Abstract
Vertebrate tubulin is encoded by a multigene family that produces distinct gene products, or isotypes, of both the α- and β-tubulin subunits. The isotype sequences are conserved across species supporting the hypothesis that different isotypes subserve different functions. To date, however, most studies have demonstrated that tubulin isotypes are freely interchangeable and coassemble into all classes of microtubules. We now report that, in contrast to other isotypes, overexpression of a mouse class V β-tubulin cDNA in mammalian cells produces a strong, dose-dependent disruption of microtubule organization, increased microtubule fragmentation, and a concomitant reduction in cellular microtubule polymer levels. These changes also disrupt mitotic spindle assembly and block cell proliferation. Consistent with diminished microtubule assembly, there is an increased tolerance for the microtubule stabilizing drug, paclitaxel, which is able to reverse many of the effects of class V β-tubulin overexpression. Moreover, transfected cells selected in paclitaxel exhibit increased expression of class V β-tubulin, indicating that this isotype is responsible for the drug resistance. The results show that class V β-tubulin is functionally distinct from other tubulin isotypes and imparts unique properties on the microtubules into which it incorporates.
INTRODUCTION
Microtubules are essential filamentous structures in eukaryotic cells where they are responsible for the directed movement of vesicles, the organization of the endoplasmic reticulum and Golgi apparatus in the cytoplasm, and the equipartitioning of chromosomes before cell division. The organelles are assembled from heterodimers of α- and β-tubulin, which polymerize in a head-to-tail manner to form linear protofilaments, and these associate laterally into tubular structures that normally consist of 13 protofilaments. Vertebrate α- and β-tubulins are each encoded by a 6- to 7-member multigene family that produces highly homologous and conserved gene products that differ most radically in their last 10-15 amino acids (Sullivan, 1988; Luduena, 1998). These carboxyl-terminal sequences have been used to assign β-tubulin gene products to seven distinct classes (Lopata and Cleveland, 1987). Each of these classes (hereafter referred to as β1, β2, β3, β4a, β4b, β5, and β6) defines a β-tubulin isotype that differs significantly from other isotypes within the same organism, but differs very little from the same isotype in other vertebrate species.
With the discovery that tubulin proteins in an organism are heterogeneous, a hypothesis was formulated suggesting that different tubulin proteins might perform different functions in the cell (Fulton and Simpson, 1976). In the intervening years, however, most of the experimental and genetic evidence has argued that different β-tubulins coassemble freely into all cellular microtubules (see Joshi and Cleveland, 1990; Luduena, 1998 for reviews). In gene replacement experiments using the fungal organism, Aspergillus nidulans, for example, May demonstrated that a β-tubulin that is predominantly produced during conidiation can substitute for the major β-tubulin isoform that is used during hyphal growth even though the two proteins are only 83% homologous (May, 1989). In cultured mammalian cells, a variety of studies have demonstrated that all microtubules are copolymers of all the available β-tubulin isotypes produced in the cell (Lewis et al., 1987; Lopata and Cleveland, 1987; Sawada and Cabral, 1989). In contrast to these results, genetic studies in Drosophila have provided evidence that not all β-tubulins in the fly are functionally equivalent. Using quantitative gene expression experiments, Raff and coworkers demonstrated that any level of coexpression of a somatic β-tubulin with the testis-specific β-tubulin allowed all cytoplasmic microtubules to assemble and function normally. When the amount of somatic β-tubulin exceeded 20%, however, axoneme assembly was disrupted and the flies became sterile (Hoyle and Raff, 1990). Taken together, the results to date suggest that all β-tubulins can coassemble to form functional cytoplasmic microtubules, but that specific isotypes may be needed to form specialized microtubule-containing structures such as axonemes.
In vitro studies have more recently shown that microtubules composed of different β-tubulin isotypes may have differences in drug binding and microtubule dynamics (Banerjee and Luduena, 1992; Panda et al., 1994; Derry et al., 1997), but the changes measured were relatively small and the in vivo significance of the observations is uncertain. Over the last few years, additional publications have appeared reporting that increased expression of various β-tubulin isotypes was found in cells selected for resistance to paclitaxel, a microtubule stabilizing drug, and to estramustine, a microtubule inhibitory drug (reviewed in Burkhart et al., 2001). Although the results are tantalizing, they are only correlative in nature and do not demonstrate that the increased expression of β-tubulin was responsible for drug resistance. Moreover, contrary to logic, most of the β-tubulin isotypes were implicated in increased drug resistance, arguing against specific functions for the different isotypes.
Chinese hamster ovary (CHO) cells express β1-, β4b-, and β5-tubulins in a ratio of 70:25:5 (Sawada and Cabral, 1989; Ahmad et al., 1991). In previous studies with these cells, we demonstrated that overexpression of β1-, β2-, or β4b-tubulin had no discernible effect on microtubule assembly or paclitaxel resistance (Blade et al., 1999). Moreover, assembly into all classes of microtubules was observed for all of the transfected tubulins including the β2 isotype, even though it is not a normal constituent of CHO microtubules. The results suggested that all three transfected isotypes are largely interchangeable in CHO cells. Subsequent to those studies, however, we reported that very high overexpression of β3-tubulin, a neuronal and testis specific tubulin isotype, could inhibit proliferation, reduce microtubule assembly, and confer weak resistance to paclitaxel (Hari et al., 2003b). We now report that even modest overexpression of β5-tubulin, a minor isotype produced by most vertebrate tissues (Sullivan et al., 1986), has very dramatic effects on the microtubule cytoskeleton and cell proliferation.
MATERIALS AND METHODS
Construction of an Epitope-tagged β5-tubulin Gene
Mouse β5-tubulin cDNA (GenBank accession No. BC008225) cloned into a pMCV-SPORT6 vector was obtained from ATCC (Manassas, VA) and a fragment containing the complete β-tubulin coding sequence was excised using the restriction enzymes EcoRI and NotI. This fragment was gel purified and subcloned into the tetracycline-regulated expression vector pTOPneo (Gonzalez-Garay et al., 1999) to produce a new plasmid named pTOP/β5.
A 9-amino acid epitope sequence derived from hemagglutinin antigen (HA) was inserted at the carboxy terminus of the β5-tubulin coding sequence using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The mutagenic primers used were 5′GGATGAAGAAGAGATCAACGAATACCCCTACGACGTGCCCGATTACGCTTAACTGCTCTTTTCTTAGCCTTGAT3′ and its reverse complement. As the reaction required the insertion of 30 nucleotides not present in the template (underlined sequence), the (+) and (-) strand primers were designed to place these nonhybridizing nucleotides in the center with 22 nucleotides of hybridizing sequence on either side. The presence of such a large insertion led to a reduced affinity of the primers to the template compared with affinity between the two primers that are completely matched. To overcome this problem, pTOP/β5 was first amplified with one primer for 16 cycles before the second primer was added and amplification was continued for 16 additional cycles. The resulting plasmid pTOP/HAβ5 was sequenced to be sure the HA tag was correctly placed and that no other changes in the β5 coding sequence had occurred.
