Abstract
Background and purpose
Intraventricular thrombolysis (IVT) is a promising treatment in facilitating intraventricular clot resolution after intraventricular hemorrhage. We examined in-hospital outcomes and resource utilization after thrombolysis in patients with intraventricular hemorrhage requiring ventriculostomy in a 'real-world' setting.
Methods
Retrospective cohort. We identified adult patients with primary diagnosis of non-traumatic intracerebral hemorrhage requiring ventriculostomy from the Nationwide Inpatient Sample (NIS) from 2002–2011. We compared demographic and hospital characteristics, comorbidities, inpatient outcomes, and resource utilization measures between patients treated with IVT and those managed with ventriculostomy, but without IVT. Population estimates were extrapolated using standard NIS weighting algorithms.
Results
We included 34,044 patients in the analysis, of whom 1,133 (3.3%) received IVT. The thrombolysis group had significantly lower inpatient mortality (32.4% vs 41.6%, P=0.001) and it remained lower after controlling for baseline demographics, hospital characteristics, comorbidity, case severity and withdrawal of care status (adjusted odds ratio [OR]: 0.670; 95% confidence interval [CI]: 0.520–0.865; P=0.002). There was a trend toward favorable discharge (home or rehabilitation) among the thrombolysis cohort (adjusted OR: 1.335; 95% CI: 0.983–1.812, P=0.064). The adjusted rates of bacterial meningitis and ventricular shunt placement were similar between groups. The thrombolysis group had longer length of hospital stay (LOS) and higher inflation adjusted cost of care, but cost of care per day LOS was similar to the non-IVT group.
Conclusions
IVT for intracerebral hemorrhage requiring ventriculostomy resulted in lower inpatient mortality and a trend toward favorable discharge outcome with similar rates of inpatient complications compared to the non-IVT group.
Keywords: intraventricular hemorrhage, intracerebral hemorrhage, hemorrhagic stroke, thrombolysis, intraventricular thrombolysis, ventriculostomy, shunting, meningitis, mortality, outcomes, real-world, nationwide inpatient sample
Introduction
Intraventricular extension of intracerebral hemorrhage (ICH) is common; occurring in 40% cases of non-traumatic ICH, and is a strong independent predictor of mortality after ICH.1, 2 Intraventricular hemorrhage (IVH) is historically treated by insertion of a ventriculostomy catheter to allow for monitoring of intracranial pressure and drainage of hemorrhagic cerebrospinal fluid (CSF). However, ventriculostomy catheter alone does not promote clot resolution and may become obstructed by intraventricular blood. Intraventricular injection of fibrinolytic agents has been shown to facilitate clearing of ventricular blood clot, decrease the rate of hydrocephalus in animal models and improve mortality in case-series and meta-analysis.3–6
Efficacy of intraventricular thrombolysis (IVT) with recombinant tissue plasminogen activator (tPA) is being evaluated in a large multicenter clinical trial (Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage, CLEAR III).7 Efficacy in clinical trials may not always be reflected at a population level due to limited generalizability of trials using strict study protocols and variations in clinical practice. Moreover, clinical trials, due to their limited sample size, may not have enough power to study differences in infrequent treatment-related adverse events. Therefore, large-scale population studies are necessary to substantiate the results of clinical trials and evaluate the effectiveness of the delivery of treatment in clinical practice to a broader target. Although not yet approved by the United States (US) Food and Drug Administration, intraventricular tPA is already used off-label for the treatment of IVH associated hydrocephalus in the US.8 Frequency of utilization and outcomes of such treatment outside the context of clinical trials is largely unknown. Therefore, we aimed to study in-hospital outcomes and resource utilization after IVT for ICH patients requiring ventriculostomy in a population-based, retrospective cohort study from a large national health database.
