Abstract
Mice double deficient in LAMP-1 and -2 were generated. The embryos died between embryonic days 14.5 and 16.5. An accumulation of autophagic vacuoles was detected in many tissues including endothelial cells and Schwann cells. Fibroblast cell lines derived from the double-deficient embryos accumulated autophagic vacuoles and the autophagy protein LC3II after amino acid starvation. Lysosomal vesicles were larger and more peripherally distributed and showed a lower specific density in Percoll gradients in double deficient when compared with control cells. Lysosomal enzyme activities, cathepsin D processing and mannose-6-phosphate receptor expression levels were not affected by the deficiency of both LAMPs. Surprisingly, LAMP-1 and -2 deficiencies did not affect long-lived protein degradation rates, including proteolysis due to chaperone-mediated autophagy. The LAMP-1/2 double-deficient cells and, to a lesser extent, LAMP-2 single-deficient cells showed an accumulation of unesterified cholesterol in endo/lysosomal, rab7, and NPC1 positive compartments as well as reduced amounts of lipid droplets. The cholesterol accumulation in LAMP-1/2 double-deficient cells could be rescued by overexpression of murine LAMP-2a, but not by LAMP-1, highlighting the more prominent role of LAMP-2. Taken together these findings indicate partially overlapping functions for LAMP-1 and -2 in lysosome biogenesis, autophagy, and cholesterol homeostasis.
INTRODUCTION
One crucial role of the membrane limiting late endosomes and lysosomes is to separate the potent activities of lysosomal acid hydrolases from other cellular constituents. Protein components of the lysosomal membrane mediate a number of essential functions of this compartment, including the acidification of the lysosomal lumen, transport of amino acids, fatty acids, and carbohydrates resulting from the hydrolytic degradation as well as other nutrients generated by lysosomal hydrolases. In addition, lysosomal membrane proteins may be involved in the interaction and fusion of the lysosomes with themselves as well as with other cell components, including endosomes, phagosomes, and the plasma membrane (Fukuda, 1991). Several highly glycosylated proteins of the lysosomal membrane have been identified (Peters and von Figura, 1994; Hunziker et al., 1996) but their specific functions are largely unknown.
Lysosome associated membrane protein-1 (LAMP-1) and LAMP-2 are major protein components of the lysosomal membrane. They are type I transmembrane proteins with a large luminal domain, one transmembrane domain, and a C-terminal cytoplasmic tail. The conserved cytosolic tails of LAMP-1 and -2 are 11 residues long and contain necessary information for their intracellular targeting after biosynthesis (Hunziker et al., 1996). Despite their 37% amino acid sequence homology, LAMP-1 and -2 are distinct proteins which most likely diverged relatively early in evolution as evidenced by their localization on different chromosomes (Fukuda, 1991). To address the specific functions of LAMP-1 and -2, we previously generated mice deficient in each of these proteins. LAMP-1-deficient mice were viable and fertile. Apart from a mild regional astrogliosis and altered immunoreactivity against cathepsin D in the brain, all tissues of these mice were normal (Andrejewski et al., 1999). Although LAMP-1 is an abundant protein component of the lysosomal membrane, lysosomal properties including enzyme activities, pH, osmotic stability, density, morphology, subcellular distribution, and lysosomal enzyme processing were similar to controls in LAMP-1-deficient cells. An upregulation of LAMP-2 protein was observed in kidney, spleen, and heart of LAMP-1-deficient mice, whereas the levels of another lysosomal membrane protein, LIMP-2, were unaffected. This upregulation was also evident in tissues lacking only one LAMP-1 allele, suggesting compensation of LAMP-1 by LAMP-2.
In contrast to the relatively mild phenotype in LAMP-1 knockout mice, deficiency of LAMP-2 caused a more severe phenotype. Fifty percent of the mice died at the age of 20-40 days (Tanaka et al., 2000). Electron microscopy revealed a massive accumulation of autophagic vacuoles in several tissues including liver, pancreas, spleen, kidney, skeletal muscle, heart, capillary endothelium, intestinal wall, lymph nodes, and neutrophilic leukocytes. Both skeletal and cardiac muscle cells showed an accumulation of large autophagic vacuoles. Autophagy is an intracellular bulk degradation pathway that plays an important role in the cellular protein economy (Mizushima et al., 2002). Autophagic vacuoles originate from isolation membranes that finally close to form sealed autophagosomes, which then fuse with late endosomes/lysosomes (Mizushima et al., 2003). The physiological importance of LAMP-2 is supported by the finding that LAMP-2 deficiency is the primary defect in Danon disease (Nishino et al., 2000), a lysosomal glycogen storage disease with normal acid maltase activity (Danon et al., 1981).
Reduced degradation of long-lived proteins, which mainly occurs via autophagy, and quantitative electron microscopy indicated that retarded consumption rather than increased formation of autophagic vacuoles was the cause for the accumulation of autophagic vacuoles in LAMP-2-deficient hepatocytes (Tanaka et al., 2000; Eskelinen et al., 2002a). We further detected an elevated secretion of a subset of lysosomal enzymes and impaired processing of cathepsin D in LAMP-2-deficient hepatocytes. We proposed these defects to be due to a reduced half-life of the 46 kDa mannose-6-phosphate receptor (MPR). In addition, we found that the localization of this receptor was shifted from the trans-Golgi network (TGN) toward autophagic vacuoles.
One of the three isoforms of LAMP-2, LAMP-2a, was proposed to be the receptor for a lysosomal pathway of selective uptake and degradation of cytosolic proteins (Cuervo and Dice, 1996). This pathway is known as chaperone-mediated autophagy because it requires various chaperones for the transport through the lysosomal membrane (Agarraberes and Dice, 2001). Chaperone-mediated autophagy was shown to be activated in confluent cells including human lung fibroblasts by serum withdrawal and in rat tissues including liver by prolonged starvation (reviewed in Dice, 2000).
We now describe the generation of mice, double deficient in both LAMP-1 and LAMP-2. The double-deficient mice died between embryonic days 14.5 and 16.5, with a variable overall pathomorphology mainly featuring facial dysgenesis. Electron microscopy revealed that most tissues including Schwann cells and endothelium accumulated autophagic vacuoles. In this article we address the specific functions of LAMP-1 and -2 in lysosomal biogenesis, autophagy, chaperone-mediated autophagy, and cholesterol homeostasis using single- and double-deficient fibroblast cell lines isolated from the knockout mouse embryos.
MATERIALS AND METHODS
Generation of LAMP-1 and -2 Double-deficient Mice
LAMP-1-deficient mice (Andrejewski et al., 1999) and LAMP-2-deficient mice (Tanaka et al., 2000) were mated. Doubly heterozygote (LAMP-1+/-; LAMP-2+/-) females of the following generation were used for further breeding with LAMP-1-deficient males. Neonatal and postnatal offspring of this generation were genotyped using established PCR protocols for the targeted LAMP-1 and -2 gene loci (Andrejewski et al., 1999; Tanaka et al., 2000). In addition immunoblot analysis was performed to confirm the complete absence of LAMP-1 and -2 protein.
Isolation and Culture of Fibroblasts from Mouse Embryos
Mouse embryonic fibroblasts (MEF) were isolated from 12.5-13.5-day-old embryos by trypsin digestion. Independent cell lines derived from individual embryos were used for all experiments. Primary cells, at passage below 10, were used in most of the experiments. Some of the cell lines underwent a spontaneous immortalization around passage 10-20. Some cell lines were immortalized by transfection with a plasmid containing the Simian Virus 40 large T antigen. At least two independent cell lines with identical genotype were used for most experiments. Cells were grown in Dulbecco's MEM (DMEM) containing 10 or 20% fetal calf serum and antibiotics. Amino acid starvation of cells was performed using serum-free EBSS medium (Invitrogen, Karlsruhe, Germany) or Krebs-Henseleit buffer.
Antibodies
Antibodies used in this article have been described earlier (Eskelinen et al., 2002a). In addition, we used rabbit anti-LC3 (Isei Tanida and Takashi Ueno, Juntendo University, Tokyo, Japan), rat anti-mouse LAMP-1 and rat anti-mouse LAMP-2 (Developmental Studies Hybridoma Bank, Iowa), rabbit anti-rat LIMP-2/LGP-85 (Kuronita et al., 2002), rabbit anticathepsin L (Yoshitaka Tanaka), mouse antilysobisphosphatidic acid (Jean Gruenberg, University of Geneva, Switzerland), rabbit anti-mouse NPC1 (William Garver, University of Arizona), rabbit anti-human NPC2 (Shutish Patel, New England Biomedical Research Center, Newington), rabbit anti-rab7 (Suzanne Pfeffer, Stanford University), and rabbit antidinitrophenol (ICN ImmunoBiologicals, Lisle, IL).