Transfection and Isolation of Stable Transfected Cell Lines
CHO tTA 6.6a cells expressing the tetracycline regulated transactivator (Gonzalez-Garay et al., 1999) were seeded into a 35-mm tissue culture dish containing a sterile coverslip and transfected with pTOP/HAβ5 using Lipofectamine reagent (Invitrogen, Carlsbad, CA) as described by the manufacturer. After transfection, the coverslip was removed into alpha modification of minimum essential medium (αMEM; Sigma-Aldrich Co., St. Louis, MO) for later immunofluorescence analysis of transient expression. The remaining cells in the dish were incubated overnight in αMEM containing 1 μg/ml tetracycline to repress expression of the HAβ5-tubulin, and the cells were then trypsinized and replated in 100-mm dishes containing αMEM, 1 μg/ml tetracycline (Sigma-Aldrich), and 2 mg/ml G418 (Invitrogen). After 7-8 days a few G418-resistant colonies were isolated and the remaining colonies were pooled and stored as a total G418-resistant population. Mouse NIH 3T3 fibroblasts and human HeLa carcinoma cells were transiently transfected using a similar procedure except that a cotransfection was performed using pTOP/HAβ5 and pTOP/tTA, the latter plasmid containing cDNA for the tetracycline regulated transactivator.
Immunofluorescence
Cells were grown on sterile glass coverslips for 48-72 h. The coverslips were removed from media and washed in PBS, and soluble proteins were extracted by incubating in MTB buffer (20 mM Tris-HCl, pH 6.8, 1 mM MgCl2, 2 mM EGTA, 0.5% Nonidet P-40) containing 4 μg/ml (4.7 μM) paclitaxel (Sigma-Aldrich) for 2 min at 4°C. After fixing in methanol at -20°C for 15 min, the fixed cells were rehydrated in PBS for 10-15 min and then incubated in PBS containing a 1:50 dilution of mouse mAb DM1A (Sigma-Aldrich) directed against α-tubulin and 1:50 dilution of affinity-purified rabbit polyclonal HA antibody (Bethyl Laboratories, Montgomery, TX) for 1 h at 37°C in a humid chamber. The coverslips were then washed in PBS and incubated for an additional hour in 1:50 dilution of Alexa 488-conjugated goat anti-rabbit IgG, 1:50 dilution of Alexa 594-conjugated goat anti-mouse IgG (Molecular Probes, Eugene, OR) and 1 μg/ml DAPI. After washing in PBS, the coverslips were inverted onto 3 μl of Gel/Mount (BioMeda Corp., Foster City, CA) and were viewed by epifluorescence using an Optiphot microscope (Nikon, Inc., Melville, NY) equipped with a Plan Apochromat 60×, 1.4 n.a. oil objective (Nikon, Inc.) and filters to minimize overlap between fluorescence emission from the fluorescent tags. Images were acquired with a MagnaFire color digital camera (Optronics, Goleta, CA) attached to a MacIntosh G4 computer (Apple Computer, Cupertino, CA).
Measurement of Drug Resistance
A cloning efficiency assay was performed to measure the sensitivities of different cell lines to various drugs. Approximately 200 cells from each culture were seeded into 6 replicate wells of a 24-well dish containing increasing concentrations of paclitaxel in αMEM with or without tetracycline. After 7 days the medium was removed, and cells were stained with a solution of 0.05% methylene blue in water as described previously (Cabral et al., 1980). The plates were rinsed gently with water to remove excess stain, dried at room temperature, and photographed using a Coolpix 990 digital camera (Nikon Inc.).
Electrophoretic Techniques
Nontransfected CHO tTA cells or cells expressing HA-tagged beta tubulin proteins were grown in 24-well dishes and lysed in 1% SDS. Proteins were precipitated with 5 vol of acetone, resuspended in SDS sample buffer (0.0625 M Tris-HCl, pH 6.8, 2.5% SDS, 5% 2-mercaptoethanol, 10% glycerol), fractionated on a 7.5% polyacrylamide SDS minigel, and transferred to PROTRAN nitrocellulose membranes (Schleicher & Schuell, Keene, NH). The membranes were blocked by incubating in PBST (PBS with 0.05% Tween 20) containing 3% dry milk. After washing in PBST three times, the membranes were incubated in 1:2000 dilution of a mouse mAb specific for the N-terminal amino acids of all β-tubulin isoforms (Theodorakis and Cleveland, 1992) or mouse mAb DM1A. A 1:2000 dilution of actin-specific mouse mAb C4 (Chemicon International Inc., Temecula, CA) was also added to act as an internal control for sample loading. Incubation was carried out for 1 h at room temperature and the membranes were again washed three times in PBST. The membranes were then incubated 1 h in a 1:2000 dilution of goat anti-mouse IgG coupled to Cy5 (Chemicon). Reacting protein bands were detected by capturing fluorescence emission on a STORM 860 imager (Molecular Dynamics Inc., Sunnyvale, CA).
Measurement of Polymerized and Unpolymerized Tubulin
Cells were grown in 24-well dishes for 2 days in αMEM to allow the expression of plasmid-encoded genes and then lysed in 100 μl MTB containing 0.14 M NaCl and 4 μg/ml (4.7 μM) paclitaxel to keep polymerized microtubules intact (Minotti et al., 1991). After lysis, cell remnants were scraped from the wells and transferred to 1.5-ml microcentrifuge tubes; any residual soluble material remaining in the wells was removed by washing with 100 μl of the same lysis buffer. To solubilize any potential insoluble residue not removed during the wash, 100 μl of 1% SDS was added to the wells for later addition to the cytoskeletal fraction. The lysates were briefly vortexed and centrifuged at 12,000 × g for 15 min at 4°C. The supernatants containing the unpolymerized tubulin were transferred to fresh tubes. The pellets containing the polymerized tubulin were resuspended in 50 μl water and combined with the residues solubilized in SDS from the corresponding wells. A precise amount (4 μl) of bacterial lysate containing a glutathione-S-transferase (GST)/α-tubulin fusion protein was added to each supernatant and pellet to control for potential losses in later steps of the procedure. Proteins were precipitated using 5 vol acetone and resuspended in 30 μl of SDS sample buffer. Equal volumes from each sample were fractionated on a 7.5% polyacrylamide SDS minigel (Bio-Rad, Hercules, CA) and transferred to nitrocellulose membranes. Blots were incubated with mouse monoclonal antibodies to α-tubulin (DM1A, Sigma-Aldrich) or to the N-terminus of β-tubulin, followed by a goat anti-mouse secondary antibody coupled to Cy5 (Chemicon) as described above. Bands corresponding to α-tubulin and GST-α-tubulin in each fraction were quantified using NIH Image analysis software. The percent of total tubulin polymerized into microtubules was calculated by normalizing tubulin in the supernatant and pellet fractions to the amount of GST-α-tubulin, dividing the normalized value from the pellet by the sum of the values from supernatant and pellet and multiplying the fraction by 100.
RESULTS
Overexpression of β5-tubulin Disrupts Microtubule Organization
To assess the effects of β5-tubulin overexpression, a full-length mouse β5-tubulin cDNA was modified to encode a 9-amino acid hemagglutinin antigen (HA) tag at the carboxy terminus of the protein so that it could be distinguished from endogenous cellular tubulin and was cloned into a pTOP vector that allows tetracycline regulated expression in mammalian cells (Gonzalez-Garay et al., 1999). Transfection was carried out in tTApur 6.6a, a CHO cell line that stably expresses the tetracycline-regulated transactivator (Gonzalez-Garay et al., 1999). At 3 days posttransfection the cells were triple stained for immunofluorescence with an antibody specific for the HA tag, an antibody specific for endogenous α-tubulin, and a dye that binds DNA. We have observed that using a high titer of HA antibody inhibits the binding of α-tubulin antibodies to the microtubules when incorporation of HAβ5-tubulin is high. The greatly reduced α-tubulin staining allows cells with high HAβ5-tubulin production to appear green in the merged image and cells with lower HAβ5-tubulin production (and correspondingly higher α-tubulin staining) to appear yellow (mixture of green and red fluorescence). Cells that produce little or no HAβ5-tubulin appear red.