Methods
Data-source
We analyzed data from the Nationwide Inpatient Sample (NIS) of the Healthcare Cost and Utilization Project (HCUP) from 2002–2011. NIS is a 20% stratified random sample of all admissions to non-federal hospitals in the US. It contains information regarding demographics, hospital characteristics, primary and secondary diagnoses, inpatient procedures, comorbidities and case-severity measures. All diagnoses and procedures are recorded using International Classification of Diseases version 9 Clinical Modification (ICD-9-CM) codes. Discharge weights are provided to permit extrapolation of population estimates from the sampled cases. Detailed information regarding the design and the contents of NIS is available at the HCUP website.9
Case-selection
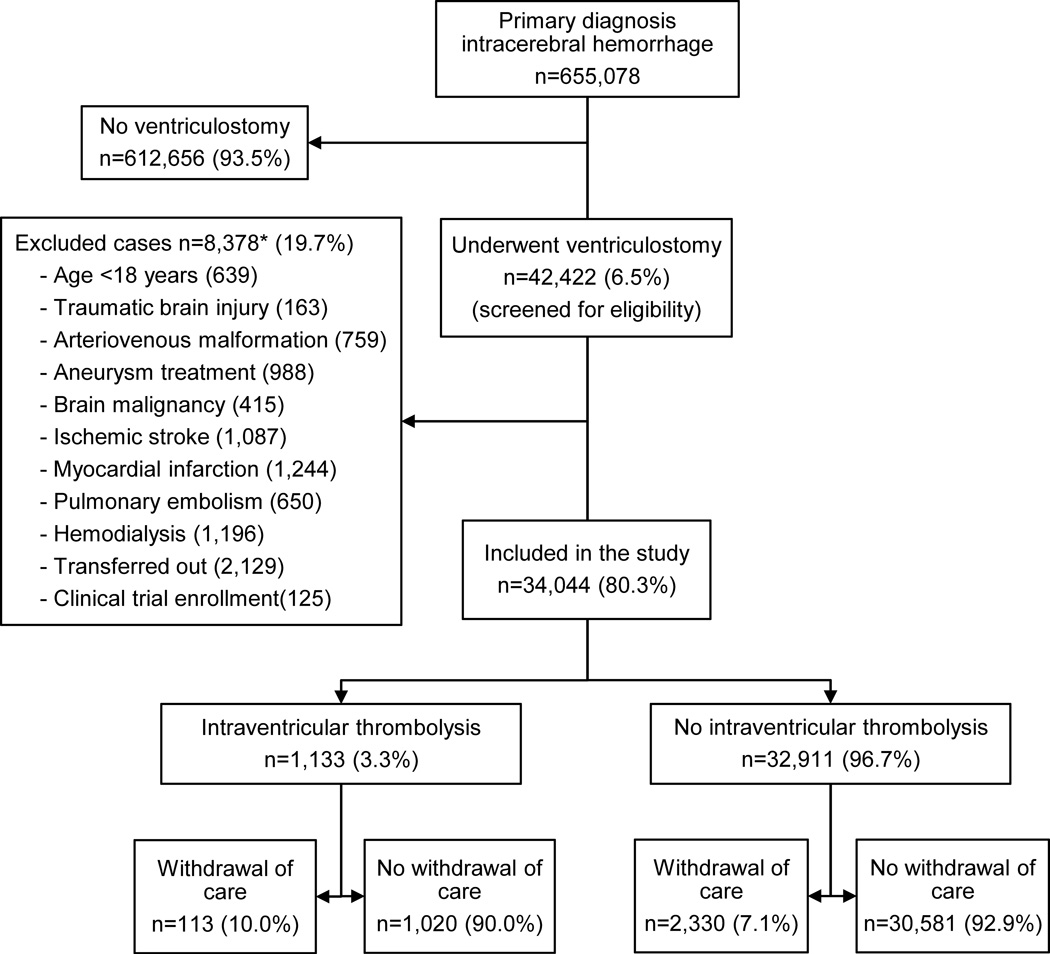
We first identified cases with primary diagnosis of non-traumatic ICH with or without IVH by using ICD-9-CM code 431.10, 11 Only patients with IVH requiring ventriculostomy were selected, using procedure code 02.2 (prior to October, 1, 2011) and 02.21 (from October, 1, 2011).12 We excluded cases with age <18 years, traumatic brain injury, brain malignancy, cerebral vascular malformations and those undergoing aneurysm clipping or coiling to restrict our population to those with primary ICH. Thrombolytic treatment was ascertained by procedure code 99.10. As the ICD-9-CM code does not distinguish the indication and route of thrombolytic treatment delivery, cases with acute stroke, myocardial infarction, pulmonary embolism, and end-stage renal disease requiring dialysis with possibility of access catheter thrombosis were excluded to minimize the uncertainty of indication for thrombolytic treatment. As the unit of the NIS database is discharge after hospitalization, rather than an individual patient, cases transferred to another hospital were excluded to prevent double counting of the same patient. Patients enrolled in a clinical trial (ICD-9-CM code V70.7) were also excluded. (Figure 1)
Figure 1.
Case selection
* Not mutually exclusive
Comorbidity and severity adjustment
We calculated the modified Charlson comorbidity index,13 a weighted score of 17 different comorbidities validated for outcome adjustment for analyses of administrative datasets using ICD-9-CM codes.14 Case severity was determined using the all patient refined diagnosis-related groups (APR-DRGs) to assess risk of mortality using an algorithm developed by 3M™ Health Information Systems. This proprietary 4 point ordinal scale (minor, moderate, major and extreme risk of mortality) is derived from age, primary and secondary diagnoses, and procedures.15, 16 The APR-DRG methodology has been validated to predict mortality more reliably than other severity measures using administrative datasets and has been used as a severity indicator in prior studies, including those relating to hemorrhagic stroke.17–19
Outcomes measures
The primary outcome of interest was inpatient mortality. Secondary outcomes studied were a composite favorable outcome of discharge to home/self-care or rehabilitation, and a composite unfavorable outcome of discharge to skilled nursing facility, hospice or death. Discharge disposition has been shown to correlate with 90-day and 1-year modified Rankin Scale with discharge to home or rehabilitation indicating higher functional potential than discharge to skilled nursing facility.20 Other safety outcomes studied were rates of bacterial meningitis, permanent ventricular shunting, gastrointestinal bleeding, gastrostomy and tracheostomy. Resource utilization measures used in the study were length of stay (LOS), overall cost of care and cost of care per day LOS. Cost of care was obtained by using hospital charges and HCUP cost-to-charge ratios, and was adjusted for inflation to obtain 2013 US dollar values by using yearly inflation rates published by US Department of Labor-Bureau of Labor statistics.21
We also compared the outcomes between the IVT group and the non-IVT group among the following sub-cohorts to assess robustness of the primary results: (1) cases excluding withdrawal of care (ICD-9-CM code V66.7),22 (2) survival beyond 48 hours from admission, (3) high ICH case volume hospitals (>47 cases/year comprising top 2 quartiles), (4) coding for obstructive hydrocephalus, (5) prolonged mechanical ventilation (>96 hours), (6) transferred in from another hospital.
