Summary
Neurotransmitter release probability (Pr) largely determines the dynamic properties of synapses. While much is known on the role of presynaptic proteins in transmitter release, their specific contribution to synaptic plasticity is unclear. One such protein, tomosyn, is believed to reduce Pr by interfering with the SNARE complex formation. Tomosyn is enriched at hippocampal mossy fiber-to-CA3 pyramidal cell synapses (MF-CA3), which characteristically exhibit low Pr, strong synaptic facilitation and pre-synaptic PKA-dependent LTP. To evaluate tomosyn's role in MF-CA3 function, we used a combined knockdown (KD)-optogenetic strategy whereby presynaptic neurons with reduced tomosyn levels were selectively activated by light. Using this approach in mouse hippocampal slices we found that facilitation, LTP, and PKA-induced potentiation were significantly impaired at tomosyn-deficient synapses. These findings not only indicate that tomosyn is a key regulator of MF-CA3 plasticity, but also highlight the power of a combined KD-optogenetic approach to determine the role of presynaptic proteins.
Keywords: CA3, hippocampus, mossy fiber terminals, short-term plasticity, LTP, presynaptic release probability, optogenetics
Introduction
Exocytosis of neurotransmitter-containing vesicles is a multistep process that requires proper SNARE protein complex assembly (Jahn and Fasshauer, 2012). While this core mechanism is shared with little to no variation by different types of synaptic terminals, its assembly is tightly regulated by a host of synaptic proteins that interact with the SNARE complex, and ultimately control basal neurotransmitter release probability (Pr) (Südhof, 2012). Differential expression and distribution of these proteins may account for the great variability in basal Pr observed across central synapses (Dobrunz and Stevens, 1997). Importantly, basal Pr determines the efficacy and dynamic properties of these synapses. In response to repetitive activation, high Pr synapses tend to depress while low Pr synapses tend to facilitate (Trommershauser et al., 2003; Zucker and Regehr, 2002). These forms of short-term plasticity (STP; i.e. depression and facilitation) in turn underlie the type of computation the synapse performs and the reliability of its information transfer (Abbott and Regehr, 2004; Klug et al., 2012). In addition, enduring changes in Pr underlie presynaptic forms of long-term plasticity in the CNS (Castillo, 2012; Yang and Calakos, 2010). However, the precise contribution of specific presynaptic proteins to basal Pr and to short- and long-term plasticity is not fully understood.
The hippocampal mossy fiber to CA3 pyramidal cell synapse (MF-CA3) has a very low basal Pr and is commonly used as a model synapse for investigating several unique forms of presynaptic plasticity. MF-CA3 displays robust frequency-dependent facilitation (Salin et al., 1996) and presynaptic, PKA-dependent LTP (Huang et al., 1994; Nicoll and Schmitz, 2005; Weisskopf et al., 1994). However, the molecular mechanisms underlying these forms of plasticity are still largely unknown. Tomosyn is a regulatory protein highly expressed in MF-CA3 terminals (Barak et al., 2010; Sakisaka et al., 2008). It is believed that tomosyn interaction with syntaxin (Fujita et al., 1998; Hatsuzawa et al., 2003) and with SNAP-25 (Bielopolski et al., 2014) hinders the formation of the SNARE complex. Tomosyn over-expression led to reduction in Pr and inhibition of vesicle priming in neuroendocrine cells and neurons (Fujita et al., 1998; Hatsuzawa et al., 2003; Williams et al., 2011; Yizhar et al., 2004). Tomosyn down regulation enhanced synaptic transmission in C. elegans and in the mouse hippocampus (Gracheva et al., 2007; McEwen et al., 2006; Sakisaka et al., 2008) but led to opposite effects in superior cervical ganglion neurons and in insulin secreting cells (Baba et al., 2005; Cheviet et al., 2006). In addition, tomosyn has an evolutionarily conserved PKA phosphorylation site at serine 724 whose phosphorylation decreases tomosyn-syntaxin interaction (Baba et al., 2005). The high expression levels of tomosyn in the mossy-fiber pathway, suggest that this protein might contribute significantly to the low Pr and robust presynaptic forms of plasticity displayed by the MF-CA3 synapse.
To determine the role of tomosyn in synaptic transmission and plasticity, we developed a unique approach that combines an in vivo shRNA-mediated knockdown (KD) strategy, with optogenetics. Using this approach, we were able to selectively activate tomosyn-deficient synapses in mouse hippocampal slices. We report that tomosyn KD strongly reduces MF-CA3 short- and long-term plasticity. Our findings strongly suggest that tomosyn plays a key role in setting the characteristically low Pr at MF-CA3 synapses (Lawrence et al., 2004; Salin et al., 1996). In addition, we show that a KD-optogenetic strategy overcomes common limitations associated with low transduction rates, as well as developmental effects that often accompany the use of transgenic animals.
Results
Normal synaptic physiology in optically-activated mossy fibers
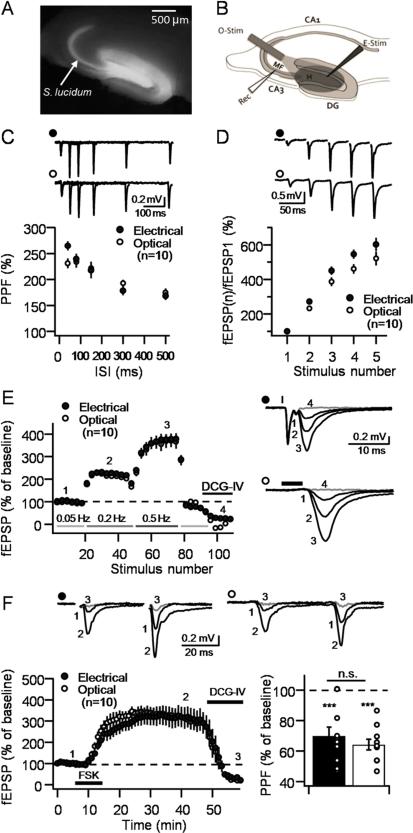
We first set to examine whether optogenetic activation of MFs elicited normal MF-CA3 synaptic responses, including the uniquely robust STP observed at these synapses by electrical stimulation. To this end, we lentivirally transduced presynaptic dentate granule cells (DGCs) in vivo with the fast kinetic channelrhodopsin2 variant ChIEF (Lin et al., 2009) fluorescently tagged with mCitrine. Hippocampal slices were prepared from injected animals exhibiting strong fluorescence exclusively in dentate gyrus (DG) and S. lucidum (Figure1A). In all experiments, synaptic responses (extracellular field EPSPs, or fEPSPs) were alternately elicited by electrical or optical stimulation delivered to the hilar region of the DG (Figure 1B). STP was assessed by analyzing: 1) paired-pulse facilitation (PPF) with two pulses at various inter-stimulus intervals (ISI); 2) burst-induced facilitation with a five-stimulus, 25 Hz burst; and 3) low-frequency facilitation (LFF) by stepping baseline frequency from 0.05 Hz to 0.2 Hz, and to 0.5 Hz. PPF revealed no differences between optically and electrically-elicited responses (n = 10, Figure 1C). Likewise, synaptic facilitation elicited by a five-stimulus burst was similar following electrical or optical MF stimulation (n = 10, Figure 1D). Slight reductions in synaptic facilitation at 25 Hz are likely due to action potential failure as a result of ChIEF activation failure (Lin et al., 2009). Consistent with this interpretation, LFF (0.2 and 0.5 Hz) was indistinguishable between optically and electrically-induced responses (Figure 1E). We thus conclude that optical stimulation can be reliably used to elicit MF-CA3 STP.