Using these antibody conditions, nontransfected CHO cells (red) exhibited dense microtubule networks (Figure 1A). In contrast, cells with low-to-moderate HAβ5-tubulin expression (yellow) had somewhat less dense microtubule networks and cells with high HAβ5-tubulin expression (green) had very few microtubules. The reasons for these cell-to-cell differences in expression are uncertain but probably relate to variability in transcriptional activity associated with the random sites at which the plasmid integrates into genomic DNA as well as the number of copies of plasmid that are integrated. In addition to having fewer microtubules, cells with high HAβ5-tubulin production also exhibited extensive fragmentation of the microtubule cytoskeleton (arrows). Similar microtubule disruption was seen with β5-tubulin lacking the HA tag, but because CHO cells already have low endogenous levels of β5-tubulin, it was more difficult to distinguish transfected from nontransfected cells (our unpublished results). To be certain that the microtubule disrupting effects of HAβ5-tubulin overexpression were not restricted to CHO cells, additional transfections were carried out in mouse NIH 3T3 fibroblasts (Figure 1B) and in human HeLa carcinoma cells (Figure 1C) with similar results.
Figure 1.
HAβ5-tubulin overexpression disrupts microtubules. CHO (A), NIH 3T3 (B), and HeLa (C) cells were transfected with pTOP/HAβ5 plasmid DNA and grown 3 days in αMEM. The cells were then processed for immunofluorescence using antibodies specific for the HA-tag (green fluorescence) and α-tubulin (red fluorescence). The specimens were also stained for DNA (blue fluorescence). Nontransfected cells are red (α-tubulin staining only), but cells that express HAβ5-tubulin are yellow (low-to-moderate HAβ5-tubulin content) or green (high HAβ5-tubulin content). Note that high levels of HAβ5 expression frequently cause extensive fragmentation of microtubules (arrows). Bar (A), 20 μm.
Clones That Stably Produce HAβ5-tubulin Exhibit Varying Degrees of Microtubule Disruption
The immunofluorescence data suggested that the extent of microtubule disruption was related to levels of HAβ5-tubulin expression. To study this relationship in a more quantitative manner, stably transfected CHO cells were selected using G418 in the presence of tetracycline to inhibit expression of the transgene and thereby limit potential toxicity. Western blot analysis was then used to screen random G418-resistant clones for their level of HAβ5-tubulin production 24 h after the removal of tetracycline. Two clones were chosen for further study: one, HAβ5c12, in which HAβ5-tubulin represented ∼15% of total β-tubulin (Figure 2, lane 5), and a second, HAβ5c8, in which the transgene product was ∼50% of total β-tubulin (Figure 2, lane 3). A clone expressing HAβ1-tubulin (55% of total β-tubulin) is also shown and was used as a negative control for some later experiments (Figure 2, lane 1). A fourth clone, HAβ1(L215H), expressing HAβ1-tubulin (40% of total β-tubulin) with a previously described paclitaxel-resistance mutation (Gonzalez-Garay et al., 1999) was used as a positive control for a tubulin protein known to reduce microtubule assembly (Figure 2, lane 7). Note that in all four cell lines, the presence of tetracycline almost completely repressed production of the transfected tubulin (Figure 2, lanes 2, 4, 6, and 8).
Figure 2.
Western blot analysis of HAβ5-tubulin expression in stably transfected cell lines. Clonal CHO cell lines with stable expression of HAβ1-tubulin (lanes 1 and 2), HAβ5-tubulin (clone HAβ5c8, lanes 3 and 4; clone HAβ5c12, lanes 5 and 6), and mutant HAβ1(L215H)-tubulin (lanes 7 and 8) were selected in G418 and analyzed for tubulin production using a mAb that recognizes the amino terminal region of both transfected (HAβ) and endogenous (β) β-tubulin. An antibody recognizing actin (A) was also included to act as a control for loading. The cells were maintained in 1 μg/ml tetracycline and then grown 24 h in the presence (+) or absence (-) of the antibiotic before analysis. Note that there is little or no expression of the HA-tagged proteins in presence of tetracycline.
Microtubule organization in each of the four cell lines was examined by immunofluorescence microscopy 3 days after removing tetracycline (Figure 3). As we previously reported (Gonzalez-Garay and Cabral, 1995; Blade et al., 1999), high expression of HAβ1-tubulin had no obvious effect on the number of microtubules or their organization in the cell (Figure 3A). Similarly, HAβ5c12, which has lower expression of HAβ5-tubulin, also exhibited no major changes in microtubule density or organization (Figure 3B). In contrast, HAβ5c8, which has a level of HAβ5-tubulin production similar to the production of HAβ1-tubulin in control cells, exhibited extensive disruption of the microtubule cytoskeleton (Figure 3C). Most cells had only a few, mostly long microtubules, and broken and fragmented microtubules were commonplace (arrows). In addition to these changes in the cytoplasmic microtubules, cells producing high amounts of HAβ5-tubulin also had defects in mitotic spindle assembly. In place of normal bipolar spindles with well-organized chromosomes (e.g., inset, Figure 3A), multipolar spindles with few spindle fibers and disorganized groups of chromosomes were common (e.g., inset, Figure 3C). Similar defects in CHO cell spindle assembly have been previously reported to inhibit chromosome segregation and subsequent cytokinesis leading to the formation of large polyploid cells with abnormal nuclear morphologies such as those seen here (Abraham et al., 1983; Kung et al., 1990; Cabral and Barlow, 1991). To rule out the possibility that these effects might be due to some other abnormality in clone HAβ5c8, several other clones with similar levels of expression were examined and all showed a similar phenotype (our unpublished results). Transfection with mutant HAβ1-tubulin encoding an L215H substitution led to a more modest reduction in microtubule density and did not produce the extensive fragmentation seen with clone 8 (Figure 3D). Thus, β5-tubulin disrupts microtubules more potently than a known microtubule disrupting mutation in the β1-tubulin isotype. The microtubule fragments seen in HAβ5c8 are also occasionally seen in wild-type cells but their frequency is at least an order of magnitude higher in HAβ5-tubulin transfected cells. It should be noted that these microtubule fragments have also been observed in cells directly fixed with methanol and in living cells (our unpublished results), ruling out the possibility that fragmentation might have occurred during the preextraction procedure used to obtain the immunofluorescence images shown in Figures 1 and 3.
Figure 3.
Microtubule organization in stably transfected cells. Cell lines HAβ1 (A), HAβ5c12 (B), HAβ5c8 (C), and HAβ1(L215H) (D) were grown 3 days in the absence of tetracycline to induce expression of the transfected cDNA. The cells were extracted in a microtubule stabilizing buffer and stained with an antibody specific for transfected HA-tagged β-tubulin (green fluorescence) and with a dye for nuclear DNA. Although the DNA stained blue in immunofluorescence as in Figure 1, here we pseudocolored it red to improve the contrast of the figure. Note the sparse microtubule network and fragmented microtubules (arrows) in the cell line (C) with high HAβ5-tubulin expression. Insets in A and C show mitotic cells from the same cultures. Bar (A), 20 μm.
