Abstract
Ever since its first description in 1918, Dientamoeba fragilis has struggled to gain recognition as a significant pathogen. There is little justification for this neglect, however, since there exists a growing body of case reports from numerous countries around the world that have linked this protozoal parasite to clinical manifestations such as diarrhea, abdominal pain, flatulence, and anorexia. A number of studies have even incriminated D. fragilis as a cause of irritable bowel syndrome, allergic colitis, and diarrhea in human immunodeficiency virus patients. Although D. fragilis is most commonly identified using permanently stained fecal smears, recent advances in culturing techniques are simplifying as well as improving the ability of investigators to detect this organism. However, there are limitations in the use of cultures since they cannot be performed on fecal samples that have been fixed. Significant progress has been made in the biological classification of this organism, which originally was described as an ameba. Analyses of small-subunit rRNA gene sequences have clearly demonstrated its close relationship to Histomonas, and it is now known to be a trichomonad. How the organism is transmitted remains a mystery, although there is some evidence that D. fragilis might be transmitted via the ova of the pinworm, Enterobius vermicularis. Also, it remains to be answered whether the two distinct genotypes of D. fragilis recently identified represent organisms with differing virulence.
INTRODUCTION
Since the first description of Dientamoeba fragilis by Jepps and Dobell in 1918 (65) this ameboid organism has escaped the interest of most clinicians and diagnostic microbiologists. This is reflected in a variety of descriptions conferred on the organism, such as: “a neglected cause of diarrhea” (49), “an unusual intestinal pathogen” (12), “an emerging protozoal infection” (129), and “an enigma shrouded in the mysteries of clinical parasitology” (140).
Jepps and Dobell (65) considered the nucleus of D. fragilis to be the characteristic feature of the organism, since they observed that the predominant form was binucleate, a feature which readily differentiated it from other human intestinal amebas. Interestingly, although they had isolated D. fragilis from seven persons, six of whom had a history of dysentery or chronic diarrhea, they felt that this observation was of no clinical significance. This conclusion was based on their observation that D. fragilis had a similar mode of nutrition to the nonpathogenic organisms Entamoeba coli and Endolimax nana, in contrast to Entamoeba histolytica, which was then considered to be a “tissue parasite.” They proposed that humans were aberrant hosts, in which cysts did not develop, and suggested that D. fragilis had a true animal host in which it was capable of encystation. Unfortunately, there is still no evidence to support the existence of a natural host besides humans nor has a cystic stage of D. fragilis ever been convincingly demonstrated. Furthermore, the lack of a suitable animal model that is capable of supporting the life cycle of D. fragilis and that develops similar clinical symptoms has severely hindered more detailed studies of the biology of the organism.
The seeds of doubt concerning the pathogenicity of D. fragilis were unfortunately planted by Jepps and Dobell (65) and nourished by Dobell and O'Conner (38) and account at least partially for the lingering resistance in many clinical circles to accept the disease-causing potential of this organism. However, the evidence supporting the pathogenicity of D. fragilis is too convincing to justify the continued neglect of this parasite as a cause of diarrhea, abdominal pain, flatulence, fatigue, and anorexia, the symptoms most commonly observed in patients infected with this organism (68). Indeed, the organism has been isolated from and associated with clinical symptoms in patients from numerous countries throughout the world (140). Perhaps the most striking reason to consider D. fragilis a potential pathogen is that it can be easily treated and that the great majority of patients show significant clinical improvement thereafter (35, 49).
TAXONOMY
The name Dientamoeba fragilis refers to the fact that it is an enteric ameba with the curious characteristic of being binucleate and that it tends to degenerate rapidly after excretion in stool (65). It was also classified as an ameba by Chatton (20), who included it in the family Entamoebidae, where it remained for the best part of 50 years. However, doubts about its affinities were raised by Dobell (36), when he noted the presence of the “centrodesmus” and the great similarity of D. fragilis at the light microsopic level to Histomonas meleagridis (see also “Morphology” below). Histomonas was, and is, accepted as a trichomonad flagellate despite its tendency to lose its flagellum in culture or when it invades tissues. Dobell (36) strongly suggested that Dientamoeba and Histomonas were closely related and that D. fragilis was an aberrant flagellate. This relationship was formalized when Grassé (51) removed Dientamoeba from the Entamoebidae and created the family Dientamoebidae to contain these two genera.
Further morphological support for the classification of D. fragilis within the trichomonad flagellates had to await the transmission electron microscopy studies by Camp et al. (13), who confirmed Dobell's observations. Molecular support for the relationship was first obtained by Dwyer (39, 40), who showed substantial antigenic similarities among Dientamoeba, Histomonas, and Trichomonas to the exclusion of Entamoeba species.
Another 20-year gap ensued until DNA studies of Dientamoeba commenced. Phylogenetic analysis of the D. fragilis small-subunit rRNA gene sequence (115), using the same strain that had been studied at the morphological level by Camp et al. (13) (strain Bi/pa; ATCC 30948), unequivocally confirmed its trichomonad affinities; more recently, analysis of the same gene from H. meleagridis confirmed the close and specific relationship between Histomonas and Dientamoeba (48). The latter investigation hinted at a link between Histomonas/Dientamoeba and the genus Tritrichomonas, but this remains to be confirmed.
So where is Dientamoeba classified today? The organism presently resides in the phylum Parabasala, class Trichomonadea, family Trichomonadidae, and possibly the subfamily Tritrichomonadinae (48). The systematics of the parabasalids is, however, in need of revision, and the specifics of Dientamoeba classification may change. Its affinities to Histomonas and other trichomonads will not.
At the other end of the taxonomic scale, there are very different questions. How many species of Dientamoeba are there? 2. Is D. fragilis of human origin a single species?
At the morphological level, amoeboid organisms present a serious problem because there are very few phenotypic characteristics on which to base a species diagnosis. Indeed, morphology has been superseded by molecular markers in the description and identification of species in some genera of amoeboid organisms, for example Entamoeba (34) and Naegleria (31). Although D. fragilis has been found in nonhuman primates, this diagnosis is based only on morphology (59, 73, 83). Whether these organisms represent distinct species within the genus awaits further information. However, attempts to infect a macaque intrarectally with human D. fragilis were unsuccessful (36). As far as we are aware, no other species in the genus Dientamoeba have been described.
Some molecular data do exist to address the second question. Using the approach of examining restriction fragment length polymorphisms of the D. fragilis small-subunit rRNA gene (riboprinting), Johnson and Clark (66) identified the existence of two genetically distinct types of D. fragilis among 12 isolates from humans. An additional 90 or more isolates have subsequently been studied using the same method (J. J. Windsor, unpublished data). In the latter set of samples only one genotype has been found. The rarer of the two genotypes was found in only two cases; one of these strains happens to be the isolate Bi/pa studied by Camp et al. (13) and Silberman et al. (115). This indicates that the results of studies using isolate Bi/pa may not be representative of the species as a whole. The degree of sequence divergence between the ribosomal genes of the two genotypes is estimated to be approximately 2% (66, 94). Whether this constitutes a species level of divergence in protozoa is a matter for debate, and no consensus exists among researchers in the field.
The significance of the existence of two genetically distinct forms of D. fragilis deserves to be investigated further. Is one form more virulent than the other, and could this possibly contribute to the differences in clinical perceptions regarding the organism's pathogenicity? Results from the largest study so far have found only one genotype in both symptomatic and asymptomatic individuals (94). The rarity of the second genotype will make investigation of its role in disease difficult.
MORPHOLOGY
Most of the detailed light microscopic descriptions of D. fragilis date back to the early and mid-1900s. Using a camera lucida, the parasitologists of the time produced surprisingly detailed plates (8, 26, 65, 133, 134, 135, 136, 137). There are subtle differences in the descriptions since some workers prepared material direct from feces (57, 133, 134, 135, 136, 137) whereas others used material from culture (36). Dobell (36) considered D. fragilis in feces to be degenerate and therefore not a true morphological representation.
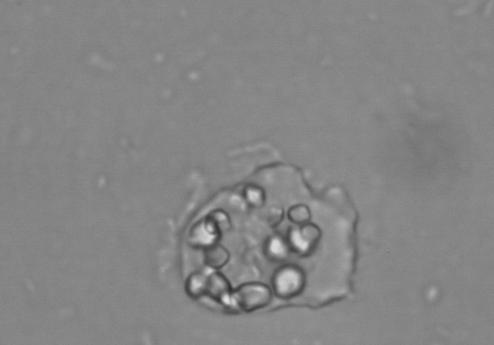
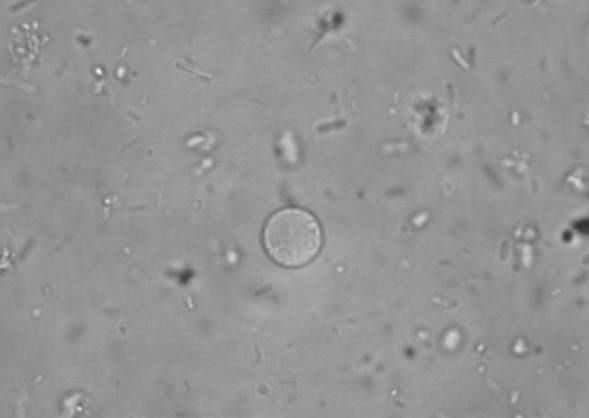
In direct saline preparations, D. fragilis usually appears rounded and shows a wide variation in size. In the original description by Jepps and Dobell (65), the size range given was 3.5 to 12 μm. Much larger sizes have been found in culture (20 to 40 μm) (114). The size range of D. fragilis in culture overlaps that of E. histolytica, E. hartmanni, and Endolimax nana (102, 107). The nuclei of D. fragilis are not visible in saline or iodine preparations, although food vacuoles or inclusions may be seen. D. fragilis moves by using thin, hyaline, leaf-like pseudopodia, which are irregularly lobed (Fig. 1) (65). Hakansson (55), examining his own freshly evacuated stool specimen, found rounded trophozoites of D. fragilis. Only after 5 to 10 min at room temperature did they recover from this temporary “paralysis” and display the characteristic fan-shaped motility, with lobes and indentations. Unlike Entamoeba trophozoites, no flow of endoplasm into the pseudopodia has been observed in D. fragilis, and while the edge is constantly changing, with sharp points appearing, no progression is seen (55, 56).
FIG. 1.
D. fragilis growing in Robinson's culture, showing ingested rice starch and fine leaf-like pseudopodia. magnification, ×400.
