Abstract
After invasion of epithelial cells, Salmonella enterica Typhimurium resides within membrane-bound vacuoles where it survives and replicates. Like endocytic vesicles, the Salmonella-containing vacuoles (SCVs) undergo a maturation process that involves sequential acquisition of Rab5 and Rab7 and displacement toward the microtubule-organizing center. However, SCVs fail to merge with lysosomes and instead develop subsequently into a filamentous network that extends toward the cell periphery. We found that the initial centripetal displacement of the SCV is due to recruitment by Rab7 of Rab7-interacting lysosomal protein (RILP), an effector protein that can simultaneously associate with the dynein motor complex. Unlike the early SCVs, the Salmonella-induced filaments (Sifs) formed later are devoid of RILP and dynein, despite the presence of active Rab7 on their membranes. Kinesin seems to be involved in the elongation of Sifs. SifA, a secreted effector of Salmonella, was found to be at least partly responsible for uncoupling Rab7 from RILP in Sifs and in vitro experiments suggest that SifA may exert this effect by interacting with Rab7. We propose that, by disengaging RILP from Rab7, SifA enables the centrifugal extension of tubules from the Salmonella-containing vacuoles, thereby providing additional protected space for bacterial replication.
INTRODUCTION
Salmonella enterica serovar Typhimurium is a facultative intracellular pathogen that causes gastroenteritis in humans and a fatal typhoid-like affliction in susceptible mice (Tsolis et al., 1999). Salmonella have the ability to evade the immune response by entering a variety of host cells, including macrophages and epithelial cells. The bacteria gain access to the intracellular compartment by means of a type three secretion system (TTSS) that is encoded within the Salmonella pathogenicity island (SPI)-1. By injecting a defined set of bacterial effectors into the cells through the TTSS, Salmonella induce in the host cells a process akin to macropinocytosis, whereby the bacteria become trapped within a membrane-bound compartment called the Salmonella-containing vacuole (SCV) (Amer and Swanson, 2002). The bacteria remain for many hours within the SCV, and after a lag period replicate within this intracellular compartment. To survive within the SCV Salmonella must avoid exposure to the bactericidal contents of lysosomes and to reactive oxygen metabolites, a process that is facilitated by the injection of a different set of effectors (Waterman and Holden, 2003).
In cells of epithelial origin the SCV undergoes a drastic change over time, acquiring filamentous protrusions (Garcia-del Portillo et al., 1993). These Salmonella-induced filaments (Sifs) are elongated tubules that extend from the original SCV along microtubules. Sif formation is driven by products of a second pathogenicity island, SPI-2 (Stein et al., 1996; Brumell et al., 2001a), which are delivered to the cytosol of the host cells across the membrane of the SCV (Hensel, 2000). Expression of one of these effectors, SifA, was found to be sufficient to generate Sif-like filamentous structures in otherwise untreated, i.e., uninfected, cells (Brumell et al., 2001a). That Sif formation is an important contributor to bacterial virulence is suggested by several observations. First, SifA-defective Salmonella displayed significantly attenuated virulence in the mouse typhoid model (Stein et al., 1996). Moreover, SifA mutants failed to replicate in murine macrophages, the host cell niche during infection (Stein et al., 1996; Beuzon et al., 2002). Last, disruption of microtubules not only prevents the formation of Sifs but also impairs intracellular bacterial replication (Garcia-del Portillo et al., 1993).
It is unclear how the early SCV, as well as the Sifs formed subsequently, avoid fusion with the lysosomal compartment and or delivery of lysosomal enzymes from the trans-Golgi network via the mannose-6-phosphate receptor. In normal cells, fusion of late endosomes with lysosomes is controlled by Rab7 (Bucci et al., 2000), which also contributes to the regulation of fusion of phagosomes with lysosomes (Harrison et al., 2003). However, the inability of SCV to merge with lysosomes is not due to exclusion of Rab7, which was reported to be present on the membrane of both early and late vacuoles containing Salmonella (Meresse et al., 1999; Brumell et al., 2001b). Moreover, the Rab7 recruited to the SCV is most likely active, because both the acquisition of LAMP by the SCV and the formation of Sifs are inhibited by inactive forms of Rab7 (Meresse et al., 1999; Brumell et al., 2001b). Thus, fusion of lysosomes with SCV fails to occur despite the recruitment of seemingly active Rab7 to the vacuoles, suggesting that the block is exerted at a later, downstream stage in the fusion sequence.
The only effector known to date to be engaged by active Rab7 is RILP (Rab7-interacting lysosomal protein). RILP possesses two distinct domains: one that binds to the GTP-bound form of Rab7, and another that recruits the dynein/dynactin complex (Cantalupo et al., 2001; Jordens et al., 2001). By simultaneously associating with both targets, RILP promotes the interaction of the microtubule motor complex with vesicles bearing active Rab7. As a result, the vectorial nature of the dynein motor promotes the centripetal displacement of vesicles with active Rab7 toward the microtubule-organizing center (MTOC), where lysosomes are concentrated (Cantalupo et al., 2001; Jordens et al., 2001). It is therefore believed that RILP induces the apposition of late endosomes to lysosomes, fostering their fusion.
In view of its fusogenic role downstream of Rab7, we hypothesized that RILP may be targeted by Salmonella to prevent delivery of lysosomal contents to the SCV. To test this notion, we analyzed the distribution and activity of RILP during the course of invasion by S. enterica Typhimurium. Our data revealed that whereas RILP associated with early SCVs and mediated their centripetal displacement, it was not present on Sifs. The ability of Sifs to extend centrifugally correlated with a paucity of dynein and was dependent on kinesin activity. We found that SifA is (one of) the components responsible for interfering with RILP recruitment to the active Rab7 present on Sifs.
