Abstract
Background
Rhythm control with antiarrhythmic drugs (AADs) is not superior to rate control in patients with heart failure (HF) and atrial fibrillation (AF), but AF ablation may be more successful at achieving rhythm control than AADs. However, risks for both ablation and AADs are likely higher and success rates lower in patients with HF.
Objective
To compare rate control versus AF catheter ablation strategies in patients with AF and HF.
Methods
We conducted a meta-analysis of trials which randomized HF patients (LVEF<50%) with AF to a rate control or AF catheter ablation strategy and reported change in LVEF, quality of life, 6-minute walk test, or peak oxygen consumption. Study quality and heterogenity were assessed using Jadad scores and Cochran’s Q statistics, respectively. Mantel Haenszel relative risks and mean differences were calculated using random effect models.
Results
Four trials (N=224) met inclusion criteria; 82.5% (n=185) had persistent AF. AF ablation was associated with an increase in LVEF (mean difference 8.5%; 95%CI 6.4,10.7%; P<0.001) compared to rate control. AF ablation was superior in improving quality of life by Minnesota Living with Heart Failure (MLWHF) questionnaire scores (mean difference −11.9; 95%CI −17.1, −6.6; P<0.001). Peak oxygen consumption and 6-minute walk distance increased in AF ablation compared to rate control patients (mean difference 3.2; 95%CI 1.1,5.2; P=0.003; mean difference 34.8; 95%CI 2.9, 66.7; P = 0.03, respectively). In the persistent AF subgroup LVEF and MLWHF were significantly improved with AF ablation. Major adverse event rates (RR 1.3; 95% CI, 0.4, 3.9; p=0.64) were not significantly different. No significant heterogeneity was evident.
Conclusions
In patients with HF and AF, AF catheter ablation is superior to rate control in improving LVEF, quality of life and functional capacity. Prior to accepting a rate control strategy in HF patients with persistent or drug refractory AF, consideration should be given to AF ablation.
Keywords: Atrial fibrillation, heart failure, catheter ablation, pulmonary vein isolation
Atrial fibrillation (AF) and heart failure (HF) are two common cardiac conditions associated with substantial morbidity, mortality and cost on health care systems (1–4). The two conditions frequently coexist and may promote the other. AF is present in up to 50% of patients with HF (5). AF in HF patients is associated with increased hospital stay, stroke and mortality (6–8). This may be at least partially attributed to the hemodynamic effects of AF due to loss of atrial contraction along with irregular and/or rapid ventricular rates, which can lead to left ventricular dysfunction and decreased cardiac output (9, 10).
Rhythm control with antiarrhythmic drugs (AADs) has failed to be superior to rate control in patients with HF and AF in terms of cardiovascular mortality or worsening of HF (11). The risk of adverse events associated with AADs and their limited efficacy in restoring sinus rhythm have triggered an increased interest in AF catheter ablation (12, 13).
Several observational studies of AF catheter ablation in patients with HF have reported that maintenance of sinus rhythm by catheter ablation can significantly improve cardiac function (14–16).
The aim of our study was to determine if AF catheter ablation is superior to rate control in patients with AF and HF. We performed a meta-analysis of randomized controlled trials that compared AF catheter ablation to rate control in patients with HF and AF.
METHODS
This meta-analysis of clinical trials was performed according to the guidelines of The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (17).
Literature Review
Relevant studies were selected by searching Pubmed, Medline, Embase, Central, ClinicalTrials.gov, The Cochrane Library and ISI Web of Science (Jan 1980 – Feb 2015). The search was independently conducted by two investigators (S.A and M.Q.). Search key terms were atrial fibrillation, persistent atrial fibrillation, pulmonary venous isolation, catheter ablation, heart failure, left ventricular dysfunction, impaired left ventricular systolic function, reduced left ventricular systolic function, low ejection fraction, heart failure with reduced ejection fraction, functional capacity and quality of life. Bibliographies of retrieved studies were hand-searched to identify relevant studies.
Selection Criteria and Quality Analysis
Prospective randomized controlled trials published in English that compared rate control to AF catheter ablation were included.
Trials that included patients with left ventricular ejection fraction (LVEF) <50% randomized to a rate control strategy or AF catheter ablation and that reported at least one of the studied outcomes were included. Studies had to fulfill the following criteria for inclusion: 1) prospective randomized controlled trial design; 2) patients enrolled with LVEF <50% and history of AF; 3) randomization to AF catheter ablation versus rate control (pharmacologic or atrioventricular-node ablation with biventricular or right ventricular pacing); 4) study follow-up of at least six months. Studies that utilized AADs as rhythm control or which did not report at least one outcome of interest were excluded.
