Abstract
There is now wide acceptance of the concept that the similarity between many acute infectious diseases, be they viral, bacterial, or parasitic in origin, is caused by the overproduction of inflammatory cytokines initiated when the organism interacts with the innate immune system. This is also true of certain noninfectious states, such as the tissue injury syndromes. This review discusses the historical origins of these ideas, which began with tumor necrosis factor (TNF) and spread from their origins in malaria research to other fields. As well the more established proinflammatory mediators, such as TNF, interleukin-1, and lymphotoxin, the roles of nitric oxide and carbon monoxide, which are chiefly inhibitory, are discussed. The established and potential roles of two more recently recognized contributors, overactivity of the enzyme poly(ADP-ribose) polymerase 1 (PARP-1) and the escape of high-mobility-group box 1 (HMGB1) protein from its normal location into the circulation, are also put in context. The pathogenesis of the disease caused by falciparum malaria is then considered in the light of what has been learned about the roles of these mediators in these other diseases, as well as in malaria itself.
Four species of malarial parasite, members of a genus of protozoa within the suborder Haemosporidiidea, infect humans, and all are spread by female Anopheles mosquitoes. In practice, only one of these parasites, Plasmodium falciparum, causes fatal disease. It is the organism targeted in attempts to develop a malarial vaccine and is also the focus of research on antimalarial drug resistance. Therefore, references to malaria in this review are to falciparum malaria. Malaria stands out among the systemic infectious diseases of humans for many reasons, apart from the sheer scale of the problems it causes (344). Although it has been recognized as an entity for thousands of years, it was still thought to be noninfectious long after Pasteur had demonstrated that a number of other major diseases were caused by bacteria, and knowledge of its life cycle was not complete until 1951 (370). The relatively large P. falciparum is easy to locate within blood vessels in fixed tissue sections, and from the late 19th century (40), this characteristic allowed a plausible explanation of falciparum malarial disease, particularly its coma, in terms of poor oxygen delivery through partly obstructed blood vessels. This appeared to give malarial disease a unique pathogenesis, still with some currency today.
It is our view that focusing on malaria in isolation will never provide the insights required to understand the pathogenesis of this disease. How can the illnesses caused by a spirochete and a virus be so clinically identical (184), typhoid readily diagnosed as malaria (296), and malaria in returning travelers so commonly dismissed as influenza? Why do former military personnel with much personal experience of malaria invariably believe that the onset of brucellosis or Q fever is a relapse of malaria when they first seek out medical advice? An adequate explanation of malaria disease has to answer these questions. Understanding why these clinical confusions occur entails appreciating the sequence of events that led up to the cytokine revolution that has transformed this field over the last 15 years. To explain this adequately, we must outline the pathogenesis of these clinically overlapping systemic diseases caused by infectious agents other than malaria, as well as that of conditions such as burns, trauma, hemorrhagic shock, snakebite and heatstroke, which are not caused by specific microorganisms but which apparently have a similar molecular pathogenesis. An additional layer of complexity is provided by the fact that these molecules, often referred to as the proinflammatory cytokines, are also the basis of much cell-mediated immunity against infectious agents. We only touch on the recognition end of the spectrum of events that leads to the mediators of innate immunity and disease, which was comprehensively reviewed for bacterial infections recently in this journal (420). Instead, we concentrate on the current state of knowledge at the effector end of the process, where disease is actually generated. The pathogenesis of the more clinical aspects of falciparum malaria, such as hyperlactatemia, metabolic acidosis, hypoglycemia, respiratory distress, impaired consciousness, and anemia, has been reviewed recently (91).
CYTOKINE THEORY OF DISEASE
The Pre-rTNF Era
To our knowledge, Paul Cannon was the first to argue, in 1941, that the diseases caused by malaria and bacterial infections would prove to be governed by the same pathologic principles (63). Later that decade, Brian Maegraith, quoting Cannon, reasoned that a range of infectious agents, including Plasmodium, probably all caused disease by generating acute general inflammation (p. 367 of reference 253). The opinion that infectious organisms do not harm us directly, but do so indirectly through our response to them, was nicely put by Lewis Thomas in an erudite 1972 essay (410). But it was an essay of its time, about complement activation, epinephrine, and lysozymal enzymes, not the soluble mediators we would now call proinflammatory cytokines. These, now so numerous as to be grouped in sets of families, had already begun to be described by this time, with the advent of macrophage migration inhibitory factor (MIF) (42, 113), lymphotoxin (LT) (342), and tumor necrosis factor (TNF) (66). However, no link was made between these mediators and systemic disease pathogenesis or protection against pathogenic organisms, but they were linked to delayed hypersensitivity (42, 113, 342) and protection against tumors by bacterial immunostimulants (66). The newly described roles for MIF, and its detection in human sepsis and malaria tissues, has been extensively reviewed recently (91).
TNF acquired its name because Carswell et al. (66) had collected serum 2 h after mice (infected 14 to 21 days earlier with live BCG, the bacillus Calmette-Guérin strain of Mycobacterium tuberculosis) had received an injection of gram-negative enterobacterial wall endotoxin, i.e., lipopolysaccharide (LPS), and found that this serum, and defined fractions of it, caused necrosis of established tumors in other mice. A little earlier, it had been observed that mouse hemoprotozoa died inside circulating red cells in undisturbed infections at higher parasite densities (96) and at very low parasite densities, in the absence of a parasite-specific antibody, in mice that had been infected with BCG (85).
This implied the activity of a novel soluble circulating mediator. TNF was plausibly involved. First, the interval between BCG and infection with hemoprotozoa was the same as the Sloan-Kettering group had used before administering LPS (66), and second, other protectants against mouse hemoprotozoa (80, 81, 85, 93), all acknowledged macrophage activators, were all known to protect against tumors. Some had been shown to prime for TNF generation. Moreover, the ability of these agents to protect against hemoprotozoa could be predicted by how well they sensitized mice to LPS (81). A collaboration with the original laboratory studying TNF showed that TNF-rich serum fractions would indeed inhibit malaria parasite growth in vivo (99), that malaria infection would replace BCG as a primer for TNF production (99), and that the ability of a priming agent in our system paralleled its effectiveness, at the same dose, to prime for TNF generation (E. A. Carswell, personal communication). In addition, interleukin-1 (IL-1) (then called lymphocyte-activating factor) was present in the TNF-rich fractions, and roles for it in malaria were proposed (99). Thus developed the argument that TNF and similar mediators were involved in the host response to malaria parasites, a field previously the province of antibody-based mechanisms. TNF had no previously proposed role in immunity against any infectious agent—nor, indeed, in disease pathogenesis.
This work on malaria was confirmed and considerably extended by researchers at the Middlesex Hospital in London, who were the first to show that LPS induces serum factors that kill malaria parasites in vitro (400). They also showed, by using murine models, that TNF-rich serum would kill Plasmodium yoelii, a non-lethal parasite; but not Plasmodium berghei, one of the parasites lethal to mice (399), a pattern that corresponded to that found in the original BCG protection experiments (85).
During these studies, we observed many parallels between the pathology caused by enterobacterial LPS and malaria infection in mice (79), a similarity (the LPS source being the typhoid vaccine of 1945) previously noted for human malarial illness (p. 345 of reference 253). The late 1970s was the era in which it was being realized that the many host responses to LPS, including its capacity to induce illness and pathology, were not caused by the direct effects of this molecule but instead were caused indirectly, by inducing host cells to release soluble mediators. To our knowledge, this was first demonstrated (in Joe Berry's laboratory in Austin, Tex.) by showing that C3H/HeJ mice, while refractory to the ability of LPS to block gluconeogenesis, were susceptible to the transfer of this activity via peritoneal exudate cell supernatants from wild-type mice exposed to LPS (285, 286). A few years later, the idea of the effects of LPS in mammals being mediated by soluble factors generated by host cells had become a widely accepted principle (273). Thus, it was reasoned that this group of cytokines would explain much of the illness and pathology of malaria, the Jarisch-Herxheimer syndrome (discussed below), and the clinical similarity between malaria and typhoid (99). This seemed inevitable for this disease and other infections caused by gram-negative bacteria, since in the 1970s the Sloan-Kettering group had often used Salmonella infection instead of BCG, and LPS from Salmonella strains instead of Escherichia coli, to generate TNF (Carswell, personal communication).
One indirect approach to understanding this similarity took advantage of the unique characteristic of hemoprotozoan infections that allows counts of Giemsa-stained peripheral blood smears to provide information, not available for bacterial or viral infections, about the total pathogen load in an infected host. If the arguments for a key role for the mediators of endotoxicity in malaria and similar diseases had validity, it would follow that the parasite density at which a host species begins to exhibit illness on a first exposure to malaria or babesiosis would be in proportion to the amount of E. coli LPS that the host could withstand before the onset of toxicity. This proved to be the case for all hosts, including reptiles, birds, and a range of mammals including humans (82). Sometimes only a 50% lethal dose was available in the literature, but the range of responses is so extreme that the correlation is still obvious. Since P. falciparum and Babesia microti, another hemoprotozoan protozoan, infect both humans and another host (the owl monkey, Aotus sp., and the mouse, respectively), it was possible to establish that the relationship depended on a characteristic of the host, not the parasite species. This provided, for the first time, a plausible explanation for the long-standing puzzle that, although very low parasite densities cause onset of illness in first infections of human malaria and babesiosis and bovine babesiosis, mice withstand high parasite densities of several species of either causative genus before onset of illness. In other words, previously unexposed humans become ill after exposure to very few hemoprotozoan parasites whereas mice do not become ill until exposed to many organisms. Similarly inexplicable had been the observation that incredibly high malaria parasite densities (sometimes reaching a peak of 35,000 parasites per 10,000 red cells) do not cause illness in reptiles (161). Thus, the pursuit of the LPS-inducible soluble factors (later called cytokines) appeared to be the best way to understand disease pathogenesis in malaria and babesiosis. Since individuals recently recovered from malaria withstand high doses of both malaria parasites and LPS (186, 341), it was reasoned (79, 83, 99) that this approach to innate malarial tolerance in different host species offered insight, in terms of reduced levels of TNF and related cytokines, into understanding the acquired malarial tolerance seen in children in malarial areas.
A corollary to these ideas was that the long-postulated malaria toxin did not cause illness directly, as had been assumed since the late 19th century, but did so through inducing the host to release a shower of LPS-inducible cytokines that, at lower concentrations, are an essential part of the host immune response. Thus, it was noted that TNF induction could be used as an in vitro assay for the presence of the active principle of malaria toxin (83, 99). This has proved to be so for P. falciparum (363) and also for Trypanosoma brucei (393), another hemoprotozoan in which low parasite densities can cause onset of human (231) and bovine (263) illness but, as in malaria and babesiosis, high densities are required in rodents (424). Malaria toxins are discussed later in this review.
The Post-rTNF Era
TNF.
When Chiron (courtesy of Bruce Beutler) and later Asahi recombinant TNF became available to us, we found that this cytokine, when continuously released in small quantities from intraperitoneal osmotic pumps, would inhibit the in vivo growth of the rodent malaria parasite, Plasmodium chabaudi (95). This result was extended by the Middlesex group to P. yoelii, with the additional information that it did not have this activity in vitro, implying that an additional mediator had to be induced in vivo by TNF (401). When we injected rTNF into mice carrying low loads of another rodent malaria parasite, Plasmodium vinckei, it caused changes that completely mimicked the hypoglycemia, mid-zonal liver damage, and pulmonary accumulation of neutrophils seen in the terminal stages of this infection (92). Also, the dyserythropoiesis and erythrophagocytosis (89) and fetal death (88) observed in P. vinckei-infected mice, as well as in human falciparum malaria, were induced by injecting a small dose of rTNF into mice carrying a light, subclinical load of P. vinckei. Others, by now interested in the role of TNF in bacterial sepsis, showed that the increased serum lactate level, hypotension, adrenal medullary necrosis, renal tubular necrosis (413, 414), and hepatomegaly (143), all seen in falciparum malaria, occur in animals given parenteral rTNF.
The availability of rTNF also ushered in a period when it was used use in therapeutic trials in human tumor patients, and its side effects strikingly mimicked many of the clinical changes seen in malaria. These included fatigue, fever, chills, headache, thrombocytopenia, anorexia, hypotension, myalgia, nausea, vomiting, and diarrhea (206, 380). Symptoms were worse with higher doses and were often dose-limiting. Biochemical changes recorded in malaria, such as hypertriglyceridemia (304) and low serum iron levels (316), were also observed in patients to whom TNF had been administered (108, 206, 274). Likewise, the clotting cascade, which is often activated in malaria patients (322), was activated in TNF recipients (32, 421). Altered mental states were also recorded, particularly in patients receiving infusions of IL-2, a cytokine that induces TNF (276). In one IL-2 study, 15 of 44 patients developed behavioral changes sufficiently severe to warrant acute intervention and 22 had severe cognitive defects (117). All recovered without residual neurological deficit, which is reminiscent of cerebral malaria. Even the labial herpes often seen in malaria may be reproduced by infusing TNF into tumor patients (119, 397), implying a role for TNF in malarial immunosuppression. We subsequently attributed this to its ability to induce inducible nitric oxide synthase (iNOS) (331). Since these changes were often induced by doses of rTNF too small to cause a detectable rise in serum TNF levels (206, 436), it is not surprising that patients with clinical falciparum malaria, in whose serum high levels of TNF can be detected (see below), often exhibit these changes in a severe and protracted form.
A technical advance made possible by the availability of rTNF was an enzyme-linked immunosorbent assay to assay the serum of malaria patients for the presence of this molecule. Systematic studies, comparing serum TNF levels with complexity of disease and outcome, began soon thereafter. For example, it was found that the serum TNF level in East African (165) and West African (229) children at time of admission correlated with the severity of disease and mortality, even though serum TNF levels varied greatly. This highlights a problem inherent in studying malaria in tropical countries, where variations in previous malarial exposure through malarial tolerance and thus variable degrees of inhibition of the ability to generate TNF make the interpretation of data difficult. A clearer pattern was detected in a group of nonimmune Europeans who had not previously traveled to tropical areas (215). Patients with complicated malaria (combined organ dysfunction, hypotension, thrombocytopenia, and the highest parasite densities) and the longest durations between onset of clinical symptoms and diagnosis had significantly higher TNF levels than did those in whom malaria ran a more benign course. In another contrasting setting (the Solomon Islands), we found that serum TNF levels correlated directly with both fever and parasite density (58). Since then, TNF has become a standard cytokine assay for malaria, and its study has generated over 750 papers.
IL-1.
As noted above, we proposed roles for IL-1 in malaria in 1981 (99) and showed that macrophages from malaria-infected mice were primed for the release of IL-1 (437). Circulating levels are increased in septic shock and experimental endotoxin fever in humans (62). In our view, the impression that IL-1 is a much less important cytokine than TNF in malaria and sepsis pathogenesis largely stems from the fact that IL-1, unlike TNF, has never been made available in amounts sufficient to generate a critical mass of literature on its in vivo use. Undoubtedly the main champion of IL-1 has been Charles Dinarello, who began working on this mediator when it was still called endogenous pyrogen, then an undefined entity. Credit for linking this undefined pyrogen to IL-1 must go to Keith McAdam, who, in collaboration with Dinarello, showed that serum amyloid A inducer copurified with both endogenous pyrogen and lymphocyte-activating factor, an earlier name for IL-1 (266). As often noted in reviews (120, 121), it would be a mistake not to take IL-1, which is just as pleotropic as TNF, into equal consideration when investigating diseases such as malaria.
We later demonstrated that the presence of very low concentrations of either rIL-1α or rIL-1β can greatly enhance the effectiveness of a fixed dose of TNF to generate nitric oxide and to induce hypoglycaemia in malaria-primed mice (330). Increased concentrations of IL-1β, as well as TNF, in serum have been associated with the severity of falciparum malaria in West Africa (229), and were also generated in cocultures containing monocytes or unfractionated peripheral blood mononuclear cells and P. falciparum-infected red cells (426). In addition, glycosylphosphatidylinositol (GPI), a major malarial toxin, induces IL-1 from macrophages (364). Moreover, infusion with rIL-1 generates the same fever, rigors, fatigue, headache, and nausea as does administration of rTNF (427). Nevertheless, sepsis patients receiving recombinant human IL-1 receptor antagonist in a phase III trial derived little benefit (220). As Dinarello has stressed in summarizing some of his group's laboratory work, simultaneously blocking both IL-1 and TNF is a logical therapeutic direction (120, 442), but to date the realities of biotechnology patents appear to be retarding enthusiasm for testing this combined treatment in patients.
Lymphotoxin.
As with IL-1, LT has generated only a limited literature because of its poor availability for in vivo experiments. One of the very first cytokines described (342), LT was cloned (166) and generated in recombinant form (6) before rTNF was available. LT was termed TNF-β for a period (when TNF was TNF-α) before reverting to its original name of lymphotoxin. Since then, it has been further investigated and now has alpha and beta categories of its own. LT upregulates TNF expression (306) and shares at least one of the TNF receptors (359), yet data obtained in experiments with mice support the notion that TNF and LT have largely nonredundant functions in vivo (228). While LT has been described in a pig model of sepsis (235), its detection in human sepsis has been more equivocal (382). Like TNF, LT increases the expression of intercellular cell adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) (200) and thus enhances cellular adhesion to endothelium.
The work that led to the identification of the malaria toxin, GPI, was based on its capacity to induce TNF (discussed below), but formal investigations of whether this molecule induces LT have not yet been done. Some years ago, we showed that LT was present in human malarial serum and that injecting the recombinant form into malaria-primed mice induced the same high levels of IL-6 and hypoglycemia (both of which are seen in the severe disease) as did administration of TNF (94). It now seems clear-cut, from experiments with knockout mice, that LT is the key mediator of the mouse model of cerebral malaria, the ANKA strain of P. berghei (137). The prevention of this cerebral pathology through immunization against malaria GPI is therefore difficult to interpret other than indicating that GPI induces LT. Moreover, LT shares with TNF and IL-1 the ability to induce nitric oxide generation in vivo (329). It has been known for some years that a crude extract of P. falciparum-infected red cells induces LT (146), and GPI has been shown to be responsible for all of the iNOS-inducing activity in crude P. falciparum extracts (391). Taken together, these data imply that GPI does indeed induce LT as well as TNF and IL-1. An earlier claim that anti-TNF antibody prevented the development of cerebral symptoms in this mouse cerebral malaria model (163) is probably best explained by the polyclonal antibody then available neutralizing LT as well as TNF. In summary, despite its small literature, LT may well be as important in practice as TNF in initiating many inflammatory pathways, and ignoring it may have caused a number of surprising negative in vivo results with TNF neutralization.
HMGB1 RELEASE INTO THE CIRCULATION
The cytokine response that leads to malarial disease is still not fully explained. For example, the mediators thus far incriminated are released early and have short lives in the circulation, yet untreated malaria is a chronic disease. HMGB1, one of the high-mobility group box (HMGB) proteins, which is now accepted to enhance the severity of sepsis pathogenesis and to extend its duration, may well elucidate this. This family of nuclear molecules was named in the 1960s (as HMG proteins) for their electrophoretic mobility properties on acrylamide gels. HMGB1 is a ubiquitous, abundant, and evolutionarily conserved nonhistone DNA-binding molecule that has been known for decades to be present in all vertebrate cell nuclei and to play roles in DNA transcription, replication, bending, and repair (47). In passing, we note that HMGB2 and HMGB3 are widespread during embryogenesis, with only the former existing in adults cells, and then in limited locations (57). Well before its additional role in sepsis was appreciated, HMGB1 was unwittingly supporting a thriving area of research in neuronal development and neurite outgrowth under the name amphoterin (272) when sequencing data revealed unexpectedly, in 1993, that amphoterin and HMGB1 were identical (310). Although HMGB proteins are normally intracellular, work published in 1999 by Haichao Wang and Kevin Tracey showed that a novel protein circulating in mice during the later stages of the lethal toxicity of enterobacterial LPS was, like amphoterin, unexpectedly found, when sequenced, to be HMGB1 (428). This paper also reported the presence of HMGB1 circulating in the blood of sepsis patients and mimicry of the in vivo pathological effects of LPS with rHMGB1. Accordingly, the authors suggested that HMGB1 could act as an inflammatory mediator downstream of TNF and IL-1, and enthusiasm for this concept, as well as evidence to support it, has spread (see below).
The identity of HMGB1 as an unusual and complex cytokine that also binds to DNA has recently been reviewed (13). Because it lacks a classical signal sequence, the regulation of its release is not clear. In monocytes, its LPS-induced secretion occurs through exocytosis of cytoplasmic granules containing HMGB1 (153). LPS operates in collaboration with lysophosphatidylcholine, a bioactive lipid generated at the site of inflammation (153). As evidence that HMGB1 has now become acceptably “mainstream” in sepsis, we note that references to its origins, activities, and clinical implications have been included in recent prominent general reviews of this condition (45, 102, 326).
HMGB1 consists of two homologous DNA-binding sequences, termed box A and box B. The DNA-binding domain within box B (localized to 20 amino acids, from positions 89 to 108) contains the proinflammatory function of the full-length molecule (242); paradoxically, the A-box protein, when administered alone in recombinant form, has a strong anti-inflammatory activity (13). In vitro studies have shown that HMGB1 is secreted by activated monocytes and macrophages (13, 153, 428). This has been confirmed in vivo through its detection by immunohistochemistry during experimental arthritis in rats (224) and rheumatoid arthritis in humans (224, 396). As well as being found in the nucleus of synovial macrophages (as in controls), HMGB1 was abundant in the cytoplasm of these cells, in keeping with its secretion via a nonclassical, vesicle-mediated secretory pathway (153). It was also present in the adjacent extracellular matrix (224).
In contrast to the rapid rise and fall of TNF and IL-1 release, HMGB1 is released from macrophages only after they have been exposed to LPS, TNF, or IL-1 for 8 h, and secretion continues for an unusually long period (20 h) (428). In its turn, HMGB1 generates a further wave of these inflammatory cytokines, with a slower onset and a longer duration, than does LPS (14). This is consistent with the presence of raised TNF levels for up to 24 h after onset of illness in the mouse cecal ligation and puncture model (376), a condition in which HMGB1 is found in the circulation (418) and against which anti-HMGB1 treatments protect (13, 418). When injected, the recombinant form of HMGB1 produces the changes characteristic of endotoxicity (428), and a single injection of 1 μg into joints of mice generates histologically confirmed arthritis that lasts for up to 28 days (323). In addition, TNF and IL-6 have been found in the brain after HMGB1 has been introduced into the cerebral ventricles of mice (7). HMGB1 has also been reported to be present in plasma in ischemia/reperfusion injury (373), human hemorrhagic shock (303), and, in mice, LPS toxicity (418), cecal ligation and puncture (418), and brain trauma (221). Delayed injection of neutralizing anti-HMGB1 antibodies significantly protects mice against a 100% lethal dose of LPS (428), and antibody specific for the B box is just as effective (242).
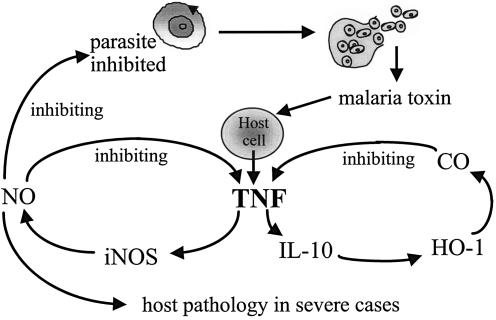
It was first shown in the amphoterin literature that HMGB1 binds to RAGE, the receptor for advanced glycation end products (AGE) (197). This receptor is a member of the immunoglobulin superfamily and is unusual in that it has a repertoire of ligands, including amyloid fibrils and S100, a chemokine, as well as HMGB1 and AGE. AGE is relevant to diabetes pathology. Its expression is upregulated at a number of cellular locations, including endothelial cells, in various examples of inflammation. When engaged with AGE, RAGE increases the permeability of endothelial cells to albumin-sized molecules (353), and the presence of the soluble form of RAGE (sRAGE) prevents this in vivo (432). There is, not surprisingly, considerable interest in the usefulness of sRAGE in various disease states mediated by a number of the ligands of RAGE, but the possibility of its relevance to sepsis and malaria has not yet been exploited. Following the engagement of RAGE on human endothelial cells with rHMGB1, there is a dose- and time-dependent increase in the expression of ICAM-1 and VCAM-1, TNF, IL-8, monocyte chemotactic protein 1, plasminogen activator inhibitor 1, and tissue plasminogen activator, as well as RAGE itself (149). Apparently TNF can also upregulate RAGE (395). These consequences of RAGE-ligand interaction are likely to reinforce, from a malaria and sepsis perspective, the proposal (354, 355) that HMGB1 is an important amplification signal in inflammation. This property makes it a worthwhile therapeutic target. The place of HMGB1 within the inflammation process is depicted in Fig. 1.
FIG. 1.
The proposed central role of HMGB1 in the amplification and perpetuation of inflammation. As well as excessive TNF and IL-1 inducing HMGB1 secretion, they can be predicted to run down cellular energy through overactivating PARP-1, thus favoring necrosis, which releases HMGB1 from the nucleus, over apoptosis, which is energy dependent and does not cause HMGB1 release. By increasing the expression of RAGE (a receptor for which HMGB1 is one of several ligands) and then activating it, HMGB1 induces a further wave of inflammatory cytokines, including the TNF and IL-1 that induced it in the first place. Thus, HMGB1 is thought to amplify and extend inflammatory reactions.
In collaboration with Kevin Tracey and coworkers, we have found that circulating levels of HMGB1 are increased in falciparum malaria (L. M. Alleva et al., unpublished data). In addition, the translocation of HMGB1 from the nucleus to the cytoplasm, as occurs after LPS (153) and within synovial fluid macrophages of rat and human rheumatoid arthritis (224) has been observed in several organs in fatal human malaria (A. C. Mills et al., unpublished data). Thus, it appears that this new development in understanding inflammation applies equally to malaria as to sepsis, as has been found recently for MIF and iNOS (78, 87). Its links to adhesion and coagulopathy mediators, as outlined above (149), are particularly intriguing for the pathogenesis of malaria disease. This is explored further later in this review.
We also note a report that human platelets, of which HMGB1 is a normal constituent, present this molecule on their surface once they become activated (338). Should this HMGB1 be directly donated to RAGE (as expected, since activated platelets adhere to endothelial cells), it would not have contributed to the levels of this cytokine in plasma, assays for which could therefore underestimate total activity in circumstances where platelet activation occurs, such as malaria (281) and sepsis (156). Circulating TNF, whose level is increased in malaria, has been reported since 1988 to activate platelets (112), and such activation has since been proposed to be associated with malarial vascular pathology (164). A recent immunohistochemical study has shown platelets to have aggregated in brain blood vessels in a selected group of human cerebral malaria cases (164). Presumably, these platelets were activated and therefore can be expected to carry much HMGB1 on their cell surface (338), thus upregulating adhesion and coagulopathy activity (149). Unfortunately, a complete interpretation of this platelet immunohistochemistry (164) is not yet possible, since the one-third of the patients in that series whose brain sections did not show significant inflammatory change or sequestration of parasitized red cells or monocytes in the cerebral vasculature (87) were not included in this study (164).
Therapeutic Approaches Targeting HMGB1
Two possible therapeutic approaches arise from the HMGB1 literature. Parenteral ethyl pyruvate (a common food additive) has been reported to rescue mice from models of sepsis and systemic inflammation, whether administered before or 4 or 24 h after the onset of inflammatory insult (418). This has been argued to occur because it is a potent water-soluble radical scavenger. When given before LPS, it inhibits the usual increase in the levels of both TNF and HMGB1 in plasma due to LPS, whereas when given 4 or 24 h after LPS, when the early TNF peak had come and gone, HMGB1 release was still inhibited (418). This implies a more important role for HMGB1 than for TNF in these model diseases. Cecal ligation and puncture is the hardest model of systemic inflammation to influence experimentally. The ability of ethyl pyruvate (418), recombinant Box A protein (13), and neutralizing antibody specific for the B box of HMGB1 (13, 242) to significantly enhance survival in this model when treatment is delayed for 24 h after cecal ligation and puncture, by which time 10% of mice had already died, provides the best example yet of a clinically relevant therapeutic window for sepsis. In other words, these results meet, for the first time, a goal that would be required for practical intervention in patients who are already ill with a range of systemic infectious conditions, including falciparum malaria. A similar analysis, with the same conclusions, has been performed with ethyl pyruvate in a rat LPS shock model (423). Ethyl pyruvate (402) and sodium pyruvate (284) have been reported to protect against experimental hemorrhagic shock. This is further discussed in the section on PARP-1 activation (see below). In addition, recombinant HMGB1 A-Box protein and neutralizing antibody specific for the B box of HMGB1 are both efficacious in treating established arthritis in mice and rats (223). This result, seen in conjunction with the outcome when these agents are used in experimental sepsis (13), demonstrates the essential similarity between local inflammation and these systemic diseases.
sRAGE is effective in vivo in various models, its efficacy includes blocking the onset of increased vascular permeability (432) and accelerating wound closure in diabetic animals (162). As the functions of the HMGB1-RAGE complex become elucidated in malaria and sepsis, it may become increasingly plausible that sRAGE is a therapeutic approach with much potential across the circumstances in which HMGB1, as well as AGE and the other ligands of RAGE, cause pathology. By binding to HMGB1, sRAGE inhibits HMGB1-induced neurite outgrowth (197) and release of TNF from human macrophages in vitro (396). At the time of writing, we are not aware of any in vivo data on whether sRAGE might act, when administered after the onset of models of sepsis illness, as does ethyl pyruvate (418). One unknown dimension is the degree to which RAGE monopolizes the functional capacity of its wide range of ligands. For example, S100B, a well-accepted ligand for RAGE has also been reported to act independently of it (375). It has also been demonstrated that when extracellular HMGB1 stimulates the differentiation of a murine erythroleukemia cell line, it does so independently of RAGE (378). Nevertheless, since HMGB1 is a late-onset mediator downstream from TNF, both of these approaches, ethyl pyruvate and sRAGE, are exciting practical possibilities in the treatment of systemic inflammatory disease, including malaria, sepsis, and the range of noninfectious states described later in this review. Knowledge about the proinflammatory actions of this mediator is still in its infancy, and, as clearly set out at the end of a recent review, many key questions about its interactions with the rest of the inflammatory response remain to be answered (13).
