Abstract
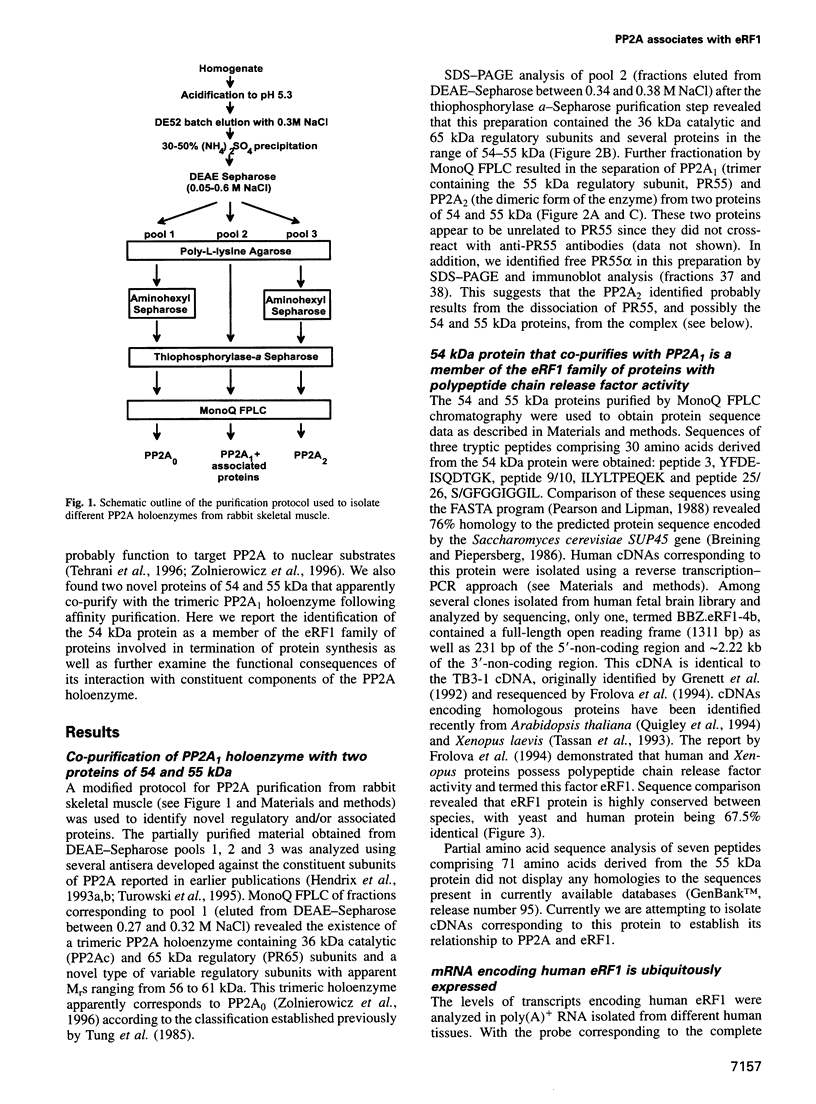
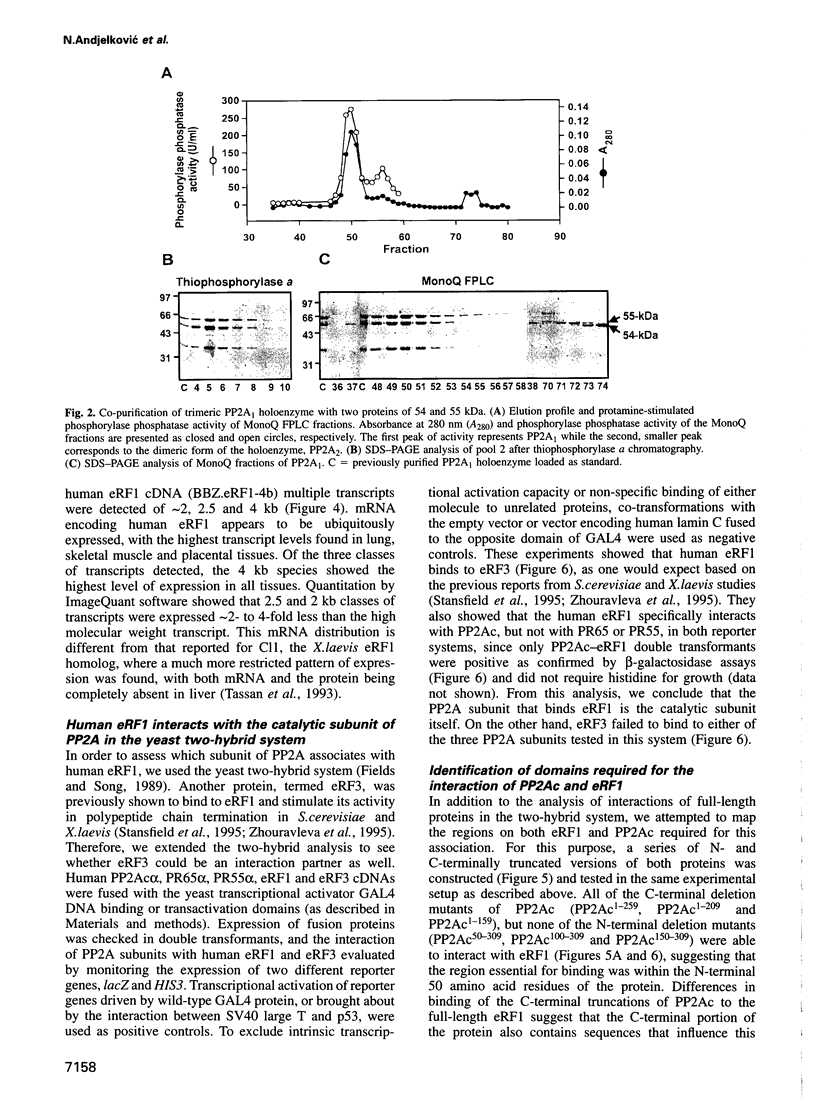
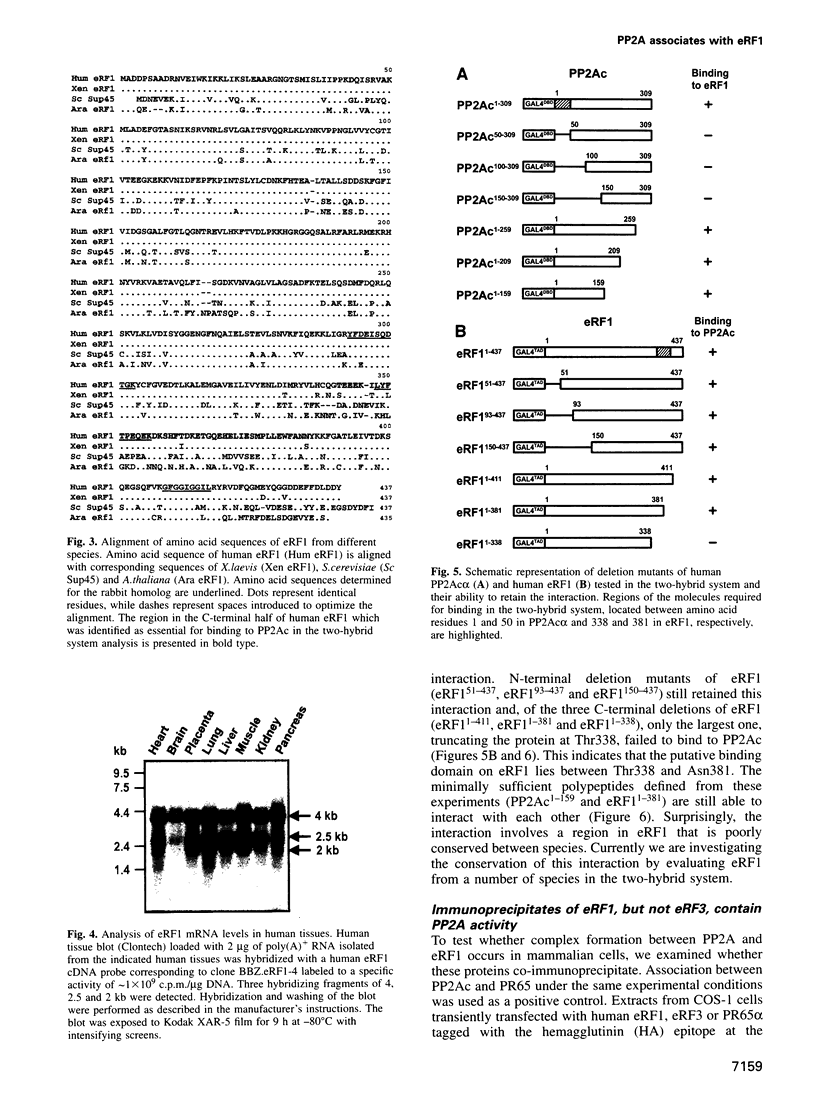
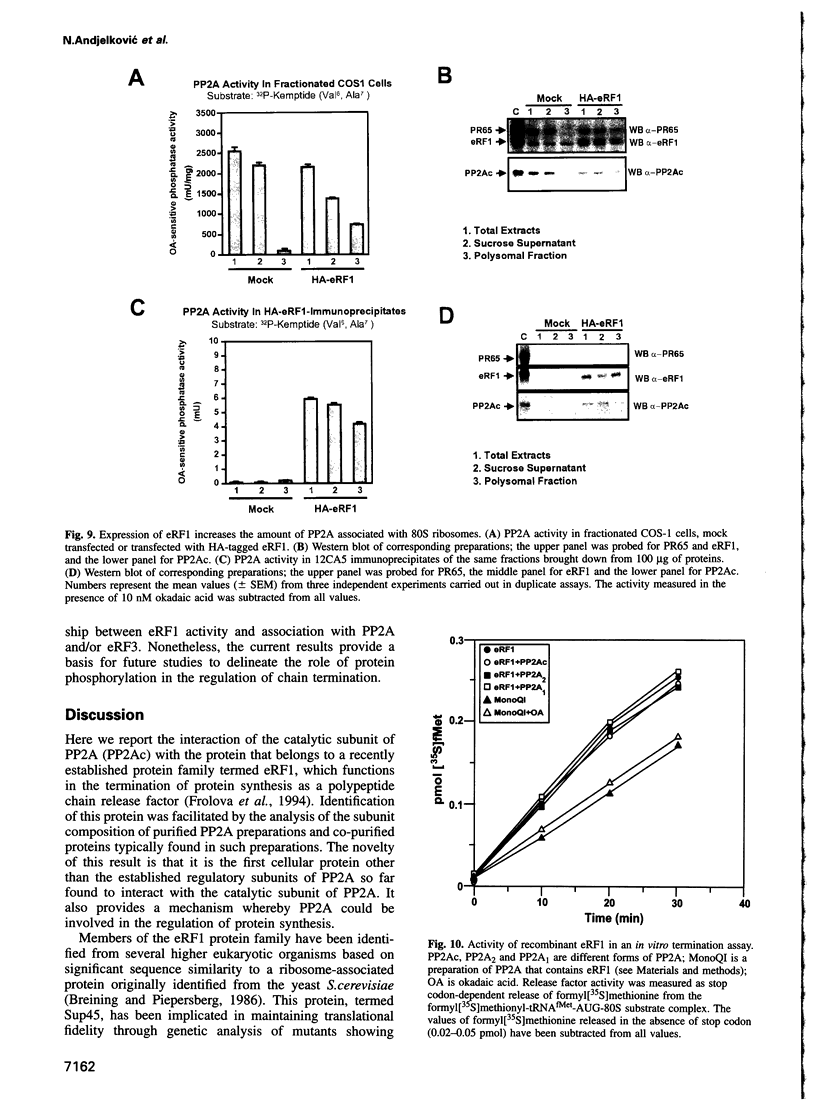
By a number of criteria, we have demonstrated that the translation termination factor eRF1 (eukaryotic release factor 1) associates with protein phosphatase 2A (PP2A). Trimeric PP2A1 was purified from rabbit skeletal muscle using an affinity purification step. In addition to the 36 kDa catalytic subunit (PP2Ac) and established regulatory subunits of 65 kDa (PR65) and 55 kDa (PR55), purified preparations contained two proteins with apparent Mrs of 54 and 55 kDa. Protein microsequencing revealed that the 55 kDa component is a novel protein, whereas the 54 kDa protein was identified as eRF1, a protein that functions in translational termination as a polypeptide chain release factor. Using the yeast two-hybrid system, human eRF1 was shown to interact specifically with PP2Ac, but not with the PR65 or PR55 subunits. By deletion analysis, the binding domains were found to be located within the 50 N-terminal amino acids of PP2Ac, and between amino acid residues 338 and 381 in the C-terminal part of human eRF1. This association also occurs in vivo, since PP2A can be co-immunoprecipitated with eRF1 from mammalian cells. We observed a significant increase in the amount of PP2A associated with the polysomes when eRF1 was transiently expressed in COS1 cells, and eRF1 immunoprecipitated from those fractions contained associated PP2A. Since we did not observe any dramatic effects of PP2A on the polypeptide chain release activity of eRF1 (or vice versa), we postulate that eRF1 also functions to recruit PP2A into polysomes, thus bringing the phosphatase into contact with putative targets among the components of the translational apparatus.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudet A. L., Caskey C. T. Mammalian peptide chain termination. II. Codon specificity and GTPase activity of release factor. Proc Natl Acad Sci U S A. 1971 Mar;68(3):619–624. doi: 10.1073/pnas.68.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowsky R. T., Kamibayashi C., Mumby M. C., Hannun Y. A. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993 Jul 25;268(21):15523–15530. [PubMed] [Google Scholar]
