Abstract
Background:
There is considerable evidence which suggests that Omega 3 polyunsaturated fatty acids may have a potential use in the treatment of epilepsy.
Aim:
The study was to investigate the effect of Omega 3 polyunsaturated fatty acids (as fish oil supplementation) in reducing the frequency and severity of epileptic seizures in children with medically resistant epilepsy.
Materials and Methods:
In the case-control study, a total of 70 children with medically resistant epilepsy underwent assessment of the frequency and severity of the epileptic attacks at baseline, after one month, two months and three months from the beginning of the study; 35 children received fish oil and the other 35 children received placebo.
Results:
The number of children who received fish oil, having 0 epileptic attacks increased from 0%, before starting the study, up to 57.1% at the end of the third month, while the improvement was minimal in the placebo group, with a significant difference in the improvement between the intervention and the control groups. There was no statistically significant difference in improvement in the severity of the seizures either between cases and control or between the beginning and the end of the study.
Conclusion:
Omega 3 polyunsaturated fatty acids elevated the seizure threshold in epileptic patients and may help in achieving seizure control.
Keywords: Epilepsy, fish oil, omega 3 polyunsaturated fatty acids (n-3 PUFA), seizures
Introduction
Epilepsy is one of the most prevalent noncommunicable neurologic conditions and an important cause of disability and mortality.[1] Diet has been demonstrated to be an effective treatment in the management of seizure disorders.[2] There is evidence suggesting that dietary Omega 3 polyunsaturated fatty acids (n-3 PUFA) may have a potential use in the treatment of epilepsy.[3,4,5]
PUFAs modulate the structure and function of lipid rafts and neuronal membranes, including raft and membrane-incorporated proteins.[6] The lipid bilayer tends to exist at an optimum transition point between gel and liquid crystal, and the maintenance of this state, often referred to as fluidity, is of physiological importance and can be strongly influenced by fatty acid composition. Transmembrane and peripheral proteins of various shapes, molecular masses and charges regulate important cellular functions; but their position, integration and functioning are all affected by membrane fluidity and thus fatty acid composition, especially the double bonds in PUFA, have strong influence and the replacement of even a single double bond in these PUFAs is sufficient to exert a profound effect on the physical properties of the membrane.[7]
The primary n-3 PUFA in the brain is Docosahexaenoic acid (DHA); it is involved in the regulation of neuronal function via several different mechanisms, like interaction with ion channels, modulation of membrane biophysical properties, regulation of neurotransmitter release, neurotransmitter signaling, and synthesis of biologically active oxygenated derivatives and nuclear-receptor-mediated transcription of genes.[8] Other n-3 PUFA, such as α-linolenic acid (ALA) or Eicosapentaenoic acid (EPA), constitute a very small fraction of brain total fatty acids (<1% for ALA, and <0.1% for EPA).[9] The fundamental importance of DHA for brain development is beyond dispute. DHA is the most fluidizing element in cell membranes.[10]
Diet is the important source of n-3 PUFA. The n-3 PUFA derived from marine animals primarily consist of EPA and DHA. The n-3 PUFAs derived from plants consists of ALA. Although DHA can be endogenously synthesized from ALA in the liver,[11] the conversion efficiency of ALA to DHA is very low, being only approximately 7% or less in humans.[12] Most DHA, therefore, must be obtained from marine dietary sources or fish-oil based nutraceutical supplements.
With respect to epilepsy, there is evidence suggesting that n-3 PUFAs may have a potential use in the treatment of epilepsy; recent studies in animal models have shown that they can raise the threshold of epileptic seizures.[3] In the following study, the effect of n-3 PUFAs in fish oil on epileptic seizures' frequency and severity was examined.
Materials and Methods
This single-blinded, randomized, pretest post-test controlled group study was conducted in the Neurology Department at Alexandria Pediatric Center from September 2013 to April 2014. Ethical approval was obtained from the ethical committee of the High Institute of Public Health. Written informed consent was obtained from the parents of the participants. Children of both sexes, between 4 and 12 years of age, with average weight of 26.76 kg, suffering from medically resistant epilepsy,[13] regardless of the type of seizure (partial or generalized) were randomly divided into 2 groups: 1) the intervention or fish oil-supplemented group and 2) the control or placebo assigned group. In both groups, children continued to take their anti-epileptic treatments. Evaluation of the frequency and severity of the epileptic seizures was done before starting the intervention, after one month, after two months and at the end of the third month of the study.
