Abstract
Background:
Hyperglycemia and hypoglycemia are associated with adverse clinical outcomes in intensive care patients. In product development studies at 4 ICUs, the safety and performance of an intravascular continuous glucose monitoring (IV-CGM) system was evaluated in 70 postsurgical patients.
Methods:
The GluCath System (GluMetrics, Inc) used a quenched chemical fluorescence mechanism to optically measure blood glucose when deployed via a radial artery catheter or directly into a peripheral vein. Periodic ultrasound assessed blood flow and thrombus formation. Patient glucose levels were managed according to the standard of care and existing protocols at each site. Reference blood samples were acquired hourly and compared against prospectively calibrated sensor results.
Results:
In all, 63 arterial sensors and 9 venous sensors were deployed in 70 patients. Arterial sensors did not interfere with invasive blood pressure monitoring, sampling or other aspects of patient care. A majority of venous sensors (66%) exhibited thrombus on ultrasound. In all, 89.4% (1383/1547) of arterial and 72.2% (182/252) of venous measurements met ISO15197:2003 criteria (within 20%), and 72.7% (1124/1547) of arterial and 56.3% (142/252) of venous measurements met CLSI POCT 12-A3 criteria (within 12.5%). The aggregate mean absolute relative difference (MARD) between the sensors and the reference was 9.6% for arterial and 14.2% for venous sensors.
Conclusions:
The GluCath System exhibited acceptable accuracy when deployed in a radial artery for up to 48 hours in ICU patients after elective cardiac surgery. Accuracy of venous deployment was substantially lower with significant rates of intravascular thrombus observed using ultrasound.
Keywords: continuous glucose monitoring, intravascular, hospital, intensive care
Hyperglycemia is common in critically ill patients, and is associated with increased mortality.1 Insulin therapy to maintain glycemic control is the recommended standard of care for both postcardiac surgery patients and more broadly in critical care.2,3 However, intensive glucose control has been shown to result in an increased incidence of severe hypoglycemia, which is independently associated with mortality.4,5 The currently available methods for intermittently monitoring blood glucose may contribute to the risk of hyper and hypoglycemia. Continuous glucose monitoring holds the promise of reducing these risks while allowing more intensive glucose control, which may improve clinical outcomes.6
The GluCath IV-CGM System was an investigational device designed to monitor blood glucose accurately, conveniently, and continuously in critically ill patients. The objective of the product development studies described in this report was to assess the safety and performance of the device in postcardiac surgery ICU patients, who are already managed with a glycemic control protocol. Composite data are presented from 70 patients across 4 inpatient clinical sites. Data have previously been reported from outpatient studies and 2 of the 4 inpatient sites.7-10
Methods
Study Design and Patient Selection
Patients were enrolled in a 2 cohorts. In the first cohort initiated September 2011, an arterial sensor was deployed for up to 24 hours at 3 sites: Thomas Jefferson University (TJU) in Philadelphia, PA, USA, Royal North Shore Hospital (RNSH) in Sydney, Australia, and Onze Lieve Vrouwe Gasthuis (OLVG) in Amsterdam, the Netherlands. In the second cohort initiated October 2012, an arterial sensor was deployed for up to 48 hours at 2 sites (RNSH, OLVG) and a venous sensor was deployed for up to 48 hours at 2 sites: RNSH and Saint Luke’s Mid America Heart Institute (MAHI) in Kansas City, MO, USA. System enhancements were introduced over the course of the study.
Study protocols were approved as nonsignificant risk investigations by each site’s Institutional Review Board or Ethics Committee. Common inclusion criteria were age ≥ 18 years; planned ICU admission after elective surgery; consent to periodic ultrasound examinations, blood sampling, and insertion of a GluCath sensor through a standard radial artery catheter or into a peripheral vein. Exclusion criteria were an expected ICU stay of < 24 hours; known pregnancy; or a known contraindication to heparin.
Study Device
The GluCath System used a quenched chemical fluorescence sensing mechanism to optically measure glucose in blood.11 Blue light was transmitted via an optical fiber to illuminate the sensing chemistry at the distal tip of the sensor. The sensing chemistry fluoresced green (530 nm) in proportion to the glucose level. This response was sensitive to both temperature (Figure 1) and pH (Figure 2). Local temperature was measured with a thermocouple deployed adjacent to the optical fiber. Differences in the fluorescent response at 2 excitation wavelengths (420 and 470 nm) were used to correct for the effect of pH on the glucose response.12 Sensors lots were characterized during the manufacturing process to determine parameters for a Michaelis-Menten calibration curve, individual sensor parameters were then prospectively calibrated in vivo using glucose and pH values obtained from a reference analyzer.13
Figure 1.
