Abstract
One-fourth of all deaths in industrialized countries result from coronary heart disease. A century of research has revealed the essential causative agent – cholesterol-carrying low density lipoprotein (LDL). LDL is controlled by specific receptors (LDLRs) in liver that remove it from blood. Mutations that eliminate LDLRs raise LDL and cause heart attacks in childhood, whereas mutations that raise LDLRs reduce LDL and diminish heart attacks. If we are to eliminate coronary disease, lowering LDL should be the primary goal. Effective means to achieve this goal are currently available. The key questions are: Who to Treat, When to Treat, and How Long to Treat.
INTRODUCTION
Among adults in industrialized countries, one-fourth of all deaths result from arterial blockage caused by atherosclerotic plaques (Heron, 2013). Most of these deaths are attributable to occlusion of the coronary arteries which produces heart attacks. Against this background, it is quite surprising that heart attacks were recognized as clinical entities a mere 100 years ago. Within 50 years, the disease had increased to epidemic proportions. Only in recent years has the incidence of heart attacks in the United States begun to decline (Levy, 2012).
The multifaceted path by which cholesterol became linked to coronary heart disease is one of the great biomedical stories of the 20th century. Table 1 shows major events in the century of cholesterol and coronaries. It summarizes the scientific evidence that converged from multiple disciplines to implicate cholesterol-carrying low density lipoprotein (LDL) as the instigator of atherosclerotic plaques and high dietary fat as the major cause of pathologic LDL levels. We have divided the discoveries into two sequential eras – first, the era of cholesterol and then the era of LDL.
Table 1.
A Century of Cholesterol and Coronaries
| First Half - The Era of Cholesterol | |
| 1910 | Human Atherosclerotic Plaques Contain Cholesterol |
| 1913 | High Cholesterol Diet Causes Atherosclerosis in Rabbits |
| 1919 | Heart Attacks Recognized in Humans |
| 1933 | Feedback Inhibition of Cholesterol Synthesis Demonstrated |
| 1938 | Familial Hypercholesterolemia Described |
| 1950 | Cholesterol Biosynthetic Pathway Elucidated |
| 1951 | High Fat Diets Raise Plasma Cholesterol in Humans |
| 1953 | Risk Factor Concept Advanced |
| Second Half - The Era of LDL | |
| 1955 | LDL Identified as Risk Factor for CHD |
| 1973 | LDL Receptor Discovered |
| 1976 | HMG CoA Reductase Inhibitors (Statins) Discovered |
| 1981 | Statins Increase LDL Receptors in vivo |
| 1987 | First Statin (Mevacor) Approved for Human Use |
| 1994 | Statins Decrease Heart Attacks and Prolong Life |
| 1997 | SREBP Pathway Elucidated |
| 2006 | PCSK9: Destroyer of LDL Receptors |
This review focuses on selected discoveries that we consider to be milestones in the century of cholesterol and coronaries. In order to cover 100 years of a robust field in a 6000-word essay, we were forced to limit the literature that could be cited. We recognize the important contributions of many uncited scientists whose work deepened our understanding of cholesterol, LDL, and coronary atherosclerosis. The reader should be aware that our discussion of the SREBP pathway draws conclusions about human liver physiology that represent extrapolations from experiments in rats, mice, hamsters, and dogs. Inasmuch as measurements of similar depth cannot be made on the livers of human subjects, we believe that these extrapolations are justified by their explanatory potential for clinical and epidemiologic observations.
Cholesterol-Rich Plaques in Humans and Rabbits
Atherosclerotic plaques on the surface of the aorta were described by German pathologists in the 19th century (Leibowitz, 1970). The word atherosclerosis is derived from the Greek word atheros, which means gruel. It describes the cheesy substance that exudes from the plaques on sectioning. The first hint that the cheesy substance was cholesterol came in 1910 when the German chemist Adolf Windaus found that plaques from human aortas contained 25-fold more cholesterol than normal aortas. Shortly thereafter, in 1913, Nikolaj Anitschkow, a Russian pathologist, fed pure cholesterol to rabbits and produced profound atherosclerosis, thereby raising the possibility that dietary cholesterol is the source of the gruel (Anitschkow and Chalatow, 1913; Steinberg, 2005).
Heart Attacks Diagnosed in Living Patients
Although pathologists recognized that coronary arteries could be occluded by atherosclerotic plaques, this was considered to be a universally fatal event. Transient episodes of nonfatal chest pain were attributed to indigestion or other non-cardiac causes. This dogma was overturned in 1919 when the American clinician James Herrick used the recently invented electrocardiograph machine to demonstrate changes in the electrical pattern of the heart in patients who were experiencing severe nonfatal chest pain (Herrick, 1919; Herrick, 1944). By providing a method for the firm diagnosis of heart attacks, the electrocardiogram ushered in the modern era of cardiology.
Familial Hypercholesterolemia (FH) Described
The connection between plasma cholesterol and heart attacks was put on firm genetic footing in 1938 when a Norwegian physician, Carl Müller, described families in which high plasma cholesterol levels were transmitted as an autosomal dominant trait (Müller, 1938). The disease was named familial hypercholesterolemia (FH), and it was soon recognized that the elevated cholesterol levels were associated with a 20-fold increase in the incidence of heart attacks in middle age.
First Description of Cholesterol Feedback
In 1933, Rudolph Schoenheimer, then working in Germany, placed mice in sealed bottles, fed them a cholesterol-free diet, and found that the cholesterol content of the bottles increased. When the diet contained cholesterol, there was no longer a contribution from the mice. This landmark study demonstrated not only that animals can synthesize cholesterol, but also that synthesis is inhibited when cholesterol is present in the diet (Schoenheimer and Breusch, 1933). This was the first demonstration of the fundamental principle of end-product feedback inhibition of a biosynthetic pathway, prefiguring the classic work of Jacob and Monod (1961), Pardee (2003), and Umbarger (1992). Schoenheimer's discovery also laid the groundwork for the discovery of the LDL receptor in the 1970's (Goldstein and Brown, 2009) and the Scap/SREBP pathway in the 1990's (Brown and Goldstein, 2009).
Cholesterol Biosynthetic Pathway Elucidated
In the 1950's, a small group of talented biochemists worked out the complex pathway by which the 27-carbon, 4-ring cholesterol molecule is synthesized through repeated polymerizations from acetate, a simple 2-carbon building block attached to CoA. Most prominent in this endeavor were Konrad Bloch and Feodor Lynen who shared the 1964 Nobel Prize in Physiology or Medicine for their discoveries (Bloch, 1965; Zetterström, 2009). Of particular importance was the identification by Bucher and Lynen of the 6-carbon compound 3-hydroxy-3-methylglutarate attached to CoA (HMG CoA) as the first intermediate committed solely to synthesis of cholesterol and other isoprenoids (Bucher et al., 1960). This finding focused attention on HMG CoA reductase as the rate-controlling enzyme in the cholesterol biosynthetic pathway.
Risk Factor Concept Advanced
In 1951 Paul Dudley White and his colleague, Menard M. Gertler, cardiologists at the Massachusetts General Hospital in Boston, made careful observations on 100 people under age 40 who had suffered heart attacks. They identified a series of risk factors that predisposed to the disease (Gertler and White, 1954). These consisted of male sex, high blood cholesterol, elevated blood pressure, cigarette smoking, positive family history, and a mesomorphic body build. All of these are recognized as risk factors today.
