Abstract
Despite a gradual decrease in prevalence, clonorchiasis is still prevalent in East Asia. A large and compelling body of evidence links clonorchiasis and cholangiocarcinoma, although the mechanisms involved are not completely understood. Clonorchiasis induces biliary epithelial hyperplasia and metaplasia, and this could facilitate at least one stage of the carcinogenesis, which is promoting effect. In areas of endemic infection, more clonorchiasis cases are now diagnosed incidentally during radiological examinations such as cholangiography, ultrasonography, and computed tomography. Radiological findings are regarded as pathognomonic for clonorchiasis since they reflect the unique pathological changes of this disorder. These radiological examinations currently play important roles in the diagnosis, staging, and decision-making process involved in the treatment of cholangiocarcinoma. The morphological features and radiological findings of clonorchiasis-associated cholangiocarcinoma are essentially combinations of the findings for the two diseases. The morphological features of clonorchiasis- associated cholangiocarcinoma, observed in radiological examinations, do not differ from those of the usual cholangiocarcinoma. In patients diagnosed with or suspected to have clonorchiasis, radiological findings should be carefully scrutinized for occult cholangiocarcinoma.
INTRODUCTION
Several trematode parasites in humans are known to be epidemiologically linked with malignancy; notable examples are the associations between the blood-fluke Schistosoma haematobium and tumors of the bladder urothelium (79) and between the liver-flukes Clonorchis sinensis and Opisthorchis viverrini and cholangiocarcinoma. The areas where human clonorchiasis, a disease caused by chronic C. sinensis infection, is endemic are confined largely to the Far East. In these regions, clonorchiasis is considered an important cause of recurrent pyogenic cholangitis and cholangiocarcinoma. Several well-documented epidemiological, histopathological, and experimental studies of C. sinensis have provided convincing evidence of a relationship between this trematode infection and the tendency for malignant transformation of the biliary epithelium in humans and experimentally infected animals (11, 42, 46).
In this paper, evidence indicating that C. sinensis is an etiological factor in the pathogenesis of human cholangiocarcinoma and morphological features of clonorchiasis and cholangiocarcinoma, with particular reference to imaging diagnosis, are reviewed.
LIFE CYCLE OF CLONORCHIS SINENSIS
The life cycle of C. sinensis has been well documented (78,93). The definitive hosts of C. sinensis are humans, dogs, hogs, cats, martens, badgers, mink, weasels, and rats. The eggs, shed by adult worms, are deposited in the biliary tree of these mammalian hosts, enter the intestine, and are passed with the feces. On reaching water, the eggs are ingested by snails. Although several species of snails serve as the first intermediate hosts, Parafossarulus and Bithynia are the most suitable. Within the snail, the eggs undergo metamorphosis and asexual reproduction for 4 to 5 weeks, after which cercariae are shed into the water. These free-swimming forms penetrate the skin between the scales of freshwater fish. Numerous species of freshwater fish, mostly belonging to the family Cyprinidae, serve as the second intermediate host. After a few days in the fish muscle, the cercariae become encysted and form metacercariae. Humans and other fish-eating mammals then acquire the infection by ingesting raw or inadequately cooked fish. As a result of the digestive processes in the stomach and intestines, the metacercariae eventually excyst in the duodenum and migrate though the ampulla of Vater into the bile duct, where they mature into adult worms over a period of a month. In humans, the adult fluke inhabits the biliary tract, generally localizing within the intrahepatic bile ducts. The adult worm is a small trematode with an elliptical shape and an average length of 10 to 25 mm (78, 93). The trematode is a true hermaphrodite and lays fully embryonated eggs. The adult fluke has a life span of 20 to 25 years, which explains the persistent infection for a long duration. The completion of this life cycle is restricted to areas of endemic infection, which reflect the geographic distribution of the essential snail species (108).
CHLONORCHIASIS
Pathophysiology
C. sinensis causes inflammation around the biliary tree, severe hyperplasia of epithelial cells, metaplasia of mucin-producing cells in the mucosa, and progressive periductal fibrosis (39, 66, 78, 90, 109). The severity of these pathological changes tends to correlate with the duration of infection, the parasite burden, and the susceptibility of the host (34, 77).
In humans, the histopathological responses to C. sinensis infection are distinctive. A classical description of the responses has been written by Hou (41). Gross examination of an infected liver reveals slight irregularities at the capsular surface in mild to moderate cases; however, in severe cases, pale cystic areas can be seen where the dilated peripheral bile ducts are directly seen through the liver capsule as the dilated and fibrosed bile ducts approach the surface. The cut surface of the liver reveals dilatation of the medium-sized bile ducts with thickened walls. The histopathological findings of clonorchiasis are characterized by bile duct epithelial proliferation, which is followed by periductal fibrosis (Fig. 1). Biliary hyperplasia is the distinctive lesion of early Clonorchis infection, but the portal tracts do not become so damaged as to lead to portal venous hypertension or biliary cirrhosis in most clinical cases. In addition to biliary hyperplasia, the biliary epithelium frequently becomes edematous and desquamation may be seen in areas of tissue in close proximity to the flukes. Periductal infiltrates of mononuclear cells are frequently found; however, inflammation of the bile duct walls is generally only slight in uncomplicated cases. Metaplasia of the biliary epithelial cells into mucin- producing cells occurs during early infection, and these cells may proliferate to produce many small gland-like structures in the mucosa, leading to a persistent and excessively high mucus content in the bile. Chronic and persistent infections result in a gradual increase in the amount of fibrous tissues, which may eventually engulf some of the proliferating glands, giving the appearance of cholangiofibrosis. As this fibrosis proceeds, the epithelial proliferation becomes milder. In such chronic cases, fecal egg counts may drop markedly. These histopathological changes are distinctive features of clonorchiasis; therefore, when proliferation of the ductal epithelium with metaplastic cells (described as adenomatous hyperplasia) (66) and periductal fibrosis are observed in patients in an area of endemic infection, they are highly suggestive of clonorchiasis on histological grounds, even though the parasite is unobserved in specimen sections (4).
FIG. 1.
Histopathological findings of clonorchiasis (hematoxylin and eosin stain). Note the flukes (arrows) within the dilated bile ducts, biliary epithelial hyperplasia (arrowheads), and periductal fibrosis.
The histopathological features of infection in experimental models are in many respects similar to those described in humans. Several researchers (66, 77,110) have described the histopathological responses of rats and guinea pigs to C. sinensis infection over a period of 11 to 12 weeks. These documented histopathological changes have been divided into several phases. The first phase is characterized by edema and desquamation of bile duct epithelium. This is followed by epithelial hyperplasia, pseudostratification of the biliary epithelium, and mucin-secreting cell metaplasia (66). Metaplastic squamous cells may appear in conjunction with glandular proliferation, giving an appearance suggestive of adenomatous hyperplasia (66). Heavy periductal infiltration of inflammatory cells, including eosinophils, is observed during the first 2 weeks of infection. After 12 weeks, these infiltrates are composed of plasma cells, lymphocytes, and other mononuclear cell types (66). The final phase is marked by development of periductal fibrosis.
The complications of clonorchiasis are the results of biliary obstruction. Parasite-induced mucin-secreting cells create bile with a high mucin content, which, combined with adult flukes and eggs, serves as a nidus for bacterial superinfection and intrahepatic stone formation. Bacterial infections are of enteric origin, with Escherichia coli being identified most frequently as a pathogen (109). The ectasia of intrahepatic bile ducts may progress to pyogenic cholangitis, liver abscess, and hepatitis (109).
Clinical Manifestations
The clinical manifestations of clonorchiasis are protean (11, 90, 93) and tend to reflect the worm burden. Most patients with mild infections, i.e., with fewer than 100 flukes, have few symptoms. Early symptoms may include general malaise, abdominal discomfort, and diarrhea. In 10 to 40% of patients, peripheral eosinophilia accompanies a fluctuating jaundice that is usually obstructive. Moderate infection (generally fewer than 1,000 flukes) presents with fever and chills, as well as fatigue, anorexia, diarrhea, weight loss, discomfort, and abdominal distension. Up to 20,000 flukes may be present in patients with severe disease, which presents as acute right upper quadrant pain, often superimposed on the signs and symptoms seen in moderate infections. In the late stage of severe cases, jaundice, diarrhea, portal hypertension, hepatosplenomegaly, a scites, and edema can occur (34, 42, 77, 89, 93, 97). Pyogenic cholangitis, cholelithiasis, chronic cholecystitis, pancreatitis, and cholangiocarcinoma have been described as potential long-term complications of clonorchiasis (42).
Many hepatic and biliary diseases can mimic clonorchiasis in their clinical presentation. Differential diagnoses of clonorchiasis include acute or chronic hepatitis, cancer along the bile ducts, hepatocholedocholithiasis with recurrent pyogenic cholangitis, sclerosing cholangitis, Caroli's disease, and Fasciola hepatica infection (70).
Epidemiology
Clonorchiasis is endemic in the Far East, especially in southern and northeastern China, eastern Russia, Vietnam, and Korea (90). The custom of eating raw freshwater fish contributes to the high incidence of infection in these areas (14).
In 1947, Stoll (106) estimated that 19 million Asians harbored this liver fluke. Despite a gradual decrease in its prevalence over the decades, the International Agency for Research on Cancer Working Group estimated in 1994 that about seven million people were infected in areas of endemic infection (46). The national survey in Korea in 1997 revealed that the prevalence of clonorchiasis was still 1.4% (77a). The difficulty of eliminating clonorchiasis in areas of endemic infection has been attributed mainly to the difficulty in detecting infected cases, although other contributory factors, including reinfection after treatment, have been discussed (37, 64). C. sinensis is currently the most prevalent human parasitic helminth detected by fecal examination in Korea (52, 76).
The rate of clonorchiasis in areas of endemic infection is greater in aged people and in men than in the younger age groups and in women (93, 99, 102). The rate of positive results in fecal examination generally reaches a maximum in the age group from 50 to 59 years. In addition, the same age-related pattern has been observed for worm burden. The higher infection rates and heavier worm burden in older people suggests that humans have little protective immunity and are superinfected throughout life. The infection rate decreases in the seventh decade, which might reflect an elevated death rate among the infected population. The higher percentage of clonorchiasis in men and in old people is probably related to dietary habits. In areas of endemic infection, people traditionally prefer to eat raw freshwater fish, soaked simply in vinegar or red-pepper mash, as an appetizer when drinking liquor at social gatherings. In some areas, fermented raw fish is a favorite side dish. Because women infrequently participate in such rituals, they are less frequently exposed to the infection.