Figure 1. Characterization of optogenetically-induced MF-CA3 plasticity.
A. Representative hippocampal slice expressing ChIEF in the dentate gyrus, hilus and S. lucidum. B. Diagram illustrating the positioning of the recording electrode (Rec) electrical stimulation electrode (E-Stim) and the optic fiber (O-Stim). For all panels in this figure, synaptic responses were elicited using intermittent optical and electrical stimulation at intervals of 10 seconds, delivered to the same slices. C. Paired-pulse facilitation at 40, 80, 150, 300 and 500 ms inter-stimulus intervals. Representative traces (top); summary plot (bottom). D. Burst-induced facilitation (5 pulses at 25 Hz). Representative traces (top); summary plot (bottom). E. Low-frequency facilitation (LFF; 0.05 to 0.2 Hz and to 0.5 Hz). Summary plot (left); representative traces (right). Black lines above the traces indicate the duration of the stimulation. F. Forskolin application (25 μM for 10 min) induced long-lasting potentiation (left panel) that was associated with a reduction in paired-pulse facilitation (PPF) (right panel). Stimulus artifact was removed from traces in panels B,D and F for better visualization. 1 μM DCG-IV was applied at the end of all experiments (gray traces). Numbers in the time-course plots E and F indicate the time points when the representative traces were taken. Number of slices for each condition is shown in brackets. Data are presented as mean ± S.E.M; n.s., not significant; KD, Knock-down; Scr, scrambled, H, Hilus; DG, dentate gyrus; MF, mossy fiber pathway.
MF-CA3 synapses are also known to exhibit robust long-lasting potentiation of glutamate release following transient activation of the cAMP/PKA cascade (Weisskopf et al., 1994). We therefore compared the potentiation of optically and electrically-induced responses following bath application of the adenylyl-cyclase activator forskolin (25 μM for 10 minutes). We found that both types of responses were equally potentiated by forskolin and, consistent with an increase in Pr, this potentiation was accompanied by a similar reduction in PPF following both electrical and optical activation (p>0.05; Figure 1F). Finally, as expected for MF-elicited responses, bath application of the group II metabotropic glutamate receptor agonist DCG-IV (1 μM), at the end of each experiment, virtually abolished synaptic transmission evoked either optically or electrically (see Experimental Procedures). Together, these results demonstrate that optical activation of ChIEF-expressing MFs reproduces typical properties of MF-CA3 synapses commonly studied by electrical stimulation.
Tomosyn knock-down reduces short-term plasticity at mossy fiber synapses
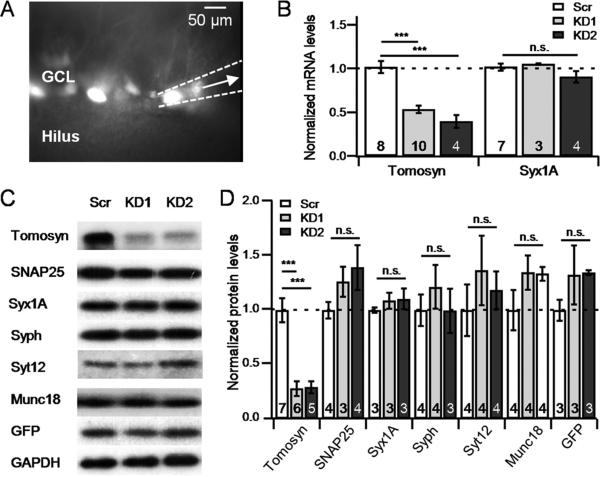
We next sought to determine the role of tomosyn at MF-CA3 synapses by introducing an anti-tomosyn1 shRNA sequence into DGCs. To control for potential off-targets effects, we used two different shRNA sequences (KD1/KD2), and compared them to a scrambled, non-targeting control sequence (Scr). To validate the efficacy of the shRNA sequences in reducing tomosyn levels, we first transduced DGCs in vivo (P21-22) with lentiviral particles that included KD1, KD2 or Scr under control of the U6 promoter. The lentiviral construct also included EGFP to visualize the transduced neurons. We then prepared hippocampal slices 19-21 days post-injection, and performed single cell quantitative RT-PCR (qRT-PCR) by suctioning primarily EGFP-expressing cells using a glass pipette (Figure 2A), and measuring tomosyn mRNA from these neurons. Results from this analysis confirmed that tomosyn mRNA levels dropped by roughly 50% in slices expressing either one of the two KD sequences, in comparison with slices expressing Scr (p<0.0001; Figure 2B). No change in the mRNA levels of syntaxin1A (Figure 2B) was found following tomosyn KD. In parallel, we verified by Western blot that the reduction in tomosyn mRNA levels translates to reduced protein levels (p<0.01; Figure 2C,D) but does not alter the expression level of other presynaptic proteins such as SNAP25, syntaxin1A, synaptophysin, synaptotagmin12 or munc18 (Figure 2C,D). It is therefore likely that our knockdown strategy specifically reduces tomosyn1 levels from DGCs.
