Abstract
The burden of falciparum malaria remains as great as ever, and, as has probably always been the case, it is carried mainly by tropical Africa. Of the various means available for the control of malaria, the use of effective drugs remains the most important and is likely to remain so for a considerable time to come. Unfortunately, the extensive development of resistance by the parasite threatens the utility of most of the affordable classes of drug: the development of novel antimalarials has never been more urgently needed. Any attempt to understand the vast complexities of falciparum malaria in Africa requires an ability to think “from molecule to policy.” In consequence, the review ambitiously tries to examine the current pharmacopeia, the process by which new drugs are developed and the ways in which drugs are actually used, in both the formal and informal health sectors. The informal sector is particularly important in Africa, where around half of all antimalarial treatments are bought from informal outlets and taken at home without supervision by health care professionals: the potential impact of adherence on clinical outcome is discussed. Given that the full costs are carried by the patient in a large proportion of cases, the importance of drug affordability is explored. The review also discusses the splicing of new drugs into national policy. The various parameters that feed into deliberations on changes in drug policy are discussed.
INTRODUCTION: THE BURDEN OF FALCIPARUM MALARIA
Indigenous malaria has been recorded as far north as 64°N latitude and as far south as 32°S latitude (Cordoba in Argentina). Within these limits, there are large areas free of malaria, which is essentially a focal disease, and the extent and intensity of transmission of malaria depend greatly on local environmental and other conditions (141, 162).
Over 90% of the world's public health burden from malaria is borne by populations in Africa. The most striking features of Plasmodium falciparum under transmission conditions common to most of Africa is that almost everyone develops a new infection every year and everyone experiences a disease event at some stage in their lives. Falciparum malaria is usually a clinical febrile event, most commonly in young children. Death is comparatively rare as a direct result of infection (but is nonetheless a major health problem) — perhaps only 0.25% of infections result in death (53). Exposure to infection from birth onward results in the rapid development of an immune response to the fatal consequences of infection. However, public health cost to communities is still high. The available evidence suggests that P. falciparum infection is probably directly responsible for approximately one million deaths on the African continent each year (138). Over 75% of these deaths are among young children, representing one of the most significant infectious challenges a child is likely to encounter during infancy and early childhood. From detailed demographic studies in Africa, approximately 25% of the deaths during the first 5 years of life have been directly attributed to malaria (140).
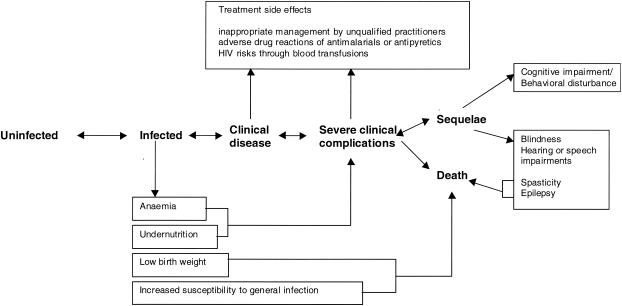
Focusing on the direct consequences of clinical infection and subsequent death provides only part of the overall public health equation. There are morbid and fatal consequences allied to each step of the infection and disease process (141) (Fig. 1). Chronic subclinical infections render an individual anemic (88). It has also been argued that subclinical infections affect the severity and outcome of other infectious diseases (89). Pregnant mothers exposed to malaria infection may suffer severe anemia, and the child, if he or she survives, is often born with low birth weight. About 25% of all neonatal mortality may be related to malaria infection during pregnancy in some parts of Africa (49). Patients who survive the severe pathological consequences of infection may be left with debilitating sequelae such as epilepsy, spasticity, blindness or more subtle behavioral or cognitive impairments (17, 60, 156). Drug interventions may also carry a risk of adverse events, the importance of which is discussed below. The overall public health impact of P. falciparum infection in Africa probably extends well beyond the direct effect of a new infection on a single disease and mortality event.
FIG. 1.
Direct and indirect health consequences of P. falciparum malaria infection. Reprinted from reference 142 with permission.
PROSPECTS FOR CONTROL: DRUGS IN CONTEXT
Before the recent ‘Roll Back Malaria’ (RBM) initiative (96; Roll Back Malaria, World Health Organization, 2002 [http://mosquito.who.int/cgi-bin/rbm/rbmportal/custom/home/mal/login.jsp]), the most significant period in the history of malaria control in Africa was the outcome of the conference held by the World Health Organization (WHO) in Kampala, Uganda, in 1950 (30). Supporters of global eradication were adamant that Africa could achieve the successes achieved by coordinated mass action (particularly by use of DDT) in South America, India, and some parts of Africa such as Freetown, Sierra Leone. The 20 years following the Kampala conference witnessed limited success of several small projects in Africa, none of which was sustained. For whatever reason, the guiding principle that the parasite's cycle between human and vector could be forever broken was recognized as impracticable for most of the African continent. The agenda was redefined from parasite eradication to disease control. At this juncture, despite our detailed understanding of the mechanics of infection between primary and secondary hosts, the understanding of the broad public health implications of infection and disease in Africa had been largely neglected. This may have contributed to the complex reasons for the failure of malaria control in Africa, by hampering the construction of an epidemiological framework that would allow the effective targeting of cost-effective strategies, particularly the potential for drug intervention (140).
Africa includes the majority of the world's poorest countries, which are unable to finance basic services and sustainable infrastructure. In global terms malaria, poverty, and development are intrinsically linked, and sub-Saharan Africa epitomizes this vicious cycle (126). During the 1990s, malaria control returned to the international public health agenda with the announcement of the global RBM initiative. It is widely accepted that the single most important contribution to reducing global malaria mortality will be the development of a safe, efficacious, and affordable vaccine. Even with increased investment in research, however, it seems unlikely that a vaccine will be available for widespread use for at least 15 to 20 years. Meanwhile the cornerstones of most malaria control in Africa today include (i) the provision of effective, prompt treatment for febrile events as close to the home as possible; (ii) the use of insecticide-treated bed nets (ITN); (iii) the prevention of malaria infection during pregnancy; and (iv) the early detection and containment of epidemics. These tools are being promoted within a changing delivery environment. We are moving away from viewing malaria as a “vertical” disease program within ministries of health toward managing malaria control as part of a general improvement in health service delivery, improved general clinical management, and decentralized (subnational) ownership or priority setting and resourcing.
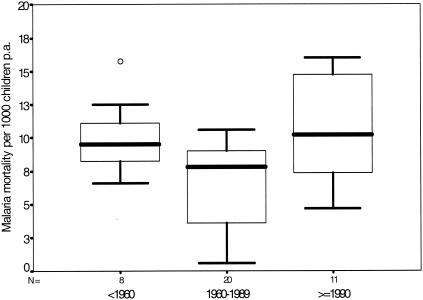
Given the extent of the public health and economic burden posed by malaria in Africa, efforts to galvanize international and governmental support for RBM in Africa have been welcomed by all African heads of state. Despite the intention of the RBM movement to reduce disease burden and mortality, there are several reasons to be cautious. Drugs, such as chloroquine, have been the mainstay of malaria case management in Africa for over 50 years. Since the late 1980s, sensitivity to chloroquine has rapidly declined across much of Eastern and Southern Africa, rendering the drug useless in many countries. Options other than chloroquine for resource-constrained African governments (which are experiencing a declining per capita expenditure on essential drugs) are few. Areas where malaria had previously been contained, such as in Southern Africa and the East African Highlands, are experiencing a resurgence of localized epidemics. Despite the overwhelming evidence of the benefits of insecticide-treated nets and “intermittent presumptive treatment” of pregnant women (see below), service delivery systems are weak and access by populations to these interventions is poor. At a time when conflicts, displaced populations, human immunodeficiency virus HIV, and global economics threaten the fragile livelihoods of most rural populations, there is growing evidence that since the mid-1980s, the burden from malaria has been increasing (53, 130, 155, 141). A review of cause-specific pediatric mortality data since the early part of the last century highlights the fact that despite a continuous decline in non-malaria mortality since before the 1960s, there has been a rise in malaria mortality since the early 1980s (141) (Fig. 2). The most parsimonious explanation for this change in mortality patterns is the declining efficacy of antimalarial drugs used to manage the disease.
FIG. 2.
Box plot showing median (central lines), 25 and 75% quartile ranges around the median (box width), and upper and lower limits (T) of malaria-specific mortality estimates per 1,000 children aged 0 to 4 years per annum recorded pre-1960, from 1960 to 1989, and from 1990 to 1995. Reprinted from reference 141 with permission.
Personal protection with ITNs or the prevention of infection through other methods of vector control will have important implications for the frequency of new clinical episodes requiring effective drug management (91). However, it is notable that even with efficacious preventative strategies, the numbers of clinical disease events in Africa will remain substantial and drugs are likely to remain one of the main tools for limiting the mortality, morbidity, and economic impact of falciparum malaria in Africa. The big challenges can be summarized in a few sentences.
(i) Chloroquine-resistant P. falciparum now causes most of the infections treated in sub-Saharan Africa, and many nations are changing first-line treatment to sulfadoxine-pyrimethamine (SP).
(ii) Resistance to SP is growing increasingly common in east Africa and is expected to spread rapidly.
(iii) Development of antimalarial drugs has been slow, mainly for economic reasons: there is less return on the investment needed to develop an antimalarial drug than is the case for a new antibiotic.
(iv) As a result, Africa is faced with a serious problem: inexpensive, safe, effective and practicable drugs are needed for treatment of uncomplicated malaria, and there are few choices.
In this article, we set out to explore the practical limitations that face public health specialists (primarily in Africa) when planning their strategy for the drug treatment of falciparum malaria. We start by reviewing the pharmacology of the main drug groups, move on to examine the promise (and limitations) of the novel technologies being used to identify lead compounds, and then briefly describe the stages of drug development. In the next section we discuss the difficulties of clinical diagnosis and how communities currently manage the disease outside the formal health sector. We go on to examine the use of drugs in the formal sector, for prophylaxis, intermittent presumptive treatment, and management of uncomplicated and severe disease. We then highlight the need for post-marketing surveillance and close the article by reviewing the decision-making process in national malaria control programs.
PHARMACOLOGY AND THERAPEUTICS OF ANTIMALARIALS
Pharmacological Basis of Antimalarial Therapeutics
As with any group of drugs, the pharmacological properties of antimalarials form the basis of their therapeutic use. Mechanisms of action, for example, have a bearing on such matters as the stage of the parasite life cycle which is most strongly affected and whether cross-resistance can be anticipated between different compounds. Pharmacokinetic properties, as another example, often determine dose interval, duration of treatment, and high-risk patient groups.
Mechanisms of action.
It would be a mistake to imagine that some sort of “strategic overview” has been taken whereby aspects of plasmodial biochemistry have been judged key targets for drug development. Drug discovery in malaria has, by and large, been serendipitous: mechanisms of action are still understood incompletely, if at all, and have been studied long after the drug has been in long-hallowed use. A few mechanisms of action are worthy of comment.
(i) Hemoglobin digestion in the food vacuole.
The 4-aminoquinolines, described below, interfere with the essential process of hemoglobin digestion. However, newer food vacuole processes, including the plasmepsin aspartic proteinases and falcipain cysteine proteinase, have been identified as possible drug targets. One would expect 4-aminoquinolines to antagonize (and themselves be antagonized by) these proteinases, because it is the digestion of hemoglobin that releases heme, the toxic moiety which, after complexing with 4-aminoquinolines, damages the parasite.
(ii) Folate pathway.
SP and the newer combination chlorproguanil-dapsone (Lapdap) are competitive inhibitors of key enzymes in the folate pathway. However, point mutations are easily acquired in the genes that encode these enzymes, making resistance to the antifolates develop quite rapidly. Furthermore, one would predict that high folate concentrations would oppose the effects of this drug group; this is the case in vitro, and there is some evidence of in vivo effects too. It is possible to antagonize folate uptake by the parasite, and the utility of this approach is being explored.
(iii) Alkylating agents.
The artemisinin drugs generate free radicals that rapidly alkylate parasite membranes (particularly near the food vacuole); this process is probably catalyzed by haemozoin (malaria pigment), and this may explain the large therapeutic index of the group. The parasite seems to find it difficult to develop resistance to this particular mechanism, and the artemisinins affect many stages in the life cycle.
(iv) Mitochondrial function.
Atovaquone works by inhibition of cytochrome c reductase, which may be the basis of its synergy with the prodrug proguanil. Unfortunately, the parasite readily develops resistance to atovaquone. New potential drug targets, including dihydroorotate dihydrogenase, are located within the mitochondrion.
(v) The apicoplast.
The antibiotics—including tetracyclines—work in the apicoplast, by interfering with protein translation.
Pharmacokinetics.
Clinical response to a drug can sometimes be directly measured with accuracy, like the effect of insulin on blood sugar or warfarin on clotting, and this measurement alone can be enough to predict how much drug is needed, how often doses should be repeated, and when toxicity is likely to occur. Such direct measurements are often impossible, and an indirect approach, using drug concentrations and their rates of change, is used to predict the magnitude and duration of action of a drug. Measurement of antimalarial drug concentrations and tailoring of doses to the individual patient are practically impossible in an African setting. Fortunately, for many antimalarial drugs (e.g., the artemisinins, antifolates, and antibiotics) the “gap” between therapeutic and toxic levels (the therapeutic index) is so wide that no such monitoring is required. Equally fortunately, for drugs with narrow therapeutic indices (e.g., quinine and the 4-aminoquinolines), the relationship between dose and steady-state concentrations is reasonably reliable, so that toxicity can usually be avoided by getting the dose right. Unfortunately, some disease states perturb drug disposition, leading to toxicity. For example, renal impairment reduces chloroquine clearance and may result in concentration-dependent toxicity.
Adverse drug reactions.
All drugs cause toxicity. Type A adverse effects (AEs) result from excessive responses to a drug; these AEs are predictable from the known effects of the drug and are dose or concentration related. In contrast, type B AEs are not predictable from the known effects of the drug; there may be an immunological basis to the AE, and there is often no clear relationship with the dose or concentration of drug. Clinical development of a new drug usually involves its administration to fewer than 2,000 people: this will show up many common AEs, but life-threatening AEs (most commonly of type B) may be rare, perhaps with an incidence of only 1 in 10,000 or less, and so are unlikely to be seen in these trials. Furthermore, certain patient groups are at particular risk of severe AEs (including the elderly, the very young, glucose-6-phosphate dehydrogenase (G6PD)-deficient people and HIV-positive people) and these may not be well represented in submissions to Regulatory authorities. The true prevalence of AEs may become apparent only after the new drug is launched. The difficulty of collecting postmarketing safety data for antimalarial drugs cannot be underestimated.
A Swift Comparative Overview of the Main Drug Classes
The main groups of drugs used for falciparum malaria are summarized in the brief descriptions that follow. However, the most important point here is that there are simply too few drugs in the armamentarium (particularly for uncomplicated malaria), and even fewer can be envisaged in practical use (for reasons of cost or complexity of dosing). Therefore, before the reader embarks on the sections describing the individual drugs, a swift overview is called for. The reader will notice, and may disapprove of, the repeated emphasis on drug cost. It could be argued that the cost of a medicine should be unimportant if it is required to save lives. This position is, of course, unassailable, and yet the harsh reality is that people are able to use only what they can afford unless drug prices are subsidised.
(i) Chloroquine was the first-line drug throughout Africa until a decade ago, and it remains in extensive use. Clinically evident resistance to chloroquine is now present all across Africa, and countries are tackling the question of which drug to replace it with.
(ii) Amodiaquine is a close congener of chloroquine, and there is cross-resistance between the two. However, a larger proportion of patients given amodiaquine will experience a cure than is the case with chloroquine. Furthermore, amodiaquine has advantages over the artemisinin drugs in combination therapy. These two properties are reawakening interest in this drug. However, people are concerned about the AE profile of amodiaquine (which was documented when the drug was being used for prophylaxis).
(iii) SP is now the first-line drug used throughout much of the continent, but resistance is developing quickly.
(iv) Chlorproguanil-dapsone (Lapdap). Chlorproguanil-dapsone works in the same way as SP but is more potent and more rapidly eliminated. There is evidence that chlorproguanil-dapsone achieves clinical cure where SP has failed and that is has a lower selection pressure for resistance than SP. Chlorproguanil-dapsone became available for clinical use in late 2003, and, as with any new drug, a period of postmarketing surveillance is needed to determine its possible role.
(v) Quinine is the drug of first choice for severe malaria syndromes, and quinine resistance is unusual in Africa. It can be used orally for uncomplicated disease, but symptomatic toxicity and a complicated dosing regimen make it impractical.
(vi) The artemisinins (including artesunate, artemether, and dihydroartemisinin) are potent, well-tolerated, and rapidly eliminated drugs. If used on their own, they must be given for at least 5 days to avoid recrudescence. They are appropriate choices for severe malaria (parenteral formulations are available), but their main role in uncomplicated malaria is in combination therapy, which is reviewed below. Artemether-lumefantrine (Coartem) is the only fixed-ratio combination therapy (developed to international standards of quality) currently available. There are concerns about the complicated dose regimen and relatively high cost of Coartem.
(vii) Mefloquine is an expensive drug and hence probably not a practicable option. It is prone to cause nausea and vomiting, which presents practical problems. Furthermore, it is eliminated very slowly, giving rise to concern that it exerts a high selection pressure for resistance.
(viii) Atovaquone-proguanil is a very expensive drug and hence not a practical option.
(ix) Halofantrine is also expensive and is associated with a reasonably high prevalence of cardiac adverse effects: it is not currently considered to be a practical option.
(x) Antibiotics, such as the tetracyclines and macrolides, are sometimes used in combination with other drugs, but they are inadequate for treatment of falciparum malaria if used alone.
Several new drug combinations are likely to become available for treatment of uncomplicated malaria in the next few years. Pyronaridine-artesunate, chlorproguanil-dapsone-artesunate, and dihdroartemisinin-piperaquine are all at some point in clinical development. All are reliant on the concept of artemisinin combination therapy, and it is unclear what advantages each will have over the others.
The 4-Aminoquinolines
Chloroquine (31) is probably still the most widely used antimalarial drug, but the extensive spread of resistance has severely limited its usefulness. It remains effective for P. ovale, P. malariae, and most cases of P. vivax infection worldwide; in restricted areas, such as parts of Central America, Haiti, and parts of the Middle East, P. falciparum remains chloroquine sensitive too. Against sensitive strains, chloroquine is rapidly effective, and patients experience symptomatic improvement speedily. If the patient is “semi-immune,” the consequences of treatment failure need not be a major clinical problem. However, in children and nonimmune travelers, such treatment failure can prove fatal. Many chloroquine-resistant strains of P. falciparum remain sensitive to its congener amodiaquine, and the utility of this drug (alone or in combination with other antimalarial drugs) is being studied in parts of Africa.
Mode of action.
Figuring out the mechanism of chloroquine action has proven to be an elusive business (14, 15). The selective accumulation of chloroquine within the parasitized red cell is generally accepted to be central, and it has been argued that the driving force for accumulation is a pH gradient into the acidic food vacuole. However, recent work provides compelling evidence that the primary driving force for drug accumulation is binding to ferriproptoporphyrin IX (heme), a by-product of hemoglobin degradation. Furthermore, the formation of chloroquine-heme complexes is also essential for drug activity. The accepted view of chloroquine action is that the drug binds to heme, preventing the detoxification of heme by crystallization into malaria pigment (or hemozoin). The chloroquine-heme complex, which retains its cytotoxic potential, thus accumulates to a level capable of killing the parasite. The finer details of this process still await elucidation.
