Abstract
CIN85 is a multidomain adaptor protein involved in Cbl-mediated down-regulation of epidermal growth factor (EGF) receptors. CIN85 src homology 3 domains specifically bind to a proline-arginine (PxxxPR) motif in Cbl, and this association seems to be important for EGF receptor endocytosis. Here, we report identification of novel CIN85 effectors, all containing one or more PxxxPR motifs, that are indispensable for their mutual interactions. These effectors include phosphatidyl-inositol phosphatases SHIP-1 and synaptojanin 2B1, Arf GTPase-activating proteins ASAP1 and ARAP3, adaptor proteins Hip1R and STAP1, and a Rho exchange factor, p115Rho GEF. Acting as a molecular scaffold, CIN85 clusters its effectors and recruits them to high-molecular-weight complexes in cytosolic extracts of cells. Further characterization of CIN85 binding to ASAP1 revealed that formation of the complex is independent on cell stimulation. Overexpression of ASAP1 increased EGF receptor recycling, whereas ASAP1 containing mutated PxxxPR motif failed to promote this event. We propose that CIN85 functions as a scaffold molecule that binds to numerous endocytic accessory proteins, thus controlling distinct steps in trafficking of EGF receptors along the endocytic and recycling pathways.
INTRODUCTION
Signaling pathways initiated by activation of receptor tyrosine kinases (RTKs) play critical roles in intercellular communication during development as well as in many physiological responses (Pawson and Scott, 1997; Schlessinger, 2000), whereas aberrations in RTKs' activity lead to various pathological processes (Aaronson, 1991). Thus, negative control of receptor signaling is critical for cell homeostasis. One of the major processes leading to RTK signaling termination is clathrin-mediated receptor endocytosis, followed by its endosomal sorting and lysosomal degradation (Sorkin and Waters, 1993). Alternatively, receptors can be recycled to the plasma membrane. These trafficking events are controlled via a complex network of protein-protein and protein-lipid interactions, as well as by receptor phosphorylation and ubiquitination (Waterman and Yarden, 2001; Dikic, 2003; Dikic and Giordano, 2003). The evolutionarily conserved family of ubiquitin ligases, represented by Cbl, Cbl-b, and Cbl-c in mammals, is responsible for mediating ubiquitination, and subsequent lysosomal degradation, of many RTKs, among them epidermal growth factor (EGF) receptors (Thien and Langdon, 2001; Dikic et al., 2003). Interestingly, Cbl directs monoubiquitination, rather than polyubiquitination, of activated EGF and platelet-derived growth factor (PDGF) receptors (Haglund et al., 2003).
Additionally, Cbl and Cbl-b control RTK internalization by binding to and ubiquitinating a multidomain protein CIN85 (Cbl-interacting protein of 85 kDa) (Haglund et al., 2002; Petrelli et al., 2002; Soubeyran et al., 2002; Szymkiewicz et al., 2002). CIN85, also known as Ruk and SETA, is a broadly expressed adaptor protein (Bogler et al., 2000; Gout et al., 2000; Take et al., 2000; Dikic, 2002). It is homologous to CMS/CD2AP (Dustin et al., 1998; Kirsch et al., 1999), sharing the same domain structure and high sequence identity, and therefore these molecules comprise one family of adaptor proteins (Dikic, 2002). The N termini of CIN85 and CMS/CD2AP are composed of three highly similar src homology 3(SH3) domains that are involved in interactions with various signaling molecules, among them CD2, AIP/Alix, BLNK, and SB-1, which suggests roles in such diverse cellular processes as T-cell receptor clustering, induction of apoptosis in glial cells or B-cell receptor signaling (Dikic, 2002). On the other hand, the proline-rich region of CIN85 acts as an interaction module for additional SH3 domain-containing proteins, which couple it, for example, to cytoskeletal rearrangements (p130Cas and cortactin), regulation of Src family kinases (Fyn, Src, and Yes), and phosphoinositide metabolism regulation (p85 subunit of phosphatidylinositol-3 kinase) (Gout et al., 2000; Dikic, 2002; Lynch et al., 2003). The C-terminal coiled-coil regions of CIN85 and CMS mediate their oligomerization, which might add an additional complexity level for formation of multi-protein CIN85/CMS-associated protein networks (Kirsch et al., 1999; Borinstein et al., 2000; Watanabe et al., 2000).
After RTKs activation, CIN85 binds to Cbl via its SH3 domains (Take et al., 2000; Soubeyran et al., 2002), and at the same time the proline-rich region of CIN85 constitutively interacts with endophilins (Petrelli et al., 2002; Soubeyran et al., 2002), implicated in regulation of early endocytic events. In this way, CIN85 recruits endophilins to complexes with activated receptors, controlling receptor internalization (Petrelli et al., 2002; Soubeyran et al., 2002). Recently, we have identified an atypical proline-arginine (PxxxPR) motif that serves as a specific recognition site for the SH3 domains of CIN85/CMS adaptor molecules (Kowanetz et al., 2003a). Mutation of the corresponding arginine in full-size Cbl abolished its association with CIN85 (Kowanetz et al., 2003a). Interestingly, it has been reported that the SH3 domain of Gads adaptor protein also recognizes arginine-orientated peptides independently of a classical polyproline type 2 peptide (Berry et al., 2002; Liu et al., 2003).
CIN85 binding to Cbl and Cbl-b was enhanced by growth factor-induced tyrosine phosphorylation of Cbl, probably due to a conformational change in the distal carboxyl termini of Cbl/Cbl-b, leading to opening and/or stabilization of the PxxxPR motif (Take et al., 2000; Soubeyran et al., 2002; Kowanetz et al., 2003a). All three SH3 domains of CIN85 bound to PxxxPR peptides of Cbl/Cbl-b with high specificity and relatively low affinity, thus enabling full-size CIN85 to simultaneously interact with multiple Cbl molecules, promoting clustering of Cbl/EGF receptor complexes in mammalian cells (Kowanetz et al., 2003a).