Seizure frequency was evaluated by recording the number of epileptic attack per month using a diary given to the parents who had to register the number of seizure attacks each day. Seizure severity was assessed by the National Hospital Seizure Severity Scale (NHS3),[14] which was administered during an interview with the parents or a witness to the seizures; it contains seven seizure-related factors and generates a score from 1 to 27. Scores on the NHS3 in the intervention and control groups were compared before, and at one month, two months and three months after the start of intake of fish oil or placebo.
The intervention group received for three months a 3-milliliter daily dose of 1200 mg fish oil providing 240 mg DHA and 360 mg EPA. Supplements used in the present study also contained Vitamin E added to prevent oxidation of the fatty acids. The control group received daily 3 milliliters of corn oil as placebo. All children known to have hypersensitivity to fish oil, with bleeding disorders, treated with Warfarin for 30 days or less prior to enrollment, taking aspirin daily or enrolled any change in antiepileptic drugs for 30 days or less prior to enrollment were excluded from the study.
Statistical analysis
Data were coded and analyzed with statistical software SPSS version 16. All statistical analysis was done using two-tailed tests and an alpha of 0.05. A P value less than or equal to 0.05 was considered to be significant. Descriptive statistics included the mean with standard deviation and percent to describe the scale and categorical data, respectively, while the median was used for skewed data and scores. P values are adjusted P values for Mann-Whitney and Friedman tests used to compare medians.
Results
The mean ages of the 70 subjects of our study were 6.9 ± 2.5 years and 6.6 ± 2.4 years in the intervention and control groups (X = 0.07 P = 0.96). All the patients were followed up monthly in the Neurology clinic by means of a seizure diary to find out the number of their seizures per month and their score in the NHS3.
At baseline (one month before fish oil supplementation), a median number of four seizures per month was obtained in both the intervention and the control group. This median decreased to 2 after one month and became 0 after two and three months of intervention with fish oil supplementation while it remained 4 in the control group. Therefore, there was a statistically significant decrease in the monthly number of seizures after three months of fish oil supplementation compared to the baseline before supplementation (P < 0.05). [Table 1 and Figure 1].
Table 1.
Distribution of children according to the number of epileptic attacks per month
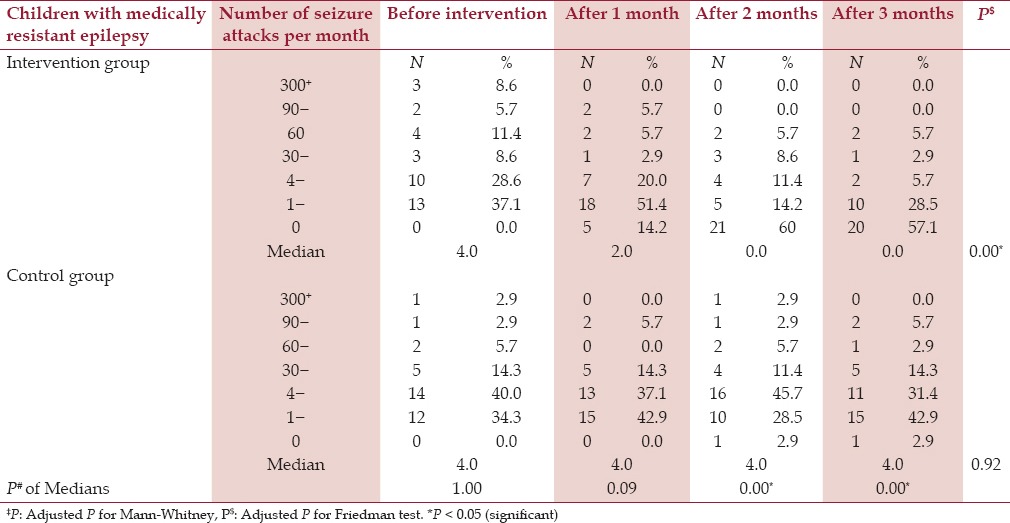
Figure 1.
