Abstract
Purpose
Cardiac glycosides affect several pathways central for tumor formation. We sought to evaluate the association between digoxin use and colorectal cancer (CRC) risk.
Methods
We conducted a nested case-control study using The Health Improvement Network (THIN), a medical records database representative of the broader United Kingdom population. Study cases were defined as those with a diagnostic code for CRC. Each case was matched to up to 4 eligible controls on age, sex, practice site, and duration of follow-up before index date using incidence density sampling. Exposure of interest was digoxin therapy before index date. The odds ratios (ORs) and 95% confidence intervals (CIs) for CRC associated with digoxin use were estimated using conditional logistic regression analysis, adjusted for BMI, alcoholism, smoking history, diabetes mellitus, heart disease, chronic NSAIDs use and previous screening colonoscopies.
Results
The case control analysis included 20,990 CRC patients and 82,054 controls whose mean follow up time before index date was 6.5 years (SD 4.0). The adjusted OR for CRC among current digoxin users was increased compared to non-users with an adjusted ORs of 1.41 (95%CI 1.25–1.59, p<0.0001) 1.45 (95%CI 1.22–1.72, p<0.0001) and 1.41 (95%CI 1.00–1.99, p=0.049) for first prescriptions 1–5 years, 5–10 years and more than 10 years before index date respectively. Similar results were observed when cumulative duration and number of digoxin prescriptions were analyzed. The risk was not elevated for past digoxin users.
Conclusions
Current digoxin use is associated with increased CRC risk.
Keywords: digoxin, colorectal, cancer, risk factor
Introduction
Digoxin is a cardiac glycoside that has been used for more than 200 years in the treatment of congestive heart failure and atrial fibrillation. The main cardiogenic effects of digoxin are mediated through inhibition of the sodium potassium ATPase pump, secondary increase in intracellular calcium concentration and as a result increased contractility of cardiac myocytes (1). Cardiac glycosides also affect several pathways central for tumor formation. Digoxin was shown to: suppress growth of hypoxic NSCLC and breast cancer cells (2) (3) (4) and possibly decrease the risk for castrate resistant prostate cancer through inhibition of hypoxia inducible factor 1 (5, 6); reduce protein synthesis of the tumor suppressor gene P53 through the Src/MAPK pathway (7); inhibit the FA/BRCA pathway (8); induce autophagy through mTOR and ERK1/2 signaling pathways in NSCLC cells (9) (10); and induce immunogenic cell death in immune-competent but not immune-deficient mice through inhibition of the Na+/K+-ATPase (11). Furthermore, the alpha 1 isoform of the sodium potassium ATPase mediates cell migration and growth in addition to the pumping activities and is down regulated in colon, pancreas, kidney, bladder and prostate cancers (12, 13).
In recent years, additional effects of cardiac glycosides on cancer cells have been described. Digoxin is a phytoestrogen that can bind to estrogen receptor (ER) and stimulate estrogen sensitive tumors in the breast and uterus (14, 15). In a Danish study, the use of digoxin among women was associated with a 30% increase in risk of invasive breast cancer. The risk increased modestly with increased duration of therapy up to 40% (16, 17). Another work from Denmark, demonstrated higher risk for ER positive breast cancers and relapse during the first year after diagnosis in women treated with digoxin (18). Similar results were seen in the Nurses’ Health Study cohort with significant risk for ER positive tumors (HR 1.46, 95%CI 1.10–1.95), especially for duration of therapy of more than 4 years. No association was observed for ER negative disease (19). Other works demonstrated an increase in ER negative breast cancers, although to a lesser extent (17). Stopping digoxin treatment reduced cancer risk in the year after treatment cessation (OR 0.63, 95%CI 0.51–0.77), and the risk increased later to levels similar to risk of non-users (17). Another support for the estrogenic effect of digoxin derives from prostate cancer research. A study using data from the Health Professionals Follow-up Study demonstrated a protective association between digoxin use and prostate cancer risk (RR 0.76, 95%CI 0.61–0.95) especially with use of more than 10 years (20). A population based case-control study in King County, Washington showed a similar risk reduction of 40% (21). Other studies showed no change in risk (22).
Recently, a small retrospective clinical analyses (1) showed improved overall survival in patients treated with digoxin during chemotherapy in breast, colorectal, head and neck, and hepatocellular carcinoma patients. The same effect was not demonstrated in patients with lung and prostate cancers. A single report described cytotoxic activity of cardiac glycosides against colorectal cancer cells at concentrations higher than the therapeutic levels (23).