In this report, we identify several novel CIN85 effectors, including synaptojanin 2B1, SHIP-1, Hip1R, p115RhoGEF, ASAP1, and ARAP3, which interact directly with CIN85 SH3 domains through their PxxxPR motifs. These proteins are implicated in the control of clathrin-mediated receptor endocytosis, receptor recycling, and cytoskeletal rearrangements. Detailed analysis of the functionality of the CIN85-ASAP1 complex revealed its important role in regulating EGF receptor recycling pathways. We propose that CIN85 acts as a cargo-specific intermediate scaffold linking these effectors with regulation of distinct steps in intracellular trafficking of EGF receptors.
MATERIALS AND METHODS
Products, Antibodies, and Expression Vectors
EGF was purchased from Serologicals (Norcross, GA). Mouse anti-myc antibodies were from Covance (Princeton, NJ) rabbit anti-GFP antibodies were from Molecular Probes (Eugene, OR), and mouse anti-FLAG M2 and M5 antibodies were from Sigma-Aldrich (St. Louis, MO). Anti-ASAP1 antibodies (642), anti-Cbl (RF), anti-CIN85 (CT), and anti-EGF receptor (RK2) or anti-autophosphorylated EGF receptor (phosphotyrosine 1173, anti-pEGFR) antibodies were used as described previously (Haglund et al., 2002; Szymkiewicz et al., 2002). Constructs of Cbl, Cbl-b, FLAG-CMS, FLAG-CIN85, and its different deletion mutants, glutathione S-transferase (GST) fusion proteins encoding the SH3 domains of CIN85, FLAG-tagged ubiquitin, FLAG-ASAP1, and synaptojanin 2B1 were described previously (Brown et al., 1998; Nemoto et al., 2001; Soubeyran et al., 2002; Szymkiewicz et al., 2002; Kowanetz et al., 2003a). Briefly, GST-SH3 A, B, or C encode individual SH3 domains of CIN85, SH3AB, or SH3BC-combination of two consecutive CIN85-SH3 domains, SH3ABC (3SH3) construct encodes all three SH3 domains of CIN85. CIN85-ΔA or CIN85-ΔAB correspond to the protein devoid of the first or of the first and second SH3 domains, respectively. CIN85-PCc contains only the proline-rich region and the coiled-coil motif of CIN85, whereas CIN85-ΔCc lacks the coiled-coil motif. Mouse Hip1R cDNA was from David Drubin (University of California, Berkeley, CA), ARAP3 (human) construct was provided by Phillip Hawkins (Inositide Laboratory, Cambridge, United Kingdom), mouse STAP-1 cDNA was from Akihiko Yoshimura (Kyushu University, Fukuoka, Japan), and SHIP-1 (human) was from Christophe Erneux (Universite Libre de Bruxelles, Brussels, Belgium).
Site-directed Mutagenesis
All mutant constructs were generated by polymerase chain reaction by using QuikChange (Stratagene, La Jolla, CA). The arginine residues were mutated to alanines in the following proteins: Hip1R-R1030A, ASAP1-R1041A, ARAP3-R99A, STAP1-R12A, SHIP-1-R1033A, R1139A, synaptojanin 2B1-R1249A, and p115 RhoGEF-S776R. The constructs were verified by sequencing. The sequences of the oligonucleotides used are available upon request.
Cell Culture and Transfections
HEK293T, NIH3T3-SAA, NIH3T3, MDA-MB-468, and CHO-EGFR cells were used as described previously (Haglund et al., 2002; Soubeyran et al., 2002; Szymkiewicz et al., 2002). Expression of wild-type CIN85 in NIH3T3-SAA cells was maintained by presence of G418 (1.2 mg/ml) in the culture medium. Cells were transfected with LipofectAMINE reagent (Invitrogen, Carlsbad, CA) following manufacturer's instructions. Thirty hours after transfection, the cells were starved for additional 12 h and stimulated with 100 ng/ml EGF for indicated times. Cells were lysed in ice-cold lysis buffer containing a cocktail of protease and phosphatase inhibitors as described elsewhere (Soubeyran et al., 2002).
Immunofluorescence
NIH3T3 cells seeded on collagen-coated coverslips were transfected with indicated constructs for 24 h and starved for additional 12 h. Cells were fixed in 4% paraformaldehyde. After permeabilization and blocking, cells were incubated with primary antibodies and then with Alexa Fluor-conjugated secondary antibodies (Dako, Denmark). The preparations were mounted using Fluoromount G, and the images were taken with an Axioplan 2 microscope (Carl Zeiss, Jena, Germany).
Gel Filtration Analysis
MDA-MB-468, NIH-3T3-SAA, or HEK293T cells were washed with phosphate-buffered saline (PBS) and scraped from the dish and lysed in PBS containing 0.1% Tween 20, 1 mM Pefablock (Fluka, Buchs, Switzerland), and 1 mM Na3VO4. The lysates were incubated on ice for 15 min and centrifuged at 15,000 × g for 60 min. Resulting cytosolic extracts (2-5 mg of protein) were loaded on a Superdex 200 13/30 fast-performance liquid chromatography column (Amersham Biosciences, Piscataway, NJ), and separated in the presence of PBS and 0.1 mM Pefablock. Collected fractions (0.5 or 1 ml) were subjected to immunoprecipitation with indicated antibodies.
Biochemical Assays
GST binding assays, immunoprecipitation, and immunoblotting were performed as described previously (Szymkiewicz et al., 2002).
