Abstract
Giardia lamblia is an early branching protist that possesses peripheral vacuoles (PVs) with characteristics of lysosome-like organelles, located underneath the plasma membrane. In more evolved cells, lysosomal protein trafficking is achieved by cargo recognition involving adaptor protein (AP) complexes that recognize specific amino acid sequences (tyrosine and/or dileucine motifs) within the cytoplasmic tail of membrane proteins. Previously, we reported that Giardia has a tyrosine-based sorting system, which mediates the targeting of a membrane-associated cysteine protease (encystation-specific cysteine protease, ESCP) to the PVs. Here, we show that Giardia AP1 mediates the transport of ESCP and the soluble acid phosphatase (AcPh) to the PVs. By using the yeast two-hybrid assay we found that the ESCP tyrosine-based motif interacts specifically with the medium subunit of AP1 (Giμa). Hemagglutinin-tagged Giμa colocalizes with ESCP and AcPh and coimmunoprecipitates with clathrin, suggesting that protein trafficking toward the PVs is clathrin-adaptin dependent. Targeted disruption of Giμa results in mislocalization of ESCP and AcPh but not of variant-specific surface proteins. Our results suggest that, unlike mammalian cells, only AP1 is involved in anterograde protein trafficking to the PVs in Giardia. Moreover, even though Giardia trophozoites lack a morphologically discernible Golgi apparatus, the presence of a clathrin-adaptor system suggests that this parasite possess a primitive secretory organelle capable of sorting proteins similar to that of more evolved cells.
INTRODUCTION
In the early branching protist Giardia lamblia, the endomembrane system is reduced to the presence of an endoplasmic reticulum (ER) and lysosome-like peripheral vacuoles (PVs). The property of the PVs to accumulate exogenous macromolecules and, at the same time, lysosome-like soluble hydrolases, suggests that this parasite possesses an endosomal/lysosomal system represented in this single organelle (Lanfredi-Rangel et al., 1998). Although there are obvious differences, protein transport in Giardia mimics that of higher eukaryotes because proteins can be secreted constitutively (e.g., variant-specific surface proteins; VSPs) or in regulated manner (e.g., cyst wall proteins; CWPs) (Lujan et al., 1995; Mowatt et al., 1995; Nash et al., 1995). However, unlike yeast and mammalian cells, the lysosomal sorting pathway is not well defined in Giardia. In more evolved cells, membrane proteins carrying a sorting motif in their cytoplasmic tails are routed to lysosomes (Bonifacino and Traub, 2003), whereas soluble hydrolases are sorted from the trans-Golgi network (TGN) to the endosome/lysosome system through a receptor-mediated process (e.g., mannose 6-phosphate receptors) (Ghosh and Kornfeld, 2003). To recruit these proteins, several adaptor proteins recognize tyrosine-based (YXXφ) or dileucine-based motifs in the cytoplasmic tail of transmembrane proteins (Ghosh and Kornfeld, 2003; Bonifacino and Traub, 2003) and also bind clathrin inducing the formation of the clathrin-coated vesicles (CCVs) (Ohno et al., 1995, 1996). There are four heterotetrameric adaptor protein (AP) complexes, AP1, AP2, AP3, and AP4, each composed by four subunits: two large chains (one each of γ/α/δ/ε and β1-4, respectively), one medium-sized chain (μ1-4), and one small chain (σ1-4) (Boehm and Bonifacino, 2002). These AP complexes have been described in different sub-cellular locations where they may function specifically in cargo selection (Boehm and Bonifacino, 2002). It is well known that AP2 mediates endocytosis from the plasma membrane, whereas AP1, AP3, and AP4 participate in protein sorting from the TGN and/or endosomes to lysosomes.
In Giardia, we recently found that a transmembrane PV protein (encystation-specific cysteine protease, ESCP) is transported to the PVs through a tyrosine-based motif (Touz et al., 2003). Moreover, soluble hydrolases such as acid phosphatase (AcPh) and cathepsin B also reside within Giardia PVs (Ward et al., 1997; Lanfredi-Rangel et al., 1998; Slavin et al., 2002), although no receptor involved in the traffic of these soluble proteins has been identified. Thus, it is possible that Giardia possesses a similar mechanism for lysosomal protein trafficking where adaptor proteins may be involved. The presence of several secretory protein genes in the Giardia genome database (McArthur et al., 2000), including a putative clathrin heavy chain, and two large, one medium, and one small subunits of AP1 and AP2 homologous proteins supports this hypothesis. Probably only AP1 and AP2 participate in protein transport to lysosome-like PVs in Giardia because no other adaptor complexes, including AP3 and AP4, the monomeric adaptor GGA, Dab2, epsin, or Hrs (Bonifacino and Traub, 2003) are present in the nearly complete Giardia genome.
Here, we show that AP1 is involved in the anterograde protein transport to the Giardia PVs but not in the constitutive secretion of VSPs. This finding stands in contrast to the multiple adaptor proteins required for delivery to the endosome/lysosome in more evolved organisms.
MATERIALS AND METHODS
Giardia lamblia Cultivation and Transfection
Trophozoites of the isolate WB, clone 1267 (Nash et al., 1988), were cultured as described previously (Keister, 1983). Encystation of trophozoite monolayer was accomplished by the method described by Boucher and Gillin (1990). Trophozoites were transfected with the constructs by electroporation and selected with puromycin as described previously (Yee and Nash, 1995; Singer et al., 1998; Elmendorf et al., 2001). Cotransfection selection was accomplished by adding 100 μg of puromycin and 50 μg of geneticin (Invitrogen, Carlsbad, CA) to the growth medium. For induction of μa double-stranded RNA (μa dsRNA), cells transfected with dsRNA-μa or Neo-dsRNA-μa vectors were cultured in growth medium with 10 μg of tetracycline (Sigma-Aldrich, St. Louis, MO) for 24 h. To test the role of Giμa during encystation, cells transfected with dsRNA-μa were encysted for 48 h in the presence or absence of 10 μg of tetracycline.
Immunofluorescence Assays
For fixed cells, trophozoites cultured in growth medium were harvested and processed as described previously (Lujan et al., 1995). For direct double staining, anti-hemagglutinin (HA) monoclonal antibody (mAb) (Sigma-Aldrich) was conjugated with Zenon One fluorescein and used to detect Giμa (final dilution of anti-HA 1:500). Anti-V5 mAb (Sigma-Aldrich), used for ESCP and AcPh detection, was conjugated with Zenon One Texas Red-X (final dilution of anti-V5 1:200) as suggested by the manufacturer (Molecular Probes, Eugene, OR). For VSP9B10, 9B10 mAb (1:200) was used in indirect assays. For cyst detection, the supernatant of 48-h-encysting-cells was washed twice with phosphate-buffered saline (PBS)-0.1% growth medium and incubated with the anti-CWP2-specific 7D2 mAb conjugated with Texas Red as described previously (Touz et al., 2003). Controls included omission of primary antibody and staining of nontransfected cells. The specimens were examined with an Axioplan fluorescence microscope (Carl Zeiss, Thornwood, NY) by using a 40×/0.75 (44 03 50) or a 100×/1.3 (44 03 50) Plan-NEOFLUAR lens (Carl Zeiss) or Leica TCSNT/SP confocal microscope. Digital images were recorded using a Hamamatsu digital camera (Hamamatsu, Bridgewater, NJ) and processed with QED Camera Plug-inä (QED Imaging, Pittsburgh, PA).