NITRIC OXIDE
Twenty years ago, nitric oxide (NO) was of biological interest only as an air pollutant from car exhausts, but this changed in 1987, when it was shown to be produced in living mammalian cells (201, 307). It is now the theme of a massive biomedical literature, currently generating 8,000 to 9,000 publications each year. Predominantly it has physiological signaling functions, although it can cause damage if excessively produced. It is widely studied across the entire spectrum of biology, extending from plants and insects to mammals, in which it plays roles in blood vessel tone, vision, hearing, smooth and skeletal muscle control, brain function, and the immune system. Biological NO is generated from arginine by an enzyme, NOS, with several forms, including two that are constantly present and activated by high local concentrations of calcium. Another form of the enzyme, iNOS, is induced by inflammatory cytokines and operates independently of local calcium concentration.
In the late 1980s, malaria researchers were confronted with the puzzle that TNF, which had no known way of influencing neurons, nevertheless (once hypoglycemia, which TNF was known to cause, was excluded) correlated with the degree of coma in cerebral malaria (215, 229). When it became evident that TNF could induce endothelial cells to generate NO (217), that neurons respond to NO (151, 155), and that NO, like oxygen, freely crosses cell membranes, it was proposed (97, 98) that TNF-generated iNOS in cerebral blood vessel walls could provide a plausible link between TNF and coma. This suggestion led to considerable data from various sources, much of it conflicting, in which plasma nitrogen oxide levels (a surrogate for NO generation) varied according to geographical location, the patients' ages, and malarial endemicity (10, 103, 123, 225, 297). Not only were many studies inadequately controlled for dietary nitrite and nitrate and renal function, but also the interpretation of all of them was limited because levels of nitrogen oxides in plasma, even if they were generated by endogenous iNOS, provide no clue to their precise cellular origins. This is all-important when dealing with a molecule so ephemeral that it can act in a very local area only. As described below, iNOS immunohistochemistry eventually answered many of these questions.
Homeostatic Functions
As with most molecular pathways that influence biological processes, the inflammatory cytokine cascade has various negative-feedback mechanisms built into it to minimize the chance of its getting out of hand. For example, inflammatory cytokines, via iNOS, inhibit the growth of many organisms that induce them (70, 118, 152, 170), peroxynitrite (generated when NO reacts with superoxide [34]) inhibits the activity of iNOS (311, 328), and NO downregulates TNF production (203). Such a homeostatic role for NO in severe malaria is suggested by data from Gabon (225), Tanzania (16), and Papua New Guinea (84), where healthy children, whose age and history implied that they were malaria-tolerant, had the highest circulating nitrogen oxide levels in the population. In Tanzania, this group also had the highest-peripheral blood monocyte iNOS level, an observation that, together with high serum nitrogen oxide levels, has been extended to adults in Indonesian Papua (50). This last observation is consistent with the high frequency of asymptomatic parasitemia that can be present in adults in this population (50) and in adults in tropical Africa (116). In view of the negative feedback that exists between NO and TNF, these results may shed light on NO as a contributor to malarial tolerance in humans. Complexities inherent in such field studies include gross whole-body measurement of a mediator that acts very locally and other infective sources of NO induction, such as intestinal parasites (50).
High levels of NO have been functionally linked to LPS tolerance in various animal species (141, 203, 227, 334, 377, 411). At least in mice, the phenomenon may have functional redundancy, since mice genetically deficient in iNOS (iNOS knockout [KO] mice) can be made LPS tolerant (447). Tolerance to LPS and tolerance malaria presumably have related mechanisms, since patients who are malarial tolerant are also tolerant to LPS (186, 341). Toll-like receptors (TLR) are the linchpins of the recognition by the host of the initiators of innate immunity and thus of the onset of cytokine-induced disease. Protozoal GPI (along with peptidogylcans from gram-positive bacteria, Borrelia LPS, Mycoplasma lipoproteins, Treponema glycolipids, and Mycobacterium cell wall molecules) is recognized on cell surfaces by TLR2 (61), and once enterobacterial LPS forms a complex with LPS-binding protein and CD14 (439), it is recognized by TLR4 (76). These two TLRs mutually cross-tolerize (237), so that the molecular site of tolerance lies somewhere along the downstream signaling pathways shared by TLR2 and TLR4 that lead to activation of AP-1 or NF-κB and thus to generation of inflammatory cytokines. This would explain, at a signaling level, why GPI from Trypanosoma cruzi cross-tolerizes with LPS in vitro (337). There is as yet no information on whether the malaria GPI does the same, but the presence of general similarities between GPI from these two sources (392) makes it likely. Some time ago, antisera raised against whole crude preparations of exoantigens of P. berghei, P. yoelii, or P. falciparum were shown not to neutralize the activity of LPS (27). These data are consistent with, but do not prove that, the chronically high NO production in malaria-tolerant individuals in Tanzania (16) plays a homeostatic role through TNF inhibition and is thus a major cause of the ability of these individuals to withstand large parasite loads without experiencing illness. In both LPS and malaria tolerance, a plausible way in which it might do this is to reduce the production of inflammatory cytokines along the TLR2/TLR4 signaling pathway (150, 411). LPS tolerance, with reduced TNF production, has also been demonstrated at the cellular level in chronic sepsis (181). As discussed below, IL-10-induced carbon monoxide also has credence in all of these circumstances where a control on TNF production must be explained.
Another example of NO acting to achieve homeostasis in falciparum malaria is its capacity to inhibit the adhesion of parasitized red cells to vascular endothelium (367). Such adhesion has recently been demonstrated to be necessary to maximize TNF production and hence illness (298). Hence, by reducing adhesion, NO is reducing the amount of TNF that, through iNOS, had been generating it. A further example of its homeostatic activity is the immunosuppression that accompanies malaria. Since macrophages can suppress nearby lymphocytes by generating NO (278), this concept was adopted to provide an explanation for malarial immunosuppression (8, 331). This has proved to be a general concept for diseases in which the systemic release of inflammatory cytokines occurs and has rationalized the immunosuppression that accompanies thermal injury (261, 366), graft versus host disease (GVHD) (44), and Trypanosoma cruzi infection (2), as well as malaria. It was also argued that NO-induced T-cell suppression, as a by-product of this homeostasis, explained the rarity of autoimmune diseases in the areas of Africa where falciparum malaria is hyperendemic (84). The persistence of high NO until well into adulthood (50), shown in Papua and implied in Africa by the high asymptomatic parasitemias that have been reported (116), improves this argument, since it is not just children in whom autoimmune diseases are rare in these areas (167). Where NO fits in to the broad picture of the homeostatic pattern within the inflammatory pathways that operate in disease is discussed later in this review.
Pathogenic Activity
Intuitively, homeostatic.agents cannot also be harmful, and unfortunately this assumption has become embedded, for NO, in the recent malaria literature (16, 190). Certainly the potential for cytokine-induced NO to cause pathology exists, since iNOS KO mice have been reported to be protected against a diverse range of pathology, including that caused in the lungs by LPS (226), that caused in the lungs and liver after hemorrhagic shock (187), the refractory vasodilation seen after cecal ligation and puncture (193), mortality and organ failure induced by zymosan (109), endothelial dysfunction after 15 h of treatment with enterobacterial LPS (72), and liver damage and fatal outcome after treatment with anti-Fas antibody (69). Likewise, the increased gut permeability caused by HMBG1 does not occur in iNOS KO mice (348). Testing this approach in humans has been hampered by the absence of a selective iNOS inhibitor that is safe for human use. Recently, however, results on the safety and technical efficacy of a prodrug, l-N-6-(1-iminoethyl)lysine 5-tetrazole amide (SC-51), which is rapidly converted in vivo to the active metabolite l-N-6-(1-iminoethyl)lysine (L-NIL), have been published (176). It caused no side effects in a randomized double-blind placebo-controlled crossover trial and reduced NO levels in exhaled breath by >90% relative to control levels in asthmatic patients, with the effects lasting at least 72 h.
When autopsy samples became available, iNOS could be detected in a number of organs from patients with fatal falciparum malaria, including many of the brains (87) of African children. Similar results obtained with material from a smaller number of patients were presented at the 1999 International Nitiric Oxide Meeting (I. A. Clark, M. M. Awburn, R. O. Whitten, R. A. Carr, N. G. Liomba, M. E. Molyneux, and T. E. Taylor, Acta Physiol. Scand. 167(Suppl. 645): abstract O-45, p. 14, 1999). The most dramatic concentrations of iNOS were not in the brain but in the systemic blood vessel walls and skeletal muscle. This was also true for sepsis cases (87). Others also detected this inducible form of NOS in brains of adults from Thailand with fatal cases (258), although they did not examine other organs. In our experience, only about half of a group of 32 patients with clinically defined pediatric cerebral malaria showed appreciable iNOS in cerebral vessel walls. These brains, but no other brains, contained clusters of monocytes, which also stained for iNOS. These changes were not seen in the other half of the 32 patients, and many of these (11 of the 32 patients with clinically diagnosed cerebral malaria cases) had no, or negligible, sequestered parasitized erythrocytes in the brain vessels (87). As recently reviewed (91), this may provide some appreciation of how wide the net is cast, in pathophysiological terms, when cerebral malaria is diagnosed in children compared to adults (371). This review (91) also discusses how the high levels of iNOS in ventilatory skeletal muscle (poor oxygen delivery), in combination with NO-induced mitochrondrial failure (poor oxygen utilization), provide a likely prospect of contributing to the respiratory failure seen in many cases of pediatric falciparum malaria (107, 136, 301). Documentation of mitochondrial failure during malaria infection has a long history (327).
Recent studies of human sepsis have found a positive association between high local NO levels and disease severity (51, 280), and, as noted above, they have found high levels of iNOS protein in key tissues in fatal human malaria (87) and sepsis (87, 232) cases, but not in controls. Moreover, there is a great deal of detailed literature exploring how high generation of NO from iNOS can cause pathology at a molecular level, for example in skeletal muscle (134, 232, 295, 346). As reviewed recently in a malaria context (91), inhibition of membrane Na+K+-ATPase, the cell's sodium and water expulsion pump, by NO (172, 332) can be expected to have profound effects on the integrity of cellular membranes wherever iNOS induction is strong. An additional pathological role for excessive NO production is in ATP depletion, via peroxynitrite excessively activating poly(ADP-ribose) polymerase 1 (PARP-1) (365), as discussed below. In summary, more commonly the homeostatic functions of NO prevail, but, given the opportunity, it also contributes to fatal pathology.
The widespread interest in NO from iNOS in protection against and pathology of malaria has extended to investigating variations of African populations for modifications within the NOS2A gene, which encodes iNOS. Various NOS2A polymorphisms in African populations, probably in regulatory regions controlling gene transcription, have a range of associations with severe falciparum malaria and also with a fatal outcome for this disease (54, 55, 190). There is much to be resolved, such as the possible effects of linkage disequilibrium, in which genetic variants located physically close to each other often have correlated alleles. Nevertheless, one allele described from The Gambia has been associated with susceptibility to cerebral malaria, as distinct from death from this disease (54). Another allele, possibly the same one as that associated with protection against severe malaria in Tanzania, had no association with severe malaria in The Gambia (54). The presence of this polymorphism in Tanzania and Kenya (190) was shown to be associated with increased nitrogen oxide levels in fasting urine and plasma and also with protection from severe malarial illness and anemia, two acknowledged cytokine-induced pathologies. This implies that this polymorphism provides those carrying it with the ability to become tolerant to malaria faster than those without it, through rapid generation of enough NO to inhibit TNF production (150, 411), and to be particularly efficient at maintaining that tolerance.
This raises the question of how, in the fatal cerebral malaria cases (with high iNOS levels in cerebral and systemic blood vessel walls and skeletal muscle [87]), the iNOS level became high enough to cause pathology without having shut down TNF production, and thus protect the host rather than causing severe disease. We propose here that the factor which determines whether iNOS-generated NO protects or harms the host is the rate at which it is produced. Before expanding this argument, we must recall the differences that occur when TNF is induced by either LPS or HMGB1, as described above. There appears to be good evidence that T. cruzi GPI requires TLR2 expression for induction of IL-12, TNF, and NO (61), not the TLR4 used by LPS (76). Nevertheless, LPS and GPI appear to induce TNF along similar pathways, with a similar extent of phosphorylation of the range of mitogen-activated protein kinases and with similar kinetics, and to use functionally similar receptors (337). An excellent overall review of pattern recognition molecules and innate immunity in parasitic diseases has appeared recently (268). HMGB1, in contrast, leads to a different profile of gene expression, pattern of cytokine expression, and kinetics of activation of p38 mitogen-activated protein kinase from that associated with LPS (309). This is consistent with HMGB1 inducing TNF from human peripheral blood mononuclear cells more slowly than does LPS, and levels remaining increased for longer (14).
Moreover, LPS-tolerant human monocytes can still be triggered by HMGB1 to generate TNF (14), even though the NO level would be high in these cells. This implies that NO, the main suppressor of TNF induction by LPS (and therefore probably by malaria), cannot switch off the inflammatory cytokine cascade initiated by HMGB1. Hence, if NO generation by malaria toxin occurs too slowly to downregulate the primary wave of TNF induced by malaria toxin (and thus downregulate itself); it would be powerless either to stop the secondary wave of inflammatory cytokines induced by HMGB1 or to thereby downregulate its own induction. In this way, a relatively slow buildup of iNOS-induced NO could result in its reaching the harmful levels seen in fatal sepsis (51, 87, 280) or malaria (87). We suggest that this is less likely to occur in individuals genetically endowed to induce NO rapidly through iNOS, such as the individuals described in East Africa (190). Instead, malaria tolerance would become established more readily in these people than in the rest of the population. This interpretation implies that hypoargininemia in children with cerebral malaria (247) is caused by its increased consumption as NO is generated and that arginine infusion late in the infection, when the HMGB1-induced inflammatory wave is under way and homeostasis has been lost, could occur too late for NO to switch off the inflammatory response and, indeed, could exacerbate aspects of the pathology.
CARBON MONOXIDE
Both carbon monoxide (CO) and NO are generated by enzymes that have at least one constitutive form and another form, iNOS and hemoxygenase-1 (HO-1), respectively, induced by various agents, including inflammatory cytokines. NO and CO act interactively as second messengers in many ways (178), a well-known example being that they both can activate soluble guanylate cyclase to generate cyclic GMP (356) and thus dilate blood vessel walls. They also both inhibit TNF generation (305) through their action on NF-κB (349). Hence, CO and NO provide an important degree of redundancy in inflammatory homeostasis.
This shared inhibitory activity of NO and CO against TNF duplicates that of IL-10, the prototype anti-inflammatory cytokine, which suppresses the generation of TNF and IL-1β by inhibiting NF-κB (429). Indeed, the high circulating levels of IL-10 seen in human malaria (315, 433) and sepsis (244) have been proposed to suppress disease severity by inhibiting the systemic inflammatory effects of TNF (189). The recent report of IL-10 producing its strong anti-TNF effect by inducing HO-1, and thus generating CO (233), brings these previously unconnected observations into the same chain of events. The earlier observation that TNF induces HO-1 (64) is also now explained by its ability to induce IL-10 (318). Likewise, the anti-inflammatory effect of 15d prostaglandin J2, shown to be present in tissues during inflammation (158), also operates through HO-1 induction and subsequent generation of CO (234). As in sepsis, cyclooxygenase-2 is induced in severe malaria (114). Thus, rather than still simply being a marker for heme degradation and a generator of antioxidant defenses, HO-1 is now recognized to be an integral part of the network of inflammatory mediators. Its proposed homeostatic role in malaria, helping reduce inflammatory cytokine production and thus generating malarial tolerance, is shown in Fig. 2.
FIG. 2.
The shared ability of cytokine-induced NO and CO to inhibit TNF production and therefore provide a negative-feedback loop to inhibit the illness and pathology in cytokine-release syndromes. Reprinted from reference 99a with permission from the publisher.
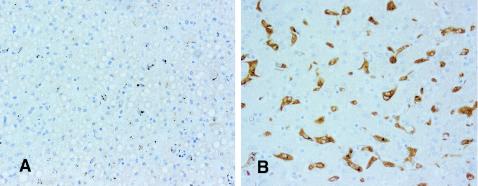
Before the IL-10 or prostaglandin links of HO-1 were appreciated, others (271, 351) had immunostained brains, but no other tissues, from adult malaria cases, for this enzyme. With this additional background for its anti-inflammatory capacity, brain, lung, and liver tissues from 40 African children with fatal falciparum malaria and sepsis or septic meningitis were stained for HO-1 to locate cellular sites where the CO-mediated anti-inflammatory activity of IL-10 might be mediated. In brief, HO-1 was strongly upregulated in Kupffer cells from virtually all malaria and sepsis patients (86). In contrast, it was absent in the Kupffer cells from all three patients in whom death was not associated with severe systemic infection or coma (Fig. 3). It was almost as frequently seen in macrophages and monocytes in lungs across all cases, but it was least frequent in brain monocytes, where it was rare or absent in 8 of the 11 brains in which none of the features (sequestered parasites and microhemorrhages) usually associated with adult cerebral malaria were observed. This study provides evidence, in addition to our findings with iNOS (87), that the entity covered by clinically diagnosed cerebral malaria in African children is primarily a systemic inflammatory disease, with coma sometimes being an encephalopathy of systemic, rather than local cerebral, origin. Moreover, this evidence of strong upregulation during malaria of the enzyme that generates CO provides, through the capacity of CO to downregulate TNF, a plausible model of an IL-10-induced pathway for malarial tolerance.
FIG. 3.
HO-1 staining of liver tissue, showing a representative field of the three cases in which death was not associated with severe systemic infection or coma (A) and and a representative field from the 37 malaria or sepsis patients who had been comatose before death. Biotin-conjugated secondary antibody and streptavidin-conjugated horseradish peroxidase from an LSAB+ kit (DAKO) were applied to sections to amplify the antigen signal for subsequent 3,3′-diaminobenzidine staining, which produces a permanent brown color. Magnification, ×400.
Although we did not directly demonstrate CO generation in malaria or sepsis cases, HO-1 provides the same quality of evidence as does iNOS for inducible NO, for which it is a widely used correlate. Our HO-1 results are consistent with endogenous CO production, measured in exhaled breath from mechanically ventilated patients suffering from severe sepsis, being significantly higher than in controls (446). Over a decade earlier, endogenous CO production was found in dogs severely ill from infection with Babesia canis (404), which has been documented to cause a disease with pathophysiology paralleling that of falciparum malaria (254).
EXCESSIVE PARP-1 ACTIVATION
Normally, PARP catalyzes the successive transfer of ADP-ribose units from its substrate NAD+ to a variety of proteins, and this is now accepted to control many biochemical processes, most notably, as reviewed recently (183), those related to genome stability. Homologues have been identified, and so a numbered sequence, in which the original PARP is now called PARP-1, was introduced. Seven PARP genes have now been cloned, and up to 16 sequences related to PARP have been identified in the human genome. We shall restrict our comments to PARP-1, the variant for which there is evidence of relevance to disease pathogenesis. PARP-1 is a DNA repair enzyme, catalyzing poly(ADP-ribosyl)ation in response to DNA damage, thereby facilitating its repair (56). As noted above, overactivity of PARP-1, which can be driven by DNA nicks generated by peroxynitrite (a product of excessive NO, and superoxide, downstream of TNF [34]), can deplete cellular NAD+ and can then deplete ATP in efforts to resynthesize NAD+ (37, 365, 387). This has the potential to reversibly inhibit many stages of aerobic respiration (what Fink [148] refers to as cytopathic hypoxia) and, as has been reviewed (369), cause bioenergetic failure. Thus, even if oxygen is plentiful, the patient's cells would utilize it very ineffectively, predisposing to the hypoglycemia, hyperlactatemia, and acidosis commonly seen in severe malaria and sepsis and traditionally attributed to poor oxygen delivery to the cells. In addition, PARP-1 activation enhances the activity of the transcription factor NF-κB (240), potentially generating more of the proinflammatory cytokines that activated it in the first place.
Three independently derived strains of PARP-1-deficient mice have been generated, and experiments with these mice provide unequivocal in vivo evidence that strong activation of this enzyme has profound consequences in disease. These mice are protected against a number of noninfectious conditions, including brain ischemia (135), hemorrhagic shock (243), and brain trauma (434), as well as illness induced by enterobacterial LPS (300), Streptococcus pneumoniae (222), and experimental cecal ligation and puncture (376). Consistent with the influence of PARP-1 activation over NF-κB activation, PARP-1-deficient mice generate much less TNF and iNOS than do wild-type mice exposed to S. pneumoniae (222) or LPS (300).
Research so far restricted to animal models implies a potential use for inhibitors of PARP-1 activation. These agents, in particular a phenanthridinone derivative N-(6-oxo-5,6-dihydrophenanthridin-2-yl)-N,N-dimethylacetamide HCl, termed PJ34 (249), have been reported to protect mice against brain ischemia (1), splanchnic ischemia and reperfusion (205, 265), LPS toxicity (205), various models of local inflammation (249), and the brain pathology caused by S. pneumoniae infection (222). They have also protected rats against the zymosan model of multiorgan failure (110) and have protected pigs against a model of sepsis in which a fibrin-thrombin clot is impregnated with E. coli and inserted intraperitoneally at surgery so that E. coli is slowly released intraperitoneally (159). The outcomes of these in vivo studies are consistent with subsequent in vitro experiments, in which cells were exposed to inflammatory cytokines with or without the addition of lysosomes containing NAD+. Supplementation with NAD+ prevented the reduction in oxygen usage usually caused by these cytokines (216). This is entirely consistent with the interpretations of the in vivo data of other groups (110, 159).
In vivo evidence of PARP-1 activation exists, with poly(ADP-ribose) (PAR)—whose presence indicates PARP-1 activation—being demonstrated immunohistochemically in ischemic brains from rats (412) and patients (248) and in brains from patients with cerebral malaria in Vietnam (271). No other organs were examined in these malaria cases, and the primary energy depletion implications of the presence of PAR were neither noted nor exploited. We are unaware of any report of tissues from human sepsis cases having been examined for PAR to date. In a recent study of skeletal muscle biopsy specimens from critically ill sepsis patients (51), concentrations of ATP (which is depleted by PARP-1 activation [37]) in muscle from those with an eventual fatal outcome were much lower than those in clinically indistinguishable patients who recovered. Samples were taken within 24 h of admission to the intensive care unit, and death occurred an average of 5 days later, so the assays did not simply monitor a terminal event. Consistent with the ability of peroxynitrite to activate PARP-1 (386), bioenergetic failure correlated well with evidence of high NO generation in the same muscle samples (51). This is in agreement with the high iNOS staining in skeletal muscle (and elsewhere) found in both falciparum malaria and sepsis patients (87).
These observations add to the accumulating evidence that deranged cellular energetics, from causes other than inadequate tissue perfusion, make important contributions to many of the harmful effects of illnesses in which excessive iNOS induction, leading to excessive peroxynitrite generation, can contribute to severe disease. One consequence is that potential apoptosis, being an energy-intensive process, instead becomes necrosis (238). As discussed above, this leads to the release of HMGB1 from the cell nucleus into the plasma (350), amplifying the inflammatory signals that activated PARP-1 in the first place. This could explain how ethyl pyruvate reduces HMBG1 release (418), in that sodium pyruvate (and therefore presumably ethyl pyruvate as well) inhibits PARP-1 fragmentation, reducing necrosis (284) and thus HMGB1 release (350) from this source. As noted previously (13), an important question to be addressed is the proportion of the HMGB1 that is secreted from activated macrophages (153) and from necrotic cells (350) in severe malaria and sepsis.
NONINFECTIOUS STATES CONCEPTUALLY SIMILAR TO MALARIAL DISEASE
An acute systemic reaction that can lead to illness and multiple-system involvement, such as is seen experimentally after LPS injection, is not restricted to acute systemic infections but is also observed in a number of noninfectious states. This was recognized some decades ago, and by the mid-1950s it was already realized that the same fundamental macrophage-dependent (451) processes were at work in these conditions as in gram-negative bacterial infections, with rats rendered tolerant to LPS also demonstrating tolerance to the posttrauma and posthemorrhage reactions (452). The range of circumstances that initiate these functionally related and similar pathologies is superficially surprising (Table 1), but the literature that binds them together unmistakably tells the one story. Their downstream pathogenesis unequivocally ties them conceptually to malarial disease. Moreover, the more these conditions appear to be governed by the same pathophysiology that governs malaria and sepsis, the more remote becomes the possibility that any of the significant pathology seen in malaria and sepsis is caused by the direct actions of molecules (excluding bacterial exotoxins) released by the infectious agents.
TABLE 1.
Cytokine release syndromes
| Noninfectious causes
|
Pathogen induced | |
|---|---|---|
| Misadventure | Iatrogenic causes | |
| Trauma | Jarisch-Herxheimer reaction | Viral diseases |
| Burns | OKT3 adverse response | Bacterial and rickettsial diseases |
| Hemorrhagic shock | Acute GVH disease | Malaria |
| Snake bite | Other protozoal diseases | |
Tissue Injury Syndromes
Tissue injury syndromes include those arising from severe blunt injury (leading to the crush syndrome) and major surgery, heatstroke, and the post-burn syndrome. References to increased circulating levels of inflammatory cytokines and subsequent NO generation dominate the literature on the multisystem syndromes seen after trauma (31, 41, 77, 101, 171, 339), major surgery (23, 59, 157, 211, 292, 339, 345), heatstroke (11, 48, 49, 75, 179), and burns (33, 259, 417). A major puzzle in understanding these conditions has been to identify the nature and the source of the trigger for this inflammatory cascade. Pancreatitis is another condition that belongs to this group, by virtue of its clinical and pathological characteristics, evidence of tissue necrosis (126), and the literature implicating inflammatory cytokines (140, 168, 336, 422) and NO (100, 111, 277, 299) in its pathogenesis. Increased gut permeability, allowing intestinal LPS to gain access to the patient's cells and thus initiating the TNF cascade, has often been considered, but is still questioned as a primary lesion (214, 239). The pathogenesis of pancreatitis has been well reviewed lately (257).
Scaffidi et al. have demonstrated that HMGB1 remains locked in the nuclei of cells that have undergone controlled death, or apoptosis, since it remains closely bound to chromatin. In contrast, when uncontrolled death, or necrosis, occurs, HMGB1 is released from the cell, causing the same inflammatory cascade that is generated by activated macrophages to be set in motion (350). Tellingly, necrotic cells from HMGB1 KO mice, but not wild-type mice, did not induce the inflammatory cascade. Likewise, PARP-1 KO mice, which are protected from the rundown of cellular energy that forces apoptosis toward necrosis (238), are protected from trauma (435). From this work, it can be inferred that the initial event in the above conditions, in which tissue injury is extensive, is probably massive HMGB1 release from damaged cells. The inflammation-enhancing actions of HMGB1, as described above, could therefore account for the increases in cytokine and NO levels that have been found in crush syndrome. Moreover, HMGB1 has been demonstrated to increase gut permeability (348), plausibly acting in part via NO (175), allowing gut LPS to enter the circulation as a secondary event, thus exacerbating systemic inflammation. A commentary article by Santangelo et al. in 1982 (347), reporting on clinical experiences with victims of an earthquake in Southern Italy, is prescient. They noted that the outcome was often fatal if a badly crushed limb was not amputated immediately and that leaving it in place exposed the patient to a high risk of tissue infection, which generated a syndrome that was, in their opinion, difficult to differentiate from the crush syndrome. Through our understanding of HMGB1, this observation may have now been rationalized at a molecular level.
It has been observed that thermal injury in mice enhances the reactivity of TLR2 and TLR4 (312), rendering them more susceptible to the systemic inflammation caused by a range of infectious agents. So far, there is apparently no evidence about whether the cytokine-like roles of HMGB1 extend to upregulating these TLRs, but a precedent has been set by the finding that MIF (333) and gamma interferon (IFN-γ) (142, 279) have this activity, so it is plausible for HMGB1 as well. Knowledge about the mechanism of tissue injury syndromes would be greatly advanced if more were understood about the interaction of TLRs and HMGB1. This appears at present to be limited to a report that HMGB1 can release inflammatory cytokines from macrophages from TLR4-deficient mice (14) and inferences that can be drawn from the report that LPS-tolerant monocytes are still susceptible to HMBG1 (13).