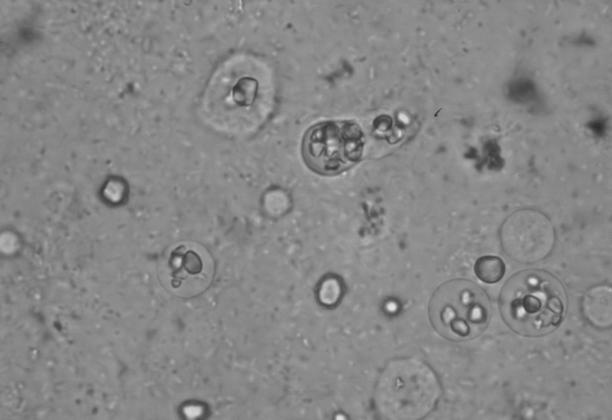
Jepps and Dobell (65) found that 80% of D. fragilis trophozoites in permanently stained fecal smears were binucleate and 20% were mononucleate. This percentage can vary considerably, even in stool samples taken from the same patient on different days (134). Although seen less frequently, some trophozoites have been described with as many as four or five nuclei (36, 81, 136). The diameter of the nuclei varies from 1 to 3 μm but depends largely on the size of the trophozoite (65, 137). Internally, the nuclei appear fragmented, usually containing four to eight granules, without peripheral chromatin (Fig. 2) (137). Often, one of the granules is larger than the others and stains more deeply (8, 65, 133). The binucleate form of D. fragilis is the typical stage observed. Mononucleated trophozoites of D. fragilis are therefore recently divided forms, produced by the process of binary fission, and are slightly smaller than the binucleates. The division is by simple constriction of the cell body. Nuclear division is found only in mononucleated trophozoites (36, 135). Dobell (36) described a “connecting thread” which joined the two nuclei together. He termed this a “centrodesmus” and could find no trace of it in mononucleated organisms. Wenrich (135) termed this structure a “post-division desmose,” believing it to arise from an intranuclear division centre. Dobell, however, thought that this organelle permanently linked the nuclei. Dobell's review (36) of the morphology of D. fragilis and its comparison to that of the turkey pathogen Histomonas meleagridis was the defining publication of the era and was the first paper to acknowledge the flagellate attributes of D. fragilis and its morphological similarities to H. meleagridis.
FIG. 2.
Iron-hematoxylin-stained smear of D. fragilis showing pleomorphic trophozoites. Note the characteristic fragmented nuclei and the very small mononucleated trophozoite in the center magnification, ×1,000.
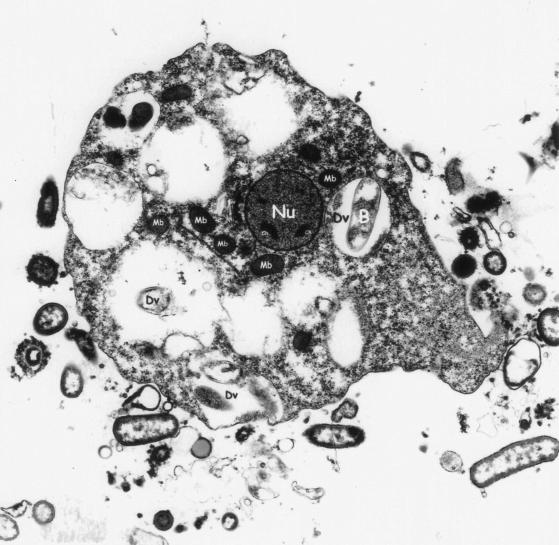
Curiously, only a handful of papers have been published on the morphology of D. fragilis on the basis of transmission electron microscopy (13, 91, 112, 113). Camp et al. (13) published a comprehensive study of the binucleate stage of D. fragilis strain Bi/pa. This paper confirmed many of the light microscopic observations of the flagellate characteristics of D. fragilis by Dobell (36). An extranuclear spindle is found between the nuclei, originating from polar complexes adjacent to one of the nuclei. These structures are homologous to the atractophores described in hypermastigotes (61). Parabasal filaments extend laterally to the external surface of the atractophores. Extensive Golgi complexes overlie the filaments and are very similar to the parabasal apparatus seen in trichomonads and hypermastigotes. This spindle is composed of two bundles of approximately 30 to 40 microtubules. One bundle appears at some distance from the nucleus, whereas the other is juxtanuclear and is often seen in a groove of the nuclear envelope. The nuclear structure of D. fragilis more closely resembles that of trichomonad flagellates rather than that of Entamoeba spp. Chromatin bodies or granules are often seen in the nucleoplasm (Fig. 3 and 4), and the nuclear envelope consists of two membranes (Fig. 5).
FIG. 3.
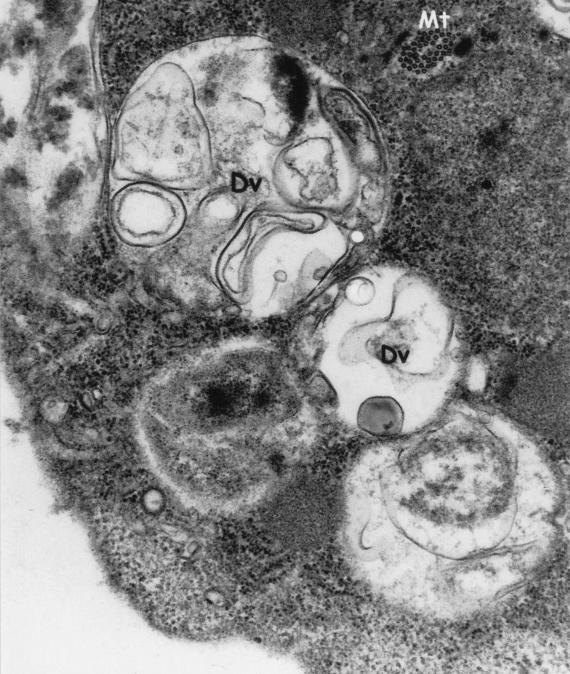
Electron micrograph of a mononucleated trophozoite of D. fragilis. The nucleus (Nu) and chromatin (Ch) are labeled. Electron-dense microbody-like inclusions surround the nucleus. Digestive vacuoles (Dv) can be clearly seen, one of which contains an ingested bacterium (B). Magnification, ×5,200.
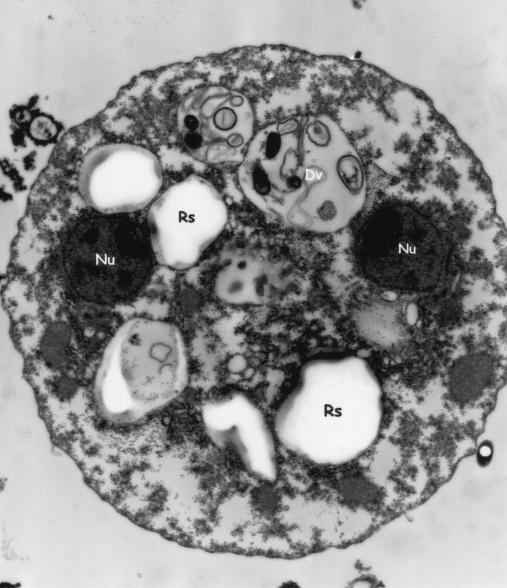
FIG. 4.
Electron micrograph of a binucleated trophozoite of D. fragilis. Digestive vacuoles (Dv) contain either myelin or rice starch (Rs). Magnification, ×25,000.
FIG. 5.
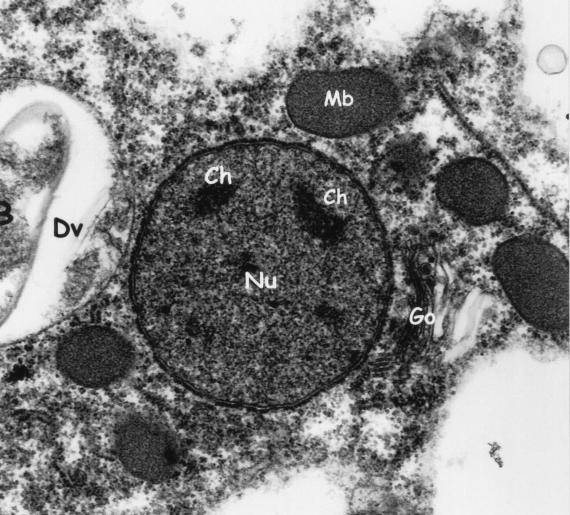
Electron micrograph demonstrating chromatin bodies (Ch) in the nucleus (Nu), surrounded by a double nuclear membrane. Note the microbody-like (Mb) inclusions, digestive vacuole (Dv), ingested bacteria (B), and Golgi apparatus (Go). Magnification, ×15,500.
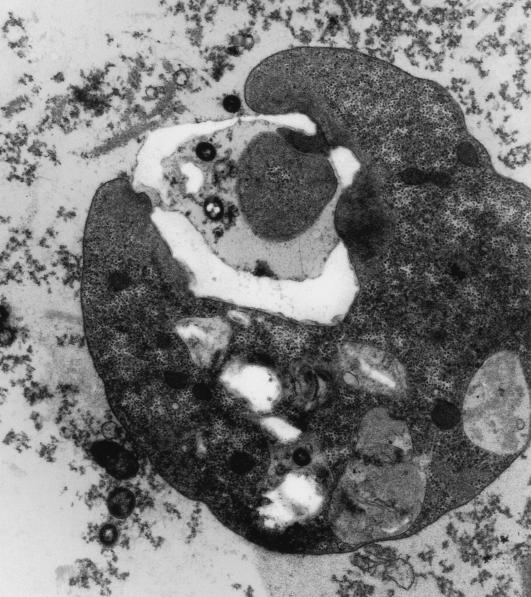
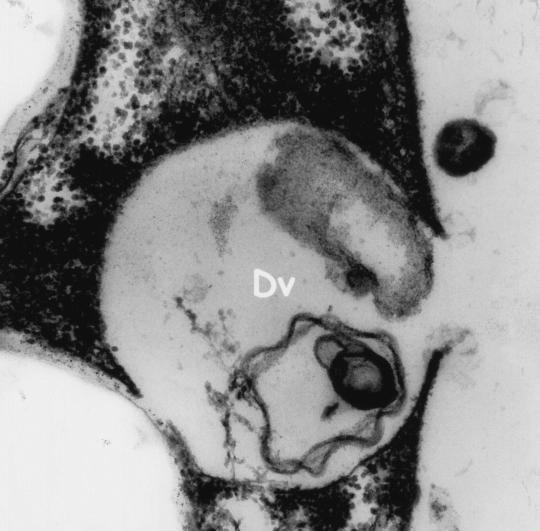
Electron microscopy revealed electron-dense rounded inclusions in the cytoplasm that were termed “microbody-like” and were presumed to be homologous to the paraxostylar granules of trichomonads (13) (Fig. 3). These inclusions were subsequently recognized as being hydrogenosomes (82). Digestive granules are also commonly found in the cytoplasm and may contain myelin, bacteria, or rice starch (Fig. 3 and 6). D. fragilis feeds by phagocytosis (Fig. 7), and waste products are released from the digestive vacuoles by exocytosis (Fig. 8).
FIG. 6.
Electron micrograph of D. fragilis demonstrating digestive vacuoles (Dv) containing myelin. Magnification, ×21,000.
FIG. 7.
Electron micrograph of D. fragilis exhibiting phagocytosis. Magnification, ×6,600.
FIG. 8.
Electron micrograph of D. fragilis exhibiting exocytosis. Magnification, ×28,500.
CLINICAL OVERVIEW
Eighty-five years after its first description, although D. fragilis is accepted as a true pathogen in some countries and infected patients are treated, it is still struggling to gain acceptance as a legitimate pathogen in many others. This is a result of D. fragilis at times being found in patients who exhibited no apparent clinical symptoms (24) and often being identified in patients coharboring other suspect pathogens. Robertson (100) described a young female patient who was admitted to hospital with a history of recurrent attacks of diarrhea and abdominal pain. D. fragilis was isolated from a series of her stool specimens, which also contained E. histolytica, Trichomonas spp., and Blastocystis hominis, as well as Endolimax nana and spirochetes. In the same year, Thomson and Robertson (128) reported a case involving a 38-year-old man who had dysentery. Although D. fragilis was isolated from this patient, other organisms were also found, including Entamoeba coli, Trichuris trichura and B. hominis. Due to the coinfections in these two patients, there was no sound basis to support a primary disease-causing role for D. fragilis. D. fragilis was subsequently found in stool samples from a healthy 3-year-old girl (125) and in epidemiological studies from Canada (96), England (64), and South Africa (42), but strong evidence suggestive of its pathogenic potential was not presented.
