Abstract
Background:
Current methods of blood glucose (BG) monitoring and insulin delivery are labor intensive and commonly fail to achieve the desired level of BG control. There is great clinical need in the hospital for a user-friendly bedside device that can automatically monitor the concentration of BG safely, accurately, frequently, and reliably.
Methods:
A 100-patient observation study was conducted at 6 US hospitals to evaluate the first generation of the Intravenous Blood Glucose (IVBG) System (Edwards Lifesciences LLC & Dexcom Inc). Device safety, accuracy, and reliability were assessed. A research nurse sampled blood from a vascular catheter every 4 hours for ≤ 72 hours and BG concentration was measured using the YSI 2300 STAT Plus Analyzer (YSI Life Sciences). The IVBG measurements were compared to YSI measurements to calculate point accuracy.
Results:
The IVBG systems logged more than 5500 hours of operation in 100 critical care patients without causing infection or inflammation of a vein. A total of 44135 IVBG measurements were performed in 100 patients with 30231 measurements from the subset of 75 patients used for accuracy analysis. In all, 996 IVBG measurements were time-matched with reference YSI measurements. These pairs had a mean absolute difference (MAD) of 11.61 mg/dl, a mean absolute relative difference (MARD) of 8.23%, 93% met 15/20% accuracy defined by International Organization for Standardization 15197:2003 standard, and 93.2% were in zone A of the Clarke error grid. The IVBG sensors were exposed to more than 200 different medications with no observable effect on accuracy.
Conclusions:
The IVBG system is an automated and user-friendly glucose monitoring system that provides accurate and frequent BG measurements with great potential to improve the safety and efficacy of insulin therapy and BG control in the hospital, potentially leading to improved clinical outcomes.
Keywords: near-continuous glucose monitoring system, Intravenous Blood Glucose (IVBG) system, glucose sensor, accuracy, safety, critical care glucose monitoring
Hyperglycemia, hypoglycemia, and glycemic variability are strongly associated with increased morbidity and mortality in hospitalized medical and surgical patients.1-8 Nondiabetic patients that develop hospital-related hyperglycemia secondary to the metabolic stress response to injury and illness may benefit the most from insulin therapy and near-normal blood glucose (BG) control.9-10 There is clinical consensus that prolonged/severe hyperglycemia and hypoglycemia lead to increased clinical risk and should be avoided.11-23 Hyperglycemia can cause glycosuria,24 fluid shifts,24 electrolyte imbalance,24 impaired wound healing,23-24 immune dysfunction,23,25-28 and oxidative stress.24,27 Hypoglycemia can cause intense activation of the sympathetic nervous system (leading to myocardial ischemia and arrhythmia in susceptible patients) and cell damage, especially neurons and glial cells within the central nervous system.29-43
Real-world methods of glucose monitoring and insulin therapy in the hospital are currently unable to avoid hypoglycemia and glycemic variability while attempting to maintain the concentration of BG in the desired target range. The 2001 prospective randomized study by Van den Berghe et al revealed a significant reduction in morbidity and mortality in surgical ICU patients managed aggressively with intravenous (IV) insulin to the target BG concentration of 80-110 mg/dl (4.4-6.1 mmol/L) when compared to a control group where IV insulin was started only after BG exceeded 215 mg/dl (> 12 mmol/L). The BG in radial artery blood was measured every 1 to 4 hours using a standardized method of blood sampling and handling, and an accurate blood gas/glucose analyzer. Despite frequent BG monitoring and adjustments in the IV infusion dose of regular insulin, patients were outside of the target BG range 30% of the time and 5% of the intensively managed patients developed severe hypoglycemia defined as a BG measurement < 40 mg/dl (< 2.2 mmol/L).44-46
Numerous follow-up studies were unable to confirm the significant improvement in clinical outcome from intensive insulin therapy (IIT) and near-normal BG control reported by Van den Berghe.47-54 The large prospective randomized NICE-SUGAR clinical trial reported that morbidity and mortality actually increased in the IIT group (mixed medical and surgical ICU patients) that targeted BG < 108 mg/dl (< 6 mmol/L), when compared to less-intense insulin therapy and a target BG of 140-180 mg/dl (8-10 mmol/L). Of interest, BG was maintained within the target range less than 50% of the time and 6.8% of the intensively managed patients developed a BG < 40 mg/dl (< 2.2 mmol/L).54 The increase in hypoglycemia associated with IIT was corroborated by Arabi et al with an adjusted odds ratio of 50.65 when compared to conventional therapy.55
Clinical trials using standardized BG measurement methods are needed to identify the optimal BG target for different patient groups. These studies require the design of safe and effective tools that can be used by the bedside nurse to maintain the BG level in the target range and minimize glycemic variability, while eliminating the risk for hypoglycemia.56-58 To be clinically useful, the tool should provide a BG measurement as frequently as every 10 minutes to capture the appropriate BG dynamics59 and be automated to decrease the amount of nursing time and effort required for glucose monitoring.60-62
There is a great clinical need in the hospital for a user-friendly bedside device that can automatically monitor the concentration of BG safely, accurately, frequently, and reliably.63-69 Edwards Lifesciences LLC and Dexcom Inc developed an Intravenous Blood Glucose (IVBG) System for monitoring patients in the operating rooms, intensive care units, and general floors of the hospital. The real-time BG measurements and BG trend information will be used by the bedside nurse to adjust insulin therapy in relation to changing nutrition, insulin sensitivity, and patient physiology.
This is the first observational study that evaluated the first-generation IVBG System in a wide variety of patient populations and hospital environments. The manufacturer used this preliminary data to optimize the next generation of this system. The second generation system, called the GlucoClear System has obtained the CE Mark in the European Union.
Materials and Methods
A large prospective non-randomized multicenter observational study was conducted to evaluate the safety, accuracy, and reliability of the IVBG System in the critical care environment of 6 US hospitals (Table 1). The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practices. Edwards Lifesciences sponsored the study and provided technical assistance to research personnel. The Institutional Review Board at each site approved the study as a nonsignificant risk. The patients were informed about the study methods and risks prior to signing the informed consent document. Patients enrolled in the study did not receive any treatment or medication outside the standard of care prescribed at each hospital for the given procedure and patient. Each patient was managed in the OR, ICU, and/or general ward of the hospital according to the site’s standard of care. And whereas some patients remained in the ICU for the duration of the study, other patients moved from the ICU to the general ward during the latter portion of the study. Both clinical and research staff were blinded to IVBG System glucose measurements during the study.
Table 1.
