Abstract
Background:
Polycystic ovary syndrome (PCOS) is of clinical and public health importance, affecting up to one in five women of reproductive age. It has significant and diverse clinical implications including reproductive, metabolic, and psychological features.
Aim:
The study was to investigate the effect of anti-inflammatory dietary combo on metabolic, endocrine, inflammatory, and reproductive profiles in overweight and obese women with PCOS.
Materials and Methods:
A total of 100 nonpregnant, overweight, and obese adult females with PCOS according to the Rotterdam criteria, were screened during the year 2012, and 75 completed the trial. At baseline and study end, fasting blood samples were drawn to measure biological markers, body fat percent (BFP), and visceral fat area (VFA) were assessed by the InBody720 device and anthropometric measurements were done for all participants who were subjected to an anti-inflammatory hypocaloric diet and physical activity for 12 weeks.
Results:
At study completion, we achieved moderate weight loss of (± 7%) and significant improvements in body composition, hormones and menstrual cyclicity, blood pressure, glucose homeostasis, dyslipidemia, C-reactive protein (CRP), and serum amyloid A (SAA) (surrogate measures of cardiovascular risk (CVR)). This was a clinically relevant weight loss that is associated with a reduced prevalence of type 2 diabetes mellitus (DM2) and metabolic syndrome (MS) in the general population and improved fertility outcomes in PCOS. We achieved 63% regain of menstrual cyclicity and 12% spontaneous pregnancy rate within 12 week.
Conclusions:
We have explored an additional dietary treatment option with good prognostic metabolic and reproductive responses to weight loss that occur in overweight and obese PCOS.
Keywords: Acute-phase serum amyloid A, diet and inflammation, lifestyle modification, polycystic ovary syndrome, SAA, surrogates of cardiovascular risk
Introduction
Polycystic ovary syndrome (PCOS) is a frustrating experience for women, often complex for managing clinicians; and is a scientific challenge for researchers. It has significant and diverse clinical implications including reproductive (infertility, hyperandrogenism, and hirsutism), metabolic (insulin resistance, impaired glucose tolerance, type 2 diabetes mellitus (DM2), and adverse cardiovascular risk (CVR) profiles), and psychological features (increased anxiety, depression, and worsened quality of life).[1]
Diagnosis of PCOS is relatively straightforward. Common criteria established by the Rotterdam Conference in 2003 include at least two of three characteristics; oligomenorrhea, clinical and/or biochemical hyperandrogenism, and ultrasound PCO criteria in the absence of other diseases.[2] Many PCOS women are overweight or obese, although adiposity is not a defining criteria for PCOS.[3] Obesity is highly prevalent in the general population and in PCOS women and is an independent risk factor for coronary artery disease (CAD). Regardless of what reasons women have for seeking diagnosis and treatment of PCOS, it is imperative for practitioners to assess a woman's risk for CAD.[4]
Indeed, inflammation is considered to be the key feature of endothelial dysfunction and atherosclerosis. Women with PCOS are predisposed to increased visceral adiposity and this appears to be across all categories of body mass index (BMI).[5] Manifestations of chronic inflammation as evidenced by increase in C-reactive protein (CRP), pro-inflammatory cytokines and chemokines, white blood cell count, oxidative stress, and various markers of endothelial inflammation are associated with PCOS.[6] Acute-phase serum amyloid A (SAA) is a novel pro-inflammatory adipokine, which increased in obese insulin resistant subjects. SAA is increased in PCOS women and is elevated by the intake of simple sugars. Metformin treatment decreases SAA in these women. SAA may be a valuable diagnostic marker in the management of dysmetabolic states including PCOS.[7]