Statistical analysis
Comparisons were made using Chi-square and Wilcoxon rank sum tests for categorical and continuous variables respectively. Multivariate logistic regression was used to adjust for available potential confounders in assessing the effect of IVT on outcomes. The following covariates were included in all regression models: age, sex, race/ethnicity, inter-institutional transfer, hospital characteristics (location, teaching status, geographic region, bedsize and ICH case volume quartile), modified Charlson comorbidity index, 3M™ APR-DRG risk of mortality subclass, coronary artery disease, diabetes mellitus, hypertension, atrial fibrillation, dyslipidemia, anemia, valvular disease, anticoagulation associated hemorrhage, thrombocytopenia, blood components transfusion, performance of cerebral angiography, craniectomy and craniotomy, prolonged mechanical ventilation and withdrawal of care status. We studied 10-year temporal trends of utilization of ventriculostomy in ICH and of IVT among the cases included in the analysis using Chi-square test for linear association. As recommended by HCUP, population estimates were obtained by complex sample analyses that consider weights, clustering, and stratification used for NIS sampling.23 All analyses were performed by using IBM SPSS version 20 (IBM Corporation, NY, USA) with statistical significance set at P<0.05. No adjustment was made for multiple comparisons due to the exploratory nature of the analysis.
Results
Of the 655,078 cases with primary diagnosis of ICH, 42,422 (6.5%) underwent ventriculostomy. Among 34,044 patients meeting eligibility criteria, 1,133 (3.3%) patients received IVT. Patients receiving IVT were slightly younger [median (interquartile range, IQR) age: 58 (51–70) vs 61 (51–72) years, P<0.001]. Sex and racial distributions were similar between the IVT and non-IVT groups. The IVT group had proportionately higher rates of inter-institutional transfer and patients were more likely to be treated in a teaching hospital and in the western US. Annual hospital ICH case volume was also higher among the treatment group. (Table 1)
TABLE 1.
Baseline patient and hospital characteristics
| No IVT (N=32,911) |
IVT (N=1,133) |
||||
|---|---|---|---|---|---|
| n | (%) | n | (%) | P value | |
| Age, year | 0.013 | ||||
| 18–64 | 19325 | (58.7) | 751 | (66.3) | |
| 65–79 | 10169 | (30.9) | 271 | (23.9) | |
| ≥80 | 3417 | (10.4) | 111 | (9.8) | |
| Female sex | 14365 | (43.7) | 502 | (44.3) | 0.806 |
| Race/ethnicity | 0.102 | ||||
| Caucasian | 13970 | (42.4) | 434 | (38.3) | |
| Black | 6712 | (20.4) | 235 | (20.7) | |
| Hispanic | 2638 | (8.0) | 131 | (11.6) | |
| Asian or Pacific Islander | 1469 | (4.5) | 85 | (7.5) | |
| Other | 1160 | (3.5) | 48 | (4.2) | |
| Missing information | 6961 | (21.2) | 200 | (17.6) | |
| Transfer from another hospital | 5763 | (17.5) | 311 | (27.4) | <0.001 |
| Hospital geographic region | 0.020 | ||||
| Northeast | 5690 | (17.3) | 182 | (16.0) | |
| Midwest | 5760 | (17.5) | 180 | (15.9) | |
| South | 14282 | (43.4) | 383 | (33.8) | |
| West | 7179 | (21.8) | 388 | (34.2) | |
| Hospital location | 0.133 | ||||
| Rural | 584 | (1.8) | ≤10* | (0.0) | |
| Urban | 32006 | (98.2) | 1122 | (100.0) | |
| Teaching hospital | 24266 | (74.5) | 995 | (88.7) | <0.001 |
| Hospital bed size | 0.428 | ||||
| Small | 1031 | (3.2) | 28 | (2.5) | |
| Medium | 5829 | (17.9) | 160 | (14.2) | |
| Large | 25730 | (79.0) | 935 | (83.3) | |
| Hospital ICH case volume quartile (cases/year) | <0.001 | ||||
| 1st (1–23) | 2754 | (8.4) | 46 | (4.1) | |
| 2nd (24–47) | 7558 | (23.0) | 170 | (15.0) | |
| 3rd (48–84) | 9869 | (30.0) | 305 | (26.9) | |
| 4th (≥85) | 12730 | (38.7) | 612 | (54.0) | |
NIS data use agreement prohibits reporting of cells with n≤10. ICH=intracerebral hemorrhage, IVT=intraventricular thrombolysis
Patients receiving IVT were more likely to have history of hypertension (P=0.030) and anemia (P<0.001). IVT group also had a higher modified Charlson comorbidity index (P=0.038), a higher rate of undergoing cerebral angiography (P<0.001), and a lower rate of craniotomy (P=0.003). Overall case severity as assessed by 3M™ APR-DRGs risk of mortality was higher in the thrombolysis group (extreme likelihood of dying: 56.6% vs. 41.5%, p<0.001). The rates of prolonged mechanical ventilation and obstructive hydrocephalus were also higher in thrombolysis group (P<0.001 and P=0.003 respectively). (Table 2)
TABLE 2.