Subject Enrollment Summary.
| Investigational site | Site # | Investigator name | Subjects enrolled | Roll-in subjects | Post-roll-in subjects | Efficacy evaluable, n (%)a |
|---|---|---|---|---|---|---|
| University of Maryland, Medical System, Baltimore, MD, USA | 180 | Grant Bochicchio | 33 | 4 | 29 | 27 (93.1) |
| Washington Hospital Center, Washington, DC, USA | 366 | Michelle Magee | 13 | 4 | 9 | 9 (100.0) |
| Jefferson Medical College, Philadelphia, PA, USA | 497 | Jeffrey Joseph | 28 | 4 | 24 | 23 (95.8) |
| Franklin Square Hospital Center, Baltimore, MD, USA | 498 | James Welker | 2 | 2 | — | — |
| International Diabetes Center, Minneapolis, MN, USA | 499 | Robert Cuddihy | 20 | 4 | 16 | 16 (100.0) |
| Providence St. Vincent’s Hospital, Portland, OR, USA | 88 | Anthony Furnary | 4 | 4 | — | — |
| Total | 100 | 22 | 78 | 75( 96.2) |
The denominator is the number of post-roll-in subjects.
Patient Population
One hundred subjects were enrolled and studied between July 2009 and April 2010, all of whom were evaluated with respect to safety (Figure 1). Of the enrolled subjects, 22 were roll-in cases. The roll-in subjects allowed research personnel at each site to gain experience using the IVBG System. The remaining 78 subjects were eligible for evaluation with respect to effectiveness (accuracy and reliability). Among these 78 subjects, 2 had inadequate data due to IVBG System issues and 1 had inadequate data because the subject’s surgical procedure was cancelled and the subject was discharged before any IVBG data could be obtained, leaving 75 subjects to be evaluated with respect to effectiveness.
Figure 1.
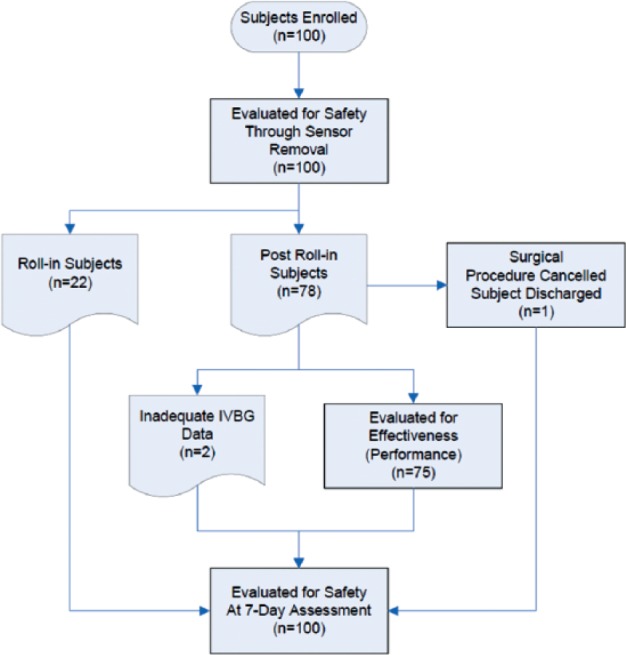
Subject accountability.
Inclusion criteria were ≥18 years of age, hospital admission for major nonemergent surgery or serious medical illness, and anticipated treatment in the ICU for at least 24 hours. Exclusion criteria were pregnancy, uncontrolled hyperglycemia, diabetic ketoacidosis, end-stage organ failure, contraindication to heparin anticoagulation (brain surgery, brain/spinal cord trauma, and history of heparin-induced thrombocytopenia), and inadequate peripheral veins for insertion of a dedicated IV catheter. Study subject demographics are summarized in Table 2. Considering the entire study population, 70% of the subjects (70/100) were admitted for elective surgical procedures. Of those, 56% were cardiac procedures (39/70), 27% were pancreatic procedures (19/70), and the remaining 17% included esophageal, hernia, intestinal, spinal, liver, lung, bladder, and bleeding. Of the subjects, 30% (30/100) were admitted for trauma (22/30) and unstable medical conditions such as bleeding, CHF, chemotherapy, COPD, hypothermia, hypoglycemia, pancreatitis, pneumonia, and poisoning.
Table 2.
Subject Demographics for the Entire Study Population and for the Subgroup Assessment for Effectiveness (Accuracy).
| Category | Safety evaluation | Effectiveness evaluation |
|---|---|---|
| Number of Subjects | 100 | 75 |
| Age (years) | 56.5 (13.6) | 54.9 (14.2) |
| Height (cm) | 175 (11.3) | 175 (11.3) |
| Weight (kg) | 89.6 (20.4) | 92.0 (19.6) |
| BMI | 29.3 (6.2) | 30.1 (6.0) |
| Male | 68.0% | 70.7% |
| Caucasian | 81.0% | 78.7% |
| Black, Hispanic, Asian | 17.0% | 18.7% |
| Systolic blood pressure (mmHg) | 130.4 (18.3) | 130.4 (18.0) |
| Diastolic blood pressure (mmHg) | 71.4 (12.9) | 71. (12.2) |
| Heart rate (bmp) | 80.4 (16.1) | 79.8 (16.0) |
| Temperature (°C) | 36.9 (0.9) | 36.9 (1.0) |
| History of diabetes | 27.0% | 28.0% |
| History cardiac disease | 62.0% | 56.0% |
| Cardiac surgery | 39.0% | 36.0% |
| Pancreas surgery | 19.0% | 20.0% |
| Other surgery | 12.0% | 12.0% |
| Trauma (no surgery) | 22.0% | 25.3% |
| Other medical condition | 8.0% | 6.7% |
Values are percentage or mean (SD).
Investigational Device: Intravenous Blood Glucose (IVBG) System
The IVBG System was developed by Edwards Lifesciences, LLC (Irvine, CA) and Dexcom Inc. (San Diego, CA) to monitor the concentration of venous BG in patients hospitalized with serious medical and surgical illnesses. A detailed description of the system and its operation has been described by Bailey et al.87 Briefly, it consists of a peripheral IV catheter, an IV glucose sensor, a flush solution, and a bedside monitor. The IV catheter (Terumo SURFLO® 20G × 1.25") used for sensor placement was standardized across sites for the study. The IVBG sensor was connected to the IVBG monitor via a light-weight flexible cable. The IV catheter and IVBG sensor were connected to the flush solution by flexible plastic tubing. The IVBG monitor contained a bidirectional rotary pump to control the flow of blood and flush solution. The IVBG monitor and flush solution were attached to an IV pole with wheels to facilitate patient ambulation. The IVBG sensor and tubing were supplied sterile from the manufacturer (Figure 2).
