Abstract
Northern ungulates acclimatize to winter conditions with restricted food supply and unfavorable weather conditions by reducing energy expenditure and voluntary food intake. We investigated in a study on red deer whether rates of peptide and glucose transport in the small intestines are also reduced during winter as part of the thrifty phenotype of winter-acclimatized animals, or whether transport rates are increased during winter in order to exploit poor forage more efficiently. Our results support the latter hypothesis. We found in a feeding experiment that total energy intake was considerably lower during winter despite ad libitum feeding. Together with reduced food intake, mass of visceral organs was significantly lower and body fat reserves were used as metabolic fuel in addition to food. However, efficacy of nutrient absorption seemed to be increased simultaneously. Extraction of crude protein from forage was higher in winter animals, at any level of crude protein intake, as indicated by the lower concentration of crude protein in feces. In line with these in vivo results, Ussing chamber experiments revealed greater electrogenic responses to both peptides and glucose in the small intestines of winter-acclimatized animals, and peptide uptake into jejunal brush-border membrane vesicles was increased. We conclude that reduced appetite of red deer during winter avoids energy expenditure for unproductive search of scarcely available food and further renders the energetically costly maintenance of a large gut and visceral organs unnecessary. Nevertheless, extraction of nutrients from forage is more efficient in the winter to attenuate an inevitably negative energy balance.
Keywords: seasonal acclimatization, nutrients, active transport, peptide transport, glucose transport
nutrient uptake in the gastrointestinal tract can be mediated by passive diffusion and/or active transport. The latter is an energy-consuming process, enabling transport against an electrochemical gradient. The molecular mechanisms and regulation of active transport of nutrients are well studied in domestic and laboratory animals, e.g., the uptake of glucose via the sodium glucose transporter 1 (SGLT1; Refs. 20 and 56) or that of peptides via peptide transporter 1 (PEPT1; Ref. 33). Many studies have found that expression of intestinal transport proteins is positively influenced by the concentration of nutrients flushing the gut. For instance, the expression of SGLT1 in the small intestine dramatically declines during the transition from the preruminant to the mature ruminant state in goats, sheep, and dairy cattle, apparently as a result of the coincident decline in the concentration of luminal monosaccharides (19, 54). In contrast, concentrate-selecting cervids like roe deer, which are supposed to bypass ruminal fermentation of soluble nutrients down the reticular groove, have, as adults, considerably higher SGLT1 expression in the small intestine than grass- and roughage-consuming ruminants (43–45). Therefore, if rates of intestinal active transport depend on the concentration of nutrients in the gut content, one would expect a change of the extent and importance of these processes with season in herbivores living in seasonal environments.
Northern latitudes or high altitudes are characterized by profoundly differing living conditions between winter and summer, particularly for herbivores. Outside of the vegetation period, the availability and quality of plant material are considerably lower and difficult to access if covered by snow. In addition, temperatures are considerably lower during winter, imposing higher energy expenditure for thermoregulation to endothermic organisms. Strategies to escape from this double-bind dilemma are hibernation or daily torpor in small mammals (17). Ungulates of northern latitudes react similarly, although they do not hibernate or show daily torpor in the classical sense. However, they also become hypometabolic during winter, as indicated by a resting heart rate reduced to approximately half of the rate found during summer. The reduction of energy expenditure is accomplished to some degree by reduced locomotor activity, but mainly results from lower endogenous heat production. Nevertheless, core body temperature of these large mammals is only slightly lower during winter compared with summer, but hypothermia is pronounced in the body's shell, particularly during cold winter nights (3, 4, 49, 51).
The thrifty winter phenotype of seasonal mammals further includes a shift from an anabolic metabolism during summer to the use of body fat reserves to fuel metabolism during winter (review in Refs. 2 and 41). Food intake ceases completely during winter in many hibernators (2) and is reduced in nonhibernating northern ungulates. A number of species are well known for a decline of voluntary food intake (VFI) during winter (32; also see Ref. 4 for a review of ruminants). Seasonal changes of VFI persist under experimental ad libitum feeding (36, 41, 51), thus representing an endogenous seasonal cycle of appetite entrained by photoperiod (22, 36). If less food is to be processed, one would expect a reduction of the mass of the gut and, hence, its maintenance cost. Shrinking of visceral organs and the alimentary tract during winter has, indeed, been reported for hibernating marmots (28), chamois (23), red deer, and roe deer (26). Further, the number and size of the papillae in the rumen were significantly lower during the winter period than during the growing season in red deer, fallow deer, roe deer, chamois, and mouflon (24, 30). This apparently lowered capacity for nutrient uptake during winter is contrasted by longer gut passage time. Prolonged digestion can extract nutrients more efficiently and is to be expected if forage is of poor quality (47). A lower dry matter intake is believed to be negatively correlated with mean retention time in the gastrointestinal tract (48), a correlation reported for cattle, horses, sheep, donkeys, and red deer (12, 29, 32). However, whether expression of nutrient-transporting proteins and, hence, rates of active transport also follow a seasonal cycle, is unknown. We firstly address this question in the present study.
Taking the available evidence together, we formulate two mutually exclusive hypotheses about seasonal changes of nutrient transport in the gut: 1) higher rates of active transport during winter increase exploitation of poor forage, and 2) rates of active transport are reduced during winter, as part of the thrifty phenotype of winter-acclimatized animals, induced by lower concentrations of nutrients in the gut lumen.
We tested these hypotheses in red deer (Cervus elaphus), an animal model well known for its profound seasonal changes of many physiological traits.
METHODS
Study Animals and Site
We studied a herd of red deer, adult females, and their progeny from several years, kept together with an adult stag in a 45-ha enclosure adjacent to the Research Institute of Wildlife Ecology in Vienna, Austria (48°13′N, 16°17′E, 360 m above sea level). This area consists of about 39 ha of deciduous oak-beech forest and a meadow of about 6 ha. In addition to natural forage available in the enclosure, the animals received hay and commercially available pellets for red deer (Trophy ST, Raiffeisen, Austria) delivered ad libitum at a computer-controlled feeding station continuously throughout a day and year. Except for the feeding experiment, pellets consisted of 13% crude protein, 3.9% crude fat, 13.7% crude fiber, and 54.5% nitrogen-free extract (nfe). All procedures and experiments were discussed and approved by the institutional ethics and animal welfare committee in accordance with guidelines for good scientific practice and national legislation.
Feeding Experiment
We performed a feeding experiment with 16 female red deer of ages 14 to 86 mo (mean 48) to quantify seasonal variation of VFI and nutrient uptake. These animals had been trained prior to the experiment to enter and feed at the computer-controlled feeding station. A two-gate system ensured that only a single animal at a time could enter the feeding station. The necessary narrowness of the gates for separating individuals made the station inaccessible to males, particularly when bearing antlers. When an animal lowered its head to the feeding trough, its ear tag transponder was recognized by a reading antenna, and the system delivered pellets at a rate of 95 g every 40 s. Pellet delivery stopped when an animal raised its head. The animals usually emptied the feeding trough before leaving the station, enabling accurate measurement of the mass of pellets eaten.
During the 22 mo of experiment, each animal received ad libitum two types of food pellets (Altromin, Lage, Germany) in a cross-over design. The two types comprised crude protein, crude fat, and crude fiber in concentrations (dry organic matter) typical for the natural diet of free-living red deer in July [“summer pellet”: crude protein 30.9% crude fat 6.9%, crude fiber 21.5%, nitrogen-free extract (nfe) 40.6%], and in January [“winter pellet”: crude protein 17.4%, crude fat 3.4%, crude fiber 31.8%, nfe 47.4%], respectively (4). The station was visited throughout the day and night, with slight peaks around dawn and dusk, as to be expected for a crepuscular animal with weak or even absent influence of the internal circadian timing mechanism on daily activity patterns (15).
Experimental feeding of pellets began 2 mo prior to the experiment with eight animals assigned randomly to the summer pellet and winter pellet group, respectively. Pellet types fed to each animal were switched at the beginning of the experiment and again 12 mo later. During the feeding experiment, the animals had unlimited access to the natural forage in the enclosure but did not receive other supplements like hay.