Hemorrhagic Shock
Hemorrhagic shock is not simply the consequence of reduced circulating blood volume but is an acute illness state that can overwhelm an apparently stable patient. It shares clinical characteristics with the conditions outlined in the above section and presumably shares their pathogenesis, since it also has generated a large literature on the relevance of inflammatory cytokines (22, 210, 449) and NO (15, 187, 290, 368, 407, 448). Moreover, as noted above, rats made tolerant to LPS are tolerant to both trauma and hemorrhagic shock (452). So too are PARP-1-deficient mice (243, 435), consistent with less ATP depletion, a predominance of apoptosis over necrosis, and consequently less HMGB1 release (350). A role for translocation of gut bacteria as the source of an LPS trigger for systemic inflammation is also well established in this literature, as has been extensively reviewed (343). However, hemorrhage in a mouse model, in the absence of significant tissue trauma, has been reported to cause enhanced TNF release that is not the result of increased endotoxin levels (20). This would be consistent with the action of HMGB1. While no pathway of HMGB1 release from necrotic cells is immediately obvious in severe hemorrhage, the level of this mediator in serum has nevertheless been found to be increased in human hemorrhagic shock (303); it has also been found that ethyl pyruvate, which reduces HMGB1 levels, improves outcome in experimental hemorrhagic shock (402, 440). A recent study of sodium pyruvate administration in a swine model of hemorrhagic shock has shown that its effects included minimizing NAD rundown and necrosis (284). This would accordingly reduce HMGB1 release from the nuclei of necrotic cells, which could contribute to the observed HMGB1 reduction after treatment with ethyl pyruvate (440). IL-6, one of the cytokines induced by HMGB1 (14), is essential for the increased gut permeability seen after hemorrhagic shock (441).
Pathology Caused by Snake Venom
The annual worldwide mortality from snakebites is said to be between 50,000 and 100,000 (430). Because the multiorgan pathology that can be caused by envenomation after bites from certain snakes shows indications of inflammatory change, over the past few years researchers have examined whether these venoms, rather than causing this pathology directly, might do so, at least in part, by inducing the production of proinflammatory cytokines in people who have been bitten. Venom from the South American rattlesnake, Crotalus durissus terrificus (74), the lanceheaded viper, Bothrops asper, and the Central American lancehead, Bothrops jararaca (314), induces TNF in mice. Bothrops venom also induces murine cells to generate IL-1, IL-6, IL-10, and IFN-γ as well as TNF. There was an early increase in TNF-α and IL-1 levels, followed by a more pronounced increment by 18 h (314), which is consistent with secondary induction by HMGB1. Venom-treated cells also produced very high NO levels, as one would expect from the presence of cytokines able to induce iNOS. Venom from the asp, Vipera aspis, increases serum TNF levels in rats (390); either anti-TNF antibody or soluble TNF receptor (p55), when injected at the same time as the venom, prevented certain harmful changes (389). Further study by this group has demonstrated that TNF is the cause of deleterious cardiac effects induced by this venom, in that anti-TNF antibody prevented these changes (388). A range of predictable downstream mediators are presumably induced in these models and patients, since prostanoids and adhesion molecules, as well as TNF, were induced in mice by the venom of B. jararaca (445). Envenomation by the Brazilian brown spider, Loxosceles intermedia (394), and a scorpion, Tiytus discrepans (125), appears to generate the same cytokine pattern as does envenomation by the snake venoms discussed above.
Iatrogenic Cytokine Release Syndromes
The three conditions summarized in this section are predictable side effects of human intervention. Hence, they allow precisely timed intervention that can preempt the pathology and clearly establish that the cytokine being neutralized most certainly does play a role in the pathology of this condition and, by inference, in those (infectious or otherwise) that it closely resembles. Such insights may not be easy to glean from infectious disease, in which a negative result with a neutralizing antibody (230) may be wrongly interpreted as meaning that the target cytokine is irrelevant. Instead, it may imply that by the time the patient is sick enough to warrant treatment, antibody against an “early” cytokine, such as TNF, is being administered too late in the cascade to be of practical value. The iatrogenic cytokine release syndromes offer an excellent confirmation of this argument.
Jarisch-Herxheimer reaction.
A few years apart, on either side of the end of the 19th century, Jarisch (207) and Herxheimer (185) published different aspects of the severe febrile reaction that accompanied the treatment of syphilis with mercury. Over the decades, this Jarisch-Herxheimer reaction, as it became known, was observed, as reviewed by Bryceson (52), after patients received treatment that killed invading spirochetes, bacteria, and trypanosomes. The reaction seen can be dramatic, leading to respiratory and neurological changes, and is sometimes fatal (52). In 1972 Borrelia recurrentis endotoxin was found in the circulation when this reaction occurred after tetracycline treatment of cases of louse-borne relapsing fever (53), and 9 years later it was proposed that TNF and IL-1 mediated this reaction after treatment of typhoid (99). A decade later, these inflammatory cytokines were detected in serum during the reactions observed after treating louse-borne relapsing fever (291) and Haemophilis influenzae (18). This group of inflammatory cytokines has also been implicated in the reaction seen after treatment of patients carrying filarial nematodes (173, 416). Intriguingly, it appears not to be material of nematode origin that triggers the TNF release in filariasis but, instead, an endotoxin released from gram-negative bacteria from the nematode gut (405). Proof of principle, and the therapeutic value of this information, were subsequently demonstrated when prior administration of anti-TNF antibody prevented the Jarisch-Herxhiemer reaction after penicillin treatment of louse-borne relapsing fever (104, 144).
Adverse reaction to OKT3 therapy.
In the early 1980s, a murine anti-human CD3 monoclonal immunoglobulin G2a antibody, OKT3, which is mitogenic for human T cells, began to be administered in association with transplant surgery. It proved to be highly effective in reversing (or preventing) renal allograft rejection (408). It was not, however, without side effects, generating headache, fever, and rigors in most patients and vomiting, diarrhea, and nausea in about 10% (409). Pulmonary, neurological, gastrointestinal, and thrombotic changes were often also observed. A link to increased release of TNF and IFN-γ into the circulation was made (438). Other inflammatory cytokines (e.g., IL-2, IL-3, IL-4, IL-6, and granulocyte-macrophage colony-stimulating factor) were also shown, as has been reviewed (209), to be produced in this illness, which began to be referred to as the post-OKT3 cytokine release syndrome. As with the Jahrisch-Herxmeimer response, prior treatment that neutralized excessive generation of TNF significantly reduced the side effects of administration of OKT3. In one study, a monoclonal anti-TNF antibody was employed (71), and in another, a recombinant human soluble TNF receptor that binds to both TNF and LT was used (127).
Acute GVHD.
As summarized above, OKT3 is administered to prevent a host-versus-graft reaction by the host immune system, leading to organ rejection. The mirror image, a GVHD, occurs when cellular components of the donor immune system comprise the graft and these immigrants try to reject the body into which they have been introduced. The essential mechanism is thought to be that donor T cells, activated by host alloantigens, proliferate and secrete a variety of cytokines—as if, indeed, OKT3 had been administered. It occurs mostly when allogeneic bone marrow is transplanted. The disease has a 40 to 60% incidence and is fatal in 50% of cases (385). As has been reviewed (174), the multiorgan involvement of its acute form reproduces the illness and pathology seen in patients suffering from trauma, burns, pancreatitis, and systemic infection.
The possibility that TNF is a key mediator in this condition was first suggested and investigated in 1987, when a rabbit polyclonal anti-TNF antibody was shown to greatly reduce mortality in a mouse model of acute GVHD (317). Four years later, TNF was shown to be released into the plasma of humans with this disease (194). While the presence and involvement of cytokine-induced NO in murine GVHD were being argued (154, 192), evidence for involvement of inflammatory cytokine pathways was consolidating (195, 340), including the first use of anti-TNF antibody to prevent the condition in patients (196). The field continued to develop along cytokine lines, with, for instance, a positive correlation being found between human GVHD and elevated levels of TNF-α, IL-6, and IL-10 (287), and with gene polymorphisms associated with some of these cytokines being found to be strongly associated with the likelihood of acute GVHD developing (67, 275). Anti-TNF alone continues to look promising as a treatment (204, 325), although recombinant IL-1 receptor (rIL-1R) did not prevent or improve the condition in a randomized, double-blind, placebo-controlled trial (17). In the mouse model, simultaneous blocking of TNF and IL-1 has given impressive protection (147), suggesting a plausible direction for further clinical studies. In summary, although a practical clinical application of this knowledge has not yet emerged, there is little doubt that the systemic illness of acute GVHD, like that induced by OKT3 therapy, is the result of excessive production of the group of cytokines during inflammation. This includes, but is not restricted to, the mediators mentioned in this brief summary of the field.
INFECTIOUS DISEASES OTHER THAN MALARIA
Other Systemic Protozoal Diseases
As the literature on malarial disease and inflammatory cytokines developed, it was not surprising that researchers studying other systemic protozoal diseases began to explore the possibilities of this approach to their field. Indeed, the general clinical similarities made it a reasonable prediction, and so it proved. In summary, the African (256) and South American (3) trypanosomes shed GPI molecules implicated in the induction of inflammatory cytokines, and these mediators have been implicated in the diseases caused by these parasites (251, 372). They have also been documented in visceral leishmaniasis, caused by Leishmania major (25). Human babesiosis follows the same pattern, with levels of TNF, IFN-γ, IL-2, IL-6, E-selectin, VCAM-1, and ICAM-1 in serum being highly elevated in the acute phase of infection with B. microti. Thus, as well as individual characteristics derived from factors such as the distribution and multiplication rate of the pathogen, these infections all have a sepsis- or malaria-like quality to the illness. Cytokine triggers have also been characterized in intramacrophage organisms that can cause human illnesses clinically similar to malaria, such as Brucella abortus and Coxiella burnetii, both of which contain LPS-like molecules that induce TNF (115, 208).
Severe Viral Diseases
The clinical similarity of influenza and malaria is demonstrated every time the latter is presumed to be the former in an ill traveler who has returned to a malaria-free country. Headache, nausea, myalgia, vomiting, and diarrhea are common to both, but the two diseases can also be very similar in their more serious manifestations. For instance, although a group of severe viral conditions are referred to as the viral hemorrhagic fevers, hemorrhage is by no means restricted to them, and severe influenza (24) and falciparum malaria (324) can also show it. To us these were intriguing similarities, because the widespread pathologies of viral diseases were generally argued to arise from a direct harmful effect of the virus on cells it invades. However, malaria caused strikingly similar symptoms, signs, and multiorgan pathology without invading any cells (if one ignores a few hepatocytes, entry into which is not associated with illness) except erythrocytes—and then often in small numbers. As evidence fitting our proposal of roles for pathogen-induced, host-origin cytokines in malarial disease accumulated, the likelihood of a role for them in the pathophysiology of viral diseases became increasingly compelling.
In the late 1970s, a role for inflammatory cytokines in viral disease was already plausible, since BCG, Corynebacterium parvum, and Coxiella burnetii, which were among the macrophage activators that protected mice against malaria (81, 85, 93) and helped formulate our thinking about the possible role of TNF in disease pathogenesis, had also been shown to protect against viruses in vivo (68, 218, 379, 450). Another observation that defied a traditional explanation, but suited one based on cytokines was that liver cells in which hepatitis B virus was replicating (as determined by immunohistochemistry and in situ hybridization) were not the ones that showed histological evidence of damage (43). The presumed cytokine origin of each of these confusable conditions became difficult to ignore when rTNF infusions into tumor patients produced either influenza-like or malaria-like side effects, depending on the observer's experience (32, 108, 206, 274, 380, 421). A virus (Sendai virus) had by this time been shown to induce TNF (5), although its presence was interpreted in terms of viral inhibition rather than disease in the host. Summarizing the above evidence, the concept of TNF and its downstream mediators being central to viral disease was proposed in 1989 (90).
In the early 1990s, it was still being argued that increased endothelial cell permeability in filovirus infections was caused directly by viral invasion of these cells and multiplication within them (358), but within a few years this group had espoused inflammatory cytokines by demonstrating that the observed increased permeability was, instead, the consequence of virally induced TNF from monocytes and macrophages (145). Since then, the literature on the pathogenesis of serious viral infections, including those caused by a hantavirus, Marburg and Ebola viruses, Lassa and Junin viruses, dengue viruses, and influenza virus, has become focused almost exclusively on arguments for the central roles of inflammatory cytokines. For instance, increased circulating levels of TNF, soluble TNF receptor, IL-6, and IL-10, which characterize a full-blown inflammatory response, have been detected in patients suffering from the hemorrhagic fever with renal syndrome caused by a hantavirus (245). Levels of nitrogen oxides, an indirect measure of NO production, were increased in this disease, in proportion to both TNF levels and hypotension (246). This was also recorded, in a study taking changes in renal function into account, for a European variant of hantavirus infection (169). These principles have also been established for Marburg and Ebola viruses, the clinically important filoviruses. Freshly isolated human monocytes have been shown to support the growth of both of these viruses and to activate them to release IL-1β, TNF, IL-6, and the chemokine IL-8 (383). In addition, treating Marburg-infected guinea pigs with an antagonist of IL-1 receptor or anti-TNF serum has been reported to result in survival of half of the treated animals but none of the untreated animals (202). The same pair of anti-cytokine treatments was found to protect all tested mice from Lassa virus (202). Recently, two fatal cases of Lassa fever imported into Europe were found to be associated with extremely high levels of IFN-γ and TNF shortly before death (357). Levels of TNF and other inflammatory cytokines have also been shown to be increased during Argentine hemorrhagic fever, the illness caused by Junin virus (182, 260).
Dengue hemorrhagic fever follows the same pattern, with culture fluids from dengue virus-infected peripheral blood monocytes being shown to produce TNF (12, 139). Several groups have now detected increased circulating levels of inflammatory cytokines (21, 191, 384) and NO (419) in this disease, and anti-TNF antibody treatment is reported to reduce mortality in experimental dengue virus infection (19). Finally, not only does the virus that causes influenza, the disease perhaps most commonly confused with malaria, stimulate the release of TNF from macrophages (188, 199), but also it has been possible to show that the avian influenza virus, H5N1, which is particularly virulent in humans, generates more TNF in human macrophages than do a range of less virulent strains (73). Again, the levels of both inflammatory cytokines and NO (180, 212) are increased in serum from patients. Finally, changes argued to be consistent with a cytokine-induced pathology have been described in tissues from severe acute respiratory syndrome patients (294), but no direct assays have as yet been performed. The rapidly progressing field of pattern recognition molecules now reports that viruses generate these cytokines by engaging with TLR3, TLR7, and TLR9 (9), not just TLR4, as was formerly thought (124).
At the time of writing, HMGB1 release or PARP-1 activation had not been found in severe viral infections, but the stage is set, with the cytokine and NO release that induce these changes in other inflammatory diseases already in place. Thus, these newly recognised aspects of disease pathogenesis may well apply as much to severe viral diseases as to malaria or sepsis, opening the possibility of therapeutic approaches that have not yet been considered.
SOME COMPARISONS WITH SEPSIS
The Pattern Recognition Molecules, GPI and LPS
Malaria toxin identification, and implications of its recognition pathway.
The idea that malaria parasites secrete a toxin that directly causes the illness and pathology seen in infected individuals is of very long standing (160). But how to identify it? As Maegraith noted in 1969 (255), it had been easy enough to isolate suspect factors from serum from humans or animals with malaria—the problem was to determine whether they were of host or parasite origin. To resolve this problem, an in vitro assay was needed in which the host element could be removed, and some years ago this was provided by the proposal that malarial illness arose because the parasite released material that functioned as though it was enterobacterial LPS, which was then the only recognized trigger for the release of TNF and IL-1 from host cells (83, 99). Later, we added LT to the list of induced mediators (94, 329). By 1988, material extracted from malaria parasites was shown to induce the release of TNF (28, 29), and the structure of these toxins was beginning to be elucidated, with evidence that the active molecule released from the parasite contained phosphatidylinositol (26, 30). The possibility of using this material to generate an anti-disease vaccine for malaria was discussed by this group from the late 1980s (28, 319, 398).
As has been reviewed (267), the GPIs are a special class of glycolipid, ubiquitous in eukaryotic cells, and their primary function, shown first in the African trypanosomes (65), is to anchor certain proteins to outer surfaces of cell membranes. They are much less abundant in animal cells than in parasites, in which amounts severalfold in excess of that required to anchor proteins are synthesized (425). Because of its ability to trigger the release of TNF, malaria GPI, a glycosylated form of phosphatidylinositol, has been incriminated as the malarial toxin (360, 363). Indeed, P. berghei ANKA-infected mice have been protected from pulmonary edema, acidosis, and cerebral pathology by being immunized with a synthesized P. falciparum GPI (361). The broader implications of this are discussed in the next section. These data do, however, provide useful evidence that the GPI molecule is the dominant malarial toxin that induces the release of the proinflammatory cytokines incriminated in malarial disease. They are also consistent with the notion that the cytokines induced by GPI are required to generate these aspects of the pathology of malaria.
It has been suggested that GPI also has significant direct effects on host cells to cause disease independently of TLR and inflammatory cytokines. One line of argument is based on the capacity of GPI to induce ICAM-1 in the absence of IL-1 and TNF (363). However, as reasoned above, malarial GPI very probably induces the production of LT, as well as these other two inflammatory cytokines. LT has been known for some time (200) to induce ICAM-1, and so further experiments with GPI are required to establish whether it is acting on endothelial cells independently of inflammatory cytokines. It is well established that the GPI from T. cruzi uses the same signaling pathways to induce cytokines as does LPS, and cross-tolerizes with it (337), implying that GPI induces the same range of inflammatory cytokines (including TNF, IL-1, and LT) as does LPS.
Another line of argument for a direct harmful effect of GPI concerns the pathogenesis of the hypoglycemia that can occur in severe falciparum malaria. As the cytokine model of malarial disease was developing, it was shown, as predicted by the basic literature, that small doses of TNF (92) or LT (94) would cause hypoglycemia in malaria-primed mice. TNF levels were subsequently shown to correlate with hypoglycemia in African children with malaria (165). These observations were consistent with the prediction that malarial toxin would cause pathology through the intermediary of inflammatory cytokines (83, 99). However, it has been reported that GPI has direct insulinomimetic activity on adipocytes in vitro (360), allowing the possibility that it contributes to malarial hypoglycemia without a requirement for the inflammatory cytokines it induces. Others have demonstrated that this in vitro activity is not specific to GPI but can be readily induced by the 1,2-diacylglycerol moieties of P. falciparum GPI (444). It was subsequently shown that the specific structure of the 1,2-diacylglycerol moiety itself is not critical for the other bioactivity of GPI (specifically TNF release), since removal of the 2-acyl group of P. falciparum GPI with phospholipase A2 produced syn-2-lyso-GPI, which was equally effective as native GPI at inducing TNF secretion (425). The in vivo relevance of the GPI molecule itself (compared to the cytokines it induces) to malarial hypoglycemia remains an open question. In passing, we note that it has been argued that malarial and trypanosomal GPIs are recognized by the CD1-natural-killer antigen 1.1-T-cell pathway of generating immunogobulin G responses, providing a general mechanism for rapid antibody responses in these diseases that would not be restricted by the major histocompatability complex (362). Others have not been able to confirm this (321, 335).
The effects of GPIs from both P. falciparum and T. cruzi on signal transduction pathways that lead to the inflammatory cascade being triggered are so similar (392) that it could reasonably be assumed that, as with T. cruzi GPI (61), P. falciparum GPI is recognized by TLR2, not TLR4. This would group it with the gram-positive bacterial and fungal cell wall components and various other bacterial, mycobacterial, and spirochetal lipoproteins. However, others have reported that P. falciparum GPI induces TNF secretion without membrane insertion and may use a novel mechanism tightly coupled to protein tyrosine kinase activation (425). The functional similarities of these pathways are indeed close, with cross-tolerance (237) and striking synergy between ligands of TLR4 and TLR2 for TNF generation (39). Moreover, while normally present at low concentrations on human endothelial cells, TRL2 is induced on their surface by TNF or IFN-γ (142). Hence, it should be expected that falciparum malaria, and a wide range of infectious agents that release molecules recognized by the host through TLR2 and thence protein tyrosine kinase, should produce diseases that are similar to each other, albeit varying because of the rate and location of release of the cytokine-triggering TLR ligand. Equally, it is predictable that these diseases share the illness and pathology induced by organisms that shed the TLR4 ligand, LPS, since the downstream signaling and thus many host-origin mediators generated by this signaling, are very similar whether TLR4 or TLR2 is engaged. Of course, some of these pathogens have their distinctive signatures. For P. falciparum, this is determined by the location of the organism in erythrocytes that, through changes wrought on their membranes by the parasite they contain, adhere readily to upregulated ICAM on endothelial cells. The implications of this are discussed in the next main section.
Prospects of vaccination against malarial toxin.
The host pattern recognition receptors, typically belonging to the TLR group, contribute to the recognition of bacterial, viral, and protozoal pathogens and also play a part in the initiation of the host immune defense. They also are involved in the signaling pathways that lead to cytokine-induced disease pathogenesis. For example, LPS recognition in mice has complex consequences and appears to be beneficial at lower doses because it is central, through cytokines such as TNF and LT, to generating cell-mediated immunity (CMI) (352) and antibody-dependent responses (236, 250). Indeed, TNF has been shown to be necessary for the development of antibody memory in a mouse model of malaria (241). LPS is also detrimental at higher doses through provoking excessive production of these same cytokines. Thus, immunizing against enterobacterial LPS, reducing the host's ability to generate cytokines such as TNF during an infection, could result in a complex outcome, inhibiting both the CMI and antibody-based immunity, as well as pathology, with the balance not necessarily in the host's favor. For example, the net outcome of treatment of Salmonella-infected mice with anti-TNF antibody is death from an overwhelming bacteremia (262, 289). Vaccinating against TNF-inducing agents such as LPS or GPI is not, as has been suggested (361), a parallel to immunizing against, for example, tetanus toxin, which exerts its harmful effect directly at the synapse and has no worthwhile attributes one might wish to preserve. Malaria-induced TNF, in contrast, does.
Nevertheless, published arguments suggest that it would be worthwhile using malaria GPI as an anti-disease antigen, designed to act through its capacity to reduce the TNF (and doubtless the IL-1 and LT)-induced pathology of malaria (361). GPI is probably the major malarial ligand for its pattern recognition receptor and was selected on its ability to generate all of the TNF that crude malaria extract can generate (364, 392). The capacity of such a vaccine to prevent examples of pathology previously attributed to TNF has been demonstrated in experiments with the mouse P. berghei ANKA model (361). There is the prospect, therefore, of this reduced pathology being accompanied by a reduction in any TNF-dependent CMI or antibody response generated by the host against malaria parasites.
We note, however, that monoclonal antibody against GPI blocks nitric oxide induction by total extracts of P. falciparum, demonstrating that GPI is the sole source of iNOS-generating cytokines induced by blood forms of P. falciparum (391). Therefore, it is reasonable to predict that the observed post-challenge increased in NO production by monocytes from P. falciparum-challenged immune volunteers (320) was provoked by GPI. Thus, GPI probably plays a role in the immunity possessed by these individuals, who, it should be noted, withstood challenge despite lacking any detectable specific antibody response (320). Thus, if a monocyte iNOS step is indeed central to the CMI that protects against parasite growth in people who have never had clinical malaria (and who are potential recipients of such a vaccine), the prospect of immunizing them against malarial disease by using GPI as an antigen (361) raises concerns that this CMI response would not develop and that the parasite would not be controled normally. This is little different in principle from the situation already observed in patients in whom rheumatoid arthritis has been treated with a neutralizing anti-TNF antibody, making latent tuberculosis (213, 264) or listeriosis (374) fulminant by inhibiting the CMI on which immunity to Mycobacterium tuberculosis and Listeria monocytogenes depends. From mouse (95) and human (320) studies, Plasmodium, the source of the GPI, readily provides a CMI-dependent infectious agent. In short, malaria is one of those infections where the cytokines induced by GPI are implicated in host defense as well as in the pathogenic processes. Such an anti-disease approach should therefore proceed with caution. If controlling death from malaria were this simple, vaccinating against enterobacterial LPS would protect against fatality from sepsis caused by most gram-negative bacteria. Much will depend on how universal in P. falciparum infections the recently described functionally important CMI in the absence of specific antibody (320) proves to be.
Is Long-Term Sepsis Different?
The comparisons in this review are between malaria and infectious or noninfectious acute diseases that are all, in effect, acute cytokine release syndromes in patients who are in a hyperimmune state. Details of tissue distribution and cytokine profile generated are very real and have discernible effects in different diseases, but there is an overall pattern that has been extensively acknowledged and studied. While modern life support systems and antibiotics are unquestionably advantageous for the patient, they do complicate the meaning of the term “sepsis” through keeping very sick patients alive longer. This increases the proportion of cases that still come under the sepsis umbrella but have a hypoimmune pathophysiology that in many ways is the mirror image of the acute cytokine release syndromes. Several recent reviews have discussed these late deaths as being the product of an overactive compensatory anti-inflammatory response that causes an immunosuppression so severe that overwhelming infection ensues (198, 293).
Undoubtedly this anti-inflammatory type of syndrome exists. To date, however, neither of these groups appear to have taken release of HMGB1 into account in their perceptions of events. Not only can HMGB1 play the role, as described above, of a cytokine released much later than those which have been more extensively studied (428), but also it is released from cell nuclei in necrotic tissue (350). New waves of necrosis, and thus HMGB1 release, can be expected to be triggered by regional severe ischemia for as long as the patient survives. As noted above, administration of rHMGB1 induces acute lung injury, intestinal barrier dysfunction, and lethal systemic inflammatory responses. The kinetics of HMGB1 release in long-term sepsis remain to be explored. In addition, the energy-depleting consequences of severe PARP-1 activation are a logical long-term consequence, through the action of peroxynitrite, of a hyperimmune state (37, 365, 387) and are consistent with the strikingly low ATP levels seen in muscle biopsy specimens from patients with long-term sepsis (51). In addition, depletion of lymphoid tissues is well recognized in falciparum malaria, as in other infections (63), but death commonly occurs, even in drawn-out cases, without overwhelming secondary septicemia. This would not be missed, since a bacteremia would show on the blood smears that are regularly Geimsa stained to monitor malaria parasites. Thus, death during the long-drawn-out hypoimmune stages of systemic infectious diseases may be largely outside the comparison between malaria and the other cytokine release syndromes that is the topic of this review. As noted above, a study of HMGB1 under these circumstances will clarify this.
ROLES FOR THESE MECHANISMS IN MALARIAL DISEASE
Sequestration
The phenomenon of erythrocytes containing malaria parasites adhering to endothelial cells is restricted, among the human malarias, to P. falciparum. It is referred to as parasite sequestration and mostly affects red cells as the parasite they contain reaches the second half of its 48-h maturation cycle. The traditional basis for disease pathogenesis, particularly the neurological symptoms, of falciparum malaria has been that these sequestered parasites hinder the flow of arterial blood to tissues particularly sensitive to such deprivation, such as the brain (252). The main difficulty that has always confronted this idea is to reconcile it with the stroke literature and explain, bearing in mind that the brain is exquisitely sensitive to hypoxia, why most patients who recover from cerebral malaria have no residual neurological deficit (431), implying negligible cellular damage. Studies of the pathogenesis of strokes have indicated that cerebral infarction, and subsequent long-term loss of function, can occur if perfusion pressure to any part of the brain is 15 to 20 mm Hg or less for a period of 20 to 30 min (4). This is inconsistent with a simple oligemic coma, perhaps lasting for several days (219), routinely resolving with a rapid and full return of neurological function.
The mature stages of P. falciparum are absent from circulating blood (i.e., absent from fingerprick blood smears) of ill patients and also of malaria-tolerant patients (i.e., those who are not ill despite having an appreciable parasite burden). However, young forms reliably appear at the start of the next cycle, so there is no question that the parasites were present. In other words, the red cells containing these mature forms are sequestered somewhere, whether the host is sick or not. Hence, should sequestration be an essential contributor to the illness due to falciparum malaria, as often stated, it must do so through its anatomical location, which has to be presumed to differ in healthy malaria-tolerant and ill malaria-sensitive infected individuals. Any worthwhile model for the events in severe falciparum malaria therefore has to include an explanation for how the sites of sequestration could differ between malaria-susceptible and malaria-tolerant individuals. In other words, how do malaria-tolerant children, who are heavily infected but seemingly healthy, manage to keep their high parasite loads sequestered in parts of the vascular tree where they do not cause harm? It is not just a question of parasite load, since counts have been shown not to differ significantly between patients with cerebral malaria and severe noncerebral malaria (302). A further complication is the evidence of the great variation (about 100-fold) found in peripheral parasite density in 6-h repeat observations in a malaria-tolerant population (116). It has also to be taken into account that in vitro adhesion of red cells parasitized with Babesia bovis (which causes in cattle a disease that closely mirror human falciparum malaria) does not select for strain virulence (283), as the literature had led people to presume. A static adhesion system employing umbilical endothelial cells, on which much malaria literature is based, was used. Finally, the notion of a primary requirement for brain sequestration in the coma of cerebral malaria has to accommodate the findings in the first large histological study of African children with falciparum malaria, in which about one-third of the 32 children clinically diagnosed as having cerebral malaria, using established criteria and examining more brain regions than is typical in the literature for adult cases, had negligible sequestration in the brain (87). This is discussed in the next section.
Toward Understanding the Coma of Severe Malaria
Septic encephalopathy.
Septic encephalopathy (46, 133, 443) is not well understood but is increasingly thought to involve the downstream effects of inflammatory cytokines leading to reduced oxygen extraction by the brain, energy depletion, cerebral edema, and disruption of the blood-brain barrier. A parallel entity, much less intensively investigated, accompanies noninfectious conditions in which the levels of these mediators are raised, such as traumatic surgery (415) and burn injury (282). As in malaria, altered mental status is highly associated with a fatal outcome in septic encephalopathy (381). An excellent experimental model has been the endotoxemic pig, in which a dramatic elevation of intracranial pressure has been observed, despite arterial hypotension (177). The field has been reviewed recently (130, 308). As noted earlier, overactivation of PARP-1 through the action of peroxynitrite can run down ATP levels (365). This would considerably affect the integrity of the membranes that maintain cellular function in the brain, since over 40% of all the energy released by cellular respiration in central neurons is consumed by the membrane-bound Na+/K+-ATPase that runs the pump that prevents sodium, and therefore water, accumulation in the brain (138). In other words, a key role of this enzyme in the brain is to prevent edema, which dramatically increases intracranial pressure. NO also inhibits this pump (172, 332). As summarized in this section, the evidence for systemic inflammation in malaria is consistent with these processes affecting brain function, irrespective of the degree of pathology visible on brain sections. As outlined below, this could, however, affect consciousness.