Seven Country Study Invokes Dietary Fat
In 1953, the American physiologist Ancel Keys launched a landmark international epidemiological study of heart attacks. Keys (1980) chose to study 16 cohorts of healthy men from specific populations in seven different countries. The cohorts were chosen mainly because their diets contained very different amounts of total and saturated fat – ranging from minimal fat in a cohort of Japanese fishermen who thrived on vegetables, rice, and fish to enormous amounts of fat in East Finnish foresters who habitually spread butter on their cheese. For each of the 16 cohorts, Keys ascertained 511 to 2517 healthy men between the ages of 40 and 59 and followed them for 10 years, recording fatal and nonfatal heart attacks (Keys, 1980). He found that serum cholesterol rose in proportion to the total fat intake (r = 0.67) and even more strikingly in proportion to the intake of saturated fatty acids (r = 0.87). Fatal coronary events rose in proportion to the serum cholesterol level (r = 0.80). At the two extremes, serum cholesterol varied from a mean of 165 mg/dl in the Japanese fishermen to 270 mg/dl in the East Finnish foresters. This difference was accompanied by a 13-fold higher incidence of coronary events in the East Finnish. The other 14 cohorts lay more or less on a line relating dietary saturated fat intake and serum cholesterol. Although certain deviations from this line have been proposed to contradict Keys’ conclusion, it is likely that the deviations reflect local environmental or genetic factors that modify the relation between fat intake, serum cholesterol, and heart attacks in specific populations. However, they do not negate Keys’ general conclusion that high fat intake leads to high cholesterol and to heart attacks.
Keys’ observation that low dietary fat produces low blood cholesterol and low heart attacks was supported by comparative studies of Japanese men living in Japan, Hawaii, and California (Keys et al., 1958). As the Japanese moved westward, their fat intake increased, their plasma cholesterol rose, and the number of heart attacks increased. Keys’ studies were performed before plasma lipoproteins could be readily fractionated, and we therefore consider them to conclude the first era in the story of coronaries – the era of cholesterol.
LDL Identified as Major Risk Factor
The era of LDL began in 1955 when John Gofman, a physician/physicist at the University of California, Berkeley used the newly invented analytical ultracentrifuge to separate the cholesterol-carrying lipoproteins of plasma according to their density (Gofman et al., 1954a). Two major fractions were identified – low density lipoproteins (LDL) and high density lipoproteins (HDL). When he studied plasma from heart attack patients, Gofman found a major increase in the cholesterol-carrying LDL. He also observed a reduced level of HDL (Gofman et al., 1954b). Gofman's pioneering discoveries have been replicated many times. The correlation between high LDL levels, low HDL levels, and heart attacks is one of the most profound epidemiologic correlations in all of medicine.
Gofman's finding of a positive correlation with LDL led to intense interest in this pathologic lipoprotein (Fredrickson et al., 1967). In contrast, his finding of a negative correlation with HDL received little attention until it was rediscovered 20 years later by epidemiologists, most notably in the Framingham study (Gordon et al., 1977). Despite the enormous amount of data correlating low HDL levels with increased heart attacks, the mechanistic role of HDL in the pathogenesis of coronary disease is poorly understood and remains controversial despite 40 years of study. In marked contrast, elevations of plasma LDL have been shown to produce atherosclerosis in every mammalian species ever studied.
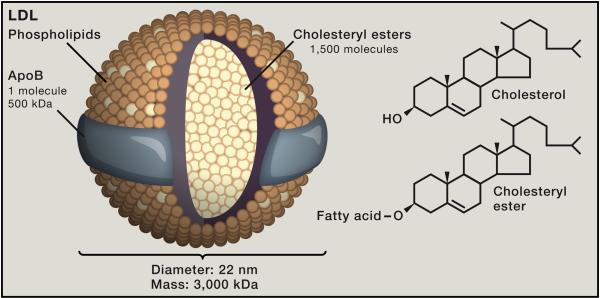
Figure 1 shows the structure of LDL. Each LDL particle contains ~1500 molecules of cholesteryl ester in a hydrophobic core surrounded by a polar phospholipid coat and a single large protein called apolipoprotein B (apoB). A prevalent theory states that LDL initiates atherosclerotic plaques when it penetrates through dysfunctional endothelium into the walls of arteries (Bonetti et al., 2003). LDL is retained there, likely by its propensity to bind to glycosaminoglycans. The lipids of LDL become oxidized and some of the reaction products modify lysine residues on apoB (Steinberg and Witztum, 2010). The modified apoB is recognized by scavenger receptors on macrophages and internalized by endocytosis, converting the macrophages to cholesterol-laden foam cells (Brown and Goldstein, 1983; Greaves and Gordon, 2009). The foam cells secrete a variety of cytokines, thereby initiating an inflammatory reaction (Hansson and Jonasson, 2009; Libby et al., 2011). In response, smooth muscle cells in the arterial intima proliferate and produce collagen. The plaque enlarges and eventually ruptures, leading to the formation of a blood clot that occludes the vessel.
Figure 1. LDL, A Cholesterol Carrier.
LDL is a spherical particle with a diameter of 220 nm and a mass of ~3000 kDa. Each particle contains ~1500 molecules of cholesteryl ester in an oily core that is shielded from the aqueous plasma by a hydrophilic coat composed of ~800 molecules of phospholipid, ~500 molecules of unesterified cholesterol, and 1 molecule of a 500-kDa protein, apoB.
All other things being equal, the higher the LDL the faster the plaques evolve. However, all other things are not equal. At any given level of LDL, plaque formation is accelerated by risk factors that include smoking, high blood pressure, and diabetes. In addition, poorly understood genetic factors affect atherosclerosis, possibly by changing the susceptibility of the endothelium to become damaged. Unless the LDL level is extraordinarily high or low, it is difficult to predict accurately whether any individual will suffer a heart attack.
LDL Receptor Pathway Elucidated
In 1972, we entered the field when we began to study the regulation of cholesterol synthesis in cultured human fibroblasts (Goldstein and Brown, 2009). We were stimulated by our encounter with two young siblings (ages 6 and 8) who were hospitalized at the National Institutes of Health because of recurrent heart attacks, owing to plasma LDL levels that were 8-fold above normal. The children had the severe homozygous form of familial hypercholesterolemia (FH), the disease that was described in 1938 in its common heterozygous form by Müller in Norway (see above) and later in its rare homozygous form by Khachadurian (1964) in Lebanon. In the homozygous children, each LDL particle was structurally normal, but they had 8-fold more particles per ml of plasma as compared with normal children. Homozygous FH is a rare disorder with a frequency of ~1 in 1 million people (Goldstein et al., 2001).
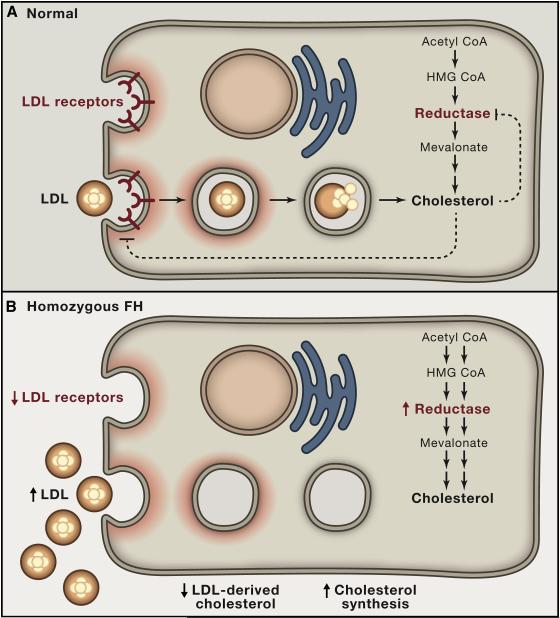
Frustrated at being unable to reduce the LDL level in these unfortunate children, we resolved to elucidate the genetic defect. In 1972, shortly after moving to the University of Texas Southwestern Medical Center in Dallas, we obtained skin biopsies from three FH homozygotes and controls. From the biopsies, we grew monolayers of fibroblasts. Like all human cells, cultured fibroblasts require cholesterol to maintain the integrity of their plasma membranes. We found that normal fibroblasts obtained cholesterol from two sources (Figure 2A). They synthesized it from acetyl CoA through the pathway described by Bloch, Lynen, and others, including the rate-controlling step catalyzed by HMG CoA reductase. In addition, normal fibroblasts somehow took up cholesterol from the LDL in the serum of the culture medium. In sharp contrast, cells from FH homozygotes could not take up cholesterol from LDL. They survived in culture because they activated the cholesterol synthesis pathway to compensate for the lack of LDL-derived cholesterol (Figure 2B). Their HMG CoA reductase activity was 100-fold above normal (Goldstein and Brown, 1973).
Figure 2. Feedback Regulation of Cholesterol Synthesis and LDL Receptors in Cultured Cells from Normal Subjects and Children with Homozygous FH.