Interestingly, Wenrich et al. (138) reported that 4.3% of college freshmen were infected with D. fragilis in a professional school in Philadelphia. This incidence of infection was similar to that of E. histolytica, but, interestingly, more students with D. fragilis had clinical symptoms than did those with E. histolytica. Das Gupta (30) also identified D. fragilis in a Bengali man in India who had intermittent attacks of diarrhea, but Hakansson (55) was the first to propose that D. fragilis was more than just an innocent commensal organism. He reported a clinical case involving a 48 year-old physician who had severe colitis. D. fragilis was observed in large numbers at the onset of the illness, and variations in its abundance corresponded to the severity of clinical symptoms. After several treatments with carbarsone, the patient's condition improved and the infection with D. fragilis disappeared. Subsequently, there have been numerous reports that have linked D. fragilis infections with clinical symptoms that subsided only after the elimination of the organism (25, 35, 56, 72, 80, 99, 103, 104, 137).
Yoeli (147) described nine patients who suffered from acute intestinal signs such as explosive diarrhea, severe abdominal pain, cramps, nausea, vomiting, mild fever, and general fatigue. In all of these patients, large numbers of D. fragilis organisms were observed in the absence of any other pathogens. Numerous reports from many different parts of the world continued to substantiate the association of D. fragilis with clinical symptoms, principally abdominal pain, diarrhea, nausea, vomiting, and fatigue (1, 3, 12, 27, 32, 53, 92, 113, 114, 143, 146).
Studies have also demonstrated links between this parasite and urticaria (146), biliary infections (127), pruritus (116), colitis (111), allergic colitis (28), irritable bowel syndrome (6), and diarrhea in people infected with human immunodeficiency virus (75, 78). Of particular significance is the observation that infections in children appear to be very common. Keystone et al. (71) reported that over 8% of 900 children studied in the Toronto area were positive for D. fragilis. Also, some authors have reported that infections in children are more often associated with clinical symptoms than are infections in adults (3, 54, 85, 117); children are also more often reported to exhibit peripheral eosinophilia (2, 93, 97, 98, 117, 134).
Despite the significant number of studies that have incriminated D. fragilis as a legitimate enteric pathogen, it is far too seldom included in the differential diagnostic repertoire of intestinal pathogens by both practicing physicians and diagnostic laboratories in many countries.
PATHOLOGY
In parallel with our poor understanding of the pathogenicity of D. fragilis, only a small number of studies have presented findings relevant to the pathological consequences of infections with this organism. The first reported pathological study performed on the appendixes of four patients (ranging in age from 20 to 28 years of age) infected with D. fragilis showed distinct similarities (11). In each case there was marked fibrosis of the subserosa of the appendixes and trophozoites were seen in the lumen, with absence of tissue invasion. Of particular relevance was the observation that in each case there were ingested red blood cells within the D. fragilis organisms. The authors considered that this was a hallmark feature of pathogenic potential, since pathogenic amebae such as E. histolytica ingest erythrocytes whereas the nonpathogenic Entamoeba coli and Endolimax nana do not. A critical analysis of these pathological findings, however, did not permit D. fragilis to be incriminated as the causative agent of the fibrosis because in three of the four cases the appendixes also contained worm ova, larvae, or adults that also could also have potentially been responsible for these lesions. In a more extensive study of appendixes containing D. fragilis, Swerdlow and Burrows (124) examined an additional 11 organs and also reported extensive fibrous connective tissue of the submucosa. They found a variety of lesions, ranging from acute suppurative appendicitis to lymphoid hyperplasia to pure fibrosis. They felt that D. fragilis probably acted as a low-grade irritant, causing a chronic inflammatory reaction that resulted in fibrosis. However, only three of the appendixes from this study had pure D. fragilis infections, making it difficult to conclude that D. fragilis was solely responsible for the underlying fibrosis.
Shein and Gelb (111) reported finding multiple punctate ulcers on endoscopic examination of a female patient who had a history of chronic abdominal pain for 1 year and a sudden production of blood-streaked diarrhea. A biopsy of her rectum revealed shallow ulcerations with evidence of acute and chronic inflammation. Trichrome staining of mucosal aspirates revealed large numbers of D. fragilis organisms in the absence of any other known pathogens. However, more recently described pathogens such as the coccidia and microsporidia might have been overlooked. Three additional studies (92, 110, 120) reported inflammatory changes of the rectum and sigmoid colon in patients with D. fragilis infections. However, many of these patients had mixed infections and many had no pathological abnormalities.
A more recent study (28) reported a case of eosinophilic colitis in a female patient harboring D. fragilis. The patient was documented as having bovine protein allergy with intermittent episodes of diarrhea and abdominal pain, despite receiving an appropriate diet. After the patient was treated with iodoquinol and the parasite was eliminated, the symptoms disappeared.
In contrast to the findings of the above studies, Cerva et al. (15) were unable to establish any relationship between the presence of D. fragilis and any pathological abnormalities in the appendixes of the patients in their study, nor were they able to detect red blood cells in any of the D. fragilis organisms contained within the lumen of the appendixes. Certainly, the lack of an animal model hampers our ability to shed light on the exact pathological manifestations caused by D. fragilis.
TRANSMISSION
The mode of transmission of D. fragilis has remained a mystery. Most intestinal protozoa that are transmitted via the fecal-oral route require a cyst stage in order for the organism to survive in the external environment. However, although a few authors have reported pseudocystic, precystic, or cystic stages of D. fragilis (52, 72, 95, 133), it is generally accepted that this parasite does not have a cyst form. It is likely that the cysts described by Kofoid (74) were merely rounded-up trophozoites. Wenrich (133) also described degenerating forms, which stained more intensely and were thought to be precystic forms or “pseudocysts.” In a later publication, Wenrich (136) found these forms to be no more frequent in older than in fresh material and subsequently dismissed the notion of “pseudocysts”. Piekarski (95) examined iron-hematoxylin-stained smears and reported the presence of precystic and cystic forms. However, closer scrutiny of the figures in that paper reveals that these forms are more likely to be degenerating trophozoites. Interestingly, Silard et al. (114) reported possible cystic forms of D. fragilis, with irregular, thick membranes, in cultured preparations examined by phase-contrast microscopy. These forms were not confirmed in permanently stained smears. It is still possible that a cyst form might be identified. Despite the larger body of research that has been undertaken on B. hominis, its cyst stage was confirmed only in 1991 (122), 79 years after its first description.
The lack of a cyst stage would cast doubt on the possibility of an effective direct fecal-oral route of transmission. Thomson and Robertson (128) stated that it appeared unlikely that an infection could occur via ingestion of the adult form of D. fragilis, since the life span of the parasite outside the body is very short. Furthermore, they doubted whether D. fragilis could survive the vicissitudes of its journey through the alimentary tract. Indeed, in a most dedicated fashion of scientific pursuit, Dobell (36) swallowed a culture containing thousands of active and healthy trophozoites of D. fragilis. After 10 years of examining his stools, he was unable to find the organism in a single sample. His attempts to infect macaques were similarly unsuccessful.
Dobell (36) compared D. fragilis with what he felt was its closest biological relative, Histomonas meleagridis. He pointed out that since H. meleagridis was known not to have a cyst form and was transmitted via the eggs of the nematode Heterakis gallinae, it was highly likely that D. fragilis was also transmitted via the ova of nematodes, and suggested Trichuris trichiura and Ascaris lumbricoides as likely candidates. Circumstantial support for this hypothesis was found in reports by a number of researchers who described helminth coinfections in large numbers of patients infected with D. fragilis (58, 128).
Burrows and Swerdlow (10) agreed with Dobell (36) and Wenrich (136) that D. fragilis was probably related to Histomonas. This was based on the fact that neither organism formed cysts, each was pathogenic to its host to various degrees, each ingested red blood cells within the host as well as in culture, each showed a lag phase of about 24 h before multiplying in cultures, and each is transmitted in the egg of a species of roundworm. However, they disagreed with Dobell regarding the intermediate host. Whereas Dobell (36) thought that the intermediate host might be Trichuris or Ascaris, Burrows and Swerdlow (10) were convinced that the incriminating intermediate host was Enterobius vermicularis. This was based on pathological analyses of 22 appendixes from which D. fragilis was isolated. They found a 20-fold greater incidence of coinfection with the pinworm E. vermicularis than the calculated expected value. In addition, they observed small amoeboid bodies, whose nuclei greatly resembled those found in uni-and binucleated D. fragilis, in the eggs of the pinworms. Further circumstantial evidence to support this assumption was provided by a number of other studies that showed a greater than expected incidence of coinfections with D. fragilis and E. vermicularis (11, 15, 19, 77, 86, 98, 124, 146). In addition, although Burrows and Swerdlow (10) were unsuccessful in their attempts to culture D. fragilis from pinworm eggs, Ockert (88, 89) not only successfully infected himself with D. fragilis by ingesting eggs of E. vermicularis, taken from a young boy who was coinfected with D. fragilis, but also successfully infected two other human subjects. It is noteworthy that the reported incidence of both D. fragilis and pinworms is higher in females than males (15, 53, 146).
Ockert and Schmidt (90) felt that they had found the definitive proof which confirmed that Enterobius served as the vector for transmitting D. fragilis. They compared the isoelectric points of trophozoites of D. fragilis in culture with ameboid-like cells that they found in the eggs of Enterobius. Interestingly, their nuclei and their cytoplasms had almost identical electrostatic charges.
Based on morphological comparisons, Dobell (36) proposed that Dientamoeba was probably related to Histomonas. Similar assumptions have been expressed by Wenrich (136, 137), Camp et al. (13), and Dwyer (39, 40, 41) as a result of their studies. More recently, the specific phylogenetic relationship between Histomonas and Dientamoeba has been determined by the analysis of small-subunit rRNAs. Both species demonstrate reduced G+C contents and longer nucleotide chain lengths compared to other parabasalids (48). Based on the totality of these similarities, it appears plausible to make an assumption that both species may also have a common modus of transmission. Certainly, from the standpoint of the parasite's ability to perpetuate itself and survive in the environment, it would be of benefit for D. fragilis to be transmitted by pinworms (23, 132).
Some authors have not been convinced of the role of Enterobius as the vector of D. fragilis since they were unable to establish any association between E. vermicularis and D. fragilis in any of their patients (69). Unfortunately, however, it is not always apparent how, or if, some of the researchers studying D. fragilis properly screened their patients for pinworms. Not only do pinworm infections often undergo spontaneous remissions, but also, unlike other nematodes that are diagnosed by the presence of fecal eggs, pinworms are diagnosed only by performing anal swabs or cellotape smears. These procedures are often not performed or are undertaken only when there are specific indications such as anal pruritus. It is therefore likely that some investigators may not have adequately looked for pinworms. However, it is also possible that other nematodes might serve as vectors. Indeed, Sukanahaketu (123) found D. fragilis-like structures in the ova of Ascaris lumbricoides in 38 patients in Thailand with D. fragilis infections. He did not find these structures in patients infected with A. lumbricoides without coinfections with D. fragilis.
Attention should be drawn to the fact that our inability to identify a cyst stage of D. fragilis does not guarantee its nonexistence. Indeed, D. fragilis infections are often associated with other intestinal parasites. Ayadi and Barri (3) investigated 1,497 confirmed D. fragilis cases and found coinfections with E. vermicularis in only 5% but with B. hominis in 40.3% of the cases and with Endolimax nana, Entamoeba coli, and Giardia intestinalis in 24, 6, and 5.7% of the cases; respectively. This high coincidence of infection with other organisms that are transmitted via the fecal-oral route suggests the possibility of a similar mode of transmission for D. fragilis.