In PCOS, a dietary trigger such as glucose is capable of inducing oxidative stress to stimulate an inflammatory response even in the absence of excess adiposity. Hyperandrogenism may be the originator of chronic low-grade inflammation. Diet-induced inflammation in particular may be the underpinning of insulin resistance in the disorder. Inflammation directly stimulates excess ovarian androgen production. Increased abdominal adiposity contributes to the inflammatory load in PCOS, and its development may be controlled by the severity of hyperandrogenism. Excess androgens encourage insulin resistance, leading to elevated insulin levels, which in turn stimulate further androgen synthesis. This vicious cycle results in a “snowball effect” worsening PCOS symptoms and making sufferers, especially susceptible to obesity and diabetes.[8,9]
As the qualitative aspects of diet may affect body composition, metabolism and may modulate the inflammatory state of this high risk group; we tried to test the hypothesis that consuming a hypocaloric low glycemic load (GL) diet with anti-inflammatory properties (as a combo diet) will reduce total and visceral adipose tissue, promote weight loss, improve the reproductive, metabolic and hormonal profiles, and attain the patients' compliance. The principal dietary components of a proposed anti-inflammatory diet should be a low GL diet, low in omega-6 fatty acids and rich in omega-3 fatty acids. For every gram of fat consumed, the individual would consume 2 grams of protein, and 3 grams of carbohydrate. This 1-2-3 ratio of macronutrients has been examined in various studies under isocaloric conditions. In each of these studies, the 1-2-3 ratio has been shown to be superior in reducing insulin and stabilizing blood lipid levels, reducing blood glucose levels, increasing weight loss in patients characterized by a high initial insulin secretion to carbohydrates, and reducing silent inflammation.[10]
Materials and Methods
This quasi-experimental trial was carried out in the Nutrition Outpatient Clinic of the High Institute of Public Health, Alexandria University; after approval by the institute's ethics committee and the university's research committee. An informed consent was obtained from all cases to participate in the study. Laboratory tests were done at Mabaret El Asafra Laboratories. Nonpregnant, overweight, and obese adult females with PCOS based on Rotterdam criteria, aged 20-40 years were referred by Obstetrics and Gynecology Department, Faculty of Medicine, Alexandria University. Subjects having type 1 or type 2 diabetes and those receiving metformin, receiving ovulation induction medications, or following a diet regimen within the last month are excluded. The sample size was calculated based on the reported changes in CRP level with lifestyle modification and accepting an α error of 0.05 and a power of 90%, the resulting minimum required sample size amounted to 75 after adjustment for a 20% dropout. As it is not possible at baseline to identify women who are likely to dropout, therefore, during the year 2012, 100 overweight and obese women with PCOS were recruited. Medical, reproductive, and dietary history and history of significant metabolic or cardiac diseases, menstrual pattern, and dietary pattern were taken from all participants. Mean systolic and diastolic blood pressure were assessed using a mercury sphygmomanometer with two readings after 5 min of rest in sitting down position (all readings were taken by the main researcher). Weight in kilograms with no shoes in a minimal clothing state by a digital scale (Beurer, Germany), height in centimeters, and waist circumference (WC) and hip circumference (HC) in centimeters were measured. At baseline and study end, body fat percent (BFP) and visceral fat area (VFA) were assessed by the InBody 720 bioelectric impedance device (Biospace Co., Ltd., Korea) and fasting blood samples were drawn to measure levels of glucose (FBG), insulin (FI), and lipids; total testosterone (TT), free testosterone (FT), and steroid hormone binding globulin (SHBG) coupled with CRP and SAA were also measured (*see data management sheet). An informed consent was obtained from all cases to participate in the study. All participants were subjected to lifestyle intervention with a hypocaloric diet and physical activity for 12 weeks. They attended the clinic once every 2 weeks. At all visits; weight, WC, and HC were measured. The prevalence for individual components of metabolic syndrome (MS) criteria in the sample was scanned according to the classification of the International Diabetes Federation (IDF).