Amodiaquine, as an analogue of chloroquine, is thought to have the same mechanism of action, and experimental data demonstrate the binding of the drug to heme and the dependence of activity on hemoglobin degradation. Amodiaquine is more potent than chloroquine in vitro, presumably reflecting increased potential for complexation with heme. It is important to note that it is the desethyl metabolite of amodiaquine which is the principal antimalarial entity in vivo following efficient hepatic metabolism.
Mechanisms of resistance.
Phenotypically, chloroquine resistance is characterized by reduced cellular accumulation of the drug (163, 164). This phenotype can be partially reversed by agents such as verapamil, so-called resistance-reversing agents (this is also the case in multidrug-resistant cancer cells). With this in mind, it was proposed that a drug efflux mechanism, analogous to that seen in multidrug-resistant cancer cells, must be responsible for chloroquine resistance; indeed, studies identified pfmdr1, a malarial homologue of human mdr1. Early studies suggested that amplification of pfmdr1 was responsible for resistance, but evidence soon surfaced to refute this simple explanation. Subsequent studies suggested an association between mutations in pfmdr1 and resistance. Again, these data are controversial. However, a definitive allelic exchange study has confirmed that pfmdr1 can influence chloroquine susceptibility in P. falciparum malaria. It was demonstrated that replacement of mutant alleles of pfmdr1 from a chloroquine-resistant parasite with wild type sequence produced a small but significant improvement in drug susceptibility and a reduction in the magnitude of the verapamil effect in the transfectants. However, it was not possible to confirm this observation with the reverse strategy, i.e., replacing wild-type sequence with mutant pfmdr1. The conclusion from these studies was that pfmdr1 had a modulatory effect but was not the major contributor to chloroquine resistance. An alternative strategy aimed at elucidating the molecular basis of chloroquine resistance was based on analysis of the progeny of a genetic cross between a chloroquine-resistant and a chloroquine-sensitive parasite clone. These studies have identified a gene; pfcrt, which encodes a 10-transmembrane-domain protein, located to the parasite's food vacuole, whose sequence appears to be predictive of chloroquine susceptibility. In comparison to chloroquine-sensitive parasites, resistant parasites carry a number of mutations in pfcrt, of which the mutation leading to the K76T amino acid mutation is predictive of the verapamil-responsive resistance phenotype. Observation with field isolates from a range of geographical settings and transfection studies provide compelling support for the claim that pfcrt is the major chloroquine resistance gene in P. falciparum. The real function of pfcrt is not clear: certainly, recent studies suggesting a role in pH modulation are fundamentally flawed. There is evidence that the protein acts as a transporter capable of either directly or indirectly allowing chloroquine movement out of the food vacuole. Recently, a number of reports have suggested that the predictability of chloroquine resistance is increased if both pfcrt and pfmdr1 are taken into consideration. Interestingly in Malawi, which stopped using chloroquine in 1993, there is evidence that the prevalence of pfcrt has declined from 85% (in 1992) to 13% (in 2000) (77), raising the question whether chloroquine might be a useful, and inexpensive, component of combination therapy.
Amodiaquine has clinical utility even in areas of chloroquine resistance. However, this assertion is now being brought into question, especially in areas of high-level chloroquine resistance. In vitro studies clearly demonstrate cross-resistance between chloroquine and amodiaquine, and the extent of cross-resistance is even greater if the desethyl metabolite is considered (16). These data suggest a potential role for pfcrt in amodiaquine sensitivity, and this claim is supported by recent transfection studies (136).
Clinical pharmacokinetics.
Chloroquine is rapidly absorbed from the gut and from intramuscular or subcutaneous injections: indeed, dangerously high peak concentrations in plasma may be reached soon after an injection of 5 mg of base/kg, and this has been linked to fatalities (167). About half of the absorbed chloroquine is cleared unchanged by the kidneys, with the rest being biotransformed in the liver to desethyl- and bisdesethylchloroquine. Although clearance is reduced in patients with renal failure, it is not usually necessary to reduce the dose. The terminal elimination half-life is very long (1 to 2 months).
In contrast to chloroquine, amodiaquine is extensively converted to its equipotent metabolite desethylamodiaquine, which is responsible for most of the antimalarial activity: desethylamodiaquine achieves much higher concentrations than its parent drug (174). Another metabolite, amodiaquine-quinoneimine, plays an important role in toxic reactions.
Therapeutic use.
Oral chloroquine is used for the treatment and prophylaxis of vivax, ovale, and malariae malarias and for uncomplicated falciparum malaria in semi-immune people. Nonimmune people with-falciparum malaria should be treated with alternative drugs because of the risks of treatment failure. Severe falciparum malaria is still treated with parenteral chloroquine in some areas where chloroquine resistance is uncommon; this practice is not recommended if alternative drugs are available.
Because of the higher efficacy of amodiaquine in much of Africa (145), the role of this drug, either alone or in combination with SP or an artemisinin-class drug, is being reexamined. Children in high-transmission areas are usually treated for malaria several times annually, and there is concern to examine the immunogenicity and AE profile of amodiaquine under such operational use.
Adverse effects and drug interactions.
Chloroquine is generally well tolerated, but when concentrations in plasma exceed 250 μg/ml, unpleasant symptoms such as dizziness, headache, diplopia, and nausea may develop. In black-skinned races pruritus of the palms, soles, and scalp is frequent. Rare toxic effects include photoallergic dermatitis, aggravation of psoriasis, skin pigmentation, leukopenia, bleaching of the hair, and aplastic anaemia. Chloroquine can exacerbate epilepsy. In cases of deliberate overdose (ingestion of 20 mg of base/kg or more at one time), toxicity is manifest rapidly (sometimes within 30 min). The main problems are coma, convulsions, hypotension, respiratory paralysis, and cardiac arrest. Electrocardiogram (ECG) changes include sinus tachycardia, bradycardia, prolonged QT interval, ectopic beats, ventricular tachycardias, and asystole. Diazepam and epinephrine have roles in treating severe chloroquine poisoning (120). Continuous weekly chloroquine use (cumulative dose, >100 g) may cause an irreversible retinopathy; while such cumulative doses may be encountered in long-term antimalarial prophylaxis, retinopathy is more usually associated with the higher anti-inflammatory doses used in collagen vascular diseases.
Hepatitis and agranulocytosis were seen in patients taking amodiaquine for prophylaxis (56), and the drug is no longer recommended for this indication. Amodiaquine-quinoneimine can be generated from amodiaquine both spontaneously, when the drugs is in aqueous solution, and as a result of enzyme activity (175). The quinoneimine is highly reactive and haptenates proteins, generating antigen to which an immune response may be mounted. Repeated exposure to this antigen may be important in the generation of organ damage.
Antifolate Drugs and Combinations
The antifolates are nearly always used in fixed-ratio combinations. The most commonly used antifolate is SP (32), and this is now the first-line drug for uncomplicated falciparum malaria in many parts of Africa. Unfortunately, resistance to SP is widespread in Asia and South America, and this is now causing concern in high-transmission areas of Africa. Proguanil (33) has been widely used for malaria prophylaxis (its popularity has fallen because of a deteriorating clinical response), and its congener chlorproguanil has just been launched in fixed ratio with dapsone (Lapdap) for malaria treatment. Of the sulfa drugs, sulfadoxine, sulfalene, and dapsone are given for their synergistic effects with pyrimethamine or chlorproguanil.
Mode of action.
This large group of drugs interferes with DNA synthesis by depleting the pool of tetrahydrofolate, an important cofactor. There are three fundamental points to grasp.
(i) One structurally diverse group of molecules acts as competitive inhibitors of the enzyme dihydrofolate reductase (DHFR). This group includes pyrimethamine and the biguanides proguanil and chlorproguanil (both of which require biotransformation to the triazines cycloguanil and chlorcycloguanil for their action on DHFR).
(ii) Another group of molecules acts as competitive inhibitors of the enzyme dihydropteroate synthetase (DHPS), an earlier enzyme of the folate pathway and one which is lacked by mammals. The group includes the sulfonamides (principally sulfadoxine and sulfalene) and sulfones (exclusively dapsone).
(iii) The DHFR inhibitors have some therapeutic utility if used alone (which is usually the case for proguanil chemoprophylaxis), but this is not the case for the DHPS inhibitors. In the treatment of malaria, DHFR inhibitors and DHPS inhibitors are used in synergistic combinations. Fixed-ratio combinations such as SP and pyrimethamine-dapsone have been available for some time; chlorproguanil-dapsone became available at the end of 2003.
Mechanisms of resistance.
Pyrimethamine and sulfadoxine both have long elimination half-lives: this equates with a strong selective pressure for resistance (162) as new infections are exposed to eventual subinhibitory drug concentrations. Resistance to DHFR inhibitors results from specific mutations in the DHFR gene (dhfr). A mutation of Ser-108 to Asn-108, the first mutation to appear in the field, reduces sensitivity to pyrimethamine. Subsequent mutations of Asn-51 to Ile-51 and Cys-59 to Arg-59 enhance resistance, and the mutation of Ile-164 to Leu-164 provides high-level resistance (114). The dhfr-164 mutation has been found at extremely low frequency in isolates from Tanzania, but it is not clear to what extent this mutation is spreading. Mutations in the DHPS gene (dhps) correlate with in vitro sulfonamide chemosensitivity. Parasite lines exhibiting high-level resistance were found to carry either a double mutation in dhps, altering both Ser-436 and Ala-613, or a single mutation, affecting Ala-581. The combination of mutations in dhfr and dhps that are required to cause clinical failure of SP is the subject of much current research (76).
Clinical pharmacokinetics.
Pyrimethamine is well absorbed after oral or intramuscular administration. The elimination half-life in children with malaria averages 81 and 124 h after oral and intramuscular injection, respectively (177).
Proguanil and chlorproguanil, which can be given only orally, reach peak concentrations in plasma after 2 to 4 h and have short elimination half-lives. Most of the antimalarial activity is due to the triazine metabolites, cycloguanil and chlorcycloguanil, respectively, which reach peak concentrations within 4 to 9 h (178). The extent of biguanide metabolism varies considerably: metabolism is catalyzed by the cytochrome P450 group (CYP 2C19 and CYP 3A4), which are subject to genetic polymorphism. Poor metabolizers of the biguanides sustain low or undetectable concentrations of the active triazines; clinical trials have failed to show diminished prophylactic efficacy in poor metabolizers.
Of the sulfonamides and sulfones, only sulfadoxine, sulfalene, and dapsone have been widely used in malaria chemotherapy. The elimination half-life of sulfadoxine is between 100 and 200 h, and that of sulfalene is significantly shorter at about 65 h. Both drugs undergo limited phase II metabolism (to the acetyl and glucuronide derivatives); certain minor phase I metabolites may contribute to idiosyncratic toxicity. The degree of acetylation varies between populations as a result of a genetic polymorphism. Dapsone is eliminated quickly in comparison with sulfadoxine, with a mean half-life of about 26 h.
Therapeutic use.
SP is in frequent use for the treatment of chloroquine-resistant falciparum malaria in Africa. However, severe skin reactions have led to its discontinuation for che-moprophylaxis.
Dapsone in combination with pyrimethamine (Maloprim) is occasionally used for prophylaxis (although the development of agranulocytosis has limited its popularity [see below]). A fixed-ratio combination of dapsone with chlorproguanil (Lapdap) has been developed for the treatment of chloroquine resistant falciparum malaria and has been available since late 2003.
Proguanil is formulated alone (for chemoprophylaxis) and also in fixed-ratio combination with atovaquone (Malarone [see below]). This drug has little public health importance in countries with endemic infection because of its high cost.
Adverse effects and drug interactions.
Severe allergic reactions to sulfa drugs are well recognized; in the case of such slowly eliminated drugs as sulfadoxine, such reactions can be life-threatening (20). Such severe reactions to sulfadoxine are reported in the setting of prophylaxis; their frequency in the setting of treatment is unknown, but current data are reassuring.
Dapsone (34) is associated with a range of concentration-related and idiosyncratic adverse reactions. (i) Patients were severe G6PD deficiency dapsone may develop severe hemolysis and methemoglobinaemia. (ii) Allergy to dapsone is recognized, with rash and fever as major clinical features. (iii) Patients given pyrimethamine-dapsone twice weekly for malaria prophylaxis developed bone marrow reactions, and Maloprim (which is recommended for use only in certain geographical areas) is now taken once weekly. The mechanism of this reaction, and the responsible drug (dapsone, pyrimethamine, or both), remains unclear.
Pyrimethamine and the biguanides proguanil and chlorproguanil seem to be associated with little serious toxicity, although folate deficiency may be exacerbated.
Quinine and Congeners
Of the four cinchona alkaloids, quinine (35) is in most frequent clinical use. Quinine is less potent than chloroquine and has a small therapeutic range. However, resistance to quinine is rare in Africa and is not a major clinical problem elsewhere (although quinine is used in combination with antibiotics, such as tetracycline, in Southeast Asia). Parenteral quinine is the drug of first choice for severe malaria, and oral quinine is an option for treatment of uncomplicated malaria where multidrug resistance is a problem.
Mode of action.
Like chloroquine, quinine interferes with parasite metabolism of heme, a toxic product of hemoglobin digestion.
Clinical pharmacokinetics.
The oral bioavailability of quinine is high. After intramuscular injection, the absorption half-life seems to vary with the drug concentration in the injectate, ranging from about 10 to 40 min. Areas under the concentration-time curve and maximum concentrations in plasma are similar following intramuscular and intravenous administration of quinine (179). Quinine is extensively bound to plasma proteins, principally to the acute phase reactant α1-acid-glycoprotein. In healthy subjects, about 80% of the total plasma quinine concentration is bound, but in patients with malaria, α1-acid-glycoprotein concentrations rise, and around 90% is bound; this may explain the apparently lower toxicity of high quinine concentrations in patients with malaria compared to that in patients who have taken a deliberate overdose.
Quinine undergoes extensive hepatic biotransformation, first to 3- and 2-hydroxyquinine; less than 20% of the drug is excreted unchanged in urine, and the impact of renal failure on the disposition of quinine does not appear to be great. Dose reductions are not recommended for patients with severe malaria complicated by either hepatic or renal impairment. In adults with uncomplicated malaria, the elimination half-time of quinine (16 h) is longer than in healthy persons (11 h); it is even longer in adults with cerebral malaria (18 h).
Therapeutic use.
Therapeutic use is reviewed below.
Adverse effects and drug interactions.
Cinchonism, comprising tinnitus, deafness, headache, nausea, and visual disturbance, affects the majority of conscious patients with therapeutic levels and does not warrant dose reduction. There are potentially life-threatening adverse events, however. These include hypersensitivity reactions, which are uncommon but involve rashes, thrombocytopenia, leukopenia, disseminated intravascular coagulation, hemolytic-uremic syndrome, bronchospasm, and pancytopenia. Quinine also stimulates the release of insulin and may exacerbate hypoglycemia. Toxicity from quinine can be seen with doses as low as 2 g of the anhydrous free base in adults, and the fatal dose ranges from 8 to 15 g. In the setting of acute poisoning, visual impairment is common and may be permanent. Serious cardiovascular compromise is less common than oculotoxicity and is usually seen in the presence of higher drug concentrations. At high concentrations, quinine can cause coma and seizures. Activated charcoal increases the clearance of quinine. Stellate ganglion block confers no benefit.
Adverse drug interactions include marked reduction in the clearance of digitalis glycosides, reduction in the clearance of flecainide, and potentiation of oral anticoagulants.
The Artemisinin Group
Artemisinin (36) is a potent antimalarial compound extracted from plant material. Artemether, artesunate, and dihdroartemisinin are semisynthetic derivatives of artemisinin that are in common clinical use. Members of the artemisinin group are used in the management of severe malaria and also, usually in combination with other drugs, in the treatment of uncomplicated falciparum malaria. The artemisinin group is potent and well tolerated, and resistance has not been encountered in the field. It would not be an exaggeration to say that many public health strategies worldwide are now dependent on this drug class; its major disadvantage is its relatively high cost, but this is falling at present.
Mode of action.
It is thought that breakdown of a labile peroxide bridge within the sesquiterpene lactone molecule generates free radicals that rapidly alkylate parasite membranes (87). Hemazoin probably catalyzes the decomposition of these drugs, which may explain the large therapeutic index of the drug group. One strain of P. berghei (an animal malaria parasite) that lacks hemozoin is resistant to the artemisinins, but resistant P. falciparum strains have not yet been encountered in the field. In contrast to other antimalarial drug groups, the artemisinins have marked effects on the circulating forms of the parasite, whose viability declines soon after the start of treatment. The artemisinins have gametocytocidal effects on P. falciparum, and this may help reduce transmission (149).
Clinical pharmacokinetics.
Artemisinin and its derivatives are rapidly hydrolyzed in vivo to dihydroartemisinin; all members of the artemisinin group have very short elimination half-lives (169). Artemether absorption is slower, more variable, and with lower biotransformation to dihydroartemisinin, when it is administered by the intramuscular or intravenous routes than when the oral route is used.
Therapeutic use.
The use of artemisinins for severe malaria and as artemisinin combination therapy (ACT) (170, 171) are reviewed in later sections.
Adverse effects.
Artemisinins form a safe and well-tolerated drug group. The main current concern centers on reproductive safety (185). The Chinese literature contains reference to the embryotoxic effects of this drug class (78, 187). Recent drug development work provides further evidence of morphological abnormalities in mammalian species; most concern focuses on long-bone shortening (171a). These effects are seen at doses and concentrations similar to those used in clinical practice. It is reassuring that the artemisinins have been extensively used without apparent problems for many years in China and Southeast Asia, and huge numbers of patients have been treated. Pharmacovigilance systems are not well established in China and Southeast Asia, but, despite this, it is important to note that no spontaneous reports of congenital abnormalities have been published. Furthermore, published data on nearly 1,000 pregnancies (nearly 100 from the first trimester) have shown no evidence of treatment-related adverse pregnancy outcomes (26, 85). While these results are encouraging, the numbers are too small to establish the safety of these compounds when used to treat malaria in pregnant women, and pharmacovigilance systems are now being established to increase the database. The WHO has concluded that (i) the artemisinins cannot be recommended for treatment of malaria in the first trimester (but should not be withheld if they are lifesaving for the mother) and (ii) they should be used in later pregnancy only when other treatments are considered unsuitable. It is salutary to remember that many women exposed to artemisinins may not know that they are pregnant and, given the inadequacy of diagnostic facilities, that these women may not have malaria.
Summary of Other Drugs Used for Malaria
Lumefantrine-artemether (Coartem).
Lumefantrine-artemether (Coartem) is the only fixed-ratio ACT currently available (43). Lumefantrine is incompletely bioavailable from the gut and is eliminated with a half life of 1 to 6 days; the disposition of artemether is briefly reviewed above. Lumefantrine is used only in combination with artemether (as Coartem) for the treatment of uncomplicated multiresistant falciparum malaria. Lumefantrine-artemether must be given twice daily, and the regimen first studied (four doses over 2 days) gave disappointing efficacy (109). A 3-day regimen is now being examined, but there are concerns about adherence with such a six-dose treatment, which might affect the operational effectiveness. There are concerns regarding the safety of the artemisinin group of drugs in the first trimester of pregnancy (see above). Lumefantrine-artemether is very expensive; WHO has negotiated a much lower price for Africa.