MATERIALS AND METHODS
Reagents
DMEM and fetal calf serum (FCS) were from Wisent (St. Bruno, PQ, Canada). FuGENE-6 was purchased from Roche Diagnostics (Indianapolis, IN). Cy5- and Cy3-conjugated donkey anti-human, -mouse, or -rabbit IgG were all from Jackson ImmunoResearch Laboratories (West Grove, PA). Rabbit anti-human LAMP2 was generously provided by Dr. M. Fukuda (La Jolla Cancer Research Foundation, La Jolla, CA). Mouse monoclonal anti-LAMP-1 antibodies were from the Developmental Studies Hybridoma Bank (Iowa City, IA). Rabbit anti-Salmonella antibodies were from Difco (Detroit, MI). Anti-c-Myc antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). The monoclonal anti-hemagglutinin (HA) antibody was purchased from Babco (Richmond, CA). Anti-dynein monoclonal antibody was from Chemicon International (Temecula, CA). MitoTracker Green FM was from Molecular Probes (Eugene, OR). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Cell Culture, Transfection, and Invasion by Salmonella
HeLa cells were cultured in DMEM with 10% FCS as described previously (Brumell et al., 2001a). Cells grown on 25-mm glass coverslips were transiently transfected with FuGENE-6 according to the manufacturer's instructions and used within 24 h of transfection. The generation of the plasmids used for expression of RILP, RILP-C33, the wild type (wt), and constitutively active (Rab7Q67L) forms of Rab7, dynamitin, and SifA has been described in detail previously (Quintyne et al., 1999; Brumell et al., 2001b; Cantalupo et al., 2001).
S. enterica serovar Typhimurium (SL 1344) and the corresponding phoP::tet and ssaR mutants were grown as described previously (Brumell et al., 2003). To induce Salmonella invasion, HeLa cells were exposed to late log-phase bacteria (∼10 Salmonella/epithelial cell) in DMEM/FCS at 37°C for 10 min (Steele-Mortimer et al., 1999). Unbound bacteria were washed off, and cells were incubated for 50 min or 2 h, as specified. To induce Sifs, infected cells were incubated for 2 h in growth medium with 50 μg/ml gentamicin followed by 12-18 h in medium with 5 μg/ml gentamicin. To block kinesin activity, cells were pretreated with 10 μM aurintricarboxylic acid (ATA) for 3 h before invasion.
Immunofluorescence and Confocal Microscopy
After invasion, HeLa cells were washed with phosphate-buffered saline (PBS) and fixed with 2.5% paraformaldehyde in PBS for 10 min at 37°C. Immunostaining was performed after permeabilization with 0.2% saponin in PBS containing 10% FCS for 10 min, followed by blocking with 5% FCS in PBS for 1 h, all at room temperature. Dynein immunostaining was performed after fixation for 10 min with methanol at -20°C. Staining with primary antibodies at recommended dilutions was for 1 h at room temperature in PBS containing 1% FCS. After washing, samples were incubated with appropriate Cy3- or Cy5-conjugated secondary antibodies. GFP, Cy5, and Cy3 fluorescence was examined using the conventional laser excitation lines and filter sets. At least 30 cells were quantified for each condition in each experiment. Student's two-tailed t tests were performed to assess the significance of differences. Staining of mitochondria in live cells was performed by incubation with 10 nM MitoTracker Green FM immediately before fluorescence imaging. Data shown represent the mean and SE of triplicate experiments.
For analysis of fluorescence recovery after photobleaching (FRAP), cells grown on coverslips were mounted in a stainless steel chamber and maintained at 37°C with a stage incubator. FRAP was estimated in cells transfected with Rab7wt-GFP or Rab7Q67L-GFP, where indicated after Sif formation in Salmonella-infected cells. The selected late endocytic organelle or Sif was bleached using the 488-nm laser line of the Zeiss LSM 510 confocal microscope at full power, resulting in a 50-80% reduction in the fluorescence intensity. Care was taken to choose individual Sifs that were not attached to other Sifs outside the bleach region. The recovery of fluorescence was then monitored over time by scanning the bleached area at the conventional (low) laser power to minimize photobleaching during sampling. To analyze the rate of recovery, we compared the fluorescence of the photobleached area to that of an adjacent unbleached area of the same cell with similar fluorescence intensity. For each time point, the fluorescence of the bleached area was normalized to that of the corresponding control (unbleached) area to correct for possible drift of the focal plane or photobleaching incurred during the low light sampling. A similar protocol was used to measure FRAP in late endocytic organelles. All FRAP measurements were performed at 37°C.
Glutathione S-Transferase (GST) Pull-Down Experiments
HeLa cells were plated at a density of 4 × 104 onto 20-cm dishes. The following day, cells were transfected for 16-20 h with plasmids encoding either green fluorescent protein (GFP) alone or GFP fused to the N terminus of Rab7 (the kind gift of Dr. J.-P. Gorvel, Institut National de la Santé et de la Recherche Médicale, France). Transfection was performed using FuGENE 6 as described above. After transfection, cells were scraped in PBS containing Ca2+ and Mg2+, centrifuged at 1500 × g for 5 min, and then resuspended in lysis buffer (1% Triton X-100, 150 mM NaCl, 2.0 mM EDTA, 1 mM NaVO4, 5 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 20 mM Tris, pH 7.5, 1 protease inhibitor tablet; Boehringer Ingelheim, Ingelheim, Germany). Insoluble debris were removed by centrifugation at 13,000 × g for 10 min and then lysates were precleared with 10 μg of GST on glutathione-agarose beads for 1 h at 4°C. Lysates were centrifuged to remove beads and transferred to a new microcentrifuge tube. Approximately 5 μg of either GST or GST-SifA on glutathione-agarose beads was added to lysates, incubated for 1 h at 4°C, and beads were sedimented. Finally, the beads were washed threefold with lysis buffer and bound proteins were solubilized in protein sample buffer. Samples were analyzed by SDS-PAGE and immunoblotting with polyclonal antibodies to GFP.