HAβ5-tubulin Incorporates Efficiently into Microtubules to Reduce Microtubule Assembly
Reduced microtubule density in HAβ5c8 was clear and dramatic but any effect of HAβ5-tubulin expression on microtubule assembly in HAβ5c12 was not obvious. Moreover, immunofluorescence observation could not reveal whether there was any preferential incorporation or exclusion of HAβ5-tubulin into or from the microtubule network. To address these questions, we turned to a modified biochemical assay we previously described that can measure small differences in cellular microtubule assembly between wild-type and mutant cell lines (Minotti et al., 1991; Hari et al., 2003a). The procedure involves lysing cells in a microtubule stabilizing buffer followed by centrifugation to separate polymerized from nonpolymerized tubulin and then quantifying tubulin in each fraction using immunoblots. Figure 4 shows a Western blot of pellet (cytoskeletal) and supernatant (soluble) fractions from each of the transfected cell lines shown in Figures 2 and 3. The ratio of transfected to endogenous tubulin in pellet (lanes 1, 3, 5, and 7) compared with supernatant fractions (lanes 2, 4, 6, and 8) was nearly identical for each of the samples, indicating that there was no preferential inclusion or exclusion of transfected tubulin from the microtubule cytoskeleton. This was true even for HAβ5c8 (lanes 3 and 4) and HAβ1(L215F) (lanes 7 and 8), which have a greatly reduced amount of tubulin in the assembled (pellet) fraction. We conclude that HAβ5-tubulin is as competent to assemble as endogenous β-tubulins, but that its incorporation destabilizes microtubules and leads to a lower fraction of total tubulin in the microtubule cytoskeleton.
Figure 4.
HAβ5-tubulin incorporates efficiently into microtubules. Cell lines HAβ1 (lanes 1 and 2), HAβ5c8 (lanes 3 and 4), HAβ5c12 (lanes 5 and 6), and HAβ1(L215H) (lanes 7 and 8) were grown 48 h in αMEM, lysed in a microtubule stabilizing buffer, and centrifuged. Pellet fractions (lanes 1, 3, 5, and 7) containing polymerized tubulin, and supernatant fractions (lanes 2, 4, 6, and 8) containing soluble tubulin were run on SDS-polyacrylamide gels, electroblotted onto nitrocellulose membranes, and probed with an antibody recognizing the amino terminus of both endogenous (β) and transfected (HAβ) β-tubulins. The ratio of HAβ/β is shown for each of the fractions. Note that the amount of polymerized tubulin is very low for HAβ5c8 (lane 3), yet the ratio of exogenous to endogenous tubulin reflects the abundance of the transfected tubulin in the cells.
Because α-tubulin was not overexpressed along with HAβ-tubulin in the transfected cell lines, we considered the possibility that some overexpressed β-tubulin might exist as monomers. Although our prior experience indicated that monomeric β-tubulin is rapidly degraded in CHO cells (Gonzalez-Garay and Cabral, 1995), it was possible that some monomeric tubulin could escape degradation, fractionate solely to the supernatant, and thereby skew measurements of the extent of tubulin assembly. We therefore measured tubulin assembly using an antibody to α-tubulin that was limiting and should only exist as heterodimers with β-tubulin. The results are summarized in Figure 5. Cells transfected with HAβ1-tubulin had ∼38% of their total tubulin in the polymerized fraction, a value that is not significantly different from nontransfected wild-type cells (40%), as shown here and in previous studies (Minotti et al., 1991; Gonzalez-Garay and Cabral, 1995; Hari et al., 2003a, 2003b). Cells transfected with mutant HAβ1(L215H)-tubulin, on the other hand, exhibited a significant reduction in polymerized tubulin (to 17% of total tubulin). As expected from immunofluorescence observation, HAβ5c8 had a drastic reduction in polymerized tubulin (to 12% of total tubulin), whereas HAβ5c12 had a much more modest reduction (to 33% of total tubulin). Thus, expression of HAβ5-tubulin produced a dose-dependent decrease in cellular microtubule assembly analogous to that seen with a mutant β1-tubulin originally identified in a paclitaxel-resistant CHO cell line.
Figure 5.
Overexpression of HAβ5-tubulin reduces tubulin assembly. The indicated cell lines were grown 48 h in absence of tetracycline, lysed in a microtubule stabilizing buffer, and centrifuged to separate polymerized tubulin from soluble heterodimers. A constant volume of a bacterial cell lysate containing GST-α-tubulin was added to each fraction to correct for any possible loss of protein in subsequent steps; then proteins were separated on SDS polyacrylamide gels, transferred to nitrocellulose membranes, and probed with an antibody to α-tubulin. Fluorescence emission from a Cy5-tagged secondary antibody was used to measure the amount of α-tubulin in each fraction. Results are expressed as the ratio of α-tubulin in the pellet divided by the total α-tubulin (pellet and supernatant) times 100%. Values in all pellet and supernatant fractions were normalized to the GST-α-tubulin in those fractions before the calculation of polymerized tubulin was made. Standard deviations were based on at least three independent experiments.
Overexpression of HAβ5-tubulin Confers Paclitaxel Resistance and Dependence
With the exception of microtubule fragmentation and the extent of disruption, the effects of HAβ5-tubulin expression on microtubule assembly mimic the phenotypes of paclitaxel-resistant cells. To determine whether HAβ5-tubulin expressing cells are, in fact, paclitaxel resistant, we examined the ability of each of the transfected cell lines to grow in varying concentrations of the drug when the transgene was expressed (minus tetracycline) and when it was not expressed (plus tetracycline). Our negative control (Figure 6A) exhibited the same sensitivity to paclitaxel regardless of whether HAβ1-tubulin was expressed or not expressed, whereas the positive control (Figure 6B) was clearly more resistant to paclitaxel when the mutant HAβ1(L215H)-tubulin was expressed. Of the two clones transfected with HAβ5-tubulin, both were more resistant to paclitaxel when tetracycline was omitted to allow transgene expression (Figure 6, C and D). Moreover, HAβ5c8, the cell line with higher expression of HAβ5-tubulin (Figure 6D), was more resistant than cell line HAβ5c12 (Figure 6C), which has lower expression. It was also observed that the higher expression of HAβ5-tubulin in HAβ5c8 made the cells paclitaxel dependent; i.e., the cells grew much better in 100 ng/ml than in 0 or 50 ng/ml paclitaxel (Figure 6D, minus tetracycline). A similar phenotype has been described in cells selected for resistance to paclitaxel that have mutations in their β1-tubulin genes (Cabral, 1983; Schibler and Cabral, 1986; Gonzalez-Garay et al., 1999; He et al., 2001).
