Abstract
Amphotericin B (Ampho B) is a fungicidal drug that causes cell wall injury. Pharmacological ascorbate induces the extracellular prooxidants, which might enter the Ampho B-induced cell wall porosity and act synergistically. We tested low-dose Ampho B with a short course of pharmacological ascorbate using a mouse model of sepsis preconditioned with an injection of Candida albicans 6 h prior to cecal ligation and puncture (CLP). In this model, candidemia reappeared as early as 6 h after CLP with a predictably high mortality rate. This characteristic mimics sepsis in the phase of immunosuppression in patients. Using the model, at 12- and 18-h post-CLP, we administered isotonic (pH neutralized) pharmacological ascorbate intravenously with low-dose Ampho B or sodium deoxycholate, vehicle-controlled, administered IP. The survival rate of low-dose Ampho B plus ascorbate was 53%, compared with <11% for low-dose Ampho B or high-dose Ampho B alone. In addition, a beneficial effect was demonstrated in terms of kidney damage, liver injury, spleen histopathology, and serum markers at 24 h after CLP. Kidney injury was less severe in low-dose Ampho B plus ascorbate combination therapy due to less severe sepsis. Moreover, ascorbate enhanced the effectiveness of phagocytosis against C. albicans in human phagocytic cells. Taken together, the data indicate that the new mouse model simulates sepsis-induced immunosuppression and that the combination of pharmacological ascorbate with an antifungal drug is a potentially effective treatment that may reduce nephrotoxicity, and perhaps also increase fungicidal activity in patients with systemic candidiasis caused by Candida albicans.
Keywords: Candida albicans, ascorbate, sepsis, amphotericin B
sepsis is a serious, generalized immune response to systemic infection, independent of the infecting organisms (42). The immune response in sepsis may present as a hyperimmune characteristic with a rapid natural course of disease and/or an immunosuppression phase responsible for nosocomial infection (55, 62). Candidiasis, especially resulting from Candida albicans, is one of the leading worldwide nosocomial infections occurring after the onset of sepsis (30, 49). The mortality rate of patients with postsepsis systemic candidiasis is more than 50%, with more than 25% of cases of candidiasis occurring in patients who had developed sepsis (17, 22). Although the incidences of infection from other fungal species are increasing in patients in the intensive care unit, C. albicans still remains the leading cause of nosocomial fungal infection (22, 48, 51). The increasing prevalence of nosocomial fungal infection in cases of sepsis may be due to the growing use of broad-spectrum antibiotics coupled with the advancement of intensive care supports (8, 29, 37, 43, 59).
Amphotericin B (Ampho B) is a traditional broad-spectrum, dose-dependent fungicidal drug, which is a pore-forming agent that acts though ergosterol inhibition. Ampho B, unfortunately, has substantial dose-dependent nephrotoxicity, and liposomal amphotericin B is expensive (11). If Ampho B can be used at a lower dose in combination with another agent, perhaps nephrotoxicity and cost will be reduced. When given intravenously as a pharmacological agent, ascorbate (ascorbic acid or vitamin C) demonstrates antineoplastic actions via the generation of extracellular hydrogen peroxide and downstream reactive oxygen species (ROS) (5–7, 37, 38). ROS formed in the presence of pharmacological ascorbate are less toxic to the host tissue, but are very toxic to cancer cells in vitro and in vivo. We hypothesized that extracellular hydrogen peroxide generated by pharmacological ascorbate could enter the fungal cell wall, which is injured or made vulnerable by the fungicidal pore-forming agent and enhanced antifungal therapeutic effectiveness (4–7). It should be noted that lower doses of ascorbate, given as a drug, may attenuate bacterial sepsis via additional mechanisms (1, 58, 63–65, 67). If ascorbate demonstrates actions that support antibacterial and antifungal activities, then ascorbate in combination with Ampho B may be an effective combined therapy.
To study ascorbate in combination with antifungal agents, an animal model system is necessary. The available model for Candida infection, characterized by Candida injection after sepsis (9), may not reflect Candida infections in humans, which mostly resulted from endogenous sources (50). As a result, we developed a new sepsis-induced candidemia reappearance model that is characterized by the reactivation of an exogenous Candida administration prior to the onset of sepsis. We used this new system to test Ampho B administered alone and in combination with pharmacological ascorbate.
METHODS
C. albicans Preparation, Characterization, and Germ Tube Test
C. albicans was isolated from blood samples (Mycology Unit, King Chulalongkorn Memorial Hospital), identified by morphology together with the API 20C AUX yeast identification kit (Biomuriex, Lyon, France), and stored in Sabouraud dextrose agar (SDA) (Thermo Scientific, Hampshire, UK) at −80°C. Before conducting all of the experiments, C. albicans was subcultured in SDA at 35°C for 24 h. The characteristics of the clinically isolated C. albicans were demonstrated by the minimal inhibitory concentration (MIC) (Table 1) and 50% lethal dose (LD50). MIC analysis followed a standard protocol (43), with all reagents purchased from Sigma-Aldrich (St. Louis, MO). The Clinical Laboratory Standard Institute recommended strains Candida krusei American Type Culture Collection (ATCC) 6258 and Candida parapsilosis ATCC 22019 were used for quality assurance. The LD50 of selected C. albicans was 4 × 105 blastospores as counted by hemocytometer (Bright-Line, Denver, CO), which was equal to the value measured by a spectrophotometer (EL×808 absorbance reader; BioTek, Shoreline, WA) by the optical density at 630 nm at 0.45 (OD 630 nm at 0.45). The procedure for LD50 was described in detail in the animal section below. For the germ tube test, 300 blastospores of C. albicans, as determined by hemocytometer, were placed in 50 μl of serum from a septic mouse (24 h after CLP) or serum from a sham mouse, incubated at 37°C for 3 h, then counted by hemocytometer. The percentage of the ratio of blastospores with germ tube formation to total blastospores was calculated.
Table 1.
The minimal inhibitory concentration that demonstrated antifungal resistance to the Candida albicans used in this study
| Drug | MIC, ng/ml |
|---|---|
| Amphotericin B | 125 |
| Fluconazole | 125 |
| Itraconazole | 31.3 |
| Ketonazole | 31.3 |
| Posaconazole | 31.3 |
| Voriconazole | 31.3 |
| Anidulafungin | 15 |
| Caspofungin | 15 |
MIC, minimal inhibitory concentration.
Animal and Animal Models
The U.S. National Institutes of Health (NIH) criteria and protocols for the use and treatment of laboratory animals were followed (NIH publication protocols no. 85-23, revised 1985). Male, 8–10-wk old BalB/C mice (National Laboratory Animal Center, Nakornpathom, Thailand) were used. The animal protocols were approved by the Institutional Animal Care and Use Committee of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. All procedures were performed under isoflurane anesthesia.
Candida albicans injection model.
Resuspended C. albicans in normal saline solution (NSS) adjusted into different doses of optical density at 630 nm in the same volume of each preparation (100 μl) was injected through the tail vein. The LD50 was extrapolated from the correlation equation of the graph of the 7-day mortality of mice, which was injected with C. albicans in different OD 630-nm doses (0.8, 0.6, 0.4, 0.2, and 0.1). The Candida dose with 50% mortality rate was calculated and identified as LD50.
Cecal ligation and puncture sepsis model.
The cecal ligation and puncture (CLP) procedure was described in detail previously (34), with some modifications. Briefly, the cecum was ligated at 12 mm from the cecal tip and punctured twice with a 23-gauge needle, leaving a small amount of fecal material exposed. The abdominal incision was closed in two layers with 6-0 nylon sutures. Antibiotic (imipenem/cilastatin 7 mg/kg in 0.3 ml NSS) was administered subcutaneously at 3 h and 8 h after CLP for 24-h end-point experiments. Additional antibiotic doses were administered at days 2, 4, and 6 after operation for 7-day survival experiments (Fig. 1).
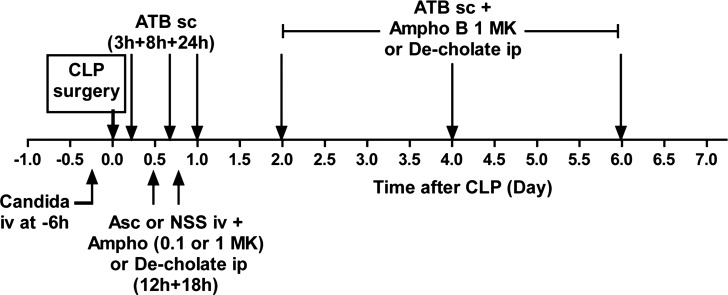
Fig. 1.
