Abstract
Secondary to the inherent limitations of both point-of-care and central laboratory glucose technologies, continuous glucose measurement has recently enjoyed a high level of investment. Because of the perceived advantages by some of measuring in the intravascular space compared to the subcutaneous tissue, a number of technologies have been developed. In this review, we evaluate nine systems that have shown promise, although only one of these has been cleared for sale in the United States. The detection methodology, regulatory status, technical issues, and company circumstance surrounding each technology are examined.
Keywords: continuous glucose monitoring, FDA approval, intravascular monitoring
In ambulatory populations and for in-hospital use, many have long felt the need for continuous glucose measurement (CGM). Point-of-care (POC) measurements are labor intensive and there is growing concern regarding POC products that are not intended for the critically ill.1 The glucose values measured with subcutaneous systems may not correspond to central laboratory values and exhibit various lag times.2,3 What is required is a system that accurately and reliably monitors continuous glucose levels. A noninvasive system that provides reliable values on a continuous basis would be ideal, but the difficulties associated with developing a clinically accurate system have been well documented.4-6 As a result, there have been many attempts to develop systems that measure intravascular glucose, but this has proved challenging.
Improved outcomes following cardiac surgery with intense insulin therapy (IIT), a version of which is known as the Portland Protocol©,7 indicated the value of closely controlling glucose levels. The enthusiasm for IIT has waned, owing to serious complications from hypoglycemic events,8 but the need for accurate perioperative monitoring has continued and many believe that intravascular systems remain the best hope. Systems that extract glucose from interstitial fluid have not found widespread acceptance, largely due to the short lifetime of the collection fibers and the need for frequent recalibration (these systems will not be described here). This article describes true intravascular blood measurement devices, either measuring in vivo or after removal of a sample of blood (ex vivo).
Although we will describe nine systems, three US companies have gone out of business, and a fourth has not yet applied for FDA approval. Products with the Conformité Européenne (CE) mark are available in the European Economic Area (EEA), but those systems have had regulatory challenges in the United States.
Issues That Apply to All Systems
These units are designed for perioperative and critical care use, and this places a substantial burden on accuracy, short lag time, and reliability. Intravascular systems are designed as alternatives to central laboratory analyses and POC strip and meter systems, and many provide continuous glucose readings. Until recently, no POC systems had been cleared by the FDA for critically ill patients, and official communications have appeared warning against “other off-label uses of glucose meters [which] include the monitoring of glycemic control of non-diabetic patients in hospitals; use on critically ill patients.”9 However, in September of 2014, Nova Biomedical’s Stat Strip was cleared for this patient population.10
Every system must have a means of vascular access—central venous catheter (CVC), arterial, or peripheral venous—and materials in contact with the bloodstream are affected to varying degrees by “foreign-body responses.” This has been described elsewhere in detail,11-13 but the clinical consequence of thrombus formation can be severe. CVC clots may result in pulmonary emboli and clots from arterial catheters can result in peripheral ischemia. Subcutaneous sensors have different challenges than intravascular catheters, but most foreign materials will cause an inflammatory reaction, and diabetes can exacerbate these effects.14 Heparin, which is sometimes added to mitigate the risk of thrombi in bloodstream catheters, may cause erroneous coagulation tests15 and may also increase the incidence of heparin-induced thrombocytopenia.
To date, the only US-cleared system for intravascular glucose measurement is the Via Medical Glucose Monitor (International Biomedical, Austin, TX).16 Several proposed systems measure glucose using either near-infrared (NIR) or mid-infrared (MIR) spectroscopy instead of the more traditional amperometric or colorimetric methodologies. Possibly as a result of the unsuccessful noninvasive glucose systems using spectroscopy, obtaining approval with this modality has been more difficult (see Luminous Medical, Inc and OptiScan Biomedical below).
Intravascular systems measure glucose using a wide variety of detection techniques, including amperometry, NIR and MIR spectroscopy, fluorescence spectroscopy, and optomechanical interferometry. See a summary of each technology in Table 1.
Table 1.
Characteristics of Intravascular Glucose Monitoring Systems.
