Abstract
Gap junction-mediated communication helps synchronize interconnected Sertoli cell activities. Besides, coordination of germ cell and Sertoli cell activities depends on gap junction-mediated Sertoli cell–germ cell communication. This report assesses mechanisms underlying the regulation of connexin 46 (Cx46) and Cx50 in mouse testis and those accompanying a “natural” seasonal and a pathological arrest of spermatogenesis, resulting from autoimmune orchitis (AIO) in mink. Furthermore, the impact of deleting Cx46 or Cx50 on the expression, phosphorylation of junction proteins, and spermatogenesis is evaluated. Cx46 mRNA and protein expression increased, whereas Cx50 decreased with adulthood in normal mice and mink. Cx46 mRNA and protein expression increased, whereas Cx50 decreased with adulthood in normal mice and mink. During the mink active spermatogenic phase, Cx50 became phosphorylated and localized to the site of the blood-testis barrier. By contrast, Cx46 was dephosphorylated and associated with annular junctions, suggesting phosphorylation/dephosphorylation of Cx46 and Cx50 involvement in the barrier dynamics. Cx46-positive annular junctions in contact with lipid droplets were found. Cx46 and Cx50 expression and localization were altered in mink with AIO. The deletion of Cx46 or Cx50 impacted on other connexin expression and phosphorylation and differently affected tight and adhering junction protein expression. The level of apoptosis, determined by ELISA, and a number of Apostain-labeled spermatocytes and spermatids/tubules were higher in mice lacking Cx46 (Cx46−/−) than wild-type and Cx50−/− mice, arguing for life-sustaining Cx46 gap junction-mediated exchanges in late-stage germ cells secluded from the blood by the barrier. The data show that expression and phosphorylation of Cx46 and Cx50 are complementary in seminiferous tubules.
Keywords: Sertoli cell, lipid droplet, connexin 43, meiosis, seasonal breeder
the seminiferous epithelium is unique in that it holds somatic Sertoli cells that divide by mitosis and germinal cells that divide, first by mitosis and then by meiosis, before differentiating into spermatids, unlike most epithelia in the body that bear a single type of cells that only undergo mitosis. The survival of the two distinct cell types is contingent upon successful Sertoli cell and germ cell physiological, symbiotic activities, where the function of one cell type impacts the function of the other and reciprocally. The establishment of occluding zonules joining adjacent Sertoli cells seals the paracellular route, establishing a physical barrier that creates and separates two superposed cellular compartments within the seminiferous epithelium (27, 77). Residing in the basal compartment, situated inferiorly to the barrier of the tubule and adjacent to the limiting membrane, the early-stage germ cells benefit from a direct access to blood-borne substances. The late-stage germ cells dwelling the lumenal compartment situated superiorly to the barrier are, in contrast, forbidden direct vascular exchanges (17, 72, 76). This particular distribution of the so-called blood-testis barrier within the seminiferous epithelium constrains the late-stage germ cells to rely on gap junction-mediated communication with adjoining Sertoli cells to carry out functions vital to their own survival yet unresolvable to germinal cells alone (73–75).
The gap junctions are made up of multimeric channels, individually built of transmembrane segments of proteins: the connexins (54, 63), which belong to a multigene family (105). The contacting cells each contribute one homomeric or heteromeric hemichannel to the cell surface. Since most cells express more than one connexin, the pairing of hemichannels may result in homotypic or heterotypic gap-junctional channels that assemble into junctional plaques joining cells (40). Because the unitary conductance of gap junctions is connexin dependent, the prevalence of one or more connexin species will impose a specific permeability to the cells, at times favoring and at others precluding the passage of regulatory molecules between cells (14, 40). In the context of spermatogenesis, the molecular diversity (10, 101), the phosphorylation status, and turnover of connexins (43) greatly impact gap junction-mediated Sertoli cell-to-Sertoli cell and Sertoli cell-to-germ cell channels and communication (75, 99). The selective intercellular traffic of regulatory molecules will condition cellular growth, differentiation, and death in the seminiferous epithelium.
The lack of a direct access to the blood supply is not restricted to the late-stage germ cells of the seminiferous epithelium. In the naturally avascular lens of the eye, the ionic and water balance of the intercellular milieu, transparency, and optical properties is conditioned by gap junction-mediated communication (58). Connexin 43 (Cx43) is primarily confined to the anterior epithelial monolayer of the lens in contrast to Cx46 and Cx50, which are coexpressed in the fiber cells of the nuclear, more central, region of the lens, where they share the same junctional plaques (26, 67). Because of the similarity of the lens fiber cells with the late-stage male germ cells in that they both lack direct access to the blood supply, the expression, localization, and interaction of Cx46, Cx50, and Cx43 amongst themselves and with other junction proteins were examined in the seminiferous epithelium. The present study is the first to evaluate the physiological significance of Cx46 and Cx50 on the outcome of spermatogenesis. This study aims to establish whether the regulation of the expression and phosphorylation of Cx46, Cx50, and Cx43 is synchronous or complementary in the seminiferous epithelium. We tested whether individually altering genes, coding for Cx46 or Cx50, impact on the expression of remaining junction proteins. The report also attempts to gain insight into the mechanisms underlying the interaction between these connexins during postnatal development in mouse and during a “natural” seasonal as well as a pathological arrest of spermatogenesis resulting from spontaneous autoimmune orchitis (AIO) in mink.
Novel evidence that the expression and phosphorylation of Cx46 and Cx50 are complementary, not only during postnatal development in the mouse and mink seminiferous epithelium but also throughout the natural seasonal as well as the pathological arrest of spermatogenesis, resulting from spontaneous AIO in mink, is presented. Moreover, we show that knocking out Cx46 results in an increase in the 51-kDa Cx50 band, accompanied by a decrease in the phosphorylated 60-kDa Cx50 band, and increases Cx43 while decreasing occludin and N-cadherin in the tubules. By contrast, the deletion of Cx50 causes a decrease in the phosphorylated 68-kDa Cx46 and in zona occuldens protein 1 (ZO-1), accompanied by an increase in PCx43 (phosphorylated in serine 368), Cx43, and claudin 11. Furthermore, we found that the phosphorylation of Cx46 and Cx50 is opposite in the seminiferous epithelium. The results advocate for a complementary expression and phosphorylation of Cx43, Cx50, and Cx46 in the mouse and mink seminiferous epithelium. In addition, the data show that when Cx46 affects the tight-junction and adhering-junction proteins, Cx50 has no effect and vice versa. Our finding that mice lacking Cx46 (Cx46−/−) are characterized by increased numbers of Apostain-positive cells, especially amongst spermatocytes and spermatids concurrent with increased intratubular apoptosis levels measured by ELISA, offers evidence of a life-sustaining role of Cx46-mediated intercellular communication on late-stage germ cells secluded by the blood-testis barrier.
MATERIALS AND METHODS
Animals
Mouse.
The mouse model provides invaluable opportunities to explore consequences of altering the coding of specific genes on precise tissue functions. However, the small size of mouse testis can provide only limited quantities of seminiferous tubule-enriched fractions (STfs). Mice were first anesthetized (urethane, 1 g/kg ip; Sigma, St. Louis, MO) before being decapitated. Animal-use protocols were approved by the University of Montreal Animal Care Committee.
Normal mouse.
Male mice were purchased from Charles River Laboratories (Saint-Constant, Quebec, Canada) of the BALB/cJ background and were housed at room temperature with food and water ad libitum and exposed to a 12:12-h light:dark cycle. Studies on development were carried out on mice aged from 7 to >60 days old or adult. Five animals were used per age group except for age 14–28 days, for which 10 animals were used, due to the small size of pups' gonads.
Cx46 and Cx50 knockout mice.
The generation of homozygous Cx46−/− and Cx50−/− has been described elsewhere (33, 85). Mice aged 16 wk old were first anesthetized, then decapitated, as stated above, and then their testes were removed and processed for different studies. Cx46+/+ and Cx46−/− mice were from the 129SvJ strain, whereas Cx50+/+ and Cx50−/− mice were from the 129SvJ × C57BL/6J mixed-strain background. Three male Cx46−/− mutant mice and three wild-type (WT) and three Cx50−/− mutant mice and three WT were used.
Mink.
The annual reproductive cycle of the mink (Mustela vison) provides a unique opportunity to consider the impact of a natural seasonal and reversible arrest of spermatogenesis on the interactive regulation of Cx46-, Cx50-, and Cx43-mediated gap junctions. Moreover, we (74, 75, 82, 97) and others (96) have established the mink as a valuable model for the study of male reproduction and autoimmunity in a seasonal breeder. AIO develops spontaneously in 30% of the black fur-coated mink (82, 98). Similarly, in humans, spontaneous AIO is characterized by a noninfectious granulomatous orchitis, spermatogenic arrest, and deposits of immunocomplexes in the basement membrane associated with infectious diseases, resulting from systemic viral infection (47, 61, 89). Immunological male infertility (55) is associated with pathological features emulating granulomatous orchitis, documented in mink with AIO (95), in which disruption of germ cell growth and Sertoli cell function results in aberrations in the processes of gene transcription and post-translational phosphorylation of Cx43 (74, 75, 82). The use of the mink model provides the distinctive advantage of identifying the difference in the impact of a pathological vs. a natural arrest of spermatogenesis on this regulation. Mink were purchased from Visonnière St. Damase (Quebec, Canada). They were caged individually with food and water ad libitum and natural lighting. Animals received a 0.9-ml/kg body wt of Na-phenobarbital (Somnotol; CDMV St-Hyacynthe, Quebec, Canada) and 0.15 ml/kg of a solution of 0.3 g/ml chloral hydrate. The right testis with its epididymis was used in microscopy and immunohistochemistry studies; the left in tissue fractions. The experiments were conducted in accordance with the Université de Montréal Animal Care Committee.
Normal mink.
The testes from a total of 135 mink were used: 10 neonatal, 10 pubertal, and five adult/mo. Harvesting was done at 30-day intervals from 90 to 270 days after birth. Tissues were collected from 2- to 3-yr-old fertile adults the last week of each month during the annual reproductive cycle.
Infertile mink with AIO.
Two- to 3-yr-old black and sapphire (genetically related to black) mink that mated and sired five or more litters the previous year, but were sterile during the current year and diagnosed with secondary infertility related to spontaneous AIO (98), were used.
Criteria of Fertility
The morphology, motility, and number of spermatozoa obtained from the ejaculated semen recovered from vaginal lavage were assessed under light microscope in March. Antisperm antibody levels in serum were measured (3, 82). A total of 20 mink with low sperm counts or immobile spermatozoa and high antibody levels and whose histopathology showed leukocyte infiltration and destruction of seminiferous tubules at autopsy and diagnosed with secondary infertility were used in this study: 10 in February and 10 in March.
Chemicals
PMSF, leupeptin, aprotinin, cell death detection ELISA kits, and Lumi-LightPLUS chemiluminescence detection kits were purchased from Roche (Laval, Quebec, Canada). Potassium bisperoxo (1, 10-phenanthroline) oxovanadate (V) [bpV (phen)] was obtained from Calbiochem (San Diego, CA) and the cell death detection ELISA kit from Boehringer-Mannheim (Laval, Quebec, Canada).
Antibodies
The following antibodies were used: rabbit polyclonal anti-caveolin-1 (Santa Cruz Biotechnology, Santa Cruz, CA); purified mouse anti-flotillin-1 (BD Biosciences, Mississauga, Ontario, Canada); MAb against single-stranded DNA F7-26 (Apostain; Alexis, San Diego, CA); rabbit polyclonal anti-Cx46 (Alpha Diagnostic International, San Antonio, TX); mouse monoclonal IgM anti-Cx50 (Invitrogen Canada, Burlington, Ontario, Canada); rabbit polyclonal anti-N-cadherin and rabbit polyclonal anti-claudin 11 (Abcam, Cambridge, MA); rabbit polyclonal anti-occludin (Zymed Laboratories, San Francisco, CA); rabbit polyclonal antibody, raised in rabbits against a synthetic peptide corresponding to the amino acid sequence 363–382 of human Cx43 that recognizes all Cx43 forms (Pan-Cx43 antibody), and myosin light chain MAb (Sigma); a rabbit polyclonal antibody recognizing PCx43 and developed against a synthetic phosphopeptide corresponding to residues surrounding Ser368 of human Cx43 (Chemicon, Temecula, CA); horseradish peroxidase (HRP)-conjugated streptavidin (Molecular Probes, Eugene, OR); and HRP-conjugated anti-mouse IgG, HRP-conjugated anti-rabbit IgG, HRP-conjugated anti-mouse IgM, biotinylated anti-rabbit F(ab′)2, and biotinylated anti-mouse F(ab′)2 (Jackson ImmunoResearch Laboratories, Mississauga, Ontario, Canada).
Isolation of Seminiferous Tubule-Enriched Fractions
Distinct anatomical and functional characteristics set apart the interstitium and the seminiferous tubules of the testis. Yet today, most assays remained carried on whole testis extracts rather than on interstitium (ITf) and seminiferous tubule-enriched (STf) fractions, as we have done here. We have shown that a before-hand exposure to even a mild enzymatic digestion hinders the detection of phosphorylated and/or glycosylated forms of proteins in the samples (2). For this reason, here, the STfs were obtained without a predigestion to preserve the integrity of the proteins in the samples using a technique that we described elsewhere (2). Briefly, the seminiferous tubules were teased apart from the interstitium from freshly decapsulated testes on ice in PBS (137 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.4), containing 2 mM PMSF, 1 mM EGTA, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 4 mM Na3VO4, 80 mM NaF, and 20 mM Na4P2O7 with 10 μM bpV (phen). The seminiferous tubule-interstitium solution was allowed to decant and then centrifuged 15 min at 400 rpm (GS-6R centrifuge, GH-3.8 rotor; Beckman Coulter, Brea, CA) at 4°C. The ITf and STf were recovered individually after a centrifugation at 1,000 rpm for 10 min (GS-6R centrifuge, GH-3.8; Beckman Coulter) at 4°C and characterized under the microscope.
Isolation of Lenses
Lenses were excised from adult normal mice and mink and put on dry ice for fragmentation. After thawing, the fragments were homogenized in PBS containing protease and phosphatase inhibitors using a Polytron PT 3100 homogenizer.
Protein Quantification
Proteins in samples were assayed using the method of Bradford (11) and materials from Bio-Rad Laboratories (Mississauga, Ontario, Canada).
Electrophoresis and Western Blot Analyses
Twenty to 30 μg total homogenate of each sample was loaded on polyacrylamide gels, separated by 10% SDS-PAGE, transferred onto nitrocellulose membranes, and subjected to Western blotting, as described previously (75, 82, 99). Briefly, membranes were blocked, 1 h at 37°C, with 5% skim milk in Tris-buffered saline (TBS; 137 mM NaCl, 27 mM KCl, 25 mM Tris·HCl, pH 7.4) and then incubated with the different antibodies: Pan-Cx43 antibody (1:20,000), PCx43 (1:400), anti-caveolin-1 (1:3,000), anti-flotillin-1 (1:250), anti-Cx46 (1:500 dilution), anti-Cx50 (1:25 dilution), anti-N-cadherin (1:1,000 dilution), anti-occludin (1:3,000 dilution), anti-claudin 11 (1:1,000 dilution). The antibody dilutions were prepared in 5% skim milk–TBS. Membranes were washed in TBS containing 0.05% Tween 20 and incubated for 1 h with a corresponding secondary antibody conjugated to HRP at room temperature. The antigen–antibody complexes were detected by chemiluminescence. The density of each antibody band was quantified by laser scanning with the Scion Image Software (Scion, Frederick, MD).
Alkaline Phosphatase Treatment
Proteins (50 μg) of STf homogenate were incubated with 10–15 units of calf intestine alkaline phosphatase (Boehringer Mannheim, Mannheim, Germany) in 30 μl digestion buffer (10 mM MgCl2, 1 mM ZnCl2 50 mM Tris·HCl, pH 8.0) for 2 h at 37°C in the presence or absence of phosphatase inhibitor. The reaction was stopped by incubating the samples with 10% TCA for 30 min at 4°C. Pellets were washed in cold acetone, dissolved in sample buffer, and boiled for 3 min. Proteins (30 μg) were applied per lane, separated by SDS-PAGE, and exposed to Western blotting with antibodies.