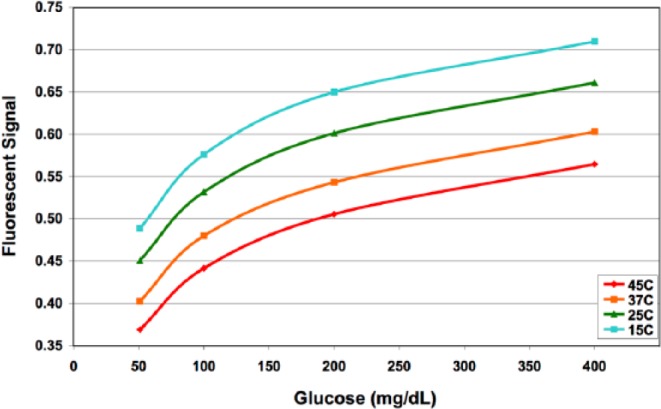
Calibration curve at varying temperatures.
Figure 2.
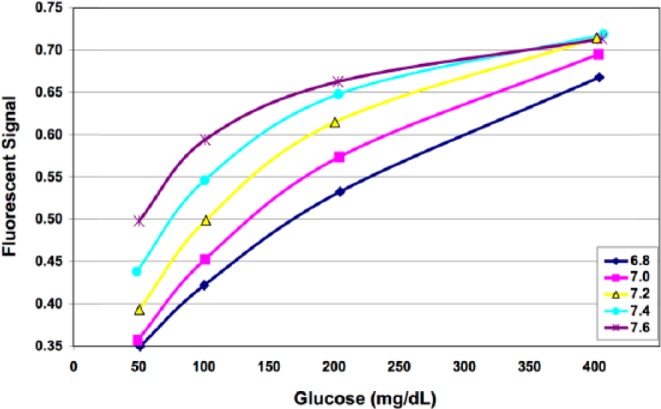
Calibration curve at varying pH.
The arterial sensor was deployed into the radial artery approximately 2 cm beyond the arterial catheter (Figure 3). The venous sensor was deployed directly in a vein extending up to 10 cm past the venous access site (Figure 4). It was inserted into a vein of the upper arm via ultrasound-guided over-the-needle direct access or modified Seldinger technique. The intended terminal position of the sensor tip was distal to the subclavian arch, approximately 2 cm below the axillary vein, similar to a midline catheter.
Figure 3.
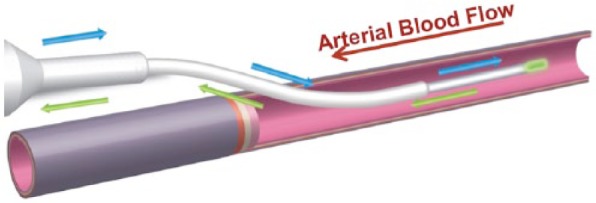
Schematic of the arterial GluCath sensor deployed via catheter.
Figure 4.
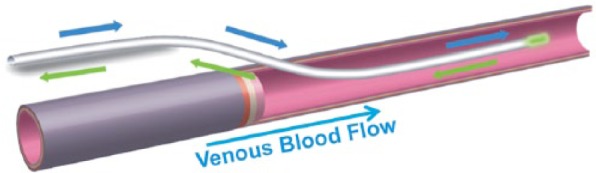
Schematic of the venous GluCath sensor deployed intravascularly.
The arterial sensor was housed in a sterile extension set that attached directly to the hub of an arterial access catheter (Figure 5), supporting pressure monitoring, blood sampling and saline flush administration. Heparin (1 U/mL) was added to the saline flush for all RNSH patients and the second half of TJU patients. The housing was modified for the 48-hour study cohort to remove a telescoping mechanism that resulted in repeat device malfunctions. The venous sensor had a suture wing for securement to the patient using a commercial device (Figure 6).
Figure 5.
Arterial GluCath sensor housing.
Figure 6.
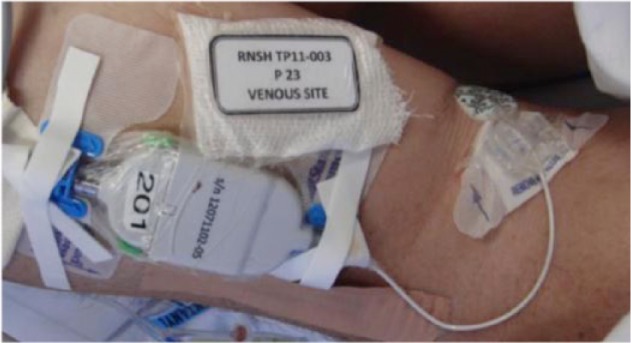
Venous GluCath sensor.
The GluCath monitor was portable and battery-operated, suitable for patient transport (Figure 7). Various software and hardware versions were used during the study. Most notably for the 48-hour study cohort, the measurement frequency was reduced from 60 to 10 seconds and the user interface for entering reference glucose and pH values to calibrate was simplified.
