Abstract
Twenty percent of very-low-birth-weight (<1500 g) preterm infants experience a serious systemic infection, and despite advances in neonatal intensive care and antimicrobials, mortality is as much as threefold higher for these infants who develop sepsis than their counterparts without sepsis during their hospitalization. Outcomes may be improved by preventative strategies, earlier and accurate diagnosis, and adjunct therapies to combat infection and protect the vulnerable preterm infant during an infection. Earlier diagnosis on the basis of factors such as abnormal heart rate characteristics may offer the ability to initiate treatment prior to the onset of clinical symptoms. Molecular and adjunctive diagnostics may also aid in diagnosing invasive infection when clinical symptoms indicate infection but no organisms are isolated in culture. Due to the high morbidity and mortality, preventative and adjunctive therapies are needed. Prophylaxis has been effective in preventing early-onset group B streptococcal sepsis and late-onset Candida sepsis. Future research in prophylaxis using active and passive immunization strategies offers prevention without the risk of resistance to antimicrobials. Identification of the differences in neonatal intensive care units with low and high infection rates and implementation of infection control measures remain paramount in each neonatal intensive care unit caring for preterm infants.
INTRODUCTION
Immature host defense mechanisms and invasive life support systems make the premature neonate particularly susceptible to overwhelming infection. Approximately 20% of very-low-birth-weight (VLBW) (birth weight <1,500 g) preterm infants experience a serious systemic infection during their initial hospital stay (441, 454, 457). While advances in neonatal intensive care have resulted in improved survival of preterm infants, mortality is as much as threefold higher for VLBW infants who develop sepsis than for those without sepsis (136, 454). In fact, sepsis accounts for approximately half of all deaths beyond the second week of life in VLBW infants (452). While the past decade has been marked by a significant decline in early-onset group B streptococcal (GBS) sepsis in both term and preterm neonates, the overall incidence of early-onset sepsis has not decreased in many centers, and several studies have found an increase in sepsis due to gram-negative organisms (95, 283, 454, 473). Infections with multidrug-resistant organisms (62, 231, 454, 473) and Candida (454) are also increasing in incidence. This review focuses on the bacterial and fungal organisms causing perinatally acquired and nosocomial sepsis in VLBW neonates and the various efforts to prevent infection in this vulnerable population. In addition, we discuss nonculture methods of predicting or detecting infection that may in the future enable clinicians caring for these infants to limit the use of empiric antibiotics and facilitate earlier detection of life-threatening infections.
MICROORGANISMS AND VERY-LOW-BIRTH-WEIGHT INFANTS: SCOPE OF THE PROBLEM
Recent data indicate that VLBW infants account for approximately 1.4% of all live births in the United States, or about 56,270 infants per year, and about one-third of this group are extremely low birth weight (ELBW) (birth weight <1,000 g) (18). While it is well known that the incidence of neonatal sepsis is inversely proportional to birth weight and gestational age, it is only recently that studies of neonatal sepsis have addressed VLBW and ELBW infants separately from other preterm and term infants. It is important to bear this in mind when interpreting the literature on neonatal sepsis, since the incidence of sepsis in term neonates is around 0.1%, compared to the incidence among all VLBW neonates of approximately 20% (454). With decreasing birth weight comes increasing risk of sepsis, since only 10% of infants with birth weights between 1,000 and 1,500 g develop sepsis compared with 35% of infants with birth weights of <1,000 g and 50% of infants with birth weights of <750 g. Some studies group infants according to gestational age, a more accurate determinant of immune function but a more subjective criterion than birth weight. While the two are usually directly related, factors such as intrauterine growth restriction may lead to a small-for-gestational-age (SGA) VLBW infant whose immune competence and risk for infection are more in line with gestational age rather than with birth weight. Immune competence and other sepsis risk factors vary widely between, for example, a 24-week 600-g infant, a 30-week 1,250-g infant, and a 37-week 1,200-g SGA infant, all of whom by definition are VLBW infants. Immature host defenses (Table 1) appear to play a larger role in risk of infection in infants of lower birth weight (<750 g) and gestational age (<28 weeks), whereas for more mature infants, other risk factors such as abdominal surgery or the presence of a central venous catheter or endotracheal tube are more important in identifying high-risk patients. Hence, studies that examine sepsis by categories of 100-g increments of birth weight or by narrow ranges of gestational age contribute the most to our understanding of sepsis in the VLBW infant.
TABLE 1.
Susceptibility of preterm infants to sepsis
| Antimicrobial defense | Preterm infant compromised defenses |
|---|---|
| Epidermal and epithelial barriers | Immature skin (3-6-cell stratum corneum and thin keratin layer) |
| Insensible water loss from skin and humidification systems, creating moist skin that favors the growth of microorganisms | |
| Skin trauma from intensive care interventions | |
| Adherence of Candida to exposed intermediate epithelial cells, favoring colonization | |
| Enhanced adherence of Candida and GBS to buccal epithelial cells of preterm versus term infants, facilitating oral colonization | |
| Invasive catheters and tubes | |
| Surface for colonization provided by intravascular catheters breaching intact epidermis | |
| Proliferation due to parenteral nutrition and biofilms on plastic catheters | |
| Colonization of endotracheal and nasogastric tubes | |
| Trauma from endotracheal, suctioning, and nasogastric tubes | |
| Intact endothelial tissues | Trauma to endothelium and endocardium from central vascular catheters |
| Injury from hyperosmolar nutrition solutions and medications | |
| Gastrointestinal mucosa | Decreased acid production |
| Immature peristalsis and reduced absorption, favoring microorganism overgrowth | |
| Thin mucin layer, leading to decreased barrier function and secretory IgA binding | |
| Diminished number of intraepithelial lymphocytes | |
| FIP | |
| NEC | |
| Microflora | Competitive bacterial microflora diminished by broad-spectrum antibiotics, favoring growth of resistant bacterial and fungal organisms |
| More commensal gram-positive organisms selected for by human milk | |
| Complement | Lower levels with decreasing gestational age |
| Cytokines | Decreased production of IL-1, IL-8, gamma interferon, TNF-α, G-CSF, and GM-CSF |
| Defensins | Diminished with decreasing gestational age |
| Neutrophils | Decreased bone marrow storage pools and G-CSF levels |
| Immature neutrophil oxidative burst | |
| Decreased numbers of azurophilic granules | |
| Diminished granular contents (lactoferrin, defensins, lysozyme, myeloperoxidase, proteases, and cathepsin G) | |
| Possible impairment of function by medications (steroids, H2 antagonists, lipid emulsions) | |
| Monocytes | Diminished number and function |
| Decreased adherence at sites of infection | |
| Decreased opsonization and phagocytosis | |
| Diminished gamma interferon, IL-3, and G-CSF release | |
| T-cells, B-cells, and antibodies | Decreased numbers of lymphocytes in gastrointestinal tract |
| Majority of transfer of maternal IgGs after 32 weeks' gestation | |
| Decreased production of antibodies by preterm lymphocytes |
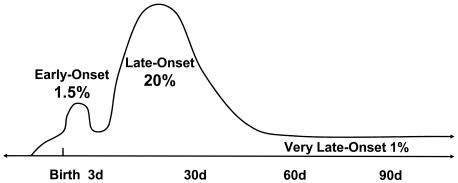
Sepsis in VLBW infants has been classified as early-onset neonatal sepsis (EONS; <72 h), late-onset neonatal sepsis (LONS; >3 days) and late-late-onset sepsis (LLOS; >3 months). These definitions have contributed greatly to diagnosis and treatment by identifying which microorganisms are likely to be responsible for sepsis during these periods and the expected outcomes of infection. Very-late-onset neonatal sepsis (VLONS), defined as sepsis starting >60 days after birth, is >2 standard deviations above the mean and may be a useful classification since sepsis at this point occurs in VLBW infants who still have a central vascular catheter, gastrointestinal disease, or chronic lung disease and appears to have better outcomes.
Recent data on the epidemiology of sepsis in preterm neonates in the United States may be obtained from several clinical consortia. The National Institute of Child Health and Human Development (NICHD) Neonatal Research Network, composed of approximately 15 neonatal intensive care units (NICUs), prospectively collects epidemiologic data on EONS and LONS sepsis in VLBW neonates (Table 2) (453, 454). In the most recent NICHD surveys, from 1998 to 2000, 1.5% of VLBW infants had EONS, defined as a positive blood culture with clinical symptoms (453). Given the widespread use of intrapartum antibiotics with preterm labor, the incidence of clinical sepsis (symptoms and abnormal laboratory findings with no growth from blood culture) is significantly higher than the incidence of culture-proven sepsis. LONS is over 10 times more common than EONS in VLBW infants (Fig. 1). The NICHD reported a 21% incidence of blood culture-proven LONS among VLBW infants (454). The incidence is higher among infants of <25 weeks gestation, with 46% of these infants suffering from LONS (454). Similar rates of sepsis in VLBW infants have been reported by other clinical consortia, with a range of 11 to 30% (61, 85, 451); (Vermont Oxford Network 2001 Database Summary). The Vermont Oxford Network reported that, among over 30,000 patients with birth weight of 501 to 1,500 g admitted to their hospitals in 2001, the incidence of EONS and LONS was 2 and 21%, respectively (Vermont Oxford Network 2001 Database Summary). The Centers for Disease Control and Prevention collect data on neonatal nosocomial infections through the Pediatric Prevention Network and the National Nosocomial Infections Surveillance (NNIS) System. In a point prevalence survey of 29 hospitals in 1999, the Pediatric Prevention Network found that the prevalence of NICU-acquired bloodstream infection was 11% among VLBW neonates (172). Relating infection to particular high-risk interventions, such as the presence of central vascular catheters, can provide useful information for comparisons of interinstitutional variations in sepsis rates. An NNIS survey of 138 high-risk nurseries, conducted between 1995 and 2001, reported the incidence of umbilical and central line-associated bloodstream infections to be 11.3 per 1,000 catheter-days for neonates with birth weight of <1,000 g and 6.9 per 1,000 catheter-days for infants with birth weight between 1,000 and 1,500 g (335a). Similar numbers were recently reported by the Canadian Neonatal Network (85).
TABLE 2.
Organisms and death rates associated with bloodstream infections in VLBW (<1,500 g) neonatesa
| Organism | EONS
|
LONS
|
||
|---|---|---|---|---|
| No. of infections (% of total) | Mortality (%)b | No. of infections (% of total) | Mortality (%)b | |
| Gram-positive bacteria (total) | 31 (36.9) | 26 | 922 (70.2) | 11.2 |
| GBS | 9 (10.7) | 30 (2.3) | 21.9 | |
| Viridans streptococcus | 3 (3.6) | |||
| Other streptococci | 4 (4.8) | |||
| Listeria monocytogenes | 2 (2.4) | |||
| Coagulase-negative Staphylococcus | 9 (10.7) | 629 (47.9) | 9.1 | |
| Staphylococcus aureus | 1 (1.2) | 103 (7.8) | 17.2 | |
| Enterococcus species | 43 (3.3) | |||
| Other | 3 (3.6) | 117 (8.9) | ||
| Gram-negative bacteria (total) | 51 (60.7) | 41 | 231 (17.6) | 36.2 |
| Escherichia coli | 37 (44.0) | 64 (4.9) | 34.0 | |
| Haemophilus influenzae | 7 (8.3) | |||
| Citrobacter | 2 (2.4) | |||
| Bacteroides | 2 (2.4) | |||
| Klebsiella | 1 (1.2) | 52 (4.0) | 22.6 | |
| Pseudomonas | 35 (2.7) | 74.4 | ||
| Enterobacter | 33 (2.5) | 26.8 | ||
| Serratia | 29 (2.2) | 35.9 | ||
| Other | 2 (2.4) | 18 (1.4) | ||
| Fungi (total) | 2 (2.4) | 160 (12.2) | 31.8 | |
| Candida albicans | 2 (2.4) | 76 (5.8) | 43.9 | |
| Candida parapsilosis | 54 (4.1) | 15.9 | ||
| Other | 30 (2.3) | |||
NICHD Neonatal Network Survey, 1998 to 2000 (453, 454). A total of 5,447 patients with EONS and 6,215 patients with LONS were studied. There were a total of 84 bloodstream infections in the EONS patients (1.5% of the total) and 1,313 bloodstream infections in the LONS patients (21.1% of the total).
All-cause mortality.
FIG. 1.
Timing of bacterial and fungal sepsis in VLBW infants. Percentages indicate the approximate number of VLBW infants with septicemia. EONS usually occurs via ascent of organisms from the birth canal to the amniotic fluid, with or without rupture of amniotic membranes. LONS occurs with vertical and horizontal spread of organisms. While the vast majority of cases of sepsis in VLBW infants occur in the first 30 days of life, VLBW infants requiring prolonged intensive care are at risk for VLONS beyond 2 months of age.
Significant interinstitution variation in the incidence of LONS has been reported. In a survey of six Boston area intensive-care nurseries, the incidence of bloodstream infection in VLBW neonates older than 2 days ranged from 8.5 to 42% (61), and among the 15 centers constituting the NICHD Neonatal Network, the rates of LONS ranged from 11 to 32% (454). Another recent study of 21 NICUs also found significant interinstitution differences in the incidence of LONS (74). Identification of clinical practices associated with the lowest rates of nosocomial sepsis in particular nurseries is an important task for these and other clinical consortia.
While the majority of VLBW infants have only one episode of culture-proven sepsis during their NICU hospitalization, 20% have two events, 6% have three, and 2% have four (454). Multiple sepsis episodes are more common in the lowest-birth-weight categories, with almost 40% of infants with birth weights of <750 g having two or more episodes. Also striking is that only one of every five evaluations for sepsis with a blood culture yielded a microorganism. This underscores the finding, that 80% of the time, empiric antibiotics will be given when no organism is isolated from culture (454).
DEFINITIONS OF SEPSIS AND FOCAL INFECTIONS IN VERY-LOW-BIRTH-WEIGHT INFANTS
Isolation of an organism from a blood culture of a neonate with clinical symptoms of infection constitutes the common definition of sepsis. Due to technical constraints, often only a single peripheral blood culture is obtained from a septic-appearing neonate, and in most studies the isolation of an organism from one blood culture is considered evidence of sepsis. In the case of coagulase-negative Staphylococcus (CoNS), which is both a common cause of sepsis and a frequent blood culture contaminant, many recent studies require either isolation from two blood cultures or a single positive blood culture with other laboratory evidence of sepsis, such as an elevated serum C-reactive protein level (CRP).
Many neonates with strong clinical indicators of sepsis, including severe apnea, lethargy, and hypotension (136), and laboratory abnormalities such as neutropenia, bandemia, and elevated CRP levels, have a negative blood culture. For this reason, some published studies of neonates include patients with the loosely defined entities “clinical sepsis” or “probable sepsis,” either as a separate group or together with culture-proven sepsis. In these patients, the blood culture may be falsely negative or the patient may be experiencing a systemic inflammatory response due to a viral infection or noninfectious process. An official classification scheme has been adopted for pediatric and adult patients, with four categories of sepsis: systemic inflammatory response syndrome, sepsis, septic shock, and severe sepsis (284). However, no similar classification scheme is in routine use for neonates. Development of a “neonatal sepsis scale” including sepsis with shock and a more rigorous definition of “clinical sepsis” in neonates will allow better correlation with outcomes and a more consistent interpretation of epidemiologic and clinical research studies and should be a priority for those performing research in the field of neonatal sepsis.
Focal infections of the skin, urinary tract, lungs, central nervous system (CNS), and intestinal tract are also common in preterm infants and may occur with or without a positive blood culture. While a comprehensive discussion of focal infections in preterm infants is beyond the scope of this review, definitions and brief descriptions of their association with sepsis are warranted.
Urinary tract infection (UTI) is generally defined as growth of at least 100 CFU per ml from a specimen obtained by suprapubic bladder aspiration or at least 10,000 CFU per ml from a sterile bladder catheterization (40, 124). Lower colony counts may be indicative of infection in preterm infants, particularly if the isolate is a gram-negative organism or Candida, and treatment should be considered due to the high risk of ascending infection and sepsis. Urine culture is not indicated in the evaluation of EONS since the urine is sterile in the first 48 to 72 h of life (464). However, urine culture should be obtained in all evaluations of sepsis beyond day 3 of life, since the clinical presentations of UTI and sepsis are similar. In a study evaluating VLBW infants with suspected sepsis, the incidence of UTI was 12.2% in ELBW infants and 5.7% in infants with birth weights of 1,000 to 1,500 g, for a combined incidence of 8.1% in all VLBW infants (124). In this study, vesicoureteral reflux was present in 14% of VLBW infants with UTI (124); therefore, a voiding cystourethrogram and renal ultrasound should be considered for preterm infants with a UTI.
Any isolation of a microorganism from a cerebrospinal fluid (CSF) culture is generally considered evidence of meningitis in preterm infants, regardless of the CSF cell count or chemistries. Diagnosis of meningitis is problematic in VLBW infants since the CSF white blood cell count is an unreliable indicator of meningitis in these infants and the lumbar puncture is often delayed until after antimicrobials have been administered, when the patient is judged to be more stable (114). There are no data in preterm infants examining trends in CSF pleocytosis after antibiotics have been administered. Some clinicians do not routinely perform lumbar puncture in preterm infants with suspected sepsis unless there is growth of an organism on blood culture (142, 497). Since meningitis may occur without septicemia and since blood cultures may be falsely negative, this places VLBW infants at risk of being undertreated for meningitis (454a).
Pneumonia remains the most difficult infection to diagnose in preterm infants (17, 34, 94). In intubated VLBW infants, bronchopulmonary dysplasia and chronic lung disease exhibit similar clinical signs and symptoms, timing, and radiographic changes compared with pneumonia. Organisms cultured from the endotracheal tube often represent colonization rather than pneumonia. Quantitative cultures from endotracheal tube aspirates may reveal a large number of organisms, and Gram stain may demonstrate many white blood cells at times when there are no radiographic or clinical symptoms of pneumonia (123, 232). Better methods of assessing the microorganisms in the lungs are needed because this is probably a very common site of infection in intubated VLBW infants (34, 94).
Necrotizing enterocolitis (NEC), while multifactorial in etiology, is frequently associated with either clinical or culture-proven sepsis in VLBW infants. NEC is commonly defined using Bell's criteria (41), which are based in large part on radiographic findings. However, several studies have found that radiography and ultrasonography have a lower sensitivity than expected in detecting pneumatosis intestinalis, portal gas, and intestinal perforation in VLBW infants with NEC (263, 293, 463). The more extremely preterm infants, as well as some VLBW infants, particularly those who have never been fed, may present with a gasless abdomen, and clinical symptoms and laboratory data may be just as vital as imaging studies in determining the medical or surgical management of these infants.
DIAGNOSIS OF SEPSIS IN VERY-LOW-BIRTH-WEIGHT INFANTS
The diagnosis of neonatal sepsis begins with clinical suspicion (136). The challenge for the neonatal practitioner is to decide which babies need empiric antibiotic therapy and for how long, a decision which is complicated by the common occurrence and nonspecific nature of sepsis-like symptoms in preterm infants. The prevalence and predictive value of common signs and symptoms of sepsis were described in a study by the NICHD Neonatal Network (136). The most common presenting symptoms in VLBW infants undergoing evaluation for suspected sepsis were increased apnea (55%), gastrointestinal problems (46%), increased need for oxygen or ventilatory support (36%), and lethargy/hypotonia (23%). The positive predictive value of these signs was low for culture-proven septicemia, ranging from 14 to 20%. The strongest predictor of septicemia in this study was hypotension, present in fewer than 5% of infants, which had only a 31% positive predictive value.
The “gold standard” for diagnosing neonatal sepsis remains the blood culture, even though, in many cases, blood cultures are negative in the face of strong clinical indicators of septicemia and even in autopsy-proven disseminated bacterial or fungal infection. In a 1999 autopsy study of ELBW infants, infection was deemed the primary cause of death by pathologists in the majority of infants (56 of 111), while sepsis was not diagnosed prior to death for 61% of these neonates (38). Maternal antibiotics given in the majority of preterm deliveries may suppress the growth of bacteria in culture, yet the neonate may have clinical symptoms and laboratory findings consistent with a diagnosis of sepsis. False-negative blood cultures in apparently septic neonates may also result from insufficient sample size. One study estimated that as many as 60% of blood cultures would be falsely negative for common neonatal pathogens if only 0.5 ml of blood is sampled in low-colony-count (<4 CFU/ml) sepsis (251). While neonates are commonly thought to have high-colony-count bacteremia compared with adults, as many as half of the neonates in one study were found to have low-colony-count bacteremia (251). Furthermore, in a prospective study of nearly 300 blood cultures drawn from critically ill neonates, 55% of culture vials contained less than 0.5 ml of blood (337). Technical difficulties associated with phlebotomy in small, sick preterm neonates often limit the volume of blood obtained and thus decrease the sensitivity of blood culture for diagnosing sepsis in this population.
Use of Adjunct Laboratory Tests To Predict Septicemia
A number of adjunctive tests, including measurements of serum interleukin-6 (IL-6) (115, 272, 341), IL-8 (145), procalcitonin (265), and CRP (77, 154, 340) levels, have been studied for their ability to predict sepsis in preterm neonates with clinical signs and symptoms of infection (Table 3). Although serum cytokine levels may rise 12 to 48 h prior to the onset of sepsis symptoms (426) and have been found to be highly predictive of neonatal sepsis, cytokine testing is not routinely performed in most clinical chemistry laboratories. Serial measurements of CRP levels appear to have the best discriminatory value for predicting septicemia among the tests currently available in many hospital laboratories, and the ability to produce CRP does not appear to be affected by gestational age (8, 377, 426). Several studies have specifically addressed the utility of CRP screening in VLBW neonates, with the sensitivity of a CRP value greater than 1 mg/dl for blood culture-proven sepsis ranging from 48 to 63% when a single test is performed and from 84 to 90% when multiple CRP screens are performed over a 24- to 48-h period following onset of symptoms (77, 487). In many studies, CRP has been shown to have higher sensitivity and negative predictive value than leukocyte indices such as the immature/total neutrophil ratio for predicting bacteremia (154). Thus, normal CRP values on serial testing may be useful in ruling out infection in preterm neonates with nonspecific symptoms and negative blood cultures (56, 120). It should be noted, however, that the sensitivity of CRP for predicting bacteremia is organism dependant. Several studies have shown that the sensitivity of serial CRP testing is lower for bacteremia due to gram-positive than to gram-negative bacteria, with the sensitivity for detecting CoNS being as low as 55% (378, 391). This may be because CoNS are relatively low-virulence pathogens and are less able to stimulate an acute-phase response or because a substantial number of infants with blood culture isolates for CoNS are not truly infected. In any case, withholding antibiotics or discontinuing antibiotic therapy less than 48 h after obtaining a blood culture in a symptomatic preterm neonate on the basis of normal CRP levels is not recommended. However, normal CRP levels have been used in the decision to discontinue empiric antibiotic therapy if cultures are negative after 48 h (249, 492). Some institutions monitor CRP levels in bacteremic neonates and discontinue antibiotic administration when the CRP level has returned to normal (377), although to our knowledge this practice has not been subjected to a rigorous clinical trial. Monitoring the trend in CRP level until it declines in a septic-appearing neonate may be of value, since a continued rise in the CRP level in a patient on antimicrobial therapy may indicate persistent septicemia, an infected central vascular catheter, infection with resistant bacteria or fungi (491), or the presence of a nidus of infection which is not reached by antibiotics, such as an abscess (223).
TABLE 3.
Diagnostic utility of CRP and cytokine measurements in predicting sepsis in VLBW infantsa
| Study | Infant population | No. of patients | No. of culture- proven sepsis events | Time of testing | Test | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|---|
| Franz et al., 2001 (145) | Preterm and term | 709 (387 preterm) | 14 | Birth | IL-8 | NR | NR | ||
| 22-26 wk | 93 (a) | 55 (a) | |||||||
| 27-29 wk | 89 (a) | 56 (a) | |||||||
| 30-32 wk | 83 (a) | 77 (a) | |||||||
| Kuster et al., 1998 (272) | VLBW | 125 | 21 | >2 days | IL-1ra | 100 (a) | 92 (a) | NR | NR |
| IL-6 | 89 (b) | 83 (b) | |||||||
| CRP | 50 (b) | 93 (b) | |||||||
| Ng et al., 1997 (340) | VLBW | 68 | 45 | >3 days | CRP + IL-6 | 98 (c) | 91 (c) | 90 (c) | 98 (c) |
| Chan and Ho, 1997 (77) | VLBW | 70 | 30 | >3 days | CRP | 48 (d) | 79 (d) | 74 (d) | 55 (d) |
| Wagle et al., 1994 (487) | <29 wk gestation | 123 | 36 | 1-87 days | CRP | 90 (a) | 81 (a) | 48 (a) | 98 (a) |
| Seibert et al., 1990 (426) | 23-31 wk gestation | 125 | 8 | Birth | CRP | 63 (a) | 70 (a) | 13 (a) | 96 (a) |
| 67 (b) | 82 (b) | 60 (b) | 86 (b) | ||||||
| 85 | 23 | >3 days | CRP | 57 (a) | 61 (a) | 30 (a) | 82 (a) | ||
| 58 (b) | 90 (b) | 93 (b) | 49 (b) |
Selected studies are summarized with sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) calculated for CRP and cytokine levels in VLBW infants. Tests were compared to different definitions of infection, which included culture-proven plus clinical sepsis (a), culture-proven sepsis (b), septicemia plus NEC plus microbiologically confirmed focal infection (c) or sepsis-like syndrome with positive blood, CSF, or joint fluid culture (d). Serial testing was performed in all studies except one (Chan et al.), but the number and timing of tests varied for the other studies. Positive values were defined as CRP ≥ 1.0 to 1.5 mg/dl, IL-6 ≥ 25 to 31 pg/ml, IL-8 ≥ 70 pg/ml, and IL-1 receptor antagonist (IL-1ra) ≥ 12,000 pg/ml. NR, not reported.
While the finding of a normal CRP level can be reassuring, the significance of an abnormal CRP value in a preterm neonate is less clear. Common conditions in premature neonates such as fetal distress, premature or prolonged rupture of membranes, hyperbilirubinemia, and respiratory distress syndrome have been associated with elevation of serum CRP levels in some (8, 86) but not all (418) studies. As many as 9% of apparently healthy neonates have been reported to have a CRP level greater than 1.0 mg/dl (374, 378). In addition, the effect on CRP levels of subacute or chronic medical conditions common to preterm infants, such as intraventricular hemorrhage and chronic lung disease, has not been elucidated. For these reasons, elevations in CRP levels should not be used as the sole indication for either starting or stopping antibiotic therapy; instead, they should be viewed in the context of blood culture results, clinical findings, and other laboratory studies. It is possible that a combination of serum markers such as CRP, IL-6, IL-8, and procalcitonin may prove to be useful adjuncts for detecting neonatal sepsis in the future. Gerdes showed that performing two or more tests at two different time points significantly improves the sensitivity and specificity of adjunct tests of neonatal sepsis (154).
In recent years, neonatal studies have reported that abnormal heart rate characteristics (HRC), defined as lack of heart rate variability and transient decelerations, occur 12 to 24 h prior to the onset of other clinical signs in infants with sepsis and sepsis-like syndrome (169, 170). There is speculation that cytokines, whose levels are elevated as much as 48 h before onset of sepsis symptoms, may be responsible for these changes in HRC (272). Griffin and coworkers have suggested that continuous monitoring of HRC may allow the earlier detection of infection and initiation of antibiotics, potentially improving outcomes. In a study of 633 NICU patients, an HRC index was developed and validated externally at another institution and was able to identify infants with a five- to six-fold-increased risk for developing sepsis or a sepsis-like illness in the following 24 h (169, 170).
Nonculture Microbiological Methods for Predicting Bloodstream Infection
Antigen detection.
Given the inherent problems with using blood culture as the sole method of detecting septicemia in premature neonates, other nonculture microbiologic methods have been developed. Antigen detection in the urine or CSF has been used in the past as an adjunctive test for the presence of GBS. The sensitivity of latex particle agglutination compared to culture for the detection of invasive GBS disease has been reported at 72 to 89% for CSF and slightly lower for urine (26, 271). However, a number of studies have shown that GBS antigen is often detectable in the urine in the absence of a positive blood culture, possibly due to surface colonization (404), absorption of GBS antigen from the gastrointestinal tract (360), or maternal treatment with antibiotics to prevent neonatal bacteremia (191). Thus, while the sensitivity and negative predictive value of urine GBS latex agglutination are high, false-positive rates as high as 30% have limited its usefulness as a screening tool for sepsis in neonates, and many laboratories have discontinued testing for urine GBS antigen (502).
Molecular diagnostics.
PCR has proved to be a valuable adjunct for detection of neonatal viral infections such as human immunodeficiency virus, herpes simplex virus, and hepatitis C virus when used in conjunction with other diagnostic testing such as serologic testing or culture. However, the use of PCR to detect bacteremia and fungemia is more challenging and thus is still under investigation. Detection of bacterial DNA in the blood has been accomplished by PCR amplification of the gene for 16S rRNA, a gene universally present in bacteria but absent in humans. Detection of as few as 10 organisms per ml of whole blood has been reported, and research into how to enhance the sensitivity and automate the PCR is under way (314). A major concern about bacterial PCR is possible contamination due to the widespread presence of bacterial DNA in the environment, which may be a major stumbling block to clinical applications. However, several studies with neonates have shown promising results. In the largest study to date of PCR for detection of neonatal bacteremia, Jordan and colleagues collected 548 paired blood specimens from patients admitted to the NICU with suspected sepsis. Of 25 specimens with positive blood cultures, 24 were positive by 16S rRNA PCR. PCR results were available within 9 h of sample acquisition, and subsequent DNA hybridization was able to distinguish gram-positive from gram-negative organisms. Only 3 of 548 samples were positive by PCR and negative by blood culture. The sensitivity, specificity, positive predictive value, and negative predictive value of this PCR were 96, 99.4, 88.9, and 99.8%, respectively (230). In this study, blood samples were cultured for 4 to 6 h prior to hydribization and PCR to increase the sensitivity of the assay. Two smaller studies of neonates have shown similar high sensitivity of whole-blood 16S rRNA PCR for detection of culture-proven bacteremia (273, 431). While these are encouraging results, further large clinical studies are necessary to determine whether PCR will be a useful adjunctive test to rapidly detect the presence or absence of bacteremia in high-risk neonates. If the high negative predictive value of PCR is substantiated in larger trials, this test may be particularly useful for avoiding the overuse of empiric antibiotics in patients with nonspecific symptoms common to both septic and nonseptic preterm neonates.
PCR detection of fungemia is another important area of investigation. Preterm infants are at high-risk of morbidity and mortality from invasive Candida disease, and earlier treatment of Candida sepsis, including central vascular catheter removal, may be associated with an improved outcome (146). PCR assays to detect a variety of genes present in multiple species of fungi have been developed and tested both in vitro and in blood samples from patients at risk for fungal sepsis. Einsele et al. used PCR for the 18S rRNA gene, which is present in nearly all clinically relevant fungal species, to test over 600 blood samples from adult cancer patients with febrile neutropenia and healthy controls (122). Compared to blood culture, PCR was 100% sensitive and 98% specific for detection of invasive fungal infection. Two studies have reported the use of PCR for detecting fungemia in pediatric patients. Jordan and colleagues reported the detection of fungal DNA in 26 of 27 pediatric blood samples that were culture positive for Candida, using PCR amplification of the chitin synthase gene involved in fungal cell wall formation (229). Our group recently reported that, in NICU and pediatric ICU patients with suspected sepsis, PCR for the panfungal 18S rRNA gene was positive in all nine paired blood samples from which blood cultures yielded Candida species (468). Furthermore, all 44 samples negative for fungal DNA by PCR were associated with a negative blood culture for Candida. We also identified three patients with positive fungal PCR and negative blood culture who had other evidence of invasive fungal disease, including two NICU patients with candiduria and signs of sepsis and one patient with NEC, intestinal perforation, and Candida peritonitis. While animal studies suggest that PCR may be more sensitive than blood culture for the detection of fungemia (480), further studies are necessary to determine whether this may be the case in humans as well. Positive fungal PCR results may indicate true invasive disease, transient fungemia that will clear without therapy, presence of dead organisms in the bloodstream, or specimen contamination during collection or processing. Jordan found that blood samples from 29 neonates at high risk for Candida sepsis, but with no clinical evidence of disease, as well as blood from individuals with mucosal colonization, were negative by fungal PCR. In contrast, our study identified seven patients with negative blood culture and no other evidence of invasive fungal disease whose blood samples were positive for fungal DNA by 18S rRNA PCR. Further refinements of PCR methods, including the use of newer methods such as quantitative real-time PCR, together with large clinical trials, are necessary to determine whether PCR will be a useful tool to rapidly predict the presence or absence of disseminated fungal or bacterial infection in high-risk patients with sepsis-like symptoms.
Another promising approach to molecular diagnosis of sepsis involves the use of DNA microarray technology (343a). Devices are being developed which incorporate automated DNA analyzers with integrated sample preparation and biosensing elements which could allow rapid and sensitive detection of small numbers of organisms in minute volumes of blood. In the near future, microchip detection of bloodstream infection may allow practitioners to tailor antibiotic therapy toward specific pathogens and avoid unnecessary antibiotic therapy and its negative consequences.
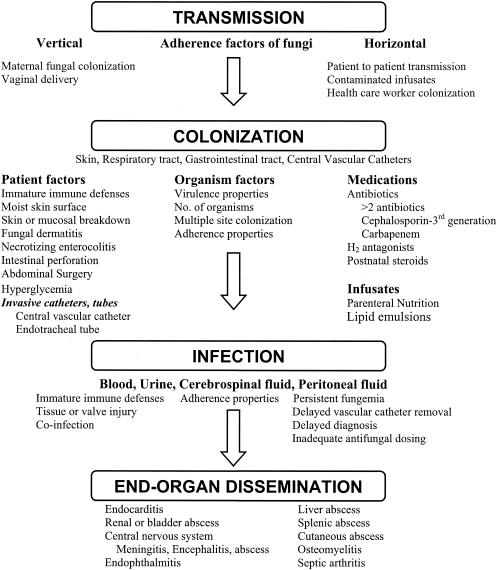
Routes of Infection
Transplacental, hematogenous transmission of bacteria is an uncommon route of EONS and occurs primarily with Listeria monocytogenes. The most common route of EONS in preterm and term infants is via an ascending amniotic infection. Members of the maternal genital flora, such as GBS and Escherichia coli, the organisms responsible for the majority of cases of EONS, may ascend through the birth canal to the amniotic fluid either through intact amniotic membranes or, more commonly, after rupture of membranes. Once infected amniotic fluid is aspirated or swallowed by the fetus, pathogens may penetrate through immature mucosal barriers, resulting in pneumonia or bacteremia, and may penetrate the blood-brain barrier, leading to meningitis. Bacteria have been implicated as a major cause of premature rupture of membranes and, consequently, of premature labor and delivery (315). Thus, prevention and timely treatment of intra-amniotic infection are important steps in preventing preterm delivery and improving neonatal outcome.
LONS most commonly occurs via horizontal or nosocomial transmission, but it may occur via vertical transmission at birth, leading to colonization and, later, to infection. Skin or mucosal colonization with potential pathogens may be acquired from the hands of health care workers or family members, from water used in incubator or ventilator humidification systems, or from contaminated fomites such as stethoscopes, which may carry organisms directly from one patient to another. Colonizing organisms may enter the bloodstream through breaks in the skin or mucosa or by gastrointestinal translocation or may be introduced through invasive devices such as vascular catheters, endotracheal tubes, or feeding tubes. Alternately, nosocomial infection may result from infusion of contaminated intravenous solutions (especially lipid-based or high-glucose solutions) or from contaminated formula or breast milk.
Susceptibility of Very-Low-Birth-Weight Infants to Infection
Susceptibility of VLBW infants to infection is presented in Table 1. The first line of defense against infection is an intact epidermis and mucous membranes, barriers that are compromised in VLBW infants. The preterm infant's stratum corneum is only 3 cell layers thick at 26 weeks' gestation (compared to 16 layers in full-term infants), with a thin keratin layer that is easily damaged by handling, adhesives applied to the skin, and alcohol and betadine applications (130). To reduce insensible water loss, the smallest preterm infants are placed in isolettes with as much as 80% humidity in the first days of life, and bacteria and fungi rapidly multiply on moist or damaged skin. Placement of peripheral and central vascular catheters, use of chest tubes, and frequent blood drawing further compromise the skin (37, 76, 130), exposing the deeper layers of the epidermis, to which organisms may adhere and then may proliferate and disseminate (425). Complications such as intravenous infiltrates with substrate-rich media such as parenteral nutrition solutions further facilitate the growth of microorganisms (99, 370, 372, 375).
In addition to the compromised skin barrier, the respiratory and gastrointestinal tracts of the VLBW infant provide surfaces for microbial colonization and invasion. These mucosal surfaces are both colonized and injured by nasogastric and endotracheal tubes and suction catheters. Mucous membrane defenses such as secretory immunoglobulin A (IgA), mucin, and defensins have been shown in some studies to be deficient in VLBW neonates (306, 387). Feeding tubes may also serve as reservoirs for pathogens. In a study of 50 preterm infants in whom orogastric feeding tubes were changed once a week, bacteria were isolated from 117 of 125 weekly feeding-tube cultures (320). The most common isolates were Staphylococcus species and members of the Enterobacteriaceae, whereas Candida was rarely isolated. All seven patients whose orogastric tube cultures yielded >100,000 CFU/ml developed NEC. More frequent changing of enteral feeding tubes may reduce colonization and risk of infection in VLBW neonates.
Once organisms have breached the mucous membranes, immaturities in both innate and adaptive immunity make preterm infants particularly vulnerable to rapid spread of infection (reviewed in references 127, 272, 286 and 376a). Low levels of serum complement, fibronectin, and defensins, as well as abnormalities in cytokine production, have been demonstrated in newborns, with preterm infants exhibiting a greater degree and longer duration of these abnormalities compared with term newborns. Most maternal-fetal transfer of IgG occurs in the third trimester of pregnancy; therefore, infants born prior to 32 weeks' gestation have low levels of passively acquired antibody, in addition to limited production of type-specific antibody in response to invading pathogens (420, 446). Cellular deficiencies of chemotaxis, phagocytosis, and microbial killing further contribute to the the vulnerability of preterm neonates to overwhelming infection (reviewed in reference 127, 272, 286, and 376a).
