Abstract
Background:
An 8-mm needle length is commonly used for insulin injections; however, recent recommendations suggest shorter needles may help patients avoid intramuscular injections and reduce pain, while maintaining adequate glucose control. The goal of these analyses was to compare the pharmacokinetics (PK) and glucodynamics (GD) of insulin lispro after a 5-mm or an 8-mm injection depth administration in 2 populations: normal weight (study 1) or obese (study 2).
Methods:
In both open-label, randomized, 2-period crossover euglycemic clamp studies, subjects received single 0.25 U/kg insulin lispro doses on 2 occasions (at 5-mm and 8-mm injection depths); samples for PK and GD analyses were collected up to 6 hours postdose. Noncompartmental PK parameters AUC0-tlast, AUC0-∞, Cmax and GD parameters Gtot, Rmax, tRmax were log-transformed prior to analysis using a mixed effects model.
Results:
There were no apparent differences between PK profiles at the 5-mm or 8-mm injection depth in either study, demonstrated by the ratios of geometric means of AUC0-tlast, AUC0-∞, and Cmax being close to 1, with 90% confidence intervals (CI) within (0.80, 1.25). There were no apparent differences between GD profiles at either injection depth with the ratios of Gtot and Rmax near unity and 90% CIs that included 1. In both studies, the tRmax values were similar between injection depths, with a small median of pairwise differences and a 90% CI that included zero.
Conclusions:
Injection depths in the 5-8 mm range did not affect the PK or GD of insulin lispro in normal weight or obese subjects.
Keywords: glucodynamics, injection depth, insulin lispro, pharmacokinetics
Patients with diabetes often need to administer multiple daily insulin injections to maintain adequate glycemic control, and for some patients, insulin injections are associated with pain, discomfort, and anxiety.1 Shorter insulin needles have been introduced in an effort to reduce the discomfort of subcutaneous (SC) insulin administration associated with longer needles.2 While the 8-mm needle length is commonly used worldwide,3 recent recommendations suggest that using shorter needles (4 mm, 5 mm, or 6 mm) may be optimal for most patients to avoid intramuscular injection and still maintain adequate glucose control.2,4,5 In clinical trials comparing 5-mm to 8-mm needles in pediatric and adult populations, the 5-mm needle was found to be as efficacious and safe as an 8-mm needle.2
However, there is still skepticism regarding the use of shorter needles to administer insulin to obese patients, who are often advised to use longer needles (≥8 mm) due to the clinical perception that insulin should be injected deep into the SC layer of tissue. Results from a study in which skin thickness and SC adipose layer thickness were measured in patients with diabetes across 3 body mass index (BMI) subgroups (<25, 25-29.9, and ≥30 kg/m2) demonstrated that skin thickness varies minimally between differing demographics, averaging approximately 1.9-2.4 mm across injection sites, ages, races, BMI, and sex, and is rarely >3 mm at injection sites for insulin.6
In an open label, randomized, crossover study comparing the metabolic control between 5-mm and 8-mm needle lengths for insulin injection in obese patients with type 1 or type 2 diabetes, the 5-mm needle was found to be similar to the 8-mm needle with respect to metabolic control.7 Similarly, in a study comparing investigator-administered injections of 20- and 60-unit (U) equivalent volumes of preserved sterile insulin diluent using 5-mm versus 8-mm needles, results demonstrated that the 5-mm needle was similar to the 8-mm needle with respect to insulin diluent leakage postinjection in obese patients with type 1 or type 2 diabetes, and there were no observed differences between the 5-mm and 8-mm needle with respect to pain intensity, bleeding, or bruising at injections sites.8
The use of needles shorter than 5 mm has been evaluated as well; an early study by Frid et al did not detect any significant differences in regular human insulin absorption between superficial and deep injections in the subcutaneous fat layer of either the abdominal wall or the thigh.9 Consistently, research by Hirsch et al demonstrated equivalent glycemic control with 4-mm versus 5-mm or 8-mm needles in subjects with a BMI between 20-49 kg/m2.10 The 4-mm needle was also found to be safe, less painful, better tolerated, and provided equivalent glycemic control in comparison to the longer 8-mm and 12.7-mm needles in a prospective, randomized, crossover trial comparing glycemic control in obese subjects.11 In addition, a study evaluating the pharmacokinetic (PK) properties of insulin lispro using 4-, 6-, and 8-mm needles in healthy Japanese males demonstrated bioequivalence between the 4- and 6-mm needles.12
Insulin lispro (Humalog®), a rapid-acting insulin analog, has been available for clinical use in the United States since 1996;13 however, the effect of injection depth on the PK and glucodynamic (GD) profile of insulin lispro in normal weight and obese patients has not been previously described. The objective of these analyses was to compare the PK and GD of insulin lispro after administration of single doses at either a 5-mm or an 8-mm injection depth in both normal weight and obese healthy subjects.