Timeline of a model of reappearance of candidemia in sepsis [Candida+cecal ligation and puncture (CLP)]. In 24-h experiments, Candida albicans was intravenously injected at 6 h before cecal ligation and puncture (CLP) (−6 h), then subcutaneously administered antibiotic (ATB sc) was administered at 3 h and 8 h. Subsequently, the intravenous (tail vein) ascorbate (Asc) or normal saline (NSS) plus intraperitoneal amphotericin B 0.1 mg·kg−1·dose−1 (0.1 MK) or 1 mg·kg−1·dose−1 (1 MK) or intraperitoneal deoxyglycolate (De-cholate) were administered at 12 h and 18 h after CLP. The mice were euthanized at 24 h after CLP. In the 7-day survival experiment, additional ATBs were injected subcutaneously at 1, 2, 4, and 6 days after CLP, and intraperitoneal amphotericin B 1 mg·kg−1·dose−1 (Ampho B 1 MK) or De-cholate was given at 2, 4, and 6 days after CLP.
A model of reappearance of candidemia during sepsis (candida+CLP).
One hundred microliters of C. albicans with the dose of OD 630 nm at 0.3 (∼2 × 105 blastospores) or reciprocal NSS was injected through the tail vein at the −6 h time point (Fig. 1). Then at 0 h, the CLP operation or sham laparotomy was performed. The schedule of antibiotic administration was demonstrated in Fig. 1. In parallel, a reciprocal volume of NSS was injected in the sham group. Blood was collected through the right retroorbital plexus under isoflurane anesthesia with a microhematocrit capillary tube (Fisher Scientific, Pittsburg, PA) and through cardiac puncture at the end-point of experiments. A blood volume of 15–50 μl was drawn at each time point, depending on the frequency of blood collection in specific models. The experiments were repeated if there were any insufficient blood sample for the analysis. At the time of euthanasia, internal organs (lungs, kidneys, spleen, and liver) and peritoneal fluids were collected in 4°C PBS for the fungal culture and fixed in 10% formalin for the organ histology.
Treatment of Ascorbate and Amphotericin B
Ascorbate (Sigma-Aldrich) was adjusted to pH 7 with sodium hydroxide (NaOH) for all experiments. Ascorbate 0.9 g/kg in sterile water or the same volume of NSS pH 7 as a control solution was administered through the tail vein at 12 h and 18 h after CLP surgery. In parallel, Ampho B (Amphotret, Maharashta, India) at 1 mg·kg−1·dose−1 and 0.1 mg·kg−1·dose−1 or sodium deoxycolate (De-cholate) (Sigma-Aldrich) 0.8 mg·kg−1·dose−1, vehicle-controlled, were injected intraperitoneally at 12 h and 18 h (Fig. 1). There was no difference in the severity of sepsis between low or high doses of De-cholate, 0.08 and 0.8 mg·kg−1·dose−1, respectively (data not shown). For the 7-day survival experiments, additional doses of Ampho B 1 mg·kg−1·dose−1 or De-cholate were administered intraperitoneally (Fig. 1). The ascorbate level after the administration on day 2 could not reach the therapeutic level, as defined by serum ascorbate more than 10 mM (data not shown).
Polymorphonuclear Cell Count, Blood Chemistry, and Cytokine Concentrations
A volume of 15 μl of blood was mixed with 250 μl of 3% acetic acid, a hemolytic solution, and the total number of leukocytes was counted with a hemocytometer. Simultaneously, 10 μl of blood was smeared on a glass slide for Wright stain and counted with ×100 magnification in 100 fields to determine the percentage of polymorphonuclear cells (PMN). The total number of PMN was calculated by total leukocyte count from hemocytometer multiplied by the percentage of PMN from the Wright stain glass slide. Serum ascorbate (Asc) and alanine transaminase (ALT) were measured with colorimetric detection by ascorbic acid assay (BioAssay U.S. Biological, Salem, MS) and ALT assay (DiaSys Diagnostic Systems, Holzheim, Germany), respectively. Serum creatinine (Scr) was measured by HPLC (32–34). Serum TNF-α, IL-6, IL-10, and IL-17 were determined by the Luminex xMap-based multiplex technology MILLIPLEX MAP (multianalyte panels) 4-plex cytokine kit (Millipore, Billerica, MA), according to the manufacturer's recommended procedure.
Microorganism Burdens in Blood, Peritoneal Fluid, and Internal Organs
Blood and peritoneal fluid in serial volume was immediately diluted in PBS, plated in SDA for 24 h at 35°C for fungal colony counting, and reciprocally placed in blood agar (Oxoid, Hampshire, UK) for 24 h at 37°C for bacterial colony counting. Internal organs were washed twice in PBS, weighed, homogenized, diluted in PBS, and incubated in SDA. The colony count in SDA after 24 h at 35°C was defined as the C. albicans burden. Moreover, to test whether there was C. albicans in feces and gastrointestinal tissues of mice without a preconditioning Candida injection, the different amounts of fecal contents were collected. Serial dilutions of gastrointestinal contents and tissue from cecum, colon, and recto-sigmoid area were collected from normal mice and septic mice at 24 h after CLP. Feces, well-mixed in PBS, and tissues were plated directly in SDA with 0.1% chloramphenicol (Bio-Rad, Hercules, CA), Sabouraud dextrose broth (SDB), and SDB with 0.05 g/l chloramphenicol (Sigma-Aldrich).
Organ Histology
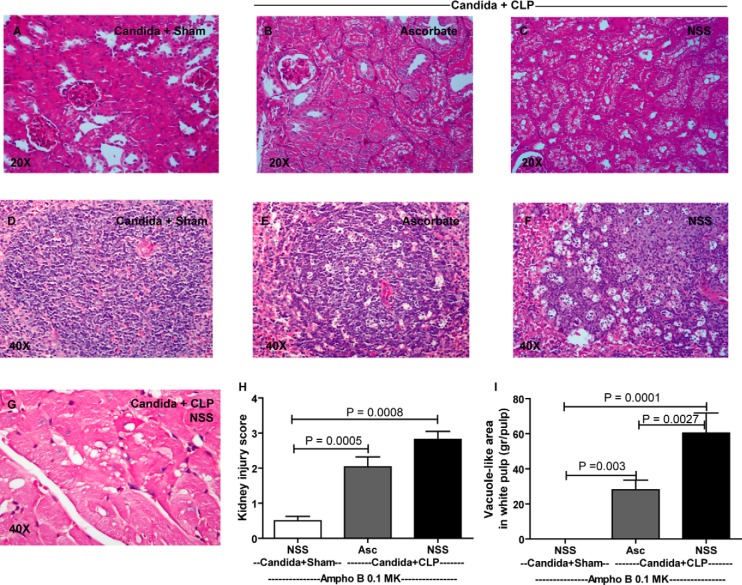
Tissue was fixed in 10% formalin and embedded in paraffin; 4-μm sections were stained with periodic acid-Schiff reagent. Kidney damage was defined as tubular epithelial swelling, loss of brush border, and vacuolar degeneration. The degree of kidney damage was estimated at ×200 magnification using 10 randomly selected fields, according to the following criteria: 0, normal; 1, area of damage <25% of tubules; 2, damage involving 25–50% of tubules; 3, damage involving 50–75% of tubules; and 4, 75–100% of the area being affected (35). Spleen damage was determined by counting the number of vacuole-like areas and groups of necrotic and apoptotic cells (44) in the white pulp of the spleen at ×400 magnification.
In Vitro Experiment for the Synergistic Effect of Ascorbate and Amphotericin B
One hundred microliters C. albicans in the dose of OD 630 nm at 0.7 diluted in Rose Park Memorial Institute (RPMI) 1640 was put into a 96-well microplate. Ampho B dissolved in dimethyl sulfoxide (DMSO) to a concentration of 125 ng/ml, 10 times lower than the MIC (Table 1), with serial concentrations of ascorbate (5–40 mM) adjusted to the neutral pH (pH 7) were mixed into each well. RPMI was added, as needed, to fully and equally fill each well. OD 630 nm was read after 24 h of incubation at 35°C to determine the number of Candida. The number of Candida was determined after 24 h of the incubation at 35°C by a spectrophotometer with OD at 630 nm.
Phagocytosis and Killing Assays
Human phagocytes from healthy donors were separated by 6% dextran solution, as previously described (41). Coincubation of 3 × 103 phagocytes/ml with 3 × 103 blastospores/ml of C. albicans in PBS with the serial concentrations of ascorbate (10–40 mM) adjusted to pH 7 at 37°C for 1 h was performed. Then, 100 μl of well-mixed solution was isolated and stained with Wright's stain according to the standard protocol. Phagocytic activity was determined by the percentage of phagocytes with Candida blastospores inside to the total phagocyte count at ×400 magnification from 100 fields per slide. Concurrently, to determine the phagocyte-killing function, 150 μl of the solution was centrifuged to separate phagocytes, then washed twice with PBS, recentrifuged, and filled with 100 μl of a nutritionally rich medium (Lysogeny broth; Sigma-Aldrich) to initiate complete cell disruption and the release of intracellular blastospores. The solution was then put in SDA at 35°C for 24 h and counted for fungal colonies (16). All experiments were performed in triplicate.
