Abstract
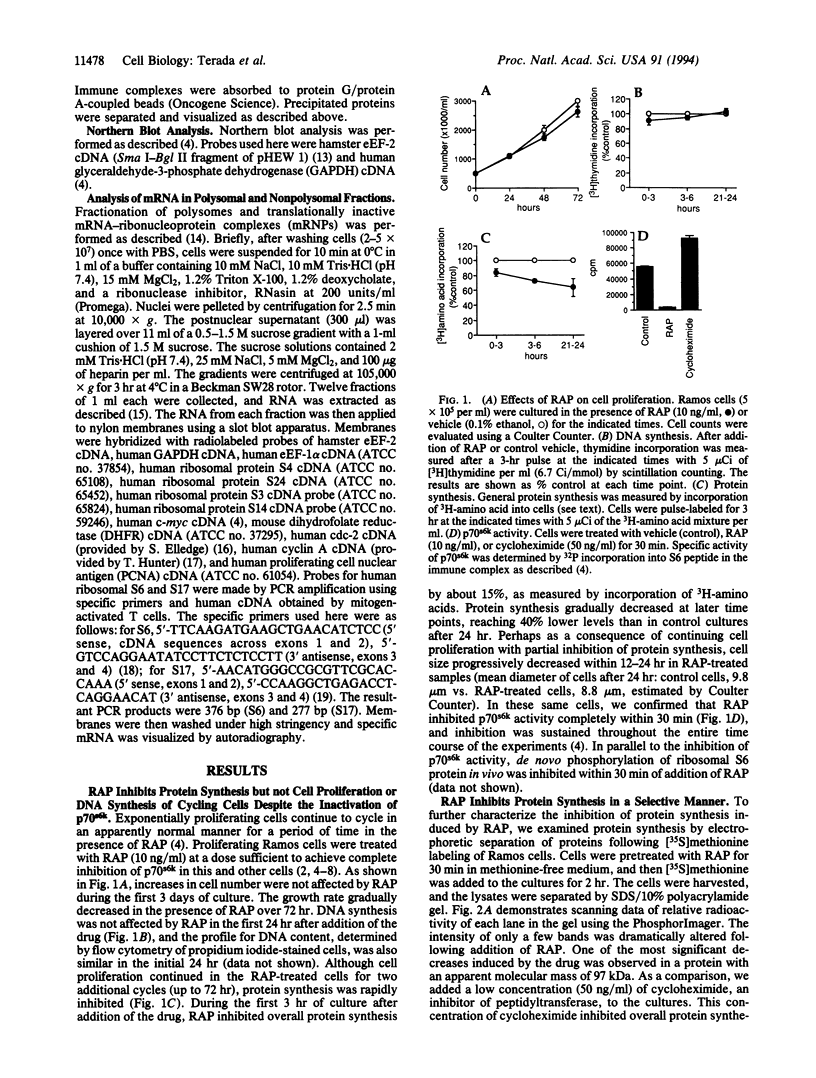
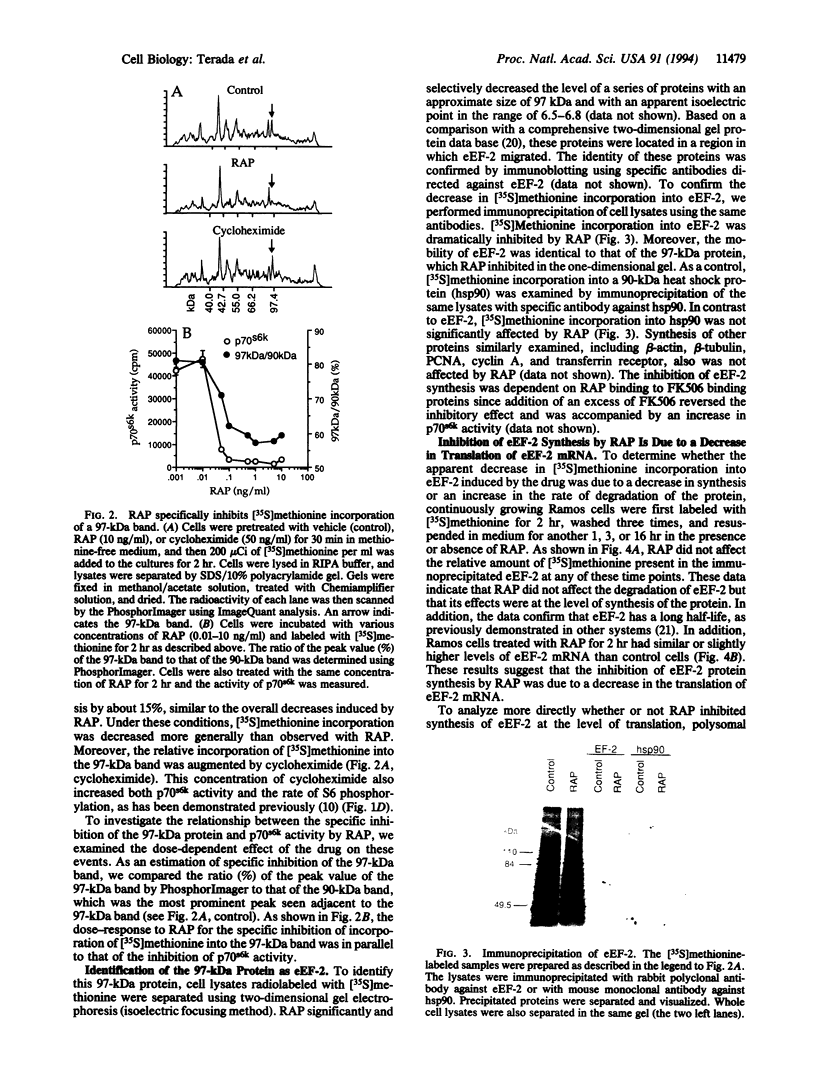
The immunosuppressant rapamycin (RAP) has been demonstrated to specifically inhibit the activity of p70 S6 kinase (p70s6k) and subsequent phosphorylation of ribosomal S6 protein in mammalian cells. Addition of RAP to proliferating lymphoid cells resulted in inhibition of protein synthesis before any changes in the rate of cell proliferation. When the cellular composition of proteins was examined by gel electrophoresis, RAP dramatically inhibited synthesis of selective proteins, particularly elongation factor 2 (eEF-2). The inhibition of eEF-2 synthesis by RAP was at the translational level. Further, RAP inhibited the polysomal association of mRNAs encoding not only eEF-2 but also elongation factor 1-alpha and ribosomal proteins without affecting mRNA translation of any of a number of nonribosomal proteins. Since levels of activity of p70s6k are correlated with the rate of biosynthesis of eEF-2, p70s6k might be involved in coordinate translational regulation of ribosomal protein mRNAs in higher eukaryotes, which have a conserved sequence at their 5' end. Specific inhibition of ribosomal protein synthesis likely explains the differential antiproliferative effect of RAP on proliferating and mitogen-activated quiescent cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal M. G., Bowman L. H. Transcriptional and translational regulation of ribosomal protein formation during mouse myoblast differentiation. J Biol Chem. 1987 Apr 5;262(10):4868–4875. [PubMed] [Google Scholar]
- Ballou L. M., Luther H., Thomas G. MAP2 kinase and 70K S6 kinase lie on distinct signalling pathways. Nature. 1991 Jan 24;349(6307):348–350. doi: 10.1038/349348a0. [DOI] [PubMed] [Google Scholar]
- Blenis J., Chung J., Erikson E., Alcorta D. A., Erikson R. L. Distinct mechanisms for the activation of the RSK kinases/MAP2 kinase/pp90rsk and pp70-S6 kinase signaling systems are indicated by inhibition of protein synthesis. Cell Growth Differ. 1991 Jun;2(6):279–285. [PubMed] [Google Scholar]
- Calvo V., Crews C. M., Vik T. A., Bierer B. E. Interleukin 2 stimulation of p70 S6 kinase activity is inhibited by the immunosuppressant rapamycin. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7571–7575. doi: 10.1073/pnas.89.16.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Madsen P., Rasmussen H. H., Leffers H., Honoré B., Gesser B., Dejgaard K., Olsen E., Magnusson N., Kiil J. A comprehensive two-dimensional gel protein database of noncultured unfractionated normal human epidermal keratinocytes: towards an integrated approach to the study of cell proliferation, differentiation and skin diseases. Electrophoresis. 1991 Nov;12(11):802–872. doi: 10.1002/elps.1150121105. [DOI] [PubMed] [Google Scholar]
- Chen I. T., Roufa D. J. The transcriptionally active human ribosomal protein S17 gene. Gene. 1988 Oct 15;70(1):107–116. doi: 10.1016/0378-1119(88)90109-6. [DOI] [PubMed] [Google Scholar]