Immunoprecipitation
HA-tagged Giμa transfected Giardia trophozoites were disrupted in lysis buffer (50 mM Tris, pH 8.0, 120 mM NaCl, 5 mM EDTA, 1% Triton X-100, and protease inhibitors) for 30 min on ice and centrifuged at 13,000 × g for 5 min at 4°C. The cell lysate was precleared by using protein A/G-Sepharose beads (Santa Cruz Biotechnology, Santa Cruz, CA) for 30 min at 4°C, and then subsequently subjected to immunoprecipitation by using 2 μl of specific anti-clathrin (kindly provided by Dr. Adrian B. Hehl, Institute of Parasitology, University of Zurich, Zurich, Switzerland) (Marti et al., 2003) or anti-aldolase (our unpublished data) mouse polyclonal antibodies. After incubation overnight at 4°C, protein A/G-Sepharose was added, and the incubation continued for 4 h. The immunoprecipitates were washed three times in lysis buffer before immunoblot analysis.
Immunoblot Analysis
Western blot assays were performed as previously reported (Lujan et al., 1995). Briefly, 10 μg of total protein/lane from transfected encysting trophozoites were resuspended in 30 μl of sample buffer (Bio-Rad, Hercules, CA) with 2-mercaptoethanol, boiled for 5 min, and electrophoresed into a 4-12% Tris-glycine polyacrylamide gel. The proteins were transblotted onto polyvinylidene difluoride membranes (Invitrogen) and probed with anti-CWP2 mAb. For pull-down assays, anti-V5 mAb and anti-HA mAb were used at 1:1000 dilution (Sigma-Aldrich). For Immunoprecipitation experiments, anti-HA or anti-VSP9B10 mAbs directed labeled with biotin (Santa Cruz Biotechnology) was used at 1:500 dilution and developed with alkaline phosphatase-conjugated streptavidin (Bio-Rad).
Yeast Two-Hybrid Screening
Construction and screening of a two-hybrid library was done by using the MATCHMAKER library construction and screening kit following the protocol suggested by the company (BD Biosciences Clontech, Palo Alto, CA). Briefly, the two-hybrid GAL4 DNA binding domain (GAL4bd) and GAL4 transcription activation domain (GAL4ad) fusion constructs were prepared by ligating ESCP cDNA or ds cDNA (obtained from total RNA of nonencysting trophozoites; GenBank accession no. AF293408) into the pGBKT7(TRP1) and pGADT7-Rec(LEU2) vectors, respectively. AH109 yeast reporter was cotransformed with pGBKT7, pGADT7-Rec, and ds cDNA by the lithium acetate procedure. For colony growth assays, AH109 transformants were resuspended in water to 0.1 OD 600/ml, and then 5 μl was spotted on plates lacking leucine and tryptophan (-L/-T) in the presence or absence of histidine (triple dropout medium, TDO) or histidine and adenine (quadruple dropout medium, QDO), and cultured at 30°C for 4-5 d. Sequencing of cDNA inserts was done using dye terminator cycle sequencing (Beckman Coulter, Fullerton, CA). For the yeast two-hybrid assays, the same strategy was used except that ds cDNA of μa, μb, cwp2, or gdpp (GenBank accession no. AACB01000112, AACB000044, U28965, and AF293412, respectively) were cloned into pGADT7(LEU2) (BD Biosciences Clontech). Amino acid substitution of ESCP-YRPI motif was made with the QuikChange mutagenesis kit (Stratagene, La Jolla, CA).
Two-Gene Plasmid Construction
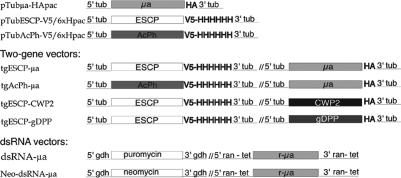
To express two tagged Giardia proteins at the same time (one carrying the V5/6 × H and the other the HA epitope at the C termini), we constructed the two-gene plasmid (tg). First, the full-length μa cDNA encoding Giμa protein (nucleotide 1-1345) was cloned in the restricted pTubH7-HApac vector (Touz et al., 2003), yielding pTubμa-HApac (Figure 1). This vector was then modified by introducing a SpeI restriction site before the 5′-tubulin promoter by using a QuikChange mutagenesis kit (Stratagene). To construct the two-gene plasmid, escp (encoding encystation-specific cystein-protease) or acph (encoding acid phosphatase, GenBank accession no. AF293415), including their 5′-tubulin and 3′-tubulin untranslated region controlling sequences, were amplified by polymerase chain reaction (PCR) from pTubESCP-V5/6 × Hpac and pTubAcPh-V5/6 × Hpac vectors, respectively (Figure 1), and cloned into the pTubμa-HApac vector by restriction and ligation into the SpeI and XbaI sites. Thus, the final constructs were tgESCP-μa and tgAcPh-μa that contain V5-tagged ESCP plus HA-tagged Giμa and V5-tagged AcPh plus HA-tagged Giμa, respectively. The same procedure was followed to create tgESCP-CWP2 and tgESCP-gDPP for V5-tagged ESCP plus HA-tagged CWP2 or HA-tagged gDPP, respectively (Figure 1). Primers used in the construction of the two-gene plasmid are listed below.
Figure 1.
Schematic representation of Giardia vectors. pTubμa-HApac contains the ORF of μa with the HA epitope sequence at the C terminus. pTubESCP-V5/6 × Hpac has the ESCP gene and the V5 epitope plus the sequence encoding six His at the C terminus. pTubAcPh-V5/6 × Hpac carries the AcPh gene and the sequences encoding the V5 epitope plus six His at the C terminus. Vectors carrying two genes (tg): tgESCP-μa contains ESCP-V5/6 × His and μa-HA; tgAcPh-μa contains AcPh-V5/6 × His and μa-HA; tgESCP-CWP2 contains ESCP-V5/6 × His and CWP2-HA; tgESCP-gDPP contains ESCP-V5/6 × His and gDPP-HA. Each vector possesses a puromycin acetyl transferase gene for selection. All genes are expressed under the control of the tubulin promoter. dsRNA vectors: dsRNA-μa and Neo-dsRNA-μa vectors contain 1-1000bp-μa sequence (r-μa) between opposing tetracycline-inducible Giardia ran promoters' and the puromycin or neomycin acetyl transferase resistant genes flanked by glutamate dehydrogenase (gdh) promoters, respectively.
Pull-Down Assays
Giardia transfected with pTubESCP-V5/6 × Hpac, tgESCP-μa, tgESCP-CWP2, or tgESCP-gDPP (Figure 1) were grown in growth medium (or encysting medium for ESCP-CWP2 protein-protein interaction positive control), harvested, and resuspended in 1 ml of lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazol, pH 8.0, 1% Triton X-100, and protease inhibitors) for 1 h at 4°C. After the lysate was centrifuged at 10,000 × g, 10 min at 4°C, the supernatant was mixed with 200 μl of Ni-agarose beads (QIAGEN, Valencia, CA) and incubated overnight at 4°C. Beads were spun down at 700 × g and washed four times with wash buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, pH 8.0, 0.1% Triton X-100, and protease inhibitors). Bound proteins were eluted four times with 100 μl of elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazol, pH 8.0, 0.1% Triton X-100, and protease inhibitors) and analyzed by immunoblotting.
