Determination of cell number in monolayer cultures. Anal Biochem 159:109–113. doi: 10.1016/0003-2697(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 23.Twentyman PR, Luscombe M. 1987. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer 56:279–285. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw RJ, Cantley LC. 2006. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 26.Metz HE, McGarry Houghton A. 2011. Insulin receptor substrate regulation of phosphoinositide 3-kinase. Clin Cancer Res 17:206–211. doi: 10.1158/1078-0432.CCR-10-0434. [DOI] [PubMed] [Google Scholar]
- 27.Dearth RK, Cui X, Kim H-J, Hadsell DL, Lee AV. 2007. Oncogenic transformation by the signaling adaptor proteins insulin receptor substrate (IRS)-1 and IRS-2. Cell Cycle 6:705–713. doi: 10.4161/cc.6.6.4035. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi CM, Emanuelli B, Kahn CR. 2006. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 29.Párrizas M, Saltiel AR, LeRoith D. 1997. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J Biol Chem 272:154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- 30.Avruch J. 1998. Insulin signal transduction through protein kinase cascades. Mol Cell Biochem 182:31–48. doi: 10.1023/A:1006823109415. [DOI] [PubMed] [Google Scholar]
- 31.Pollak M. 2012. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Yee D. 2012. Targeting insulin and insulin-like growth factor signaling in breast cancer. J Mammary Gland Biol Neoplasia 17:251–261. doi: 10.1007/s10911-012-9268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, Kane M, Kolodner R. 1993. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 34.Whitehouse CJ, Taylor RM, Thistlethwaite A, Zhang H, Karimi-Busheri F, Lasko DD, Weinfeld M, Caldecott KW. 2001. XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell 104:107–117. doi: 10.1016/S0092-8674(01)00195-7. [DOI] [PubMed] [Google Scholar]
- 35.Fishel R, Ewel A, Lee S, Lescoe MK, Griffith J. 1994. Binding of mismatched microsatellite DNA sequences by the human MSH2 protein. Science 266:1403–1405. doi: 10.1126/science.7973733. [DOI] [PubMed] [Google Scholar]
- 36.Zheng J-Y, Yu D, Foroohar M, Ko E, Chan J, Kim N, Chiu R, Pang S. 2003. Regulation of the expression of the prostate-specific antigen by claudin-7. J Membr Biol 194:187–197. doi: 10.1007/s00232-003-2038-4. [DOI] [PubMed] [Google Scholar]
- 37.Chan SW, Lim CJ, Guo K, Ng CP, Lee I, Hunziker W, Zeng Q, Hong W. 2008. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res 68:2592–2598. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- 38.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W. 2002. Mutations of the BRAF gene in human cancer. Nature 417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 39.Timmusk T, Palm K, Lendahl U, Metsis M. 1999. Brain-derived neurotrophic factor expression in vivo is under the control of neuron-restrictive silencer element. J Biol Chem 274:1078–1084. [PubMed] [Google Scholar]
- 40.Jackson JG, White MF, Yee D. 1998. Insulin receptor substrate-1 is the predominant signaling molecule activated by insulin-like growth factor-I, insulin, and interleukin-4 in estrogen receptor-positive human breast cancer cells. J Biol Chem 273:9994–10003. doi: 10.1074/jbc.273.16.9994. [DOI] [PubMed] [Google Scholar]
- 41.Byron SA, Horwitz KB, Richer JK, Lange CA, Zhang X, Yee D. 2006. Insulin receptor substrates mediate distinct biological responses to insulin-like growth factor receptor activation in breast cancer cells. Br J Cancer 95:1220–1228. doi: 10.1038/sj.bjc.6603354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Surmacz E, Burgaud J-L. 1995. Overexpression of insulin receptor substrate 1 (IRS-1) in the human breast cancer cell line MCF-7 induces loss of estrogen requirements for growth and transformation. Clin Cancer Res 1:1429–1436. [PubMed] [Google Scholar]
- 43.White MF. 1998. The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol Cell Biochem 182:3–11. doi: 10.1023/A:1006806722619. [DOI] [PubMed] [Google Scholar]
- 44.Shepherd PR. 2005. Mechanisms regulating phosphoinositide 3-kinase signalling in insulin-sensitive tissues. Acta Physiol Scand 183:3–12. doi: 10.1111/j.1365-201X.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 45.Swantek JL, Baserga R. 1999. Prolonged activation of ERK2 by epidermal growth factor and other growth factors requires a functional insulin-like growth factor 1 receptor. Endocrinology 140:3163–3169. doi: 10.1210/endo.140.7.6766. [DOI] [PubMed] [Google Scholar]
- 46.Surmacz E. 2000. Function of the IGF-I receptor in breast cancer. J Mammary Gland Biol Neoplasia 5:95–105. doi: 10.1023/A:1009523501499. [DOI] [PubMed] [Google Scholar]