Figure 2.
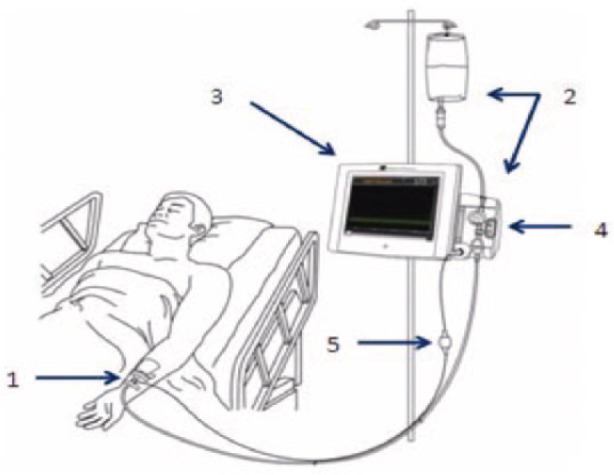
Components of the IVBG system: (1) IVBG sensor and patient IV catheter, (2) reference solution and IVBG tubing set (tubing set), (3) IVBG monitor, (4) flow control valve unit, and (5) patient cable.
Each IVBG sensor was inserted inside the lumen of a commercial 20 gauge, 1.25 inch IV catheter (Figure 3). The sensor and catheter were continuously flushed with saline solution containing dextrose (200 mg/dl) and unfractionated heparin (2 units/ml). The pharmacy at each site was responsible for preparing the flush solution. The IVBG System withdrew a 50 microliter sample of venous blood into the IV catheter lumen every 7.5 minutes, measured the glucose concentration using an electrochemical sensor, and flushed the sample back into the bloodstream. The flush solution was used to produce a 1-point calibration between each sensor measurement and to clean the sensor surface and catheter lumen. Under normal operating conditions, the IVBG System infused 4 to 10 ml of flush solution into the peripheral vein each hour (96-240 ml/day). Thus, the IVBG System infused approximately 200 to 480 units of heparin and 200 to 480 mg of glucose per day into the patient’s peripheral venous circulation. The IVBG sensor was calibrated using a patient venous blood sample and an accurate reference YSI analyzer approximately 1 hour after start-up and every 24 hours thereafter.
Figure 3.
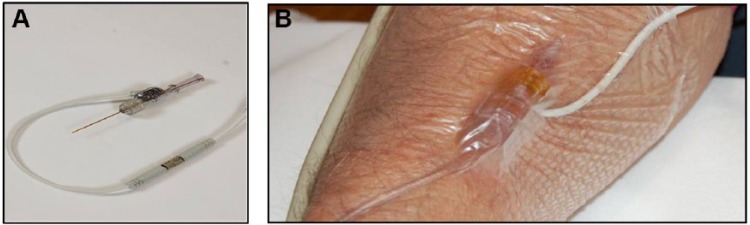
IVBG sensor (A) and IVBG sensor in situ (B).
Reference Device: YSI 2300 STAT Plus
Research personnel obtained a whole blood sample from a vascular catheter approximately every 4 hours using a standardized method. The blood was transferred to a heparinized cuvette and centrifuged at 13,400 revolutions per minute for 30 seconds. The separated plasma was presented once to the YSI 2300 STAT Plus Analyzer (YSI Life Sciences, Yellow Springs, OH). The plasma sample was mixed within the YSI sampling chamber and analyzed by 2 separate glucose-oxidase electrochemical sensors (referred to as the White and Black Channels). The YSI automatically recalibrated itself using an internal standard (180 mg/dl) every hour and external high and low standards (400 and 50 mg/dl) were tested on the YSI every 24 hours to ensure proper operation when the device was in use. The average of the glucose measurements from the White and Black Channels was used as the reference glucose measurement when calculating accuracy.
Since the IVBG sensor was inserted into a peripheral IV catheter, it would be appropriate to obtain the blood samples from the same pool of blood for both IVBG System calibrations and reference YSI measurements. However, it was not possible to reliably sample blood every 4 hours from a peripheral IV catheter for 72 hours. Therefore, whole blood for the YSI measurements was sampled from a central venous catheter (51%), a radial artery catheter (34%), and a peripheral IV catheter/venipuncture (15%); according to catheter availability, patency, and convenience.
YSI and IVBG Measurement Pairing
IVBG measurements were compared to time-matched YSI measurements. The IVBG System attempted to sample and measure peripheral venous blood every 7.5 minutes whereas a reference YSI measurement was made approximately every 4 hours. The IVBG measurement whose time was closest to the reference blood sample draw time was paired with the reference YSI measurement if the magnitude of this difference did not exceed 3.75 minutes.
Clinical Assessments by the Research Nurse
Research personnel at the bedside charted subject vital signs, movement, fluid intake/output, meals, and medications throughout the study period. The IV catheter site was observed for signs of inflammation, infection, infiltration, edema, hematoma, and venous blood flow. Additional blood was sampled twice per day to measure the partial thromboplastin time (PTT) to determine whether the heparin in the flush solution produced systemic anticoagulation.
Study Endpoints
Accuracy was evaluated by comparing the IVBG measurements to time-matched reference YSI measurements according to the 15/20% criterion described in the International Organization for Standardization (ISO) standard 15197:2003.70 This Standard required 95% of the IVBG measurements to be within ±15 mg/dl (±0.83 mmol/l) of YSI measurements <75 mg/dl (<4.17 mmol/l) and ±20% for the YSI measurements ≥ 75 mg/dl (≥ 4.17 mmol/l). IVBG System accuracy was also assessed by calculating the absolute differences and the absolute relative differences between paired IVBG and YSI measurements, and performing Bland-Altman and traditional Clarke error grid analysis.71
Accuracy was also evaluated in relation to the new ISO 15197:2013 Standard for hospital glucose monitors (blood gas/glucose analyzers and point-of-care meters). This standard was accepted and published after data acquisition for the current study was completed. It requires 95% of the IVBG System measurements to be within ±15 mg/dl (±0.83 mmol/l) of YSI measurements < 100 mg/dl (< 5.55 mmol/l) and ±15% of YSI measurements ≥ 100 mg/dl (≥ 5.55 mmol/l).72
IVBG System safety assessments were based on PTT results and on physical examination of the IV catheter insertion site during the study, on sensor/catheter removal, and at a follow-up assessment approximately 1 week (7 ± 3 days) after sensor removal. The skin, vein, and subcutaneous tissues were evaluated for the presence of inflammation, infection, edema, and hematoma.