Figure 7.
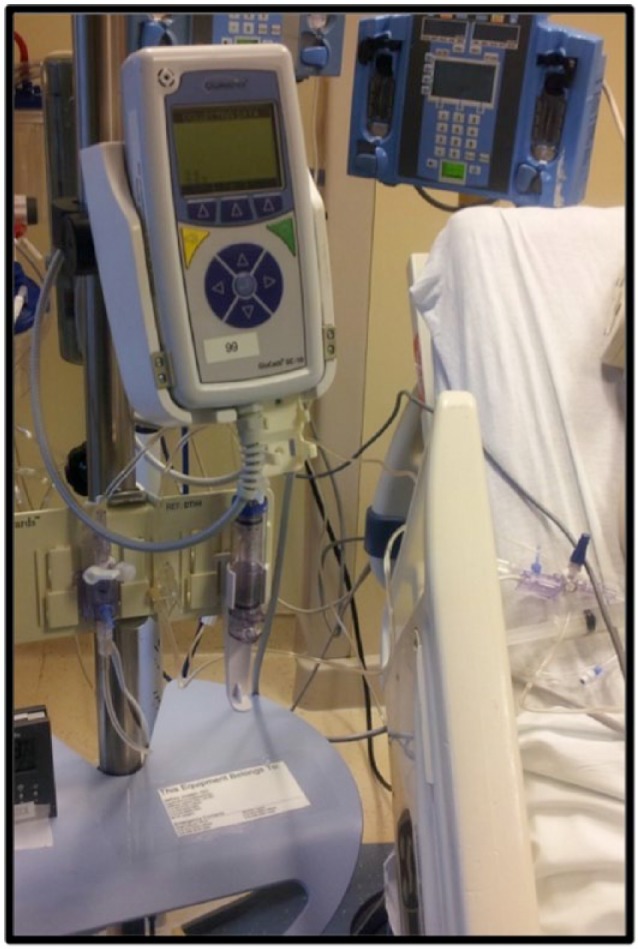
GluCath monitor.
Study Procedure
Patients were screened and consented prior to their scheduled surgery. Upon postsurgical ICU admission and stabilization, an ultrasound examination was performed and the sensor inserted using aseptic technique. A repeat ultrasound was performed after sensor insertion and again prior to removal (Figure 8). At approximately 60 and 120 minutes after sensor insertion, the system was calibrated in vivo to blood glucose and pH values measured using a reference analyzer. In 48-hour cases, sensors were recalibrated each morning of the glucose monitoring period.
Figure 8.
Ultrasound of arterial GluCath sensor.
Continuous surveillance of each patient was conducted while the GluCath sensor was indwelling, to identify factors contributing to device performance and ensure patient safety. Incidence of pressure monitoring waveform dampening or difficulty withdrawing blood samples was recorded. Patients were examined for potential adverse effects following removal of the sensor. A follow up examination was performed within 48 hours of removal. Participation in the study concluded after the follow-up examination or resolution of any adverse event.
Arterial (TJU, RNSH, OLVG) or central venous (MAHI) blood samples for performance analysis were drawn no more than once every 60 minutes, by a dedicated study nurse (TJU, RNSH, MAHI) or the bedside nurse (OLVG). The volume of blood withdrawn was minimized using a Venous Arterial Blood Management Protection (VAMP) system (Edwards Lifesciences, Irvine, CA, USA). Blood glucose and pH were measured using a point-of-care reference analyzer (Radiometer ABL at RNSH and OLVG, i-STAT 1 at MAHI and TJU). Patient blood glucose and insulin infusions were managed using the existing ICU protocol and standing orders for each patient (blood glucose target range 100-180 mg/dL or 6-10 mmol/L). The glucose values from the GluCath System were not displayed or used for patient care. Reference analyzer results were not blinded to clinical staff and were expected to influence patient care. The sensor was removed when the radial artery catheter was removed, prior to the patient’s discharge from the ICU, or after the intended 24- or 48-hour monitoring period.
Study End Points and Statistical Analysis
Performance of the system was assessed against the within 20% accuracy metric from the older ISO 15197:2003 outpatient standard (current at the time the protocols were written and widely reported in published CGM accuracy studies) and the within 12.5% metric from the current CLSI POCT 12-A3 guidance for inpatient discrete blood glucose monitoring devices. The 2003 ISO criteria specified that 95% of device results be within ± 15 mg/dL (0.83 mmol/L) for reference glucose values < 75 mg/dL (4.2 mmol/L) and within 20% for reference glucose values ≥ 75 mg/dL. CLSI POCT 12-A3 specifies that 95% of the device results be within ± 12 mg/dL (0.67 mmol/L) for reference glucose values < 100 mg/dL (5.55 mmol/L) and within 12.5% for reference glucose values ≥ 100 mg/dL. In addition, no more than 2% of device results may exceed ± 15 mg/dL (0.83 mmol/L) for reference glucose values < 75 mg/dL (4.2 mmol/L) or 20% for reference glucose values ≥ 75 mg/dL. Data were further analyzed by calculating the mean absolute relative difference (MARD) per patient, cohort and site. Data were visualized using a modified Bland–Altman plot comparing the difference between GluCath System and reference analyzer against the reference analyzer glucose.