Figure 2. Lentiviral-mediated knockdown of tomosyn effectively reduces tomosyn levels in the dentate gyrus.
A. GFP fluorescence image of a transduced hippocampal slice, showing a single transduced cell (white arrow) being collected into the pipette B. RT-PCR analysis of mRNA extracted from cells expressing GFP reveals a significant decrease in tomosyn mRNA levels in KD1/2-infected cells but not of Syntaxin1A. C-D. Western blot (C), and summary data (D) of KD1/2 or Scr-transduced hippocampal neuron in a dissociated culture confirms a significant reduction in tomosyn protein levels following tomosyn-KD, but not of other synaptic proteins. Numbers at the bottom of each bar indicate the number of individual repetitions for each measurement. Syx1A – syntaxin1A, Syph – synaptophysin, Syt12 – synaptotagmin12; Data are presented as mean ± S.E.M; n.s., not significant.
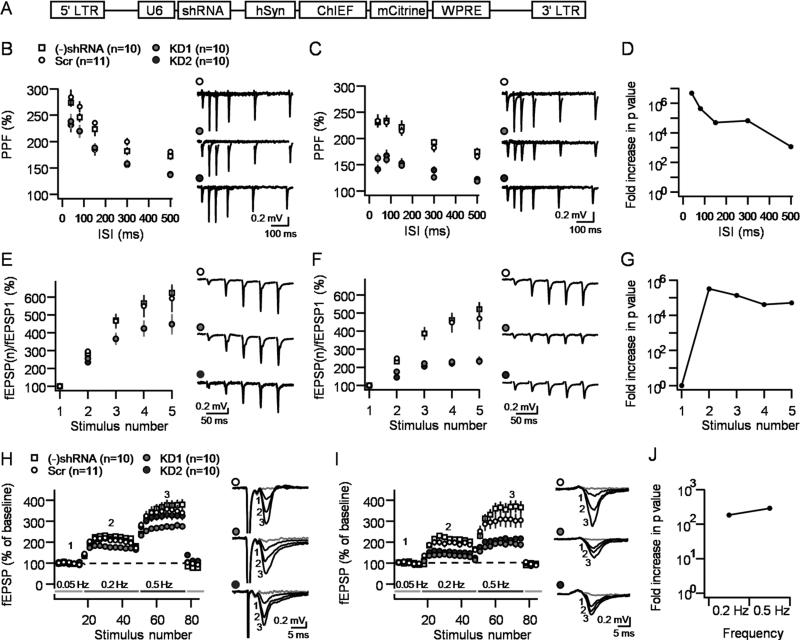
To examine the effects of tomosyn KD on MF-CA3 synaptic function, we next injected young mice (P21-35) with lentiviral vectors carrying the ChIEF construct along with KD1, KD2 or Scr sequences (Figure 3A). By this means we were able to optogenetically activate transduced DGCs and measure fEPSPs from S. lucidum (Figure 3B-J), and AMPA receptor-mediated EPSCs from individual CA3 pyramidal neurons (Figure 4). We hypothesized that if tomosyn acts as a negative regulator of Pr, then its removal from MF terminals should increase basal Pr, which would manifest as a decrease in STP (Zucker and Regehr, 2002). We also reasoned that such a decrease in facilitation would be better detected using optical stimulation given that this mode of stimulation is expected to only activate cells with reduced tomosyn levels, whereas electrical stimulation would likely recruit a mixed population of both transduced and non-transduced DGCs. Therefore, each slice was electrically and optically stimulated, allowing for the comparison between the two modes of stimulation. Consistent with both predictions, tomosyn KD was associated with reduced MF-STP compared with the Scr group and this reduction was much more prominent when optical stimulation was used. Synaptic responses elicited by electrical stimulation in tomosyn KD slices showed reduced PPF compared to controls (Figure 3B), while burst-induced facilitation showed just a trend (Figure 3E) and LFF was only reduced by KD1 but not KD2 (Figure 3H). In stark contrast, optically-elicited responses showed robust differences between both KD groups and the Scr group. PPF was strongly attenuated at all ISIs (Figure 3C), and both burst-induced facilitation and LFF were significantly reduced (Figure 3C,F,I; p<0.0001 for all three parameters). Additional analysis showed that the use of optical vs electrical stimulation in the same slices was associated with an increase in significance levels (p value) several orders of magnitude greater for all three paradigms (Figure 3.D,G and J), demonstrating the advantage of the combined optogenetics-KD strategy. No difference was observed between viruses containing the Scr sequence and those lacking any shRNA sequence in all measurements and under both forms of stimulation (Figure 3B-C,E-F,H-I). The effects of knocking down tomosyn on MF-CA3 STP were also assessed by monitoring optically-evoked, AMPA receptor-mediated EPSCs in CA3 pyramidal neurons. We found that PPF, burst-induced facilitation and LFF were all significantly reduced in both KD1 and KD2 slices compared to Scr slices (Figure 4; p<0.01 for all measurements). Together, these results indicate that tomosyn KD significantly reduces MF-STP, and also highlights the potential of a combined optogenetics-KD strategy to study the role of presynaptic proteins in transmitter release and synaptic plasticity.
Figure 3. Optogenetic activation reveals major decrease in MF-CA3 short-term plasticity following tomosyn KD.
A. Diagram showing the genetic sequence packaged by the lentivirus used in this study. B,C. PPF of extracellular field potentials (fEPSPs) elicited by electrical (B) or optical (C) stimulation in the same slice under four conditions: naïve slices, non-targeting shRNA (Scr), KD1 and KD2. D. Dividing the p values of each ISI in the electrical stimulation by its optical counterpart reveals an increase of 3-7 orders of magnitude in significance levels. E,F. Reduced burst-induced facilitation in tomosyn KD slices under electrical (E) or optical (F). G. The statistical significance levels increased by more than five orders of magnitude for each stimulus in the burst. H,I. Reduced LFF in tomosyn KD slices observed under electrical (H) or optical (I) stimulation. Numbers in the representative traces correspond to numbers in the summary plots. J. The statistical significance levels increased by more than two orders of magnitude for each frequency step. Stimulus artifact was removed from traces in panels B and E for better visualization. fEPSP responses 10 minutes after DCG-IV application are denoted in gray. Number of slices for each condition is shown in brackets. Data are presented as mean ± S.E.M.
Figure 4. Reduction in optically-induced STP is confirmed by whole-cell voltage clamp recordings.
