Abstract
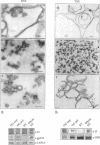
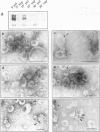
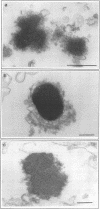
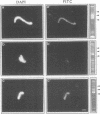
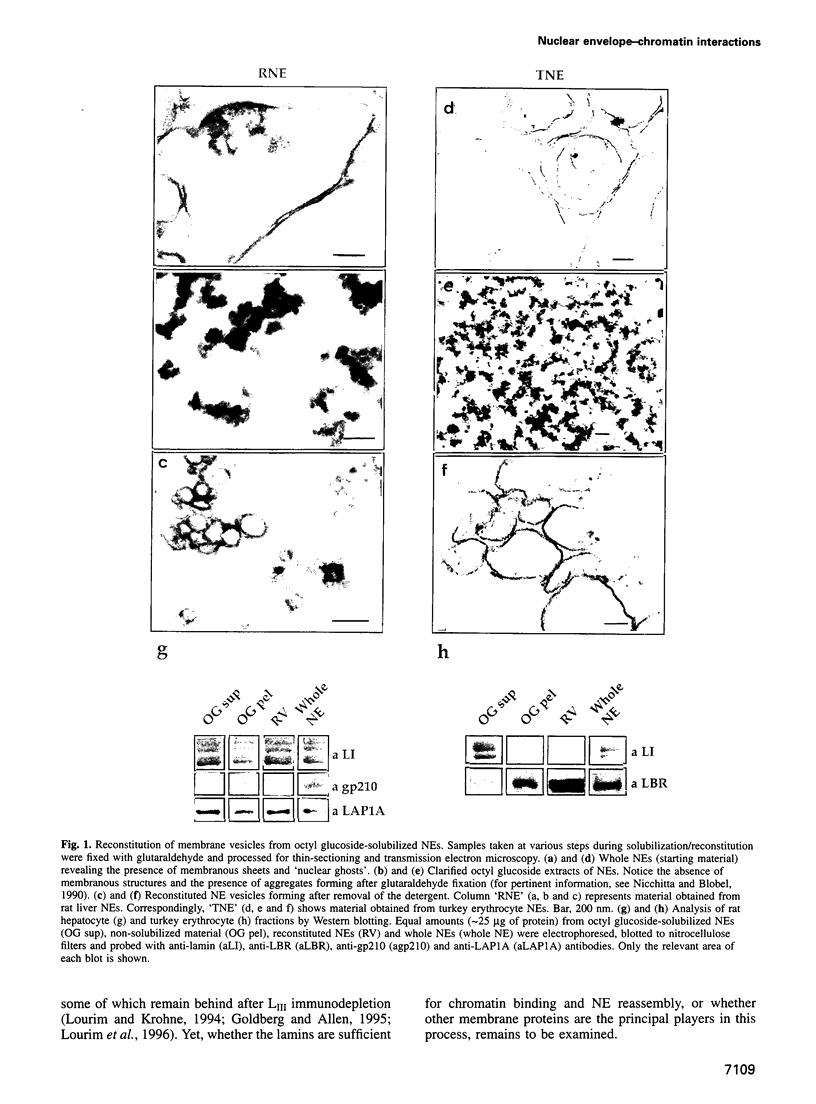
Morphological studies have established that peripheral heterochromatin is closely associated with the nuclear envelope. The tight coupling of the two structures has been attributed to nuclear lamins and lamin-associated proteins; however, it remains to be determined which of these elements are essential and which play an auxiliary role in nuclear envelope-chromatin interactions. To address this question, we have used as a model system in vitro reconstituted vesicles assembled from octyl glucoside-solubilized nuclear envelopes. Comparing the chromosome binding properties of normal, immunodepleted and chemically extracted vesicles, we have arrived at the conclusion that the principal chromatin anchorage site at the nuclear envelope is the lamin B receptor (LBR), a ubiquitous integral protein of the inner nuclear membrane. Consistent with this interpretation, purified LBR binds directly to chromatin fragments and decorates the surface of chromosomes in a distinctive banding pattern.