Assessment of Natural Forage Intake and Composition
Intake and composition of natural forage were assessed with the n-alkane method (14, 22, 37). The method takes advantage of similar fecal recoveries of naturally occurring tritriacontane (C33) and artificial dotriacontane (C32), fed as an external marker. Both summer and winter pellets contained 0.085 mg C32/g. Because pellet consumption was measured, we also knew the amount of C32 ingested per day. Daily intake of natural forage by a given individual was inferred from the dilution of ingested C32 in fecal samples obtained the day after measuring C32 intake, compared to the concentration of C33 in the same sample ingested with herbage.
Plants contain in their cuticular waxes predominantly n-alkanes of odd carbon chain lengths. Concentrations on n-alkanes differ remarkably among species and can be used to quantify the proportions of different plant species in the forage intake of herbivores from fecal material. For determining the number of detectable n-alkanes and their concentrations in the food plants available to our deer, we sampled prior to the study the 10 most common plant species representing ∼80% of ground coverage of the meadow in the enclosure. Altogether, 10 different n-alkanes (odd-chain C21–C35 and even-chain C28 and C30) could be identified. This enabled reliable quantification of the relative proportions of 10 species in an animal's intake of natural forage during the day before sampling of feces (14), assuming a gut passage time of 24 h.
Every other month, fecal samples were collected from each experimental animal following the grazing herd. During the day, before sampling of feces, we noted which plants were consumed by an animal and roughly estimated an order of preference. The 10 most frequently eaten plant species and plant parts were then sampled for analysis of n-alkanes.
Plant and fecal samples were dried at 60°C for at least 48 h to constant weight and grounded to a particle size of ∼1 mm with a mixer. Prepared samples were analyzed quantitatively for n-alkanes, according to Mayes et al. (37) by gas chromatography (Autosystem XL; Perkin Elmer, Rodgau, Germany) and for dry matter crude protein (Kjedahl method), crude fat (Soxhlet method), nfe, crude ash, and crude fiber content by Weender analysis (39).
Diet composition was estimated with a maximum-likelihood optimization procedure (R-package “nlminb”), finding the relative proportions of sampled food plants that optimally fit the found n-alkane concentrations in a fecal sample (40). The total intake of nutrients from natural forage was calculated for each animal and sampling day from the estimated dry matter intake, the percentages of various food plant species in the ingested material, and the percentages of nutrients in these plants. Energy content of pellets and natural forage was calculated using energetic values of nutrients given in Refs. 34 and 35.
Tissue and Organ Sampling
During a time period of 4 years, we euthanized animals for tissue and organ sampling. Between July 17 and 22 (summer), 3 males and 11 females of ages 7–181 mo were euthanized, and between January 14 and February 19 (winter), 1 male and 11 females of ages 13–157 mo were euthanized, either by gun shot from a distance or by pin shot after capture. Subsequently, blood was drained through opening of the Arteria carotis and the Vena jugularis. Seven of these animals had been in the feeding experiment before but were sacrificed 6 years after its termination. Thus, any carry-over effects are unlikely. The considerable age differences of sampled individuals were due to the fact that we continuously removed offspring at various ages to limit the number of deer in the enclosure before eventually sacrificing the whole herd. All males used for the study were clearly nonreproductive (3 were 7 mo old, one 13 mo); 6 females killed during winter were pregnant, and 4 that were killed in July had given birth in May. These females were considered “reproductive” in our analyses. We have no exact information about their previous reproductive history. However, females in the enclosure seemed to reproduce at rates typical for free-living red deer (38).
Immediately after shooting, each animal was weighed to the nearest kilogram with a portable balance. Carcasses were then moved to the institute's pathology for dissection. Heart, liver, spleen, kidney, and the fat depot around a kidney were removed and weighed to the nearest gram. Rumen and abomasum were also removed, emptied, rinsed, and weighed to the nearest gram. The rumen was then filled with water to determine the volume in liters.
A section of ∼1 m from the distal jejunum and from the mid-jejunum was opened along the mesenteric line, rinsed with ice-cold saline, and transferred to the laboratory for Ussing chamber studies. While samples for Ussing chamber experiments were kept in cold, modified Krebs-Henseleit buffer aerated with carbogen, tissues for later preparation of isolated brush-border membrane vesicles were snap-frozen in liquid nitrogen and stored at −80°C.
Ussing Chamber Measurements
As preliminary experiments had shown that the distal jejunum can be stored in cooled buffer solution aerated with carbogen without any negative effects on integrity and responsiveness, Ussing chamber experiments were carried out in two series. In the first series, the mid-jejunum was mounted, while the distal jejunum was investigated in the second series of experiments, ∼140 to 160 min later. Apart from different time periods between the death of the animal and start of the measurements, both segments were incubated using the same conditions.
After removal of the serosal muscle layers, stripped epithelia were mounted into six chambers with an open surface of 1.13 cm2 and incubated at 37°C in aerated buffer solutions with 0.1 mM indomethacin to inhibit endogenous synthesis of prostaglandins. The buffer solution on the serosal side of the epithelia contained 113.6 mM NaCl, 5.4 mM KCl, 0.4 mM HCl, 1.2 mM MgCl2, 1.2 mM CaCl2, 21.0 mM NaHCO3, 1.2 mM Na2HPO4, 0.3 mM NaH2PO4, 10.0 mM glucose, and 23.0 mM mannitol. The pH was adjusted to 7.4. On the mucosal side, the buffer contained no glucose (113.6 mM NaCl, 5.4 mM KCl, 1.2 mM MgCl2, 1.2 mM CaCl2, 2.0 mM NaHCO3, 0.37 mM Na2HPO4, 1.13 mM NaH2PO4, 19.83 mM Na-gluconate, 32.94 mM mannitol, 0.2 mM HCl), and the pH was adjusted to 6.4. The addition of amastatine to a final concentration of 10 μM to the mucosal buffer inhibited brush-border membrane peptidase activity.
Potential difference across the epithelia was clamped to 0 mV by application of a short-circuit current (Isc) that counterbalanced the potential difference generated by electrogenic net ion transport. Under these conditions, the addition of glucose or dipeptides like glycyl-glutamine (Gly-Gln) results in an increase in Isc due to net electrogenic ion transport.
Six epithelia from the middle and the distal jejunum were measured in parallel. A single mean value for each animal and gut section was calculated for the basal Isc recorded after an equilibration period of 30 min, for the increase after the addition of Gly-Gln to a final concentration of 12 mM, and for the increase after the addition of glucose to a final concentration of 10 mM. Only these means were used for statistical analyses. At the end of each experiment, forskolin (10 μM) was added to the serosal side to check whether chloride secretion, an indicator of the viability of the epithelia, could be induced. Single epithelia that did not respond to forskolin were excluded. Therefore, statistical analysis was done with mean values of five to six epithelia per animal and segment. For one winter and two summer animals, the mid-jejunum was excluded completely because four out of six preparations did not react properly.
Brush-Border Membrane Vesicles
Transport kinetics (Vmax, Km) of glucose or glycyl-sarcosine (Gly-Sar) uptake into isolated intestinal brush-border membrane vesicles (BBMV) was studied in a subset of six winter animals (all female, ages 6–8 mo), and 6 summer animals (2 males, 4 females, ages 13–25 mo). We choose Gly-Sar instead of Gly-Gln, as used for Ussing chamber experiments, because at the time of BBMV experiments, only Gly-Sar was available with the necessary 3H-labeling at a reasonable price, and a former study with porcine jejunum had found no relevant differences between Gly-Gln and Gly-Sar transport (53).