It is well established that the incidence of sepsis is inversely proportional to birth weight and gestational age (453, 454). Infants who are SGA have been found to be at decreased risk for EONS (454) but at higher risk for LONS than their appropriately grown gestational age-matched peers (82, 437). This may be because SGA infants are susceptible to neutropenia associated with maternal preeclampsia or because they may require central venous catheters and parenteral nutrition for long periods due to a predisposition to feeding intolerance and NEC. Another factor that may contribute to sepsis risk is gender, with male infants showing a disadvantage in some studies. In a 1988 to 1991 survey of 2416 VLBW infants, the NICHD Neonatal Network found that males were nearly 50% more likely to develop LONS than were females (odds ratio [OR], 1.48; 95% confidence interval [CI], 1.17 to 1.88) (136). Of note, however, the more recent Neonatal Network surveys did not find a preponderance of males among VLBW infants with either EONS or LONS. Male gender is a risk for sepsis in adults, attributed at least in part to the immunostimulatory properties of estrogen and the immunosuppressive properties of testosterone (15). Neonates have low levels of sex hormones; therefore, any predisposition to sepsis among male preterm infants it is likely to be attributable to other factors. Adult females generally exhibit a more robust humoral and cell-mediated immune response, leading to increased risk of autoimmune disease and possibly providing some advantage against sepsis compared with males (445), although this has not been thoroughly studied in infants. Some have postulated that genes on the X chromosome regulating thymic development and antibody production may lead to a sepsis gender gap (416). The absence of a sepsis gender gap in more recent studies of VLBW infants may reflect the fact that with increasing survival of the smaller, more vulnerable preterm infants, other risk factors such as prolonged invasive life support systems have overshadowed the male gender risk.
In addition to endogenous risk factors, exogenous factors associated with intensive care, such as parenteral nutrition and medications, further contribute to the vulnerability of preterm infants to infection. Several in vitro studies have shown that exposure to lipid emulsion solutions impairs the chemotaxis of neonatal leukocytes (129, 264, 477), although ex vivo studies of serum or cells from neonates given infusions of lipid emulsion compared to nonexposed patients showed no differences in various immune functions (376a). Lipid solutions support the growth of microorganisms (481), and a number of epidemiologic surveys have found an association between lipid infusions and CoNS bacteremia (21, 148, 311) and fungemia (215, 402) (particularly with Malassezia furfur) (294, 384, 460). Although severe sepsis is associated with altered lipid metabolism, it is not known whether discontinuation of intravenous lipid therapy for 1 to 2 days (except for Malassezia infections [see below]) at the onset of sepsis will decrease the duration of septicemia or otherwise improve the immune response or outcome in VLBW neonates.
A number of pharmacologic agents commonly used in NICU patients have been implicated as risk factors for sepsis. A single course of maternal antenatal corticosteroids, which reduces pulmonary and neurologic morbidity in preterm neonates, has been associated with a decreased risk of EONS, even with premature rupture of membranes (OR, 0.52; 95% CI, 0.31 to 0.88) (507). The effect of antenatal steroids on LONS is less clear. While the NICHD Neonatal Network found an increased risk for LONS in infants exposed to antepartum corticosteroids (OR, 1.29; CI, 1.10 to 1.51) (507), other studies have not found this association (212, 499). In any case, the benefits of antenatal steroids, including decreased mortality, intraventricular hemorrhage, respiratory distress syndrome, and EONS, strongly outweight the risk for LONS. Postnatal systemic steroid treatment has been clearly associated with a higher risk of sepsis in preterm infants, with the incidence of LONS increasing to 33% in steroid-treated VLBW infants (228, 455, 511). While the use of dexamethasone for acute or chronic lung disease has declined due to concerns about adverse neurodevelopmental effects, hydrocortisone use in the most immature VLBW infants with pressor-resistant hypotension and adrenal insufficiency has increased. Practitioners must balance the potential benefits of glucocorticoids with the increased risk of infection in this population.
Preterm infants are commonly treated with histamine type 2 receptor (H2) antagonists for prevention or treatment of stress-induced gastritis or for treatment of gastroesophageal reflux, yet these agents have immunomodulatory properties as well. H2 antagonists impair neutrophil rolling in vitro (510), while other studies have shown the presence of immunostimulatory properties of H2 antagonists (280, 343, 448). In preterm neonates, effects of H2 antagonists and proton pump inhibitors on neutrophil function or cytokine production are probably overshadowed by their effect on pH and gastrointestinal tract colonization with potential pathogens. Gastric alkalinization increases bacterial and fungal proliferation in the small bowel (39, 455), and administration of H2 antagonists has been associated with increased microbial growth in enteral feeding tubes (320) and hence with an increased risk of sepsis and NEC (402) in preterm neonates.
Another pharmacologic agent that may affect neutrophil function is indomethacin, which is commonly used in preterm neonates for closure of a patent ductus arteriosus and for prevention of intraventricular hemorrhage (IVH). Indomethacin has been associated with impaired neutrophil motility and chemotaxis, particularly in preterm infants (236). Although one prospective study found a temporal association of indomethacin treatment and sepsis (200), a more recent meta-analysis (144) and multicenter study found no association (144, 417). Vitamin E therapy has also been shown in a meta-analysis to decrease the risk of severe IVH and rationopathy of prematurity, but may be associated with an increased risk of sepsis in VLBW infants (60).
Finally, the frequent use of broad-spectrum antibiotics, particularly third-generation cephalosporins, in preterm infants alters the intestinal milieu, favoring colonization and infection with multidrug-resistant bacteria and fungi (11, 401, 439). The microflora of the skin and gastrointestinal tract limits fungal proliferation through its direct antifungal properties as well as through competition for adhesion sites and micronutrients (218). Efforts to minimize exposure to broad-spectrum antibiotics in preterm infants are warranted.
BACTERIA
Gram-Positive Organisms
Group B Streptococcus
As a result of intensive efforts at chemoprophylaxis, Streptococcus agalactiae, or GBS, is declining in incidence, but it still remains a major cause of sepsis in preterm and term infants in the United States (421). The pathogenicity of GBS has been attributed to a number of virulence factors, including lipoteichoic acid, a thick polysaccharide capsule, capsular sialic acid, and the enzyme C5a-ase, which inhibits neutrophil accumulation at the site of infection (reviewed in reference 344). In the United States, GBS colonizes the genital and lower gastrointestinal tracts of 15 to 40% of pregnant women (385). Factors that increase the risk of maternal GBS carriage include diabetes, age younger than 20 years, and African American race, and these factors also increase the risk of preterm delivery. Approximately half of all neonates born to GBS-colonized women acquire surface colonization at delivery, and without intrapartum antibiotic therapy, about 1% of colonized full-term infants develop EONS. Compared to term infants, preterm infants are much more susceptible to invasive GBS disease, in particular LONS and VLONS. In a recent case-control study of 122 infants with GBS LONS, 84% of patients were born at <34 weeks of gestation, and the risk for GBS LONS increased by a factor of 1.34 (95% CI, 1.15 to 1.56) for each week of decreasing gestation (290). This is probably due in part to low levels of maternal antibodies, which cross the placenta in the third trimester of pregnancy. A number of studies have shown low levels of GBS type-specific antibodies in infants with GBS sepsis and in their mothers (162). Other risk factors for early-onset GBS disease, which are common in preterm deliveries, include prolonged rupture of the amniotic membranes (>18 h before delivery), maternal intrapartum fever greater than 38°C, and maternal GBS UTI during the pregnancy or at delivery, which may reflect a high level of colonization or the presence of a particularly virulent strain.
GBS sepsis was the first neonatal infection to be defined as EONS, LONS, and LLOS. The majority of LLOS GBS sepsis occurs in preterm infants at an age when the immune system is more mature; thus, mortality due to LLOS GBS sepsis is much lower than that presenting at earlier ages (220, 509). Infants may have persistent colonization from birth or may acquire the organism through nosocomial routes. Transmission of GBS from breast milk, patient-to-patient spread, and colonized nursery personnel has been reported (119, 350). Increased adherence of GBS to buccal epithelial cells from preterm compared to term infants may also be an contributing factor (97). Recurrent GBS sepsis after appropriate antibiotic therapy has also been documented, since treatment fails to eradicate colonization in up to 50% of infants, infants can be repeatedly exposed through breast milk or horizontal contact, and systemic infection does not stimulate the production of protective levels of type-specific antibodies, particularly in preterm infants (166).
In 1996, consensus guidelines aimed at reducing the incidence of GBS EONS were issued. Intrapartum antibiotic prophylaxis of women with GBS rectovaginal colonization or with specific risk factors for sepsis significantly decreased the incidence of GBS EONS among both term and preterm neonates. The Neonatal Network found that the incidence of GBS EONS in VLBW infants declined from 5.9 per 1,000 in 1991-1993 to 1.7 per 1,000 in 1998-2000 (450, 453). A recent study has shown that the screening-based approach is more effective than the risk-factor-based approach in preventing GBS EONS (419), and in 2002 the Centers for Disease Control and Prevention released new recommendations for universal GBS screening of pregnant women and intrapartum penicillin prophylaxis for carriers (419). While this approach is estimated to prevent over 85% of cases of GBS EONS, a number of concerns regarding widespread intrapartum antibiotic prophylaxis have been raised. With over 25% of all pregnant women and over half of those delivering preterm receiving intrapartum antibiotics, the incidence of infections with gram-negative bacteria and antibiotic resistance among gram-negative pathogens has increased in some centers (62, 231, 473). Emergence of penicillin resistance among GBS has not become a problem in the United States; however, approximately 20 to 40% of GBS isolates in some centers are resistant to clindamycin or erythromycin (particularly type V strains) (261, 307). A recent report from Japan describes clinical GBS isolates with intermediate sensitivity and with resistance to penicillin (329), but the clinical significance of this finding is unclear. Because of the risk of emerging resistance and the failure of intrapartum antibiotics to prevent GBS LONS as well as some cases of EONS, efforts to develop a multivalent GBS vaccine are ongoing (24).
Another research priority in the field of GBS disease prevention is the development of a rapid, sensitive, and inexpensive test to detect GBS colonization in women who present to the hospital in labor. A number of commercially developed assays for GBS antigen have been tested, including a recently developed optical immunoassay that appears to have higher sensitivity than enzyme immunoassays. While these tests have high sensitivity for detecting heavy GBS colonization, the overall sensitivity is much lower than that of selective broth culture (23, 403). Since approximately 15% of cases of neonatal GBS sepsis occur when mothers have only light GBS colonization, immunoassays do not currently have adequate sensitivity to be clinically useful. Molecular biology-based assays such as fluorescence in situ hybridization and PCR for rapid GBS screening are under development (20, 250).
Staphylococcus aureus.
Staphylococcus aureus is a much less common cause of neonatal sepsis in recent decades than at its peak incidence in the 1950s through the 1970s. However, it can be a highly virulent pathogen in immunocompromised patients such as premature neonates. Extensive research has focused on the pathogenesis of S. aureus infection and is the subject of a recent review (205). Although S. aureus is more commonly associated with nosocomial sepsis, maternal-fetal infections have been reported. In a case series spanning 3 years from a single institution, seven preterm infants with congenital S. aureus infection were identified, including one with methicillin-resistant S. aureus (MRSA) (14). In all seven cases, amniotic fluid culture as well as initial blood culture of a sample from the infant yielded S. aureus, and in three cases, antenatal invasive procedures (amniocentesis or amnioinfusion) performed within a day of delivery were presumed to have contributed to infection of the fetus. Late-onset S. aureus infections in neonates include skin and deep-seated tissue abscesses, bacteremia/sepsis, endocarditis, septic arthritis, osteomyelitis, pneumonia, and meningitis (19, 224, 334, 365, 368). In addition, S. aureus is one of the most common etiologic agents of ventriculoperitoneal shunt infections in preterm infants with posthemorrhagic hydrocephalus (63, 106). Compared with other neonatal pathogens, S. aureus is associated with a relatively high incidence of deep-seated infection and suppurative complications. Of the pathogens responsible for pneumonia in preterm infants, S. aureus is the most likely to cause pneumatoceles and empyema, sometimes requiring chest tube drainage. S. aureus meningitis may be associated with brain abscesses, and neuroimaging is recommended to determine the duration of therapy. S. aureus endocarditis may occur in the absence of clinical signs such as a heart murmur, and some researchers have advocated routine echocardiography in preterm infants with S. aureus bacteremia, particularly those with a central venous catheter in or near the heart and those with two or more positive blood cultures.
S. aureus toxin-associated diseases have been reported in preterm neonates. Staphylococcal scalded skin syndrome (SSSS), in which S. aureus exfoliative toxins A or B split the granular layer of the skin, resulting in sloughing and erythema, has been found in NICU patients (301, 400). Although SSSS is not associated with severe systemic illness or bacteremia, nosocomial spread among NICU patients has been reported (400), and strict infection control measures should be implemented when a case is suspected. In contrast to the relatively benign nature of SSSS, toxic shock syndrome (TSS) due to S. aureus enterotoxins presents a more fulminant clinical picture. Criteria for diagnosing TSS include fever, hypotension, multiorgan system dysfunction, a diffuse macular rash leading to desquamation, and evidence against an alternative diagnosis. Two cases of mother-infant transmission of a TSS-like illness associated with S. aureus surface colonization have been reported (87, 167), although neither case met the strict definition of TSS. A Japanese group reported 20 infants with exanthema and thrombocytopenia in the first week of life, all of whom were colonized with TSS toxin 1 (TSST-1)-producing strains of MRSA, and they named this disease entity neonatal toxic shock syndrome-like exanthematous disease (NTED) (462). Further studies revealed that seven infants colonized with MRSA, but without NTED, had high titers of anti-TSST-1 IgG, compared with negligible titers in all four patients with NTED. These titers were measured during the acute phase of disease, suggesting that maternal anti-TSST-1 antibodies may be protective against TSS-like disease in neonates. Preterm infants born before transplacental transfer of maternal antibody may thus be at higher risk of acquiring S. aureus toxin-associated disease.
The vast majority of S. aureus clinical isolates produce β-lactamases and are resistant to penicillin. Most S. aureus strains causing colonization and infection in NICUs have remained sensitive to extended-spectrum penicillins, and treatment with oxacillin or nafcillin usually eradicates infection. Persistent or deep-seated infections may require the addition of an aminoglycoside or rifampin for effective clearance (465). Methicillin resistance among S. aureus strains in NICUs has been reported and is commonly associated with episodic outbreaks from a single clone (486). Epidemics of MRSA infection have been associated with understaffing, overcrowding, improper cleaning of equipment and hands, and mixing of patients in the NICU (13, 182). Successful eradication of MRSA outbreaks has been accomplished by scrupulous attention to infection control measures as well as by intranasal mupiricin treatment of colonized patients and health care workers (22, 184, 202). Hexacholoraphene hand washing has also been used to control an MRSA outbreak in a NICU (383).
Vancomycin- or glycopeptide-intermediate S. aureus isolates with drug MICs in the 8-μg/ml range, have been detected since the late 1980s in adults (151, 291, 424); since 2002, vancomycin-resistant S. aureus strains have also been found (766). To date, there are no published reports of vancomycin-intermediate or vancomycin-resistant S. aureus isolated in neonates. With increasing use of vancomycin for treating documented MRSA and CoNS infection and as empiric therapy for suspected LONS, it is possible that vancomycin resistance among S. aureus strains will evolve in the NICU. One approach to preventing the emergence of resistant S. aureus strains is prevention of infection through active and passive immunization of susceptible hosts. Vaccination with S. aureus capsular polysaccharides 5 and 8, which account for up to 90% of infections (128, 295), has proven efficacious in adults (434), and hyperimmune intravenous immune globulin (IVIG) preparations against staphylococcal surface proteins are under investigation for use in VLBW infants.
Coagulase-negative staphylococci.
CoNS are the etiologic agents of the majority of nosocomial infections in premature neonates. Although CoNS are common commensal organisms with little pathogenicity in immunocompetent hosts, premature neonates are particularly susceptible to invasive infection. The first step in the pathogenicity of CoNS involves adherence of the bacteria to skin, mucosal surfaces, or indwelling artificial devices, such as intravascular catheters and CNS shunts, which are commonly used in preterm infants. Adherence of CoNS is facilitated by a capsular polysaccharide adhesin consisting of poly- N-succinyl glucosamine (435, 469). Once adherence and colonization have been established, some CoNS produce an exopolysaccharide “slime,” which allows the organisms to form a biofilm and evade host defense mechanisms and antibiotic activity. The major component of slime is polysaccharide intercellular adhesin (PIA), a linear homoglycan composed of N-acetylglucosamine residues (163). In one study of 179 strains of S. epidermidis, 51% produced PIA and most of these strains formed a biofilm (299). Genes encoding PIA are located in a gene cluster termed the ica (for “InterCellular Adhesion”) operon.
The ability of CoNS to produce slime and biofilms has been linked to increased virulence in preterm infants. One longitudinal study of colonizing strains of CoNS found in 18 preterm neonates demonstrated slime production in 68% of strains on day 1 of life, 89% on day 4, and 95% on day 7 and later (104). In contrast, another study found an overall incidence of slime production of 50% in 105 nasopharyngeal colonizing strains of CoNS from 28 VLBW infants (187). This study did not find an increase in slime production over time in infants hospitalized from 4 to 15 weeks. Another study of 180 clinical isolates of CoNS from NICU patients found that although the ica operon was present in all isolates, quantitative biofilm production was greater among isolates from patients with sepsis than among those associated only with colonization (111). Mixed-species biofilms of S. epidermidis and Candida albicans may be particularly pathogenic to preterm infants. A recent report demonstrated that the slime produced by S. epidermidis inhibited the penetration of fluconazole into mixed fungal and bacterial biofilms and, conversely, that C. albicans protected staphylococci from the action of vancomycin (6). This may play a role in the large number of concomitant CoNS and Candida infections in VLBW infants, with one microbe facilitating colonization and infection with the other (see below).
Although slime and other virulence factors are important to the pathogenicity of CoNS, several studies did not find evidence of hypervirulent clones of CoNS causing disease in neonates (111, 335). However, in a study of 97 blood isolates of CoNS (29 considered sepsis isolates and 68 considered contaminants) from a single center over a 15-year period, sepsis isolates were phenotypically and genotypically more homogeneous than contaminating isolates, suggesting that disease-causing strains of CoNS may have a higher invasive capacity (53).
Of the 31 species of CoNS and the 13 known to colonize human skin, species reported to cause disease in infants include S. epidermidis, S. haemolyticus, S. hominis, S. warneri, S. saprophyticus, S. cohnii, and S. capitis. The major species involved in neonatal infection is S. epidermidis, which accounts for approximately 50 to 80% of CoNS colonization (52, 185, 407) and 60 to 93% of CoNS bloodstream infection (104). S. epidermidis colonization rates of 86 to 100% have been reported among NICU patients (104, 138, 187, 228). Occasionally CoNS is acquired from the mother at birth. Hall et al. showed that 51% of pregnant women were colonized with CoNS, and the proportion of slime-positive strains increased from 40 to 68% from the first to the third trimester of pregnancy (186). In this study, although 30% of neonates were colonized with CoNS at birth, in only three of these cases were the maternal and infant species identical based on biotype, antibiotic sensitivity pattern, slime production, phage type, and plasmid pattern profile. The majority of CoNS colonization is acquired nosocomially, predominantly from the hands of health care workers. In a survey using multiple molecular typing techniques, 62% of NICU nurses were colonized with methicillin-resistant CoNS, with similar species distribution to that of bacteremic strains in the unit (364).
Identification of intraspecies strains is important in investigating outbreaks of CoNS sepsis in the NICU, and a number of epidemiologic methods have been used in the past for strain identification, including antibiotic susceptibility testing, phage typing, and analysis of slime production. Newer studies have employed molecular typing of chromosomal or plasmid DNA using techniques such as restriction endonuclease fingerprinting, random amplification of polymorphic DNA, and pulsed field gel electrophoresis (10, 100, 339, 380). Since none of these tests has proven to have sufficient discriminatory ability when used alone, most epidemiologic studies have used a combination of two to four tests to identify related strains. For the most part, multiple strains of CoNS are found in nurseries and in individual patients at one time and over time (235, 338). However, endemic strains of CoNS can persist in NICUs for many years (217, 298, 440, 485, 486). One study found a higher proportion of distinct chromosomal patterns for S. epidermidis in blood cultures (7 of 11) than in mucocutaneous cultures (6 of 18), suggesting that the more invasive strains are more likely to become endemic (51). This study also found that S. haemolyticus strains were genotypically much more homogeneous than S. epidermidis strains, with 4 of 4 blood cultures and 15 of 15 surface cultures falling into two distinct chromosomal patterns.
While reports of S. epidermidis bacteremia on the first day of life suggest that the organism may be perinatally acquired (7, 449), it is more commonly a nosocomial pathogen. Neonates with intravascular catheters, particularly those with central vascular catheters which remain in place for prolonged periods, are at high risk for CoNS bacteremia. Another significant risk factor for CoNS septicemia is the administration of intravenous lipid infusions, which provide a growth medium for the organism (21, 148). Sepsis with CoNS is often indolent rather than fulminant, although fatalities have been reported (240). Neonates with CoNS bacteremia generally present with nonspecific symptoms such as decreased activity, increased apnea, and feeding intolerance. Since these symptoms are common even in nonseptic preterm neonates, a positive blood culture for CoNS may represent either contamination or true bacteremia, and for this reason, many studies require more than one positive blood culture (458) or other laboratory evidence of infection, such as an elevated CRP level (454), to distinguish CoNS bacteremia from contamination. In one prospective study of positive blood cultures for CoNS in infants younger than 6 months, only 25 of 59 episodes were considered to represent true infection, and hematologic indices were not found to be helpful in distinguishing between infected and uninfected neonates (335). A more recent study compared the use of two versus one peripheral blood culture for the diagnosis of CoNS sepsis in 100 neonates with suspected sepsis (458). While CoNS was isolated from 26 patients, in only 16 cases were cultures from two sites positive, and the other 10 cases were considered to represent contamination. Of note, three patients had only one of the two cultures positive for other organisms (S. aureus, Serratia marcescens, and E. coli), highlighting the challenge in isolating microorganisms from blood culture. This study, requiring two positive cultures for a diagnosis of CoNS sepsis, resulted in an 8.2% reduction in vancomycin usage, but 20 babies underwent a second blood collection and culture for every “false-positive” case of CoNS detected. In addition, the authors noted that due to the added time, expense, and discomfort involved in obtaining two peripheral-blood samples for culture, on completion of the study they reverted to the practice of using a single blood culture for preterm neonates with suspected sepsis.
In addition to being the most common cause of nosocomial bacteremia in NICU patients, CoNS may cause focal infections. The organism has been isolated from the CSF of septic preterm neonates, sometimes in absence of CSF pleocytosis (174). CoNS is among the common causes of ventriculoperitoneal shunt infection in VLBW infants (63, 106). An association of CoNS infection and NEC has been reported by some groups (174, 412), while other studies have not found this association (179, 392). Likewise, studies of CoNS delta-like toxin in neonates with NEC have yielded conflicting results (179, 413). Another nidus of infection with CoNS is the endocardium. Right-sided endocarditis due to S. epidermidis may be associated with placement of an umbilical venous catheter in the right atrium (347).
Resistance to antibiotics such as penicillin, semisynthetic penicillins, and gentamicin is common among clinical isolates of CoNS. One NICU study found an increase in multiple antibiotic resistance among colonizing CoNS strains from 32% at birth to 82% after the first week of life (104). Similarly high rates were reported by another group (187). Of particular concern is the emergence of strains of CoNS with decreased sensitivity to vancomycin (424). While this has not yet been described for neonates, heteroresistance to vancomycin has been reported. A recent report described a preterm neonate with persistent S. capitis bacteremia for 3 weeks despite treatment with vancomycin and replacement of all central venous catheters (479). The authors screened the patient's strain and 218 other strains of CoNS isolated from neonates over the previous 4 years. Using population analysis, the patient strain and 47 others proved to be heteroresistant to vancomycin. It is possible that heteroresistance to vancomycin is more common in the NICU than is currently realized, since standard susceptibility testing fails to detect subpopulations of the organism for which the MICs are higher (486). Development of vancomycin resistance has been linked to prolonged antibiotic pressure. In an adult oncology patient, for example, a colonizing strain of S. haemolyticus which was initially susceptible to vancomycin (MIC, 1 to 2 μg/ml) developed increased resistance (MIC, 8 to 32 μg/ml) and caused bacteremia after 34 days of vancomycin therapy (482). Given the potential severe sequelae associated with the emergence of vancomycin resistance, efforts should be made to limit vancomycin use in the NICU. Several studies have shown that initial therapy of suspected LONS with nafcillin or oxacillin and an aminoglycoside, rather than vancomycin and broad-spectrum cephalosporin, are not associated with increased morbidity or mortality, even in units in which the majority of CoNS are oxacillin resistant (240, 268, 312).
Enterococcus species.
Although accounting for only a small proportion of neonatal sepsis, Enterococcus species deserve special mention because of the increasing incidence of neonatal enterococcal sepsis in several studies (89, 317) and the emergence of vancomycin resistance among enterococci. Both Enterococcus fecalis and E. faecium cause sepsis in preterm neonates, with E. fecalis accounting for over 80% of cases. McNeely et al. reviewed all cases of enterococcal bacteremia in NICU patients in a large metropolitan teaching hospital and found a three-fold increase in cases in 1983 to 1993 compared with the previous decade (318). In 100 cases reviewed, the mean age of onset was approximately 45 days and the mean gestational age was 31 weeks. Of note, 64% of patients had other organisms isolated concurrently from blood culture. Another study also found a significant proportion of polymicrobial bacteremia associated with enterococcal sepsis in 83 pediatric patients, including 16 neonates (89). This may be due to the common association with central vascular catheters or NEC, found in 77 and 33% of cases, respectively.
For reasons that are unclear at present, vancomycin resistance among enterococci has not become a significant problem in most NICUs (470), yet several studies have reported endemic or epidemic vancomycin-resistant enterococci (VRE) among hospitalized neonates. In the review by McNeely et al., six neonates had bacteremia with VRE, and one died of the infection (317). Interestingly, none of the six had been given prior therapy with vancomycin, although they all had prolonged NICU stays prior to the VRE infection (mean age, 100 days). Other studies have found an association of prior antibiotic use and colonization or infection with VRE. In one study, 68% of pediatric patients infected with VRE had been treated with vancomycin within 90 days of detection of the organism (317). A recent case report describes a 4-month old ELBW infant with a central venous catheter who, after three 10-day courses of vancomycin for various infections including CoNS and E. faecalis bacteremia, developed endocarditis with vancomycin-resistant E. faecium. The infection was successfully cleared by intravenous followed by oral linezolid therapy. Limited studies of neonates suggest that linezolid is well tolerated and as effective as vancomycin for the treatment of infections with resistant gram-positive bacteria (112, 233). Rare resistance to linezolid has been observed with prolonged therapy, and its use in neonates is still under investigation (237).
Rapid spread of VRE among NICU patients was documented by Malik et al., who reported the spread of related strains of VRE to 40% of the NICU patient population (305). Two preterm infants developed bloodstream infection, and 33, including 11 of 13 babies who shared a room with the bacteremic babies, became colonized with VRE. Compared to noncolonized babies, those with colonization were more premature, had been in the hospital longer (requiring more intensive care interventions), and had more exposure to antibiotics including vancomycin. In both of these studies, outbreaks were successfully controlled after implementation of strict infection control measures including limiting the use of vancomycin. In another study, active surveillance and barrier precautions reduced VRE colonization in an NICU from 2.2 to 0.5% (358). With longer hospitalization of extremely premature neonates, particularly those with surgical complications and prolonged central venous access, it is likely that VRE will become a more significant burden in the NICU in the future. Restricting the use of vancomycin should be a high priority for those caring for these patients.
Group A, C, D, and G Streptococcus species.
Species of streptococci other than GBS are infrequent agents in EONS in premature neonates and even less common in LONS (Table 2). In the recent NICHD survey, viridans streptococci accounted for 3.6% of cases of EONS (453), and other studies have also reported pathogenicity of these organisms, particularly in premature neonates (147, 198). In one study from France, viridans streptococci were associated with neonatal septicemia more frequently than were all other types of streptococci, including GBS (444). Group A Streptococcus, historically a major agent in puerperal sepsis, has only infrequently been implicated in neonatal sepsis in the last decade (168). Cases of neonatal sepsis caused by group C, D, and G streptococci are also occasionally reported (259). Early-onset S. pneumoniae sepsis is uncommon but is more likely to occur in preterm than in term infants (57, 152). In a review of 50 cases of neonatal pneumococcal sepsis, 60% of infants were born prematurely, 91% of infants developed symptoms within 48 h of birth, pneumonia and meningitis were present in 64 and 38% of patients, respectively, and mortality was 50% (152).
Listeria monocytogenes.
Listeria monocytogenes, a gram-positive bacillus, is a well-known and well-studied neonatal pathogen. Although neonates account for approximately one-third of cases of invasive listeriosis (153), the organism accounts for less than 5% of cases of EONS in preterm neonates in most studies. L. monocytogenes is commonly found in soil as well as other environmental sources, and farm animals may become infected through ingestion of spoiled silage. Most human cases of listeriosis are associated with ingestion of contaminated food such as undercooked or processed meats, unwashed vegetables, and unpasteurized dairy products (415).
The incidence of L. monocytogenes sepsis in neonates is approximately 13 per 100,000 live births in the United States as well as in Europe (153). The vast majority of cases represent perinatal transmission, although nosocomial transmission has been reported (203, 422). Listeria infection during pregnancy may result in miscarriage, stillbirth, or chorioamnionitis, often with placental abscesses (47). Infection occurring after the fifth month of pregnancy commonly leads to premature labor and delivery, with up to 70% of cases delivering at less than 35 weeks' gestation (342). Listeria may infect the fetus through the ascending or hematogenous route, often leading to signs of severe sepsis at delivery (390). In contrast to nearly all other organisms causing neonatal sepsis, Listeria is an intracellular pathogen and primarily targets cells of the monocyte-macrophage lineage. Impaired cell-mediated immunity, characterized by deficient production of gamma interferon and IL-12, decreased number and function of natural killer cells, and immature chemotaxis, phagocytosis, and killing by mononuclear phagocytes predispose the VLBW infant to overwhelming infection with this intracellular pathogen (504).
Gram-Negative Organisms
While gram-negative organisms are responsible for a smaller fraction of neonatal sepsis than are gram-positive organisms, they are associated with the highest mortality. In the recent NICHD surveys, gram-negative bacteria accounted for 61% of cases of EONS and 18% of cases of LONS in VLBW infants, with respective mortality rates of 41 and 36% (454) (Table 1). Considerable geographic differences exist in the distribution of gram-negative organisms causing neonatal sepsis. In the United States, E. coli is the most frequent cause of both EONS and LONS due to gram-negative bacteria, including in neonates born prior to term, while in India (231), Africa (332), and Israel (270), Klebsiella pneumoniae accounts for the major proportion of cases. Gram-negative bacteria, particularly members of the Enterobacteriaceae, are normal inhabitants of the intestinal tract. Neonates may acquire early infection from the maternal gram-negative flora or may develop intestinal colonization after birth with organisms that may subsequently translocate across immature or injured intestinal mucosa, resulting in LONS, sometimes associated with NEC. Other gram-negative organisms such as Pseudomonas may be acquired through the respiratory tract, particularly in patients requiring mechanical ventilation.
Enterobacteriaceae. (i) Escherichia coli
Since E. coli is the most common cause of neonatal sepsis by gram-negative bacteria, both organism and host response have been investigated. A number of E. coli virulence factors have been identified and linked to neonatal sepsis, including the K1 capsule, fimbriae, hemolysin, rough lipopolysaccharide, Ibe (invasion of brain endothelium) proteins, and cytotoxic necrotizing factor 1. Recently, a pathogenicity island, or cluster of genes present in pathogenic but not in avirulent strains, was found in E. coli C5, a strain commonly implicated in neonatal meningitis. Mutant strains lacking this pathogenicity island were less able to induce high-level bacteremia in a neonatal-rat model (253). In a study by Friesen and Cho, E. coli isolates from term infants with sepsis were more likely to express multiple virulence factors than were those from preterm infants with sepsis implying that bacterial factors contribute to the infectivity of E. coli in term infants while host factors contribute to disease susceptibility in preterm neonates (150).
EONS with E. coli often presents at delivery and is characterized by bacteremia with or without meningitis. Septic shock due to endotoxemia may be a presenting sign. Alternatively, neonates may become colonized with E. coli at birth or through contact with colonized caregivers while in the NICU and may develop infection later. Environmental sources include ventilation systems and storage shelves (12). Outbreaks of both enteropathogenic and nonenteropathogenic strains of E. coli have been described in the NICU (12, 427, 442).
While increasing use of intrapartum antibiotics has brought about a decline in the number of cases of early-onset GBS sepsis in neonates, several studies have shown an increase in the incidence of sepsis due to gram-negative bacteria, particularly E. coli and particularly among preterm infants (453, 473). In the Neonatal Network surveys of VLBW infants, while the incidence of GBS sepsis decreased from 5.9 to 1.7 per 1,000, the incidence of sepsis from E. coli increased from 3.2 to 6.8 per 1,000 between 1991-1993 and 1998-2000 (450, 453). Of the 33 E. coli isolates causing EONS in the more recent survey, 28 (85%) were resistant to ampicillin and 1 was resistant to gentamicin. While a higher percentage of infants with E. coli EONS were born to women who received intrapartum ampicillin prior to delivery than to those who did not (1.1 versus 0.4%), this difference was not statistically significant after adjusting for gestational age and the interval between rupture of membranes and delivery. Other studies have not found an increase in gram-negative or resistant organisms among EONS isolates in full-term infants (35, 81, 95). Furthermore, the proportion of community-acquired ampicillin-resistant E. coli infections has increased (178), suggesting that factors other than increased intrapartum antibiotic use may play a role in the increasing ampicillin resistance among organisms causing EONS in preterm infants. It will be particularly important to track the trends in gram-negative bacterial resistance in the coming decade as the revised GBS prophylaxis guidelines are adopted (419). It is also important that practitioners adhere to the recommendation of using penicillin as a first choice for GBS prophylaxis since it may minimize the risk of emerging resistance among gram-negative pathogens (419).
(ii) Enterobacter, Klebsiella, and Serratia species.
Gram-negative enteric organisms of the Enterobacteriaceae family, notably Enterobacter (140), Klebsiella (50), and Serratia (88) species, are common inhabitants of the neonatal intestine which may cause nosocomial sepsis. Like the other well-known member of the family, E. coli, these organisms are surrounded by a capsule and fimbriae that contribute to their virulence in neonates. This capsular polysaccharide prevents activation of the alternative complement system protecting the bacteria from opsonization, phagocytosis, and bacteriolysis (276).
In a 1999 Centers for Disease Control and Prevention-sponsored point prevalence survey of nosocomial infection in 29 NICUs (including both term and preterm neonates), Enterobacter cloacae, Klebsiella pneumoniae, and Serratia marcescens each accounted for 5 to 6% of the total pathogens causing various types of infection (441). These three organisms together accounted for 5.1% of bloodstream infections in this survey and 8.7% of LONS in the NICHD Neonatal Network survey (454). These organisms spread rapidly in the NICU, and outbreaks with each of these pathogens have been reported in the literature. Epidemiologic studies utilizing techniques such as pulsed-field gel electrophoresis and Enterobacteriaceae repetitive intergenic consensus PCR, have shown that most nursery outbreaks are due to a limited number of clones transmitted from patient to patient on the hands of health care workers. Intensive efforts at reducing nosocomial transmission of members of the Enterobacteriaceae have successfully eradicated colonization and disease with virulent enteric strains, although in some cases closing a NICU to new admissions has been deemed necessary to prevent life-threatening infections during outbreaks.
Another mode of transmission of nosocomial pathogens is through contaminated powdered infant formula, which recently caused a fatal case of Enterobacter sakazakii sepsis and meningitis in a preterm infant (76a). Further epidemiologic investigation revealed two other suspected infections and seven infants colonized with the same organism. Cultures of both opened and unopened cans of a specific powdered formula also yielded E. sakazakii. Several other outbreaks of E. sakazakii sepsis and NEC have also been traced to contaminated powdered formula (90, 478, 495). It is recommended that whenever possible, preterm infants for whom breast milk is not available be fed with commercially sterilized, ready-to-feed liquid formula.
(iii) Citrobacter species.
Another uncommon but notable gram-negative pathogen found in VLBW neonates is Citrobacter. Invasive infections with Citrobacter koseri, formerly C. diversus, are much more common in neonates than in other patient groups, while C. freundii rarely causes disease in neonates. C. koseri is responsible for less than 5% of cases of sepsis in preterm VLBW infants but is well known because of its propensity for CNS invasion and its association with epidemic outbreaks in both well-baby and intensive care nurseries. C. koseri is not a normal inhabitant of the maternal urogenital tract, but it is occasionally present, causing maternal UTI and chorioamnionitis. Vertical transmission may lead to a neonate with severe sepsis at birth or in the first days of life (194, 361). In two reviews describing a total of 128 newborns with C. koseri sepsis and/or meningitis, approximately 35% of infants were born prematurely (117, 165). In the more recent review, 71% of infants with proven vertically acquired Citrobacter sepsis were premature compared with 26% of infants with late-onset sporadic or epidemic disease (117).
C. koseri infection is notorious for causing meningitis with cerebral abscesses and necrosis, leading to a poor neurodevelopmental outcome. In a report from the Neonatal Meningitis Cooperative Study Groups published in 1981, brain abscesses developed in 41 (77%) of 53 neonates with Citrobacter meningitis (165), and a more recent review noted a similar incidence of abscesses (117). The organism appears to have a tropism for the CNS, and recent research with a neonatal rat model demonstrated that C. koseri is able to survive phagolysosomal fusion and replicate within macrophages, causing chronic infection and brain abscess formation (474). Of note, however, brain abscess formation appears to be uncommon in vertically transmitted, Citrobacter EONS, possibly because of earlier institution of antibiotic therapy (117).
Horizontal transmission of C. koseri from patient to patient via the hands of infant caregivers has been documented. In the early 1980s, a number of outbreaks of C. koseri meningitis in well-baby nurseries were reported (156, 289, 363). Surveillance culturing in these epidemics revealed a low case-to-colonization ratio and traced outbreak sources to one or a small number of health care workers with hand and/or rectal colonization. Because of the tendency for nosocomial spread and the potentially devastating nature of the infection, contact isolation should be instituted for both preterm and term neonates with known C. koseri colonization or infection.
Members of other families. (i) Pseudomonas species.
In contrast to H. influenzae and Citrobacter, Pseudomonas infection is rarely perinatally acquired (397); however, it is among the more common gram-negative organisms causing nosocomial sepsis in NICU patients (172, 454). Most Pseudomonas infections are due to P. aeruginosa, although cases of Burkholderia cepacia (formerly Pseudomonas cepacia) infection have been reported (267, 285, 382). P. aeruginosa is commonly thought of as a “water bug,” thriving in moist environments such as humidified incubators (190), sinks (173), and ventilator circuits (304). Hands of health care workers have also been identified as an important reservoir. Foca et al. reported that 10% of nurses and 5% of clinicians in a NICU had hand cultures positive for P. aeruginosa (143). Both this group and another group investigating an outbreak of Pseudomonas infection in NICU described the possible role of long and artificial fingernails of health care workers in transmission of the organism (143, 328). P. aeruginosa is a particularly virulent pathogen for VLBW infants. Focal P. aeruginosa infections include pneumonia, conjunctivitis, and, rarely, a disease entity termed noma neonatorum, a gangrenous process affecting the nose, mouth, eyelids, and perineal area and often associated with bacteremia (155). P. aeruginosa conjunctivitis should be rapidly diagnosed and treated since it may lead to rapidly progressive invasive eye infection, as well as to systemic complications including bacteremia, meningitis, brain abscess, and death (430). In several studies, Pseudomonas had the highest mortality of all organisms causing LONS, with 50 to 75% of patients dying (240, 282, 451).
(ii) Haemophilus influenzae.