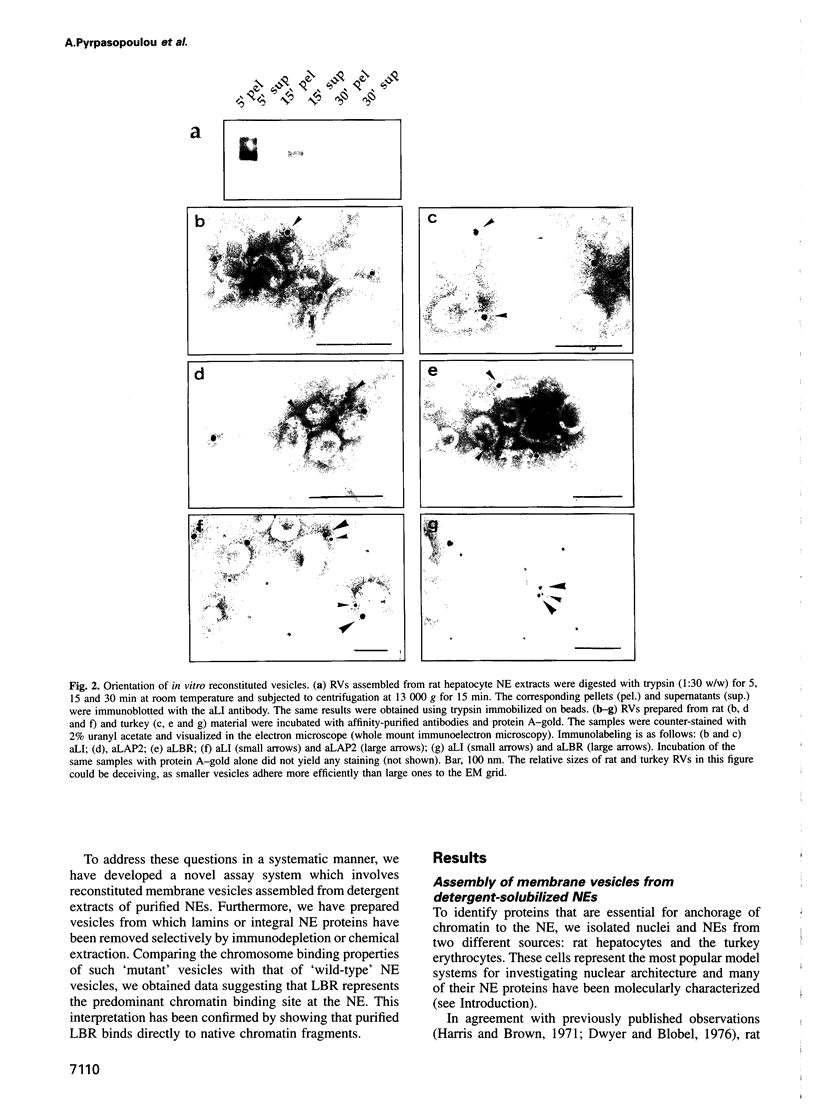
Full text
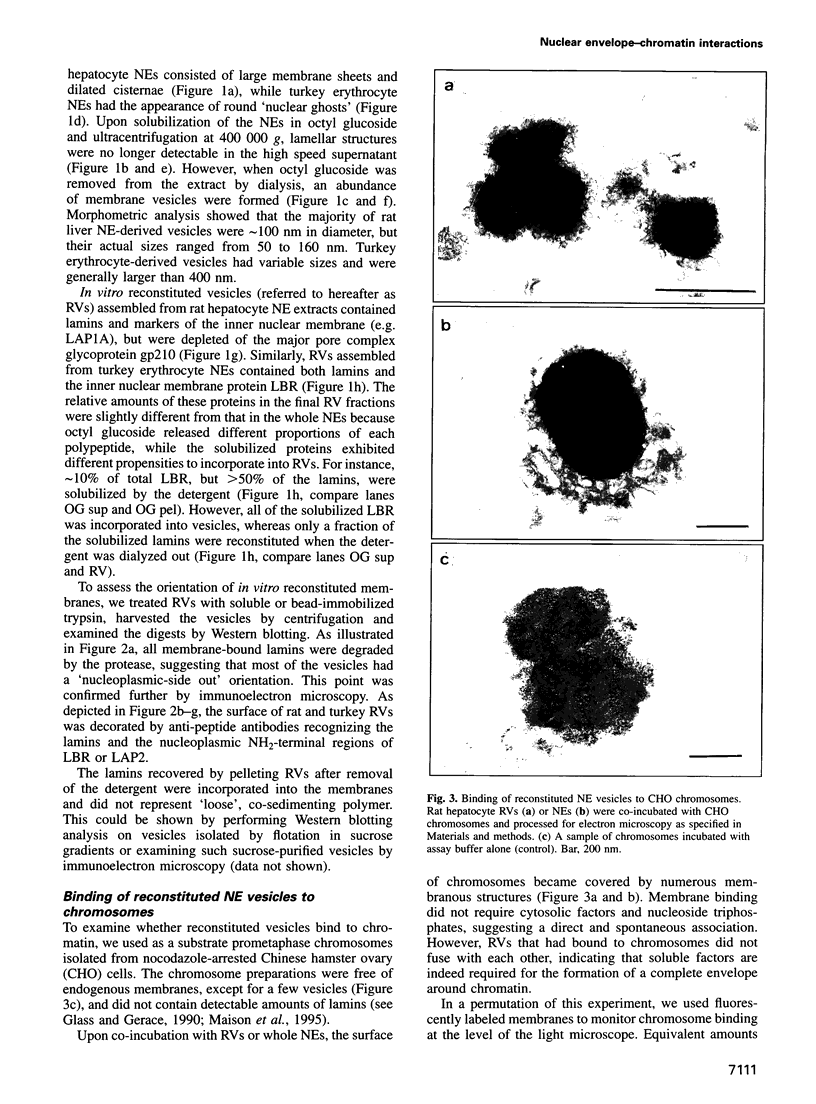
PDF
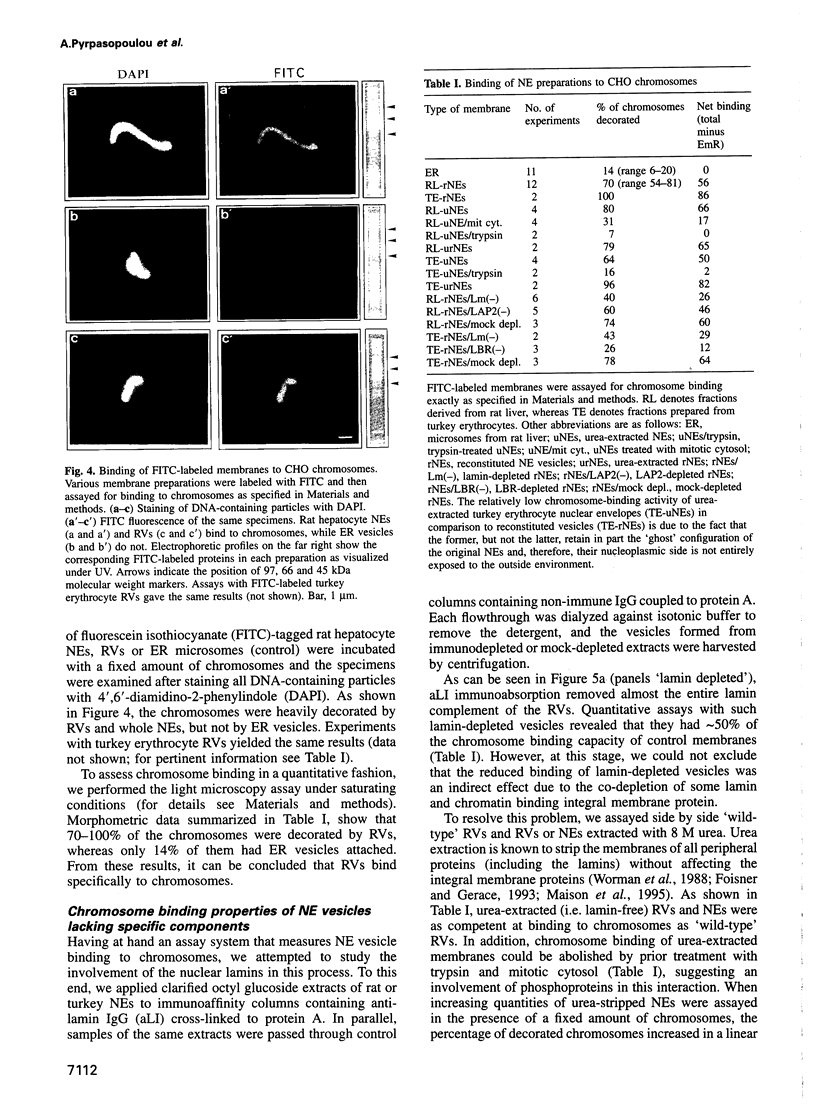
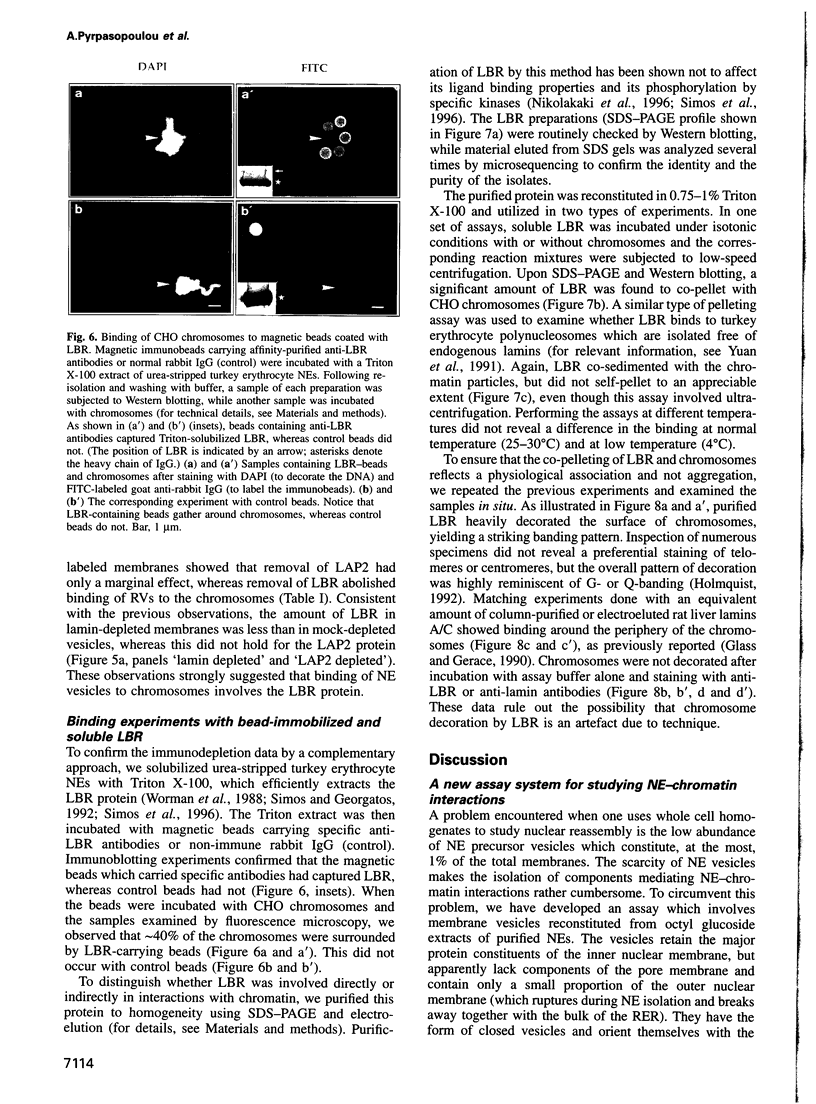
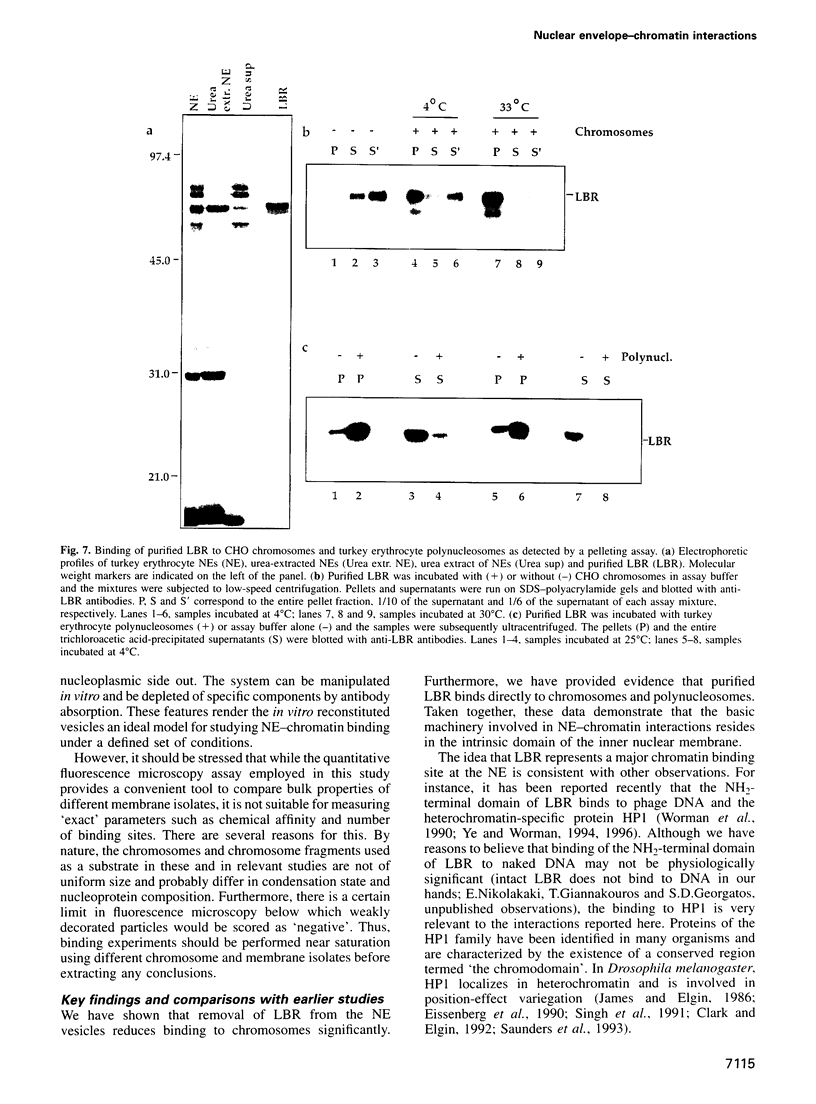
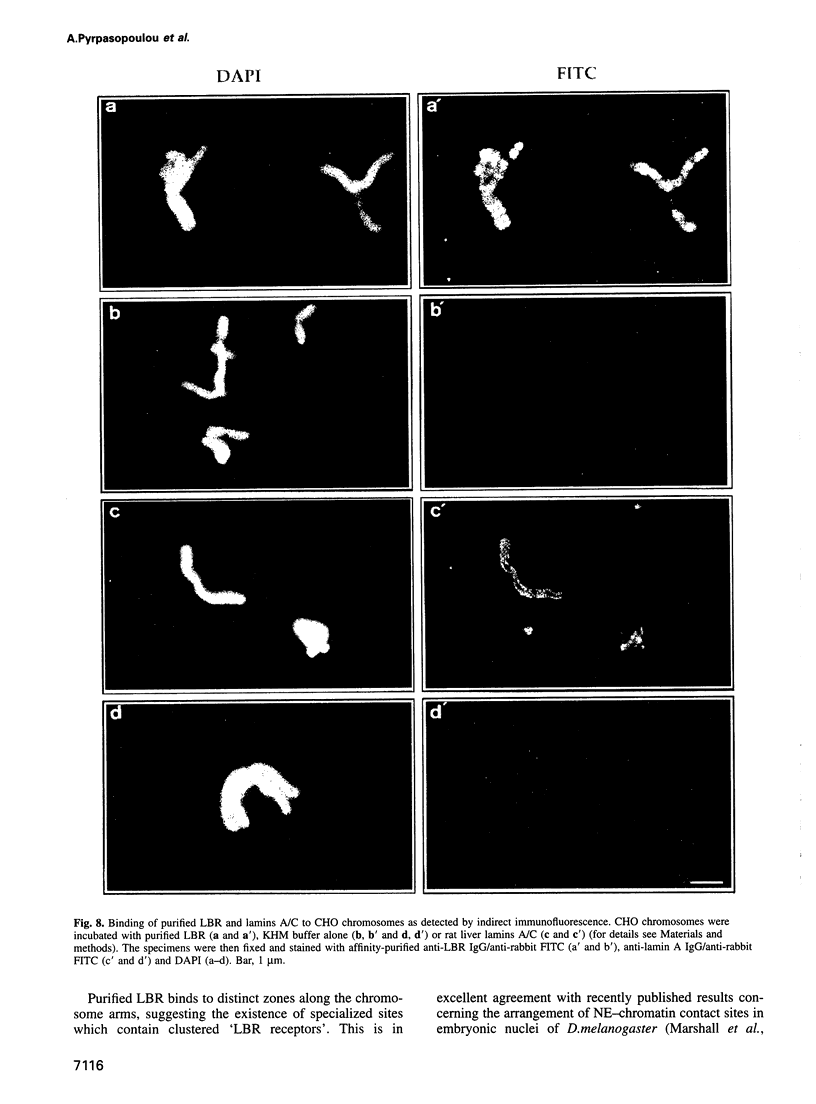



Images in this article
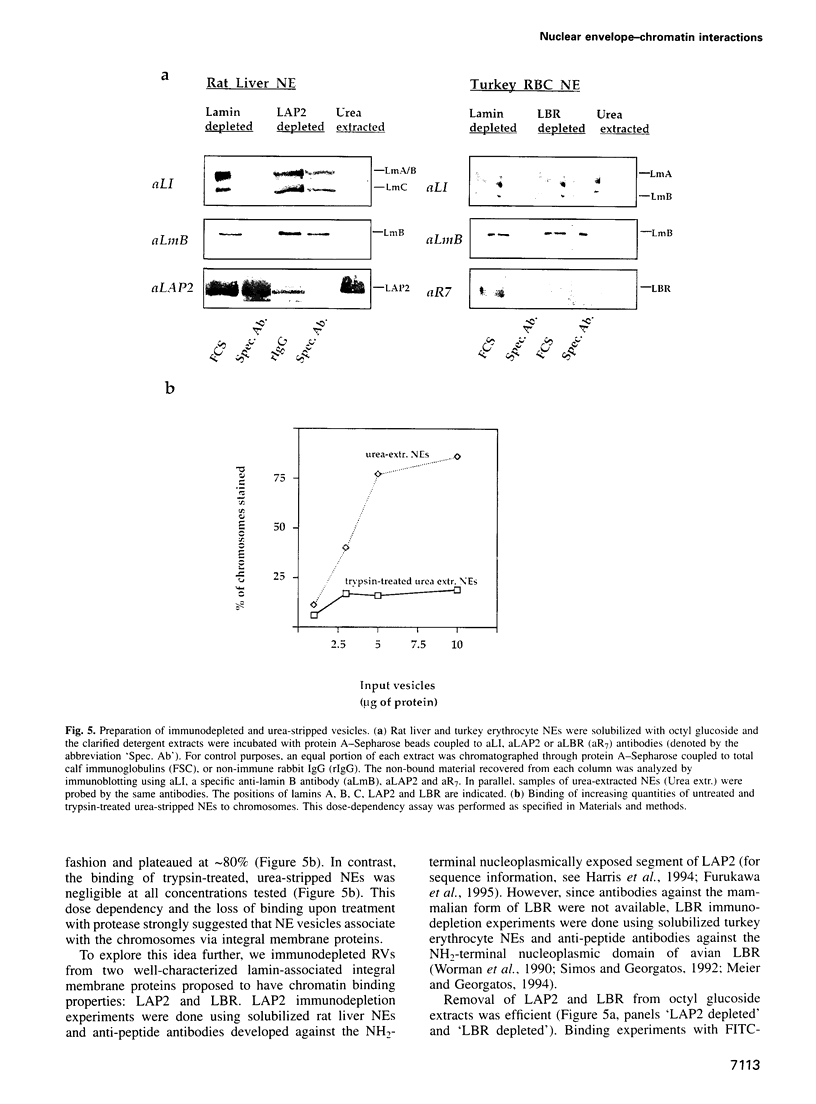
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailer S. M., Eppenberger H. M., Griffiths G., Nigg E. A. Characterization of A 54-kD protein of the inner nuclear membrane: evidence for cell cycle-dependent interaction with the nuclear lamina. J Cell Biol. 1991 Aug;114(3):389–400. doi: 10.1083/jcb.114.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont A. S., Zhai Y., Thilenius A. Lamin B distribution and association with peripheral chromatin revealed by optical sectioning and electron microscopy tomography. J Cell Biol. 1993 Dec;123(6 Pt 2):1671–1685. doi: 10.1083/jcb.123.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Burke B., Gerace L. A cell free system to study reassembly of the nuclear envelope at the end of mitosis. Cell. 1986 Feb 28;44(4):639–652. doi: 10.1016/0092-8674(86)90273-4. [DOI] [PubMed] [Google Scholar]
- Burke B. On the cell-free association of lamins A and C with metaphase chromosomes. Exp Cell Res. 1990 Jan;186(1):169–176. doi: 10.1016/0014-4827(90)90223-w. [DOI] [PubMed] [Google Scholar]
- Clark R. F., Elgin S. C. Heterochromatin protein 1, a known suppressor of position-effect variegation, is highly conserved in Drosophila. Nucleic Acids Res. 1992 Nov 25;20(22):6067–6074. doi: 10.1093/nar/20.22.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin J. C., Lassoued K., Worman H. J., Blobel G. Identification and characterization of autoantibodies against the nuclear envelope lamin B receptor from patients with primary biliary cirrhosis. J Exp Med. 1990 Sep 1;172(3):961–967. doi: 10.1084/jem.172.