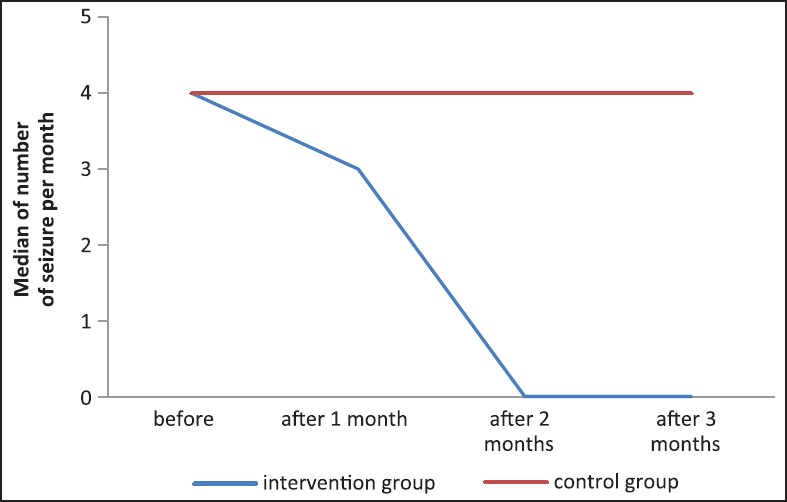
Data showing the decrease in the medians of the seizure attacks per month. After one month of starting the fish oil and reaching zero after two months of supplementation
Table 1 and Figure 2 compare the distribution of children according to the number of seizure attacks per month, before the intervention, after one month, after two months, and after three months of the study. In the intervention group, it is quite obvious that the cases are significantly improving and the number of epileptic attacks per month is decreasing after starting the fish oil supplementation. The percentage of children having zero attacks per month increased from 0% to 57.1% at the end of the third month in the intervention group, while it only reached 2.9% in the control group.
Figure 2.
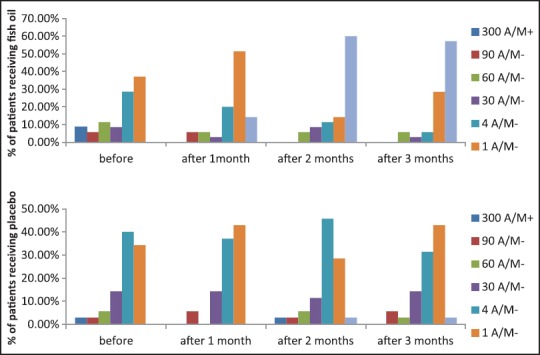
Data showing the difference in the frequency of epileptic attacks per month (A/M) between patients receiving fish oil and patients receiving placebo. There is significant improvement in the response of the fish oil group (P = 0.0)
As to seizure severity, at baseline (one month before fish oil supplementation) a median score of 13 and 12 of the NHS3 was obtained in intervention and control group, respectively. This median decreased into 11 after one month of intervention then increased to 13 again after two months and became 10 after three months of fish oil supplementation, while it remained 12 in the control group throughout the study. So in term of the median, there is no statistically significant decrease in the monthly NHS3 score after 3 months of fish oil supplementation compared to the baseline before supplementation (P > 0.05). [Table 2, Figure 3]
Table 2.
Distribution of children according to the seizure severity taking the NHS3 as reference
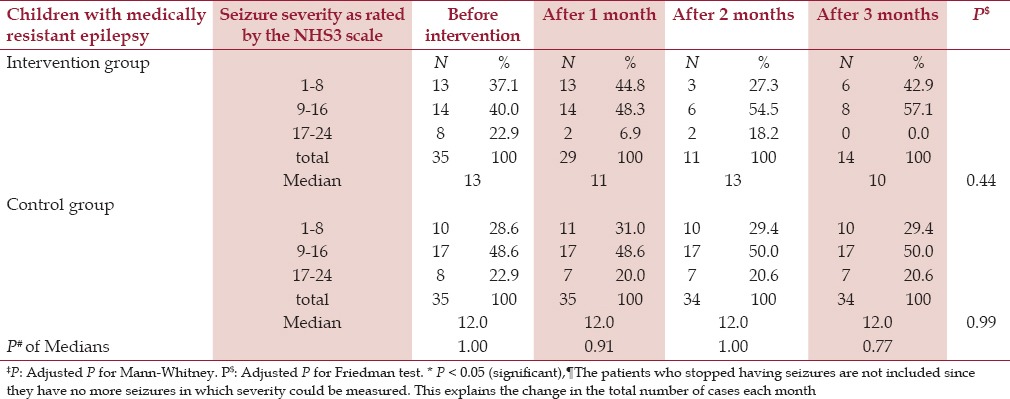
Figure 3.
