Abstract
Objective
To analyse the differences between patients with granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA) entered into randomised clinical trials (RCTs) and those followed in large observational cohorts.
Methods
The main characteristics and outcomes of patients with generalised and/or severe GPA or MPA with a five-factor score ≥1 enrolled in the French Vasculitis Study Group (FVSG) or the US-Canadian-based Vasculitis Clinical Research Consortium cohorts were compared to those enrolled in one of 2 FVSG clinical RCTs (WEG91, WEGENT) or 3 European Vasculitis Society clinical trials (CYCLOPS, CYCAZAREM, IMPROVE).
Results
657 patients (65.3% with GPA) in RCTs were compared to 437 in cohorts (90.6% with GPA). RCT patients were older at diagnosis than the cohort patients (56.6±13.9 vs. 46.8±17.3 years), had higher Birmingham vasculitis activity score (19.5±9.1 vs. 16.9±7.4), and more frequent kidney disease (84.0% vs. 54.9%) but fewer ear, nose, and throat symptoms (56.8% vs. 72.2%). At 56 months post-diagnosis, mortality and relapse rates, adjusted for age and renal function, were higher for patients with GPA in RCTs vs. cohorts (10.7% vs. 2.5% [p=0.001] and 22.5% vs. 15.6% [p=0.03], respectively) but similar for patients with MPA (6.2% vs. 6.6% [p=0.92] and 16.6% vs. 10.1% [p=0.39], respectively).
Conclusion
Patients with GPA or MPA in RCTs and those in observational cohorts show important differences that should be remembered when interpreting results based on these study populations.
Keywords: ANCA, vasculitis, granulomatosis with polyangiitis (Wegener’s granulomatosis), microscopic polyangiitis
Introduction
Patients entered into randomised controlled trials (RCTs) intuitively differ from those enrolled in observational cohorts but to what extent is a recurring and important question (1). Multicentre collaborative efforts have led to RCTs in vasculitis with samples sizes that are routinely >100 patients per trial. In parallel, several national and international longitudinal cohort studies exist, some running for >20 years and including >1,000 patients.
This study aimed to identify and list the main differences in the characteristics and outcomes of patients with generalised and/or severe granulomatosis with polyangiitis (GPA, Wegener’s) or microscopic polyangiitis (MPA) with five-factor score (FFS) ≥1, who were entered in cohort studies or RCTs.
Materials and methods
Study population
Study patients had to have generalised and/or severe GPA (involvement of ≥2 major organs or one major organ with constitutional symptoms) or MPA with a FFS ≥1 and participated in a cohort study or RCT.
Patients in cohorts were enrolled in the French Vasculitis Study Group (FVSG) database or the US-Canadian- based Vasculitis Clinical Research Consortium (VCRC) Longitudinal Study of GPA/MPA. Patients in RCTs were enrolled in the FVSG WEG91 or WEGENT trials (2, 3) or one of the 3 European Vasculitis Study Group trials (EUVAS) – CYCLOPS (4), CYCAZAREM (5), or IMPROVE (6) (details in Table I). The FFS is a prognostic score and comprises the following items: serum creatinine >1.58 mg/dl, proteinuria >1 g/day, severe gastrointestinal tract involvement, cardiomyopathy and/or central nervous system involvement; the presence of each factor is accorded one point (7). Patients with MPA with FFS=0 or localised and/or limited GPA were excluded from the study (their treatment and outcomes differ from those with more severe disease). After the end of the FVSG RCTs, all RCT patients were enrolled in the FVSG cohort but were analysed in this study as RCT patients only; none of the VCRC cohort patients participated in the RCTs included in this study.
Table I.
