Abstract
Proton pump inhibitors (PPIs) have been widely used since their introduction in the late 1980s because they are highly effective for acid-related conditions. However, some recent epidemiological studies have suggested a positive association between PPI therapy and the risk of osteoporotic fractures. The potential mechanisms underlying this association may be related to the physiologic effects of chronic acid suppression on calcium metabolism. First, chronic hypergastrinemia induced by PPI therapy may lead to parathyroid hyperplasia, resulting in increased loss of calcium from the bone. Second, profound gastric acid suppression may reduce the bioavailability of calcium for intestinal absorption. I will review the published evidence regarding these potential links and discuss their clinical implications.
Keywords: Proton pump inhibitors, hypergastrinemia, hyperparathyroidism, calcium absorption, osteoporosis, PPIs and calcium metabolism, PPI-induced hypergastrinemia, Parathyroid glands, Bone metabolism, Calcium balance
Introduction
Osteoporotic fractures represent an enormous public health issue worldwide. In the United States alone, an estimated 10 million Americans aged over 50 years have osteoporosis, while another 34 million have low bone mass. Each year, an estimated 1.5 million people in the United States suffer an osteoporosis-related fracture, an event that can lead to decreased quality of life and increased risk of death [1, 2].
PPIs are potent acid-suppressing medications that have proven efficacy against acid-related diseases. It is becoming increasingly common for patients to take these drugs on a chronic basis to prevent recurrent GERD symptoms, avoid potential complications such as peptic stricture and Barrett’s esophagus, and prevent complications related to NSAIDs [3, 4]. Furthermore, because they are perceived to be safe, these agents are often prescribed inappropriately, and patients are maintained on treatment for extended periods of time. As a result, PPIs have become one of the most commonly prescribed classes of medication since their introduction in the late 1980s, with a high prevalence of chronic use [5]. With the recent availability of both over-the-counter and generic formulations, PPI use continues to escalate [6].
Since 2006, a number of epidemiologic studies have evaluated the association between PPI therapy and risk of osteoporotic fractures [7–16]. While some of these studies reported a positive association, others failed to demonstrate this effect. Since none of these studies was a randomized controlled trial, unmeasured confounding may be a potential source of bias. Furthermore, most of these studies did not account for nutritional status and use of vitamin/calcium supplements. Nevertheless, the US Food and Drug Administration issued a warning about this potential association and called for more research on this issue [17]. The primary potential mechanisms underlying this association may be related to the physiologic effects of chronic acid suppression on calcium metabolism. In this review, I will evaluate the published evidence regarding these mechanistic links.
Potential mechanisms Linking PPIs and Calcium Metabolism
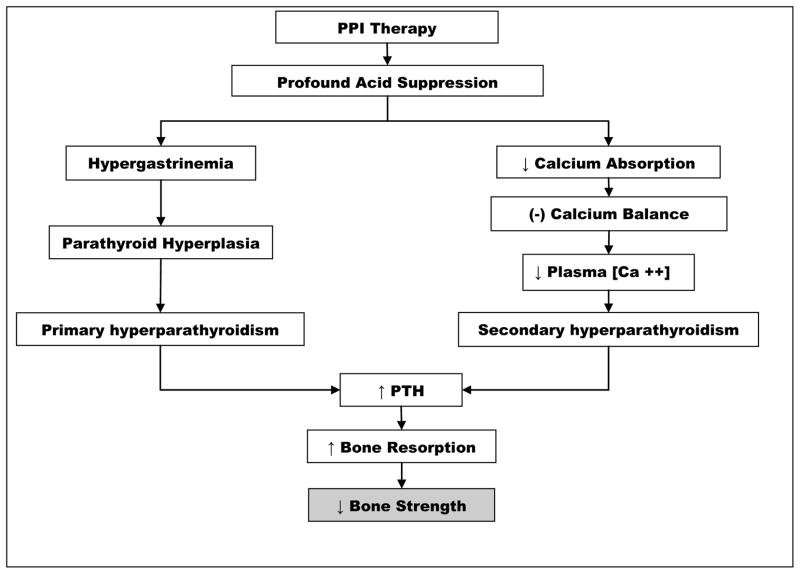
The main physiologic change induced by PPI therapy is profound suppression of gastric acid secretion. Gastric acid suppression results in hypergastrinemia, and may cause malabsorption of calcium. Both hypergastrinemia and calcium malabsorption may negatively influence bone and mineral metabolism, at least in part through induction of hyperparathyroidism (Figure 1).