The aim of the current work was to evaluate the effect of digoxin therapy on CRC risk. Since previous works demonstrated decreased CRC risk among women that used hormone replacement therapy (HRT) (24) we assumed that digoxin might decrease CRC risk due to activation of estrogenic pathways.
Methods
Study Design
We conducted a nested case-control study using The Health Improvement Network (THIN), a large population-based electronic medical record database from the United Kingdom (UK). The study was approved by the Institutional Review Board at the University of Pennsylvania and by the Scientific Review Committee of THIN.
Data source
The THIN database contains comprehensive medical records on approximately 10 million patients treated by general practitioners in 566 practice sites, with demographic and geographic distributions that are broadly representative of the general UK population. THIN includes information on patient demographics, socioeconomic status, medical diagnoses, lab results, preventative healthcare and drug prescriptions. Each medical diagnosis is defined using Read diagnostic codes (25). Each medication is coded using multilex codes. All practices contributing data to THIN following a standardized protocol of entering information. Data quality is monitored through routine analysis of the entered data (26, 27). The database has been previously used for pharmacoepidemiology studies, showing excellent quality of information on prescriptions and medical diagnoses, including cancer (28, 29, 30).
Study cohort
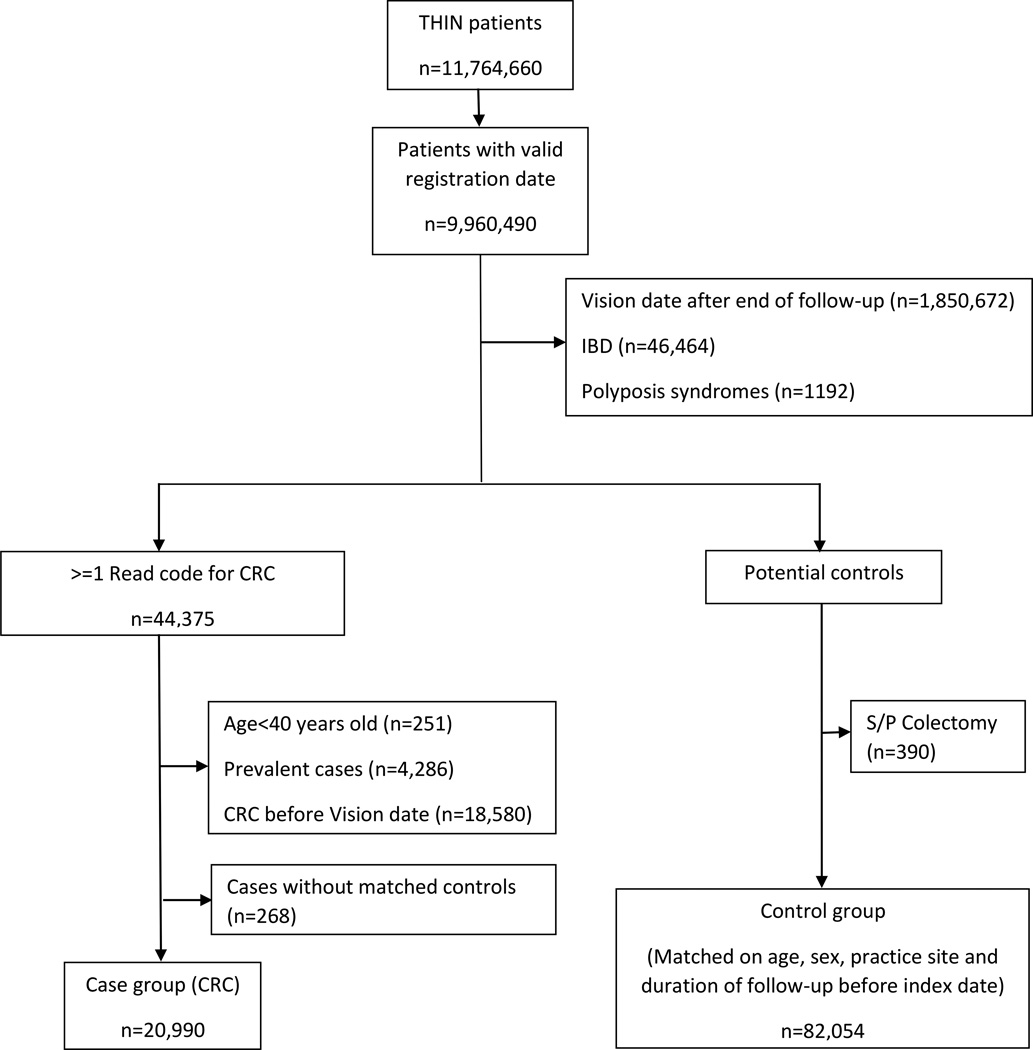
All people receiving medical care from 1995 to 2013 from a THIN practitioner were potentially eligible for inclusion (figure 1). Subjects with a diagnosis of familial CRC syndromes, IBD or age above 40 years old at the time of diagnosis were excluded in order to focus on an average risk population. Follow-up started at the later of either the date the THIN practice started using the electronic medical record (Vision software) or 183 days after the date at which the patient registered with their general practitioner (31), and ended on the index date (defined below).