Dietary treatment
We adopted a Mediterranean-inspired low glycemic load anti-inflammatory diet based on combinations of nutrients (a combo diet) encouraging the consumption of legumes, fish, and low-fat dairy products in a Mediterranean context; with a composition of 25% proteins, 25% fat, and 50% carbohydrates. Diets were designed as reduced-energy, low-fat, low-saturated fat, and moderate-to-high fiber diets. The GL was reduced by lowering sugar content in favor of more complex carbohydrates. Estimated energy requirements were calculated for each subject according to the Institute of Medicine Equation, then subtracting 500 kcal. Meal plans were previously designed using the Diabetic Exchange Calculation Forum; combo diets of 2,000; 1,800; 1,600; 1,400; 1200; 1,000; and 800 kcal content were prepared with multiple options to suit a variety of personal taste and preference,[11] using the Diabetic Exchange List. By evenly spacing carbohydrate foods through the day and by eating about the same amount at each meal or snack, blood glucose control stays within targets. We encouraged the consumption of a multigrain commercial bread brand (Rich Bake multigrain toast - six grains), a low GI bread. Menu plans and shopping lists were provided at each visit. They were directed to consume unsaturated sources of fat such as flaxseeds (40 g/day),[12,13,14] and olive oil while limiting overall fat intake.
Due to cultural, environmental, and sometimes religious barriers; we instructed all participants to use the stairs to the upper floor up and down for 30 min/day, and three times 10 min/day of sit-ups or abdominal crunches. Red meat was limited to once every 2 weeks, chickens once weekly, and fish at least twice weekly; and we encouraged the consumption of legumes. As consuming small, frequent meals rather than large, less frequent meals has been associated with improved glycemic control and lipid profiles; five small meals, 3 h apart were constructed. Ginger, chili peppers, black pepper, curcumin, bay leaves, fennel, anise, caraway, cumin, coriander, clove, cinnamon, marjoram, rosemary, and thyme were recommended for use in daily food preparation and salad seasoning. Finally, five cups of green tea intake daily.
Statistical analysis
Data analysis was performed using the Statistical Package for Social Sciences (SPSS) software version 16. For descriptive statistics, mean and standard deviation were used for normally distributed quantitative data and the median for non-normally distributed data. For analysis of numeric data, one sample Kolmogorov-Smirnov test was used. To test the association between two categorical variables, Pearson's chi-square test, Monte Carlo exact test, and Fisher's exact test were used. Mc Nemar chi-square test was used for comparing paired results from related samples pre-intervention-post intervention difference. Z-test was used for comparison between two proportions. Mann-Whitney U test for comparing two independent quantitative non-normally distributed variables. Wilcoxon signed-rank test is a nonparametric test equivalent to paired t-test, and is used to test the hypothesis that two related quantitative variables have the same distribution (% mean change calculated as: mean after intervention - baseline mean)/baseline mean ×100).
Results
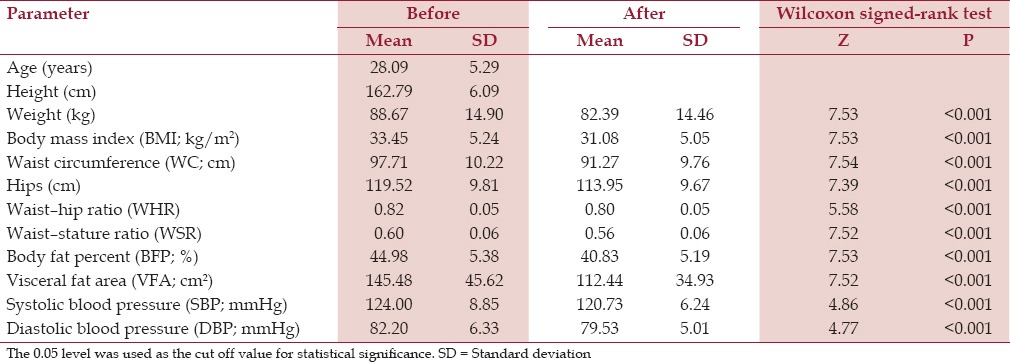
Out of the 94 women recruited, two subjects conceived; after 5 and 7 weeks, respectively, and discontinued the intervention and 17 dropped out (18%), while 75 completed the 12 weeks of the study. Comparing the data before and after intervention, there was a mean weight loss of 6.3 kg or 7.9%. Changes in BMI were 7.1% and WC decreased by 6.6%, these were statistically significant (P ≤ 0.001). Changes in BFP were -9.2% and this was statistically significant (P ≤ 0.001). VFA decreased by 21.7%, and this was statistically significant as well (P ≤ 0.001) [Table 1].