RESULTS
RILP Is Recruited to the Early SCV
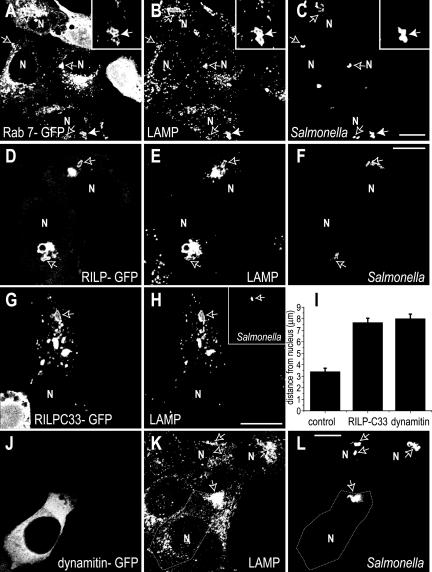
Acquisition of Rab7 and its effector protein RILP to the SCV were studied using confocal fluorescence microscopy. Because available antibodies are inadequate to detect the endogenous proteins by immunostaining, we transfected HeLa cells with GFP-tagged chimeric constructs of these proteins. When detectable levels of expression were reached, the cells were infected with S. enterica Typhimurium, and the resulting SCV were allowed to mature for 50 min to 2 h before analysis. The location of the bacteria was verified using anti-Salmonella antibodies, and the contour of the SCV was visualized by immunostaining for LAMP-1. In accordance with earlier findings (Meresse et al., 1999; Scott et al., 2002), Rab7 was present on the early SCV by 50 min (Figure 1A). As shown in Figure 1, D-F, RILP also was associated with the SCV. This recruitment of RILP implies that Rab7 is in the active, GTP-bound form on the early SCV membrane, as inferred earlier from the inhibitory effects of Rab7 (T22N) (Meresse et al., 1999; Brumell et al., 2001b). Thus, neither exclusion nor inactivation of Rab7 can account for the failure of the SCV to fuse with the lysosomal compartment.
Figure 1.
Rab7 and RILP are recruited to early SCVs. HeLa cells were transiently transfected with Rab7-GFP (A-C), RILPGFP (D-F), RILPC33-GFP (G-H), or dynamitin-GFP (J-L) before invasion with wild-type Salmonella. Cells were fixed 50 min (A-F) or 2 h (G-I) after infection, and immunostaining was used to reveal the location of LAMP (B, E, H, and K) and of the bacteria (C, F, H, inset, and L). GFP fluorescence is shown in A, D, G, and J. Arrows indicate the position of bacteria. The area noted by the solid arrow was magnified in the corresponding inset. N, nucleus. Data in I are measurements of the distance between the intracellular bacteria and the nearest edge of the nucleus in control, RILPC33- or dynamitin transfected cells. Data are means ± SE of 50 cells for each condition, from three separate experiments. Bars, 10 μm.
The Dynein-recruiting Domain of RILP Is Required for Retrograde Movement of SCV in HeLa Cells
After 50 min, most of the intracellular Salmonella were located in the vicinity of the nucleus (Figure 1, A-C). In cells expressing RILP-GFP, the bacteria were routinely found near the juxtanuclear cluster of LAMP-1-positive organelles, thought to reflect the location of the MTOC (Wubbolts et al., 1999). To investigate whether bridging of the SCV to dynein via Rab7/RILP was responsible for the centripetal displacement of the bacteria, we transfected the cells with RILPC33, a truncated form of RILP lacking the dynein-recruiting domain (Cantalupo et al., 2001; Jordens et al., 2001; Harrison et al., 2003). As shown in Figure 1, G-H, the truncated RILP was still effectively recruited to the SCV, as was LAMP-1. However, unlike untransfected cells or cells expressing full-length RILP, in cells transfected with RILPC33 the SCVs were randomly distributed throughout the cytosol. The average distance between the bacteria and the nuclear membrane was significantly greater (7.7 ± 0.4 μm; n = 150) for RILPC33-transfectants, compared with controls (3.4 ± 0.3 μm; n = 150) (Figure 1, G-I). The failure of the SCV to accumulate near the nucleus in RILPC33-transfected cells is likely due to their inability to recruit dynein/dynactin, as reported for lysosomes and phagosomes (Jordens et al., 2001). This was verified by transfection of dynamitin, a component of the dynactin complex that has been documented to uncouple dynein from its cargo (Quintyne et al., 1999). As shown in Figure 1, J-L, in cells overexpressing dynamitin the LAMP-1-containing organelles, including the SCV, tended to relocate near the margin of the cell, distant from the nucleus. The average distance between the SCV and the nuclear membrane in these cells was 8.0 ± 0.4 μm (n = 150; Figure 1I)
RILP Is Not Recruited to Sifs
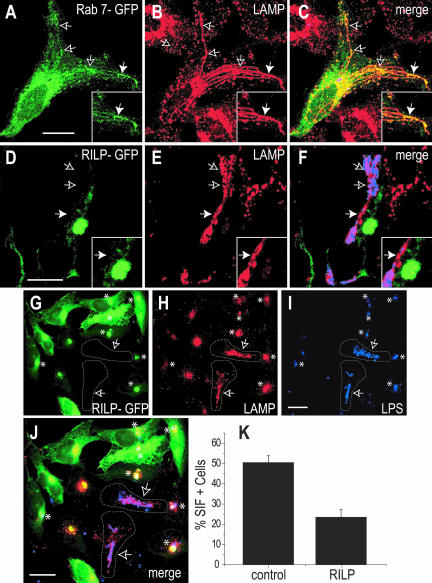
The presence of Rab7 and RILP on Sifs extending from late SCV was investigated next. Sifs were readily apparent in ≈50% of HeLa cells 6 h after infection, as judged by LAMP staining. Multiple filaments often extended from a perinuclear SCV containing a single bacterium (Figure 2A), whereas in other cells a strand of several Salmonella filled the Sif (Figure 2D). As described previously (Brumell et al., 2001b), Rab7 was detected along many of the Sifs (Figure 2, A-C). Remarkably, RILP was consistently absent from such Sifs; fewer than 1% of the Sifs examined were associated with RILP-GFP (Figure 2, D-F), and similar results were obtained with HA-tagged RILP (our unpublished data). Cells expressing low levels of ectopically expressed RILP were used for these determinations, to minimally disturb the system (see below).