Another way in which mental status could be affected in these conditions is through inflammatory mediators released from activated macrophages inducing indoleamine-2,3-dioxygenase and thus converting l-tryptophan into quinolinic acid, a brain excitotoxin. The interest in the harmful effect of quinolinic acid on neurons comes from the work of Heyes and coworkers on AIDS dementia in 1991 (185a). Depending on the degree of severity of mental change in these patients, quinolinic acid levels were increased 2- to 20-fold in the cerebrospinal fluid (CSF) and generally paralleled the severity of cognitive and motor dysfunction. Arguments have been proposed for the relevance of quinolinic acid to the pathology of brain trauma (36), and interest has now moved to its possible involvement in cerebral malaria in children in Kenya, with kynurenine pathway metabolites in the CSF being increased in proportion to the degree of severity (122). Subsequently, several metabolites of this pathway were measured in the CSF of adults with cerebral malaria in Thailand, but no correlation with convulsions or depth of coma was found (270). Being downstream of inflammatory activity and acting, at least in part, through NO (288, 313), this pathway, whether it proves crucial to the outcome or not, is an additional indication of the relevance of inflammation in these diseases.
Malaria encephalopathy.
If sequestration is the essential difference between falciparum malaria and other infections in which TLR2 recognizes the presence of an infectious agent, how different in principle is malaria from these other diseases? As outlined below, and based on the findings of the first large-scale immunohistochemistry study of African pediatric cases (86, 87), any difference may initially be very small, with some of these children dying, after a coma, in this stage. One-third of the fatal clinically defined cerebral malaria cases for which autopsies were performed [11 of 32, termed CM(A)] had little or nothing to see in the brain (6 to 13 different brain regions examined) by way of sequestered parasites, let alone monocytes clusters, microhemorrhages, iNOS or HO-1 staining that would have indicated the advent of vascular inflammatory change at the time of death. Yet they were clinically diagnosed as cerebral malaria on the same criteria as were groups CM(B) and CM(C). Two of the CM(A) group had severe anemia, and others, on autopsy, proved to have nonmalarial changes, such as bacterial pneumonia or patchy hepatic necrosis. These changes can be expected to have contributed to a fatal outcome and caused it to have occurred sooner that it would have done otherwise. Thus, this CM(A) group, making up one-third of the patients, possibly gives us an instructive insight into what had been happening inside the cerebral vasculature of the CM(B) and CM(C) patients after they had become comatose, but some time before death (Fig. 4).
FIG. 4.
HO-1 staining of brain (A to C) and liver (D to F) tissue of each of the three categories used to group the 32 clinically defined cases of cerebral malaria, on the basis of their histological and immunohistochemistry findings, as CM(A), (A and D), CM(B), (B and E); and CM(C) (C and F). Panel A typifies the 11 cases with isolated or undetectable parasites or HO-1-stained monocytes in brain sections. Panels B and C are examples from the 7 and 14 cases, respectively, with increasing parasite and cellular involvement in cerebral blood vessels: Panel B shows an influx of young intraerythrocytic malaria parasites (para) but negligible monocytes, and panel C illustrates the more mature parasites, monocyte accumulations (mono), and microhemorrhages (hemorrh) typical of CM(C). All three categories showed systemic inflammation, as demonstrated by strongly staining Kupffer cells (D to F). Dilution of primary anti-HO-1 antibody (StressGen) was 1:1,000. Staining was done as described for Fig. 3. Magnification, ×200 in all cases.
These CM(A) patients showed appreciable evidence of systemic inflammation, as demonstrated by the presence of iNOS, MIF (87), and HO-1 (86). These systemic changes were shared with those found in the comatose sepsis patients in the series — indeed, there was nothing to tell them apart immunohistochemically, and the depth of coma was of the same order in both groups. From an inflammatory perspective, the causes of coma and death in both this subset of the malaria patients and the sepsis patients were not fundamentally different. From the broader literature (91), the outcome in all cases was most probably based on the acute downstream effects of inflammatory cytokines, including energy depletion and metabolic acidosis, although data that could have provided this degree of detail were unavailable for these individual cases.
We interpret another seven patients with fatal pediatric cerebral malaria [CM(B) in reference 87] as having survived long enough for sequestration to have occurred in the brain, but not as long as did the CM(C) group, discussed below. These seven CM(B) patients showed the same systemic evidence of inflammation as did the CM(A) patients and, in addition, sequestered parasites in cerebral blood vessels, sometimes (in three of the seven patients) in very large numbers. Almost all were young trophozoites, uniformly containing only a small fleck of malarial pigment. Sections from three of the seven CM(B) brains were shown, by immunohistochemistry, to have intravascular platelet accumulations (164). However, evidence for the onset of vascular inflammatory change in the cerebral vasculature (microhemorrhages, iNOS, or HO-1-stained monocyte clusters [see the next paragraph]), was, at least in the up to 11 blocks per brain examined, almost entirely lacking (Fig. 4). As noted earlier, once platelets become cytokine activated, they export HMGB1 to their surfaces (338), and this has the capacity, through engaging (and upregulating) RAGE on endothelial cells, to cause a local amplification of the inflammatory cascade, including an increase in the expression of ICAM-1 and VCAM-1 (149). Hence, platelet-origin HMGB1 can be expected to provide a bridge between the start of accumulations of activated platelets and a change in local conditions that allow parasitized red cells to colonize new territory. In our view, this CM(B) group died late enough to allow this colonization to happen but did not live long enough to allow the adhering parasitized red cells to complete their 48-h cycle, terminating in red cell rupture and GPI release and the initiation of local inflammatory events. To us, the logic is that coma occurred in this CM(B) group for essentially the same systemic reasons as in the 11 CM(A) cases, with a probable contribution from cerebral hypoxia in those with particularly heavy loads of sequestered parasites. In addition to presumed cerebral hypoxia, these patients had as massive a degree of systemic inflammation as any in the study (87), so that a major contribution from this cannot be discounted. There was, however, very little if any evidence for the possibility of a contribution to coma from local post-schizogony inflammation within the cerebral vasculature.
The third category of clinically defined cerebral malaria cases, CM(C), comprised 14 of the 32 patients described (87). Our interpretation of these, again taking the platelet accumulation data into consideration (164), is that they survived the progression of their disease long enough for the cerebrally sequestered parasitized red cells to have completed a 48-h cycle and, at the post-schizogony burst, to have initiated new local inflammatory hot spots within the brain through GPI release. Hence, sequestered parasites, monocyte clusters, microhemorrhages, vascular wall iNOS, and monocyte HO-1 staining were well in evidence in the cerebral vasculature at the time of death (87). Most brain sections from CM(C) patients readily showed evidence of the microhemorrhages and intravascular inflammation that are generally regarded as widespread in adult Asians with fatal cerebral malaria (252, 269, 271, 371), and the impression from this literature is that, as in our CM(C) group, virtually every adult brain section examined by these investigators (252, 269, 271, 371) showed these changes. This has obvious relevance to the degree to which hypoxia from impaired blood flow could have contributed to these different subpopulations of African pediatric patients. Insufficient oxygen has many consequences, some more obvious than others. For example, it enhances nearby inflammation: a recent report provides evidence that the protein hypoxia-inducible factor 1-α upregulates the expression of at least 30 genes that govern inflammation when oxygen levels are low (106). It should be noted, however, that several treatments known to inhibit and reverse the adherence of parasitized red cells in vitro were inactive in double-blind trials with cerebral malaria patients (105, 406). We have recently reviewed the arguments that hypoxia (which we think is important in the pathogenesis of falciparum malaria) may well be caused more by ineffective utilization of oxygen, through the effects of inflammatory cytokines on energy depletion and therefore mitochondrial function, than by poor oxygen delivery (91).
Considering our CM(A), CM(B) and CM(C) groups collectively, we think there is every reason to assume that the systemic causes of encephalopathy that were acting in the CM(A) and CM(B) cases were acting even more strongly on the CM(C) brains, so that coma was probably present before the obvious changes in the brain had had time to develop. It seems reasonable to us to suggest that once these secondary changes in the local cerebral vasculature develop to the degree observed, there is little chance of recovery without neurological deficit. Moreover, we see little evidence for assuming that these obvious brain changes were involved in the onset of coma. The unanswerable question is whether the patients who experience coma, but do not develop local brain changes, have a higher chance of survival. In other words, are they underrepresented in the fatal cases compared to the total number who develop coma? Descriptions of most fatal adult cerebral malaria cases (252, 371) fit, in terms of sequestered parasites and microhemorrhages, into our CM(C) category. Conceivably, this is because these patients had enjoyed a more robust premalaria physical state than do many African children, and this contributed to their surviving long enough for the sequence of brain changes to mature to the degree observed in our CM(C) group. Unfortunately, immunohistochemistry to detect inflammatory change elsewhere in the body has not yet been performed for such adult patients. We have previously summarized the implications of high iNOS levels in the walls of brain blood vessels and also the logic of the observed coassociation of cerebral vascular wall iNOS and cerebral microhemorrhages (91). In addition, these CM(C) cases showed the strongest evidence of systemic inflammation. Thus, while the cerebral changes accumulate in patients who live long enough, and begin to affect brain function locally, the effects of systemically generated proinflammatory cytokines, including effects on the brain, would continue to increase (78).
On average, the nonmalarial encephalopathies examined for platelet accumulation in this patient series showed accumulation as strongly as did the cerebral malarias in the CM(B) or CM(C) categories (164), but of course in the absence of parasitized red cells to adhere to any upregulated ICAM-1. Nevertheless, the patients were comatose and showed immunohistochemical evidence of systemic inflammation as strongly as did the malaria cases (86, 87). This emphasizes that key underlying changes of malaria pathophysiology are shared, just as intensely, with a range of other conditions in which TLR2 recognizes the presence of an infectious agent.
Of course, the changes that distinguished CM(A), CM(B), and CM(C) and the suggested reasons for their differences, the progression that might link them together, and as the changes they share with sepsis would have been missed in the absence of immunohistochemistry of samples collected at autopsy. Unfortunately, most clinically diagnosed cases of cerebral malaria are unavoidably placed into a single category, as, indeed, were the nonautopsied two-thirds of fatal cases in this scientific study, let alone the many other patients for whom a clinical diagnosis of cerebral malaria is made in Africa daily. Little progress in understanding this condition is possible in such circumstances, and additional autopsy-based studies, bringing clinical and scientific expertise together, are the best way forward. The more molecular aspects of the impaired consciousness of falciparum malaria have been reviewed recently (91).
HOMEOSTASIS VERSUS PATHOLOGY IN INFLAMMATORY STATES
Efficacy of Anti-Inflammatory Agents in Systemic Disease States
In view of the disappointing outcome of all the expenditure on anti-inflammatory agents to treat sepsis, are the inflammatory cytokines really the way to understand the various sepsis states, including malaria? We think so. First, it is easy to reproduce the essential changes of the disease states discussed in this review by injecting TNF, IL-1, or LT. Second, these cytokines, and other elements of the complex cascades they set in motion, can be detected in the cells and circulation of affected patients. These two sets of observations have led to the logical (and, we believe, correct) argument that these molecules are probably central to the pathogenesis of these diseases. A key additional and practical piece of evidence is to know whether these diseases can be prevented or reversed by inhibiting the action of a target cytokine. This reassurance is readily obtained by studying the iatrogenic conditions, such as the Jarisch-Herxheimer reaction (104), adverse reaction to OKT3 (127), or GVHD (204), where it is easy, through pretreatment, to stop the illness before it starts. The close parallels between these conditions and sepsis states, along with the vanishingly small chance that the body would have two unrelated systems to produce this common set of complex changes, is very good circumstantial evidence for cytokine involvement across the board.
Nevertheless, obtaining this type of evidence becomes increasingly difficult once the systemic disease state is already under way, and the test becomes whether illness can be reversed by, for example, an antibody that neutralizes TNF. This carries two dangers with it, the first being suppression of the immune response against pathogens. The limitations that this puts on the possible clinical usefulness of anti-TNF were recognized early, when it not only failed to protect mice against cecal puncture peritonitis but actually made the outcome worse (128). Indeed, TNF was a requirement for survival in these animals (129). This is entirely consistent with the role of this cytokine in CMI against various pathogens and has had a more recent echo in the exacerbation of dormant tuberculosis in arthritis patients treated with anti-TNF agents (213), as discussed above. Conceptually, this is a simple negative-feedback homeostatic loop, with TNF and iNOS suppressing the growth of the organism that increased their production in the first place.
The second danger was that anti-TNF (and other approaches aimed at the early events of sepsis) would be administered at a time and circumstance when its main action would be, through neutralizing TNF, to inhibit a range of other homeostatic negative-feedback loops, directed at other mediators rather than infectious agents, that are built into the inflammatory cascade and switch them off after they have done their necessary tasks. Examples are NO (411) and CO (233) inhibiting the TNF that induced the iNOS and HO-1 that generated these gaseous mediators of homeostasis. Thus, inhibiting these homeostatic pathways in the less severe sepsis states could explain how anti-TNF and similar approaches can make these cases worse. This problem appears to be a general principle across a number of anti-inflammatory agents that have been tested in patients with sepsis. For example, recombinant activated protein C (38) has broken new ground as the first Food and Drug Administration-approved drug for severe sepsis in adults. However, despite providing a sufficient advantage over what had gone before to attract conditional approval, it too (131) is prey to the same problems that have bedeviled the other systemic anti-inflammatory agents subjected to trials in treatment of human sepsis (132), being on average harmful to more mildly affected patients and improving outcome only in those with more severe disease before treatment. Indeed, when the animal experiments that provided the encouragement for human trials with this class of agent were repeated with conditions changed so that the risk of death without treatment was lower, the same relationship as has been found in many human trials (harm when the risk of death is low, improvement when the risk is high) was found (132). Some active process such as interference with homeostasis has to be invoked, since fluid therapy, when run in parallel, did not decrease the chance of survival in low-risk groups (132). This implies that as the severity of the disease increases, a point is reached at which anti-TNF, and by inference similar agents, changes from being harmful to being helpful.
What might that point be? The obvious possibility is that this point is reached when the inflammatory state becomes so severe that its capacity to cause downstream harm exceeds the capacity of the suppressive effects of the iNOS-induced NO and the HO-1-induced CO, in other words, when the inflammatory state starts to lose homeostasis. Unfortunately, this critical point does not immediately appear to lend itself to definition in the individual patient. An alternative philosophy is to regard this point as the time when the “late” mediators, induced in their turn by TNF and IL-1, start to dominate. Unlike TNF and IL-1, MIF (35) and HMGB1 (428) levels remain increased for a long period. Again unlike anti-TNF (128), agents that inhibit MIF and HMGB1 will rescue mice from cecal puncture peritonitis even when administered 8 h (60) or 24 h (242, 418), respectively, after onset of the lesion. It will be instructive to see whether, on testing in models with low risk of death, MIF- and HMGB1-blocking agents exacerbate illness in the way in which the other agents have done. As far as we are aware, there is no reason to expect this. It is plausible that much of the TNF suppressed when its inhibitors produce a successful outcome in severe sepsis is actually generated in the “second wave” of inflammatory cytokines generated by HMGB1 acting through RAGE receptors (149). As first proposed by Schmidt et al. (355), these changes have the characteristics of a self-perpetuating and amplifying system of functional derangement. This is in contrast to many of the effects mediated through TLRs that are the conduit to the earlier generation of these cytokines by LPS or malaria toxin. This difference is presumably what causes these same cytokines, when induced by HMGB1, to have a slower onset and considerably longer duration (14, 396) than when they are induced by LPS, implying that the RAGE pathway is not susceptible to inhibition by NO or CO. This remains to be tested. If it proves so, any anti-TNF that targets HMGB1-induced TNF would encounter no homeostatic potential to be inhibited and therefore would have no pathway by which to exacerbate illness.
The other novel therapeutic approach that has considerable potential is to inhibit PARP-1. As discussed above, overactivation of PARP-1 through peroxynitrite generated from NO and superoxide downstream of TNF can lead to bioenergetic failure (365), enhancement of proinflammatory cytokine generation (240), and dramatic effects in a model infection (222). The capacity of pyruvate to prevent PARP-1 activation in a swine model of hemorrhagic shock (284) and the usefulness of ethyl pyruvate as an HMGB1 inhibitor (418) imply that these new approaches to understanding sepsis may be bound together functionally and perhaps will prove to form a single therapeutic entity.
CONCLUDING REMARKS
Once it began to be accepted that many disease processes were initiated by overenthusiastic production of the cytokines that comprise the inflammatory cascade, there was considerable optimism in some quarters that these molecules could be neutralized to achieve a useful therapeutic effect. This hope had substance in chronic conditions such as rheumatoid arthritis, where inflammatory cells are constantly being recruited, allowing treatment to be initiated at any time. This concept could also be harnessed to preempt the onset of iatrogenic systemic inflammatory states, such as the Jarisch-Herxheimer syndrome. The use of this application in acute life-threatening circumstances, such as severe sepsis and falciparum malaria, has been less rewarding. This is probably because the relatively explosive nature of these conditions makes them an inconstant target, with a series of changes quickly suppressing the one that preceded them but creating their own survival challenges for the host (403). In particular, the importance of preserving the homeostatic functions of these cytokines, which dominate in the less severely affected patients who probably would survive without interference in the inflammatory cascades, needs more recognition. It seems clear that if a practical therapeutic agent is going to emerge for these acute systemic conditions, it will come from a better appreciation of the late-stage mediators of these diseases, including MIF upregulation, PARP-1 activation, and HMGB1 release, all of which are still being explored and have considerable promise. Inhibition of these later pathways, probably in conjunction with some of the approaches currently being tested, seems to us the most promising way forward.
Finally, the theme of this review has a direct relevance to the historic controversy at the heart of the era of the onset, in France, of awareness of the infectious nature of disease. Long after both players had left the scene, the cytokine approach to disease pathogenesis can be said to have eventually reconciled Claude Bernard's opinion that all these conditions were disturbances of the patient's internal cellular environment and Louis Pasteur's subsequent view that each disease was, instead, the direct result of specific microbial invaders and their toxins. Of course Pasteur's approach was essential for vaccine development, for the concept of asepsis, and for microbiology to become a science, but it had two inferences that did not advance the understanding of disease mechanisms: that acute systemic diseases caused by different microorganisms harmed the host in fundamentally different ways, and that noninfectious diseases, such as hemorrhagic shock, were very different from those caused by infectious agents. Inflammatory cytokines are the link that allows these two views to be seen as different stages of the same story, with Pasteur's infectious agents leading to very similar proinflammatory cytokine pathways being excessively stimulated in various organs, altering cellular functions in ways not unique to that disease, as visualized by Bernard.
Acknowledgments
Kirk Rockett, Dominic Kwiatkowski, Jean-Louis Virelizier, and Craig Boutlis are thanked for helpful discussions. Funded through the Wellcome Trust and NIH respectively, Malcolm Molyneux and Terrie Taylor provided a unique bank of human tissue, as well as their clinical expertise.
Funding from the Australian National Health and Medical Research Council, the Tropical Disease Research section of the World Health Organization, and at a crucial time from the Ben Brown Anti-Malaria Fund, is also gratefully acknowledged.
REFERENCES
- 1.Abdelkarim, G. E., K. Gertz, C. Harms, J. Katchanov, U. Dirnagl, C. Szabo, and M. Endres. 2001. Protective effects of PJ34, a novel, potent inhibitor of poly(ADP-ribose) polymerase (PARP) in in vitro and in vivo models of stroke. Int. J. Mol. Med. 7:255-260. [PubMed] [Google Scholar]
- 2.Abrahamsohn, I. A., and R. L. Coffman. 1995. Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J. Immunol. 155:3955-3963. [PubMed] [Google Scholar]
- 3.Abuin, G., A. S. Couto, R. M. Delederkremer, O. L. Casal, C. Galli, W. Colli, and M. J. M. Alves. 1996. Trypanosoma cruzi: the TC-85 surface glycoprotein shed by trypomastigotes bears a modified glycosylphosphatidylinositol anchor. Exp. Parasitol. 82:290-297. [DOI] [PubMed] [Google Scholar]
- 4.Adams, J. H. 1989. Cerebral infarction — its pathogenesis and interpretation. J. Pathol. 157:281-282. [DOI] [PubMed] [Google Scholar]
- 5.Aderka, D., H. Holtmann, L. Toker, T. Hahn, and D. Wallach. 1986. Tumor necrosis factor induction by Sendai virus. J. Immunol. 136:2938-2942. [PubMed] [Google Scholar]
- 6.Aggarwal, B. B., B. Moffat, and R. N. Harkins. 1984. Human lymphotoxin: production by a lymphoblastoid cell line, purification and initial characterization. J. Biol. Chem. 259:686-691. [PubMed] [Google Scholar]
- 7.Agnello, D., H. Wang, H. Yang, K. J. Tracey, and P. Ghezzi. 2002. HMGB-1, a DNA-binding protein with cytokine activity, induces brain TNF and IL-6 production, and mediates anorexia and taste aversion. Cytokine 18:231-236. [DOI] [PubMed] [Google Scholar]
- 8.Ahvazi, B. C., P. Jacobs, and M. M. Stevenson. 1995. Role of macrophage-derived nitric oxide in suppression of lymphocyte proliferation during blood-stage malaria. J. Leukoc. Biol. 58:23-31. [DOI] [PubMed] [Google Scholar]
- 9.Akira, S., and H. Hemmi. 2003. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85:85-95. [DOI] [PubMed] [Google Scholar]
- 10.Al Yaman, F., B. Genton, D. Mokela, K. A. Rockett, M. P. Alpers, and I. A. Clark. 1996. Association between serum levels of reactive nitrogen intermediates and coma in children with cerebral malaria in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 90:270-273. [DOI] [PubMed] [Google Scholar]
- 11.Alzeer, A. H., A. Al-Arifi, A. S. Warsy, Z. Ansari, H. Zhang, and J. L. Vincent. 1999. Nitric oxide production is enhanced in patients with heat stroke. Intensive Care Med. 25:58-62. [DOI] [PubMed] [Google Scholar]
- 12.Anderson, R., S. L. Wang, C. Osiowy, and A. C. Issekutz. 1997. Activation of endothelial cells via antibody-enhanced dengue virus infection of peripheral blood monocytes. J. Virol. 71:4226-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersson, U., H. Erlandsson Harris, H. Yang, and K. J. Tracey. 2002. HMGB1 as a DNA-binding cytokine. J. Leukoc. Biol. 72:1084-1091. [PubMed] [Google Scholar]
- 14.Andersson, U., H. C. Wang, K. Palmblad, A. C. Aveberger, O. Bloom, H. Erlandsson-Harris, A. Janson, R. Kokkola, M. H. Zhang, H. Yang, and K. J. Tracey. 2000. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 192:565-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aneman, A., V. Backman, J. Snygg, C. Vonbothmer, L. Fandriks, and A. Pettersson. 1994. Accumulation of an endogenous inhibitor of nitric oxide synthase during graded hemorrhagic shock. Circ. Shock 44:111-114. [PubMed] [Google Scholar]
- 16.Anstey, N. M., J. B. Weinberg, M. Hassanali, E. D. Mwaikambo, D. Manyenga, M. A. Misukonis, D. R. Arnelle, D. Hollis, M. I. McDonald, and D. L. Granger. 1996. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J. Exp. Med. 184:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antin, J. H., D. Weisdorf, D. Neuberg, R. Nicklow, S. Clouthier, S. J. Lee, E. Alyea, C. McGarigle, B. R. Blazar, S. Sonis, R. J. Soiffer, and J. L. M. Ferrara. 2002. Interleukin-1 blockade does not prevent acute graft-versus-host disease: results of a randomized, double-blind, placebo-controlled trial of interleukin-1 receptor antagonist in allogeneic bone marrow transplantation. Blood 100:3479-3483. [DOI] [PubMed] [Google Scholar]
- 18.Arditi, M., W. Kabat, and R. Yogev. 1993. Antibiotic-induced bacterial killing stimulates tumor necrosis factor-alpha release in whole blood. J. Infect. Dis. 167:240-244. [DOI] [PubMed] [Google Scholar]
- 19.Atrasheuskaya, A., P. Petzelbauer, T. M. Fredeking, and G. Ignatyev. 2003. Anti-TNF antibody treatment reduces mortality in experimental dengue virus infection. FEMS Immunol. Med. Microbiol. 35:33-42. [DOI] [PubMed] [Google Scholar]
- 20.Ayala, A., M. M. Perrin, D. R. Meldrum, W. Ertel, and I. H. Chaudry. 1990. Hemorrhage induces an increase in serum TNF which is not associated with elevated levels of endotoxin. Cytokine 2:170-174. [DOI] [PubMed] [Google Scholar]
- 21.Azeredo, E. L., S. M. O. Zagne, M. A. Santiago, A. S. Gouvea, A. A. Santana, P. C. F. Neves-Souza, R. M. R. Nogueira, M. P. Miagostovich, and C. F. Kubelka. 2001. Characterisation of lymphocyte response and cytokine patterns in patients with Dengue fever. Immunobiology 204:494-507. [DOI] [PubMed] [Google Scholar]
- 22.Bahrami, S., Y. M. Yao, G. Leichtfried, H. Redl, I. Marzi, and G. Schlag. 1997. Significance of TNF in hemorrhage-related hemodynamic alterations, organ injury, and mortality in rats. Am. J. Physiol. 41:H2219-H2226. [DOI] [PubMed] [Google Scholar]
- 23.Baigrie, R. J., P. M. Lamont, D. Kwiatkowski, M. J. Dallman, and P. J. Morris. 1992. Systemic cytokine response after major surgery. Br. J. Surg. 79:757-760. [DOI] [PubMed] [Google Scholar]
- 24.Banks, R. A., and J. Tinker. 1977. Fulminating haemorrhagic influenza. Br. Med. J. 1:1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barral-Netto, M., R. Badaro, A. Barral, R. P. Almeida, S. B. Santos, F. Badaro, D. Pedral-Sampaio, E. M. Carvalho, E. Falcoff, and R. Falcoff. 1991. Tumor necrosis factor (cachectin) in human visceral leishmaniasis. J. Infect. Dis. 163:853-857. [DOI] [PubMed] [Google Scholar]
- 26.Bate, C. A., J. Taverne, H. J. Bootsma, R. C. Mason, N. Skalko, G. Gregoriadis, and J. H. Playfair. 1992. Antibodies against phosphatidylinositol and inositol monophosphate specifically inhibit tumour necrosis factor induction by malaria exoantigens. Immunology 76:35-41. [PMC free article] [PubMed] [Google Scholar]
- 27.Bate, C. A. W., J. Taverne, A. Dave, and J. H. L. Playfair. 1990. Malaria exoantigens induce T-independent antibody that blocks their ability to induce TNF. Immunology 70:315-320. [PMC free article] [PubMed] [Google Scholar]
- 28.Bate, C. A. W., J. Taverne, and J. H. L. Playfair. 1988. Malarial parasites induce TNF production by macrophages. Immunology 64:227-231. [PMC free article] [PubMed] [Google Scholar]
- 29.Bate, C. A. W., J. Taverne, and J. H. L. Playfair. 1989. Soluble malarial antigens are toxic and induce the production of tumour necrosis factor in vivo. Immunology 66:600-605. [PMC free article] [PubMed] [Google Scholar]
- 30.Bate, C. A. W., J. Taverne, E. Román, C. Moreno, and J. H. L. Playfair. 1992. Tumour necrosis factor induction by malaria exoantigens depends upon phospholipid. Immunology 75:129-135. [PMC free article] [PubMed] [Google Scholar]
- 31.Baue, A. E., R. Durham, and E. Faist. 1998. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock 10:79-89. [DOI] [PubMed] [Google Scholar]
- 32.Bauer, K. A., H. T. Cate, S. Barzeger, D. R. Spriggs, M. L. Sherman, and R. D. Rosenberg. 1989. Tumor necrosis factor infusions have a procoagulant effect on the hemostatic mechanism of humans. Blood 74:165-172. [PubMed] [Google Scholar]
- 33.Becker, W. K., R. L. Shippee, A. T. McManus, A. J. Mason, and B. J. Pruitt. 1993. Kinetics of nitrogen oxide production following experimental thermal injury in rats. J. Trauma 34:855-862. [DOI] [PubMed] [Google Scholar]
- 34.Beckman, J. S., and J. P. Crow. 1993. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem. Soc. Trans. 21:330-334. [DOI] [PubMed] [Google Scholar]
- 35.Beishuizen, A., L. G. Thijs, C. Haanen, and I. Vermes. 2001. Macrophage migration inhibitory factor and hypothalamo-pituitary-adrenal function during critical illness. J. Clin. Endocrinol. Metab. 86:2811-2816. [DOI] [PubMed] [Google Scholar]
- 36.Bell, M. J., P. M. Kochanek, M. P. Heyes, S. R. Wisniewski, E. H. Sinz, R. S. B. Clark, A. R. Blight, D. W. Marion, and P. D. Adelson. 1999. Quinolinic acid in the cerebrospinal fluid of children after traumatic brain injury. Crit. Care Med. 27:493-497. [DOI] [PubMed] [Google Scholar]
- 37.Berger, N. A., and S. J. Berger. 1986. Metabolic consequences of DNA damage: the role of poly (ADP-ribose) polymerase as mediator of the suicide response. Basic Life Sci. 38:357-363. [DOI] [PubMed] [Google Scholar]
- 38.Bernard, G. R., J. L. Vincent, P. F. Laterre, S. P. LaRosa, J. F. Dhainaut, A. Lopez Rodriguez, J. S. Steingrub, G. E. Garber, J. D. Helterbrand, E. W. Ely, and C. J. Fisher. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699-709. [DOI] [PubMed] [Google Scholar]
- 39.Beutler, B., T. Gelbart, and C. West. 2001. Synergy between TLR2 and TLR4: a safety mechanism. Blood Cells Mol. Dis. 27:728-730. [DOI] [PubMed] [Google Scholar]
- 40.Bignami, A., and A. Bastianelli. 1889. Observations of estivo-autumnal fever. Reforma Med. 6:1334-1335. [Google Scholar]
- 41.Bitterman, H., A. Kinarty, H. Lazarovich, and N. Lahat. 1991. Acute release of cytokines is proportional to tissue injury induced by surgical trauma and shock in rats. J. Clin. Immunol. 11:184-192. [DOI] [PubMed] [Google Scholar]
- 42.Bloom, B. R., and B. Bennett. 1966. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science 153:80-82. [DOI] [PubMed] [Google Scholar]
- 43.Blum, H. E., A. T. Haase, and G. N. Vyas. 1984. Molecular pathogenesis of hepatitis B virus infection: simultaneous detection of viral DNA and antigens in paraffin-embedded liver sections. Lancet ii:771-774. [DOI] [PubMed] [Google Scholar]
- 44.Bobe, P., K. Benihoud, D. Grandjon, P. Opolon, L. L. Pritchard, and R. Huchet. 1999. Nitric oxide mediation of active immunosuppression associated with graft-versus-host reaction. Blood 94:1028-1037. [PubMed] [Google Scholar]
- 45.Bochud, P.-Y., and T. Calandra. 2003. Pathogenesis of sepsis: new concepts and implications for future treatment. Br. Med. J. 326:262-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolton, C. F., G. B. Young, and D. W. Zochodne. 1993. The neurological complications of sepsis. Ann. Neurol. 33:94-100. [DOI] [PubMed] [Google Scholar]
- 47.Bonne Andrea, C., F. Harper, E. Puvion, M. Delpech, and A. M. De Recondo. 1986. The intracellular distribution and function of the high mobility group chromosomal proteins. Biol. Cell 58:185-194. [DOI] [PubMed] [Google Scholar]
- 48.Bouchama, A., S. Alsedairy, S. Siddiqui, E. Shail, and M. Rezeig. 1993. Elevated pyrogenic cytokines in heatstroke. Chest 104:1498-1502. [DOI] [PubMed] [Google Scholar]
- 49.Bouchama, A., R. S. Parhar, A. El-Yazigi, K. Sheth, and S. Al-Sediary. 1991. Endotoxemia and release of tumor necrosis factor and interleukin-1alpha in acute heatstroke. J. Appl. Physiol. 70:2640-2644. [DOI] [PubMed] [Google Scholar]
- 50.Boutlis, C. S., E. Tjitra, H. Maniboey, M. A. Misukonis, J. R. Saunders, S. Suprianto, J. B. Weinberg, and N. M. Anstey. 2003. Nitric oxide production and mononuclear cell nitric oxide synthase activity in malaria-tolerant Papuan adults. Infect. Immun. 71:3682-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brealey, D., M. Brand, I. Hargreaves, S. Heales, J. Land, R. Smolenski, N. A. Davies, C. E. Cooper, and M. Singer. 2002. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360:219-223. [DOI] [PubMed] [Google Scholar]
- 52.Bryceson, A. D. 1976. Clinical pathology of the Jarisch-Herxheimer reaction. J. Infect. Dis. 133:696-704. [DOI] [PubMed] [Google Scholar]
- 53.Bryceson, A. D., K. E. Cooper, D. A. Warrell, P. L. Perine, and E. H. Parry. 1972. Studies of the mechanism of the Jarisch-Herxheimer reaction in louse-borne relapsing fever: evidence for the presence of circulating Borrelia endotoxin. Clin. Sci. 43:343-354. [DOI] [PubMed] [Google Scholar]
- 54.Burgner, D., S. Usen, K. Rockett, M. Jallow, H. Ackerman, A. Cervino, M. Pinder, and D. P. Kwiatkowski. 2003. Nucleotide and haplotypic diversity of the NOS2A promoter region and its relationship to cerebral malaria. Hum. Genet. 112:379-386. [DOI] [PubMed] [Google Scholar]
- 55.Burgner, D., W. M. Xu, K. Rockett, M. Gravenor, I. G. Charles, A. V. Hill, and D. Kwiatkowski. 1998. Inducible nitric oxide synthase polymorphism and fatal cerebral malaria. Lancet 352:1193-1194. [DOI] [PubMed] [Google Scholar]
- 56.Burkle, A. 2001. Physiology and pathophysiology of poly(ADP-ribosyl)ation. Bioessays 23:795-806. [DOI] [PubMed] [Google Scholar]
- 57.Bustin, M. 1999. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol. Cell. Biol. 19:5237-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butcher, G. A., T. Garland, A. B. Adjukiewicz, and I. A. Clark. 1990. Serum TNF associated with malaria in patients in the Solomon Islands. Trans. R. Soc. Trop. Med. Hyg. 84:658-661. [DOI] [PubMed] [Google Scholar]
- 59.Cabie, A., C. Fitting, J. C. Farkas, C. Laurian, J. M. Cormier, J. Carlet, and J. M. Cavaillon. 1992. Influence of surgery on in vitro cytokine production by human monocytes. Cytokine 4:576-580. [DOI] [PubMed] [Google Scholar]
- 60.Calandra, T., B. Echtenacher, D. Le Roy, J. Pugin, C. N. Metz, L. Hultner, D. Heumann, D. Mannel, R. Bucala, and M. P. Glauser. 2000. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 6:164-170. [DOI] [PubMed] [Google Scholar]
- 61.Campos, M. A., I. C. Almeida, O. Takeuchi, S. Akira, E. P. Valente, D. O. Procopio, L. R. Travassos, J. A. Smith, D. T. Golenbock, and R. T. Gazzinelli. 2001. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167:416-423. [DOI] [PubMed] [Google Scholar]
- 62.Cannon, J. G., R. G. Tompkins, J. A. Gelfand, H. R. Michie, G. G. Stanford, J. W. M. van der Meer, S. Endres, G. Lonnemann, J. Corsetti, B. Chernow, D. W. Wilmore, S. M. Wolff, J. F. Burke, and C. Dinarello. 1990. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J. Infect. Dis. 161:79-84. [DOI] [PubMed] [Google Scholar]
- 63.Cannon, P. R. 1941. Some pathological aspects of human malaria, p. 214-220. In F. R. Moulton (ed.), A symposium on human malaria. American Association for the Advancement of Science, Washington, D.C.