Statistical and Survival Analysis
SPSS version 17.0 (SPSS, Chicago, IL) was used for all statistical analyses. Data are presented as mean values and the standard error of the means (means ± SE). Differences among groups of results were examined using one-way ANOVA, followed by Bonferroni analysis. The two-tailed Student's t-test was used to examine the difference of the optical density at 630 nm between Asc and Ampho B+Asc and also used to demonstrate the difference in serum creatinine between Candida plus CLP and sham. A P value <0.05 was considered statistically significant. Survival analyses were evaluated using the log-rank test by observation and recorded every 6–12 h for the first 72 h after Candida injection or CLP surgery, and then once daily to 7 and 14 days for CLP survival and Candida injection survival, respectively. Mice exhibiting extreme morbidity were euthanized. Some of the mice with the manifestations of the extreme morbidity, such as abnormal breathing patterns or no response to stimuli, were euthanized, and their internal organs were examined for organ histology.
RESULTS
Characteristics of Candida Injection Model
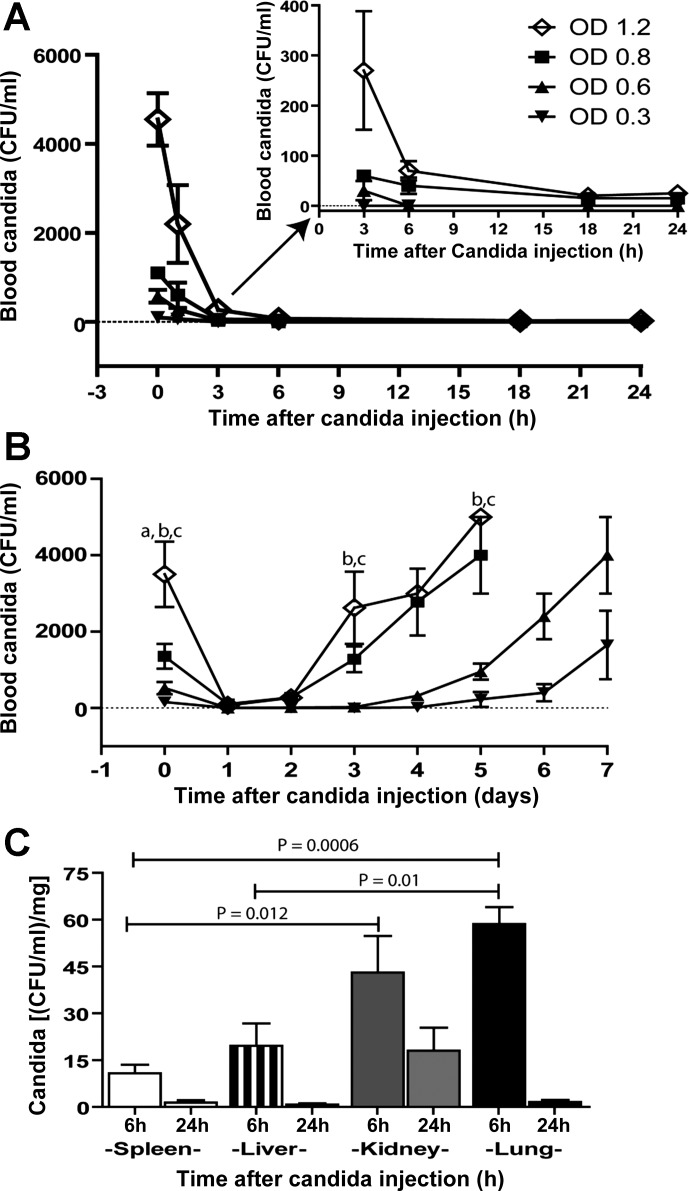
After the injection of different doses of Candida, candidemia presented after all of the selected doses of the injection. Candidemia became undetectable after 3–6 h for low-dose injections (OD 630 nm 0.3 and 0.6) (Fig. 2A, inset) and then reappeared (Fig. 2B). Despite the disappearance of Candida from the blood after 6 h and 24 h after injection with a dose of OD 630 nm 0.3, Candida was present in all measured organs (Fig. 2C). At 6 h after injection, Candida was predominantly presented in the lung and kidney 59 ± 5 and 43 ± 12 CFU·ml−1·mg−1, respectively. The presence of Candida was predominant in the liver and spleen, 20 ± 7 and 11 ± 3 CFU·ml−1·mg−1, respectively (Fig. 2C). At 24 h, the organ in which Candida was most presented was the kidney, at 18 ± 7 CFU·ml−1·mg−1 (Fig. 2C). These results indicated that Candida infection remained in tissues despite clearance from the blood after this inoculation dose. The candidemia clearance after the inoculation dose of OD 630 nm 0.3 (2 × 105 CFU/ml) facilitated the identification of this dose level for the further experimental study of the reactivation of candidiasis.
Fig. 2.
Characteristics of Candida injection model. Blood candidemia after administration of various doses of Candida at 24 h is demonstrated. A, inset: graph magnification is shown that indicates zero Candida blood level. B: candidemia at 7 days observation (B) (n = 5/time point). Candida burdens in different organs at 6 h and 24 h after Candida injection at the dose of optical density (OD) 630 nm 0.3 are shown (C) (n = 4–6/group). Measured parameters (means ± SE) were significantly different among the absorbance for fixed time point using one-way ANOVA followed by Bonferroni analysis with alpha set at 0.05 and four comparisons for A and B and eight comparisons for C. (a,b,cP < 0.05; a is OD 1.2 vs. OD 0.8, b is OD 1.2 vs. OD 0.6, and c is OD 1.2 vs. OD 0.3).
Characteristics of a Model of Reappearance of Candidemia During Sepsis (Candida+CLP)
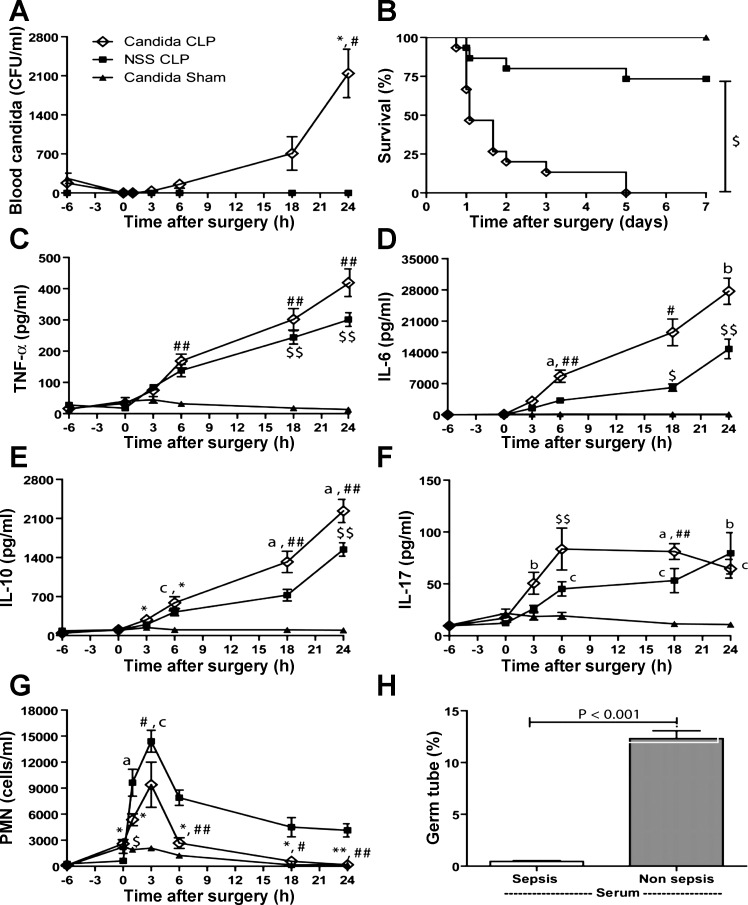
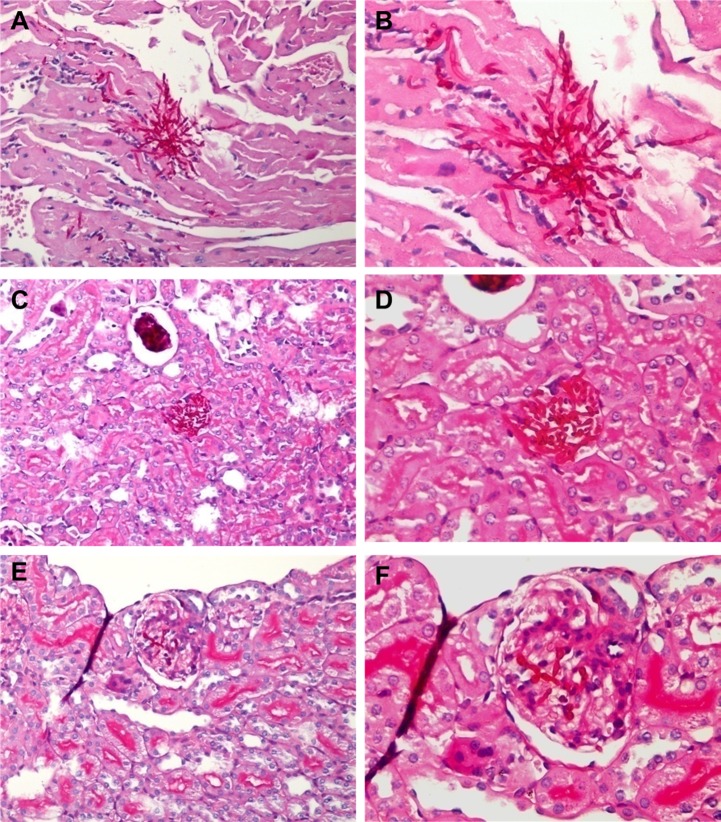
The goal of a superimposed Candida infection in a sepsis model is to have reappearance of Candida, so as to mimic Candida infection as it occurs after sepsis in humans. Therefore, the reappearance of candidemia was demonstrated. Candidemia spontaneously reappeared from the 4th day after Candida injection alone (Fig. 2B), but reappeared as early as 6 h after Candida+CLP (Fig. 3A), ∼93 h earlier. Moreover, candidemia in Candida+CLP increased very rapidly from 34 ± 16 CFU/ml at 3 h to 2,143 ± 434 CFU/ml at 24 h after CLP (Fig. 3A). In contrast, Candida injection alone without CLP showed a delayed and more modest increase in candidemia from 15 ± 10 CFU/ml at day 4 to 1,650 ± 897 CFU/ml at day 7 after injection (Fig. 2B). Of note, CLP alone did not produce candidemia at 24 h after CLP (Fig. 3A) and at 7 days (data not shown). Moreover, C. albicans could not be detected by culture (see methods) of cecal contents and gastrointestinal tissues of normal and septic mice. Sepsis from CLP without Candida, coupled with postsurgery treatment, showed a survival rate of 73% (11 of 15 mice) at day 7 (NSS+CLP), indicating a modest severity of sepsis in our CLP model (Fig. 3B). The addition of Candida infection before sepsis (Candida+CLP) produced 100% mortality at 5 days (Fig. 3B). Moreover, mice with Candida+CLP died rapidly, with >50% mortality within 1 day and >67% mortality within 2 days (Fig. 3B). Rapid mortality in the Candida+CLP model is likely due to the severity of sepsis. Compared with controls, within the first 24-h Candida+CLP mice had higher cytokines and more pronounced neutropenia than CLP alone (Fig. 3, C–G), as well as higher fungal burden (Fig. 3A). Limited leukocytosis