Preparation of Lipid Raft-Enriched Membrane Fractions
Lipid raft/caveolae-enriched membrane fractions were purified according to a variation of the method described by Schubert et al. (91) as follows. Tubule-enriched fractions from adult, >60 days old, mouse and mink, obtained in February and in November, were washed in cold PBS and homogenized in 750 μl morpholinoethanesulfonic acid-buffered saline (MBS; pH 6.5) containing 1% Triton X-100 in a 5-ml ultracentrifuge tube. Each sample was mixed with an equal volume of 80% sucrose (prepared in MBS without Triton X-100) and overlaid with a discontinuous sucrose gradient of 1.5 ml 30% sucrose and 1.5 ml 5% sucrose (prepared with MBS without Triton X-100). The samples were centrifuged at 4°C for 18 h at 200,000 g (XL-70 ultracentrifuge; Beckman Coulter). The rafts came out as a hazy, whitish band, floating at the 5–30% sucrose interface. Aliquots of each fraction were subjected to SDS-PAGE and immunoblotting.
Real-Time Quantitative PCR
Total RNA was isolated from the tubule-enriched fractions using Trizol (Life Technologies, Grand Island, NY), per the manufacturer's instructions. After isolation, 1 μg total RNA was reverse transcribed into cDNA at 42°C for 1 h using oligo(dT) and 1 μl iScript RT (iScript cDNA Synthesis Kit; Bio-Rad Laboratories, Hercules, CA) in a 20-μl final volume. The mRNA expression of Cx46, Cx50, and Cx43 was measured using primers designed for mouse or human by Primer 3 software (Premier Biosoft, Palo Alto, CA; Table 1). The primers were purchased from Invitrogen (Carlsbad, CA). The real-time quantitative PCR (Q-PCR) reactions were performed using iQ SYBR Green Supermix, according to the manufacturer's instructions, and 0.5 μM of each primer. In all real-time Q-PCR reactions, a negative control corresponding to RT reaction without the reverse-transcriptase enzyme and a blank sample was carried out (data not shown). Amplification of the housekeeping gene GAPDH was used as internal control to quantify the expression of a given gene in real-time Q-PCRs.
Table 1.
Specific primers designed for mouse and mink Cx46, Cx50, and Cx43
| Gene | Protein | GenBank Accession Number | Sense | Antisense | Product Size, bp |
|---|---|---|---|---|---|
| Mouse primers | |||||
| Gja3 | Cx46 | NM_016975.2 | AATCGGACTTCACCTGCAAC | TGCTGAGGGTTGTCTCTCCT | 217 |
| Gja8 | Cx50 | NM_008123.2 | CTGCCCCAATGTGGTAGACT | AGTGGAGGGACTTCTCAGCA | 200 |
| Gja1 | Cx43 | NM_010288.3 | GAACACGGCAAGGTGAAGAT | GAGCGAGAGACACCAAGGAC | 247 |
| Human primers | |||||
| GJA3 | Cx46 | NM_021954.3 | GCCGGCCAGTACTTTCTGTA | CCTGCTTGAGCTTCTTCCAG | 205 |
| GJA8 | Cx50 | NM_005267.4 | GAGACACTGCCTTCCTACGC | CGGGGGTCTCTACTTTCTCC | 175 |
| GJA1 | Cx43 | NM_000165.3 | ATGAGCAGTCTGCCTTTCGT | TCTGCTTCAAGTGCATGTCC | 249 |
| Human primer | |||||
| GAPDH | GAPDH | NM_002046 | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG | 87 |
Cx46/50/43, connexin 46/50/43; Gja, gap-junction protein, α.
Preparation of Tissues for Immunolocalization of Cx46, Cx50, and Apostain
Testes were perfusion fixed with Bouin's fixative (71, 79). Endogenous peroxidase activity was inhibited with 0.6% H2O2 in 70% ethanol (80). Nonspecific binding was blocked with 0.5% skim milk in TBS containing 0.1% Tween 20 (TBST) for 1 h at 37°C. Next, tissue sections were incubated with the different antibodies using the method described elsewhere (80). The tissue sections were washed in TBST after each incubation and exposed to TBS containing 0.01% H2O2, 0.05% diaminobenzidine tetrachloride (pH 7.7), for 10 min at room temperature (37). The sections were washed in water and counterstained in 0.05% aqueous methylene blue, mounted in Permount, and examined on a Carl Zeiss Axiophot 2 fluorescence microscope. The stage-dependent distribution of the different antibodies was evaluated using the identification method of the stages of the cycle of the seminiferous epithelium of Pelletier (68) in mink.
Electron Microscopy
Testicles were perfused through the testicular artery, first with PBS (154 mM NaCl, 100 mM Na phosphate, pH 7.3), followed by a diluted mixture of Karnovsky's fixative (38), 0.1 M Na cacodylate buffer, pH 7.3, and then processed as described previously (69). Briefly, tissues were postfixed in an aqueous solution of 1% osmium tetroxide–1.5% potassium ferrocyanate, processed in a 1% solution of tannic acid buffered to pH 7.8 with 0.1 M Na cacodylate, and stained en bloc in a solution of 5% uranyl acetate in Veronal acetate buffer, pH 4.5–5.2, dehydrated in ethanol, and embedded in Poly/Bed 812 (Polysciences, Warrington, PA). Silver-to-gold thin sections, collected on formvar-coated, carbon-stabilized grids, were examined at 80 kV with a Philips 300 electron microscope.
Freeze-Fracture Replication
Aldehyde-fixed testes were washed in 0.1 M Na cacodylate buffer, pH 7.3; cryoprotected in a solution of 30% glycerol in 0.2 M Na cacodylate, pH 7.35 (69, 70, 77); and fractured in a BAF Balzer apparatus at −115°C. The replicas were collected on formvar-coated, carbon-stabilized grids and examined at 80 kV with the electron microscope.
Cholesterol Labeling With Filipin
The mapping of cholesterol with filipin (Polysciences) was achieved as described earlier (78, 81).
Data and Statistical Analysis
Analyses were done with Stata software (StataCorp LP, College Station, TX). The data were evaluated with the Student's t-test or ANOVA, followed by the Tukey honestly significant difference test.
RESULTS
Characterization of the Seminiferous Tubule-Enriched Fractions
The STfs were obtained without enzymatic digestion. The rationale for our using this approach to generate the tubule-enriched fractions was based on our earlier report that the avoidance of the use of a before-hand enzymatic digestion preserves the integrity of proteins in the samples under study and allows detection of otherwise undetectable isoforms (2). Furthermore, the characterization of tubule fractions resulting from the use of our approach under the light microscope reveals that the cellular elements contained are apparently well preserved. All biochemical analyses in this study have been realized on STfs obtained without the use a before-hand enzymatic digestion and as described previously (2).
Cx46, Cx50, and Cx43 mRNA in Mouse During Development
The Cx46 mRNA levels roughly doubled from 14 to 21 days after birth and then increased further by adulthood (>60 days old) in mouse STfs (Fig. 1A). The profiles of tubular Cx50 and Cx43 mRNA levels were opposite that of the Cx46 mRNA levels during the same time period. In contrast to Cx46 mRNA levels, Cx50 mRNA levels were highest by 14 days after birth and then dropped significantly fivefold by 21 days before stabilizing after 60 days (Fig. 1A). The Cx43 mRNA profiles emulated Cx50 mRNA levels by being elevated 14 days after birth and then significantly fell fourfold with the completion of spermatogenesis in >60-day-old mice (Fig. 1A).
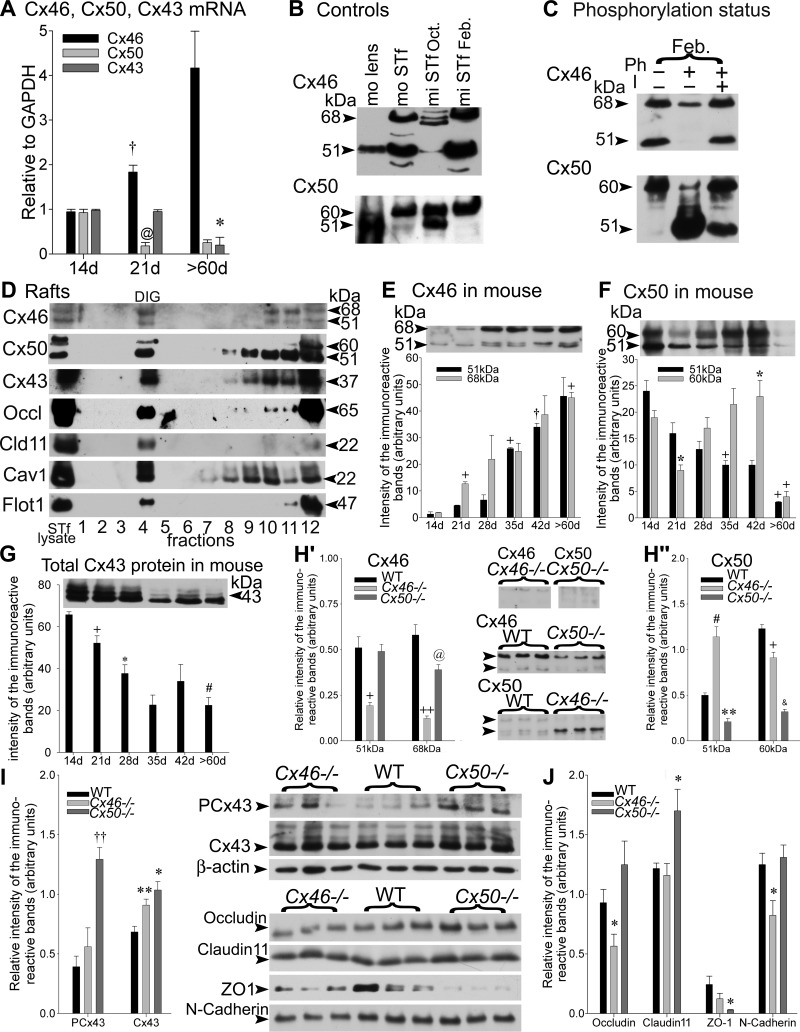
Fig. 1.
A: the samples were subjected to RT-PCR using primers specific for mouse connexin 45 (Cx46), Cx50, and/or Cx43 genes. The Cx46, Cx50, and Cx43 mRNA levels in normal mouse seminiferous tubule-enriched fractions (STfs) during mouse postnatal development are shown. The values are the means ± SE of 3 independent experiments for each age group, expressed in arbitrary units. Cx46 mRNA levels increased significantly from 14 to 21 days (d; †P < 0.03 21 days vs. 14 days) and then raised further by >60 days, in contrast to Cx50 mRNA levels that decreased significantly from 14 to 21 days (@P < 0.02 21 days vs. 14 days) and then remained low by >60 days. The Cx43 mRNA levels decreased steadily throughout development, reaching lowest values by >60 days (*P < 0.05 >60 days vs. 21 days). B: when exposed to the polyclonal Cx46 antibody (Alpha Diagnostic International), a single, intense, ∼51-kDa band was detected in an adult mouse (mo) lens used as a positive control. An additional ∼68-kDa band was, however, recognized in mouse and mink (mi) STfs. The Cx50 antibody detected a 51-kDa-immunoreactive band, accompanied by an ∼60 kDa in the adult mouse lens used as a positive control. The 51- and ∼60-kDa Cx50-immunoreactive bands were detected in mouse STf and mink STf. Oct., October; Feb., February. C: phosphorylation-status studies of Cx46 and Cx50: normal adult mink tubule-enriched fractions in February were incubated in PBS, alone or containing alkaline phosphatase (Ph) in the absence (−) or presence (+) of a phosphatase inhibitor (I). Following treatment, total protein aliquots from each sample were subjected to SDS-PAGE, followed by Western blotting with Cx46 or Cx50 antibodies. Approximately 68- and 51-kDa bands were detected by Cx46 antibodies. Both bands diminished after alkaline phosphatase treatment, revealing the presence of Cx46 phosphorylated forms. The strong 60-kDa band detected by Cx50 antibodies was decreased following alkaline phosphatase treatment, but the 51-kDa band increased, suggesting the presence of phosphorylated forms in the 60-kDa Cx50-immunoreactive band. D: experiments carried out using detergents to isolate the detergent-insoluble glycolipid (DIG) fractions from adult mink tubule-enriched fractions obtained in February: caveolin-1 (Cav1) and flotillin-1 (Flot1) served to identify the DIG fraction by Western blotting. The gap-junction proteins Cx46, Cx50, and Cx43, as well as the tight-junction proteins, occludin (Occl) and claudin 11 (Cld11), were recovered in the DIG fraction. E–G: representative Western blot analyses and quantification of individual levels of the 51- and ∼68-kDa (E) Cx46-immunoreactive bands and (F) Cx50-immunoreactive bands and (G) total Cx43, measured in mouse tubule-enriched fractions during development, are shown. The values are the means ± SE of 3 independent experiments expressed in arbitrary units. E: the changes in Cx46 protein levels were significant as follows: 51 kDa, +P < 0.01 35 days vs. 28 days and †P < 0.03 42 days vs. 35 days; 68 kDa, +P < 0.01 21 days vs. 14 days and >60 days vs. 21 days. F: the following changes in Cx50 levels were significant: 51 kDa, +P < 0.01 35 days vs. 14 days and >60 days vs. 42 days; 60 kDa, *P < 0.05 21 days vs. 14 days and 42 days vs. 21 days and +P < 0.01 60 days vs. 42 days. G: the following changes in Cx43 protein levels were significant: +P < 0.01 21 days vs. 14 days, *P < 0.05 28 days vs. 21 days, and #P < 0.0001 >60 days vs. 14 days. H–J: compensatory studies carried out in mice lacking Cx46 (Cx46−/−), wild-type (WT) mice, and Cx50−/− tubule-enriched fractions. The values shown are the means ± SE of 3 independent experiments and are expressed in arbitrary units. H′ and H“: representative Western blots, accompanied by histograms of the quantification of Cx46 and Cx50 levels in each animal group, are presented. As expected, the intensity of the Cx46 (H′)- and Cx50 (H”)-immunoreactive band levels was reduced to insignificant traces in Cx46−/− (51 kDa band: +P < 0.01 Cx46−/− vs. WT; 68 kDa band: ++P < 0.001 Cx46−/− vs. WT) and Cx50−/− (51 kDa band: **P < 0.005 Cx50−/− vs. WT; 60 kDa band: &P < 0.0005 Cx50−/− vs. WT) mice, respectively. H′: the 68-kDa Cx46 band levels decreased significantly (@P < 0.02 Cx50−/− vs. WT) in Cx50−/− mice. H“: the 51-kDa Cx50-immunoreactive band levels increased significantly (#P < 0.0001 Cx46−/− vs. WT), whereas 60 kDa levels were reduced (+P < 0.001 Cx46−/− vs. WT) in the Cx46−/− mice. Representative Western blots of (I) PCx43 (phosphorylated in serine 368), Cx43, and (J) occludin, claudin 11, zona occulden protein 1 (ZO-1), and N-cadherin levels, accompanied by histograms of the quantification of the levels measured for each junction protein in WT, Cx46−/−, and Cx50−/− mice tubule-enriched fractions. The following changes in the levels of each junction protein were significant: ††P < 0.003 PCx43 Cx50−/− vs. WT; **P < 0.005 Cx43 Cx46−/− vs. WT; *P < 0.05 Cx43 Cx50−/− vs. WT; *P < 0.05 occludin Cx46−/− vs. WT; *P < 0.05 claudin 11 Cx50−/− vs. WT; *P < 0.05 ZO-1 Cx50−/− vs. WT; *P < 0.05 N-cadherin Cx46−/− vs. WT.