(A) Normal cells obtain cholesterol from two sources: 1) endogenous synthesis and 2) receptor-mediated uptake and lysosomal hydrolysis of LDL.
(B) Lacking LDL receptors, FH cells maintain normal levels of cholesterol by increasing synthesis of cholesterol, leaving excess LDL in the culture medium.
Working intensely for several years, we found the mechanism for LDL uptake. The key is a cell surface receptor that binds apoB. With our colleague Richard Anderson, we showed that LDL receptors cluster in coated pits, regions of the cell surface that are adapted for rapid internalization by endocytosis (Anderson et al., 1977; Goldstein et al., 1979). The internalized LDL is delivered to lysosomes where the cholesteryl esters are hydrolyzed, and the cholesterol is released for new membrane synthesis. We also found that the production of LDL receptors and the enzyme HMG CoA reductase are subject to coordinate feedback suppression, as indicated by the dashed arrows in Figure 2A. When cell cholesterol levels are low, the cells produce abundant LDL receptors and HMG CoA reductase. When cellular cholesterol levels rise, the levels of these two proteins decrease. The net result is to keep the level of cholesterol in cell membranes constant (Brown and Goldstein, 1986).
Defective LDL Receptors Cause FH
We found that the defect in FH lies in the gene for the LDL receptor (Brown and Goldstein, 1974). Depending on the site of the mutation in the LDL receptor gene, some FH homozygotes are unable to produce any functional receptors (Figure 2B), whereas others produce receptors with 5-25% of normal activity (Goldstein et al., 2001). FH heterozygotes produce half the normal number of functional receptors. Their LDL levels are elevated by approximately 2-fold, and they have heart attacks in their fifth and sixth decades. The heterozygous form of FH is relatively common for a single-gene disease, occurring in at least 1 in 500 people in all populations (Goldstein et al., 2001).
LDL is secreted from the liver as a larger particle called very low density lipoprotein (VLDL) that contains triglyceride as well as cholesterol. The triglycerides are extracted in adipose tissue and muscle, causing the particle to shrink and become LDL. Most LDL is removed from the circulation by LDL receptors located in the liver. When LDL receptors are deficient, LDL particles circulate for a prolonged time and they build up to high levels in plasma, eventually depositing in arteries, thereby creating atherosclerotic plaques (Brown and Goldstein, 1986).
Our laboratory purified the LDL receptor from bovine adrenal glands, which use the receptor to supply cholesterol for synthesis of steroid hormones (Schneider et al., 1982). We obtained a partial amino sequence and used it to clone the cDNA and the gene (Yamamoto et al., 1984; Südhof et al., 1985). Located on the short arm of chromosome 19, the 18 exons of the LDL receptor gene encode a mature protein of 839 amino acids. The receptor spans the plasma membrane with its NH2-terminus facing the exterior. The external segment contains seven cysteine-rich repeats that constitute the LDL binding site. The cytoplasmic COOH-terminal segment of 50 amino acids contains the sequence that guides the receptor to coated pits (Chen et al., 1990). To date, more than 1,200 different allelic mutations in the LDL receptor gene have been described in different families with FH (Usifo et al., 2012). Some mutations destroy all function, while others allow some residual activity.
Statins Inhibit HMG CoA Reductase and Increase LDL Receptors
A therapeutic milestone occurred in 1976 when Akira Endo at the Sankyo Company in Tokyo identified the first inhibitor of HMG CoA reductase, thereby inaugurating the class of cholesterol-lowering drugs known as statins (Endo et al., 1976). Endo's statin, called compactin, was isolated from a penicillium mold. Knowing about the feedback suppression of LDL receptors, we reasoned that an HMG CoA reductase inhibitor would deprive liver cells of endogenous synthesis as a source of cholesterol. This deprivation would relieve the feedback repression of LDL receptors, and the resultant increase in LDL receptors would lower plasma LDL (Brown et al., 1978). In 1981, we tested this hypothesis by treating dogs with mevinolin, another fungal HMG CoA reductase inhibitor, closely related to compactin, that was discovered by Alfred W. Alberts and colleagues at Merck (Alberts et al., 1980). As predicted, mevinolin increased LDL receptors in the dog liver, and this led to a marked fall in plasma LDL (Kovanen et al., 1981). Subsequent studies by others showed that mevinolin (soon known as lovastatin or Mevacor) and other statins lowered plasma LDL in humans with diet-induced hypercholesterolemia (Vega and Grundy, 1991) and also in FH heterozygotes whose single functional LDL receptor gene is susceptible to activation by cholesterol deprivation (Mabuchi et al., 1981). Consistent with the LDL receptor theory, those FH homozygotes who had no functional receptors showed no cholesterol lowering when treated with mevinolin (Uauy et al., 1988).
First Statin Approved for Lowering LDL
In 1987 Merck's Mevacor became the first statin approved for human use (Brody, 1987; Byrne, 1987). The FDA approved Mevacor based on studies showing that it lowers plasma LDL and is well tolerated. At the time of approval, there was no evidence that a statin could reduce heart attacks. This evidence came in 1994 when Merck's 4S Study (Scandinavian Simvastatin Survival Study Group, 1994) demonstrated that a second-generation statin, simvastatin, not only reduced heart attacks, but actually prolonged life in middle-aged people at high risk of a coronary event.
SREBP Discovered
The statin studies showed that the feedback regulation of LDL receptors is of clinical importance, yet nothing was known of the molecular mechanism by which cholesterol regulated the synthesis and supply pathways. Essentially all cellular cholesterol is contained in membranes. The feedback system optimizes the level of cholesterol in those membranes. The puzzle is: How does a cell measure the level of cholesterol in its membranes, and how is this information transmitted to the nucleus to regulate gene transcription? The first clue came in 1993 when we isolated the key regulator – a transcription factor called Sterol Regulatory Element-binding Protein-1 (SREBP-1) (Yokoyama et al., 1993).
We purified SREBP-1 from the nuclei of cultured cells that were deprived of cholesterol so that they actively transcribed the LDL receptor gene. Our isolation procedure was based on the ability of the protein to bind to a DNA sequence required for LDL receptor transcription (Wang et al., 1993). The purified protein had a molecular mass of ~60 kDa; yet when we cloned its cDNA, we were greeted with two surprises: first, the protein purified from nuclei was only a fragment of the full length SREBP-1, which had a predicted molecular mass of 125 kDa (Yokoyama et al., 1993); and second, the newly synthesized protein was bound to membranes by virtue of two transmembrane helices (Wang et al., 1994; Hua et al., 1995). We were confronted with a problem: how does a membrane bound transcription factor move to the nucleus?
SREBP Pathway Elucidated
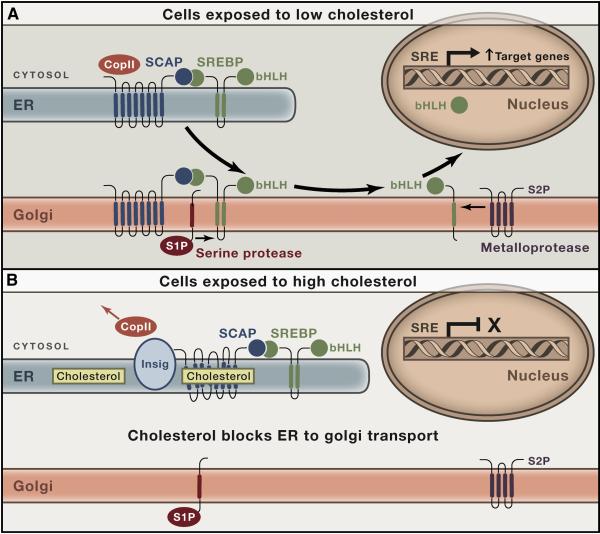
Over the next few years, we solved the movement problem, and the result is the SREBP pathway shown in Figures 3A and 3B (Brown and Goldstein, 1997; Goldstein et al., 2006). SREBPs are synthesized as integral membrane proteins of the endoplasmic reticulum (ER). The cytosolic NH2-terminal segment (molecular mass, 60 kDa) contains the basic helix-loop-helix-leucine zipper sequence by which the protein binds to DNA and activates transcription. Next is a hairpin membrane-attachment domain comprising two transmembrane helices separated by a 50-amino acid loop that projects into the ER lumen. At the COOH-terminal end is a 65-kDa regulatory domain that projects into the cytosol.