Figure 2.
Differential recruitment of Rab7 and RILP to Sifs. HeLa cells were transfected with either Rab7-GFP (green; A and C) or RILP-GFP (green; D, F, G, and J) 2 h after invasion by Salmonella. After an overnight (12- to 16-h) incubation, the cells were fixed and stained for LAMP (red; B, C, E, F, H, and J) or Salmonella (blue; C, F, I, and J). Arrows point to Sifs, identified as filamentous, LAMP-positive structures. The area noted by the solid arrow was magnified in the corresponding inset. In G-J, arrows point to Sif-containing nontransfected cells (outlined), whereas asterisks indicate the location of juxtanuclear aggregates of LAMP- and RILP-positive vesicles in transfected cells. K compares the percentage of Sif-positive HeLa cells in control cells versus RILP-overexpressing cells. Data are means ± SE of 50 cells from five individual experiments. Bars, 10 μm.
Whereas LAMP, Rab7, and RILP colocalize extensively in uninfected cells (Cantalupo et al., 2001; Jordens et al., 2001) and in cells containing early SCV (Figure 1), it is noteworthy that the juxtanuclear compartment containing RILP was largely devoid of LAMP in cells at later stages of infection (Figure 2, D-F). To identify this compartment, lysosomes were labeled with a fluid phase marker, followed by a prolonged chase of 16-18 h. The chase period included the time required for expression of RILP, Salmonella infection, and formation of Sifs. As described previously (Garcia-del Portillo and Finlay, 1995), we failed to detect lysosomal contents in Sifs, but the juxtanuclear compartment expressing RILP was rich in lysosomal marker, despite its paucity of LAMP (our unpublished data). Therefore, in Salmonella-infected cells bona fide lysosomes retain active Rab7 and RILP, yet are largely devoid of LAMP-1, which is diverted to the Sifs.
Together with the absence of RILP from the Sifs, this finding suggests that factors produced by Salmonella uncouple RILP from Rab7 in LAMP-containing compartments, possibly enabling the Sifs to extend away from the MTOC. This hypothesis was then tested in the following experiments.
Overexpression of RILP Impairs Sif Formation
The extension and maintenance of tubular structures from the juxtanuclear SCV toward the cell periphery during Sif formation implies that the centripetal action of dynein is absent or is antagonized by a predominant centrifugal motor. This may result from the inability of Rab7 to engage RILP, possibly due to competition with a factor secreted by Salmonella. To test this hypothesis, we attempted to overcome this competitive effect by overexpression of RILP. Typical results are shown in Figure 2, G-K. Sif formation was markedly depressed in cells expressing high levels of RILPGFP; only 26.1 ± 4.3% of the overexpressors displayed Sifs, compared with 56.0 ± 3.7% in controls. In cells with the lowest levels of RILP expression, where Sifs were still observable, the filaments were thicker and less branched than in control cells (Figure 2E).
Because Rab7 is required for Sif formation, it may be argued that overexpression of RILP may have scavenged active Rab7, diverting it from performing the function(s) necessary for filament extension. However, overexpression of comparable levels of RILPC33, which is fully capable of interacting with Rab7, did not prevent the formation of Sifs (our unpublished data). Therefore, the centripetal actions of RILP/dynein, rather than a nonspecific scavenging effect, seem to have antagonized the extension of Sifs.
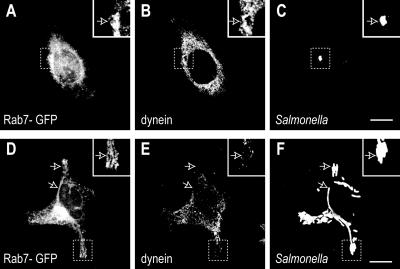
Dynein Is Reduced on Sif Membranes
RILP has been directly implicated in recruiting the microtubule motor dynein to membranes (Jordens et al., 2001). Because RILP was present on the SCV membrane yet was scarce on Sifs, despite the presence of Rab7, we investigated whether dynein followed a similar pattern. Immunostaining with a dynein-specific antibody was used for this purpose. As shown in Figure 3, dynein was indeed recruited to early SCVs, where it colocalized with Rab7-GFP (Figure 3, A-C). Two hours after invasion, 54.3 ± 6.0% of the SCVs were stained by antibodies to dynein. By contrast, little accumulation of dynein was noted on Sifs, even though Rab7 was clearly detectable surrounding most of these structures (Figure 3, D-F). When samples were fixed and stained 16-18 h postinvasion, only 5.1 ± 0.8% of the Sifs were associated with dynein. Therefore, the absence of RILP correlated with the inability of Sifs to recruit dynein. The reduced association of the membrane with the centripetal motors may have facilitated the extension of tubules toward the cell periphery.
Figure 3.
Recruitment of dynein to SCVs and Sifs. HeLa cells transfected with Rab7-GFP and infected with by Salmonella. The cells were fixed either 2 h (A-C) or 16 h after invasion (D-F), permeabilized, and stained with monoclonal antibodies to dynein and polyclonal antibodies to the bacterial LPS. (A and D) Rab7-GFP fluorescence. (B and E) Dynein staining. (C and F) Staining for LPS. Insets show areas delineated in main panels. Arrows indicate additional Sifs (D-F). Bars, 10 μm. Images are representative of three similar experiments.