Cx46 and Cx50 in Lens and STfs
The ocular lens that expresses Cx46 and Cx50 (103) was used as a positive control (Fig. 1B). The Cx46 antibody detected an intense 51-kDa band in both mouse (Fig. 1B) and mink (results not shown) lenses. In the STf of both species, the 51-kDa band appeared flanked by a heavier 68-kDa band (Fig. 1B).
The Cx50 antibody detected a band ∼51 kDa and another ∼60 kDa in mouse (Fig. 1B) and mink (results not shown) lenses. The two bands were also detected in the STfs of both species (Fig. 1B).
Phosphorylation Status of the Cx46 and Cx50
To determine the phosphorylated status of the Cx46- and Cx50-immunoreactive bands identified above (Fig. 1B), tubule-enriched fraction samples were incubated in buffer alone or containing alkaline phosphatase in the absence or presence of phosphatase inhibitor (Fig. 1C). After treatments, the samples were subjected to SDS-PAGE and then probed with Cx46 or Cx50 antibodies. The intensity of the 68- and 51-kDa Cx46-immunoreactive bands decreased after treatment (Fig. 1C). The results indicate that the 68-kDa band contains phosphorylated forms of Cx46. This notwithstanding, that the 51-kDa band contains phosphorylated forms of Cx46, besides the nonphosphorylated Cx46, cannot be ruled out. The apparent decrease in the 51-kDa band's intensity suggests that Cx46 may be rapidly degraded upon dephosphorylation. Alkaline phosphatase treatment decreased the intensity of the 60-kDa Cx50-immunoreactive band while increasing that of the 51-kDa band (Fig. 1C), indicating that the 60-kDa band contains phosphorylated forms of Cx50. However, that the 51-kDa band contains phosphorylated forms of Cx50, in addition to nonphosphorylated forms, cannot be ruled out.
Cx46, Cx50, Cx43, Occludin, Claudin 11, and Lipid Rafts
Rationale.
The high concentration of sphingolipids and cholesterol of the lipid rafts promotes the gathering or the exclusion constituents from microdomains (83) and controls the delivery, assembly, and removal of individual proteins from selected membrane domains (44). Cx46- and Cx50-mediated gap junctions are enriched in cholesterol and sphingomyelin (4, 34), suggesting that connexin channels could segregate into lipid rafts. Furthermore, we have reported elsewhere that formation/dismantling of gap junctions arises in lipid-rich microdomains and that their maturation appears in cholesterol-poor domains (74, 76, 77, 81), advocating in favor of a sorting of connexin-mediated channels, according to their species, or pairing characteristics through differences in lipid/protein ratios in Sertoli cell membranes. At present, Cx43 has been reported to interact with caveolin-1 and -2 (45) in other cell systems, whereas Cx46 and Cx50 have been documented in lens caveolae (49), but the presence or absence of these three connexins in lipid rafts of the testis has never been established. This study aims to test this. The information is of relevance because of the documented contribution of the rafts to intracellular signaling interactions in other cell systems (21).
Because tubule-enriched fractions were obtained in larger amounts from mink than mouse testes, the lipid raft studies were carried out in mink. Detergent-insoluble glycolipid (DIG) microdomains were separated by sucrose-density centrifugation in 12 equal-volume fractions [1 = top of the gradient; 12 = bottom of the gradient (pellet)]. The STf lysate was used as a control. The fractions were collected and then first probed with different antibodies before being subjected to Western blotting (Fig. 1D). The STf caveolin-1 and flotillin-1 were both used to identify the DIG fractions (DIG = fraction 4; Fig. 1D). The gap-junction proteins Cx46, Cx50, and Cx43 were recovered in the DIG fraction (Fig. 1D). As well, the tight-junction proteins occludin and claudin 11 were recovered in the DIG fraction (Fig. 1D).
Cx46, Cx50, and Cx43 Protein Expression During Mouse Development
The studies begin at 14 days after birth (Fig. 1, E–G), because before day 14 after birth, the size of the developing mouse testes is too small to yield relatively pure tubule-enriched fractions in satisfactory amounts.
Cx46.
The intensity of the 51- and ∼68-kDa Cx46-immunoreactive bands increased throughout development, reaching highest levels by adulthood, albeit the increase in the 68-kDa occurred earlier than that of the 51-kDa band (Fig. 1E). The 51-kDa levels increased significantly by 35 days compared with 28 days and then again by 42 days compared with 35 days after birth. The ∼68-kDa Cx46-immunoreactive band levels increased significantly by 21 days compared with 14 days, as well as by >60 days compared with 21 days (Fig. 1E). A 14-kDa band was observed to decrease with development when whole membranes were exposed (not shown), in contrast to the 51- and 68-kDa Cx46-immunoreactive bands that both increased throughout development.
Cx50.
In contrast to Cx46, the 51-kDa Cx50-immunoreactive band showed a decrease in intensity in adulthood (>60 days; Fig. 1F). The 51-kDa band levels declined progressively, first from 28 to 35 days and then from 42 to >60 days (Fig. 1F). The 60-kDa Cx50-immunoreactive band levels decreased significantly, first from 14 to 21 days, then increased significantly from 21 to 42 days, and then again decreased in >60 days (Fig. 1F). Together, these results show that the 51- and 68-kDa Cx46 (Fig. 1E) and the 51-kDa Cx50 levels varied in a complementary fashion in normal adult mice STfs (Fig. 1F).
Cx43.
The total Cx43 levels measured using the Pan-Cx43 antibody steadily and significantly decreased, first from 14 to 21 days and again from 21 to 28 days (Fig. 1G). Total Cx43 levels reached lowest values by adulthood in mouse tubule-enriched fractions compared with 14-day values (Fig. 1G). Total Cx43 levels and the levels of the 51-kDa Cx50-immunoreactive band both decreased by adulthood (Fig. 1F), in contrast to the levels of the 51- and 68-kDa Cx46 (Fig. 1E) that increased instead.
Effects of Knocking out Cx46 or Cx50 on Other Connexins
Cx46.
As expected, the 51- and 68-kDa Cx46-immunoreactive bands both decreased significantly to trace levels in Cx46−/− mouse tubule-enriched fractions compared with WT (Fig. 1H′; Cx46). The 68-kDa Cx50-immunoreactive band levels decreased significantly in Cx50−/− mice compared with WT (Fig. 1H′; Cx46).
Cx50.
Similarly, no Cx50-immunoreactive bands were detected in Cx50−/− mice (Fig. 1H″; Cx50). The 51-kDa Cx50-immunoreactive band levels more than doubled, in contrast to the 60-kDa Cx50-immunoreactive band levels that decreased significantly in Cx46−/− compared with WT (Fig. 1H″; Cx50). Together, these observations are evidence of the interdependent complementarity in the regulation of the expression of these two connexin species within the seminiferous tubules.
Cx43.
PCx43 levels increased significantly in Cx50−/− compared with WT mice (Fig. 1I). Total Cx43 levels augmented significantly in Cx46−/− and Cx50−/−mice tubule-enriched fractions compared with WT (Fig. 1I).
Effects of Knocking out Cx46 or Cx50 on Tight-Junction Proteins
The occludin levels were diminished significantly, whereas claudin 11 and ZO-1 levels showed little changes in Cx46−/− mice compared with WT (Fig. 1J). In contrast, occludin changed little, claudin 11 augmented significantly, but ZO-1 decreased in Cx50−/− mouse tubule-enriched fractions compared with WT (Fig. 1J).
Effects of Knocking out Cx46 or Cx50 on N-Cadherin
The N-cadherin levels diminished significantly in Cx46−/− mice but not in Cx50−/−, where they showed little changes compared with WT mice (Fig. 1J).
Together, the results suggest that in addition to an apparent complementarity in the expression of Cx46 and Cx50, the silencing of Cx46 or Cx50 affected differently, not only the expression of other connexin gap-junction isoforms but also the expression of the tight- and adhering-junction proteins in mouse seminiferous tubules. Furthermore, the data show that when Cx46 affects the tight- and adhering-junction proteins, Cx50 has no effect and vice versa (Fig. 1I). Table 2 provides a summary of the compensatory effects of deleting Cx46 or Cx50 on remaining junction protein in the tubule fractions.
Table 2.
Summary of the changes in junction proteins in Cx46−/− and Cx50−/−
| Protein | Cx46−/− | Cx50−/− |
|---|---|---|
| Cx46, 51 kDa | ↓ | = |
| Cx46, 68 kDa | ↓ | ↓ |
| Cx50, 51 kDa | ↑ | ↓ |
| Cx50, 60 kDa | ↓ | ↓ |
| Cx43, total | ↑ | ↑ |
| Cx43, phosphorylated in serine 368 | = | ↑ |
| Occludin | ↓ | = |
| Claudin 11 | = | ↑ |
| ZO-1 | = | ↓ |
| N-Cadherin | ↓ | = |
Cx46/50−/−, mice lacking Cx46/50; ZO-1, zona occuldens protein 1.
Cx46 and Cx50 in Mink STfs
Mink reproduction.
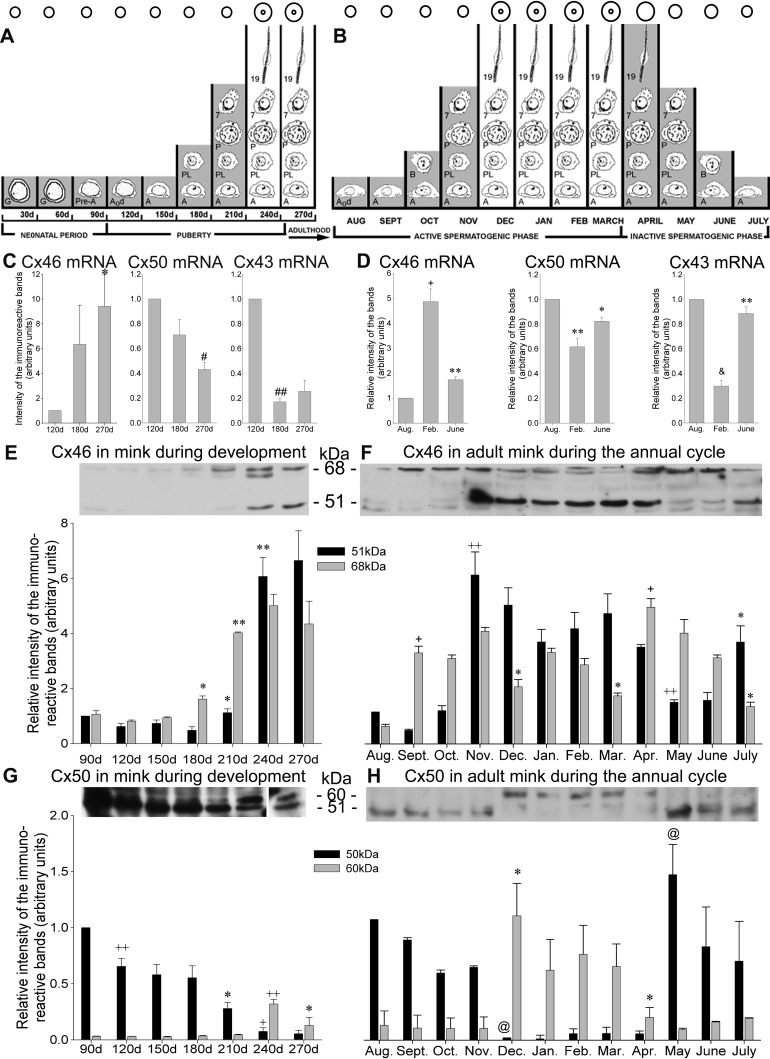
Mink (M. vison) breed in March in the Northern hemisphere. The female mink have a delayed implantation, and pups are born in April–May (62). Figure 2, A and B, respectively, depict a calendar of the germ cell's population recorded monthly during mink postnatal development and annual seasonal reproductive cycle. Figure 2B shows that the active spermatogenic phase is being followed by an inactive spermatogenic phase. The time periods when the blood-testis barrier is incompetent in blocking vascularly infused permeability tracers are identified in contrast to those that mark the periods when the barrier blocks entry of blood-borne content in seminiferous tubules (Fig. 2, A and B) (68, 74, 75, 82). We have presented strong evidence that the endowment of impermeability of this barrier coincides with the canalization of a lumen in the seminiferous tubules, not with the colonization of the epithelium by a particular generation of germ cells (68, 74).
Fig. 2.
Variations in the Cx46, Cx50, and Cx43 mRNA and protein levels in mink STfs during postnatal development and the annual reproductive cycle. A calendar of the germ cell population recorded during the (A) postnatal development and (B) annual seasonal reproductive cycle in mink seminiferous tubule cross-sections is provided. The mink breed in the 2nd and 3rd wk of March. The pups are born in April–May. Each month is represented by a vertical column in the 2 diagrams. The columns either span (A) from birth to adulthood by 270 days after birth or (B) cover the 12-mo seasonal reproductive cycle of the adult mink. The shading refers to time periods when the blood-testis barrier is permeable to vascularly infused permeability tracers. Conversely, the months when the barrier is competent in blocking tracers are not shaded. The inner circles apposed atop indicate the presence of a lumen. G, gonocytes; Pre-A, pre-type A spermatogonia; A0d, type A0 spermatogonia dividing; A, type A spermatogonia; PL, preleptotene spermatocyte; P, pachytene spermatocyte; 7, step 7 spermatid; 19, step 19 spermatid; B, type B spermatogonia. After a 90-day neonatal period, puberty encompasses the colonization of the tubules by type A spermatogonia until reaching the 1st spermatozoa in the epididymis, ∼250 days after birth. This is followed, first by an ”active“ and then by an ”inactive“ phase of the annual reproductive cycle in the adult mink. A reduction in mitotic and meiotic activities yields spermatids in reduced abundance. By August (AUG), the division of spermatogonial stem cells (A0d) marks the onset of the active phase of the annual seasonal reproductive cycle [after Pelletier (68) and modified by Pelletier et al. (82)]. The individual Cx46, Cx50, and Cx43 mRNA levels, measured by RT-PCR in tubule-enriched fractions, are plotted in arbitrary units during (C) development and (D) the annual seasonal reproductive cycle in the adult mink. The values are the means ± SE of 3 independent experiments and are normalized to the 120-day values (C) and August values (D). C: the Cx46 mRNA levels increased significantly (*P < 0.05 270 days vs. 120 days); however, Cx50 decreased (#P < 0.0001 270 days vs. 120 days) by adulthood. Similarly, Cx43 fell significantly (##P < 0.00001 180 days vs. 120 days) by 180 days after birth and then remained low in the adult. D: during the seasonal reproductive cycle, Cx46 mRNA levels were highest in February (+P < 0.001 Feb. vs. Aug.) but then decreased significantly by June (**P < 0.005 June vs. Feb.). By contrast, Cx50 mRNA levels were lowest in February (**P < 0.005 Feb. vs. Aug.) before increasing significantly by June (*P < 0.05 June vs. Feb.). The Cx43 levels were lowered significantly by February (&P < 0.0005 Feb. vs. Aug.) but increased significantly by June (**P < 0.005 June vs. Feb.). E and F: representative Western blots with histograms showing the monthly changes in Cx46 protein levels in tubule-enriched fractions during (E) development and (F) the annual reproductive cycle in mink. Quantification of the levels of the 51- and 68-kDa Cx46-immunoreactive bands is provided. The values are the means ± SE of 3 independent experiments expressed in arbitrary units and normalized to the 51-kDa band in 90-day-old mink for studies on development and normalized to August during the annual reproductive cycle. The changes in Cx46 protein levels were significant during (E) development (51 kDa band: *P < 0.05 210 days vs. 180 days and **P < 0.005 240 days vs. 210 days; 68 kDa band: *P < 0.05 180 days vs. 150 days and **P < 0.005 210 days vs. 180 days) and (F) the seasonal reproductive cycle (51 kDa band: ++P < 0.001 Nov. vs. Sept. and May vs. April and *P < 0.05 July vs. June; 68 kDa: +P < 0.01 Sept. vs. Aug. and April vs. March and *P < 0.05 Dec. vs. Nov., March vs. Feb., and July vs. June). G and H: representative Western blots of Cx50 in mink tubule-enriched fractions during (G) development and (H) the annual reproductive cycle. The quantification of the 51- and 60-kDa Cx50-immunoreactive band levels are plotted monthly. The values are the means ± SE of 3 independent experiments expressed in arbitrary units and normalized to the 51-kDa band in the 90 day old during development and to August during the annual reproductive cycle. The following changes in Cx50 levels were significant during development [G; 51 kDa band (black bars): ++P < 0.001 120 days vs. 90 days, *P < 0.05 210 days vs. 180 days, and +P < 0.01 240 days vs. 210 days; 60 kDa band (gray bars): ++P < 0.001 240 days vs. 210 days and *P < 0.05 270 days vs. 240 days] and the annual reproductive cycle (H; 51 kDa band: @P < 0.02 Dec. vs. Nov. and May vs. April; 60 kDa band: *P < 0.05 Dec. vs. Nov. and April vs. March).