Liver flukes have a life span of 20 to 25 years; this creates a problem for Asian immigrants to other areas, who may develop clinical symptoms several years after leaving an area of endemic infection (90, 109). Clonorchiasis in North America has been reported in recent decades, reflecting the immigration of people from areas of endemic infection (49, 83, 89, 97, 98, 117). The prevalence of clonorchiasis among these immigrants varies between studies, ranging from 15.5 to 26% (9, 97, 98); however, most of these reports were published more than 10 years ago. Although clonorchiasis does not have a great impact on public health in North America, recognition of this parasite and its associated complications continues to be important for the correct diagnosis of disease in immigrants or travelers from areas of endemic infection (91). Unfortunately, the common clinical features of clonorchiasis, along with the increased risk of developing long-term sequelae such as cholangiocarcinoma, remain largely unknown to many physicians providing care for this population.
Diagnosis
Clonorchiasis should be suspected in patients who develop manifestations of hepatic or biliary disease, if they have a history of ingesting raw freshwater fish in an area of endemic infection. The diagnosis of clonorchiasis is usually established by microscopic examination of the feces for eggs. The formalin-ether sedimentation technique is known to be more reliable than the direct-smear method for detecting eggs in feces. The cellophane thick-smear method has usually been used for mass screening (94). The eggs of C. sinensis are oval and measure 27 to 35 μm by 12 to 20 μm. They have an operculum at the slender end, with prominent shoulders. The opposite (abopercular) end is broad and has a small spine-like prominence (23).
Although clonorchiasis is easily diagnosed by fecal examination, mass screening by fecal examination recently became more difficult because of poor voluntary cooperation (38). A number of serological techniques have been developed as supplementary diagnostic methods. The intradermal test, which uses a diluted extract of adult C. sinensis antigens, was once used widely (93) but is not recommended any more because of its very low specificity. Enzyme-linked immunosorbent assay for the detection of antibodies against C. sinensis has also been used as an alternative serological test (8). However, unfortunately, the serological methods currently available exhibit considerable cross-reactivity and therefore are not widely accepted as screening techniques (3).
At present, clonorchiasis is commonly diagnosed incidentally during radiological screening, especially by ultrasonography of the abdomen for other purposes, since symptoms of clonorchiasis are vague and nonspecific in most cases (11, 70, 73). Ultrasonography is widely accepted as an accurate and feasible diagnostic method for clonorchiasis (11, 37, 70, 73). However, although helpful, none of these various serological tests or radiological examinations has been reported to surpass fecal examination because of their limited sensitivity, specificity, or applicability (38).
Once diagnosed, clonorchiasis is treated very effectively with praziquantel (three 25-mg/kg doses daily for 1 to 3 days) with few side effects (10, 78,94, 115). The published efficacy of this treatment is invariably over 90% (10, 93). The early diagnosis and the availability of safe and effective treatment for clonorchiasis might suggest that the cholangiocarcinoma associated with this infection can be prevented (51,91, 97). However, to the best of our knowledge, there is no published evidence that early eradication of C. sinensis reduces the occurrence of clonorchiasis-related complications, such as cholangiocarcinoma, in areas of endemic infection. An experimental study (39) showed that the mucin-secreting cell metaplasia and adenomatous hyperplasia remain 6 months after treatment and that biliary epithelial cells maintain a higher proliferative activity than controls. Another experimental study (62) revealed that hamsters with combined clonorchiasis and N-nitrosodimethylamine treatment developed cholangiocarcinoma in spite of praziquantel treatment.
Imaging Findings
The radiological findings in clonorchiasis have been summarized by Lim (70) in 1990. In spite of the remarkable advances in medical-imaging technology over the last decade, there are few developments in terms of the imaging of clonorchiasis.
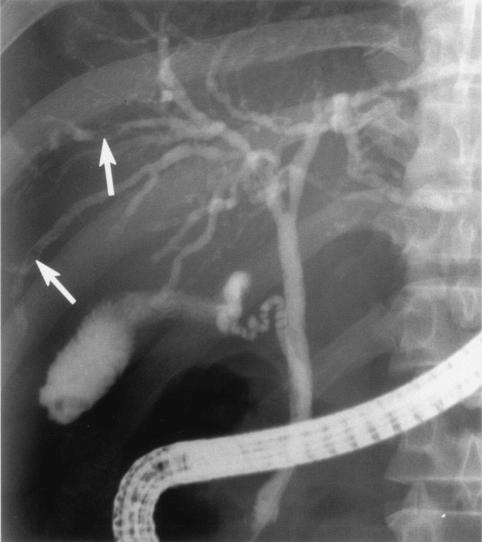
The characteristic radiological findings of clonorchiasis include diffuse and uniform dilatation of the peripheral intrahepatic bile ducts with no or minimal dilatation of the extrahepatic bile duct and without focal obstructing lesions in the larger bile ducts. This characteristic appearance of the biliary tree was first described on the basis of cholangiographic findings. Cholangiography usually shows many elliptical or filamentous filling defects within the peripheral intrahepatic ducts; dilatation of the intrahepatic ducts, particularly in the periphery; and a hazy appearance of the intrahepatic ducts (16, 86). Filling defects are several millimeters in diameter, i.e., compatible with the width of the fluke (Fig. 2). The predominant dilatation of the peripheral ducts is related to the location of the worms. The haziness of the intrahepatic ducts is probably caused by the increased production of mucinous material and incomplete mixing of the contrast media (11).
FIG. 2.
Posteroanterior endoscopic retrograde cholangiography in a 54-year-old man with clonorchiasis. Note the diffuse and uniform dilatation of the peripheral intrahepatic bile ducts with minimal dilatation of the extrahepatic bile duct and elliptical filling defects within the peripheral intrahepatic ducts, which correspond to the flukes (arrows).
On ultrasonography, characteristic findings of clonorchiasis are summarized as diffuse, mild, uniform dilatation of the small intrahepatic bile ducts with no or minimal dilatation of larger bile ducts and without an obstructing lesion (12, 70, 73). The ductal wall is often thickened, and its echogenicity is increased. Occasionally, flukes or aggregates of eggs are shown as nonshadowing echogenic foci or casts within the bile duct (70) (Fig. 3). These findings are regarded as pathognomonic for clonorchiasis in areas of endemic infection (11,70). The accuracy of ultrasonography in the diagnosis of clonorchiasis varies widely, depending mainly on the prevalence in the study population. In 1993, Sung et al. (111) reported a sensitivity of 3.8% and a specificity of 99.6% by analyzing 9,063 screening ultrasonographic studies in a patient group with a prevalence rate of 1.8%, and they concluded that ultrasonography is not an adequate screening method for the diagnosis of clonorchiasis due to its low sensitivity. More recent studies (38, 59) also have shown that ultrasonography has a low diagnostic accuracy for clonorchiasis. According to a study carried out in an area of endemic infection (38), the sensitivity of ultrasonography was 52% and its specificity was 51%. The low sensitivity was attributed to false- negative cases with mild infection, and the low specificity was attributed to false- positive cases with residual pathology after cure. This low specificity is of particular interest, since the number of cases cured has been continuously increasing over recent decades due to nationwide control and changes in the ecological environment (38, 64). Ultrasonography is less useful in the differentiation between cured clonorchiasis and active infection, since it reflects the pathological changes in the bile ducts (73), which may persist for years after cure (15, 37, 65), rather than reflecting the presence of the worm itself.
FIG. 3.
Hepatic ultrasonography (right intercostal oblique plane) in a 60-year-old woman (a) and a 41-year-old man (b) with clonorchiasis. Note the diffuse, uniform dilatation of the intrahepatic bile ducts and the increased echogenicity of the ductal wall (arrowheads). Flukes or aggregates of eggs are shown as nonshadowing echogenic foci within the bile ducts (arrowheads in panel b).
Computed tomography findings of clonorchiasis are essentially the same as those observed by ultrasonography, i.e., mild, uniform dilatation of the peripheral intrahepatic bile ducts without a focal obstructing lesion. The extrahepatic duct has a normal diameter, and no definite obstructing lesion is seen, even by thin-section helical computed tomography (Fig. 4). These findings are considered pathognomonic for clonorchiasis (11, 12, 14, 70). Computed tomography was previously thought to be of little value in delineating the fluke itself (12, 70); however, a recent experimental study (64) revealed that the fluke can be visualized by thin-section helical computed tomography. This study also demonstrated that disease activity can be assessed by dynamic contrast- enhanced computed tomography during experimental canine clonorchiasis, suggesting the possibility of distinction between active clonorchiasis and cured cases with residual pathology.
FIG. 4.
Transverse hepatic computed tomography in a 45-year-old woman (a) and a 57-year-old man (b) with clonorchiasis. Note the mild, uniform dilatation of the intrahepatic bile ducts (arrowheads).
CHOLANGIOCARCINOMA
Cholangiocarcinoma is a malignant tumor that arises from any portion of the bile duct epithelium, i.e., anywhere from the terminal ductules (canals of Hering) to the ampulla of Vater, as well as at the peribiliary glands (intramural and extramural) (5, 81). Cholangiocarcinoma is the second most prevalent liver cancer after hepatocellular carcinoma. The tumor shows a slight male predominance, but the male-female ratio is much closer to unity than for hepatocellular carcinoma (14, 42, 85, 87). The tumor usually occurs in the sixth or seventh decade of life, and its occurrence in patients younger than 40 years is rare (87).
The majority of cholangiocarcinomas are adenocarcinomas with variable grades of differentiation (mainly well-differentiated adenocarcinomas), although several uncommon types are also encountered, such as adenosquamous, squamous, mucinous, and anaplastic carcinomas (80). Local and metastatic tumor growth characteristics, not histology, govern surgical resectability.
Cholangiocarcinomas are usually classified as intrahepatic, hilar (Klatskin tumor), or extrahepatic although their precise definitions and classification are controversial (32). Peripheral intrahepatic cholangiocarcinoma is generally thought to originate from the intrahepatic bile ducts (i.e., the bile ducts distal to second-order branches), while hilar cholangiocarcinoma is thought to originate from the right or left hepatic ducts (i.e., first-order bile duct branches) or the bifurcation of the two hepatic ducts (5, 14, 53, 81, 103). These three types of cholangiocarcinoma—peripheral intrahepatic, hilar intrahepatic, and extrahepatic—have been traditionally regarded as distinct disease entities clinically, therapeutically, and radiologically (53, 81).