- Chung J., Kuo C. J., Crabtree G. R., Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992 Jun 26;69(7):1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Spottswood M. R. A new human p34 protein kinase, CDK2, identified by complementation of a cdc28 mutation in Saccharomyces cerevisiae, is a homolog of Xenopus Eg1. EMBO J. 1991 Sep;10(9):2653–2659. doi: 10.1002/j.1460-2075.1991.tb07808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer P. K., Meyuhas O., Perry R. P., Johnson L. F. Regulation of ribosomal protein mRNA content and translation in growth-stimulated mouse fibroblasts. Mol Cell Biol. 1982 Jun;2(6):685–693. doi: 10.1128/mcb.2.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond M. L., Bowman L. H. Insulin stimulates the translation of ribosomal proteins and the transcription of rDNA in mouse myoblasts. J Biol Chem. 1988 Nov 25;263(33):17785–17791. [PubMed] [Google Scholar]
- Henriksen O., Smulson M. E. Function and properties of aminoacyl transferase II. II. Subcellular distribution during asynchronous and synchronous growth. Arch Biochem Biophys. 1972 May;150(1):175–182. doi: 10.1016/0003-9861(72)90024-0. [DOI] [PubMed] [Google Scholar]
- Kaspar R. L., Kakegawa T., Cranston H., Morris D. R., White M. W. A regulatory cis element and a specific binding factor involved in the mitogenic control of murine ribosomal protein L32 translation. J Biol Chem. 1992 Jan 5;267(1):508–514. [PubMed] [Google Scholar]
- Kohno K., Uchida T., Ohkubo H., Nakanishi S., Nakanishi T., Fukui T., Ohtsuka E., Ikehara M., Okada Y. Amino acid sequence of mammalian elongation factor 2 deduced from the cDNA sequence: homology with GTP-binding proteins. Proc Natl Acad Sci U S A. 1986 Jul;83(14):4978–4982. doi: 10.1073/pnas.83.14.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. J., Chung J., Fiorentino D. F., Flanagan W. M., Blenis J., Crabtree G. R. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature. 1992 Jul 2;358(6381):70–73. doi: 10.1038/358070a0. [DOI] [PubMed] [Google Scholar]
- Lane H. A., Fernandez A., Lamb N. J., Thomas G. p70s6k function is essential for G1 progression. Nature. 1993 May 13;363(6425):170–172. doi: 10.1038/363170a0. [DOI] [PubMed] [Google Scholar]
- Levenson R. M., Nairn A. C., Blackshear P. J. Insulin rapidly induces the biosynthesis of elongation factor 2. J Biol Chem. 1989 Jul 15;264(20):11904–11911. [PubMed] [Google Scholar]
- Levy S., Avni D., Hariharan N., Perry R. P., Meyuhas O. Oligopyrimidine tract at the 5' end of mammalian ribosomal protein mRNAs is required for their translational control. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3319–3323. doi: 10.1073/pnas.88.8.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreni F., Amaldi F. Translational regulation of ribosomal protein synthesis in Xenopus cultured cells: mRNA relocation between polysomes and RNP during nutritional shifts. Eur J Biochem. 1992 May 1;205(3):1027–1032. doi: 10.1111/j.1432-1033.1992.tb16870.x. [DOI] [PubMed] [Google Scholar]