Kinesin Activity is Required for Sif Formation
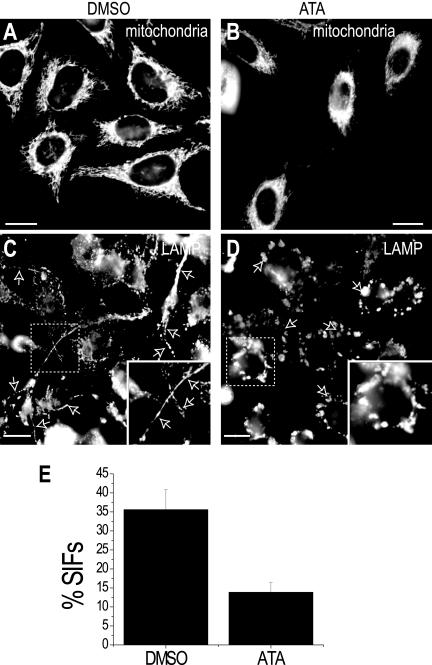
Whereas loss of dynein from the membrane of the late SCV would reduce the tendency of the vacuoles to be retained near the MTOC, it cannot by itself account for the centrifugal extension of tubular structures along microtubules to generate Sifs. In most cells, the predominant centrifugal motors are the kinesins. We therefore investigated whether kinesin activity was required for extension of the Sifs, in combination with the reduced centripetal effect of dynein. This was accomplished using ATA, an agent reported earlier to be a potent inhibitor of kinesin activity (Hopkins et al., 2000). The effectiveness of ATA (10 μM) was first confirmed by analyzing the distribution of mitochondria, which are known to extend to the cell periphery in a kinesin-dependent manner. As shown in Figure 4, whereas mitochondria are readily detectable throughout untreated HeLa cells, they tend to cluster near the nucleus in cells treated with ATA (cf. Figures 4, A and B). Retention of the voltage-sensitive dye MitoTracker by the ATA-treated cells attests to the metabolic well-being of the cells and argues against a nonspecific deleterious effect of the kinesin inhibitor. Figure 4, C and D, compare the pattern of LAMP staining of infected cells that were either treated with ATA or left untreated. As before, 16-18 h after infection many of the control cells displayed Sifs (Figure 4C). In this series of experiments 35.6 ± 5.3% of the control cells contained one or more Sifs. Remarkably, a reduced number of Sifs was detectable in ATA-treated cells, where the LAMP surrounding the bacteria was found predominantly in vesicular structures that tended to cluster in the vicinity of the nucleus (Figure 4D). Only 13.8 ± 2.5% of the ATA-treated cells had Sifs (Figure 4E).
Figure 4.
Effect of kinesin inhibitors on Sif formation. (A and B) Distribution of mitochondria in control and ATA-treated cells. HeLa cells were pretreated with either vehicle (DMSO) or with 10 μM ATA for 3 h, and mitochondria were then stained using 10 nM of MitoTracker Green FM. Note that mitochondria are dispersed throughout the cell in the control (DMSO-treated) cells (A) but clustered around the nucleus after treatment with ATA (B). (C and D) Analysis of the effect of ATA on Sif formation. Cells pretreated with ATA or DMSO were infected with Salmonella. After 16-18 h, LAMP immunostaining revealed typical Sif formation (arrows) in the control (DMSO-treated) cells (C), whereas ATA-treated cells showed reduced Sif formation (D). Arrows indicate the position of bacteria, identified by LPS staining. Bars, 10 μm. Images are representative of three similar experiments. Data in E are the summary of measurements of Sif formation in control or ATA-treated cells from experiments like those in C and D. Data are means ± SE of three separate experiments, each counting 50 infected cells.
Rab7 Is Active on the Sif Membrane
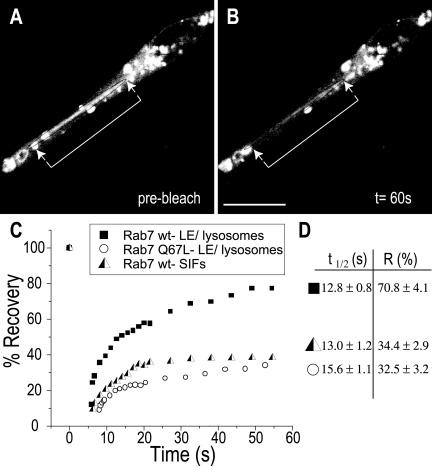
The failure of Rab7 to associate with RILP on the membrane of Sifs may indicate that the GTPase is inactive, i.e., in the GDP-bound state. Alternatively, it may result from occupancy of the effector site of active, GTP-bound Rab7 by a competing molecule, possibly a bacterial product. Two approaches were used to test the state of Rab7 activation. Because the rate of dissociation of Rab GTPases from membranes is thought to be determined by the conversion of the GTP-bound to the GDP-bound forms, measurements of the rate of Rab7 exchange from the Sif should provide an indication of its activation state. Because at the steady state the rates of association and dissociation of Rab7 are identical, the dissociation rate can be inferred from the rate of association. The latter can be estimated in intact cells from the kinetics of fluorescence recovery after an organelle expressing Rab7-GFP has been photobleached. This approach was recently used successfully to assess the effect of RILP on the activation state of Rab7 (Jordens et al., 2001). To validate the sensitivity of the assay, we compared the rate and extent of FRAP of late endosomes/lysosomes of uninfected cells expressing wild-type Rab7-GFP or Rab7 (Q67L), a constitutively active mutant. After bleaching of wild-type Rab7, recovery was fast (t1/2 = 12.8 ± 0.8 s) and extensive (fractional recovery = 70.8 ± 4.1%; Figure 5). This implies that most of the endosomal Rab7 turns over rapidly, as found earlier by Jordens et al. (2001). On the other hand, when constitutively active Rab7-GFP was expressed, recovery after photobleaching was very poor. Only 32.5 ± 3.2% had recovered after 180 s, the longest time studied. Having validated the use of FRAP, we proceeded to analyze the behavior of Rab7 on Sifs. As shown in Figure 5, A-D, the recovery of fluorescence was poor in Sifs (34.4 ± 2.9%), suggesting that Rab7 is trapped in the active state by a slowly dissociating effector.
Figure 5.