- Dunphy W. G., Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991 Oct 4;67(1):189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Foulkes J. G., Ernst V., Levin D. H. Separation and identification of type 1 and type 2 protein phosphatases from rabbit reticulocyte lysates. J Biol Chem. 1983 Feb 10;258(3):1439–1443. [PubMed] [Google Scholar]
- Frolova L., Le Goff X., Rasmussen H. H., Cheperegin S., Drugeon G., Kress M., Arman I., Haenni A. L., Celis J. E., Philippe M. A highly conserved eukaryotic protein family possessing properties of polypeptide chain release factor. Nature. 1994 Dec 15;372(6507):701–703. doi: 10.1038/372701a0. [DOI] [PubMed] [Google Scholar]
- Grenett H. E., Bounelis P., Fuller G. M. Identification of a human cDNA with high homology to yeast omnipotent suppressor 45. Gene. 1992 Jan 15;110(2):239–243. doi: 10.1016/0378-1119(92)90655-9. [DOI] [PubMed] [Google Scholar]
- Healy A. M., Zolnierowicz S., Stapleton A. E., Goebl M., DePaoli-Roach A. A., Pringle J. R. CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol Cell Biol. 1991 Nov;11(11):5767–5780. doi: 10.1128/mcb.11.11.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings B. A., Adams-Pearson C., Maurer F., Müller P., Goris J., Merlevede W., Hofsteenge J., Stone S. R. alpha- and beta-forms of the 65-kDa subunit of protein phosphatase 2A have a similar 39 amino acid repeating structure. Biochemistry. 1990 Apr 3;29(13):3166–3173. doi: 10.1021/bi00465a002. [DOI] [PubMed] [Google Scholar]
- Hendrix P., Mayer-Jackel R. E., Cron P., Goris J., Hofsteenge J., Merlevede W., Hemmings B. A. Structure and expression of a 72-kDa regulatory subunit of protein phosphatase 2A. Evidence for different size forms produced by alternative splicing. J Biol Chem. 1993 Jul 15;268(20):15267–15276. [PubMed] [Google Scholar]
- Hendrix P., Turowski P., Mayer-Jaekel R. E., Goris J., Hofsteenge J., Merlevede W., Hemmings B. A. Analysis of subunit isoforms in protein phosphatase 2A holoenzymes from rabbit and Xenopus. J Biol Chem. 1993 Apr 5;268(10):7330–7337. [PubMed] [Google Scholar]