Quality of the studies was assessed independently by two reviewers (S.A., M.Q.). Jadad’s method was used to assess quality of the studies (18). The items assessed were blinding, randomization, and description of withdrawals or dropouts. All included studies had a quality score of three. No studies attempted double blinding. Therefore, weighting of the results was not performed.
Data Extraction and Outcome Measures
Data were independently extracted by two investigators (S.A. and M.Q.) and discrepancies were resolved by unanimous consensus. Extracted data included number of patients in each intervention arm, characteristics of included patients, procedure characteristics, LVEF, quality of life parameters and complications. Primary authors were contacted when data on studied outcomes was not reported.
The primary clinical endpoint was change in LVEF after 6 months. Secondary end points were Minnesota Living with Heart Failure (MLWHF) questionnaire scores, 6-minute walk test distance and peak oxygen consumption. Complications, adverse events, and deaths were also summarized.
Major adverse events were defined as death, intracranial hemorrhage, cardiac tamponade, pericardial effusion, pneumothorax, hemothorax, deep venous thrombosis or pulmonary embolism, sepsis or pulmonary vein stenosis (>50%) requiring intervention. Procedural complications were defined as any complication that occurred in the 30-day post procedural period. AF ablation was defined as pulmonary vein isolation with or without additional substrate modification and excluding AV junction ablation. Rate control was defined as the use of pharmacological therapy or AV junction ablation for rate control.
Statistical Analysis
The results are presented as mean difference for continuous outcome measures with 95% confidence intervals. Random-effect models were used for all reported outcomes (19).
Heterogeneity among studies was assessed with the inconsistency index (I2) statistic, which ranges from 0% to 100% and is defined as the percentage of the observed inter-trial variability that is due to heterogeneity rather than chance for each outcome (I2 > 60% denotes significant heterogeneity) (20). Potential publication bias was evaluated by Begg’s funnel plots method (21).
In order to further detect any clinical heterogeneity, several sensitivity analyses were performed for the LVEF and MLWHF outcomes:
Trials including only patients with persistent AF were analyzed, excluding a trial which included patients with both persistent and paroxysmal AF.
Trials using only pharmacologic rate control were analyzed, excluding a study which used atrioventricular-node ablation with biventricular pacing as a rate control strategy
One trial assessed LVEF by 2 methods with different results. Both results were used in a sensitivity analysis.
The LVEF for inclusion in 3 trials was <40% and in one trial was <50%. The trial with LVEF criterion of <50% was excluded as a sensitivity analysis.
All trials except one had >80% of patients free of AF after ablation, except one trial, which had > 50% of patients free of AF. This trial was excluded as a sensitivity analysis.
All statistical analyses were performed using REVMAN software version 5.3. Two-tailed p values <0.05 were considered significant.
RESULTS
Study selection
Of 1144 papers originally retrieved by searching the databases, 4 met the inclusion criteria (Figure 1).
Figure 1.
PRISMA diagram showing search strategy results and exclusion steps.
Characteristics of included studies and patients
The 4 RCTs were published between 2008 and 2014 and involved 224 patients (22–25). Three studies were conducted in Europe and one in both Europe and the United States. All trials were published in English.
The mean age of patients included in the trials ranged from 57 to 63 years. The proportion of males in the studies was 89%. Ischemic cardiomyopathy was the most common etiology for HF in the included patients. Three of the trials included patients with only persistent AF (23–25), whereas one trial included both paroxysmal and persistent AF (22). All but 39 of the included 224 patients had persistent AF. The mean duration of persistent AF was over one year. The mean LVEF of the included patients was 26.1% and all patients had New York Heart Association (NYHA) functional classification of II or III. Further patient characteristics are listed in Table 1.
Table 1.