Summary of the main inclusion and exclusion criteria and relapse definitions of RCTs and cohorts included in this comparative study.
| Study | Study aim/description | Main eligibility criteria* | Main exclusion/restrictive criteria | Relapse definition/details/comments¶ |
|---|---|---|---|---|
| Trials | ||||
| WEG91 (2) | Oral vs. IV cyclophosphamide for induction of remission |
|
|
|
| WEGENT (3) | Azathioprine vs. methotrexate for remission maintenance, after IV cyclophosphamide for induction |
|
|
|
| CYCLOPS (4) | Oral vs. IV cyclophosphamide for induction of remission |
|
|
|
| CYCAZAREM (5) | Continued oral cyclophosphamide vs. early switch to azathioprine for remission maintenance |
|
|
|
| IMPROVE (6) | Azathioprine vs. mycophenolate mofetil for maintenance, after IV or oral cyclophosphamide for induction |
|
|
|
| Cohorts | ||||
| FVSG | French multicentric observational cohort |
|
|
|
| VCRC-GPA-MPA | North American multicentric longitudinal protocol |
|
|
|
Patients with renal limited vasculitis and no general or constitutional symptoms were not included in this comparative study.
Although definitions of minor relapses are reported in this table, those (minor) relapses that did not lead to a change in immunosuppressant therapy and/or patient RCT withdrawal were not included this comparative study.
The FFS prognostic score includes 5 parameters, each of them scoring for 1 point if present: elevated serum creatinine levels (140 moles/liter or 1.58 mg/dl), proteinuria (1 gm/day), severe gastrointestinal tract involvement, cardiomyopathy, and/or central nervous system (CNS) involvement.
Data form these 7 patients were not available in the RCT report or for this study.
ACR: American College of Rheumatology; ANCA: antineutrophil cytoplasm antibody; BVAS: Birmingham Vasculitis Activity Score; FVSG: French Vasculitis Study Group; FFS: five-factor score; GPA: granulomatosis with polyangiitis; IV: intravenous; MPA: microscopic polyangiitis; RCT: randomised controlled trial; VCRC: Vasculitis Clinical Research Consortium.
As a prerequisite for entry in the studied cohorts or RCTs, patients had to satisfy the 1990 American College of Rheumatology (ACR) classification criteria and/or 1994 Chapel Hill nomenclature definitions of disease (8, 9); patients in the VCRC cohort had to satisfy modified ACR criteria, with a positive antineutrophil cytoplasm antibody (ANCA) test result being an additional parameter (10). In the FVSG longitudinal database, patients can be entered at any time, including at diagnosis; patients in the VCRC database are usually enrolled later during the course of their disease, during a follow-up visit. None of the RCTs were blinded, and all included post-RCT follow-up (for this study, follow-up data for the IMPROVE RCT were not available).
Study schedules and clinical data elements
In the FVSG longitudinal database, there are no scheduled follow-up visits, but information on survivors is updated at least every 2 years. Patients in the VCRC have follow-up visits every 3 or 12 months, according to the patient’s preference, and at the time of a disease flare, if any occurs.
In both cohorts, as well as for the WEG91, WEGENT 91, CYCAZAREM and CYCLOPS RCTs, data are collected from the time of diagnosis to last follow-up assessment, death or first relapse since study entry. In the VCRC cohort, date of relapse before enrolment, if it had occurred, is not recorded. For the IMPROVE RCT, only follow-up times from the date of remission rather than diagnosis were available (i.e. 3 to 6 months after starting cyclophosphamide induction therapy – average, 4.27 months), and ANCA results were not available because of different recording and extraction systems.
Outcome definitions
Relapse definitions differed slightly in each of the studies (Table I) but largely corresponded to new or recurrent manifestations due to active vasculitis, thus leading to a Birmingham vasculitis activity score (BVAS) >0. This analysis focused on major relapses or relapses that led to a change in the immunosuppressant therapy for studies with no pre-established definition for major relapse (cohorts, WEG91 and WEGENT).
Statistical analysis
The main demographics and clinical characteristics of cohort and RCT patients were compared at the time of diagnosis. Clinical outcomes (relapses and deaths) were compared on the basis of last available study visit until September 2010. Categorical variables were compared using a chi-square test or, when appropriate, Fisher’s exact test, and continuous variables using Student’s t-test. Mortality and relapse rates were also compared after adjustment for age and glomerular filtration rate. For all analyses, p≤0.05 was considered significant. Statistical analyses were conducted using Stata Statistical Software: Release 12 (StataCorp, 2011, College Station, TX: StataCorp LP).