Giardia dsRNA Vector Construction
A dsRNA vector (our unpublished data) was constructed based on a tetracycline-inducible plasmid made by Sun and Tai (2000) later modified by Elmendorf et al. (personal communication) exchanging puromycin acetyl transferase for neomycin acetyl transferase gene (Singer et al., 1998). To silence the μa gene, nucleotides 1-1000 of μa open reading frame (ORF) (1-1345 nucleotides) were introduced into dsRNA vector by using dsRNAμaF and dsRNAμaR primers for μa amplification (see below). In this plasmid, the 1000-base pair-μa sequence was introduced between opposing tetracycline-inducible Giardia ran promoters, yielding the vector dsRNA-μa (Figure 1). To cotransform trophozoites and be able to select for each vector, the neomycin acetyl transferase was exchanged for puromycin acetyl transferase gene present in dsRNA-μa by using the QuikChange mutagenesis kit (Figure 1) (Stratagene).
Slot-Blot Assay
Two micrograms of total RNA extracted from growing trophozoites were immobilized onto a Nytran SuperCharge nylon membrane (Schleicher & Schuell, Keene, NH) by using the MINIFOLD II slot-blot system, according to the manufacturer's instructions. Membranes were first hybridized with anti-sense probes specific for the last 350 base pairs of μa gene and then stripped and reprobed by using an antisense specific for tubulin as control. The density of each band was measured using image analysis software EagleSight (Stratagene).
Brefeldin A (BFA) Treatment
To investigate the effect of BFA (Molecular Probes; stock solution 10 mg/ml dimethyl sulfoxide) in the transport of ESCP and Giμa, trophozoites were allowed to attach to the slides for 30 min in PBS-0.1% growth medium at 37°C. The PBS-0.1% growth medium was then discarded and fresh solution containing 50 μg/ml BFA (or dimethyl sulfoxide alone) was added for 1 h at 37°C. After fixation with 4% formaldehyde, the location of ESCP and Giμa was determined by immunofluorescence assays as described above.
Statistics
Descriptive statistics included calculation of mean and SD of control (-Tet) and experimental (+Tet) groups. A comparison of the means was performed using independent-samples Student's t test. A p ≤ 0.05 was considered significant.
Oligonucleotide Primers Used (5′-3′ Orientation)
For yeast-two hybrid assay, the following primers were used: ESCPXmaI-A T T C C C G G G T C T T G G G G A C C A A G T T C T G G G C G T A T G G, ESCP-SalI-G G G A C A A A A T A C C G T C C A A T A A T T G C A C G T C G A C C T G, ESCP(-YRPI)s-T G C G T A G T T A A G T C C C G C G G G A C A A A A g c c g c t g c a g c a A T T G C A C G G A T C C G T C G A C C T G C A G C G, ESCP(-YRPI)as-C G C T G C A G G T C G A C G G A T C C G T G C A A T t g c t g c a g c g g c T T T T G T C C C G C G G G A C T T A A C T A C G C A, μa-NcoI-G T T A C C A T G G T A A G C T C T C T G C T A A T C A T T, μa-EcoRI-G T T G T C A T A A A A C A C A C C A T C G T G G A A T T C C A G, μb-NdeI-G T T C A T A T G A T C A A G G C G G T C A T T C T T T T G, μb-BamHI-T G C G C G G C C T G T A A A C C C A A T C T T G G A T C C A T C, CWP2-NdeI-G T T C A T A T G T G C C C T G C C A C C G A G G A G G A G G C C, CWP2-BamHI-A A C A A G C C T A T T G T C C G C A G A A G G G G A T C CA T C, DPP-NdeI-G T T C A T A T G A T G A C G C T A T C G G C C T G G A T T A T A, and DPP-BamHI-T T T G A C T G G C T A G A T A C T T A C C T T G G A T C C A T C.
For two-gene vector construction, the following primers were used: μa-NcoI-G A CT CCATGG TAAGCTCTCTGCTAATCATTCACGGG, μa-SfoI-A T G G T T G T C A T A A A A C A C A C C A T C G T G G G C G C C T A C G, CWP2-NcoI-G A C T C C A T G G T C G C A G C C C T T G T T C T A G G A C T T C T T, CWP2-SfoI-A A C A A G C C T A T T G T C C G C A G A A G G G G C G C C T A C G, DPP-NcoI-G A C T C C A T G G C G C T A T C G G C C T G G A T T A T A, DPP-SfoI-T T T G A C T G G C T A G A T A C T T A C C T T G G C GCC T A CG, Tub5′-SpeI-GTTACTAGTATGCAATGACGCAGCG G C A C A A A G A G C, and Tub3-XbaI-T G C T T G T T C C T G G G C C T C A A A T G G T T A T C T A G A T A C G.
For dsRNA production, the following primers were used: dsRNAμaF-(BamHI)CACGGATCCATGATAAGCTCTCTGCTAATCATTCAC, and dsRNAμaR(SphI)TATTGCCCTCATGAGCAAGTTGTCAAGGCATGCTGG.
Restriction sites are denoted in bold or italics.
RESULTS
Lysosomal-Membrane Protein ESCP Interacts with the Medium Subunit of Giardia Adaptor Protein 1 (Giμa)
The yeast two-hybrid method was used to screen a Giardia cDNA library to detect putative proteins involved in the transport of ESCP to the PVs. The bait strain expressing pGBKT7-ESCP-(TRP1) was cotransfected with a nonencysting Giardia trophozoite cDNA library in which the inserts were expressed as fusion proteins with the pGADT7-Rec(LEU2) transcription activation domain (see MATERIALS AND METHODS). The initial screen produced 27 positive clones that grew in the selective medium TDO, and 24 positive clones that grew in the selective medium QDO. PCR and restriction digest analysis revealed 10 unique groups among these 24 clones. BLAST search and sequence analysis identified a number of proteins involved in different pathways, including three sequences encoding to a dynein-like protein (GenBank accession no. EAA37885), three encoding a Rab6-interacting protein (GenBank accession no. EAA42818), and, most significantly, five sequences corresponding to the ORF of the medium subunit of Giardia adaptor protein 1 (GenBank accession no. EAA38136), called Giμa (Marti et al., 2003). These results suggested that the polymeric adaptor protein AP1 is involved in binding and transport of ESCP to PVs in Giardia.
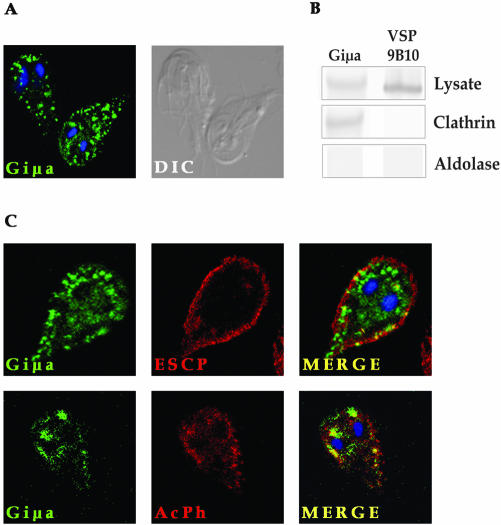
Giμa Localizes around Giardia Nuclei and Colocalizes with ESCP and AcPh in Lysosome-like Peripheral Vacuoles
In mammalian cells, lysosomal-protein transport from the TGN to the endosome/lysosome system is accomplished by inclusion of these proteins into clathrin-coated vesicles in an adaptin-mediated manner (Bonifacino and Traub, 2003). Immunofluorescence assays of constitutively low-expressed HA-tagged Giμa (Figure 1) showed this protein surrounding the nuclei and also close to the plasma membrane (Figure 2A). To address the question of whether HA-tagged Giμa is integrated into the AP1 complex, specific polyclonal antibodies (pAbs) were added to protein extracts obtained from Giardia trophozoites expressing Giμa. The results showed that HA-tagged Giμa but not VSP9B10 coimmunoprecipitates with the coatomer clathrin isolated using anti-clathrin heavy chain pAb. On the other hand, neither HA-tagged Giμa nor VSP9B10 was nonspecifically coimmunoprecipitated when an unrelated anti-aldolase pAb was added (Figure 2B).