Study Design and Methods
Study Design
Two separate open-label, randomized, 2-period, crossover, euglycemic clamp studies were conducted. Study 1 was conducted in healthy, normal weight subjects and study 2 in healthy, obese subjects. In both studies, subjects received single doses of insulin lispro (0.25 U/kg) on 2 different occasions, with a washout time between study periods that ranged from 3 to 20 days. Both studies were approved by local investigational review boards and were performed in compliance with the principles of Good Clinical Practice and in accordance with the Declaration of Helsinki.
In each study period, subjects were dosed with insulin lispro after an 8-hour fast and underwent a euglycemic clamp procedure. Insulin lispro doses were administered subcutaneously into alternate lower abdominal quadrants. Injections were administered at an approximately 90-degree angle into a raised skinfold for the 8-mm injection depth (31-gauge pen needles; Becton, Dickinson and Company, Durham, NC, USA), and without raising a skinfold for the 5-mm injection depth.
Subjects
Inclusion criteria required subjects to be overtly healthy males or females, as determined by medical history and physical examination. In study 1, subjects were required to be between 21-50 years of age (inclusive) and to have a BMI between 18.5-29.9 kg/m2 (inclusive). In study 2, subjects were required to be between 21-60 years old (inclusive) and to have a BMI ≥30 kg/m2. Subjects in both studies were excluded if fasting venous blood glucose (plasma) was >108 mg/dL (6 mmol/L). All subjects provided written informed consent.
Sample Size
Minimum sample sizes of 12 completers in study 1 and 16 completers in study 2 provided at least 80% probability to demonstrate that the 90% CI for the ratio of geometric means of insulin AUC and Cmax between the 5-mm and the 8-mm injection depths was within the reference range of (0.75, 1.33). The sample size for study 1 assumed a ratio of the means of 1.05 and a 20% intrasubject coefficient of variation (CV) for the PK parameters, based on previous insulin lispro PK studies (Eli Lilly and Company, Data on File). As study 2 was completed after study 1, the sample size calculation for study 2 assumed an anticipated ratio of the means of 1.00 and a 26% intrasubject CV for the PK parameters.
Euglycemic Clamp Procedure
Euglycemia (determined by the subject’s baseline on each respective study dosing day) was maintained using a glucose clamp procedure for up to approximately 6 hours after insulin lispro administration (defined as time 0). Glucose infusion rates (GIR) were adjusted to maintain euglycemia, and blood samples were drawn for determination of blood glucose and serum insulin lispro concentrations throughout the clamp. For both studies, the clamp was discontinued if the GIR was 0 for at least 30 minutes after the clamp had been underway for at least 4 hours. For each study period, subjects remained in the fasting state until after the glucose clamp procedure was completed.
In both studies, a 20% dextrose solution was administered during the clamp procedure. In study 1, the GIR was adjusted manually to maintain the blood glucose concentrations within ±5% of the predose target value, defined as 5 mg/dL below the mean of predose fasting blood glucose concentrations measured on the day of the glucose clamp. Glucose concentrations were measured every 5 to 30 minutes throughout the clamping procedure using an automated glucose analyzer (Yellow Springs Instruments 2300 STAT PLUS [YSI] analyzer; Yellow Springs, OH, USA), and GIR changes were recorded. In study 2, GIRs were adjusted automatically via a Biostator® (Life Science Instruments; Elkhart, IN, USA) to maintain the blood glucose concentration at 5% below the baseline glucose concentration, defined as the mean of 3 predose fasting blood glucose concentrations measured on the day of the glucose clamp. A blood sample for blood glucose determination was taken at least every 30 minutes for Biostator calibration. Glucose concentrations and GIR changes were electronically recorded using the Biostator.