Figure 1.
Potential mechanistic links between PPI therapy and decreased bone strength
PPI therapy and PTH levels
Parathyroid hormone (PTH) is the principal calcium-regulating hormone and plays a pivotal role in calcium and bone metabolism. As the primary calciotropic hormone, PTH maintains serum calcium concentrations by stimulating bone resorption, increasing renal tubular calcium re-absorption, and stimulating renal calcitriol production, which leads to increased active transport of calcium in the upper intestine. PTH also plays a major role in bone remodeling, and recent evidence has shown that PTH has both catabolic and anabolic effects on the skeleton [18, 19]. PTH stimulates bone formation when given intermittently and stimulates bone resorption when administered continuously. In patients with hyperparathyroidism caused by hyperplasia or an adenoma, PTH secretion is inappropriately and persistently elevated in relation to the serum calcium concentration. In these settings, PTH induces excessive bone remodeling characterized by a rate of bone resorption that exceeds the rate of bone formation [20].
PPI-induced Hypergastrinemia, the Parathyroid Glands, and Bone Metabolism
Because PPIs are such potent inhibitors of acid secretion, they cause a significant increase in serum gastrin. By blocking gastric acid output and raising gastric pH, the PPIs inhibit somatostatin release from mucosal D cells located in the gastric antrum. Somatostatin suppresses antral G cells, which release the hormone gastrin. Thus, PPIs indirectly cause hypergastrinemia by suppressing somatostatin release, which normally acts as a brake on gastrin release. Omeprazole, for example, causes a 2- to 6-fold increase in serum gastrin levels in 80–100 % of the patients receiving long-term therapy [21–25]. About 20–30 % of patients can have serum gastrin levels greater than 500 ng/L (>6× upper limit of normal) [23, 24, 26]. In most cases, the increase occurs during the first few months of PPI therapy and plateaus thereafter [22]. However, in two studies of GERD patients with a long follow-up period (up to 42 months), trends towards steadily increasing serum gastrin levels were observed [24, 27].
In addition to its well-known trophic effects on gastrointestinal tissues [28, 29], hypergastrinemia has been shown to have a stimulatory effect on the parathyroid glands. In rats, hypergastrinemia induced by antral exclusion led to hyperparathyroidism and increased parathyroid gland volume and weight, owing to hyperplasia of the parenchymal cells [30]. Furthermore, 5 weeks of omeprazole administration induced hypergastrinemia and resulted in hyperplasia and hypertrophy of the parathyroid glands and increased PTH gene expression in chickens [31, 32]. The omeprazole-evoked increase in the weight of the parathyroid glands and PTH gene expression was not affected by concurrent administration of ergocalciferol at a dose that increased serum calcium [32]. These changes were also coupled with reduced femur density in the chickens [32]. Furthermore, direct infusion of gastrin increased the weight of the parathyroid glands and reproduced the effect of omeprazole on PTH gene expression in the same animal model, suggesting that the trophic effect of omeprazole on the parathyroid glands was mediated through the induction of hypergastrinemia [32]. The same mechanism may also be responsible for the osteopenia observed in young rats that received long-term omeprazole treatment [33].