Figure 2.
Giμa colocalizes with PV-resident proteins. (A) Indirect immunofluorescence assays show the HA-tagged Giμa surrounding both nuclei and close to the plasma membrane by confocal microscopy. (B) Analyses of immunoprecipitates of Giardia lysates formed using Giardia polyclonal antibodies to clathrin and aldolase in HA-tagged Giμa-expressing organisms. Only immunoprecipitates formed using anti-heavy chain clathrin specifically associates with HA-tagged Giμa. (C) Confocal microscopy shows colocalization of HA-tagged Giμa (green) and V5-tagged ESCP (red) in the PVs (yellow, merge). Double staining of HA-tagged Giμa (green) and V5-tagged AcPh (red) show colocalization of these two proteins around the nuclei and in the PVs in yellow (merge). Magnification, 1000×.
Direct double immunostaining of trophozoites transfected with two-gene plasmids expressing HA-tagged Giμa and V5-tagged ESCP or AcPh (Figure 1) revealed that AP1 partially colocalizes with both PV-resident proteins (Figure 2C). The colocalization of AP1 with ESCP and AcPh suggests that Giμa is involved in the recruitment from a sorting region that remains undefined (Golgi-like organelle?) and the transport of cargo proteins to the PVs.
ESCP Interacts Specifically with Giμa through the YRPI Tyrosine-based Motif
In higher eukaryotes, AP complexes bind to the tyrosine-based or dileucine-based motifs of membrane-associated proteins (Bonifacino and Traub, 2003). Previously, we showed that deletion or mutation of the ESCP tyrosine-based motif (YRPI) causes missorting of ESCP from the PVs to the surface of the trophozoite (Touz et al., 2003). Here, we used the two-hybrid assay to determine whether the YRPI sequence is responsible for the interaction with Giμa and whether this motif interacts with the medium subunit of adaptor protein 2 (Giμb). Thus, the μa, μb, cwp2, or gdpp genes were cloned into pGADT7(LEU2) and full-length escp or escp(-yrpi) (ESCP where the YRPI sorting motif is replaced by alanines) were cloned into the two-hybrid bait pGBKT7(TRP1). Consistent with our previous findings (Touz et al., 2003), Giμa interacts with the full-length ESCP through its tyrosine-based motif (Figure 3, A and B). In contrast, Giμb did not bind ESCP (Figure 3, A and B), suggesting that AP1 but not AP2 is involved in transport of ESCP to the PVs of Giardia. The cyst wall protein CWP2 (Lujan et al., 1995) and the surface Giardia dipeptidilpeptidase gDPP (Touz et al., 2002c) were used as positive and negative controls, respectively; confirming that ESCP interacts specifically with its substrate CWP2 but in a manner independent of the YRPI motif (Figure 3, A and B) (Touz et al., 2003).
Figure 3.
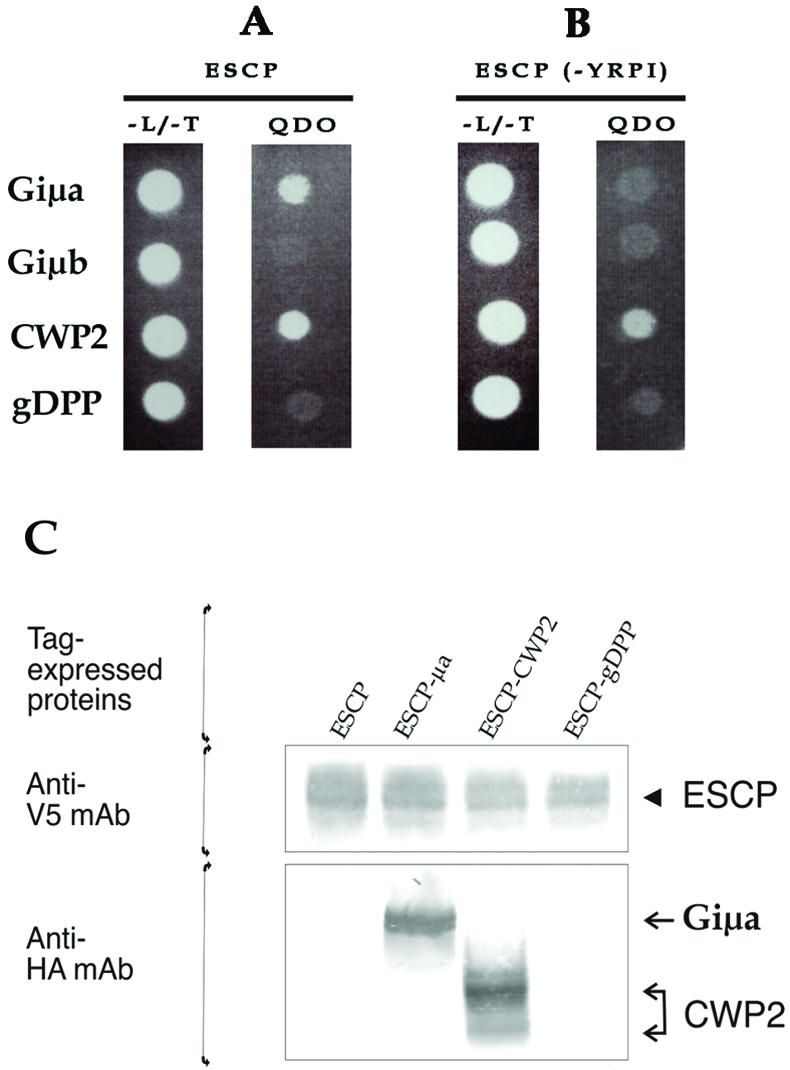
The μ subunit of AP1 interacts specifically with ESCP tyrosine-based motif. (A) Yeast two-hybrid assay shows that the μ subunit of AP1 (Giμa) as well as CWP2 (positive control) is able to interact with ESCP, allowing the yeast to grow in selective medium QDO. In contrast, neither the μ subunit of AP2 (Giμb) or gDPP (negative control) interacts with ESCP restricting yeast growth to the nonselective medium lacking LEU and TRP (-L/-T). (B) When the YRPI sorting motif of ESCP is mutated to alanines (ESCP-YRPI), Giμa is unable to bind to ESCP, showing that the Giμa-ESCP interaction depends on YRPI tyrosine-based motif. CWP2 still interacts with the mutated ESCP, indicating that this interaction is independent of the tyrosine-based motif. (C) V5/6 × His-tagged ESCP was purified using Ni-agarose beads and detected by immunoblotting by using anti-V5 mAb (arrowhead). The presence of associated proteins was analyzed by immunoblotting by using anti-HA mAb. Only Giμa and CWP2 were pulled down by ESCP (arrows).
