Abstract
Background:
The relationship between depression, hippocampus (HC), and executive dysfunctions seems complex and has been the focus of research. Recent evidence indicates a possible role of HC in executive dysfunction seen in depression. No such studies on Indian population have been done.
Aim:
To look for changes in HC and executive functions in depression.
Settings and Design:
A cross-sectional analytical controlled study. Sample size 50 (controls 50).
Materials and Methods:
Hippocampal volume and executive dysfunction was measured using structural magnetic resonance imaging (MRI) and Wisconsin Card Sorting Test (WCST), respectively. Findings on these two parameters were compared between depressives and healthy matched controls as well as between first episode (FE) and recurrent depressives and across the severity of depression (mild, moderate, and severe).
Statistical Analysis:
Statistical Package for Social Sciences (SPSS) version 17 was used for analysis. Normally distributed continuous variables were analyzed with independent t-tests. Analysis of variance (ANOVA) was used for multiple comparisons. Categorical data were compared with χ2 or Fisher's exact test. Clinical correlations were conducted using Pearson correlations.
Result:
Depressed patients had a smaller left (Lt) hippocampal volume as well as poor performance on several measures of executive functions. Smaller hippocampal volume was found even in FE. Those who had a past burden of depressive illness had an even smaller hippocampal volume. No direct correlation was found between the HC volume and cognitive dysfunction.
Conclusion:
Depressive illness appears to be toxic to the HC. The relationship between HC and executive dysfunction in depression may be indirect through its functional connections.
Keywords: Depression, executive deficits, hippocampus, magnetic resonance imaging, wisconsin card sorting test
The relationship between hippocampus (HC) and major depression seems to be complex. Studies have found hippocampal atrophy in depression.[1,2] The HC, apart from its well-established role in memory,[3] appears to have a possible role in regulation of executive functions.[4,5,6] Also, executive dysfunction is common in depression. In the present study, we compared the hippocampal volume and executive functions in depression with healthy controls. The relation of these parameters with severity of current episode and history of past depressive episodes was explored. This, to our knowledge, is the first such study on Indian population.
Many magnetic resonance imaging (MRI) studies of HC in patients with major depressive disorder (MDD) have shown reduction in HC volume,[7,8,9,10,11,12] while some others have not found any change.[13,14] A smaller left (Lt) HC[9,11] or right (Rt) HC[15] was reported in few studies. Structural changes in the HC related to total lifetime duration of illness have been reported in recurrent depressives.[16,17,18,19] In contrast, other studies have found no evidence for an association between HC volume and number of depressive episodes.[9,11] Of the few studies of first episode (FE) depression, either no change in HC was found[20] or volume reductions were reported.[21,22] As far as the relation of HC volume with depression severity is concerned Vakili et al., observed a positive correlation,[14] but subsequent studies found no relation of HC volume with the severity of depressive episode.[22,23]
Executive deficits seen in depression include problems with planning, organizing, sequencing, shifting, information processing speed, and maintaining information in working memory.[24,25,26,27,28] The test commonly used to examine executive function in people with psychiatric disorders is the Wisconsin Card Sorting Test (WCST).[29] Studies have shown that WCST scores may vary as a function of the severity of depression.[30,31,32] However, some studies found no such relationship between severity of depression and the overall cognitive performance.[33] Moreover, only a few studied executive dysfunctions in young depressives.[25,34]
MATERIALS AND METHODS
This cross-sectional analytical controlled study included cases of major depression with or without history of past depression, diagnosed as per International Classification of Diseases-10 Diagnostic Criteria for Research (ICD-10-DCR) F32 and F33 using structured interview Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM IV), between ages 21 and 50 years presenting to psychiatry outpatient department (OPD). Comorbid psychiatric illness, bipolar disorder, hypo/hyperthyroidism, head injury, substance abuse were excluded. Local ethical committee gave clearance. Beck's Depression Inventory (BDI) was administered to quantify the depression severity into mild, moderate, or severe. Structural MRI of the brain for morphometric study was done for all the patients and the hippocampal volumes measured. WCST (64 card, computerized version) was administered to all patients for the assessment of cognitive functions. For comparison, 50 healthy subjects matched with respect to age, gender, handedness, and educational level were selected. Controls were also screened for family history of depression. They were administered the General Health Questionnaire (GHQ - 60, with an inclusion score of < 12) to rule out any significant psychiatric morbidity. A 1.5 T MRI machine (Siemens Magnetom Symphony Maestro Class with an additional workstation running Lenovo software) was used for imaging and all imaging and volumetric processing was carried outby the same operator (HS), blinded to participant identity and diagnosis. Standard protocols and procedures were followed.[23,35,36] The details of the methods adopted in WCST and MRI (including the radiological boundaries) can be accessed from the author (shahbaaz323@yahoo.com). The interrater reliability of hippocampal volume was high (r = 0.94, degrees of freedom (df) =31, P < 0.05).