Cx46, Cx50, and Cx43 mRNA Expression in Normal Mink
During development.
Cx46 mRNA levels increased steadily by 270 days in adulthood when the values recorded were significantly higher compared with 120-day-old mink (Fig. 2C). In contrast, Cx50 mRNA levels decreased significantly by 270 days compared with the 120 day olds (Fig. 2C). Cx43 mRNA profiles emulated the Cx50 profiles by being highest by 120 days, then dropping abruptly by 180 days, and remaining low by 270 days (Fig. 2C).
During the annual reproductive cycle in adult mink.
By February, Cx46 mRNA levels were highest, whereas Cx50 and Cx43 reached lowest levels in mink tubule fractions (Fig. 2D). As well, the low Cx46 mRNA values recorded by June were accompanied by relatively high Cx50 and Cx43 mRNA levels (Fig. 2D). Thus Cx46 mRNA showed profiles opposite to those of Cx50 and Cx43 mRNA, not only during postnatal development but also during the annual seasonal reproductive cycle.
Cx46 Protein Expression in Normal Mink
During development.
The 51- and 68-kDa Cx46-immunoreactive band levels both increased with development (Fig. 2E). The increase in 51-kDa levels was significant, first by 210 days compared with 180 days and again by 240 compared with 210 days; thereafter, the 51-kDa levels remained elevated by 270 days. When taking into consideration only the 68-kDa band, these levels raised significantly by 180 days compared with 150 days and again by 210 days compared with 180 days; after that, these levels remained elevated in adulthood (270 days; Fig. 2E).
During the annual reproductive cycle in the adult mink.
The 51-kDa Cx46-immunoreactive band levels reached low values from August to October. However, these levels peaked by November and then remained elevated until April (Fig. 2F). By May, 51-kDa levels dropped significantly compared with April, albeit they transiently increased by July compared with June (Fig. 2F). The profiles of the 68-kDa Cx46-immunoreactive band levels were virtually the opposite of the 51-kDa Cx46-immunoreactive band levels. The 68-kDa levels were elevated from September to November and again from April to June, that is, during the time periods when the 51-kDa levels were low. By contrast, 68-kDa levels diminished from December to March (Fig. 2F), which corresponds to the time periods when the 51-kDa levels were elevated. The 68-kDa increases were significant from August to September and then from March to April, whereas the decreases were significant by December compared with November and by March compared with February (Fig. 2F). The data indicate that Cx46 is dephosphorylated during the active spermatogenic phase (Fig. 2F).
Cx50 Protein Expression in Normal Mink
During development.
The 51-kDa Cx50-immunoreactive band levels were highest by 90 days and then decreased steadily by adulthood (Fig. 2G). The decreases of 51-kDa levels were significant from 90 to 120, 180 to 210, and 210 to 240 days (Fig. 2G). The levels of the 60-kDa Cx50-immunoreactive band remained low during most of development, except when they significantly but transiently raised by 240 days (Fig. 2G). Thereafter, 60-kDa levels then decreased by 270, while still remaining higher than at 90 days after birth (Fig. 2G).
During the annual reproductive cycle in the adult mink.
The 51-kDa Cx50 levels were augmented from August to November and then from May to July; by contrast, only traces of 51 kDa Cx50 were detected from December to April (Fig. 2H). There were traces of 60 kDa Cx50 from August to November, and then these levels increased from December to April, but they decreased again from May to July (Fig. 2H). For the 51-kDa Cx50 band, the decrease recorded was significant from November to December, whereas the increase was significant from April to May. For the 60-kDa band, the increase was significant from November to December, whereas the decrease was significant from March to April (Fig. 2H). The data indicate the phosphorylation of Cx50 during the active spermatogenic phase (Fig. 2H).
These results show that dephosphorylation of Cx46 and phosphorylation of Cx50 take place during the active spermatogenic phase, whereas the opposite takes place during the seasonal testicular regression. The phosphorylation of Cx46 and Cx50 is opposite.
Cx46 Immunolabeling
Mouse.
CONTROLS.
No reaction product was detected in Cx46−/−mice testes paraffin sections exposed to Cx46 antibodies (Fig. 3A).
Fig. 3.
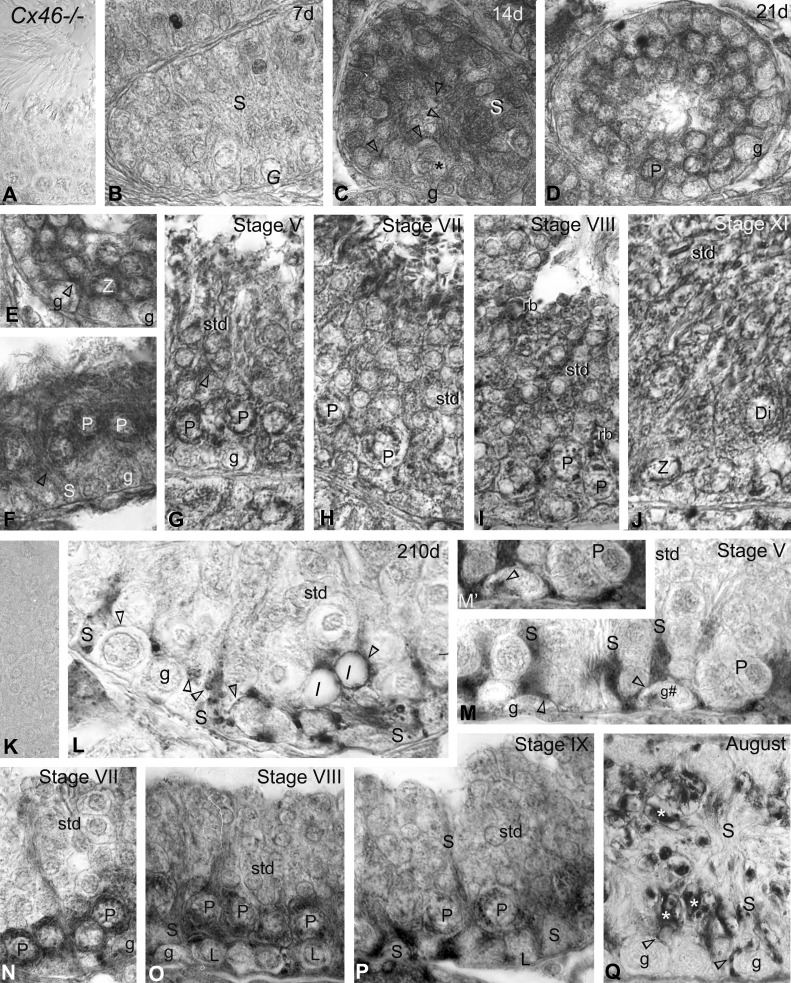
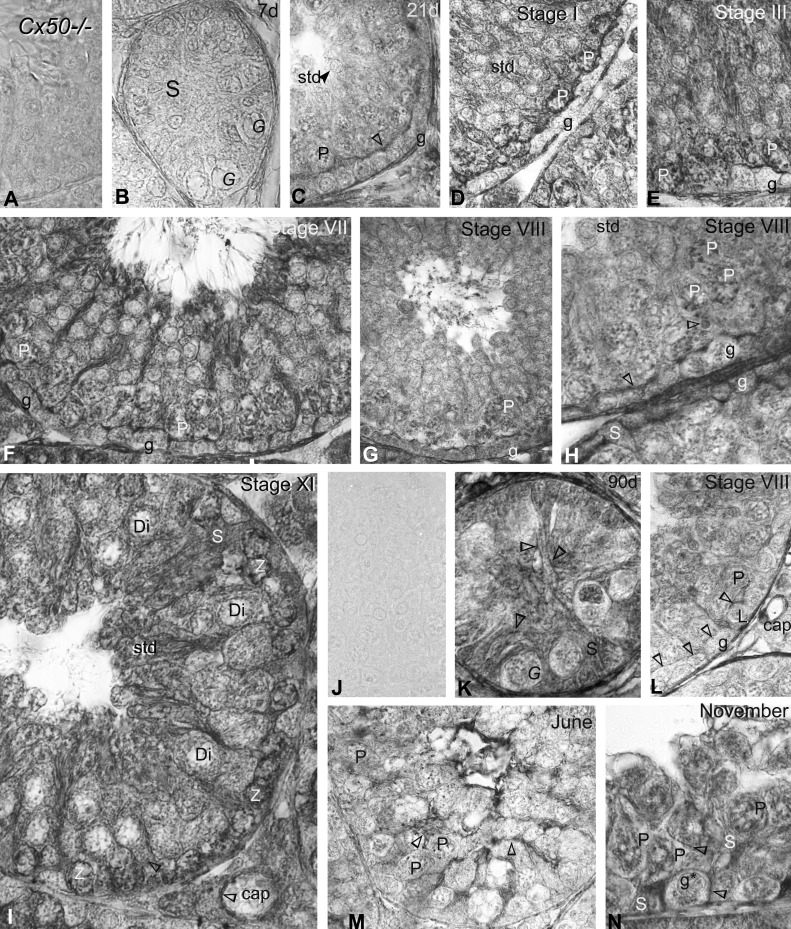
Immunoperoxidase labeling with anti-Cx46 in (A–J) mice and (K–Q) mink. A: no reaction product is detected with Cx46 antibodies used on Cx46−/− mice testis sections. B: Cx46 labeling was virtually undetectable in this 7-day-old testis section. G, undifferentiated spermatogonia; S, Sertoli cell. C: Sertoli cell plasma membranes, whether facing each other near the center of this developing tubule or engaged in Sertoli cell-to-Sertoli cell and Sertoli cell-to-germ cell (*) contacts near the basal 3rd, are heavily labeled (open arrowheads) by 14 days. g, spermatogonia. D–F: the colonization by spermatocytes of the seminiferous tubules and canalization of a lumen are seen in this 21-day-old mouse testis section. The open arrowheads point to labeling at (F) Sertoli cell plasma membranes and (E) intercellular contacts. The endoplasmic reticulum and Golgi apparatus of (E) zygotene (Z) and (D and F) pachytene (P) spermatocytes are heavily labeled. G–J: as well, Sertoli plasma membranes (open arrowhead in G) and the endoplasmic reticulum and Golgi apparatus of pachytene, zygotene, and diplotene (Di) spermatocytes are labeled in adult mice. The stages of the cycle of seminiferous epithelium appear at the top of the micrographs. std, spermatid; rb, residual body. K: no immunostaining is detected in Cx46 controls done on a normal adult mink testis section obtained in February when using the primary or secondary antibody alone. L: Cx46 immunolabeling is shown in a 210-day-old and (E–I) adult mink testes in (M–P) February and (Q) August. Labeling (open arrowheads) is identified in variously sized vacuoles scattered within the trunk of Sertoli cells, as well as in Sertoli cell contacts with the germ cells (spermatogonia; pachytene spermatocytes). Lipid droplets (l), near the base of Sertoli cells, are surrounded by an intense, Cx46-positive halo (open arrowheads). M–P: in February, Cx46 labeling is seen (open arrowheads) in regions of Sertoli cells and in their contacts between themselves and with spermatogonia, leptotene (L), and pachytene spermatocytes during the different stages of the mink seminiferous cycle, appearing in roman numerals atop the figures. M′: a higher magnification of Cx46 labeling in the Golgi region of the spermatogonium, identified g# in M. The endoplasmic reticulum and the Golgi zone of the leptotene and pachytene spermatocytes are labeled. Cx46 labeling decreased in pachytene spermatocytes from stage VIII (O) to stage IX (P). Q: during the seasonal testicular regression, Cx46 labels (arrowheads) thin Sertoli cell processes, some of which are in contact with spermatogonia and pachytene spermatocytes, remaining in seminiferous tubules by August. Lipofuscin pigments are identified (asterisks) in regressed Sertoli cells. Original magnification, ×860 (A–M and N–Q); ×980 (M′).
DEVELOPMENT AND ADULTHOOD.
Cx46 labeling was apparent at the sites of Sertoli cell-to-Sertoli cell and Sertoli cell-to-germ cell contacts by 14 days after birth (Fig. 3C) but not in 7-day-old seminiferous tubules (Fig. 3B). The canalization of a lumen and the colonization by spermatocytes of most seminiferous tubules were concomitant events that took place typically ∼21 days after birth in the mouse (Fig. 3, D–F). The Sertoli cell intercellular contacts were labeled (Fig. 3F). In addition, the endoplasmic reticulum and Golgi apparatus of spermatocytes exhibited heavy labeling (Fig. 3, D–F), and Cx46-positive minuscule dots were detected in round spermatids (Fig. 3, D–F). In the adult mouse, Cx46 labeled contacting Sertoli plasma membranes, as well as the endoplasmic reticulum and Golgi apparatus of spermatocytes during the stages of the seminiferous epithelium cycle (Fig. 3, G–J).
Mink.
CONTROLS.
The controls using either the primary or the secondary antibody alone showed no reaction product (Fig. 3K).
DEVELOPMENT.
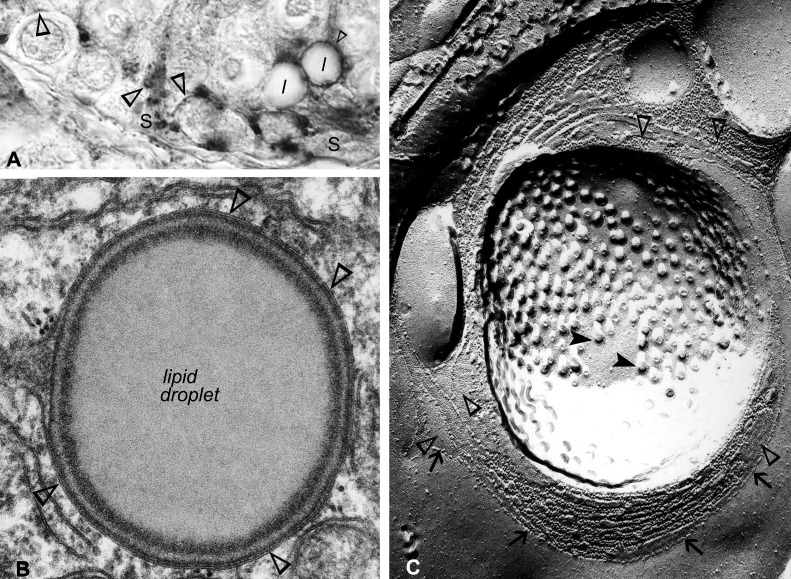
The colonization by spermatocytes of the seminiferous tubules takes place ∼180 days after birth in mink. However, in contrast to rodents, the canalization of a lumen occurs after the completion of spermatogenesis, ∼240 days after birth in mink (Fig. 2, A and B). Sertoli cell–Sertoli cell and Sertoli cell-to-spermatogonia or Sertoli cell-to-young spermatocytes contacts established near the base of the epithelium were labeled (Fig. 3L). Minute, Cx46-positive halos, corresponding to annular junctions, were contained within the Sertoli cells' trunk (Fig. 3L). Several lipid droplets were surrounded individually by a Cx46-positive halo (Fig. 3L). A Cx46-positive annular junction wrapped around a lipid droplet is shown in Figs. 3L and 4A. The annular junction, establishing contacts with the lipid droplet surface, is clearly apparent in the electron microscopy of thin sections (Fig. 4B) and exposed freeze-cleaved membranes (Fig. 4C). Our novel observation here of Cx46-positive Sertoli cell annular junctions enfolding lipid droplets evidences not only the ability of the droplets to communicate with other organelles in Sertoli cells through surface contacts but also indicates that Cx46 may be an actor in the lipid droplet–cell junction communication process.