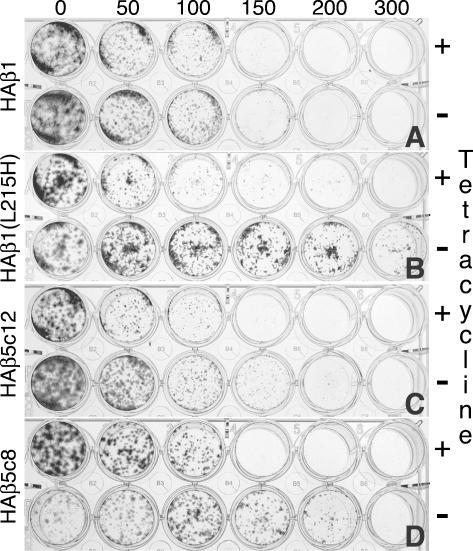
Figure 6.
Overexpression of HAβ5 confers paclitaxel resistance. Approximately 200 cells were plated in replicate wells of 24-well dishes containing the indicated concentrations of paclitaxel (in ng/ml) and grown 7 days in the presence (+) or absence (-) of 1 μg/ml tetracycline. The surviving colonies were then stained with methylene blue. All cell lines have similar sensitivity to paclitaxel when grown in presence of tetracycline, but in the absence of tetracycline only cells expressing the mutant HAβ1-tubulin or the HAβ5-tubulin show increased resistance. The cell lines are indicated at the left of each panel. Note that the molecular weight of paclitaxel is 854. Therefore, ng/ml can be converted to nM by simply multiplying the concentrations in the figure by 1.17.
The poor growth of HAβ5c8 in the absence of tetracycline is likely to result from the large reduction in cellular microtubule assembly produced by HAβ5-tubulin overproduction, and paclitaxel could be restoring growth by promoting microtubule assembly. Consistent with this interpretation, microtubule organization and density in HAβ5c8 cells grown in tetracycline appeared normal and the cells had single largely symmetrical nuclei (Figure 7A). After 3 days in the absence of tetracycline, however, during which time HAβ5-tubulin accumulated, few microtubules remained and cell and nuclear morphology changed (Figure 7B). The cells became large and flat, and they exhibited larger, misshapen nuclei that frequently had micronuclei of varying sizes. Similar morphological changes are commonly seen in paclitaxel-dependent CHO cells that have mutations in their β1-tubulin genes (Cabral et al., 1983; Schibler and Cabral, 1986). Those cells also have large reductions in microtubule assembly that lead to defective spindle formation, a delay in mitosis, scattered chromosomes, and reentry into the G1 phase of the cell cycle without prior cytokinesis (Cabral and Barlow, 1991). Repeated cell cycle progression in the absence of cell division then leads to the observed cell and nuclear morphology. Growing HAβ5c8 in the absence of tetracycline, but in the presence of 100 ng/ml (117 nM) paclitaxel, prevented these changes; i.e., it resulted in cells with normal nuclear morphology and an abundance of microtubules that failed to show evidence of the bundling normally associated with paclitaxel treatment (Figure 7D). Addition of paclitaxel to cells grown in tetracycline to inhibit HAβ5-tubulin expression, on the other hand, did produce extensive bundling of the microtubules (Figure 7C). In these cells the drug was able to interfere with spindle function leading to the mis-segregation of chromosomes and inhibition of cytokinesis, thus producing large multinucleated cells as described before in CHO cells (Kung et al., 1990). It should be noted that all microtubules in cells grown in absence of tetracycline were stained by the HA antibody, thus ruling out the possibility of differential incorporation of tubulin isotypes into microtubules in presence or absence of paclitaxel (our unpublished results). We conclude that the addition of a drug like paclitaxel, that stabilizes microtubules and promotes microtubule assembly, can counteract the microtubule destabilizing effects of HAβ5-tubulin expression.
Figure 7.
Microtubule assembly in cells overexpressing HAβ5-tubulin is restored by paclitaxel. Cell line HAβ5c8 was grown 3 days in αMEM with or without tetracycline and paclitaxel and processed for triple-label immunofluorescence with antibodies to the HA tag, antibodies to α-tubulin, and a DNA stain. (A) 1 μg/ml tetracycline; (B) no tetracycline; (C) 1 μg/ml tetracycline plus 100 ng/ml (117 nM) paclitaxel; (D) 100 ng/ml paclitaxel but no tetracycline. Note that images acquired with the α-tubulin antibody are shown but that identical images were acquired with the HA antibody except for cells in A and C, which had no HAβ5-tubulin production or staining. Insets show nuclear morphologies of the cells indicated by arrows. Bar (A), 20 μm.
Paclitaxel Selects for Cells With Increased Expression of HAβ5-tubulin
To provide additional evidence that expression of HAβ5-tubulin is responsible for the paclitaxel resistance observed in cell lines HAβ5c8 and HAβ5c12, we reasoned that paclitaxel should efficiently select for cells that express HAβ5-tubulin. The results of such an experiment are shown in Figure 8. Cells transfected with HAβ5-tubulin and selected in G418 exhibited a low level of HAβ5-tubulin expression (Figure 8, lane 1). Because of differences in sites of integration and number of copies integrated among the transfected cells, this G418-resistant cell population consisted of a heterogeneous mixture of cells with variable levels of HAβ5 expression, including cells with little or no expression. When the G418-resistant cell population was reselected in 100 ng/ml (117 nM) paclitaxel, the surviving cells exhibited a much higher level of HAβ5-tubulin expression (Figure 8, lane 2), and all the cells were positive for HAβ5-tubulin expression (our unpublished results). The results indicate that paclitaxel only allowed survival of cells that expressed higher levels of HAβ5-tubulin than the starting population and argue strongly that the expression of the transgene was responsible for the paclitaxel resistance of the cells.
Figure 8.
Expression of HAβ5-tubulin in paclitaxel selected cells. CHO tTApur 6.6a cells were transfected with pTOP/HAβ5 and selected in G418 in presence of tetracycline to obtain stably transfected cells. A portion of the total G418-resistant population (lane 1) was then reselected in αMEM without tetracycline but containing 100 ng/ml (117 nM) paclitaxel for 10 days (lane 2). Cells surviving the paclitaxel selection were compared with the G418-resistant cells by growing both cell populations 24 h in αMEM, separating the proteins on SDS gels, and staining Western blots with an antibody that recognizes the amino terminal region of both transfected (HAβ5) and endogenous (β) β-tubulin. The ratio of transfected to endogenous β-tubulin (HAβ5/β) for both cell populations is shown.
DISCUSSION
Although β-tubulin is a highly conserved protein, significant differences exist in the primary structure even among different gene products within the same species. Most of the differences are found in the carboxyl terminal 15 amino acids, and these differences are well conserved across vertebrate species thus forming the basis for classification of β-tubulin into distinct classes or isotypes (Lopata and Cleveland, 1987). Using specific antibody and molecular probes, a number of laboratories have shown that some isotypes (β1, β2, and β4) are abundantly expressed in a variety of tissues, whereas other isotypes exhibit tissue restricted expression (e.g., β3 in neurons and Sertoli cells; β6 in platelets and avian erythrocytes) or low but ubiquitous expression (e.g., β5; see Sullivan, 1988; Luduena, 1998 for review).