To analyze the interaction between Giμa and ESCP, we transfected Giardia trophozoites with the following vectors: 1) pTubESCP-V5/6 × Hpac (expressing V5/His-tagged ESCP alone), 2) tgESCP-μa (expressing V5/His-tagged ESCP plus HA-tagged μ1-AP1), 3) tgESCP-CWP2 (expressing V5/His-tagged ESCP plus HA-tagged CWP2), and 4) tgESCP-gDPP (expressing V5/His-tagged ESCP plus HA-tagged gDPP) (Figure 1), and were later subjected to protein pull-down assays. Affinity purified His-tagged ESCP and associated proteins were initially analyzed by immunoblotting with anti-V5 mAb to detect the presence of ESCP (Figure 3C, arrowhead) or with anti-HA mAb to detect associated proteins (Figure 3B, arrows). The analysis showed that the interaction of ESCP with Giμa and its substrate CWP2, is both specific and stable in vivo (Figure 3B, ESCP-μa).
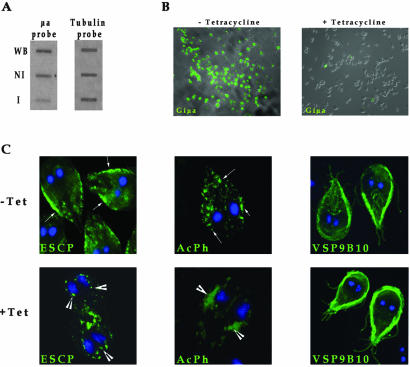
Lower Expression of Giμa Changes ESCP and AcPh Subcellular Localization
The role of AP1 in transport was further tested by inhibition of Giμa expression by using the dsRNA vectors dsRNA-μa and Neo-dsRNA-μa (Figure 1). After selection and induction, semiquantitative reverse transcription-PCR revealed an increase of the μa-antisense RNA production (our unpublished data) as well as a fivefold decrease in μa-sense RNA as determined by slot-blot analysis (Figure 4A). The ability of dsRNA-μa to decrease Giμa expression was confirmed by inhibition of constitutively expressed HA-tagged Giμa by cotransfecting pTubμa-HApac with the inducible Neo-dsRNA-μa vector. Selection was performed by adding puromycin for pTubμa-HApac and geneticin for Neo-dsRNA-μa. After induction of the μa dsRNA with tetracycline, the expression of Giμa was dramatically reduced (Figure 4B). These data indicate that both dsRNA-μa and NeodsRNA-μa vectors decreased Giμa expression.
Figure 4.
Depletion of Giμa alters ESCP and AcPh but not VSP subcellular localization. AP1 expression is reduced after production of μa dsRNA. (A) Two micrograms of total RNA was used for slot-blot assay by using μa-antisense or tubulin-antisense probes. WB, total RNA of nontransfected trophozoites; NI, total RNA of noninduced transfected trophozoites; I, total RNA of induced transfected trophozoites. (B) Immunofluorescence assay shows Giμa expression in noninduced (-tetracycline) and induced transfected trophozoites (+tetracycline). Magnification, 200×. (C) Confocal microscopy shows that the normal distribution of ESCP in the PVs (-Tet, arrows) is altered after the production of μa dsRNA (+Tet, arrowheads). AcPh is located around nuclei and in the PVs (-Tet, arrows) but is retained in the sorting place in Giμa-depleted cells (+Tet, arrowheads). VSP9B10 does not change its surface localization after Giμa depletion. Magnification, 630×.
To determine how depletion of Giμa affects the sorting and transport to PVs and the surface of Giardia, trophozoites were cotransfected with either pTubESCP-V5/6 × Hpac (expressing V5-tagged ESCP) or pTubAcPh-V5/6 × Hpac (expressing V5-tagged AcPh) and the Neo-dsRNA-μa vector. After growing transfected trophozoites with 10 μg of tetracycline for 72 h, ESCP and AcPh mostly localized to regions close to the nuclei (Figure 4C, arrowheads). In some cases, ESCP was also localized on the surface and colocalized with VSPs (our unpublished data). In contrast, no change was seen in the surface location of VSP9B10 (Figure 4C). Despite changes in location of ESCP and AcPh, there was no effect in the protein levels of ESCP, AcPh, and VSP9B10 in these cells, as determined by immunoblotting (our unpublished data). These data support the active participation of Giμa in the transport of PV-resident proteins.
Giμa Disruption Affects the Last Step of the Encystation Process
To survive outside the host, Giardia trophozoites differentiate into infective cysts, which are excreted in the feces (Adam, 2001). After the reception of the stimulus that triggers encystation, Giardia undergoes several metabolic and morphological changes comprising the synthesis, processing, and transport of proteins (CWPs) that will be secreted and assembled to form a protective cyst wall (Lujan et al., 1995a; Mowatt et al., 1995).
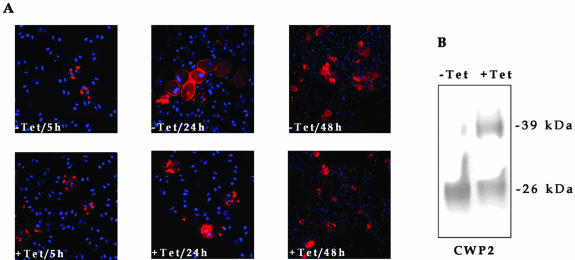
No effect was observed in the growth of Giμa-depleted trophozoites; however, these cells fail to accomplish encystation. When dsRNA-μa transfected trophozoites were induced with tetracycline for 48 h during encystation, the cyst production was significantly reduced (13.9%) compared with noninduced cells (40.3%) (Table 1 and Figure 5A). Analysis of CWP2 (a cyst wall protein highly induced during encystation) by immunofluorescence showed that this protein is present at high level in both cultures.
Table 1.
Targeted depletion of AP1 medium subunit (μa) decreases cyst production
| Giardia trophozoites | Tetracycline induction | No. attached cells/ml | No. nonattached cells/ml | % Encysting trophozoites | % Cyst production |
|---|---|---|---|---|---|
| dsRNAμa | 550.000 ± 10.500 | 1.00 × 106 ± 1.000 | 50.1 ± 10.5 | 40.4 ± 12.5 | |
| (I) dsRNAμa | 10μg | 400.000 ± 4.500 | 1.10 × 106 ± 1.500 | 80.2 ± 11.8 | 13.9 ± 7.8 |
Giardia trophozoites transfected with dsRNA-μa vector were induced (I) to express μa dsRNA by the addition of 10 μg of tetracycline to the pre- and encysting medium. After 48 h, the medium was decanted and attached and nonattached cells (including encysting trophozoites and cyst) were harvested separately and counted. The percentage of encysting cells and cysts were determined by immunofluorescence (Touz et al., 2002a). Results represent the mean of 10 independent experiments. For cyst production Student's t-test was performed: t(18) = 5.69; p < 0.001. The 95% confidence interval of difference is from 16.72 to 36.30.
Figure 5.
Depletion of Giμa reduces cyst formation. (A) Immunofluorescence assay using direct labeled anti-CWP2 7D2 mAb. Five hours after initiation of encystation both noninduced (-Tet, trophozoites nonexpressing μa dsRNA) and induced cultures (+Tet, trophozoites expressing μa dsRNA) show the presence of CWP2 in the ESVs. At 24 h and 48 h of encystation induced cultures show large decreases in cyst production. (B) Immunoblotting by using anti-CWP2 7D2 mAb shows that in noninduced transfected trophozoites (NI) CWP2 is a 26-kDa protein, whereas in induced trophozoites (I), a nonprocessed 39-kDa protein is additionally detected.