Sample Collection and Assay
In both studies, serial blood samples were collected predose and up to 6 hours after the administration of each insulin lispro dose (10, 20, 30, 45, 60, 90, 120, 180, 240, 300, and 360 minutes relative to dosing), for the determination of serum insulin (lispro) concentrations.
Serum concentrations of insulin lispro were measured by validated radioimmunoassay (RIA) methods, in which samples were pretreated with 20% polyethylene glycol (PEG) to remove any endogenous anti-insulin antibodies. In study 1, PEG-treated samples were combined with [125I]-labeled despentapeptide insulin (DPI) and DPI antiserum, the bound complex was precipitated, and 125I counts in the pellet were determined with a gamma counter. The concentration of insulin was determined by interpolation from a standard curve prepared from insulin lispro, with lower and upper limits of quantitation of 20.0 and 2500.0 pM, respectively. The interassay accuracy (% relative error) during validation ranged from 90.8% to 103.9% and the interassay precision (% relative standard deviation) during validation ranged from 2.0% to 9.6%. In study 2, a validated RIA method using a specific insulin lispro antiserum was used to measure free immunoreactive insulin lispro (IRI). PEG-treated serum samples were combined with [125I]-labeled insulin lispro and insulin lispro antiserum, and the bound complex was precipitated. 125I counts in the pellet were determined with a gamma counter. The concentration of insulin lispro was determined by interpolation from a standard curve prepared from insulin lispro. This assay was demonstrated to be specific for insulin lispro, without notable cross-reactivity to endogenous human insulin. The lower limit of quantification was 96 pM, and the upper limit of quantification was 1721 pM. The interassay accuracy (% relative error [% nominal − 100]) during validation ranged from −4.8% to −0.5%, and the interassay precision (% coefficient variation) during validation ranged from 3.0% to 13.5%. For both studies, serum insulin concentration samples were assayed at MDS Pharma Services Saint Laurent, Quebec, Canada.
Pharmacokinetic and Glucodynamic Analyses
The PK and GD parameters estimated in both studies are described below. In both studies, PK parameters were evaluated by standard noncompartmental methods of PK analysis using PKS/WinNonlin Enterprise version 5.0.1. Primary PK parameters included area under the insulin lispro concentration-time curve from time zero to the last time point with a measurable concentration (AUC0-tlast), area under the insulin lispro concentration-time curve from time to zero to infinity (AUC0-∞), maximum insulin lispro concentration (Cmax), and time to maximum insulin lispro concentrations (tmax).
The primary GD parameters of interest were the cumulative amount of glucose infused from dosing until clamp cessation (Gtot) and the maximum glucose infusion rate (Rmax). Other GD parameters, such as time of maximum glucose infusion rate (tRmax) and the time of half maximal glucose infusion rate before or after Rmax (early tRmax50% or late tRmax50%), were also calculated. A locally weighted scatter plot smoothing (LOESS) function was applied to the individual raw data to obtain a fit from which the GD parameters were estimated.
Statistical Analysis
In both studies, PK parameters (AUC0-tlast, AUC0-∞, and Cmax), and GD parameters (Gtot, Rmax) were log-transformed prior to analysis using a mixed effects model. The model included fixed effects for the categorical variables injection depth, period, and sequence. A random subject’s effect was included in the model. Time variables such as tmax and tRmax were analyzed using nonparametric statistical methods.
Safety
Safety was assessed via physical examination, vital signs, adverse event (AE) monitoring, and clinical laboratory evaluations. A follow-up visit was conducted within 14 days of the subject’s last study drug dose.
Results
Demographics
Eighteen subjects entered study 1; 17 were randomly assigned to treatment, and 16 completed both treatment periods and were included in the PK evaluation. A total of 16 healthy subjects entered and completed study 2. Demographic characteristics for study 1 and study 2 are shown in Table 1.