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer N., Blobel G. A modified procedure for the isolation of a pore complex-lamina fraction from rat liver nuclei. J Cell Biol. 1976 Sep;70(3):581–591. doi: 10.1083/jcb.70.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C., James T. C., Foster-Hartnett D. M., Hartnett T., Ngan V., Elgin S. C. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9923–9927. doi: 10.1073/pnas.87.24.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R., Gerace L. Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell. 1993 Jul 2;73(7):1267–1279. doi: 10.1016/0092-8674(93)90355-t. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Panté N., Aebi U., Gerace L. Cloning of a cDNA for lamina-associated polypeptide 2 (LAP2) and identification of regions that specify targeting to the nuclear envelope. EMBO J. 1995 Apr 18;14(8):1626–1636. doi: 10.1002/j.1460-2075.1995.tb07151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D., Blobel G. Two distinct attachment sites for vimentin along the plasma membrane and the nuclear envelope in avian erythrocytes: a basis for a vectorial assembly of intermediate filaments. J Cell Biol. 1987 Jul;105(1):105–115. doi: 10.1083/jcb.105.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D. Towards an understanding of nuclear morphogenesis. J Cell Biochem. 1994 May;55(1):69–76. doi: 10.1002/jcb.240550108. [DOI] [PubMed] [Google Scholar]
- Gerace L., Foisner R. Integral membrane proteins and dynamic organization of the nuclear envelope. Trends Cell Biol. 1994 Apr;4(4):127–131. doi: 10.1016/0962-8924(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Gilchrist J. S., Pierce G. N. Identification and purification of a calcium-binding protein in hepatic nuclear membranes. J Biol Chem. 1993 Feb 25;268(6):4291–4299. [PubMed] [Google Scholar]