| System, company | Detection technology | Sampling site | Measurement site | Regulatory status | System status | Technical issues |
|---|---|---|---|---|---|---|
| GlucoScout, Via Medical | Electrochemical, glucose oxidase | Peripheral venous | Ex vivo | FDA 510(k) cleared | On market, EEA and US | Unheparinized glucose-containing infusion volume |
| GlucoClear, Edwards LifeSciences | Dexcom electrochemical, glucose oxidase | Peripheral venous | Ex vivo | CE Mark | On market, EEA | Corporate support? |
| Automated blood glucose monitor, Luminous Medical | Near-infrared; then electrochemical glucose oxidase | Peripheral venous, arterial | Ex vivo | None | Company closed | Regulatory approval; detection technology |
| OptiScanner 5000, OptiScan Biomedical | Mid-infrared, plasma | CVC, peripheral venous | Ex vivo | CE mark | On market, EEA | Unheparinized infusion, drug interferences? |
| OPTImus, IntelliDX | Electrochemical | Peripheral venous | Ex vivo | None | Company closed | Funding? |
| GlySure CGMS, GlySure | Boronate fluorescence | CVC | In vivo | CE Mark | In development | Nonthrombic sensor; interferences? |
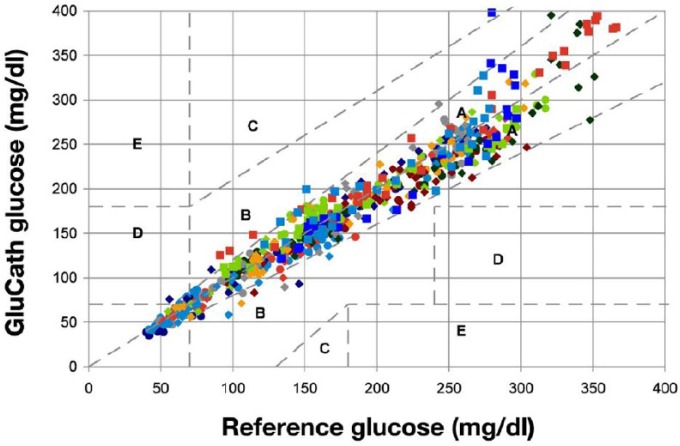
| Glucath, Glumetrics | Boronate-quenched fluorescence | Peripheral venous, arterial | In vivo | None | Company closed | Thrombus formation |
| Glucoset, (originally Invivosense) | Boronate optomechanical | Peripheral venous | In vivo | None | Under development | Interferences? |
| Autosampler, Cascade Metrix | Electrochemical | Peripheral venous | Ex vivo | None | Under development | Development progress? |
FDA-Cleared System
Via Medical
The sole device currently cleared in the United States is the GlucoScout (Via Medical).16 It accesses blood from a peripheral vein, withdraws 1.2 ml of blood for glucose oxidase electrochemical analysis, and then returns the blood together with 6 ml of nonheparinized calibration solution (Figure 1). It initially performs a 2-point calibration, and then calibrates at 82 mg/dl before each measurement.17
Figure 1.
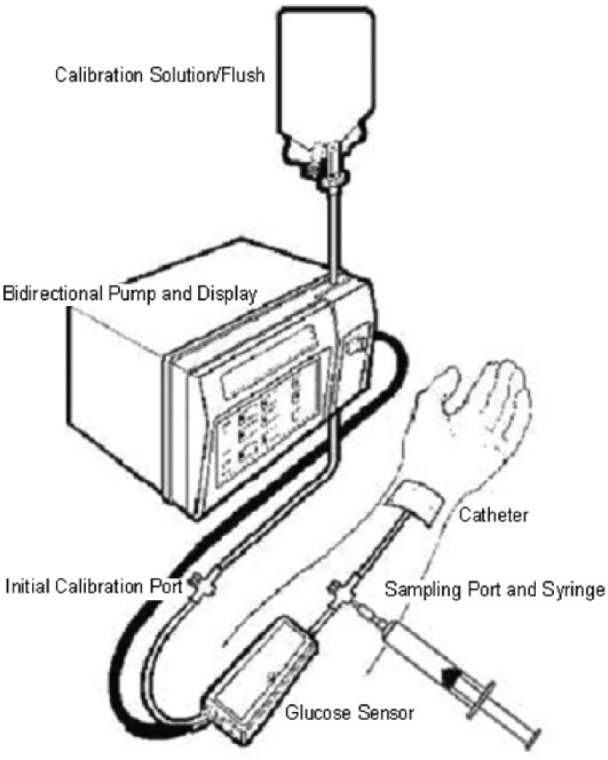
Via Medical GlucoScout.18
Ganesh et al17 described the GlucoScout performance compared to the HemoCue® β-glucose analyzer and showed 86% of points in the Clarke error grid18 “A” zone, 11% of points in the “B” zone, and 2% of points in the “D” zone (Figure 2).
Figure 2.