Table 1.
Anthropometric characteristics before and after intervention in the studied sample
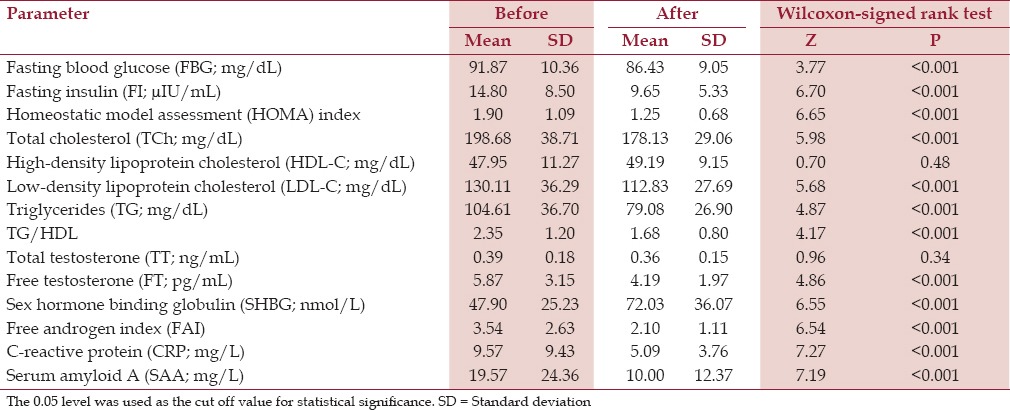
Our combo diet-related weight loss resulted in the following changes; FBG decreased by 5.15%, FIdecreased by 27.86%, and homeostatic model assessment (HOMA) showed a decrease of 27.50%; these were statistically significant (P ≤ 0.001). There was an 8.9% reduction in total cholesterol, an 18.02% decline in triglycerides, and 10.6% reduction in LDL cholesterol. But the 2.6% increase in HDL was insignificant (P = 0.48), as was the drop in TT (P = 0.34). But the drop in free androgen index (FAI) by 31% and the very large increase in mean SHBG levels by 65.6%, were both statistically significant (P ≤ 0.001). There was a remarkable drop in the levels of the two chosen inflammatory markers and CVR indices; CRP and SAA, the basic levels of which were abnormally high, with 35 and 38% improvement from the baseline, respectively (P ≤ 0.001) [Table 2].
Table 2.
Metabolic, hormonal, and inflammatory markers before and after intervention
Studying the correlation between anthropometric characteristics and lipid profile indices at baseline, it was noted that a moderate inverse correlation existed between HDL and BMI and waist and WSR. TG had a weak correlation with WHR only, while TG/HDL correlated with BMI, waist, WHR, and WSR.
Studying the correlations between the anthropometric characteristics and hyperandrogenemia indices at baseline showed that SHBG correlated moderately and significantly with BMI, waist, WSR, BFP, and VFA in an inverse manner. FAI correlated significantly (stronger than SHBG and better than TT or FT) with; weight, BMI, waist, hips, WHR, WSR, BFP, and VFA in a positive relationship. Please note: After intervention FAI kept relationships with waist, WHR, and WSR in a lesser degree of strength and significance, while SHBG correlated negatively only with BFP.