and/or rapid neutropenia could be factors in the severity of Candida+CLP. In contrast, germ tube production in septic serum was lower than in normal serum (Fig. 3H). High blood Candida levels in CLP mice (Fig. 3A), despite less Candida growth in vitro with septic serum, is consistent with an immunologic cell role in the control of candidemia. Candida+CLP mice at 3 days after injection showed multiple Candida pseudohyphae lesions on the heart, renal glomeruli, and renal interstitium (Fig. 4). These findings are consistent with disseminated candidiasis. The reappearance of Candida from internal sources in the Candida+CLP mice resembles human sepsis in terms of the reactivation of latent Candida, although from different sources of Candida (21, 50). Subsequently, we used the Candida+CLP model for further studies due to the Candida reactivation property of the model.
Fig. 3.
Characteristics of a model of reappearance of candidemia in sepsis (Candida+CLP). Serial blood candidemia within 24 h after Candida injection at 6 h before CLP (Candida+CLP) or NSS injection then CLP (NSS+CLP) or Candida injection with sham operation (Candida+Sham) (A) (n = 7/group) was demonstrated. The survival of different groups (B) (n = 15/group), cytokine responses including, proinflammatory cytokines (TNF-α, IL-6) (C and D), anti-inflammatory cytokines (IL-10) (E), fungal response cytokines (IL-17) (F) (n = 7/group), neutrophil count (G) (n = 5/group) are shown. Additionally, the comparison of germ tube growth in normal mouse serum and septic mouse serum is shown (H) (n = 3–5/group). Measured parameters (mean ± SE) were significantly different among the Candida+CLP, NSS+CLP, and Candida+Sham groups for fixed time point using one-way ANOVA followed by Bonferroni analysis with alpha set at 0.05 and three comparisons. (a,b,cP < 0.05, *,#,$P < 0.005, **,##,$$P < 0.0005; a, *, and ** denote Candida+CLP vs. NSS+CLP; b, #, and ## denote Candida+CLP vs. Candida+Sham; and c, $, and $$ denote NSS+CLP vs. Candida+Sham).
Fig. 4.
Representative histology from a model of reappearance of candidemia in sepsis (Candida+CLP). Pseudohyphae and mononuclear infiltration at ×200 and ×400 magnification by periodic acid-Schiff (PAS) stain in cardiac tissue in (A and B), renal interstitium (C and D), and renal glomeruli (E and F) from mice that died after 3 days of a model of reappearance of candidemia in sepsis (Candida+CLP).
The In Vitro Synergistic Effect of Amphotericin B with Ascorbate and In Vivo Ascorbate Concentrations
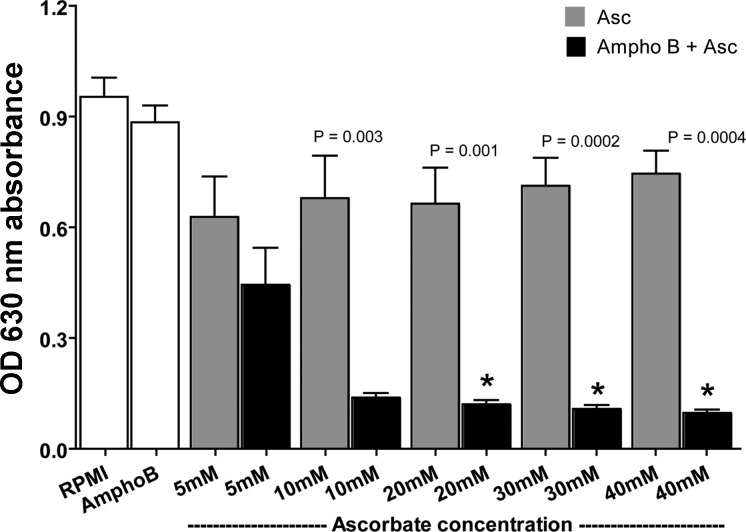
We used the Candida+CLP model to test the synergistic properties of Ampho B and ascorbate, beginning with in vitro experiments. The Ampho B concentration was selected at 10 times lower than the minimal inhibitory concentration (MIC), and incubations were performed with different pharmacological ascorbate concentrations. The Ampho B concentration was chosen such that no fungicidal effect was predicted with its use alone. Also, a very low dose of Ampho B could reduce nephrotoxicity in vivo. Ascorbate concentrations were chosen to reflect those measured in humans after intravenous administration (66, 67). As predicted, neither low-dose Ampho B (125 ng/ml) alone nor ascorbate alone had any effect on Candida growth. Ampho B combined with ascorbate concentrations ranging from 10 to 40 mM demonstrated growth inhibition of ∼85% (Fig. 5). Alternatively, there was no synergistic effect of ascorbate at these concentrations with fluconazole at a dose 10 times lower than the MIC (125 ng/ml) (data not shown). It appears that the synergistic effects of high-dose ascorbate may be limited to only specific antifungal drugs.
Fig. 5.
In vitro synergy of low-dose amphotericin B and high-dose ascorbate. Synergistic effect of nontherapeutic dose Amphotericin B with different ascorbate concentration, in vitro, is shown. Data (means ± SE) were obtained from five to seven independent experiments. P values were compared between doses using the independent Student's t-test.
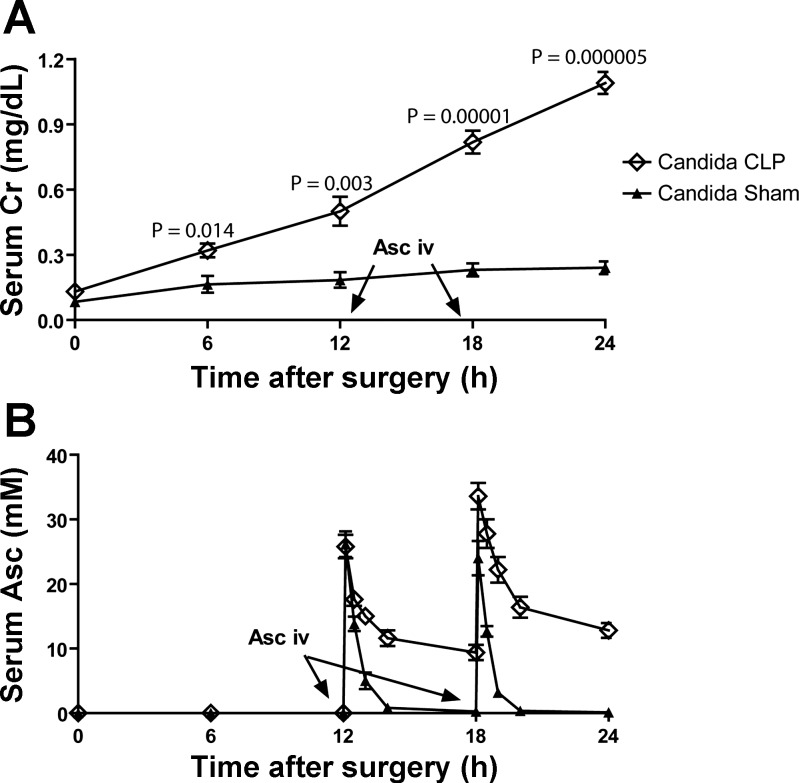
Subsequently, ascorbate concentration at or above 10 mM was chosen as the target level of serum ascorbate for further experiments. We take advantage of renal dysfunction in the Candida+CLP model to maintain serum ascorbate (Fig. 6A). While ascorbate is rapidly cleared in mice without renal insufficiency, ascorbate concentrations are maintained at or above 10 mM for many hours in Candida+CLP mice with ascorbate injection at 12 h and 18 h after surgery (Fig. 6B). Ascorbate injections administered prior to 12 h post-CLP were cleared more rapidly (data not shown). In addition, the treatment of patients is usually performed after the onset of the diseases, which is similar to the delayed treatment in the mouse model. For these reasons, we conducted experiments with treatment beginning 12 h after sepsis to test the synergy of pharmacologic ascorbate with low-dose amphotericin B.