However, the tumor types classified according to the traditional classification scheme, based on the location of the involved ducts, have overlapping features in practice. Cholangiocarcinoma, regardless of the location of the involved ducts, can show any of the typical exophytic, infiltrative, or polypoid growth patterns or combinations of the three patterns (68). Cholangiocarcinomas arising from the peripheral intrahepatic duct to the distal common duct may have similar morphological features. Furthermore, some cholangiocarcinomas involve both the intrahepatic and extrahepatic ducts, which makes a clear-cut classification difficult. Therefore, it appears that different biological behaviors of these tumors are caused by their different locations and sizes at the time of diagnosis. All cholangiocarcinomas seem to be biologically the same tumors originating from the same biliary epithelium (32). Tumors originating from the large bile duct are discovered early due to their critical location, causing jaundice or cholangitis even as small tumors. Conversely, tumors originating from the small bile ducts do not cause significant biliary obstruction until the late stage, when the tumor itself or metastatic hilar lymphadenopathy causes obstruction of the common hepatic duct (32).
Because of its peculiar growth patterns, polypoid cholangiocarcinoma is thought to be a distinct entity rather than an early manifestation of the more typical mass-forming intrahepatic cholangiocarcinoma (32). Polypoid cholangiocarcinoma is infrequently found in both the intra- and extrahepatic ducts. Histologically, this tumor is mostly of the papillary adenocarcinoma type, which shows intraluminal growth (2, 56, 63). This type of cholangiocarcinoma has a better prognosis than other types of cholangiocarcinoma.
In terms of treatment, complete resection with negative histological margins provides the only hope for long-term survival. At this time, there is no effective adjuvant therapy for this tumor. Cholangiocarcinoma responds poorly to chemotherapy. Radiation therapy may be indicated postoperatively (6, 88).
Imaging Findings
Cholangiocarcinoma is a biliary carcinoma that arises from the intra- or extrahepatic bile ducts and manifests as various histological types and growth patterns. Accordingly, it has a wide spectrum of radiological appearances, which may overlap with those of other hepatobiliary diseases, including benign diseases (68). The radiological findings of the various types of cholangiocarcinoma have been summarized recently in articles by Han et al. (32) and Lee et al. (68).
Although computed tomography provides better anatomical detail (e.g., by depicting vascular or extrahepatic invasion) and more accurate staging (118), magnetic resonance imaging allows greater contrast which facilitates detection of the tumor and evaluation of hepatic parenchymal changes peripheral to the tumor. However, the two modalities are equally effective at detecting and correctly diagnosing the tumor (32).
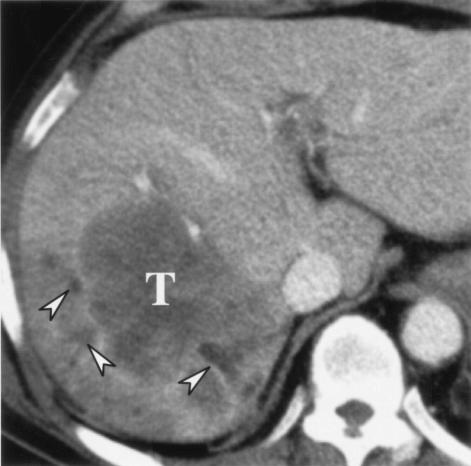
Peripheral cholangiocarcinoma classically manifests as a large, well- defined hepatic mass with lobulated margins (32). At computed tomography, peripheral cholangiocarcinoma appears as an irregular mass with markedly low attenuation and internal stippled hyperattenuating foci (14, 53, 103). Dynamic computed tomography and magnetic resonance imaging reveal rim-like or band-like peripheral contrast enhancement of variable thickness around the tumor during the early phase, with progressive and concentric filling of contrast material at later phase (25, 53, 72, 75). This phenomenon is explained by slow diffusion of contrast materials into the interstitial spaces of the tumor (47). A fibrotic component within this type of cholangiocarcinoma also contributes to the delayed contrast enhancement of the tumor (58). Frequently noted ancillary findings in peripheral cholangiocarcinoma include capsular retraction and dilatation of the peripheral intrahepatic ducts (especially when associated with clonorchiasis) (53, 73) (Fig. 5).
FIG. 5.
Intrahepatic peripheral cholangiocarcinoma in a 56-year-old woman. Transverse hepatic computed tomography shows a low-attenuation mass (T) in the right lobe of the liver. Note the dilated intrahepatic ducts peripheral to the tumor (arrowheads).
Most hilar and extrahepatic cholangiocarcinomas are infiltrative, causing a focal stricture of the bile duct, whereas papillary carcinoma is occasionally found in the form of an intraductal polypoid mass (13, 32, 95, 116). By thin-section computed tomography, the tumor tissue of the infiltrative type can be detected as an ill-defined focal wall thickening, usually with early or late enhancement or both (13, 33). By ultrasonography, the tumor can appear as a mural thickening or as an encircling mass along the bile duct wall (95). However, in many cases, the infiltrating tumor is frequently seen by either ultrasonography or computed tomography as nonunion of the dilated right and left hepatic ducts without an identifiable mass (13, 104). Cholangiography shows a focal or diffuse stricture or complete obstruction of the bile ducts and thus allows the extent of the disease to be determined (22, 104, 116) (Fig. 6).
FIG. 6.
Hilar cholangiocarcinoma in a 58-year-old man. Transverse hepatic computed tomography (a) and right anterior oblique cholangiography (b) using a percutaneous transhepatic biliary drainage tube (curved arrow) show narrowing of the right (open arrows) and left (solid arrows) main ducts and abnormal enhancement of the ductal wall, which correspond to the tumor. Peripheral intrahepatic ducts are dilated (arrowheads).
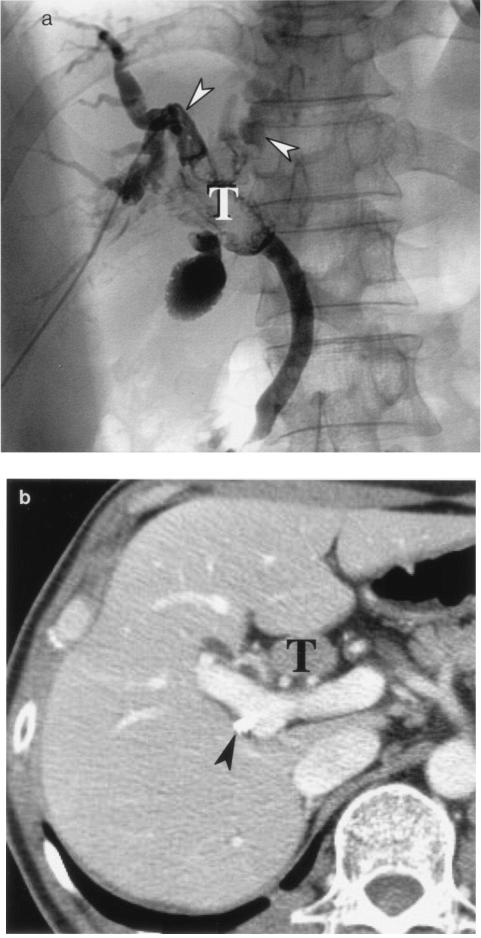
Polypoid cholangiocarcinoma is depicted as an intraluminal polypoid mass by both ultrasonography and computed tomography (13, 33, 95, 116) and as a polypoid filling defect by cholangiography (22, 104, 116). By computed tomography, its characteristic features include regional dilatation of the bile duct and an obstructing mass with higher attenuation than that of bile (63) (Fig. 7). In some instances, a peculiar growth pattern characterized by superficial mucosa spreading is observed. The true extent of this type of cholangiocarcinoma is known to be difficult to determine, even by high-quality cholangiography. Therefore, choledochoscopy and biopsy are often necessary before curative resection is performed (32).
FIG. 7.
Intraductal polypoid cholangiocarcinoma in the common bile duct of a 50-year-old woman. Posteroanterior percutaneous transhepatic cholangiography (a) and transverse hepatic computed tomography (b) show an intraductal tumor (T) within the proximal common bile duct. Arrowheads in panel a indicate the dilated intrahepatic ducts; the arrowhead in panel b indicates a percutaneous transhepatic biliary drainage tube.
Etiology
Cholangiocarcinomas frequently develop in patients with preexisting bile duct diseases, some of which are considered precursors of cholangiocarcinoma. Such preexisting bile duct diseases include liver fluke infection, recurrent pyogenic cholangitis, cholelithiasis, primary sclerosing cholangitis, choledochal cyst, benign biliary tumors, exposure to thallium oxide, choledochoenteric anastomosis, and the so-called ductal plate malformations (e.g., biliary hamartoma, polycystic disease, congenital hepatic fibrosis, and Caroli's disease) (5, 22, 56, 81, 103, 104).
In areas where cholangiocarcinoma is not endemic, primary sclerosing cholangitis is one of the most important etiological factors in cholangiocarcinogenesis. Occult cholangiocarcinoma has been identified in up to 40% of autopsy specimens and in 9 to 36% of liver explants after liver transplantation in patients with primary sclerosing cholangitis (6, 92, 100). Another important risk factor for cholangiocarcinogenesis is that of congenital biliary cystic disease, such as choledochal cysts and Caroli's disease, which have been reported to have a 2.5 to 28% rate of malignant transformation (6, 92). In the Far East and in Southeast Asia, liver fluke infections due to C. sinensis and O. viverrini are the most frequently cited causes of cholangiocarcinoma (11, 96).
Chronic biliary infection or stasis, regardless of the underlying cause, is considered a contributory factor in cholangiocarcinogenesis. Several authors (56, 84) have hypothesized that the lining epithelium of the bile duct, when persistently exposed to biochemically altered bile, may undergo a carcinomatous transformation through the stages of mucosal adenomatous hyperplasia and dysplasia.