Patient Demographics
| First Author (Reference) | Khan (22) | Jones (24) | Macdonald (23) |
Hunter (25) |
|---|---|---|---|---|
| Year of publication | 2008 | 2013 | 2010 | 2014 |
| Mean Age (years) | 60.5 ± 8.0 | 63 ± 9.5 | 63.3 ± 7.5 | 57.4 ± 11.0 |
| Randomized arm (AF catheter ablation /rate control) | 41 / 40 | 26 / 26 | 22 / 19 | 26 / 24 |
| Mean Follow up, Months | 6 | 12 | ≥6 | 6 |
| Sex, N (male/female) | 74 / 7 | 45 / 7 | 32 / 9 | 48 / 2 |
| Coronary Artery Disease, % | 70.5 | 46.0 | 51.4 | 25.9 |
| Persistent AF, % | 48.5 | 100.0 | 100.0 | 100.0 |
| Duration of continuous AF, months | n/a | 23.5 ± 25.5 | 53.3 ± 42.7 | 24 (range 12-48) |
| Time since HF diagnosis, months | n/a | 58 ± 60 | n/a | 26.3 (range 14-56) |
| Time Since first AF diagnosis, months | 47.4 ± 31.1 | 51 ± 60 | n/a | n/a |
| Body Mass Index | n/a | 29.4±4.6 | 30 ± 5.6 | n/a |
| Hypertension, % | n/a | 33.3 | 61.0 | n/a |
| Diabetes Mellitus, % | n/a | 23.5 | 26.8 | n/a |
| Prior TIA/ Stroke, % | n/a | 11.8 | 9.8 | n/a |
| Chronic Lung disease, % | n/a | 13.7 | 22.0 | n/a |
| NYHA Class | 2.48 ± 0.5 | |||
| 1, % | 0 | 0 | 0 | |
| 2, % | 52 | 10 | 45 | |
| 3, % | 2 or 3 100% | 48 | 90 | 55 |
| 4, % | 0 | 0 | 0 | |
| Cardiomyopathy | n/a | |||
| Ischemic, % | 32.5 | 48.6 | 26 | |
| Non-ischemic % | 67.5 | 51.4 | 74 | |
| QRS duration at ECG, msec | 91 ± 9.5 | 116 ± 20 | 103 ± 14 | n/a |
| Baseline medical therapy | ||||
| - Amiodarone, % | n/a | 12 | n/a | |
| - ACE I/ ARB, % | 100 | 98 | 95 | |
| - Beta-blockers, % | 100 | 92 | 88 | n/a |
| - Aldosterone antagonist % | 100 in NYHA | 36.5 | 31.6 | |
| - Digoxin, % | 3 n/a |
54 | 51.3 | |
| LVEF at baseline, % | 28.0 ± 7.5 | 23.5 ± 7.8 | 17.7 ± 6.9 | 32.7 ± 10 |
| Left atrial diameter, cm | 4.8 ± 0.6 | 4.8 ± 0.7 | n/a | 5.1 ± 1 |
| Baseline 6 minute walk, meters | 275 ± 49 | 413.5 ± 94 | 333.7 ± 121 | n/a |
| Baseline pro BNP, pg/ml | n/a | 347.5 ± 309 | 2216.5 ± 1951 | 500 ± 1255 |
| MLWHF score at baseline | 89 ± 11.4 | 45.5 ± 22.0 | 57.4 ± 20.1 | 44.4 ± 89 |
| Peak VO2 at baseline | n/a | 17.25 ± 5 | n/a | 20.8 ± 13 |
| AF Free Survival post AF catheter ablation, % | 88 | 88 | 50 | 81 |
| Prior antiarrhythmic drugs | n/a | n/a | ||
| - AF catheter ablation group % | 34.6 | 53.8 | ||
| - Rate Control group % | 34.6 | 41.7 | ||
Results are reported as mean ± SD or median (interquartile range), unless otherwise stated.
All of the included trials were of high quality (≥3/5) according to the Jadad quality assessment score. None of the included trials attempted double blinding. Dropouts and withdrawals were described appropriately in the included trials. The blanking period ranged from two to three months. The percentage of patients requiring a repeat procedures ranged from 19.5 to 53.7%. Only one study had crossover of patients and intention to treat analysis was used. Please refer to Table 2 for further details.
Table 2.
Interventions Characteristics
| First Author (Reference) | Khan (22) | Jones (24) | Macdonald (23) |
Hunter (25) |
|---|---|---|---|---|
| Blanking Period (Months) | 2 | 2 | 3 | 3 |
| Frequency of monitoring (Months) | 2, 3*, 6 | 2, 3, 6, 12 | 3*, 6 | 1, 3, 6 |
| Modality of assessing heart rhythm | Loop Recorder | 48 hour Holter Monitor Existing implanted devices |
24 hour Holter Monitor | 48-hour Holter Monitor |
| AAD strategy post ablation | AAD for 2 months | AAD stopped post ablation | Amiodarone for 3 months | AAD stopped post ablation |
| Number of patients undergoing repeat procedures (%) | 8 (19.5) | 5 (20.1) | 6 (30.0) | 14 (53.7) |
| Crossover | None | 2** | None | None |
| Ablation strategy of AF | PVI +/− Linear lesions and sources of complex fractionated electrograms | PVI +/− Linear lesions +/− left atrial complex fractionated electrograms +/− Cardioversion and cavotricuspid isthmus ablation. | PVI +/− Linear lesions and sources of complex fractionated electrograms +/− Cardioversion +/− cavotricuspid isthmus ablation | PVI with ablation of complex or fractionated electrograms +/− Linear lesions +/− Cavotricuspid isthmus ablation |
| Follow up (Months) | 6 | 12 | 6 | 6 – 12 |
Only in the AF catheter ablation group
1 patient in AF catheter ablation group and 1 patient in the rate control group. Intention to treat analysis used.