Immunofluorescence
Cells were grown on coverslips and fixed in cold methanol or 4% paraformaldehyde in phosphate-buffered saline (PBS). After aldehyde fixation, cells were permeabilized in 0.1% Triton X-100 or 0.2% saponin in PBS. Primary and secondary antibodies were diluted in 3% bovine serum albumin (BSA) in PBS and incubated on the cells for 1 h. Goat anti-rabbit, -rat, or -mouse conjugated to Alexa Fluor 488 or 594 (Molecular Probes, Eugene, OR) were used as secondary antibodies. For filipin or Nile Red staining, paraformaldehyde-fixed cells were incubated with 0.5 mg/ml filipin (Sigma, Munich, Germany) or 5 μg/ml Nile Red (Molecular Probes) in PBS for 60 min and washed with PBS. After filipin staining, immunofluorescence labeling was performed without a separate permeabilization step. Acidic compartments were labeled by incubating cells in Lysotracker Red or DAMP (N-(3-((2,4-dinitrophenyl)amino) propyl)-N-(3-aminopropyl) methylamine) (Molecular Probes). The coverslips were mounted with Mowiol containing the antifading reagent DABCO (1,4 diazobicyclo-(2.2.2) octane), and viewed with a Zeiss Axiovert 200M fluorescence microscope (Göttingen, Germany) with or without an Apotome device for optical sectioning.
Morphology and Electron Microscopy of Embryos
For electron microscopic investigation three controls (LAMP-1+/-, LAMP-2+/-) and three double-knockout embryos at embryonic day 12.5 were used. They were fixed by immersion in 6% glutaraldehyde (in 0.1 M phosphate buffer, pH 7.4), dissected in transversal or sagittal plain, postfixed with 2% osmium tetroxide, and embedded in Araldite according to standard methods. Semithin sections were stained with toluidine blue. For ultrathin sections the tissue blocks were trimmed to obtain sections from the following organs: neural tube, dorsal root ganglia, and roots of spinal nerves, heart, and liver. Ultrathin sections were stained with uranyl acetate and lead citrate and viewed with a Zeiss EM 900.
Electron Microscopy of Cultured Cells
Cells were grown on 6-cm cell culture dishes to subconfluency. Fixation was performed with 2.5% glutaraldehyde in 0.2 M HEPES, pH 7.4, for 2 h. The cells were scraped off the dish after 30-min fixation and pelleted. Postfixation was done using 1% osmium tetroxide in water for 1 h. The pellets were then dehydrated and embedded in Epon. The number of autophagic vacuole profiles per cell area was counted under the microscope using 12,000 × g magnification. Cell area was estimated by point counting from negatives taken at 400 × g magnification, covering the area of one grid square. Two to five grid squares were counted for each sample.
Subcellular Fractionation
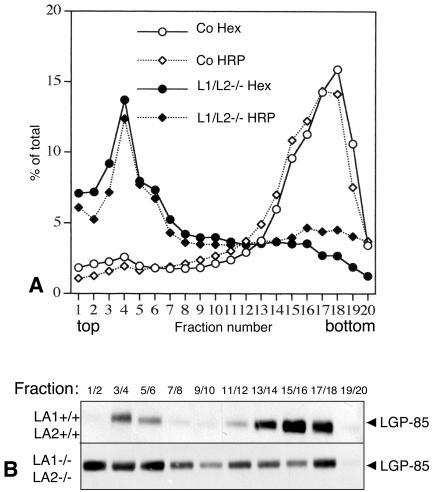
Tissue homogenates of fibroblasts were prepared in 0.25 M sucrose, 3 mM imidazole/HCl, pH 7.4. Postnuclear supernatant (0.8 ml) was applied onto 11.2 ml of Percoll solution to obtain a final Percoll concentration of 27%, and the gradient was centrifuged for 30 min at 20,000 rpm in the vertical rotor VTi 65.1 (Beckmann Instruments, Berkeley, CA). Density as well as horseradish peroxidase (HRP) and β-hexosaminidase activities were determined in the collected fractions. LIMP-2/LGP-85 content was estimated by Western blotting by combining two adjacent fractions into one sample.
Western Blotting
Cells were extracted using Tris-buffered saline (TBS), proteinase inhibitor cocktail (Roche, Mannheim, Germany), and 1 or 0.5% Triton X-100. For LC3 Western blotting, cells were extracted with PBS containing 2% NP-40, 0.2% SDS, and proteinase inhibitor cocktail. After SDS-PAGE, the proteins were transferred to PVDF membranes using a semidry blotting system. Transfer efficiency was checked with Ponceau staining. The membranes were subsequently blocked with 5% skimmed milk powder and incubated with the primary antibody. After washing in PBS, 0.1% Tween 20, the blots were incubated with HRP-coupled secondary antibodies. Signals were visualized using the ECL-Detection System (Amersham, Freiburg, Germany). LC3 Western blotting was done using 15% SDS-PAGE according to (Kabeya et al., 2000). Quantitation of the bands was performed by densitometry using AIDA 3.2.1 and Gel-ProAnalyser 3.1 Software.
Cholesterol Determination
The amount of cholesterol was measured in cell extracts prepared using 50 mM Tris, pH 8.0, 2 mM CaCl2, 80 mM NaCl, 1% Triton X-100, with the Amplex Red Cholesterol Assay kit (Molecular Probes). For the fluorometric quantification, 25 μl of cell extracts and cholesterol standards were incubated in 96-well plates according to the instructions of the manufacturer, and fluorescence was detected using Fluorolite1000 (Dynatech Laboratories, Alexandria, VA).
Protein Degradation
Growth curves were determined for each cell culture before and parallel to the protein degradation experiments. To label proteins, MEFs were incubated at 37°C for 48 h in complete fresh DMEM (Sigma) containing 20% (vol/vol) FCS (fetal calf serum, Invitrogen) and with 5 μCi/ml l-[3H]leucine or 5 μCi/ml l-[3H]valine (Amersham Pharmacia Biotech Europe, Freiburg, Germany). Before the proteolysis experiments were sarted, the cells were washed once with PBS containing 2 mM l-leucine or 10 mM l-valine and chased at 37°C for 24 h in complete DMEM containing 20% (vol/vol) FCS and 2 mM l-leucine or 10 mM l-valine to eliminate short-lived proteins. For prolonged starvation, the cells were washed and chased as above but in DMEM without FCS.
Proteolysis experiments and measurements of intracellular protein degradation were carried out as described previously (Fuertes et al., 2003a, 2003b). According to previous results with human fibroblasts (Fuertes et al., 2003a, 2003b) and with MEFs (our unpublished results), NH4Cl (20 mM) + leupeptin (0.1 mM) were used to inhibit all lysosomal proteolytic pathways and 3-methyladenine (3MA; 10 mM) to specifically inhibit macroautophagic degradation. The contribution of the nonmacroautophagic lysosomal pathways (i.e., microautophagy, chaperone-mediated autophagy, etc.) was calculated by subtracting the inhibition obtained with 3MA from the inhibition obtained with NH4Cl + leupeptin. The contribution of the nonlysosomal pathways to intracellular protein degradation was calculated from the degradation remaining after inhibition with NH4Cl + leupeptin. Protein degradation was analyzed 1 h after the addition of the different inhibitors and for a period of only 3 additional hours to ensure optimal inhibition and to avoid possible secondary effects of the inhibitors. All experiments were performed at least six times with duplicate or triplicate samples and using three independent cell lines (for control and single-deficient cells) or one cell line (for double-deficient cells). Student's t test analyses were performed using GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA).
Plasmids and Transfection:
Mouse NPC1 in pEGFP-N3 (Clontech, Palo Alto, CA) and mouse NPC2 in pcDNA3.1 (Invitrogen, Carlsbad, CA) were from Matthew Scott, Stanford University. Dog rab7 in pEGFP-C1 (Clontech) was from Cecilia Bucci, University of Lecce, Italy. Rat-LAMP1 cDNA in pcDNA 3.1 was from Stefan Höning, University Göttingen, Germany. Mouse LAMP-2a cDNA was prepared by RT-PCR from mouse liver and subcloned into pcDNA 3.1. Transfections were performed with Fugene 6 (Roche, Mannheim, Germany) according to the manufacturer's instructions.