- 47.Peruzzi F, Prisco M, Dews M, Salomoni P, Grassilli E, Romano G, Calabretta B, Baserga R. 1999. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol Cell Biol 19:7203–7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hollestelle A, Nagel JHA, Smid M, Lam S, Elstrodt F, Wasielewski M, Ng SS, French PJ, Peeters JK, Rozendaal MJ, Riaz M, Koopman DG, Ten Hagen TL, de Leeuw BH, Zwarthoff EC, Teunisse A, van der Spek PJ, Klijn JGM, Dinjens WNM, Ethier SP, Clevers H, Jochemsen AG, den Bakker MA, Foekens JA, Martens JWM, Schutte M. 2010. Distinct gene mutation profiles among luminal-type and basal-type breast cancer cell lines. Breast Cancer Res Treat 121:53–64. doi: 10.1007/s10549-009-0460-8. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka S, Wands JR. 1996. A carboxy-terminal truncated insulin receptor substrate-1 dominant negative protein reverses the human hepatocellular carcinoma malignant phenotype. J Clin Invest 98:2100–2108. doi: 10.1172/JCI119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D'Ambrosio C, Keller SR, Morrione A, Lienhard GE, Baserga R, Surmacz E. 1995. Transforming potential of the insulin receptor substrate 1. Cell Growth Differ 6:557–562. [PubMed] [Google Scholar]
- 51.DeAngelis T, Chen J, Wu A, Prisco M, Baserga R. 2006. Transformation by the simian virus 40 T antigen is regulated by IGF-I receptor and IRS-1 signaling. Oncogene 25:32–42. [DOI] [PubMed] [Google Scholar]
- 52.Cristofanelli B, Valentinis B, Soddu S, Rizzo MG, Marchetti A, Bossi G, Morena AR, Dews M, Baserga R, Sacchi A. 2000. Cooperative transformation of 32D cells by the combined expression of IRS-1 and V-Ha-Ras. Oncogene 19:3245–3255. doi: 10.1038/sj.onc.1203664. [DOI] [PubMed] [Google Scholar]
- 53.Rocha RL, Hilsenbeck SG, Jackson JG, VanDenBerg CL, Weng CN, Lee AV, Yee D. 1997. Insulin-like growth factor binding protein-3 and insulin receptor substrate-1 in breast cancer: correlation with clinical parameters and disease-free survival. Clin Cancer Res 3:103–109. [PubMed] [Google Scholar]
- 54.Zhu H, Shyh-Chang N, Segrè AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, Urbach A, Thornton JE, Triboulet R, Gregory RI, Altshuler D, Daley GQ. 2011. The Lin28/let-7 axis regulates glucose metabolism. Cell 147:81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, O'Sullivan M, Lu J, Phillips LA, Lockhart VL, Shah SP, Tanwar PS, Mermel CH, Beroukhim R, Azam M, Teixeira J, Meyerson M, Hughes TP, Llovet JM, Radich J, Mullighan CG, Golub TR, Sorensen PH, Daley GQ. 2009. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet 41:843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. 2007. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet 39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 57.Nolan MK, Jankowska L, Prisco M, Xu S, Guvakova MA, Surmacz E. 1997. Differential roles of IRS-1 and SHC signaling pathways in breast cancer cells. Int J Cancer 72:828–834. [DOI] [PubMed] [Google Scholar]
- 58.Jackson JG, Zhang X, Yoneda T, Yee D. 2001. Regulation of breast cancer cell motility by insulin receptor substrate-2 (IRS-2) in metastatic variants of human breast cancer cell lines. Oncogene 20:7318–7325. doi: 10.1038/sj.onc.1204920. [DOI] [PubMed] [Google Scholar]
- 59.de Blaquiere GE, May FEB, Westley BR. 2009. Increased expression of both insulin receptor substrates 1 and 2 confers increased sensitivity to IGF-1 stimulated cell migration. Endocrine Relat Cancer 16:635–647. doi: 10.1677/ERC-08-0216. [DOI] [PubMed] [Google Scholar]
- 60.Nagle JA, Ma Z, Byrne MA, White MF, Shaw LM. 2004. Involvement of insulin receptor substrate 2 in mammary tumor metastasis. Mol Cell Biol 24:9726–9735. doi: 10.1128/MCB.24.22.9726-9735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gibson SL, Ma Z, Shaw LM. 2007. Divergent roles for IRS-1 and IRS-2 in breast cancer metastasis. Cell Cycle 6:631–637. doi: 10.4161/cc.6.6.3987. [DOI] [PubMed] [Google Scholar]
- 62.Sachdev D, Hartell JS, Lee AV, Zhang X, Yee D. 2004. A dominant negative type I insulin-like growth factor receptor inhibits metastasis of human cancer cells. J Biol Chem 279:5017–5024. [DOI] [PubMed] [Google Scholar]
- 63.Westbrook TF, Hu G, Ang XL, Mulligan P, Pavlova NN, Liang A, Leng Y, Maehr R, Shi Y, Harper JW, Elledge SJ. 2008. SCFbeta-TRCP controls oncogenic transformation and neural differentiation through REST degradation. Nature 452:370–374. doi: 10.1038/nature06780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fox EM, Miller TW, Balko JM, Kuba MG, Sanchez V, Smith RA, Liu S, Gonzalez-Angulo AM, Mills GB, Ye F, Shyr Y, Manning HC, Buck E, Arteaga CL. 2011. A kinome-wide screen identifies the insulin/IGF-I receptor pathway as a mechanism of escape from hormone dependence in breast cancer. Cancer Res 71:6773–6784. doi: 10.1158/0008-5472.CAN-11-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yee D. 2012. Insulin-like growth factor receptor inhibitors: baby or the bathwater? J Natl Cancer Inst 104:975–981. doi: 10.1093/jnci/djs258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robertson JF, Ferrero J-M, Bourgeois H, Kennecke H, de Boer RH, Jacot W, McGreivy J, Suzuki S, Zhu M, McCaffery I. 2013. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol 14:228–235. doi: 10.1016/S1470-2045(13)70026-3. [DOI] [PubMed] [Google Scholar]