Rab7 dissociates slowly from Sifs. Control or infected HeLa cells were transfected with Rab7-GFP, and mobility was estimated by FRAP. (A and B) Typical experiment where Sifs are shown immediately before (A) and 60 s after photobleaching (B). The position of the Sifs is bracketed by arrows. (C) Representative quantitation of the fluorescence recovery in uninfected cells transfected with wild-type Rab7-GFP (▪) or Rab7-Q67L (○) or in Sifs from Salmonella-infected HeLa cells transfected with wild-type Rab7-GFP ( ). (D) Summary of t1/2 and fractional recovery (R) for each condition. Data are means ± SE of 20 cells. Bars, 10 μm.
). (D) Summary of t1/2 and fractional recovery (R) for each condition. Data are means ± SE of 20 cells. Bars, 10 μm.
The preceding findings suggest that the inability of Rab7 to interact with RILP on Sifs is not due to its inactivation, because GDP-bound Rab7 would be expected to dissociate rapidly from the membrane. Consistent with this interpretation, we also found that RILP remained excluded from Sifs in cells transfected with either wild-type Rab7 or with Rab7Q67L, the constitutively active mutant (our unpublished data).
SPI-2 Effectors Are Responsible for Diminished RILP Recruitment to Sifs
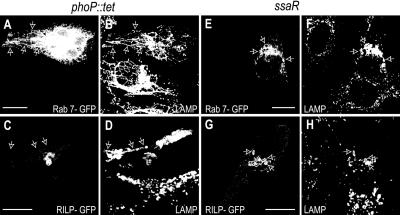
Our observations indicated that infection by Salmonella resulted in uncoupling of a subpopulation of the cellular Rab7 from RILP. In an effort to identify the responsible factor(s), we tested various mutant strains of Salmonella. We first evaluated the role of the phoP/Q locus, which regulates at least 40 genes that are important for bacterial metabolism and contribute to the ability of early SCV to evade lysosomes (Miller and Mekalanos, 1990). HeLa cells were exposed to PhoP-defective (phoP::tet) Salmonella and, following invasion and SCV formation, the cells were transfected with either Rab7-wt-GFP or RILP-GFP before overnight incubation. As illustrated in Figure 6, A-D, phoP::tet Salmonella induced the formation of Sifs that were indistinguishable from those formed by wild-type bacteria, that is, the Sifs associated with Rab7 but lacked detectable RILP. Therefore, products of the PhoP/Q locus are not required for formation of Sifs, nor are they needed to exclude RILP.
Figure 6.
Effect of mutant Salmonella strains. HeLa cells were infected with the phoP::tet (A-D) or ssaR (E-H) mutant strains of Salmonella, and then transfected with either Rab7-GFP (A and E) or RILP-GFP (C and G) and left overnight. Cells were then fixed and stained for LAMP (B, D, F, and H) and Salmonella (our unpublished data). Arrows point to Sifs in (A-D) and nonfilamentous late SCV in E-H. Bars, 10 μm. Images are representative of four similar experiments.
ssaR encodes a structural protein essential for assembly of the SPI-2 TTSS (Pfeifer et al., 1999). Deletion of this gene product consequently affects the delivery of all SPI-2 proteins into the host cytosol (Pfeifer et al., 1999). ssaR mutants invade HeLa cells normally and reside within SCV, yet are incapable of forming Sifs (Brumell et al., 2001a). We found that, even at the late stages of the infection (18 h), the SCV formed by ssaR Salmonella remained tightly clustered near the nucleus. Moreover, such SCV were not only rich in Rab7 but also in RILP (Figure 6, E-H). This observation implies that the factor(s) responsible for uncoupling Rab7 from RILP on the membrane of Sifs are delivered to the host cells by the SPI-2 TTSS.
SifA Generates RILP-deficient Filaments
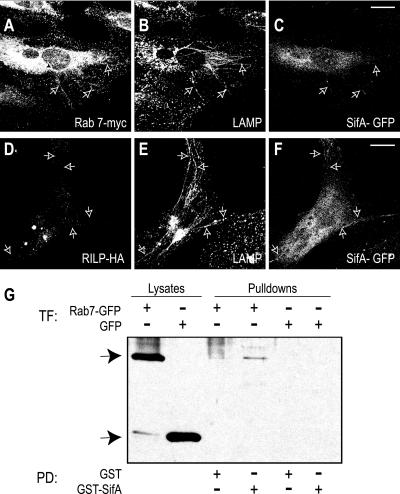
SifA has been shown to be required for the formation and/or maintenance of Sifs (Stein et al., 1996). We were unable to study the effects of selective ablation of SifA on the association of Rab7 and RILP with the SCV, because SifA-deficient bacteria escape from the vacuole into the host cell cytosol (Beuzon et al., 2002; Brumell et al., 2002). Instead, we expressed SifA in uninfected cells by transfection of the corresponding cDNA. As described recently (Brumell et al., 2001a; Boucrot et al., 2003), expression of SifA-GFP induced the formation of filamentous, LAMP-positive organelles indistinguishable from the Sifs observed upon bacterial infection (Figure 7, A-F). Cotransfection of SifA-GFP with Myc-tagged Rab7 enabled visualization of the distribution of the GTPase in such cells. As illustrated in Figure 7, A-B, Rab7 was present in a sizable fraction of Sif-like tubules. By contrast, HA-tagged RILP was not detectable on the filaments, although it was readily visualized in its customary juxtanuclear location (Figure 7, D and E). These data imply that SifA activity suffices to uncouple RILP from Rab7.
Figure 7.
Distribution of Rab7 and RILP in SifA-induced Sifs. HeLa cells were cotransfected with SifA-GFP (C and F) and Rab7-myc (A) or RILP-HA (D) before immunostaining with LAMP (B and E). Arrows indicate Sifs. Bars, 10 μm. Representative of three experiments. (G) Beads coated with SifA-GST or GST alone were used to precipitate (pull-down, PD) interacting proteins extracted from HeLa cells transfected (TF) with Rab7-GFP or GFP alone. Precipitated proteins were analyzed by SDS-PAGE alongside with samples of the whole cell lysates, transferred onto nitrocellulose, and blotted with anti-GFP antibody. Arrows indicates the location of Rab7-GFP (top arrow) or GFP (bottom arrow). Representative of two experiments.