Figure 3. The SREBP Pathway for Cholesterol Homeostasis in Animal Cells.
(A) Cholesterol deficiency. When the cholesterol content of ER membranes falls below 5 mol% of its total lipids, Scap binds COPII proteins, which incorporate the Scap/SREBP complex into COPII-coated vesicles that move to the Golgi. In the Golgi, SREBPs are cleaved by two proteases, S1P and S2P, allowing the active portion to enter the nucleus where it activates genes that increase cholesterol synthesis and uptake.
(B) Cholesterol excess. When ER cholesterol rises above 5 mol% of membrane lipids, cholesterol binds to Scap, thereby causing Scap to bind to Insig. This releases COPII proteins, abrogating transport to the Golgi. The fall in nuclear SREBP reduces transcription of genes for cholesterol synthesis and uptake.
Immediately after its translation on membrane-bound ribosomes, SREBP binds to Scap, a polytopic protein of the ER membrane (Hua et al., 1996). Scap has eight transmembrane helices and a COOH-terminal extension that projects into the cytosol where it binds to the COOH-terminal regulatory domain of SREBP (Sakai et al., 1998a). The cytosolic loop between helices 6 and 7 of Scap contains a sequence of six amino acids that binds to a complex of proteins called COPII (Sun et al., 2007), which were known previously to cluster certain ER proteins into COPII-coated vesicles that bud from ER membranes (Antonny and Schekman, 2001). These vesicles transport the Scap/SREBP complex to the Golgi apparatus.
In the Golgi, SREBP is processed sequentially by two proteases. The first, called Site-1 Protease (S1P), is a membrane bound serine protease that clips the SREBP in the luminal loop (Sakai et al., 1998b). Cleavage separates the SREBP into two membrane-bound halves. The NH2-terminal segment remains bound to the membrane, owing to its single membrane-spanning helix. It is released from the membrane by Site-2 Protease (S2P), a hydrophobic zinc metalloprotease with its active-site zinc buried in the membrane (Rawson et al., 1997; Feng et al., 2007). S2P cleaves the NH2-terminal half of SREBP within its membrane-spanning helix, releasing the transcriptionally active segment so that it can enter the nucleus to activate transcription of the LDL receptor gene. Intramembrane zinc metalloproteases related to S2P are found as far back as archaea. We coined the term Regulated Intramembrane Proteolysis (RIP) to designate the process by which membrane-bound proteins are released by cleavage within a transmembrane helix (Brown et al., 2000). Multiple examples of this regulatory mechanism are now known (Urban, 2013).
Scap: A Sterol Sensor
Its location in the ER membrane allows Scap to sense the level of cholesterol in the membrane (Radhakrishnan et al., 2004; Radhakrishnan et al., 2008). When ER cholesterol rises above a threshold of 5% of total lipids, the cholesterol binds to luminal Loop 1 of Scap (Motamed et al., 2011). This binding triggers a conformational change in luminal Loop 6 (Brown et al., 2002) that occludes the binding site for COPII proteins (Sun et al., 2007). In its new conformation, Scap binds to Insig, another polytopic protein of ER membranes (Yang et al., 2002). Insig locks Scap into a conformation that cannot bind COPII proteins, trapping the Scap/SREBP complex in the ER, preventing Golgi processing and thereby decreasing transcription of the LDL receptor gene. Transcription remains low until ER cholesterol falls, and cholesterol-free Scap/SREBP complexes can again bind COPII proteins.
Although SREBP-1 was isolated by virtue of its ability to activate transcription of the LDL receptor gene, we soon found that the protein activates the gene for HMG CoA reductase as well as the genes for all of the enzymes in the cholesterol biosynthetic pathway, thereby allowing cells to synthesize cholesterol in addition to taking it up from LDL (Shimano et al., 1996; Horton et al., 2003). SREBP-1 has two isoforms, SREBP-1a and -1c, which are derived from alternate promoters that give rise to different first exons (Shimomura et al., 1997). SREBP-1a is highly expressed in rapidly growing normal and cancer cells; it activates fatty acid synthesis as well as cholesterol synthesis, thereby supplying the major lipid components of cell membranes. SREBP-1c is expressed primarily in liver where it activates fatty acid synthesis in response to insulin (Shimomura et al., 1999), thereby contributing to fatty liver in type 2 diabetes (Moon et al., 2012). A third isoform, SREBP-2, is produced by a different gene. It is a relatively specific activator of the genes encoding the LDL receptor and cholesterol biosynthetic enzymes (Horton et al., 2003). All three SREBPs require processing by the SREBP pathway (Horton et al., 2002).
Regulation of LDL Receptors by Diet, Drugs, and Genes
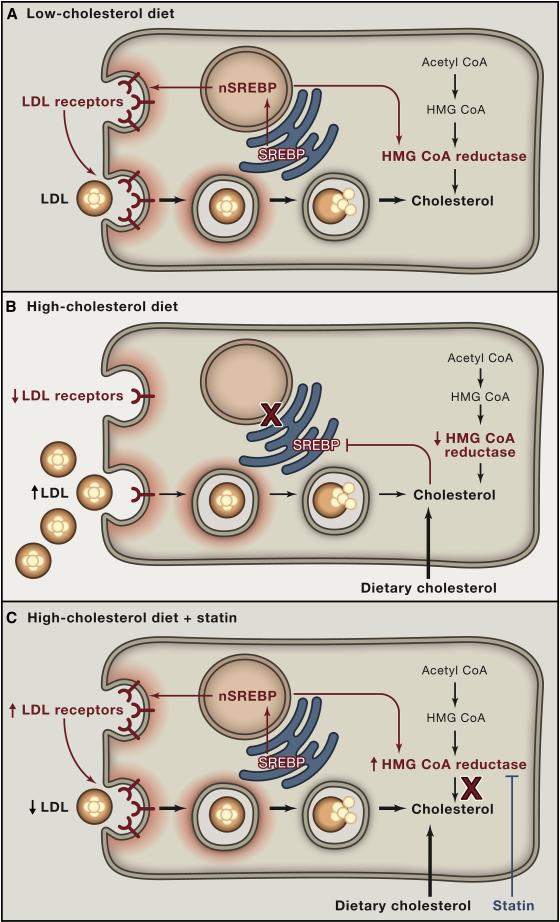
Figure 4 shows a model to explain how the regulation of SREBP-2 in the liver is responsible for the LDL elevation produced by high cholesterol/high fat diets and the LDL-lowering produced by statins. The model is based on experiments in hamsters and mice (Sheng et al., 1995; Horton et al., 2002). When the diet is low in cholesterol, liver cholesterol levels are low and SREBP-2 enters the nucleus where it activates the LDL receptor gene, the HMG CoA reductase gene, and other genes required for cholesterol synthesis (Figure 4A). The LDL receptors keep plasma LDL low. High cholesterol diets cause cholesterol to accumulate in hepatic ER membranes, blocking SREBP-2 processing, reducing LDL receptors, and raising plasma LDL (Figure 4B). In humans, the diet-mediated receptor reduction is not complete or everyone would look like FH homozygotes. It is not even 50% as in FH heterozygotes. However, it is sufficient to raise LDL from the low levels seen in Japanese fishermen to the higher levels accepted as normal in the U.S. and other industrialized countries. Statins have the opposite effect. By blocking cholesterol synthesis, statins lower ER cholesterol, activating SREBP-2, increasing LDL receptors and lowering plasma LDL (Figure 4C). Nuclear SREBP-2 also increases HMG CoA reductase but cholesterol synthesis does not increase, owing to statin inhibition. Strongly in favor of the receptor induction mechanism is the observation that statins do not lower LDL significantly in those FH homozygotes who have null mutations in both copies of the LDL receptor gene (Uauy et al., 1988).
Figure 4. Hepatic Response to Diet and Statins Mediated by the SREBP Pathway.