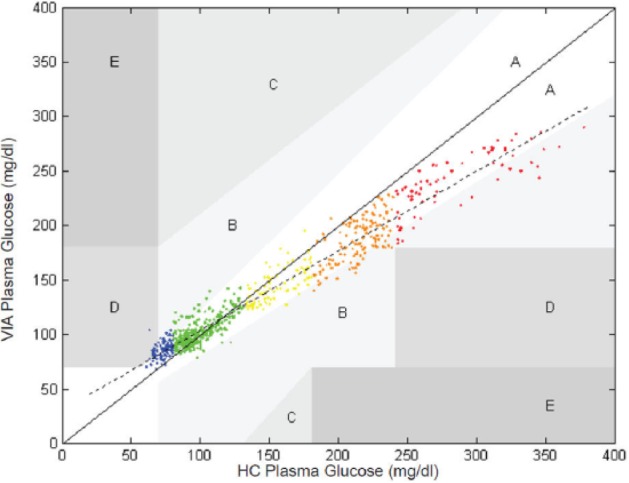
Via Medical GlucoScout (uncorrected) Clarke error grid.17
Abandoned Systems
Luminous Medical
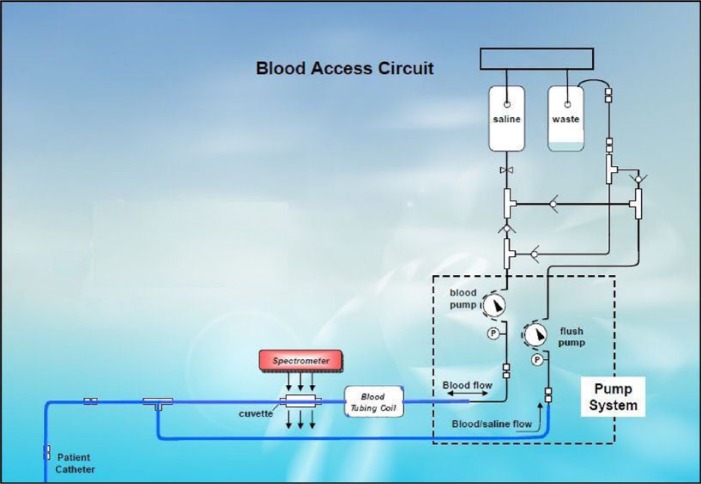
Luminous Medical, Inc (Carlsbad, CA), a spin-off of InLight Solutions, Inc (Albuquerque, NM), developed an ex vivo system that withdrew a blood sample from a peripheral vein into a cuvette, measured the glucose concentration using NIR spectrometry, and then returned the blood with about 6 ml of heparinized saline (personal communication, R. Robinson). Following each patient measurement, the system was flushed with saline that was pulled into a waste reservoir. Partial least squares (PLS) analysis was used to calculate the glucose results for each sample. Their results are shown in Figure 3 with the fluid management system shown in Figure 4 (personal communication, R. Robinson).
Figure 3.
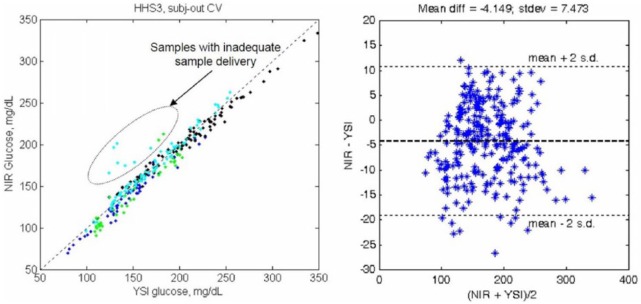
Performance of Luminous Medical automated blood glucose monitor.
Figure 4.
Luminous Medical automated blood glucose monitor blood access circuit.
In 2009, company representatives met with the FDA in a “pre-IDE” meeting19 to gain feedback for 510(k) premarket notification for the system as a class II device, but were unsuccessful.20 Proposed “predicate devices” for the system included the Via Medical Glucose Monitor (see above), the SureStep Pro Glucose Monitor (LifeScan, Inc, Milpitas, CA), a traditional POC meter, and the Avoximeter 4000 Co-oximeter (International Technidyne Corporation, Edison, NJ), an NIR blood oxygen monitor.
The FDA reportedly20 indicated that none of these devices were suitable as predicate, that they had deemed the device “truly novel,” and that unless a more traditional method of detection was employed, a premarket approval (PMA) application would be required. Even though Luminous Medical had secured the rights to a glucose oxidase-based detector in 2009,21 the time and cost needed to comply with the requirements of either a new detection system or a PMA proved to be beyond the company’s resources, and it closed operations in 2011.20
While pulse oximetry using visible/NIR measurement is well established (leading to the FDA clearance of the Avoximeter 4000 Co-oximeter in 199622 along with many earlier devices), no glucose measurement system using this technology has yet been approved by the FDA,5 and any future systems using this technology would be expected to require a PMA as a class III device.23
Glumetrics
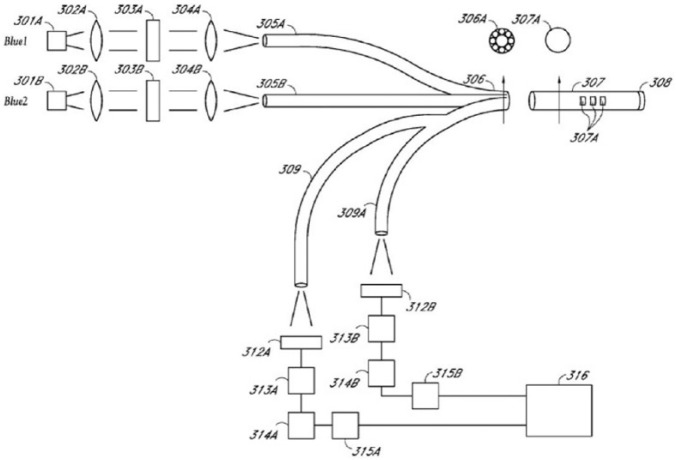
Glumetrics, Inc (Irvine, CA) employed technology based on a boronate chemical quenched fluorescence sensing element (Figure 5), originally developed at the University of California, Santa Cruz.24 Their in-vivo sensor, called Glucath, had the sensing reagent attached to the tip of a fiber optic probe (308 in Figure 6).