Fig. 4.
A: this is a higher magnification of a portion of the field shown in Fig. 3L, in which the attention is drawn here to Cx46 labeling, detected (open arrowheads) not only in Sertoli cell-to-germ cell junctions but also in annular junctions found either wrapped around a lipid droplet or scattered within the trunk of the Sertoli cell. B: electron microscopy of a thin section of an annular junction, corresponding to the one shown in A, is shown, establishing contacts with the surface of a lipid droplet. C: a corresponding image in electron microscopy of freeze fracture. In this micrograph, an impressive circular array of Sertoli cell annular gap junctions (open arrowheads), intercalated amongst strands of tight-junctional particles (arrows), is seen surrounding a cluster of filipin-cholesterol complexes (closed arrowheads). Original magnification, ×1,000 (A); ×1,000,000 (B); ×116,000 (C).
Annual reproductive cycle.
In addition to the Cx46 labeling detected in Sertoli cell-to-germ cell contacts (Fig. 3M), some spermatogonia exhibited faint, Cx46-positive dots in the Golgi zone adjacent to the nucleus during stage V of the seminiferous epithelium cycle (Fig. 3, M and M′). The perinuclear region, the Golgi zone, and the endoplasmic reticulum in leptotene and pachytene spermatocytes were intensely labeled during stage VII (Fig. 3N) and stage VIII (Fig. 3O), which, respectively, precedes and follows spermiation in the mink. Cx46 labeling was detected in the perinuclear region in spermatocytes, and the reaction product was viewed in the basal third of the Sertoli cell–Sertoli cell and in Sertoli cell–germ cell contacts by stage IX (Fig. 3P). The Sertoli cell-to-Sertoli cell contacts and Sertoli cell processes abutting germ cells remaining in the seminiferous tubules were labeled during the seasonal testicular regression (Fig. 3Q).
Cx50 Immunolabeling
Mouse.
CONTROLS.
No reaction product was detected when the specificity of Cx50 antibodies was tested in Cx50−/− mice testes paraffin sections (Fig. 5A).
Fig. 5.
Immunoperoxidase labeling with anti-Cx50 in (A–I) mice and (J–N) mink testis sections. A: no reaction product was detected when Cx50 antibodies were used on Cx50−/− mice testis sections. B: no reaction product was detectable in 7-day-old mice testis sections. C: this 21-day-old mouse testis section shows Cx50 labeling (open arrowhead) in the cell contacts settled at the site of the blood-testis barrier. D–H: at this site, Cx50 labeling is seen in cell contacts established above spermatogonia and young spermatocytes in the basal 3rd of the seminiferous epithelium during the stage cycles in adult mice. D–J: in addition, zygotene and pachytene spermatocytes show intense Cx50 labeling. I: the wall of the capillary (cap) is labeled. J: Cx50 control, done using either the 1st or the 2nd antibody in adult mink testis sections obtained in February, shows no immunostaining. K: the Sertoli cell membranes show delicate labeling (open arrowheads) in a 60-day-old mink. L: in February, the distribution of Cx50 (open arrowheads) above spermatogonia and young spermatocytes in the tubules closely coincides with that of junctional complexes established at the site of the blood-testis barrier in mink. M: the lumen of the seminiferous tubule is collapsed in June during testicular regression, and contacting Sertoli cell plasma membranes are labeled; however, the distribution of Cx50 labeling no longer coincides with that of the junctional complexes at the site of the blood-testis barrier. N: contacts between Sertoli cells and spermatogonia pachytene spermatocytes are labeled in November. As well, the Golgi zone of the spermatogonium identified g*; spermatid contains minuscule Cx50-positive dots. Original magnification, ×860.
DEVELOPMENT AND ADULTHOOD.
Cx50 was virtually undetectable in 7-day-old mouse testis sections (Fig. 5B). However, inter-Sertoli cell contacts, set up in the basal third of the seminiferous epithelium at the site of the blood-testis barrier, were intensely Cx50 positive in 21-day-old (Fig. 5C) as well as adult mice (Fig. 5, D–I). In addition, the perinuclear region of spermatocytes contained Cx50-positive material during all stages of the cycle (Fig. 5, D–I). Minute, Cx50-positive dots were also seen in round spermatids (Fig. 5, E and F). The wall of blood capillaries in the interstitial tissue of the testis was Cx50 positive (Fig. 5I).
Mink.
CONTROLS.
Controls done with either the primary or the secondary antibody alone showed no reaction product (Fig. 5J).
DEVELOPMENT AND ANNUAL REPRODUCTIVE CYCLE.
A faint and delicate Cx50 labeling was seen along Sertoli cell membranes and in the zones of contacts between them by 60 days after birth (Fig. 5K). By adulthood, Cx50 labeling was apparent in similar locations as the ones described above in the adult mouse, namely in Sertoli cell-to-germ cell contacts and inter-Sertoli cell contacts established at the site of the blood-testis barrier, which is located in the basal third of the seminiferous epithelium (Fig. 5L). The distribution of Cx50 remained apparent in Sertoli membranes, whether facing Sertoli cells or germ cells remaining in the seminiferous epithelium, but was no longer observed at the site of the blood-testis barrier by June (Fig. 5M) and by November (Fig. 5N).
Morphological Studies in Cx46−/− and Cx50−/− Mouse Testes and Epididymides
Testis.
The seminiferous epithelium contained few germ cells in apoptosis, and spermatogenesis appeared normal in both WT (Fig. 6A) and Cx50−/− mice (Fig. 6B). In contrast, Cx46−/− testes sections were characterized by plentiful apoptotic bodies (Fig. 6, C–E), typically observed amongst spermatocytes of the first and second meiotic divisions (Fig. 6, C–E) and amongst spermatids (Fig. 6D). Multinucleated giant cells were, however, infrequent (Fig. 6, C–E). The lumen of some seminiferous tubules was collapsed; other contained cellular debris (Fig. 6E).
Fig. 6.
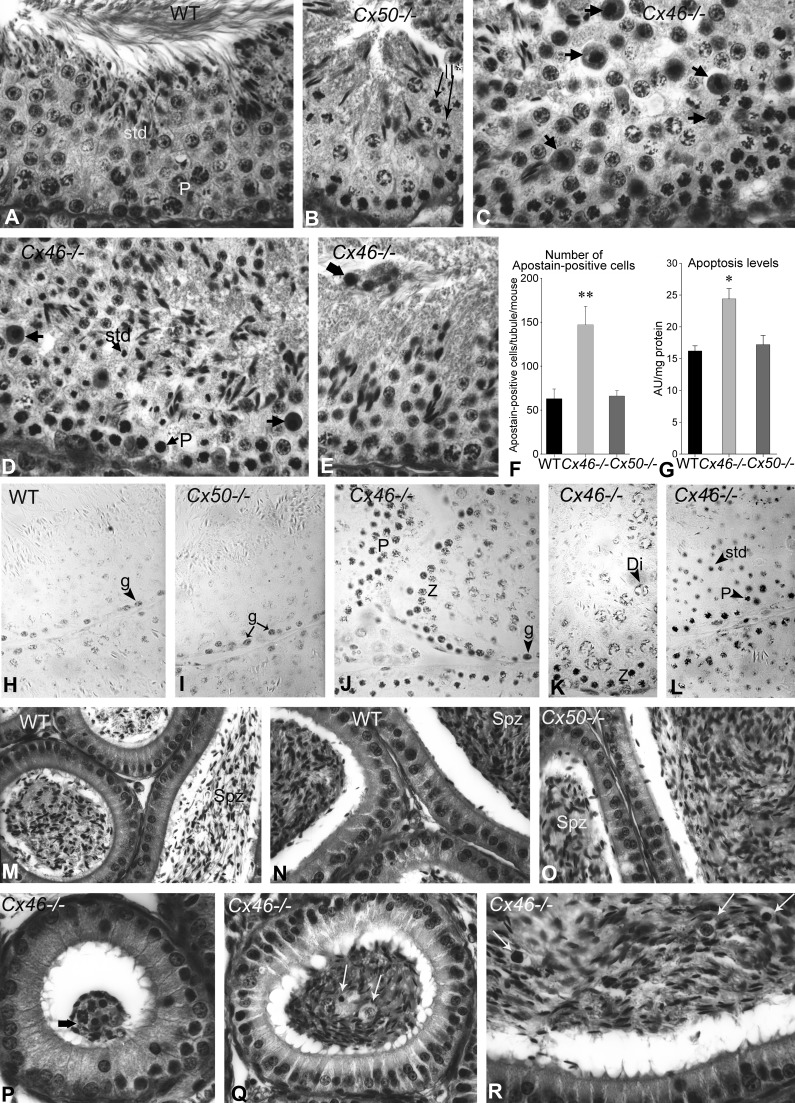
A–E: Bouin's-fixed periodic acid Schiff (PAS)-stained paraffin testis sections from (A) normal, >60-day-old WT, (B) Cx50−/−, and (C–E) Cx46−/− adult mice. A and B: spermatogenesis appeared normal in WT and Cx50−/− mice testis sections, in which pachytene spermatocytes and dividing secondary spermatocytes are identified (II*). C and D: however, Cx46−/− testis sections showed numerous tubules with plentiful apoptotic cells, particularly pachytene and diplotene spermatocytes, involved in the meiotic division (arrows). In other stages of the cycle, apoptotic spermatids were observed. E: the lumen was collapsed in some tubules, whereas in others, cellular debris or clusters of apoptotic cells (wide arrow) were observed. F: a histogram of the quantification of immunoperoxidase Apostain-labeled cells is presented. The bars represent the means ± SE of Apostain-positive cells, counted in 25 tubules from 3 different mice per experimental group. Apostain-labeled cells were significantly more numerous in Cx46−/− than in WT seminiferous tubules (**P < 0.005 Cx46−/− vs. WT). The number of labeled cells in WT and Cx50−/− mice did not differ significantly. G: a histogram of nucleosome release measured by cell death detection ELISA in the cytoplasmic fraction of WT, Cx46−/−, and Cx50−/− mice tubule-enriched fractions obtained is shown. The data are expressed in optical density at 410 nm, and the bars represent the means ± SE of measurements in 3 different mice per experimental group. The increase in nucleosome release in Cx46−/− is significantly different (*P < 0.05) compared with WT but not between WT and Cx50−/−. H–L: immunoperoxidase labeling of cells in apoptosis with antibody F7-26 (Apostain) is shown. Apostain-positive spermatogonia, zygotene, pachytene, and diplotene spermatocytes and spermatids were found in comparable quantities in (H) WT and (I) Cx50−/− mice. J–L: in Cx46−/− mice, Apostain labeling involved spermatogonia and spermatids. In addition, zygotene, pachytene, and diplotene spermatocytes were labeled in larger quantities in Cx46−/− than in WT and Cx50−/− mice. M–R: Bouin's-fixed PAS-stained epididymis paraffin sections from (M and N) WT, (O) Cx50−/−, and (P–R) Cx46−/− mice are shown. The (M) body and (N and O) tail of the epididymides of (M and N) WT and (O) Cx50−/− exhibited similar histological features, and both contained spermatozoa (Spz) in comparable amounts. However, (P and Q) the epididymides from Cx46−/− showed spermatozoa in reduced quantities. P: a cluster of apoptotic cells, reminiscent of the one shown in the testis in Fig. 3E, is identified (wide arrow). Apoptotic spermatocytes and other young germ cells are identified by arrows in the (P) head, (Q) body, and (R) tail of Cx46−/− mice epididymides. Original magnification, ×720 (A–E); ×860 (H and I and K–O); ×950 (J and P–R).
Quantification of the Number of Apoptotic Germ Cells in Seminiferous Tubule Cross-Sections
A significantly higher number of Apostain-labeled apoptotic cells per seminiferous tubule cross-section were recorded in Cx46−/− than in WT and Cx50−/− mice (Fig. 6F). However, the numbers of labeled cells per tubule did not differ significantly between WT and Cx50−/− (Fig. 6F). When measurements of nucleosome release in STfs by ELISA were carried out, significantly higher nucleosome release incidences were found in Cx46−/− than in WT tubule fractions (Fig. 6G), whereas there were no significant differences between Cx50−/− and WT (Fig. 6G).
Immunoperoxidase Labeling of Apoptotic Germ Cells With MAb F7-26 (Apostain)
The adult WT (Fig. 6H) and Cx50−/− (Fig. 6I) mouse testis sections hosted occasional Apostain-labeled germ cells, in contrast to the Cx46−/− that exhibited multiple aggregations of labeled pachytene and diplotene spermatocytes (Fig. 6, J and K) and spermatids (Fig. 6, K and L).
Epididymis.
The different regions of the epididymides exhibited comparable histological features and showed spermatozoa in similar abundance at the light microscope in WT (Fig. 6, M and N) and Cx50−/− mice (Fig. 6O). However, spermatozoa seem to be in lesser density in Cx46−/− mice epididymides (Fig. 6, P–R). Apoptotic, young germ cells, particularly spermatocytes, either solitary or clustered, were encountered frequently in the lumen of Cx46−/− mice epididymides (Fig. 6, P–R).
Cx46, Cx50, and Cx43 mRNA Expression in Mink With AIO
The Cx46 mRNA levels dropped significantly in mink tubule-enriched fractions with AIO by February compared with normal mink (Fig. 7A); however, they were increased significantly by March compared with normal mink (Fig. 7A). Cx50 mRNA levels in mink with AIO and normal mink were not significantly different (Fig. 7B). The Cx43 mRNA levels increased significantly in mink with AIO by February. The levels fell significantly by March compared with normal values (Fig. 7C).
Fig. 7.