Respective BBMV were prepared from enterocytes by Mg2+-EGTA precipitation and differential centrifugation (6, 7, 9, 52). Briefly, 30 g of tissue (wet weight) were thawed on ice in 60 ml BBMV buffer I (300 mM mannitol, 12 mM Tris, 5 mM EGTA, pH 7.1). Enterocytes were separated from the underlying tissue by 10 min of vibration using a vibromixer type E1 (Chemap, Volketswil, Switzerland). The cell suspension was then diluted with distilled water (1:5) and homogenized for 3 min with a blender (Moulinex, Germany; type 530.02). A sample was taken from the first homogenate (sample 1, S1). In the following, basolateral membranes were precipitated by the addition of MgCl2 to a final concentration of 10 mM. The pellet obtained by a first centrifugation (18 min, 3,295 g, 4°C) was discarded, and the supernatant was centrifuged again (40 min, 26,890 g, 4°C). After the second centrifugation, the pellet was homogenized in 35 ml of BBMV buffer II (60 mM mannitol, 5 mM EGTA, pH 7.1) using a Potter-Elvehjem (S30; Braun, Melsungen, Germany). Precipitation of basolateral membranes by the addition of MgCl2 and a two-step centrifugation was repeated, as described above, followed by resuspension of the pellet in 35 ml of BBMV buffer IIIa (BBMV for glucose uptake, mid-jejunum: 100 mM mannitol, 100 mM KCl, 10 mM HEPES, pH 7.4) or buffer IIIb (BBMV for dipeptide uptake, distal jejunum: 100 mM mannitol, 100 mM KCl, 35 mM HEPES, 1 mM MgSO4, pH 7.8). After one more centrifugation (45 min, 34,540 g, 4°C), the pellet containing enriched brush-border membranes was resuspended in 2 ml of buffer IIIa or IIIb by passing the suspension 10 times through a 26-gauge needle in order to allow the membranes to form vesicles.
Total protein content of S1 and the final BBMV suspension (S2) was determined according to Bradford (10). The integrity of BBMV preparations was tested in samples from one summer and winter animal, respectively. In both samples, we recorded so-called “glucose overshoot”, i.e., a rapid glucose influx into vesicles exceeding diffusional balance of glucose concentrations within vesicles and the surrounding medium. Glucose overshoot indicates the presence of intact closed vesicles (27).
For determining the degree of enrichment of brush-border membranes in the preparations, we measured the activities of alkaline phosphatase, a key enzyme of the brush-border membrane of enterocytes, and of the Na+/K+-ATPase, a key enzyme of the basolateral membrane, respectively. Enrichment was considered sufficient if alkaline phosphatase activity was at least 18 times higher than that of Na+/K+-ATPase. Other preparations were excluded from statistical analyses. No differences between summer and winter animals occurred with respect to the enrichments.
The kinetic parameters Vmax (nmol·mg protein−1·15 s−1) and Km (mmol/l) for glucose or Gly-Sar uptake into BBMV were calculated using the Michaelis-Menten equation (46). BBMV from the distal jejunum were used for studying peptide transport, while those from the mid-jejunum were used for studying glucose transport. Uptake of glucose and of Gly-Gln were quantified by the rapid filtration technique, as described earlier (46). A BBMV suspension (20 μl) was incubated in buffer solution (80 μl) containing 74 kBq (2 μCi) of [3H]-d-glucose (Perkin Elmer) or 37 kBq (1 μCi) [3H]-Gly-Sar (Hartmann Analytic, Braunschweig, Germany), and nonlabeled glucose (8 concentration steps: 0.01, 0.025, 0.05, 0.1, 0.2, 0.4, 0.8, and 1.5 mM; Sigma-Aldrich, St. Louis, MO), or nonlabeled Gly-Sar (six concentration steps: 0.25, 0.5, 1, 2.5, 5, and 10 mM; Sigma-Aldrich), respectively. The buffer solution used for measuring uptake of glucose contained either 100 mM NaCl or 100 mM KCl along with 100 mM mannitol and 10 mM HEPES (pH 7.4). The incubation buffer used for uptake of Gly-Sar consisted of 100 mM KCl, 100 mM mannitol, 1 mM MgSO4, 15 mM HEPES, and 50 mM MOPS (pH 5.9) or 100 mM KCl, 100 mM mannitol, 1 mM MgSO4, and 15 mM HEPES (pH 7.8). All buffer solutions used for Gly-Gln uptake studies contained 10 μM amastatine to prevent hydrolysis of dipeptides (13, 53). BBMV suspensions were incubated at room temperature (glucose) or 37°C (Gly-Sar), and incubation was stopped after 15 s by the addition of 1 ml ice-cold stop buffer solution. The stop buffer solution consisted of either 150 mM KCl and 10 mM HEPES (pH 7.4) (for glucose uptake studies) or 100 mM mannitol, 100 mM KCl, 35 mM HEPES, and 1 mM MgSO4 (pH 7.8) (for Gly-Sar uptake studies). The solution was immediately filtered by vacuum suction, and filters were washed twice with 4 ml of stop buffer solution to remove extravesicular radioactivity. Blanks were obtained from incubation of respective buffer solutions without vesicular membranes. Total radioactivity was counted in 80 μl of buffer solution at the end of each experiment. All measurements were performed in triplicate. Filters were dissolved in scintillation fluid (Perkin Elmer) for at least 30 min before radioactivity was measured using a liquid scintillator counter with a counting accuracy >95% (Packard Tricarb liquid scintillation analyzer, Dreieich, Germany).
Statistical Analyses
Statistical analyses were performed using software R (42) with P values derived from two-tailed tests. Data were analyzed by linear modeling and are presented as means ±95% confidence intervals (CI). In analyses of Ussing chamber measurements, we included a factor “gut section” in the model and adjusted for this double measurement of each individual by including “individual ID” as a random intercept factor in the mixed effects model (function “lme” in R). Furthermore, we included a factor “type of killing” in order to correct for possible differences caused by the two different methods used. Justification of parametric testing was checked with Shapiro-Wilk tests of normality, diagnostic tools available in R, and visual inspection of residuals. If necessary, data were transformed to obtain normal distribution of model residuals.
Kinetic values Vmax and Km for glucose and Gly-Sar uptake as a function of substrate concentration were calculated by fitting respective uptake rates vs. respective concentrations in extravesicular buffer solution with a GraphPad (GraphPad Prism version 5 for Windows GraphPad Software, San Diego, CA) algorithm to a rectangular hyperbola relationship obtained from the law of mass action (46).
RESULTS
Seasonal Variation of Energy Intake
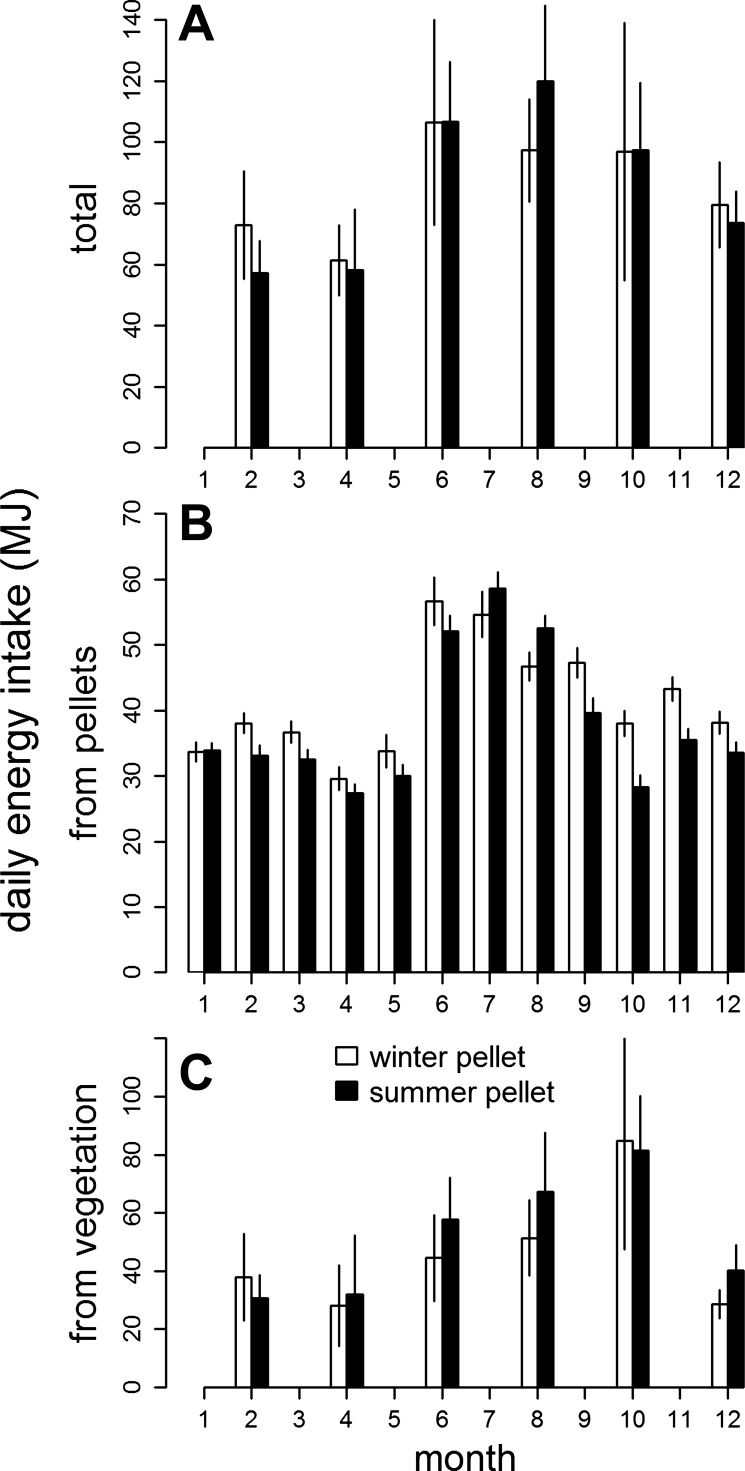
The total amount of energy intake varied considerably over the year with a nadir in late winter and early spring. Thereafter, energy intake more than doubled in a few weeks to reach a peak plateau during June to October, before it began to decline again in late autumn/early winter (Fig. 1A, differences between months, F5,60 = 8.33; P < 0.001). The type of pellet fed had no significant influence (interaction of factors months and pellet type, F5,60 = 0.93; P = 0.466).