Fig. 6.
Time course of serum creatinine (serum Cr) and in vivo ascorbate level after intravenous injection. With Candida+CLP and Candida+Sham model, progression of kidney injury as measured by serum Cr (A), and serum ascorbate (serum Asc) level at baseline and time course after injection (B) is shown (n = 5 or 6/time point). Of note, serum ascorbate level at 24 h was 12.8 ± 1.2 mM. P values of the data (means ± SE) were compared between doses by the independent Student's t-test.
Low-Dose Amphotericin B Plus Pharmacologic Ascorbate In Vivo: Effects in a Model of Reappearance of Candidemia During Sepsis (Candida+CLP Model)
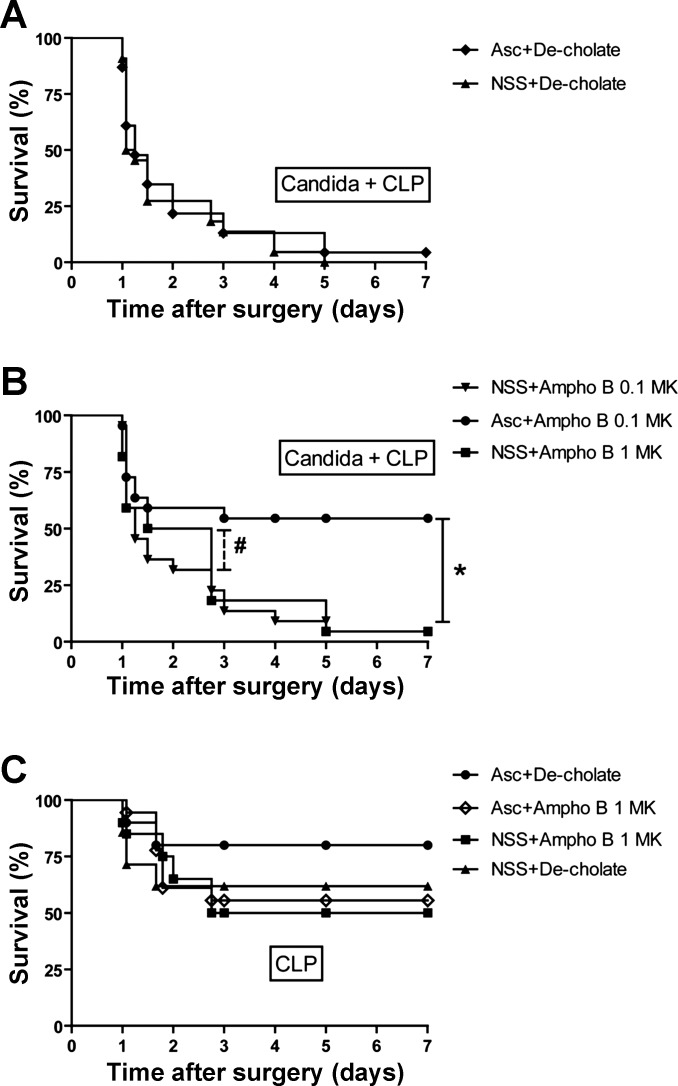
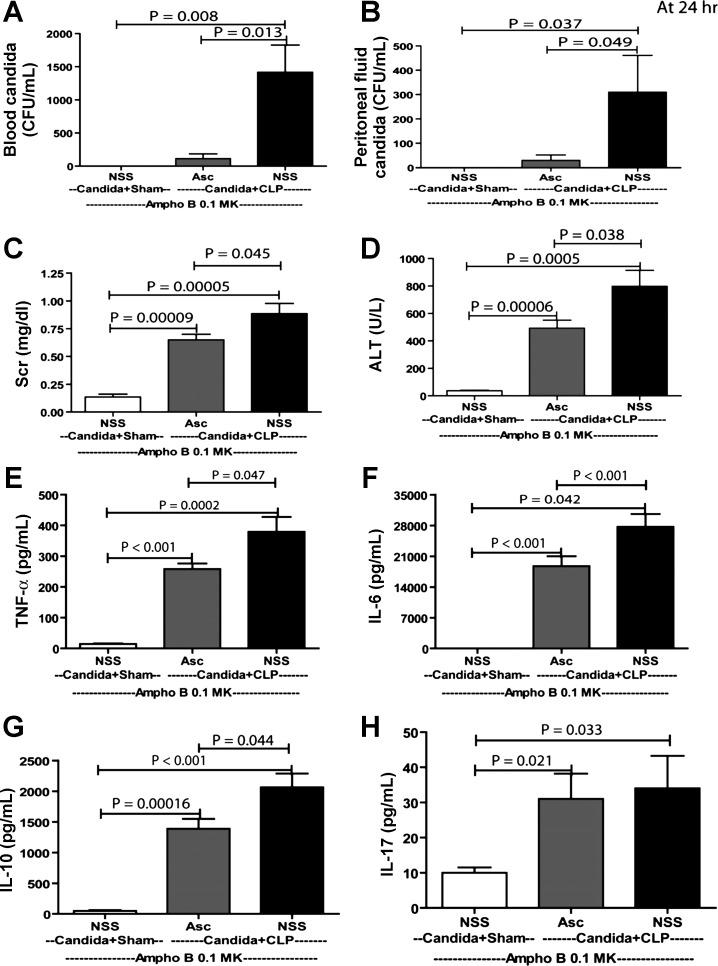
The objective of these experiments was to test the therapeutic effects of low-dose Ampho B (0.1 mg·kg−1·dose−1, which is 10 times lower than the MIC) combined with pharmacological ascorbate. As controls, deoxycholate alone (NSS+De-cholate), pharmacologic ascorbate with deoxycholate (Asc+De-cholate), low-dose Ampho B alone (NSS+Ampho B 0.1 MK), and full-dose Ampho B alone (NSS+Ampho B 1 MK) showed no survival benefit at 7 days (Fig. 7, A and B). Only ascorbate combined with low-dose Ampho B (Asc+Ampho B 0.1 MK) improved the 7-day survival, from 9% (2/22) to 54% (12/22) (Fig. 7B). It is both notable and interesting that full-dose Ampho B alone improved the survival in days 1 and 2, but was reversed on day 3, leading to high mortality and a low 7-day survival rate (Fig. 7B). In bacterial sepsis alone without Candida injection (CLP), there was a tendency toward improved sepsis survival in the ascorbate administration group, but the improvement was not statistically significant (Asc+De-cholate vs. NSS+Decholate) (Fig. 7C). Although Ampho B administration in CLP mice did not noticeably worsen the severity of sepsis (NSS+De-cholate vs. NSS+Ampho B 1 MK), it seemed to neutralize the potential benefit of ascorbate administration (Asc+Ampho B) (Fig. 7C). To determine the microorganism loads, blood and peritoneal fluid were collected at 24 h for Candida and bacterial cultures. As expected, candidemia and peritoneal Candida was lower with low-dose Ampho B plus ascorbate, but not with low-dose Ampho B alone (Fig. 8, A and B), consistent with the in vitro data (Fig. 5). However, bacterial burden was not different between groups (data not shown). In addition, we evaluated sepsis severity in the Candida+CLP model at 24 h, based on the following serum markers: Scr for renal function, ALT for liver function, and cytokines for immune responsiveness. We also obtained histopathology data of the kidney, spleen, and heart (Fig. 9). Low-dose Ampho B combined with pharmacological ascorbate attenuated organ injury: kidney injury as determined by Scr (Fig. 8C) and renal histopathology (Fig. 9H), liver injury as measured by ALT (Fig. 8D), and spleen injury as evaluated by vacuole-like lesions in the white pulp of the spleen (Fig. 9I). Moreover, there was cardiac vacuolization in some mice receiving low-dose Ampho B treatment alone, but not in mice with the combined treatment (Fig. 9G). Ampho B combined with pharmacological ascorbate reduced IL-6, TNF-α, and IL-10, but not IL-17 (Fig. 8, E–H).
Fig. 7.