Results
We compared 437 patients enrolled in cohorts (182 in the FVSG, 255 in the VCRC) to 657 patients enrolled in RCTs (220 in the FVSG RCTs, 437 in the EUVAS RCTs) (Table II). In the VCRC cohort, the mean time from diagnosis to enrolment was 31.8±54.5 months (data not available for the FVSG cohort). All RCT patients were newly diagnosed, except for 6 included in the WEG91 trial.
Table II.
Main characteristics at diagnosis of patients in observational cohorts and RCTs.
| Characteristics | Observational cohorts n= 437 |
Clinical RCTs n=657 |
p-value |
|---|---|---|---|
| Diagnosis | |||
| GPA | 396 (90.6) | 429 (65.3) | <0.001 |
| MPA | 41 (9.4) | 228 (34.7) | |
| Age at diagnosis, years, mean±SD | 46.8 ± 17.3 | 56.6 ± 13.9 | <0.001 |
| GPA | 46.0 ± 17.3 | 54.3 ± 14.6 | <0.001 |
| MPA | 55.4 ± 14.9 | 59.9 ± 12.9 | 0.06 |
| Male* | 225 (51.5) | 361 (54.9) | 0.26 |
| Constitutional symptoms* | 387/436 (88.8) | 532/622 (85.5) | 0.13 |
| Cutaneous manifestations | 124/436 (28.4) | 169/622 (27.2) | 0.65 |
| Eye involvement | 96/436 (22.0) | 169/622 (27.2) | 0.06 |
| Ear, nose and throat involvement | 314/435 (72.2) | 355/625 (56.8) | <0.001 |
| GPA | 306/394 (77.7) | 314/412 (76.2) | 0.63 |
| MPA | 8/41 (19.5) | 41/213 (19.3) | 0.96 |
| Lung involvement | 280/433 (64.7) | 378/625 (60.5) | 0.17 |
| Cardiovascular involvement | 31/432 (7.2) | 56/621 (9.0) | 0.29 |
| Gastrointestinal manifestations | 28/430 (6.5) | 48/621 (7.7) | 0.45 |
| Renal involvement | 237/432 (54.9) | 524/624 (84.0) | <0.001 |
| GPA | 206/391 (52.7) | 329/411 (80.1) | <0.001 |
| MPA | 31/41 (75.6) | 195/213 (91.6) | 0.003 |
| MDRD-GFR (ml/min/1.73 m2 for all patients, mean±SD | 74.4 ± 34.1 (n=316)† | 48.2 ± 30.2 (n=637) | <0.001 |
| GPA | 76.5 ± 33.4 (n=288)† | 55.3 ± 31.4 (n=413) | <0.001 |
| MPA | 52.1 ± 34.0 (n=28)† | 35.3 ± 22.8 (n=224) | <0.001 |
| Neurologic involvement | 113/432 (26.2) | 159/622 (25.6) | 0.83 |
| ANCA positivity (IF and/or ELISA) | 368/415 (88.7) | 441/495 (89.1) | 0.84 |
| Anti-PR3 positive (ELISA) | 252/385 (65.5) | 268/475 (56.4) | 0.007 |
| Anti-MPO positive (ELISA) | 68/384 (17.7) | 154/475 (32.4) | <0.001 |
| FFS; for patients with MPA‡ | |||
| FFS =1 | 24/41 (58.5) | 17/41 (41.5) | 0.12 |
| FFS ≥2 | 14/41 (41.5) | 24/41 (58.5) | |
| BVAS, mean±SD | 16.9 ± 7.4 (n=396)† | 19.5 ± 9.1 (n=587) | <0.001 |
| GPA | 16.7 ± 7.4 (n=359)† | 20.7 ± 9.1 (n=395) | <0.001 |
| MPA | 18.0 ± 15.5 (n=37)† | 17.0 ± 15.8 (n=192) | 0.50 |
Data are no. of patients with the characteristic/total no. of patients with the data available (%) unless indicated
Number of patients with data available.
FFS: five factor score; the original 1996 FFS was calculated and recorded in several trials and cohorts.
This score is not applicable to GPA (only the revised 2011 FFS can be used for GPA).