Statistical analysis was performed by GluMetrics, Inc staff using MatLab (MathWorks, Natick, MA, USA) and Excel (Microsoft, Redmond, WA, USA). Prospectively calibrated glucose records were recorded by the monitor every 60 (first cohort) or 10 seconds (second cohort). GluCath System results over a 5-minute time interval immediately prior to the recorded blood sample draw time were averaged and paired with the reference analyzer glucose value. This procedure minimized artifacts introduced by the arterial blood sampling and saline flush process. Automation of this pairing and averaging process for consistency across study cohorts resulted in minor variance from previously reported single-site data.
Results
Baseline Characteristics
Seventy patients were enrolled from September 2011 thru April 2013 across the 4 sites. Except for 4 initial general surgery patients at the TJU site, all patients were postcardiac surgery (45%, CABG, 40% valve, 9% both). The mean age was 67 years, 73% were male, 93% were Caucasian, and 28% had diabetes. APACHE II scores at RNSH ranged from 7-20 with a mean of 12.4 and SD of 3.1. At OLVG, scores ranged from 8-26 with a mean of 17.5 and SD of 5.6. Severity assessments were not assessed for MAHI or TJU patients.
Procedural Success
In total, 63 arterial and 9 venous sensors were successfully deployed (Table 1). The majority of sensors were deployed at RNSH in sequential 20 patient cohorts of 24- then 48-hour durations. During the initial cohort at RNSH, an additional 7 sensors were deployed then removed and/or replaced due to device deficiencies, use errors or radial artery catheter failure upon insertion. One patient who did not receive a sensor after 2 unsuccessful deployment attempts was replaced with ethics committee consent. Changes to the study procedure and device design increased the procedural success rate of the subsequent arterial cohort at RNSH and other sites to 100%. A total of 14 patients consented to venous sensor placement, of which 5 (36%) did not receive a sensor due to inadequate or inaccessible vasculature observed via ultrasound during the sensor insertion procedure.
Table 1.
Sensor Deployments and Results by Study Site.
| Deployment | Site | Duration | Patients | Paired Points | MARD (%) | ISO15197: 2003 (%) | POCT 12-A3 (%) |
|---|---|---|---|---|---|---|---|
| Arterial | TJU | 24 hours | 10 | 84 | 11.4 | 83.3 | 69.0 |
| OLVG | 24 hours | 5 | 98 | 16.5 | 69.4 | 44.9 | |
| 48 hours | 8 | 207 | 11.9 | 86.5 | 63.8 | ||
| RNSH | 24 hours | 20 (1)a | 400 | 12.4 | 82.8 | 60.5 | |
| 48 hours | 20 | 758 | 6.4 | 97.0 | 85.5 | ||
| Arterial total | 63 | 1547 | 9.6 | 89.4 | 80.8 | ||
| Venous | MAHI | 48 hours | 7 (3)a | 161 | 15.1 | 67.7 | 50.9 |
| RNSH | 48 hours | 2 (2)a | 91 | 12.7 | 80.2 | 65.9 | |
| Venous total | 9 | 246 | 14.2 | 71.5 | 55.7 |
Indicates consented patients in whom a sensor was not deployed. Four RNSH patients consented to deployment of both an arterial and venous sensor, but only 2 venous sensors were successfully deployed.
Arterial Safety and Reliability
Initially, 3 of 4 sensors deployed in general surgery patients at TJU did not achieve the intended monitoring duration. The radial artery catheter failed within 4-8 hours for each patient, with intravascular thrombus and decreased blood flow observed via ultrasound. Attempts to restore the pressure monitoring waveform with bursts of flush solution resulted in local dilution with lowered sensor glucose and temperature readings.
In contrast, only 6 of 42 (14%) arterial catheters deployed in postcardiac surgery patients at RNSH failed (includes 2 catheters that failed prior to sensor insertion). At this site, the flush solution infused over the sensor was heparinized with 1 unit of unfractionated heparin per mL to help maintain catheter patency. After heparinized flush was introduced at the TJU site, no further catheter failures were observed in postcardiac surgery patients. However, at the OLVG site a heparinized flush was not used and only 2 of 13 (15%) catheters failed (1 due to failure to maintain the flush solution).