Figure 1.
Patient selection flowchart
Case selection
Cases were defined as all individuals in the cohort with at least one Read code for CRC during the follow-up period (32, 27). The index date was the date of first CRC diagnosis. Subjects who had a recorded diagnosis for CRC within 183 days after initiation of the follow-up period were excluded in order to avoid prevalent cases (31).
Selection of controls
Selection of the control group was based on incidence density sampling (33). The eligible control pool for a case comprised all individuals who remained at risk for CRC on the index date of the case (i.e., no prior diagnosis of CRC or colectomy). Up to four controls were matched with each case on age (using age group categories of 5 years), sex, practice site, and duration of follow-up. Controls were assigned the same index date as their matched cases.
Exposures and Covariates
The primary exposure of interest was current use of digoxin defined as a last prescription for digoxin within a year before index date. Patients with last digoxin prescription more than a year before index date were defined as past users. Since colorectal tumorigenesis is a multistep process occurring over a period of 10–15 years, we analyzed the effect of first prescription timing before the index date (<6 months, 6 months- <1 year, 1– <5 years, 5– <10 years and >10 years) compared to unexposed subjects. In addition, we evaluated the association between CRC risk and cumulative duration of digoxin therapy divided to quartiles (Q1: 0–196 days, Q2: 197–579 days, Q3: 580–1301 days and Q4: more than 1301 days).
As potential confounders, we examined a comprehensive list of variables that are either known or suspected risk factors for CRC: lifestyle parameters including BMI (<25, 25–29, 30–39 and >40), smoking history (current, past or never), alcohol consumption (any use and alcoholism/alcohol dependence) and Townsend deprivation score as a surrogate for socio-economic status; presumed previous CRC screening colonoscopies (i.e. 2 years before index date); medical co-morbidities including diabetes mellitus (34) and heart disease (35); and medications which may influence CRC risk such as aspirin and NSAIDs. Covariates were measured prior to the index date.
Statistical Analysis
The baseline characteristics of cases and controls were compared using chi square test for categorical variables and t-test for continuous variables. The primary analysis was a multivariable conditional logistic regression to estimate odds ratios (OR) and 95% confidence interval for the association between digoxin use and CRC. In all the analyses the reference exposure group were individuals without documented digoxin therapy. The analysis was adjusted to all potential confounders (diabetes mellitus, heart disease, BMI, alcohol consumption, smoking history and chronic NSAIDs use as well as previous events of screening colonoscopy). All calculations were done using STATA 13.
Results
The study population consisted of 20,990 CRC patients and 82,054 matched controls with a median duration of follow up of 6.5 years (SD 4.0). Consortium diagram (Figure 1) and characteristics of cases and controls are presented (Table 1). As expected, cases were more likely to be former or current smokers, alcohol users, with an elevated BMI and a medical history of diabetes mellitus. Controls were more likely to undergo screening colonoscopy before index date, and chronic users of NSAIDs or aspirin had lower risk for CRC compared to non-users. There was no statistically significant difference in Townsend score, as a surrogate for socio-economic status, between cases and controls.
Table 1.