Quantification of Receptor Recycling by Flow Cytometry
Chinese hamster ovary (CHO) cells stably expressing epidermal growth factor receptor (EGFR) were transfected with the indicated constructs, starved for 6 h, and incubated with 100 ng/ml EGF for 30 min at 37°C to induce internalization of EGF receptor. Then, cells were rinsed twice with PBS to remove unbound ligand and subsequently incubated at 37°C in serum-free medium to allow receptor recycling. At the indicated time points cells were chilled on ice to stop membrane trafficking, harvested, resuspended in 5% bovine serum albumin/PBS, and blocked for 45 min on ice. Surface resident EGFR was detected by incubation with anti-EGFR antibody, conjugated with pycoerythrin (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at + 4°C. Cells were washed with ice-cold PBS and analyzed with an Epics XL flow cytometer (Beckman Coulter, Fullerton, CA). For each sample, 10,000 cells were analyzed and GFP-expressing cells were gated for determining the amount of EGF receptor at the plasma membrane. Mean fluorescence intensity of each sample was calculated using Expo 32 ADC software. Equal expression of the transfected proteins was checked by Western blotting (our unpublished data).
Constitutive recycling of the transferrin receptor was analyzed using a transferrin-Alexa Fluor488 conjugate (Molecular Probes). Cell monolayers of CHO cells cotransfected with either of the indicated constructs, and transferrin receptor were chilled on ice to stop membrane trafficking and incubated with 50 μg/ml transferrin-Alexa Fluor488 for 30 min on ice. Subsequently, cells were washed with ice-cold PBS to remove unbound ligand and then incubated at 37°C to restore membrane trafficking. At the indicated time points cells were collected, resuspended in PBS, and analyzed with an Epics XL flow cytometer. For each sample, 10,000 cells were analyzed and fluorescein isothiocyanate-positive cells were gated for determining the rate of transferrin release by measuring the decrease in green fluorescence intensity. Mean fluorescence intensity of each sample was calculated using Expo 32 ADC software. Equal expression of the transfected proteins was checked by Western blotting (our unpublished data).
RESULTS
Identification of Novel CIN85-interacting Partners
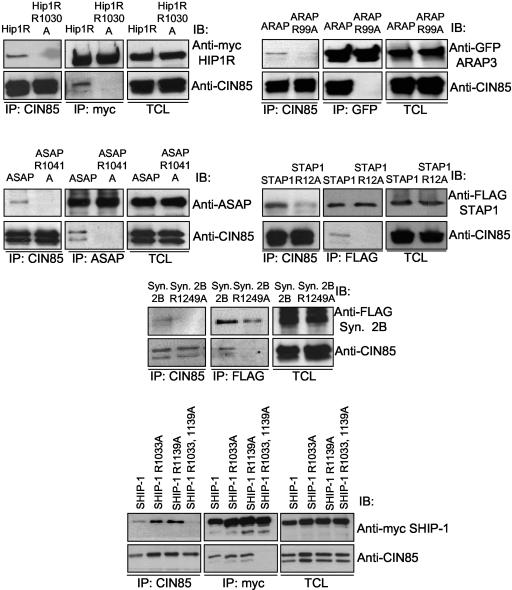
The SH3 domains of CIN85 and CMS selectively recognize a PxxxPR motif of Cbl/Cbl-b, the sequence conserved in all proteins previously shown to bind to the SH3 domains of CIN85 (Kowanetz et al., 2003a). Moreover, our yeast two-hybrid screens revealed two PxxxPR motif-containing proteins potentially implicated in receptor trafficking: Dab2 and ASAP1, as binding partners for the SH3 domains of CIN85 (Kowanetz et al., 2003b; our unpublished data). These observations suggested that additional molecules containing PxxxPR sequences could associate with CIN85. We therefore searched public databases for proteins containing the identified proline-arginine motif. This approach led to identification of numerous endocytic proteins containing one or more PxxxPR sequence as potential CIN85 effectors (Table 1). These molecules included inositol 5′ phosphatases (synaptojanin 2B1 and SHIP-1); p115RhoGEF, which couples the heterotrimeric G proteins to Rho GTPases; Arf GTPase-activating protein ASAP1; ARAP3, a protein with dual Rho and Arf GTPase activities; clathrin scaffold protein Hip1R, as well as an adaptor STAP1 (Table 1). The interactions between these proteins and CIN85 in mammalian cells were confirmed by coimmunoprecipitation studies (Figure 1), and substitution of arginine to alanine in the PxxxPR regions of Hip1R, ARAP3, ASAP1, STAP1, synaptojanin 2B1, and SHIP-1 completely blocked their coprecipitation with CIN85 in mammalian cells (Figure 1). SHIP-1 contains two PxxxPR motifs, both of which were involved in CIN85 binding (Figure 1). In the case of p115RhoGEF, a single-base polymorphism in the nucleotide 1240 that codes for serine instead of arginine within the PxxxPR motif led to a protein incapable of interacting with CIN85 (Figure 2A; our unpublished data).
Table 1.