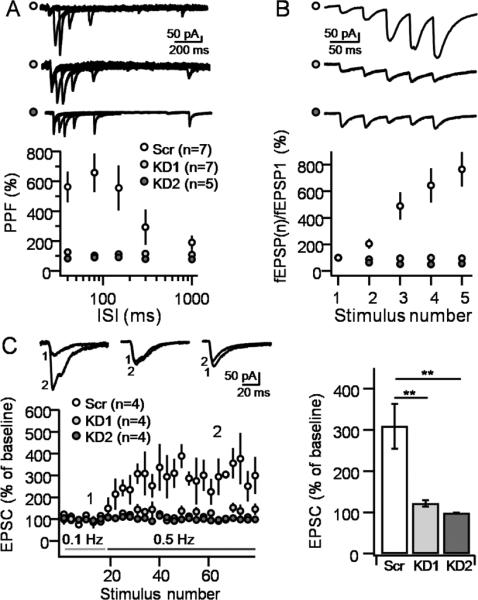
A. PPF, measured as the ratio of two optically-evoked EPSCs, was significantly reduced at all inter-stimulus intervals (ISIs) in tomosyn-KD slices. HFF (B) and LFF (C) were also significantly reduced following tomosyn knock-down. Traces in plot (C) were taken at times indicated by numbers in the time course plot. Number of slices for each condition is shown in brackets. Data are presented as mean ± S.E.M.
Tomosyn and long-term synaptic plasticity
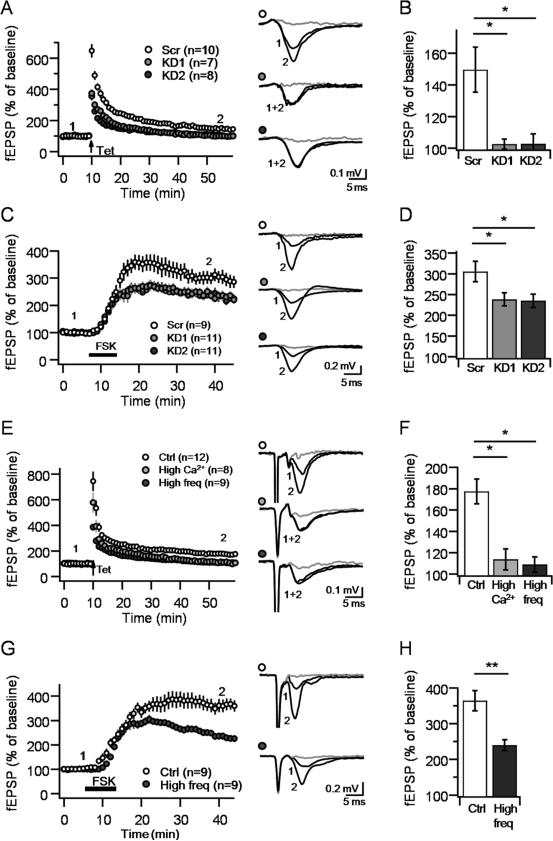
Previous studies have shown that PKA activation is necessary and sufficient to induce long-lasting increases of transmitter release at MF-CA3 synapses (Huang et al., 1994; Nicoll and Schmitz, 2005; Weisskopf et al., 1994). Tomosyn can be phosphorylated in vitro by PKA at S724, which could increase neurotransmitter release presumably by reducing tomosyn affinity to syntaxin (Baba et al., 2005). To test whether tomosyn contributes to PKA-mediated long-term plasticity, we examined both synaptically-induced MF-LTP (triggered by optical stimulation, see Experimental Procedures) and forskolin-induced potentiation (FSK-LTP) in hippocampal slices transduced with KD1, KD2 or Scr-ChIEF viruses. MF-LTP was robust in Scr slices but abolished in KD1 and KD2 slices (Scr: 150.3 ± 14.5%, n = 10; KD1: 104.1 ± 3.2%, n = 7, KD2: 107.5 ± 5%, n = 8; p<0.05, Figure 5A,B). Consistent with an increase in Pr, post-tetanic potentiation (PTP), calculated as the first three minutes after repetitive stimulation, was also reduced in tomosyn KD synapses (Scr: 517.1 ± 32.5%, KD1: 327.6 ± 17.2%, KD2: 279 ± 16.2%; p<0.0001). FSK-LTP was markedly reduced in both KD conditions, compared to Scr slices, but unlike MF-LTP, robust potentiation was still observed in these slices (Scr: 305.65 ± 24.8%, n = 9; KD1: 238.45 ± 15.63%, n = 11, KD2: 235.05 ± 16.25%, n = 11; p<0.05, Figure 5C,D). Thus, while both MF-LTP and forskolin-induced potentiation are impaired following tomosyn-KD, PKA-induced potentiation can still occur under reduced levels of presynaptic tomosyn.
Figure 5. Tomosyn KD impairs MF-LTP and PKA-induced synaptic potentiation.
A-B. Optically-induced MF LTP in Scr and KD1/2 slices (A). Bar plots of fEPSP changes 40-50 min after LTP show LTP was abolished in tomosyn KD slices. C-D. Forskolin-induced potentiation in Scr and KD1/2 slices (C). Bar plots of fEPSP changes 20-30 min after forskolin application show significantly weaker potentiation in tomosyn KD slices (D). E-H. Artificial increase in Pr by elevating extracellular Ca2+/Mg2+ ratio or baseline stimulation frequency abolished MF-CA3 LTP (E,F) and reduced FSK-LTP (G,H). Averaged representative traces are shown to the right of each time-course plot; traces were taken at times indicated by numbers in the panel, fEPSP responses 10 minutes after DCG-IV application are denoted in gray. Number of slices for each condition is shown in brackets. n.s., not significant. Number of slices for each condition is shown in brackets. Data are presented as mean ± S.E.M.