- 64.Cantoni, L., C. Rossi, M. Rizzardini, M. Gadina, and P. Gheezi. 1991. Interleukin-1 and tumor necrosis factor induce hepatic haem oxygenase. Biochem. J. 279:891-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cardoso de Almeida, M. L., and M. J. Turner. 1983. The membrane form of variant surface glycoproteins of Trypanosoma brucei. Nature 302:349-352. [DOI] [PubMed] [Google Scholar]
- 66.Carswell, E. A., L. J. Old, R. L. Kassel, S. Green, N. Fiore, and B. Williamson. 1975. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl Acad. Sci. USA 72:3666-3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cavet, J., P. G. Middleton, M. Segall, H. Noreen, S. M. Davies, and A. M. Dickinson. 1999. Recipient tumor necrosis factor-alpha and interleukin-10 gene polymorphisms associate with early mortality and acute graft-versus-host disease severity in HLA-matched sibling bone marrow transplants. Blood 94:3941-3946. [PubMed] [Google Scholar]
- 68.Cerutti, I. 1974. Propriétés antivirales du C. parvum. C. R. Acad. Sci. Paris 279:963-966. [PubMed] [Google Scholar]
- 69.Chang, B. J., M. Nishikawa, E. Sato, and M. Inoue. 2003. Mice lacking inducible nitric oxide synthase show strong resistance to anti-Fas antibody-induced fulminant hepatitis. Arch. Biochem. Biophys. 411:63-72. [DOI] [PubMed] [Google Scholar]
- 70.Chao, C. C., W. R. Anderson, S. Hu, G. Gekker, A. Martella, and P. K. Peterson. 1993. Activated microglia inhibit multiplication of Toxoplasma gondii via a nitric oxide mechanism. Clin. Immunol. Immunopathol. 67:178-183. [DOI] [PubMed] [Google Scholar]
- 71.Chatenoud, L. 1993. OKT3-induced cytokine-release syndrome-preventive effect of anti-tumor necrosis factor monoclonal antibody. Transplant. Proc. 25(Suppl. 1):47-51. [PubMed] [Google Scholar]
- 72.Chauhan, S. D., G. Seggara, P. A. Vo, R. J. MacAllister, A. J. Hobbs, and A. Ahluwalia. 2003. Protection against lipopolysaccharide-induced endothelial dysfunction in resistance and conduit vasculature of iNOS knockout mice. FASEB J. 17:773-775. [DOI] [PubMed] [Google Scholar]
- 73.Cheung, C. Y., L. L. M. Poon, A. S. Lau, W. Luk, Y. L. Lau, K. F. Shortridge, S. Gordon, Y. Guan, and J. S. M. Peiris. 2002. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 360:1831-1837. [DOI] [PubMed] [Google Scholar]
- 74.Chisari, A., E. Spinedi, M. J. Voirol, A. Giovambattista, and R. C. Gaillard. 1998. A phospholipase a(2)-related snake venom (from Crotalus durissus terrificus) stimulates neuroendocrine and immune functions—determination of different sites of action. Endocrinology 139:617-625. [DOI] [PubMed] [Google Scholar]
- 75.Chiu, W. T., T. Y. Kao, and M. T. Lin. 1996. Increased survival in experimental rat heatstroke by continuous perfusion of interleukin-1 receptor antagonist. Neurosci. Res. 24:159-163. [DOI] [PubMed] [Google Scholar]
- 76.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 77.Cinat, M. E., K. Waxman, G. A. Granger, W. Pearce, C. Annas, and K. Daughters. 1994. Trauma causes sustained elevation of soluble tumor necrosis factor receptors. J. Am. Coll. Surgeons 179:529-537. [PubMed] [Google Scholar]
- 78.Clark, I., and M. Awburn. 2002. Migration inhibitory factor in the cerebral and systemic endothelium in sepsis and malaria. Crit. Care Med. 30:S263-S267. [DOI] [PubMed] [Google Scholar]
- 79.Clark, I. A. 1978. Does endotoxin cause both the disease and parasite death in acute malaria and babesiosis? Lancet ii:75-77. [DOI] [PubMed] [Google Scholar]
- 80.Clark, I. A. 1979. Protection of mice against Babesia microti with cord factor, COAM, zymosan, glucan, Salmonella and Listeria. Parasite Immunol. 1:179-196. [DOI] [PubMed] [Google Scholar]
- 81.Clark, I. A. 1979. Resistance to Babesia spp. and Plasmodium spp. in mice pretreated with an extract of Coxiella burnetii. Infect. Immun. 24:319-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clark, I. A. 1982. Correlation between susceptibility to malaria and babesia parasites and to endotoxin. Trans. R. Soc. Trop. Med. Hyg. 76:4-7. [DOI] [PubMed] [Google Scholar]
- 83.Clark, I. A. 1982. Suggested importance of monokines in pathophysiology of endotoxin shock and malaria. Klin. Wochenschr. 60:756-758. [DOI] [PubMed] [Google Scholar]
- 84.Clark, I. A., F. M. Al Yaman, W. B. Cowden, and K. A. Rockett. 1996. Does malarial tolerance, through nitric oxide, explain the low incidence of autoimmune disease in tropical Africa? Lancet 348:1492-1494. [DOI] [PubMed] [Google Scholar]
- 85.Clark, I. A., A. C. Allison, and F. E. Cox. 1976. Protection of mice against Babesia and Plasmodium with BCG. Nature 259:309-311. [DOI] [PubMed] [Google Scholar]
- 86.Clark, I. A., M. M. Awburn, R. O. Whitten, C. G. Harper, N. G. Liomba, and M. E. Molyneux. 2003. Induction of HO-1 in tissue macrophages and monocytes in fatal falciparum malaria and sepsis. Malaria J. 2:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clark, I. A., M. M. Awburn, R. O. Whitten, C. G. Harper, N. G. Liomba, M. E. Molyneux, and T. E. Taylor. 2003. Tissue distribution of migration inhibitory factor and inducible nitric oxide synthase in falciparum malaria and sepsis in African children. Malaria J. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clark, I. A., and G. Chaudhri. 1988. Tumor necrosis factor in malaria-induced abortion. Am. J. Trop. Med. Hyg. 39:246-249. [DOI] [PubMed] [Google Scholar]
- 89.Clark, I. A., and G. Chaudhri. 1988. Tumour necrosis factor may contribute to the anaemia of malaria by causing dyserythropoiesis and erythrophagocytosis. Br. J. Haematol. 70:99-103. [DOI] [PubMed] [Google Scholar]
- 90.Clark, I. A., and W. B. Cowden. 1989. Is TNF a key to acute infectious illness? Today's Life Sci. 1:26-29. [Google Scholar]
- 91.Clark, I. A., and W. B. Cowden. 2003. The pathophysiology of falciparum malaria. Pharmacol. Ther. 99:221-260. [DOI] [PubMed] [Google Scholar]
- 92.Clark, I. A., W. B. Cowden, G. A. Butcher, and N. H. Hunt. 1987. Possible roles of tumor necrosis factor in the pathology of malaria. Am. J. Pathol. 129:192-199. [PMC free article] [PubMed] [Google Scholar]
- 93.Clark, I. A., F. E. Cox, and A. C. Allison. 1977. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology 74:9-18. [DOI] [PubMed] [Google Scholar]
- 94.Clark, I. A., K. M. Gray, E. J. Rockett, W. B. Cowden, K. A. Rockett, A. Ferrante, and B. B. Aggarwal. 1992. Increased lymphotoxin in human malarial serum, and the ability of this cytokine to increase plasma interleukin-6 and cause hypoglycaemia in mice—implications for malarial pathology. Trans. R. Soc. Trop. Med. Hyg. 86:602-607. [DOI] [PubMed] [Google Scholar]
- 95.Clark, I. A., N. H. Hunt, G. A. Butcher, and W. B. Cowden. 1987. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-gamma or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J. Immunol. 139:3493-3496. [PubMed] [Google Scholar]
- 96.Clark, I. A., J. E. Richmond, E. J. Wills, and A. C. Allison. 1975. Immunity to intra-erythrocytic protozoa. Lancet ii:1128-1129. [DOI] [PubMed] [Google Scholar]
- 97.Clark, I. A., K. A. Rockett, and W. B. Cowden. 1991. Proposed link between cytokines, nitric oxide, and human cerebral malaria. Parasitol. Today 7:205-207. [DOI] [PubMed] [Google Scholar]
- 98.Clark, I. A., K. A. Rockett, and W. B. Cowden. 1992. Possible central role of nitric oxide in conditions clinically similar to cerebral malaria. Lancet 340:894-896. [DOI] [PubMed] [Google Scholar]
- 99.Clark, I. A., J.-L. Virelizier, E. A. Carswell, and P. R. Wood. 1981. Possible importance of macrophage-derived mediators in acute malaria. Infect. Immun. 32:1058-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99a.Clark, I. A., K. A. Rockett, and D. Burgner. 2003. Genes, nitric oxide and malaria in African children. Trends Parasitol. 19:335-337. [DOI] [PubMed] [Google Scholar]
- 100.Closa, D., G. Hotter, N. Prats, O. Bulbena, J. Rosellocatafau, L. Fernandezcruz, and E. Gelpi. 1994. Prostanoid generation in early stages of acute pancreatitis: a role for nitric oxide. Inflammation 18:469-480. [DOI] [PubMed] [Google Scholar]
- 101.Cobbs, C. S., A. Fenoy, D. S. Bredt, and L. J. Noble. 1997. Expression of nitric oxide synthase in the cerebral microvasculature after traumatic brain injury in the rat. Brain Res. 751:336-338. [DOI] [PubMed] [Google Scholar]
- 102.Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420:885-891. [DOI] [PubMed] [Google Scholar]
- 103.Cot, S., P. Ringwald, B. Mulder, P. Miailhes, J. Yapyap, A. K. Nussler, and W. M. C. Eling. 1994. Nitric oxide in cerebral malaria. J. Infect. Dis. 169:1417-1418. [DOI] [PubMed] [Google Scholar]
- 104.Coxon, R. E., D. Fekade, K. Knox, K. Hussein, A. Melka, A. Daniel, G. G. Griffin, and D. A. Warrell. 1997. The effect of antibody against TNF alpha on cytokine response in Jarisch-Herxheimer reactions of louse-borne relapsing fever. Q. J. Med. 90:213-221. [DOI] [PubMed] [Google Scholar]
- 105.Craig, A. G., R. Pinches, S. Khan, D. J. Roberts, G. D. H. Turner, C. I. Newbold, and A. R. Berendt. 1997. Failure to block adhesion of Plasmodium falciparum-infected erythrocytes to ICAM-1 with soluble ICAM-1. Infect. Immun. 65:4580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cramer, T., Y. Yamanishi, B. E. Clausen, I. Forster, R. Pawlinski, N. Mackman, V. H. Haase, R. Jaenisch, M. Corr, V. Nizet, G. S. Firestein, H. P. Gerber, N. Ferrara, and R. S. Johnson. 2003. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112:645-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crawley, J., M. English, C. Waruiru, I. Mwangi, and K. Marsh. 1998. Abnormal respiratory patterns in childhood cerebral malaria. Trans. R. Soc. Trop. Med. Hyg. 92:305-308. [DOI] [PubMed] [Google Scholar]
- 108.Creagan, E. T., J. S. Kovach, C. G. Moertel, S. Frytak, and L. K. Kvols. 1988. A phase 1 clinical trial of recombinant human tumor necrosis factor. Cancer 62:2467-2471. [DOI] [PubMed] [Google Scholar]
- 109.Cuzzocrea, S., E. Mazzon, L. Dugo, A. Barbera, T. Centorrino, A. Ciccolo, M. T. Fonti, and A. P. Caputi. 2001. Inducible nitric oxide synthase knockout mice exhibit resistance to the multiple organ failure induced by zymosan. Shock 16:51-58. [DOI] [PubMed] [Google Scholar]
- 110.Cuzzocrea, S., B. Zingarelli, G. Costantino, A. Sottile, D. Teti, and A. P. Caputi. 1999. Protective effect of poly(ADP-ribose) synthetase inhibition on multiple organ failure after zymosan-induced peritonitis in the rat. Crit. Care Med. 27:1517-1523. [DOI] [PubMed] [Google Scholar]
- 111.Dabrowski, A., and A. Gabryelewicz. 1994. Nitric oxide contributes to multiorgan oxidative stress in acute experimental pancreatitis. Scand. J. Gastroenterol. 29:943-948. [DOI] [PubMed] [Google Scholar]
- 112.Dammoneville, M., J. Wietzerbin, V. Pancre, A. Delanoye, A. Capron, and C. Auriault. 1988. Recombinant tumor necrosis factors mediate platelet cytotoxicity to Schistosoma mansoni larvae. J. Immunol. 140:3962-3965. [PubMed] [Google Scholar]
- 113.David, J. 1966. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc. Natl. Acad. Sci. USA 56:72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deininger, M. H., P. G. Kremsner, R. Meyermann, and H. J. Schluesener. 2000. Focal accumulation of cyclooxygenase-1 (COX-1) and COX-2 expressing cells in cerebral malaria. J. Neuroimmunol. 106:198-205. [DOI] [PubMed] [Google Scholar]
- 115.Dellacasagrande, J., C. Capo, D. Raoult, and J. L. Mege. 1999. IFN-gamma-mediated control of Coxiella burnetii survival in monocytes: the role of cell apoptosis and TNF. J. Immunol. 162:2259-2265. [PubMed] [Google Scholar]
- 116.Delley, V., P. Bouvier, N. Breslow, O. Doumbo, I. Sagara, M. Diakite, A. Mauris, A. Dolo, and A. Rougemont. 2000. What does a single determination of malaria parasite density mean? A longitudinal survey in Mali. Trop. Med. Int. Health 5:404-412. [DOI] [PubMed] [Google Scholar]
- 117.Denicoff, K. D., D. R. Rubinow, M. Z. Papa, C. Simpson, C. A. Seipp, M. T. Lotze, A. E. Chang, D. Rosenstein, and S. A. Rosenberg. 1987. The neuropsychiatric effects of treatment with interleukin-2 and lymphokine-activated killer cells. Ann. Intern. Med. 107:293-300. [DOI] [PubMed] [Google Scholar]
- 118.Denis, M. 1991. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell. Immunol. 132:150-157. [DOI] [PubMed] [Google Scholar]
- 119.Diehl, V., M. Pfreundschuh, H. T. Steinmetz, and M. Schaadt. 1988. Phase 1 studies of recombinant human tumor necrosis factor in patients with malignant disease, p. 183. In B. Bonavida, G. E. Gifford, H. Kirchner, and L. J. Old (ed.), Tumor necrosis factor/cachectin and related cytokines. Karger, Basel, Switzerland.