- Hershey J. W. Protein phosphorylation controls translation rates. J Biol Chem. 1989 Dec 15;264(35):20823–20826. [PubMed] [Google Scholar]
- Hoshino S., Miyazawa H., Enomoto T., Hanaoka F., Kikuchi Y., Kikuchi A., Ui M. A human homologue of the yeast GST1 gene codes for a GTP-binding protein and is expressed in a proliferation-dependent manner in mammalian cells. EMBO J. 1989 Dec 1;8(12):3807–3814. doi: 10.1002/j.1460-2075.1989.tb08558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard M. J., Cohen P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem Sci. 1993 May;18(5):172–177. doi: 10.1016/0968-0004(93)90109-z. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Bylund D. B., Huang T. S., Krebs E. G. Substrate specificity of the cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3448–3452. doi: 10.1073/pnas.72.9.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khew-Goodall Y., Mayer R. E., Maurer F., Stone S. R., Hemmings B. A. Structure and transcriptional regulation of protein phosphatase 2A catalytic subunit genes. Biochemistry. 1991 Jan 8;30(1):89–97. doi: 10.1021/bi00215a014. [DOI] [PubMed] [Google Scholar]
- Kleinberger T., Shenk T. Adenovirus E4orf4 protein binds to protein phosphatase 2A, and the complex down regulates E1A-enhanced junB transcription. J Virol. 1993 Dec;67(12):7556–7560. doi: 10.1128/jvi.67.12.7556-7560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnirov V. V., Ter-Avanesyan M. D., Telckov M. V., Surguchov A. P., Smirnov V. N., Inge-Vechtomov S. G. Nucleotide sequence of the SUP2 (SUP35) gene of Saccharomyces cerevisiae. Gene. 1988 Jun 15;66(1):45–54. doi: 10.1016/0378-1119(88)90223-5. [DOI] [PubMed] [Google Scholar]
- Law B., Rossie S. The dimeric and catalytic subunit forms of protein phosphatase 2A from rat brain are stimulated by C2-ceramide. J Biol Chem. 1995 May 26;270(21):12808–12813. doi: 10.1074/jbc.270.21.12808. [DOI] [PubMed] [Google Scholar]