Mefloquine.
Mefloquine acts against the asexual stages of all species of human malaria parasite. Clinically resistant strains of P. falciparum are common in Southeast Asia. The clinical pharmacokinetics of mefloquine have been extensively reviewed (69); the key point is that this drug is very slowly eliminated, with the half-life ranging from 15 to 33 days and with steady state being reached after 8 weeks of weekly dosing (in the setting of prophylaxis). Mefloquine is used for prophylaxis and treatment of uncomplicated multidrug-resistant falciparum malaria. Mefloquine coadministered with artesunate has proved effective against uncomplicated falciparum malaria in areas where there is a high level of resistance to mefloquine alone (103). Dose-related adverse reactions are common, usually mild, and most frequently gastrointestinal. Serious central nervous system CNS events, including seizures, are estimated to occur in about 1 in 10,000 prophylactic users, which is about the same reported rate as that for chloroquine. The estimated frequency of nonserious CNS events (including headache, dizziness, insomnia, and depression) varies between 1.8 and 7.6% (and is generally higher in females than males); these proportions are similar to those for chloroquine but about fivefold higher than reported by patients taking no prophylaxis. Mefloquine use during pregnancy increases the risk of stillbirth, and the British National Formulary advises that pregnancy should be excluded before mefloquine treatment is started. The manufacturer has recently revised its advice on the relative contraindications of mefloquine. (U.S. Food and Drug Administration, Prescribing information for Lariam, September 2002 posting [http://www.rocheuk.com/ProductDB/Documents/rx/pil/Lariam_PIL.pdf]).
Atovaquone-proguanil.
Atovaquone is thought to inhibit mitochondrial respiration. Atovaquone synergizes with proguanil (interestingly, this is not synergy with proguanil's active metabolite, cycloguanil) (19) and is formulated in a fixed-ratio tablet. Resistance of P. falciparum to atovaquone is selected readily; whether resistance to the combination atovaquone- proguanil will appear when this drug is widely used remains to be seen. Atovaquone is poorly and variably absorbed and is almost entirely eliminated unchanged via the bile into the gut (there is enterohepatic circulation). The elimination half-life is long (50 to 70 h). Atovaquone-proguanil is used for treatment and prophylaxis of multidrug-resistant falciparum malaria: it is a very expensive drug and has little relevance to public health in tropical countries.
Halofantrine.
Halofantrine is a drug sometimes used for uncomplicated, but multidrug-resistant, falciparum malaria. Its absorption is incomplete and variable (its bioavailability increases after ingestion of fatty food). Halofantrine is eliminated with a terminal half-life of 1 to 2 days. The most important adverse effect is prolongation of the ECG QT interval and resulting ventricular arrhythmias (102). The need for ECG to exclude preexisting QT prolongation, has greatly limited the usefulness of this drug.
Antibiotics.
Certain antibiotics, particularly clindamycin and the tetracyclines, have useful antimalarial activity. They are never used alone but are most frequently added to quinine for treatment of patients who can take oral medication; this practice is most commonly needed in areas of intense drug resistance, where clearance of parasitemia by quinine may be prolonged.
DEVELOPING NEW ANTIMALARIAL DRUGS AND COMBINATIONS
Impact of the “Plasmodium Genome Project” on Target Identification
Our most effective antimalarials did not result from rational drug design (with the possible exception of the antifolates). For example, the quinolines (including chloroquine, amodiaquine, mefloquine, and quinine) and the endoperoxides (including the artemisinins) were developed without any clear understanding of their molecular target, and we are still far from sure how these drugs kill parasites. As we move into the 21st century, there is an expectation that target identification, validation, and rational drug design will be the pathway to the next generation of effective antimalarials. This optimism is based on the recent completion of the P. falciparum and mosquito genome projects to complement data available from the human genome project. This optimism probably needs to be treated with a degree of caution.
We are now apparently in the “postgenomic era,” in which we have global technologies capable of investigating all the genes and proteins within an organism. Although this is a little simplistic, it is argued that, by better understanding the complex interactions between molecules within an organism under defined conditions (life cycle stage, drug exposure, etc.) and by comparing key molecules in the host and the pathogen, it should be possible to identify potential chemotherapeutic targets for validation.
The challenge remains for the scientific community to distill this mass of information into more manageable data sets and to develop strategies capable of improving our ability to identify good targets. It will be essential that the application of stringent target validation criteria, high-throughput screening technologies, and lead identification technologies be embraced if we are to fully exploit this new information.
It is accepted that there will be many more opportunities for rational drug design and development in the postgenomic era. However, as outlined below, the drug development process is expensive, relatively slow, and high risk. Development of novel antimalarial drugs will demand the commitment of the malaria community and the pharmaceutical industry; it will also require adequate funding.
Economic Realities
Whatever means are used to identify a potential antimalarial drug and demonstrate its potency in vitro, the compound must then be developed into a medicine (119), during which process its benefits and risks start to be evaluated.
Although the ideas underpinning development projects often emanate from academia, almost all drugs are developed by the pharmaceutical industry (mostly in the industrialized nations) at high financial risk. Thus, although industrial research and development teams are motivated by scientific and medical concerns, funds are usually provided on the expectation of profit, and antimalarial drugs compete poorly with most other drug categories. As a result, many of our present compounds were discovered outside the pharmaceutical industry (often in academia). However, the development of these compounds to registration has always needed industrial expertise and adequate funding. Such commitment from pharmaceutical companies has usually resulted from a favorable balance between costs and the goodwill of national and/or international bodies. Contributions from the Medicine for Malaria Venture (MMV; a public venture capital fund) have been very welcome. MMV is new on the scene, and it would be unrealistic to expect it to have had a chance to make an impact yet. Therefore, the pace of antimalarial drug development is too slow, even though antimalarial drug resistance is now a major threat to global health. Our need for industrial collaborations in antimalarial drug development has never been keener, but maintaining long-term public-private partnerships (and squaring the agendas of public bodies and their industrial partners) can be something of a tightrope walk.
Stages of Drug Development
The regulation of drug development has become tighter because of past disasters, including thalidomide. The public now demands that drugs meet established standards and that safeguards are in place: the main watchdogs are regulatory authorities such as the Food and Drug Administration (FDA), the Medicines and Healthcare Products Regulatory Agency (MHRA), and European Agency for the Evaluation of Medicinal Products (EMEA). Naturally, drugs used in the tropics must meet the same standards; most countries have their own regulatory authority but usually rely on preceding evaluation by the FDA, MHRA, EMEA, or equivalent organizations.
The manufacturer of a new medicine is obliged to demonstrate the quality, safety, and efficacy of the product. The process of developing a dossier for regulatory submission commonly takes a decade and may cost many multiples of $100M. One fundamental precept underlies all stages of drug development: assertions in the dossier must be supported by valid statistical analysis, the raw data must be available for reanalysis, and the data listings must be traceable back to original source material (such as laboratory daybooks or clinical record forms). What follows is a very brief and simplistic description of the main stages of drug development; in fact, many of the separate stages are run in parallel.
Pharmaceutical development.
Development of bulk primary manufacture of the active compound is followed by pharmaceutical development (formulations for oral, rectal, or parenteral administration), so-called secondary manufacture. International good manufacturing practice regulations are used to ensure that medicines are consistently produced to accepted quality standards. In essence, the regulators need to know the chemical composition of the medicine (including the identity of contaminants) and its pharmaceutical characteristics (such as dissolution). They also need to be reassured that (i) the medicine is chemically and physically stable and (ii) its quality is reliably reproducible.
Preclinical testing.
Before a drug can be studied in humans, much time is devoted to examining its toxicology in animals. Good laboratory practice regulations ensure transparency of the data presented in the Dossier. Studies may include (i) studies of the mode of action of the drug and its detailed pharmacology; (ii) single-dose toxicology studies, which may help to outline the major features of overdosage; (iii) repeated-dose toxicology studies in more than one species (these are often more informative than single-dose experiments, since they often help describe the features of chronic toxicity; histopathological examination of tissues and close behavioral observations of the animals are often key features of both single- and repeat-dose studies); (iv) reproduction studies of both sexes, examining fertility, reversibility of any observed effects, and evidence of embryotoxicity and teratogenicity; and (v) mutagenicitiy studies, which are usually required (in many cases, lifetime carcinogenicity data are also needed).
Phase I human studies.
All the phases of human study must conform to good clinical practice regulations. Phase I may be carried out with healthy adults (usually of both sexes) or patients with the disease (depending on the potential toxicity of the drug). The total number of people studied is often under 100. The main aims are usually (i) investigation of safety (relying on objective laboratory and physiological measurements (e.g., the ECG) and tolerability (usually relying on subjective assessments made by the volunteer), often in a variety of doses; and (ii) pharmacokinetics studies, comparing human data with observations made with animals.
Phase II human studies.
Phase II human studies involve open-label assessment of the new drug in patients. During phase II, detailed studies are performed by skilled clinicians familiar with the disease being treated. One of the main elements of phase II is often description of the relationship between drug dose and effect. Specific studies may be needed for special groups such as patients at the extremes of age and patients with liver or kidney impairment. There is usually a pharmacokinetic element to phase II studies, and drug safety and tolerability remain important aspects. Study of interactions between the new drug and those with which it may be coadministered is an important part of phase II. Multiples of 100 patients are usually studied in phase II.
Phase III human studies.
Phase III human studies are are large, randomized clinical trials in which the efficacy, safety, and tolerability of the new drug is compared with those of either placebo or standard medication. In studies of antimalarial drugs, the use of a placebo is clearly impossible, and most trials compare the new medicine with the standard treatment. Patient numbers in phase III usually total 1,000 to 2,000. Therefore, in most cases, the AE profile of a new medicine is incompletely known at the time of launch. Furthermore, the efficacy data, measured by a randomized clinical trial, may have little bearing on the real world. Compliance with administration of medication is usually closely monitored during phase III. In contrast, under normal conditions, patient often comply poorly with drug regimens, especially if they are complicated. Phase III studies usually recruit well-defined, homogeneous populations and restricts patients with comorbid conditions and their concomitant medications. Phase III studies usually have a short follow-up period. Thus, if the drug effect is lost with time, a phase III study may fail to raise the alert.
Phase IV human studies (with emphasis on antimalarial drugs).
The regulatory dossier will have established that the new drug meets required quality standards and that it is efficacious and tolerable; its safety profile will have been rigorously examined in around 2,000 patients. This is insufficient information on which national authorities can base decisions about splicing the new antimalarial drug into policy, because operational conditions have not been simulated and studied. The main areas of interest when assessing the operational use of a new drug in an African setting are safety, compliance, effectiveness, and drug resistance.
Its worth noting at this point that the new drug will almost certainly be marketed as a prescription-only medicine, meaning that it is to be used under professional supervision within the formal health sector. Despite this, in an African setting, the drug is frequently used as if it were on the general sales list; i.e., it will be sold by shops without professional supervision.
(i) Safety.
Most regulatory dossiers—in any disease area, not just malaria—contain data on fewer than 2,000 people exposed to the new medicine. However, the prevalence of life-threatening but idiosyncratic AE is often lower than 1:10,000. Therefore, there is little chance of describing the AE profile of a new drug adequately until after it has been marketed. Systems of pharmacovigilance have been established in the industrialized nations, such as the “yellow card system” in the United Kingdom. However, few functioning pharmacovigilance systems operate in Africa, and gathering high-quality but numerous postmarketing data represents a major challenge.
(ii) Optimizing adherence.
Only a proportion of patients, anywhere in the world, manages complete adherence to their drug regimen even when the need for this is carefully explained. For some drugs, incomplete compliance may not threaten therapeutic outcome, but for most antimalarial drugs, close adherence to the regimen is important. Certain factors can be identified that are relatively independent of the circumstances of drug use (formal versus informal sector).
(i) On empirical grounds, problems could be expected from regimens that are protracted (beyond 3 days), require more than once-daily dosing, or involve treatment with two or more separately formulated drugs.
(ii) Most patients with malaria are poor, and drug cost may affect adherence to the regimen; part of a treatment regimen might be retained for future use even if the cash saving is tiny.
(iii) Inappropriate formulation may also have a bearing. Perhaps counterintuitively, dosing of young children in Africa with liquid formulations is less likely to be accurate than the use of a solid formulation. This mainly reflects the difficulty experienced by many mothers when estimating volumes of liquid.
(iv) If a drug frequently causes symptomatic adverse reactions (e.g., chloroquine-induced pruritus or mefloquine-induced nausea), adherence with the ideal dose regimen may be poor.
(v) Prepackaging of antimalarials into “unit doses” has been shown to influence adherence in an African setting. Furthermore, attention to the detailed design of patient information leaflets (perhaps incorporating cartoons demonstrating correct usage) may help to improve adherence.
However, even though the drug might seem optimal (a simple regimen, low cost, little symptomatic toxicity, appropriate formulation, and prepackaged unit doses), there remains tremendous scope for misuse among prescribers, dispensers, and patients. This area has not been studied extensively until recently, but there is a growing acceptance of the need for formal information education and communication work to clarify how best to generate understanding of appropriate usage. Such work is very circumstance dependent and needs to tackle both formal and informal health care sectors (83).
Effectiveness and drug resistance.
In randomized trials, the backbone of the clinical section of the drug dossier, carefully selected patients take their drugs under supervision. This is a highly artificial situation in an African context; even in the setting of formal health care, most antimalarial treatment involves outpatients. There is therefore a large scope for poor adherence to therapy and poor clinical outcome. When anti-infective agents are being used, another possible consequence of suboptimal drug use is drug resistance. In consequence, the operational effectiveness of the new drug is critical to its possible utility in public health.
Challenges of Drug Combinations
If previously licensed drugs are to be used in combination (perhaps as antimalarial ACTs), whether coformulated or copackaged, further data are required by the regulators. The question whether the benefits of combining the drugs outweigh the added risks is central. If the drugs can be shown to have synergistic properties, as is the case for SP, then the case may be relatively easy to make: the use of the isolated drugs pyrimethamine and sulfadoxine would present problems of efficacy (especially for the sulfonamide). In addition, the regulators will usually need to know the stability of the pharmaceutical preparation and the effects of the combination on preclinical toxicology and on clinical pharmacology (including pharmacokinetics).
USE OF DRUGS FOR MALARIA IN AFRICA
Who Needs Antimalarial Drugs?
Falciparum malaria is endemic, with high frequencies of transmission, throughout much of sub-Saharan Africa. Some patient groups are in danger of their lives if treatment is delayed, whereas for other groups the disease is no more than an unpleasant illness and a cause of lost earnings. Most malaria mortality is among young children and pregnant women. Children need prompt recognition of febrile illness followed by prompt and appropriate treatment. Pregnant women, in contrast, are often asymptomatic (indeed, were a microscope to be available, one would often be hard pressed to find parasites, since most infest the placenta) and need either prophylaxis or presumptive treatment. Both groups are described more fully below.
Defining Malaria Fevers in Clinical Practice
Fever is the most common clinical feature of malaria in the human host. The best descriptions of fevers due to P. falciparum derive from work on induced malaria used to treat neurosyphilis patients (65). Inoculation with P. falciparum led to certain prodromal symptoms days or hours before the first febrile attack, which were more severe in people with low malaria immunity. These symptoms may include malaise, headache, dizziness, shivering, sweating, myalgia, gastrointestinal disturbances and a transient fever (usually not exceeding ≥37.7°C). P. falciparum fevers do not follow the classical paroxysm pattern typical of other Plasmodium infections, tending to be irreg-ular fevers with a long paroxysm.
The major clinical features of falciparum malaria are not related to the level of the fever and have no grouping that allows for clear classification. Clinical features range from mild problems (such as low-level fever and headache) to more severe problems (such as prostration, coma, and acidosis). Severity is not necessarily associated with a higher parasitemia but often relates to immune status. Descriptive studies of children and adults with fever and high parasitemia have indicated that, in addition to headaches, myalgia, and vomiting, other symptoms include diarrhea, cough, and rapid breathing. Malaria disease has a very similar clinical presentation to other infections, making it hard to differentiate from conditions such as typhoid, pneumonia, and bacteremia (2, 105, 116-118).
Nevertheless, given the predominance of fever within the clinical presentation of malaria in nonimmune persons, this sign has historically dominated the diagnosis and management of the disease. In Africa, where infection and fever are common, several investigators have proposed diagnostic algorithms that broaden the clinical criteria to include other signs and symptoms. In their review of clinical algorithms Chandramohan et al. (21) conclude that, under most conditions prevalent in Africa, none of the algorithms was sufficiently accurate. These combined findings provide some justification for the adoption by the integrated management of childhood illnesses (IMCI) of fever as a single prompt for malaria case management in children in Africa (51, 180a).
Community Definitions of Malaria and Fever
African communities, plagued with infectious disease, have developed an elaborate combined biomedical and biocultural vernacular to describe fever. Anthropological and social-epidemiological studies have examined the ways in which “malaria” fevers are defined in communities in Liberia (64), Uganda (70, 81), Burkina Faso (153), Guinea Conakry (28), Ghana (9), Nigeria (127), Tanzania (82, 173), and Kenya (94). In Uganda a study of mothers' diagnoses of malaria was compared to “gold standard” clinical and parasitological observations: mothers' diagnoses achieved a sensitivity of 37% and a specificity of 58% (81). In northern Ghana, the words “pua” or “paa” are used to define febrile illnesses including variously headaches, skin rashes, diarrhea, and/or vomiting. Comparisons of these definitions of “malaria” with raised body temperatures and a parasite density greater than or equal to 4,000 μl−1 provided a sensitivity of 42% and a specificity of 79% during the peak malaria season (9). “Soumaya” is a word used to describe “malaria” in the Sikasso region of Burkina Faso (153). When mothers were asked to rank the main symptoms associated with this condition, vomiting, fever, dark urine, and jaundice were reported most frequently. However, equal numbers of mothers reported vomit, fever, dark urine, and jaundice among their sick children without soumaya during the previous 6 months. Diallo et al. reported similar findings for the various ethnically distinct words for malaria in Guinea Conakry (28). The almost universal observation is that specific terms used by communities to describe malaria fevers appear to lack both sensitivity and specificity when compared against subsequent clinically and parasitologically defined malaria.
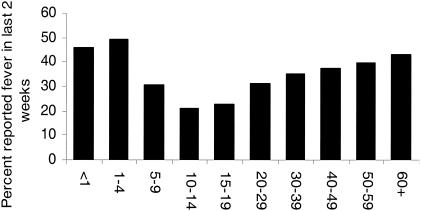
In most settings of endemic infection, adults have developed a functional immune response to infection, and the incidence of measured elevated body temperatures associated with significant increases in parasite density is low compared to that in pediatric populations (57, 93, 113, 121). Nevertheless, the term “fever” is often used by adults to describe clinical conditions subsequently managed as malaria. In rural Benin, the mean annual number of reported febrile events among children was 1.93, compared to 1.79 in adults (115). Retrospective investigations of fever frequency during the previous 14 days, undertaken in households across Malawi in 1992, provide similar findings (41). Figure 3 presents the Malawian data and shows clearly that the period prevalence of recalled fever rises with increasing age in adult populations to a level similar to that in children aged younger than 5 years. In Kenya, approximately half of all the antimalarial drug prescriptions made at rural government health facilities in malaria-endemic areas are for adult patients (Kenya Malaria Information Service, Sentinel sites for the measurement of drug sensitivity reports, 2003 posting [http://www.kmis.org/]). It is likely that pediatric populations constitute over 75% of the fatal outcomes of malaria across Africa and 30% of the “fever” burden but only 20% of the drug budget. The consequence is that there is a huge wastage of antimalarial drugs owing to the presumptive nature of malaria diagnosis and drug use among patient populations at very low risk of a poor clinical outcome.