- 120.Dinarello, C. A. 1997. Blocking interleukin-1 and tumor necrosis factor in disease. Eur. Cytokine Netw. 8:294-296. [PubMed] [Google Scholar]
- 121.Dinarello, C. A., and S. M. Wolff. 1993. The role of interleukin-1 in disease. N. Engl. J. Med. 328:106-113. [DOI] [PubMed] [Google Scholar]
- 122.Dobbie, M., J. Crawley, C. Waruiru, K. Marsh, and R. Surtees. 2000. Cerebrospinal fluid studies in children with cerebral malaria: an excitotoxic mechanism? Am. J. Trop. Med. Hyg. 62:284-290. [DOI] [PubMed] [Google Scholar]
- 123.Dondorp, A. M., T. Planche, E. E. Debel, B. J. Angus, K. T. Chotivanich, K. Silamut, J. A. Romijn, R. Ruangveerayuth, F. J. Hoek, P. A. Kager, J. Vreeken, and N. J. White. 1998. Nitric oxides in plasma, urine, and cerebrospinal fluid in patients with severe falciparum malaria. Am. J. Trop. Med. Hyg. 59:497-502. [DOI] [PubMed] [Google Scholar]
- 124.Doyle, S. E., R. O'Connell, S. A. Vaidya, E. K. Chow, K. Yee, and G. Cheng. 2003. Toll-like receptor 3 mediates a more potent antiviral response than Toll-like receptor 4. J. Immunol. 170:3565-3571. [DOI] [PubMed] [Google Scholar]
- 125.D'Suze, G., S. Moncada, C. Gonzalez, C. Sevcik, V. Aguilar, and A. Alagon. 2003. Relationship between plasmatic levels of various cytokines, tumour necrosis factor, enzymes, glucose and venom concentration following Tityus scorpion sting. Toxicon 41:367-375. [DOI] [PubMed] [Google Scholar]
- 126.Dugernier, T., P. Starkel, P. F. Laterre, and M. S. Reynaert. 1996. Severe acute pancreatitis — pathophysiologic mechanisms underlying pancreatic necrosis and remote organ damage. Acta Gastro-Enterol. Belg. 59:178-185. [PubMed] [Google Scholar]
- 127.Eason, J. D., M. Pascual, S. Wee, M. Farrell, J. Phelan, S. Boskovic, C. Blosch, K. M. Mohler, and A. B. Cosimi. 1996. Evaluation of recombinant human soluble dimeric tumor necrosis factor receptor for prevention of OKT3-associated acute clinical syndrome. Transplantation 61:224-228. [DOI] [PubMed] [Google Scholar]
- 128.Echtenacher, B., W. Falk, D. N. Mannel, and P. H. Krammer. 1990. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J. Immunol. 145:3762-3766. [PubMed] [Google Scholar]
- 129.Echtenacher, B., D. N. Mannel, and L. Hultner. 1996. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381:75-77. [DOI] [PubMed] [Google Scholar]
- 130.Eggers, V., A. Schilling, W. J. Kox, and C. Spies. 2003. Septic encephalopathy. Diagnosis and therapy. Anaesthetist 52:294-303. [DOI] [PubMed] [Google Scholar]
- 131.Eichacker, P. Q., and C. Natanson. 2003. Recombinant human activated protein C in sepsis: inconsistent trial results, an unclear mechanism of action, and safety concerns resulted in labeling restrictions and the need for phase IV trials. Crit. Care Med. 31:S94-S96. [DOI] [PubMed] [Google Scholar]
- 132.Eichacker, P. Q., C. Parent, A. Kalil, C. Esposito, X. Cui, S. M. Banks, E. P. Gerstenberger, Y. Fitz, R. L. Danner, and C. Natanson. 2002. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am. J. Respir. Crit. Care Med. 166:1197-205. [DOI] [PubMed] [Google Scholar]
- 133.Eidelman, L. A., D. Putterman, C. Putterman, and C. L. Sprung. 1996. The spectrum of septic encephalopathy: definitions, etiologies, and mortalities. JAMA 275:470-473. [PubMed] [Google Scholar]
- 134.Eldwairi, Q., A. Comtois, Y. Guo, and S. N. A. Hussain. 1998. Endotoxin-induced skeletal muscle contractile dysfunction—contribution of nitric oxide synthases. Am. J. Physiol. 275:C770-C779. [DOI] [PubMed] [Google Scholar]
- 135.Eliasson, M. J., K. Sampei, A. S. Mandir, P. D. Hurn, R. J. Traystman, J. Bao, A. Pieper, Z. Q. Wang, T. M. Dawson, S. H. Snyder, and V. L. Dawson. 1997. Poly (ADP-ribose)polymerase gene disruption renders mice resistant to cerebral ischemia. Nat. Med. 3:1089-1095. [DOI] [PubMed] [Google Scholar]
- 136.English, M., C. Waruiru, and K. Marsh. 1996. Transfusion for respiratory distress in life-threatening childhood malaria. Am. J. Trop. Med. Hyg. 55:525-530. [DOI] [PubMed] [Google Scholar]
- 137.Engwerda, C. R., T. L. Mynott, S. Sawhney, J. B. De Souza, Q. D. Bickle, and P. M. Kaye. 2002. Locally up-regulated lymphotoxin alpha, not systemic tumor necrosis factor alpha, is the principal mediator of murine cerebral malaria. J. Exp. Med. 195:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Erecinska, M., and I. A. Silver. 1994. Ions and energy in mammalian brain. Prog. Neurobiol. 43:37-71. [DOI] [PubMed] [Google Scholar]
- 139.Espina, L. M., N. J. Valero, J. M. Hernandez, and J. A. Mosquera. 2003. Increased apoptosis and expression of tumor necrosis factor-alpha caused by infection of cultured human monocytes with dengue virus. Am. J. Trop. Med. Hyg. 68:48-53. [PubMed] [Google Scholar]
- 140.Exley, A. R., T. Leese, M. P. Holliday, R. A. Swann, and J. Cohen. 1992. Endotoxaemia and serum tumour necrosis factor as prognostic markers in severe acute pancreatitis. Gut 33:1126-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fahmi, H., D. Charon, M. Mondange, and R. Chaby. 1995. Endotoxin-induced desensitization of mouse macrophages is mediated in part by nitric oxide production. Infect. Immun. 63:1863-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Faure, E., L. Thomas, H. Xu, A. E. Medvedev, O. Equils, and M. Arditi. 2001. Bacterial lipopolysaccharide and IFN-gamma induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-kappa B activation. J. Immunol. 166:2018-2024. [DOI] [PubMed] [Google Scholar]
- 143.Feingold, K. R., M. Soued, and C. Grunfeld. 1988. Tumor necrosis factor stimulates DNA synthesis in the liver of intact rats. Biochem. Biophys. Res. Commun. 153:576-582. [DOI] [PubMed] [Google Scholar]
- 144.Fekade, D., K. Knox, K. Hussein, A. Melka, D. G. Lalloo, R. E. Coxon, and D. A. Warrell. 1996. Prevention of Jarisch-Herxheimer reactions by treatment with antibodies against tumor necrosis factor alpha. N. Engl. J. Med. 335:311-315. [DOI] [PubMed] [Google Scholar]
- 145.Feldmann, H., H. Bugany, F. Mahner, H. D. Klenk, D. Drenckhahn, and H. J. Schnittler. 1996. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol. 70:2208-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ferrante, A., R. E. Staugas, K. B. Rowan, S. Bresatz, L. M. Kumaratilake, C. M. Rzepczyk, and G. R. Adolf. 1990. Production of tumor necrosis factors alpha and beta by human mononuclear leukocytes stimulated with mitogens, bacteria, and malarial parasites. Infect. Immun. 58:3996-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ferrara, J. L. M. 2002. Cellular and cytokine effectors of acute graft versus host disease. Int. J. Hematol. 76(Suppl. 1):195-198. [DOI] [PubMed] [Google Scholar]
- 148.Fink, M. 1997. Cytopathic hypoxia in sepsis. Acta Anaesthesiol. Scand. 110:87-95. [DOI] [PubMed] [Google Scholar]
- 149.Fiuza, C., M. Bustin, S. Talwar, M. Tropea, E. Gerstenberger, J. H. Shelhamer, and A. F. Suffredini. 2003. Inflammatory promoting activity of HMGB1 on human microvascular endothelial cells. Blood 101:2652-2660. [DOI] [PubMed] [Google Scholar]
- 150.Florquin, S., Z. Amraoui, C. Dubois, J. Decuyper, and M. Goldman. 1994. The protective role of endogenously synthesized nitric oxide in staphylococcal enterotoxin b-induced shock in mice. J. Exp. Med. 180:1153-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gally, J. A., P. R. Montague, G. N. J. Reeke, and G. M. Edelman. 1990. The NO hypothesis: possible effects of a short-lived, rapidly diffusible signal in the development and function of the nervous system. Proc. Natl. Acad. Sci. USA 87:3547-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Garcia, I., R. Guler, D. Vesin, M. L. Olleros, P. Vassalli, Y. Chvatchko, M. Jacobs, and B. Ryffel. 2000. Lethal Mycobacterium bovis bacillus Calmette Guérin infection in nitric oxide synthase 2-deficient mice: cell-mediated immunity requires nitric oxide synthase 2. Lab. Investig. 80:1385-1397. [DOI] [PubMed] [Google Scholar]
- 153.Gardella, S., C. Andrei, D. Ferrera, L. V. Lotti, M. R. Torrisi, M. E. Bianchi, and A. Rubartelli. 2002. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway? EMBO Rep. 3:995-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Garside, P., A. K. Hutton, A. Severn, F. Y. Liew, and A. M. Mowat. 1992. Nitric oxide mediates intestinal pathology in graft-vs-host disease. Eur. J. Immunol. 22:2141-2145. [DOI] [PubMed] [Google Scholar]
- 155.Garthwaite, J., S. L. Charles, and R. Chess-Williams. 1988. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 336:385-388. [DOI] [PubMed] [Google Scholar]
- 156.Gawaz, M., S. Fateh Moghadam, G. Pilz, H. J. Gurland, and K. Werdan. 1995. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur. J. Clin. Investig. 25:843-851. [DOI] [PubMed] [Google Scholar]
- 157.Gilliland, H. E., M. A. Armstrong, and T. J. McMurray. 1998. Tumour necrosis factor as predictor for pulmonary dysfunction after cardiac surgery. Lancet 352:1281-1282. [DOI] [PubMed] [Google Scholar]
- 158.Gilroy, D. W., P. R. Colville-Nash, D. Willis, J. Chivers, M. J. Paul-Clark, and D. A. Willoughby. 1999. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 5:698-701. [DOI] [PubMed] [Google Scholar]
- 159.Goldfarb, R. D., A. Marton, E. Szabo, L. Virag, A. L. Salzman, D. Glock, I. Akhter, R. McCarthy, J. E. Parrillo, and C. Szabo. 2002. Protective effect of a novel, potent inhibitor of poly(adenosine 5′-diphosphate-ribose) synthetase in a porcine model of severe bacterial sepsis. Crit. Care Med. 30:974-980. [DOI] [PubMed] [Google Scholar]
- 160.Golgi, C. 1886. Sull infezione malarica. Arch. Sci. Med. (Torino) 10:109-135. [Google Scholar]
- 161.Goodwin, M. H., and T. K. Stapleton. 1952. The course of natural and induced infections of Plasmodium floridense in Scelorporus undulatus undulatus. Am. J. Trop. Med. Hyg. 1:773-783. [DOI] [PubMed] [Google Scholar]
- 162.Goova, M. T., J. Li, T. Kislinger, W. Qu, Y. Lu, L. G. Bucciarelli, S. Nowygrod, B. M. Wolf, X. Caliste, S. F. Yan, D. M. Stern, and A. M. Schmidt. 2001. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am. J. Pathol. 159:513-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Grau, G. E., L. F. Fajardo, P.-F. Piguet, B. Allet, P.-H. Lambert, and P. Vassali. 1987. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 237:1210-1212. [DOI] [PubMed] [Google Scholar]
- 164.Grau, G. E., C. D. Mackenzie, R. A. Carr, M. Redard, G. Pizzolato, C. Allasia, C. Cataldo, T. E. Taylor, and M. E. Molyneux. 2003. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J. Infect. Dis. 187:461-466. [DOI] [PubMed] [Google Scholar]
- 165.Grau, G. E., T. E. Taylor, M. E. Molyneux, J. J. Wirima, P. Vassalli, M. Hommel, and P.-H. Lambert. 1989. Tumor necrosis factor and disease severity in children with falciparum malaria. N. Engl. J. Med. 320:1586-1591. [DOI] [PubMed] [Google Scholar]
- 166.Gray, P. W., B. B. Aggarwal, C. V. Benton, T. S. Bringman, W. J. Henzel, J. A. Jarrett, D. W. Leung, B. Moffatt, P. Ng, L. P. Svedersky, M. A. Palladino, and G. A. Nedwin. 1984. Cloning and expression of the cDNA for human lymphotoxin: lymphokine with tumor necrosis activity. Nature 312:721-724. [DOI] [PubMed] [Google Scholar]
- 167.Greenwood, B. M. 1968. Autoimmune disease and parasitic infections in Nigerians. Lancet ii:380-382. [DOI] [PubMed] [Google Scholar]
- 168.Grewal, H. P., A. M. Eldin, L. Gaber, M. Kotb, and A. O. Gaber. 1994. Amelioration of the physiologic and biochemical changes of acute pancreatitis using an Anti-TNF-alpha polyclonal antibody. Am. J. Surg. 167:214-219. [DOI] [PubMed] [Google Scholar]
- 169.Groeneveld, P. H. P., P. Colson, K. M. C. Kwappenberg, and J. Clement. 1995. Increased production of nitric oxide in patients infected with the European variant of hantavirus. Scand. J. Infect. Dis. 27:453-456. [DOI] [PubMed] [Google Scholar]
- 170.Gross, A., S. Spiesser, A. Terraza, B. Rouot, E. Caron, and J. Dornand. 1998. Expression and bactericidal activity of nitric oxide synthase in Brucella suis-infected murine macrophages. Infect. Immun. 66:1309-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Grzelak, I., W. L. Olszewski, M. Zaleska, M. Durlik, B. Lagiewska, M. Muszynski, and W. Rowinski. 1996. Blood cytokine levels rise even after minor surgical trauma. J. Clin. Immunol. 16:159-164. [DOI] [PubMed] [Google Scholar]
- 172.Guzman, N. J., M. Z. Fang, S. S. Tang, J. R. Ingelfinger, and L. C. Garg. 1995. Autocrine inhibition of Na+/K+-ATPase by nitric oxide in mouse proximal tubule epithelial cells. J. Clin. Investig. 95:2083-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Haarbrink, M., G. K. Abadi, W. A. Buurman, M. A. Dentener, A. J. Terhell, and M. Yazdanbakhsh. 2000. Strong association of interleukin-6 and lipopolysaccharide-binding protein with severity of adverse reactions after diethylcarbamazine treatment of microfilaremic patients. J. Infect. Dis. 182:564-569. [DOI] [PubMed] [Google Scholar]
- 174.Haire, W. D., E. I. Ruby, B. G. Gordon, K. D. Patil, L. C. Stephens, G. D. Kotulak, E. C. Reed, J. M. Vose, P. J. Bierman, A. Kessinger, and J. O. Armitage. 1995. Multiple organ dysfunction syndrome in bone marrow transplantation. JAMA 274:1289-1295. [PubMed] [Google Scholar]
- 175.Han, X. N., M. P. Fink, and R. L. Delude. 2003. Proinflammatory cytokines cause NO-dependent and -independent changes in expression and localization of tight junction proteins in intestinal epithelial cells. Shock 19:229-237. [DOI] [PubMed] [Google Scholar]
- 176.Hansel, T. T., S. A. Kharitonov, L. E. Donnelly, E. M. Erin, M. G. Currie, W. M. Moore, P. T. Manning, D. P. Recker, and P. J. Barnes. 2003. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. FASEB J. 17:334-353. [DOI] [PubMed] [Google Scholar]
- 177.Hariri, R. J., J. B. G. Ghajar, K. Bahramian, S. Sharif, and P. S. Barie. 1993. Alterations in intracranial pressure and cerebral blood volume in endotoxemia. Surg. Gynecol. Obstet. 176:155-166. [PubMed] [Google Scholar]
- 178.Hartsfield, C. L. 2002. Cross talk between carbon monoxide and nitric oxide. Antioxid. Redox Signal. 4:301-307. [DOI] [PubMed] [Google Scholar]
- 179.Hashim, I. A., A. Alzeer, S. Alshohaib, M. Alahwal, and A. Shenkin. 1997. Cytokine changes in patients with heatstroke during pilgrimage to Mecca. Mediators Inflamm. 6:135-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hayden, F. G., R. S. Fritz, M. C. Lobo, W. G. Alvord, W. Strober, and S. E. Straus. 1998. Local and systemic cytokine responses during experimental human influenza A virus infection—relation to symptom formation and host defense. J. Clin. Investig. 101:643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Heagy, W., C. Hansen, K. Nieman, M. Cohen, C. Richardson, J. L. Rodriguez, and M. A. West. 2000. Impaired ex vivo lipopolysaccharide-stimulated whole blood tumor necrosis factor production may identify “septic” intensive care unit patients. Shock 14:271-276. [DOI] [PubMed] [Google Scholar]
- 182.Heller, M. V., M. C. Saavedra, R. Falcoff, J. I. Maiztegui, and F. C. Molinas. 1992. Increased tumor necrosis factor-alpha levels in argentine hemorrhagic fever. J. Infect. Dis. 166:1203-1204. [DOI] [PubMed] [Google Scholar]
- 183.Herceg, Z., and Z. Q. Wang. 2001. Functions of poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat. Res. 477:97-110. [DOI] [PubMed] [Google Scholar]
- 184.Heron, L. G., E. A. Reiss Levy, T. C. Jacques, D. J. Dickeson, L. D. Smythe, and T. C. Sorrell. 1997. Leptospirosis presenting as a haemorrhagic fever in a traveller from Africa. Med. J. Aust. 167:477-479. [DOI] [PubMed] [Google Scholar]
- 185.Herxheimer, K. 1902. Krause Ueber eine bei Syphilitischen vorkommende Quecksilberreaktion. Dtsch. Med. Wochenschr. 28:895-897. [Google Scholar]
- 185a.Heyes, M. P., B. J. Brew, A. Martin, R. W. Price, A. M. Salazar, J. J. Sidtis, J. A. Yergey, M. M. Mouradian, A. E. Sadler, J. Keilp, D. Rubinow, and S. P. Markey. 1991. Quinolinic acid in cerebrospinal fluid and serum in HIV infection. Ann. Neurol. 29:202-209. [DOI] [PubMed] [Google Scholar]
- 186.Heyman, A., and P. B. Beeson. 1949. Influence of various disease states upon the febrile response to intravenous injection of typhoid bacterial pyrogen. J. Lab. Clin. Med. 34:1400-1403. [PubMed] [Google Scholar]
- 187.Hierholzer, C., B. Harbrecht, J. M. Menezes, J. Kane, J. Macmicking, C. F. Nathan, A. B. Peitzman, T. R. Billiar, and D. J. Tweardy. 1998. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J. Exp. Med. 187:917-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hinder, F., A. Schmidt, J. H. Gong, A. Bender, H. Sprenger, M. Nain, and D. Gemsa. 1991. Influenza A virus infects macrophages and stimulates release of tumor necrosis factor-alpha. Pathobiology 59:227-231. [DOI] [PubMed] [Google Scholar]
- 189.Ho, M., M. M. Sexton, P. Tongtawe, S. Looareesuwan, P. Suntharasamai, and H. K. Webster. 1995. Interleukin-10 inhibits tumor necrosis factor production but not antigen-specific lymphoproliferation in acute Plasmodium falciparum malaria. J. Infect. Dis. 172:838-844. [DOI] [PubMed] [Google Scholar]
- 190.Hobbs, M. R., V. Udhayakumar, M. C. Levesque, J. Booth, J. M. Roberts, A. N. Tkachuk, A. Pole, H. Coon, S. Kariuki, B. L. Nahlen, E. D. Mwaikambo, A. L. Lal, D. L. Granger, N. M. Anstey, and J. B. Weinberg. 2002. A new NOS2 promoter polymorphism associated with increased nitric oxide production and protection from severe malaria in Tanzanian and Kenyan children. Lancet 360:1468-1475. [DOI] [PubMed] [Google Scholar]
- 191.Hober, D., L. Poli, B. Roblin, P. Gestas, E. Chungue, G. Granic, P. Imbert, J. L. Pecarere, R. Vergezpascal, P. Wattre, and M. Maniezmontreuil. 1993. Serum levels of tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), and interleukin-1-beta (IL-1-beta) in dengue-infected patients. Am. J. Trop. Med. Hyg. 48:324-331. [DOI] [PubMed] [Google Scholar]
- 192.Hoffman, R. A., J. M. Langrehr, and R. L. Simmons. 1992. The role of inducible nitric oxide synthetase during graft-versus-host disease. Transplant. Proc. 24:2856. [PubMed] [Google Scholar]
- 193.Hollenberg, S. M., M. Broussard, J. Osman, and J. E. Parrillo. 2000. Increased microvascular reactivity and improved mortality in septic mice lacking inducible nitric oxide synthase. Circ. Res. 86:774-778. [DOI] [PubMed] [Google Scholar]
- 194.Holler, E. 1991. TNF-alpha in allogeneic bone marrow transplantation. Significance for immunologic reconstitution, acute graft-versus-host disease and risk of complications after bone marrow transplantation. Fortschr. Med. 109:263-266. [PubMed] [Google Scholar]
- 195.Holler, E., H. J. Kolb, R. Hintermeierknabe, J. Mittermuller, S. Thierfelder, M. Kaul, and W. Wilmanns. 1993. Role of tumor necrosis factor-alpha in acute graft-versus-host disease and complications following allogeneic bone marrow transplantation. Transplant. Proc. 25:1234-1236. [PubMed] [Google Scholar]
- 196.Holler, E., H. J. Kolb, J. Mittermuller, M. Kaul, G. Ledderose, T. Duell, B. Seeber, M. Schleuning, R. Hintermeier Knabe, and B. Ertl. 1995. Modulation of acute graft-versus-host-disease after allogeneic bone marrow transplantation by tumor necrosis factor alpha (TNF alpha) release in the course of pretransplant conditioning: role of conditioning regimens and prophylactic application of a monoclonal antibody neutralizing human TNF alpha (MAK 195F). Blood 86:890-899. [PubMed] [Google Scholar]
- 197.Hori, O., J. Brett, T. Slattery, R. Cao, J. Zhang, J. X. Chen, M. Nagashima, E. R. Lundh, S. Vijay, D. Nitecki, J. Morser, D. Stern, and A.-M. Schmidt. 1995. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of RAGE and amphoterin in the developing nervous system. J. Biol. Chem. 270:25752-15761. [DOI] [PubMed] [Google Scholar]
- 198.Hotchkiss, R. S., and I. E. Karl. 2003. Medical progress: the pathophysiology and treatment of sepsis. N. Engl. J. Med. 348:138-150. [DOI] [PubMed] [Google Scholar]
- 199.Houde, M., and D. J. S. Arora. 1990. Stimulation of tumor necrosis factor secretion by purified influenza virus neuraminidase. Cell. Immunol. 129:104-111. [DOI] [PubMed] [Google Scholar]
- 200.Husson, H., S. M. Lugli, P. Ghia, A. Cardoso, A. Roth, K. Brohmi, E. G. Carideo, Y. S. Choi, J. Browning, and A. S. Freedman. 2000. Functional effects of TNF and lymphotoxin alpha1beta2 on FDC-like cells. Cell. Immunol. 203:134-143. [DOI] [PubMed] [Google Scholar]
- 201.Ignarro, L. J., R. E. Byrns, G. M. Buga, and K. S. Wood. 1987. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ. Res. 61:866-879. [DOI] [PubMed] [Google Scholar]
- 202.Ignatyev, G., A. Steinkasserer, M. Streltsova, A. Atrasheuskaya, A. Agafonov, and W. Lubitz. 2000. Experimental study on the possibility of treatment of some hemorrhagic fevers. J. Biotechnol. 83:67-76. [DOI] [PubMed] [Google Scholar]
- 203.Iuvone, T., F. Dacquisto, R. Carnuccio, and M. Dirosa. 1996. Nitric oxide inhibits LPS-induced tumor necrosis factor synthesis in vitro and in vivo. Life Sci. 59:207-211. [DOI] [PubMed] [Google Scholar]
- 204.Jacobsohn, D. A., and G. B. Vogelsang. 2002. Novel pharmacotherapeutic approaches to prevention and treatment of GVHD. Drugs 62:879-889. [DOI] [PubMed] [Google Scholar]
- 205.Jagtap, P., F. G. Soriano, L. Virag, L. Liaudet, J. Mabley, E. Szabo, G. Hasko, A. Marton, C. B. Lorigados, F. Gallyas, B. Sumegi, D. G. Hoyt, E. Baloglu, J. VanDuzer, A. L. Salzman, G. J. Southan, and C. Szabo. 2002. Novel phenanthridinone inhibitors of poly(adenosine 5′-diphosphateribose) synthetase: potent cytoprotective and antishock agents. Crit. Care Med. 30:1071-1082. [DOI] [PubMed] [Google Scholar]
- 206.Jakubowski, A. A., E. S. Casper, J. L. Gabrilove, M.-A. Templeton, S. A. Sherwin, and H. F. Oettgen. 1989. Phase 1 trial of intramuscularly administered tumor necrosis factor in patients with advanced cancer. J. Clin. Oncol. 7:298-303. [DOI] [PubMed] [Google Scholar]
- 207.Jarisch, A. 1895. Therapeutische Versuche bei Syphilis. Wien. Med. Wochenschr. 45:721-724. [Google Scholar]
- 208.Jarvis, B. W., T. H. Harris, N. Qureshi, and G. A. Splitter. 2002. Rough lipopolysaccharide from Brucella abortus and Escherichia coli differentially activates the same mitogen-activated protein kinase signaling pathways for tumor necrosis factor alpha in RAW 264.7 macrophage-like cells. Infect. Immun. 70:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jeyarajah, D. R., and J. R. Thistlethwaite. 1993. General aspects of cytokine-release syndrome: timing and incidence of symptoms. Transplant. Proc. 25(Suppl. 1):16-20. [PubMed] [Google Scholar]
- 210.Karakozis, S., M. Hinds, J. W. Cook, D. Kim, H. Provido, and J. R. Kirkpatrick. 2000. The effects of interleukin-10 in hemorrhagic shock. J. Surg. Res. 90:109-112. [DOI] [PubMed] [Google Scholar]
- 211.Kawamura, T., R. Wakusawa, K. Okada, and S. Inada. 1993. Elevation of cytokines during open heart surgery with cardiopulmonary bypass—participation of interleukin-8 and interleukin-6 in reperfusion injury. Can. J: Anaesth. 40:1016-1021. [DOI] [PubMed] [Google Scholar]
- 212.Kawashima, H., Y. Watanabe, T. Ichiyama, M. Mizuguchi, N. Yamada, Y. Kashiwagi, K. Takekuma, A. Hoshika, and T. Mori. 2002. High concentration of serum nitrite/nitrate obtained from patients with influenza-associated encephalopathy. Pediatr. Int. 44:705-707. [DOI] [PubMed] [Google Scholar]
- 213.Keane, J., S. Gershon, R. P. Wise, E. Mirabile Levens, J. Kasznica, W. D. Schwieterman, J. N. Siegel, and M. M. Braun. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098-1104. [DOI] [PubMed] [Google Scholar]
- 214.Kelly, J. L., C. O'Sullivan, M. Oriordain, D. Oriordain, A. Lyons, J. Doherty, J. A. Mannick, and M. L. Rodrick. 1997. Is circulating endotoxin the trigger for the systemic inflammatory response syndrome seen after injury. Ann. Surg. 225:530-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kern, P., C. J. Hemmer, J. Van Damme, H.-J. Gruss, and M. Dietrich. 1989. Elevated tumour necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am. J. Med. 87:139-143. [DOI] [PubMed] [Google Scholar]
- 216.Khan, A. U., R. L. Delude, Y. Y. Han, P. L. Sappington, X. Han, J. A. Carcillo, and M. P. Fink. 2002. Liposomal NAD+ prevents diminished O2 consumption by immunostimulated Caco-2 cells. Am. J. Physiol. 282:L1082-L1091. [DOI] [PubMed] [Google Scholar]
- 217.Kilbourn, R. G., and P. P. Belloni. 1990. Endothelial cell production of nitrogen oxides in response to interferon-γ in combination with tumor necrosis factor, interleukin-1, or endotoxin. J. Natl. Cancer Inst. 82:772-776. [DOI] [PubMed] [Google Scholar]
- 218.Kirchner, H., H. M. Hirt, and K. Munk. 1977. Protection against herpes simplex virus infection in mice by Corynebacterium parvum. Infect. Immun. 16:9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kitchen, S. F. 1949. Falciparum malaria, p. 966-994. In M. F. Boyd (ed.), Malariology. The W. B. Saunders Co., Philadelphia, Pa.
- 220.Knaus, W. A., F. E. Harrell, Jr., J. F. LaBrecque, D. P. Wagner, J. P. Pribble, E. A. Draper, C. J. Fisher, L. Soll, and The rhIL-1ra Phase III Sepsis Syndrome Study Group. 1996. Use of predicted risk of mortality to evaluate the efficacy of anticytokine therapy in sepsis. Crit. Care Med. 24:46-56. [DOI] [PubMed] [Google Scholar]
- 221.Kobori, N., G. Clifton, and P. Dash. 2002. Altered expression of novel genes in the cerebral cortex following experimental brain injury. Mol. Brain Res. 104:148-158. [DOI] [PubMed] [Google Scholar]
- 222.Koedel, U., F. Winkler, B. Angele, T. Fontana, and H. W. Pfister. 2002. Meningitis-associated central nervous system complications are mediated by the activation of poly(ADP-ribose) polymerase. J. Cereb. Blood Flow Metab. 22:39-49. [DOI] [PubMed] [Google Scholar]
- 223.Kokkola, R., J. Li, E. Sundberg, A. C. Aveberger, K. Palmblad, H. Yang, K. J. Tracey, U. Andersson, and H. E. Harris. 2003. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis. Rheum. 48:2052-2058. [DOI] [PubMed] [Google Scholar]
- 224.Kokkola, R., E. Sundberg, A. K. Ulfgren, K. Palmblad, J. Li, H. Wang, L. Ulloa, H. Yang, X. J. Yan, R. Furie, N. Chiorazzi, K. J. Tracey, U. Andersson, and H. E. Harris. 2002. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum. 46:2598-2603. [DOI] [PubMed] [Google Scholar]
- 225.Kremsner, P. G., S. Winkler, E. Wildling, J. Prada, U. Bienzle, W. Graninger, and A. K. Nussler. 1996. High plasma levels of nitrogen oxides are associated with severe disease and correlate with rapid parasitological and clinical cure in Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 90:44-47. [DOI] [PubMed] [Google Scholar]
- 226.Kristoff, A. S., P. Goldberg, V. Laubach, and S. N. A. Hussain. 1998. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am. J. Respir. Crit. Care Med. 158:1883-1889. [DOI] [PubMed] [Google Scholar]
- 227.Kumins, N. H., J. Hunt, R. L. Gamelli, and J. P. Filkins. 1997. Molsidomine increases endotoxic survival and decreases cytokine production. Shock 7:200-205. [DOI] [PubMed] [Google Scholar]
- 228.Kuprash, D. V., M. B. Alimzhanov, A. V. Tumanov, S. I. Grivennikov, A. N. Shakhov, L. N. Drutskaya, M. W. Marino, R. L. Turetskaya, A. O. Anderson, K. Rajewsky, K. Pfeffer, and S. A. Nedospasov. 2002. Redundancy in tumor necrosis factor (TNF) and lymphotoxin (LT) signaling in vivo: mice with inactivation of the entire TNF/LT locus versus single-knockout mice. Mol. Cell. Biol. 22:8626-8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kwiatkowski, D., A. V. S. Hill, I. Sambou, P. Twumasi, J. Castracane, K. R. Manogue, A. Cerami, D. R. Brewster, and B. M. Greenwood. 1990. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 336:1201-1204. [DOI] [PubMed] [Google Scholar]
- 230.Kwiatkowski, D., M. E. Molyneux, S. Stephens, N. Curtis, N. Klein, P. Pointaire, M. Smit, R. Allan, D. R. Brewster, G. E. Grau, and B. M. Greenwood. 1993. Anti-TNF therapy inhibits fever in cerebral malaria. Q. J. Med. 86:91-98. [PubMed] [Google Scholar]
- 231.Kyambadde, J. W., J. C. Enyaru, E. Matovu, M. Odiit, and J. F. Carasco. 2000. Detection of trypanosomes in suspected sleeping sickness patients in Uganda using the polymerase chain reaction. Bull. W. H. Or. 78:119-124. [PMC free article] [PubMed] [Google Scholar]
- 232.Lanone, S., A. Mebazaa, C. Heymes, D. Henin, J. J. Poderoso, Y. Panis, C. Zedda, T. Billiar, D. Payen, M. Aubier, and J. Boczkowski. 2000. Muscular contractile failure in septic patients—role of the inducible nitric oxide synthase pathway. Am. J. Respir. Crit. Care Med. 162:2308-2315. [DOI] [PubMed] [Google Scholar]
- 233.Lee, T. S., and L. Y. Chau. 2002. Heme oxygenase-1 mediates the anti-inflammatory effect of IL-10 in mice. Nat. Med. 8:240-246. [DOI] [PubMed] [Google Scholar]
- 234.Lee, T. S., H. L. Tsai, and L. Y. Chau. 2003. Induction of heme oxygenase-1 expression in murine macrophages is essential for the anti-inflammatory effect of low dose 15-deoxy-delta(12,14)-prostaglandin J(2). J. Biol. Chem. 278:19325-19330. [DOI] [PubMed] [Google Scholar]
- 235.Leeper-Woodford, S. K., P. D. Carey, K. Byrne, J. K. Jenkins, B. J. Fisher, C. Blocher, H. J. Sugerman, and A. A. Fowler. 1991. Tumor necrosis factor. Alpha and beta subtypes appear in circulation during onset of sepsis-induced lung injury. Am. Rev. Respir. Dis. 143:1076-1082. [DOI] [PubMed] [Google Scholar]
- 236.Lehir, M., H. Bluethmann, M. H. Koscovilbois, M. Muller, F. Dipadova, M. Moore, B. Ryffel, and H. P. Eugster. 1996. Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor-1 signaling. J. Exp. Med. 183:2367-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lehner, M. D., S. Morath, K. S. Michelsen, R. R. Schumann, and T. Hartung. 2001. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different toll-like receptors independent of paracrine mediators. J. Immunol. 166:5161-5167. [DOI] [PubMed] [Google Scholar]
- 238.Leist, M., B. Single, H. Naumann, E. Fava, B. Simon, S. Kuhnle, and P. Nicotera. 1999. Inhibition of mitochondrial ATP generation by nitric oxide switches apoptosis to necrosis. Exp. Cell Res. 249:396-403. [DOI] [PubMed] [Google Scholar]
- 239.Lemaire, L. C., J. J. Vanlanschot, C. P. Stoutenbeek, S. J. Vandeventer, C. L. Wells, and D. J. Gouma. 1997. Bacterial translocation in multiple organ failure—cause or epiphenomenon still unproven. Br. J. Surg. 84:1340-1350. [PubMed] [Google Scholar]
- 240.Le Page, C., J. Sanceau, J. C. Drapier, and J. Wietzerbin. 1998. Inhibitors of ADP-ribosylation impair inducible nitric oxide synthase gene transcription through inhibition of NF kappa B activation. Biochem. Biophys. Res. Commun. 243:451-457. [DOI] [PubMed] [Google Scholar]
- 241.Li, C., and J. Langhorne. 2000. Tumor necrosis factor alpha p55 receptor is important for development of memory responses to blood-stage malaria infection. Infect. Immun. 68:5724-5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Li, J., R. Kokkola, S. Tabibzadeh, R. Yang, M. Ochani, X. Qiang, H. E. Harris, C. J. Czura, H. Wang, L. Ulloa, H. Wang, H. S. Warren, L. L. Moldawer, M. P. Fink, U. Andersson, K. J. Tracey, and H. Yang. 2003. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol. Med. 9:37-45. [PMC free article] [PubMed] [Google Scholar]
- 243.Liaudet, L., F. G. Soriano, E. Szabo, L. Virag, J. G. Mabley, A. L. Salzman, and C. Szabo. 2000. Protection against hemorrhagic shock in mice genetically deficient in poly(ADP-ribose)polymerase. Proc. Natl Acad. Sci. USA 97:10203-10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lin, R. Y., M. E. Astiz, J. C. Saxon, D. C. Saha, and E. C. Rackow. 1994. Relationships between plasma cytokine concentrations and leukocyte functional antigen expression in patients with sepsis. Crit. Care Med. 22:1595-1602. [PubMed] [Google Scholar]
- 245.Linderholm, M., C. Ahlm, B. Settergren, A. Waage, and A. Tarnvik. 1996. Elevated plasma levels of tumor necrosis factor (TNF)-alpha, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J. Infect. Dis. 173:38-43. [DOI] [PubMed] [Google Scholar]
- 246.Linderholm, M., P. H. P. Groeneveld, and A. Tarnvik. 1996. Increased production of nitric oxide in patients with hemorrhagic fever with renal syndrome—relation to arterial hypotension and tumor necrosis factor. Infection 24:337-340. [DOI] [PubMed] [Google Scholar]
- 247.Lopansri, B. K., N. M. Anstey, J. B. Weinberg, G. J. Stoddard, M. R. Hobbs, M. C. Levesque, E. D. Mwaikambo, and D. L. Granger. 2003. Low plasma arginine concentrations in children with cerebral malaria and decreased nitric oxide production. Lancet 361:676-678. [DOI] [PubMed] [Google Scholar]
- 248.Love, S., R. Barber, and G. K. Wilcock. 1999. Neuronal accumulation of poly(ADP-ribose) after brain ischaemia. Neuropathol. Appl. Neurobiol. 25:98-103. [DOI] [PubMed] [Google Scholar]
- 249.Mabley, J. G., P. Jagtap, M. Perretti, S. J. Getting, A. L. Salzman, L. Virag, E. Szabo, F. G. Soriano, L. Liaudet, G. E. Abdelkarim, G. Hasko, A. Marton, G. J. Southan, and C. Szabo. 2001. Anti-inflammatory effects of a novel, potent inhibitor of poly(ADP-ribose) polymerase. Inflamm. Res. 50:561-569. [DOI] [PubMed] [Google Scholar]
- 250.Mackay, F., G. R. Majeau, P. Lawton, P. S. Hochman, and J. L. Browning. 1997. Lymphotoxin but not tumor necrosis factor functions to maintain splenic architecture and humoral responsiveness in adult mice. Eur. J. Immunol. 27:2033-2042. [DOI] [PubMed] [Google Scholar]
- 251.MacLean, L., M. Odiit, and J. M. Sternberg. 2001. Nitric oxide and cytokine synthesis in human African trypanosomiasis. J. Infect. Dis. 184:1086-1090. [DOI] [PubMed] [Google Scholar]
- 252.MacPherson, G. G., M. J. Warrell, N. J. White, S. Looareesuwan, and D. A. Warrell. 1985. Human cerebral malaria. A quantitative ultrastructural analysis of parasitised erythrocyte sequestration. Am. J. Pathol. 119:385-401. [PMC free article] [PubMed] [Google Scholar]
- 253.Maegraith, B. 1948. Pathological processes in malaria and blackwater fever, p. 367-369. Blackwell, Oxford, United Kingdom.