Characteristics of cases and controls
| Cases (n=20,990) |
Controls (n=82,054) |
Unadjusted OR (95% CI) |
P-value | |
|---|---|---|---|---|
| Age at index date (year SD) | 71.3 (11.1) | 71.0 (11.2) | NA | NA |
| Male sex (% SD) | 55.1% (0.5) | 55.1% (0.5) | NA | NA |
| Duration of follow-up before index date (years SD) | 6.5 (4.0) | 6.5 (4.0) | NA | NA |
| diabetes mellitus (% SD) | 12.6% (0.3) | 10.1% (0.3) | 1.28 (1.22–1.34) | <0.0001 |
| BMI 25–29 (% SD)1 | 28.1% (0.4) | 26.7% (0.4) | 0.97 (0.93–1.02) | 0.24 |
| BMI 30–39 (% SD) | 19.4% (0.4) | 17.6% (0.4) | 1.03 (0.98–1.08) | 0.3 |
| BMI >40 (%SD) | 2.1% (0.1) | 1.7% (0.1) | 1.20 (1.07–1.34) | 0.002 |
| Smoking (ever) (% SD) | 48.4% (0.5) | 42.8% (0.5) | 1.30 (1.25–1.34) | <0.0001 |
| Current smokers (% SD) | 7.8% (0.3) | 7.3% (0.3) | 1.22 (1.15–1.3) | <0.0001 |
| Alcohol use (% SD) | 52.9% (0.5) | 49.9% (0.5) | 1.18 (1.13–1.22) | <0.0001 |
| Alcoholism (% SD) | 0.8% (0.09) | 0.5% (0.07) | 1.80 (1.49–2.17) | <0.0001 |
| Chronic NSAIDs/Aspirin use (% SD)2 | 26.3% (0.4) | 27.2% (0.4) | 1.00 (0.97–1.04) | 0.82 |
| Previous screening colonoscopy (% SD)3 | 1.5% (0.1) | 1.7% (0.1) | 0.88 (0.78–0.99) | 0.04 |
Information regarding BMI was available for 14,621 cases and 52,814 controls.
First Prescription at least 12 month before index date and last prescription within 6 months before index date, cumulative duration of therapy more than 365 days.
more than two years before index date.
Current digoxin use was associated with an increase in CRC risk with adjusted OR of 1.52 (95% CI 1.40–1.65, p<0.0001). The risk was elevated both in patients with treatment initiation less than one year before index date as well as treatment initiation more than one year before index date with adjusted ORs of 1.92 (95%CI 1.63–2.28, p<0.0001) and 1.42 (95%CI 1.29–1.56, p=0.001) respectively. Similar results were observed among current users in analyses that accounted for different timing of digoxin treatment initiation (<6 months, 6 months-<1 year, 1– <5 years, 5– <10 years and >10 years before index date) with adjusted ORs of 1.41 (95%CI 1.25–1.59, p<0.0001) 1.45 (95%CI 1.22–1.72, p<0.0001) and 1.41 (95%CI 1.00–1.99, p=0.049) for first prescription 1–5, 5–10 and more than 10 years before index date, respectively (Table 2). The risk was not elevated among past digoxin users with last prescription more than one year before index date (adjusted OR of 1.07, 95%CI 0.90–1.28, p=0.452) or at any time interval (Table 3).
Table 2.
multivariable analysis among the current digoxin users, looking at five different levels of first prescription timing before index date
| First digoxin treatment before index date |
Cases (20,990) N (%) |
Controls (82,054) N (%) |
Unadjusted OR (95% CI, p-Value) |
Adjusted OR1 (95% CI, p-Value) |
|---|---|---|---|---|
| No digoxin Rx. | 20,147 (96.0%) | 79,936 (97.4%) | Ref. | Ref. |
| <6 months | 110 (0.5%) | 190 (0.2%) | 2.36 (1.86–2.99, <0.0001) | 2.28 (1.80–2.90, <0.0001) |
| 6 months – <1 year | 103 (0.5%) | 238 (0.3%) | 1.77 (1.40–2.24, <0.0001) | 1.64 (1.30–2.08, <0.0001) |
| 1– <5 years | 403 (1.9%) | 1,083 (1.3%) | 1.49 (1.32–1.67, <0.0001) | 1.41 (1.25–1.59, <0.0001) |
| 5– <10 years | 181 (0.9%) | 480 (0.6%) | 1.50 (1.26–1.78, <0.0001) | 1.45 (1.22–1.72, <0.0001) |
| >10 years | 46 (0.2%) | 127 (0.2%) | 1.47 (1.05–2.07, =0.027) | 1.41 (1.00–1.99, =0.049) |
Adjusted to diabetes mellitus, BMI, smoking history, alcohol consumption, chronic use of Aspirin/NSAIDs, and performance of screening colonoscopy.
Table 3.
multivariable analysis among the former digoxin users, looking at five different levels of first prescription timing before index date
| First digoxin treatment before index date |
Cases (20,990) N (%) |
Controls (82,054) N (%) |
Unadjusted OR (95% CI, p-Value) |
Adjusted OR1 (95% CI, p-Value) |
|---|---|---|---|---|
| No digoxin Rx. | 20,147 (96.0%) | 79,936 (97.4%) | Ref. | Ref. |
| 1– <5 years | 83 (0.4%) | 302 (0.4%) | 1.08 (0.85–1.39, =0.52) | 1.06 (0.83–1.35, =0.66) |
| 5– <10 years | 64 (0.3%) | 210 (0.2%) | 1.25 (0.94–1.65, =0.13) | 1.21 (0.91–1.61, =0.18) |
| >10 years | 14 (0.1%) | 73 (0.1%) | 0.76 (0.43–1.36, =0.36) | 0.73 (0.41–1.30, =0.29) |
Adjusted to diabetes mellitus, BMI, smoking history, alcohol consumption, chronic use of Aspirin/NSAIDs, and performance of screening colonoscopy.