- Glass C. A., Glass J. R., Taniura H., Hasel K. W., Blevitt J. M., Gerace L. The alpha-helical rod domain of human lamins A and C contains a chromatin binding site. EMBO J. 1993 Nov;12(11):4413–4424. doi: 10.1002/j.1460-2075.1993.tb06126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass J. R., Gerace L. Lamins A and C bind and assemble at the surface of mitotic chromosomes. J Cell Biol. 1990 Sep;111(3):1047–1057. doi: 10.1083/jcb.111.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. W., Allen T. D. Structural and functional organization of the nuclear envelope. Curr Opin Cell Biol. 1995 Jun;7(3):301–309. doi: 10.1016/0955-0674(95)80083-2. [DOI] [PubMed] [Google Scholar]
- Harris C. A., Andryuk P. J., Cline S., Chan H. K., Natarajan A., Siekierka J. J., Goldstein G. Three distinct human thymopoietins are derived from alternatively spliced mRNAs. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6283–6287. doi: 10.1073/pnas.91.14.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. R., Brown J. N. Fractionation of the avian erythrocyte: an ultrastructural study. J Ultrastruct Res. 1971 Jul;36(1):8–23. doi: 10.1016/s0022-5320(71)80085-0. [DOI] [PubMed] [Google Scholar]
- Holmquist G. P. Chromosome bands, their chromatin flavors, and their functional features. Am J Hum Genet. 1992 Jul;51(1):17–37. [PMC free article] [PubMed] [Google Scholar]
- Höger T. H., Krohne G., Kleinschmidt J. A. Interaction of Xenopus lamins A and LII with chromatin in vitro mediated by a sequence element in the carboxyterminal domain. Exp Cell Res. 1991 Dec;197(2):280–289. doi: 10.1016/0014-4827(91)90434-v. [DOI] [PubMed] [Google Scholar]