Competition of a bacterial product with RILP for binding to active Rab7 was proposed above to account for the uncoupling. The extreme C terminus of SifA bears some similarity to Rab family members (Brumell et al., 2001b), and this domain is required for SifA to bind to host cell membranes (Guignot et al., 2004). Because of its reported location on Sifs (Brumell et al., 2001b; Figure 7), SifA was suspected to exert this competitive effect. To analyze this possibility, we tested the ability of SifA to interact with Rab7 in vitro. Recombinant, GST-conjugated SifA was coupled to beads and used to probe lysates of HeLa cells. The latter were previously transfected with Rab7-GFP to use the fluorescent protein as a sensitive epitope for immunoblotting. As illustrated in Figure 7G, SifA-GST, but not GST alone, cosedimented with Rab7. These experiments suggest that interaction between these proteins may result in the exclusion of RILP and may promote the extension of Sifs.
DISCUSSION
Based on the observations reported above, we propose that the early SCV recruits Rab7, which in turn associates with RILP and the dynein motor complex, promoting the displacement of the early vacuoles toward the MTOC. While the present manuscript was under review, Guignot et al. (2004) reported that microtubules accumulate around Salmonella SCVs. Moreover, these authors found that the redistribution of microtubules depends on Rab7 association with the SCV and that RILP is recruited to the vacuole. Our data are in good agreement with these findings. Because Rab7 can only associate with RILP when bound to GTP, the observations of Guignot et al. (2004) and those reported here imply that Rab7 is active on the membrane of the SCV. Therefore, the failure to fuse with lysosomes cannot be attributed to the inability of the SCV to recruit and activate Rab7 and must instead be due to the action of competing or inhibitory factors, likely of bacterial origin.
At later stages of development of the infection, Salmonella effectors delivered by the SPI-2 TTSS elicit the formation of Sifs at least in part by preventing the association of Rab7 with RILP. As described above, the uncoupling between RILP and Rab7 is likely produced by interaction of the latter with bacterial products. Because of its reported location on Sifs (Brumell et al., 2001b; Figure 7), SifA is a likely candidate in this process, because it can recapitulate both the tubulation of LAMP-containing organelles and the dissociation between Rab7 and RILP. The observed effects could be explained most simply by direct interaction between SifA and Rab7, resulting in competitive displacement of RILP. Indeed, the feasibility of this mechanism is suggested by the interaction of these two components in vitro.
The detachment of RILP and its associated centripetal motor complex by SifA and/or other bacterial factors would facilitate the extension of Sifs away from the centrosome in a microtubule-dependent manner. We propose that centrifugal extension of filaments is due to unmasking of constitutive kinesin activity. This notion is based on the observed effects of ATA, a kinesin antagonist, which depressed the formation of Sifs. Of note, a similar inhibitory effect was very recently reported in cells expressing the tetratricopeptide repeat of kinesin light chain-2, which like ATA inhibits kinesin activity (Guignot et al., 2004). Thus, when unopposed by the centripetal force exerted by dynein, kinesin motor activity seems to promote the extension of tubular Sifs. The resulting tubules not only provide a protected volume for the proliferation of Salmonella but also may bring the bacteria close to the surface of the cell for their eventual escape for reinfection of neighboring cells.
We consider it unlikely that SifA acts merely as a competitive inhibitor of Rab7, because transfection of HeLa cells with RILPC33, the truncated inhibitory form of RILP, disperses LAMP compartments to the periphery of cells (Cantalupo et al., 2001; Jordens et al., 2001) yet does not cause the extensive tubulation characteristic of Sifs. Instead, we suspect that SifA, perhaps acting in concert with other SPI-2 translocated effectors, may additionally direct the fusion of vesicles into the long tubules that constitute the Sifs. This hypothesis remains to be tested experimentally.
Note added in proof. While this manuscript was under review, Marsman et al. (Marsman, M., Jordens, I., Kuijl, C., Janssen, L., and Neefjes, J. [2004]. Dynein-mediated vesicle transport controls intracellular Salmonella replication. Mol. Biol. Cell 15, 2954-2964) reported that overexpression of RILP impairs Salmonella replication in HeLa cells. Growth inhibition was attributed to increased fusion of lysosomes with the SCV.
Acknowledgments
The dynamitin-GFP was a kind gift from Dr. T.A. Schroer. This work was supported by grants from the Canadian Institutes of Health Research, the Canadian Arthritis Society the National Sanatorium Association, Ministero dell'Istruzione, dell'Università e della Ricerca-Programmi di Ricerca di Interesse Nazionale 2001054232 and 2002054531, and the Howard Hughes Medical Institute. S.G. is the current holder of the Pitblado Chair in Cell Biology and is cross-appointed to the Department of Biochemistry, University of Toronto. B.B.F. is a Howard Hughes International Research Scholars, the University of British Columbia Peter Wall Distinguished Professor, and a Canadian Institutes of Health Research Distinguished Scientist. J.B. is a Canadian Institutes of Health Research New Investigator and is cross-appointed to the Department of Molecular Medical Genetics, University of Toronto
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-02-0092. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-02-0092.
Abbreviations used: GFP, green fluorescent protein; HA, hemagglutinin; RILP, Rab7-interacting lysosomal protein; SCV, Salmonella-containing vacuole; Sif, Salmonella-induced filament.