Statistical analyses were performed using Matlab 2012b (Mathworks, Inc, Natick, MA), SAS version 9.1.3, and S-Plus version 8.0.4.
Results and Discussion
Overall, 44 135 IVBG measurements were recorded with 30 231 of those measurements in the 75 patients evaluated for effectiveness. In the patients evaluated for effectiveness, the mean glucose concentration of the IVBG System measurements was 119 ± 45 mg/dl (with a measurement range 40 mg/dl to 400 mg/dl); 2.9% of the measurements were less than 75 mg/dl, 66.7% were between 75 and 140 mg/dl, 25.1% were between 140 and 200 mg/dl, and 5.1% were greater than 200 mg/dl.
IVBG System Accuracy
In all, 996 paired IVBG and YSI measurements were obtained from 75 subjects. When compared to the YSI measurements, 93.3% of the IVBG measurements (929/996) met the ISO 15197:2003 15/20% criteria whereas 86.7% (865/996) met the ISO 15197:2013 15/15% criteria. The IVBG System produced a mean absolute difference (± SD) of 11.61 ± 25.09 mg/dl and a mean absolute relative difference (± SD) of 8.23% ± 10.51%. Clarke error grid analysis comparing the IVBG System to the YSI revealed 93.2% of the paired values were in zone A, 5.8 % in zone B, 0.2% in zone C, and 0.8% in zone D (Figure 4).
Figure 4.
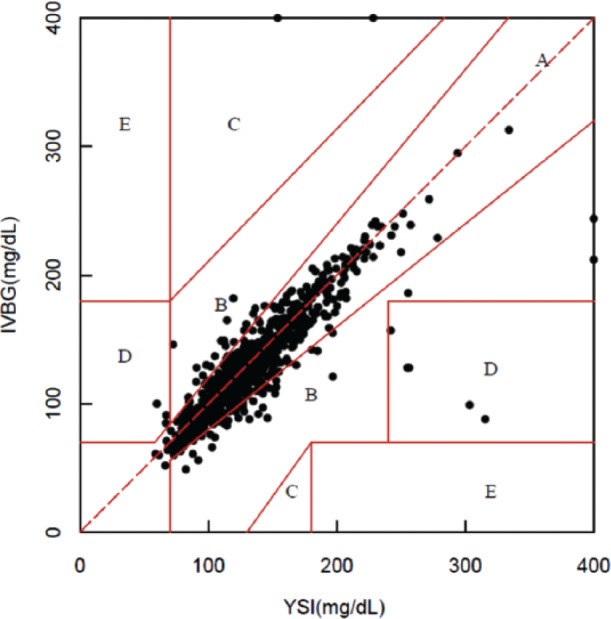
Clarke error grid.71
The IVBG sensors were exposed to more than 200 different IV and oral medications in the operating rooms, intensive care units, and general floors of the hospital. All IV medications were infused through a central venous catheter or a peripheral IV catheter inserted in the arm contralateral to the IVBG sensor. No medications were infused via the peripheral IV catheter that housed the sensor. The medications which included multiple doses of acetaminophen had no observable effect on IVBG System accuracy.
IVBG System Safety
A total of 116 IV catheters and IVBG sensors were inserted into the peripheral arm veins of 100 patients. The IVBG sensors were easily inserted into the lumen of the IV catheters without incident. System set-up was uncomplicated and required less than 10 minutes of a clinician’s time.
The IVBG System measured BG for more than 5500 hours in 100 patients without causing infection or significant inflammation of the surrounding tissue and vein. The mild edema, erythema, and bruising noted on the physical examination of some patients were consistent with routine clinical use of a peripheral IV catheter. No patient developed a serious adverse event directly related to the study device or study methods.
The IVBG System infused 10 to 20 units of unfractionated heparin per hour through the sensor and 20G IV catheter into a peripheral vein. No patient developed evidence of heparin-induced thrombocytopenia, despite being exposed to 200-480 units of heparin per day for 1 to 3 days. Of interest, 72% of the patients received additional large doses of heparin as part of their routine clinical care. The small dose of heparin infused by the IVBG System did not significantly increase the PTT in any patient.
IVBG System Reliability
The IVBG System consistently measured the concentration of BG every 7.5 minutes for 60 to 72 hours in the majority of patients studied. Most of the missed measurements were single skips, followed by a successful measurement. Overall, 85% of all IVBG System measurement attempts would have resulted in a displayed BG value on the bedside monitor. Reliability was satisfactory in the majority of patients studied, even in the critical care patients that developed dehydration and/or required catecholamine therapy for low cardiac output and blood pressure.
The average dwell time for the 116 sensors was 57.7 ± 19.2 hours per patient. Fifty-four sensors (46.6%) were inserted for the full 72 hours of the study period. Ten sensors (8.6%) were prematurely removed due to suspected sensor failure and 13 sensors (11.2%) were removed due to suspected loss of peripheral IV catheter integrity. Eight sensors (6.9%) were removed when research staff could not resolve problems related to the system. Fourteen sensors (12%) were prematurely removed because the patient withdrew consent, 12 sensors (10%) were removed due to hospital logistic issues. The IV catheter and sensor were accidentally removed by the patient in 5 cases (4%).
Discussion
The major weakness of this 72-hour study was the need to sample blood from a variety of blood sources. Ideally, blood would have been sampled exclusively from a peripheral venous catheter for calibration and correlation to match the blood sampled by the IVBG sensor. However, maintaining a functional peripheral venous catheter for the duration of the study was not possible. And, although more reliable, the radial artery catheter was typically removed within 24 to 36 hours after the surgical procedure. Sampling from a central venous catheter was limited at some sites due to the risk of infection.