- Mayer-Jaekel R. E., Hemmings B. A. Protein phosphatase 2A--a 'ménage à trois'. Trends Cell Biol. 1994 Aug;4(8):287–291. doi: 10.1016/0962-8924(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Mayer-Jaekel R. E., Ohkura H., Ferrigno P., Andjelkovic N., Shiomi K., Uemura T., Glover D. M., Hemmings B. A. Drosophila mutants in the 55 kDa regulatory subunit of protein phosphatase 2A show strongly reduced ability to dephosphorylate substrates of p34cdc2. J Cell Sci. 1994 Sep;107(Pt 9):2609–2616. doi: 10.1242/jcs.107.9.2609. [DOI] [PubMed] [Google Scholar]
- Mayer R. E., Hendrix P., Cron P., Matthies R., Stone S. R., Goris J., Merlevede W., Hofsteenge J., Hemmings B. A. Structure of the 55-kDa regulatory subunit of protein phosphatase 2A: evidence for a neuronal-specific isoform. Biochemistry. 1991 Apr 16;30(15):3589–3597. doi: 10.1021/bi00229a001. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science. 1995 Apr 14;268(5208):247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- Mumby M. C., Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol Rev. 1993 Oct;73(4):673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- Pallas D. C., Shahrik L. K., Martin B. L., Jaspers S., Miller T. B., Brautigan D. L., Roberts T. M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990 Jan 12;60(1):167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- Paushkin S. V., Kushnirov V. V., Smirnov V. N., Ter-Avanesyan M. D. Propagation of the yeast prion-like [psi+] determinant is mediated by oligomerization of the SUP35-encoded polypeptide chain release factor. EMBO J. 1996 Jun 17;15(12):3127–3134. [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruediger R., Hentz M., Fait J., Mumby M., Walter G. Molecular model of the A subunit of protein phosphatase 2A: interaction with other subunits and tumor antigens. J Virol. 1994 Jan;68(1):123–129. doi: 10.1128/jvi.68.1.123-129.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D., Erlichman J., Rubin C. S. Identification of a calmodulin-binding protein that co-purifies with the regulatory subunit of brain protein kinase II. J Biol Chem. 1984 Aug 10;259(15):9840–9846. [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacter E. Organic extraction of Pi with isobutanol/toluene. Anal Biochem. 1984 May 1;138(2):416–420. doi: 10.1016/0003-2697(84)90831-5. [DOI] [PubMed] [Google Scholar]