ANCA: anti-neutrophil cytoplasm antibody; BVAS: Birmingham vasculitis activity score; ELISA: enzyme-linked immunosorbent assay; FFS: five-factor score; GPA: granulomatosis with polyangiitis; IF: immunofluorescence; MDRD-GFR: modification of diet in renal disease - glomerular filtration rate; MPA: microscopic polyangiitis; MPO: myeloperoxidase; PR3: proteinase 3; RCT: randomised controlled trial.
Characteristics at diagnosis
GPA and anti-proteinase 3 (PR3) ANCA-positive patients represented the majority of patients in both cohorts and RCTs. RCT patients were older at diagnosis by almost 10 years than patients in cohorts, had a higher BVAS, and more frequent and severe kidney disease. Ear, nose and throat symptoms were more frequent in cohorts, which included a higher proportion of patients with GPA than MPA.
Outcomes
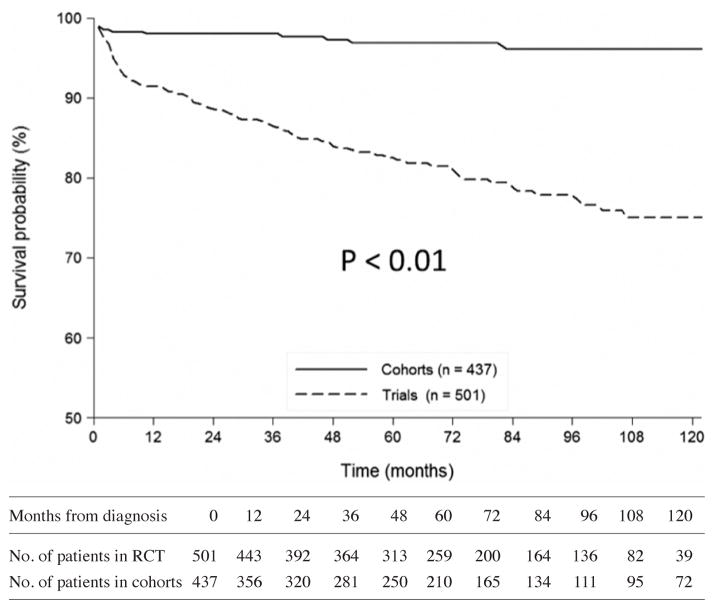
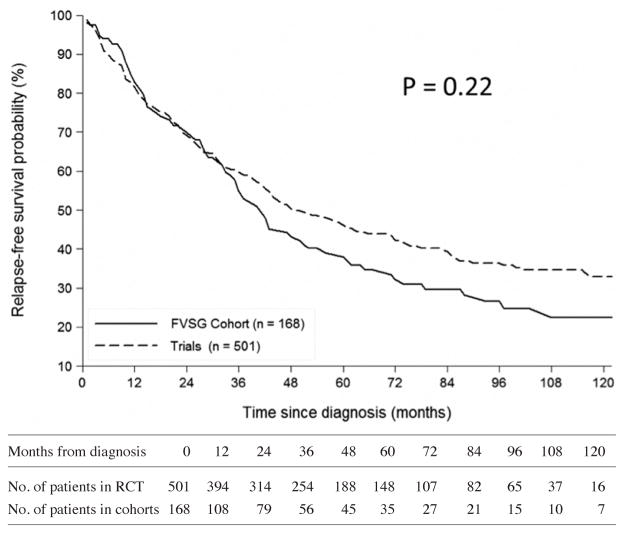
The mean follow-up duration in RCTs (including post-RCT follow-up) was shorter by 15 months than in cohorts (56.9 ± 38.6 months vs. 71.9 ± 63.4 months, respectively). At 56 months post-diagnosis (i.e. the median for RCTs) and after adjusting for age and glomerular filtration rate, mortality and relapse rates were higher for patients in RCTs than cohorts (Table III; Fig. 1–2). For patients with GPA but not MPA, adjusted relapse rates and mortality at 56 months post-diagnosis were higher for patients in RCTs than cohorts.
Table III.