Low-dose amphotericin B with high-dose ascorbate improved survival of a model of reappearance of candidemia in sepsis (Candida+CLP). Survival analysis of mice treated with high-dose ascorbate alone (Asc+De-cholate), saline alone [normal saline solution (NSS)+De-cholate], and a therapeutic dose of amphotericin B (NSS+Ampho B 1 MK) (A) and the treatment with low-dose amphotericin B with and without ascorbate (Asc+Ampho B 0.1 MK and NSS+Ampho B 0.1 MK) or full-dose Ampho B without ascorbate (NSS+Ampho B 1 MK) in Candida+CLP model (B) is demonstrated. In addition, survival analysis is shown of reciprocal treatment in CLP mice in the absence of prior C. albicans inoculation (C) using the log-rank (Mantel-Cox) test (n = 20–22/group) *P < 0.01, #P < 0.05 between indicated time point.
Fig. 8.
Low-dose Ampho B with high-dose Asc attenuated severity of a model of reappearance of candidemia in sepsis (Candida+CLP). Sepsis severity parameters at 24 h of sham surgery models (Candida+Sham, NSS administered at 12 h and 18 h), Asc, or NSS administered with CLP surgery models (Candida+CLP with NSS or Asc administered at 12 h and 18 h), as measured by candidemia (A), peritoneal Candida (B), serum creatinine (Scr) (C), alanine transminase (ALT) (D), cytokines [TNF-α (E), IL-6 (F) IL-10 (G), and IL-17 (H)] are demonstrated (n = 5–7/group). Blood ascorbate level at 24 h of Acs injection was 15.2 ± 3.1 mM. Measured parameters (means ± SE) were significantly different among the Candida+Sham with NSS, Candida+CLP with Asc, and Candida+CLP with NSS groups for a fixed time point using one-way ANOVA, followed by Bonferroni analysis with alpha set at 0.05 and making three comparisons.
Fig. 9.
Low-dose Ampho B with high-dose Asc attenuated organ histology of a model of reappearance of candidemia in sepsis (Candida+CLP). The representative figures of renal histology at 24 h of the model with PAS staining in sham surgery models (Candida+Sham, NSS administered at 12 h and 18 h) (A), Asc or NSS administered with CLP surgery models (Candida+CLP with Asc or NSS administered at 12 h and 18 h; B and C). In parallel, spleen histology in individual group (D–F) and cardiac histology in non-Asc-treated Candida+CLP (G) is demonstrated. The original magnification in all of histology figures was ×400. The histological scoring from renal (H) and spleen histology (I) is shown (n = 5 or 6/group). Measured parameters (means ± SE) were significantly different among the Candida+Sham with NSS, Candida+CLP with Asc, and Candida+CLP with NSS groups for fixed time point using one-way ANOVA, followed by Bonferroni analysis with alpha set at 0.05 and three comparisons.
Higher Ascorbate Concentration Improved Phagocytosis and Intracellular Killing of Candida by Human Phagocytic Cells
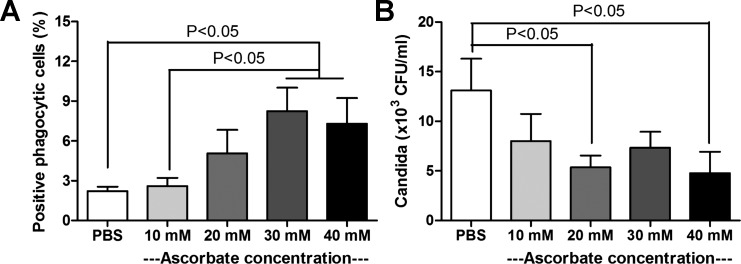
Effective phagocytosis and phagocyte killing are mechanisms for the control of candidiasis, especially in the setting of neutropenia (32). The phagocyte killing depends on oxidative stress production, which might be associated with ascorbate (61). We tested the ability of human phagocytic cells to phagocytize and kill Candida, in the presence of different ascorbate concentrations. Phagocytic activity was enhanced with pharmacological ascorbate concentrations of 30 and 40 mM (Fig. 10A), and Candida killing activity improved at concentrations of 20 and 40 mM (Fig. 10B).
Fig. 10.
Ascorbate-enhanced effective phagocytosis of human phagocytes. The positive phagocytic cells after incubation with Candida blastospore (A) and the Candida killing activity (B) with different ascorbate concentrations are shown. Data (means ± SE) were obtained from five or six independent experiments. Measured parameters were significantly different among the ascorbate concentration groups for using one-way ANOVA, followed by Bonferroni analysis with alpha set at 0.05 and five comparisons.
DISCUSSION
Prior mouse models of Candida used Candida injection after sepsis (9), but this may not be the ideal method to mimic reactivation of candidemia. In this study, we injected Candida before sepsis, which was induced by CLP surgery 6 h after Candida injection. We demonstrated that sepsis reactivated candidemia, resembling the alteration of intrinsic Candida microbiota in patients with candidiasis (50). We found more severe injuries and a much higher mortality rate in Candida+CLP, compared with CLP alone. Using this model, we were able to investigate a new treatment modality and the combination of pharmacological ascorbate with low-dose amphotericin B (Ampho B). This combination improved survival and attenuated disease severity, compared with the use of either modality alone.
The severity of intravenous Candida albicans infection depends on the injected doses and the properties of C. albicans used in different studies (36, 56). With high-dose Candida injection, candidemia persisted at all time points. As for low-dose Candida injection, mice cleared up candidemia very rapidly within the first 24 h after injection, with no spontaneous reappearance until after 4 days. Even with rapid clearance of candidemia in the low-dose model, tissue Candida persisted in the lung, kidney, and liver, consistent with other reports (36, 66). For these reasons, low-dose injections seemed to suitably mimic the immunosuppression phase, despite the more rapid natural course in mice, compared with human patients with sepsis. It is interesting to note that Candida injection alone without CLP did not cause renal injury at 24 h after injection, as measured by serum creatinine (Scr) (Figs. 8C and 9H), despite Candida being detected in the kidney at 24 h (Fig. 2C).
Decreasing the time interval needed for Candida reappearance would improve the experimental model of sepsis immunosuppression. Candida reappeared as early as 6 h after sepsis initiation in a model of reappearance of candidemia during sepsis (Fig. 3A). In comparison, more than 4 days were needed in nonseptic mice for Candida reappearance (Fig. 2B). Several sources of Candida reactivation were demonstrated in several organs (Fig. 2C) for mice in the Candida+CLP model, which was different from the translocation across the gut mucosa in patients with superimposed candidiasis (14). However, the reactivation of the existing internal sources of Candida may more closely resemble the human condition, compared with other current models. Sepsis immunosuppression is initially defined as a condition in which patients or experimental animals are susceptible to opportunistic infection in sepsis patients (40, 55). There are at least two hypotheses related to the sepsis-immunosuppressive phase. The serial theory states that immunosuppression takes place after the hyperinflammatory phase. The parallel theory states that immunosuppression is concomitant with hyperinflammation (3, 52). Generally, immune compromise after sepsis can be manifested by the reactivation of endogenous infections, such as cytomegalovirus, herpes virus, mycobacteria, and candidosis. We used candidemia reactivation as a marker of sepsis immunosuppression. It was necessary to inject Candida to be able to study its reactivation as part of sepsis immunosuppression. Without prior Candida injection, we could not identify C. albicans by culture methodology in gastrointestinal tissue and contents, the natural reservoir of Candida (14), from either normal or septic mice. Several explanations for this failure to identify C. albicans are possible, including 1) conventional culturing methods may be insufficient to detect Candida as part of normal mouse flora; 2) Candida may not be part of normal mouse flora; or 3) the presence of Candida may be dependent on mouse environment or strain. More sensitive detection methods and/or longer observation times after sepsis (60) might be necessary to demonstrate spontaneous candidemia after sepsis alone. Nevertheless, the reactivation of candidemia in Candida+CLP was very rapid, within hours after sepsis, compared with days or weeks in patients in the intensive care unit. This difference might, at least in part, be due to more focused and appropriate treatments and life support systems for patients, a notable absence in experimental studies using mice.