THERAPY
There is only a limited amount of information available on the efficacy of therapeutic agents against this organism. Successful reported treatments for D. fragilis infections include diphetarsone (70; S. S. Desser and Y. J. Yang, Letter, Can. Med. Assoc. J. 114:290, 293, 1976), tetracycline (29, 69), carbarsone (69, 72), metronidazole (28, 117, 120), iodoquinol (28, 79, 116, 117), erythromycin (98) hydroxychinoline (98), paromomycin (28), and secnidazole (49).
Advancements in our understanding of the effects of protozoicidal agents on D. fragilis is technically limited by our inability to maintain this organism in axenic cultures. Drug susceptibility testing is consequently undertaken in the presence of supporting bacteria (17). Accordingly, it is impossible to determine whether the antimicrobial agent is active against D. fragilis or the accompanying bacteria supporting its survival. The argument, however, can be made that if bacteria are necessary for the survival of D. fragilis, testing should be done under conditions which most closely mirror those conditions. It has also been difficult to interpret minimal amebecidal in vitro concentrations of the drugs presently available, since their concentrations in the gastrointestinal tract have not been determined (17).
Despite the limited number of studies of the treatment of D. fragilis and the still unresolved issues pertaining to in vitro testing methods, it cannot be denied that a substantial body of information exists indicating that the elimination of D. fragilis from symptomatic patients results in clinical improvement. Indeed, in a recent study performed in Australia, all 21 patients with a 2-month to lifelong history of irritable bowel syndrome symptoms (including diarrhea [2 to 15 motions/day], constipation, abdominal cramping, bloating, flatulence, nausea, fatigue, and anorexia) and concurrent D. fragilis infections who were treated with iodoquinol and doxycycline showed complete elimination of D. fragilis. Clinical improvement was achieved in 67% of these patients (6). Some of the patients, however, experienced side effects, including dizziness, headaches, nausea, lethargy, and pruritus. These findings, besides demonstrating the effectiveness of this treatment regimen, also suggested a possible pathogenic role for D. fragilis as a cause of irritable bowel syndrome.
Side effects have also been reported for other therapeutic agents used to treat D. fragilis infections. Transient liver function abnormalities were observed in several patients treated with diphetarsone (70). Tetracycline has limited usefulness in children because of its well-established deleterious effect on dental development. Presently, iodoquinol and tetracycline are the most commonly employed medications, but a recent study found the antiamebic drug secnidazole to be highly effective. D. fragilis was eradicated in 34 of 35 patients after receiving a single dose of secnidazole. A second dose was required only for one patient (49). Clearly, however, more work is required to establish effective and safe therapeutic protocols. In the United States, all therapy for D. fragilis is considered investigational by the Food and Drug Administration (94).
EPIDEMIOLOGY AND DIAGNOSIS
Studies from a large number of countries have substantiated the worldwide distribution of D. fragilis (139). Prevalence rates have been reported to vary from 0% in Prague (14) to as high as 42% in children in Germany (86, 87). Infection rates are probably influenced by population density and levels of hygiene, since rates of infection have been shown to be higher in mental institutions (56), among selected military personnel (105), among parasitology students (119), and in missionaries (127). In a semicommunal group of adults in the United States, an infection rate of 52.5% was reported (79). The diagnostic methods employed have a profound effect on the successful detection of D. fragilis and consequently on the accuracy and interpretation of such reports. In addition to the level of competence of the person evaluating the fecal samples, a number of other factors influence the diagnostic pursuit. The binucleate structure of D. fragilis cannot be appreciated if the sample is in a saline preparation (142), and so permanently stained fecal smears should be made (116). Grendon et al. (54) surveyed detection methods for D. fragilis in State Public Health Laboratories in the United States and found that permanent staining of all stools, rather than only loose and watery ones, resulted in a fivefold increase in the detection rate. The numbers of D. fragilis organisms shed in feces may vary considerably from day to day (146; Desser and Yang, Letter), as is the case for many intestinal protozoa. Yang and Scholten (145) examined the stool distribution of D. fragilis in one patient and found that more than twice as many organisms were present in the last portion evacuated. Also, increasing the number of fecal samples to three has been reported to increase the detection rate by over 30% (60). Detection rates have been reported to double when culture results were compared to stained smears from the same cohort of patients (141). It is clear that accurate diagnosis of D. fragilis requires the use of suitable staining or culture techniques and examination of more than one fecal sample.
Microscopy
Permanently stained fecal smears are commonly used in North America and are appreciated as an essential aid in the diagnosis of intestinal protozoa. In Europe, however, fresh unpreserved stool specimens are generally used for examination while stained smears are used in reference centers. Laboratories that examine stools by direct microscopy should be aware that D. fragilis trophozoites may be encountered as refractile, rounded forms, varying in size from 5 to 15 μm (142) (Fig. 9). The nuclear structure cannot be seen in saline or iodine preparations, and consequently the cells may be dismissed as artifacts. It is essential that permanent stained smears be performed on every stool sample to properly identify trophozoites of D. fragilis. The crystal violet hematoxylin method of Velat et al. (131) can be used to stain D. fragilis and other trophozoites of flagellates in fresh wet preparations. Although the preparation of the stain is complex, the method is simple and the results are excellent. Another simple method of staining without using specialized fixatives is to air dry a fecal smear, fix it in industrial methylated spirit, and stain it with either Giemsa stain (43) or Field's stain (81). However, it is not possible to see the typical fragmented nuclei when using these simple, rapid methods since the nuclear contents often coalesce (86). Much better cytological results can be obtained by using a suitable fixative (see below) in combination with a permanent staining method. Concentration methods are not generally recommended for the recovery of D. fragilis, although trophozoites are sometimes found in concentrated stools (145).
FIG. 9.
Direct microscopy of an unstained saline fecal preparation showing the typical rounded trophozoite of D. fragilis. Magnification, ×400. Reprinted from reference 141 with permission from the publisher.
Fixatives
Once D. fragilis trophozoites degenerate, they become harder to recognize. Therefore, for optimal results, fecal specimens should be placed in a fixative immediately (145). Dobell (36) employed Schaudinn's fixative followed by staining with Heidenhain's iron-alum hematoxylin. Generally, all fixatives used for intestinal protozoa are suitable for D. fragilis, these include polyvinyl alcohol (PVA) (50), modified Schaudinn's fixative (109), phenol-alcohol-formalin (9), and sodium acetate-acetic acid-formalin (SAF) (145). SAF has the advantages that it is simple to make and relatively nontoxic (compared to other fixatives) and can also be used for concentration methods. The merthiolate-iodine-formalin method (106) is a combined fixative and stain technique; however, it is not very stable and does not stain the nuclei of D. fragilis well (146). Both Schaudinn's fixative and PVA (which is a plastic powder dissolved in Schaudinn's fixative) contain mercuric chloride. Concerns about safety and problems with the disposal of mercury led researchers to look for substitutes for mercuric chloride that are more environmentally friendly (47, 62). Studies using copper sulfate (CuSO4) were controversial. Horen (62) found that copper sulfate gave results comparable to those obtained with the original formula, whereas Garcia et al. (47) described inferior results, for protozoa in general, with this substitute. They compared the original PVA with a formulation in which a zinc sulfate base was used to replace the mercuric chloride and found it to be a viable substitute, although the overall morphology was not as good. To date, none of the mercury substitute fixatives produce results equal to those obtained with mercuric chloride.
Staining
According to Dobell (36) “all good cytological methods yield good preparations of D. fragilis if they are employed with appropriate precautions.” Over the years, many different stains have been used to detect D. fragilis, after appropriate fixation, including iron-haematoxylin (36), Mayer's haemalum (127), Lawless' stain (76), Celestine Blue (144), and Wheatley's trichrome (139). Ockert (86) found that fast methods of preparing permanent stained smears, such as Lawless' technique, often gave poor results. This could be overcome by using thin fecal smears, rapidly overlaying the smear with a mixture of stain and fixative, complete rinsing out of the fixative, and using very pure reagents (86). The combination of fixative and stain is important since this can greatly affect the quality of protozoan morphology. Generally, the trend has been to use PVA or Schaudinn's fixative with Wheatley's trichrome (7, 53, 79, 116, 117, 118) or SAF fixative with iron-hematoxylin (67, 71, 145). SAF has been used with Wheatley's trichrome (84, 143), but this may not give optimal results (46). Garcia and Shimizu (46) compared specimens fixed in a commercial zinc sulfate-based Schaudinn's fixative (EcoFix) (Meridian Diagnostics, Inc.) and stained with either Wheatley's trichrome or a commercial EcoStain (Meridian Diagnostics, Inc.). The commercial stain produced a gray-green or gray-blue monotone, with very little pink tone, and the contrast was lower than that achieved with trichrome stain. Nevertheless, the combination of EcoFix and EcoStain provided a better alternative than EcoFix and Wheatley's trichrome. For optimal results, without the inherent safety problems associated with mercuric chloride fixatives, we favor the combination of SAF and iron-hematoxylin.
Van Gool et al. (130) in the Netherlands described a “triple feces test” that combined the sampling of patient stools collected on three consecutive days, the use of SAF fixative, and the use of the stain chlorazol black. The test was described as highly effective in identifying intestinal protozoa, including D. fragilis, was relatively fast and easy to perform, and did not require dehydration steps with xylene. Although this technique was found to be a better detection method for fecal protozoa than direct microscopy and concentration methods, it has not been compared to the more established permanent staining methods used in North America.
Technical difficulties can arise when D. fragilis chromatin granules are covered with stain deposits, when the nuclear fragmentation is not obvious, or when the majority of trophozoites are mononucleated (145). In these circumstances, they can be confused with trophozoites of Endolimax nana (44, 45, 145).
Immunological Diagnosis
Specific immunological tests for D. fragilis are not currently available. Chan et al. (18) were the first to develop an immunofluorescence assay to identify D. fragilis trophozoites in preserved fecal specimens. They produced anti-D. fragilis antiserum in rabbits by using the dixenic D. fragilis strain Bi/pa. After absorption of the antiserum with Klebsiella pneumoniae and Bacteroides vulgatus, the two bacteria present in the culture, it was used in an indirect fluorescent-antibody assay to detect D. fragilis in preserved fecal samples. There were no cross-reactions with any of the 4 species of helminths or 10 species of protozoa encountered in their study. The authors considered the indirect fluorescent-antibody assay to be highly specific for D. fragilis and were able to identify the organism in seven of nine confirmed positives. Two samples with only very small numbers of D. fragilis trophozoites gave questionable results.
In a later study, Chan et al. (16) employed an immunoblot assay and found that serum samples from patients with confirmed D. fragilis infections reacted with a 39-kDa D. fragilis protein. It is unclear what this protein may be, what significance it may have in the pathogenesis of the disease, and whether it has any immunoprophylactic properties.
DNA-Based Diagnosis
Only one study to date has investigated the potential of detecting DNA in feces for diagnosis of D. fragilis infection (94) by amplifying a portion of the small-subunit rRNA gene by PCR. However, the sensitivity of this PCR cannot be compared directly to microscopy because different samples were used for the two detection methods. The development of a quick and accurate immunodiagnostic test would be of great benefit to the diagnosis of D. fragilis infections.