RESULTS
Deficiency of Both LAMPs Caused Embryonic Lethality
We used LAMP-1- and LAMP-2-deficient mice for the generation of mice lacking both LAMP proteins. LAMP-1-/- females were mated with LAMP-2y/- males. In the offspring of these matings LAMP-1+/-, LAMP-2+/- mice were obtained and females were further used to breed with LAMP-1-/- males. In the next generation eight different combinations of LAMP genotypes were observed (Supplement 1A). In one of eight offspring (12.5%) LAMP double-deficient (LAMP-1-/-, LAMP-2y/-) mice were expected. However, after monitoring and genotyping 90 mice at ∼2-3 weeks of age, no viable LAMP-1-/-, LAMP-2y/- offspring were observed (Supplement 1B), indicating that the lack of both LAMP proteins resulted in embryonic lethality. We also observed that the number of male mice with only one intact LAMP-1 allele (LAMP-1+/-, LAMP-2y/-) was much lower than expected. All three mice obtained with this genotype died within 10 days of age suggesting a dose-dependent effect of LAMP expression on the viability (Supplement 1B). To determine the stage where embryonic development ceased, pregnancies were terminated starting at day 13.5 pc, the last day of organogenesis in mouse development, to 16.5 pc. At day 13.5 pc, 5 (11.3%) of 44 embryos were double deficient. Also at day 14.5 pc, 11 (12.5%) of 87 embryos were LAMP-1-/-, LAMP-2y/-. At this age the majority of these embryos (7 of 11) looked less developed compared with control or single-deficient littermates. At day 16.5 pc, no viable LAMP-1-/-, LAMP-2y/- embryos were identified. Taken together these data suggested that LAMP double deficiency lead to prenatal death between embryonic days 14.5 and 16.5. To verify the absence of LAMP proteins, we derived embryonic fibroblast cell lines from day 12.5 and 13.5 pc embryos. The absence of both LAMP proteins was confirmed by immunoblot experiments (Figure 1A) and immunofluorescence labeling (our unpublished results).
Figure 1.
(A) Immunoblot analysis of extracts from mouse embryonic fibroblasts derived from E12.5 embryos. The expression of lysosomal membrane proteins LAMP-1, LAMP-2, and LIMP-2/LGP-85 was analyzed. (B-G) Macroscopic phenotype of LAMP-1/LAMP-2-deficient embryos (C, D, E, and G) in comparison with control (LAMP-1+/+/LAMP-2+/+ littermates; B and F). (H) Microscopic organization of the organs of a double-deficient embryo (E12) as seen in a transversal section. NT, neural tube. DRG, dorsal root ganglion. Br, bronchus. H, heart. Semithin section, toluidine blue. Bars in B-E, 5 mm; F and G, 2 mm; H, 0.5 mm.
LAMP-1/LAMP-2 double-deficient mouse embryos exhibited a variable macroscopic phenotype, ranging from virtually normal (compare wild type in Figure 1, B and F, with LAMP-1/LAMP-2 double deficient in Figure 1, C and D) to a complex pattern of predominantly craniofacial (Figure 1, E and G) abnormalities. In the latter case, the main features consisted of a foreshortened forebrain and head and a severely reduced length of both the maxillary and mandibular portions of the jaws combined with the absence of detectable vibrissal anlagen. In several cases, the closure of the ocular choroid fissure appeared incomplete (Figure 1, E and G), and in these “severe” cases the developing external ear was likewise missing.
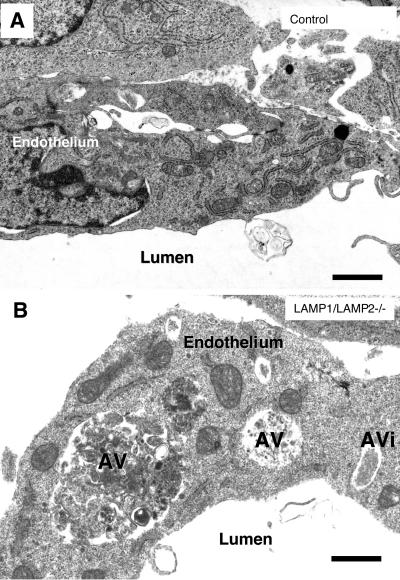
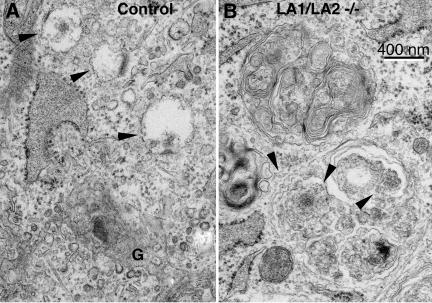
The general microscopic appearance of the organs was essentially similar in controls and double knockouts (Figure 1H). However, on the cellular level, double-knockouts showed a much higher frequency of cytoplasmic vacuoles, which could often be identified as autophagic vacuoles. These vacuoles were most regularly seen in vascular endothelium (Figure 2B compared with a control embryo in Figure 2A) and Schwann cells (Supplement 2A), furthermore they occurred in the neuroepithelium (Supplement 2, B and C) and occasionally in hepatocytes (our unpublished results), whereas they were not detected in the myocardium.
Figure 2.
Autophagic vacuoles accumulated in cells of LAMP-1/LAMP-2 double-deficient embryos. (A) Endothelium of a wild-type embryo. (B) Endothelium of a blood vessel in the neural tube in a LAMP-1/LAMP-2 double-deficient embryo. The endothelial cells displayed several cytoplasmic vacuoles with polymorphous contents. Some of the vacuoles could be identified as autophagic vacuoles (AV) or autophagosomes (AVi). Bars, 0.5 μm.
Autophagic Vacuoles Accumulated in Double-deficient Fibroblasts during Starvation
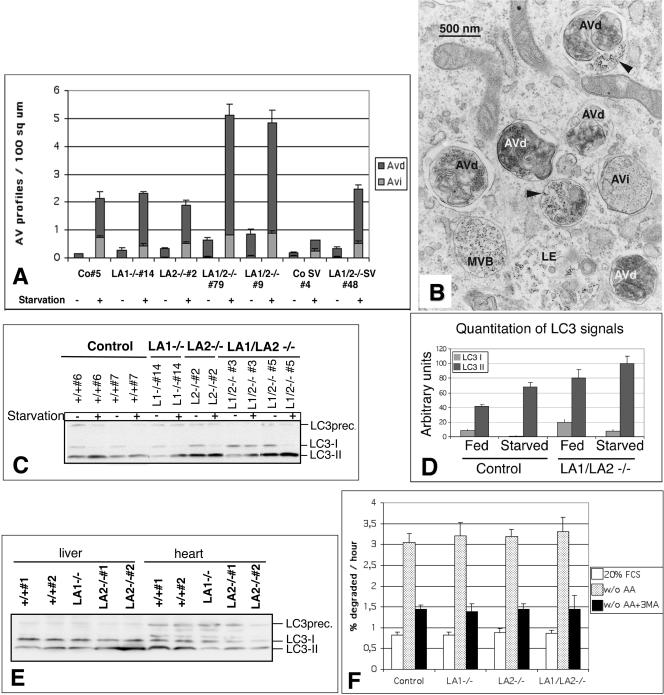
To get a deeper insight into the cellular functions in fibroblasts lacking both LAMP-1 and -2, we analyzed these cells morphologically and biochemically. First we investigated whether the autophagic accumulation observed in situ was also preserved in fibroblast cells derived from LAMP-double-deficient embryos. Quantitative electron microscopy revealed that after 2 h of starvation in serum- and amino acid-free medium, ∼2.5 times more autophagic vacuoles accumulated in the double-deficient cell lines than in control cell lines or single-deficient cell lines (Figure 3A). Also without starvation the number of autophagic vacuoles was higher in the double-deficient cells as compared with single knockout and control cells (Figure 3A). Interestingly, in the double-deficient cells the amount of early autophagic vacuoles was comparable to control cells, but the amount of late vacuoles, containing partially degraded cytoplasmic material and also a fluid-phase endocytic marker (Figure 3B), was increased. This suggested that although initial maturation of autophagosomes including fusion with endosomes was functional, the final maturation step of the late autophagic vacuoles was retarded. The autophagic vacuole accumulation was confirmed by analyzing the levels of the autophagosome marker protein LC3 by Western blotting (Kabeya et al., 2000). The ratio of the cytoplasmic form (LC3I) to the membrane-bound form (LC3II) was comparable in all genotypes with and without starvation (Figure 3C). However, in both starved and nonstarved LAMP-1/LAMP-2 double-deficient cells (Figure 3D), and also in LAMP-2 single-deficient cells (our unpublished results), the absolute amounts of LC3I and LC3II were increased as compared with controls. We showed earlier LAMP-2 single-deficient liver and heart muscle tissues to have an excessive autophagic vacuole accumulation (Tanaka et al., 2000). In liver tissues LC3II level was clearly increased compared with controls, whereas in heart muscle, where a less severe accumulation of vacuoles was observed by electron microscopy, LC3I and LC3II levels were not increased (Figure 3E). This suggested that electron microscopy was the most sensitive way to detect autophagic vacuole accumulation, particularly when mainly late autophagic vacuoles were accumulating.
Figure 3.