Figure 5.
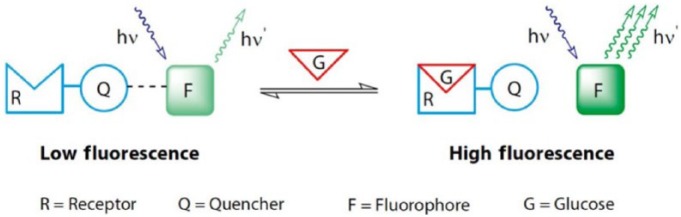
Glumetrics chemistry scheme.26
Figure 6.
Glumetrics fiber optic sensor.25
The sensor was deployed through a peripheral venous catheter, with blue light traveling down an optical fiber to the sensor. Green fluorescence increased in proportion to increasing glucose concentration. Glucose results (Figure 7) for 20 patients were reported, showing 95% of all points in the “A” Clarke zone, and a mean absolute relative deviation (MARD) of 7.9%.26
Figure 7.
Glumetrics venous Clarke error grid plot.26
In a corresponding study with the sensor implanted in a radial artery, of 437 paired sensor and reference values, 353 (80.8%) were within 20% of the reference value when the blood glucose was ≥4.2mmol/l (75 mg/dl). The aggregate MARD was 13.0%, with the MARD for individual sensors ranging from 4.7% to 33.5%.27
Even though the Glucath sensor was coated with a heparin-based coating to inhibit thrombus formation, the sensor had problems with thrombus formation in one cohort of patients,28 while a second study detected only clinically insignificant thrombus in five of 21 patients.29 In a small observational study,30 the authors state, “Poor IA-CGM (intra-arterial continuous glucose monitoring) system performance (> 11% MARD) in 3 subjects was attributed to optical signal variability associated with routine patient care activities and the administration of 3 interfering medications (mannitol, citrate, glubionate). The company did not obtain additional funding and has since closed shop.”
IntelliDX
A system named OPTImus (IntelliDX, Santa Clara, CA) was reviewed in 2007,31 when it was reported to have a “MRAD” of 15.0% compared to reference, but no description of the measurement technology was provided; the system was identified in that report only as the “Glucon OPTImus under development.” Another report32 described the company as being out of business.
Systems in Development
OptiScan Biomedical
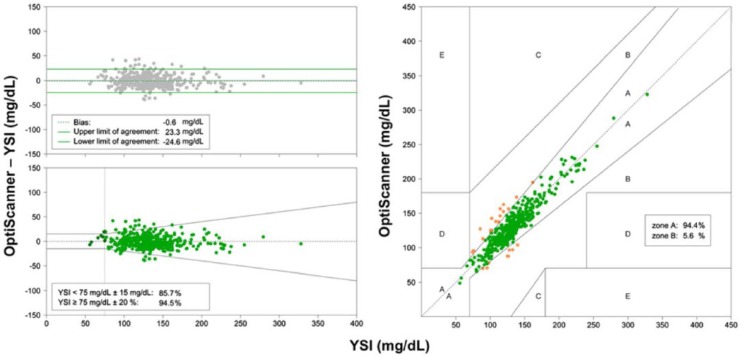
OptiScan Biomedical (Hayward, CA) has an intravascular system, the OptiScanner 5000®33 (CE mark granted in 2011), and announced in April 2014 three-site-pivotal studies.34,35 Results obtained with an earlier version appeared in 200536 and 2006,37 and a new system, the OptiScanner 6000, which the company says “will be designed to automatically measure and trend glucose, hemoglobin and central venous oxygen saturation levels,”38 has been announced.39
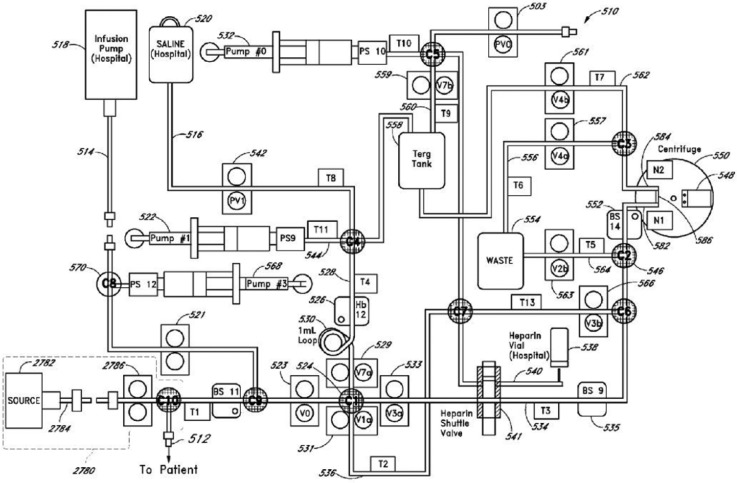
The OptiScanner connects to a venous catheter40 and withdraws 3.0 ml of blood, of which 120 μl is heparinized, separated to plasma by centrifugation, and analyzed in a cassette by MIR spectroscopy, with approximately 2.8 ml returned to the patient (Figure 8). There is no heparin in the returned blood.40 With measurement every 15 minutes, patient blood loss is approximately 10 ml per day, while an additional 144 ml per day of saline is infused.