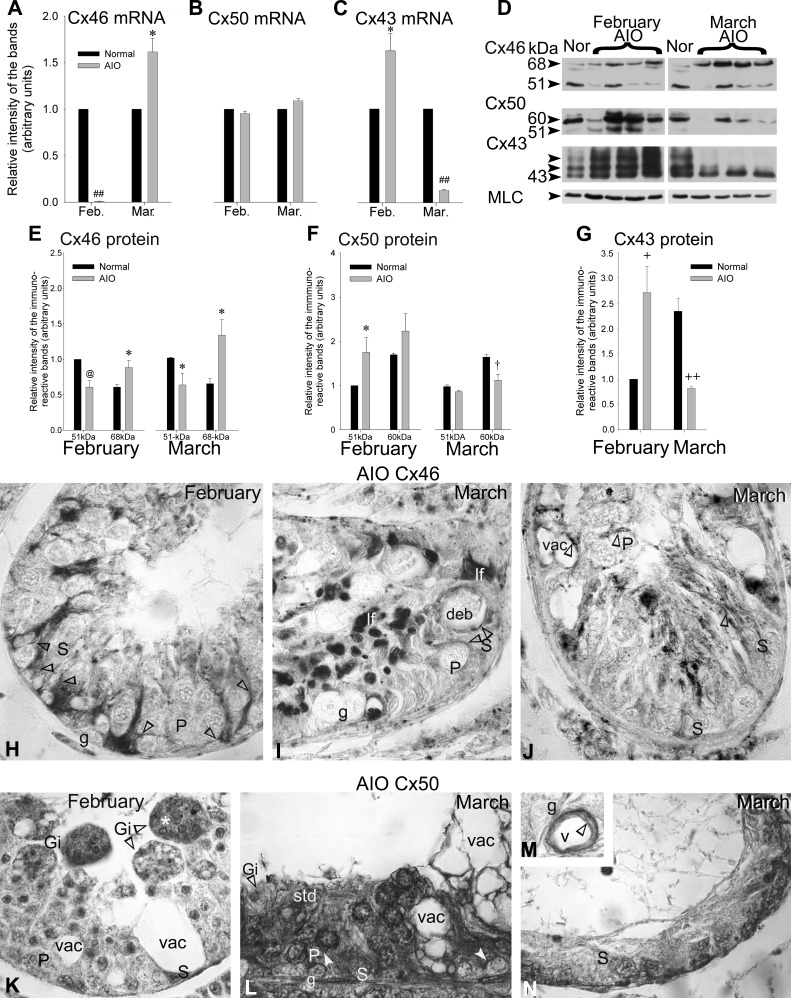
A–C: the seminiferous tubule-enriched samples were subjected to RT-PCR. The (A) Cx46, (B) Cx50, and (C) Cx43 mRNA levels measured in normal adult mink were compared with those in mink with autoimmune orchitis (AIO) in February and March (Mar.). The data in arbitrary units are expressed as the means ± SE of 3 independent experiments and normalized to the normal value for each month. The differences measured between normal mink and mink with AIO were statistically significant for (A) Cx46 mRNA (##P < 0.00001 Feb. AIO vs. Feb. Normal and *P < 0.05 Mar. AIO vs. Mar. Normal) and for (C) Cx43 mRNA (*P < 0.05 Feb. AIO vs. Feb. Normal and ##P < 0.00001 Mar. AIO vs. Mar. Normal). B: the differences in Cx50 mRNA levels measured in normal mink and mink with AIO show no significant difference. Representative Western blots of (D) Cx46, Cx50, and Cx43 in normal (Nor) adult mink and mink with AIO in February and March are shown. The quantification of (E) Cx46, (F) Cx50, and (G) Cx43 protein levels, measured in normal mink and mink with AIO, is provided. The values are the means ± SE of 4 independent experiments, expressed in arbitrary units, normalized to the 51-kDa Cx46 for February, the 51-kDa Cx50 for February, and total Cx43 for February. The myosin light chain (MLC) was used as the internal loading control and did not change significantly in the different experimental conditions of the study. Significant differences were measured in the junction protein levels between normal mink and mink with AIO: Cx46, February, 51 kDa band: @P < 0.02 AIO vs. Normal, 68 kDa band: *P < 0.05 AIO vs. Normal; March, 51 kDa band: *P < 0.05 AIO vs. Normal, 68 kDa band: *P < 0.05 AIO vs. Normal; Cx50, February, 51 kDa band: *P < 0.05 AIO vs. Normal; March, 60 kDa band: †P < 0.03 AIO vs. Normal; Cx43: February, +P < 0.01 AIO vs. Normal; March, ++P < 0.001 AIO vs. Normal. Immunoperoxidase labeling with anti-Cx46 in (H) February and (I and J) March and with anti-Cx50 in (K) February and (L–N) March of orchitic adult mink testis paraffin sections, respectively. H: Cx46 labeling (open arrowheads) is seen amongst Sertoli cells and Sertoli cell–Sertoli cell, Sertoli cell–spermatogonia, and pachytene spermatocytes contacts. I and J: the membrane of vacuoles (vac) remaining behind the exfoliation of germ cells is Cx46 positive. I: some of these vacuoles contain cellular debris (deb). IF, lipofuscin pigment. K: giant cells remaining within or being released from the seminiferous epithelium are identified (Gi). The cells within the giant cell (Gi*) show signs of apoptosis. L: Cx50 labeling (open arrowhead) is observed amongst Sertoli cells, Sertoli cell contacts to germ cells, as well in the perinuclear zone of pachytene spermatocytes in the early phase of AIO but not in (N) tubules, where destruction is massive in the late phase of the disease. M: Cx50 labeling (open arrowhead) is seen in the wall of a blood vessel (v). Original magnification, ×860.
Cx46 Protein Expression in Mink With AIO
The 51-kDa Cx46-immunoreactive band levels decreased significantly in contrast to the 68-kDa band levels that augmented in mink with AIO by February compared with normal mink (Fig. 7, D and E), suggesting an increase in phosphorylated Cx46. As well, by March, the 51-kDa Cx46 levels decreased significantly, and the 68-kDa Cx46 levels augmented in orchitic tubules compared with normal, suggesting again an increase in phosphorylation (Fig. 7, D and E).
Cx50 Protein Expression in Mink With AIO
The levels of the 51-kDa Cx50-immunoreactive bands increased significantly in tubules of mink with AIO compared with normal by February (Fig. 7, D and F). The 60-kDa band levels fell significantly by March in mink with AIO compared with normal values (Fig. 7, D and F). Profiles of Cx46 and Cx50 protein levels and phosphorylation status were opposite in mink with AIO (Fig. 7, D and F).
Cx43 Protein Expression in Mink With AIO
The levels of Cx43 increased significantly by February but dropped by March in mink with AIO compared with the normal (Fig. 7, D and G), thus emulating the Cx50 profiles (Fig. 7, D and F).
Morphological Studies Mink Testes With AIO
Spontaneous AIO was characterized by germ cell exfoliation (Fig. 7H), multinucleate giant cell formation (Fig. 7K), vacuolization of Sertoli cells (Fig. 7, I–L and N), and arrest of spermatogenesis at different stages of germinal cell development (Fig. 7, I–L and N) (75, 82). The release of cohorts of germ cells gave a vacuolated appearance to seminiferous epithelium (Fig. 7, I–L and N). The lumen was collapsed in some tubules (Fig. 7, I and J); others were infiltrated by cells from the immune system. Only Sertoli cells and spermatogonia remained in severely damaged tubules (Fig. 7, I, J, and N).
Cx46 immunolabeling.
Labeling was observed in minute vesicles in Sertoli cells (Fig. 7H), the cell membrane of large vacuoles, and facing Sertoli cell membranes (Fig. 7, H–J).
Cx50 immunolabeling.
Sertoli cell membranes facing early-stage germ cells were labeled (Fig. 7L). In addition, the perinuclear region of remaining spermatocytes was Cx50 positive (Fig. 7L). As well, the wall of blood vessels (Fig. 7M) was labeled. However, severely damaged tubules exhibited insignificant labeling (Fig. 7N).
DISCUSSION
Cx46 and Cx50 in the Seminiferous Epithelium
Cx46 has been studied in osteoblastic cells (41), astrocytes (24), alveolar epithelial cells (1), bone (90), human breast tumor (7), heart (20), and lens (5, 32, 33). Cx50 has also been studied in the lens (31, 103). However, this is the first report on Cx46 and Cx50 in the mouse and mink seminiferous epithelium.
Cx46 and Cx50 Expression and Phosphorylation
Cx46.
Our finding of a 51- and 68-kDa phosphorylated, Cx46-immunoreactive band in mouse and mink seminiferous tubule fractions agrees with the report of bands of similar molecular mass in other rodent tissues and cultured cells (19, 41). As well, our observation of a phosphorylated form of Cx46 in tubule fractions is consistent with the report of the involvement of casein kinase and PKC (88) in the phosphorylation of Cx46 in threonine and serine residues in the lens (9, 100).
Cx50.
Our observation of a 51-kDa Cx50-immunoreactive band in mouse and mink lens and tubule fractions agrees with the report of a similar band in the murine lens (93). Moreover, our finding of a phosphorylated 60-kDa Cx50 form is consistent with the report of the phosphorylation of Cx50 on serine 395 and threonine residues by PKA, PKC-γ, and extracellular signal-regulated kinase in the lens (49, 51, 93).
Evidence for an Interactive Regulation of Cx46, Cx50, and Cx43 in the Seminiferous Epithelium
The 51-kDa, Cx46-level profiles were opposite of those of the 51-kDa Cx50 and Cx43 in mouse-developing tubule fractions. As well, the 51-kDa Cx46 and 51-kDa Cx50 profiles were opposite during development in mink. Moreover, we found that the phosphorylation of Cx46 and Cx50 is also opposite during mouse development, as well as during the normal mink annual seasonal reproduction cycle. The change in the phosphorylation status of Cx46 and Cx50, in addition to being opposite, takes place from November to April, that is, during the active spermatogenic phase. This advocates for a timely and interdependent regulation of not only the expression but also the phosphorylation of Cx43, Cx50, and Cx46 in the seminiferous epithelium. In mouse, deleting Cx50 results in a decrease in phosphorylated 68 kDa Cx46, whereas deletion of Cx46 causes an increase in 51 kDa Cx50, accompanied by a decrease in the phosphorylated 60-kDa Cx50. Moreover, deletion of Cx50 is accompanied by a dramatic increase in PCx43. Together, these findings evidence a complementary expression and phosphorylation of Cx46 and Cx50 and show that the expression and phosphorylation of one connexin influence the expression and phosphorylation of other connexins in the seminiferous epithelium. Our observations are in line with the report of an upregulation of Cx46 in regions of the lens where Cx43 was downregulated (60) and with the inverse relation in Cx43 and Cx46 protein amounts reported in Y79b retinoblastoma cell xenografts in humans (15).
Evidence for an Influence of Cx46, Cx50, and Cx43 on Other Junction Constituents in the Sertoli Cell Junctional Complexes
Our observation of Cx46 interacting with other cell junction components, besides or in addition to Cx50 and Cx43, reflects the reciprocal influence of individual cell junction constituents on each other within Sertoli cell junctional complexes. Cx46 deficiency causes a total Cx43-level increase and an occludin and N-cadherin decrease. Cx50 deficiency results in increased total Cx43 and claudin 11 levels and dropping ZO-1 levels in mice. Thus when tight-junction and adhering-junction proteins are affected by Cx46 deletion, they are not affected by the deletion of Cx50 and vice versa. Our finding of an interaction of Cx46 and Cx50 with ZO-1 in the seminiferous epithelium agrees with the report of a Cx46- and Cx50-ZO-1 interaction in other cell systems (65). As well, our observation is also in agreement with an earlier report of a Cx43 increase accompanied by a decrease in N-cadherin, occludin, and claudin 11 protein levels in the seminiferous tubules during development and the annual reproductive cycle in mink (74). In whole mouse testis extracts, the conditional invalidation of Cx43 in mouse Sertoli cells was found to increase N-cadherin, β-catenin, and occludin but to decrease ZO-1 protein levels (18). The blocking of gap junction-mediated cell coupling with gap-junction inhibitors and small interfering RNA to decrease Cx43 levels causes similar effects in a Sertoli cell line (18). The interdependence amongst individual constituents within the Sertoli cell junctional complex entails that modifications targeted to a single constituent alter the expression and phosphorylation state of other junction constituents and likely the function that these constituents regulate.
The Timely Transition of Cx43 to Cx46 and Cx50 Expression and Phosphorylation
The regulation of the expression and phosphorylation of Cx46, Cx50, and Cx43 is timely with the collection of particular generation germ cells in the seminiferous epithelium. Our finding here, that total Cx43 protein levels decrease with adulthood in the tubules, confirms earlier observations in the mouse (99). Moreover, we have documented a peak in tubular Cx43 levels concurrent with the onset of meiosis and the colonization of the tubules by spermatocytes (74, 75), an observation consistent with the localization of Cx43 mRNA to primary spermatocytes (86). This study localizes Cx50 and Cx46 in the perinuclear region of spermatocytes and found that the Cx43 levels elevated by the beginning of mouse and mink development, during cellular growth and proliferation, dropped during maturation in adulthood. The profile of Cx50 expression emulates that of Cx43. By contrast, the low 51- and 68-kDa Cx46 expression early in development augments with adulthood. In the mink, we found that the phosphorylation status of Cx46 and Cx50 is opposite during the active spermatogenic phase. Together, these observations are indicative of a stage-specific transition of Cx43 and Cx50 to Cx46 expression, taking place during the switch from the mitotic to the meiotic division, the colonization of the tubules by spermatocytes, and their translocation into the lumenal compartment of the seminiferous epithelium.
The Regional Distribution of Cx46, Cx50, and Cx43 in the Seminiferous Epithelium
We have reported earlier that PCx43 is localized to gap junctions established at the site of the blood-testis barrier (99). Our observation here, that phosphorylated Cx50 increased during the active spermatogenic phase and that during this period, Cx50 has a distribution reminiscent of that of PCx43, is indicative of an implication of Cx43 and Cx50 phosphorylation in the dynamics of this barrier in contrast to Cx46 that exhibits a different distribution within the seminiferous epithelium. During the mink active spermatogenic phase, Cx50 became phosphorylated (Fig. 2H) and localized to the site of the blood-testis barrier (Fig. 5L) in contrast to Cx46, which was dephosphorylated (Fig. 2F) and associated with annular junctions during the same period, suggesting the involvement of Cx46 and Cx50 phosphorylation/dephosphorylation in the dynamics of this barrier. Cx46-positive annular junctions, establishing contacts with the lipid-droplet surface, were found, indicating the participation of Cx46 in the touch and degradation process. We have reported that the blood-testis barrier is competent in blocking entry of blood-borne substances from December to March in this species (Fig. 2B) (68, 69). Phosphorylation of Cx43 has been shown to influence junction permeability by regulating the connexin-degradation process (30). Phosphorylation of Cx50 by PKC-γ uncouples cortical fiber cells in the lens and reduces Cx50-mediated communication, in contrast to phosphorylation by PKA that augments it (51). Thus transitory changes in the phosphorylation status of Cx50 could, in theory, momentarily impede Cx50-mediated junction communication established at the site of the blood-testis barrier while uncoupling germ cells and Sertoli cells in accordance with the physiological requirements of the spermatogenic activity.
In addition, we localized Cx50 and Cx46 to the perinuclear region of spermatocytes. This study also reveals a transition of Cx43 to Cx46 expression during spermatogenesis. Significantly, this transition to Cx46 expression coincides with a drop in the oxygen levels available to spermatocytes in transit from the normo-oxygenated basal compartment into the hypoxic lumenal compartment of the seminiferous epithelium. Whether the upregulation of Cx46 in late-stage germ cells is causally related to the drop in oxygen supply inflicted to these cells by the impediment of a direct access to circulation imposed by the blood-testis barrier remains to be established. This notwithstanding, this view is compatible with the report that hypoxia upregulated Cx46 in epithelial cells and that exogenous Cx46 prolonged mouse N2A cells, outliving from hypoxia-induced cell death (7). Here, Cx46-deficient mice show significant increases in apoptosis levels measured by ELISA, as well as in the number of Apostain-positive spermatocytes and spermatids, indicating that Cx46 gap junction-mediated exchanges are life sustaining for these late-stage germ cells. In addition, our finding of Cx46 in the perinuclear region of selected germ cells raises the possibility that Cx46 could serve as a marker for an intermediate germ cell phenotype, the meiosis. The spermatocytes may be unique in their capacity to house transiently, besides or in addition to Cx43 (86), a pool of Cx46 and Cx50. Because the spermatocyte stage extends over several days, opportunities for the Cx46, “retained” in the perinuclear compartment to interact with other cell junction proteins, such as Cx50 and Cx43, available within the cell or neighboring cells, are favored. Whether this inter-relation is carried out, awaiting Cx46 oligomerization, is unknown, but the view is not incompatible with the report of connexin oligomerization, required for the transport from an intracellular compartment to the cell surface (42, 64). Our observation of Cx46 in vesicles, as well as in junctional plaques at the surface of Sertoli cells, argues favorably for Cx46, at times accumulating in vesicles and at other times, transported to and retained at the plasma membrane in response to various stimuli. Together, these observations raise the possibility of a transit of Cx46 between the perinuclear region and the cell surface in spermatocytes before disposal in Sertoli cells. Cx46 has been shown to be retained in a trans-Golgi compartment in several cell systems, including cultured osteoblastic cells (41), lens cells (6), and canine bone tissue (90).
In addition, our observation evidences the biological significance of Cx46, not only on spermatocytes but also on Sertoli cell physiology as well. The infrequency of apoptotic figures that we typically observed in WT normal mouse testis sections, despite the documentation of 75% of the potential number of spermatozoa being lost through apoptosis-mediated germ cell death (35, 39), is proof of the efficient removal and disposal of cellular debris by Sertoli cells. However, our observation of an overload of apoptotic germ cells in Cx46-deficient mice indicates that imbalances in Cx46 gap junction-mediated exchanges result in the phagocytic clearance ability of Sertoli cells being exceeded or in an alteration of the Sertoli cell phagocytic function. Here, the targeted deletion of Cx46 increases nucleosome releases measured by ELISA and causes an overload of dying Apostain-positive cells in situ, that is, of damaged cells that are nonengulfed germ cells by Sertoli cells. By contrast, AIO was characterized by an overload of apoptotic and giant cells without modification of apoptosis levels in the early stages of the disease (82).