We further analyzed the cumulative duration of digoxin use before index date, divided to quartiles (Q1: 0–196 days, Q2: 197–579 days, Q3: 580–1301 days and Q4: more than 1301 days) and CRC risk. For current digoxin users, there was an elevated CRC risk in all quartiles with an adjusted ORs of 1.97 (95%CI 1.63–2.38, p<0.0001), 1.52 (95%CI 1.29–1.78, p<0.0001), 1.44 (95%CI 1.23–1.68, p<0.0001) and 1.38 (95%CI 1.18–1.61, p<0.0001) for Q1–Q4 compared to individuals not treated with digoxin, respectively. The risk remained stable in Q2–Q4 (more than 196 days after treatment initiation) (Table 4). Among past users there was no impact for treatment duration on CRC risk in all duration quartiles (Table 4). Similar results were observed when number of digoxin prescriptions was analyzed (data not shown).
Table 4.
multivariable analysis of cumulative durations of digoxin treatment and colorectal cancer risk
| Digoxin therapy1 | Cases (20,990) N (%) |
Controls (82,054) N (%) |
Unadjusted OR (95% CI, p-Value) |
Adjusted OR2 (95% CI, p-Value) |
|---|---|---|---|---|
| Current users | ||||
| No digoxin Rx. | 20,147 (96.0%) | 79,936 (97.4%) | Ref. | Ref. |
| Q1 | 169 (0.8%) | 332 (0.4%) | 2.07 (1.72–2.50, <0.0001) | 1.97 (1.63–2.38, <0.0001) |
| Q2 | 212 (1%) | 533 (0.6%) | 1.59 (1.35–1.87, <0.0001) | 1.52 (1.29–1.78, <0.0001) |
| Q3 | 232 (1.1%) | 617 (0.8%) | 1.51 (1.30–1.77, <0.0001) | 1.44 (1.23–1.68, <0.0001) |
| Q4 | 230 (1.1%) | 636 (0.8%) | 1.44 (1.24–1.68, <0.0001) | 1.38 (1.18–1.61, <0.0001) |
| Former users | ||||
| No digoxin Rx. | 20,147 (96.0%) | 79,936 (97.4%) | Ref. | Ref. |
| Q1 | 103 (0.5%) | 347 (0.4%) | 1.18 (0.94–1.47, =0.15) | 1.14 (0.91–1.42, =0.26) |
| Q2 | 35 (0.2%) | 119 (0.1%) | 1.19 (0.81–1.73, =0.37) | 1.16 (0.80–1.70, =0.43) |
| Q3 | 10 (0.05%) | 70 (0.09%) | 0.58 (0.30–1.13, =0.11) | 0.59 (0.31–1.16, =0.13) |
| Q4 | 13 (0.06%) | 49 (0.06%) | 1.08 (0.59–2.00, =0.80) | 1.02 (0.55–1.89, =0.95) |
Q1: 0–196 days, Q2: 197–579 days, Q3: 580–1301 days and Q4: more than 1301 days
Adjusted to diabetes mellitus, ischemic heart disease, BMI, smoking history, alcohol consumption, chronic use of Aspirin/NSAIDs, and performance of screening colonoscopy
Additionally, a secondary analysis was performed only among women in order to evaluate possible estrogenic effects of digoxin. The association between digoxin use and CRC remained similar to the overall analysis with no risk in past users (adjusted OR 1.00, 95%CI 0.74–1.36, p=0.99) and increased risk in current users with an adjusted OR of 1.41 (95%CI 1.22–1.62, p<0.0001) for treatment more than one year before index date (for comparison, among males the adjusted OR was 1.42 with 95%CI 1.25–1.62 and p<0.0001).The risk increased in all quartiles of cumulative durations for current users with adjusted ORs of 1.91 (95%CI 1.44–2.52, p<0.0001), 1.40 (95%CI 1.09–1.80, p=0.008), 1.63 (95%CI 1.30–2.03, p<0.0001) and 1.31 (95%CI 1.03–1.67, p=0.03) for Q1–Q4 respectively. There was no increase in risk for any of the duration levels among women with past digoxin treatment more than one year before index date.