(A) Low cholesterol diet. Proteolytic cleavage of SREBP is increased. The cleaved SREBP enters the nucleus to activate genes controlling cholesterol synthesis (including HMG CoA reductase) and uptake (LDL receptor). nSREBP, nuclear portion of cleaved SREBP.
(B) High cholesterol diet. Proteolytic cleavage of SREBPs is decreased, resulting in decreased nuclear SREBP and decreased activation of target genes. The decrease in LDL receptors produces an increase in plasma LDL.
(C) High cholesterol diet plus statin therapy. Statins inhibits HMG CoA reductase, causing a transient decrease in ER cholesterol. In response, SREBP cleavage is increased, and the resulting nuclear SREBP activates the genes for HMG CoA reductase and LDL receptor. The increased HMG CoA reductase is inhibited by the statin, and the increased LDL receptors lower plasma LDL.
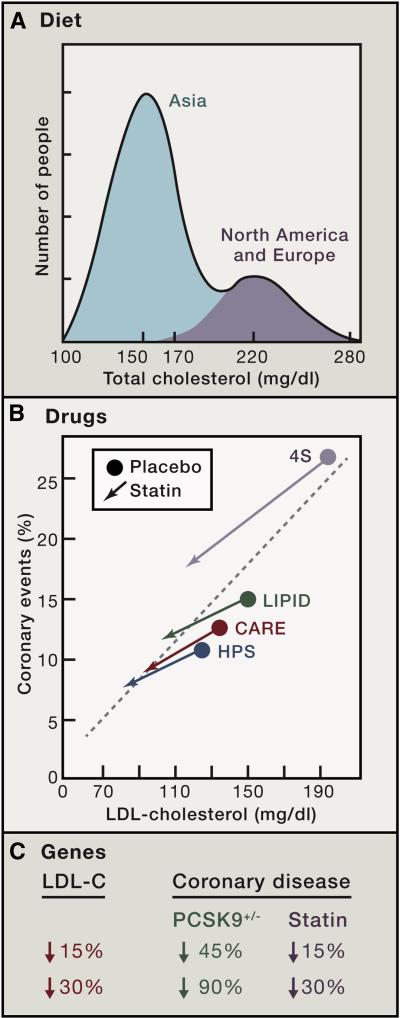
Figure 5 illustrates the effects of diet, drugs, and genes on plasma LDL and the consequent effects on coronary disease. The upper panel (Figure 5A) is a graphic representation of plasma cholesterol levels in the human species as extrapolated from surveys of middle-aged people in major populations of the world. We use total cholesterol values because there are more measurements of total cholesterol than of LDL. However, differences in total cholesterol closely reflect differences in LDL. The distribution of total cholesterol levels is highly skewed. The data predict that the median cholesterol level for the human species is approximately 170 mg/dl. This is attributable to the large number of people in China and other Asian populations where the historical mean cholesterol level is about 150 mg/dl (Chen et al., 1991; Junshi et al., 1990). In North America and Europe, the mean value is approximately 220 mg/dl. The incidence of heart attacks correlates with these levels. Almost unheard of in China and Japan before recent increases in their fat intake, heart attacks are much more frequent in Europe and the U.S., especially when plasma cholesterol exceeds 220 mg/dl (graduated red shading in Figure 5A).
Figure 5. Diagram Illustrating the Effects of Diet, Drugs, and Genes on Plasma LDL and Coronary Disease.
(A) Diet. Idealized depiction of the frequency distribution of plasma cholesterol levels in the human species as extrapolated from surveys of middle-aged people in major populations of the world. The higher the cholesterol level, the higher the risk for coronary disease, as denoted by the graded red shading.
(B) Drugs. Frequency of coronary events plotted against plasma level of LDL-cholesterol in four double-blind, placebo-controlled trials in which middle-aged people at risk for heart attacks were treated for five years with a statin or placebo. The number of subjects in each study were as follows: 4S Study, 4444; LIPID, 9014; CARE, 4159; and HPS, 20,536.
(C) Genes. Difference in risk for coronary disease in middle-aged people depending on whether plasma LDL-cholesterol level is reduced over a lifetime (heterozygous loss-of-function of PCSK9) or for only five years (statin therapy).
Statins Reduce Heart Attacks and Prolong Life
Figure 5B summarizes the results of four double-blind, placebo-controlled trials in which middle-aged people at high risk for heart attacks were treated for five years with a statin or placebo (38,153 total subjects). The results are remarkably consistent. In each case lowering LDL reduced heart attacks with no evidence for a lower threshold (Scandinavian Simvastatin Survival Study Group, 1994; Sacks et al., 1996; The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group, 1998; Heart Protection Study Collaborative Group, 2002). In the largest study, the Heart Protection Study (HPS), 20,536 subjects were treated with simvastatin for five years. The study was large enough to allow multiple subgroup analyses. These showed that the reduction in relative risk was similar even when subjects had risk factors including diabetes, hypertension, and smoking. A recent meta-analysis of 22 statin trials involving 134,000 participants concluded that for each reduction of LDL-cholesterol of 1 mmol/l (40 mg/dl) cardiovascular events are reduced by 20%, even in people who were considered to be at low risk (Cholesterol Treatment Trialists' (CTT) Collaborators, 2012). Considered together, these statin trial results strongly support the dominant role of LDL in coronary disease.
PCSK9: A Protein that Destroys LDL Receptors and Raises LDL
The genetic connection between LDL and heart attacks was established by mutations in LDL receptors that raise LDL and increase coronary events (Brown and Goldstein, 1986). Recently, the other side of the coin was exposed – namely, mutations that increase LDL receptors, lower LDL and reduce coronary events (Cohen et al., 2006; Abifadel et al., 2014). The mutations occur in a gene that encodes an enzyme called PCSK9, which stands for proprotein convertase subtilisin/kexin type 9 (Horton et al., 2009). Secreted into plasma by liver and other organs, PCSK9 binds to hepatic LDL receptors and disrupts the recycling mechanism that returns the receptors to the cell surface after internalization (Lagace et al., 2006; Zhang et al., 2007). As a result, the number of cell surface LDL receptors declines and LDL rises. Inasmuch as PCSK9 circulates in the plasma of normal individuals, plasma LDL is never as low as it would be if LDL receptors were functioning maximally.
The relation between PCSK9 and LDL receptors was discovered by Abifadel, et al. (2003) in studies of rare patients with gain-of-function mutations that increase the activity of the protein. Affected individuals have high LDL levels that are transmitted as an autosomal dominant trait. The reverse phenomenon was discovered by Cohen and Hobbs, who identified people with common loss-of-function mutations in PCSK9 that lead to low LDL levels and protect against heart attacks (Cohen et al., 2006). Three percent of Caucasians are heterozygous for a missense mutation (R46L) that partially inactivates PCSK9. On average, these individuals have a 15% reduction in plasma LDL when compared with age-matched controls. Remarkably, they have a 46% reduction in early coronary events. Even more striking, 2% of African-Americans are heterozygous for one of two nonsense mutations (either Y142X or C679X) that inactivate one copy of the PCSK9 gene. Their LDL levels are reduced by 28%, and they have an 88% reduction in heart attacks when studied at a mean age of 54 years (Cohen et al., 2006).
Cohen and Hobbs’ finding of a 45% lowering of coronary events in Caucasians with missense mutations in PCSK9 was rapidly confirmed in two other studies (McPherson and Kavaslar, 2007; Kathiresan, 2008). The reduction in coronary disease in individuals with diminished PCSK9 occurs despite the presence of other risk factors, including hypertension, diabetes, and smoking. This finding correlates with the finding in the HPS study that LDL lowering with a statin can reduce heart attacks even when other risk factors are present (Heart Protection Study Collaborative Group, 2002).