Table 1.
Subject Demographics.
| Study 1: Normal weight subjects (n = 16) | Study 2: Obese subjects (n = 16) | |
|---|---|---|
| Age (years), mean (SD) | 31.1 (9.4) | 40.8 (11.2) |
| BMI (kg/m2), mean (SD) | 22.9 (2.3) | 33.8 (3.2) |
| Gender, n (%) male | 13 (81.3) | 11 (68.8) |
| Race, n (%) | ||
| East Asian | 13 (81.3) | 0 (0.0) |
| Caucasian | 3 (18.7) | 12 (75.0) |
| African American | 0 (0.0) | 3 (18.8) |
| American Indian or Alaska Native | 0 (0.0) | 1 (6.3) |
Abbreviations: BMI, body mass index; n, number of subjects; SD, standard deviation.
Pharmacokinetics
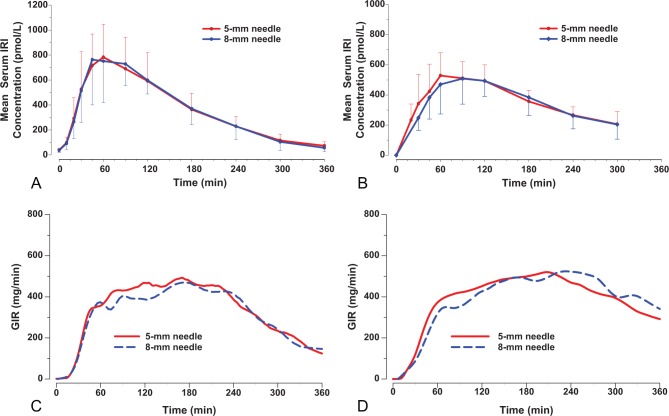
PK results for both studies are summarized in Table 2, and the concentration versus time profiles are shown in Figures 1A and 1B. In study 1, there were no apparent differences between the PK profiles following administration of insulin lispro via the 5-mm and 8-mm injection depth, as demonstrated by the ratios of geometric means for PK parameters of AUC0-tlast, AUC0-∞, and Cmax being very close to 1, with 90% CIs within (0.80, 1.25). Similarly, no apparent differences were observed between the PK profiles of the 2 injection depths in study 2. In both studies, the statistical analysis of tmax showed similar results for the 2 injection depths, with narrow 90% CI for median of pairwise differences that included zero.
Table 2.
Pharmacokinetic and Glucodynamic Parameters (Studies 1 and 2).
| Study 1: Normal weight subjects |
Study 2: Obese subjects |
|||||
|---|---|---|---|---|---|---|
| Parameter (unit) | 5-mm injection depth | 8-mm injection depth | Ratio of LS meanse (90% CI) | 5-mm injection depth | 8-mm injection depth | Ratio of LS meanse (90% CI) |
| N | 16 | 16 | 16 | 16 | 16 | 16 |
| Pharmacokinetics | ||||||
| AUC0-tlast (pmol•min/L) | 125000 (25) | 127000 (22) | 0.99 (0.93, 1.05) | 105000 (26) | 102000 (27) | 0.97 (0.92, 1.03) |
| AUC0-∞ (pmol•min/L) | 134000 (26) | 133000 (22) | 1.00 (0.94, 1.07) | 135000 (26) | 134000 (29) | 1.00 (0.95, 1.05) |
| Cmax (pmol/L) | 831 (30) | 822 (36) | 1.01 (0.94, 1.09) | 569 (24) | 560 (29) | 0.99 (0.91, 1.07) |
| tmax (min) | 60 (45-120)a | 60 (45-120)a | 0.00 (−15, 15)b | 90 (30-120)a | 90 (60-180)a | −15.0 (−30, 0)b |
| Glucodynamics | ||||||
| Gtot (g) | 116 (24) | 111 (23) | 1.04 (0.95, 1.14) | 129 (41.9) | 130 (40.9) | 0.99 (0.92, 1.08) |
| Rmax (mg/min) | 635 (24) | 592 (19) | 1.07 (0.98, 1.17) | 572 (39.8) | 589 (37.2) | 0.97 (0.88, 1.07) |
| tonset (min) | 25 (5-45)a | 23 (10-35)a | 2.5 (−5, 10)b | 21 (5-43)a | 15 (4-47)a | 5.00 (−20,15)b |
| tRmax (min) | 93 (45-220)a | 150 (50-240)a | −33 (−90, 0)b | 204 (48-360)a | 237 (144-360)a | −21 (−96, 24)b |
| early tRmax50% (min) | 41 (32-95)a | 46 (31-86)a | 0.6 (−12, 6)b | 49 (32-119)a | 55 (41-251)a | −6 (−10, 10)b |
| late tRmax50% (min)c,d | 278 (165-330)a | 272 (187-350)a | −21.4 (−35, 5)a | 305 (77-354)a | 338 (257-359)a | −26 (−282, 17)b |