AAD=antiarrhythmic drug
Outcomes
LVEF
Data for LVEF were available from all included trials. There was no significant heterogeneity (I2 = 0%) nor detectable publication bias.
AF catheter ablation compared to rate control was associated with an 8.5% increase in LVEF at 6 to 12 months (mean difference 8.53; 95% CI 6.4, 10. 7; P<0.001). The improvement in LVEF in the AF catheter ablation arm compared to rate control was evident in all of the included trials (Figure 2A).
Figure 2. Changes in Functional Outcomes.
A. Change in LVEF. B. Change in MLWHF. C. Change in 6-Minute Walk Test Distance. D. Change in Peak VO2. Mean difference (MD) and 95% confidence intervals (CI) in studies comparing AF catheter ablation to rate control in HF patients.
Quality of life and functional capacity measures
AF Catheter ablation was superior to rate control strategy in improving quality of life. Data on MLWHF were available from all the included trials. Across the included trials, there was no evidence of significant heterogeneity (I2 = 8%) or publication bias. There was a significant improvement in MLWHF questionnaire scores in the AF catheter ablation intervention group versus the rate control group (mean difference −11.9; 95%CI −17.1, −6.6; P<0.001) (Figure 2B).
Data on 6-minute walk tests were available from three prospective clinical trials. Across the included trials, there was evidence of moderate heterogeneity (I2 =45%). Significant improvement in performance on 6-minute walk tests were observed in patients undergoing AF catheter ablation compared to a rate control strategy (mean difference 34.8; 95%CI 2.9, 66.7; P = 0.03) (Figure 2C).
Data on peak oxygen consumption (VO2) were available from two prospective clinical trials. Across the included trials, there was no evidence of significant heterogeneity (I2 = 0%). Peak VO2 significantly increased in AF catheter ablation compared to rate control patients (mean difference 3.2; 95% CI 1.1, 5.3; P=0.003). (Figure 2D).
Sensitivity analyses
One trial included patients with both paroxysmal and persistent AF, in addition to using AV node ablation with biventricular pacing as a rate control strategy (22). Upon exclusion of that trial, AF catheter ablation was still associated with significant improvements in LVEF (mean difference 7.8; 95% CI 4.2, 11.5; P<0.001) and MLWHF (mean difference −11.7; 95% CI −20.3, −3.8; P=0.007).
One trial assessed LVEF by radionuclide ventriculography and cardiovascular magnetic resonance (CMR) (23). Both results were used in separate analyses, both yielding statistically significant increases in LVEF in the AF catheter ablation group versus the rate control group. The same trial included patients with LVEF <50%, whereas the other 3 trials included patients with LVEF <40%. It also reported 50% of patients free of AF post ablation, whereas the other trials had >80% of patients free of AF post ablation. Upon exclusion of that trial (23), AF catheter ablation was still associated with significant improvements in LVEF (mean difference 8.8; 95% CI 6.3, 11.3; P<0.001) and MLWHF (mean difference −13. 8; 95% CI −19.3, 8.3; P<0.001).
Procedural Complications and Adverse Events
We were able to obtain previously unpublished data on complications and incorporate them into our analysis from two randomized controlled trials (23, 24). Two strokes, four cardiac tamponades and one pericardial effusion were seen in the AF catheter ablation, culminating in a procedural complication rate of 6.3%. Details of complications and adverse events are listed in Table 3.
Table 3.
Adverse Events
| Complications | Khan (22) | Jones (24) | Macdonald (23) | Hunter (25) | ||||
|---|---|---|---|---|---|---|---|---|
| AF Catheter Ablation |
Rate Control |
AF Catheter Ablation |
Rate Control |
AF Catheter Ablation |
Rate Control |
AF Catheter Ablation |
Rate Control |
|
| Major | ||||||||
| Death | - | - | 1** | - | - | - | - | 1 |
| Stroke | - | - | - | 1 | 1 | - | 1 | - |
| Intracranial Hemorrhage | - | - | - | - | - | - | - | 1 |
| Myocardial Infarction | - | - | - | 1 | - | - | - | - |
| Cardiac tamponade | - | - | 1 | - | 2 | - | 1 | - |
| Pericardial effusion | 1 | - | - | - | - | - | - | |
| Pneumothorax | - | 1 | - | - | - | - | - | |
| Minor | ||||||||
| Pulmonary vein stenosis | 2* | - | - | - | - | - | - | - |
| Temporary worsening of HF | 1 | - | 2 | 3 | 3 | 1 | - | - |
| Groin bleeding | 3 | - | 1 | - | - | - | - | - |
| Pocket hematoma | - | 2 | - | - | - | - | - | - |
| Chest infection | - | - | 1 | - | - | - | - | - |
| Ventricular lead dislodgement | - | 2 | - | - | - | - | - | - |
Mild asymptomatic pulmonary vein stenosis that did not require intervention
Death occurred 11 months post-ablation from progressive worsening of heart failure and end-stage lung disease.