Multiple proteins containing the PxxxPR motif function as effectors of CIN85 in mammalian cells
| Protein | Motif | Interacts with | Selected functions |
|---|---|---|---|
| ASAP1 | PVPLPR aa 1036 | SH3-ABC | -GTPase activating protein for Arf1 and Arf 5, phosphatidylinositol 4,5-biphosphate dependent (Brown et al., 1998; Kam et al., 2000) |
| constitutively | -involved in focal adhesion assembly and regulation of actin cytoskeleton (Randazzo et al., 2000a; Liu et al., 2002) | ||
| ARAP3 | PVPKPR aa 94 | SH3-AB | -an effector of PI3 kinase |
| PEPSPR aa 129 | constitutively | -a potential link between Rho and Arf signaling (Krugmann et al., 2002) | |
| PPQPPR aa 389 | |||
| Hip 1R | PSPAPR aa 1025 | SH3-ABC | -binds clathrin and F-actin (Engqvist-Goldstein et al., 1999) |
| PSIAPR aa 1044 | constitutively | -a potential link between endocytosis and actin cytoskeleton rearrangements (Engqvist-Goldstein et al., 1999, 2001) | |
| Synaptojanin 2B1 | PVPKPR aa 1244 | SH3-ABC | -an isoform of synaptojanin 1 (De Camilli et al., 1996) |
| increased upon | -binds amphiphysin, endophilin and Grb2 (Nemoto et al., 2001) | ||
| stimulation | - an effector of Rac, functions at early stages of EGFR endocytosis (Malecz et al., 2000) | ||
| SHIP-1 | PKPAPR aa 1028 | SH3-ABC | -an inositol 5′ phosphatase, negative regulator of the immune system |
| PTPTPR aa 1135 | constitutively | -regulates calcium flux, activation of Erk signaling cascade, activation of the kinase PKB/Akt (reviewed in March and Ravichandran, 2002) | |
| STAP1 | PKPAPR aa 7 | SH3-ABC | -PH and SH2 domains containing adaptor, functions downstream of c-kit in hematopoietic cells, tyrosine-phosphorylated by activated c-kit (Masuhara et al., 2000) |
| constitutively | |||
| p115-RhoGEF | PKPRPR aa 771 | SH3-AC | -a link between heterotrimeric G proteins and RhoGTPases (Hart et al., 1998) |
The binding partners of the SH3 domains of CIN85 are listed. The PxxxPR motifs in these proteins are indicated and the amino acid numbering refers to the first amino acid in the proline-arginine sequence. Previously reported functions of the identified proteins are summarized. The specificity of binding to the SH3 domains of CIN85 and dependence on the growth factor stimulation as determined by immunoprecipitation assays is indicated.
Figure 1.
SH3 domains of CIN85 bind multiple PxxxPR-containing proteins. (A) HEK293T cells were transiently transfected with CIN85 together with indicated proteins or their arginine mutants. Lysates were subjected to immunoprecipitation (IP) and immunoblotting (IB) with indicated antibodies.
Figure 2.
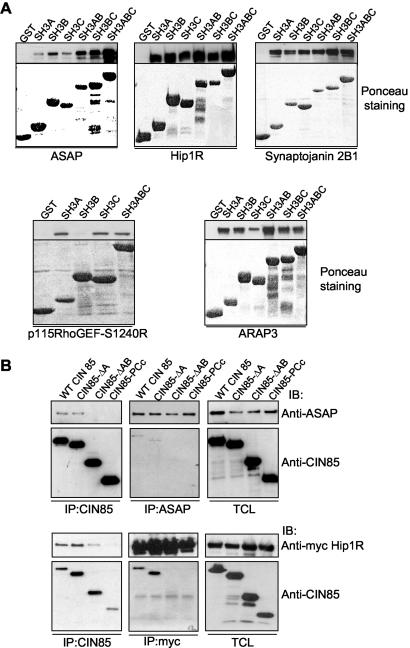
CIN85 SH3 domains can cluster numerous effectors. (A) Equal amounts of lysates from HEK293T cells transiently transfected with indicated proteins were incubated with GST alone or GST-fusion proteins encoding the single (SH3A, SH3B, and SH3C), double (SH3AB and SH3BC), or triple (SH3ABC) SH3 domains of CIN85. Top, amounts of precipitated proteins. Bottom, levels of used GST constructs stained with Ponceau. (B) FLAG-tagged constructs encoding wild-type CIN85 or its deletion isoforms were transiently expressed together with FLAG-ASAP or myc-Hip1R in HEK293T cells. Lysates were immunoprecipitated (IP) and immunoblotted (IB) with indicated antibodies.
On the other hand, the PxxxPR sequence is also present in numerous other proteins found in the databases, only a small percentage of which might constitute bona fide CIN85 binding partners in mammalian cells. For example, PAK2, ZO-2, and TAFII70 that were previously shown to interact with CIN85 SH3 domains in vitro were unable to interact with CIN85 in cells (Kurakin et al., 2003). Moreover, ARAP3 contains three PxxxPR motifs (Table 1); however, it is sufficient to mutate a single arginine (R99) to prevent binding to CIN85 (Figure 1). Similarly, only the motif containing arginine 1031, but not the PxxxPR sequence containing arginine 1049, in Hip1R, mediates its binding to CIN85 (Figure 1; our unpublished data). These observations suggest that the presence of a core PxxxPR motif in a given molecule does not suffice to mediate interaction with the SH3 domains of CIN85. Additional factors, such as preference for some “x” amino acids in the context of the surrounding residues, as well as structural availability of the motif, might determine their binding.