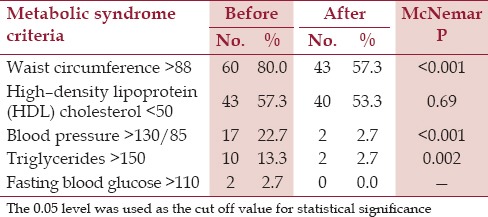
The prevalence for individual components of MS criteria in the sample is shown in Table 3. Fifteen subjects presented with the full blown picture of MS (20.0%). The improvement after intervention was 80%.
Table 3.
Metabolic syndrome criteria among subjects before and after intervention
Forty-three cases of those who completed the study were oligomenorrheic (<8 menses/year) of those 27 had regained ≥2 menses within the study period (62.8%). There were seven cases of simple ovarian cysts (<5 cm), all showed the disappearance of these cysts within the study period. Seven women conceived by the end of the study period out of 58 eligible for spontaneous pregnancy (12%), that is, calculated after excluding male factor infertility, tubal factor, and intrauterine device (IUD) use.
Discussion
While lifestyle management is recommended as first-line treatment of PCOS, the optimal dietary composition is unclear. Weight loss improved the presentation of PCOS regardless of dietary composition in the majority of studies.[15]
Anti-inflammatory nutrition is the understanding how individual nutrients affect the same molecular targets affected by pharmacological drugs. Pharmacological agents often work downstream from the true primary molecular target of inflammation, whereas anti-inflammation nutrition works upstream to reduce the dietary factors that activate nuclear factor kappa B (NF-kB) to generate silent inflammation. The success of this anti-inflammatory diet can be measured clinically by various markers of silent inflammation as fibrinogen, CRP, and SAA, as well as improvement of dysmetabolic conditions (i. e., DM2, MS, cardiovascular disease (CVD), etc.) that are associated with obesity.[16]
Inspired by Sears and Ricordi,[16] the dietary portfolio,[17] Moran et al.,[15] and Marsh et al.,[18] actions that seemed logic were: energy restriction; anti-inflammatory approach with low GI foods in a Mediterranean style; decreasing red meat and processed meat; prohibiting added sugar and decreasing saturated fat; encouraging herbs, spices, phytochemicals, antioxidants, and omega 3 intake; small frequent meals; and an achievable form of exercise. To the best of our knowledge, this is the first clinical trial adopting the use of anti-inflammatory dietary approach with pharmacological targeting in the management of an overweight and obese PCOS population.
Compliance was high and the dropout rate was relatively low as 77% of patients were motivated by a desire to conceive. According to Moran et al.,[19] out of 45 randomized subjects in a trial of two different dietary interventions in PCOS over 4 months, 17 discontinued the trial (37.8%). Hoeger et al.,[20] reported that dropout rate was 39%.
At study completion, we achieved mean weight loss of 6.3 kg or 7.2% and reductions in WC by 6.6%, reduction in BFP by 9.2%, and a 21.7% reduction in VFA. This was a clinically relevant weight loss that is associated with a reduced prevalence of DM2 and MS in the general population and improved fertility outcomes in PCOS.[21]
We achieved an 8.9% reduction in total cholesterol, an 18.02% decline in triglycerides, and 10.6% reduction in LDL cholesterol. FBG decreased by 5.15%, FIdecreased by 27.86%, and HOMA showed a decrease of 27.50%. The reduction in blood pressure was 4.3/2.7 mmHg. Moran et al.,[19] achieved a 7.6% weight reduction over 16 weeks, which resulted in an 8.8% reduction in total cholesterol, a 12.5% decline in triglycerides, and 9.8% reduction in LDL.
In the present study, TG/HDL correlated with BMI, WC, WHR, and WSR; denoting that it could be a better marker for CVR in this population than TG alone, which was already not very high in our sample.
Many related studies have used different cut off points, to define insulin resistant subjects, for example, fasting insulin >15 mU/L and HOMA >2. In our study FI>15 in 38.7% and HOMA > 2 in 33.3% of the studied sample, denoting that measuring FIalone may be as sensitive and more cost effective than HOMA in this specific population.