Figure 8.
One version of the OptiScanner fluidic diagram.41
A University of Amsterdam42 report of the system with CVC access, indicated a number of problems with the system, including occlusion alarms in 32 of 101 patients (24 of which could not be resolved), and interferences that required elimination with additions to an “interference library” for hydroxyethyl starch, IgG, propofol, albumin, cephalosporins, lactate, phosphate, and mannitol. The authors note, “While the device was designed for critically ill patients it had not yet been tested in vivo, and specifically, in critically ill patients at the ICU bedside. The present study expands the experience with the devices and shows that the devices could perform accurate glucose level measurements.”
Following corrections for all the identified interferences, the 463 results for the remaining 71 patients are shown in Figure 9. The authors concluded, “The blood glucose measuring devices we used needed calibration for several previously unrecognized interferences. Thereafter, accuracy of the device for measuring plasma glucose levels in “our cohort” of critically ill patients improved, but external validation is highly recommended.”
Figure 9.
OptiScanner performance after interference corrections.42
GlucoClear
Edwards LifeSciences (Irvine, CA) has developed the GlucoClear System (CE mark for a first generation system in 200943) and a second-generation system, GlucoClear 2, in 2013.44 It uses a dual electrochemical detector developed by Dexcom (San Diego, CA), and withdraws a 40-µl blood sample that is returned with a heparinized glucose solution with about 1.25 ml of 200 mg/dl (or 100 mg/dl45) heparinized glucose solution. A study of the first-generation system46 reported 99.5% of the results in the Clarke error grid “A” and “B” zones. An ICU study using the newer GlucoClear2 indicated 99% “A” zone and 1% “B” zone points with 4578 results compared to reference, and a MARD of 4.9%.47 At the quoted 200 mg/dl glucose concentration, less than 20 IU of heparin and 20 mg of glucose are delivered to the patient per hour.
In October 2014, Edwards stated, “…the company is redirecting the resources from its Glucose monitoring program to its [Enhanced Surgical Recovery] initiative, and The Company recorded a $5.0 million charge in the third quarter of 2014 to write-down assets related to its automated glucose monitoring program. The charge related primarily to intellectual property, fixed assets, inventory and severance expenses.”48 In response, Edwards’s CEO and chairman Michael Mussallem stated, “We are still discussing what might happen with that program. We do think it’s very valuable. We have accomplished a great deal. We have a commercial product, clear path to commercialization in the U.S., a CE mark in Europe, and so we are considering strategic options in that, including partnering.”49
GlySure
The GlySure system (Oxfordshire, UK) uses a direct fluorescence boronate-sensing element (Figure 10) at the tip of a fiber optic probe inserted into the jugular vein (rather than the quenched fluorescence chemistry employed by Glumetrics). Results reported from a 34-patient study stated “The 456 sample values recorded by the monitor based on 8-hour calibrations were correlated . . . and the MARD for the study was 9.40%. . . . 89.23% of the data fell within zone ‘A’ . . . with the rest falling within zone ‘B.’”50 An earlier abstract reported a 24-patient study at what appears to be the same institution and quoted a MARD of 7.70%, with 92.7% of the data zone “A,” 6.8% in zone “B,” and by difference, 0.5% in zone “D.”51
Figure 10.
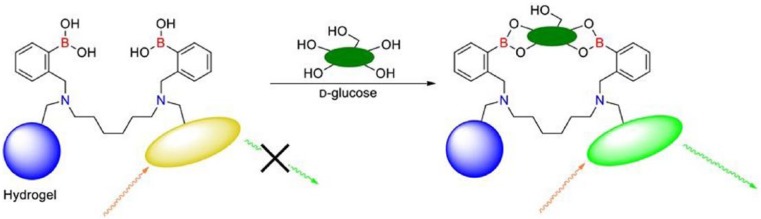
GlySure fluorescent sensor system.52
Glucoset
The Glucoset system (Glucoset AS, Trondheim, Norway) is an in-vivo hydrogel matrix sensor at the end of an optical fiber that responds to changes in the diameter of the hydrogel by an interferometric optical measurement (Figure 11). When 3-phenylboronic acid complexes with glucose, it causes cross-linking, which changes the diameter, causing a change in the path of light sent down the fiber.
Figure 11.