Overall in this study, 10 of 63 (15.8%) arterial catheters and GluCath sensors were removed prior to completion of the intended glucose-monitoring period due to clinically significant sampling or waveform difficulty. Ultrasound evidence of intraarterial thrombus was observed in a total of 13 patients (20.6%), of whom 5 were those from whom catheters were subsequently removed, 5 exhibited nonsignificant waveform dampening, and 3 exhibited no dampening or sampling difficulty.
Venous Safety and Reliability
Ultrasound evidence of thrombus was observed in 6 of 9 patients (66%) in whom venous sensors were deployed. Four of these recorded adverse device effects were not clinically significant, partial occlusions that did not require treatment. However, the remaining 2 resulted in total venous occlusion requiring treatment, and were reported as probable or possible device-related serious adverse events. One patient experienced total occlusion of the basilic vein with nonocclusive thrombus extending to the axillary vein. The second patient showed no evidence of thrombus during the period in which the sensor was indwelling, but later developed a systemic prothombotic condition (unrelated to the GluCath sensor or study procedure) after sensor removal and full occlusion of the cephalic vein was observed upon follow-up.
Device deficiencies were observed in the thermocouple of 5 sensors (55%), resulting in erratic temperature and glucose readings. One sensor also exhibited optical failure. As a result of these deficiencies, only 200 hours of usable glucose data were available from the intended 336 hours of monitoring time at the MAHI site (of which sensors were actually indwelling for 294 hours). Evaluation of the failed devices resulted in a change to sensor design and securement method.
Performance Data Set
Of 2113 reference blood samples collected, 1799 (85.1%) were paired with GluCath System readings for performance analysis. The first reference sample was used to calibrate the system, requiring 72 samples (3.4%). An additional 80 samples (3.8%) were excluded per protocol if they were collected for nonstudy reasons, within 60 minutes of the prior sample, showed evidence of dilution or were out of the sensor measurement range (>240 mg/dL or 13.3 mmol/L glucose, <7.2 or >7.5 pH). Device deficiencies resulted in 162 samples (7.7%) where a GluCath System reading was unavailable for performance analysis. A table of excluded points by objective criteria is available in the supplemental data (available at dst.sagepub.com/supplemental).
Device Accuracy
The aggregate MARD for arterial sensors was 9.6%. Individual patient MARDs ranged from 3.6% to 33.0%, with 26 patients ≤ 8%, 15 patients 8-12%, 14 patients 12-16%, and 7 patients > 16%. Performance in the final 48-hour arterial cohort at RNSH was improved, with 15 of 20 patients exhibiting an MARD ≤ 8% for an aggregate MARD of 6.4%. This site and cohort represented 49.0% of the total paired arterial data points collected. The aggregate MARD for venous sensors was 14.2% with individual patient MARDs ranging from 7.8% to 26.9%.
Overall, 89.4% (1383/1547) of arterial and 72.2% (182/252) of venous GluCath System measurements met the ISO 15197:2003 system accuracy criteria. 72.7% (1124/1547) of arterial and 56.3% (142/252) of venous measurements met the tighter POCT 12-A3 criteria. Only 2.1% (33/1547) of arterial and 14.6% (37/246) of venous reference samples were < 100 mg/dL. Only 1 reference glucose was < 75 mg/dL. Within the RNSH 48-hour arterial cohort 97.0% (735/758) of results met the ISO criteria and 85.5% (648/758) met the POCT criteria.