Discussion
Treatment with digoxin and other cardiac glycosides was previously described as a risk factor for breast and uterine cancers and potentially a protective factor against prostate cancer, possibly through estrogenic effects (14, 21). Despite the fact that estrogen and progestin hormone replacement therapy was shown to decrease CRC risk (24) only a single limited retrospective work, focusing on a small number of CRC patients demonstrated possible cytotoxic activity and increased overall survival in patients treated with digoxin along with chemotherapy (1).
In this nested case-control study we aimed to investigate the association between digoxin use and CRC risk in a large, average risk population. Current digoxin users had increased risk for CRC with an OR of 1.92 (1.63–2.28, <0.0001) for patients with treatment initiation less than one year before index date and an OR of approximately 1.4 for treatment initiation at different time interval from one year up to more than ten years prior to diagnosis. The risk dropped to normal among former digoxin users with an OR of 1.07 (0.90–1.28, p=0.45) at least one year after treatment cessation, resembling previous results in breast cancer (17). Similar results were observed when cumulative duration and number of digoxin prescriptions were analyzed. There was no difference in risk when the results were stratified according to sex. As mentioned previously, digoxin is a phytoestrogen, but while in the Women health initiative (WHI), use of estrogen and progestin hormone replacement therapy (HRT) decreased CRC risk (24) in our study the risk increased among current digoxin users, thus suggesting other biological mechanisms rather than activation of estrogenic pathways.
The higher risk demonstrated in patients with treatment initiation during the year before CRC diagnosis, might possibly be due to protopathic bias secondary to drug prescription prior to index date related to increased use of medical services (36). Since previous works demonstrated direct effects of certain isoforms of the sodium potassium ATPase on tumorigenic pathways, such as the Src/MAPK, in tissue from CRC patients (12, 13) an immediate increase in risk due to direct effects of digoxin cannot be ruled out.
The main advantage of the current study was using a large computerized database with detailed information on drug prescriptions. Since 98% of the UK population receive their healthcare through general practitioners, this database reflects the general healthcare delivery pattern for the entire UK population. Cases and controls were matched on age, sex, practice site and duration of follow-up with no additional socio-economic differences between the groups as expressed in Townsend score. The study also included only patients with at least 6 months of follow-up prior to diagnosis in order to avoid prevalent CRC cases. However, the current work had several limitations. The THIN database lacks information on digoxin dosing for the majority of users. Nevertheless, due to the narrow therapeutic range of digoxin we assume that duration of treatment was an appropriate surrogate for cumulative dose. The fact that the risk in our study resembles previous results regarding the risk for breast cancer among digoxin users support this assumption (19). Information regarding BMI levels as possible confounder for CRC risk was missing in up to 30% of the study population. We categorized those individuals with missing BMI data as a separate category in our analysis. Additionally we’ve done a second analysis restricted among patients with BMI data that did not change our results. Additionally, THIN lack information regarding polyp detection, tumor location and stage, thus we were unable to investigate possible differential effects of treatment on tumor invasiveness. The THIN database has no information regarding compliance with digoxin treatment, however all current users in our study renewed prescriptions at least once every 3 months and used digoxin as long term treatment. Furthermore we analyzed separately current and past users. If compliance was lower, we expect a non-differential misclassification that would bias the results towards the null.
Since CRC develops over a period of 10–15 years and the median follow up in the study was approximately 6 years, it is possible that some patients already had a colonic adenoma at the time of initiation of digoxin therapy. Taken together with the additional small number of patients that were treated with digoxin more than 10 years before index date, we were limited in analyzing the impact of digoxin on cancer initiation. Patients on digoxin therapy may also be less healthy than those who were not treated with digoxin and may be more likely to be followed by their general practitioner and undergo CRC screening. For this reason, we controlled the analysis for previous screening colonoscopies. The use of screening colonoscopy in the UK general population is extremely low, thus differences in health care received, particularly CRC screening services, were unlikely to confound the results. Additionally, we performed a restriction analysis only to cases and controls that weren’t screened. There was no change in results among this subset, supporting no effect of screening on outcome.
In summary, we demonstrated a higher risk for CRC associated with current digoxin use. The risk increased with treatment initiation and returned to normal one year after treatment cessation. Further work should evaluate the biological pathways behind this association focusing on the role of the sodium potassium ATPase in tumor formation. This association, if confirmed, could have clinical implications. It might suggest that physicians need to consider alternative treatment for heart failure in patients with known CRC risk.