- Sontag E., Fedorov S., Kamibayashi C., Robbins D., Cobb M., Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993 Dec 3;75(5):887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- Stansfield I., Grant G. M., Akhmaloka, Tuite M. F. Ribosomal association of the yeast SAL4 (SUP45) gene product: implications for its role in translation fidelity and termination. Mol Microbiol. 1992 Dec;6(23):3469–3478. doi: 10.1111/j.1365-2958.1992.tb01782.x. [DOI] [PubMed] [Google Scholar]
- Stansfield I., Jones K. M., Kushnirov V. V., Dagkesamanskaya A. R., Poznyakovski A. I., Paushkin S. V., Nierras C. R., Cox B. S., Ter-Avanesyan M. D., Tuite M. F. The products of the SUP45 (eRF1) and SUP35 genes interact to mediate translation termination in Saccharomyces cerevisiae. EMBO J. 1995 Sep 1;14(17):4365–4373. doi: 10.1002/j.1460-2075.1995.tb00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield I., Tuite M. F. Polypeptide chain termination in Saccharomyces cerevisiae. Curr Genet. 1994 May;25(5):385–395. doi: 10.1007/BF00351776. [DOI] [PubMed] [Google Scholar]
- Strubin M., Newell J. W., Matthias P. OBF-1, a novel B cell-specific coactivator that stimulates immunoglobulin promoter activity through association with octamer-binding proteins. Cell. 1995 Feb 10;80(3):497–506. doi: 10.1016/0092-8674(95)90500-6. [DOI] [PubMed] [Google Scholar]
- Tassan J. P., Le Guellec K., Kress M., Faure M., Camonis J., Jacquet M., Philippe M. In Xenopus laevis, the product of a developmentally regulated mRNA is structurally and functionally homologous to a Saccharomyces cerevisiae protein involved in translation fidelity. Mol Cell Biol. 1993 May;13(5):2815–2821. doi: 10.1128/mcb.13.5.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani M. A., Mumby M. C., Kamibayashi C. Identification of a novel protein phosphatase 2A regulatory subunit highly expressed in muscle. J Biol Chem. 1996 Mar 1;271(9):5164–5170. doi: 10.1074/jbc.271.9.5164. [DOI] [PubMed] [Google Scholar]
- Tuite M. F., Stansfield I. Translation. Knowing when to stop. Nature. 1994 Dec 15;372(6507):614–615. doi: 10.1038/372614a0. [DOI] [PubMed] [Google Scholar]