CULTIVATION
General Considerations
The culture methods used for D. fragilis are xenic, in which the parasite is grown in an undefined bacterial flora. The balance required in controlling the bacterial flora while providing for the needs of the parasite is crucial for the successful culture of intestinal protozoa (21). The intestinal bacterial flora provides D. fragilis with a food source. In all xenic culture media, rice starch provides the carbohydrate essential for bacterial growth. Antibiotics such as erythromycin, penicillin, and streptomycin are often used to suppress gram-positive organisms (101, 108, 114). Usually xenic culture media are biphasic, with a slant of agar or egg and a liquid-phase overlay, although monophasic media have also been successfully used. Many different xenic media can be used to grow D. fragilis from clinical specimens, although we prefer to use Robinson's medium (101).
Historical Background
Some early parasitologists maintained that D. fragilis was easily isolated and grew abundantly in certain media (22, 36). This is in contrast to the experiences of many present-day investigators (21). Dobell (36) credits Thomson and Robertson with the first culture of D. fragilis, using Boeck and Drbohlav's E. histolytica medium (Locke egg serum) (5). It was heavily contaminated with Blastocystis hominis, and they were able to maintain it for only a short period. Dobell and Svensson are credited with having produced the first culture of D. fragilis free from other protozoa in 1929 (36).
Dobell (36) used two different biphasic media, one (HSre + S) with a solid slope of inspissated horse serum and the other (Ehs + S) made with an inspissated egg slope. The former gave the best results when overlaid with dilute egg white in Ringer's fluid and supplemented with rice starch. Interestingly, Dobell (36) found an optimum growth temperature of 40°C for some strains of D. fragilis. Cleveland and Collier (22) isolated D. fragilis while attempting to improve their cultivation of E. histolytica. The medium they used was Loeffler's dehydrated beef serum slants covered with fresh horse serum saline.
Balamuth (4) described a monophasic liquid medium containing dehydrated egg yolk and liver infusion. This medium permitted the growth of amebae (Entamoeba histolytica, Entamoeba coli, Iodamoeba bütschlii, and Endolimax nana), flagellates (Trichomonas spp. and Chilomastix mesnili), and D. fragilis. Balamuth used this medium to study the effects of drugs, amebicides, and antibiotics on amebae and D. fragilis. While experimenting on a xenic culture of D. fragilis, Balamuth inadvertently eliminated all but two of the bacterial species and produced a dixenic culture. Balamuth designated the subline Bi/pa, and the bacteria were identified as Clostridium perfringens and Aerobacter aerogenes. Klebsiella pneumoniae and Clostridium perfringens are now listed as the bacterial species, although Chan et al. (18) used this strain and found the anaerobic species to be Bacteroides vulgatus.
Jacobs (63) performed many experiments on the cultivation of D. fragilis but was unable to support its growth without viable bacteria. Using crude xenic cultures transplanted into a medium containing Clostridium perfringens with penicillin, streptomycin, and sulfadiazine, he was able to produce a monoxenic culture of D. fragilis. However, all attempts to produce an axenic culture (without other organisms) of D. fragilis have failed to date (17, 18, 21).
Robinson (101) formulated a biphasic medium for the diagnosis of human parasitic amebae that had a saline agar slope as the solid phase. The liquid phase was complex and included Escherichia coli growing in a defined medium (R). Other additives included erythromycin, horse serum, potassium phthalate, Bacto Peptone, and rice starch (21). Robinson's medium supported the growth of all intestinal amebae including D. fragilis. This medium was used by Johnson and Clark (66) to grow D. fragilis prior to performing riboprinting. Diamond (33) developed a monophasic medium, TYSGM-9 (Trypticase, yeast extract, serum, gastric mucin), which supported the growth of lumen-dwelling protozoa, including D. fragilis.
Surveys Using Culture
The most sensitive method of detecting D. fragilis is by using culture techniques, compared directly to stained smears (86, 141). Although cultivation of intestinal protozoa is not usually attempted in routine diagnostic laboratories (21, 140), recent data have shown that it can be successfully employed outside a research environment (141). The use of Robinson's medium in a small diagnostic laboratory doubled the detection rate from 1.3 to 2.6% compared with the rate for trichrome-stained smears. It was considered less laborious than staining and required a smaller amount of feces, and culture lysates can provide material for subsequent genotyping. On the negative side, cultures take 48 h or longer and cannot be used for fecal samples that are submitted in fixative. They are unlikely to replace staining methods in diagnostic parasitology laboratories because they do not detect all intestinal protozoa. However, cultures may play a role in laboratories that do not have the expertise to detect D. fragilis in stained smears but want to exclude this parasite. Positive cultures were even obtained from stool samples stored at room temperature or 4°C for 24 h. A previous study (108) had reported that D. fragilis could be cultured from feces stored for up to 24 h at room temperature but only for 10 h at 4°C.
Ockert (86) reported two studies undertaken in his research laboratory where all stool samples were both stained and cultured. In the first study, involving 576 children, 3% of the stained smears were positive whereas 35% were positive in culture. In the second study, involving 1,066 persons, 1.97% of the stained smears were positive while 39.3% were positive in culture. The medium used was a modification of that of Dobell and Laidlaw (37), using coagulated human serum for the solid phase. The primary cultures were subcultured twice into fresh medium. Of the D. fragilis-positive samples identified, only 40% were detected by the primary culture whereas over 80% were detected following the first subculture and the rest were detected after the second subculture. Silard et al. (114) also favored the use of Dobell and Laidlaw medium and isolated D. fragilis from 2.8% of clinical samples. Using culture over a 10-year period in Israel, Talis et al. (127) detected D. fragilis in 30,609 (15.2%) of 201,750 specimens. The medium used was a diphasic egg medium formulated in their laboratory. Using samples from a patient with known D. fragilis infection, Sawangjaroen et al. (108) compared three media, modified Boeck and Drbohalav (BD) medium, TYSGM-9 (33), and Cleveland and Collier medium (22). Modified Boeck and Drbohalav medium was found to be the most suitable since it was the only medium that supported the initial growth and subculture of D. fragilis. The authors then surveyed consecutive samples from patients with diarrhea. A surprisingly low incidence of 1.5% (4 of 260) was found; however, culture doubled the detection rate.
Appearance in Culture
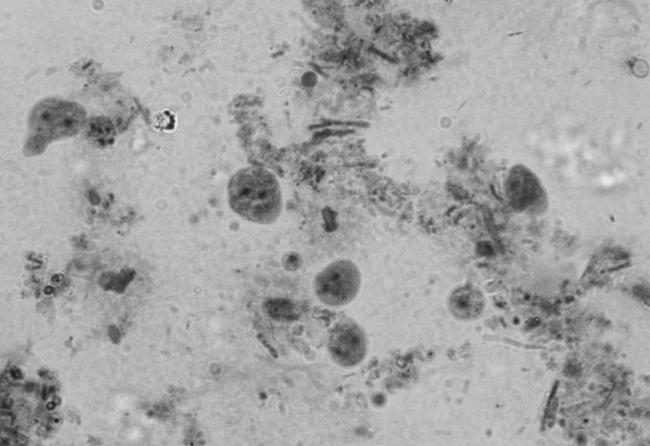
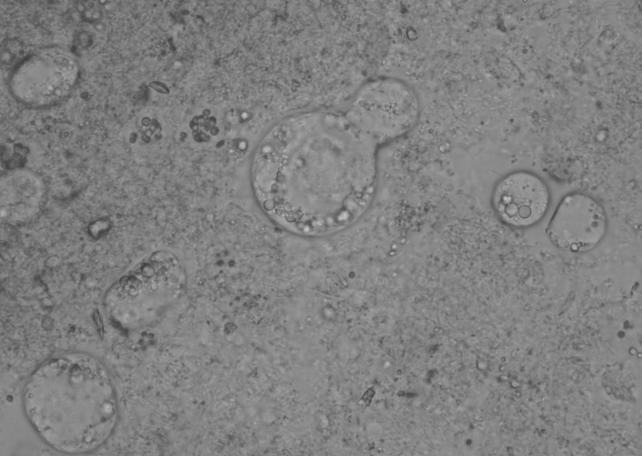
Trophozoites of D. fragilis initially appear as rounded refractile bodies, containing many rice starch granules, in freshly mounted culture preparations after 48 h of incubation (Fig. 10). After approximately 10 min at room temperature, they begin to produce sluggish, small, irregular pseudopodia (108) (Fig. 1). The size of D. fragilis in culture varies considerably, and some workers have described a range of 6 to 40 μm (114). Robinson and Ng (102) found that with experience, this size range became a good guide to the presumptive identification of D. fragilis after addition of a drop of iodine. Moreover, the appearance of circular brown-red forms filled with starch grains aided this diagnosis. Several intestinal amebae grow well in culture media used for D. fragilis and also ingest rice starch. Although none of the amebae demonstrate the characteristic motility of D. fragilis, it is nevertheless recommended that the diagnosis be confirmed by using a suitable staining method (101). Robinson (101) fixed culture-positive amebae in a mixture of acetic acid and phosphotungstic acid and stained them with a hematoxylin stain. Windsor et al. (141) confirmed the positive amebae by fixing in Schaudinn's fixative and staining with trichrome, whereas Silard et al. (114) preferred a simpler method of fixing in methanol and staining with Giemsa. Diagnostic uncertainties can arise for cultures containing granular forms of B. hominis and D. fragilis (J. J. Windsor, unpublished data). However, these species can be differentiated on the basis that the granules of B. hominis are distinctly rounded (Fig. 11) whereas D. fragilis simply contains ingested rice starch. Blastocystis grows freely in nearly all xenic media suitable for D. fragilis and Entamoeba spp. This was of considerable concern for investigators attempting to isolate these parasites (21, 37).
FIG. 10.
Rounded trophozoites of D. fragilis in Robinson's culture. Magnification, ×400.
FIG. 11.
Granular form of Blastocystis hominis in Robinson's culture. Magnification, ×400.
CONCLUSIONS
Significant progress has been made in the biological classification of D. fragilis. Although D. fragilis was initially thought to be an ameba, phylogenetic analysis of small-subunit rRNA gene sequences has confirmed morphological observations and antigenic analyses showing that it has an extremely close relationship with Histomonas, and today D. fragilis is classified as among the trichomonads.
Unfortunately, there still exists a difference of opinion regarding the clinical significance of this organism as a human pathogen. The overwhelming circumstantial evidence, however, strongly suggests that D. fragilis is a bona fide pathogen. This is based primarily on the observation that there are a large number of case reports from many parts of the world that describe patients whose clinical symptoms subsided only after therapeutic intervention and elimination of the organism. However, it cannot be denied that there are patients who harbor this organism but do not exhibit clinical signs. Recently undertaken molecular studies may help to shed light on these differing observations. Using riboprinting, two genetically distinct types of D. fragilis have been identified. It certainly would not be unreasonable to speculate that different genetic types may demonstrate different degrees of virulence.
The accuracy of diagnostic testing for D. fragilis has traditionally relied on permanently stained fecal smears, since the characteristic binucleate appearance of the organism cannot be appreciated in saline or iodine preparations. A number of studies have substantiated the necessity of using this methodology. The fairly recent introduction of fecal cultures for diagnosis indicates that infection rates are significantly higher than those found using stained smears and that cultures are less laborious to perform. However, culture cannot be done on fecal samples received in fixative, and it does not detect all intestinal protozoa. Consequently, it is unlikely to replace permanent staining in the diagnostic parasitology setting, but it might appeal to laboratories that want to exclude D. fragilis but do not have sufficient expertise in reading stained smears.
There are great gaps in our present state of knowledge concerning the virulence, pathogenicity, and mode of transmission of D. fragilis. However, neglecting to include D. fragilis on the list of potential culprits in unexplained cases of chronic diarrhea, abdominal pain, fatigue, flatulence, and anorexia, and even in patients with irritable bowel syndrome-like syndromes, can no longer be justified.