Comorbidities, inpatient procedures and case severity
| No IVT (N=32,911) |
IVT (N=1,133) |
||||
|---|---|---|---|---|---|
| n | (%) | n | (%) | P value | |
| Modified Charlson comorbidity index | 0.038 | ||||
| 0 | 16742 | (51.3) | 539 | (47.5) | |
| 1 | 8699 | (26.7) | 286 | (25.2) | |
| 2 | 4155 | (12.7) | 207 | (18.2) | |
| ≥3 | 3042 | (9.3) | 102 | (9.0) | |
| Hypertension | 24669 | (75.6) | 925 | (81.6) | 0.030 |
| Diabetes mellitus | 6749 | (20.7) | 280 | (24.7) | 0.071 |
| Dyslipidemia | 4724 | (14.4) | 186 | (16.4) | 0.303 |
| Coronary artery disease | 3798 | (11.5) | 135 | (11.9) | 0.829 |
| Congestive heart failure | 2609 | (8.0) | 101 | (8.9) | 0.579 |
| Atrial fibrillation | 4309 | (13.1) | 142 | (12.6) | 0.774 |
| Valvular disease | 1154 | (3.5) | 55 | (4.8) | 0.291 |
| Anemia | 5311 | (16.1) | 286 | (25.2) | <0.001 |
| Thrombocytopenia | 1610 | (4.9) | 55 | (4.8) | 0.961 |
| Alcohol abuse | 2873 | (8.8) | 97 | (8.6) | 0.902 |
| Drug abuse | 1923 | (5.9) | 85 | (7.5) | 0.288 |
| Chronic kidney disease | 1852 | (5.6) | 83 | (7.3) | 0.185 |
| Transfusion of packed red blood cells | 2812 | (8.5) | 122 | (10.7) | 0.315 |
| Transfusion of platelets | 1758 | (5.3) | 58 | (5.1) | 0.884 |
| Transfusion of fresh frozen plasma | 2837 | (8.6) | 124 | (10.9) | 0.199 |
| Cerebral angiogram | 3498 | (10.6) | 217 | (19.2) | <0.001 |
| Craniotomy | 1113 | (3.4) | ≤10* | (0.4) | 0.003 |
| Craniectomy | 515 | (1.6) | ≤10* | (0.5) | 0.164 |
| Mechanical ventilation >96hrs | 14773 | (44.9) | 662 | (58.4) | <0.001 |
| Obstructive hydrocephalus | 20189 | (61.3) | 801 | (70.7) | 0.003 |
| 3M™ APR-DRG risk of mortality | <0.001 | ||||
| Minor likelihood of dying | 399 | (1.2) | ≤10* | (0.9) | |
| Moderate | 1235 | (3.8) | 45 | (3.9) | |
| Major | 17449 | (53.5) | 437 | (38.6) | |
| Extreme | 13555 | (41.5) | 641 | (56.6) | |
NIS data use agreement prohibits reporting of cells with n≤10. APR-DRG = all patient refined diagnosis related groups, IVT=intraventricular thrombolysis
The rate of ventriculostomy utilization in ICH increased from 5.7% in 2002–2003 to 7.0% in 2010–2011 (trend P<0.001) and the rate of IVT among the cases included in this analysis also showed an upward trend from 0.6% to 5.6% across the same interval (trend P<0.001). (Figure 2)
Figure 2.