- Morris R. E. Rapamycin: FK506's fraternal twin or distant cousin? Immunol Today. 1991 May;12(5):137–140. doi: 10.1016/S0167-5699(05)80040-4. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Bhagat B., Palfrey H. C. Identification of calmodulin-dependent protein kinase III and its major Mr 100,000 substrate in mammalian tissues. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7939–7943. doi: 10.1073/pnas.82.23.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T., Kohno K., Ishiura M., Ohashi H., Uchida T. Complete nucleotide sequence and characterization of the 5'-flanking region of mammalian elongation factor 2 gene. J Biol Chem. 1988 May 5;263(13):6384–6391. [PubMed] [Google Scholar]
- Pata I., Hoth S., Kruppa J., Metspalu A. The human ribosomal protein S6 gene: isolation, primary structure and location in chromosome 9. Gene. 1992 Nov 16;121(2):387–392. doi: 10.1016/0378-1119(92)90149-j. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Price D. J., Grove J. R., Calvo V., Avruch J., Bierer B. E. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992 Aug 14;257(5072):973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- Proud C. G. Protein phosphorylation in translational control. Curr Top Cell Regul. 1992;32:243–369. doi: 10.1016/b978-0-12-152832-4.50008-2. [DOI] [PubMed] [Google Scholar]
- Rao T. R., Slobin L. I. Regulation of the utilization of mRNA for eucaryotic elongation factor Tu in Friend erythroleukemia cells. Mol Cell Biol. 1987 Feb;7(2):687–697. doi: 10.1128/mcb.7.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobin L. I., Rao M. N. Translational repression of EF-1 alpha mRNA in vitro. Eur J Biochem. 1993 May 1;213(3):919–926. doi: 10.1111/j.1432-1033.1993.tb17836.x. [DOI] [PubMed] [Google Scholar]
- Terada N., Franklin R. A., Lucas J. J., Blenis J., Gelfand E. W. Failure of rapamycin to block proliferation once resting cells have entered the cell cycle despite inactivation of p70 S6 kinase. J Biol Chem. 1993 Jun 5;268(16):12062–12068. [PubMed] [Google Scholar]
- Terada N., Lucas J. J., Szepesi A., Franklin R. A., Domenico J., Gelfand E. W. Rapamycin blocks cell cycle progression of activated T cells prior to events characteristic of the middle to late G1 phase of the cycle. J Cell Physiol. 1993 Jan;154(1):7–15. doi: 10.1002/jcp.1041540103. [DOI] [PubMed] [Google Scholar]
- Terada N., Lucas J. J., Szepesi A., Franklin R. A., Takase K., Gelfand E. W. Rapamycin inhibits the phosphorylation of p70 S6 kinase in IL-2 and mitogen-activated human T cells. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1315–1321. doi: 10.1016/s0006-291x(05)81549-9. [DOI] [PubMed] [Google Scholar]
- Uetsuki T., Naito A., Nagata S., Kaziro Y. Isolation and characterization of the human chromosomal gene for polypeptide chain elongation factor-1 alpha. J Biol Chem. 1989 Apr 5;264(10):5791–5798. [PubMed] [Google Scholar]