Abbreviations: AUC0-∞, area under the concentration versus time curve from zero to infinity; AUC0-tlast, area under the insulin lispro concentration time curve from time zero to the last time point with a measurable concentration; CI, confidence interval; Cmax, maximum observed drug concentration; Gtot, total amount of glucose infused; LS, least squares; n, number of observations; Rmax, maximum glucose infusion rate; tmax, time of maximum observed drug concentration; tonset, the time from insulin dosing to the first change of glucose infusion; tRmax, time of Rmax. Data are shown as geometric means and coefficients of variation unless otherwise indicated.
Median (range).
Median of pairwise differences (90% CI).
For study 1, late tRmax50% results are based on a sample size of 15 at the 5-mm injection depth, and a sample size of 14 at the 8-mm injection depth.
For study 2, late tRmax50% results are based on a sample size of 10 at the 5-mm injection depth, and a sample size of 6 at the 8-mm injection depth.
Test = 5-mm injection depth, reference = 8-mm injection depth.
Figure 1.
Mean (± SD) serum immunoreactive insulin (IRI) concentration versus time profiles in normal weight subjects (A) and obese subjects (B), and locally weighted scatter plot smoothing (LOESS) fits of glucose infusion rate (GIR) versus time data in normal weight subjects (C) and obese subjects (D) following the administration of 0.25 U/kg insulin lispro at 5-mm and 8-mm injection depths.
Glucodynamics
GD results for study 1 and study 2 are summarized in Table 2. Figure 1C and 1D shows the LOESS fits of GIR versus time profiles following the administration of 0.25 U/kg doses of insulin lispro via the 5-mm and 8-mm injection depth for both studies. In study 1, there were no apparent differences between the GD profiles following administration of insulin lispro via the 5-mm and 8-mm injection depth, with the ratios (90% CI) of Gtot and Rmax near unity and 90% CIs that included 1. Early time measures (tonset and early tRmax50%) showed no evidence of a statistical difference between the 5-mm and 8-mm depths, and the statistical analysis of tRmax demonstrated similar results for the 2 injection depths, with a small median of pairwise differences and a 90% CI that included zero. The late tRmax50% had a median difference of 21 minutes and a narrow 90% CI.
Likewise, in study 2, there were no apparent differences between the GD profiles following administration of insulin lispro at either injection depth with the ratios (90% CI) of Gtot and Rmax near unity and 90% CIs that included 1. The early time measures (tonset and early tRmax50%) had a median difference of 5-6 minutes and narrow 90% CIs. The comparison of tRmax values showed a difference of 21 minutes and a wider CI. Glucose infusion rates did not decline to 50% of Rmax for several subjects at the end of the clamp (6 subjects at the 5-mm injection depth and 10 subjects at the 8-mm injection depth); therefore it was not possible to calculate the late tRmax50% in those cases.