The calculation of IVBG System point accuracy was decreased because the glucose concentration measured by the IVBG sensor in peripheral venous blood was compared to the reference YSI glucose concentration measured in blood sampled from radial artery, central venous, and peripheral venous catheters. The concentration of plasma glucose in peripheral venous blood tends to be 3 to 6 mg/dl lower than in the radial artery. The difference may increase to greater than 10 to 15 mg/dl during ambulation, skeletal muscle shivering, low tissue blood flow, and high plasma insulin levels. The concentration of plasma glucose in central venous (superior vena cava) blood tends to be 4 to 10 mg/dl lower than the concentration of glucose in radial artery blood. The arterial-central venous BG difference may widen significantly (>20 mg/dl) in patients with increased cellular metabolism, increased skeletal muscle blood flow, high insulin sensitivity, and high plasma insulin levels. These differences directly impact the calculation of point accuracy with a glucose monitoring device.73-80
The calculation of point accuracy of a glucose monitoring device may be further decreased due to contamination and/or dilution of reference blood samples with glucose-free or glucose-containing parenteral solutions. Dilution of the reference blood sample with a glucose-free solution tends to lower the plasma glucose concentration by a small percentage.64,81 Contamination of a reference blood sample with any glucose-containing solution may be problematic because a D5W solution (5% dextrose in water) has a 5000 mg/dl glucose concentration. There is a high probability that many of the paired data points with low correlation were caused by preanalytical and analytical error in the reference YSI glucose measurement, and not an error in the IVBG System glucose measurement.
Prospective randomized trials attempting to evaluate the clinical effects of insulin therapy and BG control highlight the technical challenges related to obtaining an accurate BG measurement in the real-world environment of the ICU, and the importance of standardizing the methods of blood sample acquisition, handling, and analysis.82-86 For example, the true BG measurement of the 2 groups in the NICE-SUGAR Study will never be known because blood was sampled from multiple blood sources (radial artery, peripheral vein, central vein, and finger-stick capillary), handled using a variety of methods, and analyzed using a variety of point-of-care meters, blood gas/glucose analyzers, and central laboratory methods. The large total BG measurement error (preanalytical plus analytical error) makes interpretation of the NICE-SUGAR trial data and other clinical trial outcome data problematic.80
Conclusions
This article describes the first large prospective observational study to evaluate the safety, accuracy and reliability of the first-generation IVBG System when used in the operating rooms, intensive care units, and general floors of 6 US hospitals. Overall, the first-generation IVBG System was easy to set up, calibrate, and utilize in a variety of patient populations and hospital environments. It was found to be safe and effective when studied for more the 5500 hours in 100 critically ill patients.
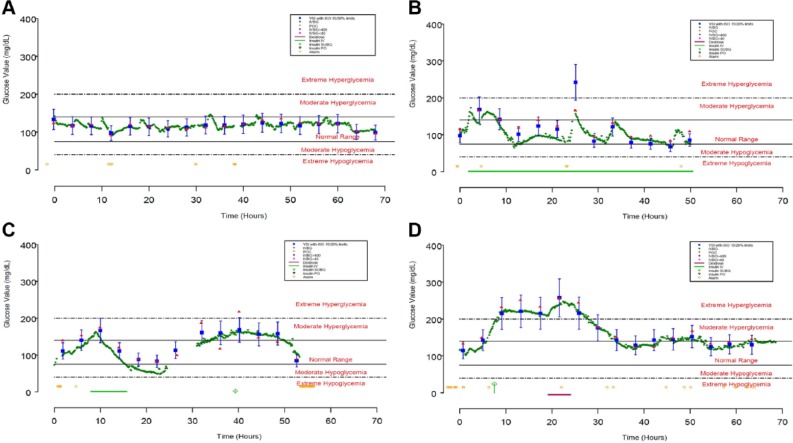
The first-generation IVBG System accurately and reliably measured BG when evaluated in a variety of patient populations and hospital environments. The data from 4 subjects is presented in Figure 5. These examples represent the extremes in sensor accuracy with MARD values ranging from 2.49 to 15.56% (additional performance data is provided in Table 3). In each case, the IVBG System trends with reference YSI values although consistent biases were observed in subjects 0057 and 0158. These biases could be the result of either sampling site differences or error introduced through calibration.
Figure 5.
Four sensors that represent acceptable (0021 and 0162, panels A and D) and unacceptable (0057 and 0158, panels B and C) accuracy relative to the 95% criterion in the ISO Standard 15197:2003. Closed blue squares with whiskers represent YSI measurements with limits imposed by 95% criterion in ISO standard 15197:2003, closed green diamonds represent IVBG measurements, closed red triangles represent concurrent point-of-care glucose meter measurements, yellow open circles represent IVBG alerts and alarms, green bars represent IV insulin infusions, open green diamonds represent subcutaneous insulin injections, and magenta bars represent dextrose infusions.
Table 3.
Duration of Use and Metrics for Accuracy and Reliability for the 4 Sensors Depicted in Figure 5.
| Sensor ID | Site | Duration (hr) | ISO 15/20% | MAD (mg/dl) | MARD (%) | Reliability (%) |
|---|---|---|---|---|---|---|
| 0021 | UMD | 68.01 | 100 | 2.88 | 2.46 | 89.08 |
| 0057 | WHC | 50.54 | 62 | 20.84 | 15.56 | 90.71 |
| 0158 | TJU | 56.40 | 75 | 16.47 | 14.81 | 74.47 |
| 0162 | TJU | 69.08 | 100 | 5.13 | 3.19 | 82.96 |
This study demonstrated the need to improve reliability of the IVBG System. Engineers used this data to develop a second-generation IVBG System (under the trade name GlucoClear®) with an optimized blood sampling and flushing mechanism. While maintaining reliability, the second-generation IVBG System has demonstrated improved accuracy (Table 4).
Table 4.
Summary of the published studies of the First- and Second-Generation IVBG Systems describing the number of subjects, number of sensor/reference pairs, MAD (mg/dl), MARD (%), and reliability (percentage of measurement attempts would have resulted in a displayed BG value on the bedside monitor).
| Study type | Study dates | Generation | Subjects | Pairs | MAD | MARD | Reliability | Reference |
|---|---|---|---|---|---|---|---|---|
| In-clinic, multicenter | 06/2009-07/2009 | 1 | 50 | 2815 | 10.70 | 6.60 | 79.0 | 87 |
| Perioperative, multicenter | 07/2009-04/2010 | 1 | 75 | 996 | 11.61 | 8.23 | 85.0 | Current study |
| In-clinic, single site | 12/2011 | 2 | 10 | 1725 | 7.19 | 5.04 | n/a | 88 |
| ICU, single site | 01/2012-02/2012 | 2 | 10 | 1393 | 5.17 | 5.05 | 90.0 | 88 |
A safe and user-friendly glucose monitoring system that provides accurate and frequent BG measurements has great potential to decrease nursing time/effort while improving the safety and efficacy of insulin therapy and BG control in the hospital.89 Clinicians in the future may utilize this near-continuous glucose monitoring system to perform a prospective randomized clinical trial to determine whether clinical outcome can be improved (decreased morbidity, mortality, length of stay, and hospital cost) as a result of optimized insulin therapy and BG control (avoidance of hyperglycemia, hypoglycemia, and glycemic variability).61,79,90 A near-continuous IV glucose monitoring system that is safe, accurate, reliable, and user-friendly has great potential to be integrated with a computer controller and infusion pumps for IV insulin and IV glucose to produce a closed-loop in-hospital artificial pancreas.63-69,91
Key Messages
The IVBG System was able to safely, automatically, and near-continuously monitor BG concentrations when attached to a peripheral IV catheter for more the 5500 hours in the intended-use patient populations and critical care environments of 6 US hospitals.