The only adult human study that has assessed the effect of PPI therapy on serum levels of PTH was conducted 18 years ago among a small group (7 males, 12 females; mean age 67, standard deviation 13 years) of Japanese patients with gastric ulcers [34]. The study showed that, after 8 weeks of omeprazole therapy, the mean PTH level increased by 28 % compared to baseline among these patients. The increased PTH level was also accompanied by increases in several markers of bone turnover including serum osteocalcin, alkaline phosphatase, and tartrate-resistant acid phosphatase. However, urinary excretion of hydroxyproline and calcium decreased. Several factors made it difficult to definitively interpret these data. PTH was only measured at a single time-point, which may be misleading as it does not reflect the dynamic effect of PTH over a 24-h period. Second, the dose of omeprazole was only 20 mg po qd. The effect of such a relatively small dose of omeprazole on gastric acid secretion and gastrin level is uncertain. Gastrin levels were not measured. Furthermore, the patients were maintained on only 500–700 mg of calcium intake daily during the study, which could have affected PTH levels. Finally, the bone turnover markers used in this study, particularly urinary hydroxyproline excretion, had poor specificity, which might have contributed to the modest changes in bone remodeling observed. Despite these limitations, this study provided preliminary evidence supporting an effect of PPI therapy on PTH and bone turnover in humans.
PPI and Calcium Balance
Calcium is the major cation of bone mineral in human. The typical calcium content of the adult human body is 1 kg. Nearly all is found in the skeleton, After peaking at 35–40 years, bone mass decreases in both sexes. The decline occurs at an accelerated rate in women during the first 10 years after menopause. Thereafter, the rate of calcium decreases is comparable in both men and women.
Calcium balance in the elderly
A negative calcium balance in mid- to late adulthood has been postulated to be the main cause of osteoporosis. Calcium absorption is the most important determinant of calcium balance [35]. In fact, decreased calcium absorption has been shown to directly lead to increased risk of greater bone resorption and osteoporotic fractures, particularly vertebral and hip fractures, among elderly women [36, 37]. Elderly patients are also less able to increase calcium absorption efficiency to compensate for low calcium intake compared to younger individuals [35]. Furthermore, it is well reported that the dietary calcium intake among Americans is low. Using 1999–2000 NHANES data, Ervin et al. found median daily calcium intake from food sources for adults women aged ≥60 years to be 563 mg, well below the national adequate intake of 1,200 mg [38].
Calcium Supplementation
Given the low dietary intake of calcium, calcium supplementation is widely advocated to achieve the target calcium intake in the elderly population. Data from the 1999–2000 National Health and Nutrition Examination Survey indicated that nearly 20 % of Americans aged ≥60 years take calcium supplements [39]. The prevalence increased to 33.5 % when calcium-containing antacids were included. Calcium carbonate is the most commonly used form of calcium supplement. Calcium carbonate is the most commonly used and the least expensive form of calcium supplement, at approximately one-third the cost of the more expensive food source, which includes skim milk and calcium-fortified orange juice made from frozen concentrate. Cost is a consideration for many patients. Another reason for its popularity is that calcium carbonate supplements provide greater amounts of elemental calcium and consequently require fewer tablets than other forms of calcium.
Calcium Absorption
The absorption of ingested calcium takes place primarily in the small bowel and to a lesser extent in the colon. The absorption occurs via two mechanisms. Active transport of calcium occurs in the proximal duodenum. It is a transcellular process that requires metabolic energy and is dependent on vitamin D. Passive calcium transport of calcium involves paracellular diffusion down a chemical gradient. It occurs throughout the length of the small intestine.
If the calcium content of the chyme is relatively low, much of the calcium in solution is absorbed in the duodenum by active transport. Passive calcium transport accounts for most calcium absorption when calcium intake is adequate or high, largely in the more distal portions of the small intestine.