A potential explanation for these observations is that tomosyn phosphorylation is required for MF-LTP and FSK-LTP. Against this possibility, using FRET imaging in PC12 cells and tomosyn phosphomutants (Gladycheva et al., 2007), we found that constitutively phosphorylated (tomosynS724D) and non-phosphorylated (tomosynS724A) mutants did not affect tomosyn interaction with either syntaxin1A or SNAP25, as measured by changes in apparent FRET efficiency (p>0.05 for both proteins; Supplementary Figure 1). Alternatively, the increase in basal Pr at tomosyn-deficient synapses may occlude presynaptic MF-LTP and FSK-LTP. In support of this possibility, we found that increasing Pr artificially, either by elevating the extracellular (Ca2+/Mg2+) ratio from (2.5/1.3) to (3.5/0.3), or by changing the baseline stimulation frequency from 0.05 to 0.2 Hz (Supplementary Figure 2), abolished MF-LTP (Ctrl: 177.5 ± 11.6%, n=12; High Ca2+: 113.8 ± 9.8%, n=8; High frequency: 108.9 ± 7.2%, n=9; p<0.01; Figure 5E,F) and reduced FSK-LTP, as seen under tomosyn KD conditions (Ctrl: 364.1 ± 28.1%, n=9; High frequency: 239.4 ± 15.2%, n=9; p<0.01; Figure 5G,H). Together, these results indicate that an increase on basal Pr likely accounts for the MF-LTP and FSK-LTP deficits observed at tomosyn-deficient MF-CA3 synapses.
Discussion
In this study, we combined optogenetics with a shRNA KD strategy to selectively activate MF-CA3 synapses with reduced levels of tomosyn. We report that presynaptic forms of plasticity such as STP, LTP and FSK-induced potentiation, are all impaired at tomosyn deficient synapses. The most plausible explanation for these results is that tomosyn deficient synapses exhibit increased basal Pr. Our findings suggest that the high expression of tomosyn, normally observed in MFs, likely plays a crucial role in setting the characteristically low Pr at the MF-CA3 synapse (Lawrence et al., 2004; Salin et al., 1996), and that changes in tomosyn levels or function may have a significant impact in controlling synaptic transmission and plasticity.
Numerous studies have aimed to characterize the role of presynaptic proteins in neurotransmitter release. For the last two decades this task has largely relied on the use of global KO mice (Südhof, 2012). While certainly informative, major drawbacks of this approach are the risk of compensation during early development and the difficulty in focusing the manipulation on a specific region. More recently, in vivo KD using viral vectors have emerged as a complimentary strategy which can overcome both of these aforementioned limitations (Hommel et al., 2003). However, high transduction rates are required since electrical stimulation likely recruits both transduced and non-transduced cells. To circumvent this problem, we have developed an optogenetic-KD technique that selectively activates neurons expressing the shRNA sequence. This strategy enables a rapid, cell type-specific manipulation of protein levels, thereby eliminating the risk for developmental compensatory changes. Our findings demonstrate that optical stimulation can be efficiently used to study MF-CA3 function (Figure 1), but also, that the combined optogenetic-KD approach is an efficient way to investigate the role of presynaptic proteins in synaptic transmission and plasticity.
Previous studies have shown that tomosyn over-expression reduces synaptic transmission at various synapses (Barak et al., 2013a; Hu et al., 2013) however, the effects of tomosyn KO and KD have produced conflicting results (Baba et al., 2005; Cheviet et al., 2006; Gracheva et al., 2007; McEwen et al., 2006; Sakisaka et al., 2008; Zhang et al., 2006). Consistent with the high expression levels of tomosyn in MFs (Barak et al., 2010; Sakisaka et al., 2008), our findings showing a robust reduction in the magnitude of STP at tomosyn-deficient MF-CA3 synapses, strongly suggest that tomosyn is an important negative regulator of Pr at this synapse. We also found that MF-LTP is abolished in tomosyn-deficient synapses. Our observations that MF-LTP is also abolished under high Pr conditions, further support the notion that tomosyn down-regulation likely impairs MF-LTP through a similar mechanism. Of note, a previous study reported normal MF-LTP in tomosyn KO mice (Sakisaka et al., 2008). This apparent discrepancy could be due to compensatory changes likely occurring in KO mice. Another study showed that tomosyn over-expression via lentiviral transduction of DGCs did not alter MF-LTP or LFF (Barak et al., 2013a). However, as seen in our new study, electrical stimulation is sub-optimal when compared to optical stimulation and could have underestimated the actual effect of tomosyn on MF-CA3 synaptic function. It is precisely because of these limitations that we developed a combined KD/optogenetic strategy that allows us to assess transmission and plasticity at tomosyn-deficient synapses selectively.
Several factors determine Pr at synapses, including the amount of presynaptic Ca2+ influx via voltage-gated Ca2+ channels (VGCCs), the coupling between Ca2+ and the release sensors and the number of docked and primed vesicles (Atwood and Karunanithi, 2002). Our findings strongly suggest that tomosyn is a major player in determining the characteristically low Pr at MF-CA3 synapses. A previous study using KO mice reported that the presynaptic protein involved in vesicle priming Munc13-2 also plays a key role in regulating Pr at MF terminals (Breustedt et al., 2010). More recently, loose coupling between VGCCs and release sensors has emerged as an additional mechanism regulating Pr at MF terminals (Vyleta and Jonas, 2014). These presumably complementary mechanisms likely provide exquisite control of transmitter release at the MF-CA3 synapse, although their relative contribution under physiological conditions remains to be determined. The characteristically low basal Pr at MF-CA3 synapses is associated with robust short-term facilitation (Henze et al., 2000; Nicoll and Schmitz, 2005) that enables single granule cells to discharge post-synaptic CA3 during bouts of high-frequency activity. Our findings, showing that tomosyn down-regulation completely abolishes this facilitation, suggest that high expression levels of tomosyn are likely required to maintain a proper flow of information from the dentate gyrus to the CA3 area.
PKA activation potentiates MF-CA3 synaptic transmission (Weisskopf et al., 1994) but until recently, the PKA target(s) remained elusive. Down regulation of numerous presynaptic proteins, which are direct or indirect PKA targets, such as synapsin (Spillane et al., 1995), Rab3a (Castillo et al., 1997), Rim1α (Castillo et al., 2002; Kaeser et al., 2008; Yang and Calakos, 2010) and rabphillin (Schluter et al., 1999) did not alter forskolin-induced potentiation. A notable exception can be found in a recent study showing impaired forskolin-induced potentiation in synaptotagmin12 KO mice (Kaeser-Woo et al., 2013). PKA phosphorylation of tomosyn at serine 724 was shown to decrease its interaction with syntaxin (Baba et al., 2005) but the effect on synaptic transmission was unresolved (Baba et al., 2005; Sakisaka et al., 2008). In the present study we found no evidence that tomosyn phosphorylation at S724 affects the interaction with syntaxin1A and SNAP-25. Furthermore, by artificially elevating Pr, we were able to mimic the effects of tomosyn KD on both MF-LTP and FSK-LTP. Based on these findings we conclude that tomosyn does not significantly participate in PKA-mediated potentiation of MF-CA3 transmission.