FIG. 3.
Age-structured, period prevalence of reported fevers during the preceding 2 weeks, recorded during a national household survey in Malawi (41).
Despite the ambiguities of defining fever in rural African communities, this is what serves as the prompt for treatment. Using estimates of fever period prevalence from demographic and health surveys undertaken over the last 10 years in Africa, it is estimated that pediatric populations experience circa seven fever events each year, representing 0.85 billion fevers in 2000 areas across of endemic infection in Africa (143; MEASURE Program, Demographic and health surveys for sub-Saharan Africa databases derived using STATCompiler, 2002 posting [http://www.measuredhs.com/]). Extrapolation of these estimates to older children and adults suggests that approximately 2.8 billion fevers occur annually among the total population exposed to malaria risks on the continent.
Management of Fever
Treatment-seeking behavior.
The management of fever by most African communities is one of polypharmacy (46, 84). Combinations of home-based treatments, either traditional remedies or over-the-counter purchased drugs (95), and formal clinic consultations all form part of a matrix of treatment-seeking behavior prompted by the local perception of the presenting illnesses. The use and sources of and the compliance with existing antimalarial drugs in Africa remains a priority area for behavioral science research since it provides insights into the landscape of drug use and entry points for future changes in case management and drug policy.
Here we review the available literature for a number of African countries to highlight some of the basic differences in access to, use of, and compliance with drug regimens to manage fever. For the purposes of this review, we have selected household studies investigating fevers among household members during a fixed recall period during the preceding 2 to 4 weeks. Definitions of formal service access or home-based management differ between study sites. Largely, we have regarded the formal health sector to reflect services provided by government-run, private, or mission sector hospitals, health centers, or dispensaries or community-based health care providers. We have expanded the definition of home-based management, often defined as shop-bought medicines, to include all drugs used at the household level. These may have included medicines from previous formal-sector consultations or shop purchases, drugs provided by neighbors or other household members, or drugs purchased from registered pharmacies without prescription. It is often impossible to define precisely the origin of the home-based drugs used for fever management. We have focused on the use of drugs to manage morbidity, and thus the use of traditional medicines in the home has not been classified as a “home-based” management strategy. Clearly, there are inherent definition difficulties in making strict comparisons between studies of the frequencies of public, private, and home-based consultations, but we have used the review more as means of estimating the expected frequency of antimalarial drug contacts from formal and informal sources in febrile populations.
Tables 1 and 2 show the percentage of fevers where mothers or guardians of young children adopted a home-based drug strategy across Africa. The studies (n = 20) were undertaken at different times, the earliest being from 1983 to 1985 and the most recent being in 2000. The use of drugs as part of the home-based care of febrile events is common across Africa, with a median of 67% (interquartile range, 48, 78%) of fevers in young children being managed in this way. Tables 3 and 4 show the percentage of pediatric fevers managed within a formal health service context. Among the 20 studies where estimates of formal health sector use for fevers has been presented in the original reports, the median percentage of fevers managed by government, private, or mission service providers was 34% (IQR, 25, 48%). In other words, approximately half as many fevers are managed by the formal health services as are managed by self-medication strategies at the household level.
TABLE 1.
Proportions of fevers managed at home through drugs purchased from shops, mobile vendors, or available in the household
| Location | % of fevers managed at home | Yr of study | Age of patients (yr) | Refer- ence |
|---|---|---|---|---|
| Bagamoyo, Tanzania | 18 | 1983-1984 | 0-4 | 96 |
| Kabale, Uganda | 26 | 1998 | 0-4 | 79 |
| Dar es Salaam, Tanzania | 29 | 1993 | 0-4 | 184 |
| Tougan, Nouna, and Solenza, Burkina Faso | 44 | 1994 | 0-4 | 73 |
| Idere, Mbaugwu, and Ukehe, Nigeria | 47 | 1999 | 0.5-6 | 126 |
| Latebiokorshie, Accra, Ghana | 48 | 1998-1999 | 0-16 | 8 |
| Nationwide, Malawi | 53 | 1992 | 0-10 | 41 |
| Kilifi, Kenya | 58 | 1992 | 0-4 | 93 |
| Blantyre, Malawi | 63 | 2000 | 0-4 | 61 |
| Mombasa, Kenya | 64 | 1997 | 0-4 | 89 |
| Naga-Eboko, Cameroon | 69 | 1987 | 0-14 | 67 |
| Siaya, Kenya | 71 | 1990-1991 | 0-6 | 124 |
| Chokor, Accra, Ghana | 72 | 1998-1999 | 0-16 | 8 |
| Bomi & Grand Cape, Liberia | 76 | 1984-1989 | 0-7 | 45 |
| Bungoma, Kenya | 78 | 1996 | 0-4 | 55 |
| Conakry, Guinea | 79 | 1986 | 0-4 | 24 |
| Chonyi, Kenya | 79 | 1997 | 0-4 | 89 |
| Plateuax region, Togo | 83 | 1984 | 0-4 | 27 |
| Yaounde, Cameroon | 91 | 1987 | 0-14 | 67 |
| Edea, Cameroon | 93 | 1987 | 0-14 | 67 |
TABLE 2.
Proportions of fevers managed at home by administering antimalarials
| Location | % of patients given antimalarials | Reference |
|---|---|---|
| Machakos, Kenya | 12 | 128 |
| Kilifi Town, Kenya | 28 | 93 |
| Siaya, Kenya | 29 | 124 |
| Guinea Conakary | 37 | 24 |
| Bungoma, Kenya | 47 | 55 |
| Kampala, Uganada | 50 | 80 |
| Kilifi, Kenya | 53 | 137 |
| Yaounde, Cameroon | 69 | 67 |
| Accra, Ghana | 78 | 109 |
| Kwale, Kenya | 84 | 68 |
| Yaounde, Cameroon | 91 | 67 |
| Yaounde, Cameroon | 93 | 67 |
| Plateau, Togo | 100 | 27 |
TABLE 3.
Proportions of fevers managed by formal public and/or private health care facilities
| Location | % of fevers managed at facilities | Yr of studya | Age of patients (yr) | Refer- ence | |
|---|---|---|---|---|---|
| Mombasa, Kenya | 14 | 1997 | 0-4 | 89 | |
| Plateuax region, Togo | 20 | 1984 | 0-4 | 27 | |
| Siaya, Kenya | 20 | 1990-1991 | 0-6 | 124 | |
| Bomi and Grand Cape, Liberia | 23 | 1984-1989 | 0-7 | 45 | |
| Kilifi, Kenya | 25 | 1992 | 0-4 | 93 | |
| Chonyi, Kenya | 25 | 1997 | 0-4 | 89 | |
| Chokor, Accra, Ghana | 27 | 1998-1999 | 0-16 | 8 | |
| Tougan, Nouna, and Solenza, Burkina Faso | 27 | 1994 | 0-4 | 73 | |
| Idere, Mbaugwu, and Ukehe, Nigeria | 32 | 1999 | 0.5-6 | 126 | |
| Telimele and Kindia, Guinea (rural) | 33 | NA | 0-5 | 47 | |
| Blantyre, Malawi | 34 | 2000 | 0-4 | 61 | |
| Latebiokorshie, Accra, Ghana | 42 | 1998-1999 | 0-16 | 8 | |
| Conakry, Guinea | 43 | 1986 | 0-4 | 24 | |
| Bungoma, Kenya | 43 | 1996 | 0-4 | 55 | |
| Kingandu, Democratic Republic of Congo | 45 | 1984-1989 | 0-6 | 157 | |
| Nationwide, Malawi | 56 | 1992 | 0-10 | 41 | |
| Kabale, Uganda | 62 | 1998 | 0-4 | 79 | |
| Telimele and Kindia, Guinea (urban) | 69 | NA | 0-5 | 47 | |
| Dar es Salaam, Tanzania | 84 | 1993 | 0-4 | 184 | |
| Bagamoyo, Tanzania | 85 | 1983-1984 | 0-4 | 96 |
NA, not available.
TABLE 4.
Proportions of fevers managed through formal health sector by administering antimalarials
There are fewer studies where details of the drugs administered to children have been presented. Table 3 summarizes the frequency of actions undertaken that included an antimalarial drug at the household level and the formal health sector level, respectively. The median estimates of antimalarial drug use in the informal sector were 53% (IQR, 37, 84%), compared to nearly twice as many antimalarial drug prescriptions in the formal sector, i.e., 92% (IQR, 73, 95%).
Several studies included in Tables 1 and 3 also examined the use of formal and informal sectors and antimalarial drug consumption in older children and adults. Notably, the study undertaken in Dar es Salaam suggested that use of government, private, or self-medication facilities varied little between children and adults (186). Other studies in Malawi and Uganda provide comparable evidence of similar treatment-seeking patterns between adults and children (41, 80). One study in Burkina Faso did demonstrate a difference in service preference for fever management between adults and children: 27% of children were taken to formal services, compared to 15% of adults who used them (73). Perhaps the most striking and detailed reports of drug use by populations derives from a community-based study undertaken at Saradidi, Kenya, during the early 1980s (144). The frequency of chloroquine consumption per person per annum is shown in Fig. 4 and is consistent with the patterns of reported fever described in Malawi (41) (Fig. 3). The Saradidi study was an experimental trial of chloroquine administration and, as such, does not provide us with any sense of “normal” drug access and use. Nevertheless, the study does highlight that antimalarial drug consumption is as common in adult as childhood populations living in areas of Africa that in theory support the acquisition of clinical immunity by the end of childhood.
FIG. 4.
Age pattern of annual chloroquine treatments per person during a community-based study of improving access to antimalarial drugs in Saradidi, Kenya (144).
Adherence.
Poor patient adherence with more than single-day regimens of drugs is a common enough phenomenon for most disease systems in the developed and developing world (54, 62). This poses a particular threat to effective self-medication for malaria in Africa (46, 84). Among the studies included in this review, failure to complete full 3-day courses of chloroquine ranged from 25 to 85% (24, 42, 55, 61, 67, 71, 83, 94, 107, 153). A variety of reasons for this have been explored and include early patient recovery, purchasing of drugs according to limited budgets, and poor patient awareness of correct doses. Such characteristics are not limited to self-medication; poor adherence is also common with prescribed medicines in the formal health sector (1, 58, 73, 79, 106).
Overdosing is probably less frequent than underdosing but is not uncommon. In Kenya, 14% of children were reported to have received more than the recommended dose of chloroquine (83), and in Burkina Faso, as many as 30% of children received more than the recommended dose of chloroquine (153). A study of formal health service providers in Kenya revealed that SP was prescribed over several days rather than as a single dose since service providers felt that the drug lacked potency when given as a single dose (58). The extent of repeated dosing with the same drug within the same illness episode has not been well enough described, although is important to define this factor, given the extent of polypharmacy adopted by most communities.
Recent studies of improved practice through shopkeeper training (83), consumer behavioral change programs (111), or improved drug packaging (5, 188) may provide some hope for improving on the current poor adherence to recommended doses.
Implications of fever incidence and treatment for effective antimalarial drug policies.
The presumptive diagnosis of malaria in Africa has been widely promoted on the basis that any potentially, rapidly fatal malaria infections should not be missed in a semi-immune child (21). This clinical position has been largely acceptable in areas where the drugs used to manage fevers are both cheap and safe. Inevitably, combinations of new and old pharmaceuticals increase costs demonstrably. Given the extent to which populations in Africa consume antimalarials, predominantly chloroquine and SP, it would appear that this is an affordable option for many households. Costs may, however, restrict compliance with existing therapies, with patients purchasing only a few tablets rather than complete treatment courses. As new drugs, such as ACTs, become more widely promoted as first-line therapeutics for fever management, this may either exclude the informal sector or increase the extent to which cost impinges on compliance. Over two-thirds of fevers are self-medicated in Africa and thus cannot be excluded from the development of strategies to use ACT in Africa.
Drugs with lower safety margins will continue to be beneficial to communities where the greater number of fevers are likely to be due to the disease and where many of these infections carry a high risk of poor disease outcome (for example, much of Southeast Asia). In sharp contrast, safety margins must remain high in Africa, and may argue in favor of a massive investment in diagnostics to guide prescription practices. It is hard to estimate the costs required to introduce microscopy at every level of malaria case management or whether this would be feasible. Two things are certain, however: first, this would exclude the informal sector, and second, it would require a major departure from the IMCI advocacy for presumptive treatment of all fevers with antimalarials (180a).
The number of antimalarial drug-patient versus drug-parasite contacts occur under the varied transmission conditions that characterize Africa remains largely unknown. These epidemiological parameters are important to provide a more rationale basis on which to define the future application and utility of new antimalarial drug strategies currently being considered, particularly ACTs. Perhaps of greater significance to current debates on antimalarial drugs for patients in Africa are the anticipated costs to both national essential-drugs programs and households in Africa. Currently, the incidence of perceived malaria illness, presumptive diagnosis, and antimalarial drug consumption are huge for the African continent and distinguish it from malarious areas of Southeast Asia and Latin America.
Drug Use by the Formal Health Care Sector
Although self-medication in the community accounts for about half of all antimalarial drug use, we must not forget the continuing pivotal role played by the formal health care sector (community health centers, private clinics, and district hospitals). The strategies available to the formal sector are currently chemoprophylaxis, intermittent presumptive treatment (IPT), case management of uncomplicated malaria, and case management of severe malaria syndromes.
Most countries with endemic infection have a National Malaria Control Program (NMCP) that advises ministers of health on the drugs that ought to be used. Consequently, in publicly funded health facilities, the range of available drugs is often limited. It should be noted that publicly funded facilities are not usually free at source: patients generally have to pay for their drugs. In contrast, although private clinics and hospitals are generally encouraged to follow NMCP guidelines, they usually have access to a wider range of drugs, although often at a higher cost than in the public sector.
Chemoprophylaxis.
Readers in the industrialized nations will be familiar with the provision of chemoprophylaxis to travelers (which we have touched on in the sections describing the individual drugs) but will perhaps be less familiar with the issues relevant to populations in countries where infection is endemic. In summary, only a very small proportion of African populations takes chemoprophylaxis. The main reason for this lies in practical economics: people are unwilling to use their sparse and overcommitted resources in this way. Further, we should remember that risk-versus-benefit analyses do not always favor chemoprophylaxis. If one lives permanently in a high-transmission setting, protective immunity reduces the mortality and morbidity of falciparum malaria after early childhood whereas the cumulative risks of drug exposure were lifelong chemoprophylaxis to be taken, would remain. There are special groups for whom the benefits would probably outweigh the cumulative risks: these include children younger than 5 years who reside in high-transmission areas and patients with hemoglobinopathies (infants and pregnant women merit separate consideration and are discussed below). In fact, few of these at-risk patient groups actually receive chemoprophylaxis—in much the same way as few diabetics in malaria-endemic countries receive comprehensive care—for reasons of economics and logistics.
We may describe a country as being in a malaria-endemic region, but this is a simplification. Geographical factors (such as altitude) may render malaria transmission infrequent in some localities within that country. Being infrequently exposed to transmission, all age groups may lack protective immunity under such circumstances. In such areas, epidemics of falciparum malaria can occur (perhaps caused by unusual variations in climate), resulting in predictable mortality and morbidity. Provision of early warning and early detection are research goals, and some advances (using satellite data) have been made. Chemoprophylaxis would probably be a practicable intervention in such circumstances.
Intermittent presumptive treatment (for pregnant women and infants).
In high-transmission regions, immunity to malaria develops in the first few years of life, and older people rarely suffer severe malaria. However, during pregnancy the risks of maternal anemia, maternal death, and low-birth-weight infants are increased by P. falciparum infection (12, 13) and are further exacerbated if there is coincident HIV infection (146, 156). In HIV-negative mothers, risk is greatest in primigravidae and declines with succeeding pregnancies; in HIV-positive mothers, the risks persist with succeeding pregnancies. It is important to understand that infection is usually asymptomatic; furthermore, blood slides may be negative, despite placental infection. As a result, case management of febrile illness will miss the majority of infections. Trials of weekly chloroquine chemoprophylaxis showed an impact on maternal anemia and birth weight. However, compliance with a weekly regimen is poor, and the sensitivity of the parasite to chloroquine is declining throughout Africa. Thus, SP has been studied for its effects in this setting, but instead of being given at frequent intervals as a form of chemoprophylaxis, SP has been given intermittently, on the assumption of infection, in the second and third trimesters (135). This IPT has been shown to improve both maternal and fetal outcomes and is being introduced operationally. It is important to understand two points: (i) we do not know whether the success of IPT relies on the prolonged chemoprophylactic effect of SP (which is eliminated very slowly) or whether IPT is successful simply because of periodic “clearance” of placental infection, and (ii) sensitivity of malaria parasites to SP is declining in Africa, and it is unclear for how much longer IPT with SP will continue to work.
The same sort of concept is also being applied to children younger than 12 months, in whom clinical malaria and severe anemia are major causes of pediatric hospital admission and death (128). Case management (see below) is currently the main strategy, but it has its limitations: (i) very young children are, effectively, nonimmune, and their condition may deteriorate within hours of the onset of symptoms; and (ii) in rural areas, parents may be unable to reach health centers or hospitals in time to prevent death. A randomized trial has been done with infants in a rural area of Tanzania to examine the efficacy of intermittent SP dosing on the rate of malaria and severe anemia. Children were given SP (or placebo) and iron supplementation at 2, 3, and 9 months of age. SP gave a protective efficacy of 59% against malaria and 50% against severe anemia. This new approach to malaria control is currently being examined by WHO; however, once again, it is important to be aware that the parasite may be moving more rapidly that the authorities: resistance to SP threatens this strategy.
Case management of uncomplicated malaria.
Numerically, case management of uncomplicated malaria is the principal way in which antimalarial drugs are employed (there are perhaps 120 million cases treated annually in Africa), and it has a large impact on morbidity and mortality. Efficient case management of uncomplicated malaria relies heavily on the availability of effective and accessible drugs.
In high-transmission areas, the main objectives of treatment are to limit morbidity and produce symptomatic improvement. Since early reinfection is almost inevitable, one is not aiming to maintain a negative blood slide in the medium or long term. In contrast, the presence of P. falciparum parasitemia in nonimmune patients (be they travelers to malarious areas or residents in low-transmission areas) demands urgent antimalarial treatment, aiming to eliminate infection. Such patients are at high risk of developing life-threatening complications.
The individual antimalarial drugs are described in their own sections above. In the setting of country where malaria is endemic, the choice of drug is determined mainly by availability, cost, and resistance patterns. Most countries now have NMCPs that seek to coordinate national effort and establish policy. Resistance to chloroquine is common throughout the tropics, and the WHO has published guidance on when countries should consider changing their first-line drug (182). Although chloroquine remains in widespread use throughout Africa, many African countries have now changed their first-line drug, and chloroquine is little used in South America and Southeast Asia. In places where chloroquine has been replaced, SP has generally been the next choice. Unfortunately, SP resistance is now so severe in Southeast Asia that this drug is little used; more alarmingly, SP resistance in Africa seems to be becoming more prevalent; NMCP managers are urgently seeking options.