Haemophilus influenzae may be vertically transferred from mother to infant at the time of delivery and occasionally causes EONS in preterm infants. H. influenzae accounted for 8.3% of EONS cases in the most recent NICHD survey (453), and the incidence of H. influenzae sepsis in preterm neonates appears to be increasing in some centers (150). Most cases involve non-typable strains, with fewer than 10% caused by H. influenzae type b, presumably due to maternal immunity to the latter. The presentation of H. influenzae sepsis in preterm neonates is generally quite fulminant and often includes pneumonia simulating severe respiratory distress syndrome. Mortality has been reported as high as 90% (150).
Anaerobic Bacteremia
In most recent surveys, anaerobes accounted for less than 5% of cases of neonatal bacteremia (345, 453), with premature infants representing a substantial proportion of these cases (345). In a review of 178 cases of neonatal anaerobic bacteremia reported in the literature, 73 isolates were Bacteroides species (69 were B. fragilis), 57 were Clostridium species (mostly C. perfringens), 35 were Peptostreptococcus species, 5 were Propionibacterium acnes, 3 were Veillonella species, 3 were Fusobacterium species, and 2 were Eubacterium species. Other reports have confirmed this predominance of B. fragilis and C. perfringens among anaerobic isolates from cultures of blood samples from neonates.
Two distinct clinical scenarios have been associated with neonatal anaerobic sepsis. Presentation within the first 2 days of life is often associated with chorioamnionitis. Anaerobic organisms are commonly cultured from amniotic fluid of women presenting with signs of intraamniotic infection prior to term (443). In an 18-year survey of neonatal anaerobic bacteremia at a single institution, gram-positive organisms, predominatly C. perfringens and Peptostreptococcus species, were responsible for the majority of EONS cases. In the second scenario, anaerobic LONS in preterm infants is usually associated with bowel pathology such as severe NEC or focal intestinal perforation. Gram-negative anaerobes such as B. fragilis predominate in these cases, and infection may lead to abscess formation. Compared with other anaerobes, Bacteroides species possess virulence factors such as pili, fimbriae and capsular polysaccharide that allow them to compete with members of the aerobic gastrointestinal flora and cause infection (393). It is important to consider the possibility of anaerobic infection in preterm neonates with NEC, since the standard antibiotic regimens used for LONS may not provide adequate coverage for anaerobes and these infections are associated with high mortality. Due to these risk factors for anaerobic sepsis, both aerobic and anaerobic blood culture bottles should be considered in the evaluation of suspected cases of EONS and when LONS has gastrointestinal pathology.
Necrotizing Enterocolitis and Infection
Approximately 10% of VLBW infants develop NEC, often leading to infectious complications such as peritonitis, abscess formation, and septicemia. NEC is a multifactorial disease, and infection may be a predisposing factor or a secondary consequence. Gastrointestinal dysmotility, which is common in preterm infants, allows prolonged contact of pathogens, toxins, and irritants with the mucosa, increasing the chance of proliferation of microorganisms. Preterm infants also have a thinner mucin layer and decreased lymphocyte pool and levels of secretory IgA, permitting adherence of microorganisms to intestinal cells and subsequent growth (222). Microorganisms are not typically found in early pathology specimens at the onset of NEC (406), and efforts to identify the predominant pathogens contributing to the disease have generally been unsuccessful (58, 179). However, a role for bacteria in the development of NEC is suggested by the following: (i) NEC does not occur in the sterile in utero environment; (ii) intestinal necrosis is absent in bacterium-free animal models (331); (iii) bacterial urinary d-lactate levels and breath hydrogen excretion increase 8 to 28 h prior to onset of NEC (84); and (iv) organisms that commonly colonize the gastrointestinal tract have been associated with temporal and geographic epidemics of NEC. Microorganisms may lead to stimulation of an inflammatory cascade with elaboration of cytotoxic cytokines and other necrosis-inducing agents such as platelet-activating factor (PAF) (214).
Bacteremia may be present in neonates with NEC. In a retrospective multicenter analysis of 269 cases of NEC in 2,681 VLBW infants, bacteremia due to gram-negative pathogens was more common infants with Bell's stage III compared with stage II NEC (24 and 8%, respectively), while bacteremia due to gram-positive pathogens occurred equally (19% and 27%, respectively) and fungemia was uncommon in this study (2 and 3%, respectively) (476).
Important in the diagnosis of NEC in a VLBW infant is that both rotavirus and adenovirus infection may present with bloody mucoid stools, ileus and abdominal distension, and not watery diarrhea, due to the immaturity of the gastrointestinal tract and to diminished active chloride secretion and passive sodium secretion (188, 432).
Focal Intestinal Perforation
Isolated intestinal perforation is a relatively newly described entity among preterm infants and previously was included in the category of stage III NEC. Its frequency has increased due to the survival of younger preterm infants and increased antenatal and postnatal treatment with steroids and indomethacin (160, 161). Intestinal perforation occurs in an area of the small bowel (usually the terminal ileum) without signs of necrosis. Clinical symptoms and laboratory findings are generally more benign and the outcome is more favorable in focal intestinal perforation (FIP) than in NEC. In a study comparing 36 cases of FIP to 80 cases of NEC at one institution over a 12-year period (1989 to 2000), FIP patients were of lower birth weight than NEC patients (772 and 1,273 g, respectively; P < 0.0001) and younger gestational age (26 and 29.5 weeks, respectively, P = 0.007) (92a). The median age of presentation in this study was 2 weeks for both entities. Candida and CoNS species were isolated from peritoneal cultures in approximately 50% of the FIP cases compared to 14% of the NEC cases (P ≤ 0.001), whereas members of the Enterobacteriaceae were isolated in 75% of the NEC cases compared to 25% of the FIP cases (P = 0.007). Enterococcus species and anaerobes were isolated in the peritoneal fluid of approximately 25 and 5% of patients, respectively, for both entities. Since the gastrointestinal tract is commonly colonized with CoNS and Candida species in the first weeks of life, peritoneal cultures may help guide antibiotic decisions about the management of these patients with gastrointestinal perforations.
Concurrent Bacteremia and Fungemia
Simultaneous infection with more than one organism is not uncommon in VLBW infants. In one recent multicenter study of LONS, two or more microorganisms were isolated from 4% of positive blood cultures (55 of 1,368) (454), while in an earlier study of sepsis, UTI, and meningitis, 24% of the cultures yielded multiple organisms (137). Studies have documented a high incidence of concurrent fungemia and bacteremia, most commonly with CoNS and Enterococcus. From 1994 to 1998 at a single center, 30% of infants with Candida sepsis (32 of 107) had growth of bacteria from the same blood culture or during the period of candidemia (242). In another study in two NICUs, among 58 infants with Candida sepsis, 19% had concurrent bacteremia and fungemia (131). Most cases of mixed-species septicemia occur in VLBW patients with central venous catheters or those with NEC, abdominal surgery, or other conditions compromising the gastrointestinal mucosa. Formation of mixed-species biofilms on vascular catheters, particularly with Candida and CoNS, may account in part for the relatively high incidence of mixed-species septicemia associated with these organisms. Both these organisms form biofilms that impede the penetration of antibiotic and antifungal agents (6).
Simultaneous bacteremia and candidemia has been associated with endocarditis in neonates. Noyola reported an incidence of endocarditis of 35.2% in patients with concomitant bacterial and candidal sepsis compared to 9.2% in patients with fungemia alone (P = 0.02) (352). In another study of endocarditis, 31% of the cases (5 of 16) involved two or more organisms, and Candida and CoNS were responsible for 3 of the 5 endocarditis cases caused by multiple organisms (101). Central venous catheters are often positioned within the right atrium in VLBW infants, simultaneously providing a surface for biofilm formation and organism proliferation and a source of potential local trauma to the endocardium and valvular tissue, creating a nidus for thrombus formation and infection. Whenever possible, the following measures may minimize infection-associated morbidity and mortality in VLBW infants: avoidance of central venous catheter use, care during insertion to place the catheter in the superior or inferior vena cava rather than within the heart, continued surveillance for proper positioning, and prompt removal of catheters when they are no longer essential or when fungemia or persistent bacteremia develops.
Antibiotic Resistance in Gram-Negative Pathogens
Antimicrobial resistance in gram-negative pathogens associated with both EONS and LONS is an increasing problem in NICU patients (11,471,483). Infection with these microorganisms is significantly associated with a birth weight of <1,000 g and with the duration of antibiotic exposure. Resistance among early-onset bloodstream isolates in neonates may reflect antepartum antibiotic exposure in colonized mothers or may be attributable to the increase in antibiotic resistance in community-acquired organisms. In a recent study of 1,410 NICU patients, 17% had gastrointestinal tract colonization with cephaolosporin-resistant gram-negative bacilli and one-third of these colonized patients were VLBW infants. Of the colonizing resistant organisms, 30% had activity against extended-spectrum β-lactamase (ESBL) and 35% demonstrated gentamicin resistance (439). Another study of 426 neonates reported that colonization with resistant gram-negative bacilli occurred in 41 neonates who were treated with intravenous amoxicillin and cefotaxime, compared to only 3 infants treated with penicillin and tobramycin (108). While expression of ESBLs has not been shown to increase the virulence of an organism, it may result in delayed institution of appropriate antimicrobial therapy and possibly in increased morbidity and mortality. In addition, eradication of infection with ESBL-producing organisms requires antimicrobial agents such as imepenem that have not been thoroughly studied in preterm neonates and may increase risk for fungal sepsis (43, 177, 398, 399). Broad-spectrum cephalosporin use may induce chromosomal ESBLs in gram-negative bacilli, and efforts to minimize empiric use of these cephalosporins are warranted. In one center, restriction of the use of cephalosporins by using ampicillin and amikacin for empiric treatment of EONS and oxacillin and amikacin for empiric treatment of LONS pending culture results was associated with a reduction of colonization with resistant gram-negative bacilli from 32 to 11% (67).
Mortality and Morbidity Associated with Bacterial Sepsis
As the death rate from respiratory causes has declined among preterm infants in the last two decades, sepsis has become the leading cause of mortality in these vulnerable patients. In two recent NICHD surveys of 15 NICUs in the United States, all-cause mortality was 37% among VLBW infants with EONS and 18% among those with LONS (453, 454) (Table 1). Mortality was approximately threefold higher in patients with either EONS or LONS than in those without. While these surveys report mortality from all causes, in the case of EONS 54% of the deaths (17 of 31) occurred within 3 days of birth, and thus it is possible that sepsis was directly related to death. For LONS, 49% of deaths occurred within 3 days and 60% occurred within 7 days of a positive blood culture, and clinicians attributed the death to infection in 87% of these cases (454). Both the type of organism and the species greatly affect mortality (see Table 1). Infections with gram-negative organisms were associated with a worse outcome; 41% of cases of EONS and 36% of cases of LONS associated with gram-negative organisms ended in death.
Neurologic morbidity has been attributed to both EONS and LONS in preterm infants. Intra-amniotic fluid infection often leads to overproduction of inflammatory cytokines that may be cytotoxic to the developing brain, and chorioamnionitis has been linked to cerebral palsy in term and near-term infants (503, 508). This association is less clear in preterm infants, since it has been estimated that chorioamnionitis (histologic and/or clinical) is present in as many as 85% of preterm deliveries (157). Chorioamnionitis and neonatal sepsis have been linked to periventricular leukomalacia, a form of white matter injury that is found in approximately 4% of VLBW infants and is highly correlated with cerebral palsy (367). The 2002 Neonatal Network survey reported a higher rate of severe IVH and periventricular leukomalacia in VLBW infants with EONS (32 and 12%, respectively; OR, 3.2; 95% CI, 1.9 to 5.5) (453), and a recent retrospective case control study of VLBW infants also found that occurrence of EONS was a predictor of high-grade IVH (OR, 8.19; 95% CI, 1.55 to 43.1) (292). An association between LONS and brain ultrasound abnormalities is less clear. While a 1988 to 1991 Neonatal Network survey of 2,416 VLBW infants found an association between LONS and severe IVH (451), the 1998 to 2000 survey by the same group did not find this association (454). Other studies have found associations between neonatal sepsis and clinical evidence of brain injury in preterm infants. In a study of 59 neonates of <32 weeks' gestation with cerebral palsy compared with 234 controls (330), sepsis was associated with an increased risk of cerebral palsy after adjustment for gestational age and other known antepartum and intrapartum risks (OR, 3.6; 95% CI, 1.8 to 7.4). However, another study of ELBW infants observed to 20 months' corrected age, using multiple logistic regression, found that sepsis was associated with deafness (OR, 3.15; 95% CI, 1.05 to 9.48) but not mental or motor disability or blindness (181). Long-term follow-up studies are required to further define the impact of LONS on the neurodevelopmental outcome in VLBW and ELBW infants.
Meningitis is associated with a worse neurodevelopmental outcome in preterm infants than in term infants. In a 10-year study of neonatal meningitis in the United Kingdom, moderate or severe disability was detected in 12% of those with birth weight of >2,500 g, 31% of those with birth weight of 1,500 to 2,499 g, and 44% of those with birth weight of <1,500 g (204). Similarly, another group found that 41% of 39 VLBW survivors of neonatal meningitis suffered major neurologic sequelae, compared with 11% of matched control infants (114).
In addition to adverse neurologic outcome, sepsis has been associated with other morbidities in preterm infants. If the intra-amniotic fluid infection is present for a longer period before delivery, a fetal inflammatory response may develop, with elevation in the levels of serum cytokines including IL-6, IL-1β, and tumor necrosis factor alpha (TNF-α). This inflammatory process has been associated with septic shock at birth and with long-term pulmonary and neurologic morbidity (reviewed in reference 159). In the 1998 to 2000 survey of 7,606 VLBW infants at 15 Neonatal Network centers, after adjustment for gestational age, the 84 infants with EONS had a significantly higher risk of respiratory distress syndrome and chronic lung disease (453). The same group found that VLBW infants with LONS had a significantly longer mean duration of hospitalization than did those without sepsis (79 and 60 days respectively) (454).
Treatment of Bacterial Sepsis
Although a full discussion of the treatment of sepsis is beyond the scope of this review, a brief mention of issues germane to preterm infants is warranted. The majority of VLBW infants have risk factors for sepsis such as maternal chorioamnionitis and prolonged rupture of membranes, as well as clinical signs possibly consistent with sepsis, such as respiratory distress. For this reason, most are treated with empiric antibiotics after birth, pending blood culture results. While the mainstay of empiric therapy for EONS for both term and preterm infants in most centers is ampicillin and gentamicin, the emergence of gentamicin-resistant gram-negative bacteria in some centers may prompt the use of other aminoglycosides. In cases of known or suspected sepsis due gram-negative bacteria, and particularly if meningitis is present, addition of a broad-spectrum cephalosporin may be beneficial. Concentrations in CSF are more easily and safely achieved by broad-spectrum cephalosporins and are 50 to 100 times the MIC, compared to aminoglycoside levels in CSF, which reach only approximately 2.5 times the MIC (195).
For empiric treatment of suspected LONS presenting after 3 to 7 days of age, nafcillin or oxacillin and an aminoglycoside may provide sufficient initial coverage. Most CoNS are resistant to β-lactam antibiotics, and many centers use empiric vancomycin for LONS. However, several studies have shown that limiting vancomycin use to culture-proven CoNS infection does not result in increased morbidity or mortality in preterm infants (240). The majority of gram-negative organisms have remained sensitive to aminoglycosides; however, knowledge of the susceptibility patterns in individual NICUs should be used to guide the choice of antibiotic. As discussed above, to minimize the risk of emergence of ESBL-producing bacteria as well as the risk of fungal sepsis, empiric cephalosporin use should be limited in the NICU. However, suspected or proven sepsis with gram-negative bacteria warrants initial double-antibiotic coverage for synergy, and an aminoglycoside and a broad-spectrum cephalosporin form a reasonable combination. When choosing treatment for LONS, consideration should also be given to the possibility of Pseudomonas and to nonbacterial etiologies such as Candida and herpes simplex virus. These infections are becoming increasingly common among preterm infants and are associated with very high morbidity and mortality. In addition, in neonates with intestinal perforation, anaerobic coverage may be appropriate pending the results of blood and peritoneal fluid cultures.
Aside from antimicrobial therapy, septic VLBW infants may require blood pressure support with crystalloid, blood products, and vasopressors. In VLBW infants with hypotension unresponsive to conventional pressors, a short course of stress-dose hydrocortisone has been shown to raise the blood pressure, although effects on the short- or long- term outcome have not been established (428, 493). Use of hydrocortisone plus a fluorinated steroid improved the outcome in septic adults with low cortisol levels but worsened the outcome if cortisol levels were high (16). Consideration should be given to evaluating the serum cortisol level prior to initiating therapy with stress-dose hydrocortisone in hypotensive preterm infants.
Use of adjunct therapies for neonatal sepsis is limited. Several small trials and a meta-analysis of IVIG treatment for suspected neonatal sepsis have shown a small reduction in mortality among patients with subsequently culture-proven sepsis (197, 356); however, the majority of patients in these studies were term neonates. A recent meta-analysis of several small randomized trials has also shown that granulocyte colony-stimulating factor (G-CSF) treatment may reduce mortality in neutropenic septic neonates (both term and preterm) (OR, 0.34, 95% CI, 0.12 to 0.92) but not in neonates with normal neutrophil counts (36, 71, 72). Recombinant activated protein C (102) and PAF acetylhydrolase (423) have shown promise in reducing mortality in adults with sepsis but have not been tested in neonates. Although there is overlap in the pathophysiology of sepsis in adults and neonates, there are significant differences, and one cannot assume that therapies proven efficacious in adults will improve the outcome of septic preterm infants.
Another area for further study relates to whether prompt removal of central vascular catheters in septicemic VLBW infants improves outcome. As discussed below, several studies suggest that this is the case for candidemia, but the data are less convincing for bactermia. Although one retrospective cohort study found an improved outcome in bacteremic infants whose catheters were removed promptly (44), this may simply reflect the fact that catheters are more likely to be left in place in the smallest, sickest infants, in whom obtaining other vascular access is difficult. Other studies have reported that 46% of cases of CoNS bacteremia in NICU patients were successfully cleared without removing central lines (241), and 45% of cases of neonatal Enterobacteriaceae bactermia were cleared without removal of central venous catheters and with no increase in morbidity or mortality (336). Neither of these studies was restricted to VLBW neonates. Bacterial infections can initially be treated through central venous catheters, but if the organism is isolated on two or more blood cultures despite appropriate antibiotic therapy and no other source such as an abscess is found, central vascular catheters may be infected and should be removed at that time.
The duration of antibiotic treatment is highly variable because of the issue of “clinical sepsis” discussed above. The vast majority of true bacterial pathogens are detected in blood culture within 48 h, and in the absence of other strong clinical indicators of infection, empiric antibiotic use may be discontinued at this time (249, 492). Once a pathogen is identified, antibiotic coverage should be narrowed based on susceptibility testing. Repeat culture is important for documenting adequate treatment and determining the duration of therapy. Treatment of bacteremia due to gram-positive organisms has traditionally lasted 7 to 10 days, although shorter courses may be adequate for uncomplicated CoNS bacteremia. Bacteremia due to gram-negative organisms, deep-seated infections, abscesses, meningitis, endocarditis, and osteomyelitis require longer courses of antibiotic treatment. Practitioners should refer to the most recent edition of the Red Book report of the Committee on Infectious Diseases published by the American Academy of Pediatrics for specific therapeutic recommendations (374a).
FUNGAL ORGANISMS
With increasing survival of smaller, more immunocompromised preterm infants, the incidence of invasive fungal infection is increasing among NICU patients, with high associated morbidity and mortality (454). The early NICU course favors colonization and proliferation of fungi since many VLBW infants have central vascular catheters and are exposed to broad-spectrum antibiotics and parenteral nutrition. The vast majority of fungal infections in preterm neonates are due to Candida species, with a small number being due to Malassezia and other rare fungi. Candida is an opportunistic pathogen; the major factor predisposing VLBW infants to colonization and invasive infection is the ability of Candida species to adhere to skin, mucosal, and catheter surfaces as commensal organisms. With damage to the skin or mucosal membranes and with the diminished immune defenses of preterm infants, Candida can disseminate to the bloodstream. Acute mortality from Candida infection may be associated with septic shock due to the production of endotoxin-like substances, hemolysin, and pyrogens by the organism (300, 308). Finally, owing to its adherence properties, Candida may seed tissues and form abscesses, which are difficult to eradicate (Fig. 2) (109, 206-210, 258).
FIG. 2.
Pathogenesis of fungal colonization and infection.
In VLBW infants, C. albicans accounts for 50 to 97% of cases of fungal colonization (Table 4) and about 50% of cases of fungal sepsis (30, 183, 215, 246, 255, 362, 395, 402). Compared to other Candida species, C. albicans demonstrates increased adherence to and penetration of vascular endothelium, possibly accounting for its higher incidence as a cause of sepsis, end-organ dissemination, and mortality (158, 225, 352, 494). C. parapsilosis is the second most prevalent species in VLBW infants, being present in 13 to 83% of patients with fungal colonization, and may initially colonize the gastrointestinal tract (126). In two large multicenter NICU studies, this species accounted for 26% of cases of fungal sepsis (57 of 219) from 1991 to 1993, increasing to 34% of cases of fungal sepsis (57 of 160) from 1998 to 2000 (451, 454). In a study of six NICUs, C. parapsilosis colonization in preterm infants was strongly associated with the presence of a central venous catheter and exposure to H2 antagonists and cephalosporins (133, 401). C. parapsilosis is able to form biofilms on catheters and may contaminate glucose-containing solutions such as parenteral nutrition mixtures (99, 370, 372, 375). In contrast to C. albicans, diminished host immunity alone does not appear to increase C. parapsilosis dissemination. In an animal study of gastrointestinal fungal colonization with C. albicans, C. parapsilosis, and C. tropicalis, oral antibiotic and glucose supplementation led to high concentrations of C. albicans and C. parapsilosis in the intestine, with intraepithelial microabscesses (323). When immunosuppression was added, using cyclophosphamide and prednisolone, C. albicans and C. tropicalis sepsis developed in 62 and 24% of the animals, respectively, but C. parapsilosis did not disseminate.
TABLE 4.
Fungal colonization in VLBW and ELBW infantsa
| Characteristic | Results for study (study period)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Baley et al. (30) (1983) | Hageman et al. (183) (1985) | Pappu-Katikaneni et al. (362) (1991) | Rowen et al. (395) (1992) | Saiman et al. (402) (1993-1995) | Huang et al. (215) (1996) | Kicklighter et al. (255) (1998-1999)b | Kaufman et al. (246) (1998-2000)b | |
| Birth wt (gr) | <1,500 | <1,500 | <1,500 | <1,500 | <1,500 | <1,500 | <1,500 | <1,000 |
| No. of patients | 146 | 38 | 40 | 116 | 780 | 116 | 52b | 50b |
| % of patients with colonization | 27 | 47 | 63 | 34 | 31 | 22 | 46 | 60 |
| % of patients colonized with: | ||||||||
| C. albicans | 16 | 24 | 40 | 26 | 18 | 13 | 44 | 44 |
| C. papapsilosis | 5 | 24 | 10 | 4 | 13 | 6 | 37 | 45 |
| C. glabrata | 0 | 0 | 0 | 3 | <1 | 4 | 3 | |
| C. guilliermondii | 0 | 0 | 0 | 0 | <1 | 0 | 3 | |
| C. lusitaniae | 0 | 0 | 0 | 0 | 0 | 11.1 | 5.1 | |
| C. tropicalis | 2.1 | 0 | 2.5 | 1.7 | 0 | 3.7 | 0 | |
| Other | 3.4 | 0 | 0 | 0 | 2.7c | 0.8 | 0 | 1.1 |
| % of total no. of patients colonized by site: | ||||||||
| Skin | ||||||||
| Groin or axilla | 13.7 | 12.9 | 0 | 48 | ||||
| Umbilicus | 8 | |||||||
| Gastrointestinal tract | ||||||||
| Rectal | 19.2 | 33.6 | 31 | 12.9 | 45 | 54 | ||
| Stool | 62.5 | |||||||
| Respiratory tract | ||||||||
| Oropharyngeal | 11 | 11.2d | 6 | |||||
| Endotracheal | 4.8 | 11.2d | 2.6 | 42 | ||||
| Urine | 8.3 | 7.7 | ||||||
| % of total no. of patients with fungal sepsis | 2.1 | 7.9 | 12.5 | 6.0 | 3.1 | 2.6 | 3.9 | 16 |
C. glabrata was historically thought to be less pathogenic than other species of Candida because it does not form hyphae, but the prevalence of C. glabrata invasive infection is increasing among immunocompromised patients including VLBW neonates (131, 141). In studies of adult immunocompromised patients, risk factors for C. glabrata sepsis include prolonged hospitalization, prior antibiotic and fluconazole exposure, hand carriage, and infection with other fungal organisms (141). A factor contributing to an increased incidence of C. glabrata infection in preterm neonates may be the azole antifungal treatment of genital candidiasis, which is common during pregnancy. This may lead to selection of the relatively azole-resistant C. glabrata, which may colonize preterm infants following rupture of membranes or during vaginal delivery. In VLBW infants, gastrointestional disease (NEC or focal intestinal perforation) or multiple-site colonization is often present prior to invasive C. glabrata infection (9, 131, 248). This implies that a breakdown of skin or mucosal barriers or a large inoculum of C. glabrata contributes to invasive infection in these infants.
C. tropicalis is also reported to be an increasingly common cause of sepsis in VLBW infants (302, 333, 388). Invasive infection is associated with damage to the gastrointestinal mucosa, antibiotic suppression, and neutropenia in other immunocompromised hosts, but in a recent study of neonates, use of parenteral nutrition was the only identified risk factor (388, 405, 505, 506). Other Candida species reported to cause sepsis in VLBW infants include C. krusei, C. lusitaniae, C. guilliermondii, and C. dubliniensis.
Host Defense against Fungal Infection
The immature lymphocyte and antibody system predisposes VLBW infants to skin and mucosal fungal colonization, while deficient innate host defense mechanisms predispose them to dissemination and overwhelming infection (Table 1) (308, 376a). With compromise of the barrier defense system, invasive fungal infection may occur due to deficient neutrophil number and function. Neutrophils play a major role in antifungal defense, ingesting and killing Candida through the production of oxygen metabolites in a process requiring antibodies, cytokines, and activation of the C3 complement component, all of which are decreased in preterm infants compared with term infants and adults. Neutrophil granules and cytokines play a critical role in the lysis of Candida hyphae and pseudohyphae, which are too large to be engulfed by phagocytosis (113, 389). Macrophages are also important in the control of fungal colonization and infection, since they ingest and kill Candida without a requirement for complement activation; however, their adherence, phagocytosis, and oxidative killing are impaired in preterm infants, affecting their ability to contain fungal infection (247, 308). In addition to cell-mediated defenses, cytokines contribute to innate immunity to fungal infection, through direct inhibition of fungal proliferation and through enhancement of cell-mediated fungicidal activity (257).
Fungal Colonization
In VLBW infants, invasive fungal infection is usually preceded by colonization, which may occur via vertical or horizontal transmission. In studies of VLBW and ELBW infants, most fungal colonization occurs by 2 weeks of life (31, 215, 395). Fungal colonization and subsequent infection depend on exposure, size of inoculum, host susceptibility, and properties of the pathogen (Fig. 2).
Candida yeast cells adhere preferentially to intermediate layers of the vaginal tract that are increased during pregnancy, increasing maternal fungal colonization and exposure of vaginally delivered infants (425). The incidence of vaginal fungal colonization during pregnancy has been reported to be between 25 and 46%, with 85 to 90% of these cases being due to C. albicans and the remainder being due predominantly to C. glabrata and C. tropicalis (175, 501). Despite frequent fungal colonization, chorioamnionitis caused by Candida is a rare event, with almost all vertical transmission occurring when the infant passes through the birth canal via cutaneous contact with or swallowing or aspiration of fungi (313, 501). Treatment of maternal Candida vaginosis and UTIs during pregnancy may decrease the inoculum to which the infant is exposed and may potentially prevent vertical transmission (386).
Candida colonization may also be acquired horizontally, primarily from the hands of health care workers. In a multicenter trial examining fungal colonization in six NICUs, Candida species were isolated on the hands of 29% of health care workers (859 of 2,989) (401). While C. albicans was a more common fungal isolate than C. parapsilosis in all NICU patients (14 and 7%, respectively), C. parapsilosis was the most common species isolated from the hands of NICU staff. C. parapsilosis was isolated from 19% (similar to a single-center incidence of 20% [216]) and C. albicans was isolated from 5% of the cultures of samples from health care personnel (p < 0.001). C. lusitaniae (2%), C. guilliermondii (1%), C. tropicalis (<1%), and C. glabrata (<1%) were also recovered from hand cultures. The potential for spread and infection is demonstrated in a case report of a NICU health care worker colonized with fungi of the same genotype isolated from the blood cultures of nine VLBW infants with C. albicans sepsis over a 3-month period (216). Careful attention to hand hygiene may play a major role in reducing the transmission of Candida to vulnerable patients.
Increased handling required by sicker preterm infants increases the risk of acquiring fungal colonization from health care workers' hands, contaminated infusates, catheters, or catheter-related devices. A neonatal acute physiology score of greater than 10 was a risk factor for fungal colonization by C. albicans and C. parapsilosis in a multicenter study (401). A recent study of hand washing in NICUs reported that there are an average of 78 touches per infant during a 12-h shift (93). Colonization may occur in the sickest infants due to the increased physical assessment of the child, handling of central and peripheral intravenous lines with multiple medication administration, frequent suctioning and repositioning of endotracheal tubes and respiratory tubing, and more exposure to invasive procedures such as intubation, vascular catheter insertion, and urinary catheterization.
Intravenous infusates may transmit fungi directly to the bloodstream. In vitro growth curves demonstrate that Candida species have a selective growth advantage compared to bacteria in parenteral nutrition fluid (433). In some cases, intravenous fluid delivery systems or transducers rather than the infusates themselves may be the source of fungal contamination. In an epidemiologic study of an outbreak of five cases of Candida sepsis in one NICU, retrograde medication syringe systems were the source of the Candida contamination (433). The outbreak developed after the frequency of changing intravenous tubing was decreased from every 24 h to every 72 h, and it was terminated by using syringes only once and resuming intravenous tubing changes every 24 h. Additionally, parenteral nutrition should not be infused via a catheter with a transducer, to avoid potential contamination.
Candida species causing invasive infection rarely colonize environmental sources (30, 409). Despite the tendency of fungus to grow in moist environments, the water reservoirs of isolettes (n = 172), walls of incubators (n = 183), and distal ends of respiratory tubing (n = 106) were evaluated weekly for 6 months and only 1 isolette water reservoir culture yielded C. tropicalis a week after colonization was detected in an infant (30). This was confirmed in other studies of VLBW infants, where there was no detection of fungi from isolettes, incubators, washbasins, water faucets, chest tubes, intravenous drug pumps, ventilators, pumped breast milk, inanimate objects, or radiant warmers (30, 388, 409).
Risk factors.
Risk factors for fungal colonization and infection are similar to those for bacterial colonization and infection, as discussed in an earlier section. In a multicenter trial of six NICUs, changes in the microflora of the gastrointestinal tract due to the use of cephalosporins selected for both C. albicans and C. parapsilosis intestinal colonization, whereas central vascular catheters and vaginal delivery were risk factors for C. albicans and H2 antagonist exposure was a risk factor for C. parapsilosis intestinal colonization (401). These findings imply that C. parapsilosis colonization may be promoted by factors that facilitate proliferation while C. albicans colonization is enhanced by conditions favoring adherence. This study also found a number of variables that were not associated with rectal fungal colonization, including premature rupture of membranes, maternal antibiotics, endotracheal intubation, surgical procedures, and exposure to postnatal steroids or antimicrobial agents other than broad-spectrum cephalosporins. It is possible, however, that these factors are associated with fungal colonization at sites other than the gastrointestinal tract which was examined in this study.
Incidence.
Fungal colonization of the skin, respiratory tract, or gastrointestinal tract occurs in 10% of full-term infants compared to 26.7 to 62.5% of VLBW infants in the first weeks of life (Table 4). In a single-site study, fungal colonization was three times more likely to occur in VLBW infants, being found in 47% of the VLBW infants (18 of 38) compared to 14% of infants with birth weight of >1,500 g (31 of 219) (P < 0.001) (183).
The skin and gastrointestinal tract are the most common sites of fungal colonization in VLBW infants. In the largest study to date, fungal rectal colonization was detected in approximately 30% of 780 VLBW infants, with the majority of isolates being C. albicans and C. parapsilosis (401). In the same study, 37% of 370 infants with birth weight of <1,000 g were colonized compared to 21% of 410 infants with birth weight between 1,000 and 1,500 g (P < 0.001). While C. albicans colonization was more common than C. parapsilosis colonization in infants with birth weight between 1,000 and 1,500 g (P < 0.02) and in those with birth weight of >1,500 g (P < 0.001), no difference was found in infants with birth weight of <1,000 g. A second study also found equivalent rates of C. albicans and C. parapsilosis colonization in ELBW infants (246).
(i) Skin.
In VLBW infants, the prevalence of fungal skin colonization is approximately 13% and increases to as high as 48% in ELBW infants (Table 4). Candida species produce proteases that may be lytic for the thin keratin layer of the preterm infant and phospholipases against lipid membranes; both enzymes may facilitate epithelial invasion (130, 308). Increased transepidermal water loss from preterm skin creates a moist environment that facilitates fungal colonization and growth. Due to the increased permeability of the preterm skin, substrates such as glucose may diffuse to the epithelial surface, facilitating Candida growth. Candida may alter its surface structure in the presence of high glucose, increasing its adherence and proliferative properties (99, 370, 372, 375). Skin maturation occurs by 2 weeks of life in extreme preterm infants, after which new fungal skin colonization occurs less frequently (30, 183, 215).
(ii) Gastrointestinal tract.
The microflora of the gastrointestinal tract plays an important role in fungal colonization and infection in VLBW infants. Buccal candidal adherence is increased in preterm compared to term infants, facilitating colonization (96). A normal bacterial flora inhibits Candida growth by competing for both adhesion sites and nutrients (196, 252). In a study of athymic mice given an oral challenge with Candida, fungal colonization was attenuated in mice with a normal gut flora compared to those with a germ-free microflora (196). This highlights the fact that the intestinal microflora may be as important as an intact immune system in preventing fungal colonization.
The incidence of rectal fungal colonization from several single-site studies of VLBW infants is between 19.2 and 45% (30, 183, 255, 395) and is higher (63%) when stool is cultured directly (362). In a study that examined rectal colonization from birth until day 56 of life in VLBW infants, there was no new rectal fungal colonization after day 28 of life (255).
Direct translocation across the bowel wall may occur when the number of yeast microorganisms overwhelms the mucosal and immune defense of the preterm gastrointestinal tract. In a prospective study of 40 VLBW infants during the first 6 weeks of life, serial twice-weekly quantitative fungal stool cultures were examined (362). The gastrointestinal colonization rate was 63% (25 of 40), with 66% (17 of 25) having more than 8 × 106 Candida CFU per g of stool. At a concentration of greater than 8 × 106 Candida CFU per g of stool, 50% of these infants developed gastrointestinal symptoms and 28.5% developed systemic sepsis within 1 to 3 weeks of this heavy colonization. Combining the studies by Chapman and Faix and Noyola et al., it was found that NEC preceded or occurred concurrently with sepsis in 16.5% of cases of fungal sepsis (30 of 182) (80, 352). Candida is sometimes seen at the site of perforation with NEC or FIP on pathologic examinations probably due to its ability to adhere to deep epithelial and tissue layers (325, 326, 349, 425). Additionally, Candida binds to fibrin, fibronectin, platelets, and integrin sites on leukocytes, which all are present at the nidus of injury in these gastrointestinal diseases (109, 164, 199, 210, 475).
(iii) Respiratory tract.
The presence of an endotracheal tube, mechanical ventilation, and suctioning predisposes intubated VLBW infants to respiratory tract fungal colonization and infection. Pulmonary host defense occurs primarily through alveolar macrophages, cilia and mucus, and surfactant, all of which are deficient in preterm infants. The presence of the endotracheal tube and suction catheter leads to flattening of the respiratory epithelial cells (278), while mechanical ventilation and high oxygen concentrations lead to loss of goblet cells and cilia (91); restoration of normal histology does not occur for weeks after a significant injury (297).
Endotracheal fungal colonization is present in approximately 6.5% of full-term intubated infants (55), 8.3 to 11% of VLBW intubated infants (30, 215, 395), and 42% of ELBW intubated infants (246). In infants with fungal colonization at any site, endotracheal colonization occurred in 24.1% (7 of 28) and 33.3% (13 of 39) of VLBW and 70% (21 of 30) of ELBW colonized infants (30, 246, 395). In the largest analysis of intubated VLBW infants, Rowen et al. demonstrated endotracheal fungal colonization in 11% (13 of 116) and found that, when present, fungemia was 15.4 times more likely to occur than in infants without any fungal colonization (95% CI, 4.0 to 55.7) (395). Controlling for fungal colonization at other sites, endotracheal colonization alone increased the risk of fungal sepsis (RR, 5.9; 95% CI, 1.34 to 26). This study did not demonstrate a correlation between nasopharyngeal and endotracheal fungal colonization.
Fungal Infections
Congenital candidiasis.
Congenital candidiasis is uncommon and may occur as an isolated skin infection or in conjunction with systemic fungal disease. In one series of cases of chorioamnionitis, Candida was isolated from the fetal surface of the placenta in only 0.8% of infections (313). Candida chorioamnionitis and congential infection has been associated with the presence of an intrauterine contraceptive device in about 25% of cases, probably due to heavy fungal colonization (107, 226, 501). Congenital fungal infection was first described in term infants who presented with pustules, vesicles, skin abscesses, and an erythematous maculopapular rash of the trunk and extremities, at times leading to desquamation (42, 105). Subamniotic fungal abscesses form beadlike discrete yellow plaques on the umbilical cord or placenta. Histologic evaluation of the placenta demonstrates hyphae, microabcsesses, and granulomas and is often helpful in identifying the cause of these skin manifestations.
Congenital candidiasis is more commonly associated with systemic spread and worse outcome in preterm than in term neonates. For this reason, term infants with congenital candidiasis should be evaluated for an immunodeficiency. Early reviews demonstrate that half of the patients were preterm infants and that without systemic therapy, these patients, as well as full-term infants with pneumonia, died (107, 226, 501). Whyte et al. described similar findings for 18 cases of histopathologic Candida chorioamnionitis, with 15 leading to neonatal infection (all in infants with birth weight of <2,000 grams), and for 12 cases at <28 weeks' gestation (501). VLBW infants with congenital Candida infection are more likely to present with severe infection, such as pneumonia and widespread dermatitis with focal areas of superficial erosion and desquamation (226, 501). The autopsy findings for the three infants who died within 1 h of birth demonstrated a diffuse pneumonitis with budding yeast in the airways, middle ear, stomach, and syncytial giant cells as well as pseudohyphae in the esophagus and stomach, all suggesting a contiguous spread of Candida along mucosal surfaces. In another early review, only 5 of 27 cases occurred in infants for whom the rupture of membranes occurred >12 h prior to delivery; therefore, the congenital infection may occur via intact membranes, a break in the amniotic membranes, or a large inoculum present once the membranes had ruptured or at delivery (107). Candida can infect and penetrate human fetal and chick chorioallantoic membranes, but animal studies have shown that it cannot pass through the placental barrier (79).
