Abstract
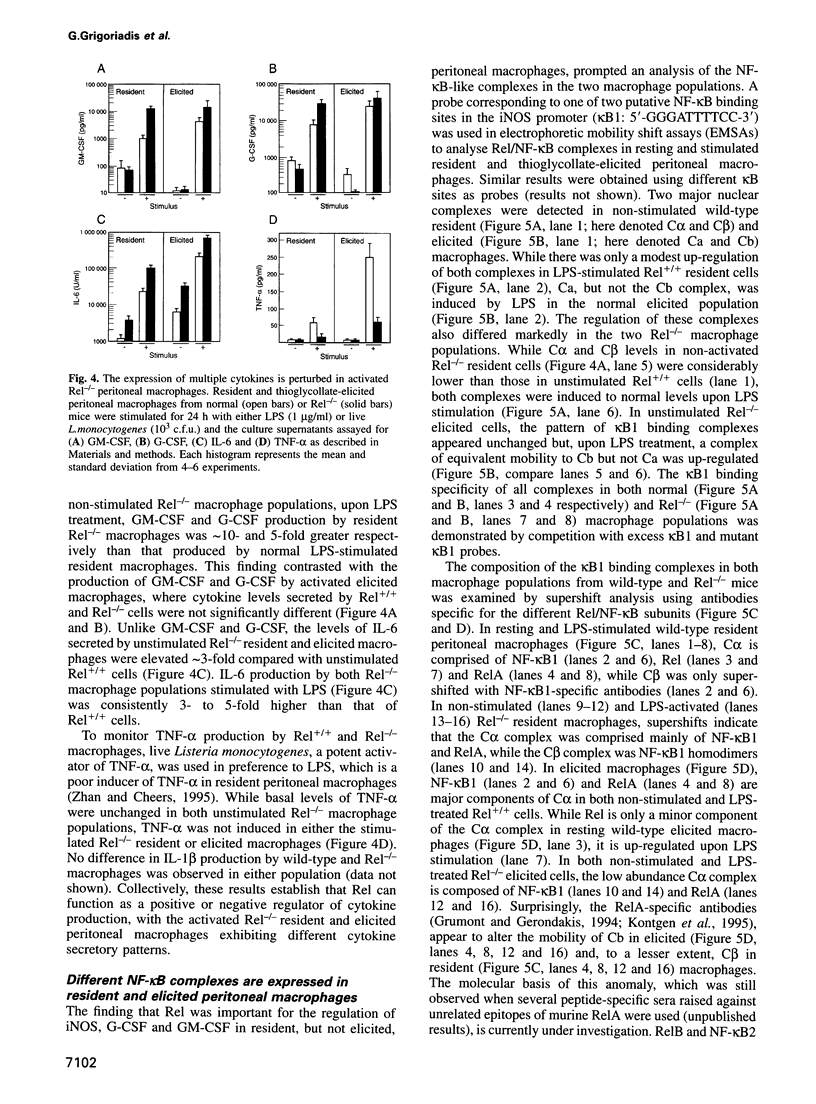
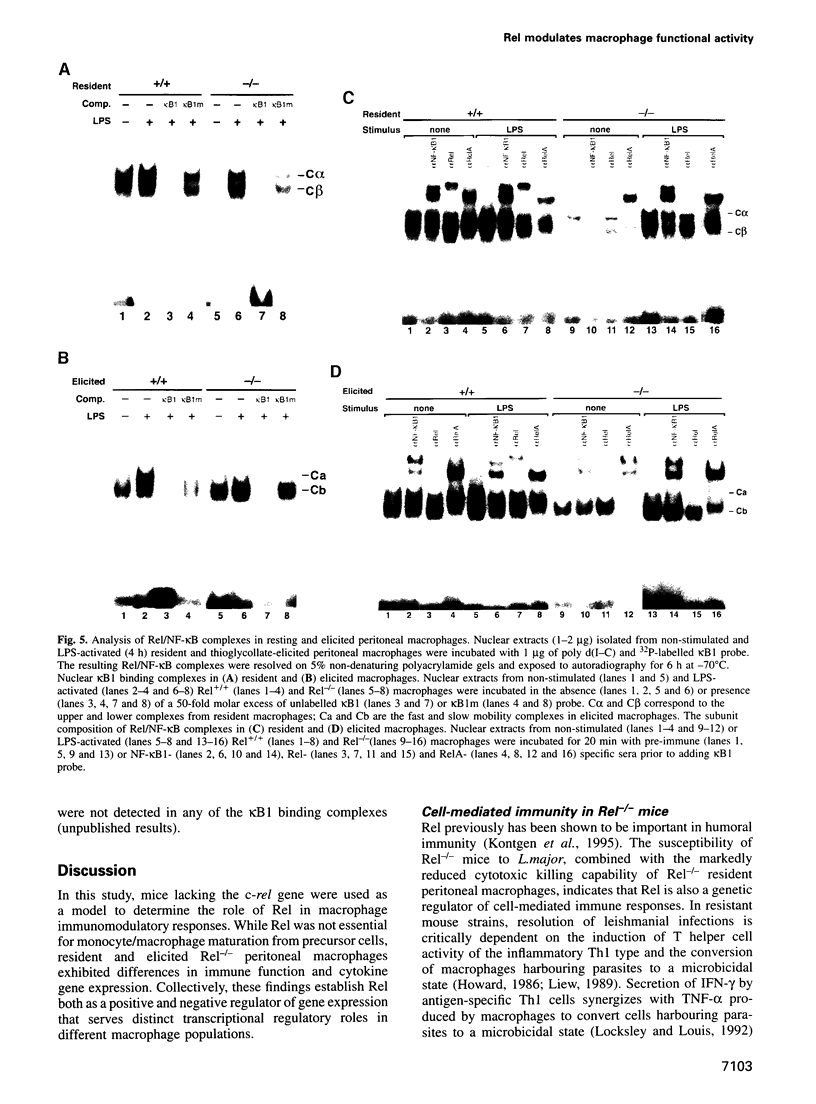
The role of Rel in the monocyte/macrophage lineage was examined in mice with an inactivated c-rel gene. Although the frequency of monocytic cells was normal in Rel-/- mice, we show that Rel serves distinct roles in regulating gene expression and immune effector function in different mature macrophage populations. Stimulated Rel-/- resident peritoneal macrophages produced higher than normal levels of granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF) and interleukin-6 (IL-6), but tumour necrosis factor-alpha (TNF-alpha) production was not induced. Diminished cytotoxic activity exhibited by resident Rel-/- macrophages was consistent with reduced nitric oxide production resulting from impaired up-regulation of inducible nitric oxide synthase expression. While a similar altered pattern of IL-6 and TNF-alpha expression was observed in stimulated Rel-/- peritoneal effusion macrophages, cytotoxic activity, nitric oxide, GM-CSF and G-CSF production by these cells was normal. The alternate regulation of certain genes in the two macrophage populations coincided with different patterns of nuclear Rel/NF-kappaB complexes expressed in normal resident and elicited cells. Collectively, these results establish that Rel is a positive or negative regulator of transcription in macrophages and that Rel has distinct roles in different macrophage populations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Blackwell T. S., Holden E. P., Blackwell T. R., DeLarco J. E., Christman J. W. Cytokine-induced neutrophil chemoattractant mediates neutrophilic alveolitis in rats: association with nuclear factor kappa B activation. Am J Respir Cell Mol Biol. 1994 Oct;11(4):464–472. doi: 10.1165/ajrcmb.11.4.7917314. [DOI] [PubMed] [Google Scholar]
- Brownell E., Mathieson B., Young H. A., Keller J., Ihle J. N., Rice N. R. Detection of c-rel-related transcripts in mouse hematopoietic tissues, fractionated lymphocyte populations, and cell lines. Mol Cell Biol. 1987 Mar;7(3):1304–1309. doi: 10.1128/mcb.7.3.