Combination therapy (104, 171) (of which ACT is a subdivision), which is mentioned above, is a topical strategy that is being explored at the moment. The logic underpinning combination therapy is compelling: during treatment with two drugs, the chance that a mutant resistant to both drugs will emerge can be calculated from the product of the individual per-parasite mutation rates (assuming that the resistance mutations are not linked). The artemisinin derivatives reduce the parasite biomass by around 4 log units for each asexual cycle, and this makes them the most rapidly efficacious antimalarial drugs in use and makes ACT an attractive proposition. This rapid reduction of the parasite biomass plays a major theoretical role when artemisinin derivatives are combined with another antimalarial drug: the parasite population available to develop mutations to the second drug is reduced by several log units. Thus, when mefloquine was used in combination with artemisinins in Thailand, the rate of development of mefloquine resistance was reduced.
WHO is recommending combination therapy as part of the ideal strategy for malaria control in Africa, but there are practical concerns.
(i) Combination therapy has been shown to reduce the rate of spread of drug resistance in Thailand, but it is not clear that the same will apply in an African setting, where drug use will be difficult to regiment.
(ii) Coartem (see above) is the only fixed-ratio combination at the moment (others, mostly ACTs, are under development), its efficacy in randomized trial is disappointing unless six doses are given over 3 days (108), and its operational effectiveness is unknown. Thanks to an agreement brokered by the WHO, Coartem will be made available for $2.40 per adult treatment course—brave and generous of the manufacturer, and WHO should be commended for this achievement. However, the price may still be too high for first-line treatment in Africa, although it is possible that the new Global Fund can help in this regard.
(iii) If combination therapy is to be introduced as coadministration of two separate drugs (for example, amodiaquine plus artesunate in a blister pack), a great deal of operational work will be needed to ensure smooth implementation.
(iv) If fixed-ratio alternatives to Coartem are to be used (and assuming that these are developed to internationally recognised standards of quality and safety), then there will be a lead-in time of several years of development.
(v) There are concerns about the safety of the artemisinins in pregnancy (especially the first trimester), as described above.
Uncomplicated malaria requires little supportive care, and, in high-transmission areas, patients are usually managed without admission to hospital. Rehydration (by mouth) is important because febrile patients (particularly young children) may become dehydrated rapidly; oral rehydration solutions are appropriate for outpatient care. Fever (a risk factor for seizures in young children [160]) should be managed by removing clothing, tepid sponging, and fanning; mothers can be told how to do this. Acetaminophen (Tylenol) is the safest antipyretic drug and can be given orally or rectally (although suppositories are expensive). Aspirin is contraindicated in children because of its association with Reye's syndrome.
Case management of severe malaria.
It is estimated that about 12 million cases of severe malaria are treated annually in Africa, with a mortality rate exceeding 10% (assuming competent management). Effective management of severe malaria syndromes is expensive and relies heavily on hospital and human resources (which are often not equal to the task in tropical countries).
The main antimalarial drugs used for severe falciparum malaria are quinine and the artemisinin class. Resistance to these drugs is not a major problem (although some resistance to quinine is seen in Southeast Asia). The principal clinical challenge is keeping the patient alive long enough to permit the antiparasitic drugs to work.
The basic principles of managing severe malaria can be summarized as follows. (i) The objective of treatment is to save life: secondary considerations, such as parasite clearance and fever clearance, are relegated until the patient has been resuscitated. (ii) Treatment should be started immediately the diagnosis is proved (in some cases, it is appropriate to start as soon as the diagnosis is suspected). (iii) A drug regimen should be chosen that is appropriate for the known local pattern of drug resistance. Thus, although quinine remains appropriate in Africa, the artemisinins may be preferred in Southeast Asia. (iv) Drug dosage should be calculated by body weight rather than estimated. This is especially important with quinine and quinidine, which have small therapeutic indices. (v) The antimalarial drug should be given parenterally wherever possible. Oral (or nasogastric) dosing is best avoided: there may be gastric stasis, and drug absorption is unreliable. (vi) Loading doses of quinine or quinidine should be given unless the patient has received parenteral quinine/quinidine or oral halofantrine within the previous 24 h. (vii) The response to therapy should be monitored frequently. (viii) Drugs should be given orally as soon as patients are able to swallow and retain tablets.
(i) Quinine.
The only contraindication to the use of quinine is reliable evidence of serious quinine allergy. Hemolysis, pregnancy, jaundice, and renal failure are not contraindications (160). Quinine has a narrow therapeutic range, and doses should always be adjusted for body weight. A loading dose should be given to achieve therapeutic concentrations more rapidly; there is some evidence from clinical trials of the clinical benefit of a loading dose (112), but the practice is based largely on sound pharmacokinetic data. Contraindications to the use of a loading dose are treatment with quinine or quinidine in the previous 24 h and treatment with halofantrine in the previous 24 h. If patients develop severe malaria following mefloquine treatment, a full dose of quinine should be given (148). Quinine must never be given as a bolus intravenous injection: the risk of serious adverse reactions is high. The preferred means of administration is by slow, constant-rate infusion of the drug diluted in crystalloid solution (166). If intravenous administration is impossible, quinine may be given intramuscularly. Maintenance doses of quinine are usually given every 8 h, but in African children 12-h maintenance doses are effective. In Southeast Asia, quinine is given for 7 days. Courses are generally shorter in semi-immune African patients (with 5 days being standard in many countries). To prevent the accumulation of quinine, the maintenance dose should be reduced to one-half after 48 h of parenteral treatment. If possible, blood slides should be examined during treatment; after 24 h of treatment, counts usually fall in a log-normal manner and asexual parasitemia should disappear within 5 days (gametocytes may persist, but they are nonpathogenic and of no clinical importance, although they are of some public health relevance). A rising, or unchanging, parasite count after 24 h of quinine treatment may indicate drug resistance: this is particularly likely in infections acquired in Southeast Asia.
(ii) Artemisinins.
Intramuscular artemether is at least as efficacious as intravenous quinine and may be preferable in Southeast Asia, where sensitivity to quinine is declining (154). An initial loading dose is recommended, followed by once-daily administration of the maintenance dose for at least 3 days or until an oral antimalarial drug can be taken. Suppositories of artemisinin and artesunate (75) have proved effective in adults and children with cerebral and other forms of severe malaria in China and Southeast Asian countries. This is a particularly promising route of administration for use at the most peripheral level of the health service. If malaria is suspected or confirmed in a patient with an acute fever who is unable to swallow tablets, these suppositories may prevent the evolution to severe disease.
(iii) Supportive care.
A complete description of the various syndromes of severe malaria, and their management, is outside the scope of the present review (4). The more common features of severe disease are discussed briefly.
(a) Encephalopathy. Patients may be unconscious for a variety of reasons (92, 98, 99, 151, 161). However, prolonged unconsciousness suggests cerebral malaria, which results from sequestration of parasites in the cerebral vasculature (18). There are specific retinal abnormalities in a proportion of patients (59). The airway should be protected, a nasogastric tube should be passed, and these unconscious patients should be nursed on their sides to avoid aspiration pneumonia. Patients should be turned regularly at intervals. There are no ancillary treatments proven to reduce the mortality of cerebral malaria. However, some patients develop brain swelling (100) and are at risk of brain shift; mannitol may be useful in these cases.
(b) Seizures. Seizures are a common feature of cerebral malaria in both adults and children. In addition, children may have febrile seizures, from which they recover quickly, without prolonged encephalopathy. Because seizures are so common in patients with cerebral malaria people have tried a single dose of phenobarbitone to reduce the frequency of seizures. In Thai adults, 3.5 mg/kg seemed to have an effect, but this work probably bears repetition (168). In African children, 10 mg/kg did not achieve therapeutic drug levels (176), and, while 20 mg/kg halved the incidence of seizures, mortality was doubled, presumably due to respiratory depression (23). Seizures must be treated promptly by following standard guidelines (they are usually treated with a benzodiazepine).
(c) Hypoglycemia. Hypoglycemia is a particularly common feature of severe malaria in children and pregnant women. In patients with cerebral malaria, correction of hypoglycemia sometimes improves the level of consciousness; more often, it has no obvious effect on coma. Hypoglycemia may develop or recur despite continuous intravenous infusions of dextrose. Octreotide and glucagon may also be used if they are available.
(d) Lactic acidosis. Lactic acidosis complicates other severe malaria syndromes and may occur in isolation in children (39). It is best treated by correcting hypovolemia and improving oxygenation. Sodium bicarbonate may be used if the pH is below 7.0, but the value of this treatment remains controversial. Dichloroacetate can reduce lactate levels, but its role in severe malaria remains to be established (74).
(e) Anemia. Severe anemia is especially common in young children and pregnant women. Where blood screened for HIV and hepatitis viruses is freely available, transfusion is usual if the hematocrit falls toward 20%. In much of the tropics, where screened blood is less freely available, the criteria for transfusion are stricter (40). Even so, a hematocrit of 15% is probably an absolute indication for transfusion.
(f) Hemoglobinuria and blackwater fever. Nowadays, hemoglobinuria is most commonly seen in patients with glucose-6-phosphate deficiency who are being treated with oxidant drugs such as chloroquine, dapsone, and primaquine. This massive intravascular hemolysis may lead to oliguric renal failure and severe anemia. It should be noted that although quinine can cause hemolysis, its benefits outweigh its risks and it should be used at standard doses.
(g) Renal failure and disturbances of fluid balance. Renal dysfunction is seen in about one-third of adults with severe malaria but is uncommon in children. Specialist advice should be sought if available, and dialysis may be needed. Fluid overload should be avoided, since the development of pulmonary edema carries a grave prognosis.
(h) Disseminated intravascular coagulation. If there is evidence of coagulopathy (spontaneous bleeding or prolonged prothrombin time), vitamin K should be given by slow intravenous injection. Fresh frozen plasma or, preferably, cryoprecipitates should be given, and platelet transfusion should be considered if the platelet count is less than 25,000 or the bleeding time is prolonged.
The reader will have been struck that, in clinical terms, there is little out of the ordinary in these serious syndromes: many other diseases cause a similar range of multisystem failure. Similarly, antimalarial drugs apart, the management of these syndromes follows standard practice. In the industrialized nations, where a full range of supportive care and expertise is readily available, death usually results from late presentation; the diagnosis of malaria may have been missed because of unfamiliarity and failure to enquire about foreign travel. In most countries where malaria is endemic, the diagnosis is reached swiftly because it is common. Death most usually results from a lack of resources. However, late presentation plays a part, with poverty and lack of transportation being the usual main factors.
SPLICING NEW DRUGS INTO NATIONAL POLICY
Defining New Drug Policies
Changing a drug policy in any country is not a trivial exercise. It should be stressed at the outset that decisions of this nature need to be taken by national authorities; not by drug developers or international bodies (although, when the system is working well, these two groups can make extremely helpful contributions to the process). It is increasingly apparent that a working knowledge of the key properties of a new drug (price, effectiveness, safety, practicability, and tendency to select resistance) needs to be obtained by policy makers (which is where drug developers and international bodies can prove helpful, in setting out information in a user-friendly manner).
For antimalarial drugs in Africa, the features of the disease and the patterns of drug use increase the complexity of the decision making required to define change. First, almost everyone has several illness episodes referred to as “malaria” every year. Second, almost everyone will seek a drug treatment for this illness through self-medication from poorly regulated informal sectors or at a formal clinic without basic diagnostic facilities. Finally, households and governments on the African continent are poor. In this section, we have drawn heavily from the WHO report on an informal consultation on antimalarial drugs (181), the WHO Africa Regional Office's guidelines on antimalarial policy frameworks (182), and experiences from countries in East Africa and southern Africa which have undergone recent changes to their national antimalarial drug policy (132, 180; R. Shretta, Background paper for Fourth RBM Global Partners Meet., 2001).
The WHO states that a national antimalarial drug policy is a set of recommendations and regulations concerning the availability and rational use of antimalarial drugs. The national antimalarial drug policy, states WHO, should be part of the national essential-drug policy and the NMCP and in line with the overall national health policy (181). The basic principle of an antimalarial drug policy is to provide safe, effective, high-quality, accessible, and affordable antimalarial drugs to the populations at risk of malaria according to the epidemiological setting but, at the same time, to promote rational drug use in order to delay or prevent the development of antimalarial drug resistance.
In essence, promotion of rational drug use policies must be developed in concert with definition of effective, appropriate case management guidelines. Malaria drug policy is therefore a composite of drug selection and clinical guidelines. The treatment categories include (i) drugs for first-line treatment (for probable or confirmed cases), (ii) drugs for second- and third-line treatment (given to treatment failures), (iii) drugs for severe malaria, (iv) drugs for pregnant women (including IPT), (v) self-treatment/over-the-counter treatment, and (vi) mass treatment (sometimes recommended during epidemics). The effects of HIV on the efficacy and side effects of antimalarial drugs remain poorly understood. Some preliminary data indicate a higher risk of AE with SP in HIV-positive patients (22). An antimalarial drug policy must provide for all these treatment categories, but the choice of first-line treatment has the greatest economic implications and the greatest public health implications.
A number of factors influence a national government's decision to abandon current drug policies in favor of new, revised strategies. The emphasis and significance of these factors have changed over time. Currently, there is greater emphasis on prolonging useful therapeutic lives of drugs through ACT (171, 183) and on changing international financing to support the selection of more expensive drugs (152; Global Fund To Fight AIDS, Tuberculosis and Malaria, 7 August 2003 update [http://www.fundthefund.org/gfatm.html]). Despite the dynamics of international opinion, the principles behind changing drug policy remain the same. These are described in the following sections.
Drug Efficacy
Knowledge of the efficacy of existing drugs within a range of epidemiological conditions provides the main evidence for the performance of current policy. A number of criteria have been proposed to define in vivo drug efficacy, the most widely accepted being the WHO in vivo test (182). This is a test of clinical efficacy (under conditions of supervised dosing) that is designed to estimate the outcome of malaria treatment over a 14-day period: a time chosen primarily for practicability in Africa. If the patient's condition deteriorates in the first 3 days or if parasitemia fails to fall at the desired rate, an outcome of early treatment failure is recorded. Persistence of parasitemia, with fever, after 3 days is recorded as late treatment failure. If the clinical condition of the patient meets standard criteria, including the presence of either parasitemia or fever (but not both), up to day 14, the test outcome is an adequate clinical response. More recently, there has been a general acceptance that these data do not capture the importance of persistent infections in the absence of fever, and they have been rerevised to allow for the definition of late parasitological failures (184).
These tests require only a small number of patients but must be undertaken across broad ranges of malaria ecology. The pitfalls of restricting site selection are illustrated by the Kenyan experience. Implementation of the recommendation that chloroquine should be abandoned in favor of SP as first-line treatment was delayed because all initial data were derived from only intensely malaria-endemic areas (132). Long-term surveillance of drug efficacy is also critical to allow for temporal changes in sensitivity; the same framework should be used to provide head-to-head comparisons with proposed new options for case management.
The credibility and ownership of drug efficacy data also pose problems when presented to national “stakeholders” who must decide on essential drugs, clinical case management, etc. A departure from the traditional view that drug efficacy testing is the realm of the research fraternity is gaining acceptance. The establishment of Ministry of Health sentinel sites for monitoring drug sensitivity has elevated the credibility of drug efficacy data within government decision-making processes in East Africa. This has been implemented through the East African Network for Monitoring Antimalarial Drug Therapy (EANMAT), a coalition between six ministries of health and local research groups to maintain long-term, quality-assured data at national sentinel sites (37).
There remains a considerable degree of uncertainty about when a drug should be replaced. This is largely a result of our poor understanding of the consequences of treatment failure among semi-immune patients who are reinfected frequently following initial drug exposures. Ideally, the in vivo clinical and parasitological efficacy data would be interpreted against temporal data on changing malaria morbidity and mortality risks at the community level. However, national health information systems in Africa are often unequal to the task: the diagnostic criteria remain vague, and when improved diagnostics and quality reporting exist, these capture only the events presenting to facilities, whereas most morbid and fatal events occur at home. Despite this, a number of models have been proposed to assist policy revision by using data derived from efficacy studies and based on a variety of assumptions including expert opinion of changing disease burden (including development of anemia through persistent infection, development of severe complications, and death), costs (including costs of retreatment), and compliance (10, 11, 49, 50, 131, 147).
A cutoff level of 25% treatment failures is widely quoted to prompt change based upon the above models. This cutoff level has been promoted by WHO and has been adopted by a number of countries in their general thinking of “acceptable” levels of resistance. It is, however, a variable level, depending on the degree to which patient populations have already acquired a degree of functional immunity. It would be hard to accept a drug with such a high failure rate in populations exposed to epidemics and with little immunity. Several relatively rich countries in Asia and South America have decided not to accept a level higher than 25%, while in Kenya, Tanzania, and Uganda, changes in treatment policies have not occurred until the frequency of treatment failures reached much higher levels. The extent to which populations have access to effective second-line therapies might influence the decision about the level at which changes in first-line therapies are made. The WHO Africa Regional Office has proposed a dynamic process of change which allows a stepwise analysis and interpretation of clinical failures over time (72, 182). The various proportions of clinical failure rates have been classified as the grace period, alert period, action period, and change period. These are discussed in more detail below.
(i) During the grace period, low levels of drug failures occur (≤5%). Countries may build consensus by conducting a wide range of research on epidemiological, social, behavioral situation and health systems analysis without urgency. Reliable mechanisms for malaria data collection and analysis can be established during this period. Baseline data and trends of drug efficacy should be determined.
(ii) During the alert period, treatment failure rates are between 6 and 15%. Mechanisms for the process of change should be set up, and discussions about the rate of change of drug efficacy to the current first-line drug should be initiated. The discussions should address when to implement change. The expected adverse effects of increased drug resistance should be evaluated.
(iii) During the action period, treatment failures are between 16 and 24%. Action for change should take place based on already set strategies. Treatment failure, potential alternatives, and the channels of distribution should be evaluated. This will provide a plan for intervention when resistance to the first-line drug is intolerable.
(iv) During the change period, the rate of treatment failure has reached 25% or above. Consensus for change must already have been reached, so that the change is made within the shortest time as possible. The actual cutoff point will depend on many factors.
Making sure that policy makers and stakeholders in national drug and case management policies are familiar with efficacy data, their interpretation, and their validity is paramount to effectively moving the evidence-based data into policy action. This is often a long and difficult process, demanding multiple meetings with relevant divisions within ministries of health drug manufacturers, prescribers, and consumers. This process of consensus building is of value only when there are viable alternatives for which comparative data can be simultaneously presented against the complex matrix of other considerations described below.
Acceptability and Adherence
We have already highlighted the importance of understanding the context in which drugs are used through detailed behavioral studies. Such evidence in some ways is as important to developing new drug policies as efficacy data. In the previous section, we dealt with efficiacy data. Efficacy is measured as part of a randomized clinical trial (generally as part of phase III clinical testing), where drug use is directly observed: in other words, this is a highly artificial circumstance. Adherence to drug regimens is often critical to the operational effectiveness of a drug and is increasingly important when considering ACT. Issues related to the duration of treatment, number of daily doses, speed of response to the drug, minor side effects, price versus affordability, packaging, and reputation of the drugs all play a significant role in patient adherence. New drug policies must be underpinned by research into consumer education, the training of drug providers, and the packaging of drugs (188), and this research needs to be built into the implementation phases of policy change.