Recurrent pyogenic cholangitis (also called Oriental cholangiohepatitis or intrahepatic pigmented-stone disease), which is also endemic in the Far East, is associated with cholangiocarcinoma in 5 to 10% of cases (9, 81). Moreover, stone formation in recurrent pyogenic cholangitis has been closely linked with Clonorchiasis infection (5, 7, 21, 70, 71). It has been suggested that C. sinensis causes recurrent pyogenic cholangitis by acting as a nidus for stone formation, which predisposes a patient to stasis and secondary bacterial infection, or by damaging the bile ducts, resulting in strictures and stone formation (21, 71).
ASSOCIATION BETWEEN CLONORCHIASIS AND CHOLANGIOCARCINOMA
Reports that clonorchiasis predisposes the host to cholangiocarcinoma appeared early in the last century (50, 82, 114). Later, on the basis of autopsy findings, Hou (42) described clonorchiasis to be present in 58% of cases of cholangiocarcinoma in Hong Kong and stated that it was responsible for 15% of all liver cancers in areas of endemic infection. Many studies in these areas have since documented high incidences of concurrent cholangiocarcinoma and clonorchiasis (4, 12, 20, 27, 96).
In the 1980s, several authors documented that the association between clonorchiasis and cholangiocarcinoma was debatable and commented that the significance of this combination remained to be confirmed (24, 85), even though other researchers had already concluded that an etiologic relationship existed (20, 42, 44, 45, 54). As Belamaric (4) described, some uncertainty about this relationship persists because of the following observations. First, in Southeast Asia, where cholangiocarcinoma is endemic, clonorchiasis is also exceedingly common; therefore, the development of cholangiocarcinoma in a patient with clonorchiasis may be coincidental rather than a cause-and-effect phenomenon. Second, cholangiocarcinomas are encountered in the absence of clonorchiasis. Finally, only rarely does a patient, among many infected individuals, develop a cholangiocarcinoma. Nevertheless, in 1994 the International Agency for Research on Cancer Working Group (46) concluded that clonorchiasis is probably carcinogenic in humans, and the causal relationship between clonorchiasis and cholangiocarcinoma is now generally accepted by most researchers since epidemiological, experimental, and pathological data have accumulated that suggest a positive correlation.
Epidemiological Evidence
The relationship between clonorchiasis and the development of cholangiocarcinoma has been established by many epidemiological studies. It is undisputed that the prevalence of cholangiocarcinoma is significantly higher in areas where liver fluke infestation is endemic than in other areas (48, 54, 74). Even within Korea, where clonorchiasis was once very prevalent, the prevalence of cholangiocarcinoma in areas of endemic infection, i.e., the southeastern part of Korea, is much higher than that in other areas (20). In 1994, the International Agency for Research on Cancer Working Group (46) analyzed three previous case-control studies (20, 31, 55) carried out in Korea and Hong Kong. In these studies and another, more recent, study (101), the relative risk of clonorchiasis for cholangiocarcinoma ranged from 2.7 to 6.5. It was also reported that cholangiocarcinoma is associated with heavy C. sinensis infections (55).
Comparison of the ratio of hepatocellular carcinoma to cholangiocarcinoma in areas with a high incidence of primary liver cancer also provides useful information. In Hong Kong and Canton, where infection with liver flukes is common, the ratio of cholangiocarcinoma to hepatocellular carcinoma is roughly 1:5 (42, 69). However, in areas where liver fluke infections are absent but the primary liver cancer rate is still high, the ratio is much higher; for example, in Java it was 1:56, in Africa generally it was 1:38, and in Johannesburg it was 1:20 (49, 105). These statistics clearly show that the presence of liver fluke infection in a population with a high incidence of primary liver cancer is associated with an overall increase in the incidence of cholangiocarcinoma (27). In Korea, the rate of C. sinensis infection in patients with cholangiocarcinoma was also found to be two to five times greater than in those with hepatocellular carcinoma (20, 55).
Experimental Studies
Experimental clonorchiasis has been generated in guinea pigs, rabbits, rats, hamsters, cats, and dogs (23, 30, 43, 44, 66). In the 1960s Hou reported that cholangiocarcinomas developed in cats and dogs which were either spontaneously or experimentally infected with C. sinensis (43,44). Another study of experimental animals by Hou (42) showed that only prolonged severe infection results in cholangiocarcinoma. Hou found that in cats, which develop the same ductal lesions as humans, adenomatous hyperplasia finally progressed into cholangiocarcinoma in 3 of 93 infected animals whereas none of the animals without adenomatous hyperplasia developed cancer. However, to our knowledge, this observation has not been reproduced by other researchers. Many other experimental studies have found that other animals infected with C. sinensis alone have never developed cholangiocarcinoma, although the livers of animals experimentally infected with C. sinensis developed adenomatous hyperplasia, periductal fibrosis, inflammatory infiltration, and mucin-secreting cell metaplasia (66, 67). Therefore, it has been suggested that the induction of cholangiocarcinoma from Clonorchiasis may be species dependent (61) and that the liver fluke per se does not provide the sole carcinogenic stimulus leading to malignancy (28).
Some investigations (28, 61, 110, 113) have reported synergism between liver fluke infection and exogenous chemical carcinogens with respect to cholangiocarcinoma development in hamsters. Lee et al. (61) demonstrated a high incidence (75%) of cholangiocarcinoma in hamsters that were treated with N-nitrosodimethylamine and then infected with C. sinensis. They also observed that hamsters treated with N-nitrosodimethylamine prior to C. sinensis infection and hamsters exposed to N-nitrosodimethylamine and C. sinensis simultaneously developed cholangiocarcinoma in the liver whereas hamsters infected with C. sinensis prior to N- nitrosodimethylamine treatment and hamsters treated with either N-nitrosodimethylamine or C. sinensis, but not both, did not develop cholangiocarcinoma (62). Their results suggest that clonorchiasis in hamsters may have a promoting effect on cholangiocarcinoma development by inducing replication and fixing of N- nitrosodimethylamine-damaged DNA before repair.
Histopathological Evidence
Several researchers (42, 54) have documented histopathological observations of the relationship between clonorchiasis and cholangiocarcinoma in humans. In an autopsy study (42) of 28 cases in patients with concomitant clonorchiasis and cholangiocarcinoma, Hou observed the direct transformation of hyperplastic bile ducts in human clonorchiasis to cholangiocarcinoma and reported that all transitional stages in the development of the carcinoma were demonstrable. He found that carcinomas arose most frequently in association with adenomatous changes in the wall of the bile duct. Kim (54) described that the lining epithelium of the bile duct, when persistently exposed to biochemically altered bile or irritated with C. sinensis adult worms, may undergo a carcinomatous transformation through a stage of dysplasia.
SUGGESTED MECHANISMS OF CARCINOGENESIS
In spite of a large body of evidence about the causative relationship between clonorchiasis and cholangiocarcinoma, the actual mechanism of this carcinogenesis is not completely understood. It seems plausible that cholangiocarcinogenesis associated with clonorchiasis is the cumulative end result of a multifactorial carcinogenic mechanism (27). Although the exact mechanism has yet to be determined, bile duct hyperplasia irritated by the worms undoubtedly plays an important role (probably promotion) in cholangiocarcinogenesis through a stage of dysplasia. The proliferating bile ducts may be susceptible to the action of a carcinogen(s) present at levels insufficient to induce bile duct tumors in noninfected individuals. Such carcinogens may occur naturally, may be formed in vivo from ingested precursor molecules, may be endogenous substances, or may be formed by the worm.
Biliary Hyperplasia Induced by C. sinensis
Experimental studies have suggested that C. sinensis stimulates biliary epithelial hyperplasia (40), a process which is thought to play an important role in the promotion of carcinogenesis (61, 62). This hypothesis was described earlier in the work by Thamavit et al. (112) concerning another liver fluke, O. viverrini, in which infected hamsters that were fed with the carcinogen N-nitrosodimethylamine invariably developed cholangiocarcinoma.
However, the mechanism by which the parasite invokes such biliary hyperplasia and metaplasia is unknown. According to an experimental study (40) using bromodeoxyuridine staining to observe the proliferation of biliary epithelial cells in rats, epithelial proliferation was confined to the hepatic region containing the worms, suggesting that the proliferation of epithelial cells is provoked only by direct stimulation by the worms. The worms move continuously in the duct (93), and their suckers and tegument come into intimate contact with the biliary epithelial cells. However, it is still plausible that the host cells may receive immunological or chemical stimuli in addition to mechanical stimuli (40, 109).
Exogenous and Endogenous Chemical Carcinogens
The observation that cholangiocarcinomas arise in a relatively small percentage of individuals who have clonorchiasis implies that other factors may be also necessary for malignant transformation to occur.
In 1956, Hou speculated that some carcinogenic substance might act synergistically with C. sinensis to induce cholangiocarcinoma and suggested that such carcinogens may be the metabolic or degenerative products of the parasite or a bile component altered chemically by the parasite (42). A similar hypothesis was proposed by Hong et al. (40), who suggested that certain secreted metabolites from C. sinensis may alter the cellular nature of the host. Chou and Gibson (18) have suggested that the mucosal hyperplasia and mucin-secreting cell metaplasia in human clonorchiasis may be a step in tumor formation, and Chou et al. (19) further postulated that the deficiency of sulfomucin biosynthesis by the neoplastic biliary epithelium may represent the regression of transformed cells to a less differentiated level. Ohta et al. (84) hypothesized that the lining epithelium of the bile duct, when persistently exposed to chemically altered bile, may undergo a carcinomatous transformation through a stage of mucosal dysplasia.
While these propositions seem plausible, it now appears that exogenous environmental carcinogens, which are ubiquitous in nature, are more important. The most extensively studied of these carcinogens in terms of the cholangiocarcinogenesis associated with liver fluke infection are N-nitroso compounds (28, 61, 62, 112,113).
Two-Stage Carcinogenesis
In a number of models of chemical carcinogenesis, a multistep mechanism gives rise to a two-step theory, which can probably best be divided into initiation and promotion (26). Initiation represents a rapid, permanent change induced in the target tissue by exposure to a carcinogen, whereas promotion is the slow process that shows a progression of reversible effects; of course, initiation must precede promotion.