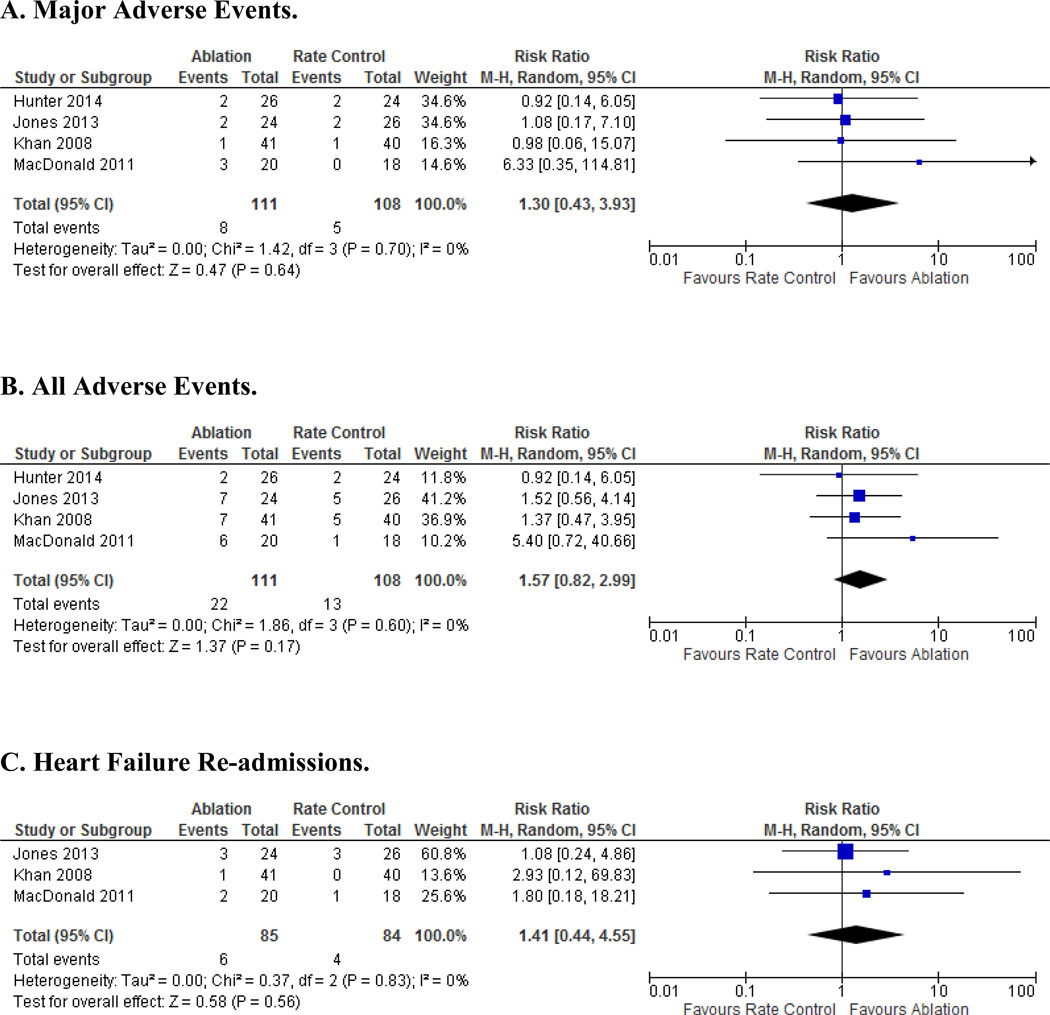
Overall, no statistically significant difference was found in the major adverse event rates between the AF catheter ablation (7.2%) versus the rate control (4.6%) arms (RR 1.3; 95% CI, 0.4, 3.9; P=0.64) (Figure 3A). Upon examining all reported adverse events, there was a trend toward more events in the AF catheter ablation (18.9%) compared to the rate control arm (12.0%), but this did not reach statistical significance (RR 1.6; 95% CI 0.82, 2.99; P=0.17) (Figure 3B).
Figure 3. Adverse Outcomes.
A. Major Adverse Events. B. All Adverse Events. C. Heart Failure Re-admissions. Odds ratios (OR) and 95% confidence intervals (CI) in studies comparing AF catheter ablation to rate control in HF patients.
No difference in HF re-admissions was seen between the two treatment groups (RR 1.4; 95% CI 0.44, 4.55; P= 0.56) (Figure 3C).