The evaluation of CVR in women at midlife should include markers of androgens, such as SHBG, and measures of free androgen activity.[22,23] Many references have used the term, hyperandrogenism defined as an elevated TT (>0.5 ng/mL) or a FAI (ratio of (testosterone/SHBG) × (100)) >1.5.[24,25,26,27] In our sample, TT >0.5 ng/mL was present in 26.7% and FAI >1.5 was present in 81.3%. As the FAI correlated significantly with weight, BMI, waist, hips, WHR, WSR, BFP, and VFA in a positive relationship; FAI is to be considered as a better marker for hyperandrogenism.
In the Study of Women's Health Across the Nation (SWAN) study,[28] low SHBG and high FAI were strongly related to elevated CV risk factors even after controlling for BMI. Moran et al.,[19] demonstrated a 13.7% decrease in TT, 18.2% reduction in FAI, and an 11.4% increase in SHBG. In the current study, while the decrease in TT was insignificant, the drop of FAI was dramatic (31%) as was the increase of SHBG (65.6%).
We achieved 63% regain of menstrual cyclicity and 12% spontaneous pregnancy rate within 12 weeks.
A case-control study indicates a high prevalence of the MS in Caucasian PCOS women and low HDL is the criterion which best explains this.[29] In our study population, WC >88 cm presented in 80.0% and HDL level <50 mg/dL in 57.3%, were the two criteria which best explains the 20% prevalence of MS in our PCOS population.
Considering baseline CRP concentrations when prescribing dietary interventions to lower lipid concentrations may be useful.[30] Our combo diet caused 35% drop in CRP levels, which was highly significant from baseline P ≤ 0.001. In the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial,[31,32] statin therapy lowered high-sensitivity CRP (hs-CRP) levels and achieved reduction of 62% in cardiovascular events in patients with successfully lowered serum levels of hs-CRP.
In patients with acute coronary syndromes, baseline CRP and SAA levels are associated with a greater risk for a stroke.[33] In the Women's Ischemia Syndrome Evaluation (WISE) study,[34] 25% of the women had SAA values >10.0 mg/L and 58% had hs-CRP values >3 mg/L, values that are considered abnormally high. In our sample, 52% of women had SAA values >10.0 mg/L and 77.3% had hs-CRP values >3 mg/L. Fenofibrate therapy or good omega-3 status–whether achieved with flaxseed, fish, or fish oil supplement–appear to be helpful in controlling elevated SAA.[28] Our combo diet achieved significant drop in levels of SAA; with a decrease of 38.25% from baseline. SAA may be useful as a parameter for monitoring the progress of obesity intervention and anti-inflammatory therapy.[35]
Conclusion
Our dietary strategy resulted in moderate weight loss and significant improvements in body composition, hormones and menstrual cyclicity, blood pressure, glucose homeostasis, dyslipidemia, and CRP and SAA (surrogate measures of CVR). The literature on weight loss options in PCOS has thus been expanded to include an anti-inflammatory dietary approach with pharmacological targeting for achieving a clinically relevant weight loss in PCOS patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Data management sheet
References
- 1.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rotterdam ESHRE/ASRM Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 3.Cho LW, Randeva HS, Atkin SL. Cardiometabolic aspects of polycystic ovarian syndrome. Vasc Health Risk Manag. 2007;3:55–63. [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander CJ, Tangchitnob EP, Lepor NE. Polycystic ovary syndrome: A major unrecognized cardiovascular risk factor in women. Rev Obstet Gynecol. 2009;2:232–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Duleba AJ, Dokras A. Is PCOS an inflammatory process? Fertil Steril. 