Analysis of data
Using Statistical Package for Social Sciences (SPSS) 17, between and within group comparisons were made—between group comparison being between the controls and the depressed and within group between the FE and the multiple episode (ME) as well as between mild, moderate, and severe depressives. Normally distributed continuous variables were analyzed with independent t-tests. Analysis of variance (ANOVA) was used for multiple comparisons. Categorical datawere compared with χ2 or, when the expected cell frequency fell below 5, Fisher's exact test. Clinical correlations were conducted using Pearson correlations. Multiple regression analysis was done wherever there was a significant correlation; taking depressed/controls as dependent variable and the correlated parameters as independent variables using stepwise regression method.
RESULTS
Patients with major depression and healthy controls did not differ significantly in age, marital status, education, or handedness. All patients and control subjects were males.
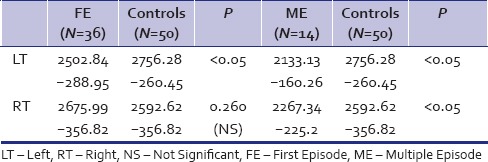
The depressives had a smaller Lt hippocampal volume (mean = 2,399.33; standard deviation (SD) =307.53) as compared to the healthy controls (mean 2,756.28; SD = 260.45), P < 0.001). The Rt hippocampal volume did not differ significantly between the patients and control groups. The ME had smaller Lt and RtHC as compared to the FE depressives (P < 0.001). When compared with the controls independently, the FE had significant hippocampal volume reduction only on the Lt side, while the ME had significantly lower HCvolume on both Rt and Lt side. In the recurrent depressive group, the average number of past episodes of depression was 2.305 with a history of electroconvulsive therapy (ECT) in three patients. The hippocampal volume did not differ significantly between the mild, moderate, and severe depressives either on the Lt or the Rt side.
There was a significant difference between the depressed and the nondepressed healthy controls on total errors, persevervative errors, perseverative responses, number of complete categories, and trials for the first category domains. The FE and ME depressives did not differ on any WCST parameters significantly. There was a significant difference between the mild, moderate, and severe depressives in terms of perseverative responses. No other difference was noted. In depressed and control groups, Pearson correlation coefficient analysis revealed significant correlation between the Rt hippocampal volume and the number of complete categories on WCST (R = 0.337, P < 0.05 and R2 = 0.11). No significant correlation was seen between the other WCST parameter and hippocampal volume in any of the subgroups.
To summarize the results, the main findings of this study were:
Depressed and control groups comparison - The depressed group had a lower Lt hippocampal volume as compared to the nondepressed healthy controls. The RtHC did not differ significantly between the two groups. The depressed group also performed poorly on several measures of executive functions
ME and FE depressives’ comparison - Currently, depressed patients who had a past burden of depressive illness (ME) had a lower Lt and Rt hippocampal volume as compared to depressives in their FE. The FE depressives when compared to healthy controls also had hippocampal volume reduction, but only on the Lt side. However, the ME did not differ from the FE depressives in terms of performance on WCST
Mild, moderate, and severe depressives comparison - When seen across the ICD-10 severity specifiers (mild, moderate, and severe), the depressives did not differ in their hippocampal volume [Tables 1-5].
Table 1.
Sociodemographical data compared
Table 5.