Accumulation of autophagic vacuoles in LAMP-1/LAMP-2 double-deficient MEFs. (A) Primary and SV-40 large T-antigen immortalized (CoSV, LA1/2-/-SV) MEFs were cultured in the presence or absence of serum and amino acids for 2 h. The amounts of early (Avi) and late autophagic vacuoles (Avd) were determined by quantitative electron microscopy. Co, control cells; LA1-/-, LAMP-1-deficient cells; LA2-/-, LAMP-2-deficient cells, LA1/2-/- double-deficient cells. The numbers (5, etc.) indicate individual cell lines. (B) Electron micrograph of LAMP-1/LAMP-2 double-deficient cells (79), which were fed with BSA gold in serum-free medium for 2 h to label endosomes and lysosomes. Early (AVi) and late autophagic vacuoles (AVd) were abundant. Multivesicular bodies (MVB) and late endosomes (LE) containing BSA gold are also indicated. Arrowheads indicate late autophagic vacuoles which have fused with BSA-gold positive endosomes. (C) Western blotting of the autophagic marker protein LC3 in nonstarved cells and in cells incubated in serum and amino acid free medium for 2 h. The locations of LC3 precursor (LC3prec.) as well as LC3I and LC3II are indicated on the right. (D) Quantitation of the absolute levels of LC3I and LC3II in the Western blot shown in C for control and double-deficient cells. The values represent the average and SD of cell lines with identical genotype. (E) LC3 Western blot of LAMP-1 and LAMP-2 single-deficient liver and heart extracts. (F) Degradation of long lived proteins in the absence or presence of 3-methyladenine (3MA). MEFs were metabolically labeled with [3H]valine or [3H]leucine and then chased for 24 h in full medium. Cells were then switched to culture medium with serum (20% FCS) or to serum and amino acid free medium (w/o AA) for 4 h. TCA-soluble and -precipitable radioactivities were measured from the culture medium. Protein degradation was measured as the net release of TCA-soluble radioactivity, expressed as percentage of the total TCA-precipitable radioactivity present in cells at the beginning of the incubation. The results are mean and SD from 9-14 (20% FCS) or 6 experiments (w/o AA) with 2-3 parallel samples. Three (control, LA1-/-, LA2-/-) or one (LA1/LA2-/-, 79) independent cell lines were used.
To clarify whether a decreased proteolysis caused the accumulation of autophagic vacuoles in double-deficient MEFs, we analyzed the degradation of long-lived proteins in starved and nonstarved MEFs in the presence or absence of 3MA, an autophagy inhibitor (Figure 3F). Surprisingly, LAMP- deficiencies did not affect the overall rates of protein degradation. The rate of 3MA-sensitive protein degradation in amino acid-starved cells (which represented autophagic degradation) was also unaltered. Thus, it is unlikely that decreased proteolysis was the cause for accumulation of late autophagic vacuoles.
In agreement with the normal proteolytic rates in the double-deficient MEFs, we observed no differences in the steady state protein levels of two major lysosomal proteases, cathepsin L and cathepsin D (our unpublished results). Lysosomal enzyme activities were also unaltered (our unpublished results) in LAMP-single and double-deficient MEFs. Further, the processing of newly synthesized cathepsin-D appeared to be normal as evaluated by pulse-chase labeling followed by immunoprecipitation from cell extracts and medium (our unpublished results). Acidification of the lysosomal compartments also appeared normal as judged by vital staining with the acidotropic drug Lysotracker Red, or by DAMP (N-(3-((2,4-dinitrophenyl)amino) propyl)-N-(3-aminopropyl) methylamine) followed by immunofluorescence staining with antidinitrophenol antibodies.
Lysosomal hydrolases are targeted from the TGN to endosomes by mannose-6 phosphate receptors (MPRs). We therefore studied the steady state levels of the 46 and 300 kDa MPRs (MPR46 and MPR300, respectively) by Western blotting. Both receptors mediate Golgi-to-endosome transport, whereas MPR300 only is able to mediate transport from the cell surface to endosomes. The levels of MPR300 in the double knockout MEFs were similar to those in control and single-deficient cell lines (our unpublished results). MPR46 levels tended to be lower in primary MEF lines deficient in LAMP-1/LAMP-2, but this difference was lost in double-deficient lines immortalized with the SV large T antigen (our unpublished results). However, MPR300 subcellular localization was altered in LAMP-double-deficient MEFs. In control and single-deficient cells MPR300 localized to a vesicular-reticular network around the nucleus (Supplement 3, A-C). In the double-deficient cells MPR300 tended to localize to more vesicular structures in the perinuclear area (Supplement 3D), although control cell-like staining pattern was also observed in some of the cells. The immunofluorescence localization of MPR46 was not affected in any of the knockout MEF lines (Supplement 4).
Lysosomes Were More Peripherally Located and Had a Lower Density in LAMP-deficient Cells
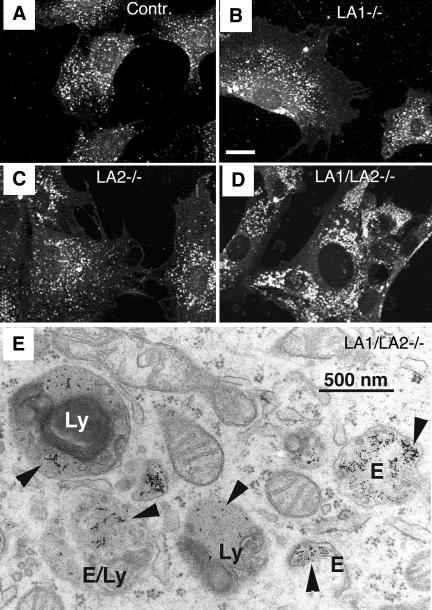
We investigated lysosome distribution and morphology in LAMP-deficient cells using immunofluorescence staining. Labeling of LIMP-2/LGP-85 (Figure 4, A-D), cathepsin-D (our unpublished results), or lysobisphosphatidic acid (LBPA), a late endosomal/lysosomal marker (see Figure 7, E and F, red), revealed an altered size and distribution of late endosomes/lysosomes in LAMP-1/LAMP-2-deficient fibroblasts. In these cells (Figure 4D) lysosomes were slightly larger and more peripherally localized than the endo/lysosomes in the other genotypes. However, electron microscopy revealed that typical dense lysosomes, containing electron-dense grainy material and some internal lamellar membranes, still existed in the LAMP-1/LAMP-2 double-deficient cells. These structures were additionally identified as lysosomes by accumulation of BSA-gold, a fluid-phase endocytic marker fed to the cells for 2 h (Figure 4E). The only obvious abnormality of the LAMP-deficient endo/lysosomes was a slightly increased content of internal lamellar membranes (Figure 4E).
Figure 4.
The lysosomal compartment in LAMP double-deficient MEFs. Immunofluorescence staining of LIMP-2/LGP-85 in (A) control, (B) LAMP-1-deficient, (C) LAMP-2-deficient, and (D) LAMP-1/LAMP-2 double-deficient MEFs. Note the larger size and the more peripheral location of lysosomes in LAMP double knockout cells. Bar, 20 μm. The images show representative cells, based on experiments with at least two independent cell lines for each genotype. (E) Electron micrograph of LAMP-1/LAMP-2 double-deficient cells fed with the fluid-phase endocytic marker BSA-gold for 2 h. Structures with a typical morphology of dense lysosomes (Ly) or endosomes (E) contain the endocytic marker (arrowheads). Note also the multilamellar membranes inside the lysosome on the left.
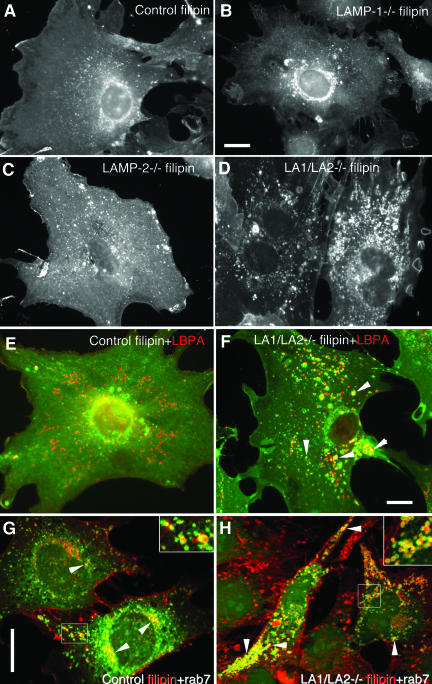
Figure 7.
Cholesterol accumulation in LAMP-1/LAMP-2 double-deficient MEFs. Filipin staining revealed distribution of unesterified cholesterol in (A) control, (B) LAMP-1-/-, (C) LAMP-2-/-, and (D) LAMP-1/LAMP-2-/- cells. Note increased vesicular staining in LAMP-2 single-deficient cells and prominent vesicular accumulation in LAMP double-deficient cells. (E and F) Double labeling with filipin (green) and the late endosomal marker LBPA (red) in control (E) and LAMP double-deficient cells (F). Yellow color (arrowheads) indicates colocalization in a subset of the endosomes of LAMP double knockout cells (F). (G and H) Double labeling of filipin (red) and GFP-rab7 (green) in control (G) and LAMP-1/LAMP-2 double-deficient cells (H). Rab7 colocalization in filipin positive vesicles is indicated by arrowheads and shown at higher magnification in the inserts. Bars, 20 μm.