The Candida injection changed a modest severity of CLP model with a 30% mortality rate into more severe injury with a 100% mortality rate (Fig. 3B). A number of factors could be responsible. The germ tube findings that we report here show that the serum of sepsis mice was less effective than normal mouse serum as for fungal growth, despite an exponential increase in Candida in sepsis. This finding suggests that changes in immune response elements could be responsible for accelerated candidemia in Candida+CLP septic mice. One contributing factor may be a decreased neutrophil response in Candida+CLP compared with CLP alone, consistent with the neutrophil depletion occurring more rapidly when sepsis is combined with candidemia (31, 32). Rapid neutrophil depletion in Candida+CLP might be due to the higher sepsis severity, which leads to the higher rate of neutrophil apoptosis, as previously mentioned (18). Another factor may be associated with IL-17 responsiveness in Candida+CLP mice, as IL-17 is a cytokine responsible for the fungal infection (25, 26). While Candida+CLP mice had an increase in IL-17 compared with CLP mice, the increase may have been insufficient for host protection. IL-17 was the only cytokine, among others we tested, with a similar level at 24 h in Candida+CLP, compared with CLP alone. Moreover, IL-17 was the earliest cytokine to increase as early as 3 h after Candida+CLP, but IL-17 did not increase in CLP alone. This characteristic suggested the importance of IL-17 in controlling the severity of sepsis in the Candida+CLP model. We propose that IL-17 synthesis in Candida+CLP was more strongly stimulated by both bacterial and fungal infection (25, 26). The dual importance of IL-17 in both bacterial and fungal infection was previously published (19, 20, 25, 28). Still another contributing factor of high mortality in Candida+CLP mice could be injury to the spleen. In this study, we found groups of apoptotic/necrotic cells in the white pulp of the spleen, previously described as a “vacuole-like area”, were more prominent in Candida+CLP spleens, consistent with the description in severe sepsis (44). Apoptosis of immune cells, especially apoptosis in the spleen as reported in patients with sepsis (24) and mouse models of sepsis (34, 35, 44), was hypothesized to be one of the responsible factors for the immunosuppression and more severe injury in sepsis. The severe destruction of the spleen might be an additional contributing factor to the immunosuppression observed in the Candida+CLP model.
In animals and humans, concentrations of ascorbate (vitamin C) are normally tightly controlled when it is ingested orally, via transporter saturation (45–47). In humans, intestinal transporters saturate at doses above 200 mg, with decreased bioavailability. Tissue transporters saturate when ingested doses are above 100 mg. Likewise, the renal reabsorptive ascorbate transporter saturates at doses above 100 mg, with urinary excretion of unabsorbed ascorbate. Together, these mechanisms produce orchestrated ascorbate concentrations. When ascorbate is administered either intraperitoneally in animals or intravenously in animals or humans, tight control mechanisms are transiently saturated, until renal excretion restores homeostasis. With parenteral administration of pharmacological doses of ascorbate, plasma concentrations can reach 500 times those possible with oral administration (47). Only these pharmacological concentrations produce hydrogen peroxide in the extracellular fluid, which serves as a pro-drug for the formation of reactive oxygen species that are toxic to cancer cells, but harmless to normal tissues (4, 7, 23). It has been postulated that ROS formed from pharmacological ascorbate could also be harmful to bacteria (6). Lower doses of pharmacological ascorbate have effects that attenuate the severity of sepsis (1, 2, 58, 63–65, 67) that are not bactericidal.
Our findings showed that while pharmacological ascorbate alone was ineffective for candidemia, pharmacological ascorbate combined with Ampho B, even when the latter was reduced to 10 times lower than the MIC, was more effective than Ampho B alone. Furthermore, full-dose Ampho B showed minimal survival at 7 days in the Candida CLP model, compared with more than 50% survival at 7 days using pharmacological ascorbate with low-dose Ampho B. In vitro, for maximum synergy with low-dose Ampho B, 10 mM ascorbate was sufficient (Fig. 5). This concentration was not only easily achieved in mice but also achieved easily and safely in humans (23). Synergy between pharmacological ascorbate and Ampho B may be a consequence of each having separate actions on Candida membranes. Ampho B is a pore-forming agent that destabilizes the Candida membrane. Reactive oxygen species generated by pharmacological ascorbate from hydrogen peroxide, could also act independently to destabilize Candida membranes. In addition, hydrogen peroxide may also diffuse into Candida, with the subsequent formation of internal reactive oxygen species leading to internal membrane or protein damage. These potential actions of pharmacological ascorbate are independent of Ampho B, and synergy is reasonably predictable. Additional independent effects of ascorbate on neutrophils, phagocytosis, and killing could also contribute to the benefits of pharmacological ascorbate (15, 27, 53, 61).
Because Ampho B is nephrotoxic, lower-dose regimens are preferable. Pharmacological ascorbate was permissive for lower dosing of Ampho B in the Candida+CLP model. The CLP model induces renal insufficiency, such that only two doses of intravenous ascorbate as the delayed treatment were sufficient. In contrast, most of the successful sepsis treatments in mouse models need to be administered at the onset or the early phase of sepsis (10, 12, 13, 33–35).
A concern relating to this model is whether or not additional nephrotoxicity is induced by pharmacologic ascorbate. In the setting of renal insufficiency, intrarenal oxalate formation and crystallization are concerns with pharmacological ascorbate. Oxalic acid, an end product of ascorbate metabolism, can crystallize as calcium oxalate in urine and renal interstitial tissues. In Candida+CLP mice treated with ascorbate, we did not find any crystal deposits in kidney histology, after 1 day and from autopsy (data not shown). We also found that serum creatinine was lower in mice treated with pharmacological ascorbate combined with low-dose Ampho B, compared with Ampho B alone. Moreover, we demonstrated that ascorbate injection in Candida+CLP did not alter the kidney function. On the other hand, full-dose Ampho B improved the mortality rate at day 2 of survival but not at day 7 of survival, a finding that suggests its toxicity in full-dose treatment (Fig. 6B). The findings from the combined treatment group demonstrate that the treatment effects of adding pharmacological ascorbate were beneficial for renal function. Another concern involved the limitations of mouse models and whether or not they are able to sufficiently represent human patient conditions and responses. The debate on this topic is still ongoing (54, 57). Nevertheless, we postulated that our two-hit models are more complex and might more accurately mimic the patient's conditions. However, validation in patients with reciprocal sepsis condition is required.
Perspectives and Significance
In our new mouse model demonstrating an immunosuppressive phase with Candida reappearance, a synergistic effect of pharmacological ascorbate with low-dose Ampho B was demonstrated in vitro and in vivo, without apparent toxicity. Taken together, the data provide a proof of concept for pharmacological ascorbate as an adjuvant for antifungal treatment. Pharmacological ascorbate is inexpensive and can be easily available. Pharmacological ascorbate in combination with existing antifungals may indicate the use of lower doses with reduced toxicity.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.L. and A.C. conception and design of research; A.L., P.S., H.T., P.T., R.N., and N.W. performed experiments; A.L., P.S., T.B., H.T., P.T., R.N., N.W., and N.S. analyzed data; A.L., P.S., T.B., H.T., P.T., R.N., N.W., M.L., A.C., and N.S. interpreted results of experiments; A.L. and P.T. prepared figures; A.L. drafted manuscript; A.L., P.S., H.T., K.T., S.E.-o., M.L., A.C., and N.S. edited and revised manuscript; A.L., P.S., K.T., S.E.-o., M.L., and A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors would like to gratefully acknowledge the Center of Excellence in Immunology and Immune-Mediated Diseases, Department of Microbiology, Faculty of Medicine, Chulalongkorn University for its support of this research study. This study was funded by the following grants: the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University; Grant for Development of New Faculty Staff, Chulalongkorn University, as well as a grant for Chula Research Scholar, Ratchadaphiseksomphotch Endowment Fund. We also thank Kevin P. Jones for the English language editing of the manuscript.