More recently, 63 cases of congenital cutaneous candidiasis (1960 to 1997) were reviewed (105). Since all cases diagnosed in the first 6 days of life were considered, there may have been overlap with postnatally acquired mucocutaneous infection (see below). Twenty-seven infants (43%) had birth weight of >2,500 g, 21 (33%) had birth weight between 1,000 and 2,500 g, and 15 (24%) had birth weight of less than 1,000 g. Infants with diffuse burn-like erythematous macular dermatitis with skin exfoliation were more likely to have blood, urine, or CSF involvement (9 of 12 infants) compared to a papulopustular rash at any gestational age (105). ELBW infants with congenital cutaneous candidiasis had a greater risk of invasive fungal infection (10 of 15) and mortality, although only one-third of these patients received systemic therapy (105). In full-term infants, this infection was unlikely to disseminate. A more descriptive diagnosis of congenital cutaneous candidiasis would be a skin rash in the first 2 days of life with or without placental histology.
Mucocutaneous candidiasis.
Severe mucocutaneous fungal infections may occur in preterm infants, typically in the second to third week of life (135). Invasive Candida dermatitis presents as a diffuse burn-like erythematous rash that may be papulopustular and lead to exfoliation. Risk factors include extreme prematurity, vaginal birth, postnatal steroids, and hyperglycemia (33, 135, 394). Invasive fungal dermatitis in VLBW infants has a high rate of dissemination to the bloodstream, and evaluation for sepsis (blood, urine, and CSF cultures) and treatment with systemic antifungals should be considered.
Faix et al. reported an incidence of fungal mucocutaneous infection of 7.8% (28 of 358) in VLBW infants over a 4-year period (1983 to 1987) with dissemination to invasive fungal infection in 32% of cases (9 of 28) despite topical and enteral nystatin therapy (135). Similar to congenital cutaneous candidiasis, the nature of the rash affects the risk of dissemination. In a study by Baley and Silverman, invasive fungal infection developed in seven of eight ELBW infants with severe mucocutaneous infection, described as a scalding dermatitis (33). C. albicans was isolated from the skin scrapings of all eight patients. Rowen et al. reported severe mucocutaneous infection described as invasive fungal dermatitis in 6% of ELBW infants (16 of 271) over a 2-year period (1991 to 1993) (394). Invasive fungal dermatitis developed earlier in these ELBW infants, at a mean age of 9 days (range, 6 to 14 days). The seven cases confirmed by biopsy were due to C. albicans (n = 3), C. parapsilosis (n = 1), Curvularia sp. (n = 1), Aspergillus spp. (n = 2), and Trichosporon sp. (n = 1). Bloodstream dissemination occurred in 70% of cases (11 of 16), all involving Candida species. Biopsies demonstrated invasion into the epidermis, with an inflammatory process ranging from dermal invasion and granuloma formation to focal necrosis and hemorrhage. This study also demonstrated that invasive fungal dermatitis may spread from the skin to the bloodstream or from the bloodstream to the skin (fungemia leading to dermal involvement) (394). In five cases, fungus was isolated from the bloodstream 1 to 4 days after severe skin lesions developed, while in two cases fungal sepsis preceded the skin manifestations by 24 to 48 h.
Fungal sepsis.
Candida accounts for approximately 12% of LONS in VLBW infants (Table 2) (37, 402, 454). In most centers, the incidence of fungal sepsis among VLBW infants ranges from 2 to 6.8% and is inversely proportional to gestational age and birth weight (30, 183, 215, 246, 255, 302, 362, 395, 402). The incidence of fungal sepsis among preterm infants has increased considerably over the last two decades. In a single-center study (1989 to 1998) of VLBW infants, the incidence increased from 3.8% in 1989 to 6.8% in 1998 (302). The Neonatal Network, reporting on over 13,000 VLBW infants, showed that the proportion of LONS caused by fungi increased from 9% in 1991 to 1993 to 12% in 1998 to 2000, while the associated mortality increased from 28 to 32% (451, 454). This increase may be due to the survival of smaller and sicker preterm infants exposed to invasive therapies for long periods.
In a multicenter study of six NICUs from 1993 to 1995, the incidence of fungal sepsis was 0.26% (3 of 1,139) in infants with birth weight of >2,500 g, 3.1% (29 of 926) in infants with birth weight of <1,500 g, and 5.5% (23 of 421) in infants with birth weight of < 1,000 g (402). A later five-NICU retrospective cohort study of ELBW infants (1994 to 1998) demonstrated a higher incidence of fungal sepsis of 10% (136 of 1,372) (M. G. Karlowicz, J. L. Rowen, M. L. Barnes-Eley, B. L. Burke, M. L. Lawson, C. M. Bender, K. E. Shattuck, M. Horgan, and W. L. Albritton, Abstract, Pediatr. Res. 51:301A, 2002). This is similar to the results of a single-center study of 1,407 ELBW infants, which showed an increase in fungal sepsis from 1.0% in 1981 to 1985 to 9.9% in 1991 to 1995 (266). In the five-NICU study, the incidence of fungal sepsis increased linearly with decreasing gestational age: from 3% at 28 weeks to 28% at 23 weeks (Karlowicz et al., abstract, 2002). There was also considerable interinstitution variability in incidence of fungal sepsis, ranging from 4 to 14%.
(i) Risk factors.
Risk factors for fungal sepsis overlap with those for fungal colonization, as discussed previously (43, 402) (Fig. 2). In two multicenter studies, lower gestational age (<28 weeks), birth weight (<1,000 g), and cephalosporin or carbapenem use were the strongest risk factors for fungemia after multivariate analysis (43, 402). Multiple-site fungal colonization (two or more sites) is also a risk factor for invasive fungal infection, with each additional site increasing the risk by a factor of 3 (95% CI, 1.4 to 6.8) (246). In a multicenter study in which rectal colonization cultures were performed for 76% of preterm infants, univariate analysis demonstrated prior colonization as a risk factor for fungal sepsis, but multivariate analysis controlling for birth weight and abdominal surgery demonstrated that prior rectal colonization was not a risk factor (402). Other single sites may have greater sensitivity, such as endotracheal and stool cultures compared to swabs of the rectum. In two smaller studies of VLBW infants, each with five cases of fungal sepsis, Candida was isolated from endotracheal and stool cultures of all patients prior to the onset of fungal sepsis (362, 395). Bloodstream fungal infection often is preceded by colonization, but it may also occur in the absence of colonization via direct seeding of the bloodstream from infected catheters, infusates, feedings, or feeding tubes (215, 246, 402).
Some studies have found differences between colonizing and infecting Candida species. Studies have reported multisite C. albicans colonization, at the same time as C. glabrata sepsis was diagnosed in preterm neonates (215, 246). In another study, one infant with C. albicans rectal colonization developed C. dubliniensis sepsis (246). This may be due to undetected colonization with multiple fungi or may indicate that bloodstream infection occurred from another source.
Vascular catheters create a unique surface for proliferation of microorganisms, with a matrix of coagulation factors, including platelets and fibrinogen, that favor fungal adherence. Parenteral nutrition is often given through these catheters, providing a substrate for growth (433). Candida species tend to form biofilms on catheter surfaces that shield the organism from the host immune response and from antifungal drugs (6). For this reason, removal of central vascular catheters is recommended for any preterm infant with candidemia in order to eradicate the infection as rapidly as possible and reduce the risk for deep-seated infection (46, 59, 80, 370, 372, 375).
Hyperglycemia may increase the risk of fungal sepsis by providing a substrate for proliferation and by upregulating the expression of genes responsible for pathogenicity (180, 211). In one study of 370 VLBW infants, 7 of 12 infants with fungal sepsis were hyperglycemic and required insulin therapy (32). Sepsis in VLBW infants may result in insulin resistance and hyperglycemia; therefore, high serum glucose levels may be secondary to sepsis in addition to being a risk factor for fungal proliferation.
(ii) Candida species causing fungal sepsis.
In three large multicenter studies of LONS in VLBW infants, C. albicans and C. parapsilosis were the most common species causing candidemia (303, 451, 454). These studies cover the periods of 1991 to 1993 (n = 6,911, 12 centers, United States), 1995 to 1998 (n = 5,555, 28 centers, Israel), and 1998 to 2000 (n = 6,215, 15 centers, United States). In these studies, fungal sepsis due to C. albicans occurred in 51, 23, and 48% of cases, respectively, whereas C. parapsilosis was the cause in 26, 17, and 34%. In the 1995 to 1998 study, one NICU reported using amphotericin B as empiric therapy for evaluation of LONS in all NICU patients, which may select for Candida species other than C. albicans and C. parapsilosis (302).
The prevalence and species distribution of Candida appears to be similar in adult ICU and NICU patients. A study comparing Candida species in seven surgical ICUs (SICUs) and six NICUs between 1993 and 1995 described an incidence of fungal sepsis of 9.8 per 1,000 admissions and 0.99 per 1,000 patient-days in SICU patients and 12.3 per 1,000 admissions and 0.64 per 1,000 patient-days for NICU patients (381). The Candida species causing bloodstream infections in the SICU patients included C. albicans (48%), C. glabrata (24%), C. tropicalis (19%), C. parapsilosis (7%), and other Candida species, (2%). Comparatively, in the NICU the distribution for fungal sepsis was C. albicans (63%), C. parapsilosis (29%), C. glabrata (6%), and other Candida species (3%). The species variations may reflect differences in empiric or prophylaxis practices.
Although C. albicans and C. parapsilosis are the predominant Candida species in VLBW infants, an increasing number of other Candida species have been isolated recently, with C. glabrata being one the more common emerging species. Features of neonatal C. glabrata sepsis were compared to those of C. albicans and C. parapsilosis sepsis in a two-center retrospective review from 1991 through 1998 (Table 5) (131). Of all cases of candidemia in the two centers, 15.5% were due to C. glabrata. This high incidence of C. glabrata could not be attributed to the use of fluconazole for prophylaxis or treatment of fungal infection in these patients, since this agent was rarely used during or before the study period in these units. Interestingly, C. glabrata sepsis occurred in patients with a significantly higher gestational age and birth weight compared to sepsis with non-glabrata Candida species. While the clinical presentation of C. glabrata infection was somewhat less severe, the number of positive blood cultures and the incidence of meningitis, UTI, and NEC were not significantly different, indicating that C. glabrata is capable of causing invasive disease in preterm infants. Also of note in this study, five of the six patients with birth weight of >1,500 g who developed fungemia had a history of gastrointestinal surgery (two had NEC with subsequent short-gut syndrome, two had jejunal atresia, and one had gastroschisis), further demonstrating this important association in higher-birth-weight infants.
TABLE 5.
Comparison of C. albicans, C. parapsilosis, and C. glabrata sepsisa
| Characteristic | Results for:
|
P value | ||
|---|---|---|---|---|
| C. albicans | C. parapsilosis | C. glabrata | ||
| Baseline characteristics | ||||
| No. of patients | 41 | 8 | 9 | |
| Gestational age (wk) | 26.6 ± 0.5 | 27.3 ± 1.3 | 29.7 ± 1.2 | 0.01 |
| Birth wt (g) | 932 ± 75 | 965 ± 194 | 1,442 ± 292 | 0.02 |
| No. (%) of VLBW (<1,500 g) infants | 38 (93) | 7 (88) | 7 (78) | NS |
| No. (%) born via vaginal delivery | 26 (63) | 4 (50) | 7 (78) | NS |
| Factors present at the diagnosis of fungemia | ||||
| Age at diagnosis (days) | 17 (11-31) | 25 (17-87) | 14 (10-21) | NS |
| No. (%) on antibiotics | 12 (38) | 3 (38) | 6 (67) | NS |
| No. (%) with apnea | 28 (68) | 4 (50) | 2 (22) | 0.01 |
| Lowest platelet count (103/mm3) | 74 ± 9 | 39 ± 2 | 124 ± 34 | 0.04 |
| No. of positive blood cultures for fungus | 2.1 ± 1.1 | 1.8 ± 0.71 | 2.0 ± 1.3 | NS |
| No. (%) with candiduria | 15 (37) | 2 (25) | 2 (22) | NS |
| No. (%) with meningitis | 5 (12) | 1 (13) | 1 (11) | NS |
| No. with renal abscessc/total no. tested (%) | 5/38 (13) | 1/7 (14) | 0/7 (0) | NS |
| No. with cardiac vegetations/total no. tested (%) | 2/30 (7) | 0/6 (0) | 0/8 (0) | NS |
| No. (%) with necrotizing enterocolitis | 6 (15) | 0 | 1 (11) | NS |
| No. (%) dying of Candida infection | 0 | 0 | 0 | NS |
| No. (%) who died, all-cause mortality | 3 (7) | 0 | 0 | NS |
Data from reference 131. Plus-minus values are mean ± standard deviation. Median is given as 25th to 75th percentile.
Significant difference between glabrata and non-glabrata groups (P < 0.05). NS, not significant.
Urinary tract ultrasound and echocardiogram were not performed on every patient.
(iii) Timing.
The age of onset of disseminated fungal infection among NICU patients has a wide range, with some cases occurring in the first week of life and others occurring later than 6 months of age. Among VLBW infants, the mean onset of infection ranges from 15 to 33 days of age (31, 234, 366, 402). Fungal sepsis may occur earlier in infants with lower gestational age and birth weight. One small study found fungal sepsis occurring at a median age of 7 days in infants younger than 26 weeks' gestation (324, 366). ELBW infants have a broader range of time when they are susceptible to fungal sepsis compared with older-gestational-age preterm infants, with one study reporting onset from 1 to 76 days of age (Karlowicz et al., abstract, 2002). The pattern of earlier fungal sepsis in ELBW infants probably reflects their extremely immature host defenses, while VLONS may occur if the ELBW infants continue to have significant risk factors such as a central vascular catheter, endotracheal tube, or gastrointestinal disease.
(iv) Clinical presentation.
The symptoms of fungal sepsis are similar to those of bacterial sepsis in VLBW infants (43, 136, 402). The majority of preterm infants with fungal sepsis develop thrombocytopenia, but this is a common laboratory finding in patients with sepsis due to other organisms as well. In a study of 154 infections in 943 VLBW infants, 84% of patients (11 of 13) with fungal sepsis, 75% of patients (18 of 24) with gram-negative bacterial sepsis, and 48% of patients (56 of 117) with gram-positive bacterial sepsis had a platelet count of <100,000/mm3 (176).
(v) End-organ dissemination.
At the time fungal infection is clinically apparent, the organisms have often disseminated from the blood, urine, or CSF to adhere to and proliferate in body fluids, tissues, and organs. Candida species can cause endocarditis, endophthalmitis, dermatitis, peritonitis, osteomyelitis, and septic arthritis, and fungal abscesses may form in the CNS, kidneys, liver, spleen, skin, bowel, and peritoneum (Fig. 2). Fungal end-organ dissemination has been noted since the earliest reports of Candida sepsis in neonates (201, 260). In an early review (1972 to 1982) of 31 VLBW infants with invasive fungal infection, dissemination occurred to the bones or joints (26%), eyes (23%), lungs (13%), skin (10%), heart (6%), and peritoneum (3%) (227). More than one organ system was involved in 21 of these cases. The high prevalence of end-organ dissemination in earlier studies of neonatal fungal sepsis reflects prolonged periods of fungemia, since most of the diagnoses were made late or by autopsy. A meta-analysis of studies reporting fungal end-organ dissemination in neonates, spanning both older and more recent practices from 1979 to 2002, found that the median prevalence was 5% for cardiac vegetations or thrombi, 3% for endophthalmitis, 5% for renal involvement, and 4% for CNS abscesses (45). In a more recent study (1989 to 1999) of 86 cases of fungal sepsis in neonates, cardiac vegetations or thrombi occurred in 15.2%, endophthalmitis occurred in 6%, renal involvement occurred in 4.5%, and liver abscesses occurred in 3% (352). Dissemination may be higher in lower-birth-weight infants. In a 1988 to 1995 study examining 46 ELBW infants with fungal sepsis or meningitis, fungal abscesses of the CNS were found in 13 patients (28%) (149).
Chapman and Faix (80) have reported increased fungal end-organ dissemination with persistently positive blood cultures for more than 7 days (1981 to 1999). In a more recent review (1989 to 1999), Noyola et al. also found that infants with candidemia for more than 5 days were more likely to demonstrate ophthalmologic, renal, or cardiac abnormalities than those with a shorter duration of candidemia (352). However, when amphotericin B was given and central vascular catheters were removed within 2 days of the first positive blood culture, outcomes such as end-organ dissemination and mortality were not decreased (352). With improved blood culture methods, the majority of blood cultures grow Candida within 2 days. One study of neonates demonstrated that isolation of fungus in blood cultures occurred at 37 ± 14 h, with 97% of blood cultures positive by 72 h if the patient was not exposed to antifungal therapy (414) and 91% positive with antifungal exposure.
(a) Endocarditis and infected vascular thrombi. Candida endocarditis has been found in 5.5 to 15.2% of cases of fungal sepsis, with equal prevalence for C. albicans and C. parapsilosis. Fungal endocarditis may be associated with higher mortality than fungemia alone (46, 80, 302, 352). Noyola et al. reported that of 11 patients with fungal endocarditis, 3 died acutely and 2 died later of unrelated complications (352). Central vascular catheters place neonates at increased risk for endocarditis and infected vascular thrombi (319, 346, 461). As discussed above, they can cause local trauma to valvular, endocardial, or endothelial tissue, creating a nidus for thrombus and infection. To minimize the risk of trauma, care must be taken to avoid placing umbilical or peripherally inserted central catheters past the superior or inferior vena cava. Removal of central vascular catheters as soon as is reasonably feasible may also reduce the risk of vascular injury, microbial colonization, and disseminated infection. In addition, prompt removal of central catheters in patients with fungal sepsis is likely to reduce the incidence of endocarditis and infected vascular thrombi.
(b) Endophthalmitis and retinopathy of prematurity. Endophthalmitis begins as a chorioretinal lesion that gradually elevates and breaks free in the vitreous, appearing as a white fluffy ball. These solitary or multiple white lesions are most often seen in the posterior retina (zone I) and vitreous. In adults and children, the clear cell-free vitreous becomes hazy due to an influx of inflammatory cells. This vitreous reaction is more difficult to recognize in preterm infants due to the vitreous haze that is present in the first weeks of life. The most immature infants appear to be at highest risk for fungal endophthalmitis (reviewed in reference 29). In an early study of VLBW infants, endophthalmitis occurred in four (50%) of eight cases of fungal sepsis, with excellent prognosis after prompt treatment with amphotericin B and 5-fluorocytosine (28). More recent studies have reported a much lower incidence of retinal endophthalmitis, probably due to more rapid diagnosis, treatment, and surveillance of systemic candidemia. In a retrospective study from 1989 to 1999, 4 of 67 (6%) of preterm infants with fungal sepsis who underwent indirect ophthalmoscopy examination had endophthalmitis (352). Severe fungal eye pathology may develop in ELBW infants with fungal sepsis. A recent report describes a 24-week-gestation infant with Candida sepsis at 4 weeks of age who, on examination at the time fungemia was detected, had markedly immature fundi with limited vascularization. Five weeks later, despite antifungal therapy, bilateral cataract and intraocular inflammation were present, with progressive retinopathy of prematurity (ROP) and tractional retinal detachment (429). On vitrectomy and lensectomy, an intralenticular Candida abscess was found.
Even in the absence of visible retinal abscesses or chorioretinitis, there is some epidemiologic evidence that Candida sepsis may predispose VLBW infants to severe ROP. Kremer first described 15 preterm infants with C. albicans sepsis and ROP (269). Since then, several studies have examined this association. In a study of ELBW infants, threshold ROP developed in 33% of infants (19 of 58) with a history of candidemia compared to 10% (39 of 391) without candidemia, but this was not statistically significant when controlled for gestational age (242). Conversely, Noyola and colleagues, in a case-control study of VLBW infants <28 weeks' gestation, found that 52% of infants with candidemia (24 of 46) developed threshold ROP compared to 24% of controls without candidemia (11 of 46) (P = 0.008) (351). In this series, retinal detachment occurred in 10 of the candidemic patients compared to 4 of the controls (P = 0.10) (351). In another study of 130 ELBW infants from 1996 to 1999, controlling for gestational age, race, days receiving supplemental oxygen, and cumulative postnatal dexamethasone dose, fungal sepsis was independently associated with the need for laser therapy for ROP (OR, 8.2; 95% CI, 2.0 to 33.0) (189). Of note, however, 13 of 14 patients with fungal sepsis had received postnatal steroids, implying that they were critically ill for prolonged periods; thus, other factors than Candida sepsis may have contributed to the development of ROP. While the data regarding an association between fungal sepsis and ROP are conflicting, early and frequent screening for retinal pathology is recommended in VLBW infants with candidemia.
(c) Renal system and fungal infection. Renal factors that favor Candida growth are hypertonicity and acidity of the tubular lumen as well as a delayed renal inflammatory response. In VLBW infants, urine pH is increased in the first weeks of life due to decreased bicarbonate absorption at the proximal tubule, so that a UTI at this time may be due to local immune factors and hematogenous spread. Prematurity, antibiotic treatment, and immunosuppressive therapy are also risk factors for renal fungal infections. In six studies of VLBW infants, the incidence of fungal UTI was 2.4% among all 1,322 infants and 58.3% (28 of 48) in infants with fungal sepsis (31, 135, 227, 255, 395, 490). In one study of ELBW infants, the incidence of fungal UTI was 6% (3 of 50), and 1 of 8 infants with fungal sepsis had Candida UTI (246).
Renal abscess formation in preterm infants may occur by dissemination of candidemia or as an ascending infection with candiduria (reviewed in reference 239). Hurley and Winner observed in animal studies that large doses (>2 × 106 organisms) of Candida given intravenously produced systemic candidiasis, with systemic fungal lesions and renal involvement in all cases (219). Smaller intravenous doses resulted in isolated renal infection with acute pyelonephritis and multiple cortical abscesses developing within 48 h, followed by cortical scarring 1 week later, with papillary necrosis and mycelia isolated in the tubular lumens of the medulla. Additionally, in these animals, large fungal abscesses in the renal pelvis led to obstructive nephropathy and renal failure.
Renal fungal abscess formation may complicate Candida UTIs in preterm infants. Fungal UTI may occur alone or in conjunction with fungal sepsis. In one study, 41 (0.5%) of 8,790 neonates admitted to the NICU with median birth weight of 890 g and gestational age of 27 weeks developed candiduria (64). Candiduria occurred in the more preterm infants, and renal abscesses developed in 36.6% of the infants with candiduria (15 of 41). These 15 patients had a median birth weight of 770 g and gestational age of 26 weeks. Initial ultrasound examinations were normal in six patients who developed renal abscesses 8 to 39 days later. This study suggests that for infants with candiduria, renal imaging studies should be performed at the time of infection and also at the end of antifungal treatment.
Renal fungal abscess formation may cause acute renal failure and long-term morbidity in preterm neonates. In the same study that is described above, Bryant et al. found that 2 of 15 preterm infants with candiduria developed acute renal failure (64). One patient required percutaneous aspiration of the renal fungal abscess. The clinical course of the 15 cases of renal fungal abscesses included persistent renal echogenic foci at hospital discharge in 6 patients (40%), calcification in 4 (27%), partial obstruction in 2 (13%), and hydronephrosis in 2 (13%). Without antifungal treatment papillary necrosis may occur (472).
(d) Central nervous system candidiasis. CNS fungal infection may involve meningitis, ventriculitis, or abscess formation in VLBW infants (83, 134). In six studies from 1979 to 1994, the overall incidence of culture-proven fungal meningitis among VLBW infants was 1.6% (17 of 1,048 infants). In these studies, 36% of VLBW infants with fungal sepsis (23 of 64) had evidence of meningitis by culture or abnormal CSF indices (31, 132, 135, 227, 395, 490). In contrast, other studies have reported a lower incidence of fungal meningitis among preterm and term neonates with fungal sepsis, ranging from 5.7 to 22% (45, 80, 139, 149).
Culture of the CSF is important in diagnosing fungal meningitis in VLBW infants since CSF cell counts and chemistries may not be abnormal. In one study of 25 infants with Candida sepsis or meningitis, spinal fluid abnormalities occurred in only 25% of infants with culture-proven Candida meningitis (277). In another study of 12 VLBW infants with Candida meningitis detected by culture, the most common CSF abnormality was low glucose (<30 mg/dl), noted in 7 of 9 tested cases, while the CSF protein level was elevated in only half of the patients (range, 30 to 480 mg/dl) (288). CSF white blood cell counts ranged from 3 to 1030 cells/μl, with varying percentages of neutrophils and mononuclear cells in these cases of culture-proven fungal meningitis (288).
Fungal abscesses of the CNS have been reported to be microscopic and not readily detectable by ultrasonography or computed tomography (193, 467). In a study of 46 ELBW patients with fungal sepsis and/or meningoencephalitis, only 6 of 13 patients with fungal CNS abscesses (detected by ultrasound or computed tomography or on autopsy) had abnormal results on lumbar puncture (149). Two studies have found an association between invasive fungal infection and periventricular leukomalacia in preterm infants, possibly related to the release of cytokines which may damage the periventricular white matter (149, 512). These findings demonstrate the need for cranial imaging such as utrasonography in all patients with fungal sepsis, regardless of the results of CSF studies.
Outcome of invasive fungal infection. (i) Neurodevelopmental outcome.
In the largest analysis (1988 to 1994) of fungal infection and morbidity in ELBW infants, 46 infants with Candida sepsis and/or meningoencephalitis were compared to a cohort of 470 ELBW infants without fungal sepsis (149). Six cases were not diagnosed until autopsy. C. albicans and C. parapsilosis accounted for 78 and 20% of the cases, respectively. Comparing 27 Candida-infected patients to the 303 control patients who survived and were available for analysis, there was increased severe neurodevelopmental impairment (41 and 12%, respectively; P = 0.005), less intact neurodevelopment (41 and 65%, respectively; P = 0.02), and a trend toward increased periventricular leukomalacia (P = 0.06) in the Candida-infected patients. Infected infants had more chronic lung disease (100 and 11%, respectively; P = 0.0001) and severe ROP (stage 3 or 4) (22 and 9%, respectively; P = 0.04). In this study, neurodevelopmental outcome was related to timing of initiation of antifungal therapy. Antifungal therapy was initiated 5.1 ± 3.0 days after blood culture was drawn for those infants who had severe disabilities or died compared to 2.1 ± 1.3 days for those who were normal or mildly impaired (P < 0.0001).
In a smaller retrospective study of infants with birth weight of <1,250 g, neurodevelopmental outcome was assessed between 2 and 3 years of age in 14 infants who had fungal sepsis or meningitis compared to 21 matched controls (277). The mental developmental index was similar (83 ± 20 and 90 ± 20, respectively), but the performance developmental index was lower in the patients who experienced fungal sepsis (71 ± 21 and 87 ± 18, respectively P < 0.05). However, not all studies have demonstrated adverse neurodevelopmental outcome in preterm survivors of fungal sepsis compared with their gestational age-matched peers. In a retrospective study of VLBW infants with systemic candidiasis compared to matched controls. Baley found no difference in the mental developmental index (89 ± 16 and 83 ± 22, respectively) or the performance developmental index (91 ± 18 and 85 ± 22, respectively) scores or neurologic impairment at 2 years of age (27).
(ii) Mortality.
Early studies reported autopsy evidence of disseminated or focal fungal disease in up to 30% of patients who died of suspected sepsis but had no growth of fungus from blood, urine, or CSF cultures prior to death (31, 227, 354). More recently, with improved blood culture techniques, and with increasing awareness of the high incidence and severity of fungal sepsis among preterm infants, therapy is instituted earlier and outcome has improved. In a 1989 to 1999 review of fungal sepsis in preterm infants, only 3 (2.7%) of 110 cases of invasive fungal infection were not diagnosed until autopsy (352).
All-cause mortality among VLBW infants who experienced fungal sepsis has been reported in several multicenter studies to range from 28 to 32%, compared to a mortality of approximately 7 to 9% in VLBW infants who do not have an infection during their NICU stay (303, 450, 454). Mortality is higher in ELBW infants who develop fungal sepsis and has been reported at between 37 and 40% (149, 246), while fungal sepsis occurring after 4 weeks of life has been associated with lower mortality (133). Studies reporting high mortality associated with Candida sepsis generally present mortality from all causes, and it is likely that the number of deaths directly attributable to fungal infection is smaller. In the most recent VLBW NICHD study, which included 160 cases of fungal sepsis, 48% of fungus-related deaths occurred in the first 72 h and 73% occurred with 7 days from the time of blood culture. Several centers have reported, for smaller samples of patients, a lower mortality from fungal sepsis in preterm infants (131, 302). One study reported no mortality in 49 NICU patients with fungal sepsis, including 35 cases in VLBW infants (302), and in another study involving 52 VLBW neonates with Candida sepsis, only 3 patients died prior to NICU discharge and none of the deaths was directly attributed to fungal sepsis (131).
A number of studies have shown that C. albicans is associated with significantly higher mortality than are other Candida species (450, 454). This was first noted by Faix in a single-center study (1980 to 1990) of 45 cases of invasive fungal infection in term and preterm infants, which demonstrated a higher associated mortality due to C. albicans (24%) than to C. parapsilosis (0%) (133). In the recent NICHD Neonatal Network survey of VLBW infants, patients with C. albicans sepsis had a mortality of 44% compared to 16% for those with C. parapsilosis sepsis (454). This may be related in part to the timing of infection, with vertically transmitted C. albicans causing infection earlier, when the immune system is more compromised, and horizontally transmitted C. parapsilosis causing infection in an older, more immunocompetent host.
Treatment of Candida sepsis. (i) Antifungals.
The mainstay of therapy for neonates with disseminated fungal infection remains amphotericin B deoxycholate, with some exceptions, although newer antifungal agents are under investigation for use in neonates (reviewed in references 54, 171, and 254). Amphotericin B is generally better tolerated in neonates than in adults, allowing therapy to begin at 1 mg/kg without administering test doses. Liposomal amphotericin B preparations were shown to be comparable in efficacy to amphotericin B in a limited number of small neonatal trials and may be used in patients with renal impairment or toxicity from amphotericin B (54, 254). In adult studies, amphotericin B levels in CSF are 5 to 10% of the levels in plasma, but in one study of 13 preterm infants, concentrations in CSF were 40 to 90% of those in the plasma. In patients with meninigitis, enteral flucytosine may be added if tolerated, but resistance emerges if this drug is used as monotherapy. Animal studies have demonstrated better penetration of liposomal amphotericin than of amphotericin into CSF, although this has not been studied in preterm infants.
Several small studies have found similar efficacy of azoles compared to amphotericin B for treatment of preterm infants with fungal sepsis (118, 171, 221, 489). Of the azoles, fluconazole has undergone the most extensive study in VLBW infants. Currently, the strength of fluconazole used in preterm infants is for prophylaxis (intermittent 3-mg/kg dosing) in high-risk ELBW infants (see below). With prolonged daily treatment of fungal sepsis with azoles at doses of 6 to 12 mg/kg, resistance may develop, particularly among C. krusei and C. glabrata species; thus, amphotericin is generally preferred as a first-line agent for treatment of fungal sepsis in neonates. There is some concern about ophthalmologic side effects of voriconazole use in preterm infants with a developing retina, and this requires further study (171). A new class of antifungal agents, the echinocandins, acts through noncompetitive inhibition of 1,3-β-glucan synthesis of the fungal cell wall and is licensed for use in adults. Studies are under way to determine their efficacy in pediatric and neonatal patients.
Factors to consider in deciding the duration of therapy include the species of fungus involved, whether a central line was present and was removed, number of positive blood cultures, CSF indices, and findings of the evaluation for end-organ dissemination, including echocardiogram, renal ultrasound, ophthalmologic examination, and neuroimaging studies. Generally, 2 to 4 weeks of intravenous therapy after negative cultures is recommended for treatment of candidemia in preterm neonates, with longer courses being appropriate for meningitis and deep-seated infections. Despite prolonged courses of appropriate therapy, recurrence of Candida infection, including severe osteoarthritis, has been reported in preterm neonates (192).
(ii) Central vascular catheter removal.
In addition to treatment with antifungal medications, central vascular catheters should be removed in preterm infants with fungemia. A new central catheter should not be placed until fungemia is cleared from the bloodstream, as demonstrated by two or more negative cultures. A number of retrospective studies have found that delayed removal of central venous catheters in neonates and children with candidemia significantly increases morbidity and mortality (80, 352, 352, 365). For VLBW infants with candidemia, delayed catheter removal increases the risk of prolonged candidemia, end-organ dissemination, poor neurodevelopmental outcome, and increased mortality (243, 359, 447). In a recent retrospective cohort study of patients with candidemia in a single NICU from 1994 to 1998, 50 infants had central venous catheters removed within 3 days of detection of fungemia while another 54 infants retained their catheters for more than 3 days after the first positive blood culture for Candida (243). All infants were treated with amphotericin B. The “early-removal” patients had a significantly shorter duration of candidemia (median, 3 days; range, 1 to 14 days) compared with the “late-removal” patients (median, 6 days; range, 1 to 24 days). In cases of C. albicans bloodstream infection, mortality was lower in patients whose catheters were removed within 3 days of diagnosis. However, even with early removal and antifungal therapy, three patients with C. albicans sepsis died within 72 h of presentation, pointing to the virulence of C. albicans in neonates. The results of this and other retrospective noncontrolled studies may be confounded by the fact that it may be difficult to obtain other vascular access in the most critically ill preterm infants. However, because of the adherence properties of Candida and the tendency to form biofilms that shield the organism from antifungal agents, rapid removal of central catheters should result in faster resolution of infection and may improve outcomes.
(iii) Empirical treatment
In high-risk ELBW patients, who are not receiving antifungal prophylaxis, empiric therapy may be considered if infants have central vascular access, an endotracheal tube, thrombocytopenia (<100,000/mm3), exposure to broad-spectrum cephalosporins or carbapenem, and gestational age less than 28 weeks (43, 302, 402). Empiric therapy in high-risk ELBW infants has not been prospectively studied, and in studies of other immunocompromised patients such as bone marrow transplant patients, outcomes have not been improved (386). Amphotericin B should be chosen for empiric therapy, and fluconazole should be reserved for prophylaxis. Antifungal prophylaxis has demonstrated efficacy in these high-risk ELBW patients and is discussed below (246).
Malassezia, Trichosporin, Aspergillus, and Zygomycetes
Malassezia furfur, M. pachydermatis, and Trichosporin species are not highly virulent but have been associated with nosocomial infections in preterm infants. M. furfur is a lipid- dependent fungus which can colonize the skin, gastrointestinal tract, and Intralipid solutions of NICU patients and can be spread from patient to patient via the hands of health care workers. Bloodstream infection with M. furfur is more common in infants with lower birth weight, younger gestational age and longer NICU stays (279, 459). The infection may clear simply with discontinuation of lipid infusion and/or removal of central vascular catheters (78). However, for preterm infants with significant symptoms and documented M. furfur fungemia, treatment with systemic antifungal therapy is warranted. M. pachydermatis invasive infections have also been detected in neonates, including an outbreak of 10 cases in a NICU (78). Extensive surveillance cultures found a single strain of M. pachydermatis on nine other infants, one health care worker, and three dogs of health care workers. Education on hand washing and treatment of infections was successful in eradicating this organism from the NICU.
Aspergillus spp. and zygomycetes are extremely rare filamentous fungi but can cause severe infections in preterm infants. Aspergillus spp. may cause cutaneous, pulmonary, or disseminated disease and are susceptible to amphotericin B or itraconazole. The major host defense against aspergillosis is macrophage chemotaxis and phagocytosis, both of which are diminished in preterm infants. Infections are generally the result of environmental contamination such as dust from hospital construction or faulty cleaning practices that can carry spores that may settle in wounds or be inhaled. Regular cleaning of the ventilation systems in the NICU to avoid buildup of dust contaminated with spores, as well as appropriate containment of dust during hospital renovation and construction, can help to prevent aspergillosis infection in high-risk neonates.
Zygomycotic infections initially present as a black eschar at site of local trauma, intravenous catheter, or infiltrate and progress to necrotizing soft tissue infections (355, 411). These fungi may contaminate adhesive tape, monitor leads, and wooden tongue blades used for splints in the NICU (110, 327). Early diagnosis, amphotericin B, and surgical debridement are needed to prevent ulceration, necrosis, and rapid fatal dissemination. A high degree of suspicion is needed, and a tissue biopsy specimen must be obtained to diagnose these right-angle-branched, nonseptated hyphae (355, 411). Mortality from the infection is reported to occur in 61% of infants (11 of 18). Recently, there have been more reports of these infections in preterm infants.
PREVENTION OF NOSOCOMIAL SEPSIS IN VERY-LOW-BIRTH-WEIGHT INFANTS
Bacterial Infections
Given the high incidence and high morbidity and mortality of sepsis in preterm infants, a number of preventative strategies have been tested or are currently under investigation. Vancomycin prophylaxis against CoNS has been tested in five neonatal trials and reviewed in a meta-analysis (495). While the infusion of low-dose vancomycin has been shown to reduce the incidence of CoNS bacteremia in VLBW neonates, the meta-analysis found no difference in clinically important end points such as mortality or length of hopitalization. Given these results and the risk of emergence of vancomycin-resistant pathogens, this strategy is not recommended. Use of a vancomycin flush or lock for central catheters has been shown to reduce colonization and sepsis in immucompromised children and adults (73, 345, 443) without resulting in detectable antibiotic levels in the bloodstream, and this deserves further study in neonates with particular attention to the emergence of resistance. Additionally, the use of vascular catheters impregnated with antibiotics and/or antiseptics has reduced nosocomial bloodstream infection in adults (98) but has not yet been tested in neonates. Local antimicrobial dressings for central vascular catheters have also been studied with a limited number of patients but are associated with contact dermatitis.
IVIG was administered to preterm infants in a large number of randomized, controlled prophylaxis trials in the 1980s and 1990s, with disappointing results. Although the rationale for IVIG administration was sound, with infants of less than 32 weeks' gestation having approximately 50% as much IgG as term infants, a meta-analysis of approximately 5,000 patients enrolled in 19 trials showed only a 3% reduction in sepsis and 4% reduction in serious infection in IVIG-treated compared with placebo-treated infants (357). Moreover, there was no significant reduction in mortality, length of hospital stay, or clinically important morbidities such as IVH, NEC, or bronchopulmonary dysplasia. There were also no significant benefits in the effect on any particular pathogens such as gram-negative bacilli. Several investigators did find benefit with certain lots of IVIG that contained high titers of antibodies against specific neonatal pathogens (25, 137). Since most standard IVIG preparations contain low titers of type-specific antibodies against common neonatal pathogens, hyperimmune globulin may prove to be more efficacious in preventing or treating sepsis in preterm neonates. Anti-staphylococcal IVIG preparations have also been developed, with high titers of antibody against surface antigens such as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules), capsular polysaccharides, and lipoteichoic acid. Clinical trials are under way to determine the efficacy of hyperimmune IVIG in prevention of nosocomial staphylococcal sepsis in VLBW infants. Hyperimmune globulins against GBS (379) and against gram-negative organisms (116) are also being developed and tested.