In summary, we report a simple, but powerful approach to study the role of presynaptic proteins in basal transmission and synaptic plasticity. Tomosyn emerges as an important regulator of both basal synaptic transmission and presynaptic forms of plasticity. In support of this role in vivo is the recent observation that tomosyn levels are down-regulated in animals exposed to enriched environment and up-regulated in an Alzheimer's disease mouse model (Barak et al., 2013b).
Experimental Procedures
Generation of lentiviral particles
Lentiviral vectors were generated by insertion of the anti-tomosyn RNAi (KD1-GCACTGAGCGAGGAAACATAC, KD2- GGAACCATATGCTGTGGTTGT), or a scrambled control (Scr- CAGGAACGCATAGACGCATGA), into the 3rd generation lenti backbone pLL3.7 (Addgene #11795), using the XhoI and HpaI restriction sites, immediately following the U6 promoter. The sequence coding for the channelrhodopsin variant ChIEF (generously provided by Roger Tsien), tagged with a mCitrine fluorescent protein, was also inserted into the same plasmids and expressed under the human synapsin promoter. For lentivirus-mediated silencing of tomosyn in DGCs of intact mice, high-titer lentiviral stocks pseudotyped with the vesicular stomatitis virus G-protein (VSV-G) were produced in HEK-293T cells as previously described (Kutner et al., 2009). In brief; HEK-293T cells were transfected with lentiviral transfer construct pLL3.7.hSyn.ChIEF.mCitrine.WPRE, pLL3.7.U6.tomosynKD1/2.hSyn.ChIEF. mCitrine.WPRE or pLL3.7.U6.Scr.hSyn.ChIEF.mCitrine.WPRE and packaging constructs pMDLg-pRRE and pRSV-REV and pMD2.G envelope protein construct, by means of calcium phosphate transfection. Titers were determined by transducing HEK-293T cells with serial dilutions of concentrated lentivirus. Citrine fluorescence was evaluated by flow cytometry (FACSCalibur, Becton Dickinson Immunocytometry Systems) at 72 h after transduction; titers were 1 × 109 TU/ml.
Stereotactic injection of lentiviral constructs
All mice were treated in accordance with the principles and procedures of the Israel National Institute of Health and the United States National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals. Protocols were approved by the Institutional Animal Care and Use Committee of the Tel Aviv University and the Albert Einstein College of Medicine. For in vitro experiments viruses were delivered by stereotactic injection into the hippocampus of live C57BL6J mice (postnatal day 21-35) obtained from Harlan Laboratories or Charles River Laboratories, for experiments performed in Tel Aviv or New York, respectively. Mice were placed in a stereotaxic frame (RWD or Stoelting, IL), and anesthetized with isoflurane. Concentrated viral solution (1-2μL) was injected into two locations of the right dorsal hippocampus at a flow rate of 0.2 μL/min using a microinjection pump (Chymex, TX or Stoelting, IL). The injection siteswere defined by the following coordinates: anterior, 2.1 mm posterior to bregma, 1.6 mm from midline, 2.1 mm ventral from dura; and posterior, 3.1 mm posterior to bregma, 2.4 mm from midline, 2.7 mm ventral from dura. The needle was left in place for an additional 1 minute post-injection and gently withdrawn.
Hippocampal slice preparation
Acute transverse hippocampal slices (400 μm thick) were prepared from mice 8-14 days post injection (dpi). Briefly, the hippocampi were isolated and cutin a VT1000S or VT1200S vibrotome (Leica) in an extracellular solution containing (in mM) 215 sucrose, 2.5 KCl, 20 glucose, 26 NaHCO3, 1.6 NaH2PO4, 1 CaCl2, 4 MgCl2 and 4 MgSO4. Thirty minutes after sectioning, the cutting medium was gradually switched to an artificial cerebrospinal (ACSF) recording solution containing (in mM): 124 NaCl, 2.5 KCl, 26 NaHCO3, 1 NaH2PO4, 2.5 CaCl2, 1.3 MgSO4 and 10 glucose. All solutions were equilibrated with 95% O2 and 5% CO2 (pH 7.4). Slices were incubated for at least 60 min in ASCF solution before recordings.
Electrophysiology
Hippocampal slices were visualized using infrared differential interference contrast (IR/DIC) and mCitrine fluorescence. All slices included in this study exhibited strong fluorescence in dentate gyrus, hilus and stratum lucidum (Fig. 1A). Experiments were performed at 26.0 ± 1.0 °C in a submersion-type rec ording chamber perfused at ~1.5 ml/min with ASCF. All recordings were acquired while blind to the experimental condition, with the exception of three KD1 LTP recordings and all KD2 recordings.
MFs were activated either by electrical or optical stimulation. Electrical monopolar stimulation (square-wave voltage or current pulses, 0.1 ms pulse width) was delivered using a stimulus isolator (Isoflex, AMPI) connected to a patch-type pipette (~5 μm tip size) filled with ACSF and placed at the border between the dentate granule cell layer and hilus. In extracellular field recordings, optical stimulation was performed using a blue LED (470nm) connected to a 200 μm thick optic fiber (Prizmatix) that was directed toward the hilus and dentate gyrus, and away from the CA3 area (Fig. 1B). In whole-cell patch clamp experiments, a 473 nm laser beam (Prairie Technologies, Solid State laser) was fed through a 60× objective and directed at the S. Lucidum. The duration of light stimulation ranged from 0.5-5.0ms. Field excitatory postsynaptic potentials (fEPSPs) were recorded from stratum lucidum of the CA3 sub-region of the hippocampus using a patch pipette filled with 1M NaCl (2.5 MΩ) and an EPC10 amplifier (HEKA). Stimulation intensity was calibrated in order to elicit ~0.2 mV fEPSPs. Whole-cell recordings were obtained using a Multiclamp 700B amplifier (Molecular Devices), and borosilicate glass electrodes (2.8-3.8 MΩ resistance when filled with intracellular solution) filled with (in mM): 123 Cesium gluconate, 8 NaCl, 1 CaCl2, 10 EGTA, 10 HEPES, and 10 glucose; pH 7.3 (adjusted with KOH) and 280-290 mOsm. To block inhibitory transmission, 1 mM picrotoxin was included in the intracellular solution, and 0.5 μM GYKI 53655 was added to the ACSF in order to reduce recurrent activation of the CA3 network (Kwon and Castillo, 2008). To assess cell stability, series and input resistances were monitored with a −5 mV, 80 ms hyperpolarizing test-pulse and cells with >15% change in series resistance were excluded from analysis. In all experiments, baseline responses were collected at 0.05 Hz for all recordings, except for 5-pulse bursts, after which baseline was reduced to 0.025 Hz in order to avoid augmentation. MF-LTP was optically induced with 2 bouts of 125 pulses at 25 Hz separated by 20 seconds. Forskolin (25 μM) was bath-applied for 5 minutes after establishing at least 15 min stable baseline. At the end of each experiment, the group II metabotropic glutamate receptor agonist DCG-IV (1 μM) was added to the bath. Only synaptic responses showing more than 80% suppression by this agonist were included in the final analysis.