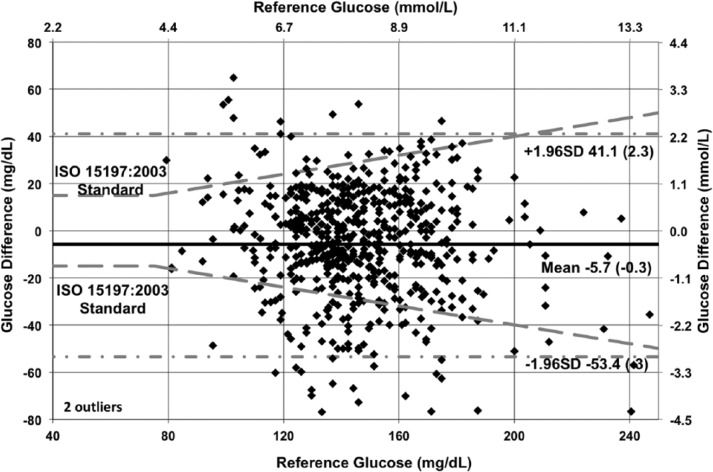
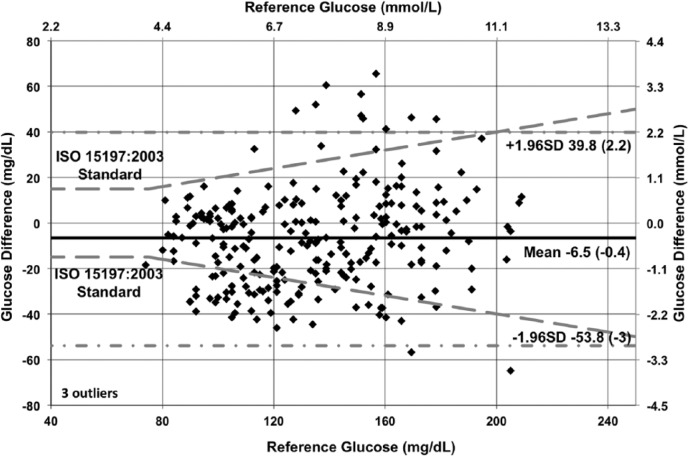
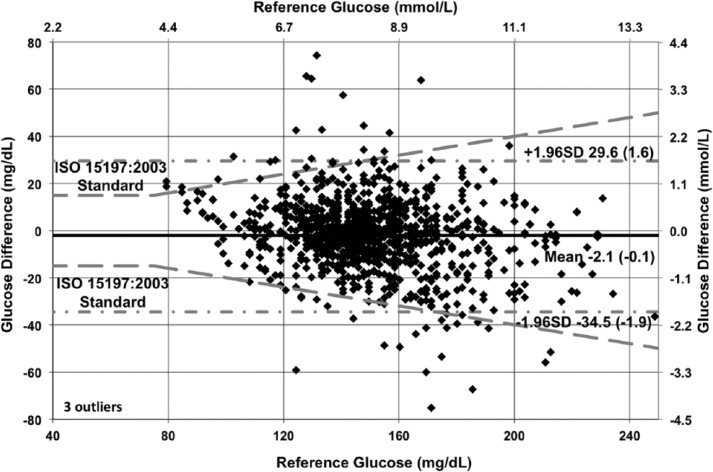
The modified Bland–Altman plots (with overlaid ISO 15197:2003 criteria) in Figures 9 to 11 show the glucose difference between GluCath System and reference analyzer versus the reference analyzer glucose for arterial and venous sensors by cohort. The mean bias ranged from −2.1 mg/dL (−0.1 mmol/L) for the 48-hour arterial cohort to −6.5 mg/dL (−0.4 mmol/L) for the venous cohort. This small negative offset appears data-specific, and a small positive bias has been observed in other experiments. No correlation was evident between the glucose difference and the reference glucose level. The limits of agreement (twice the standard deviation) were lower in the 48-hour arterial cohort relative to both the 24-hour arterial or venous cohort. This is attributable to both the greater number of data points and device enhancements incorporated in the later cohort.
Figure 9.
Bland–Altman plot of 24-hour arterial cohort (N = 35, RNSH, OLVG, TJU).
Figure 11.
Bland–Altman plot of 48-hour venous cohort (N = 9, RNSH, MAHI).
Figure 10.
Bland–Altman plot of 48-hour arterial cohort (N = 28, RNSH, OLVG).
Analysis of Outlier Results
The 164 arterial and 70 venous paired points with > 20% difference between GluCath System and reference analyzer glucose values were analyzed to determine probable cause of the difference. Recorded optical and temperature signals, patient activity, and medication logs were reviewed. The attributed causes and frequency of occurrence are shown in Table 2. As multiple causes were determined to contribute to total error, percentages add to more than 100%.
Table 2.
Outlier Analysis.
| Probable cause | Number | % |
|---|---|---|
| Device calibration | 90 | 25.2 |
| Optical signal variability | 94 | 26.3 |
| Temperature/pH changes | 50 | 14.0 |
| Patient activity | 39 | 10.9 |
| Physiological effect | 22 | 6.2 |
| Device malfunction/use error | 10 | 2.8 |
| Interfering medications | 9 | 2.5 |
| Suspect reference sample | 1 | 0.3 |
| No cause attributed | 42 | 11.8 |
The majority of outliers were attributed to initial GluCath System calibration and the stability of optical signals at the time the user marked the blood sample. Accessing the arterial line affected the sensor’s optical signals and the process of drawing warm, fresh blood over the sensor followed by cold saline flush resulted in a signature temperature and optical signal pattern. Overall optical signal variability was greater than anticipated, often correlating with routine patient care activities (eg, bathing, transitions from bed to chair, physical therapy). No signal stability checks were active in the device at the time of the study; however, these were planned for future clinical use. A temperature and pH correction algorithm was active during the study, but the physiological variability encountered in the patient population was broader than the original design parameters. In general, sensors responded slowly at peripheral temperatures under 30°C and exhibited a bias after multidegree temperature or multipoint pH changes. Physiological causes of outliers included local thrombus formation with reduced local blood flow and hypothesized saline accumulation. Acute glucose spikes were observed to correlate with bolus infusions of medications in 5% Dextrose, for cardiac output calculations or after infusions of 20% Dextrose. Finally, 3 medications were identified as being present during outlier periods that were capable of producing a clinically relevant (≥10%) interferent response: mannitol, citrate, and calcium glubionate. Specific examples of outlier results are discussed in the supplemental material.