Recombinant colony-stimulating factors (rCSF) have been tested for their ability to prevent nosocomial sepsis in high-risk neonates, and are the subject of a recent review (356). Both granulocyte-macrophage (GM)-CSF and G-CSF raise neutrophil counts and enhance neutrophil and macrophage functions that may be deficient in preterm newborns. Some studies have shown reduced expression of colony-stimulating factors from neonatal leukocytes exposed to infectious stimuli (356), further supporting the possibility that treatment with rCSF may be useful in the prevention or treatment of sepsis in preterm infants. On the other hand, colony-stimulating factors increase the expression of leukocyte adhesion molecules that could, at least theoretically, promote lung inflammation. Concerns have also been raised that rCSF-induced production of inflammatory cytokines such as TNF-α may promote tissue damage or shock. Several studies have addressed the use of rCSFs for prevention of neonatal nosocomial sepsis. In a study of 75 infants of less than 32 weeks' gestation, administration of GM-CSF was associated with a trend toward decreased nosocomial bloodstream infection from 46 to 31% (496). However, in a larger randomized, blinded, placebo-controlled trial of 264 VLBW infants, GM-CSF had no significant effect on nosocomial sepsis (36). It is possible that rCSF will play a more important role in the prevention or treatment of infection in a subset of preterm infants with neutropenia and reduced bone marrow neutrophil storage pools. Due to the multiple immunodeficiences of preterm infants, a combination of immunotherapies, such as rCSF and IVIG, may have a stronger effect than any one therapy alone, and this approach should be considered during initial clinical trials of new therapies to prevent infection.
Application of topical emollient ointment for the first 2 weeks of life to minimize skin breakdown was shown in several small trials to reduce transepidermal water loss and decrease dermatitis without changing colonization with bacteria or yeast (66, 262, 274). Although one single-center study found significantly fewer positive blood or CSF cultures in infants treated with topical emollient compared with controls (48), a multicenter randomized, controlled trial of nearly 1,200 ELBW infants found that topical emollient actually increased LONS, particularly with CoNS. Another study linked application of topical petrolatum ointment with an increased risk of systemic candidiasis (68); this indicates that this practice should not be continued.
Several other practices may significantly decrease LONS among preterm neonates. Human milk contains a number of immune modulators, including secretory IgA, lactoferrin, lysozyme, and acetylhydrolase, which breaks down PAF (reviewed in reference 348). Feeding with human milk has been shown to reduce the risk of sepsis and NEC in preterm infants (68, 296). Skin-to-skin contact between mother and infant has been shown to promote maternal production of antibodies specific for the nosocomial flora of the neonate (410). Thus, strategies to promote breast milk feeding may significantly improve the outcome of preterm infants. The Vermont Oxford Network has also demonstrated that careful attention to infection control measures (“potentially better practices”) may reduce the incidence of nosocomial infections. The better practices included measures to improve hand hygiene and patient skin care, minimize the number of days of artificial ventilation and central venous access as well as entry into these systems, and improve unit “culture” so that all health care providers feel responsible for patient outcomes. These preventative strategies are the subject of a recent review (376). In a study of these practices in 6 NICUs compared to 66 control units, the rate of infection with CoNS dropped from 25 to 16.6% (relative risk, 0.67; 95% CI, 0.51 to 0.87) (256). Of note, a major change in practice in this study involved obtaining two blood cultures rather than one from infants with suspected sepsis and discontinuing antibiotic therapy at 48 h if no organism was isolated from culture. Diagnosis of CoNS sepsis was based on growth from two blood cultures or antibiotic treatment for 5 or more days, and this change along may account for the observed decrease in CoNS sepsis, which, at 16.6%, is closer to the reported Neonatal Network rate using the same definition.
Necrotizing Enterocolitis
Given the strong association between NEC and infection, prevention would probably affect the incidence of morbidity and mortality from sepsis in VLBW infants. A great deal of research with animals and humans has been aimed at preventive measures and is the subject of recent reviews (69, 214). Some possible preventive strategies include administration of antenatal steroids, slow initiation of enteral feeding (49), human milk feeding (296), acidification of feeds (75, 321, 322), oral immunoglobulin supplementation (121), administration of phospholids (arachidonic acid and choline) (70), and feeding of prebiotics (oligosaccharides) and probiotics (Lactobacillus and Bifidobacterium species) (213). Since PAF appears to play a role in intestinal necrosis in animal models of NEC (92, 466), the use of recombinant PAF-acetylhydrolase is also under investigation for the prevention or treatment of NEC (214).
Invasive Fungal Infections
Strategies for the prevention of neonatal fungal infections have been recently reviewed (245). Preventing horizontal transmission of Candida by hand washing and use of gloves is important but has had only limited success in decreasing colonization and sepsis in critically ill neonates (215, 369, 401, 436). Hand washing is effective in reducing transient, but not permanent, colonizing members of the hand flora, thereby diminishing the number of microorganisms that may be transferred to the patient during direct contact. In a recent NICU study of 16 nurses, comparing a chlorhexidine wash and a 60% isopropanol gel, C. albicans was isolated in 8.0 and 1.3% of the cultures, respectively, after hand washing (275). Since a low inoculum is sufficient to transfer yeast to patients, some fungal transmission still may occur (65, 216, 353).
Oral antifungal prophylaxis.
Prophylaxis targeting gastrointestinal tract fungal colonization by using oral nonabsorbable antifungal agents has been tested in a number of trials. Nystatin was studied in a randomized placebo-controlled trial of 67 ventilated preterm infants with birth weight of <1,250 g, from 1985 to 1986 (438). Patients received 1 ml (100,000 U) of nystatin or placebo every 8 h until 1 week after extubation. Cultures were taken weekly from multiple sites, and fungal colonization was present in 12% of the nystatin-treated patients compared to 44% of the control patients (P < 0.05). Fungemia occurred in 2 of 34 placebo-treated patients and none of the 33 nystatin-treated patients (P = 0.16). Fungal UTI (diagnosed by suprapubic bladder aspiration) occurred in 2 nystatin-treated patients (6%) and 10 control patients (29%) (P = 0.01), implying that urine colonization may be affected by gastrointestinal colonization. Fungal resistance and potential side effects from nystatin were not evaluated in this study. Oral nystatin also does not have efficacy once Candida colonization is established, since several small studies involving a total of over 100 patients reported the failure of oral nystatin to prevent fungal sepsis in colonized ELBW and VLBW infants (103, 125, 227, 281, 498). A combination of oral and topical nystatin has also failed to prevent fungal sepsis in ELBW infants (125). In addition, the highest-risk ELBW infants often are felt to be too unstable to tolerate oral nystatin therapy (103, 438).
Prophylactic oral miconazole gel was studied in a placebo-controlled trial with 600 ventilated preterm infants with birth weights between 1,000 and 1,750 g (488). Miconazole was administered at a dose of 0.75 ml of gel on a gloved fingertip to the oral cavity three times a day during the infant's NICU hospitalization. Fungal rectal colonization was lower in the miconazole-treated (19.5%) group than in the control group (36.7%) (P < 0.0001). The study demonstrated no effect on the incidence of invasive fungal infection, which occurred in 2.0% of the miconazole-treated group and 2.6% of the control group. Prior colonization was present in 50% of these infections, and the majority of the infections were due to C. albicans (12 of 14 infections). No infants with birth weight of <1,000 g were included in this study, and azole resistance and drug-induced side effects were not evaluated.
Factors contributing to the failure of nystatin or miconazole to prevent fungal sepsis may be the agent itself, insufficient dosage, or inadequate delivery to target regions in the bowel as a result of immature gastrointestinal motility and absorption in VLBW infants. Oral prophylaxis with nonabsorbable agents may also be ineffective since many cases of invasive fungal infection occur via the skin, respiratory tract, central venous catheter, or infected infusates. Since many of the studies were performed with preterm infants at lower risk of invasive fungal infection (focusing on VLBW rather than ELBW patients), an inadequate number of patients may have been studied to demonstrate efficacy.
Fluconazole prophylaxis.
Fluconazole is a potent inhibitor of the fungal cytochrome p450 and sterol C-14 α-demethylation. It alters cell membranes, resulting in increased membrane permeability and impairment of purine and pyrimidine precusor uptake for DNA synthesis. Fluconazole is an excellent drug for prophylaxis due to its long half-life, high CSF (70 to 90%) and pulmonary (120%) penetration, low lipophilicity, and low protein binding.
In a randomized, double-blind, placebo-controlled study of 103 VLBW infants, fluconazole prophylaxis was successful in decreasing rectal colonization from 46 to 15.1% (P = 0.005) (255). It is important to note that the study was designed only to evaluate the effect of antifungal prophylaxis on rectal colonization and did not have sufficient sample size to evaluate the effect of prophylaxis on fungal sepis due to the low incidence in VLBW infants. This study did demonstrate that intravenous fluconazole was safe and effective in decreasing mucosal fungal colonization.
In a prospective, randomized, double-blind clinical trial with high-risk ELBW infants who either were intubated or had a central venous catheter present in the first 5 days of life, intravenous fluconazole prophylaxis was successful in preventing invasive fungal infection (Table 6) (246). A total of 100 patients were randomized to receive intravenous fluconazole (n = 50) or placebo (n = 50) from enrollment until they no longer required either central or peripheral intravenous access for up to 6 weeks. This approach to prophylaxis covered the period during which these patients had additional risk factors for fungal sepsis that require a central vascular catheter or intravenous access, such as administration of parenteral nutrition, lipid infusions, or broad-spectrum antibiotics (46, 402). Dosing of fluconazole was 3 mg/kg every 72 h (weeks 1 and 2) followed by every 48 h (weeks 3 and 4) and then by every 24 h (weeks 5 and 6). Invasive fungal infection (fungus isolated from the blood, urine, or CSF) developed in none of the 50 fluconazole-treated patients compared to 10 (20%) of 50 placebo-treated patients (difference in risk, 0.20; 95% CI, 0.04 to 0.36; P = 0.008). Of 50 placebo-treated patients, 8 (16%) had fungal bloodstream infection, compared to none of the fluconazole-treated patients (P = 0.007), while two additional placebo-treated patients had fungal UTIs alone. There were no differences in liver function tests between the two groups, and no significant azole resistance occurred during the study. The favorable patient side effect and fungal resistance profiles may have been due to the fluconazole dose (3 mg/kg) and intermittent dosing schedule, which was based on previous pharmacokinetic studies of fluconazole in preterm infants (408). Follow-up of the patients in this study to a median age of 14.75 months (range, 5 to 46 months) also revealed no adverse outcomes in liver function or neurodevelopmental outcome. (D. Kaufman, R. Boyle, M. Robinson and L. B. Grossman, Abstract, Pediatr. Res. 53:484A, 2003).
TABLE 6.
Summary of fluconazole prophylaxis study in ELBW infantsa
| Characteristic | Results for:
|
P | |
|---|---|---|---|
| Fluconazole patients (n = 50) | Placebo patients (n = 50) | ||
| No. (%) of patients with fungal infectionb | |||
| Blood, urine, or cerebrospinal fluid infection | 0 (0) | 10 (20) | 0.008 |
| Bloodstream infection | 0 (0) | 8 (16) | 0.007 |
| No. (%) of patients with fungal colonization | |||
| One or more sites | 11 (22) | 30 (60) | 0.002 |
| Two or more sites | 9 (18) | 26 (52) | 0.003 |
| Skin | 10 (20) | 24 (48) | 0.008 |
| Gastrointestinal tract | 9 (18) | 27 (54) | 0.003 |
| Respiratory tract | 1 (2) | 21 (42) | 0.002 |
| Resistance (MIC50) (μg/ml) | |||
| First 6 mo of study | ≤2.0 | ≤1.0 | |
| Last 6 mo of study | ≤2.0 | ≤1.0 | |
| Patient safety (liver function tests, mean ± SD) | |||
| Aspartate aminotransferase (IU/liter) | 27 ± 13 | 29 ± 16 | 0.67 |
| Alanine aminotransferase (IU/liter) | 21 ± 16 | 21 ± 19 | 0.85 |
| Direct bilirubin (mg/dl) | 0.6 ± 1.4 | 1.0 ± 1.8 | 0.34 |
| Alkaline phosphatase (IU/liter) | 305 ± 125 | 318 ± 182 | 0.70 |
| No. (%) patients who died | 4 (8) | 10 (20) | 0.22 |
Data from reference 246.
Growth of fungus in culture for a patient with symptoms of infection.
Azole resistance.
Development of drug resistance in Candida is influenced by the duration of exposure and the dosage of the antifungal agent, as described in an extensive review (500). Mechanisms of resistance include overexpression of drug efflux pumps, point mutations, and overexpression of the C-14 demethylase gene. The vast majority of fungi causing infection in preterm infants are sensitive to azole agents. Clinical and colonization fungal isolates were studied in 90 patients from 17 NICUs who had never been treated with fluconazole (396). The mean gestational age was 26 weeks, and samples for culture were taken from the skin, nasopharynx, catheter tip, urine, CSF, or blood. This study found that the major fungal species responsible for fungal sepsis in neonates were sensitive to fluconazole (MIC ≤ 2 for C. albicans in 55 isolates, MIC ≤ 4 for C. parapsilosis in 33 isolates). Higher MICs were found for other fungal species (MIC ≤ 16 for C. glabrata in 3 isolates, MIC ≤ 32 for C. guilliermondii in 2 isolates, MIC > 64 for C. tropicalis in 5 isolates). In another study of 6 NICUs, bloodstream fungal isolates demonstrated a broad range of sensitivity to fluconazole, while all isolates were susceptible to amphotericin B (MIC90 [MIC for 90% of isolates] range, 0.25 to 2.0 μg/ml), demonstrating that even in the face of azole resistance, fungi remain susceptible to other antifungal agents (402).
The use of azole antifungals for prophylaxis or therapy has the potential to induce resistance. Despite the use of azole prophylaxis in immunocompromised adults, Candida species causing bloodstream infection in North America and Europe have demonstrated a relatively constant level susceptibility to fluconazole between 1992 and 2000 (371, 373). Studies of antifungal prophylaxis in bone marrow transplant recipients receiving fluconazole for 75 days have demonstrated colonization with drug-resistant Candida of low virulence that rarely cause invasive infection and have been successfully treated with other antifungal agents (287, 310). Azole resistance may also be induced outside the hospital through broad use of over-the-counter or prescription azoles for genital, mucosal, or cutaneous yeast infections.
The VLBW and ELBW clinical trials of fluconazole prophylaxis extensively monitored azole resistance over time, and fungal isolates remained sensitive to fluconazole (246, 255). The VLBW infant study by Kicklighter et al. did not find any significant resistance over the study period of 4 weeks or during the following 4 weeks after the treatment period when they continued to evaluate their patients for rectal colonization (255). One infant in the fluconazole-treated group remained colonized with C. albicans during the entire 56-day screening period, when rectal cultures were done, and the fluconazole MIC for the isolates remained ≤0.25 μg/ml. In the ELBW study (246), fungus was isolated in 42 (4.9%) of 861 surveillance cultures in the fluconazole-treated group and 177 (23%) of 769 surveillance cultures in the placebo group. Fungal isolate sensitivities to fluconazole also did not change significantly during the 6-week treatment or the 30-month study period. There was also no difference in the MIC for the fungal isolates that caused invasive infection compared to the fungal isolates in patients in whom invasive infection did not occur. It is possible that the lower dose and intermittent dosing schedule of fluconazole may be responsible for this favorable susceptibility profile.
Mortality associated with fluconazole prophylaxis.
A Cochrane review of analysis of both the VLBW (255) and ELBW (246) prophylaxis studies demonstrated a reduced risk of mortality in the fluconazole-treated patients compared with the placebo-treated patients (relative risk, 0.44; 95% CI, 0.21, 0.91), with the number needed to treat of 9 (95% CI 5, 50) (316). The reduced mortality may be due to the effect of fluconazole on C. albicans colonization and infection. In the ELBW prophylaxis study, not only was C. albicans colonization significantly decreased, but also no C. albicans colonization was detected after the second week of life. Since there is a higher infection-associated mortality with C. albicans sepsis in VLBW infants, decreasing fungal colonization and sepsis due to C. albicans with intravenous fluconazole would significantly decrease mortality in these high-risk infants (454). These are similar to the findings of reduced invasive fungal infection and mortality of fluconazole prophylaxis in bone marrow transplant patients (309).
CONCLUSION
Because of the high incidence and high-risk nature of sepsis among VLBW infants, “sepsis phobia” is a common phenomenon in the NICU. Nearly all VLBW infants are exposed to courses of antimicrobial agents, and, in part due to lack of confidence in currently available methods for predicting or detecting sepsis, these courses of antibiotics or antifungals are often prolonged even in the absence of a positive blood culture. Since the emergence of drug resistance among neonatal pathogens is a growing threat, appropriate choice of antimicrobial agents and duration of therapy remains an important challenge for neonatal practitioners. Given the poor outcomes associated with neonatal sepsis despite current optimal antimicrobials and intensive care, concentrated research efforts should focus on prevention, reliable detection methods, and adjunctive therapies for septic preterm infants.
REFERENCES
- 1.Reference deleted.
- 2.Reference deleted.
- 3.Reference deleted.
- 4.Reference deleted.
- 5.Reference deleted.
- 6.Adam, B., G. S. Baillie, and L. J. Douglas. 2002. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 51:344-349. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal, M., P. Chaturvedi, S. K. Dey, and P. Narang. 1990. Coagulase negative staphylococcal septicemia in newborns. Indian Pediatr. 27:163-169. [PubMed] [Google Scholar]
- 8.Ainbender, E., E. E. Cabatu, D. M. Guzman, and A. Y. Sweet. 1982. Serum C- reactive protein and problems of newborn infants. J. Pediatr. 101:438-440. [DOI] [PubMed] [Google Scholar]
- 9.Aisner, J., S. C. Schimpff, J. C. Sutherland, V. M. Young, and P. H. Wiernik. 1976. Torulopsis glabrata infections in patients with cancer. Increasing incidence and relationship to colonization. Am. J. Med. 61:23-28. [DOI] [PubMed] [Google Scholar]
- 10.Almeida, R. J., J. H. Jorgensen, and J. E. Johnson. 1983. Evaluation of the automicrobic system gram-positive identification card for species identification of coagulase-negative staphylococci. J. Clin. Microbiol. 18:438-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almuneef, M. A., R. S. Baltimore, P. A. Farrel, P. Reagan-Cirincione, and L. M. Dembry. 2001. Molecular typing demonstrating transmission of gram-negative rods in a neonatal intensive care unit in the absence of a recognized epidemic. Clin. Infect. Dis 32:220-227. [DOI] [PubMed] [Google Scholar]
- 12.Alos, J. I., T. Lambert, and P. Courvalin. 1993. Comparison of two molecular methods for tracing nosocomial transmission of Escherichia coli K1 in a neonatal unit. J. Clin. Microbiol. 31:1704-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen, B. M., R. Lindemann, K. Bergh, B. I. Nesheim, G. Syversen, N. Solheim, and F. Laugerud. 2002. Spread of methicillin-resistant Staphylococcus aureus in a neonatal intensive unit associated with understaffing, overcrowding and mixing of patients. J. Hosp. Infect. 50:18-24. [DOI] [PubMed] [Google Scholar]
- 14.Andre, P., B. Thebaud, M. Guibert, F. Audibert, T. Lacaze-Masmonteil, and M. Dehan. 2000. Maternal-fetal staphylococcal infections: a series report. Am. J. Perinatol. 17:423-427. [DOI] [PubMed] [Google Scholar]
- 15.Angus, D. C., W. T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M. R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303-1310. [DOI] [PubMed] [Google Scholar]
- 16.Annane, D., V. Sebille, C. Charpentier, P. E. Bollaert, B. Francois, J. M. Korach, G. Capellier, Y. Cohen, E. Azoulay, G. Troche, P. Chaumet-Riffaut, and E. Bellissant. 2002. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288:862-871. [DOI] [PubMed] [Google Scholar]
- 17.Apisarnthanarak, A., G. Holzmann-Pazgal, A. Hamvas, M. A. Olsen, and V. J. Fraser. 2003. Ventilator-associated pneumonia in extremely preterm neonates in a neonatal intensive care unit: characteristics, risk factors, and outcomes. Pediatrics 112:1283-1289. [DOI] [PubMed] [Google Scholar]
- 18.Arias, E., M. F. MacDorman, D. M. Strobino, and B. Guyer. 2003. Annual summary of vital statistics—2002. Pediatrics 112:1215-1230. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong, D., M. R. Battin, D. Knight, and J. Skinner. 2002. Staphylococcus aureus endocarditis in preterm neonates. Am. J. Perinatol. 19:247-251. [DOI] [PubMed] [Google Scholar]
- 20.Artz, L. A., V. A. Kempf, and I. B. Autenrieth. 2003. Rapid screening for Streptococcus agalactiae in vaginal specimens of pregnant women by fluorescent in situ hybridization. J. Clin. Microbiol. 41:2170-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avila-Figueroa, C., D. A. Goldmann, D. K. Richardson, J. E. Gray, A. Ferrari, and J. Freeman. 1998. Intravenous lipid emulsions are the major determinant of coagulase-negative staphylococcal bacteremia in very low birth weight newborns. Pediatr. Infect. Dis. J. 17:10-17. [DOI] [PubMed] [Google Scholar]
- 22.Back, N. A., C. C. Linnemann, Jr., J. L. Staneck, and U. R. Kotagal. 1996. Control of methicillin-resistant Staphylococcus aureus in a neonatal intensive-care unit: use of intensive microbiologic surveillance and mupirocin. Infect. Control Hosp. Epidemiol. 17:227-231. [DOI] [PubMed] [Google Scholar]
- 23.Baker, C. J. 1996. Inadequacy of rapid immunoassays for intrapartum detection of group B streptococcal carriers. Obstet. Gynecol. 88:51-55. [DOI] [PubMed] [Google Scholar]
- 24.Baker, C. J., and M. S. Edwards. 2003. Group B streptococcal conjugate vaccines. Arch. Dis. Child. 88:375-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker, C. J., M. E. Melish, R. T. Hall, D. T. Casto, U. Vasan, L. B. Givner, and The Multicenter Group for the Study of Immune Globulin in Neonates. 1992. Intravenous immune globulin for the prevention of nosocomial infection in low-birth- weight neonates. N. Engl. J. Med. 327:213-219. [DOI] [PubMed] [Google Scholar]
- 26.Baker, C. J., and M. A. Rench. 1983. Commercial latex agglutination for detection of group B streptococcal antigen in body fluids. J. Pediatr. 102:393-395. [DOI] [PubMed] [Google Scholar]
- 27.Baley, J. E. 1991. Neonatal candidiasis: the current challenge. Clin. Perinatol. 18:263-280. [PubMed] [Google Scholar]
- 28.Baley, J. E., W. L. Annable, and R. M. Kliegman. 1981. Candida endophthalmitis in the premature infant. J. Pediatr. 98:458-461. [DOI] [PubMed] [Google Scholar]
- 29.Baley, J. E., and F. J. Ellis. 2003. Neonatal candidiasis: ophthalmologic infection. Semin. Perinatol. 27:401-405. [DOI] [PubMed] [Google Scholar]
- 30.Baley, J. E., R. M. Kliegman, B. Boxerbaum, and A. A. Fanaroff. 1986. Fungal colonization in the very low birth weight infant. Pediatrics 78:225-232. [PubMed] [Google Scholar]
- 31.Baley, J. E., R. M. Kliegman, and A. A. Fanaroff. 1984. Disseminated fungal infections in very low-birth-weight infants: clinical manifestations and epidemiology. Pediatrics 73:144-152. [PubMed] [Google Scholar]
- 32.Baley, J. E., R. M. Kliegman, and A. A. Fanaroff. 1984. Disseminated fungal infections in very low-birth-weight infants: therapeutic toxicity. Pediatrics 73:153-157. [PubMed] [Google Scholar]
- 33.Baley, J. E., and R. A. Silverman. 1988. Systemic candidiasis: cutaneous manifestations in low birth weight infants. Pediatrics 82:211-215. [PubMed] [Google Scholar]
- 34.Baltimore, R. S. 2003. The difficulty of diagnosing ventilator-associated pneumonia. Pediatrics 112:1420-1421. [DOI] [PubMed] [Google Scholar]
- 35.Baltimore, R. S., S. M. Huie, J. I. Meek, A. Schuchat, and K. L. O'Brien. 2001. Early-onset neonatal sepsis in the era of group B streptococcal prevention. Pediatrics 108:1094-1098. [DOI] [PubMed] [Google Scholar]
- 36.Banerjea, M. C., and C. P. Speer. 2002. The current role of colony-stimulating factors in prevention and treatment of neonatal sepsis. Semin. Neonatol. 7:335-349. [DOI] [PubMed] [Google Scholar]
- 37.Barker, D. P., and N. Rutter. 1995. Exposure to invasive procedures in neonatal intensive care unit admissions. Arch. Dis. Child. Fetal Neonatal Ed. 72:F47-F48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barton, L., J. E. Hodgman, and Z. Pavlova. 1999. Causes of death in the extremely low birth weight infant. Pediatrics 103:446-451. [DOI] [PubMed] [Google Scholar]
- 39.Basaran, U. N., S. Celayir, N. Eray, R. Ozturk, and O. F. Senyuz. 1998. The effect of an H2-receptor antagonist on small-bowel colonization and bacterial translocation in newborn rats. Pediatr. Surg. Int. 13:118-120. [DOI] [PubMed] [Google Scholar]
- 40.Bauer, S., A. Eliakim, A. Pomeranz, R. Regev, I. Litmanovits, S. Arnon, H. Huri, and T. Dolfin. 2003. Urinary tract infection in very low birth weight preterm infants. Pediatr. Infect. Dis. J. 22:426-430. [DOI] [PubMed] [Google Scholar]
- 41.Bell, M. J., J. L. Ternberg, R. D. Feigin, J. P. Keating, R. Marshall, L. Barton, and T. Brotherton. 1978. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benirschke, K., and S. I. Raphael. 1958. Candida albicans infection of the amniotic sac. Am. J. Obstet. Gynecol. 75:200-202. [DOI] [PubMed] [Google Scholar]
- 43.Benjamin, D. K., Jr., E. R. DeLong, W. J. Steinbach, C. M. Cotton, T. J. Walsh, and R. H. Clark. 2003. Empirical therapy for neonatal candidemia in very low birth weight infants. Pediatrics 112:543-547. [DOI] [PubMed] [Google Scholar]
- 44.Benjamin, D. K., Jr., W. Miller, H. Garges, D. K. Benjamin, R. E. McKinney, Jr., M. Cotton, R. G. Fisher, and K. A. Alexander. 2001. Bacteremia, central catheters, and neonates: when to pull the line. Pediatrics 107:1272-1276. [DOI] [PubMed] [Google Scholar]
- 45.Benjamin, D. K., Jr., C. Poole, W. J. Steinbach, J. L. Rowen, and T. J. Walsh. 2003. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics 112:634-640. [DOI] [PubMed] [Google Scholar]
- 46.Benjamin, D. K., Jr., K. Ross, R. E. McKinney, Jr., D. K. Benjamin, R. Auten, and R. G. Fisher. 2000. When to suspect fungal infection in neonates: a clinical comparison of Candida albicans and Candida parapsilosis fungemia with coagulase-negative staphylococcal bacteremia. Pediatrics 106:712-718. [DOI] [PubMed] [Google Scholar]
- 47.Benshushan, A., A. Tsafrir, R. Arbel, G. Rahav, I. Ariel, and N. Rojansky. 2002. Listeria infection during pregnancy: a 10 year experience. Isr. Med. Assoc. J. 4:776-780. [PubMed] [Google Scholar]
- 48.Bernstein, H. M., B. H. Pollock, D. A. Calhoun, and R. D. Christensen. 2001. Administration of recombinant granulocyte colony-stimulating factor to neonates with septicemia: a meta-analysis. J. Pediatr. 138:917-920. [DOI] [PubMed] [Google Scholar]
- 49.Berseth, C. L., J. A. Bisquera, and V. U. Paje. 2003. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 111:529-534. [DOI] [PubMed] [Google Scholar]
- 50.Berthelot, P., F. Grattard, H. Patural, A. Ros, H. Jelassi-Saoudin, B. Pozzetto, G. Teyssier, and F. Lucht. 2001. Nosocomial colonization of premature babies with Klebsiella oxytoca: probable role of enteral feeding procedure in transmission and control of the outbreak with the use of gloves. Infect. Control Hosp. Epidemiol. 22:148-151. [DOI] [PubMed] [Google Scholar]
- 51.Bialkowska-Hobrzanska, H., D. Jaskot, and O. Hammerberg. 1990. Evaluation of restriction endonuclease fingerprinting of chromosomal DNA and plasmid profile analysis for characterization of multiresistant coagulase-negative staphylococci in bacteremic neonates. J. Clin. Microbiol. 28:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bialkowska-Hobrzanska, H., D. Jaskot, and O. Hammerberg. 1993. Molecular characterization of the coagulase-negative staphylococcal surface flora of premature neonates. J. Gen. Microbiol. 139:2939-2944. [DOI] [PubMed] [Google Scholar]
- 53.Bjorkqvist, M., B. Soderquist, E. Tornqvist, L. Sjoberg, H. Fredlund, I. Kuhn, P. Colque-Navarro, and J. Schollin. 2002. Phenotypic and genotypic characterisation of blood isolates of coagulase-negative staphylococci in the newborn. APMIS 110:332-339. [DOI] [PubMed] [Google Scholar]
- 54.Bliss, J. M., M. Wellington, and F. Gigliotti. 2003. Antifungal pharmacotherapy for neonatal candidiasis. Semin. Perinatol. 27:365-374. [DOI] [PubMed] [Google Scholar]
- 55.Blum, M., and I. Elian. 1978. Tracheal aspirate screening for the detection of intrauterine or intrapartum candidiasis in the newborn. Mykosen 21:95-98. [DOI] [PubMed] [Google Scholar]
- 56.Bomela, H. N., D. E. Ballot, B. J. Cory, and P. A. Cooper. 2000. Use of C-reactive protein to guide duration of empiric antibiotic therapy in suspected early neonatal sepsis. Pediatr. Infect. Dis. J. 19:531-535. [DOI] [PubMed] [Google Scholar]
- 57.Bortolussi, R., T. R. Thompson, and P. Ferrieri. 1977. Early-onset pneumococcal sepsis in newborn infants. Pediatrics 60:352-355. [PubMed] [Google Scholar]
- 58.Boyle, R., J. S. Nelson, B. S. Stonestreet, G. Peter, and W. Oh. 1978. Alterations in stool flora resulting from oral kanamycin prophylaxis of necrotizing enterocolitis. J. Pediatr. 93:857-861. [DOI] [PubMed] [Google Scholar]
- 59.Branchini, M. L., M. A. Pfaller, J. Rhine-Chalberg, T. Frempong, and H. D. Isenberg. 1994. Genotypic variation and slime production among blood and catheter isolates of Candida parapsilosis. J. Clin. Microbiol. 32:452-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brion, L., E. Bell, and T. Raghuveer. 2003. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 4:CD003665. [DOI] [PMC free article] [PubMed]
- 61.Brodie, S. B., K. E. Sands, J. E. Gray, R. A. Parker, D. A. Goldmann, R. B. Davis, and D. K. Richardson. 2000. Occurrence of nosocomial bloodstream infections in six neonatal intensive care units. Pediatr. Infect. Dis. J. 19:56-65. [DOI] [PubMed] [Google Scholar]
- 62.Bromiker, R., I. Arad, O. Peleg, A. Preminger, and D. Engelhard. 2001. Neonatal bacteremia: patterns of antibiotic resistance. Infect. Control Hosp. Epidemiol. 22:767-770. [DOI] [PubMed] [Google Scholar]
- 63.Bruinsma, N., E. E. Stobberingh, M. J. Herpers, J. S. Vles, B. J. Weber, and D. A. Gavilanes. 2000. Subcutaneous ventricular catheter reservoir and ventriculoperitoneal drain-related infections in preterm infants and young children. Clin. Microbiol. Infect. 6:202-206. [DOI] [PubMed] [Google Scholar]
- 64.Bryant, K., C. Maxfield, and G. Rabalais. 1999. Renal candidiasis in neonates with candiduria. Pediatr. Infect. Dis. J. 18:959-963. [DOI] [PubMed] [Google Scholar]
- 65.Burnie, J. P. 1986. Candida and hands. J Hosp. Infect. 8:1-4. [DOI] [PubMed] [Google Scholar]
- 66.Cairo, M. S., J. Agosti, R. Ellis, J. J. Laver, B. Puppala, R. deLemos, L. Givner, M. Nesin, J. G. Wheeler, T. Seth, C. van de Ven, and A. Fanaroff. 1999. A randomized, double-blind, placebo-controlled trial of prophylactic recombinant human granulocyte-macrophage colony-stimulating factor to reduce nosocomial infections in very low birth weight neonates. J. Pediatr. 134:64-70. [DOI] [PubMed] [Google Scholar]
- 67.Calil, R., S. T. Marba, A. von Nowakonski, and A. T. Tresoldi. 2001. Reduction in colonization and nosocomial infection by multiresistant bacteria in a neonatal unit after institution of educational measures and restriction in the use of cephalosporins. Am. J. Infect. Control 29:133-138. [DOI] [PubMed] [Google Scholar]
- 68.Campbell, J. R., E. Zaccaria, and C. J. Baker. 2000. Systemic candidiasis in extremely low birth weight infants receiving topical petrolatum ointment for skin care: a case- control study. Pediatrics 105:1041-1045. [DOI] [PubMed] [Google Scholar]
- 69.Caplan, M. S., and T. Jilling. 2001. New concepts in necrotizing enterocolitis. Curr. Opin. Pediatr. 13:111-115. [DOI] [PubMed] [Google Scholar]
- 70.Carlson, S. E., M. B. Montalto, D. L. Ponder, S. H. Werkman, and S. B. Korones. 1998. Lower incidence of necrotizing enterocolitis in infants fed a preterm formula with egg phospholipids. Pediatr. Res. 44:491-498. [DOI] [PubMed] [Google Scholar]
- 71.Carr, R., N. Modi, and C. Dore. 2003. G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst. Rev. CD003066. [DOI] [PMC free article] [PubMed]
- 72.Carr, R., N. Modi, C. J. Dore, R. El Rifai, and D. Lindo. 1999. A randomized, controlled trial of prophylactic granulocyte-macrophage colony-stimulating factor in human newborns less than 32 weeks gestation. Pediatrics 103:796-802. [DOI] [PubMed] [Google Scholar]
- 73.Carratala, J., J. Niubo, A. Fernandez-Sevilla, E. Juve, X. Castellsague, J. Berlanga, J. Linares, and F. Gudiol. 1999. Randomized, double-blind trial of an antibiotic-lock technique for prevention of gram-positive central venous catheter-related infection in neutropenic patients with cancer. Antimicrob. Agents Chemother. 43:2200-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carrieri, M. P., I. Stolfi, and M. L. Moro. 2003. Intercenter variability and time of onset: two crucial issues in the analysis of risk factors for nosocomial sepsis. Pediatr. Infect. Dis. J. 22:599-609. [DOI] [PubMed] [Google Scholar]
- 75.Carrion, V., and E. A. Egan. 1990. Prevention of neonatal necrotizing enterocolitis. J. Pediatr. Gastroenterol. Nutr. 11:317-323. [DOI] [PubMed] [Google Scholar]
- 76.Cartlidge, P. H., P. E. Fox, and N. Rutter. 1990. The scars of newborn intensive care. Early Hum. Dev. 21:1-10. [DOI] [PubMed] [Google Scholar]
- 76a.Centers for Disease Control and Prevention. 2002. Enterobacter sakazakii infections associated with the use of powdered infant formula—Tennessee, 2001. Morb. Mortal. Wkly. Rep. 51:297-300. [PubMed] [Google Scholar]
- 76b.Centers for Disease Control and Prevention. 2002. Vancomycin-resistant Staphylococcus aureus—Pennsylvania, 2002. Morb. Mortal. Wkly. Rep. 51:902. [PubMed] [Google Scholar]
- 77.Chan, D. K., and L. Y. Ho. 1997. Usefulness of C-reactive protein in the diagnosis of neonatal sepsis. Singapore Med. J. 38:252-255. [PubMed] [Google Scholar]
- 78.Chang, H. J., H. L. Miller, N. Watkins, M. J. Arduino, D. A. Ashford, G. Midgley, S. M. Aguero, R. Pinto-Powell, C. F. von Reyn, W. Edwards, M. M. McNeil, and W. R. Jarvis. 1998. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers' pet-dogs. N. Engl. J. Med. 338:706-711. [DOI] [PubMed] [Google Scholar]
- 79.Chapel, T. A., C. Gagliardi, and W. Nichols. 1982. Congenital cutaneous candidiasis. J. Am. Acad. Dermatol. 6:926-928. [DOI] [PubMed] [Google Scholar]
- 80.Chapman, R. L., and R. G. Faix. 2000. Persistently positive cultures and outcome in invasive neonatal candidiasis. Pediatr. Infect. Dis. J. 19:822-827. [DOI] [PubMed] [Google Scholar]