The Three SH3 Domains of CIN85 Can Cluster Multiple Effectors
Because each SH3 domain of CIN85 can bind the Cbl-derived PxxxPR peptide with comparable affinities (Kowanetz et al., 2003a), CIN85 should be able to simultaneously interact with multiple effectors, depending on their local availability in the cell and/or the accessibility of PxxxPR motifs. Potentially, the three SH3 domains of CIN85 could aggregate specific proteins, analogously to previously described clustering of Cbl molecules (Kowanetz et al., 2003a). This would increase local concentrations of CIN85 effectors and facilitate their specific functions in a particular compartment in the cell. Consistent with this hypothesis, we could show that whereas the majority of the PxxxPR-containing proteins interacted potently with all SH3 domains of CIN85 in GST pull-down assays (SH3A, SH3B, and SH3C), the GST fusion proteins encoding double (SH3AB and SH3BC) or triple (SH3ABC) SH3 domains of CIN85 precipitated significantly more of ASAP1, Hip1R, or synaptojanin (Figure 2A). In another approach to demonstrate that CIN85 clusters its effectors via the multiple SH3 domains, we compared associations of PxxxPR-containing proteins with the wild-type CIN85 or its variants lacking individual SH3 domains. Sequential deletion of SH3 domains in CIN85 (CIN85-ΔA and CIN85-ΔAB) led to a consecutive decrease in binding to Hip1R or ASAP1, whereas deletion of all three SH3 domains of CIN85 (CIN85-PCc) abolished their interactions (Figure 2B).
In contrast to the proteins recognized by all three SH3 domains of CIN85, some CIN85 effectors interacted only with specific SH3 domains, and these proteins were not efficiently clustered by CIN85. This property would rather enable them to engage in heterologous protein complexes tethered by the adaptor CIN85. For example, ARAP3 bound to SH3 A and B, whereas p115Rho GEF encoding arginine at position 1240 interacted most potently with SH3 domains A and C of CIN85 (Figure 2A). Because, for example, ARAP3 and synaptojanin possess exactly the same core PVPKPR motif, the observed specificity is likely to be dependent on residues flanking the PxxxPR sequence.
Sizing Separation of CIN85-associated Complexes under Native Conditions in Mammalian Cells
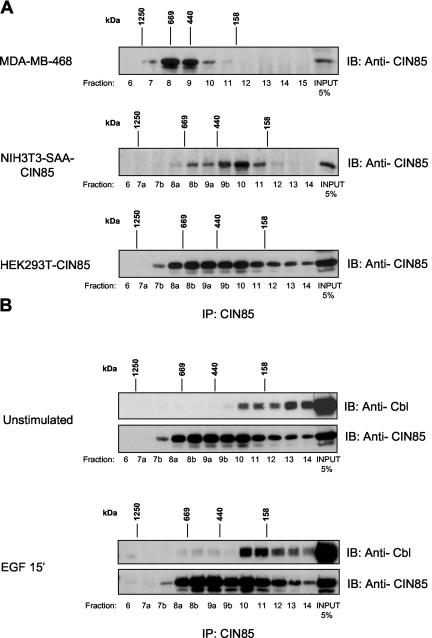
CIN85 contains numerous protein-protein interaction modules, and therefore belongs to the family of scaffold proteins. Their general property is the ability to recruit different repertoires of signaling proteins into correct cellular localization (Pawson and Scott, 1997). Importantly, such multidomain structure could allow for simultaneous association of CIN85 with different effectors and formation of large protein oligomers, and indeed, many signal transduction molecules occur as components of high-molecular-weight aggregates. Therefore, we examined whether CIN85 exists in large protein complexes in mammalian cells by using native gel filtration analysis of cytosolic extracts of mammalian cells. The 85-kDa CIN85, endogenously present in MDA or stably expressed in NIH3T3-SAA cells, is detected at a molecular weight ranging from 300 kDa to 1 MDa (Figure 3A, top). A similar, however more spread, sizing profile of CIN85 was observed in HEK293T cells overexpressing CIN85 (Figure 3A, bottom). Interestingly, Cbl was coseparated with CIN85 in high-molecular-weight protein complexes upon their coexpression in HEK293T cells treated with EGF (Figure 3B), pointing out that CIN85 is implicated in assembly of larger, Cbl-associated protein aggregates, after receptor activation.
Figure 3.
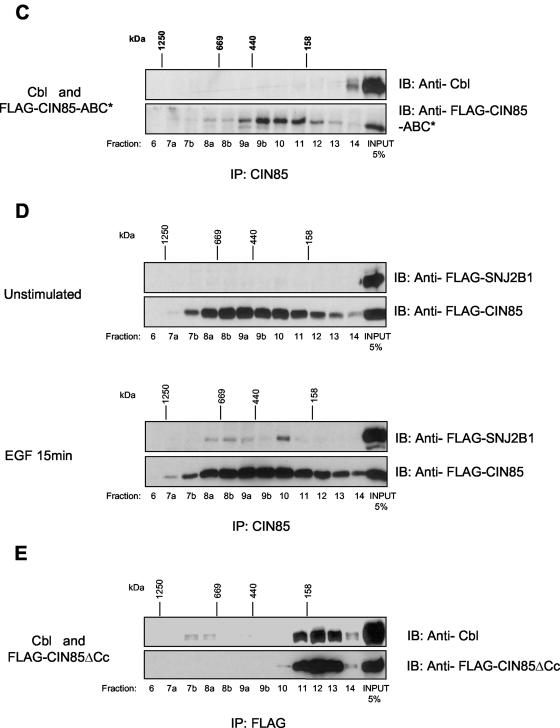
Formation of CIN85-associated complexes in mammalian cells. (A) Cytosolic extracts of MDA-MB-468, NIH3T3-SAA cells stably expressing wild-type CIN85, and HEK293T cells overexpressing CIN85 were run on Superdex 200 filtration columns. Separated proteins were collected in fractions and subjected to immunoprecipitation (IP) and immunoblotting (IB) with anti-CIN85 antibodies. TCL, total cell lysate. (B) HEK293T cells expressing CIN85 and Cbl were untreated or treated with 50 ng/ml EGF for 15 min at 37°C. Cytosolic extracts were separated by Superdex 200 filtration columns, and fractions were subjected to immunoprecipitation with anti-CIN85 antibodies followed by immunoblotting with anti-Cbl or anti-CIN85 antibodies. (C) HEK293T cells expressing CIN85-ABC* together with Cbl were analyzed as described in Figure 3B. (D) HEK293T cells expressing CIN85 and FLAG-synaptojanin 2B1 were left untreated or treated with 50 ng/ml EGF for 15 min at 37°C. Fractions of cytosolic lysates were subjected to immunoprecipitation (IP) with anti-CIN85 antibodies, followed by immunoblotting (IB) with anti-FLAG antibodies. (E) HEK293T cells expressing FLAG-CIN85-ΔCc and Cbl were analyzed as described in the Figure 3B.