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Hagler J., Palombella V. J., Melandri F., Scherer D., Ballard D., Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995 Jul 1;9(13):1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Baeuerle P., Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990 Apr;10(4):1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonato J. A., Mercurio F., Karin M. Phosphorylation of I kappa B alpha precedes but is not sufficient for its dissociation from NF-kappa B. Mol Cell Biol. 1995 Mar;15(3):1302–1311. doi: 10.1128/mcb.15.3.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
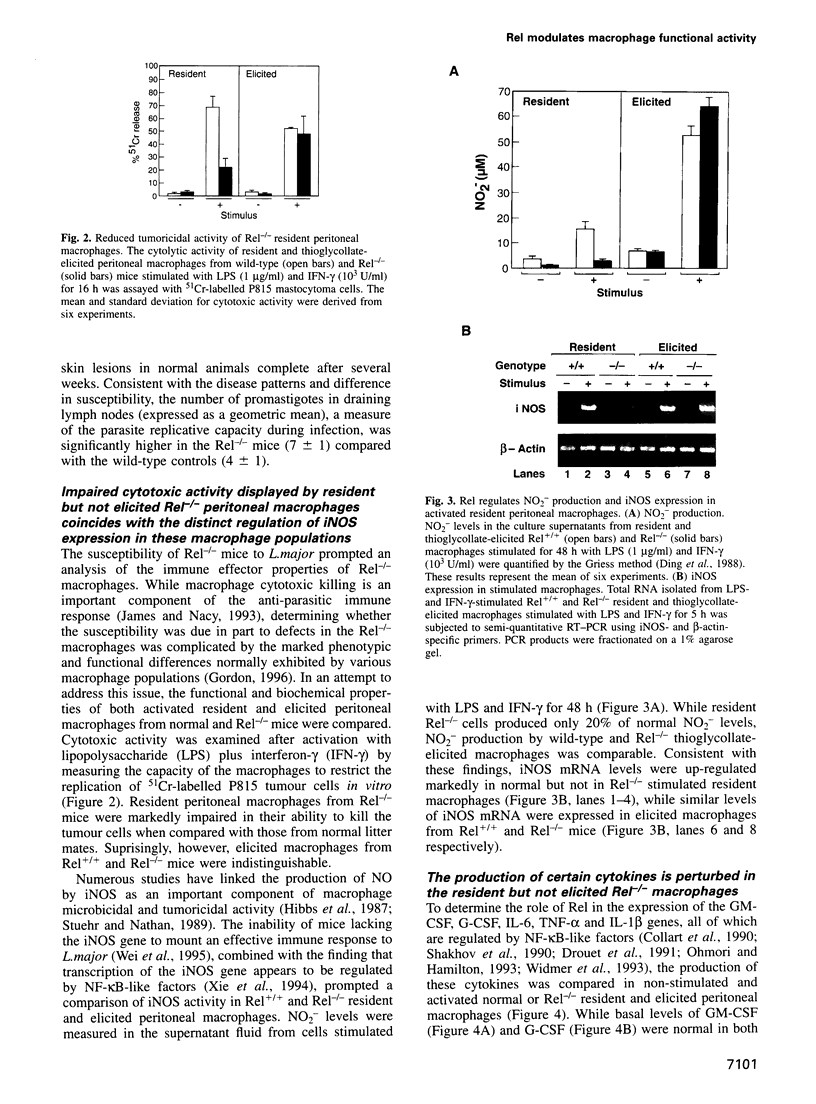
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Drouet C., Shakhov A. N., Jongeneel C. V. Enhancers and transcription factors controlling the inducibility of the tumor necrosis factor-alpha promoter in primary macrophages. J Immunol. 1991 Sep 1;147(5):1694–1700. [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Bird C. R., Bristow A., Poole S., Thorpe R. A simple sensitive bioassay for interleukin-1 which is unresponsive to 10(3) U/ml of interleukin-2. J Immunol Methods. 1987 May 4;99(1):7–11. doi: 10.1016/0022-1759(87)90025-1. [DOI] [PubMed] [Google Scholar]
- Gerondakis S., Strasser A., Metcalf D., Grigoriadis G., Scheerlinck J. Y., Grumont R. J. Rel-deficient T cells exhibit defects in production of interleukin 3 and granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3405–3409. doi: 10.1073/pnas.93.8.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Tan T. H., Rice N. R., Sica A., Young H. A. The interleukin 2 CD28-responsive complex contains at least three members of the NF kappa B family: c-Rel, p50, and p65. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1696–1700. doi: 10.1073/pnas.90.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Gordon S. The macrophage. Bioessays. 1995 Nov;17(11):977–986. doi: 10.1002/bies.950171111. [DOI] [PubMed] [Google Scholar]
- Green S. J., Crawford R. M., Hockmeyer J. T., Meltzer M. S., Nacy C. A. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990 Dec 15;145(12):4290–4297. [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Grumont R. J., Gerondakis S. The murine c-rel proto-oncogene encodes two mRNAs the expression of which is modulated by lymphoid stimuli. Oncogene Res. 1990;5(4):245–254. [PubMed] [Google Scholar]