- James T. C., Elgin S. C. Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Mol Cell Biol. 1986 Nov;6(11):3862–3872. doi: 10.1128/mcb.6.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins H., Hölman T., Lyon C., Lane B., Stick R., Hutchison C. Nuclei that lack a lamina accumulate karyophilic proteins and assemble a nuclear matrix. J Cell Sci. 1993 Sep;106(Pt 1):275–285. doi: 10.1242/jcs.106.1.275. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C., Levin A., Branton D. Copper staining: a five-minute protein stain for sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1987 Nov 1;166(2):308–312. doi: 10.1016/0003-2697(87)90579-3. [DOI] [PubMed] [Google Scholar]
- Lourim D., Kempf A., Krohne G. Characterization and quantitation of three B-type lamins in Xenopus oocytes and eggs: increase of lamin LI protein synthesis during meiotic maturation. J Cell Sci. 1996 Jul;109(Pt 7):1775–1785. doi: 10.1242/jcs.109.7.1775. [DOI] [PubMed] [Google Scholar]
- Lourim D., Krohne G. Lamin-dependent nuclear envelope reassembly following mitosis: an argument. Trends Cell Biol. 1994 Sep;4(9):314–318. doi: 10.1016/0962-8924(94)90228-3. [DOI] [PubMed] [Google Scholar]
- Ludérus M. E., de Graaf A., Mattia E., den Blaauwen J. L., Grande M. A., de Jong L., van Driel R. Binding of matrix attachment regions to lamin B1. Cell. 1992 Sep 18;70(6):949–959. doi: 10.1016/0092-8674(92)90245-8. [DOI] [PubMed] [Google Scholar]
- Maison C., Horstmann H., Georgatos S. D. Regulated docking of nuclear membrane vesicles to vimentin filaments during mitosis. J Cell Biol. 1993 Dec;123(6 Pt 1):1491–1505. doi: 10.1083/jcb.123.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison C., Pyrpasopoulou A., Georgatos S. D. Vimentin-associated mitotic vesicles interact with chromosomes in a lamin B- and phosphorylation-dependent manner. EMBO J. 1995 Jul 17;14(14):3311–3324. doi: 10.1002/j.1460-2075.1995.tb07338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W. F., Dernburg A. F., Harmon B., Agard D. A., Sedat J. W. Specific interactions of chromatin with the nuclear envelope: positional determination within the nucleus in Drosophila melanogaster. Mol Biol Cell. 1996 May;7(5):825–842. doi: 10.1091/mbc.7.