Main outcomes for patients in observational cohorts and RCTs.
| Characteristics | Observational cohorts n= 437 |
Clinical RCTs n=657 |
p-value |
|---|---|---|---|
| Follow-up since diagnosis, months, mean±SD | 71.9 ± 63.4 | 56.9 ± 38.6 | <0.001 |
| Relapses* | 259 (59.3) | 276 (42.1) | <0.001 |
| GPA patients | 244 (61.6) | 211 (49.2) | <0.001 |
| MPA patients | 15 (36.6) | 65 (28.5) | 0.30 |
| Relapses at 56 months after diagnosis† | 78 (17.9) | 136 (20.7) | 0.24 |
| GPA patients | 72 (18.2) | 101 (23.5) | 0.06 |
| MPA patients | 6 (14.6) | 35 (15.4) | 0.91 |
| Relapse rates at 56 months after diagnosis, adjusted for age and MDRD-GFR | (14.3) | (20.2) | 0.03‡ |
| GPA patients | (15.6) | (22.5) | 0.03 |
| MPA patients | (10.1) | (16.6) | 0.39 |
| Deaths | 16 (3.7) | 99 (15.1) | <0.001 |
| GPA patients | 12 (3.0) | 63 (14.7) | <0.001 |
| MPA patients | 4 (9.8) | 36 (15.8) | 0.32 |
| Deaths at 56 months after diagnosis † | 11 (2.5) | 80 (12.2) | <0.001 |
| Survival for GPA patients | 7 (1.8) | 49 (11.4) | <0.001 |
| Survival for MPA patients | 4 (9.8) | 31 (13.6) | 0.50 |
| Death rates at 56 months after diagnosis, adjusted for age and MDRD-GFR | (3.4) | (9.7) | 0.004‡ |
| GPA patients | (2.5) | (10.7) | 0.001 |
| MPA patients | (6.6) | (6.2) | 0.92 |
Data are no. of patients with the characteristic/total no. of patients with the data available (%) unless indicated.
Follow-up was censored at 56 months (i.e. the mean follow-up for RCTs), shorter than that for cohorts.
Odds ratio to relapse in RCTs compared to cohorts, at month 56 and adjusted for age and MDRD-GFR, was 1.52 (95% confidence interval, 1.04–2.23). Odds ratio for dying in RCTs compared to cohorts, at month 56 and adjusted for age and MDRD-GFR, was 3.04 (95% confidence interval, 1.42–6.54).
GPA: granulomatosis with polyangiitis; MDRD-GFR: modification of diet in renal disease - glomerular filtration rate; MPA: microscopic polyangiitis; RCT: randomised controlled trial.
Fig. 1.
Comparisons of the curves for survival since diagnosis of patients in RCTs versus cohorts. At month 56, survival rate was 96.8% [CI 95%; 94.3–98.3] in the cohorts versus 83.1% [CI 95%; 79.3–86.3] in the RCTs (HR=9.21 [CI 95%; 4.47–18.97]). IMPROVE patients are not included, as the precise date of their vasculitis diagnosis was not available.
Fig. 2.
Comparisons of the curves for relapse-free survival since diagnosis of patients in the FVSG cohort (the date of the first relapse was not available for the VCRC patients who experienced ≥1 relapse prior to enrolment in the cohort) versus RCTs (IMPROVE patients are not included, as the precise date of their vasculitis diagnosis was not available). At Month 56, relapse-free survival rate was 39.1% [CI 95%; 29.9–48.1] in the cohorts versus 47.8% [CI 95%; 42.9–52.4] in the RCTs (HR=0.85 [CI 95%; 0.67–1.09]).
Discussion
As expected, several clinical differences at diagnosis exist between patients with generalised and/or severe GPA or MPA enrolled in observational cohorts and RCTs. We identified that RCT patients were older and had more severe disease, mainly because of their more frequent and severe renal involvement, as compared to cohort participants.
There are multiple possible explanations for the differences observed in our study, with selection biases the most important, including over-representation of GPA/anti-PR3 ANCA-positive patients in cohorts (11). However, because GPA and MPA are uncommon and the RCT goals were to inform standard treatment in a pragmatic way, selection criteria for the RCTs included in this comparative analysis were broad and inclusive. RCTs enrolled both patients with anti-PR3 and anti-MPO ANCA-associated diseases, who may need to be studied separately in view of the accumulating evidence of their different pathogenesis and outcomes (12–14). RCTs might also have selected only a subset of patients with GPA or MPA and RCT patients, who exhibited more frequent but also severe kidney disease than those in cohorts.