LDL Reduction: Better Sooner than Later
The observations on subjects with PCSK9 mutations suggest strongly that lifelong LDL reductions are much more effective than late LDL reductions in preventing coronary events. Figure 5C compares the reduction in coronary disease in PCSK9 heterozygotes and the reduction observed in people in 5-year clinical trials whose LDL was lowered by a statin. When statins lower LDL by 15%, they lower coronary events by 15%, which is much less than the 45% reduction observed when LDL is reduced to a similar degree by a PCSK9 mutation. Similarly, when statins lower LDL by 30%, they lower coronary events by 30% – far weaker than the nearly 90% event-reduction observed when LDL is lowered by 30% as a result of a PCSK9 mutation. Why is the protective effect of the PCSK9 mutations so much greater than can be achieved by statins? The most likely explanation lies in the duration of the LDL lowering (Brown and Goldstein, 2006). People with PCSK9 mutations have low LDL levels throughout life, and they may never have developed atherosclerotic plaques. In the statin trials, patients were not treated until they were at high risk of a coronary event, which means that they already had established atherosclerosis. Once the disease is established, patients may require much more severe LDL lowering to prevent an event.
It is noteworthy that the 90% reduction in coronary events in people with null mutations in PCSK9 is similar to the 90% reduction observed by Ancel Keys when he studied Japanese fishermen who had low LDL levels throughout life (Keys, 1980). The advantage of lifelong low LDL is further illustrated by a recent study of subjects who absorb reduced amounts of cholesterol from the intestine as a result of loss-of-function mutations in NPC1L1, the intestinal cholesterol transporter. Lifelong reductions of only 11% in LDL produced a 53% reduction in heart attacks (The Myocardial Infarction Genetics Consortium Investigators, 2014). Thus diet, genes, and drugs all yield the same conclusion, namely, that LDL lowering can prevent heart attacks, especially when the lowering is begun before atherosclerotic plaques have developed.
Based on the high affinity of the LDL receptor for LDL, in 1977 we postulated that the human body is designed to maintain LDL-cholesterol levels in the range of 25 mg/dl (Goldstein and Brown, 1977). Indeed, plasma LDL-cholesterol levels in adults from most other animals so far studied, including primates, are lower than 60 mg/dl (Mills and Taylaur, 1971; Chapman and Goldstein, 1976). In newborn humans, LDL-cholesterol is less than 50 mg/dl (Kwiterovich, Jr. et al., 1973). LDL levels above 100 mg/dl are frequent only in humans or other animals that have consumed the typical western diet, which is high in fat and cholesterol.
The dramatic reductions in coronary events in people with PCSK9 mutations stimulated several pharmaceutical companies to produce monoclonal antibodies that bind to PCSK9 and inactivate it. Data from two early trials show that these antibodies can reduce plasma LDL by as much as 40% when given alone and by 60% when added to a statin regimen (Stein et al., 2012; Raal et al., 2012). Just like statins, anti-PCSK9 antibodies fail to reduce LDL significantly in those FH homozygotes who lack all LDL receptor activity (Stein et al., 2013), further supporting the role of LDL receptors in lowering LDL. Studies are under way to determine the long term safety of anti-PCSK9 antibodies and their efficacy in preventing heart attacks (Young and Fong, 2012; Vogel, 2012).
Accomplices of LDL
The data reviewed here focus on LDL as the sine qua non of atherosclerosis. However, LDL does not act alone. Evidence from genetically-manipulated mice indicates that the atherogenic effect of LDL requires macrophages and inflammatory cytokines (Glass and Witztum, 2001; Libby et al., 2011). In humans, at any given level of LDL the probability of a myocardial infarction is increased by well-documented risk factors that include cigarette smoking, hypertension, diabetes, and low HDL. Although these contributory factors are important, they all require LDL to instigate the lesion. The epidemiologic data in China and Japan, the genetic data with PCSK9 and NPC1L1 deficiency, and the therapeutic data with statins demonstrate that myocardial infarctions can be markedly diminished by LDL-lowering even when macrophages, cytokines, and risk factors are present. Fortunately, we now have the ability to lower LDL with diet and with drugs.
The Next Century
The past 100 years have witnessed the crest of the coronary disease epidemic in Western countries and its beginning recession. Scientists have made dramatic advances in understanding cholesterol, the lipoproteins that carry it in plasma, and the manner in which genes and diets alter lipoprotein levels. The century of cholesterol research produced four lines of evidence – experimental, genetic, epidemiologic, and therapeutic – that converge upon LDL as the primary cause of atherosclerosis. Few, if any, chronic diseases have been subjected to such intensive scrutiny, and rarely has the cause and the approach to prevention been documented so convincingly.
It does not seem an exaggeration to state that targeted application of an LDL-lowering regimen may eventually curtail one of the major killers of the last century. The key questions for the 21st century are: Who to target? and When? Ideally, LDL-lowering therapy should be initiated before atherosclerotic plaques develop, or at least before they develop their most threatening features. Currently, we base our decisions for LDL lowering on factors such as the absolute level of LDL, the presence of other risk factors, and a family history of early coronary disease. In the future, we may be able to use genetic polymorphisms that render arteries susceptible or resistant to LDL entry and susceptible or resistant to the ensuing inflammation. Such genetic polymorphisms may increase our assessment of risk, but they are unlikely to be absolute. Our decisions would be greatly helped if someone develops a noninvasive method to detect the earliest atherosclerotic plaques in the coronary arteries, to monitor their growth, and to detect the earliest signs of the inflammation and instability that lead to thrombosis. Given such a method for early detection and given our powerful methods to lower LDL, we should surely be able to end the epidemic of heart attacks that killed so many over the last century.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the many talented students, postdoctoral fellows, and collaborators who have conducted the experiments described from our laboratory. Their names are included in the cited references. Our experimental work over the last 40 years has been supported by grants from the NIH (HL-20948), the Perot Foundation, Moss Heart Foundation, and the O'Donnell Foundation. Drs. Goldstein and Brown are members of the Board of Directors and shareholders in Regeneron Pharmaceuticals.
REFERENCES
- Abifadel M, Elbitar S, El Khoury P, Ghaleb Y, Chémaly M, Moussalli M-L, Rabès J-P, Varret M, Boileau C. Living the PCSK9 adventure: from the identification of a new gene in familial hypercholesterolemia towards a potential new class of anticholesterol drugs. Curr. Atheroscler. Rep. 2014;16:439–461. doi: 10.1007/s11883-014-0439-8. [DOI] [PubMed] [Google Scholar]
- Abifadel M, Varret M, Rabes J-P, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derre A, Villeger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf J-M, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nature Genetics. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E, Patchett A, Monaghan R, Currie S, Stapley E, Albers-Schonberg G, Hensens O, Hirshfield J, Hoogsteen K, Liesch J, Springer J. Mevinolin, a highly potent competitive inhibitor of HMG-CoA reductase and cholesterol lowering agent. Proc. Natl. Acad. Sci. USA. 1980;77:3957–396l. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RGW, Brown MS, Goldstein JL. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell. 1977;10:351–364. doi: 10.1016/0092-8674(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Anitschkow N, Chalatow S. Ueber experimentelle cholesterinsteatose und ihre bedeutung für die entstehung einiger pathologischer prozesse. Zentralbl. Allg. Pathol. Anat. 1913;24:1–9. [Google Scholar]
- Antonny B, Schekman R. ER export: public transportation by the COPII coach. Curr. Opin. Cell Biol. 2001;13:438–443. doi: 10.1016/s0955-0674(00)00234-9. [DOI] [PubMed] [Google Scholar]
- Bloch K. The biological synthesis of cholesterol. Science. 1965;150:19–28. doi: 10.1126/science.150.3692.19. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- Brody JE. New type of drug for cholesterol approved and hailed as effective. New York Times; Sep 2, 1987. [Google Scholar]