Quantitative real-time PCR and Western blot
qtPCR and western blot experiments were performed according to standard procedures and are described in the supplementary information.
Reagents
Reagents were bath applied following dilution into ACSF from stock solutions prepared in water or DMSO. DCG-IV (2S,2′R,3′R-2-[2′,3′-dicarboxycyclopropyl]glycine), forskolin, and GYKI 53655 were purchased from Tocris Bioscience (Bristol, UK). Picrotoxin and all salts for making ACSF and internal solution were obtained from Sigma-Aldrich. The final DMSO concentration was <0.01% total volume.
Data acquisition and statistical analysis
Electrophysiology data were acquired and analyzed with custom-written routines in Igor Pro 6.22A (Wavemetrics). Statistical significance was set to p<0.05 (*** indicates p<0.001, ** indicates p<0.01, and * indicates p<0.05 in all figures). Results are reported as the mean ± SEM. Student's paired and unpaired t-tests were used to assess differences between electrical and optical stimulation and one-way ANOVA test was used to assess all other differences. Significant differences in the ANOVA test were followed by a Tukey post-hoc test. The magnitude of LTP was determined by comparing baseline-averaged responses before induction with responses 40–50 min after induction. Forskolin-induced potentiation was determined by comparing baseline-averaged responses before and 20-30 min after forskolin washout. Averaged fEPSP and EPSC frequency facilitation was determined by comparing baseline-averaged responses to the average of the last 15 responses of the train (30 stimulations for fEPSPs and 60 stimulations for EPSCs).
Supplementary Material
Acknowledgements
This work was supported by the Israel Science Foundation Grant 730/11 (U.A), the BSF (Grant no. 2009279; U.A. and P.E.C.), and NIH grants MH081935 and DA017392 to P.E.C., and NS053978 to E.S. and U.A. A.R.R. was partially supported by a Ford Foundation Fellowship, and K.A. was supported by NIH-T32 NS007439. We thank Drs. Inna Slutsky, Ofer Yizhar and Thomas Younts for their helpful comments on the manuscript and Dr. Irit Gottfried for assistance throughout this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Y.B.S designed and produced viruses, and performed all extracellular recordings; A.R.R. assessed the efficiency of tomosyn-KD by quantitative real-time PCR; K.A. performed whole-cell recordings; A.D.L and E.L.S preformed FRET measurements; Y.B.S., U.A. and P.E.C. designed research; Y.B.S., A.R.R. K.A. and A.D.L analyzed data; Y.B.S., U.A. and P.E.C. wrote the paper. All authors read and edited the paper.
References
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- Atwood HL, Karunanithi S. Diversification of synaptic strength: presynaptic elements. Nature reviews. 2002;3:497–516. doi: 10.1038/nrn876. [DOI] [PubMed] [Google Scholar]
- Baba T, Sakisaka T, Mochida S, Takai Y. PKA-catalyzed phosphorylation of tomosyn and its implication in Ca2+-dependent exocytosis of neurotransmitter. The Journal of cell biology. 2005;170:1113–1125. doi: 10.1083/jcb.200504055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak B, Okun E, Ben-Simon Y, Lavi A, Shapira R, Madar R, Wang Y, Norman E, Sheinin A, Pita MA, et al. Neuron-specific expression of tomosyn1 in the mouse hippocampal dentate gyrus impairs spatial learning and memory. Neuromolecular Med. 2013a;15:351–363. doi: 10.1007/s12017-013-8223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak B, Shvarts-Serebro I, Modai S, Gilam A, Okun E, Michaelson DM, Mattson MP, Shomron N, Ashery U. Opposing actions of environmental enrichment and Alzheimer's disease on the expression of hippocampal microRNAs in mouse models. Transl Psychiatry. 2013b;3:e304. doi: 10.1038/tp.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak B, Williams A, Bielopolski N, Gottfried I, Okun E, Brown MA, Matti U, Rettig J, Stuenkel EL, Ashery U. Tomosyn expression pattern in the mouse hippocampus suggests both presynaptic and postsynaptic functions. Front Neuroanat. 2010;4:149. doi: 10.3389/fnana.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielopolski N, Lam AD, Bar-On D, Sauer M, Stuenkel EL, Ashery U. Differential interaction of tomosyn with syntaxin and SNAP25 depends on domains in the WD40 beta-propeller core and determines its inhibitory activity. The Journal of biological chemistry. 2014;289:17087–17099. doi: 10.1074/jbc.M113.515296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breustedt J, Gundlfinger A, Varoqueaux F, Reim K, Brose N, Schmitz D. Munc13-2 differentially affects hippocampal synaptic transmission and plasticity. Cereb Cortex. 2010;20:1109–1120. doi: 10.1093/cercor/bhp170. [DOI] [PubMed] [Google Scholar]
- Castillo PE. Presynaptic LTP and LTD of excitatory and inhibitory synapses. Cold Spring Harbor perspectives in biology. 2012;4 doi: 10.1101/cshperspect.a005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Janz R, Sudhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature. 1997;388:590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Cheviet S, Bezzi P, Ivarsson R, Renstrom E, Viertl D, Kasas S, Catsicas S, Regazzi R. Tomosyn-1 is involved in a post-docking event required for pancreatic beta-cell exocytosis. J Cell Sci. 2006;119:2912–2920. doi: 10.1242/jcs.03037. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Shirataki H, Sakisaka T, Asakura T, Ohya T, Kotani H, Yokoyama S, Nishioka H, Matsuura Y, Mizoguchi A, et al. Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20:905–915. doi: 10.1016/s0896-6273(00)80472-9. [DOI] [PubMed] [Google Scholar]