We further tested the relative contribution of the SH3 domains in formation of CIN85-associated protein complexes in these cells. To this aim, we analyzed the sizing profile of a triple mutant of SH3 domains (CIN85-ABC*), unable to bind to Cbl or to other PxxxPR motif-containing proteins (Kowanetz et al., 2003a). CIN85-ABC* was shifted to smaller protein complexes and was not coseparated with Cbl in the same fractions (Figure 3C). The above-mentioned data suggested a scaffolding function for CIN85 via its ability to interact with numerous PxxxPR-containing proteins in cells. Accordingly, in Figure 3D we demonstrate that synaptojanin 2B1, similarly to Cbl, was recruited into large, CIN85-containing complexes, after growth-factor stimulation. On the other hand, additional CIN85-SH3 domain effectors, including ASAP1, SHIP-1, or Hip1R, were cofractioned in higher molecular weight complexes with CIN85, independently on ligand stimulation (our unpublished data). We noted that CIN85 is present in high-molecular weight complexes both in unstimulated, as well as EGF-stimulated cells, which could be explained by the fact that its SH3 domains' effectors can be dynamically exchanged during receptor trafficking. CIN85 could be therefore simultaneously engaged in aggregating different signaling or trafficking proteins at specific locations in a cell.
An additional level of complexity is achieved by the fact that CIN85 contains a coiled-coil domain at the carboxy terminus, implicated in its oligomerization upon addition of chemical cross-linking reagents (Borinstein et al., 2000; Watanabe et al., 2000). To investigate the importance of the coiled-coil domain in formation of CIN85 protein complexes, we analyzed the sizing profile of CIN85 lacking the coiled-coil domain (CIN85-ΔCc). The elution profile of CIN85-ΔCc was largely compressed to small molecular weight complexes, which contained Cbl (Figure 3E).
These observations suggest that in addition to the SH3-mediated interactions, CIN85 acts as an endocytic adaptor protein via its coiled-coil-mediated protein oligomerization. Possibly coiled-coil-oligomerized CIN85 serves as a more efficient clustering unit for the SH3-domains' effectors. It also might be engaged in hetero-oligomerization with other coiled-coil-containing proteins, and importantly this motif is common among molecules involved in vesicular trafficking. Remarkably, the CIN85 mutant devoid of the coiled-coil domain lacks punctuate vesicular localization, observed for the wild-type CIN85 (our unpublished data; Watanabe et al., 2000) and cannot function properly in endocytic trafficking.
CIN85 Promotes Interactions between Some PxxxPR-containing Proteins, whereas Other Effectors Are Present in Separate CIN85-associated Complexes
We have shown that CIN85 is able to cluster its effectors via the multiple SH3 domains, each of them binding to PxxxPR peptides with similar affinities (Figure 2), (Kowanetz et al., 2003a). Subsequently, we were interested to investigate whether different PxxxPR containing effectors can be also heterogenously clustered on the same CIN85 molecule. This would enable CIN85 to tether endocytic molecules, ensuring their coordinated, possibly sequential, functions. Similar property has been ascribed to several scaffold proteins that create multienzyme complexes in mitogen-activated protein kinase pathways, both in yeast and in mammals. These multidomain proteins seem to facilitate MAP-kinase activation and to ensure signaling specificity (Whitmarsh and Davis, 1998).
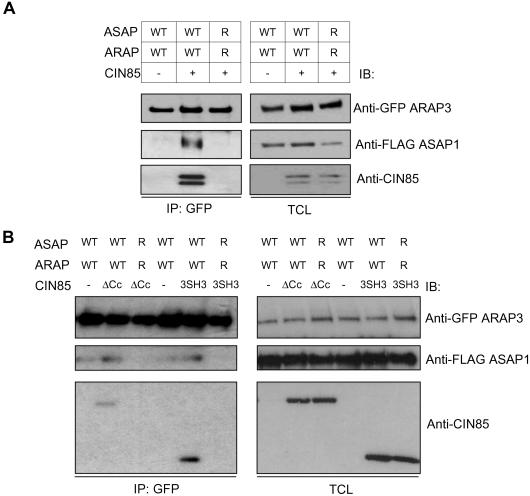
Interestingly, we observed that the two ArfGAPs, ASAP1 and ARAP3, are indeed found in one complex only in the presence of CIN85 (Figure 4A) and that these interactions were dependent on the intact CIN85 binding sites in both proteins. In addition, in Figure 4B we demonstrate that the delta-coiled-coil construct of CIN85, devoid of the ability to form dimers, still mediates clustering of ASAP1 and ARAP3. Moreover, ASAP1 and ARAP3 can be brought together via the CIN85-3SH3 domain construct, which encodes solely the three SH3 domains of CIN85 (Figure 4B). These data clearly demonstrate that one CIN85 molecule can be simultaneously complexed with multiple effectors of its SH3 domains.
Figure 4.