We used Percoll gradient centrifugation to study the lysosomal density of LAMP-1/LAMP-2 double-deficient cells. Control and LAMP double-deficient cells were fed with HRP for 5 min and then chased for 3 h to allow transport of HRP from early endosomes to lysosomes. The cells were then homogenized and postnuclear supernatants were centrifuged in 27% Percoll gradients. Activities of HRP and the lysosomal marker enzyme β-hexosaminidase were determined from the collected fractions. Western blotting was used to estimate the amount of the lysosomal membrane protein LIMP-2/LGP-85. In control cells HRP and β-hexosaminidase activities, as well as LGP-85, were located at the bottom fractions (Figure 5, A and B), indicating transport of HRP to dense lysosomes. LAMP-1 and -2 single-deficient cell lines gave results similar to the control cells (our unpublished results). However, in LAMP-1/LAMP-2-/- cells both the lysosomal markers and HRP were located in the light fractions of the gradient (Figure 5, A and B). Only a small fraction of LIMP-2/LGP-85 was found in the dense fractions from the double knockout MEFs (Figure 5B). These results suggested that the lysosomal density was decreased in LAMP-deficient cells compared with the control and single-deficient cells.
Figure 5.
Lysosomal density in LAMP-1/LAMP-2 double-deficient MEFs. (A) The lysosomal density from control and LAMP-1/LAMP-2 (L1/L2-/-) double-deficient cells was determined in 27% Percoll gradients. β-hexosaminidase activity and the activity of endocytosed horseradish peroxidase (HRP, 5-min feeding and 3-h chase) were measured in the fractions. (B) LIMP-2/LGP-85 content of the fractions was estimated by Western blotting.
Protein Degradation via Chaperone-mediated Autophagy Was Apparently Normal in LAMP-deficient Cells
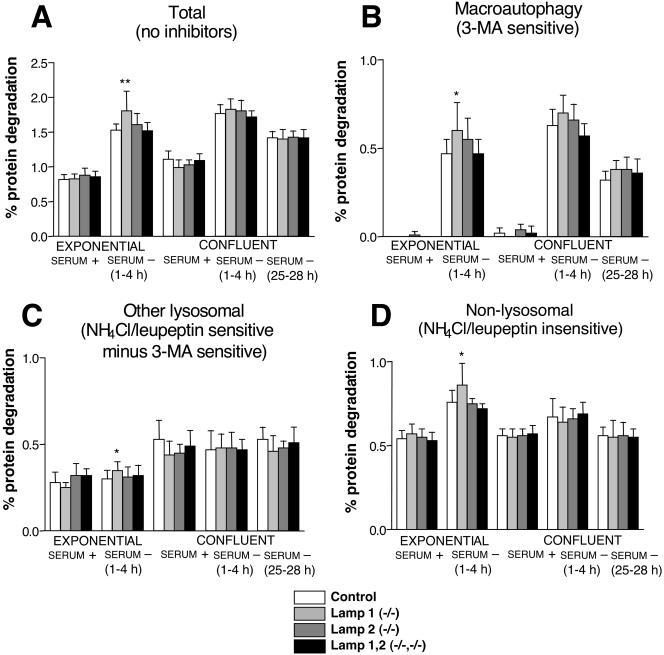
LAMP-2a has been suggested to act as a receptor for chaperone-mediated autophagy (Cuervo and Dice, 1996, 2000). We therefore wanted to check whether the activity of this pathway was decreased in the single- and double-deficient cell lines. There are no known specific inhibitors of this pathway. However, all lysosomal proteolysis pathways can be blocked using a combination of a weak base such as NH4Cl and an inhibitor of lysosomal cathepsins such as leupeptin (Fuertes et al., 2003a). Therefore, these inhibitors, together with the macroautophagy inhibitor 3MA (Seglen and Gordon, 1982), allow a separate analysis of the contribution of macroautophagy, other lysosomal pathways (which should include chaperone-mediated autophagy), and the nonlysosomal pathways, as we have shown with human fibroblasts (Fuertes et al., 2003a). No significant differences in the degradation of long-lived proteins (Figure 6A), nor in the protein degradation by nonlysosomal (Figure 6D), macroautophagic (Figure 6B), or other lysosomal pathways (Figure 6C) could be found under any conditions and for any cell line tested, except for a moderate increase in total, lysosomal, and nonlysosomal degradation in LAMP-1-deficient cell lines. Even under conditions where chaperone-mediated autophagy was supposed to be more active (prolonged starvation in confluent cells, the last group of columns in Figure 6C), we were unable to find any significant difference in the protein degradation by lysosomes in LAMP-2-deficient cell lines or in a LAMP-1/LAMP-2 double-deficient cell line, when compared with the control cell lines. The difference found in exponentially growing LAMP-1-deficient cells subjected to serum withdrawal (Figure 6A) appeared to be due to a general increase in the protein degradation because it affected all pathways of protein degradation (Figure 6). Taken together, these data with LAMP-2-deficient and LAMP1/LAMP2 double-deficient cell lines did not support a role for LAMP-2 as a receptor for chaperone-mediated autophagy in MEFs.
Figure 6.
Contribution of proteolytic pathways in MEFs under different growth conditions. MEFs were metabolically labeled and chased as described in MATERIALS AND METHODS. Exponentially growing or confluent cells were then switched to serum-containing (serum +) or serum-deficient (serum -) media, with or without proteolysis inhibitors. To allow optimal inhibition and to avoid secondary effects, protein degradation was measured at different time points starting after 1 h and continuing for a period of 3 additional hours. The total protein degradation per hour is presented in A. The contribution (percentage of the labeled protein whose degradation was inhibited per hour) of macroautophagy (B), other lysosomal pathways (C), and nonlysosomal pathways (D), of total protein degradation was calculated as described in MATERIALS AND METHODS. 3-Methyladenine was used to inhibit macroautophagy and NH4Cl + leupeptin to inhibit both macroautophagy and other lysosomal pathways (i.e., microautophagy, chaperone-mediated autophagy, etc.). The results are the mean and SD from 6-15 separate experiments with duplicate samples. Three (control, LA1-/-, LA2-/-) or one (LA1/LA2-/-, 79) independent cell lines were used. Stars indicate differences from control cell values which were found to be statistically significant at *p < 0.05 and **p < 0.005.
Unesterified Cholesterol Accumulated in Endo/lysosomes of LAMP-deficient Cells
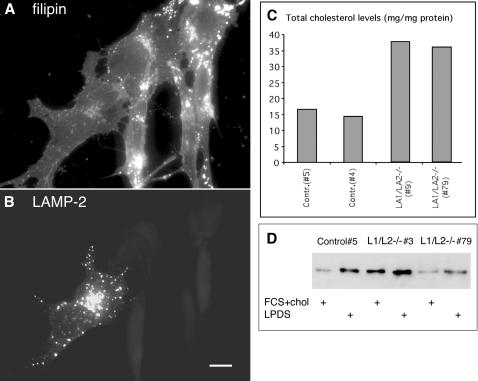
Because we observed decreased lysosomal density but no significant swelling of lysosomes in the double-deficient cells, we hypothesized that accumulation of lipids may have influenced the lysosome density. Therefore we analyzed the lipid distribution in more detail. Filipin staining was used to detect unesterified cholesterol. The staining pattern of LAMP-1 single-deficient cells was comparable to the control cells (Figure 7, A and B), whereas LAMP-2 single-deficient cells (Figure 7C) showed increased staining in small vesicles scattered around the cytoplasm. Interestingly, LAMP-1/LAMP-2 double-deficient cells (Figure 7D) showed a massive accumulation of brightly stained large vesicles around the cytoplasm. This vesicular filipin staining pattern was observed in all LAMP double-deficient cell lines tested including primary, spontaneously immortalized, and SV40 large T antigen immortalized MEF lines. The filipin-stained vesicles in double-deficient cells showed a partial colocalization with the late endosomal marker lysobisphosphatidic acid (LBPA; Figure 7F, yellow) as well as with cathepsin D or LIMP-2/LGP-85 (our unpublished results). In addition, the filipin-positive vesicles showed colocalization with transfected GFP-rab7 (Figure 8, G and H, yellow), as well as with endogenous rab7 (our unpublished results). In many vesicles rab7 signals were surrounding the filipin-positive contents (Figure 7, G and H, insets). This indicated that in LAMP double-deficient cells cholesterol accumulated in rab7 containing late endosomal/lysosomal compartments. Interestingly, filipin-positive structures almost completely colocalized with MPR46 (Supplement 4A) in control fibroblasts, whereas much less colocalization with MPR46 was found in LAMP-1/LAMP-2 double-deficient cells (Supplement 4B). Similar results were obtained in double labeling of MPR300 with filipin. Although the distribution of MPR300 tended to be more vesicular in the double-deficient MEFs (Supplement 3D), there was no colocalization of MPR300 with filipin in these vesicular structures (our unpublished results). These findings suggested that although in control cells highest concentrations of cholesterol were located to the MPR46/MPR300-positive organelles including TGN and late endosomes, in LAMP double-deficient cells cholesterol accumulated in endo/lysosomal compartments, which were negative for MPRs and positive for rab7.