Calcium Solubility and Gastric Acid
In addition to factors such as intake, vitamin D status, and estrogen level, gastric acid-mediated solubilization of dietary calcium salts has been thought to be essential for the absorption of calcium [35, 40–42]. An acidic environment in the stomach facilitates the release of ionized calcium from insoluble calcium salts such as calcium carbonate [41]. Even if a calcium salt is precipitated in the small bowel, some calcium ions are still in solution. In rats, Chonan et al. have shown that gastrectomy or omeprazole therapy led to malabsorption of calcium phosphate and impaired BMD [43, 44]. Lowering gastrointestinal pH with dietary lactate reversed the calcium malabsorption in both cases [43, 44]. In human subjects with normal acid secretion, insoluble calcium salts (e.g., calcium carbonate), taken with or without food, are absorbed at similar rates as soluble calcium salts [41]. In contrast, in achlorhydric patients, the absorption of insoluble calcium salts such as calcium carbonate taken under fasting conditions virtually does not occur, while soluble calcium salts such as calcium citrate are still absorbed normally [42, 45]. Impaired calcium absorption has also been observed in post-gastrectomy patients [46, 47]. By contrast, in a study of young healthy subjects given an H2RA, the absorption rates of calcium carbonate co-ingested with a slightly acidic meal were comparable whether the gastric pH was titrated to 7.4 or 3.0 [48]. However, since the in vivo intragastric titration procedure was carried out by measuring the pH of small aliquots of gastric content obtained every few minutes, it is unclear if the pH of the entire gastric content could be maintained at a neutral level instantaneously throughout the experiment. Therefore, it is doubtful that this experiment could reproduce a pH milieu comparable to PA or PPI-induced profound acid suppression. Taken together, most of the existing data suggest gastric acid may be important for absorption of insoluble calcium salts such as calcium carbonate.
Meal Effect and Calcium Absorption
It has been reported that calcium carbonate absorption can be stimulated by coadministration with a meal [42, 49]. Therefore, it is routinely recommended that calcium carbonate supplements be taken with a meal. However, the meal used in the study of older PA patients contained juices and had a pH of 5.8, while the other study was conducted in young healthy subjects with presumably normal acid secretion [42, 49]. Furthermore, in a cross-over trial conducted in a group of apparently healthy post-menopausal females, Heller et al. found that the bioavailability of calcium carbonate given with a meal is still significantly lower than that of calcium citrate [50]. Compared with calcium carbonate, calcium citrate provided a 46 % greater peak-basal variation and 94 % higher change in AUC for serum calcium and a 41 % greater increment in urinary calcium. Moreover, the decrement in serum PTH concentration from baseline was significantly greater after calcium citrate. The authors postulated that one possible explanation for these findings may be that some of these apparently healthy post-menopausal women had impaired gastric acid secretion due to asymptomatic atrophic gastritis, which is prevalent in the elderly population. Regardless of whether the meal effect is true, it is often difficult for many individuals to take the supplement with meals.
PPI therapy and Calcium Absorption
Six studies have directly examined the effect of PPI therapy upon calcium absorption (Table 1) [51–56] Four of the studies suggested that omeprazole therapy may impair dietary calcium absorption [51–54]. Three of the studies relied on demonstrating decreased plasma total calcium concentration with omeprazole therapy as evidence of calcium malabsorption [51–53]. However, this method has been criticized for having a low signal-to-noise ratio [57]. This may be a relevant concern here because two of the studies were conducted among hemodialysis patients whose plasma calcium levels may have been influenced by other factors [52, 53]. The remaining three studies were specifically designed to examine the effect of PPI therapy on calcium absorption [54, 55]. Using a whole gut lavage method, Serfaty-Lacrosniere et al. reported that, among young healthy subjects, full-dose omeprazole therapy did not reduce the absorption of calcium contained in milk and cheese [55]. The null result may be related to the meal effect and the use of calcium contained in dairy products [35], which has very high bioavailability. In contrast, using a validated single oral radio-tracer method, O’Connell et al. showed that among women ≥65 years of age, omeprazole at a dose of 20 mg QD taken for 7 days significantly reduced the absorption of calcium carbonate taken under fasting conditions [54]. It is unclear whether such malabsorption is reversible with co-ingestion of a meal. More recently, Hansen et al. evaluated changes in the absorption of calcium ingested with a meal among postmenopausal women related to omeprazole therapy [56]. They observed no reduction in fractional calcium absorption after 30 days of PPI therapy. However, the use of a soluble calcium salt (i.e., calcium chloride) and the inclusion of a glass of acidic orange juice with the meal made it difficult to interpret the findings. Future studies should determine whether the PPI therapy alters the fractional absorption of insoluble calcium coingested with a pH neutral meal.
Table 1.