- 254.Maegraith, B., H. M. Gilles, and K. Devakul. 1957. Pathological processes in Babesia canis infections. Z. Tropenmed. Parasitol. 8:485-514. [PubMed] [Google Scholar]
- 255.Maegraith, B. G. 1969. Is there a malaria toxin? J. Gen. Microbiol. 59:xi. [PubMed] [Google Scholar]
- 256.Magez, S., R. Lucas, A. Darji, E. B. Songa, R. Hamers, and P. Debaetselier. 1993. Murine tumour necrosis factor plays a protective role during the initial phase of the experimental infection with trypanosoma-brucei-brucei. Parasite Immunol. 15:635-641. [DOI] [PubMed] [Google Scholar]
- 257.Makhija, R., and A. N. Kingsnorth. 2002. Cytokine storm in acute pancreatitis. J. Hepatobil. Pancreat. Surg. 9:401-410. [DOI] [PubMed] [Google Scholar]
- 258.Maneerat, Y., P. Viriyavejakul, B. Punpoowong, M. Jones, P. Wilairatana, E. Pongponratn, G. D. Turner, and R. Udomsangpetch. 2000. Inducible nitric oxide synthase expression is increased in the brain in fatal cerebral malaria. Histopathology 37:269-277. [DOI] [PubMed] [Google Scholar]
- 259.Marano, M. A., Y. Fong, L. L. Moldawer, H. Wei, S. E. Calvano, K. J. Tracey, P. Barie, K. Manogue, A. Cerami, T. Shires, and S. F. Lowry. 1990. Serum cachectin/tumor necrosis factor in critically ill patients with burns correlates with infection and mortality. Surg. Gynecol. Obstet. 170:32-38. [PubMed] [Google Scholar]
- 260.Marta, R. F., V. S. Montero, C. E. Hack, A. Sturk, J. I. Maiztegui, and F. C. Molinas. 1999. Proinflammatory cytokines and elastase-alpha-1-antitrypsin in Argentine hemorrhagic fever. Am. J. Trop. Med. Hyg. 60:85-89. [DOI] [PubMed] [Google Scholar]
- 261.Masson, I., J. Mathieu, X. B. Nolland, M. Desousa, B. Chanaud, S. Strzalko, Y. Chancerelle, J. F. Kergonou, J. P. Giroud, and I. Florentin. 1998. Role of nitric oxide in depressed lymphoproliferative responses and altered cytokine production following thermal injury in rats. Cell. Immunol. 186:121-132. [DOI] [PubMed] [Google Scholar]
- 262.Mastroeni, P., A. Arena, G. B. Costa, M. C. Liberto, L. Bonina, and C. E. Hormaeche. 1991. Serum TNF alpha in mouse typhoid and enhancement of a Salmonella infection by anti-TNF alpha antibodies. Microb. Pathog. 11:33-38. [DOI] [PubMed] [Google Scholar]
- 263.Maxie, M. G., H. Tabel, and G. J. Losos. 1978. Determination of volumes of Trypanosoma vivax and T. congolense separated from cattle blood. Tropenmed. Parasitol. 29:234-238. [PubMed] [Google Scholar]
- 264.Mayordomo, L., J. L. Marenco, J. Gomez-Mateos, and E. Rejon. 2002. Pulmonary miliary tuberculosis in a patient with anti-TNF-alpha treatment. Scand. J. Rheumatol. 31:44-45. [DOI] [PubMed] [Google Scholar]
- 265.Mazzon, E., L. Dugo, S. A. De, J. H. Li, A. P. Caputi, J. Zhang, and S. Cuzzocrea. 2002. Beneficial effects of GPI 6150, an inhibitor of poly(ADP-ribose) polymerase in a rat model of splanchnic artery occlusion and reperfusion. Shock 17:222-227. [DOI] [PubMed] [Google Scholar]
- 266.McAdam, K. P., J. Li, J. Knowles, N. T. Foss, C. A. Dinarello, L. J. Rosenwasser, M. J. Selinger, M. M. Kaplan, R. Goodman, P. N. Herbert, L. L. Bausserman, and L. M. Nadler. 1982. The biology of SAA: identification of the inducer, in vitro synthesis, and heterogeneity demonstrated with monoclonal antibodies. Ann. N. Y. Acad. Sci. 389:126-136. [DOI] [PubMed] [Google Scholar]
- 267.McConville, M. J., and M. A. Ferguson. 1993. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem. J. 294:305-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.McGuinness, D. H., P. K. Dehal, and R. J. Pleass. 2003. Pattern recognition molecules and innate immunity to parasites. Trends Parasitol. 19:312-319. [DOI] [PubMed] [Google Scholar]
- 269.Medana, I. M., N. P. Day, T. T. Hien, N. T. Mai, D. Bethell, N. H. Phu, J. Farrar, M. M. Esiri, N. J. White, and G. D. Turner. 2002. Axonal injury in cerebral malaria. Am. J. Pathol. 160:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Reference deleted.
- 271.Medana, I. M., N. T. H. Mai, N. P. J. Day, T. T. Hien, D. Bethell, N. H. Phu, J. Farrar, N. J. White, and G. D. H. Turner. 2001. Cellular stress and injury responses in the brains of adult Vietnamese patients with fatal Plasmodium falciparum malaria. Neuropathol. Appl. Neurobiol. 27:421-433. [DOI] [PubMed] [Google Scholar]
- 272.Merenmies, J., R. Pihlaskari, J. Laitinen, J. Wartiovaara, and H. Rauvala. 1991. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J. Biol. Chem. 266:16722-16729. [PubMed] [Google Scholar]
- 273.Michalek, S. M., R. N. Moore, J. R. McGhee, D. L. Rosenstreich, and S. E. Mergenhagen. 1980. The primary role of lymphoreticular cells in the mediation of host responses to bacterial endotoxin. J. Infect. Dis. 141:55-63. [DOI] [PubMed] [Google Scholar]
- 274.Michie, H. R., D. R. Spriggs, K. R. Manogue, M. L. Sherman, A. Revhaug, S. T. O'Dwyer, K. Arthur, C. A. Dinarello, A. Cerami, S. M. Wolf, D. W. Kufe, and D. W. Wilmore. 1988. Tumor necrosis factor and endotoxin induce similar metabolic responses in human beings. Surgery 104:280-286. [PubMed] [Google Scholar]
- 275.Middleton, P. G., P. Taylor, G. Jackson, S. J. Proctor, and A. M. Dickinson. 1998. Cytokine gene polymorphisms associating with severe acute graft-versus-host disease in HLA-identical sibling transplants. Blood 92:3943-3948. [PubMed] [Google Scholar]
- 276.Mier, J. W., G. Vachino, J. W. M. Van der Meer, R. P. Numerof, S. Adams, J. G. Cannon, H. A. Bernheim, M. B. Atkins, D. R. Parkinson, and C. A. Dinarello. 1988. Induction of circulating tumor necrosis factor (TNF-α) as the mechanism for the febrile response to interleukin-2 (IL-2) in cancer patients. J. Clin. Immunol. 8:426-436. [DOI] [PubMed] [Google Scholar]
- 277.Mikawa, K., S. Kodama, K. Nishina, and H. Obara. 2001. ONO-1714, a new inducible nitric oxide synthase inhibitor, attenuates diaphragmatic dysfunction associated with cerulein-induced pancreatitis in rats. Crit. Care Med. 29:1215-1221. [DOI] [PubMed] [Google Scholar]
- 278.Mills, C. D. 1991. Molecular basis of “suppressor” macrophages: arginine metabolism via the nitric oxide synthetase pathway. J. Immunol. 146:2719-2723. [PubMed] [Google Scholar]
- 279.Mita, Y., K. Dobashi, Y. Shimizu, T. Nakazawa, and M. Mori. 2001. Toll-like receptor 2 and 4 surface expressions on human monocytes are modulated by interferon-gamma and macrophage colony-stimulating factor. Immunol. Lett. 78:97-101. [DOI] [PubMed] [Google Scholar]
- 280.Mitaka, C., Y. Hirata, K. Yokoyama, H. Wakimoto, M. Hirokawa, T. Nosaka, and T. Imai. 2003. Relationships of circulating nitrite/nitrate levels to severity and multiple organ dysfunction syndrome in systemic inflammatory response syndrome. Shock 19:305-309. [DOI] [PubMed] [Google Scholar]
- 281.Mohanty, D., N. Marwaha, K. Ghosh, S. Sharma, G. Garewal, S. Shah, S. Devi, and K. C. Das. 1988. Functional and ultrastructural changes of platelets in malarial infection. Trans. R. Soc. Trop. Med. Hyg. 82:369-375. [DOI] [PubMed] [Google Scholar]
- 282.Mohnot, D., O. C. Snead, and J. W. Benton. 1982. Burn encephalopathy in children. Ann. Neurol. 12:42-47. [DOI] [PubMed] [Google Scholar]
- 283.Molloy, J. B., P. M. Bowles, W. K. Jorgensen, and B. M. Cooke. 2003. Babesia bovis: adhesion of parasitized red blood cells to bovine umbilical vein endothelial cells in vitro does not select for virulence. Exp. Parasitol. 103:182-184. [DOI] [PubMed] [Google Scholar]
- 284.Mongan, P. D., J. Karaian, B. M. Van Der Schuur, D. K. Via, and P. Sharma. 2003. Pyruvate prevents poly-ADP ribose polymerase (PARP) activation, oxidative damage, and pyruvate dehydrogenase deactivation during hemorrhagic shock in swine. J. Surg. Res. 112:180-188. [DOI] [PubMed] [Google Scholar]
- 285.Moore, R. N., K. J. Goodrum, and L. J. Berry. 1976. Mediation of an endotoxic effect by macrophages. J. Reticuloendothel. Soc. 19:187-197. [PubMed] [Google Scholar]
- 286.Moore, R. N., K. J. Goodrum, R. E. Couch, and L. J. Berry. 1978. Elicitation of endotoxemic effects in C3H/HeJ mice with glucocorticoid antagonizing factor and partial characterization of the factor. Infect. Immun. 19:79-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Nagler, A., R. Or, B. Nisman, I. Kalickman, S. Slavin, and V. Barak. 1995. Elevated inflammatory cytokine levels in bone marrow graft rejection. Transplantation 60:943-948. [PubMed] [Google Scholar]
- 288.Nakamura, T. A., K. Yamada, T. Hasegawa, and T. Nabeshima. 1995. Possible involvement of nitric oxide in quinolinic acid-induced convulsion in mice. Pharmacol. Biochem. Behav. 51:309-312. [DOI] [PubMed] [Google Scholar]
- 289.Nauciel, C., and F. Espinassemaes. 1992. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect. Immun. 60:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Naziri, W., J. D. Pietsch, S. H. Appel, W. G. Cheadle, T. M. Bergamini, and H. C. Polk. 1995. Hemorrhagic shock-induced alterations in circulating and bronchoalveolar macrophage nitric oxide production. J. Surg. Res. 59:146-152. [DOI] [PubMed] [Google Scholar]
- 291.Negussie, Y., D. G. Remick, L. E. DeForge, S. L. Kunkel, A. Eynon, and G. E. Griffin. 1992. Detection of plasma tumor necrosis factor, interleukins 6, and 8 during the Jarisch-Herxheimer Reaction of relapsing fever. J. Exp. Med. 175:1207-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Neilson, D., J. P. Kavanagh, and P. N. Rao. 1996. Kinetics of circulating TNF-alpha and TNF soluble receptors following surgery in a clinical model of sepsis. Cytokine 8:938-943. [DOI] [PubMed] [Google Scholar]
- 293.Netea, M. G., J. W. M. van der Meer, M. van Deuren, and B. J. Kullberg. 2003. Proinflammatory cytokines and sepsis syndrome: not enough, or too much of a good thing? Trends Immunol. 24:254-258. [DOI] [PubMed] [Google Scholar]
- 294.Nicholls, J. M., L. L. Poon, K. C. Lee, W. F. Ng, S. T. Lai, C. Y. Leung, C. M. Chu, P. K. Hui, K. L. Mak, W. Lim, K. W. Yan, K. H. Chan, N. C. Tsang, Y. Guan, K. Y. Yuen, and J. S. Peiris. 2003. Lung pathology of fatal severe acute respiratory syndrome. Lancet 361:1773-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Nishina, K., K. Mikawa, S. Kodama, and H. Obara. 2001. ONO1714, a new inducible nitric oxide synthase inhibitor, attenuates sepsis-induced diaphragmatic dysfunction in hamsters. Anesth. Analg. 92:959-966. [DOI] [PubMed] [Google Scholar]
- 296.Nsutebu, E. F., P. Martins, and D. Adiogo. 2003. Short communication. Prevalence of typhoid fever in febrile patients with symptoms clinically compatible with typhoid fever in Cameroon. Trop. Med. Int. Health 8:575-578. [DOI] [PubMed] [Google Scholar]
- 297.Nussler, A. K., W. Eling, and P. G. Kremsner. 1994. Patients with Plasmodium falciparum malaria and Plasmodium vivax malaria show increased nitrite and nitrate plasma levels. J. Infect. Dis. 169:1418-1419. [DOI] [PubMed] [Google Scholar]
- 298.O'Dea, K. P., and G. Pasvol. 2003. Optimal tumor necrosis factor induction by Plasmodium falciparum requires the highly localized release of parasite products. Infect. Immun. 71:3155-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.O'Donovan, D. A., C. J. Kelly, H. Abdih, D. Bouchier-Hayes, R. W. G. Watson, H. P. Redmond, P. E. Burke, and D. A. Bouchier-Hayes. 1995. Role of nitric oxide in lung injury associated with experimental acute pancreatitis. Br. J. Surg. 82:1122-1126. [DOI] [PubMed] [Google Scholar]
- 300.Oliver, F. J., J. Menissier-de Murcia, C. Nacci, P. Decker, R. Andriantsitohaina, S. Muller, G. de la Rubia, J. C. Stoclet, and G. de Murcia. 1999. Resistance to endotoxic shock as a consequence of defective NF-kappa B activation in poly(ADP-ribose) polymerase-1 deficient mice. EMBO J. 18:4446-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Olumese, P. E., O. Sodeinde, R. A. Gbadegesin, O. Nafiu, S. Oguche, and O. Walker. 1995. Respiratory distress adversely affects the outcome of childhood cerebral malaria. Trans. R. Soc. Trop. Med. Hyg. 89:634. [DOI] [PubMed] [Google Scholar]
- 302.Olweny, C., S. Chauhan, O. Simooya, M. Bulsara, E. Njelsani, and H. Van Thuc. 1986. Adult cerebral malaria in Zambia: preliminary report of clinical findings and treatment response. J. Trop. Med. Hyg. 89:123-129. [PubMed] [Google Scholar]
- 303.Ombrellino, M., H. Wang, M. S. Ajemian, A. Talhouk, L. A. Scher, S. G. Friedman, and K. J. Tracey. 1999. Increased serum concentrations of high-mobility-group protein 1 in haemorrhagic shock. Lancet 354:1446-1447. [DOI] [PubMed] [Google Scholar]
- 304.Onongbu, I., and E. Onyeneke. 1983. Plasma lipid changes in human malaria. Tropenmed. Parasitol. 34:193-196. [PubMed] [Google Scholar]
- 305.Otterbein, L. E., F. H. Bach, J. Alam, M. Soares, H. Tao Lu, M. Wysk, R. J. Davis, R. A. Flavell, and A. M. Choi. 2000. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 6:422-428. [DOI] [PubMed] [Google Scholar]
- 306.Owen-Schaub, L. B., W. L. Crump, G. I. Morin, and E. A. Grimm. 1989. Regulation of lymphocyte tumor necrosis factor receptors by IL-2. J. Immunol. 143:2236-2241. [PubMed] [Google Scholar]
- 307.Palmer, R. M. J., A. G. Ferridge, and S. Moncada. 1987. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327:524-526. [DOI] [PubMed] [Google Scholar]
- 308.Papadopoulos, M. C., D. C. Davies, R. F. Moss, D. Tighe, and E. D. Bennett. 2000. Pathophysiology of septic encephalopathy: a review. Crit. Care Med. 28:3019-3024. [DOI] [PubMed] [Google Scholar]
- 309.Park, J. S., J. Arcaroli, H. K. Yum, H. Yang, H. Wang, K. Y. Yang, K. H. Choe, D. Strassheim, T. M. Pitts, K. J. Tracey, and E. Abraham. 2003. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am. J. Physiol. 284:C870-C879. [DOI] [PubMed] [Google Scholar]
- 310.Parkkinen, J., E. Raulo, J. Merenmies, R. Nolo, E. O. Kajander, M. Baumann, and H. Rauvala. 1993. Amphoterin, the 30-kDa protein in a family of HMG1-type polypeptides. Enhanced expression in transformed cells, leading edge localization, and interactions with plasminogen activation. J. Biol. Chem. 268:19726-19738. [PubMed] [Google Scholar]
- 311.Pasquet, J. P. E. E., M. H. Zou, and V. Ullrich. 1996. Peroxynitrite inhibition of nitric oxide synthases. Biochimie 78:785-791. [DOI] [PubMed] [Google Scholar]
- 312.Paterson, H. M., T. J. Murphy, E. J. Purcell, O. Shelley, S. J. Kriynovich, E. Lien, J. A. Mannick, and J. A. Lederer. 2003. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J. Immunol. 171:1473-1483. [DOI] [PubMed] [Google Scholar]
- 313.Perezseveriano, F., B. Escalante, and C. Rios. 1998. Nitric oxide synthase inhibition prevents acute quinolinate-induced striatal neurotoxicity. Neurochem. Res. 23:1297-1302. [DOI] [PubMed] [Google Scholar]
- 314.Petricevich, V. L., C. F. P. Teixeria, D. V. Tambourgi, and J. M. Gutierrez. 2000. Increments in serum cytokine and nitric oxide levels in mice injected with Bothrops asper and Bothrops jararaca snake venoms. Toxicon 38:1253-1266. [DOI] [PubMed] [Google Scholar]
- 315.Peyron, F., N. Burdin, P. Ringwald, J. P. Vuillez, F. Rousset, and J. Banchereau. 1994. High levels of circulating IL-10 in human malaria. Clin. Exp. Immunol. 95:300-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Phillips, R. E., S. Looareesuwan, D. A. Warrell, S. H. Lee, J. Karbwang, M. J. Warrell, N. J. White, C. Swasdichai, and D. J. Weatherall. 1986. The importance of anaemia in cerebral and uncomplicated falciparum malaria: role of complications, dyserythropoiesis and iron sequestration. Q. J. Med. 227:305-323. [PubMed] [Google Scholar]
- 317.Piguet, P.-F., G. E. Grau, B. Allet, and P. Vassalli. 1987. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs-host disease. J. Exp. Med. 166:1280-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Platzer, C., C. Meisel, K. Vogt, M. Platzer, and H. D. Volk. 1995. Up-regulation of monocytic IL-10 by tumor necrosis factor-alpha and cAMP elevating drugs. Int. Immunol. 7:517-523. [DOI] [PubMed] [Google Scholar]
- 319.Playfair, J. H., J. Taverne, C. A. Bate, and J. B. de Souza. 1990. The malaria vaccine: anti-parasite or anti-disease? Immunol. Today 11:25-27. [DOI] [PubMed] [Google Scholar]
- 320.Pombo, D. J., G. Lawrence, C. Hirunpetcharat, C. Rzepczyk, M. Bryden, N. Cloonan, K. Anderson, Y. Mahakunkijcharoen, L. B. Martin, and D. Wilson. 2002. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet 360:610-617. [DOI] [PubMed] [Google Scholar]
- 321.Procopio, D. O., I. C. Almeida, A. C. Torrecilhas, J. E. Cardoso, L. Teyton, L. R. Travassos, A. Bendelac, and R. T. Gazzinelli. 2002. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins from Trypanosoma cruzi bind to CD1d but do not elicit dominant innate or adaptive immune responses via the CD1d/NKT cell pathway. J. Immunol. 169:3926-3933. [DOI] [PubMed] [Google Scholar]
- 322.Pukrittayakamee, S., N. J. White, R. Clemens, S. Chittamas, H. E. Karges, V. Desakorn, S. Looareesuwan, and D. Bunnag. 1989. Activation of the coagulation cascade in falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 83:762-766. [DOI] [PubMed] [Google Scholar]
- 323.Pullerits, R., I. M. Jonsson, M. Verdrengh, M. Bokarewa, U. Andersson, H. Erlandsson Harris, and A. Tarkowski. 2003. High mobility group box chromosomal protein 1, a DNA binding cytokine, induces arthritis. Arthritis Rheum. 48:1693-1700. [DOI] [PubMed] [Google Scholar]
- 324.Punyagupta, S., T. Srichaikul, P. Nitiyanant, and B. Petchclai. 1974. Acute pulmonary insufficiency in falciparum malaria: summary of 12 cases with evidence of disseminated intravascular coagulation. Am. J. Trop. Med. Hyg. 23:551-559. [DOI] [PubMed] [Google Scholar]
- 325.Reimold, A. M. 2003. New indications for treatment of chronic inflammation by TNF-alpha blockade. Am. J. Med. Sci. 325:75-92. [DOI] [PubMed] [Google Scholar]
- 326.Riedemann, N. C., R.-F. Guo, and P. A. Ward. 2003. Novel strategies for the treatment of sepsis. Nat. Med. 5:517-524. [DOI] [PubMed] [Google Scholar]
- 327.Riley, M. V., and B. G. Maegraith. 1962. Changes in the metabolism of liver mitochondria of mice infected with rapid acute Plasmodium berghei malaria. Ann. Trop. Med. Parasitol. 56:473-482. [DOI] [PubMed] [Google Scholar]
- 328.Robinson, V. K., E. Sato, D. K. Nelson, S. L. Camhi, R. A. Robbins, and J. C. Hoyt. 2001. Peroxynitrite inhibits inducible (type 2) nitric oxide synthase in murine lung epithelial cells in vitro. Free Radical Biol. Med. 30:986-991. [DOI] [PubMed] [Google Scholar]
- 329.Rockett, K. A., M. M. Awburn, B. B. Aggarwal, W. B. Cowden, and I. A. Clark. 1992. In vivo induction of nitrite and nitrate by tumor necrosis factor, lymphotoxin, and interleukin-1: possible roles in malaria. Infect. Immun. 60:3725-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Rockett, K. A., M. M. Awburn, E. J. Rockett, and I. A. Clark. 1994. Tumor necrosis factor and interleukin-1 synergy in the context of malaria pathology. Am. J. Trop. Med. Hyg. 50:735-742. [DOI] [PubMed] [Google Scholar]
- 331.Rockett, K. A., M. M. Awburn, E. J. Rockett, W. B. Cowden, and I. A. Clark. 1994. Possible role of nitric oxide in malarial immunosuppression. Parasite Immunol. 16:243-249. [DOI] [PubMed] [Google Scholar]
- 332.Roczniak, A., and K. D. Burns. 1996. Nitric oxide stimulates guanylate cyclase and regulates sodium transport in rabbit proximal tubule. Am. J. Physiol. 270:F106-F115. [DOI] [PubMed] [Google Scholar]
- 333.Roger, T., J. David, M. P. Glauser, and T. Calandra. 2001. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 414:920-924. [DOI] [PubMed] [Google Scholar]
- 334.Rojas, A., J. Padron, L. Caveda, M. Palacios, and S. Moncada. 1993. Role of nitric oxide pathway in the protection against lethal endotoxemia afforded by low doses of lipopolysaccharide. Biochem. Biophys. Res. Commun. 191:441-446. [DOI] [PubMed] [Google Scholar]
- 335.Romero, J. F., G. Eberl, H. R. Macdonald, and G. Corradin. 2001. CD1d-restricted NK T cells are dispensable for specific antibody responses and protective immunity against liver stage malaria infection in mice. Parasite Immunol. 23:267-269. [DOI] [PubMed] [Google Scholar]
- 336.Rongione, A. J., A. M. Kusske, K. Kwan, S. W. Ashley, H. A. Reber, and D. W. Mcfadden. 1997. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology 112:960-967. [DOI] [PubMed] [Google Scholar]
- 337.Ropert, C., I. C. Almeida, M. Closel, L. R. Travassos, M. A. Ferguson, P. Cohen, and R. T. Gazzinelli. 2001. Requirement of mitogen-activated protein kinases and I kappa B phosphorylation for induction of proinflammatory cytokines synthesis by macrophages indicates functional similarity of receptors triggered by glycosylphosphatidylinositol anchors from parasitic protozoa and bacterial lipopolysaccharide. J. Immunol. 166:3423-3431. [DOI] [PubMed] [Google Scholar]
- 338.Rouhiainen, A., S. Imai, H. Rauvala, and J. Parkkinen. 2000. Occurrence of amphoterin (HMG1) as an endogenous protein of human platelets that is exported to the cell surface upon platelet activation. Thromb. Haemostasis. 84:1087-1094. [PubMed] [Google Scholar]
- 339.Roumen, R. M. H., T. Hendriks, J. Van der Venjongekrijg, G. A. P. Nieuwenhuijzen, R. W. Sauerwein, J. W. M. Van der Meer, and R. J. A. Goris. 1993. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma—relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann. Surg. 218:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Rowbottom, A. W., P. G. Riches, C. Downie, and J. R. Hobbs. 1993. Monitoring cytokine production in peripheral blood during acute graft-versus-host disease following allogeneic bone marrow transplantation. Bone Marrow Transplant. 12:635-641. [PubMed] [Google Scholar]
- 341.Rubenstein, M., J. H. Mulholland, G. M. Jeffery, and S. M. Wolff. 1965. Malaria-induced endotoxin tolerance. Proc. Soc. Exp. Biol. Med. 118:283-287. [DOI] [PubMed] [Google Scholar]
- 342.Ruddle, N. H., and B. H. Waksman. 1967. Cytotoxic effect of lymphocyte-antigen interaction in delayed hypersensitivity. Science 157:1060-1062. [DOI] [PubMed] [Google Scholar]
- 343.Rush, B. F. 1992. The bacterial factor in hemorrhagic shock. Surg. Gynecol. Obstet. 175:285-292. [PubMed] [Google Scholar]
- 344.Sachs, J., and P. Malaney. 2002. The economic and social burden of malaria. Nature 415:680-685. [DOI] [PubMed] [Google Scholar]
- 345.Sakamoto, K., H. Arakawa, S. Ikei, H. Egami, S. Mita, T. Ishiko, S. Hisano, and M. Ogawa. 1994. Elevation of circulating interleukin 6 after surgery — factors influencing the serum level. Cytokine 6:181-186. [DOI] [PubMed] [Google Scholar]
- 346.Sambe, A., D. Ungureanulongrois, G. Danialou, S. Lanone, J. Benessiano, M. Aubier, and J. Boczkowski. 1998. Role of nitric oxide on diaphragmatic contractile failure in Escherichia coli endotoxemic rats. Comp. Biochem. Physiol. 119:167-175. [DOI] [PubMed] [Google Scholar]
- 347.Santangelo, M. L., M. Usberti, E. Di Salvo, G. Belli, G. Romano, C. Sassaroli, and G. Zotti. 1982. A study of the pathology of the crush syndrome. Surg. Gynecol. Obstet. 154:372-374. [PubMed] [Google Scholar]
- 348.Sappington, P. L., R. Yang, H. Yang, K. J. Tracey, R. L. Delude, and M. P. Fink. 2002. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology 123:790-802. [DOI] [PubMed] [Google Scholar]
- 349.Sarady, J. K., S. L. Otterbein, F. Liu, L. E. Otterbein, and A. M. Choi. 2002. Carbon monoxide modulates endotoxin-induced production of granulocyte macrophage colony-stimulating factor in macrophages. Am. J. Respir. Cell. Mol. Biol. 27:739-745. [DOI] [PubMed] [Google Scholar]
- 350.Scaffidi, P., T. Misteli, and M. E. Bianchi. 2002. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191-195. [DOI] [PubMed] [Google Scholar]
- 351.Schluesener, H. J., P. G. Kremsner, and R. Meyermann. 2001. Heme oxygenase-1 in lesions of human cerebral malaria. Acta Neuropathol. 101:65-68. [DOI] [PubMed] [Google Scholar]
- 352.Schluter, D., L. Y. Kwok, S. Lutjen, S. Soltek, S. Hoffmann, H. Korner, and M. Deckert. 2003. Both lymphotoxin-alpha and TNF are crucial for control of Toxoplasma gondii in the central nervous system. J. Immunol. 170:6172-6182. [DOI] [PubMed] [Google Scholar]
- 353.Schmidt, A. M., M. Hasu, D. Popov, J. H. Zhang, J. Chen, S. D. Yan, J. Brett, R. Cao, K. Kuwabara, G. Costache, N. Simionescu, M. Simionescu, and D. Stern. 1994. Receptor for advanced glycation end products (AGEs) has a central role in vessel wall interactions and gene activation in response to circulating AGE proteins. Proc. Natl Acad. Sci. USA 91:8807-8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Schmidt, A. M., S. D. Yan, S. F. Yan, and D. M. Stern. 2000. The biology of the receptor for advanced glycation end products and its ligands. Biochim. Biophys. Acta 1486:99-111. [DOI] [PubMed] [Google Scholar]
- 355.Schmidt, A. M., S. D. Yan, S. F. Yan, and D. M. Stern. 2001. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J. Clin. Investig. 108:949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Schmidt, H. H. H. W. 1992. NO., CO and OH—endogenous soluble guanylyl cyclase-activating factors. FEBS Lett. 307:102-107. [DOI] [PubMed] [Google Scholar]