The different geographical catchment areas for these RCTs and cohorts and other centre biases may also have played a role. The VCRC is primarily a network based on centres directed by rheumatologists, whereas EUVAS centres are more commonly led by nephrologists. That RCT patients had more frequent and severe renal involvement may thus be due, at least in part, to the EUVAS RCTs being geared to patients with renal disease. However, we also analysed GPA and MPA separately and adjusted our outcome analyses for glomerular filtration rate (and age).
Although study limitations prevent drawing firm conclusions, the observed differences at diagnosis may have resulted in the higher mortality and relapse rates for RCT than cohort patients with GPA. A recent study comparing patients with MPA between Europe and Japan also suggested higher mortality in RCT patients than those from the Cambridge cohort (United Kingdom). However, that difference was mainly due to inclusion in the RCT group of MEPEX patients, who all had severe renal disease. Those MEPEX patients were not included in our study, in order to limit similar biases in disease severity (15).
A survivor bias in the cohorts may have affected our results. Patients with more severe disease, when not enrolled in RCTs, may have died quickly or had severe disability and therefore not been referred to tertiary centres where patients are entered in cohorts. While this explanation may apply to the VCRC cohort, in which patients are usually entered after they have achieved remission, the FVSG cohort attempts to enroll patients close to the time of diagnosis, and all FVSG centres participate in both RCTs and cohorts. As the prognosis of GPA and MPA is worse in older patients (16, 17), the lower age at diagnosis in the cohorts may also explain the lower mortality rate in this group. In addition, patients with GPA, who were over-represented in the cohorts, may have sought early medical attention, due to initial features like rhinitis, thus been diagnosed and treated earlier, with better outcomes.
The strengths of this study include the large number of patients studied at expert centres, with a long follow-up, including after the end of RCTs. However, this retrospective study has limitations, which limits the ability to fully compare outcomes with the 2 study types. The study design and data collection differed for the 2 independent cohorts and 5 RCTs, and treatments, which were not codified after the end of the RCTs, were not analysed. Because the precise time between diagnosis and first relapse was missing for the VCRC cohort and one RCT, no valuable time-to-event survival analyses for relapse could have been conducted. Furthermore, all of the 5 RCTs enrolled almost exclusively newly diagnosed patients, at the time of the diagnosis, but relapse rates differ between newly diagnosed and relapsing patients (18). Patients with limited or less severe disease, such as those enrolled in the NORAM trial (19), were not included in this analysis, because they are few in number, have different disease characteristics and outcomes (20), and were excluded from most of the RCTs we studied. We did not analyse pre-existing comorbidities, which may have an impact on survival and were exclusion criteria in RCT but not cohorts. Many patients are managed outside of reference centres and/or do not participate in any cohorts or RCTs (21). Finally, some comparisons were made with data that spanned for a decade, during which therapeutic advances have impacted the course and outcomes of these diseases. Thus, our results may not be generalisable to all patients with GPA or MPA.
Cohort studies and RCTs provide complementary information (1, 11, 22). The differences we observed between RCT and cohort patients, especially those with generalised and/or severe GPA, have important implications for the interpretation of study results. Whether similar differences are observed in other vasculitis cohorts or RCTs or with other types of vasculitis would be interesting to study.
Acknowledgments
In addition to the authors, many other members and investigators of the FVSG, EUVAS and/or VCRC enrolled patients in the RCTs or cohorts analysed in this study. They are listed in the separate articles that reported their respective results and are thanked again.
Funding
Dr Pagnoux was supported by a VCRC Fellowship grant (2010–2012). Dr Walsh is supported by a New Investigator Award from the Kidney Scientist Core Education and National Training Program. The Vasculitis Clinical Research Consortium has received support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54AR057319 and U01AR5187404), the National Center for Research Resources (U54RR019497), the Office of Rare Diseases Research, and National Center for Advancing Translational Science. Dr Hiemstra is supported by the National Institute of Healthcare Research and the Cambridge BRC
Footnotes
Competing interests: none declared.