Several studies have provided evidence that O. viverrini (28,29) or C. sinensis infection (61, 62) in hamsters acts as a promoter of the cholangiocarcinogenesis initiated by N-nitrosodimethylamine. More recently, cholangiocarcinogenesis by C. sinensis in hamsters was proposed as an experimental model to elucidate the role of oval cells in cholangiocarcinogenesis (35, 61). Lee et al. (60) examined oval cells expressing different immunohistological phenotypes and ultrastructural appearances in hamsters during the cholangiocarcinogenesis induced by N- nitrosodimethylamine and C. sinensis infection. They concluded that activation (initiation) by N-nitrosodimethylamine and epithelial proliferation (promotion) by C. sinensis stimulate primitive oval cells or their progeny (ductular-like oval cells) to transform into cholangiocarcinomas.
In this context, the C. sinensis may act as a promoter in a two-stage carcinogenic process, which requires an initiator stimulus from an exogenous source.
Other Mechanisms
The enhancement of carcinogenesis might also be the result of biliary stone formation in clonorchiasis, although this possibility has yet to be explored. Kowalewski and Todd (57) showed that hamsters bearing intracholecystic cholesterol pellets are more susceptible to the induction of gallbladder carcinomas by chemical carcinogens. Their observation is consistent with the clinical observation of the close link between clonorchiasis, recurrent pyogenic cholangitis, and cholangiocarcinoma, which has been discussed above.
Other factors that have been implicated in the neoplasm include liver fluke-induced immunological abnormalities and underlying protein malnutrition (27), although no direct evidence is available to support these possibilities.
MORPHOLOGY OF CHOLANGIOCARCINOMA ASSOCIATED WITH CLONORCHIASIS
Hou (42) suggested that cholangiocarcinoma arising in association with clonorchiasis may be multifocal in origin; this suggestion was made after an observation of cases in which a hepatic lobe contained a well-defined cholangiocarcinoma while malignant changes were still in progress in other parts of the liver. Classically, clonorchiasis-associated cholangiocarcinomas have been described to grow in a discrete nodular or confluent massive pattern, in which smaller ducts with adenomatous hyperplasia undergoing malignant transformation are embedded (42, 55). In such cases, the larger bile ducts are only slightly dilated and fibrotic and are often plugged with adult worms or calcium bilirubinate stones (56). Another classical characteristic of clonorchiasis-associated cholangiocarcinoma is prominent mucin secretion, which is usually accompanied by extensive fibrosis (14, 17). Chou and Chan (17) reported an overall association between clonorchiasis and mucin-producing cholangiocarcinoma of 92% in Hong Kong.
However, to the best of our knowledge, no direct evidence has been published supporting the notion that clonorchiasis-associated cholangiocarcinomas has any unique morphological characteristic distinct from cholangiocarcinomas not associated with clonorchiasis. Conversely, it has been suggested that the gross pathological findings of clonorchiasis-associated cholangiocarcinomas are essentially the same as those of usual cholangiocarcinomas in Korea, Hong Kong, and China, where clonorchiasis is endemic and cholangiocarcinoma is one of the most prevalent hepatic neoplasms (56, 68). To the best of our knowledge, most of the clinical cases of clonorchiasis-associated cholangiocarcinomas reported are not multicentric in origin, and it is virtually impossible to distinguish multicentric cholangiocarcinomas from tumors with hepatic metastases in most cases. Furthermore, as well as the discrete nodular form of intrahepatic peripheral cholangiocarcinoma, which is described above, other types of carcinomatous growth patterns in different locations of the biliary tree have been found in patients with clonorchiasis, including infiltrating hilar cholangiocarcinoma (13) and intrahepatic intraductal polypoid cholangiocarcinoma (56, 63, 107).
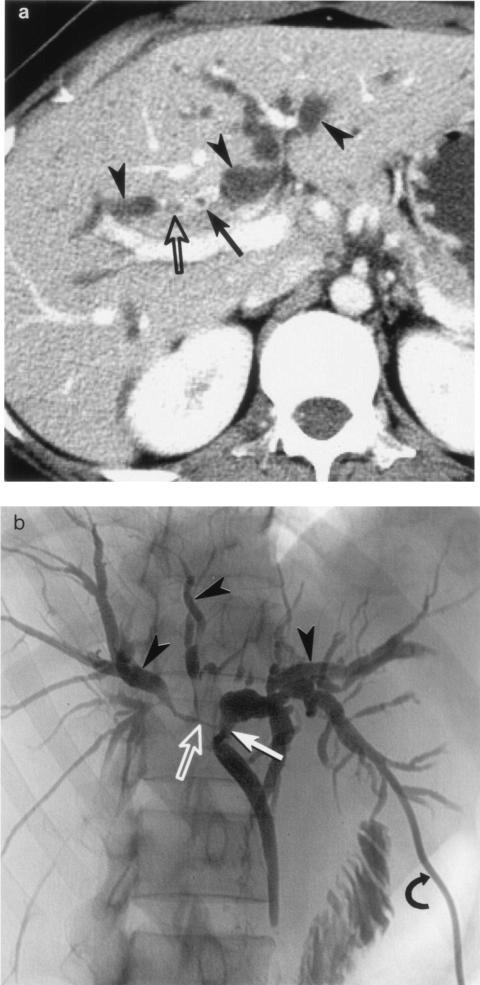
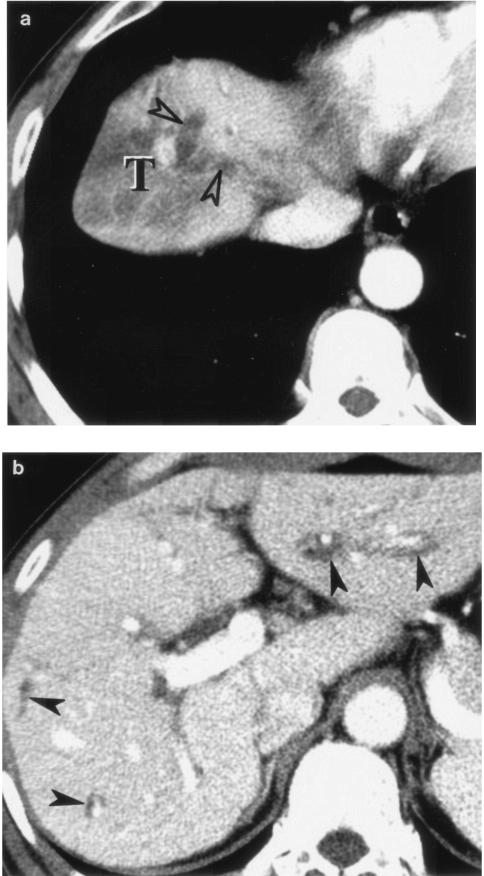
Radiological findings of clonorchiasis-associated cholangiocarcinoma are basically combinations of the findings of the two diseases. For example, intrahepatic peripheral cholangiocarcinoma associated with clonorchiasis usually presents as a discrete nodular mass, as described above. In these cases, dilatation of the intrahepatic bile ducts is usually diffuse and mild. Sometimes, severe focal dilatation is noted around the tumor. In sections prepared for pathological examination, the diffuse, mild intrahepatic ductal dilatation is attributed to changes secondary to C. sinensis infection within the bile ducts and severe dilatation around the tumor is attributed to obstruction by the tumor (12, 14, 53) (Fig. 8). In cases of hilar or extrahepatic cholangiocarcinoma associated with clonorchiasis, the entire biliary tree upstream of the obstructing tumor is diffusely dilated at both the peripheral and central intrahepatic bile ducts, differentiating it from the usual clonorchiasis without cholangiocarcinoma (Fig. 9). In these cases, the presence of clonorchiasis is not easily determined by radiological findings alone.
FIG. 8.
Intrahepatic peripheral cholangiocarcinoma associated with clonorchiasis in a 63-year-old man. Transverse hepatic computed tomography shows a low-attenuation mass (T) at the right lobe of the liver. the severe ductal dilation peripheral to the tumor (open arrowheads in panel a [more cranial section]) is due to obstruction by the tumor. The diffuse, mild intrahepatic ductal dilatation (arrowheads in panel b [more caudal section]) is secondary to clonorchiasis.
FIG. 9.
Ampulla of Vater cancer associated with clonorchiasis in a 66-year-old man. (a) Transverse hepatic computed tomography shows diffuse severe dilatation of the entire biliary tree (arrowheads). (b) Duodenoscopy shows a luminal protruding tumor (T).
CONCLUDING REMARKS
A large and compelling body of evidence suggests an etiological relationship between clonorchiasis and cholangiocarcinoma. However, the mechanisms involved are not completely understood, and many different etiologic factors are probably involved. Clonorchiasis induces biliary epithelial hyperplasia, and this could facilitate changes in at least one stage of cholangiocarcinogenesis, which is probably a promoting effect.
In areas of endemic infection, more clonorchiasis cases are now diagnosed incidentally during radiological examinations such as cholangiography, ultrasonography, and dynamic computed tomography. Radiological findings are regarded as pathognomonic for clonorchiasis because they reflect the unique pathological changes of this disease. Due to remarkable advances in imaging technology, individual flukes can now be visualized by noninvasive radiological techniques.
Radiological examinations currently play important roles in the diagnosis, staging, and decision-making process involved in the treatment of cholangiocarcinoma. The morphological features of clonorchiasis-associated cholangiocarcinoma in radiological examinations do not differ from those of other cholangiocarcinomas. The radiological findings of clonorchiasis-associated cholangiocarcinoma are essentially combinations of the findings of the two diseases. Therefore, in patients diagnosed of having or suspected to have clonorchiasis, radiological findings should be carefully scrutinized for occult cholangiocarcinoma.
Acknowledgments
We thank Jung-Ah Choi, Woo Sun Jun, and Seung Hong Choi for assistance with the manuscript.