Acknowledgments
It would be remiss of us not to extend our sincerest thanks to Alan Curry at the Manchester Royal Infirmary for his inexhaustible generosity in providing the micrographs used in the present review article.
REFERENCES
- 1.Addadi, K., Y. Le Corroller, Y. Guy, and A. Tabet-Derraz. 1972. Sur le role pathogene possible de Dientamoeba fragilis. Bull. Soc. Pathol. Exot. Fil. 65:274-275. [PubMed] [Google Scholar]
- 2.Aucott, J. N., and J. I. Ravdin. 1993. Amebiasis and “nonpathogenic” intestinal protozoa. Infect. Dis. Clin. North. Am. 7:467-485. [PubMed] [Google Scholar]
- 3.Ayadi, A., and I. Bahri. 1999. Dientamoeba fragilis: flagelle pathogene. Bull. Soc. Pathol. Exot. 5:299-301. [PubMed] [Google Scholar]
- 4.Balamuth, W. 1946. Improved egg yolk medium for cultivation of Entamoeba histolytica and other intestinal protozoa. Am. J. Clin. Pathol. 16:380-384. [DOI] [PubMed] [Google Scholar]
- 5.Boeck, W. C., and J. Drbohlav. 1925. The cultivation of Endamoeba histolytica. Am. J. Hyg. 5:371-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borody, T. J., E. F. Warren, A. Wettstein, G. Robertson, P. Recabarren, A. Fontella, K. Herdnman, and R. Surace. Eradication of Dientamoeba fragilis can resolve IBS-like symptoms. 2002. J. Gastroenterol. Hepatol. 17(Suppl.):A103. [Google Scholar]
- 7.Bruckner, D. A., L. S. Garcia, and M. Voge. 1979. Intestinal parasites in Los Angeles, California. Am. J. Med. Technol. 45:1020-1022. [PubMed] [Google Scholar]
- 8.Brug, S. L. 1936. Observations on Dientamoeba fragilis. Ann. Trop. Med. Parasitol. 30:441-452. [Google Scholar]
- 9.Burrows, R. B. 1967. A new fixative and technics for the diagnosis of intestinal parasites. Tech. Bull. Regist. Med. Technol. 37:208-212. [PubMed] [Google Scholar]
- 10.Burrows, R. B., and M. A. Swerdlow. 1956. Enterobius vermicularis as a probable vector of Dientamoeba fragilis. Am. J. Trop. Med. Hyg. 5:258-265. [DOI] [PubMed] [Google Scholar]
- 11.Burrows, R. B., M. A. Swerdlow, J. K. Frost, and C. K. Leeper. 1954. Pathology of Dientamoeba fragilis infections of the appendix. Am. J. Trop. Med. Hyg. 3:1033-1039. [PubMed] [Google Scholar]
- 12.Butler, W. P. 1996. Dientamoeba fragilis. An unusual intestinal pathogen. Dig. Dis. Sci. 41:1811-1813. [DOI] [PubMed] [Google Scholar]
- 13.Camp, R. R., C. F. Mattern, and B. M. Honigberg. 1974. Study of Dientamoeba fragilis Jepps & Dobell. I. Electronmicroscopic observations of the binucleate stages. II. Taxonomic position and revision of the genus. J. Protozool. 21:69-82. [DOI] [PubMed] [Google Scholar]
- 14.Cerva, L. 1962. Die Darmparasiten in den Kinderheimen des Prager Bezirkes. Angew. Parasitol. 2:119-124. [Google Scholar]
- 15.Cerva, L., M. Schrottenbaum, and V. Kliment. 1991. Intestinal parasites: a study of human appendices. Folia Parasitol. 38:5-9. [PubMed] [Google Scholar]
- 16.Chan, F., N. Stewart, M. Guan, I. Robb, L. Fuite, I. Chan, F. Diaz-Mitoma, J. King, N. MacDonald, and A. MacKenzie. 1996. Prevalence of Dientamoeba fragilis antibodies in children and recognition of a 39 kDa immunodominant protein antigen of the organism. Eur. J. Clin. Microbiol. Infect. Dis. 15:950-954. [DOI] [PubMed] [Google Scholar]
- 17.Chan, F. T., M. X. Guan, A. M. MacKenzie, and F. Diaz- Mitoma. 1994. Susceptibility testing of Dientamoeba fragilis ATCC 30948 with iodoquinol, paromomycin, tetracycline, and metronidazole. Antimicrob. Agents Chemother. 38:1157-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan, F. T. H., M. X. Guan, and A. M. MacKenzie. 1993. Application of indirect immunofluorescence to detection of Dientamoeba fragilis trophozoites in fecal specimens. J. Clin. Microbiol. 31:1710-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang, S. 1973. Parasitization of the parasite. JAMA 223:1510. [PubMed] [Google Scholar]
- 20.Chatton, E. P. L. 1925. Pansporella perplexa, amoebien a spores protegees, parasite des daphnies. Reflexions sur la biologie et la phylogenie des protozoaires. Ann. Sci. Nat. Zool. 10e Ser. 8:5-85. [Google Scholar]
- 21.Clark, C. G., and L. S. Diamond. 2002. Methods for the cultivation of luminal parasitic protists of clinical importance. Clin. Microbiol. Rev. 15:329-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleveland, L. R., and J. Collier. 1930. Various improvements in cultivation of Endamoeba histolytica. Am. J. Hyg. 12:606-613. [Google Scholar]
- 23.Cline, B. 1982. Drug regimens for intestinal helminth infections. Med. Clinics North Am. 66:721-742. [DOI] [PubMed] [Google Scholar]
- 24.Colea, A., R. Silard, D. Panaitescu, P. Florescu, N. Roman, and T. Capraru. 1980. Studies on Dientamoeba fragilis in Romania. II. Incidence of Dientamoeba fragilis in healthy persons. Arch. Roum. Pathol. Exp. Microbiol. 39:49-53. [PubMed] [Google Scholar]
- 25.Costa, R. S. 1948. Dientamoeba fragilis Jepps and Dobell, 1918. Primeros casos senelados en nuestro pais. Consideraciones epidemiologicas. Rev. Kuba Med. Trop. 4:115-118. [Google Scholar]
- 26.Craig, C. F. 1926. The nuclear structure of Dientamoeba fragilis. J. Parasitol. 13:137-140. [Google Scholar]
- 27.Crotti, D., and M. L. D'Annibale. 2001. Dientamoeba fragilis e dientamoebosi: aspetti di parassitologia clinicale diagnostica di laboratorio. Parassitologia 43:135-138. [PubMed] [Google Scholar]
- 28.Cuffari, C., L. Oligny, and E. G. Seidman. 1998. Dientamoeba fragilis masquerading as allergic colitis. J. Pediatr. Gastroenterol. Nutr. 26:16-20. [DOI] [PubMed] [Google Scholar]
- 29.Dardick, K. 1983. Tetracycline treatment of Dientamoeba fragilis. Conn. Med. 47:69-70. [PubMed] [Google Scholar]
- 30.Das Gupta, B. M. 1936. A case of human infection with Dientamoeba fragilis Jepps and Dobell, 1918, in Calcutta. Indian Med. Gaz. 21:528-529. [PMC free article] [PubMed] [Google Scholar]
- 31.De Jonckheere, J. B., S. Brown, P. J. Dobson, B. S. Robinson, and P. Pernin. 2001. The amoeba-to-flagellate transformation test is not reliable for the diagnosis of the genus Naegleria. Description of three new Naegleria sp. Protist 152: 115-121. [DOI] [PubMed] [Google Scholar]
- 32.Reference deleted.
- 33.Diamond, L. S. 1982. A new liquid medium for xenic cultivation of Entamoeba histolytica and other lumen-dwelling protozoa. J. Parasitol. 68:958-959. [PubMed] [Google Scholar]
- 34.Diamond, L. S., and C. G. Clark. 1993. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J. Eukaryot. Microbiol. 40:340-344. [DOI] [PubMed] [Google Scholar]
- 35.Dickinson, E. C., M. A. Cohen, and M. K. Schlenker. 2002. Dientamoeba fragilis: a significant pathogen. Am. J. Emerg. Med. 20:62-63. [DOI] [PubMed] [Google Scholar]
- 36.Dobell, C. 1940. Researches on the intestinal protozoa of monkeys and man. X. The life history of Dientamoeba fragilis: observations, experiments, and speculations. Parasitology 32:417-461. [Google Scholar]
- 37.Dobell, C., and P. P. Laidlaw. 1926. On the cultivation of Entamoeba histolytica and some other entozoic amoeba. Parasitology 18:283-318. [Google Scholar]
- 38.Dobell, C., and F. W. O'Connor. 1921. The intestinal protozoa of man. J. Bale, Sons, and Danielson, London, United Kingdom.
- 39.Dwyer, D. M. 1972. Analysis of the antigenic relationships among Trichomonas, Histomonas, Dientamoeba, and Entamoeba. I. Quantitiative fluorescent antibody methods. J. Protozool. 19:316-325. [DOI] [PubMed] [Google Scholar]
- 40.Dwyer, D. M. 1972. Analysis of the antigenic relationships among Trichomonas, Histomonas, Dientamoeba, and Entamoeba. II. Gel diffusion methods. J. Protozool. 19:316-325. [DOI] [PubMed] [Google Scholar]
- 41.Dwyer, D. M. 1974. Analysis of the antigenic relationships among Trichomonas, Histomonas, Dientamoeba, and Entamoeba. III. Immunoelectrophoresis technics. J. Protozool. 21:139-145. [DOI] [PubMed] [Google Scholar]
- 42.Fantham, H. B., and A. Porter. 1936. Some entozoa of man as seen in Canada and South Africa. Can Med. Assoc. J. 34:414-421. [PMC free article] [PubMed] [Google Scholar]
- 43.Fleck, S. L., and A. H. Moody. 1988. Faecal parasites, p. 23-24. In S. L. Fleck and A. H. Moody (ed.), Diagnostic techniques in medical parasitology. Elsevier, Amsterdam, The Netherlands.
- 44.Garcia, L. 2002. Laboratory methods for diagnosis of parasitic infection, p. 776-861. In B. A. Forbes, D. F. Sahm, and A. S. Weissfeld (ed.), Bailey and Scott's Diagnostic Microbiology, 11th ed. C. V. Mosby, St. Louis, Mo.
- 45.Garcia, L. S. 1992. Parasitology, p. 2:7.10:13:13. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook. American Society for Microbiology, Washington, DC.
- 46.Garcia, L. S., and R. Y. Shimizu. 1998. Evaluation of intestinal protozoan morphology in human fecal specimens preserved in EcoFix: comparison of Wheatley's trichrome stain and EcoStain. J. Clin. Microbiol. 36:1974-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia, L. S., R. Y. Shimizu, T. C. Brewer, and D. A. Bruckner. 1983. Evaluation of intestinal parasite morphology in polyvinyl alcohol preservative: comparison of copper sulfate and mercuric chloride bases for use in Schaudinn fixative. J. Clin. Microbiol. 17:1092-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerbod, D., V. P. Edgcomb, C. Noel, L. Zenner, R. Wintjens, P. Delgado-Viscogliosi, M. E. Holder, M. L. Sogin, and E. Viscogliosi. 2001. Phylogenetic position of the trichomonad parasite of turkeys, Histomonas meleagridis (Smith) Tyzzer, inferred from small subunit rRNA sequence. J. Eukaryot. Microbiol. 48:498-504. [DOI] [PubMed] [Google Scholar]
- 49.Girginkardesler, N., S. Coskun, I. Cuneyt Balcioglu, P. Ertan, and U. Z. Ok. 2003. Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole. Clin. Microbiol. Infect. 9:110-113. [DOI] [PubMed] [Google Scholar]
- 50.Goldman, M., and M. M. Brooke. 1953. Protozoans in stools unpreserved and preserved in PVA fixative. Public Health. Rep. 68:703-706. [PMC free article] [PubMed] [Google Scholar]
- 51.Grassé, P. P. 1953. Famille des Dientamoebidae Grasse, nov., (E. Chatton, principal author: ordre des amoebiens nus ou Amoebaea), p. 50-54. In P. P. Grasse (ed.), Traité de zoologie. Masson et Cie., Paris, France.