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L., Crimaudo C., Gerace L. cDNA cloning and characterization of lamina-associated polypeptide 1C (LAP1C), an integral protein of the inner nuclear membrane. J Biol Chem. 1995 Apr 14;270(15):8822–8828. doi: 10.1074/jbc.270.15.8822. [DOI] [PubMed] [Google Scholar]
- Meier J., Campbell K. H., Ford C. C., Stick R., Hutchison C. J. The role of lamin LIII in nuclear assembly and DNA replication, in cell-free extracts of Xenopus eggs. J Cell Sci. 1991 Mar;98(Pt 3):271–279. doi: 10.1242/jcs.98.3.271. [DOI] [PubMed] [Google Scholar]
- Meier J., Georgatos S. D. Type B lamins remain associated with the integral nuclear envelope protein p58 during mitosis: implications for nuclear reassembly. EMBO J. 1994 Apr 15;13(8):1888–1898. doi: 10.1002/j.1460-2075.1994.tb06458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J. W., Wilson K. L., Dunphy W. G. A lamin-independent pathway for nuclear envelope assembly. J Cell Biol. 1990 Dec;111(6 Pt 1):2247–2259. doi: 10.1083/jcb.111.6.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J., Dunphy W. Characterization of the membrane binding and fusion events during nuclear envelope assembly using purified components. J Cell Biol. 1992 Jan;116(2):295–306. doi: 10.1083/jcb.116.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolakaki E., Simos G., Georgatos S. D., Giannakouros T. A nuclear envelope-associated kinase phosphorylates arginine-serine motifs and modulates interactions between the lamin B receptor and other nuclear proteins. J Biol Chem. 1996 Apr 5;271(14):8365–8372. doi: 10.1074/jbc.271.14.8365. [DOI] [PubMed] [Google Scholar]