These results prompted us to analyze the molecular weight of CWP2 by immunoblotting because inhibition of ESCP is known to block the processing (but not the expression) of CWP2 and therefore the formation of the cyst wall (Touz et al., 2002b, 2003). We expected that during Giμa depletion, CWP2 would not be proteolytically processed due to the mislocation of ESCP away from the PVs. Immunoblot analysis of cells going through encystation showed that in noninduced cultures CWP2 is processed to a 26-kDa protein, whereas in induced cultures (Giμa-depleted cells) CWP2 is not completely processed, remaining mainly as a 39-kDa protein (Figure 5B). Controls of nontransfected trophozoites in the presence or absence of 10 μg of tetracycline during 48 h of encystation did not show any differences compared with noninduced dsRNA-μa transfected trophozoites. Therefore, Giμa deficiency decreases cyst production due to a failure in CWP2 processing.
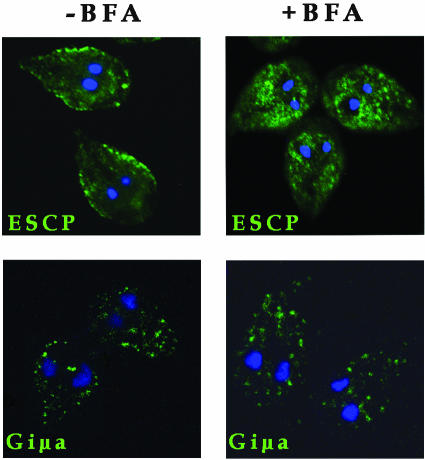
Brefeldin A Blocks ESCP Transport but Not Giμa
BFA has proved to be of great value as an inhibitor of protein trafficking in the endomembrane system of mammalian cells by inhibition of the assembly of the COPI and AP1 complexes onto membranes (Sciaky et al., 1997). To analyze whether this metabolite alters ESCP and Giμa transport, 50 μM BFA was added to trophozoites expressing V5-tagged ESCP and HA-tagged Giμa. After BFA treatment, ESCP was found close to the nuclei rather than in the PVs (Figure 6A), whereas Giμa localization was unaffected (Figure 6B).
Figure 6.
Brefeldin A alters ESCP but not Giμa localization. (A) Steady-state localization of V5-tagged ESCP in PVs underneath the plasma membrane of vegetative trophozoites (-BFA). When the cells are treated with BFA for 1 h, ESCP is retained around nuclei (+BFA). (B) HA-tagged Giμa localization around the nuclei and in the PVs (-BFA) does not change after addition of BFA (+BFA).
DISCUSSION
The protein-transport pathways to compartments of the endosomal/lysosomal system in mammalian, yeast, and protozoa are clearly related (Nothwehr and Stevens, 1994; Hirst et al., 1999). However, the presence of unusual secretory organelles in protozoa, including lysosome-like organelles, argues that there are unique features in their protein trafficking (Lanfredi-Rangel et al., 1998; Hoppe et al., 2000; Waller and McConville, 2002; Ngo et al., 2003). The early branching protist G. lamblia possesses one of the most distinctive secretory systems but on the whole is characteristically eukaryotic. For example, the transport is vesicular in nature and has both constitutive and regulated mechanisms of transport and signaling motifs that function similarly to those of higher eukaryotes. Nevertheless, unlike mammalian cells, Giardia lacks a morphologically discernible Golgi apparatus and contains an endosomal/lysosomal system concentrated in one single organelle (PVs) (Adam, 2001; Lujan and Touz, 2003). In a previous study, we reported that transport of a membrane-associated protease ESCP to the PVs is dependent upon a tyrosine-based motif (YXXØ), specifically YRPI, residing in its cytoplasmic tail. Similar motifs conforming to YXXØ operate in the transport of membrane-bound lysosomal proteins in higher eukaryotes and therefore suggested conserved mechanisms between Giardia and more evolved cells.
In mammalian cells, the medium subunits μ1, μ2, μ3, and μ4 of the adaptor complex AP1, AP2, AP3, and AP4, respectively, interact specifically with tyrosine-based sorting signals of receptors and other endosomal/lysosomal membrane proteins (Bonifacino et al., 1996; Boehm and Bonifacino, 2001; Bonifacino and Traub, 2003; Luzio et al., 2003). AP1, AP3, and AP4 participate in sorting from the TGN, whereas AP2 mediates transport to the lysosome by endocytosis. These heterotetrameric adaptor complexes, except for AP4, also take part in the recruitment of clathrin to form CCVs (Kirchhausen, 1999, 2000).
Using a variety of complementary techniques, including yeast two-hybrid assays and pull-down studies, we show that ESCP interacts with the medium subunit of Giardia AP1 complex (Giμa) through the tyrosine-based motif. In addition, we used a unique Giardia vector with a tetracycline-inducible promoter coupled with Giardia-RAN promoter that allows the inducible expression of μa dsRNA resulting in a reduction in Giμa expression. Analysis of protein sub-cellular localization in μa-depleted cells shows that the PV-resident proteins ESCP and AcPh are misplaced. Conversely, surface localization of VSP9B10 is not affected in μa-depleted cells. More than one adaptor protein is involved in the anterograde lysosomal protein trafficking in higher eukaryotes, whereas in Giardia, AP1 seems to be the only one implicated in the transport of transmembrane (ESCP) and soluble (AcPh) lysosomal enzymes from a Golgi-like organelle to the PVs. There is no evidence that other adaptor proteins are involved. First, AP3 and AP4 homologs as well as the monomeric adaptor GGA, Dab2, epsin, or Hrs (Boehm and Bonifacino, 2001) are not present in the essentially completed Giardia Genome Database (GGD, http://jbpc.mbl.edu/Giardia-HTML). Furthermore, AP2, an adaptor protein that is present in the GGD, does not seem to play a role in the transport of ESCP because AP2 fails to bind to ESCP in the yeast two-hybrid system and AP2 depletion does not alter ESCP localization (our unpublished data). However, AP2 may play a role in PV-protein transport from the plasma membrane (our unpublished data). AP1 is essential for AcPh transport although the receptor mediating transport, analogous to mannose-6-phosphate receptors that mediate transport of soluble lysosomal enzymes in mammalian cells, has not been identified in the GGD nor has any receptor been experimentally detected.
A polyclonal antibody against Giardia clathrin heavy chain was able to coimmunoprecipitate clathrin together with Giμa, suggesting that clathrin is bound to the AP1 complex through its large subunit (Giβa) forming “coated pits” (our unpublished data). The AP1-μa subunit subsequently recognizes a sorting motif within the cytoplasmic tail of the cargo protein that is packaged into CCVs at the place of sorting. Because clathrin (Marti et al., 2003) and AP1 (this work) are also localized to the PVs, it is possible that this complex remains unaltered until it reaches the PVs. Another possibility is that clathrin and adaptor protein complexes play a role in protein sorting from the PVs to different target organelles, similar to the role of the endosomal clathrin coat in protein sorting toward lysosomes (Sachse et al., 2002).