References
- Amer, A.O., and Swanson, M.S. (2002). A phagosome of one's own: a microbial guide to life in the macrophage. Curr. Opin. Microbiol. 5, 56-61. [DOI] [PubMed] [Google Scholar]
- Beuzon, C.R., Salcedo, S.P., and Holden, D.W. (2002). Growth and killing of a Salmonella enterica serovar Typhimurium sifA mutant strain in the cytosol of different host cell lines. Microbiology 148, 2705-2715. [DOI] [PubMed] [Google Scholar]
- Boucrot, E., Beuzon, C.R., Holden, D.W., Gorvel, J.P., and Meresse, S. (2003). Salmonella typhimurium SifA effector protein requires its membrane-anchoring C-terminal hexapeptide for its biological function. J. Biol. Chem. 278, 14196-14202. [DOI] [PubMed] [Google Scholar]
- Brumell, J.H., Kujat-Choy, S., Brown, N.F., Vallance, B.A., Knodler, L.A., and Finlay, B.B. (2003). SopD2 is a novel type III secreted effector of Salmonella typhimurium that targets late endocytic compartments upon delivery into host cells. Traffic 4, 36-48. [DOI] [PubMed] [Google Scholar]
- Brumell, J.H., Rosenberger, C.M., Gotto, G.T., Marcus, S.L., and Finlay, B.B. (2001a). SifA permits survival and replication of Salmonella typhimurium in murine macrophages. Cell Microbiol. 3, 75-84. [DOI] [PubMed] [Google Scholar]
- Brumell, J.H., Tang, P., Mills, S.D., and Finlay, B.B. (2001b). Characterization of Salmonella-induced filaments (Sifs) reveals a delayed interaction between Salmonella-containing vacuoles and late endocytic compartments. Traffic 2, 643-653. [DOI] [PubMed] [Google Scholar]
- Brumell, J.H., Tang, P., Zaharik, M.L., and Finlay, B.B. (2002). Disruption of the Salmonella-containing vacuole leads to increased replication of Salmonella enterica serovar typhimurium in the cytosol of epithelial cells. Infect. Immun. 70, 3264-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci, C., Thomsen, P., Nicoziani, P., McCarthy, J., and van Deurs, B. (2000). Rab 7, a key to lysosome biogenesis. Mol. Biol. Cell 11, 467-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo, G., Alifano, P., Roberti, V., Bruni, C.B., and Bucci, C. (2001). Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 20, 683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-del Portillo, F., and Finlay, B.B. (1995). Targeting of Salmonella typhimurium to vesicles containing lysosomal membrane glycoproteins bypasses compartments with mannose 6-phosphate receptors. J. Cell Biol. 129, 81-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-del Portillo, F., Zwick, M.B., Leung, K.Y., and Finlay, B.B. (1993). Salmonella induces the formation of filamentous structures containing lysosomal membrane glycoproteins in epithelial cells. Proc. Natl. Acad. Sci. USA 90, 10544-10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignot, J., Caron, E., Beuzon, C., Bucci, C., Kagan, J., Roy, C., and Holden, D.W. (2004). Microtubule motors control membrane dynamics of Salmonella-containing vacuoles. J. Cell Sci. 117, 1033-1045. [DOI] [PubMed] [Google Scholar]
- Harrison, R.E., Bucci, C., Vieira, O.V., Schroer, T.A., and Grinstein, S. (2003). Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol. Cell. Biol. 23, 6494-6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel, M. (2000). Salmonella pathogenicity island 2. Mol. Microbiol. 36, 1015-1023. [DOI] [PubMed] [Google Scholar]
- Hopkins, S.C., Vale, R.D., and Kuntz, I.D. (2000). Inhibitors of kinesin activity from structure-based computer screening. Biochemistry 39, 2805-2814. [DOI] [PubMed] [Google Scholar]
- Jordens, I., Fernandez-Borja, M., Marsman, M., Dusseljee, S., Janssen, L., Calafat, J., Janssen, H., Wubbolts, R., and Neefjes, J. (2001). The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 11, 1680-1685. [DOI] [PubMed] [Google Scholar]
- Meresse, S., Steele-Mortimer, O., Finlay, B.B., and Gorvel, J.P. (1999). The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J. 18, 4394-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, S.I., and Mekalanos, J.J. (1990). Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J. Bacteriol. 172, 2485-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer, C.G., Marcus, S.L., Steele-Mortimer, O., Knodler, L.A., and Finlay, B.B. (1999). Salmonella typhimurium virulence genes are induced upon bacterial invasion into phagocytic and nonphagocytic cells. Infect. Immun. 67, 5690-5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne, N.J., Gill, S.R., Eckley, D.M., Crego, C.L., Compton, D.A., and Schroer, T.A. (1999). Dynactin is required for microtubule anchoring at centrosomes. J. Cell Biol. 147, 321-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, C.C., Cuellar-Mata, P., Matsuo, T., Davidson, H.W., and Grinstein, S. (2002). Role of 3-phosphoinositides in the maturation of Salmonella-containing vacuoles within host cells. J. Biol. Chem. 277, 12770-12776. [DOI] [PubMed] [Google Scholar]
- Steele-Mortimer, O., Meresse, S., Gorvel, J.P., Toh, B.H., and Finlay, B.B. (1999). Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell Microbiol. 1, 33-49. [DOI] [PubMed] [Google Scholar]
- Stein, M.A., Leung, K.Y., Zwick, M., Garcia-del Portillo, F., and Finlay, B.B. (1996). Identification of a Salmonella virulence gene required for formation of filamentous structures containing lysosomal membrane glycoproteins within epithelial cells. Mol. Microbiol. 20, 151-164. [DOI] [PubMed] [Google Scholar]
- Tsolis, R.M., Kingsley, R.A., Townsend, S.M., Ficht, T.A., Adams, L.G., and Baumler, A.J. (1999). Of mice, calves, and men. Comparison of the mouse typhoid model with other Salmonella infections. Adv. Exp. Med. Biol. 473, 261-274. [PubMed] [Google Scholar]
- Waterman, S.R., and Holden, D.W. (2003). Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell Microbiol. 5, 501-511. [DOI] [PubMed] [Google Scholar]
- Wubbolts, R., Fernandez-Borja, M., Jordens, I., Reits, E., Dusseljee, S., Echeverri, C., Vallee, R.B., and Neefjes, J. (1999). Opposing motor activities of dynein and kinesin determine retention and transport of MHC class II-containing compartments. J. Cell Sci. 112, 785-795. [DOI] [PubMed] [Google Scholar]