- 81.Chen, K. T., R. E. Tuomala, A. P. Cohen, E. C. Eichenwald, and E. Lieberman. 2001. No increase in rates of early-onset neonatal sepsis by non-group B Streptococcus or ampicillin-resistant organisms. Am. J. Obstet. Gynecol. 185:854-858. [DOI] [PubMed] [Google Scholar]
- 82.Chen, S. J., B. R. Vohr, and W. Oh. 1993. Effects of birth order, gender, and intrauterine growth retardation on the outcome of very low birth weight in twins. J. Pediatr. 123:132-136. [DOI] [PubMed] [Google Scholar]
- 83.Chesney, P. J., A. Taher, E. M. Gilbert, and N. T. Shahidi. 1978. Intranuclear inclusions in megakaryocytes in congenital cytomegalovirus infection. J. Pediatr. 92:957-958. [DOI] [PubMed] [Google Scholar]
- 84.Cheu, H. W., D. R. Brown, and M. I. Rowe. 1989. Breath hydrogen excretion as a screening test for the early diagnosis of necrotizing enterocolitis. Am. J. Dis. Child. 143:156-159. [DOI] [PubMed] [Google Scholar]
- 85.Chien, L. Y., Y. Macnab, K. Aziz, W. Andrews, D. D. McMillan, and S. K. Lee. 2002. Variations in central venous catheter-related infection risks among Canadian neonatal intensive care units. Pediatr. Infect. Dis. J. 21:505-511. [DOI] [PubMed] [Google Scholar]
- 86.Chiesa, C., F. Signore, M. Assumma, E. Buffone, P. Tramontozzi, J. F. Osborn, and L. Pacifico. 2001. Serial measurements of C-reactive protein and interleukin-6 in the immediate postnatal period: reference intervals and analysis of maternal and perinatal confounders. Clin. Chem. 47:1016-1022. [PubMed] [Google Scholar]
- 87.Chow, A. W., B. K. Wittmann, K. H. Bartlett, and D. W. Scheifele. 1984. Variant postpartum toxic shock syndrome with probable intrapartum transmission to the neonate. Am. J. Obstet. Gynecol. 148:1074-1079. [DOI] [PubMed] [Google Scholar]
- 88.Christensen, G. D., S. B. Korones, L. Reed, R. Bulley, B. McLaughlin, and A. L. Bisno. 1982. Epidemic Serratia marcescens in a neonatal intensive care unit: importance of the gastrointestinal tract as a reservoir. Infect. Control 3:127-133. [DOI] [PubMed] [Google Scholar]
- 89.Christie, C., J. Hammond, S. Reising, and J. Evans-Patterson. 1994. Clinical and molecular epidemiology of enterococcal bacteremia in a pediatric teaching hospital. J. Pediatr. 125:392-399. [DOI] [PubMed] [Google Scholar]
- 90.Clark, N. C., B. C. Hill, C. M. O'Hara, O. Steingrimsson, and R. C. Cooksey. 1990. Epidemiologic typing of Enterobacter sakazakii in two neonatal nosocomial outbreaks. Diagn. Microbiol. Infect. Dis. 13:467-472. [DOI] [PubMed] [Google Scholar]
- 91.Clark, R. H., T. E. Wiswell, D. M. Null, R. A. deLemos, and J. J. Coalson. 1987. Tracheal and bronchial injury in high-frequency oscillatory ventilation compared with conventional positive pressure ventilation. J. Pediatr. 111:114-118. [DOI] [PubMed] [Google Scholar]
- 92.Claud, E. C., D. Li, Y. Xiao, M. S. Caplan, and T. Jilling. 2002. Platelet-activating factor regulates chloride transport in colonic epithelial cell monolayers. Pediatr. Res. 52:155-162. [DOI] [PubMed] [Google Scholar]
- 92a.Coates, E. W., M. G. Karlowicz, D. P. Croitoru, and E. S. Buescher. 2002. Distinctive distribution of pathogens associated with peritonitis in neonates with focal intestinal perforation versus necrotizing entercolitis. Pediatr. Res. 51:302A. [DOI] [PubMed] [Google Scholar]
- 93.Cohen, B., L. Saiman, J. Cimiotti, and E. Larson. 2003. Factors associated with hand hygiene practices in two neonatal intensive care units. Pediatr. Infect. Dis. J. 22:494-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cordero, L., L. W. Ayers, R. R. Miller, J. H. Seguin, and B. D. Coley. 2002. Surveillance of ventilator-associated pneumonia in very-low-birth-weight infants. Am. J. Infect. Control 30:32-39. [DOI] [PubMed] [Google Scholar]
- 95.Cordero, L., M. Sananes, and L. W. Ayers. 1999. Bloodstream infections in a neonatal intensive-care unit: 12 years' experience with an antibiotic control program. Infect. Control Hosp. Epidemiol. 20:242-246. [DOI] [PubMed] [Google Scholar]
- 96.Cox, F. 1986. Candida albicans adherence in newborn infants. J. Med. Vet. Mycol. 24:121-125. [PubMed] [Google Scholar]
- 97.Cox, F., and L. Taylor. 1990. Adherence of group B streptococci to buccal epithelial cells in neonates with different gestational ages. J. Perinat. Med. 18:455-458. [DOI] [PubMed] [Google Scholar]
- 98.Craft, A. P., N. N. Finer, and K. J. Barrington. 2000. Vancomycin for prophylaxis against sepsis in preterm neonates. Cochrane Database Syst. Rev. CD001971. [DOI] [PMC free article] [PubMed]
- 99.Critchley, I. A., and L. J. Douglas. 1985. Differential adhesion of pathogenic Candida species to epithelial and inert surfaces. FEMS Microbiol. Lett. 28:199-203. [Google Scholar]
- 100.Crouch, S. F., T. A. Pearson, and D. M. Parham. 1987. Comparison of modified Minitek system with Staph-Ident system for species identification of coagulase-negative staphylococci. J. Clin. Microbiol. 25:1626-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daher, A. H., and F. E. Berkowitz. 1995. Infective endocarditis in neonates. Clin. Pediatr. 34:198-206. [DOI] [PubMed] [Google Scholar]
- 102.Dalton, H. J. 2003. Recombinant activated protein C in pediatric sepsis. Pediatr. Infect. Dis. J. 22:743-745. [DOI] [PubMed] [Google Scholar]
- 103.Damjanovic, V., C. M. Connolly, H. K. van Saene, R. W. Cooke, J. E. Corkill, A. van Belkum, and D. van Velzen. 1993. Selective decontamination with nystatin for control of a Candida outbreak in a neonatal intensive care unit. J. Hosp. Infect. 24:245-259. [DOI] [PubMed] [Google Scholar]
- 104.D'Angio, C. T., K. L. McGowan, S. Baumgart, J. St Geme, and M. C. Harris. 1989. Surface colonization with coagulase-negative staphylococci in premature neonates. J. Pediatr. 114:1029-1034. [DOI] [PubMed] [Google Scholar]
- 105.Darmstadt, G. L., J. G. Dinulos, and Z. Miller. 2000. Congenital cutaneous candidiasis: clinical presentation, pathogenesis, and management guidelines. Pediatrics 105:438-444. [DOI] [PubMed] [Google Scholar]
- 106.Davis, S. E., M. L. Levy, J. G. McComb, and L. Masri-Lavine. 1999. Does age or other factor's influence the incidence of ventriculoperitoneal shunt infections? Pediatr. Neurosurg. 30:253-257. [DOI] [PubMed] [Google Scholar]
- 107.Delprado, W. J., P. J. Baird, and P. Russell. 1982. Placental candidiasis: report of three cases with a review of the literature. Pathology 14:191-195. [DOI] [PubMed] [Google Scholar]
- 108.de Man, P., B. A. Verhoeven, H. A. Verbrugh, M. C. Vos, and J. N. van den Anker. 2000. An antibiotic policy to prevent emergence of resistant bacilli. Lancet 355:973-978. [DOI] [PubMed] [Google Scholar]
- 109.DeMuri, G. P., and M. K. Hostetter. 1996. Evidence for a beta 1 integrin fibronectin receptor in Candida tropicalis. J. Infect. Dis. 174:127-132. [DOI] [PubMed] [Google Scholar]
- 110.Dennis, J. E., K. H. Rhodes, D. R. Cooney, and G. D. Roberts. 1980. Nosocomical Rhizopus infection (zygomycosis) in children. J. Pediatr. 96:824-828. [DOI] [PubMed] [Google Scholar]
- 111.de Silva, G. D., M. Kantzanou, A. Justice, R. C. Massey, A. R. Wilkinson, N. P. Day, and S. J. Peacock. 2002. The ica operon and biofilm production in coagulase-negative staphylococci associated with carriage and disease in a neonatal intensive care unit. J. Clin. Microbiol. 40:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Deville, J. G., S. Adler, P. H. Azimi, B. A. Jantausch, M. R. Morfin, S. Beltran, B. Edge-Padbury, S. Naberhuis-Stehouwer, and J. B. Bruss. 2003. Linezolid versus vancomycin in the treatment of known or suspected resistant gram-positive infections in neonates. Pediatr. Infect. Dis. J. 22:S158-S163. [DOI] [PubMed] [Google Scholar]
- 113.Diamond, R. D., R. Krzesicki, and W. Jao. 1978. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J. Clin. Investig. 61:349-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Doctor, B. A., N. Newman, N. M. Minich, H. G. Taylor, A. A. Fanaroff, and M. Hack. 2001. Clinical outcomes of neonatal meningitis in very-low birth-weight infants. Clin. Pediatr. 40:473-480. [DOI] [PubMed] [Google Scholar]
- 115.Dollner, H., L. Vatten, I. Linnebo, G. F. Zanussi, A. Laerdal, and R. Austgulen. 2001. Inflammatory mediators in umbilical plasma from neonates who develop early- onset sepsis. Biol. Neonate 80:41-47. [DOI] [PubMed] [Google Scholar]
- 116.Donta, S. T., P. Peduzzi, A. S. Cross, J. Sadoff, C. Haakenson, S. J. Cryz, Jr., C. Kauffman, S. Bradley, G. Gafford, D. Elliston, T. R. Beam, Jr., J. F. John, Jr., B. Ribner, R. Cantey, C. H. Welsh, R. T. Ellison III, E. J. Young, R. J. Hamill, H. Leaf, R. M. Schein, M. Mulligan, C. Johnson, E. Abrutyn, J. M. Griffiss, D. Slagle, et al., for The Federal Hyperimmune Immunoglobulin Trial Study Group. 1996. Immunoprophylaxis against Klebsiella and Pseudomonas aeruginosa infections. J. Infect. Dis. 174:537-543. [DOI] [PubMed] [Google Scholar]
- 117.Doran, T. I. 1999. The role of Citrobacter in clinical disease of children: review. Clin. Infect. Dis. 28:384-394. [DOI] [PubMed] [Google Scholar]
- 118.Driessen, M., J. B. Ellis, P. A. Cooper, S. Wainer, F. Muwazi, D. Hahn, H. Gous, and F. P. De Villiers. 1996. Fluconazole vs. amphotericin B for the treatment of neonatal fungal septicemia: a prospective randomized trial. Pediatr. Infect. Dis. J. 15:1107-1112. [DOI] [PubMed] [Google Scholar]
- 119.Easmon, C. S., M. J. Hastings, A. J. Clare, B. Bloxham, R. Marwood, R. P. Rivers, and J. Stringer. 1981. Nosocomial transmission of group B streptococci. Br. Med. J. (Clin. Res. Ed.) 283:459-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ehl, S., B. Gering, P. Bartmann, J. Hogel, and F. Pohlandt. 1997. C-reactive protein is a useful marker for guiding duration of antibiotic therapy in suspected neonatal bacterial infection. Pediatrics 99:216-221. [DOI] [PubMed] [Google Scholar]
- 121.Eibl, M. M., H. M. Wolf, H. Furnkranz, and A. Rosenkranz. 1988. Prevention of necrotizing enterocolitis in low-birth-weight infants by IgA-IgG feeding. N. Engl. J. Med. 319:1-7. [DOI] [PubMed] [Google Scholar]
- 122.Einsele, H., H. Hebart, G. Roller, J. Loffler, I. Rothenhofer, C. A. Muller, R. A. Bowden, J. van Burik, D. Engelhard, L. Kanz, and U. Schumacher. 1997. Detection and identification of fungal pathogens in blood by using molecular probes. J. Clin. Microbiol. 35:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.el Ebiary, M., A. Torres, J. Gonzalez, J. P. de la Bellacasa, C. Garcia, M. T. Jimenez de Anta, M. Ferrer, and R. Rodriguez-Roisin. 1993. Quantitative cultures of endotracheal aspirates for the diagnosis of ventilator-associated pneumonia. Am. Rev. Respir. Dis. 148:1552-1557. [DOI] [PubMed] [Google Scholar]
- 124.Eliakim, A., T. Dolfin, Z. Korzets, B. Wolach, and A. Pomeranz. 1997. Urinary tract infection in premature infants: the role of imaging studies and prophylactic therapy. J. Perinatol. 17:305-308. [PubMed] [Google Scholar]
- 125.El Masry, F. A., T. J. Neal, and N. V. Subhedar. 2002. Risk factors for invasive fungal infection in neonates. Acta Paediatr. 91:198-202. [DOI] [PubMed] [Google Scholar]
- 126.el Mohandes, A. E., L. Johnson-Robbins, J. F. Keiser, S. J. Simmens, and M. V. Aure. 1994. Incidence of Candida parapsilosis colonization in an intensive care nursery population and its association with invasive fungal disease. Pediatr. Infect. Dis. J. 13:520-524. [DOI] [PubMed] [Google Scholar]
- 127.English, K. B., and C. B. Wilson. 2001. Immaturity of the fetal and neonatal immune system, p. 40.1-50.1. In R. R. Rich, T. A. Fleisher, W. T. Shearer, B. L. Kotzin, and H. W. Schroeder (ed.), Clinical immunology, principles and practice, 2nd ed. Mosby, New York, N.Y.
- 128.Essawi, T., T. Na'was, A. Hawwari, S. Wadi, A. Doudin, and A. I. Fattom. 1998. Molecular, antibiogram and serological typing of Staphylococcus aureus isolates recovered from A1-Makased Hospital in East Jerusalem. Trop. Med. Int. Health 3:576-583. [DOI] [PubMed] [Google Scholar]
- 129.Etzioni, A., T. Meshulam, M. Zeltzer, A. Benderly, D. Merzbach, and P. Sujov. 1987. Intralipid effects on preterm neonate serum: chemoattractant and opsonizing capacities. J. Pediatr. Gastroenterol. Nutr. 6:105-108. [DOI] [PubMed] [Google Scholar]
- 130.Evans, N. J., and N. Rutter. 1986. Development of the epidermis in the newborn. Biol. Neonate 49:74-80. [DOI] [PubMed] [Google Scholar]
- 131.Fairchild, K. D., S. Tomkoria, E. C. Sharp, and F. V. Mena. 2002. Neonatal Candida glabrata sepsis: clinical and laboratory features compared with other Candida species. Pediatr. Infect. Dis. J. 21:39-43. [DOI] [PubMed] [Google Scholar]
- 132.Faix, R. G. 1984. Systemic Candida infections in infants in intensive care nurseries: high incidence of central nervous system involvement. J. Pediatr. 105:616-622. [DOI] [PubMed] [Google Scholar]
- 133.Faix, R. G. 1992. Invasive neonatal candidiasis: comparison of albicans and parapsilosis infection. Pediatr. Infect. Dis. J. 11:88-93. [PubMed] [Google Scholar]
- 134.Faix, R. G., and R. L. Chapman. 2003. Central nervous system candidiasis in the high- risk neonate. Semin. Perinatol. 27:384-392. [DOI] [PubMed] [Google Scholar]
- 135.Faix, R. G., S. M. Kovarik, T. R. Shaw, and R. V. Johnson. 1989. Mucocutaneous and invasive candidiasis among very low birth weight (less than 1,500 grams) infants in intensive care nurseries: a prospective study. Pediatrics 83:101-107. [PubMed] [Google Scholar]
- 136.Fanaroff, A. A., S. B. Korones, L. L. Wright, J. Verter, R. L. Poland, C. R. Bauer, J. E. Tyson, J. B. Philips III, W. Edwards, J. F. Lucey, C. S. Catz, S. Shankaran, W. Oh. 1998. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. The National Institute of Child Health and Human Development Neonatal Research Network. Pediatr. Infect. Dis. J. 17:593-598. [DOI] [PubMed] [Google Scholar]
- 137.Fanaroff, A. A., S. B. Korones, L. L. Wright, E. C. Wright, R. L. Poland, C. B. Bauer, J. E. Tyson, J. B. Philips III, W. Edwards, J. F. Lucey, et al. 1994. A controlled trial of intravenous immune globulin to reduce nosocomial infections in very low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N. Engl. J. Med. 330:1107-1113. [DOI] [PubMed] [Google Scholar]
- 138.Fanos, V., G. Verlato, A. Dal Moro, G. P. Chiaffoni, and E. M. Padovani. 1995. Staphylococcus epidermidis isolation and antibiotic resistance in a neonatal intensive care unit. J. Chemother. 7:26-29. [DOI] [PubMed] [Google Scholar]
- 139.Fernandez, M., E. H. Moylett, D. E. Noyola, and C. J. Baker. 2000. Candidal meningitis in neonates: a 10-year review. Clin. Infect. Dis. 31:458-463. [DOI] [PubMed] [Google Scholar]
- 140.Fernandez-Baca, V., F. Ballesteros, J. A. Hervas, P. Villalon, M. A. Dominguez, V. J. Benedi, and S. Alberti. 2001. Molecular epidemiological typing of Enterobacter cloacae isolates from a neonatal intensive care unit: three-year prospective study. J. Hosp. Infect. 49:173-182. [DOI] [PubMed] [Google Scholar]
- 141.Fidel, P. L., Jr., J. A. Vazquez, and J. D. Sobel. 1999. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 12:80-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fielkow, S., S. Reuter, and S. P. Gotoff. 1991. Cerebrospinal fluid examination in symptom-free infants with risk factors for infection. J. Pediatr. 119:971-973. [DOI] [PubMed] [Google Scholar]
- 143.Foca, M., K. Jakob, S. Whittier, L. P. Della, S. Factor, D. Rubenstein, and L. Saiman. 2000. Endemic Pseudomonas aeruginosa infection in a neonatal intensive care unit. N. Engl. J. Med. 343: 695-700. [DOI] [PubMed] [Google Scholar]
- 144.Fowlie, P. W., and P. G. Davis. 2002. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst. Rev. CD000174. [DOI] [PubMed]
- 145.Franz, A. R., G. Steinbach, M. Kron, and F. Pohlandt. 2001. Interleukin-8: a valuable tool to restrict antibiotic therapy in newborn infants. Acta Paediatr. 90:1025-1032. [DOI] [PubMed] [Google Scholar]
- 146.Fraser, V. J., M. Jones, J. Dunkel, S. Storfer, G. Medoff, and W. C. Dunagan. 1992. Candidemia in a tertiary care hospital: epidemiology, risk factors, and predictors of mortality. Clin. Infect. Dis. 15:414-421. [DOI] [PubMed] [Google Scholar]
- 147.Freedman, R. M., and R. Baltimore. 1990. Fatal Streptococcus viridans septicemia and meningitis: relationship to fetal scalp electrode monitoring. J. Perinatol. 10:272-274. [PubMed] [Google Scholar]
- 148.Freeman, J., D. A. Goldmann, N. E. Smith, D. G. Sidebottom, M. F. Epstein, and R. Platt. 1990. Association of intravenous lipid emulsion and coagulase-negative staphylococcal bacteremia in neonatal intensive care units. N. Engl. J. Med. 323:301-308. [DOI] [PubMed] [Google Scholar]
- 149.Friedman, S., S. E. Richardson, S. E. Jacobs, and K. O'Brien. 2000. Systemic Candida infection in extremely low birth weight infants: short term morbidity and long term neurodevelopmental outcome. Pediatr. Infect. Dis. J. 19:499-504. [DOI] [PubMed] [Google Scholar]
- 150.Friesen, C. A., and C. T. Cho. 1986. Characteristic features of neonatal sepsis due to Haemophilus influenzae. Rev. Infect. Dis. 8:777-780. [DOI] [PubMed] [Google Scholar]
- 151.Garrett, D. O., E. Jochimsen, K. Murfitt, B. Hill, S. McAllister, P. Nelson, R. V. Spera, R. K. Sall, F. C. Tenover, J. Johnston, B. Zimmer, and W. R. Jarvis. 1999. The emergence of decreased susceptibility to vancomycin in Staphylococcus epidermidis. Infect. Control Hosp. Epidemiol. 20:167-170. [DOI] [PubMed] [Google Scholar]
- 152.Geelen, S. P., L. J. Gerards, and A. Fleer. 1990. Pneumococcal septicemia in the newborn. A report on seven cases and a review of the literature. J. Perinat. Med. 18:125-129. [DOI] [PubMed] [Google Scholar]
- 153.Gellin, B. G., C. V. Broome, W. F. Bibb, R. E. Weaver, S. Gaventa, L. Mascola, and the Listeriosis Study Group. 1991. The epidemiology of listeriosis in the United States—1986. Am. J. Epidemiol. 133:392-401. [DOI] [PubMed] [Google Scholar]
- 154.Gerdes, J. S. 1991. Clinicopathologic approach to the diagnosis of neonatal sepsis. Clin. Perinatol. 18:361-381. [PubMed] [Google Scholar]
- 155.Ghosal, S. P., P. C. Sen Gupta, A. K. Mukherjee, M. Choudhury, N. Dutta, and A. K. Sarkar. 1978. Noma neonatorum: its aetiopathogenesis. Lancet 11:289-291. [DOI] [PubMed] [Google Scholar]
- 156.Goering, R. V., N. J. Ehrenkranz, C. C. Sanders, and W. E. Sanders, Jr. 1992. Long term epidemiological analysis of Citrobacter diversus in a neonatal intensive care unit. Pediatr. Infect. Dis. J. 11:99-104. [DOI] [PubMed] [Google Scholar]
- 157.Goldenberg, R. L., and D. J. Rouse. 1998. Prevention of premature birth. N. Engl. J. Med. 339:313-320. [DOI] [PubMed] [Google Scholar]
- 158.Goldstein, E., M. H. Grieco, G. Finkel, and D. B. Louria. 1965. Studies on the pathogenesis of experimental Candida parapsilosis and Candida guilliermondii infections in mice. J. Infect. Dis. 115:293-302. [DOI] [PubMed] [Google Scholar]
- 159.Goncalves, L. F., T. Chaiworapongsa, and R. Romero. 2002. Intrauterine infection and prematurity. Ment. Retard. Dev. Disabil. Res. Rev. 8:3-13. [DOI] [PubMed] [Google Scholar]
- 160.Gordon, P., J. Rutledge, R. Sawin, S. Thomas, and D. Woodrum. 1999. Early postnatal dexamethasone increases the risk of focal small bowel perforation in extremely low birth weight infants. J. Perinatol. 19:573-577. [DOI] [PubMed] [Google Scholar]
- 161.Gordon, P. V., M. L. Young, and D. D. Marshall. 2001. Focal small bowel perforation: an adverse effect of early postnatal dexamethasone therapy in extremely low birth weight infants. J. Perinatol. 21:156-160. [DOI] [PubMed] [Google Scholar]
- 162.Gotoff, S. P., C. K. Papierniak, M. E. Klegerman, and K. M. Boyer. 1984. Quantitation of IgG antibody to the type-specific polysaccharide of group B streptococcus type 1b in pregnant women and infected infants. J. Pediatr. 105:628-630. [DOI] [PubMed] [Google Scholar]
- 163.Gotz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 164.Gould, K., C. H. Ramirez-Ronda, R. K. Holmes, and J. P. Sanford. 1975. Adherence of bacteria to heart valves in vitro. J. Clin. Investig. 56:1364-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Graham, D. R., and J. D. Band. 1981. Citrobacter diversus brain abscess and meningitis in neonates. JAMA 245:1923-1925. [PubMed] [Google Scholar]
- 166.Green, P. A., K. V. Singh, B. E. Murray, and C. J. Baker. 1994. Recurrent group B streptococcal infections in infants: clinical and microbiologic aspects. J. Pediatr. 125:931-938. [DOI] [PubMed] [Google Scholar]
- 167.Green, S. L., and K. S. LaPeter. 1982. Evidence for postpartum toxic-shock syndrome in a mother-infant pair. Am. J. Med. 72:169-172. [DOI] [PubMed] [Google Scholar]
- 168.Greenberg, D., E. Leibovitz, E. S. Shinnwell, P. Yagupsky, and R. Dagan. 1999. Neonatal sepsis caused by Streptococcus pyogenes: resurgence of an old etiology? Pediatr. Infect. Dis. J. 18:479-481. [DOI] [PubMed] [Google Scholar]
- 169.Griffin, M. P., and J. R. Moorman. 2001. Toward the early diagnosis of neonatal sepsis and sepsis-like illness using novel heart rate analysis. Pediatrics 107:97-104. [DOI] [PubMed] [Google Scholar]
- 170.Griffin, M. P., T. M. O'Shea, E. A. Bissonette, F. E. Harrell, Jr., D. E. Lake, and J. R. Moorman. 2003. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr. Res. 53:920-926. [DOI] [PubMed] [Google Scholar]
- 171.Groll, A. H., J. C. Gea-Banacloche, A. Glasmacher, G. Just-Nuebling, G. Maschmeyer, and T. J. Walsh. 2003. Clinical pharmacology of antifungal compounds. Infect. Dis. Clin. North Am. 17:159-191, ix. [DOI] [PubMed] [Google Scholar]
- 172.Groskopf, W., B. Green, L. Sohn, and S. Hsu. 1991. Furosemide as a displacing agent in assay of total triiodothyronine. Clin. Chem. 37:587-588. [PubMed] [Google Scholar]
- 173.Grundmann, H., A. Kropec, D. Hartung, R. Berner, and F. Daschner. 1993. Pseudomonas aeruginosa in a neonatal intensive care unit: reservoirs and ecology of the nosocomial pathogen. J. Infect. Dis. 168:943-947. [DOI] [PubMed] [Google Scholar]
- 174.Gruskay, J., M. C. Harris, A. T. Costarino, R. A. Polin, and S. Baumgart. 1989. Neonatal Staphylococcus epidermidis meningitis with unremarkable CSF examination results. Am. J. Dis. Child. 143:580-582. [DOI] [PubMed] [Google Scholar]
- 175.Gugnani, H. C., F. K. Nzelibe, P. C. Gini, W. O. Chukudebelu, and A. N. Njoku-Obi. 1989. Incidence of yeasts in pregnant and non-pregnant women in Nigeria. Mycoses 32:131-135. [DOI] [PubMed] [Google Scholar]
- 176.Guida, J. D., A. M. Kunig, K. H. Leef, S. E. McKenzie, and D. A. Paul. 2003. Platelet count and sepsis in very low birth weight neonates: is there an organism-specific response? Pediatrics 111:1411-1415. [DOI] [PubMed] [Google Scholar]
- 177.Gupta, A., K. Ampofo, D. Rubenstein, and L. Saiman. 2003. Extended spectrum beta-lactamase-producing Klebsiella pneumoniae infections: a review of the literature. J. Perinatol. 23:439-443. [DOI] [PubMed] [Google Scholar]
- 178.Gupta, K., D. Scholes, and W. E. Stamm. 1999. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA 281:736-738. [DOI] [PubMed] [Google Scholar]
- 179.Gupta, S., J. G. Morris, Jr., P. Panigrahi, J. P. Nataro, R. I. Glass, and I. H. Gewolb. 1994. Endemic necrotizing enterocolitis: lack of association with a specific infectious agent. Pediatr. Infect. Dis. J. 13:728-734. [PubMed] [Google Scholar]
- 180.Gustafson, K. S., G. M. Vercellotti, C. M. Bendel, and M. K. Hostetter. 1991. Molecular mimicry in Candida albicans. Role of an integrin analogue in adhesion of the yeast to human endothelium. J. Clin. Investig. 87:1896-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hack, M., D. Wilson-Costello, H. Friedman, G. H. Taylor, M. Schluchter, and A. A. Fanaroff. 2000. Neurodevelopment and predictors of outcomes of children with birth weights of less than 1000 g: 1992-1995. Arch. Pediatr. Adolesc. Med. 154:725-731. [DOI] [PubMed] [Google Scholar]
- 182.Haddad, Q., E. I. Sobayo, O. B. Basit, and V. O. Rotimi. 1993. Outbreak of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. J. Hosp. Infect. 23:211-222. [DOI] [PubMed] [Google Scholar]
- 183.Hageman, J. R., J. Stenske, H. Keuler, and E. Randall. 1985. Candida colonization and infection in very low birth weight infants. J. Perinatol. 6:251-254. [Google Scholar]
- 184.Haley, R. W., N. B. Cushion, F. C. Tenover, T. L. Bannerman, D. Dryer, J. Ross, P. J. Sanchez, and J. D. Siegel. 1995. Eradication of endemic methicillin-resistant Staphylococcus aureus infections from a neonatal intensive care unit. J. Infect. Dis. 171:614-624. [DOI] [PubMed] [Google Scholar]
- 185.Hall, S. L., R. T. Hall, W. G. Barnes, and S. Riddell. 1988. Colonization with slime- positive coagulase-negative staphylococci as a risk factor for invasive coagulase-negative staphylococci infections in neonates. J. Perinatol. 8:215-221. [PubMed] [Google Scholar]
- 186.Hall, S. L., R. T. Hall, W. G. Barnes, S. W. Riddell, L. Meng, J. T. Parisi, H. W. Kilbride, and D. Maulik. 1990. Relationship of maternal to neonatal colonization with coagulase-negative staphylococci. Am. J. Perinatol. 7:384-388. [DOI] [PubMed] [Google Scholar]
- 187.Hall, S. L., S. W. Riddell, W. G. Barnes, L. Meng, and R. T. Hall. 1990. Evaluation of coagulase-negative staphylococcal isolates from serial nasopharyngeal cultures of premature infants. Diagn. Microbiol. Infect. Dis. 13:17-23. [DOI] [PubMed] [Google Scholar]
- 188.Hallstrom, M., T. Vesikari, M. Janas, S. Ikonen, and O. Tammela. 2001. Screening of rotavirus and adenovirus infections during prolonged hospitalization in a neonatal unit. Acta Paediatr. 90:1196-1198. [DOI] [PubMed] [Google Scholar]
- 189.Haroon Parupia, M. F., and R. Dhanireddy. 2001. Association of postnatal dexamethasone use and fungal sepsis in the development of severe retinopathy of prematurity and progression to laser therapy in extremely low-birth-weight infants. J. Perinatol. 21:242-247. [DOI] [PubMed] [Google Scholar]
- 190.Harpin, V. A., and N. Rutter. 1985. Humidification of incubators. Arch. Dis. Child 60:219-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Harris, M. C., C. Deuber, R. A. Polin, and I. Nachamkin. 1989. Investigation of apparent false-positive urine latex particle agglutination tests for the detection of group B streptococcus antigen. J. Clin. Microbiol. 27:2214-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Harris, M. C., G. R. Pereira, M. D. Myers, A. J. Cardin, B. Razdan, J. Pleasure, and L. M. Bell. 2000. Candidal arthritis in infants previously treated for systemic candidiasis during the newborn period: report of three cases. Pediatr. Emerg. Care 16:249-251. [DOI] [PubMed] [Google Scholar]
- 193.Haruda, F., M. A. Bergman, and D. Headings. 1980. Unrecognized Candida brain abscess in infancy: two cases and a review of the literature. Johns Hopkins Med. J. 147:182-185. [PubMed] [Google Scholar]
- 194.Harvey, B. S., T. Koeuth, J. Versalovic, C. R. Woods, and J. R. Lupski. 1995. Vertical transmission of Citrobacter diversus documented by DNA fingerprinting. Infect. Control Hosp. Epidemiol. 16:564-569. [DOI] [PubMed] [Google Scholar]
- 195.Heath, P. T., N. K. Nik Yusoff, and C. J. Baker. 2003. Neonatal meningitis. Arch. Dis. Child. Fetal Neonatal Ed. 88:F173-F178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Helstrom, P. B., and E. Balish. 1979. Effect of oral tetracycline, the microbial flora, and the athymic state on gastrointestinal colonization and infection of BALB/c mice with Candida albicans. Infect. Immun. 23:764-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Henrickson, K. J., R. A. Axtell, S. M. Hoover, S. M. Kuhn, J. Pritchett, S. C. Kehl, and J. P. Klein. 2000. Prevention of central venous catheter-related infections and thrombotic events in immunocompromised children by the use of vancomycin/ciprofloxacin/heparin flush solution: a randomized, multicenter, double- blind trial. J. Clin. Oncol. 18:1269-1278. [DOI] [PubMed] [Google Scholar]
- 198.Hensey, O. J., C. A. Hart, and R. W. Cooke. 1985. Serious infection in a neonatal intensive care unit: a two-year survey. J. Hyg. 95:289-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Herrmann, M., P. E. Vaudaux, D. Pittet, R. Auckenthaler, P. D. Lew, F. Schumacher-Perdreau, G. Peters, and F. A. Waldvogel. 1988. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J. Infect. Dis. 158:693-701. [DOI] [PubMed] [Google Scholar]
- 200.Herson, V. C., P. J. Krause, L. I. Eisenfeld, L. Pontius, and E. G. Maderazo. 1988. Indomethacin-associated sepsis in very-low-birth-weight infants. Am. J. Dis. Child. 142:555-558. [DOI] [PubMed] [Google Scholar]
- 201.Hill, H. R., T. G. Mitchell, J. M. Matsen, and P. G. Quie. 1974. Recovery from disseminated candidiasis in a premature neonate. Pediatrics 53:748-752. [PubMed] [Google Scholar]
- 202.Hitomi, S., M. Kubota, N. Mori, S. Baba, H. Yano, K. Okuzumi, and S. Kimura. 2000. Control of a methicillin-resistant Staphylococcus aureus outbreak in a neonatal intensive care unit by unselective use of nasal mupirocin ointment. J. Hosp. Infect. 46:123-129. [DOI] [PubMed] [Google Scholar]
- 203.Hof, H., R. Lampidis, and J. Bensch. 2000. Nosocomial listeria gastroenteritis in a newborn, confirmed by random amplification of polymorphic DNA. Clin. Microbiol. Infect. 6:683-686. [DOI] [PubMed] [Google Scholar]
- 204.Holt, D. E., S. Halket, J. de Louvois, and D. Harvey. 2001. Neonatal meningitis in England and Wales: 10 years on. Arch. Dis. Child. Fetal Neonatal Ed. 84:F85-F89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Honeyman A. L., H. Friedman, and M. Bendinelli. 2001. Staphylococcus aureus infection and disease, p. 1-16. In D. P. M. Projan and S. J. Projan (ed.), The regulation of virulence in the staphylococci. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 206.Hostetter, M. K. 1994. Adhesins and ligands involved in the interaction of Candida spp. with epithelial and endothelial surfaces. Clin. Microbiol. Rev. 7:29-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hostetter, M. K. 1994. Society for Pediatric Research presidential address 1994: yeast as metaphor. Pediatr. Res. 36:692-698. [DOI] [PubMed] [Google Scholar]
- 208.Hostetter, M. K. 1996. Adhesion and morphogenesis in Candida albicans. Pediatr. Res. 39:569-573. [DOI] [PubMed] [Google Scholar]
- 209.Hostetter, M. K. 1998. Linkage of adhesion, morphogenesis, and virulence in Candida albicans. J. Lab. Clin. Med. 132:258-263. [DOI] [PubMed] [Google Scholar]
- 210.Hostetter, M. K. 1999. Integrin-like proteins in Candida spp. and other microorganisms. Fungal Genet. Biol. 28:135-145. [DOI] [PubMed] [Google Scholar]
- 211.Hostetter, M. K., J. S. Lorenz, L. Preus, and K. E. Kendrick. 1990. The iC3b receptor on Candida albicans: subcellular localization and modulation of receptor expression by glucose. J. Infect. Dis. 161:761-768. [DOI] [PubMed] [Google Scholar]
- 212.How, H. Y., D. Sutler, J. C. Khoury, E. F. Donovan, T. A. Siddiqi, and J. A. Spinnato. 2001. Does the combined antenatal use of corticosteroids and antibiotics increase late-onset neonatal sepsis in the very low birth weight infant? Am. J. Obstet. Gynecol 185:1081-1085. [DOI] [PubMed] [Google Scholar]
- 213.Hoyos, A. B. 1999. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int. J. Infect. Dis. 3:197-202. [DOI] [PubMed] [Google Scholar]
- 214.Hsueh, W., M. S. Caplan, X. W. Qu, X. D. Tan, I. G. De Plaen, and F. Gonzalez- Crussi. 2003. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr. Dev. Pathol. 6:6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Huang, Y. C., C. C. Li, T. Y. Lin, R. I. Lien, Y. H. Chou, J. L. Wu, and C. Hsueh. 1998. Association of fungal colonization and invasive disease in very low birth weight infants. Pediatr. Infect. Dis. J. 17:819-822. [DOI] [PubMed] [Google Scholar]
- 216.Huang, Y. C., T. Y. Lin, H. S. Leu, H. L. Peng, J. H. Wu, and H. Y. Chang. 1999. Outbreak of Candida parapsilosis fungemia in neonatal intensive care units: clinical implications and genotyping analysis. Infection 27:97-102. [DOI] [PubMed] [Google Scholar]
- 217.Huebner, J., G. B. Pier, J. N. Maslow, E. Muller, H. Shiro, M. Parent, A. Kropec, R. D. Arbeit, and D. A. Goldmann. 1994. Endemic nosocomial transmission of Staphylococcus epidermidis bacteremia isolates in a neonatal intensive care unit over 10 years. J. Infect. Dis. 169:526-531. [DOI] [PubMed] [Google Scholar]
- 218.Hummei, R. P., E. J. Oestreicher, M. P. Maley, and B. G. MacMillan. 1973. Inhibition of Candida albicans by Escherichia coli in vitro and in the germfree mouse. J. Surg. Res. 15:53-58. [DOI] [PubMed] [Google Scholar]
- 219.Hurley, R., and H. I. Winner. 1963. Experimental renal moniliasis in the mouse. J. Pathol. 86:75. [DOI] [PubMed] [Google Scholar]
- 220.Hussain, S. M., G. S. Luedtke, C. J. Baker, P. M. Schlievert, and R. J. Leggiadro. 1995. Invasive group B streptococcal disease in children beyond early infancy. Pediatr. Infect. Dis. J. 14:278-281. [DOI] [PubMed] [Google Scholar]
- 221.Huttova, M., I. Hartmanova, K. Kralinsky, J. Filka, J. Uher, J. Kurak, S. Krizan, and V. Krcmery, Jr. 1998. Candida fungemia in neonates treated with fluconazole: report of forty cases, including eight with meningitis. Pediatr. Infect. Dis. J. 17:1012-1015. [DOI] [PubMed] [Google Scholar]
- 222.Insoft, R. M., I. R. Sanderson, and W. A. Walker. 1996. Development of immune function in the intestine and its role in neonatal diseases. Pediatr. Clin. North Am. 43:551-571. [DOI] [PubMed] [Google Scholar]
- 223.Isaacs, D., J. North, D. Lindsell, and A. R. Wilkinson. 1987. Serum acute phase reactants in necrotizing enterocolitis. Acta Paediatr. Scand. 76:923-927. [DOI] [PubMed] [Google Scholar]
- 224.Ish-Horowicz, M. R., P. McIntyre, and S. Nade. 1992. Bone and joint infections caused by multiply resistant Staphylococcus aureus in a neonatal intensive care unit. Pediatr. Infect. Dis. J. 11:82-87. [DOI] [PubMed] [Google Scholar]
- 225.Jafari, H. S., X. Saez-Llorens, E. Grimprel, J. C. Argyle, K. D. Olsen, and G. H. McCracken, Jr. 1991. Characteristics of experimental Candida albicans infection of the central nervous system in rabbits. J. Infect. Dis. 164:389-395. [DOI] [PubMed] [Google Scholar]
- 226.Johnson, D. E., T. R. Thompson, and P. Ferrieri. 1981. Congenital candidiasis. Am. J. Dis. Child. 135:273-275. [DOI] [PubMed] [Google Scholar]
- 227.Johnson, D. E., T. R. Thompson, T. P. Green, and P. Ferrieri. 1984. Systemic candidiasis in very low-birth-weight infants (less than 1,500 grams). Pediatrics 73:138-143. [PubMed] [Google Scholar]
- 228.Johnson-Robbins, L. A., A. E. el Mohandes, S. J. Simmens, and J. F. Keiser. 1996. Staphylococcus epidermidis sepsis in the intensive care nursery: a characterization of risk associations in infants <1,000 g. Biol. Neonate 69:249-256. [DOI] [PubMed] [Google Scholar]
- 229.Jordan, J. A. 1994. PCR identification of four medically important Candida species by using a single primer pair. J. Clin. Microbiol. 32:2962-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jordan, J. A., and M. B. Durso. 2000. Comparison of 16S rRNA gene PCR and BACTEC 9240 for detection of neonatal bacteremia. J. Clin. Microbiol. 38:2574-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Joshi, S. G., V. S. Ghole, and K. B. Niphadkar. 2000. Neonatal gram-negative bacteremia. Indian J. Pediatr. 67:27-32. [DOI] [PubMed] [Google Scholar]
- 232.Jourdain, B., A. Novara, M. L. Joly-Guillou, M. C. Dombret, S. Calvat, J. L. Trouillet, C. Gibert, and J. Chastre. 1995. Role of quantitative cultures of endotracheal aspirates in the diagnosis of nosocomial pneumonia. Am. J. Respir. Crit. Care Med. 152:241-246. [DOI] [PubMed] [Google Scholar]
- 233.Jungbluth, G. L., I. R. Welshman, and N. K. Hopkins. 2003. Linezolid pharmacokinetics in pediatric patients: an overview. Pediatr. Infect. Dis. J. 22:S153-S157. [DOI] [PubMed] [Google Scholar]
- 234.Juster-Reicher, A., E. Leibovitz, N. Linder, M. Amitay, O. Flidel-Rimon, S. Even-Tov, B. Mogilner, and A. Barzilai. 2000. Liposomal amphotericin B (AmBisome) in the treatment of neonatal candidiasis in very low birth weight infants. Infection 28:223-226. [DOI] [PubMed] [Google Scholar]
- 235.Kacica, M. A., M. J. Horgan, K. E. Preston, M. Lepow, and R. A. Venezia. 1994. Relatedness of coagulase-negative staphylococci causing bacteremia in low-birthweight infants. Infect. Control Hosp. Epidemiol. 15:658-662. [DOI] [PubMed] [Google Scholar]
- 236.Kamran, S., S. S. Usmani, R. A. Wapnir, R. Mehta, and R. G. Harper. 1993. In vitro effect of indomethacin on polymorphonuclear leukocyte function in preterm infants. Pediatr. Res. 33:32-35. [DOI] [PubMed] [Google Scholar]
- 237.Kaplan, S. L. 2003. Use of linezolid in children. Introduction. Pediatr. Infect. Dis. J. 22:S151-S152. [DOI] [PubMed] [Google Scholar]
- 238.Reference deleted.