References
- 1.POCOCK SJ, ELBOURNE DR. Randomized trials or observational tribulations? N Engl J Med. 2000;342:1907–9. doi: 10.1056/NEJM200006223422511. [DOI] [PubMed] [Google Scholar]
- 2.GUILLEVIN L, CORDIER JF, LHOTE F, et al. A prospective, multicenter, randomized trial comparing steroids and pulse cyclophosphamide versus steroids and oral cyclophosphamide in the treatment of generalized Wegener’s granulomatosis. Arthritis Rheum. 1997;40:2187–98. doi: 10.1002/art.1780401213. [DOI] [PubMed] [Google Scholar]
- 3.PAGNOUX C, MAHR A, HAMIDOU MA, et al. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med. 2008;359:2790–803. doi: 10.1056/NEJMoa0802311. [DOI] [PubMed] [Google Scholar]
- 4.DE GROOT K, HARPER L, JAYNE DR, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150:670–80. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 5.JAYNE D, RASMUSSEN N, ANDRASSY K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 6.HIEMSTRA TF, WALSH M, MAHR A, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA. 2010;304:2381–8. doi: 10.1001/jama.2010.1658. [DOI] [PubMed] [Google Scholar]
- 7.GUILLEVIN L, LHOTE F, GAYRAUD M, et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore) 1996;75:17–28. doi: 10.1097/00005792-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 8.JENNETTE JC, FALK RJ, ANDRASSY K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 9.LEAVITT RY, FAUCI AS, BLOCH DA, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum. 1990;33:1101–7. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 10.WGET. Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med. 2005;352:351–61. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- 11.CONCATO J, LAWLER EV, LEW RA, GAZIANO JM, ASLAN M, HUANG GD. Observational methods in comparative effectiveness research. Am J Med. 2010;123:e16–23. doi: 10.1016/j.amjmed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 12.LYONS PA, RAYNER TF, TRIVEDI S, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367:214–23. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WALSH M, FLOSSMANN O, BERDEN A, et al. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64:542–8. doi: 10.1002/art.33361. [DOI] [PubMed] [Google Scholar]
- 14.LIONAKI S, BLYTH ER, HOGAN SL, et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum. 2012;64:3452–62. doi: 10.1002/art.34562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FURUTA S, CHAUDHRY AN, HAMANO Y, et al. Comparison of phenotype and outcome in microscopic polyangiitis between Europe and Japan. J Rheumatol. 2014;41:325–33. doi: 10.3899/jrheum.130602. [DOI] [PubMed] [Google Scholar]
- 16.FLOSSMANN O, BERDEN A, DE GROOT K, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011;70:488–94. doi: 10.1136/ard.2010.137778. [DOI] [PubMed] [Google Scholar]
- 17.HOGANSON DD, FROM AM, MICHET CJ. ANCA vasculitis in the elderly. J Clin Rheumatol. 2008;14:78–81. doi: 10.1097/RHU.0b013e31816b2fbd. [DOI] [PubMed] [Google Scholar]
- 18.SPECKS U, MERKEL PA, SEO P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369:417–27. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DE GROOT K, RASMUSSEN N, BACON PA, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52:2461–9. doi: 10.1002/art.21142. [DOI] [PubMed] [Google Scholar]
- 20.STONE JH Wegener’s Granulomatosis Etanercept Trial Research Group. Limited versus severe Wegener’s granulomatosis: baseline data on patients in the Wegener’s granulomatosis etanercept trial. Arthritis Rheum. 2003;48:2299–309. doi: 10.1002/art.11075. [DOI] [PubMed] [Google Scholar]
- 21.GROSS CP, MALLORY R, HEIAT A, KRUMHOLZ HM. Reporting the recruitment process in clinical trials: who are these patients and how did they get there? Ann Intern Med. 2002;137:10–6. doi: 10.7326/0003-4819-137-1-200207020-00007. [DOI] [PubMed] [Google Scholar]
- 22.DEKKERS OM, VON ELME, ALGRA A, ROMIJN JA, VANDENBROUCKE JP. How to assess the external validity of therapeutic trials: a conceptual approach. Int J Epidemiol. 2010;39:89–94. doi: 10.1093/ije/dyp174. [DOI] [PubMed] [Google Scholar]