2012;97:7–12. doi: 10.1016/j.fertnstert.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wildman RP, Kaplan R, Manson JE, Rajkovic A, Connelly SA, Mackey RH, et al. Body size phenotypes and inflammation in the Women's Health Initiative Observational Study. Obesity (Silver Spring) 2011;19:1482–91. doi: 10.1038/oby.2010.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan BK, Adya R, Shan X, Aghilla M, Lehnert H, Keay SD, et al. The anti-atherogenic aspect of metformin treatment in insulin resistant women with the polycystic ovary syndrome: Role of the newly established pro-inflammatory adipokine Acute-Phase Serum Amyloid A; evidence of an adipose tissue-monocyte axis. Atherosclerosis. 2011;216:402–8. doi: 10.1016/j.atherosclerosis.2010.08.069. [DOI] [PubMed] [Google Scholar]
- 8.González F. Inflammation in polycystic ovary syndrome: Underpinning of insulin resistance and ovarian dysfunction. Steroids. 2012;77:300–5. doi: 10.1016/j.steroids.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esposito K, Giugliano D. Diet and inflammation: A link to metabolic and cardiovascular diseases. Eur Heart J. 2006;27:15–20. doi: 10.1093/eurheartj/ehi605. [DOI] [PubMed] [Google Scholar]
- 10.Sears B. Anti-inflammatory diets for obesity and diabetes. J Am Coll Nutr. 2009;28:482S–91S. doi: 10.1080/07315724.2009.10718115. [DOI] [PubMed] [Google Scholar]
- 11.Smith CIF, Wing RR. New directions in behavioral weight-loss programs. Diabetes Spectr. 2000;13:142–6. [Google Scholar]
- 12.Landete JM. Plant and mammalian lignans: A review of source, intake, metabolism, intestinal bacteria and health. Food Res Int. 2012;46:410–24. [Google Scholar]
- 13.Dodin S, Cunnane SC, Mâsse B, Lemay A, Jacques H, Asselin G, et al. Flaxseed on cardiovascular disease markers in healthy menopausal women: A randomized, double-blind, placebo-controlled trial. Nutrition. 2008;24:23–30. doi: 10.1016/j.nut.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Nowak DA, Snyder DC, Brown AJ, Demark-Wahnefried W. The effect of flaxseed supplementation on hormonal levels associated with polycystic ovarian syndrome: A case study. Curr Top Nutraceutical Res. 2007;5:177–81. [PMC free article] [PubMed] [Google Scholar]
- 15.Moran LJ, Ko H, Misso M, Marsh K, Noakes M, Talbot M, et al. Dietary composition in the treatment of polycystic ovary syndrome: A systematic review to inform evidence-based guidelines. J Acad Nutr Diet. 2013;113:520–45. doi: 10.1016/j.jand.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Sears B, Ricordi C. Anti-inflammatory nutrition as a pharmacological approach to treat obesity. J Obes. 2011;2011 doi: 10.1155/2011/431985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins DJ, Jones PJ, Lamarche B, Kendall CW, Faulkner D, Cermakova L, et al. Effect of a dietary portfolio of cholesterol-lowering foods given at 2 levels of intensity of dietary advice on serum lipids in hyperlipidemia. A randomized controlled trial. JAMA. 2011;306:831–9. doi: 10.1001/jama.2011.1202. [DOI] [PubMed] [Google Scholar]
- 18.Marsh KA, Steinbeck KS, Atkinson FS, Petocz P, Brand-Miller JC. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr. 2010;92:83–92. doi: 10.3945/ajcn.2010.29261. [DOI] [PubMed] [Google Scholar]
- 19.Moran LJ, Noakes M, Clifton PM, Tomlinson L, Galletly C, Norman RJ. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:812–9. doi: 10.1210/jc.2002-020815. [DOI] [PubMed] [Google Scholar]
- 20.Hoeger KM, Kochman L, Wixom N, Craig K, Miller RK, Guzick DS. A randomized, 48-week, placebo-controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: A pilot study. Fertil Steril. 2004;82:421–9. doi: 10.1016/j.fertnstert.2004.02.104. [DOI] [PubMed] [Google Scholar]