DISCUSSION
We present the first meta-analysis of high quality prospective randomized controlled trials comparing AF catheter ablation versus rate control strategies in patients with HF and AF.
Previous meta-analyses have relied mainly on data from observational studies in addition to a small number of randomized controlled trials (26 – 28). This resulted in heterogeneity that was highly significant, raising uncertainties about the consistency of the combined studies. The main outcomes studied were LVEF, BNP and complications; all concluded improvements in LVEF of 11–13%. Through collaboration with primary investigators of the included RCTs, our current meta-analysis provides similar conclusions regarding improvement in LVEF in addition to new insight into subjective and objective quality of life measures and complication rates, including heart failure readmissions. It also provides conclusions based on a larger number of randomized controlled trials, culminating in results with trivial heterogeneity among the majority of reported outcomes, suggesting strong homogeneity in clinical and methodological characteristics of the included randomized clinical trials.
We sought evidence from randomized controlled trials in an attempt to limit the influence of selection bias and control for unmeasured confounders (29). We also targeted the specific patient population of heart failure with reduced LVEF and documented AF, instead of including heart failure with preserved LVEF. At least 6 months of follow up was chosen partially to control for differences in the use of AAD after AF catheter ablation (30).
A significant improvement in LVEF was seen with the AF catheter ablation strategy in comparison to the rate control approaches. The trend in improvement of LVEF was evident among all of the included randomized clinical trials. Of note, 22 to 50% of patients were still in AF after AF catheter ablation, suggesting a role for reduction of AF burden, rather than AF cure, leading to the aforementioned benefits. Another possibility is that patients may have had better follow up and potentially better overall medical care in the AF catheter ablation group (Table 2). Several other observational studies have also shown improvement in LVEF in ablation compared to rate control arms (14–16). This highlights the importance of preserving or restoring the atrial contribution to cardiac hemodynamics, as the AF catheter ablation rhythm control strategy provides additional benefits over simple control of rapid ventricular rate in patients with HF in AF.
Novel findings from this meta-analysis included the marked improvements in the quality of life, 6-minute walk test performance and peak VO2 in the AF catheter ablation group. The improvement in peak VO2 carries particular significance given that it is a well-established prognostic indicator in HF with potential impact on survival and hospital stay (32–34). The change in distance walked on the 6-minute walk test has also been established as an independent predictor of survival (35). In one of the trials where half of patients in the AF catheter ablation remained in AF, there was no difference in the 6 minute walk test outcome between the two groups (23). This trial led to the moderate heterogeneity observed of this outcome. Nevertheless, the improvements in quality of life observed despite similar HF re-admission rates between the two groups are consistent with the overall improvements observed in the 6 minute walk test and peak VO2 (31). The concordant findings in these three outcomes provide both subjective and objective evidence for the benefits of catheter ablation for AF.
Although the inconsistency index statistic measure of heterogeneity does not capture differences in methodology between AF ablation approaches (e.g. PVI and various substrate ablation approaches), as well as between rate control approaches (e.g. AV nodal blocking medications vs. AV junction ablation), we performed sensitivity analyses, including repeating meta-analyses of LVEF and MLWHF without the one study using AV junction ablation for rate control (22) and found similar results.
Catheter ablation is an invasive procedure, which may provide benefits, but which also carries well known risks, such as stroke, pericardial tamponade, pericarditis, bleeding, pulmonary stenosis, atrioesophageal fistula, and even death, risks which may be even higher in a population with HF. The AF catheter ablation peri-procedural major complication rate in this study was 6.3%. Despite the structural changes seen in patients with reduced LVEF, the reported AF catheter ablation complication rate in this meta-analysis is comparable to, though perhaps slightly higher than, AF catheter ablation complication rates reported in a large prospective study (5.2%) and a recent meta-analysis in patients with structurally normal hearts (2.7–3.5%) (36–37). The trend toward higher adverse event rates compared to the rate control group (p=0.17) suggests there may be a price paid for improving the other outcomes measured in this study. Given the overall low adverse event rates, our study was likely under powered to detect statistically significant differences in adverse events. These risks are put in context to the rate control adverse event rates in this study of 12.5% in the pharmacological rate control studies and 10% in the study using AV node ablation and biventricular pacing. Crossover occurred in one trial in only two patients, making it less likely that crossovers can explain the comparability in the incidence of major complications. It is perhaps more plausible that AF ablation was associated with a relatively low rates of major adverse event complications, such as death or stroke (<1%), in these studies (38).