HC volume and WCST compared between Mild, moderate and, severe depressives
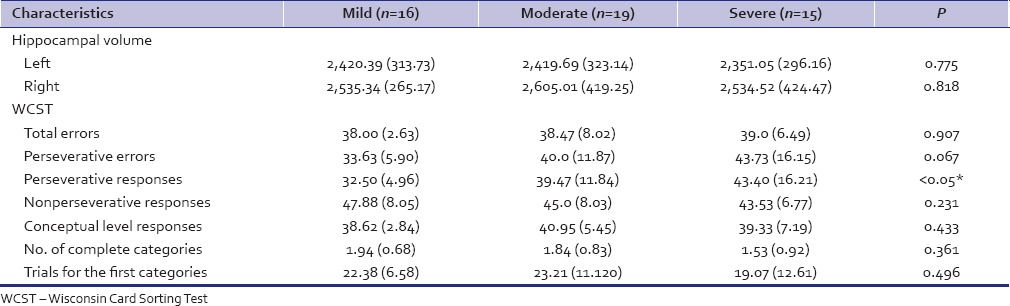
Table 2.
HC and WCST compared between depressed and controls
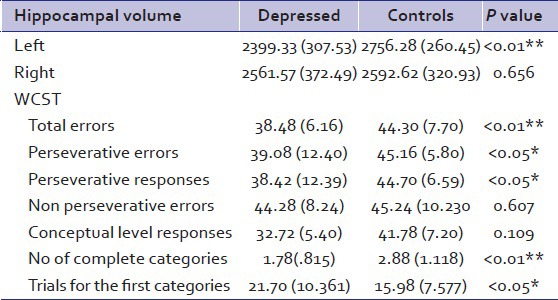
Table 3.
HC volume and WCST compared between first episode and multiple episode
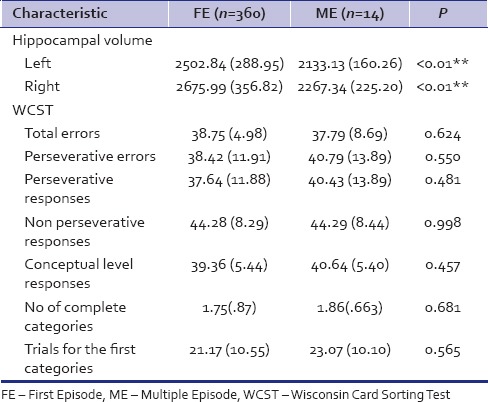
Table 4.
First episode and multiple episode independently compared with controls
DISCUSSION
Smaller HC in depressive group suggests atrophy. There are several hypothesis to explain the observed decrease in HC volume in major depression, most prominent of these being that prolonged and raised cortisol levels can produce neuronal dysfunction with decreased glucoseuptake, reduced dendritic arborization, and ultimately, neuronaldeath and cell loss in the HC in animals.[37,38] The presence of hippocampal atrophy in the FE itself along with the finding of greater volume reduction in recurrent depressives, suggests that the hippocampal atrophy goes on increasing with the burden of depressive illness. This seems to be in confirmation with the findings worldwide reported in previous such studies including a meta-analysis on HC volume loss in FE depression.[7] While this study reports that depressed patients in their FE itself have neuroanatomic changes and disease burden in the past adds to this insult, the debate whether depression causes hippocampal volume loss in a cumulative manner or a smaller hippocampal size itself predisposes these individuals to increasing number of depressive episodes, is far from settled. While this study suggests the former, it remains a fact that a cross-sectional study like this, in principle, cannot conclude about causality.
The presence of impairment on WCST performance in the depressed group as brought out in this study is in conformity with previous such studies worldwide,[32] indicating that depression affects cognition. Also, this study confirms that ME depressives and FE depressives are similar when assessed for the performance on various WCST parameters and cumulative illness did not affect their performance. But again, to conclude that cumulative illness has no effect on executive functions without doing a “before and after” serial WCST measurement study would be premature.