- 52.Greenway, D. 1928. Dientamoeba fragilis en la Argentina. Arch. Argent. Enferm. Aparato Dig. (Buenos Aires) 3:897. [Google Scholar]
- 53.Grendon, J. H., R. F. Digiacomo, and F. J. Frost. 1995. Descriptive features of Dientamoba fragilis infections. J. Trop. Med. Hyg. 98:309-315. [PubMed] [Google Scholar]
- 54.Grendon, J. H., R. F. Digiacomo, and F. J. Frost. 1991. Dientamoeba fragilis detection methods and prevalence: a survey of State Public Health Laboratories. Public Health Rep. 106:322-325. [PMC free article] [PubMed] [Google Scholar]
- 55.Hakansson, E. G. 1936. Dientamoeba fragilis, a cause of illness: report of case. Am. J. Trop. Med. 16:175-183. [Google Scholar]
- 56.Hakansson, E. G. 1937. Dientamoeba fragilis: some further observations. Am. J. Trop. Med. 17:349-362. [Google Scholar]
- 57.Halawani, A. E. 1930. Dientamoeba fragilis from a culture of human faeces. Trans. R. Soc. Trop. Med. Hyg. 23:326-327. [Google Scholar]
- 58.Haughwout, F. G., and F. S. Horrilleno. 1920. The intestinal animal parasites found in one hundred sick Filipino children. Philipp. J. Sci. 16:1-73. [Google Scholar]
- 59.Hegner, R., and H. J. Chu. 1930. A comparative study of the intestinal protozoa of wild monkeys and man. Am. J. Hyg. 12:62-108. [Google Scholar]
- 60.Hiatt, R. A., E. K. Markell, and E. Ng. 1995. How many stool examinations are necessary to detect pathogenic intestinal protozoa? Am. J. Trop. Med. Hyg. 53:36-39. [PubMed] [Google Scholar]
- 61.Hollande, A., and J. Carruette-Valentin. 1971. Les atractophores, l'induction du fuseau et la division cellulaire chez les Hypermastigines. Etude infrastructurale et revision systematique des Trichonymphines et des Spirotrichonymphines. Protistologica 7:5-100. [Google Scholar]
- 62.Horen, W. P. 1981. Modification of Schaudinn fixative. J. Clin. Microbiol 13:204-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacobs, L. 1953. The cultivation of Dientamoeba fragilis. Ann. N. Y. Acad. Sci. 56:1057-1061. [DOI] [PubMed] [Google Scholar]
- 64.Jepps, M. W. 1921. Notes on the intestinal protozoa of 971 men at the University War Hospital, Southampton. J. R. Army Med. Corps 97:366-375. [Google Scholar]
- 65.Jepps, M. W., and C. Dobell. 1918. Dientamoeba fragilis n.g., n. sp., new intestinal amoeba from man. Parasitology 10:352-367. [Google Scholar]
- 66.Johnson, J. A., and C. G. Clark. 2000. Cryptic genetic diversity in Dientamoeba fragilis. J. Clin. Microbiol. 38:4653-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kabani, A., G. Cadrain, C. Trevenen, T. Jadavji, and D. L. Church. 1995. Practice guidelines for ordering stool ova and parasite testing in a paediatric population. Am. J. Clin. Pathol. 104:272-278. [DOI] [PubMed] [Google Scholar]
- 68.Katz, D. E., and D. N. Taylor. 2001. Parasitic infections of the gastrointestinal tract. Gastroenterol. Clin. North Am. 30:797-815. [DOI] [PubMed] [Google Scholar]
- 69.Kean, B. H., and C. L. Malloch. 1966. The neglected ameba: Dientamoeba fragilis. A report of 100 “pure” infections. Am. J. Dig. Dis. 11:735-746. [DOI] [PubMed] [Google Scholar]
- 70.Keystone, J. S., E. Proctor, C. Glenn, and L. Mcintyre. 1983. Safety and efficacy of diphetarsone in the treatment of amoebiasis, non-pathogenic amoebiasis and trichuriasis. Trans. R. Soc. Trop. Med. Hyg. 77:84-86. [DOI] [PubMed] [Google Scholar]
- 71.Keystone, J. S., J. Yang, D. Grisdale, M. Harrington, L. Pillon, and R. Andreychuk. 1984. Intestinal parasites in metropolitan Toronto day-care centres. Can. Med. Assoc. J. 131:733-735. [PMC free article] [PubMed] [Google Scholar]
- 72.Knoll, E. W., and K. M. Howell. 1945. Studies on Dientamoeba fragilis. Its incidence and possible pathogenicity. Am. J. Clin. Pathol. 15:178-183. [PubMed] [Google Scholar]
- 73.Knowles, R., and B. M. Das Gupta. 1936. Some observations on the intestinal protozoa of macaques. Indian J. Med. Res. 24:547-556. [Google Scholar]
- 74.Kofoid, C. A. 1923. Amoeba and man, p. 149-174, 219-312. In University of California Chronicle for 1923. University of California Press, Berkeley.
- 75.Lainson, R., and B. A. Da Silva. 1999. Intestinal parasites of some diarrhoeic HIV-seropositive individuals in North Brazil with particular reference to Isospora belli Wenyon, 1923 and Dientamoeba fragilis Jepps & Dobell, 1918. Mem. Inst. Oswaldo Cruz 94:611-613. [DOI] [PubMed] [Google Scholar]
- 76.Lawless, D. K. 1953. A rapid permanent-mount stain technique for the diagnosis of intestinal protozoa. Am. J. Trop. Med. Hyg. 20:1137-1138. [DOI] [PubMed] [Google Scholar]
- 77.Melvin, D. M., and M. M. Brooke. 1962. Parasitologic surveys on Indian reservations in Montana, South Dakota, New Mexico, Arizona and Wisconsin. Am. J. Trop. Med. Hyg. 11:765-772. [DOI] [PubMed] [Google Scholar]
- 78.Mendez, O. C., G. Szmulewicz, C. Menghi, S. Torres, G. Gonzalez, and C. Gatta. 1994. Comparison of intestinal parasite infestation indexes among HIV positive and negative populations. Medicina (Buenos Aires) 54:307-310. [PubMed] [Google Scholar]
- 79.Millet, V., M. J. Spencer, M. Chapin, M. Stewart, J. A. Yatabe, T. Brewer, and L. S. Garcia. 1983. Dientamoeba fragilis, a protozoan parasite in adult members of a semicommunal group. Dig. Dis. Sci. 28:335-339. [DOI] [PubMed] [Google Scholar]
- 80.Mollari, M., and J. V. Anzulovic. 1938. Cultivation and pathogencity of Dientamoeba fragilis, with a case report. J. Trop. Med. Hyg. 41:246-247. [Google Scholar]
- 81.Moody, A. H., and S. L. Fleck. 1985. Versatile Field's stain. J. Clin. Pathol. 38:842-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muller, M. 1975. Biochemistry of protozoan microbodies: peroxisomes, alpha-glycerophosphate oxidase bodies, hydrogenosomes. Annu. Rev. Microbiol. 29:467-483. [DOI] [PubMed] [Google Scholar]
- 83.Myers, B. J., and R. E. Kuntz. 1968. Intestinal protozoa of the baboon Papio doguera Pucheran, 1856. J. Protozool. 15:363-365. [DOI] [PubMed] [Google Scholar]
- 84.Naiman, H. L., L. Sekla, and W. L. Albritton. 1980. Giardiasis and other intestinal parasitic infections in a Manitoba residential school for the mentally retarded. Can. Med. Assoc. J. 122:185-188. [PMC free article] [PubMed] [Google Scholar]
- 85.Norberg, A., C. E. Nord, and B. Evengard. 2003. Dientamoeba fragilis—a protozoal infection which may cause severe bowel distress. Clin. Microbiol. Infect. 9:65-68. [DOI] [PubMed] [Google Scholar]
- 86.Ockert, G. 1990. Symptomatology, pathology, epidemiology, and diagnosis of Dientamoeba fragilis, p. 394-410. In B. M. Honigberg (ed.), Trichomonads parasitic in humans. Springer Publications, New York, N.Y.