Quality
Quality assurance is an important aspect of regulatory files and postmarketing surveillance. However, many drugs in common use are manufactured by local generic companies, and not “northern-hemisphere” pharmaceutical companies. Although global standards for drug quality are becoming increasingly rigorous, the quality of drugs on the market in many countries continues to pose a major public health threat (123). In Southeast Asia, Newton et al. (101) investigated the distribution of counterfeit artesunate tablets. Of 104 shop-bought “artesunate” samples from Cambodia, Laos, Myanmar (Burma), Thailand, and Vietnam, 38% did not contain artesunate. Characteristics such as the cost and physical appearance of the tablets and packaging reliably predicted authenticity. In Cambodia, fake artesunate was sold by 71% of vendors and pharmacists and fake mefloquine was sold by 60%. Patients and village health providers frequently bought the fakes because of the lower price (124). In a recent study in Nigeria, for all groups of drugs investigated, more than 50% failed to comply with the British Pharmacopoia specifications. Some preparations contained no active ingredient; some had too much, and others had too little. One study found that 100% of chloroquine phosphate syrup samples, 22% of SP samples, and 8% of quinine samples failed basic quality assurance tests (150). In an investigation of the quality of nine brands of SP drugs marketed in Dar es Salaam, Tanzania, only four passed dissolution (an in vitro test for bioavailability) (66). In Uganda, up to 30% of the tablet samples and 33% of injectable samples contained less than the stated amount of chloroquine (108). Poor-quality drugs decrease efficacy, affect the reputation of the drug, and undermine drug policy (25, 38; http://allafrica.com/health/).
Adverse Effects
AEs following antimalarial drug use have been described in detail earlier. At one end of the spectrum, mild AEs such as pruritus and gastrointestinal upset may influence adherence to the regimen. Of greater significance are syndromes such as agranulocytosis, hepatitis, and severe skin reactions. The reproductive safety of medicines remains a major concern. These represent significant threats to the public health if new antimalarial drugs continue to be used in Africa as they are at present; since almost everyone will be exposed frequently. The balance at the policy decision level is one of gains versus risks. While it is inconceivable that drugs are retained in policy despite overwhelming evidence of lack of utility, the deployment of new drugs for which wide-scale clinical experience is lacking remains a risk. Unfortunately, reliable estimates AEs for many of the new drug combinations proposed for use in Africa are not available. This will continue to hamper effective decision making, and efforts must continue to establish postmarketing surveillance systems (see the description of phase IV human studies above).
Use in Special Groups
The various groups in which an antimalarial drug is likely to be used must be considered during the policy formation. These broad groups are described above, but pregnant women are of particular importance.
Capacity of Health Care Systems To Implement Policy
The capacity of weak health care systems and drug delivery channels to handle changes in drug policy needs to be accounted for during the development of new policy. How will essential drug packages respond to changes when these are “push” rather than “pull” delivery systems? How will poorly paid health facility staff manage newer prescription-only drugs that have a high market value when they are paid poorly (133)? How will drugs be managed within the prolific and important informal sector? These are examples of factors that must be considered when revising national drug policies. In themselves, they should not be seen as impenetrable obstacles to changing drug policy, but they must be accounted when protecting the rational and equitable use of new drugs during implementation.
Cost, Affordability, and Cost Effectiveness Analysis
Analytical approaches to defining the cost implications of changing antimalarial drugs are receiving critical attention at the moment (50, 172). Models are being developed to accommodate parameters that determine the trade-offs between public health and cost. Costs apply not only to governments, which must support essential drugs through formal health care systems, but also to patients outside the formal health sector. These are complex models and by their very nature are dynamic and theoretical, but they can be used to explore options.
Goodman et al. [50] highlight the fact that any cost-effectiveness analysis involves the consideration of the incremental costs and health effects of implementing an intervention, compared with either the status quo or a different intervention. Considering the introduction of SP as a new first-line drug, a comparison could be made with the costs and effects of continuing to use chloroquine. Alternatively, the cost of changing immediately to SP could be compared with changing after a delay of 3 or 5 years, and SP could be compared with other replacement regimens. Other parameters within a cost-effectiveness equation include changes in caseload, frequency of drug and laboratory supply stock taking, patient waiting times, costs to patients (through fewer return visits to formal health care facilities and additional visits to other treatment sources), time diverted from productive activities, and Incidence of new cases (by the use of transmission-blocking drugs such as artemisinins) (103).
Presently, all that national decision-makers have at their disposal are the costs per treatment course of each drug against the reported efficacy of the drugs. In some ways, this is all that many governments are concerned with; they are less concerned about cost-effectiveness but are interested to know whether they can afford the change. Information about the current and projected future number of first-line and second-line treatments required and the prices of different drugs remains a critical part of the decision-making process. This is particularly true for countries in Africa, where per capita expenditure on essential drugs is declining. In Kenya, for example, per capita expenditure on essential drugs has declined by as much as 40% in real terms owing to a weakening of the ability of Kenyan shilling to buy overseas raw commodities and to declining donor support.
Achieving a Consensus
Early participation of all national stakeholders in reviewing data and options is critical to building a consensus for drug policy change. This is often harder than it appears on the surface. There are a number of examples where key stakeholders were unintentionally not included in early discussions, leading to a fragmented policy discussion (132, 134). Building several layers of consensus simultaneously is difficult: drug policies must be developed in concert with policies on case management (including IMCI), management of malaria in pregnancy (safe-motherhood initiatives), and policies and legislation effecting drug registration, access, and regulation. For these reasons alone, it is not surprising that the time taken from the detection of dramatic declines in drug sensitivity through policy change to implementation of new policies can be between 2 and 5 years.
CONCLUSIONS
African nations will continue to face many health challenges in coming years, and malaria remains one the major burdens. Despite a great deal of progress in our understanding of this parasite and the pathophysiological and immune processes that it initiates, we have barely held our own against its burden of mortality and morbidity. By and large, we already possess many of the necessary tools to improve malaria control in Africa, but their implementation is currently suboptimal. This is mainly for reasons of economics (the revenue costs of the interventions to private individuals and health care systems) and logistics (the relative lack of human resources for complex interventions). So, while it is true that new tools will always be needed, the most immediate concern is obtaining the wherewithal to implement existing control measures. This message seems to have been heard by the leaders of the G8 nations, and a Global Fund has been set up. However, international political will would need to be sustained if inroads are to be made, and the Global Fund is currently unequal to its task.
For example, the transmission of falciparum malaria could be markedly reduced. We have no vaccine (although this is an area of ongoing endeavour), but ITNs have been shown to have the capability of major impact on public health. Sustainable implementation of ITNs is one of the goals of malaria control, but readers should not underestimate the effort that will be required to make widespread implementation happen.
It seems that we shall be reliant on antimalarial drugs for many decades to come. The drugs used for severe malaria are not currently threatened by resistance, but those used as first-line therapy (whether as self-medication from shops or in the formal health care system) are failing fast. Africa will certainly need access to novel antimalarial drugs in due course, and drug discovery efforts are under way. However, replacements for SP are needed immediately, and it takes many years to develop a novel molecule into drug. Therefore, for some time to come, we shall be reliant on the intelligent deployment of existing drugs and drug combinations. Thanks to the efforts of academia, international organizations (such as WHO, MMV, and the Wellcome Trust) and a few pharmaceutical companies, a variety of drugs and drug combinations will become available in the next 2 or 3 years. However, we need to keep costs and practicality in the forefront of our minds. Chloroquine and SP are the principal drugs for malaria management at present, and they cost a few cents per treatment. Resistance is making us think of using drugs that cost multiples of dollars: this in a setting where many ministries of health are faced with declining drug budgets and escalating problems in other areas (not least HIV). New approaches to case definitions/diagnosis and case management may be needed in this setting.
It is important to be aware that the data needed to pass regulatory scrutiny—the stage that the next wave of drugs is approaching—are insufficient to form a basis on which to determine the role of a new drug for the public health. Decision makers need data on operational effectiveness (efficacy data from randomized trials are simply too articifial), safety (with data sets in the tens of thousands), and impact on the evolution of drug resistance. There is an urgent need to use behavioral and epidemiological data on fever and drug exposure risks to better estimate the likely AE profiles under different endemicity and drug management scenarios. And even when such operational data are available, experience shows us that the business of splicing new drugs into the public health is extremely complicated, often taking us beyond science and into national politics.
Despite the enormity of the challenges, there may be some justification for a glimmer of cautious optimism: basic scientists, economists, sociologists, and public health scientists are probably in much closer collaboration nowadays than they have been in the past. Any attempt to understand the vast complexities of human malaria requires an ability to think “from molecule to policy,” and this will require continued multidisciplinary communication and effort.
Acknowledgments
R.S. is supported by The Wellcome Trust (U.K.) as part of the Senior Fellows Program (no. 058992). P.W. and S.W. are grateful to the Wellcome Trust for institutional support.
REFERENCES
- 1.Abuya, T. O. 2001. A descriptive account of private clinics in a malaria endemic area on the Kenyan Coast: quality of care and patient compliance. M.S. thesis, Kenyatta University Nairobi, Kenya.
- 2.Akpede, G. O., R. M. Sykes, and P. O. Abiodun. 1992. Indications for lumbar puncture in children presenting with convulsions and fever of acute onset: experience in the Children's Emergency Room of the University of Benin Teaching Hospital, Nigeria. Ann. Trop. Paediatr. 12:385-389. [DOI] [PubMed] [Google Scholar]
- 3.Reference deleted.
- 4.Anonymous. 2000. Severe falciparum malaria. World Health Organization. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-S90. [PubMed] [Google Scholar]
- 5.Ansah, E. K., J. O. Gyapong, I. A. Agyepong, and D. B. Evans. 2001. Improving adherence to malaria treatment for children: the use of pre-packed chloroquine tablets vs chlorquine syrup. Trop. Med. Int. Health 6:496-504. [DOI] [PubMed] [Google Scholar]
- 6.Barat, L., J. Chipipa, M. Kolczak, and T. Sukwa. 1999. Does the availability of blood slide microscopy for malaria at health centres improve the management of persons with fever in Zambia? Am. J. Trop. Med. Hyg. 60:1024-1030. [DOI] [PubMed] [Google Scholar]
- 7.Barat, L., and M. H. Kramer. 1999. Assessment of microscopic diagnosis of malaria at eight health facilities in Bungoma District, Kenya. Final Trip Report. Malaria Epidemiology Section, EB, DPD, Centers for Disease Control & Prevention, Atlanta, Ga.
- 8.Biritwum, R. B., J. Welbeck, and G. Barnish. 2000. Incidence and management of malaria in two communities of different socio-economic level, in Accra, Ghana. Ann. Trop. Med. Parasitol. 94:771-778. [DOI] [PubMed] [Google Scholar]
- 9.Binka, F. N., S. S. Morris, D. A. Ross, P. Arthur, and M. E. Aryeetey. 1994. Patterns of malaria morbidity and mortality in children in Northern Ghana. Trans. R. Soc. Trop. Med. Hyg. 88:381-385. [DOI] [PubMed] [Google Scholar]
- 10.Bloland, P. B., and M. Ettling. 1998. Making malaria treatment policy in the face of drug resistance. Ann. Trop. Med. Parasitol. 93:5-23. [DOI] [PubMed] [Google Scholar]
- 11.Bloland, P. B., P. N. Kazembe, A. J. Oloo, B. Himonga, L. M. Barat, and T. K. Ruebush. 1998. Chloroquine in Africa: critical assessment and recommendations for monitoring and evaluating chloroquine therapy efficacy in sub-Saharan Africa. Trop. Med. Int. Health 3:543-552. [DOI] [PubMed] [Google Scholar]
- 12.Brabin, B. J., S. O. Agbaje, Y. Ahmed, and N. D. Briggs. 1999. A birthweight nomogram for Africa, as a malaria-control indicator. Ann. Trop. Med. Parasitol. 93(Suppl. 1):S43-S57. [PubMed] [Google Scholar]
- 13.Brabin, B. J., M. Hakimi, and D. Pelletier. 2001. An analysis of anemia and pregnancy-related maternal mortality. J. Nutr. 131:604S-614S. [DOI] [PubMed] [Google Scholar]
- 14.Bray, P. G., O. Janneh, K. J. Raynes, M. Mungthin, H. Ginsburg, and S. A. Ward. 1999. Cellular uptake of chloroquine is dependent on binding to ferriprotoporphyrin IX and is independent of NHE activity in Plasmodium falciparum. J. Cell Biol. 145:363-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bray, P. G., M. Mungthin, R. G. Ridley, and S. A. Ward. 1998. Access to hematin: the basis of chloroquine resistance. Mol. Pharmacol. 54:170-179. [DOI] [PubMed] [Google Scholar]
- 16.Bray, P. G., S. R. Hawley, M. Mungthin, and S. A. Ward. 1996. Physicochemical properties correlated with drug resistance and the reversal of drug resistance in Plasmodium falciparum. Mol. Pharmacol. 50:1559-1566. [PubMed] [Google Scholar]
- 17.Brewster, D. R., D. Kwiatkowski, and N. J. White. 1990. Neurological sequelae of cerebral malaria in children. Lancet 336:1039-1043. [DOI] [PubMed] [Google Scholar]
- 18.Brown, H., S. Rogerson, T. Taylor, M. Tembo, J. Mwenechanya, M. Molyneux, and G. Turner. 2001. Blood-brain barrier function in cerebral malaria in Malawian children. Am. J. Trop. Med. Hyg. 64:207-213. [DOI] [PubMed] [Google Scholar]
- 19.Canfield, C. J., M. Pudney, and W. E. Gutteridge. 1995. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp. Parasitol. 80:373-381. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control. 1985. Adverse reactions to Fansidar and updated recommendations for its use in the prevention of malaria. Morb. Mortal. Wkly. Rep. 8:713-714. [PubMed] [Google Scholar]
- 21.Chandramohan, D., S. Jaffar, and B. M. Greenwood. 2002. Use of clinical algorithms for diagnosing malaria. Trop. Med. Int. Health 7:45-52. [DOI] [PubMed] [Google Scholar]
- 22.Coopman, S. A., R. A. Johnson, R. Platt, and R. S. Stern. 1993. Cutaneous disease and drug reactions in HIV infection. N. Engl. J. Med. 328:1670-1674. [DOI] [PubMed] [Google Scholar]
- 23.Crawley, J., C. Waruiru, S. Mithwani, I. Mwangi, W. Watkins, D. Ouma, P. Winstanley, T. Peto, and K. Marsh. 2000. Effect of phenobarbital on seizure frequency and mortality in childhood cerebral malaria. Lancet 355:701-707. [DOI] [PubMed] [Google Scholar]
- 24.Dabis, F., J. G. Breman, A. J. Roisin, F. Haba, and the ACSI-CCCD team. 1989. Monitoring selective components of the primary health care: methodology and community assessment of vaccination, diarrhoea and malaria practices in Conakry, Guinea. Bull. W. H. O. 67:675-684. [PMC free article] [PubMed] [Google Scholar]
- 25.Daily News, Tanzania. 2001. Malaria drug SP in limbo. 4 July 2001.
- 26.Deen, J. L., L. von Seidlein, M. Pinder, G. E. Walraven, and B. M. Greenwood. 2001. The safety of the combination artesunate and pyrimethamine-sulfadoxine given during pregnancy. Trans. R. Soc. Trop. Med. Hyg. 95:424-428. [DOI] [PubMed] [Google Scholar]
- 27.Deming, M. S., A. Gayibor, K. Murphy, T. S. Jones, and T. Karsa. 1989. Home treatment of febrile children with anti-malarial drugs in Togo. Bull. W. H. O. 67:695-700. [PMC free article] [PubMed] [Google Scholar]
- 28.Diallo, A. B., G. De Serres, A. H. Beavogui, C. Lapointe, and P. Viens. 2001. Home care of malaria infected children less than 5 years of age in a rural area of the republic of Guinea. Bull. W. H. O. 79:28-32. [PMC free article] [PubMed] [Google Scholar]
- 29.Reference deleted.
- 30.Dobson, M. J., M. Malowany, and R. W. Snow. 2000. Malaria control in East Africa: the Kampala conference and the Pare-Taveta scheme—a meeting of common and high ground. Parasitologia 42:149-167. [PubMed] [Google Scholar]
- 31.Dollery, C. (ed.). 1999. Chloroquine, p. C177-C182. In Therapeutic drugs. Churchill Livingstone, Inc., New York, N.Y.
- 32.Dollery, C. (ed.). 1999. Pyrimethamine, p. P296-P300. In Therapeutic drugs. Churchill Livingstone, Inc., New York, N.Y.
- 33.Dollery, C. (ed.). 1999. Proguanil, p. P232-P236. In Therapeutic drugs. Churchill Livingstone, Inc., New York, N.Y.
- 34.Dollery, C. (ed.). 1999. Dapsone, p. D13-D18. In Therapeutic drugs. Churchill Livingstone, Inc., New York, N.Y.
- 35.Dollery, C. (ed.). 1999. Quinine, p. Q16-Q21. In Therapeutic drugs. Churchill Livingstone, Inc., New York, N.Y.
- 36.Dollery, C. (ed.). 1999. Artemisinin, p. A208-A213. In Therapeutic drugs. Churchill Livingstone, Inc., New York, N.Y.
- 37.East African Network for Monitoring Antimalarial Treatment. 2001. Monitoring antimalarial drug resistance within national malaria control programmes (NMCPs): the EANMAT experience. Trop. Med. Int. Health 6:1-8. [DOI] [PubMed] [Google Scholar]
- 38.East African Standard. 1999. Pharmacy boss blames weak legislation for drugs menace, p. 8. 14, September 1999.
- 39.English, M., R. Sauerwein, C. Waruiru, M. Mosobo, J. Obiero, B. Lowe, and K. Marsh. 1997. Acidosis in severe childhood malaria. Q. J. Med. 90:263-270. [DOI] [PubMed] [Google Scholar]
- 40.English, M., M. Ahmed, C. Ngando, J. Berkley, and A. Ross. 2002. Blood transfusion for severe anaemia in children in a Kenyan hospital. Lancet 359:494-495. [DOI] [PubMed] [Google Scholar]
- 41.Ettling, M., D. A. McFarland, L. J. Schultz, and L. Chitsulo. 1994. Economic impact of malaria in Malawian households. Trop. Med. Parasitol. 45:74-79. [PubMed] [Google Scholar]
- 42.Ejezie, G. C., E. N. U. Ezedinachi, E. A. Usanga, E. I. I. Gemade, N. W. Ikpatt, and A. A. A. Alaribe. 1991. Malaria and its treatment in rural villages of Aboh Mbaise, Imo state, Nigeria. Acta Trop. 48:17-24. [DOI] [PubMed] [Google Scholar]
- 43.Ezzet, F., M. van Vugt, F. Nosten, S. Looareesuwan, and N. J. White. 2000. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob. Agents Chemother. 44:697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Font, F., M. Alonso Gonzalez, R. Nathan, J. Kimario, F. Lwilla, C. Ascaso, M. Tanner, C. Menendez, and P. L. Alonso. 2001. Diagnostic accuracy and case-management of clinical malaria in the primary health services of a rural area in south-eastern Tanzania. Trop. Med. Int. Health 6:423-428. [DOI] [PubMed] [Google Scholar]
- 45.Foster, S. O., R. A. Spiegel, A. Mokdad, S. Yeanon, S. R. Becker, J. N. Thornton, and M. K. Galakpai. 1993. Immunisation, oral rehydration therapy and malaria chemotherapy under 5 in Bomi & Grand Cape Mount Counties, Liberia, 1984-1988. Int. J. Epidemiol. 22(Suppl. 1):S50-S55. [DOI] [PubMed] [Google Scholar]
- 46.Foster, S. D. F. 1991. Pricing, distribution and use of antimalarial drugs. Bull W. H. O. 69:349-361. [PMC free article] [PubMed] [Google Scholar]
- 47.Glik, D. C., W. B. Ward, A. Gordon, and F. Haba. 1989. Malaria treatment among mothers in Guinea. J. Health Soc. Behav. 30:421-435. [PubMed] [Google Scholar]
- 48.Reference deleted.