Figure 8.
Electron microscopical visualization of filipin labeled membrane cholesterol. Arrowheads in control (A) and double-deficient fibroblasts (B) indicate the filipin labeling. In control cells labeling was detected in the limiting membranes of small vesicles close to the Golgi apparatus (G). In LAMP-1/LAMP-2 double-deficient fibroblasts (B) filipin-induced membrane alterations were seen in both the limiting and internal membranes of endo/lysosomal vesicles. Note that lamellar internal membranes of the upper endo/lysosome are not labeled by filipin.
Filipin-labeled membrane cholesterol is also visible by electron microscopy as typical membrane deformations (Punnonen et al., 1989). In agreement with the light microscopical analysis, in wild-type fibroblasts filipin-induced membrane formations were detected in small vesicles close to the Golgi apparatus (Figure 8A). In double knockout cells labeling was observed in both the limiting and internal, typically concentric, membranes of endo/lysosomal vesicles, indicating that unesterified cholesterol accumulated in both the limiting and internal membranes (Figure 8B). This labeling pattern was reminiscent of the cholesterol distribution in endo/lysosomes of NPC1 patient fibroblasts (Garver and Heidenreich, 2002).
To confirm that the cholesterol accumulation was due to the deficiency of LAMPs, we transiently transfected LAMP double-deficient cells with a mouse cDNA for LAMP2a. The proportion of cells containing filipin-positive endo/lysosomal vesicles was quantitated. Vesicular filipin staining was observed in 7% (37 of 533) of control wild-type cells and in 80% (1051 of 1314) of nontransfected LAMP double-deficient cells (Figure 9A). However, only 18% (69 of 381) of the double-deficient cells reexpressing LAMP-2a had vesicular filipin staining; instead, filipin staining was similar to control cells (Figures 7A and 9, A and B). A significantly smaller rescue effect was observed when a similar experiment was performed using LAMP-1 cDNA, 69% (34 of 49) of double-deficient cells reexpressing LAMP-1 still showed vesicular filipin staining. This suggested that LAMP-2 deficiency was the major cause for the endo/lysosomal cholesterol storage observed in LAMP-1/LAMP-2 double-deficient MEFs.
Figure 9.
Reduction of cholesterol storage in LAMP double-deficient cells after LAMP-2a re-expression. (A and B) LAMP-2a was transiently transfected to LAMP1/LAMP-2 double-deficient cells. After 1 day, cholesterol was stained with filipin (A) and LAMP-2 was detected by immunofluorescence (B). Cells not expressing LAMP-2 showed staining for cholesterol in endo/lysosomal vesicles, whereas the LAMP-2a-positive cell showed a filipin staining pattern similar to control cells (see Figure 8A). Bar, 10 μm. (C) Biochemical cholesterol assay in two control and two LAMP double-deficient primary cell lines. (D) Western blot of LDL receptor in one control and two LAMP-1/LAMP-2 double-deficient cell lines. The cells were incubated in DMEM containing 10% FCS and 10 μg/ml cholesterol (FCS+chol), or in DMEM containing 10% LPDS for 2 days.
We next analyzed the cholesterol levels per milligram of cell protein in whole cell extracts. In LAMP-1/LAMP-2-deficient primary MEFs the cholesterol concentration was about twofold higher than in control cells (Figure 9C). In LAMP-2 single-deficient cells an intermediate level of cholesterol was observed (our unpublished results). The difference in total cholesterol content was lost in many of the spontaneously immortalized or SV40-transformed double-deficient cell lines (our unpublished results); however, these cell lines still showed endo/lysosomal cholesterol accumulation in filipin staining. Thus the cholesterol localization was altered in all double-deficient cell lines, although the primary cell lines only showed an additional increase in the total cholesterol levels.
We also tested whether the double-deficient cells could be cleared of accumulated cholesterol by culturing them in the absence of exogenous cholesterol. Culturing the cells for 2 days in a medium containing lipoprotein and cholesterol-free serum (LPDS) did not change the filipin staining pattern or the overall cholesterol content of the double-deficient cells (our unpublished results). This indicated that the stored endo/lysosomal cholesterol could not be cleared in the absence of exogenous low-density lipoprotein (LDL) cholesterol. During culture in LPDS medium cells upregulate the LDL receptor levels to enhance uptake of extracellular cholesterol. This regulation is mediated by cholesterol levels in the endoplasmic reticulum (ER; Brown and Goldstein, 1986). Upregulation of LDL receptor in LPDS medium was detected in both control and LAMP double-deficient cells (Figure 9D), which suggested that intracellular LDL receptor regulation via ER was functional in the LAMP double-deficient cells. However, although some variation was observed between the individual deficient lines, the unstimulated LDL receptor levels tended to be higher in the double-deficient cell lines than in the control cell lines (Figure 9D). This might have been due to impaired transport of cholesterol from the lysosomal compartment to the ER, where the cholesterol sensors are located. Decreased cholesterol in the ER in turn is known to lead to upregulation of LDL receptor levels.
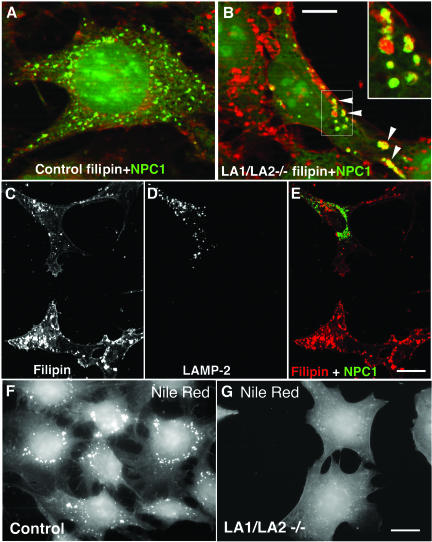
Fibroblasts derived from Niemann Pick-Type C patients show endo/lysosomal cholesterol storage (Garver and Heidenreich, 2002) similar to what we observed in LAMP double-deficient cells (Figures 7 and 8). Mutations in the transmembrane protein NPC1 (Loftus et al., 1997) or in the soluble lysosomal protein NPC2 (Naureckiene et al., 2000) cause the cholesterol storage of this disease. We therefore investigated the expression of these proteins in the double-deficient cells. Using Western blotting we found that the steady state levels of NPC1 and NPC2 proteins were not affected in LAMP-deficient cells (our unpublished results). Further, transient overexpression of GFP-tagged NPC1 or myc-tagged NPC2 did not rescue the cholesterol storage phenotype of LAMP-deficient cells (our unpublished results). However, the localization of endogenous NPC1 as well as transfected NPC1-GFP in LAMP-1/LAMP-2 double-deficient cells was different from that of control cells. In control cells NPC1-GFP localized to fine punctate, filipin-negative structures (Figure 10A), whereas in many of the LAMP double-deficient cells, enlarged, often ring-like structures were observed. In these structures NPC1-GFP colocalized with filipin (Figure 10B, arrowheads and inset). The altered distribution of NPC1 in double-deficient fibroblasts could be rescued by reexpression of LAMP2a (Figure 10, C-E).
Figure 10.
Altered NPC1 localization and Nile Red staining in LAMP-1/LAMP-2 double-deficient cells. NPC1-GFP was transiently expressed in control cells (A) and in LAMP-1/LAMP-2 double-deficient cells (B). An overlay of NPC1-GFP (green) and filipin (red) is shown. Note the increased size of NPC1 vesicles in the double-deficient cells. In these structures NPC1 colocalized with filipin (arrowheads and insert in B). Bar, 10 μm. (C-E) After parallel transfection of LAMP-2 and NPC1-GFP in LAMP-1/LAMP-2 double-deficient cells, filipin staining (C), LAMP-2 staining (D) and NPC1-GFP were analyzed. Note the reduction of filipin positive vesicles, and the distribution of NPC1-GFP in small filipin-negative structures, in the cell expressing LAMP-2. Bar, 20 μm. (F and G) Nile Red staining revealed lipid droplets as punctate structures in control cells (F), whereas almost no Nile Red-positive cytoplasmic structures were found in LAMP double-deficient MEFs (G). Bar, 20 μm.
Rab7 overexpression has been reported to abolish the endo/lysosomal cholesterol accumulation in NPC1 patient cells (Choudhury et al., 2002). In LAMP double-deficient cells, however, transient expression of GFP-rab7 did not alter the filipin staining pattern (Figure 7, G and H). Quantitation showed that 77% of cells expressing GFP-rab7 still had vesicular filipin staining. Instead the localization of both endogenous (our unpublished results) and transfected rab7 was altered. In control cells rab7 was found in the perinuclear region where it showed some colocalization with filipin (Figure 7G), whereas in LAMP double-deficient cells rab7 was almost exclusively localized in filipin-positive vesicles, which were often found in the peripheral cytoplasm (Figure 7H). We also compared the rab7 expression levels in control and LAMP double-deficient cells by Western blotting and found them to be identical. These results indicated that rab7 localization was severely altered in the LAMP double-deficient cells.