The majority (82.5%) of patients in the current study had persistent AF, so results may not be easily extrapolated to a population with paroxysmal AF. However, this high proportion of patients with persistent AF, as well as the sensitivity analyses demonstrating similar efficacy of AF catheter ablation compared to rate control for persistent AF, supports attempting an AF catheter ablation approach in this population despite persistence of AF. In current clinical practice, patients with HF and persistent AF may be more readily relegated to a rate control approach. Our findings suggest that significant additional benefit might be achieved by pursuing a rhythm control approach with AF catheter ablation. A well designed, adequately powered randomized controlled trial seems warranted to provide further data on safety, efficacy, survival and functional capacity outcomes associated with catheter ablation in patients with HF and AF. Subanalyses of patients with AF and HF in the ongoing CABANA trial of AF ablation vs antiarrhythmic drug use will also be of interest.
The current study has several limitations. First, patients were only followed up to one year, therefore limiting the assessment of long-term outcomes. Secondly, the number of patients included in these RCTs remains small. Thus, the included randomized clinical trials were not powered to assess hard outcomes, such as mortality. Thirdly, there may have been bias in selecting healthier patients, who were suitable candidates for an invasive strategy, for these randomized trials. A fourth limitation is the fact that the power of funnel plots in detecting publication bias increases with the inclusion of more studies; however, we were limited by the available trials in the literature. Nevertheless, the mean LVEF of patients was <35% in all studies, NYHA functional class was predominantly 2–3, and complication and adverse event rates were at the higher end of that reported in the literature, suggesting these patients might be representative of a HF population at large. And lastly, PVI alone was not used in the majority of cases; after PVI additional atrial substrate modification was used to varying degrees in each study. However, these approaches reflect contemporary local practice for catheter ablation of persistent AF.
CONCLUSIONS
An AF catheter ablation strategy in patients with AF and HF results in improved LV function, functional capacity, HF symptoms, and quality of life compared with a rate control strategy. Patients with HF may be at higher risk of complications with interventional approaches for both rhythm and rate control strategies. However, our analyses suggest that prior to accepting a rate control strategy in HF patients with persistent or drug refractory AF, an individualized approach should be pursued, including consideration to performing AF catheter ablation in appropriately selected patients.
PERSPECTIVES.
Competency in Medical Knowledge 1
Selecting the strategy to address atrial fibrillation in heart failure should consider several factors including the type of atrial fibrillation and patient’s preferences
Competency in Medical Knowledge 2
A strategy of AF catheter ablation may lead to improvement of atrial fibrillation with improvements in left ventricular function and functional capacity.
Competency in Interpersonal & Communication Skills
It is important to take into account the possibility of higher complication rates in a discussion of ablation versus rate control strategies.
Translational Outlook 1
The mortality rates associated with AF catheter ablation versus rate control strategy is an important potential area of research.
ACKNOWLEDGEMENTS
MKC is supported by NIH grant R01HL111314.
Abbreviations and Acronyms
- AF
atrial fibrillation
- HF
heart failure
- AADs
antiarrhythmic drugs
- PVI
pulmonary vein isolation
- LVEF
left ventricular ejection fraction
- MLWHF
Minnesota Living with Heart Failure
- NYHA
New York Heart Association
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relationships With Industry: Daniel Cantillon MD: Consulting/honoraria: St. Jude Medical
Khaldoun Tarakji MD MPH: Speaker / consulting honorarium: Medtronic, Spectranetics
REFERENCES
- 1.Chen LY, Shen WK. Epidemiology of atrial fibrillation: A current perspective. Heart Rhythm. 2007;4:S1–S6. doi: 10.1016/j.hrthm.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the united states. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 4.Berry C, Murdoch DR, McMurray JJ. Economics of chronic heart failure. Eur J Heart Fail. 2001;3:283–291. doi: 10.1016/s1388-9842(01)00123-4. [DOI] [PubMed] [Google Scholar]
- 5.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson WG, Stevenson LW, Middlekauff HR, et al. Improving survival for patients with atrial fibrillation and advanced heart failure. J Am Coll Cardiol. 1996;28:1458–1463. doi: 10.1016/s0735-1097(96)00358-0. [DOI] [PubMed] [Google Scholar]
- 7.Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: A retrospective analysis of the SOLVD trials. studies of left ventricular dysfunction. J Am Coll Cardiol. 1998;32:695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 8.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The framingham heart study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 9.Daoud EG, Weiss R, Bahu M, et al. Effect of an irregular ventricular rhythm on cardiac output. Am J Cardiol. 1996;78:1433–1436. doi: 10.1016/s0002-9149(97)89297-1. [DOI] [PubMed] [Google Scholar]