- 49.Goodman, C. A., P. G. Coleman, and A. J. Mills. 1999. Cost-effectiveness of malaria control in sub-Saharan Africa. Lancet 354:378-384. [DOI] [PubMed] [Google Scholar]
- 50.Goodman, C. A., P. G. Coleman, and A. J. Mills. 2001. Changing the first line drug for malaria treatment — cost-effectiveness analysis with highly uncertain inter-temporal trade-offs. Health Econ. 10:731-749. [DOI] [PubMed] [Google Scholar]
- 51.Gove, S. 1997. Integrated management of childhood illness by out-patient health workers: technical basis and overview. Bull. W. H. O. 75(Suppl. 1):7-24. [PMC free article] [PubMed] [Google Scholar]
- 52.Greenberg, A. E., P. Nguyen-Dinh, J. M. Mann, N. Kabote, R. L. Colebunders, H. Francis, T. C. Quinn, P. Baudoux, B. Lyamba, F. Davachi, J. M. Roberts, N. Kabeya, J. W. Curran, and C. C. Campbell. 1988. The association between malaria, blood transfusions and HIV seropositivity in a paediatric population in Kinshasa, Zaire. JAMA 259:545-549. [PubMed] [Google Scholar]
- 53.Greenwood, B. M., K. Marsh, and R. W. Snow. 1991. Why do some children develop severe malaria? Parasitol. Today 7:277-281. [DOI] [PubMed] [Google Scholar]
- 54.Griffith, S. 1990. A review of the factors associated with patient compliance and the taking of prescribed medicines. Br. J. Gen. Pract. 40:114-116. [PMC free article] [PubMed] [Google Scholar]
- 55.Hamel, M. J., A. Odhacha, J. M. Roberts, and M. S. Deming. 2001. Malaria control in Bungoma District, Kenya: a survey of home treatment of children with fever, bed net use and attendance at antenatal clinics. Bull. W. H. O. 79:1014-1023. [PMC free article] [PubMed] [Google Scholar]
- 56.Hatton, C., T. Peto, C. Bunch, G. Pasvol, S. Russel, C. Singer, G. Edwards, and P. A. Winstanley. 1986. Frequency of severe neutropaenia associated with amodiaquine prophylaxis against malaria. Lancet i:411-413. [DOI] [PubMed] [Google Scholar]
- 57.Henry, M. C., C. Rogier, I. Nzeyimana, S. B. Assy, J. Doussou-Yovo, M. Audibert, J. Mathonnat, A. Keundjian, E. Akodo, T. Teuscher, and P. Carnevale. 2003. Inland valley rice production systems and malaria infection and disease in the savannah of Cote D'Ivoire. Trop. Med. Int. Health 8:449-458. [DOI] [PubMed] [Google Scholar]
- 58.Herman, E. J. 1999. Assessment of the quality of outpatient management of childhood illness in non-government health facilities, Bungoma District, Kenya. Final report. Centers for Disease Control and Prevention, Atlanta, Ga.
- 59.Hero, M., S. P. Harding, C. E. Riva, P. A. Winstanley, N. Peshu, and K. Marsh. 1997. Photographic and angiographic characterization of the retina of Kenyan children with severe malaria. Arch. Ophthalmol. 115:997-1003. [DOI] [PubMed] [Google Scholar]
- 60.Holding, P. A., and R. W. Snow. 2001. The impact of Plasmodium falciparum malaria on performance and learning: a review of the evidence. Am. J. Trop. Med. Hyg. 64(Suppl. 1):68-75. [DOI] [PubMed] [Google Scholar]
- 61.Holtz, T. H., L. H. Marum, C. Mkandala, N. Chizani, J. M. Roberts, A. Macheso, M. E. Parise, and S. P. Kachur. 2001. Health-seeking behaviour and home treatment for febrile illness, Blantyre district, Malawi, 2000. Presented at 50th Annual Meeting of The American Society of Tropical Medicine & Hygiene. Am. J. Trop. Med. Hyg. 65(Suppl. 3):329.11693878 [Google Scholar]
- 62.Homedes, N., and A. Ugalde. 1993. Patients' compliance with medical treatments in the third world. What do we know? Health Policy Planning 8:291-314. [Google Scholar]
- 63.Reference deleted.
- 64.Jackson, L. C. 1985. Malaria in Liberian children and mothers: biocultural perceptions of illness vs clinical evidence of disease. Soc. Sci. Med. 20:1281-1287. [DOI] [PubMed] [Google Scholar]
- 65.James, S. P. 1931. Some general results of a study of induced malaria in England. Trans. R. Soc. Trop. Med. Hyg. 24:478-538. [Google Scholar]
- 66.Jande, M. B., O. Ngassapa, and I. O. Kibwage. 2000. Quality of sulfadoxine/pyrimethamine tablets marketed in Dar es Salaam, Tanzania. East Central Afr. J. Pharm Sci. 3:20-24. [Google Scholar]
- 67.Josse, R., M. Merlin, A. Combe, R. Josseran, J. Y. Le Hesran, F. Avennec, B. Ngnintedem, A. M. Nkongtso, M. Kamwa, P. Eboumbou, J. Tribouley, and C. Ripert. 1988. Etude comparee des indices paludometriques a Nanga-Eboko, Yaounde et Edea (Cameroun). Med. Trop. 48:201-208. [PubMed] [Google Scholar]
- 68.Kaburu, J. N. 2002. Malaria treatment seeking behaviour in Kwale District of coast province of Kenya. M. Phil. thesis. Kenyatta University, Nairobi, Kenya.
- 69.Karbwang, J., and N. J. White. 1990. Clinical pharmacokinetics of mefloquine. Clin. Pharmacokinet. 19:264-79. [DOI] [PubMed] [Google Scholar]
- 70.Kengeya-Kayondo, J. F., J. A. Seeley, E. Kajura-Bajenja, E. Kabunga, E. Mubiru, F. Sembajja, and D. W. Mulder. 1994. Recognition, treatment seeking behaviour and perception of cause of malaria among rural women in Uganda. Acta Trop. 58:267-273. [DOI] [PubMed] [Google Scholar]
- 71.Kidane, G., and R. H. Morrow. 2000. Teaching mothers to provide home treatment of malaria in Tigray, Ethiopia: a randomised trial. Lancet 356:550-555. [DOI] [PubMed] [Google Scholar]
- 72.Kitua, A. Y. 1999. Antimalarial drug policy: making systematic change. Lancet 354(Suppl. IV):32. [DOI] [PubMed] [Google Scholar]
- 73.Krause, G., and G. Sauerborn. 2000. Comprehensive community effectiveness of health care. A study of malaria treatment in children and adults in rural Burkina Faso. Ann. Trop. Paediatr. 20:273-282. [DOI] [PubMed] [Google Scholar]
- 74.Krishna, S., W. Supanaranond, S. Pukrittayakamee, F. T. Kuile, M. Ruprah, and N. J. White. 1996. The disposition and effects of two doses of dichloroacetate in adults with severe falciparum malaria. Br. J. Clin. Pharmacol. 41:29-34. [DOI] [PubMed] [Google Scholar]
- 75.Krishna, S., T. Planche, T. Agbenyega, C. Woodrow, D. Agranoff, G. Bedu-Addo, A. K. Owusu-Ofori, J. A. Appiah, S. Ramanathan, S. M. Mansor, and V. Navaratnam. 2001. Bioavailability and preliminary clinical efficacy of intrarectal artesunate in Ghanaian children with moderate malaria. Antimicrob. Agents Chemother. 45:509-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kublin, J. G., F. Dzinjalamala, D. Kamwendo, E. Malkin, J. Cortese, L. Martino, R. Mukadam, S. Rogerson, A. Lescano, M. E. Molyneux, P. A. Winstanley, P. Chimpeni, T. E. Taylor, and C. V. Plowe. 2001. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J. Infect. Dis. 185:380-388. [DOI] [PubMed] [Google Scholar]
- 77.Kublin, J. G., J. F. Cortese, E. M. Njunju, R. A. Mukadama, J. J. Wirima, P. N. Kazembe, A. A. Djimde, B. Kouriba, and C. V. Plowe. 2003. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J. Infect. Dis. 187:1870-1875. [DOI] [PubMed] [Google Scholar]
- 78.Li, Z. L. 1988. Teratogenicity of sodium artesunate. Zhong Yao Tong Bao 13:42-44. [PubMed] [Google Scholar]
- 79.Lin, Y., and L. M. Franco. 2000. Assessing malaria treatment and control at peer facilities in Malawi. Quality Assurance Project Case Study. Published for the USAID by the quality Assurance Project (QAP), Bethesda, Md.
- 80.Lindblade, K. A., D. B. O'Neill, D. P. Mathanga, J. Katungu, and M. L. Wilson. 2000. Treatment for clinical malaria is sought promptly during an epidemic in a highland region of Uganda. Trop. Med. Int. Health 5:865-875. [DOI] [PubMed] [Google Scholar]
- 81.Lubanga, R. G. N., S. Norman, D. Ewbank, and C. Karamagi. 1997. Maternal diagnosis and treatment of children's fever in an endemic malaria zone of Uganda: implications for the malaria control programme. Acta Trop. 68:53-64. [DOI] [PubMed] [Google Scholar]
- 82.Magnussen, P., B. Ndawi, A. K. Sheshe, J. Byskov, and K. Mbwana. 2001. Malaria diagnosis and treatment administered by teachers in primary schools in Tanzania. Trop Med Int. Health 6:273-279. [DOI] [PubMed] [Google Scholar]
- 83.Marsh, V. M., W. M. Mutemi, J. Muturi, A. Haaland, W. M. Watkins, G. Otieno, K. Marsh. 1999. Changing home treatment of childhood fevers by training shop-keepers in rural Kenya. Trop. Med. Int. Health 4:383-389. [DOI] [PubMed] [Google Scholar]
- 84.McCrombie S. C. 1996. Treatment seeking for malaria: a review of recent research. Soc. Sci. Med. 43:933-945. [DOI] [PubMed] [Google Scholar]
- 85.McGready, R., T. Cho, N. K. Keo, K. L. Thwai, L. Villegas, S. Looareesuwan, N. J. White, and F. Nosten. 2001. Artemisinin antimalarials in pregnancy: a prospective treatment study of 539 episodes of multidrug-resistant Plasmodium falciparum. Clin. Infect. Dis. 33:2009-2016. [DOI] [PubMed] [Google Scholar]
- 86.Reference deleted.
- 87.Meshnick, S. R. 2002. Artemisinin: mechanisms of action, resistance and toxicity. Int. J. Parasitol. 32:1655-1660. [DOI] [PubMed] [Google Scholar]
- 88.Menendez, C., E. Kahigwa, and R. Hirt. 1997. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxsis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet 350:844-850. [DOI] [PubMed] [Google Scholar]
- 89.Molineaux, L. 1996. Plasmodium falciparum malaria: some epidemiological implications of parasite and host diversity. Ann. Trop. Med. Parasitol. 90:379-393. [DOI] [PubMed] [Google Scholar]
- 90.Molyneux, C. S., V. Mung'ala-Odera, T. Harpham, and R. W. Snow. 1999. Maternal responses to childhood fevers: a comparison of rural and urban residents in coastal Kenya. Trop. Med. Int. Health 4:836-845. [DOI] [PubMed] [Google Scholar]
- 91.Molyneux, D. H., K. Floyd, G. Barnish, and E. M. Fevre. 1999. Transmission control and drug resistance in malaria: a crucial interaction. Parasitol. Today 15:238-240. [DOI] [PubMed] [Google Scholar]
- 92.Molyneux, M. E. 2000. Impact of malaria on the brain and its prevention. Lancet 355:671-672. [DOI] [PubMed] [Google Scholar]
- 93.Mwangi, T. W. 2003. PhD thesis. Clinical epidemiology of malaria under differing levels of transmission in Kilifi, Kenya. Open University, Milton Keynes, United Kingdom.
- 94.Mwenesi, H. R. A. 1993. Mothers' definition and treatment of childhood malaria on the Kenyan Coast. Ph.D. thesis. London School of Hygiene & Tropical Medicine, University of London, London, United Kingdom.
- 95.Mwenesi, H. 1994. The role of drug delivery systems in health care: the case of self-medication. Afr. J. Health Sci. 1:42-48. [PubMed] [Google Scholar]
- 96.Nabbarro, D. N., and E. M. Tayler. 1998. The “roll back malaria” campaign. Science 280:2067-2068. [DOI] [PubMed] [Google Scholar]
- 97.Neuvians, D., F. D. E. Mtango, and A. A. Kielmann. 1988. The burden of disease among pre-school children from rural Tanzania. Trop. Med. Parasitol. 39:9-13. [PubMed] [Google Scholar]
- 98.Newton, C. R., and D. A. Warrell. 1998. Neurological manifestations of falciparum malaria. Ann. Neurol. 43:695-702. [DOI] [PubMed] [Google Scholar]
- 99.Newton, C. R., T. T. Hien, and N. White. 2000. Cerebral malaria. J. Neurol. Neurosurg. Psychiatry 69:433-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Newton, C. R., F. J. Kirkham, P. A. Winstanley, G. Pasvol, N. Peshu, D. A. Warrell, and K. Marsh. 1991. Intracranial pressure in African children with cerebral malaria. Lancet 337:573-576. [DOI] [PubMed] [Google Scholar]
- 101.Newton, P., S. Proux, M. Green, F. Smithuis, J. Rozendaal, S. Prakongpan, K. Chotivanich, M. Mayxay, S. Looareesuwan, J. Farrar, F. Nosten, and N. J. White. 2001. Fake artesunate in Southeast Asia. Lancet 357:1948-1950. [DOI] [PubMed] [Google Scholar]
- 102.Nosten F., F. O. ter Kuile, C. Luxemburger, C. Woodrow, D. E. Kyle, T. Chongsuphajaisiddhi, and N. J. White. 1993. Cardiac effects of antimalarial treatment with halofantrine. Lancet 341:1054-1056. [DOI] [PubMed] [Google Scholar]
- 103.Nosten, F., M. van Vugt, R. Price, C. Luxemburger, K. L. Thway, A. Brockman, R. McGready, F. ter Kuile, S. Looareesuwan, and N. J. White. 2000. Effects of artesunate-mefloquine combination on the incidence of Plasmodium falciparum malaria and mefloquine resistance in Western Thailand: a prospective study. Lancet 356:297-302. [DOI] [PubMed] [Google Scholar]
- 104.Nosten, F., and P. Brasseur. 2002. Combination therapy for malaria: the way forward? Drugs 62:1315-1329. [DOI] [PubMed] [Google Scholar]
- 105.O'Dempsey, T., T. F. McArdle, B. E. Laurence, J. C. Lamont, J. E. Todd, and B. Greenwood. 1993. Overlap in the clinical features of pneumonia and malaria in African children. Trans. R. Soc. Trop. Med. Hyg. 87:662-665. [DOI] [PubMed] [Google Scholar]
- 106.Ofori-Adjei, D., and D. K. Arhinful. 1996. Effect of training on the clinical management of malaria by medical assistants in Ghana. Soc. Sci. Med. 42:1169-1176. [DOI] [PubMed] [Google Scholar]
- 107.Okonkwo, P. O., C. O. Akpala, H. U. Okafor, A. U. Mbah, and O. Nwaiwu. 2001. Compliance to correct dose of chloroquine in uncomplicated malaria correlates with improvement in the condition of rural Nigerian children. Trans. R. Soc. Trop. Med. Hyg. 95:320-324. [DOI] [PubMed] [Google Scholar]
- 108.Ogwal-Okeng, J. W., D. O. Okello, and O. Odyek. 1998. Quality of oral and parenteral chloroquine in Kampala. East Afr. Med. J. 75:692-694. [PubMed] [Google Scholar]
- 109.Omari, A. A., C. Preston, and P. Garner. 2002, posting date. Artemether-lumefantrine for treating uncomplicated falciparum malaria. Cochrane Database Syst. Rev. [Online]. [DOI] [PubMed]
- 110.Osei, L., and H. A. Beecham. 1990. Drug use before clinic attendance. Ghana Med. J. 24:249-254. [Google Scholar]
- 111.Pagnoni, F., N. Convelbo, J. Tiendrebeogo, S. Cousens, and F. Esposito. 1997. A community-based programme to provide prompt and adequate treatment of presumptive malaria in children. Trans. R. Soc. Trop. Med. Hyg. 91:512-517. [DOI] [PubMed] [Google Scholar]
- 112.Pasvol, G., C. R. Newton, P. A. Winstanley, W. M. Watkins, N. M. Peshu, J. B. Were, K. Marsh, and D. A. Warrell. 1991. Quinine treatment of severe falciparum malaria in African children: a randomized comparison of three regimens. Am. J. Trop. Med. Hyg. 45:702-713. [DOI] [PubMed] [Google Scholar]
- 113.Petersen, E., B. Hogh, N. T. Marbiah, E. Dolopaie, A. Gottschaus, A. P. Hanson, and A. Bjorkman. 1991. Clinical and parasitological studies on malaria in Liberian adults living under intense malaria transmission. Ann. Trop. Med. Parasitol. 85:577-584. [DOI] [PubMed] [Google Scholar]
- 114.Plowe, C. V., J. F. Cortese, A. Djimde, O. C. Nwanyanwu, W. M. Watkins, P. A. Winstanley, J. G. Estrada-Franco, R. E. Mollinedo, J. C. Avila, J. L. Cespedes, D. Carter, and O. K. Doumbo. 1997. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J. Infect. Dis. 176:1590-1596. [DOI] [PubMed] [Google Scholar]
- 115.Rashed, S., H. Johnston, P. Dongier, R. Moreau, C. Lee, J. Lambert, and C. Schaefer. 2000. Economic impact of febrile morbidity and the use of permethrin-impregnated bed nets in a malarious area. I. Study of demographics, morbidity and household expenditures associated with febrile morbidity in the Republic of Benin. Am. J. Trop. Med. Hyg. 62:173-180. [DOI] [PubMed] [Google Scholar]
- 116.Redd, S. C., P. B. Bloland, P. N. Kazembe, E. Patrick, R. Tembenu, and C. C. Campbell. 1992. Usefulness of clinical case-definitions in guiding therapy for African children with malaria or pneumonia. Lancet 340:1140-1143. [DOI] [PubMed] [Google Scholar]
- 117.Redd, S. C., P. N. Kazembe, S. P. Luby, O. Nwanyanwu, A. W. Hightower, C. Ziba, J. J. Wirima, L. Chitsulo, C. Franco, and M. Olivar. 1996. Clinical algorithm for treatment of Plasmodium falciparum malaria in children. Lancet 347:223-227. [DOI] [PubMed] [Google Scholar]
- 118.Richens, J., T. Smith, T. Mylius, and V. Spooner. 1992. An algorithm for the clinical differentiation of malaria and typhoid: a preliminary communication. Papua New Guinea Med. J. 35:298-302. [PubMed] [Google Scholar]
- 119.Ridley, R. G. 2002. Medical need scientific opportunity and the drive for antimalarial drugs. Nature 415:686-693. [DOI] [PubMed] [Google Scholar]
- 120.Riou, B., P. Barriot, A. Rimailho, and F. J. Baud. 1988. Treatment of severe chloroquine poisoning. N. Engl. J. Med. 318:1-6. [DOI] [PubMed] [Google Scholar]
- 121.Rogier, C., D. Commenges, and J.-F. Trape. 1996. Evidence for an age-dependent pyrogenic threshold of Plasmodium falciparum parasitaemia in highly endemic populations. Am. J. Trop. Med. Hyg. 54:613-619. [DOI] [PubMed] [Google Scholar]
- 122.Reference deleted.