Temporal trends
Error bar indicates standard error of the population estimate
The thrombolysis group had lower unadjusted inpatient mortality compared to the non-IVT group (32.4% vs 41.6%, odds ratio [OR]: 0.671; 95% confidence interval [CI]: 0.528–0.854, P=0.001). Adjusted inpatient mortality (adjusted OR: 0.670; 95% CI: 0.520–0.865, P=0.002) and the rate of composite unfavorable discharge was lower (adjusted OR: 0.670; 95% CI: 0.502–0.894, P=0.007) in the IVT group after controlling for available potential confounders. There was a trend toward higher rate of favorable discharge in the treatment group (adjusted OR: 1.335; 95% CI: 0.983–1.812, P=0.064). The adjusted rates of bacterial meningitis, permanent ventricular shunting, gastrostomy and tracheostomy were similar between the two groups. The outcome comparisons between the IVT and non-IVT groups after excluding withdrawal of care were comparable to the primary results as shown in Table 3. The outcomes of the other sub-cohorts were also largely consistent with the primary results that included all cases and are shown in Table I in the online-only Data Supplement. It is noteworthy that the proportions of patients with favorable outcome was higher for IVT vs. non-IVT patients for patients treated in high ICH case volume hospitals (adjusted OR: 1.497; 95% CI: 1.075–2.086, P=0.017) and for patients transferred from another hospital (adjusted OR: 2.159; 95% CI: 1.190–3.918, P=0.011). Overall inpatient mortality improved from 43.3% during 2002–2007 to 38.7% during 2008–2011 (P<0.001). IVT cohort had lower mortality during 2002–2007 (31.9% vs 43.5%, OR: 0.606; 95% CI: 0.398–0.924, P=0.019) and a trend toward lower mortality during 2008–2011 (32.6% vs 39.0%, OR: 0.757; 95% CI: 0.555–1.031, P=0.076).
TABLE 3.
Discharge outcomes and inpatient complications
| No IVT | IVT | |||||
|---|---|---|---|---|---|---|
| % | % | Unadjusted OR (95% CI) |
P value | Adjusted OR (95% CI) |
P value | |
| All cases | (N=1,133) | (N=32,911) | ||||
| Inpatient mortality | 41.6 | 32.4 | 0.671 (0.528–0.854) | 0.001 | 0.670 (0.520–0.865) | 0.002 |
| Favorable discharge* | 23.2 | 28.1 | 1.292 (0.969–1.724) | 0.080 | 1.335 (0.983–1.812) | 0.064 |
| Unfavorable discharge† | 61.3 | 54.1 | 0.745 (0.575–0.965) | 0.026 | 0.670 (0.502–0.894) | 0.007 |
| Bacterial meningitis | 3.0 | 2.0 | 0.674 (0.266–1.708) | 0.402 | 0.525 (0.202–1.362) | 0.185 |
| Ventricular shunting | 7.8 | 11.1 | 1.470 (1.027–2.103) | 0.034 | 1.358 (0.929–1.984) | 0.114 |
| Gastrointestinal bleeding | 1.7 | 1.3 | 0.731 (0.262–2.037) | 0.547 | 0.591 (0.204–1.719) | 0.334 |
| Tracheostomy | 2.3 | 2.9 | 1.230 (0.537–2.817) | 0.624 | 1.401 (0.592–3.317) | 0.442 |
| Gastrostomy | 26.0 | 33.3 | 1.422 (1.069–1.893) | 0.015 | 1.250 (0.947–1.650) | 0.116 |
| Withdrawal of care excluded | (N=1,020) | (N=30,581) | ||||
| Inpatient mortality | 38.7 | 26.9 | 0.583 (0.445–0.764) | <0.001 | 0.614 (0.460–0.821) | 0.001 |
| Favorable discharge* | 24.6 | 30.9 | 1.365 (1.022–1.825) | 0.035 | 1.333 (0.983–1.807) | 0.064 |
| Unfavorable discharge† | 58.8 | 49.7 | 0.693 (0.528–0.909) | 0.008 | 0.677 (0.505–0.906) | 0.009 |
| Bacterial meningitis | 3.1 | 2.2 | 0.720 (0.285–1.823) | 0.487 | 0.560 (0.217–1.449) | 0.232 |
| Ventricular shunting | 8.3 | 11.1 | 1.388 (0.948–2.033) | 0.091 | 1.248 (0.847–1.841) | 0.263 |
| Gastrointestinal bleeding | 1.8 | 1.4 | 0.774 (0.276–2.169) | 0.625 | 0.610 (0.209–1.784) | 0.367 |
| Tracheostomy | 2.5 | 2.6 | 1.041 (0.490–2.213) | 0.917 | 1.142 (0.522–2.497) | 0.739 |
| Gastrostomy | 27.3 | 34.5 | 1.404 (1.054–1.871) | 0.020 | 1.183 (0.902–1.552) | 0.224 |
Discharge to home/self-care or rehabilitation.
Discharge to skilled nursing facility, hospice or death.
Sum of favorable and unfavorable discharge rates is not 100% as other less common specified (such as home health care, law enforcement, intermediate care center) and unspecified discharge dispositions were not included in the definitions.