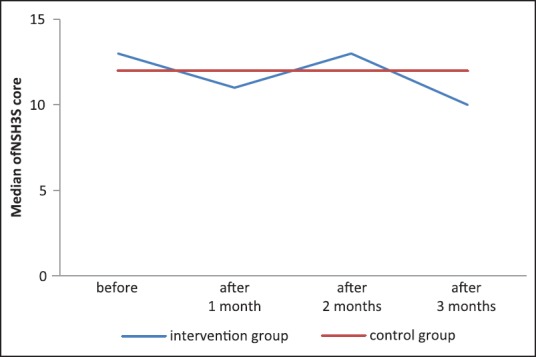
Data showing no significant decrease in the medians of the seizure severity scores, which indicates that there is no effect of the fish oil on seizure severity
At the end of the study, all of the patients in the intervention group had scores between 1 and 16, while in the control group the scores still reached 24. Although there was some change in the NHS3 scores by the end of the trial, there was no statistical significant difference, either between intervention cases and control, or between the beginning and the end of the study (P > 0.05).
Discussion
Approximately one-third of patients with epilepsy do not achieve seizure control with the available drugs, and many patients experience troublesome adverse drug effects.[15] Animal studies and preliminary clinical observations suggest that nutritional supplementation with long chain n-3 fatty acids may be useful in the nonpharmacological treatment of patients with epilepsy and n-3 PUFAs increase seizure thresholds.[15] However, there is some controversy over the effectiveness of n-3 PUFAs.[11]
Therefore, the present study was undertaken so as to ascertain whether or not n-3 PUFAs supplementation would enhance seizure control.
As to severity, there was no impressive decrease in the severity of the seizures attacks. The attacks that occurred were as strong in the intervention group as in the control group.
As to the frequency, however, the number of epileptic attacks decreased dramatically: 20 of the 35 children in the intervention group became free of seizures after three months of fish oil supplementation, while10 patients were having only 1 to 3 epileptic attacks per month and finally, only 3 children continued to have 30 + attacks per month, although 12 patients had been in this category before starting the intervention.
Impressive researches demonstrated the importance of n-3 PUFAs in epilepsy treatment in both humans and animals, and some of them were in agreement with our work. A clinical trial by Khayat et al., studying the anticonvulsant effects of n-3 PUFAs on 20 patients with intractable seizure was published in 2010 and revealed a negative correlation between DHA and EPA plasma concentration and seizure frequency, duration and severity.[4] This is similar to our successful results only regarding attacks frequency. In addition to that, the results of a recent randomized placebo-controlled trial, of low-dose and high-dose fish oil versus placebo (corn oil, linoleic acid) in 24 participants with drug-resistant epilepsy, by DeGiorgio et al., indicate that low-dose fish oil may reduce seizures and improve the health of people with epilepsy.[5] The results of our studies are stronger than those of the two recent studies mentioned, and this may be due to the low intake of food containing n-3 PUFA in Egypt, which was deduced in a specific nutrition survey done in 2010 by the department of epidemiology in Harvard School of Public Health. This survey showed that North Africa/Middle East region, including Egypt, is considered to have very low mean consumption of sea food Omega 3 fats (<100 mg per day) and low mean consumption of plants Omega 3 food fats (<600 mg per day).[16]
Conclusion
There is an obvious decrease in the number of epileptic attacks per month in the children with medically resistant epilepsy taking fish oil containing long chain n-3 PUFAs (EPA + DHA), which agrees with the theories suggesting that n-3 PUFAs elevate the seizure threshold in epileptic patients and may help in achieving seizure control.
Financial support and sponsorship
This project has been funded with support from Alexandria University, Faculty of Medicine.