- 87.Ockert, G. 1972. Zur epidemiologie von Dientamoeba fragilis Jepps et Dobell 1918. 1. Mitteilung: die Verbreitung der art in Kinderkollektiven. J. Hyg. Epidemiol. Microbiol. Immunol. 16:213-221. [PubMed] [Google Scholar]
- 88.Ockert, G. 1972. Zur epidemiologie von Dientamoeba fragilis Jepps et Dobell 1918. 2. Mitteilung: Versuch der Uebertragung der Art mit Enterobius-Eiern. J. Hyg. Epidemiol. Microbiol. Immunol. 16:222-225. [PubMed] [Google Scholar]
- 89.Ockert, G. 1975. Zur epidemiologie von Dientamoeba fragilis. 3. Weitere zur Uebertragung der Art mit Enterobius-Eiern. J. Hyg. Epidemiol. Microbiol. Immunol. 19:17-21. [PubMed] [Google Scholar]
- 90.Ockert, G., and T. Schmidt. 1976. Zur epidemiologie von Dientamoeba fragilis Jepps et Dobell. 1918. 4. Mitteilung: Nachweis von Dientamoeba fragilis in Enterobius eirn mit hilfe von IEP-bestimmungen. J. Hyg. Epidemiol. Microbiol. Immunol. 20:76-81. [PubMed] [Google Scholar]
- 91.Ockert, G., and F. Schneider. 1974. Elektronenmikroskopische Befunde bei Dientamoeba fragilis. Z. Gesamte Hyg. 20:555-557. [PubMed] [Google Scholar]
- 92.Ockert, G., and U. Schulz. 1972. Zur pathogenetischen Rolle von Dientamoeba fragilis. Dtsch. Gesundheitsw. 27:1156-1158. [PubMed] [Google Scholar]
- 93.Panosian, C. 1988. Parasitic diarrhoea. Infect. Dis. Clin. North Am. 2:685-703. [PubMed] [Google Scholar]
- 94.Peek, R., F. R. Reedeker, and T. van Gool. 2004. Direct amplification and genotyping of Dientamoeba fragilis from human stool specimens. J. Clin. Microbiol. 42:631-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Piekarski, G. 1948. Zur Frage der Cystenbildung bei Dientamoeba fragilis. Z. Hyg. Infektionskr. 127:496-500. [PubMed] [Google Scholar]
- 96.Porter, A. 1934. Remarks on intestinal parasites in Montreal and the relation of Entamoeba histolytica to colitis. Can. Med. Assoc. J. 30:134-137. [PMC free article] [PubMed] [Google Scholar]
- 97.Preiss, U., G. Ockert, S. Broemme, and A. Otto. 1990. Dientamoeba fragilis infection, a cause of gastrointestinal symptoms in childhood. Klin. Paediatr. 202:120-123. [DOI] [PubMed] [Google Scholar]
- 98.Preiss, U., G. Ockert, S. Broemme, and A. Otto. 1991. On the clinical importance of Dientamoeba fragilis infections in childhood. J. Hyg. Epidemiol. Microbiol. Immunol. 35:27-34. [PubMed] [Google Scholar]
- 99.Rachet, J., A. Busson, and G. Terrial. 1945. Le role pathogene de ‘Dientamoeba fragilis’. Arch. Mal. Appar. Dig. Mal. Nutr. 34:196-200. [Google Scholar]
- 100.Robertson, A. 1923. Notes on a case infected with Dientamoeba fragilis, Jepps and Dobell, 1917. J. Trop. Med. Hyg. 26:243-244. [Google Scholar]
- 101.Robinson, G. L. 1968. The laboratory diagnosis of human parasitic amoebae. Trans. R. Soc. Trop. Med. Hyg. 62:285-294. [DOI] [PubMed] [Google Scholar]
- 102.Robinson, G. L., and P. H. T. Ng. 1968. The size of Dientamoeba fragilis in culture. Trans. R. Soc. Trop. Med. Hyg. 62:156-158. [DOI] [PubMed] [Google Scholar]
- 103.Rothman, M. M., and H. J. Epstein. 1941. Clinical symptoms associated with the so-called non-pathogenic amoeba. JAMA 116:694-700. [Google Scholar]
- 104.Sapero, J. J. 1939. Clinical studies in non-dysenteric amoebiasis. Am. J. Trop. Med. 19:497-541. [Google Scholar]
- 105.Sapero, J. J., and C. M. Johnson. 1939. Entamoeba histolytica and other intestinal parasites: incidence in variously exposed groups of the Navy. U. S. Navy Med. Bull. 37:297-301. [Google Scholar]
- 106.Sapero, J. J., and D. K. Lawless. 1953. The ‘MIF’ stain-preservation technique for the identification of intestinal protozoa. Am. J. Trop. Med. Hyg. 2:613-619. [DOI] [PubMed] [Google Scholar]
- 107.Sargeaunt, P. G., and J. E. Williams. 1982. The morphology in culture of the intestinal amoebae of man. Trans. R. Soc. Trop. Med. Hyg. 76:465-472. [DOI] [PubMed] [Google Scholar]
- 108.Sawangjaroen, N., R. Luke, and P. Prociv. 1993. Diagnosis by faecal culture of Dientamoeba fragilis infections in Australian patients with diarrhoea. Trans. R. Soc. Trop. Med. Hyg. 87:163-165. [DOI] [PubMed] [Google Scholar]
- 109.Scholten, T. 1972. An improved technique for the recovery of intestinal protozoa. J. Parasitol. 58:633-634. [PubMed] [Google Scholar]
- 110.Schulz, U., and G. Ockert. 1971. Darmprotozoen als Ursache intestinaler Stoerungen. Dtsch. Med. Wochenschr. 51:1963-1968. [DOI] [PubMed] [Google Scholar]
- 111.Shein, R., and A. Gelb. 1983. Colitis due to Dientamoeba fragilis. Am. J. Gastroenterol. 78:634-636. [PubMed] [Google Scholar]
- 112.Silard, R., and B. Burghelea. 1986. Endosymbionts in Dientamoeba fragilis trophozoites resistant to antiprotozoal drugs. Arch. Roum. Pathol. Exp. Microbiol. 45:65-74. [PubMed] [Google Scholar]
- 113.Silard, R., B. Burghelea, D. Panaitescu, and V. Burcos. 1984. Ultrastructure of Dientamoeba fragilis: a study of the mononucleated stage. Arch. Roum. Pathol. Exp. Microbiol. 43:87-101. [PubMed] [Google Scholar]
- 114.Silard, R., A. Colea, D. Panaitescu, P. Florescu, and N. Roman. 1979. Studies on Dientamoeba fragilis in Romania. I. Dientamoeba fragilis isolated from clinical cases. Problems of diagnosis, incidence, clinical aspects. Arch. Roum. Pathol. Exp. Microbiol. 38:359-372. [PubMed] [Google Scholar]
- 115.Silberman, J. D., C. G. Clark, and M. L., Sogin. 1996. Dientamoeba fragilis shares a recent common evolutionary history with the trichomonads. Mol. Biochem. Parasitol. 76:311-314. [DOI] [PubMed] [Google Scholar]
- 116.Spencer, M. J., M. R. Chapin, and L. S. Garcia. 1982. Dientamoeba fragilis: a gastrointestinal protozoan infection in adults. Am. J. Gastroenterol. 77:565-569. [PubMed] [Google Scholar]
- 117.Spencer, M. J., L. S. Garcia, and M. R. Chapin. 1979. Dientamoeba fragilis. An intestinal pathogen in children? Am. J. Dis. Child. 133:390-393. [PubMed] [Google Scholar]
- 118.Spencer, M. J., V. E. Millet, L. S. Garcia, L. Rhee, and L. Masterson. 1983. Parasitic infections in a pediatric population. Pediatr. Infect. Dis. J. 2:110-113. [DOI] [PubMed] [Google Scholar]
- 119.Stabler, R. M. 1941. Intestinal protozoa in 106 parasitology students. J. Parasitol. 24:90. [Google Scholar]
- 120.Steinitz, H. 1973. Klinische Beobachtungen bei Infektionen mit Dientamoeba fragilis. Z. Gastroenterol. 11:179-182. [PubMed] [Google Scholar]
- 121.Steinitz, H. 1972. The treatment of chronic amoebic infection with metronidazole (flagyl). Digestion 6:75-82. [DOI] [PubMed] [Google Scholar]
- 122.Stenzel, D. J., B. Stein, and J. Lengy. 1991. A cyst-like stage of Blastocystis hominis. Int. J. Parasitol. 21:613-615. [DOI] [PubMed] [Google Scholar]
- 123.Sukanahaketu, S. 1977. The presence of Dientamoeba fragilis in the Ascaris lumbricoides ova: the first report from Thailand. J. Med. Assoc. Thail. 60:256-258. [PubMed] [Google Scholar]
- 124.Swerdlow, M. A., and R. B. Burrows. 1955. Dientamoeba fragilis, an intestinal pathogen. JAMA 158:176-178. [DOI] [PubMed] [Google Scholar]
- 125.Taliaferro, W. H., and E. R. Becker. 1924. A note on the human intestinal amoeba, Dientamoeba fragilis. Am. J. Hyg. 4:71-74. [Google Scholar]
- 126.Talis, B., and B. Stein. 1961. The presence of Dientamoeba fragilis in the gall bladder. Harefueh 61:126. [PubMed] [Google Scholar]
- 127.Talis, B., B. Stein, and J. Lengy. 1971. Dientamoeba fragilis in human feces and bile. Isr. J. Med. Sci. 7:1063-1069. [PubMed] [Google Scholar]
- 128.Thomson, J. G., and A. Robertson. 1923. Dientamoeba fragilis, Jepps and Dobell 1917. A case of human infection in England. J. Trop. Med. Hyg. 26:135-136. [Google Scholar]
- 129.Van Gool, T., and J. Dankert. 1996. Drie opkomende protozoaire infectieziekten in Nederland: Cyclospora-, Dientamoeba-en Microspora-infecties. Ned. Tijdschr. Geneeskd. 140:155-160. [PubMed] [Google Scholar]
- 130.Van Gool, T., R. Weijts, E. Lommerse, and T. G. Mank. 2003. Triple faeces test: an effective tool for detection of intestinal parasites in routine clinical practice. Eur. J. Clin. Microbiol. Infect. Dis. 22:284-290. [DOI] [PubMed] [Google Scholar]
- 131.Velat, C. A., P. P. Weinstein, and G. F. Otto. 1950. A stain for the rapid differentiation of the trophozoites of the intestinal amoeba in fresh, wet preparations. Am. J. Trop. Med. 30:43-51. [DOI] [PubMed] [Google Scholar]
- 132.Warren, K. S., and A. A. Mahmoud. 1975. Algorithms in the diagnosis and management of exotic diseases. V. Enterobiasis. J. Infect. Dis. 132:229-232. [DOI] [PubMed] [Google Scholar]
- 133.Wenrich, D. H. 1936. Studies on Dientamoeba fragilis (protozoa). I. Observations with special reference to nuclear structure. J. Parasitol. 22:76-83. [Google Scholar]
- 134.Wenrich, D. H. 1937. Studies on Dientamoneba fragilis (protozoa). II. Report of unusual morphology in one case with suggestions as to pathogenicity. J. Parasitol. 23:183-196. [Google Scholar]
- 135.Wenrich, D. H. 1939. Studies on Dientamoeba fragilis (protozoa). III. Binary fission with special reference to nuclear division. J. Parasitol. 25:43-45. [Google Scholar]
- 136.Wenrich, D. H. 1944. Nuclear structure and nuclear division in Dientamoeba fragilis (protozoa). J. Morphol 74:467-491. [Google Scholar]
- 137.Wenrich, D. H. 1944. Studies on Dientamoeba fragilis (protozoa). IV. Further observations, with an outline of present-day knowledge of this species. J. Parasitol. 30:322-337. [Google Scholar]
- 138.Wenrich, D. H., R. M. Stabler, and J. H. Arnett. 1935. Entamoeba histolytica and other intestinal protozoa in 1060 college freshmen. Am. J. Trop. Med. 15:331-345. [Google Scholar]
- 139.Wheatley, W. B. 1951. A rapid staining procedure for intestinal amoebae and flagellates. Am. J. Clin. Pathol. 21:990-991. [DOI] [PubMed] [Google Scholar]
- 140.Windsor, J. J., and E. H. Johnson. 1999. Dientamoeba fragilis: the unflagellated human falgellate: a review. Br. J. Biomed. Sci 56:293-306. [PubMed] [Google Scholar]
- 141.Windsor, J. J., L. MacFarlane, G. Hughes-Thapa, S. K. A. Jones, and T. M. Whiteside. 2003. Detection of Dientamoeba fragilis by culture. Br. J. Biomed. Sci. 60:79-83. [DOI] [PubMed] [Google Scholar]
- 142.Windsor, J. J., and A. M. Rafay. 1997. Laboratory detection of Dientamoeba fragilis. Br. J. Biomed. Sci. 54:223-224. [PubMed] [Google Scholar]
- 143.Windsor, J. J., A. M. Rafay, A.K. Shenoy, and E. H. Johnson. 1998. The incidence of Dientamoeba fragilis in faecal samples submitted for routine microbiological analysis. Br. J. Biomed. Sci. 55:172-175. [PubMed] [Google Scholar]
- 144.Yang, J., and T. Scholten. 1976. Celestin blue B stain for intestinal protozoa. Am. J. Clin. Pathol. 65:715-718. [DOI] [PubMed] [Google Scholar]
- 145.Yang, J., and T. Scholten. 1977. A fixative for intestinal parasites permitting the use of concentration and permanent staining procedures. Am. J. Clin. Pathol. 67:300-304. [DOI] [PubMed] [Google Scholar]
- 146.Yang, J., and T. H. Scholten. 1977. Dientamoeba fragilis: a review with notes on its epidemiology, pathogenicity, mode of transmission, and diagnosis. Am. J. Trop. Med. Hyg. 26:16-22. [DOI] [PubMed] [Google Scholar]
- 147.Yoeli, M. 1955. A report on intestinal disorders accompained by large numbers of Dientamoeba fragilis. J. Trop. Med. Hyg. 58:38-41. [PubMed] [Google Scholar]