Studies of the impact of omeprazole on calcium absorption
| Study | Study subjects | Type of calcium administered | Calcium absorption assay | Impaired calcium absorption? |
|---|---|---|---|---|
| Graziani et al., 1995 [51] | Young healthy subjects | Meal containing calcium | Post-meal plasma calcium | Yes |
| Serfaty-Lacrosniere et al., 1995 [55] | Young healthy subjects | Meal with calcium in dairy products | Whole gut lavage | No |
| Hardy et al., 1998 [53] | Hemodialysis patients | Calcium carbonate during and 5 min before meal | Monthly mean plasma calcium concentration | Yes |
| Graziani et al., 2002 [52] | Hemodialysis patients | Meal containing calcium | Post-meal plasma calcium | Yes |
| O’Connell et al., 2005 [54] | Healthy females >65 years | Calcium carbonate given fasting | Single tracer method | Yes |
| Hansen et al., 2011 [56] | Healthy post-menopausal females | Calcium chloride given with a meal including acidic orange juice | Dual isotope method | No |
Conclusions
A trophic effect of PPI therapy on parathyroid glands has been demonstrated in several animal models. However, its clinical relevance in human has yet to be properly investigated. It is obvious that calcium must be ionized and in solution to be absorbed. Therefore, profound acid suppression may theoretically interfere with calcium solubilization and absorption. However, the results of most the studies on this issue are difficult to interpret or have limited clinical applicability because of methodological limitations. Using a valid approach, one study did demonstrate calcium carbonate malabsorption under fasting condition with high daily dose omeprazole. However, further studies are required to adequate evaluate the role of meal effect on the absorption of calcium carbonate in this setting.
What are the clinically implications of these data? Clearly, PPIs should only be prescribed when there is an appropriate clinical indication. Since clinical circumstances may change over time, it is also important to periodically review the clinical need for PPI therapy. Furthermore, the potential link between PPI therapy and parathyroid hyperplasia is mediated by chronic hypergastrinemia. Therefore, minimizing the duration and dose of PPI therapy should mitigate this effect. For example, on-demand PPI therapy may be a good option in GERD patients without moderate to severe erosive esophagitis. In addition, while it is quite likely that PPI-induced hypochlorhydria can affect calcium solubility, it is clear that, when calcium intake is adequate, differences in solubility only play a minor role in the amount of calcium that is absorbed. Therefore, patients who require chronic PPI therapy should be instructed to ensure adequate daily calcium intake according to the daily recommended allowance corresponding to their age. Also, soluble calcium salts such as calcium citrate and calcium contained in milk and cheese have high bioavailability for absorption regardless of gastric pH. Therefore, if financially feasible, these can be the preferred sources of calcium for patient receiving chronic PPI therapy. If calcium carbonate must be used, the patient should be instructed to take it with a meal.
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
References
- 1.Leibson CL, Tosteson AN, Gabriel SE, et al. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. Journal of the American Geriatrics Society. 2002;50:1644–50. doi: 10.1046/j.1532-5415.2002.50455.x. [DOI] [PubMed] [Google Scholar]
- 2.Chrischilles EA, Butler CD, Davis CS, et al. A model of lifetime osteoporosis impact [erratum appears in Arch Intern Med 1922 Mar;152(3):655] Archives of Internal Medicine. 1991;151:2026–32. [PubMed] [Google Scholar]
- 3.Hetzel DJ. Controlled clinical trials of omeprazole in the long-term management of reflux disease. Digestion. 1992;51:35–42. doi: 10.1159/000200913. [DOI] [PubMed] [Google Scholar]