- Gladycheva SE, Lam AD, Liu J, D'Andrea-Merrins M, Yizhar O, Lentz SI, Ashery U, Ernst SA, Stuenkel EL. Receptor-mediated regulation of tomosyn-syntaxin 1A interactions in bovine adrenal chromaffin cells. The Journal of biological chemistry. 2007;282:22887–22899. doi: 10.1074/jbc.M701787200. [DOI] [PubMed] [Google Scholar]
- Gracheva EO, Burdina AO, Touroutine D, Berthelot-Grosjean M, Parekh H, Richmond JE. Tomosyn negatively regulates both synaptic transmitter and neuropeptide release at the C. elegans neuromuscular junction. The Journal of physiology. 2007;585:705–709. doi: 10.1113/jphysiol.2007.138321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsuzawa K, Lang T, Fasshauer D, Bruns D, Jahn R. The R-SNARE motif of tomosyn forms SNARE core complexes with syntaxin 1 and SNAP-25 and down-regulates exocytosis. The Journal of biological chemistry. 2003;278:31159–31166. doi: 10.1074/jbc.M305500200. [DOI] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nature medicine. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- Hu Z, Tong XJ, Kaplan JM. UNC-13L, UNC-13S, and Tomosyn form a protein code for fast and slow neurotransmitter release in Caenorhabditis elegans. Elife. 2013;2:e00967. doi: 10.7554/eLife.00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Li XC, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser-Woo YJ, Younts TJ, Yang X, Zhou P, Wu D, Castillo PE, Sudhof TC. Synaptotagmin-12 phosphorylation by cAMP-dependent protein kinase is essential for hippocampal mossy fiber LTP. J Neurosci. 2013;33:9769–9780. doi: 10.1523/JNEUROSCI.5814-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Kwon HB, Blundell J, Chevaleyre V, Morishita W, Malenka RC, Powell CM, Castillo PE, Sudhof TC. RIM1alpha phosphorylation at serine-413 by protein kinase A is not required for presynaptic long-term plasticity or learning. Proc Natl Acad Sci U S A. 2008;105:14680–14685. doi: 10.1073/pnas.0806679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A, Borst JG, Carlson BA, Kopp-Scheinpflug C, Klyachko VA, Xu-Friedman MA. How do short-term changes at synapses fine-tune information processing? J Neurosci. 2012;32:14058–14063. doi: 10.1523/JNEUROSCI.3348-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nature protocols. 2009;4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- Kwon HB, Castillo PE. Role of glutamate autoreceptors at hippocampal mossy fiber synapses. Neuron. 2008;60:1082–1094. doi: 10.1016/j.neuron.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ, Grinspan ZM, McBain CJ. Quantal transmission at mossy fibre targets in the CA3 region of the rat hippocampus. The Journal of physiology. 2004;554:175–193. doi: 10.1113/jphysiol.2003.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophysical journal. 2009;96:1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen JM, Madison JM, Dybbs M, Kaplan JM. Antagonistic regulation of synaptic vesicle priming by Tomosyn and UNC-13. Neuron. 2006;51:303–315. doi: 10.1016/j.neuron.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nature reviews. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Sakisaka T, Yamamoto Y, Mochida S, Nakamura M, Nishikawa K, Ishizaki H, Okamoto-Tanaka M, Miyoshi J, Fujiyoshi Y, Manabe T, et al. Dual inhibition of SNARE complex formation by tomosyn ensures controlled neurotransmitter release. The Journal of cell biology. 2008;183:323–337. doi: 10.1083/jcb.200805150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter OM, Schnell E, Verhage M, Tzonopoulos T, Nicoll RA, Janz R, Malenka RC, Geppert M, Sudhof TC. Rabphilin knock-out mice reveal that rabphilin is not required for rab3 function in regulating neurotransmitter release. J Neurosci. 1999;19:5834–5846. doi: 10.1523/JNEUROSCI.19-14-05834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane DM, Rosahl TW, Sudhof TC, Malenka RC. Long-term potentiation in mice lacking synapsins. Neuropharmacology. 1995;34:1573–1579. doi: 10.1016/0028-3908(95)00107-h. [DOI] [PubMed] [Google Scholar]
- Südhof TC. The presynaptic active zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommershauser J, Schneggenburger R, Zippelius A, Neher E. Heterogeneous presynaptic release probabilities: functional relevance for short-term plasticity. Biophysical journal. 2003;84:1563–1579. doi: 10.1016/S0006-3495(03)74967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyleta NP, Jonas P. Loose coupling between Ca2+ channels and release sensors at a plastic hippocampal synapse. Science (New York, NY. 2014;343:665–670. doi: 10.1126/science.1244811. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science (New York, NY. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Williams AL, Bielopolski N, Meroz D, Lam AD, Passmore DR, Ben-Tal N, Ernst SA, Ashery U, Stuenkel EL. Structural and functional analysis of tomosyn identifies domains important in exocytotic regulation. The Journal of biological chemistry. 2011;286:14542–14553. doi: 10.1074/jbc.M110.215624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Calakos N. Acute in vivo genetic rescue demonstrates that phosphorylation of RIM1alpha serine 413 is not required for mossy fiber long-term potentiation. J Neurosci. 2010;30:2542–2546. doi: 10.1523/JNEUROSCI.4285-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Matti U, Melamed R, Hagalili Y, Bruns D, Rettig J, Ashery U. Tomosyn inhibits priming of large dense-core vesicles in a calcium-dependent manner. Proc Natl Acad Sci U S A. 2004;101:2578–2583. doi: 10.1073/pnas.0308700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Lilja L, Mandic SA, Gromada J, Smidt K, Janson J, Takai Y, Bark C, Berggren PO, Meister B. Tomosyn is expressed in beta-cells and negatively regulates insulin exocytosis. Diabetes. 2006;55:574–581. doi: 10.2337/diabetes.55.03.06.db05-0015. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.