Safety and Tolerability
There were no AEs related to study drug in either study as judged by the investigator. In both studies, the most commonly reported AEs were associated with the study procedures, such as bruises and swelling or pain over cannulation, venipuncture, and infusion sites. In study 1, 12 subjects reported a total of 20 AEs that were related to study procedures, and 1 subject reported an AE that was related to both the study procedure and the device used for injection. Of the AEs related to study procedures, 14 were mild, 5 were moderate, and 2 were considered severe (aching sensation and soreness at the glucose infusion site during the clamp procedure). In study 2, 5 subjects reported a total of 7 AEs, 2 of which were related to study procedures; all were considered mild in severity. There were no clinically significant alterations in laboratory values or vital signs in either study.
Discussion
There were no statistical differences in the PK or GD profiles of insulin lispro between the 5-mm or 8-mm injection depth in either study. A minor, not statistically significant trend for an increase in tRmax was observed in both studies. Given there were no differences in tmax between needle lengths, the reason for this trend is not known. In addition, the administration of insulin lispro at both injection depths appeared to be well tolerated in healthy subjects.
Taken together, these studies indicate that injection depths in the 5- to 8-mm range do not affect the PK or GD of insulin lispro in either population and provide further evidence to support the safety and efficacy of administration of insulin lispro at a 5-mm injection depth in obese patients with diabetes.
Since these studies were performed, new injection recommendations have been published advising the use of shorter needles (4-6 mm) for insulin injection.5 More recent data in Japanese patients have showed no differences in glycemic control between 4-mm and 5-mm needles14 and no differences in the exposure to insulin between 4-mm, 6-mm, and 8-mm needles.13
Patients need to have their insulin reliably delivered into the SC tissue without discomfort, leakage, or injection site adverse effects. Needle lengths previously recommended for SC injection are now known to be too long for many adults and most children because of the increased risk of intramuscular (IM) injections. Needles of 5-mm and even 4-mm length are estimated to provide reliable subcutaneous drug delivery, with substantially reduced risk of IM injection.6,15
This is the first study comparing the PK and GD of insulin after administration of single doses at either a 5-mm or an 8-mm injection depth in both normal weight and obese healthy subjects using the euglycemic clamp procedure. The randomized crossover design was selected to allow comparison between injection depths on an intrasubject basis, allowing each subject to serve as his/her own control. The 0.25 U/kg dose was selected, as it is a clinically relevant mealtime dose of insulin lispro for patients with either type 1 or type 2 diabetes. Healthy subjects were enrolled in these studies to minimize physiologic variability as well as other confounding factors, such as concomitant medications, commonly encountered in patient populations. While the use of healthy subjects could be considered a study limitation as they are not the target patient population, it does not impact the conclusion that the PK and GD profiles observed are similar for both injection depths. A direct comparison between the normal weight and obese populations was not performed, given the multiple differences between studies such as BMI inclusion criteria, clamp technique, injection device, insulin assays, geographic location, and population. A minor methodological limitation of these studies was that the actual depth of injection was not visualized (eg, with high frequency ultrasound).
Conclusion
In summary, there were no differences in PK or GD profiles for insulin lispro administered at a 5-mm or an 8-mm injection depth, in either a normal weight or an obese population. The data from these 2 studies support the use of shorter needles regardless of BMI and corroborate previous clinical data.
Acknowledgments
The authors would like to acknowledge the contributions of Adam Scott (Eli Lilly and Company) and Lisa O’Brien (Eli Lilly and Company) for performing the PK and/or PD analyses for these studies.
Footnotes
Abbreviations: AE, adverse event; AUC0-tlast, area under the insulin lispro concentration time curve from time zero to the last time point with a measurable concentration; AUC0-∞, area under the insulin lispro concentration time curve from time zero to infinity; BMI, body mass index; BP, blood pressure; CI, confidence interval; Cmax, maximum insulin lispro concentration; CV, coefficient of variation; DPI, despentapeptide insulin; early tRmax50%, the time for GIR to reach 50% of Rmax before tRmax; GD, glucodynamic; GIR, glucose infusion rate; Gtot, cumulative amount of glucose infused from dosing until clamp cessation; IRI, immunoreactive insulin; late tRmax50%, the time for GIR to reach 50% of Rmax after tRmax; LOESS, locally weighted scatter plot smoothing; PEG, polyethylene glycol; PK, pharmacokinetics; RIA, radioimmunoassay; Rmax, maximum glucose infusion rate; SC, subcutaneous; tmax, time to maximum insulin lispro concentration; tonset, the time from insulin dosing to the first change of glucose infusion; tRmax, time of maximum glucose infusion rate; U, unit.