CIN85 SH3 domains can bridge ASAP1 and ARAP3 ArfGAP proteins. (A) HEK293T cells were transiently transfected with GFP-ARAP3 or FLAG-ASAP1, wild-type or arginine mutants (WT or R, respectively), in the presence or absence of CIN85. Cell lysates were immunoprecipitated (IP) with anti-GFP antibodies, and after recovery of the bound material, detection was performed by immunoblotting (IB) with indicated antibodies. Expression of proteins was monitored in total cell lysates (TCL). (B) Experimental procedure was the same as in A, except that ΔCc or 3SH3-CIN85 constructs were used instead of the wild-type CIN85.
Therefore, both Arf effectors might function coordinately due to CIN85 SH3 domains-mediated clustering. The heterologous clustering by the SH3 domains of CIN85 could be in this case facilitated by the fact that ARAP3 interacts specifically with SH3 domains A and B only, and is not efficiently aggregated by multiple SH3 domains of CIN85 (Figure 2A).
On the other hand, we have not noticed any significant effect of CIN85 presence on associations between other effectors (our unpublished data). These proteins seem to be components of separate complexes with the SH3 domains of CIN85, involved in distinct trafficking and signaling processes. The formation of a given complex could in turn be determined, in addition to compartmentalization of the effectors, by protein modifications, such as phosphorylation.
CIN85 and ASAP1 Complex Is Involved in EGF Receptor Recycling
CIN85-SH3 domains' mediated interaction and clustering of Cbl molecules is critical for EGF receptor internalization, trafficking, and degradation (Soubeyran et al., 2002; Szymkiewicz et al., 2002; Kowanetz et al., 2003a). We were interested to further investigate whether CIN85 associations with other effectors play important roles in receptor trafficking. We focused on ASAP1, a phospholipid-dependent Arf GTPase activating protein, which has been shown to regulate actin cytoskeletal rearrangements (Brown et al., 1998; Randazzo et al., 2000a). Importantly, a previously described interaction between ASAP1 and POB1 suggested a link to clathrin-mediated endocytosis (Oshiro et al., 2002).
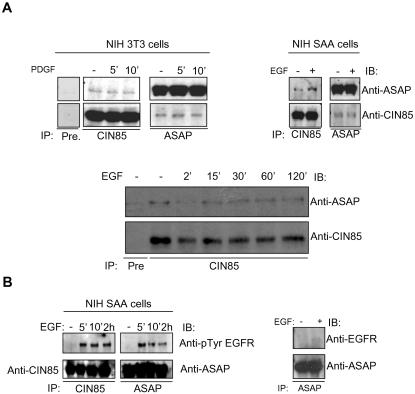
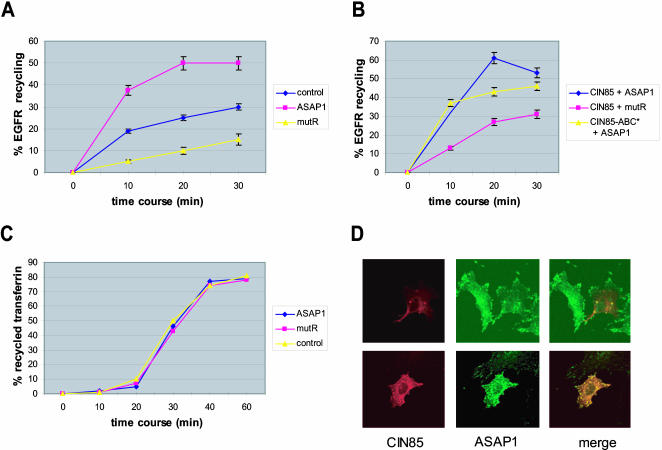
We first analyzed complex formation between endogenous proteins in NIH3T3 fibroblasts, which express high levels of both molecules. Endogenous ASAP1 and CIN85 were coprecipitated independently on prolonged PDGF or EGF stimulation from NIH3T3 and NIHSAA (a clone of NIH3T3 cells stably expressing EGF receptor) cell lysates, respectively (Figure 5A). The levels of coprecipitation also were not dependent on ASAP1 tyrosine phosphorylation by FAK or Pyk2 tyrosine kinases (our unpublished data). Moreover, both proteins were detected in complexes with activated receptors in those cells (Figure 5B) (Soubeyran et al., 2002). This prompted us to study the function of CIN85/ASAP1 interaction in EGF receptor trafficking. The formation of CIN85/ASAP1 complexes did not affect the rate of receptor internalization (our unpublished data). Instead, overexpression of ASAP1 led to an increase of receptor recycling (Figure 6A). This effect was dependent on the GTP-ase activating (GAP) activity, as well as on an intact PxxxPR motif in ASAP1 (Figure 6A; our unpublished data). To verify that the effect of ASAP1 was indeed mediated by CIN85, we compared the rate of receptor recycling in the presence ASAP1 and of either the wild-type CIN85 or the triple mutant of CIN85 SH3 domains (CIN85-ABC*) that are unable to bind proline-rich proteins (Kowanetz et al., 2003a; Figure 6B). Cotransfection of ASAP1 together with CIN85-ABC* led to a decrease in recycling compared with coexpression with wild-type CIN85. Notably, receptor recycling was even more decreased when PxxxPR-defective ASAP1 (mutR) was cotransfected with the wild-type CIN85 (Figure 6B; also see Figure 6A). This might indicate that the SH3 domains of CIN85 bind yet other proteins acting as (negative) regulators of EGFR recycling that may counterbalance the action of ASAP1. In any case, the above-mentioned data suggest that to mediate receptor recycling processes, ASAP1 has to be able to induce hydrolysis of GTP bound to Arf to GDP and, additionally, to bind to CIN85. Furthermore, this effect was EGFR-specific, because under similar experimental conditions, overexpression of ASAP1 did not influence constitutive recycling of the transferrin receptor (Figure 6C). Thus, CIN85 complexed to ASAP1 is involved in receptor recycling pathway and remarkably CIN85 homologue CMS/CD2AP has been shown to interact and colocalize with Rab4, a marker of recycling endosomes (Cormont et al., 2003). We also noted that in cells overexpressing CIN85, ASAP1 showed reduced localization to focal adhesions, and both ASAP1 and CIN85 colocalized in vesicular structures in NIH3T3 cells (Figure 6D). Therefore ASAP1, previously known to mediate cytoskeletal rearrangements might couple them via CIN85 to membrane trafficking or might function separately in either of these processes. These data expand the scaffolding role for CIN85 in controlling various stages of EGF receptor trafficking in cells.