- 357.Schmitz, H., B. Kohler, T. Laue, C. Drosten, P. J. Veldkamp, S. Gunther, P. Emmerich, H. P. Geisen, K. Fleischer, M. F. C. Beersma, and A. Hoerauf. 2002. Monitoring of clinical and laboratory data in two cases of imported Lassa fever. Microbes Infect. 4:43-50. [DOI] [PubMed] [Google Scholar]
- 358.Schnittler, H. J., F. Mahner, D. Drenckhahn, H. D. Klenk, and H. Feldmann. 1993. Replication of Marburg virus in human endothelial cells. A possible mechanism for the development of viral hemorrhagic disease. J. Clin. Investig. 91:1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Schoenfeld, H. J., B. Poeschl, J. R. Frey, H. Loetscher, W. Hunziker, A. Lustig, and M. Zulauf. 1991. Efficient purification of recombinant human tumor necrosis factor beta from Escherichia coli yields biologically active protein with a trimeric structure that binds to both tumor necrosis factor receptors. J. Biol. Chem. 266:3863-3869. [PubMed] [Google Scholar]
- 360.Schofield, L., and F. Hackett. 1993. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J. Exp. Med. 177:145-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Schofield, L., M. C. Hewitt, K. Evans, M. A. Siomos, and P. H. Seeberger. 2002. Synthetic GPI as a candidate antitoxic vaccine in a model of malaria. Nature 418:785-789. [DOI] [PubMed] [Google Scholar]
- 362.Schofield, L., M. J. McConville, D. Hansen, A. S. Campbell, R. B. Fraser, M. J. Grusby, and S. D. Tachado. 1999. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science 283:225-229. [DOI] [PubMed] [Google Scholar]
- 363.Schofield, L., S. Novakovic, P. Gerold, R. T. Schwarz, M. J. McConville, and S. D. Tachado. 1996. Glycosylphosphatidylinositol toxin of Plasmodium up-regulates intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and e-selectin expression in vascular endothelial cells and increases leukocyte and parasite cytoadherence via tyrosine kinase-dependent signal transduction. J. Immunol. 156:1886-1896. [PubMed] [Google Scholar]
- 364.Schofield, L., L. Vivas, F. Hackett, P. Gerold, R. T. Schwarz, and S. Tachado. 1993. Neutralizing monoclonal antibodies to glycosylphosphatidylinositol, the dominant TNF-alpha-inducing toxin of Plasmodium falciparum—prospects for the immunotherapy of severe malaria. Ann. Trop. Med. Parasitol. 87:617-626. [DOI] [PubMed] [Google Scholar]
- 365.Schraufstatter, I. U., D. B. Hinshaw, P. A. Hyslop, R. G. Spragg, and C. G. Cochrane. 1986. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J. Clin. Investig. 77:1312-13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Schwacha, M. G., and S. D. Somers. 1998. Thermal injury-induced immunosuppression in mice: the role of macrophage-derived reactive nitrogen intermediates. J. Leukoc. Biol. 63:51-58. [DOI] [PubMed] [Google Scholar]
- 367.Serirom, S., W. H. Raharjo, K. Chotivanich, S. Loareesuwan, P. Kubes, and M. Ho. 2003. Anti-adhesive effect of nitric oxide on Plasmodium falciparum cytoadherence under flow. Am. J. Pathol. 162:1651-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Shah, N. S., and T. R. Billiar. 1998. Role of nitric oxide in inflammation and tissue injury during endotoxemia and hemorrhagic shock. Environ. Health Perspect. 106:1139-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Shall, S., and G. de Murcia. 2000. Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat. Res. 460:1-15. [DOI] [PubMed] [Google Scholar]
- 370.Shortt, H. E., N. H. Fairley, G. Covell, P. G. Shute, and P. C. C. Garnham. 1951. The pre-erythrocytic stage of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 44:405-419. [DOI] [PubMed] [Google Scholar]
- 371.Silamut, K., N. H. Phu, C. Whitty, G. Turner, K. Louwrier, N. Mai, J. A. Simpson, T. T. Hien, and N. J. White. 1999. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am. J. Pathol. 155:395-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Silva, J. S., F. S. Machado, and G. A. Martins. 2003. The role of nitric oxide in the pathogenesis of Chagas disease. Front. Biosci. 8:S314-S325. [DOI] [PubMed] [Google Scholar]
- 373.Sims, C. A., S. Wattanasirichaigoon, M. J. Menconi, A. M. Ajami, and M. P. Fink. 2001. Ringer's ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Crit. Care Med. 29:1513-1518. [DOI] [PubMed] [Google Scholar]
- 374.Slifman, N. R., S. K. Gershon, J. H. Lee, E. T. Edwards, and M. M. Braun. 2003. Listeria monocytogenes infection as a complication of treatment with tumor necrosis factor alpha-neutralizing agents. Arthritis Rheum. 48:319-324. [DOI] [PubMed] [Google Scholar]
- 375.Sorci, G., F. Riuzzi, A. L. Agneletti, C. Marchetti, and R. Donato. 2003. S100B inhibits myogenic differentiation and myotube formation in a RAGE-independent manner. Mol. Cell. Biol. 23:4870-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Soriano, F. G., L. Liaudet, E. Szabo, L. Virag, J. G. Mabley, P. Pacher, and C. Szabo. 2002. Resistance to acute septic peritonitis in poly(ADP-ribose) polymerase-1-deficient mice. Shock 17:286-292. [DOI] [PubMed] [Google Scholar]
- 377.Soszynski, D. 2002. Inhibition of nitric oxide synthase delays the development of tolerance to LPS in rats. Physiol. Behav. 76:159-169. [DOI] [PubMed] [Google Scholar]
- 378.Sparatore, B., M. Pedrazzi, M. Passalacqua, D. Gaggero, M. Patrone, S. Pontremoli, and E. Melloni. 2002. Stimulation of erythroleukaemia cell differentiation by extracellular high-mobility group-box protein 1 is independent of the receptor for advanced glycation end-products. Biochem. J. 363:529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Spencer, J. C., R. Ganguly, and R. H. Waldman. 1977. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with bacille Calmette-Guérin. J. Infect. Dis. 136:171-175. [DOI] [PubMed] [Google Scholar]
- 380.Spriggs, D. R., M. L. Sherman, H. Michie, K. A. Arthur, K. Imamura, D. Wilmore, E. Frei, and D. W. Kufe. 1988. Recombinant human tumor necrosis factor administered as a 24-hour intravenous infusion. A phase 1 and pharmacologic study. J. Natl. Cancer Inst. 80:1039-1044. [DOI] [PubMed] [Google Scholar]
- 381.Sprung, C. L., P. N. Peduzzi, C. H. Shatney, R. M. Schein, M. F. Wilson, J. N. Sheagren, L. B. Hinshaw, and The Veterans Administration Systemic Sepsis Cooperative Study Group. 1990. Impact of encephalopathy on mortality in the sepsis syndrome. Crit. Care Med. 18:801-806. [DOI] [PubMed] [Google Scholar]
- 382.Sriskandan, S., D. Moyes, G. Lemm, and J. Cohen. 1996. Lymphotoxin-alpha (TNF-beta) during sepsis. Cytokine 8:933-937. [DOI] [PubMed] [Google Scholar]
- 383.Stroher, U., E. West, H. Bugany, H. D. Klenk, H. J. Schnittler, and H. Feldmann. 2001. Infection and activation of monocytes by Marburg and Ebola viruses. J. Virol. 75:11025-11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Suharti, C., E. C. M. van Gorp, T. E. Setiati, W. M. V. Dolmans, R. J. Djokomoeljanto, C. E. Hack, H. ten Cate, and J. W. M. van der Meer. 2002. The role of cytokines in activation of coagulation and fibrinolysis in dengue shock syndrome. Thromb. Haemostasis 87:42-46. [PubMed] [Google Scholar]
- 385.Sviland, L., and A. M. Dickinson. 1999. A human skin explant model for predicting graft-versus-host disease following bone marrow transplantation. J. Clin. Pathol. 52:910-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Szabo, C. 1996. The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia-reperfusion injury. Shock 6:79-88. [DOI] [PubMed] [Google Scholar]
- 387.Szabo, C., B. Zingarelli, M. O'Connor, and A. L. Salzman. 1996. DNA strand breakage, activation of poly(ADP-ribose) synthetase, and cellular energy depletion are involved in the cytotoxicity in macrophages and smooth muscle cells exposed to peroxynitrite. Proc. Natl Acad. Sci. USA 93:1753-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Szold, O., R. Ben-Abraham, I. Frolkis, M. Sorkine, and P. Sorkine. 2003. Tumor necrosis factor as a mediator of cardiac toxicity following snake envenomation. Crit. Care Med. 31:1449-1453. [DOI] [PubMed] [Google Scholar]
- 389.Szold, O., R. Ben-Abraham, A. A. Weinbroum, T. E. Englender, D. Ovadia, M. Sorkine, C. Bon, R. Flaison, and P. Sorkine. 2001. Antagonization of TNF attenuates systemic hemodynamic manifestations of envenomation in a rat model of Vipera aspis snakebite. Intensive Care Med. 27:884-888. [DOI] [PubMed] [Google Scholar]
- 390.Szold, O., A. A. Weinbroum, R. Ben-Abraham, T. E. Englender, D. Ovadia, and P. Sorkine. 2000. Xanthine oxidase and tumor necrosis pastor alpha: possible mediators of remote tissue injury alter viper envenomation. Isr. Med. Assoc. J. 2:816-820. [PubMed] [Google Scholar]
- 391.Tachado, S. D., P. Gerold, M. J. McConville, T. Baldwin, D. Quilici, R. T. Schwarz, and L. Schofield. 1996. Glycosylphosphatidylinositol toxin of Plasmodium induces nitric oxide synthase expression in macrophages and vascular endothelial cells by a protein tyrosine kinase-dependent and protein kinase c-dependent signaling pathway. J. Immunol. 156:1897-1907. [PubMed] [Google Scholar]
- 392.Tachado, S. D., P. Gerold, R. Schwarz, S. Novakovic, M. McConville, and L. Schofield. 1997. Signal transduction in macrophages by glycosylphosphatidylinositols of Plasmodium, Trypanosoma, and Leishmania: activation of protein tyrosine kinases and protein kinase C by inositolglycan and diacylglycerol moieties. Proc. Natl Acad. Sci. USA 94:4022-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Tachado, S. D., and L. Schofield. 1994. Glycosylphosphatidylinositol toxin of Trypanosoma brucei regulates IL-1 alpha and TNF-alpha expression in macrophages by protein tyrosine kinase mediated signal transduction. Biochem. Biophys. Res. Commun. 205:984-991. [DOI] [PubMed] [Google Scholar]
- 394.Tambourgi, D. V., V. L. Petricevich, F. C. Magnoli, S. L. M. R. Assaf, S. Jancar, and W. D. Dasilva. 1998. Endotoxemic-like shock induced by loxosceles spider venoms: pathological changes and putative cytokine mediators. Toxicon 36:391-403. [DOI] [PubMed] [Google Scholar]
- 395.Tanaka, N., H. Yonekura, S. Yamagishi, H. Fujimori, Y. Yamamoto, and H. Yamamoto. 2000. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-alpha through nuclear factor-kappa B, and by 17beta-estradiol through Sp-1 in human vascular endothelial cells. J. Biol. Chem. 275:25781-25790. [DOI] [PubMed] [Google Scholar]
- 396.Taniguchi, N., K. Kawahara, K. Yone, T. Hashiguchi, M. Yamakuchi, M. Goto, K. Inoue, S. Yamada, K. Ijiri, S. Matsunaga, T. Nakajima, S. Komiya, and I. Maruyama. 2003. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 48:971-981. [DOI] [PubMed] [Google Scholar]
- 397.Tanneberger, S., H. Lenk, U. Muller, J. Ebert, and T. Shiga. 1988. Human pharmacological investigation of a human recombinant tumor necrosis factor preparation, p. 205. In B. Bonavida, G. E. Gifford, H. L. Kirchner, and J. Old (ed.), Tumor necrosis factor/cachectin and related cytokines. Karger, Basel, Switzerland. [PubMed]
- 398.Taverne, J., C. A. W. Bate, and J. H. L. Playfair. 1989. Induction of TNF in vitro as a model for the identification of toxic malaria antigens. Lymphokine Res. 8:317-322. [PubMed] [Google Scholar]
- 399.Taverne, J., P. Depledge, and J. H. Playfair. 1982. Differential sensitivity in vivo of lethal and nonlethal malarial parasites to endotoxin-induced serum factor. Infect. Immun. 37:927-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Taverne, J., H. M. Dockrell, and J. H. L. Playfair. 1981. Endotoxin-induced serum factor kills malarial parasites in vitro. Infect. Immun. 33:83-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Taverne, J., J. Tavernier, W. Fiers, and J. H. L. Playfair. 1987. Recombinant tumour necrosis factor inhibits malaria parasites in vivo but not in vitro. Clin. Exp. Immunol. 67:1-4. [PMC free article] [PubMed] [Google Scholar]
- 402.Tawadrous, Z. S., R. L. Delude, and M. P. Fink. 2002. Resuscitation from hemorrhagic shock with Ringer's ethyl pyruvate solution improves survival and ameliorates intestinal mucosal hyperpermeability in rats. Shock. 17:473-477. [DOI] [PubMed] [Google Scholar]
- 403.Taylor, F. B. 2001. Staging of the pathophysiologic responses of the primate microvasculature to Escherichia coli and endotoxin: examination of the elements of the compensated response and their links to the corresponding uncompensated lethal variants. Crit. Care Med. 29:S78-S89. [DOI] [PubMed] [Google Scholar]
- 404.Taylor, J. H., A. J. Guthrie, and A. Leisewitz. 1991. The effect of endogenously produced carbon monoxide on the oxygen status of dogs infected with Babesia canis. J. S. Afr. Vet. Assoc. 62:153-155. [PubMed] [Google Scholar]
- 405.Taylor, M. J., H. F. Cross, and K. Bilo. 2000. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J. Exp. Med. 191:1429-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Taylor, T. E., M. E. Molyneux, J. J. Wirima, A. Borgstein, J. D. Goldring, and M. Hommel. 1992. Intravenous immunoglobulin in the treatment of paediatric cerebral malaria. Clin. Exp. Immunol. 90:357-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Thiemermann, C., C. Szabo, J. A. Mitchell, and J. R. Vane. 1993. Vascular hyporeactivity to vasoconstrictor agents and hemodynamic decompensation in hemorrhagic shock is mediated by nitric oxide. Proc. Natl Acad. Sci. USA 90:267-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Thistlethwaite, J. R., Jr., A. B. Cosimi, F. L. Delmonico, R. H. Rubin, N. Talkoff Rubin, P. W. Nelson, L. Fang, and P. S. Russell. 1984. Evolving use of OKT3 monoclonal antibody for treatment of renal allograft rejection. Transplantation 38:695-701. [DOI] [PubMed] [Google Scholar]
- 409.Thistlethwaite, J. R., Jr., J. K. Stuart, J. T. Mayes, A. O. Gaber, S. Woodle, M. R. Buckingham, and F. P. Stuart. 1988. Complications and monitoring of OKT3 therapy. Am. J. Kidney Dis. 11:112-119. [DOI] [PubMed] [Google Scholar]
- 410.Thomas, L. 1972. Germs. N. Engl. J. Med. 287:553-555. [DOI] [PubMed] [Google Scholar]
- 411.Tiao, G., J. Rafferty, C. Ogle, J. E. Fischer, and P. O. Hasselgren. 1994. Detrimental effect of nitric oxide synthase inhibition during endotoxemia may be caused by high levels of tumor necrosis factor and interleukin-6. Surgery 116:332-338. [PubMed] [Google Scholar]
- 412.Tokime, T., K. Nozaki, T. Sugino, H. Kikuchi, N. Hashimoto, and K. Ueda. 1998. Enhanced poly(ADP-ribosyl)ation after focal ischemia in rat brain. J. Cereb. Blood Flow Metab. 18:991-997. [DOI] [PubMed] [Google Scholar]
- 413.Tracey, K. J., B. Beutler, S. F. Lowry, J. Merryweather, S. Wolpe, I. W. Milsark, R. J. Hariri, T. J. Fahey, A. Zentella, J. D. Albert, G. T. Shires, and A. Cerami. 1986. Shock and tissue injury induced by recombinant human cachectin. Science 234:470-474. [DOI] [PubMed] [Google Scholar]
- 414.Tracey, K. J., S. F. Lowry, T. J. Fahey, J. D. Albert, Y. Fong, D. Hesse, B. Beutler, K. R. Manogue, S. Calvano, A. Cerami, and G. T. Shires. 1987. Cachectin/tumor necrosis factor induces lethal shock and stress hormone response in the dog. Surg. Gynecol. Obstet. 164:415-422. [PubMed] [Google Scholar]
- 415.Tufo, H. M., A. M. Ostfeld, and R. Shekelle. 1970. Central nervous system dysfunction following open-heart surgery. JAMA 212:1333-1340. [PubMed] [Google Scholar]
- 416.Turner, P. F., K. A. Rockett, E. A. Ottesen, H. Francis, K. Awadzi, and I. A. Clark. 1994. Interleukin-6 and tumor necrosis factor in the pathogenesis of adverse reactions after treatment of lymphatic filariasis and onchocerciasis. J. Infect. Dis. 169:1071-1075. [DOI] [PubMed] [Google Scholar]
- 417.Ueyama, M., I. Maruyama, M. Osame, and Y. Sawada. 1992. Marked increase in plasma interleukin-6 in burn patients. J. Lab. Clin. Med. 120:693-698. [PubMed] [Google Scholar]
- 418.Ulloa, L., M. Ochani, H. Yang, M. Tanovic, D. Halperin, R. Yang, C. J. Czura, M. P. Fink, and K. J. Tracey. 2002. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc. Natl Acad. Sci. USA 99:12351-12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Valero, N., L. M. Espina, G. Anez, E. Torres, and J. A. Mosquera. 2002. Short report. Increased level of serum nitric oxide in patients with dengue. Am. J. Trop. Med. Hyg. 66:762-764. [DOI] [PubMed] [Google Scholar]
- 420.Van Amersfoort, E. S., T. J. Van Berkel, and J. Kuiper. 2003. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 16:379-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.van der Poll, T., H. R. Buller, H. Gate, C. H. Wortel, K. A. Bauer, S. J. H. van Deventer, E. Hack, H. P. Sauerwein, R. D. Rosenberg, and J. W. Gate. 1990. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N. Engl. J. Med. 322:1622-1627. [DOI] [PubMed] [Google Scholar]
- 422.Vanlaethem, J. L., A. Marchant, A. Delvaux, M. Goldman, P. Robberecht, T. Velu, and A. Deviere. 1995. Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology 108:1917-1922. [DOI] [PubMed] [Google Scholar]
- 423.Venkataraman, R., J. A. Kellum, M. Song, and M. P. Fink. 2002. Resuscitation with Ringer's ethyl pyruvate solution prolongs survival and modulates plasma cytokine and nitrite/nitrate concentrations in a rat model of lipopolysaccharide-induced shock. Shock 18:507-512. [DOI] [PubMed] [Google Scholar]
- 424.Viens, P., G. A. Targett, E. Leuchars, and A. J. Davies. 1974. The immunological response of CBA mice to Trypanosoma musculi. I. Initial control of the infection and the effect of T-cell deprivation. Clin. Exp. Immunol. 16:279-294. [PMC free article] [PubMed] [Google Scholar]
- 425.Vijaykumar, M., R. S. Naik, and D. C. Gowda. 2001. Plasmodium falciparum glycosylphosphatidylinositol-induced TNF-alpha secretion by macrophages is mediated without membrane insertion or endocytosis. J. Biol. Chem. 276:6909-6912. [DOI] [PubMed] [Google Scholar]
- 426.Wahlgren, M., J. S. Abrams, V. Fernandez, M. T. Bejarano, M. Azuma, M. Torii, M. Aikawa, and R. J. Howard. 1995. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells and secretion of cytokines (IL-1-beta, IL-1RA, IL-6, IL-8, IL-10, TGF beta, TNF alpha, G-CSF, GM-CSF). Scand. J. Immunol. 42:626-636. [DOI] [PubMed] [Google Scholar]
- 427.Walsh, C. E., J. M. Liu, S. M. Anderson, J. L. Rossio, A. W. Nienhuis, and N. S. Young. 1992. A trial of recombinant human interleukin-1 in patients with severe refractory aplastic anaemia. Br. J. Haematol. 80:106-110. [DOI] [PubMed] [Google Scholar]
- 428.Wang, H. C., O. Bloom, M. H. Zhang, J. M. Vishnubhakat, M. Ombrellino, J. T. Che, A. Frazier, H. Yang, S. Ivanova, L. Borovikova, K. R. Manogue, E. Faist, E. Abraham, J. Andersson, U. Andersson, P. E. Molina, N. N. Abumrad, A. Sama, and K. J. Tracey. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285:248-251. [DOI] [PubMed] [Google Scholar]
- 429.Wang, P., P. Wu, M. I. Siegel, R. W. Egan, and M. M. Billah. 1995. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J. Biol. Chem. 270:9558-9563. [DOI] [PubMed] [Google Scholar]
- 430.Warrell, D. A. 2001. “To search and Study out the secret of Tropical Diseases by way of Experiment”. Lancet 358:1983-1988. [DOI] [PubMed] [Google Scholar]
- 431.Warrell, D. A., S. Looareesuwan, M. J. Warrell, P. Kasemsarn, R. Intaraprasert, D. Bunnag, and T. Harinasuta. 1982. Dexamethasone proves deleterious in cerebral malaria: a double-blind trial in 100 comatose patients. N. Engl. J. Med. 306:313-319. [DOI] [PubMed] [Google Scholar]
- 432.Wautier, J. L., C. Zoukourian, O. Chappey, M. P. Wautier, P. J. Guillausseau, R. Cao, O. Hori, D. Stern, and A. M. Schmidt. 1996. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J. Clin. Investig. 97:238-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Wenisch, C., B. Parschalk, E. Narzt, S. Looareesuwan, and W. Graninger. 1995. Elevated serum levels of IL-10 and IFN-gamma in patients with acute Plasmodium falciparum malaria. Clin. Immunol. Immunopathol. 74:115-117. [DOI] [PubMed] [Google Scholar]
- 434.Whalen, M. J., R. S. Clark, C. E. Dixon, P. Robichaud, D. W. Marion, V. Vagni, S. Graham, L. Virag, G. Hasko, R. Stachlewitz, C. Szabo, and P. M. Kochanek. 2000. Traumatic brain injury in mice deficient in poly-ADP(ribose) polymerase: a preliminary report. Acta Neurochir. 76:61-64. [DOI] [PubMed] [Google Scholar]
- 435.Whalen, M. J., R. S. B. Clark, C. E. Dixon, P. Robichaud, D. W. Marion, V. Vagni, S. H. Graham, L. Virag, G. Hasko, R. Stachlewitz, C. Szabo, and P. M. Kochanek. 1999. Reduction of cognitive and motor deficits after traumatic brain injury in mice deficient in poly(ADP-ribose) polymerase. J. Cereb. Blood Flow Metab. 19:835-842. [DOI] [PubMed] [Google Scholar]
- 436.Wiedenmann, B., P. Reichardt, U. Räth, L. Theilmann, B. Schüle, A. Ho, E. Schlick, J. Kempeni, W. Hunstein, and B. Kommerell. 1989. Phase-1 trial of intravenous continuous infusion of tumor necrosis factor in advanced metastatic carcinomas. J. Cancer Res. Clin. Oncol. 115:189-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Wood, P. R., and I. A. Clark. 1984. Macrophages from Babesia and malaria infected mice primed for monokine release. Parasite Immunol. 6:309-317. [DOI] [PubMed] [Google Scholar]
- 438.Woodle, E. S., J. R. Thistlethwaite, I. A. Ghobrial, L. K. Jolliffe, F. P. Stuart, and J. A. Bluestone. 1991. OKT3 F(ab′)2 fragments—retention of the immunosuppressive properties of whole antibody with marked reduction in T cell activation and lymphokine release. Transplantation 52:354-360. [DOI] [PubMed] [Google Scholar]
- 439.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 440.Yang, R., D. J. Gallo, J. J. Baust, T. Uchiyama, S. K. Watkins, R. L. Delude, and M. P. Fink. 2002. Ethyl pyruvate modulates inflammatory gene expression in mice subjected to hemorrhagic shock. Am. J. Physiol. 283:G212-G221. [DOI] [PubMed] [Google Scholar]
- 441.Yang, R., X. Han, T. Uchiyama, S. K. Watkins, A. Yagushi, R. L. Delude, and M. P. Fink. 2003. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am. J. Physiol. 285:G621-G629. [DOI] [PubMed] [Google Scholar]
- 442.Yoshinari, D., I. Takeyoshi, Y. Koibuchi, K. Matsumoto, Y. Kawashima, T. Koyama, S. Ohwada, and Y. Morishita. 2001. Effects of a dual inhibitor of tumor necrosis factor-alpha and interleukin-1 on lipopolysaccharide-induced lung injury in rats: involvement of the p38 mitogen-activated protein kinase pathway. Crit. Care Med. 29:628-634. [DOI] [PubMed] [Google Scholar]
- 443.Young, G. B., C. F. Bolton, T. W. Austin, Y. M. Archibald, J. Gonder, and G. A. Wells. 1990. The encephalopathy associated with septic illness. Clin. Investig. Med. 13:297-304. [PubMed] [Google Scholar]
- 444.Zakeri, S., K. Taylor, J. L. Goad, and M. Hommel. 2000. Polar Plasmodium falciparum lipids induce lipogenesis in rat adipocytes in vitro. Microbes Infect. 2:1789-1798. [DOI] [PubMed] [Google Scholar]
- 445.Zamuner, S. R., and C. F. P. Teixeira. 2002. Cell adhesion molecules involved in the leukocyte recruitment induced by venom of the snake Bothrops jararaca. Mediators Inflamm. 11:351-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Zegdi, R., D. Perrin, M. Burdin, R. Boiteau, and A. Tenaillon. 2002. Increased endogenous carbon monoxide production in severe sepsis. Intensive Care Med. 28:793-796. [DOI] [PubMed] [Google Scholar]
- 447.Zingarelli, B., P. W. Hake, and J. A. Cook. 2002. Inducible nitric oxide synthase is not required in the development of endotoxin tolerance in mice. Shock 17:478-484. [DOI] [PubMed] [Google Scholar]
- 448.Zingarelli, B., F. Squadrito, D. Altavilla, G. Calapai, G. M. Campo, M. Calo, A. Saitta, and A. P. Caputi. 1992. Evidence for a role of nitric oxide in hypovolemic hemorrhagic shock. J. Cardiovasc. Pharmacol. 19:982-986. [DOI] [PubMed] [Google Scholar]
- 449.Zingarelli, B., F. Squadrito, D. Altavilla, G. Calapai, M. Dirosa, and A. P. Caputi. 1994. Role of tumor necrosis factor-alpha in acute hypovolemic hemorrhagic shock in rats. Am. J. Physiol. 266:H1512-H1515. [DOI] [PubMed] [Google Scholar]
- 450.Zvilich, M., J. C. Williams, D. Waag, W. R. Rill, P. Bell, and M. Kende. 1995. Efficacy of Coxiella burnetii and its chloroform-methanol residue (CMR) fraction against Rift Valley fever virus infection in mice. Antiviral Res. 27:137-149. [DOI] [PubMed] [Google Scholar]
- 451.Zweifach, B. W., B. Benacerraf, and L. Thomas. 1957. The relationship between the vascular manifestations of shock produced by endotoxin, trauma, and hemorrhage II. The possible role of the reticulo-endothelial system in resistance to each type of shock. J. Exp. Med. 106:403-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Zweifach, B. W., and L. Thomas. 1957. The relationship between the vascular manifestations of shock produced by endotoxin, trauma, and hemorrhage. I. Certain similarities between the reactions in normal and endotoxin-tolerant rats. J. Exp. Med. 106:385-401. [DOI] [PMC free article] [PubMed] [Google Scholar]