Cx46 and Cx43 and Blood-Testis Barrier
Our observation that Cx46 and Cx50 mRNA and protein are expressed and localized in seminiferous tubules is novel. Most tissue cells host more than a single isotype-cloned connexin (84). Cells expressing several species of connexins have the ability to form mixed gap-junction channels (13, 102, 104). Cx46 (the α3-connexin) differs from Cx50 (the α8-connexin) in that it can exceptionally form functional heteromeric gap junctions with either Cx43, an α-connexin, or Cx32, a β-connexin, in Xenopus oocytes (104) and cultured primary alveolar epithelial cells (1). The molecular diversity of hemichannels results in heterotypic gap-junction channels that influence a wide variety of cellular functions, such as growth and differentiation (16, 40). Moreover, the identity of connexins available to cells dictates the mode of gap communication through ionic or biochemical coupling.
The seminiferous epithelium is singular, in that many germ cells undergoing distinct life stages are in contact with a unique Sertoli cell. Yet, the physiological requirements imposed by individual life stages differ from mitosis to meiosis. On the one hand, gap junction-mediated communication helps synchronize interconnected Sertoli cell activities over some distance in seminiferous tubules. On the other hand, the gap junction-mediated Sertoli cell–germ cell communication favors coordination of germ cell and Sertoli cell activities. This coordination is vital because it helps germinal cells to carry out functions unresolvable to themselves alone, timely with their individual life stages (73–75). In this context, Cx46, endogenously expressed in the spermatocytes that are involved in the translocation from one side to the other of the blood-testis barrier, may take on a physiological role. Connexins can be mechanosensitive, the response being connexin specific (8). The gap-junction channels open to allow exchanges between cell interiors, usually only after two hemichannels dock. A singularity of Cx46 and Cx50 resides in that these connexins can provide both gap-junction channels as well as nonjunctional conductances in contrast to other connexins that typically form closed hemichannels in nonjunctional cellular membranes (29). In Xenopus laevis oocytes, Cx46 (67) and Cx50 (107) form open hemichannels when expressed exogenously. Hemichannels and complete gap-junction channels share the same permeability and gating characteristics, albeit that open hemichannels can endanger the cell because they are nonselective. For instance, Xenopus oocytes, expressing Cx46 or Cx50 exogenously, will survive only if the hemichannels are maintained closed by high extracellular Ca2+ concentration (28). The increased apoptotic number of spermatocytes that we measured in Cx46−/− mice suggests that hemichannels are not maintained closed following this genetic deletion. In contrast to Cx46 and Cx50, ubiquitous Cx43 forms open hemichannels, only when challenged with a nonphysiological, extracellular Ca2+ concentration (48).
Cx43, Cx46, and Cx50 in Lipid Rafts
Our finding of Cx43 in rafts in seminiferous tubule fractions agrees with the report of Cx43 in two cholesterol-binding integral membrane proteins of a subpopulation of lipid raft domains: caveolin-1 (45, 50, 91) and caveolin-2 (45) in other cell systems. As well, our observation of Cx46 and Cx50 in rafts in seminiferous tubule fractions is in line with the localization of these two connexins in lens caveolae (49) but differs from the localization of only Cx46 in lipid rafts of the lens (91). The high concentration of sphingolipids and cholesterol of the lipid rafts favors the accumulation of specific proteins in these microdomains. Cx46- and Cx50-mediated gap junctions are enriched in cholesterol and sphingomyelin (4, 34), suggesting that connexin channels can segregate into lipid rafts. The functional significance of rafts in the regulation of membrane channels is based on their contribution to intracellular signaling interactions (21, 94) and their ability to include or exclude proteins selectively from individual membrane domains (83). The sorting controls the delivery, assembly, and removal of individual proteins from selected microdomains (44, 87, 92). Each ∼8-nm gap-junction, intramembranous particle, visualized on the P-fracture face of the plasma membrane (Fig. 4C), corresponds to one connexin or hemichannel, and the pairing of two of these units results in a channel. The filipin cytochemistry has allowed us to establish that gap-junction formation/dismantling occurs in lipid-rich microdomains, whereas junction maturation occurs in cholesterol-poor domains (74, 76, 77, 81). This observation indicates that individual connexin-mediated channels are sorted in different microdomains, according to connexin species or pairing characteristics, through differences in lipid/protein ratios in Sertoli cell membranes.
AIO Lipid Raft and Cx43 and Cx46
The TNF-α mRNA and protein were reported in round spermatids (22), whereas IL-1α and IL-6 are secreted by Leydig and Sertoli cells (66). The IFN-γ mRNA and protein were recorded in phytohemagglutinin-stimulated early spermatids but not in peritubular cells, Sertoli cells, or pachytene spermatocytes (23). Spontaneous AIO causes the release of TNF-α, IL-6 (82), and IFN-γ (unpublished observations), associated with aberrations in the gene transcription and post-translational phosphorylation of Cx43 (75). TNF-α and IL-1β have been shown to modify Cx43 turnover in folliculostellate cells (30, 59). The release of proinflammatory cytokines triggered by AIO may alter the recruitment and sorting of Cx46, Cx50, and Cx43 channels into areas of the Sertoli cell plasma membrane possessing a rigidity related to that of “rafts,” resulting in modifications in the behavior, function, and future of these gap junctions. This hypothesis will require the comparison of the collection of Cx46, Cx50, and Cx43 in the lipid rafts of cells in orchitic tubules with that of normal. This notwithstanding, the view is compatible with the following two reports in different cell systems. In Madin-Darby canine kidney cells, TNF-α-triggered translocation of TNF receptor 1 and c-Src into lipid rafts stimulates an intracellular pathway that deregulates gap-junction intercellular communication (25). Similarly, the release of IFN-γ by enterocytes during necrotizing enterocolitis displaces Cx43 from lipid rafts, impairing Cx43 in these cells and inhibiting their migration (46).
Cx46 Localizes in Junctions Around Lipid Droplets
Our earlier observation of Sertoli cell lipid droplets establishing contacts with cholesterol-positive cell junctions, cisternae of endoplasmic reticulum, and junctional segments blebbing from the cell surface has evidenced the ability of the droplets to communicate with other intracellular organelles through stable or transient surface contacts in the seminiferous epithelium (74). Lipid droplets–cell junction contacts have been documented in other cell systems (57, 106). Our finding here of Cx46-positive annular junctions establishing contacts with the lipid droplet surface in Sertoli cells is novel and is indicative of the participation of Cx46 in the process. Proteomic studies have identified several proteins on the lipid droplet surface in macrophages (36) and uncovered the traffic of membrane proteins and the implication of intracellular membranes and lipid droplet contacts (12, 52, 53, 56).
Perspectives and Significance
The establishment of a Sertoli cell occluding zonule barrier in the basal third of the seminiferous epithelium creates a basal cellular compartment superposed by a lumenal compartment. The early-stage germ cells hosted in the basal compartment benefit from a direct access to blood-borne substances, whereas the late-stage germ cells, dwelling the lumenal compartment, do not. The feature of a lack of a direct access to the blood supply is shared by the late-stage male germ cells and the fiber cells of the naturally avascular lens of the eye, where Cx46 and Cx50 have been scrutinized initially. However, this is the first report of Cx46 and Cx50 in testis. The increase recorded here in apoptosis levels and in the number of apoptotic Apostain-positive spermatocytes and spermatids in Cx46−/− mice argues in favor of life-sustaining Cx46 gap junction-mediated exchanges in the late-stage germ cells that are secluded from the blood by the barrier. Moreover, our findings of a downregulation of Cx46 concomitant with an upregulation of PCx43 in Cx50−/− mice and of an upregulation of Cx50 and Cx43 in Cx46−/− mice are evidence that the regulation of Cx46 is complementary, not synchronous, with Cx50 and Cx43 in the seminiferous epithelium. Furthermore, phosphorylation of Cx46 and Cx50 is opposite or complementary in the seminiferous epithelium. As well, the increase in PCx43 levels, recorded in Cx50−/− mouse tubule fractions, is reflective of the individual cell junction constituents' influence on the phosphorylation state and likely on the function regulated by other junction proteins within the Sertoli cell junctional complexes. The data advocate in favor of an interdependent regulation of Cx43, Cx50, and Cx46 expression and phosphorylation in the seminiferous epithelium. In addition, the data show that when Cx46 affects the tight-junction and adhering-junction proteins, Cx50 has no effect and vice versa, indicating that the deletion of Cx46 or Cx50 impacts the behavior of other constituents within the Sertoli cell junctional complex. During the mink active spermatogenic phase, Cx50 became phosphorylated and localized to the site of the blood-testis barrier, in contrast to Cx46, which was dephosphorylated and associated with annular junctions, suggesting involvement of Cx46 and Cx50 phosphorylation/dephosphorylation in the dynamic of this barrier. Our novel detection of Cx46-positive annular junctions establishing contacts with the lipid droplet surface is indicative of the participation of Cx46 in the touch and degradation process. Our finding of Cx46, Cx50, and Cx43 in lipid rafts may reflect the sorting out of individual connexin-mediated channels in Sertoli cell membrane microdomains, according to connexin species, or pairing characteristics through differences in lipid/protein ratios. In controlling the traffic of regulatory molecules responsible for these functions, the molecular diversity of connexins regulates cell and tissue growth, cell differentiation, and death during spermatogenesis. The diversity in functions of signaling, permeability, voltage gating, and interaction of channels with connexins in bordering cells is echoed by the molecular diversity of constitutive connexins. This study paves the way to invaluable opportunities to explore unsuspected consequences of altering the coding of Cx46 and Cx50, not only on the remaining junctional constituents' expression and post-translational transformation but also more importantly, on their individual functions within the Sertoli cell junctional complex that forms the anatomical basis of the blood-testis barrier.
GRANTS
Support for this work was provided by Natural Sciences and Engineering Research Council of Canada (NSERC) Grant OGP0041653 (to R.-M. Pelletier) and NSERC Grant OGP0194652 (to M. L. Vitale).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.-M.P. conception and design of research; R.-M.P., C.D.A., L.C., and N.M.K. performed experiments; R.-M.P. and M.L.V. analyzed data; R.-M.P. and M.L.V. interpreted results of experiments; R.-M.P. and M.L.V. prepared figures; R.-M.P. drafted manuscript; R.-M.P. and M.L.V. edited and revised manuscript; R.-M.P. and M.L.V. approved final version of manuscript.
REFERENCES
- 1.Abraham V, Chou ML, George P, Pooler P, Zaman A, Savani R, Koval M. Heterocellular gap junctional communication between alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 280: L1085–L1093, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Akpovi CD, Pelletier RM. A revised and improved method for the isolation of seminiferous tubule-enriched fractions that preserves the phosphorylated and glycosylated forms of proteins. In: Human Embryogenesis Methods and Protocols, edited by Lafond J, Vaillancourt C. Totowa, NJ: Humana, Springer Protocols, 2009, p. 159–168. [DOI] [PubMed] [Google Scholar]
- 3.Akpovi CD, Yoon SR, Vitale ML, Pelletier RM. The predominance of one of the SR-BI isoforms is associated with increased esterified cholesterol levels not apoptosis in mink testis. J Lipid Res 47: 2233–2247, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Alcala J, Katar M, Maisel H. Lipid composition of chick lens fiber cell gap junctions. Curr Eye Res 2: 569–578, 1982. [DOI] [PubMed] [Google Scholar]
- 5.Baldo GJ, Gong X, Martinez-Wittimghan FJ, Kumar NM, Gilula NB, Mathias RT. Gap junctional coupling in lenses from alpha(8) connexin knockout mice. J Gen Physiol 118: 447–456, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee D, Das S, Molina SA, Katz MR, Jena S, Bossmann LK, Pal D, Takemoto DJ. Investigation of the reciprocal relationship between the expression of two gap junction proteins, connexin 46 and connexin 43. J Biol Chem 286: 24519–24533, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee D, Gakhar G, Madgwick D, Hurt A, Takemoto D, Nguyen TA. A novel role of gap junction connexin46 protein to protect breast tumors from hypoxia. Int J Cancer 127: 839–848, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao L, Sachs F, Dahl G. Connexins are mechanosensitive. Am J Physiol Cell Physiol 287: C1389–C1395, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Berthoud VM, Beyer EC, Kurata WE, Lau AF, Lampe PD. The gap junction protein connexin 56 is phosphorylated in the intracellular loop and the carboxy-terminal region. Eur J Biochem 244: 89–97, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Bevans CG, Kordel M, Rhee SK, Harris AL. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem 273: 2808–2816, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 12.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3–L1 adipocytes. J Biol Chem 279: 46835–46842, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Bruzzone R, White TW, Paul DL. Expression of chimeric connexins reveals new properties of the formation and gating behavior of gap junction channels. J Cell Sci 107: 955–967, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem 238: 1–27, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Burr DB, Molina SA, Banerjee D, Low DM, Takemoto DJ. Treatment with 46 siRNA suppresses the growth of human Y79 retinoblastoma cell xenografts in vivo. Exp Eye Res 92: 259, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buzzard JJ, Wreford NG, Morrison JR. Thyroid hormone, retinoic acid, and testosterone suppress proliferation and induce markers of differentiation in cultured rat Sertoli cells. Endocrinology 144: 3722–3731, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Byers SW, Pelletier RM. Sertoli cell tight junctions and the blood-testis barrier. In: Tight Junctions, edited by Cereijido M. Boca Raton, FL: CRC, 1992, p. 279–304. [Google Scholar]
- 18.Carette D, Weider K, Gilleron J, Giese S, Dompierre J, Bergmann M, Brehm R, Denizot JP, Segretain D, Pointis G. Major involvment of connexin 43 in seminiferous epithelial junction dynamics and male fertility. Dev Biol 346: 54–67, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Chandross KJ, Kessler JA, Cohen RI, Simburger E, Spray DC, Bieiri P, Dermietzel R. Altered connexin expression after peripheral nerve injury. Mol Cell Neurosci 7: 501–518, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Chi NC, Bussen M, Brand-Arzamendi K, Ding C, Olgin RM, Shaw RM, Martin GR, Stainier DY. Cardiac conduction is required to preserve cardiac chamber morphology. Proc Natl Acad Sci USA 107: 14662–14667, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daraber P, Draberova L. Lipid rafts in mast cell signaling. Mol Immunol 38: 1247–1252, 2002. [DOI] [PubMed] [Google Scholar]