Conflicts of interest
There are no conflicts of interest
Acknowledgement
Special thanks for Dr. Shehata Farag Shehata Assistant Lecturer of Biostatistics In High Institute of Public Health, Alexandria University, for his precious help in the statistical studies.
References
- 1.International League Against Epilepsy, World Health Organization, International Bureau for Epilepsy. Epilepsy, a manual for medical and clinical officers in Africa. Revised edition. Geneva: Epicadec; 2002. p. 124. [Google Scholar]
- 2.Kane P, Cartaxo AL. Seisures. In: Kohlstadt I, editor. Food and Nutrition in Disease Management. New York: Taylor and Francis group; 2009. pp. 394–412. [Google Scholar]
- 3.Schlanger S, Shinitzky M, Yam D. Diet Rich with Omega-3 fatty acids alleviates convulsion symptoms in epilepsy patients. Epilepsia. 2002;43:103–4. doi: 10.1046/j.1528-1157.2002.13601.x. [DOI] [PubMed] [Google Scholar]
- 4.Al Khayat HA, Awadalla MM, Al Wakad A, Marzook ZA. Polyunsaturated fatty acids in children with idiopathic intractable epilepsy: Serum levels and therapeutic response. J Pediatr Neurol. 2010;8:175–85. [Google Scholar]
- 5.DeGiorgio CM, Miller PR, Harper R, Gornbein J, Schrader L, Soss J, et al. Fish oil (n-3 fatty acids) in drug resistant epilepsy: A randomised placebo-controlled crossover study. J Neurol Neurosurg Psychiatry. 2015;86:65–70. doi: 10.1136/jnnp-2014-307749. [DOI] [PubMed] [Google Scholar]
- 6.Luchtman DW, Song C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: Findings from animal and clinical studies. Neuropharmacology. 2013;64:550–65. doi: 10.1016/j.neuropharm.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Frisardi V, Panza F, Seripa D, Farooqui T, Farooqui AA. Glycerophospholipids and glycerophospholipid-derived lipid mediators: A complex meshwork in Alzheimer's disease pathology. Prog Lipid Res. 2011;50:313–30. doi: 10.1016/j.plipres.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Taha AY, Burnham WM, Auvin S. Polyunsaturated fatty acids and epilepsy. Epilepsia. 2010;51:1348–58. doi: 10.1111/j.1528-1167.2010.02654.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen CT, Liu Z, Ouellet M, Calon F, Bazinet RP. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: An in situ study. Prostaglandins Leukot Essent Fatty Acids. 2009;80:157–63. doi: 10.1016/j.plefa.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Kidd PM. Omega-3 DHA and EPA for cognition, behavior, and mood: Clinical findings and structural-functional synergies with cell membrane phospholipids. Altern Med Rev. 2007;12:207–27. [PubMed] [Google Scholar]
- 11.Trépanier MO. The anticonvulsant effects of docosahexaenoic acid in rodents. [dissertation] Toronto: University of Toronto: Library and Archives Canada Published Heritage Branch; 2011. p. 157. [Google Scholar]
- 12.Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev. 2005;45:581–97. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- 13.Krauss GL, Sperling MR. Treating patients with medically resistant epilepsy. Neurol Clin Pract. 2011;1:14–23. doi: 10.1212/CPJ.0b013e31823d07d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Donoghue MF, Duncan JS, Sander JW. The National Hospital Seizure Severity Scale: A further development of the Chalfont Seizure Severity Scale. Epilepsia. 1996;37:563–71. doi: 10.1111/j.1528-1157.1996.tb00610.x. [DOI] [PubMed] [Google Scholar]
- 15.Yuen AW, Sander JW, Fluegel D, Patsalos PN, Bell GS, Johnson T, et al. Omega-3 fatty acid supplementation in patients with chronic epilepsy: A randomized trial. Epilepsy Behav. 2005;7:253–8. doi: 10.1016/j.yebeh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Micha R, Khatibzadeh S, Shi P, Fahimi S, Li S. Global, Regional, and National consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. Br Med J. 2014;348:1–20. doi: 10.1136/bmj.g2272. [DOI] [PMC free article] [PubMed] [Google Scholar]