We next wanted to investigate whether localization of neutral lipids and cholesterol esters was affected. These lipids are components of lipid droplets, and Nile Red has been used to stain these structures (Greenspan et al., 1985). In almost all control cells we observed large spherical structures around the nucleus (Figure 10F). In contrast, no spherical structures were seen in many of the LAMP-1/LAMP-2 double knockout cells, and instead a diffuse plasma membrane staining was observed (Figure 10G). This suggested that synthesis of lipid droplets was impaired in cells deficient in both LAMPs. This may be connected to the accumulation of unesterified cholesterol in lysosomes, which likely leads to decreased cholesterol esterification. Esterified cholesterol is one of the lipids stored in lipid droplets (Aoki and Massa, 1975).
DISCUSSION
Several functions have been suggested for lysosomal membrane proteins, such as sequestration of numerous acid hydrolases, maintenance of an acidic intralysosomal environment, transport of degradation products out of lysosomes, and specific interaction and fusion events between lysosomes and other organelles (Fukuda, 1991; Peters and von Figura, 1994; Eskelinen et al., 2003). LAMP-1 and -2, the two most abundant lysosomal membrane proteins, have been estimated to contribute to ∼50% of all proteins of this membrane (Hunziker et al., 1996). The presence of LAMP molecules is one of the major definitions of the lysosomal compartment (Kornfeld and Mellman, 1989). What then was the consequence of total absence of both major lysosomal membrane proteins on lysosomal structure and function? Surprisingly, we observed that typical lysosomes, as defined by ultrastructural appearance, and capacity to take up fluid phase markers and degrade proteins, still existed in LAMP-1/LAMP-2 double-deficient MEFs. It is likely that other lysosomal membrane proteins including LIMP 1 and LIMP-2/LGP-85 were able to take over the protective function against lysosomal hydrolases.
However, the generation of LAMP-1/LAMP-2 double-deficient embryos described in this study demonstrated that these proteins fulfill at least partially overlapping functions in vivo. The tight functional association of the proteins is also highlighted by the observation that mice with only one functional LAMP allele were more susceptible to early postnatal mortality. Although no gross impairment of organ development was encountered, LAMP-1/LAMP-2 double-deficient embryos were characterized by a massive accumulation of autophagic vacuoles in many tissues, especially endothelial cells. It is possible that further embryonic development beyond embryonic day E15 was prevented due to these cellular alterations and impaired delivery of nutrients.
We showed earlier that autophagic vacuoles accumulate in LAMP-2 single-deficient liver tissue and isolated hepatocytes (Tanaka et al., 2000; Eskelinen et al., 2002a). This accumulation could be attributed to a decreased lysosomal degradation of long-lived proteins. In this study we observed an accumulation of late autophagic vacuoles in the LAMP double-deficient cells. Surprisingly, in LAMP-1/LAMP-2 double-deficient MEFs the protein degradation rates under all conditions tested were similar to control cells, suggesting that the accumulation of autophagic vacuoles was not due to retarded protein degradation. Thus, the increased accumulation could be due to impaired degradation of other macromolecules including lipids or possibly to defective export of degradation products from autophagic vacuoles to the cytoplasm. It is also possible that in the double-deficient cells, retarded protein degradation per autophagic vacuole was compensated by the increased amount of vacuoles, and therefore we did not see a difference in protein degradation rates between control cells and the double-deficient cells.
One of the three LAMP-2 isoforms, LAMP-2a, has been suggested to be the receptor for the chaperone-mediated autophagy, a selective uptake and degradation of cytosolic proteins by lysosomes (Cuervo and Dice, 1996). This pathway was shown to be active in confluent lung fibroblasts and Chinese hamster ovary cells cultured in serum-free medium (Cuervo and Dice, 1998). In this study we analyzed MEFs deficient in LAMP-2, LAMP-1, or both. In LAMP-2-deficient cells all LAMP-2 isoforms including LAMP-2a were disrupted. MEF cells deficient in LAMP-2 or both LAMP-1 and -2 did not show changes in the lysosomal protein degradation rates under six different conditions. These results suggested that deficiency of LAMP-2 or both LAMP-2 and LAMP-1 did not alter lysosomal protein degradation rates even in confluent cells under prolonged (28 h) serum starvation, a condition where chaperone-mediated autophagy is supposed to be more active. Thus, it is possible that LAMP-2 is not the only receptor in chaperone-mediated autophagy or that the pathway is not active in mouse embryonic fibroblasts, like in other cells. However, the latter alternative appears unlikely because the proteolytic rates measured in our MEFs resembled those of human fibroblasts, where the pathway is known to be active. Another possibility is that other proteolytic pathways compensate for the defective activity of chaperone-mediated autophagy in LAMP-2-deficient mouse fibroblasts. Although our experiments with various inhibitors excluded this for macroautophagy, proteasomal degradation, and nonlysosomal proteolytic pathways different from proteasomes, the possibility still exists that a lysosomal pathway that is different from macroautophagy (such as microautophagy) is activated to compensate for the deficiency in chaperone-mediated autophagy.
We observed an altered subcellular distribution of MPR300 in LAMP double-deficient MEFs. Despite this, cathepsin D delivery to the lysosomal compartment and uptake of arylsulfatase A, a mannose-6-phosphate-containing ligand, from the culture medium were not affected (unpublished data). This was in contrast to LAMP-2 single-deficient hepatocytes where we observed that lysosomal biogenesis, MPR46 expression levels (Eskelinen et al., 2002b) and MPR300 function in endocytosis (unpublished data) were affected. This may be explained by different expression levels of LAMP proteins and different requirements for lysosomal functions in embryonic fibroblasts and adult hepatocytes.
Intriguingly, we found altered lipid localization in LAMP-1/LAMP-2 double-deficient and LAMP-2 single-deficient MEFs. Prominent storage of unesterified cholesterol in endo/lysosomal compartments of LAMP-1/LAMP-2 double-deficient cells was observed. Transfection with wild-type LAMP-2a constructs reversed the cholesterol storage phenotype, whereas LAMP-1 transfection had a much smaller effect, which demonstrated the major importance of LAMP-2 for the cholesterol metabolism. The decreased amount of Nile Red-stained lipid droplets in the double-deficient MEFs also indicated that lipid metabolism was altered. Recent studies have demonstrated that lipid droplets are not passive storage structures but have an active role in lipid metabolism (Liu et al., 2004).
The pattern of cholesterol storage in LAMP-1/LAMP-2-deficient cells resembled that in fibroblasts from Niemann-Pick type C patients (Garver and Heidenreich, 2002). Similar to LAMP double-deficient cells, these cells accumulated unesterified cholesterol in late endosomal/lysosomal vesicles. Mutations in NPC1 or in NPC2 have been described to be responsible for the cholesterol storage phenotype (Garver and Heidenreich, 2002). It has been reported that after transfection of NPC1 to cells deficient in this protein, the protein first localized to the limiting membranes of cholesterol-loaded endo/lysosomes. After cholesterol had been cleared from endo/lysosomes, the localization of the reexpressed NPC1 changed to small late endosomal filipin-negative vesicles, which was identical to the localization of NPC1 in control cells (Zhang et al., 2001). We observed that in LAMP double-deficient cells NPC1 localized in the cholesterol-loaded endo/lysosomes, whereas in control cells NPC1 localized in small filipin-negative vesicles. Thus, it is likely that in the double-deficient cells NPC1 was recruited to the correct localization for cholesterol clearance, but it was not able to fulfill this function. The altered distribution of NPC1-GFP could be reversed by parallel expression of NPC1-GFP and LAMP2, indicating that LAMP-2 directly or indirectly influenced the trafficking or function of NPC1. Similar to NPC1, rab7 also localized in the cholesterol-loaded endosomes in the LAMP double-deficient cells. It has been reported that cholesterol accumulation in the lysosomal compartment increases the amount of membrane-associated rab7 (Lebrand et al., 2002) and this was suggested to interfere with the rab7 function. Thus, it is possible that at least some of the alterations observed in the LAMP double-deficient cells, such as altered localization of lysosomal vesicles in the peripheral cytoplasm, were due to impaired function of rab7. Further studies are however necessary to clarify the mechanistic reasons for the accumulation or cholesterol and decreased amount of lipid droplets in MEFs deficient of LAMP-1 and -2.
In conclusion, the viability of mice deficient in either LAMP-1 or LAMP-2, as well as the embryonic lethal phenotype of LAMP-1/LAMP-2 double-deficient mice indicated that these two major lysosomal membrane proteins share common functions in vivo. However, LAMP-2 seemed to have more specific functions because LAMP-2 single deficiency had more severe consequences than LAMP-1 single deficiency. In embryonic fibroblasts, mutual disruption of both LAMPs was associated with an increased accumulation of autophagic vacuoles, altered lysosomal distribution and appearance, and disturbed cholesterol metabolism, whereas protein degradation rates were not affected. These results clearly show that the LAMP proteins fulfill functions far beyond the initially suggested roles in maintaining the structural integrity of the lysosomal compartment.