Fig. 1.
Seasonal changes of total daily energy intake (A), energy intake from pellets (B), and from natural vegetation (C) (monthly means with 95% confidence intervals) of adult red deer hinds provided with pellets of lower vs. higher content of crude protein and energy (“winter” vs. “summer” pellets), respectively.
Over the year, total energy intake from pellets was similar to energy intake from natural vegetation (F1,4411 = 0.456, P = 0.500). However, the relative contributions of both food types to daily energy intake varied between months (interaction of factors months and source of energy, F1,4411 = 9.59, P < 0.001; cf. Fig. 1, B and C). While energy intake from pellets remained about 30 to 40 MJ per day rather constant from November to May, it increased considerably to higher values during June to August (Fig. 1B, differences between months, F11,8594 = 117.64, P < 0.001). Similarly, the relative contribution of type of pellet received to total energy uptake from pellets varied with season (interaction of factors months and pellet type, F11,8594 = 15.63, P = 0.001), although the total energy intake over the year from pellets did not significantly differ between pellet types fed (difference between pellet types, F1,8594 = 0.72, P = 0.398).
Energy intake from natural vegetation was lowest during the winter months and increased continuously from spring to late autumn (Fig. 1C, effect of month, F5,60 = 6.51, P < 0.001). Again, pellet type fed had no significant influence on this seasonal effect (interaction of factors months and pellet type, F5,60 = 0.69, P = 0.637), nor on the total amount of energy intake from natural vegetation over the year (effect of pellet type, F1,60 = 0.39; P = 0.535). During October, energy intake from vegetation reached its annual maximum of 83 MJ/day (95% CI ± 17.4) being about three times higher during this month than energy intake from pellets (F1,864 = 37.48, P < 0.001; cf. Fig. 1, B and C).
Seasonal Differences in the Extraction of Crude Protein from Forage Intake
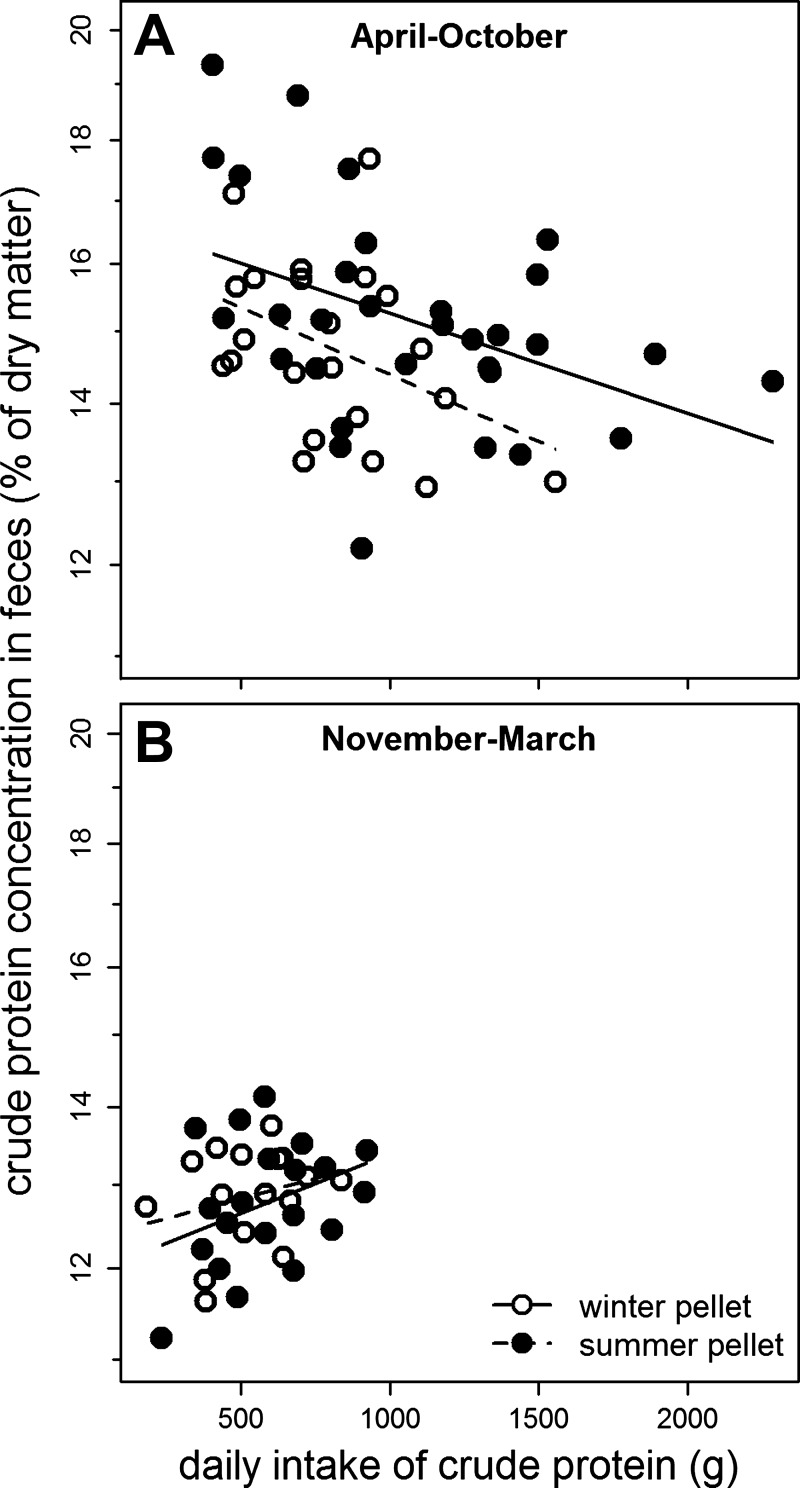
During summer, uptake of crude protein increased with the total amount of crude protein ingested per day, as indicated by the negative correlation between the amount of crude protein ingested and the concentration of crude protein found in feces sampled during the following day (Fig. 2A, F1,32 = 6.04, P = 0.020). This relation was absent during winter (Fig. 2B, F1,14 = 0.167, P = 0.689) when efficacy of protein extraction seemed to be at maximum, resulting in much lower concentrations of crude protein excreted with feces (difference of intercepts of regressions Apr–Oct vs. Nov–Mar, F1,61 = 25.55, P < 0.001). These results were not influenced by the type of pellet fed (Fig. 2A, difference of slopes F1,32 = 0.26, P = 0.612, difference of intercepts F1,11 = 0.00, P = 0.958; Fig. 2B, difference of slopes F1,14 = 0.18, P = 0.676, difference of intercepts F1,14 = 0.05, P = 0.824).