The importance of AP1 for survival and growth differs depending on the specific organism; it seems to play a more essential role in higher eukaryotes than in lower eukaryotes (Nakai et al., 1993; Yeung et al., 1999; Meyer et al., 2000; Shim and Lee, 2000; Shim et al., 2000; Gokool, 2003; Ngo et al., 2003). Although not critical for Giardia trophozoite survival and multiplication, AP1 is necessary for cyst formation. AP1 may act indirectly in this process by transporting ESCP to the PVs, allowing it to cleave the carboxyl tail of the CWP2. This event is critical for mature cyst wall formation (Touz et al., 2002b, 2003). Another less likely possibility is that AP1 is involved in the formation of encystation-specific secretory vesicles (ESVs) because secretory vesicles in many evolved cells contain clathrin-adaptor proteins together with other coat proteins at the time of vesicle formation (Tooze and Tooze, 1986; Molinete et al., 2000; Tooze et al., 2001). We do not favor the second explanation because we observed that ESV formation and CWP2 expression are not affected in AP1-deficient cells. AP1 could also be critical during the process of excystation because the AcPh seems to perform a crucial role during this step of cell differentiation (Slavin et al., 2002). AP1-deficient trophozoites are unable to encyst, implying that disruption of the AP1 function can serve as a potential target to block Giardia transmission.
Using the yeast two-hybrid system, we identified a number of proteins in nonencysting trophozoites that interacts with ESCP. Most are homologs of proteins that play a known role in the secretory processes and are associated with Golgi and Golgi functions. The interactions of ESCP and proteins known to be Golgi associated in higher eukaryotes suggest the existence of an organelle-specific membrane complex analogous to the Golgi complex in Giardia. Furthermore, cells treated with BFA, which inhibits the assembly of the COPI and AP1 complexes onto membranes in mammalian cells, show a redistribution of ESCP to the ER but not of Giμa, suggesting that ESCP is transported to the PVs first through a Golgi-like compartment in COPI-coated vesicles and then assembled into AP1/clathrin-coated vesicles in a post-Golgi compartment resistant to BFA. Our results are consistent with the presence of an organelle developed enough to select proteins to be packaged and delivered to different final destinations in the vegetative form of Giardia. Whether this organelle with sorting function is a specialized ER, a primitive Golgi, or TGN organelle or even a subdomain of the nuclear envelope remains to be determined.
In summary, we show that AP1 is specifically involved in the anterograde protein transport to the lysosome-like PVs in Giardia. Furthermore, the presence of homologues associated with Golgi that play a role in vesicular transport, the existence of similar activities of inhibitors of Golgi function as well as sorting signals analogous to higher eukaryotes, all suggest the presence of a Golgi or Golgi-like organelle in Giardia trophozoites. Because Giardia is an early branching protist, the study of vesicular transport in this parasite will lead to a clearer understanding of the minimal machinery required for protein transport in more evolved cells.
Acknowledgments
We thank John T. Conrad and Dr. Hugo D. Luján for critical reading of the manuscript and Dr. Adrian B Hehl for the gift of the anti-clathrin heavy chain polyclonal antibody.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-10-0744. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-10-0744.
Abbreviations used: AcPh, acid phosphatase; CCV, clathrin-coated vesicle; CWP2, cyst wall protein 2; ESCP, encystation-specific cysteine protease; GGD, Giardia Genome Database; gDPP, Giardia dipeptidilpeptidase; PV, peripheral vacuole; TGN, trans-Golgi network; VSP, variant-specific surface protein; YXXφ, tyrosine-based motif.
References
- Adam, R.D. (2001). Biology of Giardia lamblia. Clin. Microbiol. Rev. 14, 447-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm, M., and Bonifacino, J.S. (2001). Adaptins: the final recount. Mol. Biol. Cell 12, 2907-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm, M., and Bonifacino, J.S. (2002). Genetic analyses of adaptin function from yeast to mammals. Gene 286, 175-186. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J.S., Marks, M.S., Ohno, H., and Kirchhausen, T. (1996). Mechanisms of signal-mediated protein sorting in the endocytic and secretory pathways. Proc. Assoc. Am. Physicians 108, 285-295. [PubMed] [Google Scholar]
- Bonifacino, J.S., and Traub, L.M. (2003). Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72, 395-447. [DOI] [PubMed] [Google Scholar]
- Boucher, S.E., and Gillin, F.D. (1990). Excystation of in vitro-derived Giardia lamblia cysts. Infect. Immun. 58, 3516-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmendorf, H.G., Singer, S.M., Pierce, J., Cowan, J., and Nash, T.E. (2001). Initiator and upstream elements in the alpha2-tubulin promoter of Giardia lamblia. Mol. Biochem. Parasitol. 113, 157-169. [DOI] [PubMed] [Google Scholar]
- Ghosh, P., and Kornfeld, S. (2003). AP-1 binding to sorting signals and release from clathrin-coated vesicles is regulated by phosphorylation. J. Cell Biol. 160, 699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokool, S. (2003). sigma 1-and mu 1-Adaptin homologues of Leishmania mexicana are required for parasite survival in the infected host. J. Biol. Chem. 278, 29400-9. [DOI] [PubMed] [Google Scholar]
- Hirst, J., Bright, N.A., Rous, B., and Robinson, M.S. (1999). Characterization of a fourth adaptor-related protein complex. Mol. Biol. Cell 10, 2787-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe, H.C., Ngo, H.M., Yang, M., and Joiner, K.A. (2000). Targeting to rhoptry organelles of Toxoplasma gondii involves evolutionarily conserved mechanisms. Nat. Cell Biol. 2, 449-456. [DOI] [PubMed] [Google Scholar]
- Keister, D.B. (1983). Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 77, 487-488. [DOI] [PubMed] [Google Scholar]
- Kirchhausen, T. (1999). Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol. 15, 705-732. [DOI] [PubMed] [Google Scholar]
- Kirchhausen, T. (2000). Clathrin. Annu. Rev. Biochem. 69, 699-727. [DOI] [PubMed] [Google Scholar]
- Lanfredi-Rangel, A., Attias, M., de Carvalho, T.M., Kattenbach, W.M., and De Souza, W. (1998). The peripheral vesicles of trophozoites of the primitive protozoan Giardia lamblia may correspond to early and late endosomes and to lysosomes. J. Struct. Biol. 123, 225-235. [DOI] [PubMed] [Google Scholar]
- Lujan, H.D., Mowatt, M.R., Conrad, J.T., Bowers, B., and Nash, T.E. (1995). Identification of a novel Giardia lamblia cyst wall protein with leucine-rich repeats. Implications for secretory granule formation and protein assembly into the cyst wall. J. Biol. Chem. 270, 29307-29313. [DOI] [PubMed] [Google Scholar]