REFERENCES
- 1.Abdel-Rahim, A. Y. 2001. Parasitic infections and hepatic neoplasia. Dig. Dis. 19:288-291. [DOI] [PubMed] [Google Scholar]
- 2.Alden, M. E., F. M. Waterman, A. K. Topham, D. J. Barbot, M. J. Shapiro, and M. Mohiuddin. 1995. Cholangiocarcinoma: clinical significance of tumor location along the extrahepatic bile duct. Radiology 197:511-516. [DOI] [PubMed] [Google Scholar]
- 3.Ambroise-Thomas, P., and A. Goullier. 1984. Parasitological examinations and immunodiagnostic advances in fluke infections. Arzneimittelforschung 34:1129-1132. [PubMed] [Google Scholar]
- 4.Belamaric, J. 1973. Intrahepatic bile duct carcinoma and C. sinensis infection in Hong Kong. Cancer 31:468-473. [DOI] [PubMed] [Google Scholar]
- 5.Callea, F., C. Sergi, G. Fabbretti, M. Brisigotti, C. Cozzutto, and D. Medicina. 1993. Precancerous lesion of the biliary tree. J. Surg. Oncol. Suppl. 3:131-133. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain, R. S., and L. H. Blumgart. 2000. Hilar cholangiocarcinoma: a review and commentary. Ann. Surg. Oncol. 7:55-66. [DOI] [PubMed] [Google Scholar]
- 7.Chan, F. L., S. W. Man, L. L. Y. Leong, and S. T. Fan. 1989. Evaluation of recurrent pyogenic cholangitis with CT: analysis of 50 patients. Radiology 170:165-169. [DOI] [PubMed] [Google Scholar]
- 8.Chen, C. Y., W. C. Hsieh, H. H. Shih, and S. N. Chen. 1988. Immunodiagnosis of clonorchiasis by enzyme-linked immunosorbent assay. Southeast Asian J. Trop. Med. Public Health 19:117-121. [PubMed] [Google Scholar]
- 9.Chen, M. F., Y. Y. Jan, C. S. Wang, T. L. Hwang, L. B. Jeng, S. C. Chen, and T. J. Chen. 1993. A reappraisal of cholangiocarcinoma in patients with hepatolithiasis. Cancer 71:2461-2465. [DOI] [PubMed] [Google Scholar]
- 10.Chen, M. G., X. J. Hua, Z. R. Wan, Y. Q. Weng, M. J. Wang, P. J. Zhu, B. Z. He, and M. Y. Xu. 1983. Praziquantel in 237 cases of clonorchiasis sinensis. Chin. Med. J. 96:935-940. [PubMed] [Google Scholar]
- 11.Choi, B. I., and J. K. Han. 2001. Other parasitic diseases, p. 579-581. In K. Okuda, D. G. Mitchell, Y. Itai, and J. Ariyama (ed.), Hepatobiliary disease pathophysiology and imaging. Blackwell Science, London, United Kingdom.
- 12.Choi, B. I., H. J. Kim, M. C. Han, Y. S. Do, M. H. Han, and S. H. Lee. 1989. CT findings of clonorchiasis. Am. J. Roentgenol. 152:281-284. [DOI] [PubMed] [Google Scholar]
- 13.Choi, B. I., J. H. Lee, M. C. Han, S. H. Kim, J. G. Yi, and C. W. Kim. 1989. Hilar cholangiocarcinoma: comparative study with sonography and CT. Radiology 172:689-692. [DOI] [PubMed] [Google Scholar]
- 14.Choi, B. I., J. H. Park, Y. I. Kim, E. S. Yu, S. H. Kim, W. H. Kim, C. Y. Kim, and M. C. Han. 1988. Peripheral cholangiocarcinoma and clonorchiasis: CT findings. Radiology 169:149-153. [DOI] [PubMed] [Google Scholar]
- 15.Choi, D., J. H. Lim, S. K. Kim, E. Y. Kim, M. Lee, and S. T. Hong. 1999. Long-lasting sonographic and histopathological findings in cured clonorchiasis of rabbits. Korean J. Parasitol. 37:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi, T. K., K. P. Wong, and J. Wong. 1984. Cholangiographic appearance in clonorchiasis. Br. J. Radiol. 57:681-684. [DOI] [PubMed] [Google Scholar]
- 17.Chou, S. T., and C. W. Chan. 1976. Mucin-producing cholangiocarcinoma: an autopsy study in Hong Kong. Pathology 8:321-328. [DOI] [PubMed] [Google Scholar]
- 18.Chou, S. T., and J. B. Gibson. 1970. The histochemistry of biliary mucins and the changes caused by infestation with Clonorchis sinensis. J. Pathol. 101:185-197. [DOI] [PubMed] [Google Scholar]
- 19.Chou, S. T., C. W. Chan, and W. L. Ng. 1976. Mucin histochemistry cholangiocarcinoma. J. Pathol. 8:321-328. [DOI] [PubMed] [Google Scholar]
- 20.Chung, C. S., and S. K. Lee. 1976. An epidemiological study of primary liver carcinomas in Busan area with special to clonorchiasis. Korean J. Pathol. 10:33-46 [Google Scholar]
- 21.Dachman, A. H. 1994. Inflammatory cholangitis, parasitic disease, primary biliary cirrhosis, and papillary (ampullary) stenosis. II. The gallbladder and biliary tract, p. 633-664. In A. C. Friedman and A. H. Dachman (ed.), Radiology of the liver, biliary tract, and pancreas. Mosby, St. Louis, Mo.
- 22.Dachman, A. H. 1994. Primary biliary neoplasia. II. The gallbladder and biliary tract, p. 611-632. In A. C. Friedman and A. H. Dachman (ed.), Radiology of the liver, biliary tract, and pancreas. Mosby, St. Louis, Mo.
- 23.Dooley, J. R., and R. C. Neafie. 1976. Clonorchis and opisthorchiasis, p. 509-516. In C. H. Binford, and D. H. Connor (ed.), Pathology of tropical and extraordinary diseases, vol. 1. Armed Forces Institute of Pathology, Washington, D.C.
- 24.Edmondson, H. A., and R. L. Peters. 1985. Liver, p. 1194. In J. M. Kissane (ed.), Anderson's pathology, Mosby, St. Louis, Mo.
- 25.Fan, Z. M., Y. Yamashita, M. Harada, Y. Baba, H. Yamamoto, T. Matsukawa, A. Arakawa, T. Miyazaki, and M. Takahashi. 1993. Intrahepatic cholangiocarcinoma: spin-echo and contrast-enhanced dynamic MR imaging. Am. J. Roentgenol. 161:313-317. [DOI] [PubMed] [Google Scholar]
- 26.Farber, E. 1984. The multiple nature of cancer development. Cancer Res. 44:4217-4223. [PubMed] [Google Scholar]
- 27.Flavell, D. J. 1981. Liver-fluke infection as an aetiological factor in bile-duct carcinoma of man. Trans. R. Soc. Trop. Med. Hyg. 75:814-824. [DOI] [PubMed] [Google Scholar]
- 28.Flavell, D. J., and S. B. Lucas. 1982. Potentiation by the human liver fluke, Opisthorchis viverrini, of the carcinogenic action of N-nitrosodimethylamine upon the biliary epithelium of the hamster. Br. J. Cancer 46:985-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flavell, D. J., and S. B. Lucas. 1983. Promotion of N- nitrosodimethylamine-initiated bile duct carcinogenesis in the hamster by the human liver fluke, Opisthorchis viverrini. Carcinogenesis 4:927-930. [DOI] [PubMed] [Google Scholar]
- 30.Flavell, D. J., K. Pattanapanyasat, S. B. Lucana, and V. Vongsangnak. 1980. Opisthorchis viverrini: liver changes in golden hamsters maintained on high and low protein diets. Acta Trop. 37:337-350. [PubMed] [Google Scholar]
- 31.Gibson, J. B. 1971. Parasites, liver disease and liver cancer, p. 42-50. In Liver cancer. IARC scientific publication no. 1. IARC, Lyon, France.