Fig. 2.
Concentrations of crude protein in feces in relation to total crude protein intake during the previous day, in red deer hinds studied during summer (A) and winter months (B), and provided with pellets of lower vs. higher content of crude protein and energy (“winter” vs “summer” pellets), respectively (note logarithmic scale of y-axis).
Seasonal Variation of Organ, Fat Depot, and Body Mass
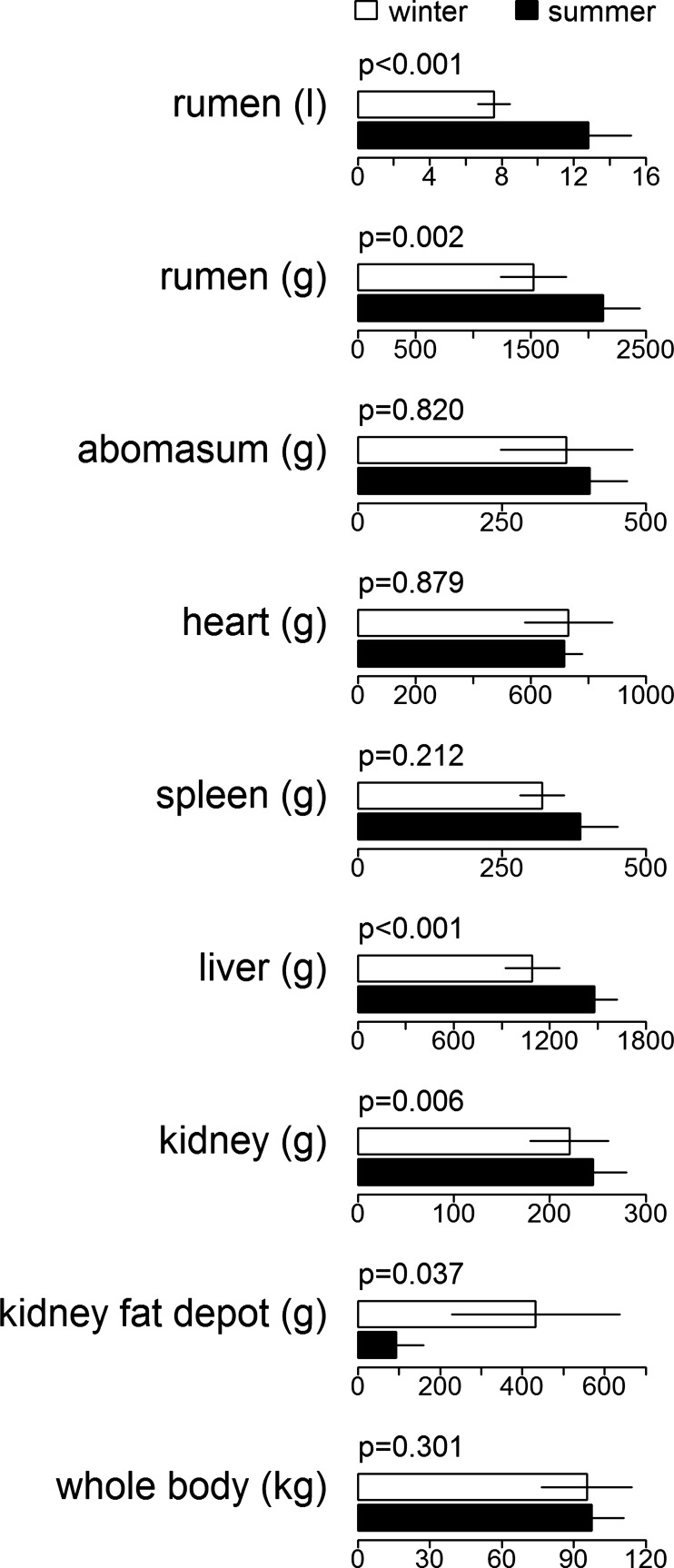
In contrast to the apparent upregulation of nutrient extraction in the gut during winter, we found, after correcting for the influence of body mass, that mass of visceral organs was significantly lower in winter animals compared to summer animals. Rumen volume, empty rumen mass, liver mass, and kidney mass were significantly reduced during winter (Fig. 3), compared to the mass in July by 41%, 28%, 26%, and 10%, respectively. One exception was the kidney fat depot, which in January/February was 78% larger than during July. We did not find significant seasonal differences in the abomasum, heart, spleen, and total body mass (Fig. 3).
Fig. 3.
Variation of organ and body mass between seasons. Rumen and abomasum mass were measured after emptying the organ, rumen volume by filling the empty rumen with water. P values for seasonal differences of organ mass or volume, given above pairs of bars, are adjusted for influences of body mass, age, and sex; comparison of body mass is adjusted for age and sex.
Electrochemical Analyses of Nutrient Transport
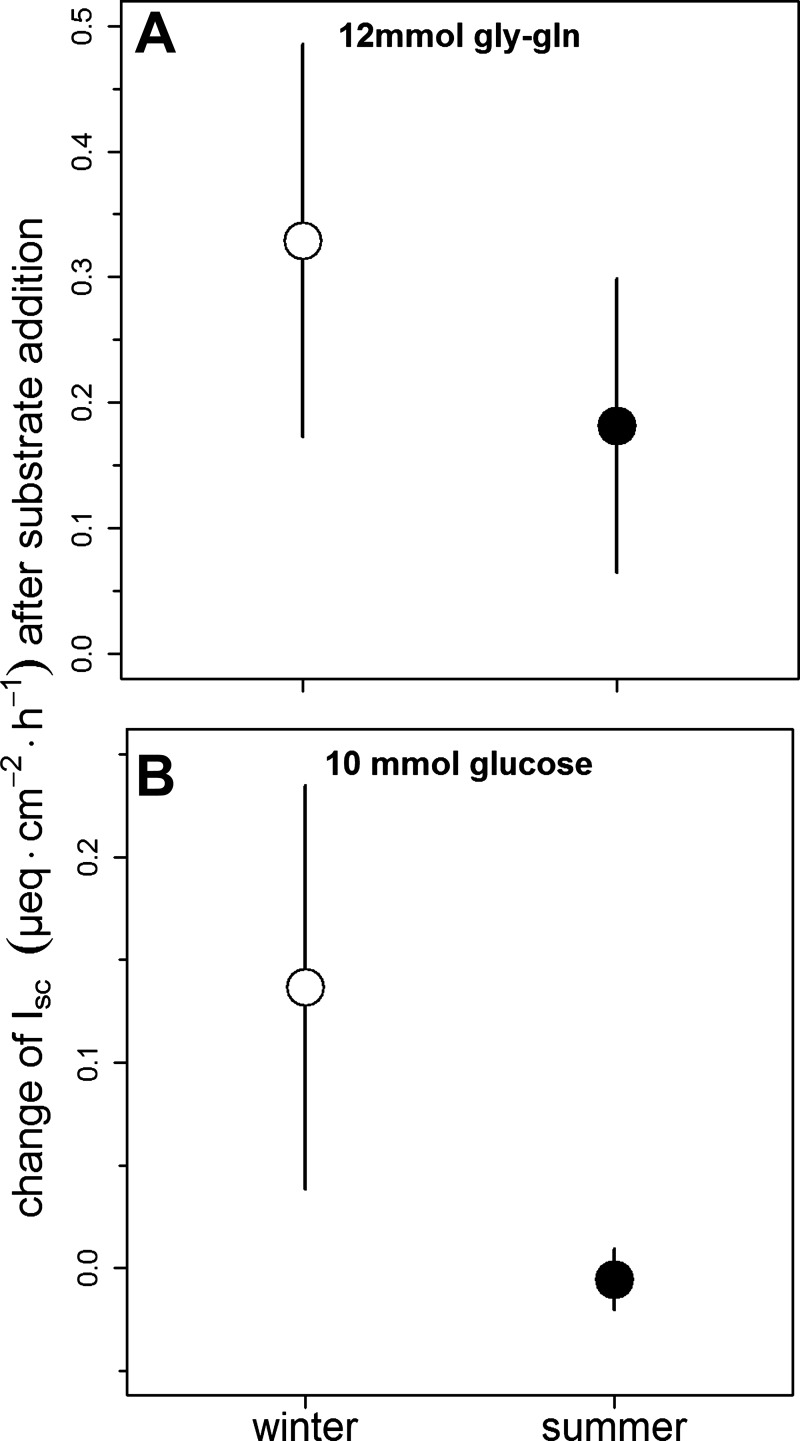
Results from electrochemical and BBMV studies were in line with differences in crude protein extraction during summer and winter found in the feeding experiment (Fig. 2). Measurements in Ussing chambers revealed significant differences between samples from winter and summer animals in the changes of the short-circuit current (Isc) across epithelia of the small intestine after adding substrates. Both, Gly-Gln and glucose increased Isc more in small intestinal segments from winter animals compared to tissues from summer animals, indicating higher electrogenic transport (Fig. 4). Furthermore, glucose increased Isc more in some of the oldest individuals in the experiment (aged 12–15 yr), and preparations from the distal jejunum seemed to have higher Isc than those from middle jejunum upon adding Gly-Gln. No significant differences were found between reproducing and not reproducing animals, or type of killing (Table 1).