- 4.DeVault KR, Castell DO, et al. American College of G. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. American Journal of Gastroenterology. 2005;100:190–200. doi: 10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 5.Bashford JN, Norwood J, Chapman SR. Why are patients prescribed proton pump inhibitors? Retrospective analysis of link between morbidity and prescribing in the General Practice Research Database [see comments] Bmj. 1998;317:452–6. doi: 10.1136/bmj.317.7156.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin RM, Lim AG, Kerry SM, et al. Trends in prescribing H2-receptor antagonists and proton pump inhibitors in primary care. Aliment Pharmacol Ther. 1998;12:797–805. doi: 10.1046/j.1365-2036.1998.00374.x. [DOI] [PubMed] [Google Scholar]
- 7.Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine h(2) receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79:76–83. doi: 10.1007/s00223-006-0021-7. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y-X, Lewis JD, Epstein S, et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–53. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 9.de Vries F, Cooper A, Logan R, et al. Fracture risk in patients receiving concomitant bisphosphonate and acid-suppressive medication or bisphosphonate alone. Osteoporosis International. 2007;18:S261. [Google Scholar]
- 10.Targownik LE, Lix LM, Metge CJ, et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures [see comment] CMAJ Canadian Medical Association Journal. 2008;179:319–26. doi: 10.1503/cmaj.071330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalili H, Huang ES, Jacobson BC, et al. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ. 2012;344:e372. doi: 10.1136/bmj.e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med. 2011;170:765–71. doi: 10.1001/archinternmed.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corley DA, Kubo A, Zhao W, et al. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139:93–101. doi: 10.1053/j.gastro.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy. 2008;28:951–9. doi: 10.1592/phco.28.8.951. [DOI] [PubMed] [Google Scholar]
- 15.Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int. 2008;83:251–9. doi: 10.1007/s00223-008-9170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pouwels S, Lalmohamed A, Souverein P, et al. Use of proton pump inhibitors and risk of hip/femur fracture: a population-based case-control study. Osteoporos Int. 2011;22:903–10. doi: 10.1007/s00198-010-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FDA. Drug safety communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. 2010 [Google Scholar]
- 18.Jilka RL, Weinstein RS, Bellido T, et al. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone [see comment] Journal of Clinical Investigation. 1999;104:439–46. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindsay R, Nieves J, Formica C, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350:550–5. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- 20.Stewart AF, Vignery A, Silverglate A, et al. Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity. Journal of Clinical Endocrinology & Metabolism. 1982;55:219–27. doi: 10.1210/jcem-55-2-219. [DOI] [PubMed] [Google Scholar]
- 21.Brunner G, Creutzfeldt W. Omeprazole in the long-term management of patients with acid-related diseases resistant to ranitidine. Scandinavian Journal of Gastroenterology - Supplement. 1989;166:101–5. doi: 10.3109/00365528909091254. discussion 11–3. [DOI] [PubMed] [Google Scholar]
- 22.Brunner G, Creutzfeldt W, Harke U, et al. Therapy with omeprazole in patients with peptic ulcerations resistant to extended high-dose ranitidine treatment. Digestion. 1988;39:80–90. doi: 10.1159/000199610. [DOI] [PubMed] [Google Scholar]