- Grumont R. J., Gerondakis S. The subunit composition of NF-kappa B complexes changes during B-cell development. Cell Growth Differ. 1994 Dec;5(12):1321–1331. [PubMed] [Google Scholar]
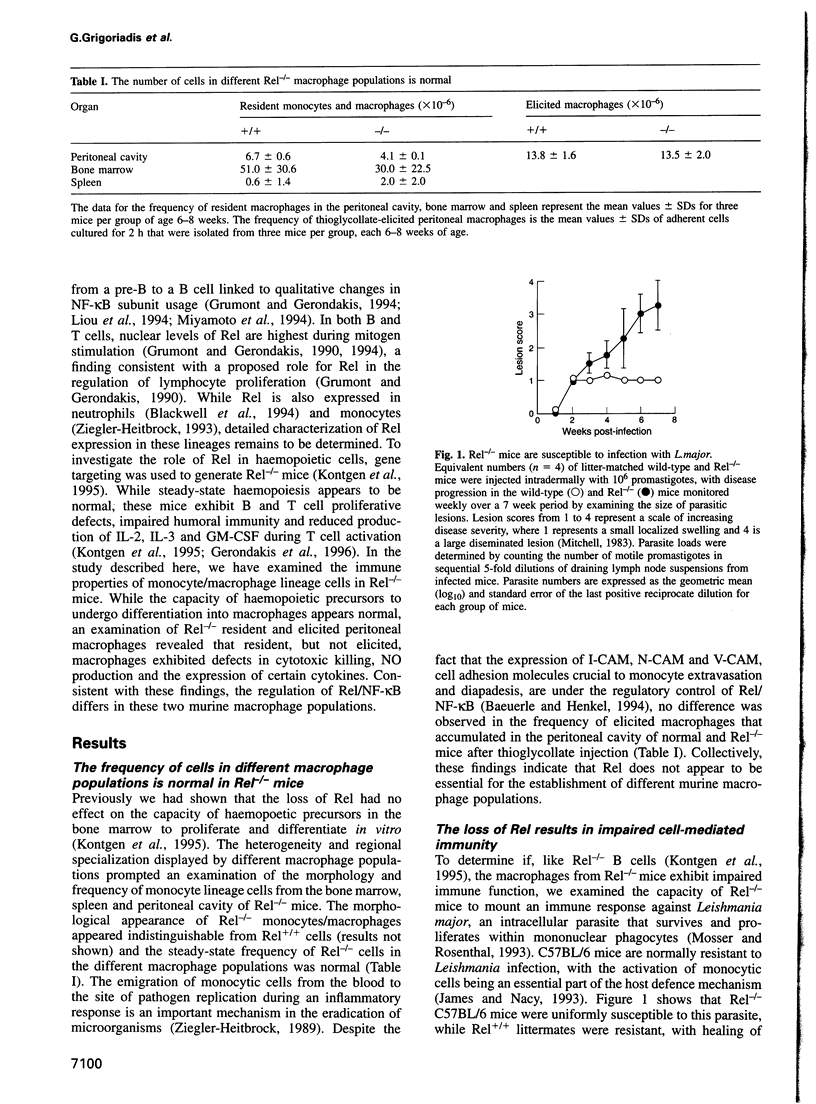
- Handman E., Hocking R. E., Mitchell G. F., Spithill T. W. Isolation and characterization of infective and non-infective clones of Leishmania tropica. Mol Biochem Parasitol. 1983 Feb;7(2):111–126. doi: 10.1016/0166-6851(83)90039-7. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Howard J. G. Immunological regulation and control of experimental leishmaniasis. Int Rev Exp Pathol. 1986;28:79–116. [PubMed] [Google Scholar]
- James S. L., Nacy C. Effector functions of activated macrophages against parasites. Curr Opin Immunol. 1993 Aug;5(4):518–523. doi: 10.1016/0952-7915(93)90032-n. [DOI] [PubMed] [Google Scholar]
- James S. L., Nacy C. Effector functions of activated macrophages against parasites. Curr Opin Immunol. 1993 Aug;5(4):518–523. doi: 10.1016/0952-7915(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Kaszubska W., Hooft van Huijsduijnen R., Ghersa P., DeRaemy-Schenk A. M., Chen B. P., Hai T., DeLamarter J. F., Whelan J. Cyclic AMP-independent ATF family members interact with NF-kappa B and function in the activation of the E-selectin promoter in response to cytokines. Mol Cell Biol. 1993 Nov;13(11):7180–7190. doi: 10.1128/mcb.13.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A. Frequency analysis of lymphokine-secreting CD4+ and CD8+ T cells activated in a graft-versus-host reaction. J Immunol. 1990 Oct 1;145(7):2167–2176. [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Köntgen F., Grumont R. J., Strasser A., Metcalf D., Li R., Tarlinton D., Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995 Aug 15;9(16):1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- Lehming N., Thanos D., Brickman J. M., Ma J., Maniatis T., Ptashne M. An HMG-like protein that can switch a transcriptional activator to a repressor. Nature. 1994 Sep 8;371(6493):175–179. doi: 10.1038/371175a0. [DOI] [PubMed] [Google Scholar]