- Brown AJ, Sun L, Feramisco JD, Brown MS, Goldstein JL. Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Mol. Cell. 2002;10:237–245. doi: 10.1016/s1097-2765(02)00591-9. [DOI] [PubMed] [Google Scholar]
- Brown MS, Faust JR, Goldstein JL, Kaneko I, Endo A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J. Biol. Chem. 1978;253:1121–1128. [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Familial hypercholesterolemia: Defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc. Natl. Acad. Sci. USA. 1974;71:788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: Implications for cholesterol deposition in atherosclerosis. Ann. Rev. Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Lowering LDL - not only how low, but how long? Science. 2006;311:1721–1723. doi: 10.1126/science.1125884. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Cholesterol feedback: from Schoenheimer's bottle to Scap's MELADL. J. Lipid Res. 2009;50:S15–S27. doi: 10.1194/jlr.R800054-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- Bucher NLR, Overath P, Lynen F. β-Hydroxy-β-methylglutaryl coenzyme A reductase, cleavage and condensing enzymes in relation to cholesterol formation in rat liver. Biochim. Biophys. Acta. 1960;40:491–501. doi: 10.1016/0006-3002(60)91390-1. [DOI] [PubMed] [Google Scholar]
- Byrne JA. The miracle company: excellence in the lab and executive suite makes Merck a powerhouse. Business Week. 1987 Oct 19;:84–90. [Google Scholar]
- Chapman MJ, Goldstein S. Comparison of the serum low density lipoprotein and of its apoprotein in the pig, rhesus monkey and baboon with that in man. Atherosclerosis. 1976;25:267–291. doi: 10.1016/0021-9150(76)90033-2. [DOI] [PubMed] [Google Scholar]
- Chen W-J, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J. Biol. Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- Chen Z, Peto R, Collins R, MacMahon S, Lu J, Li W. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. Brit. Med. J. 1991;303:276–282. doi: 10.1136/bmj.303.6797.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholesterol Treatment Trialists' (CTT) Collaborators The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JC, Boerwinkle E, Mosley TH, Jr., Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- Endo A, Kuroda M, Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976;72:323–326. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- Feng L, Yan H, Wu Z, Yan N, Wang Z, Jeffrey PD, Shi Y. Structure of a Site-2 protease family intramembrane metalloprotease. Science. 2007;318:1608–1612. doi: 10.1126/science.1150755. [DOI] [PubMed] [Google Scholar]
- Fredrickson DS, Levy RI, Lees RS. Fat transport in lipoproteins - An integrated approach to mechanisms and disorders. N. Engl. J. Med. 1967;276:32–44. 94–l03. doi: 10.1056/NEJM196701052760107. [DOI] [PubMed] [Google Scholar]
- Gertler MM, White PD. Coronary heart disease in young adults: A multidisciplinary study. Harvard University Press; Cambridge: 1954. p. 218. [Google Scholar]
- Glass CK, Witztum JL. Athersclerosis: The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Gofman JW, Delalla O, Glazier F, Freeman NK, Lindgren FT, Nichols AV, Strisower B, Tamplin AR. The serum lipoprotein transport system in health, metabolic disorders, atherosclerosis and coronary heart disease. Plasma. 1954a;2:413–484. doi: 10.1016/j.jacl.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Gofman JW, Rubin L, McGinley JP, Jones HB. Hyperlipoproteinemia. Am. J. Med. 1954b;17:514–520. doi: 10.1016/0002-9343(54)90126-6. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Anderson RGW, Brown MS. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979;279:679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Familial hypercholesterolemia: Identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc. Natl. Acad. Sci. USA. 1973;70:2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. The low-density lipoprotein pathway and its relation to atherosclerosis. Ann. Rev. Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Lipoprotein receptors: Genetic defense against atherosclerosis. Clin. Res. 1982;30:417–426. [Google Scholar]
- Goldstein JL, Brown MS. History of discovery: The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th. McGraw-Hill, Inc.; New York: 2001. pp. 2863–2913. [Google Scholar]
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease: The Framingham study. Am. J. Med. 1977;62:707–7l4. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J. Lipid Res. 2009;50:S282–S286. doi: 10.1194/jlr.R800066-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK, Jonasson L. History of discovery: the discovery of cellular immunity in the atherosclerotic plaque. Arterioscler. Thromb. Vasc. Biol. 2009;29:1714–1717. doi: 10.1161/ATVBAHA.108.179713. [DOI] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomized placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- Heron M. Deaths: leading causes for 2010. Natl.Vital Stat.Rep. 2013;62(6):1–97. [PubMed] [Google Scholar]
- Herrick JB. Thrombosis of the coronary arteries. JAMA. 1919;72:387–390. [Google Scholar]
- Herrick JB. An intimate account of my early experience with coronary thrombosis. Am. Heart J. 1944;27:1–18. [Google Scholar]
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J. Lipid Res. 2009;50:S172–S177. doi: 10.1194/jlr.R800091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL. Combined analysis of olignucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA. 2003;100:12027–12032. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Nohturfft A, Goldstein JL, Brown MS. Sterol resistance in CHO cells traced to point mutation in SREBP cleavage activating protein (SCAP). Cell. 1996;87:415–426. doi: 10.1016/s0092-8674(00)81362-8. [DOI] [PubMed] [Google Scholar]
- Hua X, Sakai J, Ho YK, Goldstein JL, Brown MS. Hairpin orientation of sterol regulatory element binding protein-2 in cell membranes as determined by protease protection. J. Biol. Chem. 1995;270:29422–29427. doi: 10.1074/jbc.270.49.29422. [DOI] [PubMed] [Google Scholar]
- Jacob F, Monod J. On the regulation of gene activity. Cold Spring Harbor Symp. Quant. Biol. 1961;26:193–211. doi: 10.1101/sqb.1961.026.01.048. [DOI] [PubMed] [Google Scholar]
- Junshi C, Campbell TC, Junyao L, Peto R. A study of the characteristics of 65 Chinese counties. In: Junshi C, editor. Diet, Life-Style, and Mortality in China. Oxford Univ Press; UK: 1990. pp. 42–53. [Google Scholar]
- Kathiresan S. A PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N. Engl. J. Med. 2008;358:2299–2300. doi: 10.1056/NEJMc0707445. [DOI] [PubMed] [Google Scholar]
- Keys A. Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease. Harvard University Press; Cambridge, Massachusetts: 1980. pp. 1–381. [Google Scholar]
- Keys A, Kimura N, Kusukawa A, Bronte-Stewart B, Larsen N, Keys MH. Lessons from serum cholesterol studies in Japan, Hawaii and Los Angeles. Ann. Int. Med. 1958;48:83–94. doi: 10.7326/0003-4819-48-1-83. [DOI] [PubMed] [Google Scholar]
- Khachadurian AK. The inheritance of essential familial hypercholesterolemia. Am. J. Med. 1964;37:402–407. doi: 10.1016/0002-9343(64)90196-2. [DOI] [PubMed] [Google Scholar]
- Kovanen PT, Bilheimer DW, Goldstein JL, Jaramillo JJ, Brown MS. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc. Natl. Acad. Sci. USA. 1981;78:1194–1198. doi: 10.1073/pnas.78.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiterovich PO, Jr., Levy RI, Fredrickson DS. Neonatal diagnosis of familial type-II hyperlipoproteinaemia. Lancet. 1973;i:ll8–l22. doi: 10.1016/s0140-6736(73)90194-3. [DOI] [PubMed] [Google Scholar]
- Lagace TA, Curtis DE, Garuti R, McNutt MC, Park SW, Prather HB, Anderson NN, Ho YK, Hammer RE, Horton JD. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J. Clin. Invest. 2006;116:2995–3005. doi: 10.1172/JCI29383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz JO. The history of coronary heart disease. University of California Press; Berkeley and Los Angeles: 1970. p. 227. [Google Scholar]
- Levy D. Combating the epidemic of heart disease. JAMA. 2012;308:2624–2626. doi: 10.1001/jama.2012.164971. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- Mabuchi H, Haba T, Tatami R, Miyamoto S, Sakai Y, Wakasugi T, Watanabe A, Koizumi J, Takeda R. Effects of an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase on serum lipoproteins and ubiquinone-l0 levels in patients with familial hypercholesterolemia. N. Engl. J. Med. 1981;305:478–482. doi: 10.1056/NEJM198108273050902. [DOI] [PubMed] [Google Scholar]