Fig. 4.
Rates of dipeptide (glycyl-l-glutamine; A) and glucose transport (B) across small intestine epithelium preparations from winter (dipeptide, n for calculating plotted means and CI = 27; glucose, n = 25) and summer animals (dipeptide, n = 22; glucose, n = 22), determined by Ussing chamber measurements. See Table 1 for results of statistical analyses.
Table 1.
Factors influencing changes in short-circuit current (Isc) across epithelia of small intestines in Ussing chambers after substrate addition
| Substrate |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 mM Glucose |
12 mM Glycyl-l-Glutamine |
|||||||||
| Fixed Effects | Coefficient | Standard Error | F | df | P | Coefficient | Standard Error | F | df | P |
| Log (Isc before substrate addition) | 0.95 | 0.042 | 519.55 | 1,19 | <0.001 | 1.13 | 0.076 | 224.72 | 1,21 | <0.001 |
| Seasona | 0.11 | 0.029 | 15.35 | 1,19 | <0.001 | 0.09 | 0.043 | 4.66 | 1,21 | 0.043 |
| Log (age in months) | 0.05 | 0.020 | 6.09 | 1,19 | 0.023 | 0.01 | 0.029 | 0.22 | 1,21 | 0.647 |
| Reproductionb | 0.06 | 0.047 | 1.88 | 1,19 | 0.186 | 0.05 | 0.079 | 0.37 | 1,21 | 0.547 |
| Gut sectionc | 0.01 | 0.063 | 0.04 | 1,19 | 0.850 | 0.10 | 0.052 | 4.06 | 1,21 | 0.057 |
| Type of killingd | 0.02 | 0.037 | 0.44 | 1,19 | 0.516 | 0.05 | 0.056 | 0.82 | 1,21 | 0.374 |
Statistics are obtained from linear mixed modeling with log-transformed-dependent variable, and “animal” as random factor to correct for repeated measurements in two gut sections of each individual.
January or February vs. July.
Gravid (winter) or lactating (summer) vs. not reproducing.
Mid-jejunum vs. distal jejunum.
Gun shot vs. pin shot.
Brush-Border Membrane Uptake Studies
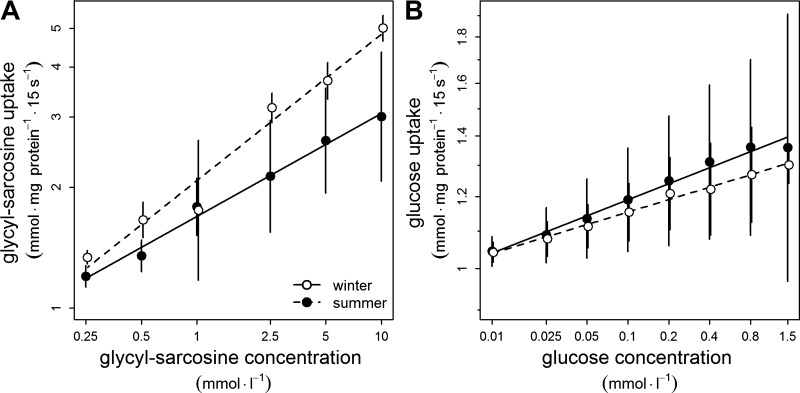
The maximal rate (Vmax) of the H+-dependent uptake of Gly-Sar into BBMV from winter animals was 5.5 mmol·mg protein−1·15 s−1 (± 0.3), significantly higher (F1,6 = 6.66, P = 0.042) than that found in BBMV from summer animals (3.0 ± 0.9 mmol·mg protein−1·15 s−1). Analysis of substrate concentration-dependent uptake confirmed this result (Fig. 5A). In contrast, concentration-dependent uptake of glucose (Fig. 5B), as well as Vmax of the Na+-dependent uptake of glucose, was not significantly different in BBMV from winter and summer animals (0.3 ± 0.1 mmol·mg protein−1·15 s−1, vs. 0.4 ± 0.3 mmol·mg protein−1·15 s−1; F1,6 = 0.40, P = 0.551).
Fig. 5.
Concentration-dependent uptake of a dipeptide (A) and glucose (B) in vesicles prepared from brush-border membranes of small intestine enterocytes (note logarithmic scales). Differences in slopes of regression for dipeptide uptake, F1,37 = 6.20, P = 0.017; for glucose uptake, F1,46 = 3.02, P = 0.089.
DISCUSSION
Our study clearly supports the hypothesis that winter-acclimatized red deer exhibit higher efficacy of nutrient absorption. Results about peptide transport across enterocytes in the jejunum were unambiguously in line. Both, electrogenic responses to the addition of nutrients during Ussing chamber experiments, as well as direct determination of peptide uptake into BBMV, indicated higher H+-dependent peptide absorption during winter. In addition, the feeding experiment demonstrated in vivo that red deer, indeed, extract crude protein to a larger degree from ingested forage during winter, and, therefore, excrete lower concentrations of crude protein in feces.
Results on glucose transport suggested that this process is regulated similarly to the transport of peptides. However, the evidence was weaker. While the electrogenic response to the addition of glucose in Ussing chamber experiments was significantly higher in winter compared to summer animals, no differences could be found with respect to Na+-dependent uptake of glucose into BBMV. This might be due to the fact the BBMV technique can only provide kinetic data of sodium-coupled glucose uptake across the isolated apical membrane, whereas Ussing chamber experiments represent data obtained from intact intestinal mucosa, with its complete cell machinery, including regulatory factors, such as protein kinases (55). Furthermore, the intestinal segments used for Ussing chamber experiments and uptake studies were not identical. For technical reasons, BBMV for the determination of peptide uptake were prepared from the distal jejunum, while for investigation of glucose uptake, the mid-jejunum was used. Although not significantly different, electrogenic responses to the addition of substrates or forskolin were slightly smaller in mid-jejunum compared to distal gut segments. Such differences in electrogenic responses and glucose uptake into BBMV have recently been shown for porcine small intestines (18, 21). It might be that the effect of season on glucose uptake was obfuscated due to the fact that values for Vmax were generally smaller in the mid-jejunum compared to the distal jejunum in red deer, too. Unfortunately, we have no additional data about glucose uptake in vivo to clarify this inconsistency.
The feeding experiment further elucidated how nutrient absorption is regulated in red deer. In summer-acclimatized animals, we found a negative correlation between crude protein intake and crude protein excretion with feces, as to be expected if the involved enzymes and both amino acid and peptide transporters are stimulated by dietary protein when fed above maintenance level (1, 31). Protein extraction increasing with intake has also been found in Przewalski horses (32). However, this relation was not found in our deer during the winter months, suggesting that a seasonal signal was superimposed, or even substituted, for transporter expression regulation by nutrient concentrations in the gut. This could have been an endogenous signal entrained by photoperiod. For instance, the ability to actively transport nutrients is maintained in intestinal tissues of hibernating ground squirrels compared with their active counterparts and shows apparent upregulation in hibernators when transport rates are normalized to tissue mass (11).