IVT=intraventricular thrombolysis
Patients receiving IVT had longer LOS [median (IQR): 18 (10–26) vs 14 (6–25) days, P<0.001]. The IVT group also incurred higher inflation adjusted cost of care [58,770 (33,379–88,434) vs 42,052 (21,757–71,481) USD, P<0.001]. These rates remained higher in the IVT group after excluding cases with withdrawal of care. The cost of care per day LOS was similar between the two groups (P=0.285 for all cases and P=0.270 after excluding withdrawal of care) (Table II in the online-only Data Supplement). LOS was similar between the two groups among the survivors [21 (15–29) vs 21 (14–31) days, P=0.931). Resource utilization measures stratified by risk of mortality subclass revealed higher cost of care and LOS in IVT cohort only among cases with major or extreme likelihood of dying (Table III in the online-only Data Supplement).
Discussion
This study, the first to our knowledge exploring the outcomes of IVT for IVH requiring ventriculostomy at the population level, showed that patients treated with intraventricular thrombolytics had lower inpatient mortality compared to those given standard care without thrombolysis. This finding is consistent with randomized studies showing decreased mortality in patients treated with IVT compared to those with standard treatment.4, 24, 25 The 33% mortality seen in the treatment group in this study is higher than that reported in the thrombolytic trials4, 25 but the thrombolysis trials excluded patients with predicted poor prognosis such as hematoma volume >30cc, age> 80 years and midbrain compression; the mortality rates in prior observational studies from clinical chart abstraction are similar to those found in the current study.26, 27 The effect size of survival benefit with thrombolytic treatment (33% lower odds of mortality) is also close to that found in a previous meta-analysis of observational studies showing decrease in odds by 56% (95% CI: 21%-75%) with IVT versus ventriculostomy alone.3 We also found a higher rate of favorable discharge among the thrombolysis group which reached statistical significance in the subgroups of high ICH case volume hospitals and transfer-in from another hospital. This finding may indicate a potential benefit of the treatment on functional outcome in addition to improved survival, especially in hospitals with experience in this procedure. It is well known, however, that functional recovery continues for months after hemorrhagic stroke 28 and a well-validated follow up functional assessment such as 180-day modified Rankin scale (not available in NIS database) is needed to confirm or refute the treatment effect on functional outcome.
The adjusted rates of permanent ventricular shunt placement were similar between the two comparison groups (adjusted OR: 1.358; 95% CI: 0.929–1.984, P=0.114). This result may indicate that thrombolytics do not prevent communicating hydrocephalus despite faster clearance of intraventricular blood. Alternatively, this finding may be driven by a higher survival rate of patients with more severe disease in IVT compared to non-IVT patients. Of note, consistent with the current study, a prior meta-analysis found no evidence that IVT reduces the need for ventricular shunting procedures.3 Prior studies have shown no increase in infectious complications with IVT.3 This was also found in our study showing similar rates of bacterial meningitis in both groups.
This is also the first large study analyzing the hospital characteristics and resource utilization measures associated with IVT. Teaching hospitals and hospitals with larger annual ICH case volume were more likely to utilize IVT, possibly due to teaching hospitals having greater availability of resources and technical expertise, higher full time in-hospital staffing and overall more experience with resultant higher comfort level of treating physicians for using this infrequent treatment. Higher overall cost associated with IVT is largely explained by longer LOS in the thrombolysis group, as the cost of care per day of hospitalization was not higher. We speculate that preferential improvement in survival of the most severe cases may have contributed to comparative higher cost of care and longer LOS in the IVT group. Patients in the non-treatment group had higher rates of craniotomy. This may reflect differences in the population considered for surgery and thrombolysis with larger ICH more likely receiving the surgical treatment and larger IVH more likely to receive thrombolysis.
The results of this study should be interpreted with caution due to inherent limitations of administrative databases, the retrospective and exploratory nature of the analysis, and a lack of well-validated ICH severity measures, and follow up data. For severity measures, the NIS database does not have critically important prognostic elements such as Glasgow coma scale and ICH/IVH volume and location. We have used a previously-validated DRG based risk of mortality algorithm to partially overcome this limitation and found APR-DRG risk of mortality to be a strong predictor of mortality in a dose-response fashion (Table IV in the online-only Data Supplement). The primary limitation in assessing any treatment effect from a non-randomized study is the risk of confounding by indication (i.e., that the choice to treat with thrombolysis was tied to the final outcomes in a non-causal way) and survivor bias (i.e. patients surviving initial days after ICH had more time to be selected for IVT thus causing spurious association of IVT with survival).29 In this case, however, patients given thrombolysis had higher APR-DRG severity scores, arguing against our findings being solely due to confounding. Additionally, more than 2/3 patients in the IVT group received thrombolysis within 2 days after ventriculostomy. Therefore, survivor bias in this study though possible, is less likely to impact the mortality significantly as suggested by lower mortality in IVT group among patients surviving at least 2 days from admission. Our method of case selection is also an important limitation. ICD-9-CM code 431 is validated to have high positive predictive value for diagnosing primary ICH from administrative datasets, but its accuracy in identifying IVH has not previously been studied.10, 11 Similarly, procedure code 99.10 has high specificity for intravenous thrombolysis in stroke.30 However, no prior study has validated the use of the code for ascertaining IVT. In order to increase the accuracy of case ascertainment, we excluded the cases with confounding diagnoses for thrombolysis indication such as ischemic stroke, myocardial infarction, pulmonary embolism, and those on hemodialysis with possibility of clotted access lines requiring thrombolytic agents. The temporal trend in the fraction of treated cases is coincident with development and publication of research data supporting the use of thrombolysis in IVH, lending some credibility to our case selection method. Nevertheless, it remains possible that our cases were not all cases of IVH and that some ‘treated’ cases were given thrombolytic agents via some other route than intraventricular. Although diagnosis coding is imperfect, random ICD-9 coding errors would bias the results toward the null, so are unlikely to account for the measured differences in mortality rates found in this study. Despite these limitations, we believe that large scale studies to analyze the effects of infrequently used treatments such as IVT by chart abstraction is not feasible and national administrative databases such as the NIS provides a more readily accessible tool to validate the effectiveness of this treatment at the population level in routine clinical practice.