Supplementary Material
Acknowledgement
Dr. Yang and Dr. Boursi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Yang YX, Boursi B, Haynes K and Mamtani R contributed to conception and design of the study; Yang YX and Boursi B acquired the data; Yang YX, Boursi B, Haynes K and Mamtani R contributed to analysis and interpretation of data, drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
The authors would also like to thank Nadir Arber M.D. M.Sc. MHA for reviewing the manuscript.
Dr. Boursi would like to thank the Djerassi family for supporting his post-doctoral fellowship.
Funding: The work was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
None of the authors has any relevant conflict of interest to declare.
References
- 1.Menger L, Vacchelli E, Kepp O, et al. Trial watch - Cardiac glycosides and cancer therapy. Oncoimmunology. 2013;2:e23082. doi: 10.4161/onci.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei D, Peng J, Gao H, et al. Digoxin downregulates NDRG1 and VEGF through the inhibition of HIF-1α under hypoxic conditions in human lung adenocarcinoma A549 cells. Int J Mol Sci. 2013;14:7273–7285. doi: 10.3390/ijms14047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Wong CCL, Wei H, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31:1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Schitoa L, Reya S, Tafanic M, et al. Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells. PNAS. 2012;109(40):E2707–E2716. doi: 10.1073/pnas.1214019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Qian DZ, Tan YS, et al. Digoxin and other cardiac glycosides inhibit HIF-1 alpha synthesis and block tumor growth. PNAS. 2008;105(50):19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranasinghe W, Sengupta S, Williams S. The effects of nonspecific HIF1a inhibitors on development of castrate resistance and metastases in prostate cancer. Cancer Medicine. 2014;3(2):245–251. doi: 10.1002/cam4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Zheng M, Zhichuan L, et al. Cardiac glycosides inhibit p53 synthesis by a mechanism relieved by Src or MAPK inhibition. Cancer Res. 2009;69(16):6556–6564. doi: 10.1158/0008-5472.CAN-09-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wha Jun D, Hwang M, Kim HJ, Hwang SK, Kim S, Lee CH. Ouabain, a cardiac glycoside, inhibits the Fanconi anemia/BRCA pathway activated by DNA interstrand cross-linking agents. PLoS ONE. 8(10):e75905. doi: 10.1371/journal.pone.0075905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Qiub Q, Shena J. Cardiac glycosides induce autophagy in human non-small cell lung cancer cells through regulation of dual signaling pathways. The International Journal of Biochemistry & Cell Biology. 2012;44:1813–1824. doi: 10.1016/j.biocel.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Zhan Y, Xu R, Shao R, Jiang J, Wang Z. Src mediates extracellular signal-regulated kinase 1/2 activation and autophagic cell death induced by cardiac glycosides in human non-small cell lung cancer cell lines. Molecular Carcinogenesis. 2014 doi: 10.1002/mc.22147. [DOI] [PubMed] [Google Scholar]
- 11.Menger L, Vacchelli E, Adjemian S, et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med. 2012;4(143) doi: 10.1126/scitranslmed.3003807. [DOI] [PubMed] [Google Scholar]
- 12.Sakai H, Suzuki T, Maeda M, et al. Up-regulation of Na(+),K(+)-ATPase alpha 3-isoform and down-regulation of the alpha1-isoform in human colorectal cancer. FEBS Lett. 2004;563:151–154. doi: 10.1016/S0014-5793(04)00292-3. [DOI] [PubMed] [Google Scholar]
- 13.Zhichuan L, Zhang Z, Xie J, et al. Na/K-ATPase mimetic pNaKtide peptide inhibits the growth of human cancer cells. J Biol Chem. 2011;286(37):32394–32403. doi: 10.1074/jbc.M110.207597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biggar RJ. Molecular pathways: digoxin use and estrogen-sensitive cancers - risks and possible therapeutic implications. Clin Cancer Res. 2012;18:2133–2137. doi: 10.1158/1078-0432.CCR-11-1389. [DOI] [PubMed] [Google Scholar]
- 15.Biggar RJ, Wohlfahrt J, Melbye M. Digoxin use and the risk of cancers of the corpus uteri, ovary and cervix. Int J Cancer. 2011;131:716–721. doi: 10.1002/ijc.26424. [DOI] [PubMed] [Google Scholar]