Declaration of Conflicting Interests: All authors, except Dr. Linda Morrow, are employees and minor shareholders of Eli Lilly and Company
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: These studies were funded by Eli Lilly and Company.
References
- 1. McKay M, Compion G, Lytzen L. A comparison of insulin injection needles on patients’ perceptions of pain, handling, and acceptability: a randomized, open-label, crossover study in subjects with diabetes. Diabetes Technol Ther. 2009;11(3):195-201. [DOI] [PubMed] [Google Scholar]
- 2. Strauss KA, Hannet I, McGonigle J, et al. Ultra-short (5 mm) insulin needles: trial results and clinical recommendations. Practical Diabetes Int. 1999;16:218-221. [Google Scholar]
- 3. De Coninck C, Frid A, Gaspar R, et al. Results and analysis of the 2008-2009 Insulin Injection Technique Questionnaire survey. J Diabetes. 2010;2(3):168-179. [DOI] [PubMed] [Google Scholar]
- 4. Thow JC, Home PD. Insulin injection technique: depth of injection is important. Brit Med J. 1990;301:3-4.2200543 [Google Scholar]
- 5. Frid A, Hirsch L, Gaspar R, et al. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36(suppl 2):S3-S18. [DOI] [PubMed] [Google Scholar]
- 6. Gibney MA, Arce CH, Byron KJ, Hirsch LJ. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin. 2010;26(6):1519-1530. [DOI] [PubMed] [Google Scholar]
- 7. Kreugel G, Keers JC, Kerstens MN, Wolffenbuttel BH. Randomized trial on the influence of the length of two insulin pen needles on glycemic control and patient preference in obese patients with diabetes. Diabetes Technol Ther. 2011;13(7):737-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ignaut DA, Fu H. Comparison of insulin diluent leakage postinjection using two different needle lengths and injection volumes in obese patients with type 1 or type 2 diabetes mellitus. J Diabetes Sci Technol. 2012;6(2):389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frid A, Linde B. Intraregional differences in the absorption of unmodified insulin from the abdominal wall. Diabet Med. 1992;9(3):236-239. [DOI] [PubMed] [Google Scholar]
- 10. Hirsch LJ, Gibney MA, Albanese J, et al. Comparative glycemic control, safety and patient ratings for a new 4 mm x 32G insulin pen needle in adults with diabetes. Curr Med Res Opin. 2010;26(6):1531-1541. [DOI] [PubMed] [Google Scholar]
- 11. Bergenstal R, Strock E, Peremislov D, Parvu V, Gibney M, Hirsch L. Insulin therapy with a 4 mm x 32G pen needle vs larger needles in obese subjects. Paper presented at: American Diabetes Association 73rd Scientific Session; June 2013; Chicago, IL. [Google Scholar]
- 12. Hirose T, Ogihara T, Tozaka S, Kanderian S, Watada H. Identification and comparison of insulin pharmacokinetics injected with a new 4-mm needle vs 6- and 8-mm needles accounting for endogenous insulin and C-peptide secretion kinetics in non-diabetic adult males. J Diabetes Investig. 2013;4(3):287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Humalog (package insert). Indianapolis, IN: Eli Lilly and Company; 2007. [Google Scholar]
- 14. Nagai Y, Ohshige T, Arai K, et al. Comparison between shorter straight and thinner microtapered insulin injection needles. Diabetes Technol Ther. 2013;15(7):550-555. [DOI] [PubMed] [Google Scholar]
- 15. Sim KH, Hwang MS, Kim SY, Lee HM, Chang JY, Lee MK. The appropriateness of the length of insulin needles based on determination of skin and subcutaneous fat thickness in the abdomen and upper arm in patients with type 2 diabetes [published correction in Diabetes Metab J 2014;38(3):244]. Diabetes Metab J. 2014;38(2):120-133. [DOI] [PMC free article] [PubMed] [Google Scholar]