In conclusion, using population-based data, we have shown that IVT in patients with primary ICH requiring ventriculostomy may be associated with higher survival to discharge and perhaps even improved favorable discharge disposition. We found no evidence that IVT increased the rate of bacterial meningitis. This finding is consistent with the results from prior randomized pilot trials and small observational studies. A more definitive conclusion regarding the effect of the treatment on outcomes requires confirmation by large randomized studies, such as the ongoing CLEAR III trial.7
Supplementary Material
Acknowledgments
Sources of Funding
National Institute of Health/National Institute of Neurological Disorders and Stroke supported this research with grant number 5U01NS062851.
Disclosures
Dr Daniel F. Hanley was awarded significant research support of grant numbers 5U01NS062851 for CLEAR III and for MISTIE III 1U01NS08082.
References
- 1.Hanley DF. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke. 2009;40:1533–1538. doi: 10.1161/STROKEAHA.108.535419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maas MB, Nemeth AJ, Rosenberg NF, Kosteva AR, Prabhakaran S, Naidech AM. Delayed intraventricular hemorrhage is common and worsens outcomes in intracerebral hemorrhage. Neurology. 2013;80:1295–1299. doi: 10.1212/WNL.0b013e31828ab2a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaberel T, Magheru C, Parienti JJ, Huttner HB, Vivien D, Emery E. Intraventricular fibrinolysis versus external ventricular drainage alone in intraventricular hemorrhage: a meta-analysis. Stroke. 2011;42:2776–2781. doi: 10.1161/STROKEAHA.111.615724. [DOI] [PubMed] [Google Scholar]
- 4.Naff N, Williams MA, Keyl PM, Tuhrim S, Bullock MR, Mayer SA, et al. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial. Stroke. 2011;42:3009–3016. doi: 10.1161/STROKEAHA.110.610949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 3. Effects of intraventricular urokinase on clot lysis and posthemorrhagic hydrocephalus. Neurosurgery. 1986;19:553–572. doi: 10.1227/00006123-198610000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Vereecken KK, Van Havenbergh T, De Beuckelaar W, Parizel PM, Jorens PG. Treatment of intraventricular hemorrhage with intraventricular administration of recombinant tissue plasminogen activator A clinical study of 18 cases. Clin Neurol Neurosurg. 2006;108:451–455. doi: 10.1016/j.clineuro.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Ziai WC, Tuhrim S, Lane K, McBee N, Lees K, Dawson J, et al. A multicenter, randomized, double-blinded, placebo-controlled phase III study of Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III) Int J Stroke. 2014;9:536–542. doi: 10.1111/ijs.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapointe M, Haines S. Fibrinolytic therapy for intraventricular hemorrhage in adults. Cochrane Database Syst Rev. 2002:CD003692. doi: 10.1002/14651858.CD003692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overview of the Nationwide Inpatient Sample (NIS) [Accessed Decemeber 1, 2012];HCUP Databases. Healthcare Cost and Utilization Project (HCUP) www.hcup-us.ahrq.gov/nisoverview.jsp.
- 10.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 11.Williams GR, Jiang JG, Matchar DB, Samsa GP. Incidence and occurrence of total (first-ever and recurrent) stroke. Stroke. 1999;30:2523–2528. doi: 10.1161/01.str.30.12.2523. [DOI] [PubMed] [Google Scholar]
- 12.Sekula RF, Cohen DB, Patek PM, Jannetta PJ, Oh MY. Epidemiology of ventriculostomy in the United States from 1997 to 2001. Br J Neurosurg. 2008;22:213–218. doi: 10.1080/02688690701832084. [DOI] [PubMed] [Google Scholar]
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 14.Bar B, Hemphill JC., 3rd Charlson comorbidity index adjustment in intracerebral hemorrhage. Stroke. 2011;42:2944–2946. doi: 10.1161/STROKEAHA.111.617639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Averill RF, Goldfield N, Hughes JS, Bonazelli J, McCullough EC, Steinbeck BA, et al. ALL PATIENT REFINED DIAGNOSIS RELATED GROUPS (APR-DRGs) Version 20.0 Methodology Overview. [Accessed December 1, 2012];3M Health Information Systems. http://www.hcup-us.ahrq.gov/db/nation/nis/APR-DRGsV20MethodologyOverviewandBibliography.pdf.