- 16.Ahern TP, Lash TL, Sørensen HT, Pedersen L. Digoxin treatment is associated with an increased incidence of breast cancer: a population-based case-control study. Breast Cancer Research. 2008;10:R102. doi: 10.1186/bcr2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biggar RJ, Wohlfahrt J, Oudin A, Hjuler T, Melbye M. Digoxin Use and the Risk of Breast Cancer in Women. JCO. 2011;29(16):2165–2170. doi: 10.1200/JCO.2010.32.8146. [DOI] [PubMed] [Google Scholar]
- 18.Biggar RJ, Andersen EW, Kroman N, Wohlfahrt J, Melbye M. Breast cancer in women using digoxin: tumor characteristics and relapse risk. Breast Cancer Research. 2013;15:R13. doi: 10.1186/bcr3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahern TP, Tamimi RM, Rosner BA, Hankinson SE. Digoxin use and risk of invasive breast cancer: evidence from the Nurses’ Health Study and meta-analysis. Breast Cancer Res Treat. 2014;144:427–435. doi: 10.1007/s10549-014-2886-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platz EA, Yegnasubramanian S, Liu JO, et al. A novel two-stage, transdisciplinary study identifies digoxin as a possible drug for prostate cancer treatment. Cancer Discovery. 2011;1:68–77. doi: 10.1158/2159-8274.CD-10-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright JL, Hansten PD, Stanford JL. Is digoxin use for cardiovascular disease associated with risk of prostate cancer? The Prostate. 2014;74:97–102. doi: 10.1002/pros.22733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flahavan EM, Sharp L, Bennett K, Barron TI. A cohort study of digoxin exposure and mortality in men with prostate cancer. BJU Int. 2014;113:236–245. doi: 10.1111/bju.12287. [DOI] [PubMed] [Google Scholar]
- 23.Felth J, Rickardson L, Rosen J, et al. Cytotoxic effects of cardiac glycosides in colon cancer cells, alone and in combination with standard chemotherapeutic drugs. J. Nat. Prod. 2009;72:1969–1974. doi: 10.1021/np900210m. [DOI] [PubMed] [Google Scholar]
- 24.Simon MS, Chlebowski RT, Wactawski-Wende J, et al. Estrogen plus progestin and colorectal cancer incidence and mortality. J Clin Oncol. 2012;30(32):3983–3990. doi: 10.1200/JCO.2012.42.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chisholm J. The Read clinical classification. Bmj. 1990;300(6732):1092. doi: 10.1136/bmj.300.6732.1092. PubMed PMID: 2344534; PubMed Central PMCID: PMC1662793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourke A, Dattani H, Robinson M. Feasibility study and methodology to create a quality-evaluated database of primary care data. Inform Prim Care. 2004;12:171–177. doi: 10.14236/jhi.v12i3.124. [DOI] [PubMed] [Google Scholar]
- 27.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Safe. 2007;16:393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 28.Hollowell J. The general practice research database: quality of morbidity data. Popul Trends. 1997:36–40. [PubMed] [Google Scholar]
- 29.Van-Staa TP, Wegman S, De-Vries F, Leufkens B, Cooper C. Use of statins and risk of fractures. JAMA. 2001;285:1850–1855. doi: 10.1001/jama.285.14.1850. [DOI] [PubMed] [Google Scholar]
- 30.Haynes K, Forde KA, Schinnar R, Wong P, Strom BL, Lewis JD. Cancer incidence in The Health Improvement Network. PDS. 2009;18:730–736. doi: 10.1002/pds.1774. [DOI] [PubMed] [Google Scholar]
- 31.Lewis JD, Bilker WB, Weinstein RB, Strom BL. The relationship between time since registration and measured incidence rates in the general practice research database. Pharmacoepidemiol Drug Saf. 2005;14:443–451. doi: 10.1002/pds.1115. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Rodriguez LA, Huerta-Alvarez C. Reduced risk of CRC among long-term users of aspirin and nonaspirin NSAIDS. Epidemiology. 2001;1(12):88–93. doi: 10.1097/00001648-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Lubin JH, Gail MH. Biased selection of controls for case-control analysis of cohort studies. Biometrics. 1984;40:63–75. [PubMed] [Google Scholar]
- 34.Jarvandi S, Davidson NO, Schootman M. Increased Risk of Colorectal Cancer in Type 2 Diabetes Is Independent of Diet Quality. Plos one. 2013 doi: 10.1371/journal.pone.0074616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan AO, Jim MH, Lam KF, Morris JS, Siu DC, Tong T, et al. Prevalence of colorectal neoplasm among patients with newly diagnosed coronary artery disease. JAMA. 2007;298(12):1412. doi: 10.1001/jama.298.12.1412. [DOI] [PubMed] [Google Scholar]
- 36.Horwitz RI, Feinstein AR. The problem of “protopathic bias” in case control studies. Am J Med. 1980;68(2):255–258. doi: 10.1016/0002-9343(80)90363-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.