- Paddy M. R., Belmont A. S., Saumweber H., Agard D. A., Sedat J. W. Interphase nuclear envelope lamins form a discontinuous network that interacts with only a fraction of the chromatin in the nuclear periphery. Cell. 1990 Jul 13;62(1):89–106. doi: 10.1016/0092-8674(90)90243-8. [DOI] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. THE ULTRASTRUCTURE OF A MAMMALIAN CELL DURING THE MITOTIC CYCLE. J Cell Biol. 1964 Jun;21:429–463. doi: 10.1083/jcb.21.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders W. S., Chue C., Goebl M., Craig C., Clark R. F., Powers J. A., Eissenberg J. C., Elgin S. C., Rothfield N. F., Earnshaw W. C. Molecular cloning of a human homologue of Drosophila heterochromatin protein HP1 using anti-centromere autoantibodies with anti-chromo specificity. J Cell Sci. 1993 Feb;104(Pt 2):573–582. doi: 10.1242/jcs.104.2.573. [DOI] [PubMed] [Google Scholar]
- Senior A., Gerace L. Integral membrane proteins specific to the inner nuclear membrane and associated with the nuclear lamina. J Cell Biol. 1988 Dec;107(6 Pt 1):2029–2036. doi: 10.1083/jcb.107.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos G., Georgatos S. D. The inner nuclear membrane protein p58 associates in vivo with a p58 kinase and the nuclear lamins. EMBO J. 1992 Nov;11(11):4027–4036. doi: 10.1002/j.1460-2075.1992.tb05496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos G., Maison C., Georgatos S. D. Characterization of p18, a component of the lamin B receptor complex and a new integral membrane protein of the avian erythrocyte nuclear envelope. J Biol Chem. 1996 May 24;271(21):12617–12625. doi: 10.1074/jbc.271.21.12617. [DOI] [PubMed] [Google Scholar]
- Singh P. B., Miller J. R., Pearce J., Kothary R., Burton R. D., Paro R., James T. C., Gaunt S. J. A sequence motif found in a Drosophila heterochromatin protein is conserved in animals and plants. Nucleic Acids Res. 1991 Feb 25;19(4):789–794. doi: 10.1093/nar/19.