- 23.Koop H, Klein M, Arnold R. Serum gastrin levels during long-term omeprazole treatment. Alimentary Pharmacology & Therapeutics. 1990;4:131–8. doi: 10.1111/j.1365-2036.1990.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 24.Jansen JB, Klinkenberg-Knol EC, Meuwissen SG, et al. Effect of long-term treatment with omeprazole on serum gastrin and serum group A and C pepsinogens in patients with reflux esophagitis. Gastroenterology. 1990;99:621–8. doi: 10.1016/0016-5085(90)90946-x. [DOI] [PubMed] [Google Scholar]
- 25.Lind T, Cederberg C, Forssell H, et al. Relationship between reduction of gastric acid secretion and plasma gastrin concentration during omeprazole treatment. Scandinavian Journal of Gastroenterology. 1988;23:1259–66. doi: 10.3109/00365528809090202. [DOI] [PubMed] [Google Scholar]
- 26.Lamberts R, Creutzfeldt W, Struber HG, et al. Long-term omeprazole therapy in peptic ulcer disease: gastrin, endocrine cell growth, and gastritis [see comments] Gastroenterology. 1993;104:1356–70. doi: 10.1016/0016-5085(93)90344-c. [DOI] [PubMed] [Google Scholar]
- 27.Koop H, Arnold R. Long-term maintenance treatment of reflux esophagitis with omeprazole Prospective study in patients with H2-blocker-resistant esophagitis. Digestive Diseases & Sciences. 1991;36:552–7. doi: 10.1007/BF01297018. [DOI] [PubMed] [Google Scholar]
- 28.Hakanson R, Sundler F. Trophic effects of gastrin. Scandinavian Journal of Gastroenterology - Supplement. 1991;180:130–6. doi: 10.3109/00365529109093190. [DOI] [PubMed] [Google Scholar]
- 29.Wang TC, Koh TJ, Varro A, et al. Processing and proliferative effects of human progastrin in transgenic mice. Journal of Clinical Investigation. 1996;98:1918–29. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimelius L, Johansson H, Lundqvist G, et al. The parathyroid glands in experimentally induced hypergastrinemia in the rat. Scandinavian Journal of Gastroenterology. 1977;12:739–44. doi: 10.3109/00365527709181713. [DOI] [PubMed] [Google Scholar]
- 31.Gagnemo-Persson R, Hakanson R, Sundler F, et al. Growth of the parathyroid glands in omeprazole-treated chickens. Scandinavian Journal of Gastroenterology. 1994;29:493–7. doi: 10.3109/00365529409092460. [DOI] [PubMed] [Google Scholar]
- 32.Gagnemo-Persson R, Samuelsson A, Hakanson R, et al. Chicken parathyroid hormone gene expression in response to gastrin, omeprazole, ergocalciferol, and restricted food intake. Calcified Tissue International. 1997;61:210–5. doi: 10.1007/s002239900325. [DOI] [PubMed] [Google Scholar]
- 33.Cui GL, Syversen U, Zhao CM, et al. Long-term omeprazole treatment suppresses body weight gain and bone mineralization in young male rats. Scandinavian Journal of Gastroenterology. 2001;36:1011–5. doi: 10.1080/003655201750422585. [DOI] [PubMed] [Google Scholar]
- 34.Mizunashi K, Furukawa Y, Katano K, et al. Effect of omeprazole, an inhibitor of H+, K(+)-ATPase, on bone resorption in humans. Calcified Tissue International. 1993;53:21–5. doi: 10.1007/BF01352010. [DOI] [PubMed] [Google Scholar]
- 35.Nordin BE. Calcium and osteoporosis. Nutrition. 1997;13:664–86. doi: 10.1016/s0899-9007(97)83011-0. [DOI] [PubMed] [Google Scholar]
- 36.Ensrud KE, Duong T, Cauley JA, et al. Low fractional calcium absorption increases the risk for hip fracture in women with low calcium intake. Study of Osteoporotic Fractures Research Group. Annals of Internal Medicine. 2000;132:345–53. doi: 10.7326/0003-4819-132-5-200003070-00003. [DOI] [PubMed] [Google Scholar]
- 37.Nordin BE, O’Loughlin PD, Need AG, et al. Radiocalcium absorption is reduced in postmenopausal women with vertebral and most types of peripheral fractures. Osteoporosis International. 2004;15:27–31. doi: 10.1007/s00198-003-1493-1. [DOI] [PubMed] [Google Scholar]
- 38.Ervin RB, Wang CY, Wright JD, et al. Dietary intake of selected minerals for the United States population: 1999–2000. Advance Data. 2004:1–5. [PubMed] [Google Scholar]
- 39.Radimer K, Bindewald B, Hughes J, et al. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. American Journal of Epidemiology. 2004;160:339–49. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 40.Champagne ET. Low gastric hydrochloric acid secretion and mineral bioavailability. Advances in Experimental Medicine & Biology. 1989;249:173–84. doi: 10.1007/978-1-4684-9111-1_12. [DOI] [PubMed] [Google Scholar]