Alternatively, the lowered VFI during winter may have induced expression of transporters. While peptide and amino acid uptake seems to be stimulated by dietary protein when daily intake is high (>18%), no changes caused by substrate concentration could be found when dietary protein intake did not meet maintenance level (16), suggesting that with very low substrate concentrations, expression is not decreased below a certain threshold. This would be in line with the rather low fecal protein excretion in winter in the present study, indicating that either the photoperiod or the reduced VFI stimulated all processes involved in protein digestion and absorption. In the rat, for example, fasting increases dipeptide transport in the intestine by increasing the abundance of PEPT1 in the brush-border membrane. The mechanism appears to be an increase in PEPT1 gene expression (50).
Further support for different regulation of transporter expression during summer and winter comes from the BBMV study. Variation of nutrient uptake seemed to be higher in preparations from summer animals for most substrate concentrations tested (Fig. 5), as one would expect if the amount of nutrient intake varies more during summer (Fig. 2) and, hence, regulates transporter expression to a larger extent than during winter. However, ultimate corroboration of whether nutrient transporter activity in red deer is, indeed, differently regulated in summer- and winter-acclimatized animals is still lacking, because the test of difference between slopes of regressions of these relations between summer and winter months did not reach statistical significance (Fig. 2; difference between slopes of regression Apr–Oct vs. Nov–Mar, F1,61 = 2.80, P = 0.100). Altogether, our study shows for the first time, to our knowledge, that nutrient transport rates can vary seasonally.
Along with increased digestive efficacy during winter, due to presumably longer gut passage time of ingested food (12, 29, 32, 48) and increased rates of absorption (this study), we found a reduction of voluntary food intake (Fig. 1) and size of visceral organs (Fig. 3), as to be expected from previous studies (23, 25). Interestingly, these changes were not associated with detectable differences in whole body mass (Fig. 3), presumably due to the ad libitum availability of pellets. However, what we did find is the expected accumulation of body fat during summer/autumn, and its consumption during the winter months, as indicated by seasonal mass changes of the kidney fat depot (Fig. 3), a good indicator of the mass of other depots, e.g., fatty tissue in the rump region (38). In line with this result, the deer showed during the summer/autumn fattening period an increasing preference for natural forage (Fig. 1C), particularly for energy-rich seeds like beechnuts and acorns, being amply available in the enclosure during autumn. The deer further showed a significant preference for winter pellets from September to March (with the exception of January, Fig. 1B), despite the lower energy content of this pellet type. However, winter pellets also contained less crude protein, causing considerably higher heat increment of feeding (8). Consequently, consumption of winter pellets was found to be associated with lower energy expenditure than consumption of summer pellets (51). Therefore, avoidance of a high intake of crude protein during the autumn fattening period and the anabolic body fat consumption during the winter time seems to maximize digestive efficacy for energy uptake.
Perspectives and Significance
The thrifty winter phenotype of red deer is apparently characterized by avoiding energy expenditure for unproductive search of scarcely available food, which, in addition, renders the energetically costly maintenance of a large gut and visceral organs unnecessary. Simultaneously, extraction of nutrients is increased during winter in order to maximally exploit forage and to attenuate an inevitably negative energy balance. This holds true for nutrient uptake in the small intestines, as indicated by our results. However, it seems at first glance questionable for nutrient uptake in the rumen, because number and size of ruminal papillae are reduced during winter (24, 30). Yet uptake of short-chain fatty acids (SCFA), the major ruminal source of energy, occurs mainly by diffusion (5). Hence, a reduced surface for SCFA absorption and a decreased rumen volume during winter may be necessary for maintaining a sufficient gradient of SCFA concentrations between rumen content and blood, because SCFA production is low due to lower intake of food and presumably diminished microbial fermentation at lower rumen temperature (51). It remains in future studies to rigorously investigate this hypothesis.
GRANTS
This work was funded by Austrian Science Fund: P15939-B06, and the governments of Vienna and Lower Austria.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: W.A. and G.B. conception and design of research; W.A. analyzed data; W.A., B.S., M.R.W., and G.B. interpreted results of experiments; W.A. prepared figures; W.A. drafted manuscript; W.A., G.B., B.S., and M.R.W. edited and revised manuscript; W.A., C.B., M.B., M.G., A.L., B.S., M.R.W., and G.B. approved final version of manuscript; C.B., M.B., M.G., A.L., B.S., and M.R.W. performed experiments.
ACKNOWLEDGMENTS
We thank Folko Balfanz, Andreas Duscher, Friedrich Reimoser and Peter Steiger for help in managing the study herd, Erich Klansek for botanical analyses, Michael Hämmerle, Minh Hien Le, Frieda Tataruch, and Raimund Winkelbauer for chemical analyses, Helmut Dier, Brunhilde Gabriel, Anna Kübber-Heiss, Ivana Nabih, and Theodora Steineck for help with pathological dissection and preparing samples, and Renate Hengsberger for help with manuscript submission.
Current address: M. Guschlbauer, Center of Experimental Medicine, University of Cologne, Robert-Koch-Straße 10, 50931 Cologne, Germany.
REFERENCES
- 1.Adibi SA. Regulation of expression of the intestinal oligopeptide transporter (Pept-1) in health and disease. Am J Physiol Gastrointest Liver Physiol 285: G779–G788, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Arnold W. Energetics of social hibernation. In: Life in the Cold: Ecological, Physiological, and Molecular Mechanisms, edited by Carey C, Florant GL, Wunder BA, and Horwitz B, Boulder, CO: Westview Press, 1993, p. 65–80. [Google Scholar]
- 3.Arnold W, Ruf T, Kuntz R. Seasonal adjustment of energy budget in a large wild mammal, the Przewalski horse (Equus ferus przewalskii) II. Energy expenditure. J Exp Biol 209: 4566–4573, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Arnold W, Ruf T, Reimoser S, Tataruch F, Onderscheka K, Schober F. Nocturnal hypometabolism as an overwintering strategy of red deer (Cervus elaphus). Am J Physiol Regul Integr Comp Physiol 286: R174–R181, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Aschenbach JR, Penner GB, Stumpff F, Gäbel G. Ruminant Nutrition Symposium: Role of fermentation acid absorption in the regulation of ruminal pH. J Anim Sci 89: 1092–1107, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Biber J, Stieger B, Haase W, Murer H. A high yield preparation for rat kidney brush border membranes Different behaviour of lysosomal markers. Biochim Biophys Acta 647: 169–176, 1981. [DOI] [PubMed] [Google Scholar]
- 7.Binder HJ, Murer H. Potassium/proton exchange in brush-border membrane of rat ileum. J Membrane Biol 91: 77–84, 1986. [DOI] [PubMed] [Google Scholar]
- 8.Blaxter K. Energy Metabolism in Animals and Man. New York: Cambridge University Press, 1989, p. 336. [Google Scholar]
- 9.Booth AG, Kenny AJ. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J 14: 575–581, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 11.Carey HV, Frank CL, Seifert JP. Hibernation induces oxidative stress and activation of NF-κB in ground squirrel intestine. J Comp Physiol B 170: 551–559, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Clauss M, Streich WJ, Schwarm A, Ortmann S, Hummel J. The relationship of food intake and ingesta passage predicts feeding ecology in two different megaherbivore groups. Oikos 116: 209–216, 2007. [Google Scholar]