- 16.Edwards N, Honemann D, Burley D, Navarro M. Refinement of the Medicare diagnosis-related groups to incorporate a measure of severity. Health Care Financ Rev. 1994;16:45–64. [PMC free article] [PubMed] [Google Scholar]
- 17.Baram D, Daroowalla F, Garcia R, Zhang G, Chen JJ, Healy E, et al. Use of the All Patient Refined-Diagnosis Related Group (APR-DRG) Risk of Mortality Score as a Severity Adjustor in the Medical ICU. Clin Med Circ Respirat Pulm Med. 2008;2:19–25. doi: 10.4137/ccrpm.s544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Y. Applying the 3M All Patient Refined Diagnosis Related Groups Grouper to measure inpatient severity in the VA. Med Care. 2003;41:II103–II110. doi: 10.1097/01.MLR.0000068423.39715.CE. [DOI] [PubMed] [Google Scholar]
- 19.Xian Y, Holloway RG, Pan W, Peterson ED. Challenges in assessing hospital-level stroke mortality as a quality measure: comparison of ischemic, intracerebral hemorrhage, and total stroke mortality rates. Stroke. 2012;43:1687–1690. doi: 10.1161/STROKEAHA.111.648600. [DOI] [PubMed] [Google Scholar]
- 20.Qureshi AI, Chaudhry SA, Sapkota BL, Rodriguez GJ, Suri MF. Discharge destination as a surrogate for Modified Rankin Scale defined outcomes at 3- and 12-months poststroke among stroke survivors. Arch Phys Med Rehabil. 2012;93:1408–1413. doi: 10.1016/j.apmr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CPI Inflation Calculator. Databases, Tables & Calculators by Subject. [Accessed January 1, 2014];Bureau of Labor Statistics. http://www.bls.gov/data/inflation_calculator.htm. [Google Scholar]
- 22.Qureshi AI, Adil MM, Suri MF. Rate of utilization and determinants of withdrawal of care in acute ischemic stroke treated with thrombolytics in USA. Med Care. 2013;51:1094–1100. doi: 10.1097/MLR.0b013e3182a95db4. [DOI] [PubMed] [Google Scholar]
- 23.Houchens R, Elixhauser A. Final Report on Calculating Nationwide Inpatient Sample (NIS) Variances, 2001. [Accessed December 1, 2012];HCUP Method Series Report # 2003-02. http://www.hcup-us.ahrq.gov/reports/methods/CalculatingNISVariances200106092005.pdf.
- 24.Gubucz I, Kakuk I, Major O, Szegedi N, Barsi P, Panczel G, et al. [Effectiveness and safety of intraventricular fibrinolysis in secondary intraventricular hemorrhages (a prospective, randomized study)] Orv Hetil. 2004;145:1609–1615. [PubMed] [Google Scholar]
- 25.Tung MY, Ong PL, Seow WT, Tan KK. A study on the efficacy of intraventricular urokinase in the treatment of intraventricular haemorrhage. Br J Neurosurg. 1998;12:234–239. doi: 10.1080/02688699845050. [DOI] [PubMed] [Google Scholar]
- 26.Fountas KN, Kapsalaki EZ, Parish DC, Smith B, Smisson HF, Johnston KW, et al. Intraventricular administration of rt-PA in patients with intraventricular hemorrhage. South Med J. 2005;98:767–773. doi: 10.1097/01.smj.0000170732.24324.ea. [DOI] [PubMed] [Google Scholar]
- 27.Torres A, Plans G, Martino J, Godino O, Garcia I, Gracia B, et al. Fibrinolytic therapy in spontaneous intraventricular haemorrhage: efficacy and safety of the treatment. Br J Neurosurg. 2008;22:269–274. doi: 10.1080/02688690701834494. [DOI] [PubMed] [Google Scholar]
- 28.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Stroke. Neurologic and functional recovery the Copenhagen Stroke Study. Phys Med Rehabil Clin N Am. 1999;10:887–906. [PubMed] [Google Scholar]
- 29.Psaty BM, Koepsell TD, Lin D, Weiss NS, Siscovick DS, Rosendaal FR, et al. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47:749–754. doi: 10.1111/j.1532-5415.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi AI, Harris-Lane P, Siddiqi F, Kirmani JF. International classification of diseases and current procedural terminology codes underestimated thrombolytic use for ischemic stroke. J Clin Epidemiol. 2006;59:856–858. doi: 10.1016/j.jclinepi.2006.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.