The absence of any significant correlation between hippocampal volumes with depression severity is in consonance with previous studies[21,23] and substantiates that DSM IV classification of depressive episode is a purely symptom-based classification and does not correlate with neurobiology findings.[39]
In the depressed group, we found smaller hippocampal volume as well as presence of significantly poor performance on WCST which would tend to suggest that the executive functions may be related to hippocampal volume loss in depressed individuals indicating thereby that those depressed who have a smaller HC also have executive dysfunction (in other words, the HC atrophy would be directly responsible for the cognitive deficits in depression) as has been suggested by Frodl et al.[23] Interestingly however, we did not find any such correlation. This may be explained by hypothesizing that the core structure of the HC may not be involved in depression. There are indications that instead of the core structure of HC, it may be the functional connections of the HC involved in executive functions which may be affected, thus explaining the executive dysfunction seen in depression.[5] This may possibly explain the WCST impairment in depressed group without any correlation with hippocampal volume loss. Wall and Messier[5] theorized that the HC–oribitomedial prefrontal circuit can efficiently contribute to the integration of cognition, emotion, and behavior; and can thus influence executive functions; thus, underlining a possible role of HC as a substrate for WCST performance.[5]
In case of the ME patients, we considered the previous burden of illness in the form of past history of depression, unlike Sheline[19] who had considered the total number of days depressed as well as treatment particulars. This was done because it was felt that history of having been depressed in the past is much more reliable and easy to collect/recollect than the precise history of the number of days depressed and the treatment taken. The present study design did not consider treatment parameters/drug naivety of the patients. However, later assessment on this point revealed that some of the depressed patients were on antidepressants when enrolled in the study. In the FE only, five (out of 36) were on antidepressants. The type of drugs/dosage was not known reliably. Overall this group can be roughly taken to be drug naïve. In the group with a history of depressive episode in the past (ME), majority (11 out of 14) had been exposed to antidepressants in the past (ranging from 3 months back to about 10 years back) and only three out of 14 were on antidepressants at the time of study. Hence, a large majority of the depressed patients were not on any treatment when enrolled. But this study deliberately kept this dimension (the treatment and its implications like duration of treatment/drug free period before index presentation, etc.) out of the major outcome because the study was not designed to look into this aspect from the very beginning and no follow-up was done. Hence, conclusive inference on drug/treatment aspect was not derived as previous studies have done.[17]
In the comparison of ME with the FE, the sample size of ME was only 14. This may be a little less number of subjects for a robust statistical analysis in this subgroup. However, previous studies world over have done similar evaluation with even lesser number of samples and come across with valuable and creditable inferences.[20] Also, the hippocampal volumes were not adjusted for total intracranial volume, but a recent meta-analysis[7] concluded that the total cerebral volume added little to any explanation of the variation of the volume of the gray matter structures in question.
The sample was all male and general health was expected to be better than general population. However, we believe that the results can still be generalized to the general Indian male population.
ACKNOWLEDGEMENTS
Dr. H. Sahni, MD radiology, Dept of Radiology, AFMC, Pune.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Campbell S, MacQueen G. An update on regional brain volume differences associated with mood disorders. Curr Opin Psychiatr. 2006;19:25–33. doi: 10.1097/01.yco.0000194371.47685.f2. [DOI] [PubMed] [Google Scholar]
- 2.Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: A selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Tamminga CA. The hippocampus. Am J Psychiatry. 2005;162:25. doi: 10.1176/appi.ajp.162.1.25. [DOI] [PubMed] [Google Scholar]
- 4.Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context and regulation: Perspectives from affective neuroscience. Psychol Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- 5.Wall PM, Messier C. The hippocampal formation – Orbitomedial prefrontal cortex circuit in the attentional control of active memory. Behav Brain Res. 2001;127:99–117. doi: 10.1016/s0166-4328(01)00355-2. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. Longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161:2081–90. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 7.Videbech P, Ravnkilde B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 8.Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–80. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steffens DC, Byrum CE, McQuoid DR, Greenberg DL, Payne ME, Blitchington TF, et al. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–9. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- 10.Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vainio P, Partanen K, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30:117–25. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- 11.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–7. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 12.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–31. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 13.Axelson DA, Doraiswamy PM, McDonald WM. Hypercortisolemia and hippocampal changes in depression. Psychiatry Res. 1993;47:163–73. doi: 10.1016/0165-1781(93)90046-j. [DOI] [PubMed] [Google Scholar]