- 10.Clark DM, Plumb VJ, Epstein AE, Kay GN. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol. 1997;30:1039–1045. doi: 10.1016/s0735-1097(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 11.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 12.Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the atrial fibrillation follow-up investigation of rhythm management (AFFIRM) study. Circulation. 2004;109:1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 13.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 14.Chen MS, Marrouche NF, Khaykin Y, et al. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J Am Coll Cardiol. 2004;43:1004–1009. doi: 10.1016/j.jacc.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 15.Hsu LF, Jais P, Sanders P, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373–2383. doi: 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- 16.Sacher F, Corcuff JB, Schraub P, et al. Chronic atrial fibrillation ablation impact on endocrine and mechanical cardiac functions. Eur Heart J. 2008;29:1290–1295. doi: 10.1093/eurheartj/ehm577. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [updated March 2011]. Available from: URL: http://www.cochrane-handbook.org. [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 22.Khan MN, Jais P, Cummings J, et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359:1778–1785. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald MR, Connelly DT, Hawkins NM, et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: A randomised controlled trial. Heart. 2011;97:740–747. doi: 10.1136/hrt.2010.207340. [DOI] [PubMed] [Google Scholar]
- 24.Jones DG, Haldar SK, Hussain W, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61:1894–1903. doi: 10.1016/j.jacc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 25.Hunter RJ, Berriman TJ, Diab I, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial) Circ Arrhythm Electrophysiol. 2014;7:31–38. doi: 10.1161/CIRCEP.113.000806. [DOI] [PubMed] [Google Scholar]
- 26.Wilton SB, Fundytus A, Ghali WA, et al. Meta-analysis of the effectiveness and safety of catheter ablation of atrial fibrillation in patients with versus without left ventricular systolic dysfunction. Am J Cardiol. 2010;106:1284–1291. doi: 10.1016/j.amjcard.2010.06.053. [DOI] [PubMed] [Google Scholar]
- 27.Dagres N, Varounis C, Gaspar T, et al. Catheter ablation for atrial fibrillation in patients with left ventricular systolic dysfunction. A systematic review and meta-analysis. J Card Fail. 2011;17:964–970. doi: 10.1016/j.cardfail.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Anselmino M, Matta M, D'Ascenzo F, et al. Catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction: A systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2014 doi: 10.1161/CIRCEP.114.001938. [DOI] [PubMed] [Google Scholar]
- 29.Odgaard-Jensen J, Vist GE, Timmer A, et al. Randomisation to protect against selection bias in healthcare trials. Cochrane Database Syst Rev. 2011;(4):MR000012. doi: 10.1002/14651858.MR000012.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leong-Sit P, Roux JF, Zado E, et al. Antiarrhythmics after ablation of atrial fibrillation (5A study): Six-month follow-up study. Circ Arrhythm Electrophysiol. 2011;4:11–14. doi: 10.1161/CIRCEP.110.955393. [DOI] [PubMed] [Google Scholar]
- 31.Flynn KE, Lin L, Moe GW, et al. Relationships between changes in patient-reported health status and functional capacity in outpatients with heart failure. Am Heart J. 2012;163 doi: 10.1016/j.ahj.2011.09.027. 88,94.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Groote P, Dagorn J, Soudan B, Lamblin N, McFadden E, Bauters C. B-type natriuretic peptide and peak exercise oxygen consumption provide independent information for risk stratification in patients with stable congestive heart failure. J Am Coll Cardiol. 2004;43:1584–1589. doi: 10.1016/j.jacc.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 33.Corra U, Mezzani A, Bosimini E, Giannuzzi P. Cardiopulmonary exercise testing and prognosis in chronic heart failure: A prognosticating algorithm for the individual patient. Chest. 2004;126:942–950. doi: 10.1378/chest.126.3.942. [DOI] [PubMed] [Google Scholar]
- 34.Swank AM, Horton J, Fleg JL, et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: Results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail. 2012;5:579–585. doi: 10.1161/CIRCHEARTFAILURE.111.965186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Passantino A, Lagioia R, Mastropasqua F, Scrutinio D. Short-term change in distance walked in 6 min is an indicator of outcome in patients with chronic heart failure in clinical practice. J Am Coll Cardiol. 2006;48:99–105. doi: 10.1016/j.jacc.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 36.Bohnen M, Stevenson WG, Tedrow UB, et al. Incidence and predictors of major complications from contemporary catheter ablation to treat cardiac arrhythmias. Heart Rhythm. 2011;8:1661–1666. doi: 10.1016/j.hrthm.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Gupta A, Perera T, Ganesan A, et al. Complications of catheter ablation of atrial fibrillation: A systematic review. Circ Arrhythm Electrophysiol. 2013;6:1082–1088. doi: 10.1161/CIRCEP.113.000768. [DOI] [PubMed] [Google Scholar]
- 38.Scheinman MM, Huang S. The 1998 NASPE prospective catheter ablation registry. Pacing Clin Electrophysiol. 2000;23:1020–1028. doi: 10.1111/j.1540-8159.2000.tb00891.x. [DOI] [PubMed] [Google Scholar]