Even though most attention has focused on the highly variable carboxyl terminal amino acids, internal differences also exist among the different isotypes. An alignment of mouse β-tubulin sequences, for example, reveals numerous amino acid mismatches distributed throughout the primary sequence, most of them associated with the highly variable β6-tubulin. Omitting β6-tubulin from the analysis gives a more modest number of scattered amino acid differences, and, as previously pointed out by Sullivan (1988), suggests that β-tubulin falls into two distinct evolutionary branches consisting of β1, β2, and β4 on the one hand, and β3 and β5 on the other. Consistent with this classification, when the highly variable carboxyl terminal sequences are omitted from the analysis, the members of the first group differ from the consensus β-tubulin sequence at only 2-3 positions, but β3 and β5 differ from the consensus at 13 and 16 positions, respectively. Moreover, β3 and β5 share 9 positions at which they differ from the consensus and have the same amino acid in 8 of those 9 positions.
The observation that tubulin isotypes differ in both sequence and tissue distribution supports the hypothesis that different tubulin proteins subserve different functions (Fulton and Simpson, 1976). Because β3- and β6-tubulins belong to the more divergent group of vertebrate β-tubulin isotypes and because both are expressed in a tissue-specific manner, these would seem to be the most likely candidates for possessing unique properties. For example, β3-tubulin is found primarily in neuronal cells, where it makes up 23% of the total β-tubulin (Banerjee et al., 1988), and is found to a lesser extent in testes. Similarly, β6-tubulin distribution is limited to marginal band microtubules in mammalian platelets and, additionally, to erythroid cells in avian species (Wang et al., 1986; Murphy et al., 1987). Despite their divergence and limited tissue distributions, several studies showed that both β-tubulins assemble into all microtubules in both normal and transfected cells (Joshi et al., 1987; Lewis et al., 1987; Ranganathan et al., 2001). On the other hand, some reports have hinted at unique properties for these two isotypes. Of all the β-tubulin isotypes, for example, only β3 and β6 are known to undergo phosphorylation (Diaz-Nido et al., 1990; Alexander et al., 1991; Rudiger and Weber, 1993). In PC12 cells β3-tubulin has been described to assemble less well than other isotypes and to have a granular appearance in developing neurites (Asai and Remolona, 1989; Joshi and Cleveland, 1989). More recently, we have demonstrated that transfected β3-tubulin assembles efficiently into all CHO cell microtubules, but that high incorporation of this isotype reduces microtubule polymer levels, confers weak paclitaxel resistance, and inhibits cell proliferation when its level reaches 80% of total β-tubulin (Hari et al., 2003b).
In vitro studies using immunological fractionation of bovine brain tubulin to obtain purified tubulin isotypes have also argued for differences in the properties of β3-tubulin. For example, it has been reported that β3-tubulin has a twofold lower affinity for colchicine than that of β2 (Banerjee and Luduena, 1992) and microtubules composed of β3-tubulin exhibit increased dynamics that are less sensitive to suppression by paclitaxel (Panda et al., 1994; Derry et al., 1997). Although most of the differences were small, the observations to date suggest that β3-tubulin may have unique characteristics that are not shared by the other β-tubulin isotypes.
Of all the vertebrate isotypes, β5-tubulin is the least studied and understood. In chickens, this isotype appears to be widely distributed among different tissues (Sullivan et al., 1986), and the same is likely to hold for mammalian species as well. One study found β5-tubulin in 3T3 and CEF cells, where it was estimated to make up 16-20% of total β-tubulin (Lopata and Cleveland, 1987), and we and others have previously demonstrated that β5-tubulin accounts for ∼5% of total β-tubulin in CHO cells (Sawada and Cabral, 1989; Ahmad et al., 1991). On the basis of these rather limited studies, we infer that β5-tubulin is probably a minor constituent in most mammalian cells. Even less is known about possible functional properties of β5-tubulin except for the observation that it is partially excluded from microtubules in developing neurites of PC12 cells (Joshi and Cleveland, 1989).
To determine whether β5-tubulin shares similarities to the major constitutive β-tubulin isotypes (i.e., β1, β2, and β4b) or whether it has some functional or assembly distinctive properties analogous to β3-tubulin, we raised the level of β5-tubulin in CHO cells by transfection of a cDNA encoding the mouse protein. To our surprise, even a modest increase in production of this isotype to 15% of total tubulin (cell line HAβ5c12) produced a small but significant decrease in microtubule assembly and a small increase in paclitaxel resistance. A further increase in HAβ5-tubulin production to 50% of total β-tubulin led to a precipitous drop in cellular microtubule assembly, often leaving only a few microtubules that could be seen with an HA antibody. Double staining with an α-tubulin antibody demonstrated that these were the only microtubules remaining in the cell. These residual microtubules are unlikely to be hyperstable because they stain poorly with antibodies to acetylated α-tubulin, a marker for microtubule stability (Piperno et al., 1987) and because preliminary experiments in our laboratory indicate that they are still dynamic (unpublished data). Elevated HAβ5-tubulin production also resulted in a twofold increase in paclitaxel resistance and a paclitaxel-dependent phenotype in which the cells grew poorly unless paclitaxel was present to stabilize the microtubules. We have not seen such dramatic effects on microtubule assembly with any other tubulin isotype that we have transfected (β1, β2, β3, and β4b), even when expression was much higher. We conclude that β5-tubulin has uniquely potent microtubule inhibitory effects and in this respect resembles some altered β1-tubulins that we previously characterized in paclitaxel-resistant and -dependent mutants (Cabral et al., 1983; Schibler and Cabral, 1986; Gonzalez-Garay et al., 1999). However, the microtubule inhibitory effects of β5-tubulin are much greater than those produced by most of the mutant β1-tubulins we have examined, and even in rare cases where mutant β1-tubulins produce a comparable reduction in microtubule assembly, we do not typically observe the extensive microtubule fragmentation seen with HAβ5-tubulin overproduction.
CHO cells that overproduce HAβ5-tubulin behave like cells that are treated with drugs like colcemid or that produce mutant tubulins that inhibit microtubule assembly (Abraham et al., 1983; Kung et al., 1990); i.e., they have severely reduced microtubule polymer, form abnormal spindles, fail to segregate chromosomes, and become large multinucleated cells. Time-lapse observation has shown that the morphological changes that occur in CHO cells with defects in microtubule assembly result from a delay, but not a block, in mitosis, followed by reentry of the cells into G1 phase without completing cytokinesis (Cabral and Barlow, 1991). Nuclear membrane formation around the missegregated chromosomes produces unusual nuclear morphologies including lobed structures and multiple nuclei of various sizes. In contrast, other cell lines such as HeLa and NIH 3T3 block more tightly in mitosis and undergo apoptosis when there are spindle defects (Kung et al., 1990; Rudner and Murray, 1996). Few of those cells escape the mitotic block and thus large multinucleated cells are uncommon. The reasons why different cell lines respond differently to a mitotic block are unclear. In all cases, however, HAβ5 overexpression inhibits cell proliferation, either because cells are lost through apoptosis (HeLa and NIH 3T3) or because the cells fail to divide, become large and multinucleated, and then eventually die (CHO).