IVBG measurements correlated closely with the reference YSI measurements. Of the IVBG measurements, 93% met the 2003 15/20% ISO Standards criteria for point accuracy, the MAD was 11.61 mg/dl, the MARD was 8.23%, and 93% of the measurements were in zone A of the Clarke error grid.
Edwards Lifesciences engineers used data from this observational study to redesign the sensor’s sampling and flushing system, improving the reliability of blood sample acquisition when attached to a peripheral IV catheter.
Clinical trials are required to determine whether a device such as the IVBG System with near-continuous BG measurements along with appropriate alerts and alarms can be used to improve the safety and efficacy of insulin therapy in the hospital and, ultimately, demonstrate an improvement in clinical outcomes.
Acknowledgments
The authors acknowledge the work of the dedicated research personnel and the kind cooperation of the patients who participated in this study.
Footnotes
Abbreviations: AD, absolute difference; ARD, absolute relative difference; BG, blood glucose; ICU, intensive care unit; ISO, International Organization for Standardization; IVBG, Intravenous Blood Glucose system; MAD, mean absolute difference; MARD, mean absolute relative difference; mg/dl, milligrams per deciliter; mmol/L, millimoles per liter; PTT, partial thromboplastin time; YSI, Yellow Springs Instrument.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The study protocol was developed by Edwards Lifesciences with advice from the study investigators and research personnel. During the execution of the observational study (July 2009 and April 2010), GVB was an employee of University of Maryland School of Medicine Baltimore, MD; MFM was an employee of Washington Hospital Center, Washington, DC; RMB was an employee of International Diabetes Center (IDC), Methodist Hospital, Minneapolis, MN; APF was an employee of Providence Heart and Vascular Institute, Portland, OR; JIJ and BRH were employees of Thomas Jefferson University, Philadelphia PA; AMG and MJH were employees of Edwards Lifesciences, Irvine, CA; and PCS was an employee of Dexcom, San Diego, CA. At the time of publication, BRH was an employee of Edwards Lifesciences, Irvine, CA. RMB has served as member of a scientific advisory board, a consultant or conducted clinical research with Medtronic, DexCom and Abbott Diabetes Care. All contracts for services were with RMB’s employer, Park Nicollet Institute and no personal compensation for these services went to RMB. JIJ was a member of the scientific/clinical advisory board of, and conducted research for, Edwards Lifesciences. All funds were paid directly to JIJ’s employer, Thomas Jefferson University.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for research personnel, study-related supplies, and study-specific procedures was provided by Edwards Lifesciences, Irvine, CA.
References
- 1. Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773-778. [DOI] [PubMed] [Google Scholar]
- 2. Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36:3008-3013. [DOI] [PubMed] [Google Scholar]
- 3. Hermanides J, Vriesendorp TM, Bosman RJ, et al. Glucose variability is associated with intensive care unit mortality. Crit Care Med. 2010;38:838-842. [DOI] [PubMed] [Google Scholar]
- 4. Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471-1478. [DOI] [PubMed] [Google Scholar]
- 5. Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007-1021. [DOI] [PubMed] [Google Scholar]
- 6. Umpierrez GE, Smiley D. Time-dependent glycemic variability and mortality in critically ill patients with diabetes. Crit Care Med. 2011;39:211-213. [DOI] [PubMed] [Google Scholar]
- 7. Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041-2047. [DOI] [PubMed] [Google Scholar]
- 8. Krinsley JS, Meyfroidt G, van den Berghe G, et al. The impact of premorbid diabetic status on the relationship between the three domains of glycemic control and mortality in critically ill patients. Curr Opin Clin Nutr Metab Care. 2012;15:151-160. [DOI] [PubMed] [Google Scholar]
- 9. Gale SC, Sicoutris C, Reilly PM, et al. Poor glycemic control is associated with increased mortality in critically ill trauma patients. Am Surg. 2007;73:454-460. [DOI] [PubMed] [Google Scholar]
- 10. Akhtar S, Barash PG, Inzucchi SE. Scientific principles and clinical implications of perioperative glucose regulation and control. Anesth Analg. 2010;110:478-497. [DOI] [PubMed] [Google Scholar]
- 11. Lazar HL, Chipkin SR, Fitzgerald CA, et al. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109:1497-1502. [DOI] [PubMed] [Google Scholar]
- 12. Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67:352-362. [DOI] [PubMed] [Google Scholar]
- 13. Mraovic B, Hipszer BR, Epstein RH, et al. Preadmission Hyperglycemia is an Independent Risk Factor for In-Hospital Symptomatic Pulmonary Embolism after Major Orthopedic Surgery. J Arthroplasty. 2010;25:64-70. [DOI] [PubMed] [Google Scholar]
- 14. Bochicchio GV, Joshi M, Bochicchio KM. Early hyperglycemic control is important in critically injured trauma patients. J Trauma. 2007;63:1353-1358. [DOI] [PubMed] [Google Scholar]
- 15. Grey NJ, Perdrizet GA. Reduction of nosocomial infections in the surgical intensive-care unit by strict glycemic control. Endocr Pract. 2004;10:46-52. [DOI] [PubMed] [Google Scholar]
- 16. Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26:57-65. [DOI] [PubMed] [Google Scholar]
- 17. Inzucchi SE. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006;355:1903-1911. [DOI] [PubMed] [Google Scholar]
- 18. Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:16-38. [DOI] [PubMed] [Google Scholar]
- 19. ACE/ADA Task Force on Inpatient Diabetes: American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control. Diabetes Care. 2006;29:1955-1962. [DOI] [PubMed] [Google Scholar]
- 20. Moghissi ES, Korytkowski MT, DiNardo M, et al. American association of clinical endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15:353-369. [DOI] [PubMed] [Google Scholar]
- 21. Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40:3251-3276. [DOI] [PubMed] [Google Scholar]