- 32.Han, J. K., B. I. Choi, A. Y. Kim, S. K. An, J. W. Lee, T. K. Kim, and S. W. Kim. 2002. Cholangiocarcinoma: pictorial essay of CT and cholangiographic findings. Radiographics 22:173-187. [DOI] [PubMed] [Google Scholar]
- 33.Han, J. K., B. I. Choi, T. K., Kim, S. W. Kim, M. C. Han, and K. M. Yeon. 1997. Hilar cholangiocarcinoma: thin-section spiral CT findings with cholangiographic correlation. Radiographics 17:1475-1485. [DOI] [PubMed] [Google Scholar]
- 34.Harinasuta T, M. Riganti, and D. Bunnag. 1984. Opisthorchis viverrini infection: pathogenesis and clinical features. Arzneimittelforschung 34:1167-1169. [PubMed] [Google Scholar]
- 35.Haswell-Elkins, S. Satarug, M. Tsuda, E. Mairiang, H. Esumi, P. Sithithaworn, P. Mairiang, M. Saitoh, P. Yongvanit, and D. B. Elkins. 1994. Liver fluke infection and cholangiocarcinoma: model of endogenous nitric oxide and extragastric nitrosation in human carcinogenesis. Mutat. Res. 305:241-252. [DOI] [PubMed] [Google Scholar]
- 36.Higginson, J. 1955. Relation of carcinoma of the liver to cirrhosis, malaria, syphilis and parasitic disease. Schweiz. Z. Allg. Pathol. Bakteriol. 18:625-643. [DOI] [PubMed] [Google Scholar]
- 37.Hong, S. T., K. H. Park, M. Seo, B. I. Choi, J. Y. Chai, and S. H. Lee. 1994. Correlation of sonographic findings with histopathological changes of the bile ducts in rabbits infected with Clonorchis sinensis. Korean J. Parasitol. 32:223-230. [DOI] [PubMed] [Google Scholar]
- 38.Hong, S. T., K. S. Yoon, M. J. Lee, M. Seo, M. H. Choi, J. S. Sim, B. I. Choi, C. K. Yun, and S. H. Lee. 1998. Control of clonorchiasis by repeated praziquantel treatment and low diagnostic efficacy of sonography. Korean J. Parasitol. 36:249-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong, S. T., S. Huh, W. G. Kho, J. R. Ryu, J. Y. Chai, S. H. Lee, and E. J. Kim. 1990. Changes of histopathological and serological findings of the liver after treatment in rabbit clonorchiasis. Seoul J. Med. 31:117-127. [Google Scholar]
- 40.Hong, S. T., W. G. Kho, W. H. Kim, J. Y. Chai, and S. H. Lee. 1993. Turnover of biliary epithelial cells in Clonorchis sinensis infected rats. Korean J. Parasitol. 31:83-89. [DOI] [PubMed] [Google Scholar]
- 41.Hou, P. C. 1955. The pathology of Clonorchis sinensis infestation of the liver. J. Pathol. Bacteriol. 70:53-64. [DOI] [PubMed] [Google Scholar]
- 42.Hou, P. C. 1956. The relationship between primary carcinoma of the liver and infestation with Clonorchis sinensis. J. Pathol. Bacteriol. 72:239-246. [DOI] [PubMed] [Google Scholar]
- 43.Hou, P. C. 1964. Primary carcinoma of bile duct of the liver of the cat (Felis catus) infested with Clonorchis sinensis. J. Pathol. Bacteriol. 87:239-244. [DOI] [PubMed] [Google Scholar]
- 44.Hou, P. C. 1965. Hepatic clonorchiasis and carcinoma of the bile duct in a dog. J. Pathol. Bacteriol. 89:365-367. [PubMed] [Google Scholar]
- 45.Hou, P. C. 1965. Pathological changes in the intrahepatic bile ducts of cats (Felis catus) infested with Clonorchis sinensis. J. Pathol. Bacteriol. 89:357-364. [DOI] [PubMed] [Google Scholar]
- 46.International Agency for Research on Cancer Working Group. 1994. Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis). IARC Monogr. Eval. Carcinog. Risks Hum. 61:121-175. [PMC free article] [PubMed] [Google Scholar]
- 47.Itai, Y., K. Ohtomo, T. Kokubo, T. Yamauchi, M. Minami, N. Yashiro, and T. Araki. 1986. CT of hepatic masses: significance of prolonged and delayed enhancement. Am. J. Roentgenol. 146:729-733. [DOI] [PubMed] [Google Scholar]
- 48.Juttijudata, P., C. Chiemchaisri, C. Palavatana, and S. Churnratanakul. 1983. A high incidence of cholangiocarcinoma in patients with biliary obstructive (malignant) disease in Thailand. J. Natl. Cancer Inst. 71:229. [PubMed] [Google Scholar]
- 49.Kammerer, W. S., J. D. Van der Decker, T. B. Keithg, and K. E. Mott. 1977. Clonorchiasis in New York City Chinese. Trop. Doct. 7:105-106. [DOI] [PubMed] [Google Scholar]
- 50.Katsurada, F. 1900. Beitrag zur kenntnis des distomum spatulatum. Beitrage zur pathologischen Anatomie und zur allgemeinen Pathologie 28:479-505. [Google Scholar]
- 51.Kim, K. H., C. D. Kim, H. S. Lee, S. J. Lee, Y. T. Jeen, H. J. Chun, C. W. Song, S. W. Lee, S. H. Um, J. H. Choi, H. S. Ryu, and J. H. Hyun. 1999. Biliary papillary hyperplasia with clonorchiasis resembling cholangiocarcinoma. Am. J. Gastroenterol. 94:514-517. [DOI] [PubMed] [Google Scholar]
- 52.Kim, S. S., M. H. Han, S. K. Park, H. S. Lim, and S. T. Hong. 1990. A survey on the epidemiological factors of clonorchiasis in the Pohang industrial belt along the Hyungsan River, Kyongsangbuk-do. Korean J. Parasitol. 28:213-219. [DOI] [PubMed] [Google Scholar]
- 53.Kim, T. K., B. I. Choi, J. K. Han, H. J. Jang, S. G. Cho, and M. C. Han. 1997. Peripheral cholangiocarcinoma of the liver: two-phase spiral computed tomography findings. Radiology 204:539-543. [DOI] [PubMed] [Google Scholar]
- 54.Kim, Y. I. 1984. Liver carcinoma and liver fluke infection. Arzneimittelforschung 34:1121-1126. [PubMed] [Google Scholar]
- 55.Kim, Y. I., D. H. Yang, and K. R. Chang. 1974. Relationship between Clonorchis sinensis infestation and cholangiocarcinoma of the liver in Korea: epidemiological and pathologic reappraisal of 495 consecutive primary carcinoma of the liver on Seoul and Busan areas. Seoul J. Med. 15:247-255. [Google Scholar]
- 56.Kim, Y. I., E. S. Yu, and S. T. Kim. 1989. Intraductal variant of peripheral cholangiocarcinoma of the liver with Clonorchis sinensis infection. Cancer 63:1562-1566. [DOI] [PubMed] [Google Scholar]
- 57.Kowalewski, K., and E. F. Todd. 1971. Carcinoma of the gallbladder induced in hamsters by insertion of cholesterol pellets and feeding dimethylnitrosamine. Proc. Soc. Exp. Biol. Med. 136:482-486. [DOI] [PubMed] [Google Scholar]
- 58.Lacomis, J. M., R. L. Baron, J. H. Oliver III, M. A. Nalesnik, and M. P. Federle. 1997. Cholangiocarcinoma: delayed CT contrast enhancement patterns. Radiology 203:98-104. [DOI] [PubMed] [Google Scholar]
- 59.Lee, H. K. 1995. Evaluation of sonography and skin test in diagnosis of clonorchiasis at the Hyongsan-gang (River) area. Korean J. Parasitol. 33:117-123. [DOI] [PubMed] [Google Scholar]
- 60.Lee, J. H., H. J. Rim, and S. Sell. 1997. Heterogeneity of the “oval-cell” response in the hamster liver during cholangiocarcinogenesis following Clonorchis sinensis infection and dimethylnitrosamine treatment. J. Hepatol. 26:1313-1323. [DOI] [PubMed] [Google Scholar]
- 61.Lee, J. H., H. J. Rim, and U. B. Bak. 1993. Effect of Clonorchis sinensis infection and dimethylnitrosamine administration on the induction of cholangiocarcinoma in Syrian golden hamsters. Korean J. Parasitol. 31:21-30. [DOI] [PubMed] [Google Scholar]
- 62.Lee, J. H., H. M. Yang, U. B. Bak, and H. J. Rim. 1994. Promoting role of Clonorchis sinensis infection on induction of cholangiocarcinoma during two- step carcinogenesis. Korean J. Parasitol. 32:13-18. [DOI] [PubMed] [Google Scholar]
- 63.Lee, J. W., J. K. Han, T. K. Kim, Y. H. Kim, B. I. Choi, M. C. Han, K. S. Suh, and S. W. Kim. 2000. Computed tomography features of intraductal intrahepatic cholangiocarcinoma. Am. J. Roentgenol. 175:721-725. [DOI] [PubMed] [Google Scholar]
- 64.Lee, K. H., S. T. Hong, J. K. Han, C. J. Yoon, S. Lee, S. H. Kim, and B. I. Choi. 2003. Experimental clonorchiasis in dogs: CT findings before and after treatment. Radiology. 228:131-138. [DOI] [PubMed] [Google Scholar]
- 65.Lee, S. H., S. T. Hong, and J. S. Kim. 1987. Histopathological changes of the liver after praziquantel treatment in Clonorchis sinensis infected rabbits. Korean J. Parasitol. 25:110-122. [DOI] [PubMed] [Google Scholar]
- 66.Lee, S. H., T. S. Shim, S. M. Lee, and J. G. Chi. 1978. Studies on pathological changes of the liver in albino rats infected with Clonorchis sinensis. Korean J. Parasitol. 16:148-155. [DOI] [PubMed] [Google Scholar]
- 67.Lee, S. Y., S. H. Lee, and J. G. Chi. 1978. Ultrastructural changes of the hepatocytes and biliary epithelia due to Clonorchis sinensis in guinea pigs. Korean J. Parasitol. 16:88-102. [DOI] [PubMed] [Google Scholar]
- 68.Lee, W. J., H. K. Lim, K. M. Jang, S. H. Kim, S. J. Lee, J. H. Lim, and I. W. Choo. 2001. Radiologic spectrum of cholangiocarcinoma: emphasis on unusual manifestations and differential diagnoses. Radiographics 21:S97-S116. [DOI] [PubMed] [Google Scholar]
- 69.Liang, P. C., and C. Tung. 1959. Morphological study and etiology of primary liver carcinoma and its incidence in China. Chin. Med. J. 79:336-347. [PubMed] [Google Scholar]
- 70.Lim, J. H. 1990. Radiologic findings of clonorchiasis. Am. J. Roentgenol. 155:1001-1008. [DOI] [PubMed] [Google Scholar]
- 71.Lim, J. H. 1991. Oriental cholangiohepatitis: pathologic, clinical, and radiologic features. Am. J. Roentgenol. 157:1-8. [DOI] [PubMed] [Google Scholar]
- 72.Lim, J. H., Y. I. Kim, and C. K. Park. 2000. Intraductal mucosal-spreading mucin-producing peripheral cholangiocarcinoma of the liver. Abdom. Imaging 25:89-92. [DOI] [PubMed] [Google Scholar]
- 73.Lim, J. H., Y. T. Ko, D. H. Lee, and S. Y. Kim. 1989. Clonorchiasis: sonographic findings in 59 proved cases. Am. J. Roentgenol. 152:761-764. [DOI] [PubMed] [Google Scholar]
- 74.Lin, A. C., S. W. Chapman, H. R. Turner, and J. D. Wofford. 1987. Clonorchiasis: an update. South Med. J. 80:919-922. [DOI] [PubMed] [Google Scholar]
- 75.Loyer, E. M., H. Chin, R. A. DuBrow, C. L. David, F. Eftekhari, and C. Charnsangavej. 1999. Hepatocellular carcinoma and intrahepatic peripheral cholangiocarcinoma: enhancement patterns with quadruple phase helical CT—a comparative study. Radiology 212:866-875. [DOI] [PubMed] [Google Scholar]
- 76.Min, D. Y., M. H. Ahn, K. M. Kim, and C. W. Kim. 1986. Intestinal parasite survey in Seoul by stool examination at Hanyang University Hospital. Korean J. Parasitol. 24:209-212. [DOI] [PubMed] [Google Scholar]
- 77.Min, H. K. 1984. Clonorchis sinensis: pathogenesis and clinical features of infection. Arzneimittelforschung 34:1151-1153. [PubMed] [Google Scholar]
- 77a.Ministry of Health and Welfare and Korean Association of Health. 1997. Prevalence of intestinal parasitic infections in Korea. The sixth report. Ministry of Health and Welfare, Seoul, Korea.
- 78.Monroe, L. S. 1995. Gastrointestinal parasites, p. 3181-3183. In W. S. Haubrich, F. Schaffner, and J. E. Berk (ed.), Bockus gastroenterology, 5th ed. The W. B. Saunders Co., Philadelphia, Pa.