- 13.Daniel H, Adibi SA. Functional separation of dipeptide transport and hydrolysis in kidney brush border membrane vesicles. FASEB J 8: 753–759, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Dove H, Mayes RW. Plant wax components: a new approach to estimating intake and diet composition in herbivores. J Nutr 126: 13–26, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Ensing EP, Ciuti S, de Wijs FALM, Lentferink DH, ten Hoedt A, Boyce MS, Hut RA. GPS-based daily activity patterns in European red deer and North American elk (Cervus elaphus): indication for a weak circadian clock in ungulates. PLoS One 9: e106997, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferraris RP, Diamond JM. Specific regulation of intestinal nutrient transporters by their dietary substrates. Annu Rev Physiol 51: 125–141, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Geiser F, Ruf T. Hibernation versus daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol Zool 68: 935–966, 1995. [Google Scholar]
- 18.Guschlbauer M, Klinger S, Burmester M, Horn J, Kulling SE, Breves G. trans-Resveratrol and ε-viniferin decrease glucose absorption in porcine jejunum and ileum in vitro. Comp Biochem Physiol A 165: 313–318, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi H, Yonezawa T, Kanetani T, Terada F, Katoh K, Obara Y. Expression of mRNA for sodium-glucose transporter 1 and fatty acid translocase in the ruminant gastrointestinal tract before and after weaning. Anim Sci J 76: 339–344, 2005. [Google Scholar]
- 20.Hediger MA, Coady MJ, Ikeda TS, Wright EM. Expression cloning and cDNA sequencing of the Na+/glucose cotransporter. Nature 330: 379–381, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Herrmann J, Schröder B, Klinger S, Thorenz A, Werner AC, Abel H, Breves G. Segmental diversity of electrogenic glucose transport characteristics in the small intestines of weaned pigs. Comp Biochem Physiol A 163: 161–169, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Heydon MJ, Sibbald AM, Milne JA, Brinklow BR, Loudon ASI. The interaction of food availability and endogenous physiological cycles on the grazing ecology of red deer hinds (Cervus elaphus). Funct Ecol 7: 216–222, 1993. [Google Scholar]
- 23.Hofmann RR. Evolutionäre und saisonbedingte Anpassung des Verdauungsapparates des Gamswildes (Rupicapra rupicapra). In: Wildbiologische Informationen für den Jäger, edited by Hofmann RR. Stuttgart: Enke, 1983, p. 85–93. [Google Scholar]
- 24.Hofmann RR. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologia (Berl) 78: 443–457, 1989. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann RR. Wildbiologische Informationen für den Jäger. Stuttgart: ENKE, 1983, p. 1–168. [Google Scholar]
- 26.Hofmann RR. Zum Ernährungsverhalten und zum wechselnden Nährstoff- und Energiebedarf von Reh-, Gams- und Rotwild in Mitteleuropa. In: Wildbiologische Informationen für den Jäger, edited by Hofmann RR. Stuttgart: Enke, 1983, p. 75–84. [Google Scholar]
- 27.Hopfer U. Tracer studies with isolated membrane-vesicles. Methods Enzymol 172: 313–331, 1989. [DOI] [PubMed] [Google Scholar]
- 28.Hume D, Beiglböck C, Ruf T, Frey-Roos F, Bruns U, Arnold W. Seasonal changes in morphology and function of the gastrointestinal tract of free-living alpine marmots (Marmota marmota). J Comp Physiol B 172: 197–207, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Z, Hudson RJ. Digestive responses of wapiti Cervus elaphus canadensis to seasonal forages. Acta Theriol 41: 415–423, 1996. [Google Scholar]
- 30.Kamler J. Morphological variability of forestomach mucosal membrane in red deer, fallow deer, roe deer and mouflon. Small Rum Res 41: 101–107, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Karasov WH, Solberg DH, Diamond JM. Dependence of intestinal amino-acid-uptake on dietary-protein or amino-acid levels. Am J Physiol Gastrointest Liver Physiol 252: G614–G625, 1987. [DOI] [PubMed] [Google Scholar]
- 32.Kuntz R, Kubalek C, Ruf T, Tataruch F, Arnold W. Seasonal adjustment of energy budget in a large wild mammal, the Przewalski horse (Equus ferus przewalskii) I. Energy intake. J Exp Biol 209: 4557–4565, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Leibach FH, Ganapathy V. Peptide transporters in the intestine and the kidney. Annu Rev Nutr 16: 99–119, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Livesey G. The energy equivalents of ATP and the energy values of food proteins and fats. Br J Nutr 51: 15–28, 1984. [DOI] [PubMed] [Google Scholar]
- 35.Livesey G, Elia M. Estimation of energy-expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J Clin Nutr 47: 608–628, 1988. [DOI] [PubMed] [Google Scholar]
- 36.Loudon ASI. Photoperiod and the regulation of annual and circannual cycles of food intake. Proc Nutr Soc 53: 495–507, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Mayes RW, Lamb CS, Colgrove PM. The use of dosed and herbage n-alkanes as markers for the determination of herbage intake. J Agric Sci 107: 161–170, 1986. [Google Scholar]
- 38.Mitchell B, McCowan D, Nicholson IA. Annual cycles of body weight and condition in Scottish Red deer, Cervus elaphus. J Zool Lond 180: 107–127, 1976. [Google Scholar]
- 39.Nehring K. Agrikulturchemische Untersuchungsmethoden für Dünge- und Futtermittel, Böden und Milch. Hamburg, Berlin: Parey, 1960, p. 310. [Google Scholar]
- 40.Newman JA, Thompson WA, Penning PD, Mayes RW. Least squares estimation of diet composition from n-alkanes in herbage and faeces using matrix mathematics. Aust J Agr Res 46: 793–805, 1995. [Google Scholar]
- 41.Parker KL, Barboza PS, Gillingham MP. Nutrition integrates environmental responses of ungulates. Funct Ecol 23: 57–69, 2009. [Google Scholar]
- 42.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2013. [Google Scholar]
- 43.Rowell-Schäfer A, Dyer J, Hofmann RR, Lechner-Doll M, Meyer HHD, Shirazi-Beechey SP, Streich WJ. Abundance of intestinal Na+/glucose cotransporter (SGLT1) in roe deer (Capreolus capreolus). J Anim Physiol Anim Nutr 82: 25–32, 1999. [Google Scholar]
- 44.Rowell-Schäfer A, Lechner-Doll M, Hofmann RR, Streich WJ, Güven B, Meyer HHD. Metabolic evidence of a ‘rumen bypass’ or a ‘ruminal escape’ of nutrients in roe deer (Capreolus capreolus). Comp Biochem Physiol A Mol Integr Physiol 128: 289–298, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Rowell A, Dyer J, Hofmann RR, Shirazi-Beechey SP. Expression of Na+/glucose cotransporter in the intestinal brush-border membrane of ruminants with different feeding habits. Biochem Soc Trans 25: 482S, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Schröder B, Breves G. Mechanisms of phosphate uptake into brush border membrane vesicles from goat jejunum. J Comp Physiol B 166: 230–240, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Sibly RM. Strategies of digestion and defecation. In: Physiological Ecology: an Evolutionary Approach to Resource Use, edited by Townsend CR, and Calow P. Saunderland, MA: Sinauer, 1981, p. 109–139. [Google Scholar]
- 48.Sibly RM, Calow P. Physiological Ecology of Animals. An Evolutionary Approach. Melbourne, Australia: Blackwell Scientific, 1986, p. IX, 179 pp. [Google Scholar]
- 49.Signer C, Ruf T, Arnold W. Hypometabolism and basking: The strategies of Alpine ibex to endure harsh over-wintering conditions. Funct Ecol 25: 537–547, 2011. [Google Scholar]
- 50.Thamotharan M, Bawani SZ, Zhou XD, Adibi SA. Functional and molecular expression of intestinal oligopeptide transporter (Pept-1) after a brief fast. Metabolism 48: 681–684, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Turbill C, Ruf T, Mang T, Arnold W. Regulation of heart rate and rumen temperature in red deer: effects of season and food intake. J Exp Biol 214: 963–970, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilmore DW, Moses A. Catabolic illness. Strategies for enhancing recovery. N Engl J Med 325: 695–702, 1991. [DOI] [PubMed] [Google Scholar]
- 53.Winckler C, Breves G, Boll M, Daniel H. Characteristics of dipeptide transport in pig jejunum in vitro. J Comp Physiol B 169: 495–500, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Wood IS, Dyer J, Hofmann RR, and Shirazi-Beechey SP. Expression of the Na+/glucose co-transporter (SGLT1) in the intestine of domestic and wild ruminants. Pflügers Arch 441: 155–162, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Wright EM, Hirsch JR, Loo DDF, Zampighi GA. Regulation of Na+/glucose cotransporters. J Exp Biol 200: 287–293, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Zhao FQ, Okine EK, Cheeseman CI, Shirazi-Beechey SP, Kennelly JJ. Glucose transporter gene expression in lactating bovine gastrointestinal tract. J Anim Sci 76: 2921–2929, 1998. [DOI] [PubMed] [Google Scholar]