- 239.Karlowicz, M. G. 2003. Candidal renal and urinary tract infection in neonates. Semin. Perinatol. 27:393-400. [DOI] [PubMed] [Google Scholar]
- 240.Karlowicz, M. G., E. S. Buescher, and A. E. Surka. 2000. Fulminant late-onset sepsis in a neonatal intensive care unit, 1988-1997, and the impact of avoiding empiric vancomycin therapy. Pediatrics 106:1387-1390. [DOI] [PubMed] [Google Scholar]
- 241.Karlowicz, M. G., P. J. Furigay, D. P. Croitoru, and E. S. Buescher. 2002. Central venous catheter removal versus in situ treatment in neonates with coagulase-negative staphylococcal bacteremia. Pediatr. Infect. Dis. J. 21:22-27. [DOI] [PubMed] [Google Scholar]
- 242.Karlowicz, M. G., P. J. Giannone, J. Pestian, A. L. Morrow, and J. Shults. 2000. Does candidemia predict threshold retinopathy of prematurity in extremely low birth weight (≤1000 g) neonates? Pediatrics 105:1036-1040. [DOI] [PubMed] [Google Scholar]
- 243.Karlowicz, M. G., L. N. Hashimoto, R. E. Kelly, Jr., and E. S. Buescher. 2000. Should central venous catheters be removed as soon as candidemia is detected in neonates? Pediatrics 106:E63. [DOI] [PubMed] [Google Scholar]
- 244.Reference deleted.
- 245.Kaufman, D. 2003. Strategies for prevention of neonatal invasive candidiasis. Semin. Perinatol. 27:414-424. [DOI] [PubMed] [Google Scholar]
- 246.Kaufman, D., R. Boyle, K. C. Hazen, J. T. Patrie, M. Robinson, and L. G. Donowitz. 2001. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N. Eng. J. Med. 345:1660-1666. [DOI] [PubMed] [Google Scholar]
- 247.Kaufman, D., L. Klipatrick, R. G. Hudson, D. E. Campbell, A. Kaufman, S. D. Douglas, and M. C. Harris. 1999. Decreased superoxide production, degranulation, tumor necrosis factor alpha secretion, and CD11b/CD18 receptor expression by adherent monocytes from preterm infants. Clin. Diagn. Lab. Immunol. 6:525-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kaufmann, C. S., and W. G. Merz. 1989. Electrophoretic karyotypes of Torulopsis glabrata. J. Clin. Microbiol. 27:2165-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kawamura, M., and H. Nishida. 1995. The usefulness of serial C-reactive protein measurement in managing neonatal infection. Acta Paediatr. 84:10-13. [DOI] [PubMed] [Google Scholar]
- 250.Ke, D., and M. G. Bergeron. 2001. Molecular methods for rapid detection of group B streptococci. Expert Rev. Mol. Diagn. 1:175-181. [DOI] [PubMed] [Google Scholar]
- 251.Kellogg, J. A., F. L. Ferrentino, M. H. Goodstein, J. Liss, S. L. Shapiro, and D. A. Bankert. 1997. Frequency of low level bacteremia in infants from birth to two months of age. Pediatr. Infect. Dis. J. 16:381-385. [DOI] [PubMed] [Google Scholar]
- 252.Kennedy, M. J., and P. A. Volz. 1985. Ecology of Candida albicans gut colonization: inhibition of Candida adhesion, colonization, and dissemination from the gastrointestinal tract by bacterial antagonism. Infect. Immun. 49:654-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Khan, N. A., Y. Wang, K. J. Kim, J. W. Chung, C. A. Wass, and K. S. Kim. 2002. Cytotoxic necrotizing factor-1 contributes to Escherichia coli K1 invasion of the central nervous system. J. Biol. Chem. 277:15607-15612. [DOI] [PubMed] [Google Scholar]
- 254.Kicklighter, S. D. 2002. Antifungal agents and fungal prophylaxis in the neonate. Neoreviews 3:e249-e255. [Google Scholar]
- 255.Kicklighter, S. D., S. C. Springer, T. Cox, T. C. Hulsey, and R. B. Turner. 2001. Fluconazole for prophylaxis against candidal rectal colonization in the very low birth weight infant. Pediatrics 107:293-298. [DOI] [PubMed] [Google Scholar]
- 256.Kilbride, H. W., D. D. Wirtschafter, R. J. Powers, and M. B. Sheehan. 2003. Implementation of evidence-based potentially better practices to decrease nosocomial infections. Pediatrics 111:e519-e533. [PubMed] [Google Scholar]
- 257.Kilpatrick, L., and M. C. Harris. 2004. Cytokines and the inflammatory response in the fetus and neonate, p. 1555-1571. In R. A. Polin, W. W. Fox, and S. H. Abman (ed.), Fetal and neonatal physiology. The W. B. Saunders Co., Philadelphia, Pa.
- 258.Kinneberg, K. M., C. M. Bendel, R. P. Jechorek, E. A. Cebelinski, C. A. Gale, J. G. Berman, S. L. Erlandsen, M. K. Hostetter, and C. L. Wells. 1999. Effect of INT1 gene on Candida albicans murine intestinal colonization. J. Surg. Res. 87:245-251. [DOI] [PubMed] [Google Scholar]
- 259.Klein, J. O. 2001. Bacterial sepsis and meningitis, p. 943-998. In J. O. Klein and J. S. Remington (ed.), Infectious diseases of the fetus and newborn infant. The W. B. Saunders Co., Philadelphia, Pa.
- 260.Klein, J. D., T. Yamauchi, and S. P. Horlick. 1972. Neonatal candidiasis, meningitis, and arthritis: observations and a review of the literature. J. Pediatr. 81:31-34. [DOI] [PubMed] [Google Scholar]
- 261.Ko, W. C., H. C. Lee, L. R. Wang, C. T. Lee, A. J. Liu, and J. J. Wu. 2001. Serotyping and antimicrobial susceptibility of group B Streptococcus over an eight-year period in southern Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 20:334-339. [DOI] [PubMed] [Google Scholar]
- 262.Kocherlakota, P., and E. F. La Gamma. 1998. Preliminary report: rhG-CSF may reduce the incidence of neonatal sepsis in prolonged preeclampsia-associated neutropenia. Pediatrics 102:1107-1111. [DOI] [PubMed] [Google Scholar]
- 263.Kodroff, M. B., M. A. Hartenberg, and R. A. Goldschmidt. 1984. Ultrasonographic diagnosis of gangrenous bowel in neonatal necrotizing enterocolitis. Pediatr. Radiol. 14:168-170. [DOI] [PubMed] [Google Scholar]
- 264.Kohelet, D., S. Peller, E. Arbel, and M. Goldberg. 1990. Preincubation with intravenous lipid emulsion reduces chemotactic motility of neutrophils in cord blood. J. Parenter. Enteral Nutr. 14:472-473. [DOI] [PubMed] [Google Scholar]
- 265.Kordek, A., S. Giedrys-Kalemba, B. Pawlus, W. Podraza, and R. Czajka. 2003. Umbilical cord blood serum procalcitonin concentration in the diagnosis of early neonatal infection. J. Perinatol. 23:148-153. [DOI] [PubMed] [Google Scholar]
- 266.Kossoff, E. H., E. S. Buescher, and M. G. Karlowicz. 1998. Candidemia in a neonatal intensive care unit: trends during fifteen years and clinical features of 111 cases. Pediatr. Infect. Dis. J. 17:504-508. [DOI] [PubMed] [Google Scholar]
- 267.Krcmery, V., J. Havlik, and L. Vicianova. 1987. Nosocomial meningitis caused by multiply resistant Pseudomonas cepacia. Pediatr. Infect. Dis. J. 6:769. [PubMed] [Google Scholar]
- 268.Krediet, T. G., M. E. Jones, L. J. Gerards, and A. Fleer. 1999. Clinical outcome of cephalothin versus vancomycin therapy in the treatment of coagulase-negative staphylococcal septicemia in neonates: relation to methicillin resistance and mecA gene carriage of blood isolates. Pediatrics 103:E29. [DOI] [PubMed] [Google Scholar]
- 269.Kremer, I., N. Naor, S. Davidson, M. Arbizo, and I. Nissenkorn. 1992. Systemic candidiasis in babies with retinopathy of prematurity. Graefes Arch. Clin. Exp. Ophthalmol. 230:592-594. [DOI] [PubMed] [Google Scholar]
- 270.Krontal, S., E. Leibovitz, M. Greenwald-Maimon, D. Fraser, and R. Dagan. 2002. Klebsiella bacteremia in children in southern Israel (1988-1997). Infection 30:125-131. [DOI] [PubMed] [Google Scholar]
- 271.Kumar, A., and G. A. Nankervis. 1980. Latex agglutination test and countercurrent immunoelectrophoresis for detection of group B streptococcal antigen. J. Pediatr. 97:328-329. [DOI] [PubMed] [Google Scholar]
- 272.Kuster, H., M. Weiss, A. E. Willeitner, S. Detlefsen, I. Jeremias, J. Zbojan, R. Geiger, G. Lipowsky, and G. Simbruner. 1998. Interleukin-1 receptor antagonist and interleukin-6 for early diagnosis of neonatal sepsis 2 days before clinical manifestation. Lancet 352:1271-1277. [DOI] [PubMed] [Google Scholar]
- 273.Laforgia, N., B. Coppola, R. Carbone, A. Grassi, A. Mautone, and A. Iolascon. 1997. Rapid detection of neonatal sepsis using polymerase chain reaction. Acta Paediatr. 86:1097-1099. [DOI] [PubMed] [Google Scholar]
- 274.Lane, A. T., and S. S. Drost. 1993. Effects of repeated application of emollient cream to premature neonates' skin. Pediatrics 92:415-419. [PubMed] [Google Scholar]
- 275.Larson, E., M. Silberger, K. Jakob, S. Whittier, L. Lai, L. P. Della, and L. Saiman. 2000. Assessment of alternative hand hygiene regimens to improve skin health among neonatal intensive care unit nurses. Heart Lung 29:136-142. [PubMed] [Google Scholar]
- 276.Lassiter, H. A., S. W. Watson, M. L. Seifring, and J. E. Tanner. 1992. Complement factor 9 deficiency in serum of human neonates. J. Infect. Dis. 166:53-57. [DOI] [PubMed] [Google Scholar]
- 277.Lee, B. E., P. Y. Cheung, J. L. Robinson, C. Evanochko, and C. M. Robertson. 1998. Comparative study of mortality and morbidity in premature infants (birth weight, <1,250 g) with candidemia or candidal meningitis. Clin. Infect. Dis. 27:559-565. [DOI] [PubMed] [Google Scholar]
- 278.Lee, R. M., C. Chambers, H. O'Brodovich, and J. B. Forrest. 1984. Trypan blue method for the identification of areas of damage to airway epithelium due to mechanical trauma. Scanning Electron Microsc. 1984:1267-1271. [PubMed] [Google Scholar]
- 279.Leeming, J. P., T. M. Sutton, and P. J. Fleming. 1995. Neonatal skin as a reservoir of Malassezia species. Pediatr. Infect. Dis. J. 14:719-721. [PubMed] [Google Scholar]
- 280.Leeper-Woodford, S. K., D. Carey, K. Byrne, C. Walsh, B. Fisher, H. J. Sugerman, and A. A. Fowler. 1998. Histamine receptor antagonists, cyclooxygenase blockade, and tumor necrosis factor during acute septic insult. Shock 9:89-94. [DOI] [PubMed] [Google Scholar]
- 281.Leibovitz, E., A. Iuster-Reicher, M. Amitai, and B. Mogilner. 1992. Systemic candidal infections associated with use of peripheral venous catheters in neonates: a 9- year experience. Clin. Infect. Dis. 14:485-491. [DOI] [PubMed] [Google Scholar]
- 282.Leigh, L., B. J. Stoll, M. Rahman, and J. McGowan, Jr. 1995. Pseudomonas aeruginosa infection in very low birth weight infants: a case-control study. Pediatr. Infect. Dis. J. 14:367-371. [DOI] [PubMed] [Google Scholar]
- 283.Levine, E. M., V. Ghai, J. J. Barton, and C. M. Strom. 1999. Intrapartum antibiotic prophylaxis increases the incidence of gram-negative neonatal sepsis. Infect. Dis. Obstet. Gynecol. 7:210-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Levy, M. M., M. P. Fink, J. C. Marshall, E. Abraham, D. Angus, D. Cook, J. Cohen, S. M. Opal, J. L. Vincent, and G. Ramsay. 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 29:530-538. [DOI] [PubMed] [Google Scholar]
- 285.Levy, R., D. T. Hindley, R. Burman, S. A. Halder, and J. Blease. 1995. Unusual cause of pseudomonal infection. Br. Med. J. 310:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lewis, D. B., and C. B. Wilson. 2001. Developmental immunology and role of host defense in fetal and neonatal susceptibility to infection, p. 25-138. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant. The W. B. Saunders Co., Philadelphia, Pa.
- 287.Lewis, R. E., and M. E. Klepser. 1999. The changing face of nosocomial candidemia: epidemiology, resistance, and drug therapy. Am. J. Health Syst. Pharm. 56:525-533. [PubMed] [Google Scholar]
- 288.Lilien, L. D., R. S. Ramamurthy, and R. S. Pildes. 1978. Candida albicans meningitis in a premature neonate successfully treated with 5-fluorocytosine and amphotericin B: a case report and review of the literature. Pediatrics 61:57-61. [PubMed] [Google Scholar]
- 289.Lin, F. C., W. F. Devoe, C. Morrison, J. Libonati, P. Powers, R. J. Gross, B. Rowe, E. Israel, and J. G. Morris, Jr. 1987. Outbreak of neonatal Citrobacter diversus meningitis in a suburban hospital. Pediatr. Infect. Dis. J. 6:50-55. [DOI] [PubMed] [Google Scholar]
- 290.Lin, F. Y., L. E. Weisman, J. Troendle, and K. Adams. 2003. Prematurity is the major risk factor for late-onset group B streptococcus disease. J. Infect. Dis. 188:267-271. [DOI] [PubMed] [Google Scholar]
- 291.Linares, J. 2001. The VISA/GISA problem: therapeutic implications. Clin. Microbiol. Infect. 7(Suppl 4):8-15. [DOI] [PubMed] [Google Scholar]
- 292.Linder, N., O. Haskin, O. Levit, G. Klinger, T. Prince, N. Naor, P. Turner, B. Karmazyn, and L. Sirota. 2003. Risk factors for intraventricular hemorrhage in very low birth weight premature infants: a retrospective case-control study. Pediatrics 111:e590-e595. [DOI] [PubMed] [Google Scholar]
- 293.Lindley, S., D. L. Mollitt, J. J. Seibert, and E. S. Golladay. 1986. Portal vein ultrasonography in the early diagnosis of necrotizing enterocolitis. J. Pediatr. Surg. 21:530-532. [DOI] [PubMed] [Google Scholar]
- 294.Long, J. G., and H. L. Keyserling. 1985. Catheter-related infection in infants due to an unusual lipophilic yeast—Malassezia furfur. Pediatrics 76:896-900. [PubMed] [Google Scholar]
- 295.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 296.Lucas, A., and T. J. Cole. 1990. Breast milk and neonatal necrotising enterocolitis. Lancet 336:1519-1523. [DOI] [PubMed] [Google Scholar]
- 297.Lundgren, R., P. Horstedt, and B. Winblad. 1983. Respiratory mucosal damage after brush biopsy—an experimental study in rabbits. Eur. J. Respir. Dis. 64:9-23. [PubMed] [Google Scholar]
- 298.Lyytikainen, O., H. Saxen, R. Ryhanen, M. Vaara, and J. Vuopio-Varkila. 1995. Persistence of a multiresistant clone of Staphylococcus epidermidis in a neonatal intensive-care unit for a four-year period. Clin. Infect. Dis. 20:24-29. [DOI] [PubMed] [Google Scholar]
- 299.Mack, D., M. Haeder, N. Siemssen, and R. Laufs. 1996. Association of biofilm production of coagulase-negative staphylococci with expression of a specific polysaccharide intercellular adhesin. J. Infect. Dis. 174:881-884. [DOI] [PubMed] [Google Scholar]
- 300.Maibach, H. I., and A. M. Kligman. 1962. The biology of experimental human cutaneous moniliasis (Candida albicans). Arch. Dermatol. 85:233. [DOI] [PubMed] [Google Scholar]
- 301.Makhoul, I. R., I. Kassis, N. Hashman, and P. Sujov. 2001. Staphylococcal scalded- skin syndrome in a very low birth weight premature infant. Pediatrics 108:E16. [DOI] [PubMed] [Google Scholar]
- 302.Makhoul, I. R., I. Kassis, T. Smolkin, A. Tamir, and P. Sujov. 2001. Review of 49 neonates with acquired fungal sepsis: further characterization. Pediatrics 107:61-66. [DOI] [PubMed] [Google Scholar]
- 303.Makhoul, I. R., P. Sujov, T. Smolkin, A. Lusky, and B. Reichman. 2002. Epidemiological, clinical, and microbiological characteristics of late-onset sepsis among very low birth weight infants in Israel: a national survey. Pediatrics 109:34-39. [DOI] [PubMed] [Google Scholar]
- 304.Malecka-Griggs, B. 1986. Microbiological assessment of 24- and 48-h changes and management of semiclosed circuits from ventilators in a neonatal intensive care unit. J. Clin. Microbiol. 23:322-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Malik, R. K., M. A. Montecalvo, M. R. Reale, K. Li, M. Maw, J. L. Munoz, C. Gedris, K. van Horn, K. A. Carnevale, M. H. Levi, and H. S. Dweck. 1999. Epidemiology and control of vancomycin-resistant enterococci in a regional neonatal intensive care unit. Pediatr. Infect. Dis. J. 18:352-356. [DOI] [PubMed] [Google Scholar]
- 306.Mallow, E. B., A. Harris, N. Salzman, J. P. Russell, R. J. DeBerardinis, E. Ruchelli, and C. L. Bevins. 1996. Human enteric defensins. Gene structure and developmental expression. J. Biol. Chem. 271:4038-4045. [DOI] [PubMed] [Google Scholar]
- 307.Manning, S. D., B. Foxman, C. L. Pierson, P. Tallman, C. J. Baker, and M. D. Pearlman. 2003. Correlates of antibiotic-resistant group B streptococcus isolated from pregnant women. Obstet. Gynecol. 101:74-79. [DOI] [PubMed] [Google Scholar]
- 308.Marodi, L., and R. B. Johnston, Jr. 2004. Developmental immunology, p. 1487-1489. In R. A. Polin, W. W. Fox, and S. H. Abman (ed.), Fetal and neonatal physiology. The W.B. Saunders Co., Philadelphia, Pa.
- 309.Marr, K. A., K. Seidel, M. A. Slavin, R. A. Bowden, H. G. Schoch, M. E. Flowers, L. Corey, and M. Boeckh. 2000. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo- controlled trial. Blood 96:2055-2061. [PubMed] [Google Scholar]
- 310.Marr, K. A., K. Seidel, T. C. White, and R. A. Bowden. 2000. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J. Infect. Dis. 181:309-316. [DOI] [PubMed] [Google Scholar]
- 311.Matlow, A., S. Camack, H. Kirpilani, E. L. Ford-Jones, D. E. Low, and B. Schmidt. 1991. Role of lipids in the pathogenesis of coagulase-negative staphylococcal bacteremia in neonates. Pediatr. Infect. Dis. J. 10:168-169. [DOI] [PubMed] [Google Scholar]
- 312.Matrai-Kovalskis, Y., D. Greenberg, E. S. Shinwell, D. Fraser, and R. Dagan. 1998. Positive blood cultures for coagulase-negative staphylococci in neonates: does highly selective vancomycin usage affect outcome? Infection 26:85-92. [DOI] [PubMed] [Google Scholar]
- 313.Maudsley, R. F., G. A. Brix, N. A. Hinton, E. M. Robertson, A. M. Bryans, and M. D. Haust. 1966. Placental inflammation and infection. A prospective bacteriologic and histologic study. Am. J. Obstet. Gynecol. 95:648-659. [DOI] [PubMed] [Google Scholar]
- 314.McCabe, K. M., G. Khan, Y. H. Zhang, E. O. Mason, and E. R. McCabe. 1995. Amplification of bacterial DNA using highly conserved sequences: automated analysis and potential for molecular triage of sepsis. Pediatrics 95:165-169. [PubMed] [Google Scholar]
- 315.McGregor, J. A., J. I. French, R. Richter, A. Franco-Buff, A. Johnson, S. Hillier, F. N. Judson, and J. K. Todd. 1990. Antenatal microbiologic and maternal risk factors associated with prematurity. Am. J. Obstet. Gynecol. 163:1465-1473. [DOI] [PubMed] [Google Scholar]
- 316.McGuire, W., L. Clerihew, and N. Austin. 2003. Prophylactic intravenous antifungal agents to prevent mortality and morbidity in very low birth weight infants. Cochrane Database Syst. Rev. CD003850. [DOI] [PubMed]
- 317.McNeeley, D. F., A. E. Brown, G. J. Noel, M. Chung, and H. De Lencastre. 1998. An investigation of vancomycin-resistant Enterococcus faecium within the pediatric service of a large urban medical center. Pediatr. Infect. Dis. J. 17:184-188. [DOI] [PubMed] [Google Scholar]
- 318.McNeeley, D. F., F. Saint-Louis, and G. J. Noel. 1996. Neonatal enterococcal bacteremia: an increasingly frequent event with potentially untreatable pathogens. Pediatr. Infect. Dis. J. 15:800-805. [DOI] [PubMed] [Google Scholar]
- 319.Mecrow, I. K., and E. J. Ladusans. 1994. Infective endocarditis in newborn infants with structurally normal hearts. Acta Paediatr. 83:35-39. [DOI] [PubMed] [Google Scholar]
- 320.Mehall, J. R., C. A. Kite, D. A. Saltzman, T. Wallett, R. J. Jackson, and S. D. Smith. 2002. Prospective study of the incidence and complications of bacterial contamination of enteral feeding in neonates. J. Pediatr. Surg. 37:1177-1182. [DOI] [PubMed] [Google Scholar]
- 321.Mehall, J. R., R. Northrop, D. A. Saltzman, R. J. Jackson, and S. D. Smith. 2001. Acidification of formula reduces bacterial translocation and gut colonization in a neonatal rabbit model. J. Pediatr. Surg. 36:56-62. [DOI] [PubMed] [Google Scholar]
- 322.Mehall, J. R., D. A. Saltzman, R. J. Jackson, and S. D. Smith. 2002. Acidification of formula with citric acid is equally effective and better tolerated than acidification with hydrochloric acid. Crit. Care Med. 30:1701-1704. [DOI] [PubMed] [Google Scholar]
- 323.Mellado, E., M. Cuenca-Estrella, J. Regadera, M. Gonzalez, T. M. Diaz-Guerra, and J. L. Rodriguez-Tudela. 2000. Sustained gastrointestinal colonization and systemic dissemination by Candida albicans, Candida tropicalis and Candida parapsilosis in adult mice. Diagn. Microbiol. Infect. Dis. 38:21-28. [DOI] [PubMed] [Google Scholar]
- 324.Melville, C., S. Kempley, J. Graham, and C. L. Berry. 1996. Early onset systemic Candida infection in extremely preterm neonates. Eur. J. Pediatr. 155: 904-906. [DOI] [PubMed] [Google Scholar]
- 325.Meyer, C. L., N. R. Payne, and S. A. Roback. 1991. Spontaneous, isolated intestinal perforations in neonates with birth weight less than 1,000 g not associated with necrotizing enterocolitis. J. Pediatr. Surg. 26:714-717. [DOI] [PubMed] [Google Scholar]
- 326.Mintz, A. C., and H. Applebaum. 1993. Focal gastrointestinal perforations not associated with necrotizing enterocolitis in very low birth weight neonates. J. Pediatr. Surg. 28:857-860. [DOI] [PubMed] [Google Scholar]
- 327.Mitchell, S. J., J. Gray, M. E. Morgan, M. D. Hocking, and G. M. Durbin. 1996. Nosocomial infection with Rhizopus microsporus in preterm infants: association with wooden tongue depressors. Lancet 348:441-443. [DOI] [PubMed] [Google Scholar]
- 328.Moolenaar, R. L., J. M. Crutcher, V. H. San Joaquin, L. V. Sewell, L. C. Hutwagner, L. A. Carson, D. A. Robison, L. M. Smithee, and W. R. Jarvis. 2000. A prolonged outbreak of Pseudomonas aeruginosa in a neonatal intensive care unit: did staff fingernails play a role in disease transmission? Infect. Control Hosp. Epidemiol. 21:80-85. [DOI] [PubMed] [Google Scholar]
- 329.Morikawa, Y., M. Kitazato, C. Katsukawa, and A. Tamaru. 2003. Prevalence of cefotaxime resistance in group B streptococcus isolates from Osaka, Japan. J. Infect. Chemother. 9:131-133. [DOI] [PubMed] [Google Scholar]
- 330.Murphy, D. J., P. L. Hope, and A. Johnson. 1997. Neonatal risk factors for cerebral palsy in very preterm babies: case-control study. B. Med. J. 314:404-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Musemeche, C. A., A. M. Kosloske, S. A. Bartow, and E. T. Umland. 1986. Comparative effects of ischemia, bacteria, and substrate on the pathogenesis of intestinal necrosis. J. Pediatr. Surg. 21:536-538. [DOI] [PubMed] [Google Scholar]
- 332.Musoke, R. N., and G. Revathi. 2000. Emergence of multidrug-resistant gram-negative organisms in a neonatal unit and the therapeutic implications. J. Trop. Pediatr. 46:86-91. [DOI] [PubMed] [Google Scholar]
- 333.Narang, A., P. B. Agrawal, A. Chakrabarti, and P. Kumar. 1998. Epidemiology of systemic candidiasis in a tertiary care neonatal unit. J. Trop. Pediatr. 44:104-108. [DOI] [PubMed] [Google Scholar]
- 334.Narang, A., K. Mukhopadhyay, P. Kumar, and O. N. Bhakoo. 1998. Bone and joint infection in neonates. Indian J. Pediatr. 65:461-464. [DOI] [PubMed] [Google Scholar]
- 335.Nataro, J. P., L. Corcoran, S. Zirin, S. Swink, N. Taichman, J. Goin, and M. C. Harris. 1994. Prospective analysis of coagulase-negative staphylococcal infection in hospitalized infants. J. Pediatr. 125:798-804. [DOI] [PubMed] [Google Scholar]
- 335a.National Nosocomial Infections Surveillance System. 2001. National Nosocomial Infections Surveillance (NNIS) System Report. Data summary from January 1992-June 2001, issued August 2001. Am. J. Infect. Control 29:404-421. [DOI] [PubMed] [Google Scholar]
- 336.Nazemi, K. J., E. S. Buescher, R. E. Kelly, Jr., and M. G. Karlowicz. 2003. Central venous catheter removal versus in situ treatment in neonates with Enterobacteriaceae bacteremia. Pediatrics 111:e269-e274. [DOI] [PubMed] [Google Scholar]
- 337.Neal, P. R., M. B. Kleiman, J. K. Reynolds, S. D. Allen, J. A. Lemons, and P. L. Yu. 1986. Volume of blood submitted for culture from neonates. J. Clin. Microbiol. 24:353-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Nesin, M., S. J. Projan, B. Kreiswirth, Y. Bolt, and R. P. Novick. 1995. Molecular epidemiology of Staphylococcus epidermidis blood isolates from neonatal intensive care unit patients. J. Hosp. Infect. 31:111-121. [DOI] [PubMed] [Google Scholar]
- 339.Neumeister, B., S. Kastner, S. Conrad, G. Klotz, and P. Bartmann. 1995. Characterization of coagulase-negative staphylococci causing nosocomial infections in preterm infants. Eur. J. Clin. Microbiol. Infect. Dis. 14:856-863. [DOI] [PubMed] [Google Scholar]
- 340.Ng, P. C., S. H. Cheng, K. M. Chui, T. F. Fok, M. Y. Wong, W. Wong, R. P. Wong, and K. L. Cheung. 1997. Diagnosis of late onset neonatal sepsis with cytokines, adhesion molecule, and C-reactive protein in preterm very low birthweight infants. Arch. Dis. Child Fetal Neonatal Ed. 77:F221-F227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Ng, P. C., K. Li, R. P. Wong, K. Chui, E. Wong, G. Li, and T. F. Fok. 2003. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch. Dis. Child Fetal Neonatal Ed. 88:F209-F213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Niels, I. S. and B. N. Walters. 1981. Neonatal listeriosis: a summer outbreak. Med. J. Aust. 2:188-191. [PubMed] [Google Scholar]
- 343.Nielsen, H. J., H. Nielsen, S. Jensen, and F. Moesgaard. 1994. Ranitidine improves postoperative monocyte and neutrophil function. Arch. Surg. 129:309-315. [DOI] [PubMed] [Google Scholar]
- 343a.Nissen, M. D., and T. P. Sloots. 2002. Rapid diagnosis in pediatric infectious diseases: the past, the present, and the future. Pediatr. Infect. Dis. J. 21:605-612. [DOI] [PubMed] [Google Scholar]
- 344.Nizet, V., P. Ferrieri, and C. E. Rubens. 2000. Molecular pathogenesis of group B streptococcal disease in newborns, p. 180-221. In D. Stevens (ed.), Streptococcal infections: clinical aspects, microbiology, and molecular pathogenesis. Oxford University Press, New York, N.Y.
- 345.Noel, G. J., D. A. Laufer, and P. J. Edelson. 1988. Anaerobic bacteremia in a neonatal intensive care unit: an eighteen-year experience. Pediatr. Infect. Dis. J. 7:858-862. [PubMed] [Google Scholar]
- 346.Noel, G. J., J. E. O'Loughlin, and P. J. Edelson. 1988. Neonatal Staphylococcus epidermidis right-sided endocarditis: description of five catheterized infants. Pediatrics 82:234-239. [PubMed] [Google Scholar]
- 347.Noel, G. J., J. E. O'Loughlin, and P. J. Edelson. 1988. Neonatal Staphylococcus epidermidis right-sided endocarditis: description of five catheterized infants. Pediatrics 82:234-239. [PubMed] [Google Scholar]
- 348.Nopper, A. J., K. A. Horii, S. Sookdeo-Drost, T. H. Wang, A. J. Mancini, and A. T. Lane. 1996. Topical ointment therapy benefits premature infants. J. Pediatr. 128:660-669. [DOI] [PubMed] [Google Scholar]
- 349.Novack, C. M., F. Waffarn, J. H. Sills, T. J. Pousti, M. J. Warden, and M. D. Cunningham. 1994. Focal intestinal perforation in the extremely-low-birth-weight infant. J. Perinatol. 14:450-453. [PubMed] [Google Scholar]
- 350.Noya, F. J., M. A. Rench, T. G. Metzger, G. Colman, J. Naidoo, and C. J. Baker. 1987. Unusual occurrence of an epidemic of type Ib/c group B streptococcal sepsis in a neonatal intensive care unit. J. Infect. Dis. 155:1135-1144. [DOI] [PubMed] [Google Scholar]
- 351.Noyola, D. E., L. Bohra, E. A. Paysse, M. Fernandez, and D. K. Coats. 2002. Association of candidemia and retinopathy of prematurity in very low birthweight infants. Ophthalmology 109:80-84. [DOI] [PubMed] [Google Scholar]
- 352.Noyola, D. E., M. Fernandez, E. H. Moylett, and C. J. Baker. 2001. Ophthalmologic, visceral, and cardiac involvement in neonates with candidemia. Clin. Infect. Dis. 32:1018-1023. [DOI] [PubMed] [Google Scholar]
- 353.Nystrom, B. 1983. Optimal design/personnel for control of intensive care unit infection. Infect. Control 4:388-390. [DOI] [PubMed] [Google Scholar]
- 354.Odds, F. C. 1993. Resistance of yeasts to azole-derivative antifungals. J. Antimicrob. Chemother. 31:463-471. [DOI] [PubMed] [Google Scholar]
- 355.Oh, D., and D. Notrica. 2002. Primary cutaneous mucormycosis in infants and neonates: case report and review of the literature. J. Pediatr. Surg. 37:1607-1611. [DOI] [PubMed] [Google Scholar]
- 356.Ohlsson, A., and J. B. Lacy. 2001. Intravenous immunoglobulin for preventing infection in preterm and/or low-birth-weight infants. Cochrane Database Syst. Rev. CD000361. [DOI] [PubMed]
- 357.Ohlsson, A., and J. B. Lacy. 2001. Intravenous immunoglobulin for suspected or subsequently proven infection in neonates. Cochrane Database Syst. Rev. CD001239. [DOI] [PubMed]
- 358.Ostrowsky, B. E., W. E. Trick, A. H. Sohn, S. B. Quirk, S. Holt, L. A. Carson, B. C. Hill, M. J. Arduino, M. J. Kuehnert, and W. R. Jarvis. 2001. Control of vancomycin- resistant enterococcus in health care facilities in a region. N. Engl. J. Med. 344:1427-1433. [DOI] [PubMed] [Google Scholar]
- 359.Pacheco-Rios, A., C. Avila-Figueroa, D. Nobigrot-Kleinman, and J. I. Santos. 1997. Mortality associated with systemic candidiasis in children. Arch. Med. Res. 28:229-232. [PubMed] [Google Scholar]
- 360.Palmer, A. L., N. K. Leos, M. Hall, G. L. Jackson, and P. J. Sanchez. 1996. Evaluation of suprapubic bladder aspiration for detection of group B streptococcal antigen by latex agglutination in neonatal urine. Am. J. Perinatol. 13:235-239. [DOI] [PubMed] [Google Scholar]
- 361.Papasian, C. J., J. Kinney, S. Coffman, R. J. Hollis, and M. A. Pfaller. 1996. Transmission of Citrobacter koseri from mother to infant documented by ribotyping and pulsed-field gel electrophoresis. Diagn. Microbiol. Infect. Dis. 26:63-67. [DOI] [PubMed] [Google Scholar]
- 362.Pappu-Katikaneni, L. D., K. P. Rao, and E. Banister. 1990. Gastrointestinal colonization with yeast species and Candida septicemia in very low birth weight infants. Mycoses 33:20-23. [DOI] [PubMed] [Google Scholar]
- 363.Parry, M. F., J. H. Hutchinson, N. A. Brown, C. H. Wu, and L. Estreller. 1980. Gram-negative sepsis in neonates: a nursery outbreak due to hand carriage of Citrobacter diversus. Pediatrics 65:1105-1109. [PubMed] [Google Scholar]
- 364.Patrick, C. H., J. F. John, A. H. Levkoff, and L. M. Atkins. 1992. Relatedness of strains of methicillin-resistant coagulase-negative Staphylococcus colonizing hospital personnel and producing bacteremias in a neonatal intensive care unit. Pediatr. Infect. Dis. J. 11:935-940. [DOI] [PubMed] [Google Scholar]
- 365.Pearlman, S. A., S. Higgins, S. Eppes, A. M. Bhat, and J. D. Klein. 1998. Infective endocarditis in the premature neonate. Clin. Pediatr. 37:741-746. [DOI] [PubMed] [Google Scholar]
- 366.Pera, A., A. Byun, S. Gribar, R. Schwartz, D. Kumar, and P. Parimi. 2002. Dexamethasone therapy and Candida sepsis in neonates less than 1250 grams. J. Perinatol. 22:204-208. [DOI] [PubMed] [Google Scholar]
- 367.Perlman, J. M. 1998. White matter injury in the preterm infant: an important determination of abnormal neurodevelopment outcome. Early Hum. Dev. 53:99-120. [DOI] [PubMed] [Google Scholar]
- 368.Persson, E., B. Trollfors, L. L. Brandberg, and I. Tessin. 2002. Septicaemia and meningitis in neonates and during early infancy in the Goteborg area of Sweden. Acta Paediatr. 91:1087-1092. [DOI] [PubMed] [Google Scholar]
- 369.Pfaller, M. A. 1994. Epidemiology and control of fungal infections. Clin. Infect. Dis. 19(Suppl. 1):S8-S13. [DOI] [PubMed] [Google Scholar]
- 370.Pfaller, M. A. 1996. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin. Infect. Dis. 22(Suppl. 2):S89-S94. [DOI] [PubMed] [Google Scholar]
- 371.Pfaller, M. A., D. J. Diekema, R. N. Jones, S. A. Messer, and R. J. Hollis. 2002. Trends in antifungal susceptibility of Candida spp. isolated from pediatric and adult patients with bloodstream infections: SENTRY Antimicrobial Surveillance Program, 1997 to 2000. J. Clin. Microbiol. 40:852-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Pfaller, M. A., S. A. Messer, and R. J. Hollis. 1995. Variations in DNA subtype, antifungal susceptibility, and slime production among clinical isolates of Candida parapsilosis. Diagn. Microbiol. Infect. Dis. 21:9-14. [DOI] [PubMed] [Google Scholar]
- 373.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, G. V. Doern, M. E. Brandt, and R. A. Hajjeh. 1999. Trends in species distribution and susceptibility to fluconazole among blood stream isolates of Candida species in the United States. Diagn. Microbiol. Infect. Dis. 33:217-222. [DOI] [PubMed] [Google Scholar]
- 374.Philip, A. G., and J. R. Hewitt. 1980. Early diagnosis of neonatal sepsis. Pediatrics 65:1036-1041. [PubMed] [Google Scholar]
- 374a.Pickering, L. K. (ed.). 2003. Red book: 2003 report of the committee on infectious diseases, 26th ed. American Academy of Pediatrics, Elk Grove Village, Ill.