- 79.Mostafa, M. H., S. A. Sheweita, and P. J. O'Connor. 1999. Relationship between schistosomiasis and bladder cancer. Clin. Microbiol. Rev. 12:97-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakajima, T., Y. Kondo, M. Mayazaki, and K. Okui. 1988. A histopathologic study of 102 cases of intrahepatic cholangiocarcinoma: histologic classification and modes of spreading. Hum. Pathol. 19:1228-1234. [DOI] [PubMed] [Google Scholar]
- 81.Nakanuma, Y., H. Minato, T. Kida, and T. Terada. 1994. Pathology of cholangiocellular carcinoma, p. 39-50. In T. Tobe, H. Kameda, M. Okudaira, and M. Ohto (ed.), Primary liver cancer in Japan. Springer-Verlag, Tokyo, Japan.
- 82.Nauck, E. G., and B. Liang. 1928. Primarer Leberkrebs und clonorchis Infektion. Arch. Schiffs-u. Tropenhyg. 32:109-112. [Google Scholar]
- 83.Noblet, G. P., and W. H. Hunter. 1983. Clonorchiasis/opisthorchiasis in South Carolina. J. S. C. Med. Assoc. 79:75-79. [PubMed] [Google Scholar]
- 84.Ohta, T., T. Nagakawa, N. Ueda, T. Nakamura, T. Akiyama, K. Ueno, and I. Miyazaki. 1991. Mucosal dysplasia of the liver and the intraductal variant of peripheral cholangiocarcinoma in hepatolithiasis. Cancer 68:2217-2223. [DOI] [PubMed] [Google Scholar]
- 85.Okuda, K., and T. Nakashima. 1985. Primary carcinomas of the liver, p. 3361-3364. In J. E. Berk (ed.), Bockus gastroenterology. The W. B. Saunders Co., Philadelphia, Pa.
- 86.Okuda, K., T. Emura, K. Morokuma, S. Kojima, and M. Yokagawa. 1973. Clonorchiasis studied by percutaneous cholangiography, and a therapeutic trial of toluene-2,4-diisothiocyanate. A case report. Gastroenterology 65:457-461. [PubMed] [Google Scholar]
- 87.Okuda, K., Y. Kubo, N. Okazaki, T. Arishima, and M. Hashimoto. 1977. Clinical aspects of intrahepatic cholangiocarcinoma including hilar carcinoma: a study of 57 autopsy-proven cases. Cancer 39:232-246. [DOI] [PubMed] [Google Scholar]
- 88.Okuda, K., and K. Takayasu. 2001. Primary malignant tumors of the liver, p. 343-389. In K. Okuda, D. G. Mitchell, Y. Itai, and J. Ariyama (ed.), Hepatobiliary disease pathophysiology and imaging. Blackwell Science, London, United Kingdom.
- 89.O'Leary, M. J., J. T. Berthiaume, and V. Sakbun. 1985. Treatment of Clonorchiasis sinensis in Hawaii's Laotian population: experience with praziquantel. Hawaii Med. J. 44:63-64. [PubMed] [Google Scholar]
- 90.Ona, F. V., and J. N. Dytoc. 1991. Clonorchis-associated cholangiocarcinoma: a report of two cases with unusual manifestations. Gastroenterology 101:831-839. [DOI] [PubMed] [Google Scholar]
- 91.Papilio, J. L., K. O. Leslie, and R. A. Dean. 1989. Cytologic diagnosis of liver fluke infestation in a patient with subsequently documented cholangiocarcinoma. Acta Cytol. 33:865-869. [PubMed] [Google Scholar]
- 92.Pitt, H. A., W. C. Dooley, C. J. Yeo, and J. L. Cameron. 1995. Malignancies of the biliary tree. Curr. Probl. Surg. 32:1-90. [DOI] [PubMed] [Google Scholar]
- 93.Rim, H. J. 1986. The current pathobiology and chemotherapy of clonorchiasis. Korean J. Parasitol. 24(Suppl.):5-141. [DOI] [PubMed] [Google Scholar]
- 94.Rim, H. J., K. S. Lyu, J. S. Lee, and K. H. Joo. 1981. Clinical evaluation of the therapeutic efficacy of praziquantel (Embay 8440) against Clonorchis sinensis infection in man. Ann. Trop. Med. Parasitol. 75:27-33. [DOI] [PubMed] [Google Scholar]
- 95.Robledo, R., A. Muro, and M. L. Prieto. 1996. Extrahepatic bile duct carcinoma: US characteristics and accuracy in demonstration of tumors. Radiology 198:869-873. [DOI] [PubMed] [Google Scholar]
- 96.Schwartz, D. A. 1980. Helminths in the induction of cancer: Opisthorchis viverrini, Clonorchis sinensis and cholangiocarcinoma. Trop. Geogr. Med. 32:95-100. [PubMed] [Google Scholar]
- 97.Schwartz, D. A. 1986. Cholangiocarcinoma associated with liver fluke infection: a preventable source of mobidity in Asian immigrants. Am. J. Gastroenterol. 81:76-79. [PubMed] [Google Scholar]
- 98.Seah, S. K. 1973. Intestinal parasites in Chinese immigrants in a Canadian city. J. Trop. Med. Hyg. 76:291-293. [PubMed] [Google Scholar]
- 99.Seo, B. S., S. H. Lee, S. Y., Cho, J. Y. Chai, S. T. Hong, I. S. Han, J. S. Sohn, B. W. Cho, S. R. Ahn, S. K. Lee, S. C. Chung, K. S. Kang, H. S. Shim, and I. S. Hwang. 1981. An epidemiologic study on clonorchiasis and metagonimiasis in riverside areas in Korea. Korean J. Parasitol. 19:137-150. [DOI] [PubMed] [Google Scholar]
- 100.Shaked, A., J. O. Colonna, L. Goldstein, and R. W. Busuttil. 1992. The interrelation between sclerosing cholangitis and ulcerative colitis in patients undergoing liver transplantation. Ann. Surg. 215:598-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shin, H. R., C. U. Lee, H. Y. Park, S. Y. Seol, J. M. Chung, H. C. Choi, Y. O. Ahn, T. Shigemastu. 1996. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int. J. Epidemiol. 25:933-940. [DOI] [PubMed] [Google Scholar]
- 102.Song, I. C., J. S. Lee, and H. J. Rim. 1983. Epidemiological studies on the distribution of Clonorchis sinensis infection in Korea. Korean Univ. Med. J. 20:165-190. [Google Scholar]
- 103.Soyer, P., D. A. Bluemke, R. Reichle, P. S. Calhoun, D. F. Bliss, A. Scherrer, and E. K. Fishman. 1995. Imaging of intrahepatic cholangiocarcinoma 1. Peripheral cholangiocarcinoma. Am. J. Roentgenol. 165:1427-1431. [DOI] [PubMed] [Google Scholar]
- 104.Soyer, P., D. A. Bluemke, R. Reichle, P. S. Calhoun, D. F. Bliss, A. Scherrer, and E. K. Fishman. 1995. Imaging of intrahepatic cholangiocarcinoma. 2. Hilar cholangiocarcinoma. Am. J. Roentgenol. 165:1433-1436. [DOI] [PubMed] [Google Scholar]
- 105.Steiner, P. E. 1960. Cancer of the liver and cirrhosis in trans-Saharan Africa and the United States of America. Cancer 13:1085-1166. [Google Scholar]
- 106.Stoll, N. R. 1947. This wormy world. J. Parasitol. 33:1-18. [PubMed] [Google Scholar]
- 107.Suh, K. S., H. R. Roh, Y. T. Koh, K. U. Lee, Y. H. Park, and S. W. Kim. 2000. Clinicopathologic features of the intraductal growth type of peripheral cholangiocarcinoma. Hepatology 31:12-17. [DOI] [PubMed] [Google Scholar]
- 108.Sun, T. 1982. Clonorchiasis and opisthorchiasis, p. 243-252. In T. Sun (ed.), Pathology and clinical features or parasitic diseases. Masson, New York, N.Y.
- 109.Sun, T. 1984. Pathology and immunology of Clonorchis sinensis infection in the liver. Ann. Clin. Lab. Sci. 14:208-215. [PubMed] [Google Scholar]
- 110.Sun, T., and L. Tung-Ma. 1973. Ultrastructural changes in the biliary tracts of guinea pigs infected with Clonorchis sinensis. J. Pathol. 109:291-293. [DOI] [PubMed] [Google Scholar]
- 111.Sung, H. K., T. M. Kim, H. Y. Choi, M. K. Lee, S. Y. Paik, K. S. Cho, and Y. H. Auh. 1993. Value of ultrasonography in the screening of clonorchiasis. Korean J. Med. Ultrasound 12:14-16. [Google Scholar]
- 112.Thamavit, W., N. Bhamarapravati, S. Sahaphong, S. Vajrasthira, S. Angsubhakorn. 1978. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 38:4634-4639. [PubMed] [Google Scholar]
- 113.Thamavit, W., R. Kongkanuntn, D. Tiwawech, and M. A. Moore. 1987. Level of Opisthorchis infestation and carcinogen dose-dependence of cholangiocarcinoma induction in Syrian golden hamsters. Virchows Arch. Abt. B 54:52-58. [DOI] [PubMed] [Google Scholar]
- 114.Watson-Wemyss, H. L. 1919. Carcinoma of the liver associated with infection of Clonorchis sinensis. Edinb. Med. J. 22:103. [Google Scholar]
- 115.Wegner, D. H. 1984. The profile of the trematoidicidal compound praziquantel. Arzneimittelforschung 34:1132-1136. [PubMed] [Google Scholar]
- 116.Yamashita, Y., M. Takahashi, S. Kanazawa, C. Charnsangavej, and S. Wallace. 1992. Hilar cholangiocarcinoma: an evaluation of subtypes with CT and angiography. Acta Radiol. 33:351-355. [PubMed] [Google Scholar]
- 117.Yellin, A. E., and A. J. Donovan. 1981. Biliary lithiasis and helminthiasis. Am. J. Surg. 142:128-136. [DOI] [PubMed] [Google Scholar]
- 118.Zhang, Y., M. Uchida, T. Abe, H. Nishimura, N. Hayabuchi, and Y. Nakashima. 1999. Intrahepatic peripheral cholangiocarcinoma: comparison of dynamic computed tomography and dynamic MRI. J. Comput. Assist. Tomogr. 23:670-677. [DOI] [PubMed] [Google Scholar]