- 22. Egi M, Finfer S, Bellomo R. Glycemic control in the ICU. Chest. 2011;140:212-220. [DOI] [PubMed] [Google Scholar]
- 23. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27:553-591. [DOI] [PubMed] [Google Scholar]
- 24. McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17:107-124. [DOI] [PubMed] [Google Scholar]
- 25. Turina M, Fry DE, Polk HC., Jr Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005;33:1624-1633. [DOI] [PubMed] [Google Scholar]
- 26. Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067-2072. [DOI] [PubMed] [Google Scholar]
- 27. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687. [DOI] [PubMed] [Google Scholar]
- 28. Yu WK, Li WQ, Li N, Li JS. Influence of acute hyperglycemia in human sepsis on inflammatory cytokine and counterregulatory hormone concentrations. World J Gastroenterol. 2003;9:1824-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of the critically ill? Diabetologia. 2006;49:1722-1725. [DOI] [PubMed] [Google Scholar]
- 30. Finfer S, Liu B, Chittock DR, et al. The Nice Sugar Study Investigators: hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108-1118. [DOI] [PubMed] [Google Scholar]
- 31. Hermanides J, Bosman RJ, Vriesendorp TM, et al. Hypoglycemia is associated with intensive care unit mortality. Crit Care Med. 2010;38:1430-1434. [DOI] [PubMed] [Google Scholar]
- 32. Krinsley JS, Schultz MJ, Spronk PE, et al. Mild hypoglycemia is independently associated with increased mortality in the critically ill. Critical Care. 2011;15:R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kosiborod M, Inzucchi SE, Goyal A, et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA. 2009;301:1556-1564. [DOI] [PubMed] [Google Scholar]
- 34. Braithwaite SS, Buie MM, Thompson CL, et al. Hospital hypoglycemia: not only treatment but also prevention. Endocr Pract. 2004;10(suppl 2):89-99. [DOI] [PubMed] [Google Scholar]
- 35. Herlein JA, Morgan DA, Phillips BG, et al. Antecedent hypoglycemia, catecholamine depletion, and subsequent sympathetic neural responses. Endocrinology. 2006;147:2781-2788. [DOI] [PubMed] [Google Scholar]
- 36. Fischer KF, Lees JA, Newman JH. Hypoglycemia in hospitalized patients. Causes and outcomes. N Engl J Med. 1986;315:1245-1250. [DOI] [PubMed] [Google Scholar]
- 37. Egi M, Bellomo R, Stachowski E, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. 2010;85:217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maynard GA, Huynh MP, Renvall M. Iatrogenic inpatient hypoglycemia: risk factors, treatment, and prevention. Diabetes Spectr. 2008;21:241-247. [Google Scholar]
- 39. Stahl M, Berger W. Higher incidence of severe hypoglycaemia leading to hospital admission in type 2 diabetic patients treated with long-acting versus short-acting sulphonylureas. Diabet Med. 1999;16:586-590. [DOI] [PubMed] [Google Scholar]
- 40. Mitrakou A, Ryan C, Veneman T, et al. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991;260:E67-74. [DOI] [PubMed] [Google Scholar]
- 41. Meneilly GS, Cheung E, Tuokko H. Counterregulatory hormone responses to hypoglycemia in the elderly patient with diabetes. Diabetes. 1994;43:403-410. [DOI] [PubMed] [Google Scholar]
- 42. Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51:724-733. [DOI] [PubMed] [Google Scholar]
- 43. Suh SW, Hamby AM, Swason RA. Hypoglycemia, brain energetic, and hypoglycemic neuronal death. Glia. 2007;55:1280-1286. [DOI] [PubMed] [Google Scholar]
- 44. Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359-1367. [DOI] [PubMed] [Google Scholar]
- 45. Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med. 2003;31:359-366. [DOI] [PubMed] [Google Scholar]
- 46. Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449-461. [DOI] [PubMed] [Google Scholar]
- 47. Vlasselaers D, Milants I, Desmet L, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373:547-556. [DOI] [PubMed] [Google Scholar]
- 48. Agus MSD, Steil GM, Wypij D, et al. Tight glycemic control versus standard care after pediatric cardiac surgery. N Engl J Med. 2012;367:1208-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35:1738-1748. [DOI] [PubMed] [Google Scholar]
- 50. Devos P, Preiser J, Melot C. Impact of tight glucose control by intensive insulin therapy on ICU mortality and the rate of hypoglycaemia: final results of the GluControl study. Intensive Care Med. 2007;33(Suppl 2):S189. [Google Scholar]
- 51. Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125-139. [DOI] [PubMed] [Google Scholar]
- 52. Gandhi GY, Nuttall GA, Abel MD, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146:233-243. [DOI] [PubMed] [Google Scholar]
- 53. Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933-944. [DOI] [PubMed] [Google Scholar]
- 54. Finfer S, Chittock D, Su SY, et al. NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283-1297. [DOI] [PubMed] [Google Scholar]
- 55. Arabi YM, Tamim HM, Rishu AH. Hypoglycemia with intensive insulin therapy in critically ill patients: predisposing factors and association with mortality. Crit Care Med. 2009;37(9):2536-2544. [DOI] [PubMed] [Google Scholar]
- 56. Lena D, Kalfon P, Preiser JC, Ichai C. Glycemic control in the intensive care unit and during the postoperative period. Anesthesiology. 2011;114:438-444. [DOI] [PubMed] [Google Scholar]
- 57. Mesotten D, Van den Berghe G. Glycemic targets and approaches to management of the patient with critical illness. Curr Diab Rep. 2012;12:101-107. [DOI] [PubMed] [Google Scholar]
- 58. Van den Berghe G, Schetz M, Vlasselaers D, et al. Clinical review. Intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target? J Clin Endocrinol Metab. 2009;94:3163-3170. [DOI] [PubMed] [Google Scholar]
- 59. Gough DA, Kreutz-Delgado K, Bremer TM. Frequency characterization of blood glucose dynamics. Ann Biomed Eng. 2003;31(1):91-97. [DOI] [PubMed] [Google Scholar]
- 60. Kovatchev BP, Clarke WL, Breton M, et al. Quantifying temporal glucose variability in diabetes via continuous glucose monitoring: mathematical methods and clinical application. Diabetes Technol Ther. 2005;7:849-862. [DOI] [PubMed] [Google Scholar]