Discussion
Novel medical devices must demonstrate that the probable benefits from use outweigh the risk of injury. Product development studies in the intended use population and environment provide valuable input on the device’s performance and extent to which known risks have been mitigated, while revealing potential new risk factors prior to pivotal studies or commercial use. Anticipated risks in this study included loss of arterial catheter function, thrombosis and inaccurate glucose readings that could result in insulin dosing errors had they been used clinically.
Arterial device point accuracy of 9.6% MARD with 89.4% meeting ISO 15197:2003 and 72.7% meeting POCT 12A-3 criteria was acceptable. A recent critical care consensus documents notes that the CLSI POCT 12A-3 standard for point-of-care glucometers may not be applicable to CGM systems and proposes that MARDs should be <14% and that values >18% represent poor accuracy.14 In this study, performance improved over time with modifications to both study procedures and device. MARDs between first and second arterial cohorts dropped from 11.9% to 6.4% at RNSH and from 16.5% to 11.9% at OLVG. No reason was definitively determined for the difference in performance between sites. Although the patient population at OLVG contained sicker patients as measured by APACHE II scores, this did not correlate with system performance. A learning effect was observed during lead-in studies and thus high sequential enrollment at the RNSH site (4 batches of 10 patients within 6 weeks each) combined with a small pool of study nurses (not responsible for clinical care) may have contributed to the lower MARDs.
Arterial catheter failure occurred in 15.8% of patients and ultrasound evidence of thrombus was observed in 20.6% of patients. A small number of outlier results (1.2% or 22/1799) were attributed to impaired blood flow, intravascular thrombus and/or saline contamination. Radial artery occlusion is a common and well-recognized complication of catheterization. Control data on arterial catheter-related thrombus were not collected, but may be necessary to assess the safety of this type of arterial sensor. One report of the related Paratrend 7 intrarterial blood gas monitoring system noted a 3.0% incidence (5/166) of waveform dampening and a 5.4% incidence (9/166) of thrombus.15 Another study including preoperative ultrasound examinations detected abnormalities of the radial artery in 27.1% of patients awaiting coronary artery surgery.16 A review article reported temporary arterial occlusion occurred following 831 of 4,217 radial artery catheterizations, an overall incidence of 19.7% with permanent occlusion occurred in 0.09% of catheterizations.17
Venous thrombus formation was observed via ultrasound in 66% of patients in whom a sensor was deployed, resulting in device-related thrombosis in 1 patient. Furthermore, 36% of consented patients were not enrolled due to inadequate or inaccessible vasculature. While accessing the deep veins of the upper arm may be routine for peripherally inserted central catheters (PICCs), this may not be practical for glucose sensor deployment. A clinical study evaluating the risks of deep venous thrombosis associated with PICCs found the following significant factors: catheter size, length of stay, prior thrombosis, duration of catheterization, bed rest, surgery, anticoagulant and vasopressor medications.18 In that study, deep vein thrombosis was observed in 0.6% (2 of 338) of cases that used a 4 Fr PICC device. Thrombosis in the basilic vein was reported in 10 cases of 1,490 PICC lines deployed in this vessel (all catheter sizes). It was hypothesized that deploying the small GluCath sensor (0.017” sensor diameter versus 0.053” for a typical 4 Fr PICC) in the larger basilic vein of the upper arm, with the tip well below the subclavian arch, would provide adequate venous blood flow for glucose sensing with minimal risk of thrombosis. In practice, thrombus formation often appeared to initiate at the vessel entry wound and propagate along the vessel wall, following the sensor.
This study was limited by the relatively small number of patients enrolled at 3 of 4 sites and variations in study protocol and procedure among sites. Postcardiac surgery patients represent a relatively healthy segment of the critical care population. Device accuracy in the hypoglycemic and severe hyperglycemia ranges could not be evaluated due to the lack of reference glucose values below 70 mg/dL (3.9 mmol/L) and the maximum 240 mg/dL (13.3 mmol/L) range of the study device. Furthermore, few reference blood samples were obtained outside the target control ranges of 100-180 mg/dL or 6-10 mmol/L, a limited range relative to outpatient CGMs. Changes introduced to the device and study procedure over the course of the studies contributed to product development process, but make aggregate results difficult to interpret clinically. Strengths of this study include the continuous surveillance and ultrasound data gathered to monitor safety and device performance, which made possible an in depth analysis of outlier results. Future studies should similarly assess the factors contributing to device reliability across differing patient populations and clinical sites with differing workflows and clinical care routines.