- Levine M. Molecular analysis of dorsal-ventral polarity in Drosophila. Cell. 1988 Mar 25;52(6):785–786. doi: 10.1016/0092-8674(88)90418-7. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990 May;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y. Functional heterogeneity of CD4+ T cells in leishmaniasis. Immunol Today. 1989 Feb;10(2):40–45. doi: 10.1016/0167-5699(89)90302-2. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., O'Donnell C. A. Immunology of leishmaniasis. Adv Parasitol. 1993;32:161–259. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- Liou H. C., Sha W. C., Scott M. L., Baltimore D. Sequential induction of NF-kappa B/Rel family proteins during B-cell terminal differentiation. Mol Cell Biol. 1994 Aug;14(8):5349–5359. doi: 10.1128/mcb.14.8.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R. M., Louis J. A. Immunology of leishmaniasis. Curr Opin Immunol. 1992 Aug;4(4):413–418. doi: 10.1016/s0952-7915(06)80032-4. [DOI] [PubMed] [Google Scholar]
- Lyons C. R., Orloff G. J., Cunningham J. M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992 Mar 25;267(9):6370–6374. [PubMed] [Google Scholar]
- MacMicking J. D., Nathan C., Hom G., Chartrain N., Fletcher D. S., Trumbauer M., Stevens K., Xie Q. W., Sokol K., Hutchinson N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995 May 19;81(4):641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Mercurio F., Didonato J., Rosette C., Karin M. Molecular cloning and characterization of a novel Rel/NF-kappa B family member displaying structural and functional homology to NF-kappa B p50/p105. DNA Cell Biol. 1992 Sep;11(7):523–537. doi: 10.1089/dna.1992.11.523. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Willson T., Rossner M., Lock P. Receptor insertion into factor-dependent murine cell lines to develop specific bioassays for murine G-CSF and M-CSF and human GM-CSF. Growth Factors. 1994;11(2):145–152. doi: 10.3109/08977199409001056. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F. Murine cutaneous leishmaniasis: resistance in reconstituted nude mice and several F1 hybrids infected with Leishmania tropica major. J Immunogenet. 1983 Oct;10(5):395–412. doi: 10.1111/j.1744-313x.1983.