REFERENCES
- 1.Armour J, Tyml K, Lidington D, Wilson JX. Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat. J Appl Physiol 90: 795–803, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Biesalski HK, McGregor GP. Antioxidant therapy in critical care—is the microcirculation the primary target? Crit Care Med 35: S577–S583, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res 12: 151–170, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Chen P, Yu J, Chalmers B, Drisko J, Yang J, Li B, Chen Q. Pharmacological ascorbate induces cytotoxicity in prostate cancer cells through ATP depletion and induction of autophagy. Anticancer Drugs 23: 437–444, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA 102: 13,604–13,609, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA 104: 8749–8754, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DB, Drisko J, Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA 105: 11,105–11,109, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collin B, Clancy CJ, Nguyen MH. Antifungal resistance in non-albicans Candida species. Drug Resist Updat 2: 9–14, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Davis CG, Chang K, Osborne D, Walton AH, Dunne WM, Muenzer JT. Increased susceptibility to Candida infection following cecal ligation and puncture. Biochem Biophys Res Commun 414: 37–43, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dear JW, Leelahavanichkul A, Aponte A, Hu X, Constant SL, Hewitt SM, Yuen PS, Star RA. Liver proteomics for therapeutic drug discovery: inhibition of the cyclophilin receptor CD147 attenuates sepsis-induced acute renal failure. Crit Care Med 35: 2319–2328, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deray G. Amphotericin B nephrotoxicity. J Antimicrob Chemother 49 Suppl 1: 37–41, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Doi K, Hu X, Yuen PS, Leelahavanichkul A, Yasuda H, Kim SM, Schnermann J, Jonassen TE, Frokiaer J, Nielsen S, Star RA. AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int 73: 1266–1274, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doi K, Leelahavanichkul A, Hu X, Sidransky KL, Zhou H, Qin Y, Eisner C, Schnermann J, Yuen PS, Star RA. Pre-existing renal disease promotes sepsis-induced acute kidney injury and worsens outcome. Kidney Int 74: 1017–1025, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis 39: 219–226, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Dwenger A, Pape HC, Bantel C, Schweitzer G, Krumm K, Grotz M, Lueken B, Funck M, Regel G. Ascorbic acid reduces the endotoxin-induced lung injury in awake sheep. Eur J Clin Invest 24: 229–235, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Du C, Calderone RA. Phagocytosis and killing assays for Candida species. Methods Mol Biol 499: 17–26, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Falagas ME, Apostolou KE, Pappas VD. Attributable mortality of candidemia: a systematic review of matched cohort and case-control studies. Eur J Clin Microbiol Infect Dis 25: 419–425, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Fialkow L, Fochesatto Filho L, Bozzetti MC, Milani AR, Rodrigues Filho EM, Ladniuk RM, Pierozan P, de Moura RM, Prolla JC, Vachon E, Downey GP. Neutrophil apoptosis: a marker of disease severity in sepsis and sepsis-induced acute respiratory distress syndrome. Crit Care 10: R155, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ferrara JL, Ward PA. Adverse functions of IL-17A in experimental sepsis. FASEB J 22: 2198–2205, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Freitas A, Alves-Filho JC, Victoni T, Secher T, Lemos HP, Sonego F, Cunha FQ, Ryffel B. IL-17 receptor signaling is required to control polymicrobial sepsis. J Immunol 182: 7846–7854, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Gosain A, Gamelli RL. Role of the gastrointestinal tract in burn sepsis. J Burn Care Rehabil 26: 85–91, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis 37: 1172–1177, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, Rousseau C, Robitaille L, Miller WH Jr. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol 19: 1969–1974, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 27: 1230–1251, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 190: 624–631, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Huppler AR, Bishu S, Gaffen SL. Mucocutaneous candidiasis: the IL-17 pathway and implications for targeted immunotherapy. Arthritis Res Ther 14: 217, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonas E, Dwenger A, Hager A. In vitro effect of ascorbic acid on neutrophil-endothelial cell interaction. J Biolumin Chemilumin 8: 15–20, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Kasten KR, Prakash PS, Unsinger J, Goetzman HS, England LG, Cave CM, Seitz AP, Mazuski CN, Zhou TT, Morre M, Hotchkiss RS, Hildeman DA, Caldwell CC. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis. Infect Immun 78: 4714–4722, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly SL, Lamb DC, Kelly DE, Manning NJ, Loeffler J, Hebart H, Schumacher U, Einsele H. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta5,6-desaturation. FEBS Lett 400: 80–82, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Klotz SA, Chasin BS, Powell B, Gaur NK, Lipke PN. Polymicrobial bloodstream infections involving Candida species: analysis of patients and review of the literature. Diagn Microbiol Infect Dis 59: 401–406, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Curr Opin Infect Dis 25: 321–327, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Kullberg BJ, Netea MG, Vonk AG, van der Meer JW. Modulation of neutrophil function in host defense against disseminated Candida albicans infection in mice. FEMS Immunol Med Microbiol 26: 299–307, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Leelahavanichkul A, Bocharov AV, Kurlander R, Baranova IN, Vishnyakova TG, Souza AC, Hu X, Doi K, Vaisman B, Amar M, Sviridov D, Chen Z, Remaley AT, Csako G, Patterson AP, Yuen PS, Star RA, Eggerman TL. Class B scavenger receptor types I and II and CD36 targeting improves sepsis survival and acute outcomes in mice. J Immunol 188: 2749–2758, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leelahavanichkul A, Huang Y, Hu X, Zhou H, Tsuji T, Chen R, Kopp JB, Schnermann J, Yuen PS, Star RA. Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing High-Mobility Group Box Protein-1. Kidney Int 80: 1198–1211, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leelahavanichkul A, Yasuda H, Doi K, Hu X, Zhou H, Yuen PS, Star RA. Methyl-2-acetamidoacrylate, an ethyl pyruvate analog, decreases sepsis-induced acute kidney injury in mice. Am J Physiol Renal Physiol 295: F1825–F1835, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leunk RD, Moon RJ. Physiological and metabolic alterations accompanying systemic candidiasis in mice. Infect Immun 26: 1035–1041, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levallois J, Nadeau-Fredette AC, Labbe AC, Laverdiere M, Ouimet D, Vallee M. Ten-year experience with fungal peritonitis in peritoneal dialysis patients: antifungal susceptibility patterns in a North-American center. Int J Infect Dis 16: e41–e43, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Levine M, Espey MG, Chen Q. Losing and finding a way at C: new promise for pharmacologic ascorbate in cancer treatment. Free Radic Biol Med 47: 27–29, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr 2: 78–88, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyn-Kew K, Standiford TJ. Immunosuppression in sepsis. Curr Pharm Des 14: 1870–1881, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Maqbool M, Vidyadaran S, George E, Ramasamy R. Optimisation of laboratory procedures for isolating human peripheral blood derived neutrophils. Med J Malaysia 66: 296–299, 2011. [PubMed] [Google Scholar]
- 42.Martin GS. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther 10: 701–706, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulu A, Kassu A, Anagaw B, Moges B, Gelaw A, Alemayehu M, Belyhun Y, Biadglegne F, Hurissa Z, Moges F, Isogai E. Frequent detection of ‘azole’ resistant Candida species among late presenting AIDS patients in northwest Ethiopia. BMC Infect Dis 13: 82, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15: 42–49, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Padayatty SJ, Levine M. New insights into the physiology and pharmacology of vitamin C. CMAJ 164: 353–355, 2001. [PMC free article] [PubMed] [Google Scholar]
- 46.Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One 5: e11414, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 140: 533–537, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, Kauffman CA, Hyslop N, Mangino JE, Chapman S, Horowitz HW, Edwards JE, Dismukes WE. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis 37: 634–643, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20: 133–163, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Picazo JJ, Gonzalez-Romo F, Candel FJ. Candidemia in the critically ill patient. Int J Antimicrob Agents 32 Suppl 2: S83–S85, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Rangel-Frausto MS, Wiblin T, Blumberg HM, Saiman L, Patterson J, Rinaldi M, Pfaller M, Edwards JE Jr, Jarvis W, Dawson J, and Wenzel RP. National epidemiology of mycoses survey (NEMIS): variations in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin Infect Dis 29: 253–258, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Ronco C, Bonello M, Bordoni V, Ricci Z, D'Intini V, Bellomo R, Levin NW. Extracorporeal therapies in non-renal disease: treatment of sepsis and the peak concentration hypothesis. Blood Purif 22: 164–174, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Sasada M, Johnston RB Jr. Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med 152: 85–98, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110: 3507–3512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shubin NJ, Monaghan SF, Ayala A. Anti-inflammatory mechanisms of sepsis. Contrib Microbiol 17: 108–124, 2011. [DOI] [PubMed] [Google Scholar]
- 56.Spellberg B, Ibrahim AS, Edwards JE Jr, and Filler SG. Mice with disseminated candiasis die of progressive sepsis. J Infect Dis 192: 336–343, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci USA 112: 1167–1172, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tyml K, Li F, Wilson JX. Delayed ascorbate bolus protects against maldistribution of microvascular blood flow in septic rat skeletal muscle. Crit Care Med 33: 1823–1828, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Valentin A, Le Guennec R, Rodriguez E, Reynes J, Mallie M, Bastide JM. Comparative resistance of Candida albicans clinical isolates to fluconazole and itraconazole in vitro and in vivo in a murine model. Antimicrob Agents Chemother 40: 1342–1345, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Deventer AJ, Goessens WH, van Belkum A, van Vliet HJ, van Etten EW, Verbrugh HA. Improved detection of Candida albicans by PCR in blood of neutropenic mice with systemic candidiasis. J Clin Microbiol 33: 625–628, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Russo TA, Kwon O, Chanock S, Rumsey SC, Levine M. Ascorbate recycling in human neutrophils: induction by bacteria. Proc Natl Acad Sci USA 94: 13,816–13,819, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wiersinga WJ. Current insights in sepsis: from pathogenesis to new treatment targets. Curr Opin Crit Care 17: 480–486, 2011. [DOI] [PubMed] [Google Scholar]
- 63.Wilson JX. Mechanism of action of vitamin C in sepsis: ascorbate modulates redox signaling in endothelium. Biofactors 35: 5–13, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu F, Wilson JX, Tyml K. Ascorbate inhibits iNOS expression and preserves vasoconstrictor responsiveness in skeletal muscle of septic mice. Am J Physiol Regul Integr Comp Physiol 285: R50–R56, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Wu F, Wilson JX, Tyml K. Ascorbate protects against impaired arteriolar constriction in sepsis by inhibiting inducible nitric oxide synthase expression. Free Radic Biol Med 37: 1282–1289, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Yordanov M, Dimitrova P, Patkar S, Falcocchio S, Xoxi E, Saso L, Ivanovska N. Ibogaine reduces organ colonization in murine systemic and gastrointestinal Candida albicans infections. J Med Microbiol 54: 647–653, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Zhou G, Kamenos G, Pendem S, Wilson JX, Wu F. Ascorbate protects against vascular leakage in cecal ligation and puncture-induced septic peritonitis. Am J Physiol Regul Integr Comp Physiol 302: R409–R416, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]