The possibility that our results are artifactual appears unlikely. Transfected CHO, HeLa, and NIH 3T3 cells all exhibited disruptions of the microtubule cytoskeleton showing that the effects are not cell type specific. Although the transfected HAβ5-tubulin cDNA encoded a mouse protein, its amino acid sequence is nearly identical to the CHO protein (GenBank Accession nos. BC008225 and X60786) and overexpression produces similar effects in the homologous NIH 3T3 cells as it does in the heterologous CHO and HeLa cells. The presence of an HA tag at the carboxy terminus of β5-tubulin also cannot explain the results because we have found similar effects by transfecting a β5-tubulin cDNA that does not encode any epitope tag (our unpublished data) and have not seen effects due to the presence of the HA tag on any of the other isotypes we have transfected (Blade et al., 1999; Hari et al., 2003b). Failure to provide an appropriate α-tubulin partner is also an unlikely cause for the diminished microtubule assembly. We and others have shown that β5-tubulin is a normal constituent of CHO cells and, even though abundance of its α-tubulin partner is only 5% of total α-tubulin, the sequence differs from that of the most abundant α-tubulin in CHO cells at only two amino acid residues, and one of those substitutions is conserved (Elliott et al., 1986). Moreover, the overexpressed HAβ5-tubulin incorporates into microtubules to the same extent as endogenous β-tubulin, implying that it competes efficiently for heterodimer formation with the available α-tubulin. Finally, transfected β2- and β3-tubulins assemble efficiently into CHO microtubules and produce no effect (β2), or only mild microtubule disruption (β3), even though they are not normally expressed in this cell line and would also not be expected to have an isotype specific α-tubulin partner available for heterodimerization (Blade et al., 1999; Hari et al., 2003b).
We can only speculate as to why cells would maintain low amounts of a β-tubulin protein that can poison microtubule assembly. Its importance is suggested by the observation that it has a widespread distribution in avian and, presumably, in mammalian cells. Moreover, it has been maintained in a CHO cell line that has been in culture since 1957 (Puck et al., 1958). During adaptation to tissue culture conditions, many chromosomal changes took place in this cell line resulting in its current karyotype of 21-22 nonidentical chromosomes (Deaven and Petersen, 1973). In this process, many genes were lost or became haploid (Siminovitch, 1976). In addition, we have shown that CHO cells produce a surfeit of tubulin and can lose half of their β1-tubulin (35% of total β-tubulin) with no apparent effect on growth of the cells (Boggs and Cabral, 1987). Yet, the low percentage of β5-tubulin has persisted, suggesting that this isotype may play a vital role in microtubule assembly. One possibility, for example, is that microtubules composed of β1-, β2- and β4b-tubulin may be insufficiently dynamic to maintain normal microtubule function; the role of β5-tubulin may be to keep microtubules in a more “plastic” state. In neuronal cells that lack β5-tubulin, β3-tubulin could be playing a similar role.
As already mentioned, vertebrate β-tubulin sequences appear to fall into at least two groups: the closely related isotypes (β1, β2, and β4) and the more divergent isotypes (β3, β5, and β6). Members of the first group tend to be expressed at high levels in many tissues, but members of the second group are usually only present at low abundance and/or are tissue restricted. Isotypes comprising the second group may be the ones that provide specialized functions. Although isotypes I, II, and IV may be used to construct the basic microtubule cytoskeleton, members from the second group might be utilized to alter the properties of the microtubules to fill a specific need in a specialized cell type. Thus, β3-tubulin may be required to maintain efficient remodeling of the bundled microtubules found in nerve axons and β6-tubulin may be required for the formation of marginal band microtubules. The fact that β5-tubulin has a more widespread distribution suggests that it plays a more basic role. Its potent effects in disrupting microtubule integrity may explain why it has only been found in low abundance in those cell lines in which it has been measured, and it is tempting to speculate that differential expression of this isotype in various cell types may be used to regulate the properties of the microtubule cytoskeleton.
Acknowledgments
We thank Dr. Tony Frankfurter for helpful discussions and an antibody to class V β-tubulin and we thank Dr. Don Cleveland for an antibody that recognizes the N-terminus of all vertebrate β-tubulin isoforms. This work was supported by National Institutes of Health Grant CA85935 to F.C.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-01-0060. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-01-0060.
Abbreviations used: CHO, Chinese hamster ovary; MTB, microtubule buffer; tet, tetracycline.
References
- Abraham, I., Marcus, M., Cabral, F., and Gottesman, M.M. (1983). Mutations in α- and β-tubulin affect spindle formation in Chinese hamster ovary cells. J. Cell Biol. 97, 1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, S., Singh, B., and Gupta, R.S. (1991). Nucleotide sequences of three different isoforms of beta-tubulin cDNA from Chinese hamster ovary cells. Biochim. Biophys. Acta 1090, 252-254. [DOI] [PubMed] [Google Scholar]
- Alexander, J.E. et al. (1991). Characterization of posttranslational modifications in neuron-specific class III β-tubulin by mass spectrometry. Proc. Natl. Acad. Sci. USA 88, 4685-4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai, D.J., and Remolona, N.M. (1989). Tubulin isotype usage in vivo: A unique spatial distribution of the minor neuronal-specific β-tubulin isotype in pheochromocytoma cells. Dev. Biol. 132, 398-409. [DOI] [PubMed] [Google Scholar]
- Banerjee, A., and Luduena, R.F. (1992). Kinetics of colchicine binding to purified beta-tubulin isotypes from bovine brain. J. Biol. Chem. 267, 13335-13339. [PubMed] [Google Scholar]
- Banerjee, A., Roach, M.C., Wall, K.A., Lopata, M.A., Cleveland, D.W., and Luduena, R.F. (1988). A monoclonal antibody against the type II isotype of beta-tubulin. Preparation of isotypically altered tubulin. J. Biol. Chem. 263, 3029-3034. [PubMed] [Google Scholar]
- Blade, K., Menick, D.R., and Cabral, F. (1999). Overexpression of class I, II, or IVb β-tubulin isotypes in CHO cells is insufficient to confer resistance to paclitaxel. J. Cell Sci. 112, 2213-2221. [DOI] [PubMed] [Google Scholar]
- Boggs, B., and Cabral, F. (1987). Mutations affecting assembly and stability of tubulin: evidence for a non-essential β-tubulin in CHO cells. Mol. Cell. Biol. 7, 2700-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhart, C.A., Kavallaris, M., and Horwitz, S.B. (2001). The role of β-tubulin isotypes in resistance to antimitotic drugs. Biochim. Biophys. Acta 1471, O1-O9. [DOI] [PubMed] [Google Scholar]
- Cabral, F. (1983). Isolation of Chinese hamster ovary cell mutants requiring the continuous presence of taxol for cell division. J. Cell Biol. 97, 22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral, F., and Barlow, S.B. (1991). Resistance to antimitotic agents as genetic probes of microtubule structure and function. Pharmacol. Ther. 52, 159-171. [DOI] [PubMed] [Google Scholar]
- Cabral, F., Sobel, M.E., and Gottesman, M.M. (1980). CHO mutants resistant to colchicine, colcemid or griseofulvin have an altered β-tubulin. Cell 20, 29-36. [DOI] [PubMed] [Google Scholar]
- Cabral, F., Wible, L., Brenner, S., and Brinkley, B.R. (1983). Taxol-requiring mutant of Chinese hamster ovary cells with impaired mitotic spindle assembly. J. Cell Biol. 97, 30-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaven, L.L., and Petersen, D.F. (1973). The chromosomes of CHO, an aneuploid Chinese hamster cell line: G-band, C-band, and autoradiographic analyses. Chromosoma 41, 129-144. [DOI] [PubMed] [Google Scholar]
- Derry, W.B., Wilson, L., Khan, I.A., Luduena, R.F., and Jordan, M.A. (1997). Taxol differentially modulates the dynamics of microtubules assembled from unfractionated and purified beta-tubulin isotypes. Biochemistry 36, 3554-3562. [DOI] [PubMed] [Google Scholar]
- Diaz-Nido, J., Serrano, L., Lopez-Otin, C., Vandekerckhove, J., and Avila, J. (1990). Phosphorylation of a neuronal-specific β-tubulin isotype. J. Biol. Chem. 265, 13949-13954. [PubMed] [Google Scholar]
- Elliott, E.M., Henderson, G., Sarangi, F., and Ling, V. (1986). Complete sequence of three α-tubulin cDNAs in Chinese hamster ovary cells: each encodes a distinct α-tubulin isoprotein. Mol. Cell. Biol. 6, 906-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton, C., and Simpson, P.A. (1976). Selective synthesis and utilization of flagellar tubulin. The multi-tubulin hypothesis. In: Cell Motility, ed. R. Goldman, T. Pollard, and J. Rosenbaum, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 987-1006.