- 41.Sheikh MS, Santa Ana CA, Nicar MJ, et al. Gastrointestinal absorption of calcium from milk and calcium salts. New England Journal of Medicine. 1987;317:532–6. doi: 10.1056/NEJM198708273170903. [DOI] [PubMed] [Google Scholar]
- 42.Recker RR. Calcium absorption and achlorhydria. New England Journal of Medicine. 1985;313:70–3. doi: 10.1056/NEJM198507113130202. [DOI] [PubMed] [Google Scholar]
- 43.Chonan O, Takahashi R, Yasui H, et al. Effect of L-lactic acid on the absorption of calcium in gastrectomized rats. Journal of Nutritional Science & Vitaminology. 1998;44:869–75. doi: 10.3177/jnsv.44.869. [DOI] [PubMed] [Google Scholar]
- 44.Chonan O, Takahashi R, Yasui H, et al. Effect of L-lactic acid on calcium absorption in rats fed omeprazole. Journal of Nutritional Science & Vitaminology. 1998;44:473–81. doi: 10.3177/jnsv.44.473. [DOI] [PubMed] [Google Scholar]
- 45.Ivanovich P, Fellows H, Rich C. The absorption of calcium carbonate. Annals of Internal Medicine. 1967;66:917–23. doi: 10.7326/0003-4819-66-5-917. [DOI] [PubMed] [Google Scholar]
- 46.Nilas L, Christiansen C, Christiansen J. Regulation of vitamin D and calcium metabolism after gastrectomy. Gut. 1985;26:252–7. doi: 10.1136/gut.26.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson J. The stomach and duodenum: postoperative complications. Philadelphia: Saunders; 1991. [Google Scholar]
- 48.Bo-Linn GW, Davis GR, Buddrus DJ, et al. An evaluation of the importance of gastric acid secretion in the absorption of dietary calcium. Journal of Clinical Investigation. 1984;73:640–7. doi: 10.1172/JCI111254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heaney RP, Smith KT, Recker RR, et al. Meal effects on calcium absorption. American Journal of Clinical Nutrition. 1989;49:372–6. doi: 10.1093/ajcn/49.2.372. [DOI] [PubMed] [Google Scholar]
- 50.Heller HJ, Greer LG, Haynes SD, et al. Pharmacokinetic and pharmacodynamic comparison of two calcium supplements in postmenopausal women. [erratum appears in J Clin Pharmacol 2001 Jan;41(1):116] Journal of Clinical Pharmacology. 2000;40:1237–44. [PubMed] [Google Scholar]
- 51.Graziani G, Como G, Badalamenti S, et al. Effect of gastric acid secretion on intestinal phosphate and calcium absorption in normal subjects. Nephrology Dialysis Transplantation. 1995;10:1376–80. [PubMed] [Google Scholar]
- 52.Graziani G, Badalamenti S, Como G, et al. Calcium and phosphate plasma levels in dialysis patients after dietary Ca-P overload. Role of gastric acid secretion. Nephron. 2002;91:474–9. doi: 10.1159/000064290. [DOI] [PubMed] [Google Scholar]
- 53.Hardy P, Sechet A, Hottelart C, et al. Inhibition of gastric secretion by omeprazole and efficiency of calcium carbonate on the control of hyperphosphatemia in patients on chronic hemodialysis. Artificial Organs. 1998;22:569–73. doi: 10.1046/j.1525-1594.1998.06200.x. [DOI] [PubMed] [Google Scholar]
- 54.O’Connell MB, Madden DM, Murray AM, et al. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. American Journal of Medicine. 2005;118:778–81. doi: 10.1016/j.amjmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Serfaty-Lacrosniere C, Wood RJ, Voytko D, et al. Hypochlorhydria from short-term omeprazole treatment does not inhibit intestinal absorption of calcium, phosphorus, magnesium or zinc from food in humans. Journal of the American College of Nutrition. 1995;14:364–8. doi: 10.1080/07315724.1995.10718522. [DOI] [PubMed] [Google Scholar]
- 56.Hansen KE, Jones AN, Lindstrom MJ, et al. Do proton pump inhibitors decrease calcium absorption? J Bone Miner Res. 2011;25:2786–95. doi: 10.1002/jbmr.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heaney RP. Factors influencing the measurement of bioavailability, taking calcium as a model. Journal of Nutrition. 2001;131:1344S–8S. doi: 10.1093/jn/131.4.1344S. [DOI] [PubMed] [Google Scholar]