Supplementary Material
Acknowledgments
We are grateful to Ellen Eckerman, Anegret Schneeman, Jenny Schröder, Stefanie Jäger, Marlies Rusch, and Katharina Stiebeling for technical assistance and to Mathew Scott, Stefan Honing, Cecilia Bucci, Isei Tanida, Takashi Ueno, Jean Gruenberg, William Garver, and Shutish Patel for plasmids or antibodies. This study was funded by a grant from the Deutsche Forschungsgemeinschaft (SA683/1-3) to P.S., the Ministry of Labor, Health, and Welfare of Japan and the Ministry of Education, Science, Sports, and Culture of Japan to Y.T., the Ministerio deCiencia y Tecnología of Spain (BMC2001-0816 and SAF2002-00206) to E.K., the German Niemann-Pick Parents Foundation, the Fonds der Chemischen Industrie and the Hensel-Stiftung, Kiel, Germany.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-02-0103. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-02-0103.
Abbreviations used: Avi, early autophagic vacuole; Avd, late autophagic vacuole; BSA, bovine serum albumin; ER, endoplasmic reticulum; FCS, fetal calf serum; GFP, green fluorescent protein; HRP, horseradish peroxidase; LAMP, lysosomal associated membrane protein; LIMP, lysosomal integral membrane protein; LBPA, lysobisphosphatidic acid; LDL, low-density lipoprotein; LPDS, lipoprotein-deficient serum; 3MA, 3-methyladenine; MEF, mouse embryonic fibroblast; MPR, mannose-6-phosphate receptor; PBS, phosphate-buffered saline; TGN, trans-Golgi network.
Online version of this article contains supporting material. Online version is available at www.molbiolcell.org.
References
- Agarraberes, F.A., and Dice, J.F. (2001). A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J. Cell Sci. 114, 2491-2499. [DOI] [PubMed] [Google Scholar]
- Andrejewski, N., Punnonen, E.L., Guhde, G., Tanaka, Y., Lullmann-Rauch, R., Hartmann, D., von Figura, K., and Saftig, P. (1999). Normal lysosomal morphology and function in LAMP-1-deficient mice. J. Biol. Chem. 274, 12692-12701. [DOI] [PubMed] [Google Scholar]
- Aoki, A., and Massa, E.M. (1975). Subcellular compartmentation of free and esterified cholesterol in the interstitial cells of the mouse testis. Cell Tissue Res. 165, 49-62. [DOI] [PubMed] [Google Scholar]
- Brown, M.S., and Goldstein, J.L. (1986). A receptor-mediated pathway for cholesterol homeostasis. Science 232, 34-47. [DOI] [PubMed] [Google Scholar]
- Choudhury, A., Dominguez, M., Puri, V., Sharma, D.K., Narita, K., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (2002). Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J. Clin. Invest. 109, 1541-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo, A.M., and Dice, J.F. (1996). A receptor for the selective uptake and degradation of proteins by lysosomes. Science 273, 501-503. [DOI] [PubMed] [Google Scholar]
- Cuervo, A.M., and Dice, J.F. (1998). Lysosomes, a meeting point of proteins, chaperones, and proteases. J. Mol. Med. 76, 6-12. [DOI] [PubMed] [Google Scholar]
- Cuervo, A.M., and Dice, J.F. (2000). Unique properties of LAMP2a compared to other LAMP2 isoforms. J. Cell Sci. 113, 4441-4450. [DOI] [PubMed] [Google Scholar]
- Danon, M.J., Oh, S.J., DiMauro, S., Manaligod, J.R., Eastwood, A., Naidu, S., and Schliselfeld, L.H. (1981). Lysosomal glycogen storage disease with normal acid maltase. Neurology 31, 51-57. [DOI] [PubMed] [Google Scholar]
- Dice, J.F. (2000). Lysosomal Pathways of Protein Degradation. Austin TX: RG Landes Co.
- Eskelinen, E.L., Illert, A.L., Tanaka, Y., Blanz, J., von Figura, K., and Saftig, P. (2002a). Role of LAMP-2 in lysosome biogenesis and autophagy. Mol. Biol. Cell 13, 3355-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen, E.L., Prescott, A.R., Cooper, J., Brachmann, S.M., Wang, L., Tang, X., Backer, J.M., and Lucocq, J.M. (2002b). Inhibition of autophagy in mitotic animal cells. Traffic 3, 878-893. [DOI] [PubMed] [Google Scholar]
- Eskelinen, E.L., Tanaka, Y., and Saftig, P. (2003). At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 13, 137-145. [DOI] [PubMed] [Google Scholar]
- Fuertes, G., Martin De Llano, J.J., Villarroya, A., Rivett, A.J., and Knecht, E. (2003a). Changes in the proteolytic activities of proteasomes and lysosomes in human fibroblasts produced by serum withdrawal, amino-acid deprivation and confluent conditions. Biochem. J. 375, 75-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes, G., Villarroya, A., and Knecht, E. (2003b). Role of proteasomes in the degradation of short-lived proteins in human fibroblasts under various growth conditions. Int. J. Biochem. Cell Biol. 35, 651-664. [DOI] [PubMed] [Google Scholar]
- Fukuda, M. (1991). Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J. Biol. Chem. 266, 21327-21330. [PubMed] [Google Scholar]
- Garver, W.S., and Heidenreich, R.A. (2002). The Niemann-Pick C proteins and trafficking of cholesterol through the late endosomal/lysosomal system. Curr. Mol. Med. 485-505. [DOI] [PubMed]
- Greenspan, P., Mayer, E.P., and Fowler, S.D. (1985). Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 100, 965-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker, W., Simmen, T., and Honing, S. (1996). Trafficking of lysosomal membrane proteins in polarized kidney cells. Nephrologie 17, 347-350. [PubMed] [Google Scholar]
- Kabeya, Y., Mizushima, N., Ueno, T., Yamamoto, A., Kirisako, T., Noda, T., Kominami, E., Ohsumi, Y., and Yoshimori, T. (2000). LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720-5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld, S., and Mellman, I. (1989). The biogenesis of lysosomes. Annu. Rev. Cell Biol. 5, 483-525. [DOI] [PubMed] [Google Scholar]
- Kuronita, T., Eskelinen, E.L., Fujita, H., Saftig, P., Himeno, M., and Tanaka, Y. (2002). A role for the lysosomal membrane protein LGP85 in the biogenesis and maintenance of endosomal and lysosomal morphology. J. Cell Sci. 115, 4117-4131. [DOI] [PubMed] [Google Scholar]
- Lebrand, C., Corti, M., Goodson, H., Cosson, P., Cavalli, V., Mayran, N., Faure, J., and Gruenberg, J. (2002). Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 21, 1289-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P., Ying, Y., Zhao, Y., Mundy, D.I., Zhu, M., and Anderson, R.G. (2004). Chinese hamster ovary k2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J. Biol. Chem. 279, 3787-3792. [DOI] [PubMed] [Google Scholar]
- Loftus, S.K. et al. (1997). Murine model of Niemann-Pick C. disease: mutation in a cholesterol homeostasis gene. Science 277, 232-235. [DOI] [PubMed] [Google Scholar]
- Mizushima, N., Ohsumi, Y., and Yoshimori, T. (2002). Autophagosome formation in Mammalian cells. Cell Struct. Funct. 27, 421-429. [DOI] [PubMed] [Google Scholar]
- Mizushima, N., Yoshimori, T., and Ohsumi, Y. (2003). Role of the Apg12 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 35, 553-561. [DOI] [PubMed] [Google Scholar]
- Naureckiene, S., Sleat, D.E., Lackland, H., Fensom, A., Vanier, M.T., Wattiaux, R., Jadot, M., and Lobel, P. (2000). Identification of HE1 as the second gene of Niemann-Pick C disease. Science 290, 2298-2301. [DOI] [PubMed] [Google Scholar]
- Nishino, I. et al. (2000). Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature 406, 906-910. [DOI] [PubMed] [Google Scholar]
- Peters, C., and von Figura, K. (1994). Biogenesis of lysosomal membranes. FEBS Lett. 346, 146-150. [DOI] [PubMed] [Google Scholar]
- Punnonen, E.L., Pihakaski, K., Mattila, K., Lounatmaa, K., and Hirsimaki, P. (1989). Intramembrane particles and filipin labelling on the membranes of autophagic vacuoles and lysosomes in mouse liver. Cell Tissue Res. 258, 269-276. [DOI] [PubMed] [Google Scholar]
- Seglen, P.O., and Gordon, P.B. (1982). 3-methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc. Natl. Acad. Sci. USA 79, 1889-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, Y. et al. (2000). Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 406, 902-906. [DOI] [PubMed] [Google Scholar]
- Zhang, M. et al. (2001). Sterol-modulated glycolipid sorting occurs in niemann-pick C1 late endosomes. J. Biol. Chem. 276, 3417-3425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.