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniura H., Glass C., Gerace L. A chromatin binding site in the tail domain of nuclear lamins that interacts with core histones. J Cell Biol. 1995 Oct;131(1):33–44. doi: 10.1083/jcb.131.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur N., Harel A., Feinstein N., Gruenbaum Y. Lamin activity is essential for nuclear envelope assembly in a Drosophila embryo cell-free extract. J Cell Biol. 1992 Oct;119(1):17–25. doi: 10.1083/jcb.119.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman H. J., Evans C. D., Blobel G. The lamin B receptor of the nuclear envelope inner membrane: a polytopic protein with eight potential transmembrane domains. J Cell Biol. 1990 Oct;111(4):1535–1542. doi: 10.1083/jcb.111.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman H. J., Yuan J., Blobel G., Georgatos S. D. A lamin B receptor in the nuclear envelope. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8531–8534. doi: 10.1073/pnas.85.22.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Worman H. J. Interaction between an integral protein of the nuclear envelope inner membrane and human chromodomain proteins homologous to Drosophila HP1. J Biol Chem. 1996 Jun 21;271(25):14653–14656. doi: 10.1074/jbc.271.25.14653. [DOI] [PubMed] [Google Scholar]
- Ye Q., Worman H. J. Primary structure analysis and lamin B and DNA binding of human LBR, an integral protein of the nuclear envelope inner membrane. J Biol Chem. 1994 Apr 15;269(15):11306–11311. [PubMed] [Google Scholar]
- Yuan J., Simos G., Blobel G., Georgatos S. D. Binding of lamin A to polynucleosomes. J Biol Chem. 1991 May 15;266(14):9211–9215. [PubMed] [Google Scholar]
- Zentgraf H., Franke W. W. Differences of supranucleosomal organization in different kinds of chromatin: cell type-specific globular subunits containing different numbers of nucleosomes. J Cell Biol. 1984 Jul;99(1 Pt 1):272–286. doi: 10.1083/jcb.99.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]