- 22.De SK, Chen HL, Pace JL, Hunt JS, Terranova PF, Enders GC. Expression of tumor necrosis factor-alpha in mouse spermatogenic cells. Endocrinology 133: 389–396, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Dejucq SK, Dugast I, Ruffault A, van der Meide PH, Jégou B. Interferon-alpha and -gamma expression in the rat testis. Endocrinology 136: 4925–4931, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, Bennett MV, Spray DC. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Rev 32: 45–56, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Dudez T, Borot F, Huang S, Kwak BR, Bacchetta M, Ollero M, Stanton BA, Chanson M. CFTR in a lipid raft-TNFR1 complex modulates gap junctional intercellular communication and IL-8 secretion. Biochim Biophys Acta 1783: 779–788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunia L, Recouvreur M, Nicolas P, Kumar NM, Bloemendal H, Benedetti EL. Assembly of connexins and MP26 in lens fiber plasma membranes studied by SDS-fracture immunolabeling. J Cell Sci 111: 109–120, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartimentation of the seminiferous epithelium. Biol Reprod 3: 308–326, 1970. [DOI] [PubMed] [Google Scholar]
- 28.Ebihara L, Steiner E. Properties of nonjunctional current expressed from a rat connexin 46 cDNA in Xenopus oocytes. J Gen Physiol 102: 59–74, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckert R, Donaldson P, Goldie K, Kisler J. A distinct membrane current in rat lens fiber cells isolated under calcium-free conditions. Invest Ophthalmol Vis Sci 39: 1280–1285, 1998. [PubMed] [Google Scholar]
- 30.Fortin ME, Pelletier RM, Meilleur MA, Vitale ML. Modulation of GJA1 turnover and intercellular communication by pro-inflammatory cytokines in the anterior pituitary folliculo-stellate cell line TtT/GF. Biol Reprod 72: 2–12, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Gerido DA, White TW. Connexin disorders of the ear, skin, and lens. Biochim Biophys Acta 1662: 159–170, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Gong X, Baldo GJ, Kumar NM, Gilula NB, Mathias RT. Gap junctional coupling in lenses lacking alpha3 connexin. Proc Natl Acad Sci USA 95: 15303–15308, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell 91: 833–843, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Henderson D, Eibi H, Weber K. Structure and biochemistry of mouse hepatic gap junctions. J Mol Biol 132: 193–218, 1979. [DOI] [PubMed] [Google Scholar]
- 35.Huckins C. The morphology and kinetics of spermatogonial degeneration in normal adult rats: an analysis using a simplified classification of the germinal epithelium. Anat Rec 190: 905–926, 1978. [DOI] [PubMed] [Google Scholar]
- 36.Itabe H, Masuda Y, Sasabe N, Kitazato K, Arai H, Takano T. Regulation of intracellular lipid storage and adipose differentiation-related protein (ADRP). In: New Frontiers in Lifestyle-Related Diseases. Tokyo: Springer Japan, 2008, Part II, p. 81–88. [Google Scholar]
- 37.Kabbaj O, Holm C, Vitale ML, Pelletier RM. Expression, activity and subcellular localization of testicular hormone-sensitive lipase (HSL) during postnatal development in the Guinea pig. Biol Reprod 65: 601–612, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27: 137–138, 1965. [Google Scholar]
- 39.Kerr JB. Spontaneous degeneration of germ cells in normal rat testis: assessment of cell types and frequency during the spermatogenic cycle. J Reprod Fertil 95: 825–830, 1992. [DOI] [PubMed] [Google Scholar]
- 40.Konig N, Zampighi GA. Purification of bovine lens cell-to-cell channels composed of connexin44 and connexin50. J Cell Sci 108: 3091–3098, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Koval M, Harley EJ, Hick K, Steinberg TH. Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. J Cell Biol 137: 847–857, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar NM, Friend DS, Gilula NB. Synthesis and assembly of human beta 1 gap junctions in BHK cells by DNA transfection with the human beta 1 cDNA. J Cell Sci 108: 3725–3734, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Kwak BR, Van Veen TA, Analbers LJ, Jongsma H. TPA increases conductance cut decrease permeability in neonatal rat cardiomyocyte gap junction channels. Exp Cell Res 220: 456–463, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Laird DW. Life cycle of connexins in health and disease. Biochem J 394: 527–543, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. Caveolin-1 and -2 interact with connexin 43 and regulate gap junctiononal intercellular communication in keratinocytes. Mol Biol Cell 19: 912–929, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leaphart CL, Dai S, Gribar SC, Richardson W, Ozolek J, Shi X, Bruns JR, Branca M, Li J, Weisz OA, Sodhi C, Hackam DJ. Interferon-inhibits enterocyte migration by reversibly displacing connexin43 from lipid rafts. Am J Physiol Gastrointest Liver Physiol 295: G559–G569, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lehmann D, Temminch D, Da Rugna D, Leibundgut B, Sulmoni A, Muller HJ. Role of immunological factors in male infertility: immunohistochemical and serological evidence. Lab Invest 57: 21–28, 1987. [PubMed] [Google Scholar]
- 48.Li H, Liu TF, Lazrak A, Peracchia C, Golberg GS, Lampe PD, Johnson RG. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J Cell Biol 134: 1019–1030, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin D, Lobell S, Jewell A, Takemoto DJ. Differential phosphorylation of connexin46 and connexin50 by H2O2 activation of protein kinase Cgamma. Mol Vis 10: 688–695, 2004. [PubMed] [Google Scholar]
- 50.Lin D, Zhou J, Zelenka PS, Takemoto DJ. Protein kinase C gamma regulation of gap junction activity through caveolin-1-containing lipid rafts. Invest Ophthalmol Vis Sci 44: 5259–5268, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, Ek-Victorin JF, Weintraub BC, Gu S, Shi Q, Burt JM, Jiang JX. Phosphorylation of connexin 50 by protein kinase A enhances gap junction and hemichannel function. J Biol Chem 286: 16914–16928, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu P, Bartz R, Zehmer JK, Ying YS, Zhu M, Serrero G, Anderson RG. Rab-regulated interaction of early endosomes with lipid droplets. Biochim Biophys Acta 1773: 784–793, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem 279: 3787–3792, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Loewenstein WR. The cell-to-cell channel of gap junctions. Cell 48: 725–726, 1987. [DOI] [PubMed] [Google Scholar]
- 55.Lustig L, Tung KS. Autoimmune orchitis and male infertility. In: The Autoimmune Diseases, edited by Rose NR, Mackay IR. Burlington, MA: Elsevier Science (USA), 2006, p. 841–847. [Google Scholar]
- 56.Martin S, Driessen K, Nixon SJ, Zerial M, Parton RG. Regulated localization of Rab18 to lipid droplets: effects of lipolytic stimulation and inhibition of lipid droplet catabolism. J Biol Chem 280: 42325–42335, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol 7: 373–378, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiol Rev 77: 21–50, 1997. [DOI] [PubMed] [Google Scholar]
- 59.Meilleur MA, Akpovi CD, Pelletier RM, Vitale ML. Tumor necrosis factor-alpha-induced anterior pituitary folliculostellate TtT/GF cell uncoupling is mediated by connexin 43 dephosphorylation. Endocrinology 138: 5374–5384, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Molina SA, Takemoto DJ. The role of connexin Cx46 promoter in lens and other hypoxic tissues. Commun Integr Biol 5: 114–117, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morgan AD. Inflammation and infestation of the testis and paratesticular structures. In: Pathology of the Testis, edited by Pugh RC. Oxford, UK: Blackwell Scientific, 1976, p. 79–138. [Google Scholar]
- 62.Murphy BD. Female reproductive system. In: Mink Biology, Health and Disease, edited by Hunter DB, Lemieux N. Guelph, Ontario, Canada: Graphic and Print Services University of Guelph, 1996, p. 9-1–9-19. [Google Scholar]
- 63.Musil LS, Goodenough DA. Biochemical analysis of connexin 43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol 115: 1357–1374, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell 74: 1065–1077, 1993. [DOI] [PubMed] [Google Scholar]
- 65.Nielsen PA, Baruch A, Shestopalov VI, Giepmans BN, Dunia I, Benedetti EL, Kumar NM. Lens connexins alpha3Cx46 and alpha8Cx50 interact with zonula occludens protein-1 (ZO-1). Mol Biol Cell 14: 2470–2481, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okuda Y, Sun XR, Morris PL. Interleukin-6 (IL-6) mRNAs expressed in Leydig and Sertoli cells are regulated by cytokines, gonadotropins and neuropeptides. Endocrine 2: 617–624, 1994. [Google Scholar]
- 67.Paul DL, Ebihara L, Takemoto DJ, Swenson KI, Goodenough DA. Connexin 46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol 115: 1077–1089, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pelletier RM. Cyclic formation and decay of the blood-testis barrier in the mink (Mustela vison): a seasonal breeder. Am J Anat 175: 91–117, 1986. [DOI] [PubMed] [Google Scholar]
- 69.Pelletier RM. Cyclic modulation of the Sertoli cell junctional complexes in a seasonal breeder: the mink (Mustela vison). Am J Anat 183: 68–102, 1988. [DOI] [PubMed] [Google Scholar]
- 70.Pelletier RM. A freeze-fracture study of cell junctions in the epididymis and vas deferens of a seasonal breeder: the mink (Mustela vison). Microsc Res Tech 30: 37–53, 1995. [DOI] [PubMed] [Google Scholar]
- 71.Pelletier RM. The distribution of connexin 43 is associated with the germ cell differentiation and with the modulation of the Sertoli cell junctional barrier in continual (guinea pig) and seasonal breeder's (mink) testes. J Androl 16: 400–409, 1995. [PubMed] [Google Scholar]
- 72.Pelletier RM. The tight junctions in the testis, epididymis and vas deferens. In: Tight Junctions. Boca Raton, FL: CRC, 2001, p. 599–628. [Google Scholar]
- 73.Pelletier RM. The tight junction in the testis. In: Recent Research in Development and Endocrinology, edited by Pandalai SG. Trivandrum, India: Transworld Research Network, 2004, p. 29–51. [Google Scholar]
- 74.Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem 46: 49–127, 2011. [DOI] [PubMed] [Google Scholar]
- 75.Pelletier RM, Akpovi CD, Chen L, Day R, Vitale ML. Cx43 expression, phosphorylation and distribution in the normal and autoimmune orchitic testis with a look at gap junctions joining germ cell-to-germ cells. Am J Physiol Regul Integr Comp Physiol 300: R121–R139, 2011. [DOI] [PubMed] [Google Scholar]
- 76.Pelletier RM, Byers SW. The blood-testis barrier and Sertoli cell junctions: structural considerations. Microsc Res Tech 20: 3–33, 1992. [DOI] [PubMed] [Google Scholar]
- 77.Pelletier RM, Friend DS. The Sertoli cell junctional complex: structure and permeability to filipin in the neonatal and adult guinea pig. Am J Anat 168: 213–228, 1983. [DOI] [PubMed] [Google Scholar]
- 78.Pelletier RM, Friend DS. Sertoli cell junctional complexes in gossypol treated neonatal and adult Guinea pigs. J Androl 7: 127–139, 1986. [DOI] [PubMed] [Google Scholar]
- 79.Pelletier RM, Okawara Y, Vitale ML, Anderson JM. Differential distribution of the tight junction associated protein ZO-1 alpha+ and alpha− isoforms in guinea pig Sertoli cells: a possible association with F-actin and G-actin. Biol Reprod 57: 367–376, 1997. [DOI] [PubMed] [Google Scholar]
- 80.Pelletier RM, Trifaró JM, Carbajal ME, Okawara Y, Vitale ML. The calcium dependent actin filament-severing protein scinderin levels and localization in bovine testis, epididymis and spermatozoa. Biol Reprod 60: 1128–1136, 1999. [DOI] [PubMed] [Google Scholar]
- 81.Pelletier RM, Vitale ML. Filipin vs enzymatic localization of cholesterol in guinea pig, mink, and mallard duck testicular cells. J Histochem Cytochem 42: 1539–1554, 1994. [DOI] [PubMed] [Google Scholar]
- 82.Pelletier RM, Yoon SR, Akpovi CD, Silvas E, Vitale ML. Defects in the regulatory clearance mechanisms favor the breakdown of self-tolerance during spontaneous autoimmune orchitis. Am J Physiol Regul Integr Comp Physiol 296: R743–R762, 2009. [DOI] [PubMed] [Google Scholar]
- 83.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res 44: 655–667, 2003. [DOI] [PubMed] [Google Scholar]
- 84.Rackauskas M, Neverauskas V, Skerberdis VA. Diversity and properties of connexin gap junction channels. Medicina (Kaunas) 46: 1–12, 2010. [PubMed] [Google Scholar]
- 85.Rong P, Wang X, Niesman I, Wu Y, Benedetti EL, Dunia I, Levy E, Gong X. Disruption of Gja8 (alpha8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development 129: 167–174, 2002. [DOI] [PubMed] [Google Scholar]
- 86.Rüttinger C, Bergmann M, Fink L, Pesch S, Seitz K, Trautmann A, Steger K, Konrad L, Brehm R. Expression of connexin 43 in normal canine testes and canine testicular tumors. Histochem Cell Biol 130: 537–548, 2008. [DOI] [PubMed] [Google Scholar]
- 87.Saez JC, Berthoud VM, Brañes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83: 1359–1400, 2003. [DOI] [PubMed] [Google Scholar]
- 88.Saleh SM, Takemoto LJ, Zoukhri D, Takemoto DJ. PKC-gamma phosphorylation of connexin 46 in the lens cortex. Mol Vis 7: 240–246, 2001. [PubMed] [Google Scholar]
- 89.Salomon F, Saremaslani P, Jakob M, Hedinger CE. Immune complexe orchitis in infertily men. Immunoelectron microscopy of abnormal basement membrane structures. Lab Invest 47: 555–567, 1982. [PubMed] [Google Scholar]
- 90.Sanches DS, Pires CG, Fukumasu H, Cogliati P, Chaible LM, Torres LN, Ferrigno CR, Dagli ML. Expression of connexins in normal and neoplastic canine bone tissue. Vet Pathol 46: 846–859, 2009. [DOI] [PubMed] [Google Scholar]
- 91.Schubert AL, Schubert W, Spray DC, Lisanti MP. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry 41: 5754–5764, 2002. [DOI] [PubMed] [Google Scholar]
- 92.Segretain D, Falk MM. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim Biophys Acta 1662: 3–21, 2004. [DOI] [PubMed] [Google Scholar]
- 93.Shakespeare TI, Sellito C, Li L, Rubinos C, Gong X, Srinivas M, White TW. Interaction between connexin50 and mitogen-activated protein kinase signaling in lens homeostasis. Mol Biol Cell 20: 2582–2592, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simons K, Vaz WI. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct 33: 269–295, 2004. [DOI] [PubMed] [Google Scholar]
- 95.Tung KS. Autoimmune disease of the testis and the ovary. In: The Autoimmune Diseases. New York: Academic, 1998, p. 687–704. [Google Scholar]
- 96.Tung KS, Ellis LC, Childs GV, Duffau M. The dark mink: a model of male infertility. Endocrinology 114: 922–929, 1984. [DOI] [PubMed] [Google Scholar]
- 97.Tung KS, Ellis LC, Childs GV, Pelletier RM, Duffau M. The dark mink. A model of male infertility. Clin Res 31: 473A, 1983. [DOI] [PubMed] [Google Scholar]
- 98.Tung KS, Ellis LC, Teuscher C, Meng A, Blaustein JC, Kohno S, Howell R. A natural model for immunologic infertility. J Exp Med 154: 1016–1032, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vitale ML, Akpovi CD, Pelletier RM. Cortactin/tyrosine-phosphorylated cortactin interactions with F-actin and connexin 43 in mouse seminiferous tubules. Microsc Res Tech 72: 856–867, 2009. [DOI] [PubMed] [Google Scholar]
- 100.Wang Z, Schey KL. Phosphorylation and truncation sites of bovine lens connexin 46 and connexin 50. Exp Eye Res 89: 898–904, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.White TW. Nonredundant gap junction functions. News Physiol Sci 18: 95–99, 2003. [DOI] [PubMed] [Google Scholar]
- 102.White TW, Bruzzone R, Wolfram D, Paul DL, Goodenough DA. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. J Cell Biol 125: 879–892, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.White TW, Goodenough DA, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulvurulent cataracts. J Cell Biol 143: 815–825, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.White TW, Paul DL, Goodenough DA, Bruzzone R. Functional analysis of selective interactions among rodent connexins. Mol Biol Cell 6: 459–470, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Willecke K, Hennermann H, Dahl E, Jungbluth S, Heykes R. The diversity of connexin genes encoding gap junctional proteins. Eur J Cell Biol 56: 1–7, 1991. [PubMed] [Google Scholar]
- 106.Wolins NE, Brasaemle DL, Bickel PE. A proposed model for fat packaging by exchangeable lipid droplets proteins. FEBS Lett 580: 5484–5491, 2006. [DOI] [PubMed] [Google Scholar]
- 107.Zampighi GA, Loo DD, Kreman M, Eskandari S, Wright EM. Functional and morphological correlates of connexin 50 expressed in Xenopus laevis oocytes. J Gen Physiol 113: 507–524, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]