- Gonzalez-Garay, M.L., and Cabral, F. (1995). Overexpression of an epitope-tagged β-tubulin in Chinese hamster ovary cells causes an increase in endogenous α-tubulin synthesis. Cell Motil. Cytoskel. 31, 259-272. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garay, M.L., Chang, L., Blade, K., Menick, D.R., and Cabral, F. (1999). A β-tubulin leucine cluster involved in microtubule assembly and paclitaxel resistance. J. Biol. Chem. 274, 23875-23882. [DOI] [PubMed] [Google Scholar]
- Hari, M., Wang, Y., Veeraraghavan, S., and Cabral, F. (2003a). Mutations in α-and β-tubulin that stabilize microtubules and confer resistance to colcemid and vinblastine. Mol. Cancer Ther. 2, 597-605. [PubMed] [Google Scholar]
- Hari, M., Yang, H., Zeng, C., Canizales, M., and Cabral, F. (2003b). Expression of class III β-tubulin reduces microtubule assembly and confers resistance to paclitaxel. Cell Motil. Cytoskel. 56, 45-56. [DOI] [PubMed] [Google Scholar]
- He, L., Yang, C.H., and Horwitz, S.B. (2001). Mutations in β-tubulin map to domains involved in regulation of microtubule stability in epothilone-resistant cell lines. Mol. Cancer Ther. 1, 3-10. [PubMed] [Google Scholar]
- Hoyle, H.D., and Raff, E.C. (1990). Two Drosophila beta tubulin isoforms are not functionally equivalent. J. Cell Biol. 111, 1009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, H.C., and Cleveland, D.W. (1989). Differential utilization of β-tubulin isotypes in differentiating neurites. J. Cell Biol. 109, 663-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, H.C., and Cleveland, D.W. (1990). Diversity among tubulin subunits: toward what functional end. Cell Motil. Cytoskel. 16, 159-163. [DOI] [PubMed] [Google Scholar]
- Joshi, H.C., Yen, T.J., and Cleveland, D.W. (1987). In vivo coassembly of a divergent β-tubulin subunit (cβ6) into microtubules of different function. J. Cell Biol. 105, 2179-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung, A.L., Sherwood, S.W., and Schimke, R.T. (1990). Cell line-specific differences in the control of cell cycle progression in the absence of mitosis. Proc. Natl. Acad. Sci. USA 87, 9553-9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, S.A., Gu, W., and Cowan, N.J. (1987). Free intermingling of mammalian β-tubulin isotypes among functionally distinct microtubules. Cell 49, 539-548. [DOI] [PubMed] [Google Scholar]
- Lopata, M.A., and Cleveland, D.W. (1987). In vivo microtubules are copolymers of available β-tubulin isotypes: localization of each of six vertebrate β-tubulin isotypes using polyclonal antibodies elicited by synthetic peptide antigens. J. Cell Biol. 105, 1707-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luduena, R.F. (1998). Multiple forms of tubulin: different gene products and covalent modifications. Int. Rev. Cytol. 178, 207-275. [DOI] [PubMed] [Google Scholar]
- May, G.S. (1989). The highly divergent β-tubulins of Aspergillus nidulans are functionally interchangeable. J. Cell Biol. 109, 2267-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minotti, A.M., Barlow, S.B., and Cabral, F. (1991). Resistance to antimitotic drugs in Chinese hamster ovary cells correlates with changes in the level of polymerized tubulin. J. Biol. Chem. 266, 3987-3994. [PubMed] [Google Scholar]
- Murphy, D.B., Wallis, K.T., Machlin, P.S., Ratrie, H., 3rd, and Cleveland, D.W. (1987). The sequence and expression of the divergent beta-tubulin in chicken erythrocytes. J. Biol. Chem. 262, 14305-14312. [PubMed] [Google Scholar]
- Panda, D., Miller, H.P., Banerjee, A., Luduena, R.F., and Wilson, L. (1994). Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc. Natl. Acad. Sci. USA 91, 11358-11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno, G., LeDizet, M., and Chang, X. (1987). Microtubules containing acetylated alpha tubulin in mammalian cells in culture. J. Cell Biol. 104, 289-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck, T.T., Ciecuira, S.J., and Robinson, A. (1958). Genetics of somatic mammalian cells. III. Long-term cultivation of euploid cells from human and animal subjects. J. Exp. Med. 108, 945-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan, S., McCauley, R.A., Dexter, D.W., and Hudes, G.R. (2001). Modulation of endogenous β-tubulin isotype expression as a result of human βIII cDNA transfection into prostate carcinoma cells. Br. J. Cancer 85, 735-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudiger, M., and Weber, K. (1993). Characterization of the post-translational modifications in tubulin from the marginal band of avian erythrocytes. Eur. J. Biochem. 218, 107-116. [DOI] [PubMed] [Google Scholar]
- Rudner, A.D., and Murray, A.W. (1996). The spindle assembly checkpoint. Curr. Opin. Cell Biol. 8, 773-780. [DOI] [PubMed] [Google Scholar]
- Sawada, T., and Cabral, F. (1989). Expression and function of β-tubulin isotypes in Chinese hamster ovary cells. J. Biol. Chem. 264, 3013-3020. [PubMed] [Google Scholar]
- Schibler, M., and Cabral, F. (1986). Taxol-dependent mutants of Chinese hamster ovary cells with alterations in α- and β-tubulin. J. Cell Biol. 102, 1522-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch, L. (1976). On the nature of hereditable variation in cultured somatic cells. Cell 7, 1-11. [DOI] [PubMed] [Google Scholar]
- Sullivan, K.F. (1988). Structure and utilization of tubulin isotypes. Annu. Rev. Cell Biol. 4, 687-716. [DOI] [PubMed] [Google Scholar]
- Sullivan, K.F., Havercroft, J.C., Machlin, P.S., and Cleveland, D.W. (1986). Sequence and expression of the chicken β5- and β4-tubulin genes define a pair of divergent β-tubulins with complementary patterns of expression. Mol. Cell. Biol. 6, 4409-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorakis, N.G., and Cleveland, D.W. (1992). Physical evidence for cotranslational regulation of β-tubulin mRNA degradation. Mol. Cell. Biol. 12, 791-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D., Villasante, A., Lewis, S.A., and Cowan, N.J. (1986). The mammalian β-tubulin repertoire: hematopoietic expression of a novel, heterologous β-tubulin isotype. J. Cell Biol. 103, 1903-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]