- Tung H. Y., Alemany S., Cohen P. The protein phosphatases involved in cellular regulation. 2. Purification, subunit structure and properties of protein phosphatases-2A0, 2A1, and 2A2 from rabbit skeletal muscle. Eur J Biochem. 1985 Apr 15;148(2):253–263. doi: 10.1111/j.1432-1033.1985.tb08833.x. [DOI] [PubMed] [Google Scholar]
- Turowski P., Fernandez A., Favre B., Lamb N. J., Hemmings B. A. Differential methylation and altered conformation of cytoplasmic and nuclear forms of protein phosphatase 2A during cell cycle progression. J Cell Biol. 1995 Apr;129(2):397–410. doi: 10.1083/jcb.129.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Newnam G., Liebman S. W. The yeast translational allosuppressor, SAL6: a new member of the PP1-like phosphatase family with a long serine-rich N-terminal extension. Genetics. 1994 Nov;138(3):597–608. doi: 10.1093/genetics/138.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelkens E., Goris J., Merlevede W. Purification and properties of polycation-stimulated phosphorylase phosphatases from rabbit skeletal muscle. J Biol Chem. 1987 Jan 25;262(3):1049–1059. [PubMed] [Google Scholar]
- Wera S., Hemmings B. A. Serine/threonine protein phosphatases. Biochem J. 1995 Oct 1;311(Pt 1):17–29. doi: 10.1042/bj3110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhouravleva G., Frolova L., Le Goff X., Le Guellec R., Inge-Vechtomov S., Kisselev L., Philippe M. Termination of translation in eukaryotes is governed by two interacting polypeptide chain release factors, eRF1 and eRF3. EMBO J. 1995 Aug 15;14(16):4065–4072. doi: 10.1002/j.1460-2075.1995.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolnierowicz S., Csortos C., Bondor J., Verin A., Mumby M. C., DePaoli-Roach A. A. Diversity in the regulatory B-subunits of protein phosphatase 2A: identification of a novel isoform highly expressed in brain. Biochemistry. 1994 Oct 4;33(39):11858–11867. doi: 10.1021/bi00205a023. [DOI] [PubMed] [Google Scholar]
- Zolnierowicz S., Van Hoof C., Andjelković N., Cron P., Stevens I., Merlevede W., Goris J., Hemmings B. A. The variable subunit associated with protein phosphatase 2A0 defines a novel multimember family of regulatory subunits. Biochem J. 1996 Jul 1;317(Pt 1):187–194. doi: 10.1042/bj3170187. [DOI] [PMC free article] [PubMed] [Google Scholar]