- 375.Plouffe, J. F., D. G. Brown, J. Silva, Jr., T. Eck, R. L. Stricof, and F. R. Fekety, Jr. 1977. Nosocomial outbreak of Candida parapsilosis fungemia related to intravenous infusions. Arch. Intern. Med. 137:1686-1689. [PubMed] [Google Scholar]
- 376.Polin, R. A., and L. Saiman. 2003. Nosocomial Infections in the Neonatal Intensive Care Unit. Neoreviews 4:e81-e88. [DOI] [PubMed] [Google Scholar]
- 376a.Polin, R. A., W. W. Fox, and S. H. Abman (ed.). 2004. Fetal and neonatal physiology, p. 1475-1674. The W. B. Saunders Co., Philadelphia, Pa
- 377.Posen, R., and R. A. deLemos. 1998. C-reactive protein levels in the extremely premature infant: case studies and literature review. J. Perinatol. 18:138-141. [PubMed] [Google Scholar]
- 378.Pourcyrous, M., H. S. Bada, S. B. Korones, V. Baselski, and S. P. Wong. 1993. Significance of serial C-reactive protein responses in neonatal infection and other disorders. Pediatrics 92:431-435. [PubMed] [Google Scholar]
- 379.Raad, I., R. Darouiche, J. Dupuis, D. Abi-Said, A. Gabrielli, R. Hachem, M. Wall, R. Harris, J. Jones, A. Buzaid, C. Robertson, S. Shenaq, P. Curling, T. Burke, C. Ericsson, and The Texas Medical Center Catheter Study Group. 1997. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. Ann. Intern. Med. 127:267-274. [DOI] [PubMed] [Google Scholar]
- 380.Raimundo, O., H. Heussler, J. B. Bruhn, S. Suntrarachun, N. Kelly, M. A. Deighton, and S. M. Garland. 2002. Molecular epidemiology of coagulase-negative staphylococcal bacteraemia in a newborn intensive care unit. J. Hosp. Infect. 51:33-42. [DOI] [PubMed] [Google Scholar]
- 381.Rangel-Frausto, M. S., T. Wiblin, H. M. Blumberg, L. Saiman, J. Patterson, M. Rinaldi, M. Pfaller, J. E. Edwards, Jr., W. Jarvis, J. Dawson, and R. P. Wenzel. 1999. National epidemiology of mycoses survey (NEMIS): variations in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin. Infect. Dis. 29:253-258. [DOI] [PubMed] [Google Scholar]
- 382.Rapkin, R. H. 1976. Pseudomonas cepacia in an intensive care nursery. Pediatrics 57:239-243. [PubMed] [Google Scholar]
- 383.Reboli, A. C., J. F. John, Jr., and A. H. Levkoff. 1989. Epidemic methicillin- gentamicin-resistant Staphylococcus aureus in a neonatal intensive care unit. Am. J. Dis. Child. 143:34-39. [DOI] [PubMed] [Google Scholar]
- 384.Redline, R. W., and B. B. Dahms. 1981. Malassezia pulmonary vasculitis in an infant on long-term Intralipid therapy. N. Engl. J. Med. 305:1395-1398. [DOI] [PubMed] [Google Scholar]
- 385.Regan, J. A., M. A. Klebanoff, R. P. Nugent, D. A. Eschenbach, W. C. Blackwelder, Y. Lou, R. S. Gibbs, P. J. Rettig, D. H. Martin, R. Edelman, and the VIP Study Group. 1996. Colonization with group B streptococci in pregnancy and adverse outcome. Am. J. Obstet. Gynecol. 174:1354-1360. [DOI] [PubMed] [Google Scholar]
- 386.Rex, J. H., T. J. Walsh, J. D. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, and J. E. Edwards. 2000. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin. Infect. Dis. 30:662-678. [DOI] [PubMed] [Google Scholar]
- 387.Rognum, T. O., S. Thrane, L. Stoltenberg, A. Vege, and P. Brandtzaeg. 1992. Development of intestinal mucosal immunity in fetal life and the first postnatal months. Pediatr. Res. 32:145-149. [DOI] [PubMed] [Google Scholar]
- 388.Roilides, E., E. Farmaki, J. Evdoridou, A. Francesconi, M. Kasai, J. Filioti, M. Tsivitanidou, D. Sofianou, G. Kremenopoulos, and T. J. Walsh. 2003. Candida tropicalis in a neonatal intensive care unit: epidemiologic and molecular analysis of an outbreak of infection with an uncommon neonatal pathogen. J. Clin. Microbiol. 41:735-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Roilides, E., A. Holmes, C. Blake, P. A. Pizzo, and T. J. Walsh. 1995. Effects of granulocyte colony-stimulating factor and interferon-gamma on antifungal activity of human polymorphonuclear neutrophils against pseudohyphae of different medically important Candida species. J. Leukoc. Biol 57:651-656. [DOI] [PubMed] [Google Scholar]
- 390.Romero, R., H. N. Winn, M. Wan, and J. C. Hobbins. 1988. Listeria monocytogenes chorioamnionitis and preterm labor. Am. J. Perinatol. 5:286-288. [DOI] [PubMed] [Google Scholar]
- 391.Ronnestad, A., T. G. Abrahamsen, P. Gaustad, and P. H. Finne. 1999. C-reactive protein (CRP) response patterns in neonatal septicaemia. APMIS 107:593-600. [DOI] [PubMed] [Google Scholar]
- 392.Rotbart, H. A., Z. T. Johnson, and L. B. Reller. 1989. Analysis of enteric coagulase- negative staphylococci from neonates with necrotizing enterocolitis. Pediatr. Infect. Dis. J. 8:140-142. [PubMed] [Google Scholar]
- 393.Rotimi, V. O., S. A. Olowe, and I. Ahmed. 1985. The development of bacterial flora of premature neonates. J. Hyg. 94:309-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Rowen, J. L., J. T. Atkins, M. L. Levy, S. C. Baer, and C. J. Baker. 1995. Invasive fungal dermatitis in the < or = 1000-gram neonate. Pediatrics 95:682-687. [PubMed] [Google Scholar]
- 395.Rowen, J. L., M. A. Rench, C. A. Kozinetz, J. M. Adams, Jr., and C. J. Baker. 1994. Endotracheal colonization with Candida enhances risk of systemic candidiasis in very low birth weight neonates. J. Pediatr. 124:789-794. [DOI] [PubMed] [Google Scholar]
- 396.Rowen, J. L., J. M. Tate, N. Nordoff, L. Passarell, and M. R. McGinnis. 1999. Candida isolates from neonates: frequency of misidentification and reduced fluconazole susceptibility. J. Clin. Microbiol. 37:3735-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Ruvalo, C. and C. R. Bauer. 1982. Intrauterinely acquired Pseudomonas infection in the neonate. Clin. Pediatr. 21:664-667. [DOI] [PubMed] [Google Scholar]
- 398.Saiman, L. 2002. Hospital-acquired infections in the neonatal intensive care unit. Introduction. Semin. Perinatol. 26:313-314. [DOI] [PubMed] [Google Scholar]
- 399.Saiman, L. 2002. Risk factors for hospital-acquired infections in the neonatal intensive care unit. Semin. Perinatol. 26:315-321. [DOI] [PubMed] [Google Scholar]
- 400.Saiman, L., K. Jakob, K. W. Holmes, S. Whittier, M. C. Garzon, J. V. Rago, P. M. Schlievert, and P. Della-Latta. 1998. Molecular epidemiology of staphylococcal scalded skin syndrome in premature infants. Pediatr. Infect. Dis. J. 17:329-334. [DOI] [PubMed] [Google Scholar]
- 401.Saiman, L., E. Ludington, J. D. Dawson, J. E. Patterson, S. Rangel-Frausto, R. T. Wiblin, H. M. Blumberg, M. Pfaller, M. Rinaldi, J. E. Edwards, R. P. Wenzel, and W. Jarvis. 2001. Risk factors for Candida species colonization of neonatal intensive care unit patients. Pediatr. Infect. Dis. J. 20:1119-1124. [DOI] [PubMed] [Google Scholar]
- 402.Saiman, L., E. Ludington, M. Pfaller, S. Rangel-Frausto, R. T. Wiblin, J. Dawson, H. M. Blumberg, J. E. Patterson, M. Rinaldi, J. E. Edwards, R. P. Wenzel, W. Jarvis, and The National Epidemiology of Mycosis Survey study group. 2000. Risk factors for candidemia in neonatal intensive care unit patients. Pediatr. Infect. Dis. J. 19:319-324. [DOI] [PubMed] [Google Scholar]
- 403.Samadi, R., A. Stek, and J. S. Greenspoon. 2001. Evaluation of a rapid optical immunoassay-based test for group B streptococcus colonization in intrapartum patients. J. Matern. Fetal Med. 10:203-208. [DOI] [PubMed] [Google Scholar]
- 404.Sanchez, P. J., J. D. Siegel, N. B. Cushion, and N. Threlkeld. 1990. Significance of a positive urine group B streptococcal latex agglutination test in neonates. J. Pediatr. 116:601-606. [DOI] [PubMed] [Google Scholar]
- 405.Sandford, G. R., W. G. Merz, J. R. Wingard, P. Charache, and R. Saral. 1980. The value of fungal surveillance cultures as predictors of systemic fungal infections. J. Infect. Dis. 142:503-509. [DOI] [PubMed] [Google Scholar]
- 406.Santulli, T. V., J. N. Schullinger, W. C. Heird, R. D. Gongaware, J. Wigger, B. Barlow, W. A. Blanc, and W. E. Berdon. 1975. Acute necrotizing enterocolitis in infancy: a review of 64 cases. Pediatrics 55:376-387. [PubMed] [Google Scholar]
- 407.Savey, A., J. Fleurette, and B. L. Salle. 1992. An analysis of the microbial flora of premature neonates. J. Hosp. Infect. 21:275-289. [DOI] [PubMed] [Google Scholar]
- 408.Saxen, H., K. Hoppu, and M. Pohjavuori. 1993. Pharmacokinetics of fluconazole in very low birth weight infants during the first two weeks of life. Clin. Pharmacol. Ther. 54: 269-277. [DOI] [PubMed] [Google Scholar]
- 409.Saxen, H., M. Virtanen, P. Carlson, K. Hoppu, M. Pohjavuori, M. Vaara, J. Vuopio-Varkila, and H. Peltola. 1995. Neonatal Candida parapsilosis outbreak with a high case fatality rate. Pediatr. Infect. Dis. J. 14:776-781. [DOI] [PubMed] [Google Scholar]
- 410.Schanler, R. J. 2001. The use of human milk for premature infants. Pediatr. Clin. North Am. 48:207-219. [DOI] [PubMed] [Google Scholar]
- 411.Scheffler, E., G. G. Miller, and D. A. Classen. 2003. Zygomycotic infection of the neonatal upper extremity. J. Pediatr. Surg. 38:E16-E17. [DOI] [PubMed] [Google Scholar]
- 412.Scheifele, D., and G. Bjornson. 1987. Staphylococcus epidermidis-associated enterocolitis. J. Pediatr. 110:330. [DOI] [PubMed] [Google Scholar]
- 413.Scheifele, D. W., G. L. Bjornson, R. A. Dyer, and J. E. Dimmick. 1987. Delta-like toxin produced by coagulase-negative staphylococci is associated with neonatal necrotizing enterocolitis. Infect. Immun. 55:2268-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Schelonka, R. L., and S. A. Moser. 2003. Time to positive culture results in neonatal Candida septicemia. J. Pediatr. 142:564-565. [DOI] [PubMed] [Google Scholar]
- 415.Schlech, W. F., III. 1991. Lowbury Lecture. Listeriosis: epidemiology, virulence and the significance of contaminated foodstuffs. J. Hosp. Infect. 19:211-224. [DOI] [PubMed] [Google Scholar]
- 416.Schlegel, R. J., and J. A. Bellanti. 1969. Increased susceptibility of males to infection. Lancet ii:826-827. [DOI] [PubMed] [Google Scholar]
- 417.Schmidt, B., P. Davis, D. Moddemann, A. Ohlsson, R. S. Roberts, S. Saigal, A. Solimano, M. Vincer, and L. L. Wright. 2001. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N. Engl. J. Med. 344:1966-1972. [DOI] [PubMed] [Google Scholar]
- 418.Schouten-Van Meeteren, N. Y., A. Rietveld, A. J. Moolenaar, and F. Van Bel. 1992. Influence of perinatal conditions on C-reactive protein production. J. Pediatr. 120:621-624. [DOI] [PubMed] [Google Scholar]
- 419.Schrag, S., R. Gorwitz, K. Fultz-Butts, and A. Schuchat. 2002. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. Morb. Mortal. Wkly. Rap. Recomm. Rep. 51:1-22. [PubMed] [Google Scholar]
- 420.Schroeder, H. W., Jr., F. Mortari, S. Shiokawa, P. M. Kirkham, R. A. Elgavish, and F. E. Bertrand III. 1995. Developmental regulation of the human antibody repertoire. Ann. N. Y. Acad. Sci. 764:242-260. [DOI] [PubMed] [Google Scholar]
- 421.Schuchat, A. 1998. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin. Microbiol. Rev. 11:497-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Schuchat, A., C. Lizano, C. V. Broome, B. Swaminathan, C. Kim, and K. Winn. 1991. Outbreak of neonatal listeriosis associated with mineral oil. Pediatr. Infect. Dis. J. 10:183-189. [DOI] [PubMed] [Google Scholar]
- 423.Schuster, D. P., M. Metzler, S. Opal, S. Lowry, R. Balk, E. Abraham, H. Levy, G. Slotman, E. Coyne, S. Souza, and J. Pribble. 2003. Recombinant platelet-activating factor acetylhydrolase to prevent acute respiratory distress syndrome and mortality in severe sepsis: phase IIb, multicenter, randomized, placebo-controlled, clinical trial. Crit. Care Med. 31:1612-1619. [DOI] [PubMed] [Google Scholar]
- 424.Schwalbe, R. S., J. T. Stapleton, and P. H. Gilligan. 1987. Emergence of vancomycin resistance in coagulase-negative staphylococci. N. Engl. J. Med. 316:927-931. [DOI] [PubMed] [Google Scholar]
- 425.Segal, E., A. Soroka, and A. Schechter. 1984. Correlative relationship between adherence of Candida albicans to human vaginal epithelial cells in vitro and candidal vaginitis. Sabouraudia 22:191-200. [PubMed] [Google Scholar]
- 426.Seibert, K., V. Y. Yu, J. C. Doery, and D. Embury. 1990. The value of C-reactive protein measurement in the diagnosis of neonatal infection. J. Paediatr. Child Health 26:267-270. [DOI] [PubMed] [Google Scholar]
- 427.Senerwa, D., O. Olsvik, L. N. Mutanda, K. J. Lindqvist, J. M. Gathuma, K. Fossum, and K. Wachsmuth. 1989. Enteropathogenic Escherichia coli serotype O111:HNT isolated from preterm neonates in Nairobi, Kenya. J. Clin. Microbiol. 27:1307-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Seri, I., R. Tan, and J. Evans. 2001. Cardiovascular effects of hydrocortisone in preterm infants with pressor-resistant hypotension. Pediatrics 107:1070-1074. [DOI] [PubMed] [Google Scholar]
- 429.Shah, G. K., J. Vander, and R. C. Eagle. 2000. Intralenticular Candida species abscess in a premature infant. Am. J. Ophthalmol. 129:390-391. [DOI] [PubMed] [Google Scholar]
- 430.Shah, S. S., and P. G. Gallagher. 1998. Complications of conjunctivitis caused by Pseudomonas aeruginosa in a newborn intensive care unit. Pediatr. Infect. Dis. J. 17:97-102. [DOI] [PubMed] [Google Scholar]
- 431.Shang, S., Z. Chen, and X. Yu. 2001. Detection of bacterial DNA by PCR and reverse hybridization in the 16S rRNA gene with particular reference to neonatal septicemia. Acta Paediatr. 90:179-183. [DOI] [PubMed] [Google Scholar]
- 432.Sharma, R., M. L. Hudak, B. R. Premachandra, G. Stevens, C. B. Monteiro, J. A. Bradshaw, A. M. Kaunitz, and R. A. Hollister. 2002. Clinical manifestations of rotavirus infection in the neonatal intensive care unit. Pediatr. Infect. Dis. J. 21:1099-1105. [DOI] [PubMed] [Google Scholar]
- 433.Sherertz, R. J., K. S. Gledhill, K. D. Hampton, M. A. Pfaller, L. B. Givner, J. S. Abramson, and R. G. Dillard. 1992. Outbreak of Candida bloodstream infections associated with retrograde medication administration in a neonatal intensive care unit. J. Pediatr. 120:455-461. [DOI] [PubMed] [Google Scholar]
- 434.Shinefield, H., S. Black, A. Fattom, G. Horwith, S. Rasgon, J. Ordonez, H. Yeoh, D. Law, J. B. Robbins, R. Schneerson, L. Muenz, S. Fuller, J. Johnson, B. Fireman, H. Alcorn, and R. Naso. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346:491-496. [DOI] [PubMed] [Google Scholar]
- 435.Shiro, H., G. Meluleni, A. Groll, E. Muller, T. D. Tosteson, D. A. Goldmann, and G. B. Pier. 1995. The pathogenic role of Staphylococcus epidermidis capsular polysaccharide/adhesin in a low-inoculum rabbit model of prosthetic valve endocarditis. Circulation 92:2715-2722. [DOI] [PubMed] [Google Scholar]
- 436.Silverman, J., J. A. Vazquez, J. D. Sobel, and M. J. Zervos. 1999. Comparative in vitro activity of antiseptics and disinfectants versus clinical isolates of Candida species. Infect. Control Hosp. Epidemiol. 20:676-684. [DOI] [PubMed] [Google Scholar]
- 437.Simchen, M. J., M. E. Beiner, N. Strauss-Liviathan, M. Dulitzky, J. Kuint, S. Mashiach, and E. Schiff. 2000. Neonatal outcome in growth-restricted versus appropriately grown preterm infants. Am. J. Perinatol. 17:187-192. [DOI] [PubMed] [Google Scholar]
- 438.Sims, M. E., Y. Yoo, H. You, C. Salminen, and F. J. Walther. 1988. Prophylactic oral nystatin and fungal infections in very-low-birthweight infants. Am. J. Perinatol. 5:33-36. [DOI] [PubMed] [Google Scholar]
- 439.Singh, N., K. M. Patel, M. M. Leger, B. Short, B. M. Sprague, N. Kalu, and J. M. Campos. 2002. Risk of resistant infections with Enterobacteriaceae in hospitalized neonates. Pediatr. Infect. Dis. J. 21:1029-1033. [DOI] [PubMed] [Google Scholar]
- 440.Sloos, J. H., A. M. Horrevorts, C. P. Van Boven, and L. Dijkshoorn. 1998. Identification of multiresistant Staphylococcus epidermidis in neonates of a secondary care hospital using pulsed field gel electrophoresis and quantitative antibiogram typing. J. Clin. Pathol. 51:62-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Sohn, A. H., D. O. Garrett, R. L. Sinkowitz-Cochran, L. A. Grohskopf, G. L. Levine, B. H. Stover, J. D. Siegel, and W. R. Jarvis. 2001. Prevalence of nosocomial infections in neonatal intensive care unit patients: results from the first national point-prevalence survey. J. Pediatr. 139:821-827. [DOI] [PubMed] [Google Scholar]
- 442.Speer, M. E., L. H. Taber, M. D. Yow, A. J. Rudolph, J. Urteaga, and S. Waller. 1976. Fulminant neonatal sepsis and necrotizing enterocolitis associated with a “nonenteropathogenic” strain of Escherichia coli. J. Pediatr. 89:91-95. [DOI] [PubMed] [Google Scholar]
- 443.Sperling, R. S., E. Newton, and R. S. Gibbs. 1988. Intraamniotic infection in low-birth- weight infants. J. Infect. Dis. 157:113-117. [DOI] [PubMed] [Google Scholar]
- 444.Spigelblatt, L., J. Saintonge, R. Chicoine, and M. Laverdiere. 1985. Changing pattern of neonatal streptococcal septicemia. Pediatr. Infect. Dis. J. 4:56-58. [DOI] [PubMed] [Google Scholar]
- 445.Spitzer, J. A. 1999. Gender differences in some host defense mechanisms. Lupus 8:380-383. [DOI] [PubMed] [Google Scholar]
- 446.Splawski, J. B., D. F. Jelinek, and P. E. Lipsky. 1991. Delineation of the functional capacity of human neonatal lymphocytes. J. Clin. Investig. 87:545-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Stamos, J. K. and A. H. Rowley. 1995. Candidemia in a pediatric population. Clin. Infect. Dis. 20:571-575. [DOI] [PubMed] [Google Scholar]
- 448.Stewart, R. M., T. C. Fabian, M. J. Fabian, L. L. Trenthem, F. E. Pritchard, M. A. Croce, and K. G. Proctor. 1995. Gastric and extragastric actions of the histamine antagonist ranitidine during posttraumatic sepsis. Surgery 117:68-82. [DOI] [PubMed] [Google Scholar]
- 449.Stoll, B. J., and A. Fanaroff. 1995. Early-onset coagulase-negative staphylococcal sepsis in preterm neonate. National Institute of Child Health and Human Development (NICHD) Neonatal Research Network. Lancet 345:1236-1237. [DOI] [PubMed] [Google Scholar]
- 450.Stoll, B. J., T. Gordon, S. B. Korones, S. Shankaran, J. E. Tyson, C. R. Bauer, A. A. Fanaroff, J. A. Lemons, E. F. Donovan, W. Oh, D. K. Stevenson, R. A. Ehrenkranz, L. A. Papile, J. Verter, and L. L. Wright. 1996. Early-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J. Pediatr. 129:72-80. [DOI] [PubMed] [Google Scholar]
- 451.Stoll, B. J., T. Gordon, S. B. Korones, S. Shankaran, J. E. Tyson, C. R. Bauer, A. A. Fanaroff, J. A. Lemons, E. F. Donovan, W. Oh, D. K. Stevenson, R. A. Ehrenkranz, L. A. Papile, J. Verter, and L. L. Wright. 1996. Late-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J. Pediatr. 129:63-71. [DOI] [PubMed] [Google Scholar]
- 452.Stoll, B. J., and N. Hansen. 2003. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin. Perinatol. 27:293-301. [DOI] [PubMed] [Google Scholar]
- 453.Stoll, B. J., N. Hansen, A. A. Fanaroff, L. L. Wright, W. A. Carlo, R. A. Ehrenkranz, J. A. Lemons, E. F. Donovan, A. R. Stark, J. E. Tyson, W. Oh, C. R. Bauer, S. B. Korones, S. Shankaran, A. R. Laptook, D. K. Stevenson, L. A. Papile, and W. K. Poole. 2002. Changes in pathogens causing early-onset sepsis in very-low- birth-weight infants. N. Engl. J. Med. 347:240-247. [DOI] [PubMed] [Google Scholar]
- 454.Stoll, B. J., N. Hansen, A. A. Fanaroff, L. L. Wright, W. A. Carlo, R. A. Ehrenkranz, J. A. Lemons, E. F. Donovan, A. R. Stark, J. E. Tyson, W. Oh, C. R. Bauer, S. B. Korones, S. Shankaran, A. R. Laptook, D. K. Stevenson, L. A. Papile, and W. K. Poole. 2002. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110:285-291. [DOI] [PubMed] [Google Scholar]
- 454a.Stoll, B. J., N. Hansen, A. A. Fanaroff, L. L. Wright, W. A. Carlo, R. A. Ehrenkranz, J. A. Lemons, E. F. Donovan, A. R. Stark, J. E. Tyson, W. Oh, C. R. Bauer, S. B. Korones, S. Shankaran, A. R. Laptook, D. K. Stevenson, L. A. Papile, and W. K. Poole. 2004. To tap or not to tap: high likelihood of meningitis without sepsis among very low birth weight infants. Pediatrics 113:1181-1186. [DOI] [PubMed] [Google Scholar]
- 455.Stoll, B. J., M. Temprosa, J. E. Tyson, L. A. Papile, L. L. Wright, C. R. Bauer, E. F. Donovan, S. B. Korones, J. A. Lemons, A. A. Fanaroff, D. K. Stevenson, W. Oh, R. A. Ehrenkranz, S. Shankaran, and J. Verter. 1999. Dexamethasone therapy increases infection in very low birth weight infants. Pediatrics 104:e63. [DOI] [PubMed]
- 456.Reference deleted.
- 457.Stover, B. H., S. T. Shulman, D. F. Bratcher, M. T. Brady, G. L. Levine, and W. R. Jarvis. 2001. Nosocomial infection rates in US children's hospitals' neonatal and pediatric intensive care units. Am. J. Infect. Control 29:152-157. [DOI] [PubMed] [Google Scholar]
- 458.Struthers, S., H. Underhill, S. Albersheim, D. Greenberg, and S. Dobson. 2002. A comparison of two versus one blood culture in the diagnosis and treatment of coagulase- negative staphylococcus in the neonatal intensive care unit. J. Perinatol. 22:547-549. [DOI] [PubMed] [Google Scholar]
- 459.Stuart, S. M. and A. T. Lane. 1992. Candida and Malassezia as nursery pathogens. Semin. Dermatol. 11:19-23. [PubMed] [Google Scholar]
- 460.Surmont, I., A. Gavilanes, J. Vandepitte, H. Devlieger, and E. Eggermont. 1989. Malassezia furfur fungaemia in infants receiving intravenous lipid emulsions. A rarity or just underestimated? Eur. J. Pediatr. 148:435-438. [DOI] [PubMed] [Google Scholar]
- 461.Symchych, P. S., A. N. Krauss, and P. Winchester. 1977. Endocarditis following intracardiac placement of umbilical venous catheters in neonates. J. Pediatr. 90:287-289. [DOI] [PubMed] [Google Scholar]
- 462.Takahashi, N., H. Nishida, H. Kato, K. Imanishi, Y. Sakata, and T. Uchiyama. 1998. Exanthematous disease induced by toxic shock syndrome toxin 1 in the early neonatal period. Lancet 351:1614-1619. [DOI] [PubMed] [Google Scholar]
- 463.Tam, A. L., A. Camberos, and H. Applebaum. 2002. Surgical decision making in necrotizing enterocolitis and focal intestinal perforation: predictive value of radiologic findings. J. Pediatr. Surg. 37:1688-1691. [DOI] [PubMed] [Google Scholar]
- 464.Tamim, M. M., H. Alesseh, and H. Aziz. 2003. Analysis of the efficacy of urine culture as part of sepsis evaluation in the premature infant. Pediatr. Infect. Dis. J. 22:805-808. [DOI] [PubMed] [Google Scholar]
- 465.Tan, T. Q., E. O. Mason, Jr., C. N. Ou, and S. L. Kaplan. 1993. Use of intravenous rifampin in neonates with persistent staphylococcal bacteremia. Antimicrob. Agents Chemother. 37:2401-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Tan, X. D., H. Chang, X. W. Qu, M. Caplan, F. Gonzalez-Crussi, and W. Hsueh. 2000. Platelet-activating factor increases mucosal permeability in rat intestine via tyrosine phosphorylation of E-cadherin. Br. J. Pharmacol. 129:1522-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Thron, A., and H. Wietholter. 1982. Cerebral candidiasis: CT studies in a case of brain abscess and granuloma due to Candida albicans. Neuroradiology 23:223-225. [DOI] [PubMed] [Google Scholar]
- 468.Tirodker, U. H., J. P. Nataro, S. Smith, L. LasCasas, and K. D. Fairchild. 2003. Detection of fungemia by polymerase chain reaction in critically ill neonates and children. J. Perinatol. 23:117-122. [DOI] [PubMed] [Google Scholar]
- 469.Tojo, M., N. Yamashita, D. A. Goldmann, and G. B. Pier. 1988. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J. Infect. Dis. 157:713-722. [DOI] [PubMed] [Google Scholar]
- 470.Toledano, H., Y. Schlesinger, D. Raveh, B. Rudensky, D. Attias, A. I. Eidelman, and A. M. Yinnon. 2000. Prospective surveillance of vancomycin-resistant enterococci in a neonatal intensive care unit. Eur. J. Clin. Microbiol. Infect. Dis. 19:282-287. [DOI] [PubMed] [Google Scholar]
- 471.Toltzis, P., M. J. Dul, C. Hoyen, A. Salvator, M. Walsh, L. Zetts, and H. Toltzis. 2001. Molecular epidemiology of antibiotic-resistant gram-negative bacilli in a neonatal intensive care unit during a nonoutbreak period. Pediatrics 108:1143-1148. [DOI] [PubMed] [Google Scholar]
- 472.Tomashefski, J. F., Jr., and C. R. Abramowsky. 1981. Candida-associated renal papillary necrosis. Am. J. Clin. Pathol. 75:190-194. [DOI] [PubMed] [Google Scholar]
- 473.Towers, C. V., M. H. Carr, G. Padilla, and T. Asrat. 1998. Potential consequences of widespread antepartal use of ampicillin. Am. J. Obstet. Gynecol. 179:879-883. [DOI] [PubMed] [Google Scholar]
- 474.Townsend, S. M., H. A. Pollack, I. Gonzalez-Gomez, H. Shimada, and J. L. Badger. 2003. Citrobacter koseri brain abscess in the neonatal rat: survival and replication within human and rat macrophages. Infect. Immun. 71:5871-5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Tsao, M. M., and D. Katz. 1984. Central venous catheter-induced endocarditis: human correlate of the animal experimental model of endocarditis. Rev. Infect. Dis. 6:783-790. [DOI] [PubMed] [Google Scholar]
- 476.Uauy, R. D., A. A. Fanaroff, S. B. Korones, E. A. Phillips, J. B. Phillips, and L. L. Wright. 1991. Necrotizing enterocolitis in very low birth weight infants: biodemographic and clinical correlates. National Institute of Child Health and Human Development Neonatal Research Network. J. Pediatr. 119:630-638. [DOI] [PubMed] [Google Scholar]
- 477.Usmani, S. S., R. G. Harper, and S. F. Usmani. 1988. Effect of a lipid emulsion (Intralipid) on polymorphonuclear leukocyte functions in the neonate. J. Pediatr. 113:132-136. [DOI] [PubMed] [Google Scholar]
- 478.van Acker, J., F. de Smet, G. Muyldermans, A. Bougatef, A. Naessens, and S. Lauwers. 2001. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J. Clin. Microbiol. 39:293-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Van Der Zwet, W. C., Y. J. Debets-Ossenkopp, E. Reinders, M. Kapi, P. H. Savelkoul, R. M. Van Elburg, K. Hiramatsu, and C. M. Vandenbroucke-Grauls. 2002. Nosocomial spread of a Staphylococcus capitis strain with heteroresistance to vancomycin in a neonatal intensive care unit. J. Clin. Microbiol. 40:2520-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.van Deventer, A. J., W. H. Goessens, A. van Belkum, H. J. van Vliet, E. W. van Etten, and H. A. Verbrugh. 1995. Improved detection of Candida albicans by PCR in blood of neutropenic mice with systemic candidiasis. J. Clin. Microbiol. 33:625-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Vasilakis, A., and K. N. Apelgren. 1988. Answering the fat emulsion contamination question: three in one admixture vs conventional total parenteral nutrition in a clinical setting. J. Parenter. Enteral Nutr. 12:356-359. [DOI] [PubMed] [Google Scholar]
- 482.Veach, L. A., M. A. Pfaller, M. Barrett, F. P. Koontz, and R. P. Wenzel. 1990. Vancomycin resistance in Staphylococcus haemolyticus causing colonization and bloodstream infection. J. Clin. Microbiol. 28:2064-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Venezia, R. A., F. J. Scarano, K. E. Preston, L. M. Steele, T. P. Root, R. Limberger, W. Archinal, and M. A. Kacica. 1995. Molecular epidemiology of an SHV-5 extended- spectrum beta-lactamase in Enterobacteriaceae isolated from infants in a neonatal intensive care unit. Clin. Infect. Dis 21:915-923. [DOI] [PubMed] [Google Scholar]
- 484.Reference deleted.
- 485.Vermont, C. L., N. G. Hartwig, A. Fleer, P. de Man, H. Verbrugh, A. J. van den, R. de Groot, and A. van Belkum. 1998. Persistence of clones of coagulase-negative staphylococci among premature neonates in neonatal intensive care units: two-center study of bacterial genotyping and patient risk factors. J. Clin. Microbiol. 36:2485-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Villari, P., C. Sarnataro, and L. Iacuzio. 2000. Molecular epidemiology of Staphylococcus epidermidis in a neonatal intensive care unit over a three-year period. J. Clin. Microbiol. 38:1740-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Wagle, S., A. Grauaug, R. Kohan, and S. F. Evans. 1994. C-reactive protein as a diagnostic tool of sepsis in very immature babies. J. Paediatr. Child Health 30:40-44. [DOI] [PubMed] [Google Scholar]
- 488.Wainer, S., P. A. Cooper, E. Funk, R. Y. Bental, D. A. Sandler, and J. Patel. 1992. Prophylactic miconazole oral gel for the prevention of neonatal fungal rectal colonization and systemic infection. Pediatr. Infect. Dis. J. 11:713-716. [DOI] [PubMed] [Google Scholar]
- 489.Wainer, S., P. A. Cooper, H. Gouws, and A. Akierman. 1997. Prospective study of fluconazole therapy in systemic neonatal fungal infection. Pediatr. Infect. Dis. J. 16:763-767. [DOI] [PubMed] [Google Scholar]
- 490.Wang, L. W., C. H. Lin, C. C. Liu, and Y. J. Lin. 1996. Systemic fungal infection in very low-birth-weight infants. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 37:272-277. [PubMed] [Google Scholar]
- 491.Warris, A., B. A. Semmekrot, and A. Voss. 2001. Candidal and bacterial bloodstream infections in premature neonates: a case-control study. Med. Mycol. 39:75-79. [DOI] [PubMed] [Google Scholar]
- 492.Wasunna, A., A. Whitelaw, R. Gallimore, P. N. Hawkins, and M. B. Pepys. 1990. C- reactive protein and bacterial infection in preterm infants. Eur. J. Pediatr. 149:424-427. [DOI] [PubMed] [Google Scholar]
- 493.Watterberg, K. L. 2002. Adrenal insufficiency and cardiac dysfunction in the preterm infant. Pediatr. Res. 51:422-424. [DOI] [PubMed] [Google Scholar]
- 494.Weems, J. J., Jr. 1992. Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations, and antimicrobial susceptibility. Clin. Infect. Dis. 14:756-766. [DOI] [PubMed] [Google Scholar]
- 495.Weir, E. 2002. Powdered infant formula and fatal infection with Enterobacter sakazakii. CMAJ. 166:1570. [PMC free article] [PubMed] [Google Scholar]
- 496.Weisman, L. E., B. F. Anthony, V. G. Hemming, and G. W. Fischer. 1993. Comparison of group B streptococcal hyperimmune globulin and standard intravenously administered immune globulin in neonates. J. Pediatr. 122:929-937. [DOI] [PubMed] [Google Scholar]
- 497.Weiss, M. G., S. P. Ionides, and C. L. Anderson. 1991. Meningitis in premature infants with respiratory distress: role of admission lumbar puncture. J. Pediatr. 119:973-975. [DOI] [PubMed] [Google Scholar]
- 498.Weitkamp, J. H., C. F. Poets, R. Sievers, E. Musswessels, P. Groneck, P. Thomas, and P. Bartmann. 1998. Candida infection in very low birth-weight infants: outcome and nephrotoxicity of treatment with liposomal amphotericin B (AmBisome). Infection 26:11-15. [DOI] [PubMed] [Google Scholar]
- 499.Wells, L. R., L. A. Papile, M. O. Gardner, C. R. Hartenberger, and L. Merker. 1999. Impact of antenatal corticosteroid therapy in very low birth weight infants on chronic lung disease and other morbidities of prematurity. J. Perinatol. 19:578-581. [DOI] [PubMed] [Google Scholar]
- 500.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Whyte, R. K., Z. Hussain, and D. deSa. 1982. Antenatal infections with Candida species. Arch. Dis. Child. 57:528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Williamson, M., S. H. Fraser, and M. Tilse. 1995. Failure of the urinary group B streptococcal antigen test as a screen for neonatal sepsis. Arch. Dis. Child. Fetal Neonatal Ed. 73:F109-F111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Willoughby, R. E., Jr., and K. B. Nelson. 2002. Chorioamnionitis and brain injury. Clin. Perinatol. 29:603-621. [DOI] [PubMed] [Google Scholar]
- 504.Wilson, C. B., and D. B. Lewis. 1990. Basis and implications of selectively diminished cytokine production in neonatal susceptibility to infection. Rev. Infect. Dis. 12(Suppl. 4):S410-S420. [DOI] [PubMed] [Google Scholar]
- 505.Wingard, J. R., J. D. Dick, W. G. Merz, G. R. Sandford, R. Saral, and W. H. Burns. 1980. Pathogenicity of Candida tropicalis and Candida albicans after gastrointestinal inoculation in mice. Infect. Immun. 29:808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Wingard, J. R., J. D. Dick, W. G. Merz, G. R. Sandford, R. Saral, and W. H. Burns. 1982. Differences in virulence of clinical isolates of Candida tropicalis and Candida albicans in mice. Infect. Immun. 37:833-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Wright, L. L., J. Verter, N. Younes, D. Stevenson, A. A. Fanaroff, S. Shankaran, R. A. Ehrenkranz, and E. F. Donovan. 1995. Antenatal corticosteroid administration and neonatal outcome in very low birth weight infants: the NICHD Neonatal Research Network. Am. J. Obstet. Gynecol. 173:269-274. [DOI] [PubMed] [Google Scholar]
- 508.Wu, Y. W., G. J. Escobar, J. K. Grether, L. A. Croen, J. D. Greene, and T. B. Newman. 2003. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA 290:2677-2684. [DOI] [PubMed] [Google Scholar]
- 509.Yagupsky, P., M. A. Menegus, and K. R. Powell. 1991. The changing spectrum of group B streptococcal disease in infants: an eleven-year experience in a tertiary care hospital. Pediatr. Infect. Dis. J. 10:801-808. [DOI] [PubMed] [Google Scholar]
- 510.Yamaki, K., H. Thorlacius, X. Xie, L. Lindbom, P. Hedqvist, and J. Raud. 1998. Characteristics of histamine-induced leukocyte rolling in the undisturbed microcirculation of the rat mesentery. Br. J. Pharmacol. 123:390-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Yeh, T. F., Y. J. Lin, W. S. Hsieh, H. C. Lin, C. H. Lin, J. Y. Chen, H. A. Kao, and C. H. Chien. 1997. Early postnatal dexamethasone therapy for the prevention of chronic lung disease in preterm infants with respiratory distress syndrome: a multicenter clinical trial. Pediatrics 100:E3. [DOI] [PubMed] [Google Scholar]
- 512.Yoon, B. H., R. Romero, C. J. Kim, J. N. Koo, G. Choe, H. C. Syn, and J. G. Chi. 1997. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am. J. Obstet. Gynecol. 177:406-411. [DOI] [PubMed] [Google Scholar]