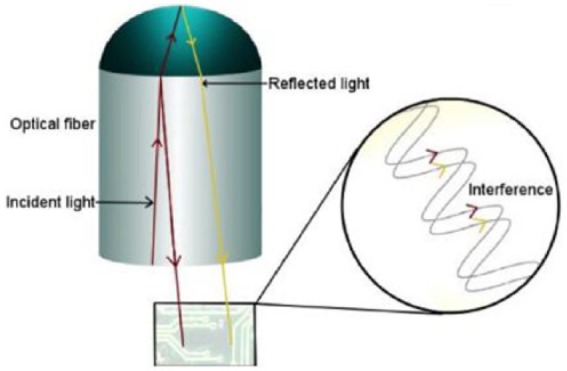
Glucoset sensing system.53
This system was originally developed by Invivosense ASA in Trondheim, Norway, which went out of business in 2009.55 Results of a porcine study were reported in 2011,55 but no human results have been published to date.
Cascade Metrix
Cascade Metrix, Inc (Indianapolis, IN) is developing the CMI Automated Glucose Monitoring System that is reported to be in the “pre-clinical prototype stage.”56 According to the company’s website, “CMI expects to obtain EU CE mark in Q1 of 2015 and to begin commercial product shipments following clearance. FDA 510k would be filed in [the second] half of 2015.”57 A 2008 publication described the automated sampling system, comparing proposed detection systems with preliminary in vitro results.58 A recent patent describes both a flow-through mid-infrared sensor and an automated electrochemical test strip and meter measuring system that is integrated with the sampling unit.59
Conclusions
Although many intravascular glucose measurement systems, employing differing access sites, sampling, and detection technology have been under development, some for many years; to date, only 1 system has been cleared or approved for sale in the United States. The difficulties encountered have been equally diverse, including difficulties in regulatory approval, thrombic events at the sensor tip for in vivo systems, unexpected drug interferences, and possibly inconsistent manufacturer support. Without doubt, the controversy regarding tight glycemic control in hospital ICUs has contributed substantial uncertainty to the development path and probable commercial success of these systems.
Acknowledgments
The authors would like to express their appreciation to Paul Strasma for providing the impetus and many of the references necessary to complete this publication and to Ries Robinson for sharing unpublished Luminous Medical information.
Footnotes
Abbreviations: CE, Conformité Européenne; CGM, continuous glucose measurement; CVC, central venous catheter; EEA, European Economic Area, IIT, intensive insulin therapy; MARD, mean absolute relative deviation; MIR, mid-infrared; NIR, near-infrared; PMA, premarket approval; POC, point of care.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JLS served as a consultant to Luminous Medical for intravascular glucose and Edwards (for noninvasive glucose measurement technologies, not intravascular glucose) in 2008. MJR is a frequent scientific advisory board member for Roche Diabetes Care, Inc and is an employee of Vanderbilt University. NIVG Consulting discloses no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Department of Anesthesiology at the University of Florida and by NIVG Consulting.
References
- 1. Klonoff DC. The Food and Drug Administration is now preparing to establish tighter performance requirements for blood glucose monitors. J Diabetes Sci Technol. 2010;4(3):499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kulcu E, Tamada J, Reach G, Potts RO, Lesho M. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care. 2003;26(8):2405-2409. [DOI] [PubMed] [Google Scholar]
- 3. Cengiz E, Tamborlane W. A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technol Ther. 2009;11(suppl 1):11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heinemann L. Noninvasive glucose monitoring systems: will we ever have such sensors for practical use? J Diabetes Sci Technol. 2007;1(6):936-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith J. The pursuit of noninvasive glucose: “hunting the deceitful turkey.” Available at: http://www.mendosa.com/The%20Pursuit%20of%20Noninvasive%20Glucose%203rd%20Edition.pdf. Accessed March 21, 2015.
- 6. Khalil O. Non-invasive glucose measurement technologies: an update from 1999 to the dawn of the new millennium. Diabetes Technol Ther. 2004;6(5):660-697. [DOI] [PubMed] [Google Scholar]
- 7. Providence Health Services. The Portland Protocol. Available at: https://appsor.providence.org/portlandprotocol/. Accessed March 21, 2015.
- 8. NICE-SUGAR Study Investigators, Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283-1297. [DOI] [PubMed] [Google Scholar]
- 9.http://www.wadsworth.org/labcert/clep/files/Glucose_meters_off_label_use_1_13_14.pdf. Accessed March 21, 2015.
- 10.http://www.fda.gov/NewsEvents/Newsroom/PressAnnounce-ments/ucm416144.htm. Last Accessed March 21, 2015.