- 14.Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, et al. Hippocampal volume in primary unipolar major depression: A magnetic resonance imaging study. Biol Psychiatry. 2000;47:1087–90. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- 15.Bell-McGinty S, Butters MA, Meltzer CC, Greer PJ, Reynolds CF, III, Becker JT. Brain morphometric abnormalities in geriatric depression: Long-term neurobiological effects of illness duration. Am J Psychiatry. 2002;159:1424–7. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- 16.McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 17.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–43. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan KR, Doraiswamy PM, Figiel GS, Husain MM, Shah SA, Na C, et al. Hippocampal abnormalities in depression. J Neuropsychiatry Clin Neurosci. 1991;3:387–91. doi: 10.1176/jnp.3.4.387. [DOI] [PubMed] [Google Scholar]
- 19.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–13. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–92. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frodl T, Meisenzahl EM, Zetzche T, Born C, Groll C, Jager M, et al. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002;159:1112–8. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- 22.MacMaster FP, Kusumakar V. Hippocampal volume in early onset depression. BMC Med. 2004;2:2. doi: 10.1186/1741-7015-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frodl T, Schaub A, Banac S, Charypar M, Jäger M, Kümmler P, et al. Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci. 2006;31:316–23. [PMC free article] [PubMed] [Google Scholar]
- 24.Fossati P, Ergis AM, Allilaire JF. Executive functioning in unipolar depression: A review. Encephale. 2002;28:97–107. [PubMed] [Google Scholar]
- 25.Stordal KI, Lundervold AJ, Egeland J, Mykletun A, Asbjørnsen A, Landrø NI, et al. Impairment across executive functions in recurrent major depression. Nord J Psychiatry. 2004;58:41–7. doi: 10.1080/08039480310000789. [DOI] [PubMed] [Google Scholar]
- 26.Must A, Szabo Z, Bodi N, Szasz A, Janka Z, Keri S. Sensitivity to reward and punishment and the prefrontal cortex in depression. J Affect Disord. 2006;90:209–15. doi: 10.1016/j.jad.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Harvey PO, Le Bastard G, Pochon JB, Levy R, Allilaire JF, Dubois B, et al. Executive functions and updating of the contents of working memory in unipolar depression. J Psychiatr Res. 2004;38:567–76. doi: 10.1016/j.jpsychires.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Pardo JV, Pardo PJ, Hume SW, I Posner M. Neurocognitive dysfunction in antidepressant-free, non-elderly patients with unipolar depression: Alerting and orienting of visuospatial attention. J Affect Disord. 2006;92:71–8. doi: 10.1016/j.jad.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 29.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. 1st ed. Odessa: Psychological Assessment Resources; 1981. Wisconsin Card Sorting Test Manual. [Google Scholar]
- 30.Feil D, Razani J, Boone K, Lesser I. Apathy and cognitive performance in older adults with depression. Int J Geriatr Psychiatry. 2003;18:479–85. doi: 10.1002/gps.869. [DOI] [PubMed] [Google Scholar]
- 31.Baudic S, Tzortzis C, Barba GD, Traykov L. Executive deficits in elderly patients with major unipolar depression. J Geriatr Psychiatry Neurol. 2004;17:195–201. doi: 10.1177/0891988704269823. [DOI] [PubMed] [Google Scholar]
- 32.Totić-Poznanović S, Marinković D, Tomić G, Paunović VR. Executive functions in young patients with unipolar depression. Srp Arh Celok Lek. 2006;134:273–7. doi: 10.2298/sarh0608273t. [DOI] [PubMed] [Google Scholar]
- 33.Merriam EP, Thase ME, Haas GL, Keshavan MS, Sweeney JA. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting test performance. Am J Psychiatry. 1999;156:780–2. doi: 10.1176/ajp.156.5.780. [DOI] [PubMed] [Google Scholar]
- 34.Purcell R, Maruff P, Kyrios M, Pantelis C. Neuropsychological function in young patients with unipolar major depression. Psychol Med. 1997;27:1277–85. doi: 10.1017/s0033291797005448. [DOI] [PubMed] [Google Scholar]
- 35.Tae WS, Hong SB. Boundary of amygdala and hippocampus. Am J Psychiatry. 2001;158:820–1. doi: 10.1176/appi.ajp.158.5.820-a. [DOI] [PubMed] [Google Scholar]
- 36.Bremner JD. Does stress damage the brain? Biol Psychiatry. 1999;45:797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 37.Duman RS. Synaptic plasticity and mood disorders. Mol Psychiatry. 2002;7:S29–34. doi: 10.1038/sj.mp.4001016. [DOI] [PubMed] [Google Scholar]
- 38.Bremner JD. Brain pathways in stress processing. Biol Psychiatry. 2002;51:91–2S. [Google Scholar]
- 39.Parker G. Classifying depression: Should paradigms lost be regained? Am J Psychiatry. 2000;157:1195–203. doi: 10.1176/appi.ajp.157.8.1195. [DOI] [PubMed] [Google Scholar]