Conclusions
The GluCath System exhibited acceptable accuracy when deployed in a radial artery for up to 48 hours in ICU patients after elective cardiac surgery. There was no evidence that the presence of the arterial sensor increased the incidence of risk beyond that of standard arterial catheterization. Accuracy of venous deployment was substantially lower with significant rates of intravascular thrombus observed using ultrasound.
Acknowledgments
The sponsor, GluMetrics, Inc., acknowledged support by the Industry University Cooperative Research Program of the University of California.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; CLSI, Clinical Laboratory Standards Institute; ICU, intensive care unit; ISO, International Standards Organization; MAHI, Mid America Heart Institute; MARD, mean absolute relative difference; OLVG, Onze Lieve Vrouwe Gasthuis; POCT, point-of-care testing; RNSH, Royal North Shore Hospital; TJU, Thomas Jefferson University.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: At the time of the study, PS was a full-time employee of GluMetrics, Inc. SF, OF, BH, MK, LM, MS, JV, PV, and JJ received research funding from GluMetrics, Inc. to conduct the clinical study. They did not own stock in GluMetrics, and they did not receive any other compensation. GluMetrics, Inc. ceased operations in 2014.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by GluMetrics, Inc.
References
- 1. Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med. 2009;37(12):3001-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lazar HL, McDonnell M, Chipkin SR, et al. The Society of Thoracic Surgeons practice guideline series: blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87(2):663-669. [DOI] [PubMed] [Google Scholar]
- 3. Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40(12):3251-3276. [DOI] [PubMed] [Google Scholar]
- 4. Finfer S, Liu B, Chittock DR, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367(12):1108-1118. [DOI] [PubMed] [Google Scholar]
- 5. Hermanides J, Bosman RJ, Vriesendorp TM, et al. Hypoglycemia is associated with intensive care unit mortality. Crit Care Med. 2010;38(6):1430-1434. [DOI] [PubMed] [Google Scholar]
- 6. Joseph JI, Hipszer B, Mraovic B, Chervoneva I, Joseph M, Grunwald Z. Clinical need for continuous glucose monitoring in the hospital. J Diabetes Sci Technol. 2009;3(6):1309-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peyser T, Zisser H, Khan U, et al. Use of a novel fluorescent glucose sensor in volunteer subjects with type 1 diabetes mellitus. J Diabetes Sci Technol. 2011;5(3):687-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Romey M, Jovanovič L, Bevier W, Markova K, Strasma P, Zisser H. Use of an intravascular fluorescent continuous glucose sensor in subjects with type 1 diabetes mellitus. J Diabetes Sci Technol. 2012;6(6):1260-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flower OJ, Bird S, Macken L, et al. Continuous intra-arterial blood glucose monitoring using quenched fluorescence sensing: a product development study. Crit Care Med. 2014;16(1):54-61. [PubMed] [Google Scholar]
- 10. Sechterberger MK, van der Voort PHJ, Strasma PJ, DeVries JH. Accuracy of intra-arterial and subcutaneous continuous glucose monitoring in postoperative cardiac surgery patients in the ICU. J Diabetes Sci Technol. 2015; 9(3): 663-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gamsey S, Miller A, Olmstead MM, et al. Boronic acid-based bipyridinium salts as tunable receptors for monosaccharides and alpha-hydroxycarboxylates. J Am Chem Soc. 2007;129(5):1278-1286. [DOI] [PubMed] [Google Scholar]
- 12. Markle DR, Suri JT, Wessling RA, Romey MA. Optical determination of ph and glucose. US 7,751,863. July 6, 2010. [Google Scholar]
- 13. Romey MA, Gamsey S, Peyser TA. Measurement devices and methods for measuring analyte concentration incorporating temperature and pH correction. US 8,473,222. June 25, 2013. [Google Scholar]
- 14. Wernerman J, Desaive T, Finfer S, et al. Continuous glucose control in the ICU: report of a 2013 round table meeting. Crit Care. 2014;18(3):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gamo M, Hirose Y, Matsuo K. [Problems in clinical use of intraarterial blood gas monitoring system, Paratrend 7]. Masui. 2000;49(12):1387-1390. [PubMed] [Google Scholar]
- 16. Rodriguez E, Ormont ML, Lambert EH, et al. The role of preoperative radial artery ultrasound and digital plethysmography prior to coronary artery bypass grafting. Eur J Cardio-Thorac Surg. 2001;19(2):135-139. [DOI] [PubMed] [Google Scholar]
- 17. Scheer B, Perel A, Pfeiffer UJ. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit Care. 2002;6(3):199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Evans RS, Sharp JH, Linford LH, et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803-810. [DOI] [PubMed] [Google Scholar]