- 11. Cunningham D, Stenken J. In Vivo Glucose Sensing. New York, NY: John Wiley; 2010. [Google Scholar]
- 12. Sefton M, Babensee J, Woodhouse K. Innate and adaptive immune responses in tissue engineering. Semin Immunol. 2008;20(2):83-85. [DOI] [PubMed] [Google Scholar]
- 13. Anderson J, Rodriguez A, Chang B. Foreign-body reaction to biomaterials. J Diabetes Sci Technol. 2008;2(5):768-777.19885259 [Google Scholar]
- 14. Ward K. A review of the foreign-body response to subcutaneously-implanted devices: the role of macrophages and cytokines in biofouling and fibrosis. J Diabetes Sci Technol. 2008;2(5):768-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoste EA, Roels NR, Decruyenaere JM, Colardyn FA. Significant increase of activated partial thromboplastin time by heparinization of the radial artery catheter flush solution with a closed arterial catheter system. Crit Care Med. 2002;30(5):1030-1034. [DOI] [PubMed] [Google Scholar]
- 16. FDA 510(k) Applications received during February, 1993. Available at: http://www.510kdecisions.com/applications/index.cfm?id=K935778. Accessed March 21, 2015.
- 17. Ganesh A, Hipszer B, Loomba N, Simon B, Torjman MC, Joseph J. Evaluation of the VIA® blood chemistry monitor for glucose in healthy and diabetic volunteers. J Diabetes Sci Technol. 2008;2(2):182-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clarke W, Cox D, Gonder-Frederick L, Carter W, Pohl S. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622-628. [DOI] [PubMed] [Google Scholar]
- 19. US Department of Health and Human Services, Food and Drug Administration. FDA decisions for investigational device exemption clinical investigations. Available at: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM311176.pdf. Accessed March 21, 2015.
- 20. Advanced technology ventures. Medical device VCs link FDA dysfunction with company shutdowns. Available at: http://www.atvcapital.com/technology-news/medical-device-vcs-link-fda-dysfunction-with-company-shutdowns. Accessed March 21, 2015.
- 21. PR Newswire. Luminous Medical, Inc. secures exclusive rights to glucose sensor for its automated glucose monitor. Available at: http://www.prnewswire.com/news-releases/luminous-medical-inc-secures-exclusive-rights-to-glucose-sensor-for-its-automated-glucose-monitor-64165897.html. Accessed March 21, 2015.
- 22. 510k clearances. Available at: https://510k.directory/clearances/K951485. Accessed March 21, 2015.
- 23. Gutman S, Bernhardt P, Pinkos A, Moxey-Mims M, Knott T, Cooper J. Regulatory aspects of noninvasive glucose measurements. Diabetes Technol Ther. 2002;4(6):779-781. [DOI] [PubMed] [Google Scholar]
- 24. University of Santa Cruz NewsCenter. Optical glucose sensor developed at UCSC holds promise for diabetics and intensive care patients. Available at: http://news.ucsc.edu/2004/03/466.html. Accessed March 21, 2015.
- 25. US patent 7,751,863.
- 26. Peyser T, Zisser H, Khan U, et al. Use of a novel fluorescent glucose sensor in volunteer subjects with type 1 diabetes mellitus. J Diabetes Sci Technol. 2011;5(3):687-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flower O, Bird S, Macken L, et al. Continuous intra-arterial blood glucose monitoring using quenched fluorescence sensing: a product development study. Crit Care Resusc. 2014;16(1):54-61. [PubMed] [Google Scholar]
- 28. Romey M, Jovanovič L, Bevier W, Markova K, Strasma P, Zisser H. Use of an intravascular fluorescent continuous glucose sensor in subjects with type 1 diabetes mellitus. J Diabetes Sci Technol. 2012;6(6):1260-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Machado P, Liu JB, Hipszer B, et al. Use of ultrasound to monitor blood flow and thrombus formation around a radial artery catheter and intravascular glucose sensor in surgical patients. Poster presented at: American Institute of Ultrasound in Medicine; March 29-April 2, 2014; Las Vegas, NV. [Google Scholar]
- 30. van der Voort P, Sechterberger M, Strasma P, Devries J. Intra-arterial versus subcutaneous continuous glucose monitoring in postoperative cardiac surgery patients. Diabetes Technol Ther. 2014;16(suppl 1):A144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Torjman M, Goldberg M, Marr A, Maldonado M, Littman J. Evaluation of an automated blood glucose monitor in cardiac surgery and critically ill ICU patients. Anesthesiology. 2007;107:A1127. [Google Scholar]
- 32. VentureDeal. Transaction record. Available at: http://www.venturedeal.com/VentureCapital/ca1ae82a-bb5b-4581-a417-82588e328137/IntelliDx-Receives-$13-million/Default.aspx. Accessed March 21, 2015.
- 33. Jax T, Heise T, Nosek L, Gable J, Lim G, Calentine C. Automated near-continuous glucose monitoring measured in plasma using mid-infrared spectroscopy. J Diabetes Sci Technol. 2011;5(2):345-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. MANAGE Automated Glucose Monitoring (MANAGE IDE). Available at: https://clinicaltrials.gov/ct2/show/NCT02211300?term=optiscanner&rank=1. Accessed March 21, 2015.