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Schmitt M. J., Verma I. M. Qualitative changes in the subunit composition of kappa B-binding complexes during murine B-cell differentiation. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5056–5060. doi: 10.1073/pnas.91.11.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B. E., Bose H. R., Jr Expression of the c-rel and c-myc proto-oncogenes in avian tissues. Oncogene. 1989 Jul;4(7):845–852. [PubMed] [Google Scholar]
- Morris L., Troutt A. B., McLeod K. S., Kelso A., Handman E., Aebischer T. Interleukin-4 but not gamma interferon production correlates with the severity of murine cutaneous leishmaniasis. Infect Immun. 1993 Aug;61(8):3459–3465. doi: 10.1128/iai.61.8.3459-3465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Shimizu H., Mitomo K., Watanabe T., Okamoto S., Yamamoto K. A lymphoid cell-specific nuclear factor containing c-Rel-like proteins preferentially interacts with interleukin-6 kappa B-related motifs whose activities are repressed in lymphoid cells. Mol Cell Biol. 1992 Apr;12(4):1736–1746. doi: 10.1128/mcb.12.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan G. P., Baltimore D. The inhibitory ankyrin and activator Rel proteins. Curr Opin Genet Dev. 1992 Apr;2(2):211–220. doi: 10.1016/s0959-437x(05)80276-x. [DOI] [PubMed] [Google Scholar]
- Ohmori Y., Hamilton T. A. Cooperative interaction between interferon (IFN) stimulus response element and kappa B sequence motifs controls IFN gamma- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J Biol Chem. 1993 Mar 25;268(9):6677–6688. [PubMed] [Google Scholar]
- Pace J. L., Russell S. W. Activation of mouse macrophages for tumor cell killing. I. Quantitative analysis of interactions between lymphokine and lipopolysaccharide. J Immunol. 1981 May;126(5):1863–1867. [PubMed] [Google Scholar]
- Perkins N. D., Edwards N. L., Duckett C. S., Agranoff A. B., Schmid R. M., Nabel G. J. A cooperative interaction between NF-kappa B and Sp1 is required for HIV-1 enhancer activation. EMBO J. 1993 Sep;12(9):3551–3558. doi: 10.1002/j.1460-2075.1993.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer D. C., Brockman J. A., Chen Z., Maniatis T., Ballard D. W. Signal-induced degradation of I kappa B alpha requires site-specific ubiquitination. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R. M., Perkins N. D., Duckett C. S., Andrews P. C., Nabel G. J. Cloning of an NF-kappa B subunit which stimulates HIV transcription in synergy with p65. Nature. 1991 Aug 22;352(6337):733–736. doi: 10.1038/352733a0. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhov A. N., Collart M. A., Vassalli P., Nedospasov S. A., Jongeneel C. V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990 Jan 1;171(1):35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B., Baldwin A. S., Jr Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-kappa B. Mol Cell Biol. 1993 Nov;13(11):7191–7198. doi: 10.1128/mcb.13.11.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos D., Maniatis T. The high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta gene. Cell. 1992 Nov 27;71(5):777–789. doi: 10.1016/0092-8674(92)90554-p. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp C. S., Wolf S. F., Unanue E. R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X. Q., Charles I. G., Smith A., Ure J., Feng G. J., Huang F. P., Xu D., Muller W., Moncada S., Liew F. Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995 Jun 1;375(6530):408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Widmer U., Manogue K. R., Cerami A., Sherry B. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 alpha, and MIP-1 beta, members of the chemokine superfamily of proinflammatory cytokines. J Immunol. 1993 Jun 1;150(11):4996–5012. [PubMed] [Google Scholar]
- Zhan Y., Cheers C. Differential induction of macrophage-derived cytokines by live and dead intracellular bacteria in vitro. Infect Immun. 1995 Feb;63(2):720–723. doi: 10.1128/iai.63.2.720-723.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Sternsdorf T., Liese J., Belohradsky B., Weber C., Wedel A., Schreck R., Bäuerle P., Ströbel M. Pyrrolidine dithiocarbamate inhibits NF-kappa B mobilization and TNF production in human monocytes. J Immunol. 1993 Dec 15;151(12):6986–6993. [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W. The biology of the monocyte system. Eur J Cell Biol. 1989 Jun;49(1):1–12. [PubMed] [Google Scholar]