- 123.Roy, J. 1994. The menace of substandard drugs. World Health Forum 15:406-407. [PubMed] [Google Scholar]
- 124.Rozendaal, J. 2001. Fake antimalaria drugs in Cambodia. Lancet 357:890. [DOI] [PubMed] [Google Scholar]
- 125.Ruebush, T. K., M. K. Kern, C. C. Campell, and A. J. Oloo. 1995. Self-treatment of malaria in a rural area of Western Kenya. Bull. W. H. O. 73:229-236. [PMC free article] [PubMed] [Google Scholar]
- 126.Sachs, J., and P. Malaney. 2002. The economic and social burden of malaria. Nature 415:680-685. [DOI] [PubMed] [Google Scholar]
- 127.Salako, L. A., W. R. Breiger, B. M. Afolabi, R. E. Umeh, P. U. Agomo, A. Asa, A. K. Adeneye, B. O. Nwankwo, and C. O. Akinlade. 2001. Treatment of childhood fevers and other illnesses in three rural Nigerian communities. J. Trop. Paediatr. 47:230-238. [DOI] [PubMed] [Google Scholar]
- 128.Schellenberg, D., C. Menendez, E. Kahigwa, J. Aponte, J. Vidal, M. Tanner, H. Mshinda, and P. Alonso. 2001. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: a randomised, placebo-controlled trial. Lancet 357:1471-1477. [DOI] [PubMed] [Google Scholar]
- 129.Schulpen, T. W. J., and W. J. A. M. Swinkels. 1980. Machakos Project Studies: Agents affecting health of mother and child in a rural area of Kenya. XIX. The utilization of health services in a rural area of Kenya. Trop. Geogr. Med. 32:340-349. [PubMed] [Google Scholar]
- 130.Shanks, G. D., K. Biomondo, S. I. Hay, and R. W. Snow. 2000. Changing patterns of clinical malaria since 1965 among a tea estate population located in the Kenyan highlands. Trans. R. Soc. Trop. Med. Hyg. 94:253-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schapira, A., P. F. Beales, and M. E. Halloran. 1993. Malaria: Living with drug resistance. Parasitol. Today 9:168-174. [DOI] [PubMed] [Google Scholar]
- 132.Shretta, R., J. A. Omumbo, B. Rapuoda, and R. W. Snow. 2000. Using evidence to change antimalarial drug policy in Kenya. Trop. Med. Int. Health 5:755-764. [DOI] [PubMed] [Google Scholar]
- 133.Shretta, R., R. Brugha, A. Robb, and R. W. Snow. 2000. Sustainability, affordability and equity of corporate drug donations: the case of Malarone®. Lancet 355:1718-1720. [DOI] [PubMed] [Google Scholar]
- 134.Reference deleted.
- 135.Shulman, C. E., E. K. Dorman, F. Cutts, K. Kawuondo, J. N. Bulmer, N. Peshu, and K. Marsh. 1999. Intermittent sulphadoxine-pyrimethamine to prevent severe anaemia secondary to malaria in pregnancy: a randomised placebo-controlled trial. Lancet 353:632-636. [DOI] [PubMed] [Google Scholar]
- 136.Sidhu, A. B., D. Verdier-Pinard, and D. A. Fidock. 2002. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science. 298:210-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Simoes, E. A. F., T. Desta, T. Tessema, T. Gerbresallassie, M. Dagnew, and S. Gove. 1997. Performance of health workers after training in integrated management of childhood illness in Gondar, Ethiopia. Bull. W. H. O. 75:43-53. [PMC free article] [PubMed] [Google Scholar]
- 138.Snow, R. W., N. Peshu, D. Forster, H. Mwenesi, and K. Marsh. 1992. The role of shops in the treatment and prevention of childhood malaria on the coast of Kenya. Trans. R. Soc. Trop. Med. Hyg. 86:237-239. [DOI] [PubMed] [Google Scholar]
- 139.Snow, R. W., M. H. Craig, U. Deichmann, and K. Marsh. 1999. Estimating mortality, morbidity and disability due to malaria among Africa's non-pregnant population. Bull. W. H. O. 77:624-640. [PMC free article] [PubMed] [Google Scholar]
- 140.Snow, R. W. 2000. The burden of malaria: understanding the balance between immunity, public health and control. J. Med. Microbiol. 49:1053-1055. [DOI] [PubMed] [Google Scholar]
- 141.Snow, R. W., J. F. Trape, and K. Marsh. 2001. The past, present and future of childhood malaria mortality in Africa. Trends Parasitol. 17:593-597. [DOI] [PubMed] [Google Scholar]
- 142.Snow, R. W., and H. M. Gilles. 2002. The epidemiology of malaria, p. 85-106. In D. A. Warrell and H. M. Gilles (ed.), Bruce Chwatt's essential malariology, 4th ed. Arnold Publishers, London, United Kingdom.
- 143.Snow, R. W., E. Eckert, and A. Teklehaimanot. 2003. Estimating the needs for malaria combination therapy for case-management in Africa. Trends Parasitol. 19:363-369. [DOI] [PubMed] [Google Scholar]
- 144.Spencer, H. C., D. C. O. Kaseje, J. M. Roberts, and A. Y. Huong. 1987. Consumption of chloroquine phosphate provided for treatment of malaria by volunteer village health workers in Saradidi, Kenya. Ann. Trop. Med. Parasitol. 81:116-123. [DOI] [PubMed] [Google Scholar]
- 145.Staedke, S. G., M. R. Kamya, G. Dorsey, A. Gasasira, G. Ndeezi, E. D. Charlebois, and P. J. Rosenthal. 2001. Amodiaquine, sulfadoxine/pyrimethamine, and combination therapy for treatment of uncomplicated falciparum malaria in Kampala, Uganda: a randomised trial: Lancet 358:368-374. [DOI] [PubMed] [Google Scholar]
- 146.Steketee, R. W., B. L. Nahlen, M. E. Parise, and C. Menendez. 2001. The burden of malaria in pregnancy in malaria-endemic areas. Am. J. Trop. Med. Hyg. 64:28-35. [DOI] [PubMed] [Google Scholar]
- 147.Sudre, P., J. G. Bremen, D. McFarland, and J. P. Koplan. 1992. Treatment of chloroquine-resistant malaria in African children: a cost-effectiveness analysis. Int. J. Epidemiol. 21:146-154. [DOI] [PubMed] [Google Scholar]
- 148.Supanaranond, W., Y. Suputtamongkol, T. M. Davis, S. Pukrittayakamee, P. Teja-Isavadharm, H. K. Webster, and N. J. White. 1997. Lack of a significant adverse cardiovascular effect of combined quinine and mefloquine therapy for uncomplicated malaria. Trans. R. Soc. Trop. Med. Hyg. 91:694-696. [DOI] [PubMed] [Google Scholar]
- 149.Targett, G., C. Drakeley, M. Jawara, L. von Seidlein, R. Coleman, J. Deen, M. Pinder, T. Doherty, C. Sutherland, G. Walraven, and P. Milligan. 2001. Artesunate reduces but does not prevent post-treatment transmission of Plasmodium falciparum to Anopheles gambiae. J. Infect. Dis. 183:1254-1259. [DOI] [PubMed] [Google Scholar]
- 150.Taylor, R. B., O. Shakoor, and R. H. Behrens. 2001. Pharmacopoeial quality of drugs supplied by Nigerian pharmacies. Lancet 357:1933-1936. [DOI] [PubMed] [Google Scholar]
- 151.Taylor, T. E., M. E. Molyneux, J. J. Wirima, K. A. Fletcher, and K. Morris. 1988. Blood glucose levels in Malawian children before and during the administration of intravenous quinine for severe falciparum malaria. N. Engl. J. Med. 319:1040-1047. [DOI] [PubMed] [Google Scholar]
- 152.Teklehaimanot, A., and R. W. Snow. 2002. Will the Global Fund help Roll Back Malaria in Africa? Lancet 360:888-889. [DOI] [PubMed] [Google Scholar]
- 153.Thera, M. A., U. D'alessandro, M. Thiero, A. Ouedraogo, J. Packou, O. A. D. Souleymane, M. Fane, G. Ade, F. Alvez, and O. Doumba. 2000. Child malaria treatment practices among mothers in the district of Yanfolila, Sikasso region, Mali. Trop. Med. Int. Health 5:876-881. [DOI] [PubMed] [Google Scholar]
- 154.Tran, T. H., N. P. Day, H. P. Nguyen, T. H. Nguyen, T. H. Tran, P. L. Pham, X. S. Dinh, V. C. Ly, V. Ha, D. Waller, T. E. Peto, and N. J. White. 1996. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N. Engl. J. Med. 335:76-83. [DOI] [PubMed] [Google Scholar]
- 155.Trape, J. F., G. Pison, M. P. Preziosi, C. Enel, A. D. Du Lou, V. Delaunay, B. Samb, E. Lagarde, J. F. Molez, and F. Simondon. 1998. Impact of chloroquine resistance on malaria mortality. C. R. Acad. Sci. Sci. Vie 321:689-697. [DOI] [PubMed] [Google Scholar]
- 156.van Eijk, A. M., J. G. Ayisi, F. O. ter Kuile, A. Misore, J. A. Otieno, M. S. Kolczak, P. A. Kager, R. W. Steketee, and B. L. Nahlen. 2001. Human immunodeficiency virus seropositivity and malaria as risk factors for third-trimester anemia in asymptomatic pregnant women in western Kenya. Am. J. Trop. Med. Hyg. 65:623-630. [DOI] [PubMed] [Google Scholar]
- 157.Van Hensbroek, M. B., A. Palmer, S. Jaffar, G. Schneider, and D. Kwiatkowski. 1997. Residual neurological sequelae after childhood cerebral malaria. J. Paediatr. 131:125-129. [DOI] [PubMed] [Google Scholar]
- 158.Veron, A. A., W. R. Taylor, A. Biey, K. M. Mundeke, A. Chahnazarian, H. Habicht, M. Mutombo, and B. Makani. 1993. Changes in use of health services in a rural health zone in Zaire. Int J. Epidemiol. 22(Suppl. 1):S20-S31. [DOI] [PubMed] [Google Scholar]
- 159.Reference deleted.
- 160.Warrell, D. A. 1999. Management of severe malaria. Parassitologia 41:287-294. [PubMed] [Google Scholar]
- 161.Waruiru, C. M., C. R. Newton, D. Forster, L. New, P. Winstanley, I. Mwangi, V. Marsh, M. Winstanley, R. W. Snow, and K. Marsh. 1996. Epileptic seizures and malaria in Kenyan children. Trans. R. Soc. Trop. Med. Hyg. 90:152-155. [DOI] [PubMed] [Google Scholar]
- 162.Watkins, W. M., and M. Mosobo. 1993. Treatment of Plasmodium falciparum malaria with pyrimethamine-sulfadoxine: selective pressure for resistance is a function of long elimination half-life. Trans. R. Soc. Trop. Med. Hyg. 87:75-78. [DOI] [PubMed] [Google Scholar]
- 163.Wellems, T. E., and C. V. Plowe. 2001. Chloroquine-resistant malaria. J. Infect. Dis. 184:770-776. [DOI] [PubMed] [Google Scholar]
- 164.Wellems, T. E., L. J. Panton, I. Y. Gluzman, V. E. do Rosario, R. W. Gwadz, A. Walker-Jonah, and D. Krogstad. 1990. Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature 345:253-255. [DOI] [PubMed] [Google Scholar]
- 165.Wernsdorfer, W. H., and I. McGregor. 1988. Malaria: principles and practice of malariology, vol. 2, p. 999-1090. Churchill Livingstone, Inc., New York, N.Y.
- 166.White, N. J., S. Looareesuwan, D. A. Warrell, M. J. Warrell, D. Bunnag, and T. Harinasuta. 1982. Quinine pharmacokinetics and toxicity in cerebral and uncomplicated Falciparum malaria. Am. J. Med. 73:564-572. [DOI] [PubMed] [Google Scholar]
- 167.White, N. J., K. D. Miller, and F. C. Churchill. 1988. Chloroquine treatment of severe malaria in children. Pharmacokinetics, toxicity and new dosage recommendations. N. Engl. J. Med. 319:1493-1500. [DOI] [PubMed] [Google Scholar]
- 168.White, N. J., S. Looareesuwan, R. E. Phillips, P. Chanthavanich, and D. A. Warrell. 1988. Single dose phenobarbitone prevents convulsions in cerebral malaria. Lancet 11:64-66. [DOI] [PubMed] [Google Scholar]
- 169.White, N. J. 1994. Clinical pharmacokinetics and pharmacodynamics of artemisinin and derivatives. Trans. R. Soc. Trop. Med. Hyg. 88(Suppl. 1):S41-S43. [DOI] [PubMed] [Google Scholar]
- 170.White, N. J., F. Norsten, S. Looareesuwan, W. M. Watkins, K. Marsh, R. W. Snow, G. O. Kokwaro, J. Ouma, T. T. Hien, M. E. Molyneux, T. E. Taylor, C. I. Newbold, T. K. Ruebush, M. Danis, B. M. Greenwood, R. M. Anderson, and P. Olliaro. 1999. Averting a malaria disaster. Lancet 353:1965-1967. [DOI] [PubMed] [Google Scholar]
- 171.White, N. J. 1999. Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia 41:301-308. [PubMed] [Google Scholar]
- 171a.White, T., S. Clode, S. Ward, I. Gaunt, C. Powell, and R. Clark. 2004. Developmental toxicity of the antimalarial artesunate in rats and rabbits. Birth Defects Res. Part A Clin. Mol. Teratol. 70:265. (Abstract.) [Google Scholar]
- 172.Wilkins, J. J., P. I. Folb, N. Valentine, and K. I. Barnes. 2002. An economic comparison of chloroquine and SP as first-line treatment for malaria in South Africa: development of a model for estimating recurrent direct costs. Trans. R. Soc. Trop. Med. Hyg. 96:85-90. [DOI] [PubMed] [Google Scholar]
- 173.Winch, P. J., A. M. Makemba, S. R. Kamazima, M. Lurie, G. K. Lwihula, Z. Premji, J. N. Minjas, and C. J. Shiff. 1996. Local terminology for febrile illnesses in Bagamoyo district, Tanzania and its impact on the design of a community-based malaria control programme. Soc. Sci. Med. 42:1057-1067. [DOI] [PubMed] [Google Scholar]
- 174.Winstanley, P. A., G. Edwards, M. L. Orme, and A. M. Breckenridge. 1987. The detection of amodiaquine in man after oral administration. Br J Clin Pharmacol. 23:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Winstanley, P. A., J. W. Coleman, J. M. Maggs, A. M. Breckenridge, and B. K. Park. 1990. The toxicity of amodiaquine and its principal metabolites towards mononuclear leukocytes and granulocyte-monocyte colony forming units. Br. J. Clin. Pharmacol. 29:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Winstanley, P. A., C. R. J. C. Newton, G. Pasvol, F. J. Kirkham, E. Mberu, N. Peshu, S. A. Ward, J. B. O. Were, D. A. Warrell, and K. Marsh. 1992. Prophylactic phenobarbitone in young children with severe falciparum malaria: pharmacokinetics and clinical effects. Br. J. Clin. Pharmacol. 33:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Winstanley, P. A., W. M. Watkins, C. R. J. C. Newton, C. Nevill, E. Mberu, P. A. Warn, C. M. Waruiru, I. N. Mwangi, D. A. Warrell, and K. Marsh. 1992. The disposition of oral and intramuscular pyrimethamine-sulfadoxine in Kenyan children with high parasitaemia but clinically non-severe falciparum malaria. Br. J. Clin. Pharmacol. 33:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Winstanley, P. A., W. M. Watkins, D. Muhia, S. Szwandt, E. Amukoye, and K. Marsh. 1997. Chlorproguanil-dapsone for uncomplicated falciparum malaria in young children: pharmacokinetics and therapeutic range. Trans. R. Soc. Trop. Med. Hyg. 91:322-327. [DOI] [PubMed] [Google Scholar]
- 179.Winstanley, P. A., C. R. Newton, W. M. Watkins, E. Mberu, S. A. Ward, P. A. Warn, I. Mwangi, C. Waruiru, G. Pasvol, D. A. Warrell, and K. Marsh. 1993. Towards optimal parenteral quinine regimens for young children with cerebral malaria: importance of unbound quinine concentration. Trans. R. Soc. Trop. Med. Hyg. 87:201-206. [DOI] [PubMed] [Google Scholar]
- 180.Wirima, J. J. W. 1999. Development of an antimalarial drug policy. Malaria Infect. Dis. Afr. 9:62-65. [Google Scholar]
- 180a.World Health Organization. 1997. Integrated management of childhood illness. WHO/CHD/97.3.A-L (generic version). World Health Organization, Geneva, Switzerland.
- 181.World Health Organization. 2000. The use of antimalarial drugs: report of a WHO informal consultation, 13-17 November 2000. WHO/CDS/RBM/2001.33. World Health Organization, Geneva, Switzerland.
- 182.World Health Organization. 2000. Framework for developing, implementing and updating antimalarial treatment policy in Africa: a guide for country malaria control programme managers. Malaria Programme, Division of Prevention and Control of Communicable Diseases, African Regional Office of World Health Organization, Harare, May 2000. World Health Organization, Geneva, Switzerland.
- 183.World Health Organization. 2001. Antimalarial drug combination therapy. Report of a technical consultation. WHO/CDS/RBM/2001.35. World Health Organization, Geneva, Switzerland.
- 184.World Health Organization. 2002. Monitoring antimalarial drug resistance: report of a WHO consultation, Geneva, 3-5 December 2001. WHO/CDS/RBM/2002.39. World Health Organization, Geneva, Switzerland.
- 185.World Health Organization. 2003. Assessment of the safety of artemisinin compounds in pregnancy. Report on two RBM/TDR informal consultations. WHO/CDS/MAL/2003.1094. World Health Organization, Geneva, Switzerland.
- 186.Wyss, K., D., Whitting, P. Kilama, D. G., McLarty, D. Mtasiwa, M. Tanner, and N. Lorenz. 1996. Utilisation of government and private health services in Dar es Salaam. East Afr. Med. J. 73:357-363. [PubMed] [Google Scholar]
- 187.Xu, J. H., and Y. P. Zhang. 1996. Contragestational effects of dihydroartemisinin and artesunate. Yao Xue Xue Bao 31:657-661. [PubMed] [Google Scholar]
- 188.Yebohah-Antwi, K., J. O. Gyapong, I. K. Asare, G. Barnish, D. B. Evans, and S. Adjei. 2001. Impact of pre-packaging antimalarial drugs on cost to patients and compliance with treatment. Bull. W. H. O. 79:394-399. [PMC free article] [PubMed] [Google Scholar]