- 35. PR Newswire. OptiScan Biomedical enrolls first patient in pivotal U.S. clinical study of OptiScanner® 5000. Available at: http://www.prnewswire.com/news-releases/optiscan-biomedical-enrolls-first-patient-in-pivotal-us-clinical-study-of-optiscanner-5000-256965261.html. Accessed March 21, 2015.
- 36. Krinsley J, Hall D, Zheng P, Braig J. Validation of the OptiScanner, a continuous glucose monitoring device. Crit Care Med. 2005;33(12):A73. [Google Scholar]
- 37. Krinsley J, Zheng P, Hall D, Magarian P. ICU validation of the OptiScanner, a continuous glucose monitoring device. Crit Care Med. 2006:34(12):A67. [Google Scholar]
- 38. OptiScan. In development. Available at: http://optiscancorp.com/technology-products/in-development/. Accessed March 21, 2015.
- 39. OptiScan Biomedical Corp press release. March 21, 2014. Available at: http://www.medicaldevice-network.com/news/newsoptiscan-biomedical-announces-launch-of-bedside-glucose-monitoring-system-4201728. Accessed March 21, 2015.
- 40. Heise T. The diabetic patient in the hospital intensive care unit (ICU) glucose monitoring measured in plasma using mid-infrared spectroscopy. Paper presented at: 46th Annual European Association for the Study of Diabetes; February 10-13, 2010; Stockholm, Sweden. [Google Scholar]
- 41. US patent 8,412,293.
- 42. van Hooijdonk RT, Winters T, Fischer JC, et al. Accuracy and limitations of continuous glucose monitoring using spectroscopy in critically ill patients. Ann Intensive Care. 2014;4(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Edwards LifeSciences. Available at: http://www.edwards.com/eu/Products/glucose/Pages/GlucoClearSpecification.aspx. Accessed November 14, 2014 (removed as of March 21, 2015).
- 44. Bailey T, Gulino A, Higgins M, Leach J, Kamath A, Simpson P. Accuracy of a first-generation intravenous blood glucose monitoring system in subjects with diabetes mellitus: a multicenter study. J Diabetes Sci Technol. 2013;7(6):1484-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Edwards LifeSciences. Edwards LifeSciences reports third quarter results. Available at: http://www.edwards.com/newsroom/Pages/NR20141023.aspx. Accessed March 21, 2015.
- 46. Edwards LifeSciences 2013. investor conference. Critical care. Available at: http://files.shareholder.com/downloads/AMDA-RAMKH/0x0x712466/c450bdb5-a1e7-46b4-88a8-787049e7fa4c/2013_04_CC_Booklet_FINAL.pdf. Accessed March 21, 2015.
- 47. Edwards LifeSciences 2013. investor conference. Available at: http://ht.edwards.com/scin/edwards/eu/sitecollectionimages/products/glucose/foubertaccuracystudyisicem2014_ar11913.pdf. Accessed March 21, 2015.
- 48. Edwards LifeSciences. Edwards LifeSciences reports third quarter results. Available at: http://www.edwards.com/newsroom/Pages/NR20141023.aspx. Accessed March 21, 2015.
- 49. Close Concerns. Interview with new JDRF interim CEO, Derek Rapp. Available at: http://www.closeconcerns.com/knowledgebase/r/3831a3cd. Accessed March 21, 2015.
- 50. Prasad M, Gopal P, Mannam G. Continuous monitoring of blood glucose using a fiber optic-based intravascular sensor during postoperative care in the ICU. Crit Care. 2014;18(suppl 1):442.25041935 [Google Scholar]
- 51. Prasad K, Gopal P, Crane B, et al. Use of an intravascular continuous blood glucose sensor during post operative ICU care of cardiac surgery patients. Paper presented at: 33rd International Symposium on Intensive Care and Emergency Medicine; 2013; Durban, South Africa Available at: http://www.glysure.com/media/23434/glysure_poster_durban_2013_updated_v2.pdf. Accessed March 21, 2015. [Google Scholar]
- 52. GlySure. Platform technology. Available at: http://www.glysure.com/technology/platform-technology/. Accessed March 21, 2015.
- 53. Glucoset continuous glucose monitoring. Available at: http://www.glucoset.com/technology-detailed/. Accessed March 21, 2015.
- 54. Bloomberg Business. Available at: http://investing.businessweek.com/research/stocks/private/snapshot.asp?privcapId=2827398. Accessed March 21, 2015.
- 55. Skjaervold N, Solligård E, Hjelme D, Aadahl P. Continuous measurement of blood glucose validation of a new intravascular sensor. Anesthesiology. 2011;114(1):120-125. [DOI] [PubMed] [Google Scholar]
- 56. Cascade Metrix, Inc. Available at: http://www.cascademetrix.com/news. Accessed March 21, 2015.
- 57. Cascade Metrix, Inc. Available at: http://www.cascademetrix.com/products. Accessed March 21, 2015.
- 58. Kunjan K, Lloyd P. Automated blood sampling and glucose sensing in critical care settings. J Diabetes Sci Technol. 2008;2(2):194-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. US patent 8,348,844.