- 21.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, et al. Diabetes Prevention Program Research Group. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: The Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611–9. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talbott EO, Zborowski JV, Sutton-Tyrrell K, McHugh-Pemu KP, Guzick DS. Cardiovascular risk in women with polycystic ovary syndrome. Obstet Gynecol Clin North Am. 2001;28:111–33. doi: 10.1016/s0889-8545(05)70189-3. [DOI] [PubMed] [Google Scholar]
- 23.Talbott EO, Guzick DS, Sutton-Tyrrell K, McHugh-Pemu KP, Zborowski JV, Remsberg KE, et al. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol. 2000;20:2414–21. doi: 10.1161/01.atv.20.11.2414. [DOI] [PubMed] [Google Scholar]
- 24.Katcher HI, Kunselman AR, Dmitrovic R, Demers LM, Gnatuk CL, Kris-Etherton PM, et al. Comparison of hormonal and metabolic markers after a high-fat, Western meal versus a low-fat, high-fiber meal in women with polycystic ovary syndrome. Fertil Steril. 2009;91:1175–82. doi: 10.1016/j.fertnstert.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356:551–66. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 26.Legro RS, Myers ER, Barnhart HX, Carson SA, Diamond MP, Carr BR, et al. Reproductive Medicine Network. The Pregnancy in Polycystic Ovary Syndrome study: Baseline characteristics of the randomized cohort including racial effects. Fertil Steril. 2006;86:914–33. doi: 10.1016/j.fertnstert.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 27.Legro RS, Zaino RJ, Demers LM, Kunselman AR, Gnatuk CL, Williams NI, et al. The effects of metformin and rosiglitazone, alone and in combination, on the ovary and endometrium in polycystic ovary syndrome. Am J Obstet Gynecol. 2007;196:402.e1–11. doi: 10.1016/j.ajog.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 28.Sutton-Tyrrell K, Wildman RP, Matthews KA, Chae C, Lasley BL, Brockwell S, et al. Sex hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–9. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 29.Gambineri A, Repaci A, Patton L, Grassi I, Pocognoli P, Cognigni GE, et al. Prominent role of low HDL-cholesterol in explaining the high prevalence of the metabolic syndrome in polycystic ovary syndrome. Nutr Metab Cardiovasc Dis. 2009;19:797–804. doi: 10.1016/j.numecd.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 30.St-Onge MP, Zhang S, Darnell B, Allison DB. Baseline serum C-reactive protein is associated with lipid responses to low-fat and high-polyunsaturated fat diets. J Nutr. 2009;139:680–3. doi: 10.3945/jn.108.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridker PM. JUPITER Study Group. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: Rationale and design of the JUPITER trial. Circulation. 2003;108:2292–7. doi: 10.1161/01.CIR.0000100688.17280.E6. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM. The JUPITER trial. Results, controversies, and implications for prevention. Circ Cardiovasc Qual Outcomes. 2009;2:279–85. doi: 10.1161/CIRCOUTCOMES.109.868299. [DOI] [PubMed] [Google Scholar]
- 33.Hermusa L, Lefrandtb JD, Tioc RA, Breek JC, Zeebregts CJ. Carotid plaque formation and serum biomarkers. Atherosclerosis. 2010;213:21–9. doi: 10.1016/j.atherosclerosis.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, et al. National Heart, Lung, and Blood Institute. Serum amyloid a as a predictor of coronary artery disease and cardiovascular outcome in women: The National Heart, Lung, and Blood Institute -Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:726–32. doi: 10.1161/01.CIR.0000115516.54550.B1. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, He X, Shi X, Huang C, Liu J, Zhou S, et al. Association between serum amyloid A and obesity: A meta-analysis and systematic review. Inflamm Res. 2010;59:323–34. doi: 10.1007/s00011-010-0163-y. [DOI] [PubMed] [Google Scholar]