- Lujan, H.D., and Touz, M.C. (2003). Protein trafficking in Giardia lamblia. Cell Microbiol. 5, 427-434. [DOI] [PubMed] [Google Scholar]
- Luzio, J.P., Poupon, V., Lindsay, M.R., Mullock, B.M., Piper, R.C., and Pryor, P.R. (2003). Membrane dynamics and the biogenesis of lysosomes. Mol. Membr. Biol. 20, 141-154. [DOI] [PubMed] [Google Scholar]
- Marti, M., Regos, A., Li, Y., Schraner, E.M., Wild, P., Muller, N., Knopf, L.G., and Hehl, A.B. (2003). An ancestral secretory apparatus in the protozoan parasite Giardia intestinalis. J. Biol. Chem. 278, 24837-24848. [DOI] [PubMed] [Google Scholar]
- McArthur, A.G., et al. (2000). The Giardia genome project database. FEMS Microbiol. Lett. 189, 271-273. [DOI] [PubMed] [Google Scholar]
- Meyer, C., Zizioli, D., Lausmann, S., Eskelinen, E.L., Hamann, J., Saftig, P., von Figura, K., and Schu, P. (2000). mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 19, 2193-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinete, M., Irminger, J.C., Tooze, S.A., and Halban, P.A. (2000). Trafficking/sorting and granule biogenesis in the beta-cell. Semin. Cell Dev. Biol. 11, 243-251. [DOI] [PubMed] [Google Scholar]
- Mowatt, M.R., Lujan, H.D., Cotten, D.B., Bowers, B., Yee, J., Nash, T.E., and Stibbs, H.H. (1995). Developmentally regulated expression of a Giardia lamblia cyst wall protein gene. Mol. Microbiol. 15, 955-963. [DOI] [PubMed] [Google Scholar]
- Nakai, M., Takada, T., and Endo, T. (1993). Cloning of the YAP19 gene encoding a putative yeast homolog of AP19, the mammalian small chain of the clathrin-assembly proteins. Biochim. Biophys. Acta 1174, 282-284. [DOI] [PubMed] [Google Scholar]
- Nash, T.E., Aggarwal, A., Adam, R.D., Conrad, J.T., and Merritt, J.W., Jr. (1988). Antigenic variation in Giardia lamblia. J. Immunol. 141, 636-641. [PubMed] [Google Scholar]
- Nash, T.E., Conrad, J.T., and Mowatt, M.R. (1995). Giardia lamblia: identification and characterization of a variant-specific surface protein gene family. J. Eukaryot. Microbiol. 42, 604-609. [DOI] [PubMed] [Google Scholar]
- Ngo, H.M., Yang, M., Paprotka, K., Pypaert, M., Hoppe, H., and Joiner, K.A. (2003). AP-1 in Toxoplasma gondii mediates biogenesis of the rhoptry secretory organelle from a post-Golgi compartment. J. Biol. Chem. 278, 5343-5352. [DOI] [PubMed] [Google Scholar]
- Nothwehr, S.F., and Stevens, T.H. (1994). Sorting of membrane proteins in the yeast secretory pathway. J. Biol. Chem. 269, 10185-10188. [PubMed] [Google Scholar]
- Ohno, H., Fournier, M.C., Poy, G., and Bonifacino, J.S. (1996). Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J. Biol. Chem. 271, 29009-29015. [DOI] [PubMed] [Google Scholar]
- Ohno, H., Stewart, J., Fournier, M.C., Bosshart, H., Rhee, I., Miyatake, S., Saito, T., Gallusser, A., Kirchhausen, T., and Bonifacino, J.S. (1995). Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269, 1872-1875. [DOI] [PubMed] [Google Scholar]
- Sachse, M., Urbe, S., Oorschot, V., Strous, G.J., and Klumperman, J. (2002). Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol. Biol. Cell 13, 1313-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaky, N., Presley, J., Smith, C., Zaal, K.J., Cole, N., Moreira, J.E., Terasaki, M., Siggia, E., and Lippincott-Schwartz, J. (1997). Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J. Cell Biol. 139, 1137-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim, J., and Lee, J. (2000). Molecular genetic analysis of apm-2 and aps-2, genes encoding the medium and small chains of the AP-2 clathrin-associated protein complex in the nematode Caenorhabditis elegans. Mol. Cells 10, 309-316. [PubMed] [Google Scholar]
- Shim, J., Sternberg, P.W., and Lee, J. (2000). Distinct and redundant functions of mu1 medium chains of the AP-1 clathrin-associated protein complex in the nematode Caenorhabditis elegans. Mol. Biol. Cell 11, 2743-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, S.M., Yee, J., and Nash, T.E. (1998). Episomal and integrated maintenance of foreign DNA in Giardia lamblia. Mol. Biochem. Parasitol. 92, 59-69. [DOI] [PubMed] [Google Scholar]
- Slavin, I., Saura, A., Carranza, P.G., Touz, M.C., Nores, M.J., and Lujan, H.D. (2002). Dephosphorylation of cyst wall proteins by a secreted lysosomal acid phosphatase is essential for excystation of Giardia lamblia. Mol. Biochem. Parasitol. 122, 95-98. [DOI] [PubMed] [Google Scholar]
- Sun, C.H., and Tai, J.H. (2000). Development of a tetracycline controlled gene expression system in the parasitic protozoan Giardia lamblia. Mol. Biochem. Parasitol. 105, 51-60. [DOI] [PubMed] [Google Scholar]
- Tooze, J., and Tooze, S.A. (1986). Clathrin-coated vesicular transport of secretory proteins during the formation of ACTH-containing secretory granules in AtT20 cells. J. Cell Biol. 103, 839-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze, S.A., Martens, G.J., and Huttner, W.B. (2001). Secretory granule bio-genesis: rafting to the SNARE. Trends Cell Biol. 11, 116-122. [DOI] [PubMed] [Google Scholar]
- Touz, M.C., Gottig, N., Nash, T.E., and Lujan, H.D. (2002a). Identification and characterization of a novel secretory granule calcium-binding protein from the early branching eukaryote Giardia lamblia. J. Biol. Chem. 277, 50557-63. [DOI] [PubMed] [Google Scholar]
- Touz, M.C., Lujan, H.D., Hayes, S.F., and Nash, T.E. (2003). Sorting of encystation-specific cysteine protease to lysosome-like peripheral vacuoles in Giardia lamblia requires a conserved tyrosine-based motif. J. Biol. Chem. 278, 6420-6426. [DOI] [PubMed] [Google Scholar]
- Touz, M.C., Nores, M.J., Slavin, I., Carmona, C., Conrad, J.T., Mowatt, M.R., Nash, T.E., Coronel, C.E., and Lujan, H.D. (2002b). The activity of a developmentally regulated cysteine proteinase is required for cyst wall formation in the primitive eukaryote Giardia lamblia. J. Biol. Chem. 277, 8474-8481. [DOI] [PubMed] [Google Scholar]
- Touz, M.C., Nores, M.J., Slavin, I., Piacenza, L., Acosta, D., Carmona, C., and Lujan, H.D. (2002c). Membrane-associated dipeptidyl peptidase IV is involved in encystation-specific gene expression during Giardia differentiation. Biochem. J. 364, 703-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller, R.F., and McConville, M.J. (2002). Developmental changes in lysosome morphology and function Leishmania parasites. Int. J. Parasitol. 32, 1435-1445. [DOI] [PubMed] [Google Scholar]
- Ward, W., Alvarado, L., Rawlings, N.D., Engel, J.C., Franklin, C., and McKerrow, J.H. (1997). A primitive enzyme for a primitive cell: the protease required for excystation of Giardia. Cell 89, 437-444. [DOI] [PubMed] [Google Scholar]
- Yee, J., and Nash, T.E. (1995). Transient transfection and expression of firefly luciferase in Giardia lamblia. Proc. Natl. Acad. Sci. USA 92, 5615-5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, B.G., Phan, H.L., and Payne, G.S. (1999). Adaptor complex-independent clathrin function in yeast. Mol. Biol. Cell 10, 3643-3659. [DOI] [PMC free article] [PubMed] [Google Scholar]