- 61. Holzinger U, Warszawska J, Kitzberger R, et al. Real-time continuous glucose monitoring in critically ill patients: a prospective randomized trial. Diabetes Care. 2010;33:467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care. 2006;15:370-377. [PubMed] [Google Scholar]
- 63. Joseph JI. Anesthesia and surgery in the diabetic patient. In: Goldstein BJ, Müller D, eds. Type 2 Diabetes: Principles and Practice. 2nd ed. New York, NY: Informa Healthcare; 2008:475-489. [Google Scholar]
- 64. Kalfon P, Chilles M. Impact of the type of glucose monitoring on the assessment of glycemic variability in critical care patients. Critical Care. 2012;16:P169. [Google Scholar]
- 65. Ganesh A, Hipszer B, Loomba N, et al. Evaluation of the VIA blood chemistry monitor for glucose in healthy and diabetic volunteers. J Diabetes Sci Technol. 2008;2:182-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schwartz SS, Horwitz DL, Zehfus B, et al. Use of a glucose controlled insulin infusion system (artificial beta cell) to control diabetes during surgery. Diabetologia. 1979;16:157-164. [DOI] [PubMed] [Google Scholar]
- 67. Albisser AM, Leibel BS, Ewart TG, et al. An artificial endocrine pancreas. Diabetes. 1974;23:389-396. [DOI] [PubMed] [Google Scholar]
- 68. Kadish AH. Automation control of blood sugar. A servomechanism for glucose monitoring and control. Trans Am Soc Artif Intern Organs. 1963;19:363-367. [PubMed] [Google Scholar]
- 69. Joseph J, Hipszer B, Mraovic B, et al. Clinical need for continuous glucose monitoring in the hospital. J Diabetes Sci Technol. 2009;3:1309-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. International Standards Organization. DIN EN ISO 15197: in vitro diagnostic test systems-requirements for blood glucose monitoring systems for self-testing in managing diabetes mellitus (ISO 15197:2003). Brussels, Belgium: European Committee for Standardization; 2003. [Google Scholar]
- 71. Clarke WL. The original Clarke error grid analysis (EGA). Diabetes Technol Ther. 2005;7(5):776-779. [DOI] [PubMed] [Google Scholar]
- 72. International Organization for Standardization. In vitro diagnostic test systems. Requirements for blood glucose monitoring system for self testing in managing diabetes mellitus. Reference number ISO 15197:2013. Geneva, Switzerland: International Organization for Standardization; 2013. [Google Scholar]
- 73. Fahy BG, Coursin DB. Critical glucose control: the devil is in the details. Mayo Clin Proc. 2008;83:394-397. [DOI] [PubMed] [Google Scholar]
- 74. Finkielman JD, Oyen LJ, Afessa B. Agreement between bedside blood and plasma glucose measurement in the ICU setting. Chest. 2005;127:1749-1751. [DOI] [PubMed] [Google Scholar]
- 75. Nayak PP, Morris K, Lang H, et al. Lack of agreement between arterial and central venous blood glucose measurement in critically ill children. Intensive Care Med. 2009;35:762-763. [DOI] [PubMed] [Google Scholar]
- 76. Nichols JH. What is accuracy and how close must the agreement be? Diabetes Technol Ther. 2005;7:558-562. [DOI] [PubMed] [Google Scholar]
- 77. Sylvain HF, Pokorny ME, English SM, et al. Accuracy of fingerstick glucose values in shock patients. Am J Crit Care. 1995;4:44-48. [PubMed] [Google Scholar]
- 78. Dungan K, Chapman J, Braithwaite SS, Buse J. Glucose measurement: confounding issues in setting targets for inpatient management. Diabetes Care. 2007;30:403-409. [DOI] [PubMed] [Google Scholar]
- 79. Torjman MC, Jahn L, Joseph JI, Crothall K. Accuracy of the Hemocue portable glucose analyzer in a large non-homogeneous population. Diabetes Technol Ther. 2001;3:591-600. [DOI] [PubMed] [Google Scholar]
- 80. Flood J, Joseph J, Kim L, et al. Glucose levels in blood simultaneously sampled from the radial artery, vena cava, and fingertip. Anesthesiology. 2007;A107:1123. [Google Scholar]
- 81. Harmsen R, Van Braam Houckgeest F, et al. Blood glucose variability, measured as mean absolute glucose, strongly depends on the frequency of blood glucose level measurements. Critical Care. 2011;15:P392. [Google Scholar]
- 82. Chakravarthy SB, Markewitz BA, Lehman C, et al. Glucose determination from different vascular compartments by point-of-care testing in critically ill patients. Chest. 2005;128(suppl):220S-221S. [Google Scholar]
- 83. Maser R, Butler M. Use of arterial blood with blood glucose reflectance meters in an intensive care unit: are they accurate? Crit Care Med. 1994;22:595-599. [DOI] [PubMed] [Google Scholar]
- 84. Kulkarni A, Saxena M, Price G, et al. Analysis of blood glucose measurements using capillary and arterial blood samples in intensive care patients. Intensive Care Med. 2005;31:142-145. [DOI] [PubMed] [Google Scholar]
- 85. Kanji S. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;12:2778-2785. [DOI] [PubMed] [Google Scholar]
- 86. Sacks D, Bruns D, Horton J, et al. Clinical Laboratory Standards Institute. POCT12-A3: point-of-care blood glucose testing in acute and chronic care facilities; approved guideline-third edition. January 2013. [Google Scholar]
- 87. Bailey T, Gulino A, Higgins MJ, Leach J, Kamath A, Simpson PC. Accuracy of a first-generation intravenous blood glucose monitoring system in subjects with diabetes mellitus: a multicenter study. J Diabetes Sci Technol. 2013;7(6):1484-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Foubert LA, Lecomte PV, Nobels FR, Gulino AM, De Decker KH. Accuracy of a feasibility version of an intravenous continuous glucose monitor in volunteers with diabetes and hospitalized patients. Diabetes Technol Ther. 2014;16(12):858-866. [DOI] [PubMed] [Google Scholar]
- 89. Torjman MC, Dalal N, Goldberg ME. Glucose monitoring in acute care: technologies on the horizon. J Diabetes Sci Technol. 2008;2:178-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ryan M, Savarese V, Hipszer B, et al. Continuous glucose monitor shows potential for early hypoglycemia detection in hospitalized patients. Diabetes Technol Ther. 2009;11:745-747. [DOI] [PubMed] [Google Scholar]
- 91. Holzinger U, Warszawska J, Kitzberger R, et al. Impact of shock requiring norepinephrine on the accuracy and reliability of subcutaneous continuous glucose monitoring. Intensive Care Med. 2009;35:1383-1389. [DOI] [PubMed] [Google Scholar]