- McPherson R, Kavaslar N. Statins for primary prevention of coronary artery disease. Lancet. 2007;369:1078. doi: 10.1016/S0140-6736(07)60516-9. [DOI] [PubMed] [Google Scholar]
- Mills GL, Taylaur CE. The distribution and composition of serum lipoproteins in eighteen animals. Comp. Biochem. Physiol. 1971;40B:489–50l. doi: 10.1016/0305-0491(71)90234-3. [DOI] [PubMed] [Google Scholar]
- Moon Y-A, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Brown MS, Goldstein JL, Horton JD. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab. 2012;15:240–246. doi: 10.1016/j.cmet.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamed M, Zhang Y, Wang ML, Seemann J, Kwon HJ, Goldstein JL, Brown MS. Identification of luminal Loop 1 of Scap protein as the sterol sensor that maintains cholesterol homeostasis. J. Biol. Chem. 2011;286:18002–18012. doi: 10.1074/jbc.M111.238311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C. Xanthomata, hypercholesterolemia, angina pectoris. Acta Med. Scand. 1938;89:75–84. [Google Scholar]
- Pardee AB, Reddy GPV. Beginnings of feedback inhibition, allostery, and multi-protein complexes. Gene. 2003;321:17–23. doi: 10.1016/s0378-1119(03)00839-4. [DOI] [PubMed] [Google Scholar]
- Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, Stein EA. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the reduction of LDL-C with PCSK9 inhibition in heterozygous familial hypercholesterolemia disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–2417. doi: 10.1161/CIRCULATIONAHA.112.144055. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: A delicate balance. Cell Metabolism. 2008;8:512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan A, Sun L-P, Kwon HJ, Brown MS, Goldstein JL. Direct binding of cholesterol to the purified membrane region of SCAP: mechanism for a sterol-sensing domain. Mol. Cell. 2004;15:259–268. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Rawson RB, Zelenski NG, Nijhawan D, Ye J, Sakai J, Hasan MT, Chang T-Y, Brown MS, Goldstein JL. Complementation cloning of S2P, a gene encoding a putative metalloprotease required for intramembrane cleavage of SREBPs. Mol. Cell. 1997;1:47–57. doi: 10.1016/s1097-2765(00)80006-4. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JMO, Wun C-C, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N. Engl. J. Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- Sakai J, Nohturfft A, Goldstein JL, Brown MS. Cleavage of sterol regulatory element binding proteins (SREBPs) at site-1 requires interaction with SREBP cleavage-activating protein. Evidence from in vivo competition studies. J. Biol. Chem. 1998a;273:5785–5793. doi: 10.1074/jbc.273.10.5785. [DOI] [PubMed] [Google Scholar]
- Sakai J, Rawson RB, Espenshade PJ, Cheng D, Seegmiller AC, Goldstein JL, Brown MS. Molecular identification of the sterol-regulated luminal protease that cleaves SREBPs and controls lipid composition of animal cells. Mol. Cell. 1998b;2:505–514. doi: 10.1016/s1097-2765(00)80150-1. [DOI] [PubMed] [Google Scholar]
- Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- Schneider WJ, Beisiegel U, Goldstein JL, Brown MS. Purification of the low density lipoprotein receptor, an acidic glycoprotein of 164,000 molecular weight. J. Biol. Chem. 1982;257:2664–2673. [PubMed] [Google Scholar]
- Schoenheimer R, Breusch F. Synthesis and destruction of cholesterol in the organism. J. Biol. Chem. 1933;103:439–448. [Google Scholar]
- Sheng Z, Otani H, Brown MS, Goldstein JL. Independent regulation of sterol regulatory element binding proteins 1 and 2 in hamster liver. Proc. Natl. Acad. Sci. USA. 1995;92:935–938. doi: 10.1073/pnas.92.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J. Clin. Invest. 1996;98:1575–1584. doi: 10.1172/JCI118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in livers of rats with streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA. 1999;96:13656–13661. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J. Clin. Invest. 1997;99:838–845. doi: 10.1172/JCI119247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EA, Honarpour N, Wasserman SM, Xu F, Scott R, Raal FJ. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation. 2013;128:2113–2120. doi: 10.1161/CIRCULATIONAHA.113.004678. [DOI] [PubMed] [Google Scholar]
- Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, Lisbon E, Gutierrez M, Webb C, Wu R, Du Y, Kranz T, Gasparino E, Swergold GD. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N. Engl. J. Med. 2012;366:1108–1118. doi: 10.1056/NEJMoa1105803. [DOI] [PubMed] [Google Scholar]
- Steinberg D. An interpretive history of the cholesterol controversy: part II: the early evidence linking hypercholesterolemia to coronary disease in humans. J. Lipid Res. 2005;46:179–190. doi: 10.1194/jlr.R400012-JLR200. [DOI] [PubMed] [Google Scholar]
- Steinberg D, Witztum JL. History of discovery: Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010;30:2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Goldstein JL, Brown MS, Russell DW. The LDL receptor gene: A mosaic of exons shared with different proteins. Science. 1985;228:815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L-P, Seemann J, Brown MS, Goldstein JL. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Insig renders sorting signal in Scap inaccessible to COPII proteins. Proc. Natl. Acad. Sci. USA. 2007;104:6519–6526. doi: 10.1073/pnas.0700907104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N. Engl. J. Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- The Myocardial Infarction Genetics Consortium Investigators Inactivating mutations in NPC1L1 and protection from coronary heart disease. N. Engl. J. Med. 2014 doi: 10.1056/NEJMoa1405386. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy R, Vega GL, Grundy SM, Bilheimer DW. Lovastatin therapy in receptor-negative homozygous familial hypercholesterolemia: Lack of effect on low-density lipoprotein concentrations or turnover. J. Pediatr. 1988;113:387–392. doi: 10.1016/s0022-3476(88)80289-0. [DOI] [PubMed] [Google Scholar]
- Umbarger HE. The origin of a useful concept - Feedback inhibition. Protein Sci. 1992;1:1392–1395. doi: 10.1002/pro.5560011019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S. Intramembrane Proteases. Biochim. Biophys. Acta. 2013;1828:2797–2800. doi: 10.1016/j.bbamem.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Usifo E, Leigh SEA, Whittall RA, Lench N, Taylor A, Yeats C, Orengo CA, Martin ACR, Celli J, Humphries SE. Low-density lipoprotein receptor gene familial hypercholesterolemia variant database: update and pathological assessment. Ann. Hum. Genet. 2012;76:387–401. doi: 10.1111/j.1469-1809.2012.00724.x. [DOI] [PubMed] [Google Scholar]
- Vega GL, Grundy SM. Influence of lovastatin therapy on metabolism of low density lipoproteins in mixed hyperlipidaemia. J. Intern. Med. 1991;230:341–350. doi: 10.1111/j.1365-2796.1991.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Vogel RA. PCSK9 inhibition: the next statin? J. Am. Coll. Cardiol. 2012;59:2354–2355. doi: 10.1016/j.jacc.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Wang X, Briggs MR, Hua X, Yokoyama C, Goldstein JL, Brown MS. Nuclear protein that binds sterol regulatory element of LDL receptor promoter: II. Purification and characterization. J. Biol. Chem. 1993;268:14497–14504. [PubMed] [Google Scholar]
- Wang X, Sato R, Brown MS, Hua X, Goldstein JL. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77:53–62. doi: 10.1016/0092-8674(94)90234-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Davis CG, Brown MS, Schneider WJ, Casey ML, Goldstein JL, Russell DW. The human LDL receptor: A cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984;39:27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, Aebersold R, Goldstein JL, Brown MS. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 2002;110:489–500. doi: 10.1016/s0092-8674(02)00872-3. [DOI] [PubMed] [Google Scholar]
- Yokoyama C, Wang X, Briggs MR, Admon A, Wu J, Hua X, Goldstein JL, Brown MS. SREBP-1, a basic helix-loop-helix leucine zipper protein that controls transcription of the LDL receptor gene. Cell. 1993;75:187–197. [PubMed] [Google Scholar]
- Young SG, Fong LG. Lowering plasma cholesterol by raising LDL receptors - revisited. N. Engl. J. Med. 2012;366:1154–1155. doi: 10.1056/NEJMe1202168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterström R. The 1964 Nobel Prize for the discovery of the biosynthesis of cholesterol. Acta Paediatrica. 2009;98:1223–1227. doi: 10.1111/j.1651-2227.2009.01282.x. [DOI] [PubMed] [Google Scholar]
- Zhang D-W, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, Cohen JC, Hobbs HH. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J. Biol. Chem. 2007;282:18602–18612. doi: 10.1074/jbc.M702027200. [DOI] [PubMed] [Google Scholar]