Figure 5.
Endogenous ASAP1 and CIN85 interact constitutively and are recruited to complexes with activated EGFR (A) NIH3T3 and NIHSAA cells were left unstimulated (-) or stimulated for indicated time points with PDGF or EGF, respectively. Cell lysates were immunoprecipitated (IP) either with preimmune serum (Pre), anti-CIN85 or anti-ASAP1 (642) antibodies, and detection was performed by immunoblotting (IB) with anti-ASAP1 or anti-CIN85 antibodies. (B) NIHSAA cells were left unstimulated (-) or stimulated with 100 ng/ml EGF for indicated time points. Cell lysates were immunoprecipitated (IP) either with anti-CIN85 or anti-ASAP antibodies, and detection was performed by immunoblotting (IB) with indicated antibodies.
Figure 6.
ASAP1 regulates EGF receptor recycling in a PxxxPR motif-dependent manner. (A) CHO-EGFR cells were mock transfected or transfected with the wild-type ASAP1 or ASAP1 R1041A (mutR). Receptor recycling assays were performed as described in MATERIALS AND METHODS using flow cytometry. (B) CHO-EGFR cells were transfected with the indicated constructs and analyzed as described in A. (C) CHO cells were cotransfected with transferrin receptor and either wild-type ASAP1 or ASAP1 R1041A (mutR). Transferrin recycling measurements were performed as described in MATERIALS AND METHODS. (D) NIH3T3 cells were transfected with FLAG-tagged CIN85 and subjected to immunofluorescence as described in MATERIALS AND METHODS.
DISCUSSION
Previous studies have demonstrated that CIN85 participates in clathrin-mediated endocytosis by linking Cbl-Cbl-b/EGF receptor complexes to endophilins (Dikic, 2002, 2003; Soubeyran et al., 2002; Szymkiewicz et al., 2002), and undergoing lysosomal degradation together with receptors (Haglund et al., 2002). In addition to participating in the Cbl/CIN85/endophilin pathway, CIN85 and its homologue CMS/CD2AP have been shown to associate with other endocytic proteins, including Grb2, p85 subunit of PI3-kinase, Crk, p130Cas, and cortactin (Dikic, 2002; Lynch et al., 2003). More recently, we have identified an unconventional proline/arginine PxxxPR motif, critical for interactions of the SH3 domains of CIN85 with Cbl and Cbl-b (Kowanetz et al., 2003a). This report provides new insights into a dynamic PxxxPR-containing protein network assembled by the scaffold protein CIN85 and emphasizes its role in controlling multiple levels of EGF receptor endocytosis. Using the yeast two-hybrid system and by screening public data bases for proteins containing specific PxxxPR motif, we have identified numerous endocytic effectors. Subsequently, we confirmed the existence of CIN85/PxxxPR-motif-containing protein complexes in mammalian cells (Figure 1). Furthermore, multiple interactions mediated via the SH3 domains of CIN85 resulted in prominent clustering of its effectors, thereby increasing their local concentrations and possibly ensuring proper coordination of receptor trafficking (Figure 2). Additionally, some of the effectors, e.g., ASAP1 and ARAP3, were heterologously clustered by the SH3 domains of CIN85 (Figure 4). Similar cooperative function of multiple SH3 domains leading to clustering of Cbl and other effectors has been demonstrated in the case of other adaptors, e.g., Nck (Wunderlich et al., 1999) and ArgBP2 (Soubeyran et al., 2003).
The scaffolding properties of CIN85 allow for formation of high-molecular-weight CIN85-associated complexes in mammalian cells (Figure 3). Whereas certain proteins constitutively associate with CIN85, binding of Cbl, Dab2, and synaptojanin 2B1 is rapidly modulated by growth factor stimulation (Figure 3D) (Soubeyran et al., 2002; Kowanetz et al., 2003b), leading to formation of distinct CIN85-associated protein networks in a cell. These various complexes assembled around CIN85-SH3 domains can differently influence the fate of internalized receptor (Figure 5C) (Soubeyran et al., 2002). Importantly, the relatively low affinity of individual CIN85 SH3 domain-PxxxPR interactions (Kowanetz et al., 2003a) also could allow for rapid exchange of CIN85 binding partners, depending on their local concentration, cellular compartmentalization, or posttranslational modifications in response to changes in the environment, such as tyrosine phosphorylation of Cbl (Soubeyran et al., 2002; Kowanetz et al., 2003b). The ability of CIN85 to dynamically exchange its effectors could ensure that cargo is appropriately moved through the different stages of the endocytic pathway.
The specific proteins with which CIN85 interacts are consistent with its function as a regulator of progress through the endocytic pathway. However, the contribution of individual CIN85-SH3 domains binding events to EGF receptor trafficking is difficult to assess, given the functional redundancy of CIN85 binding partners and their dynamic exchange on multiple SH3 domains along the endocytic compartment. The functional importance of these interactions is emphasized by the fact that presence of intact CIN85 SH3 domains is critical for sorting of activated EGF receptors for lysosomal degradation (Kowanetz et al., 2003a).
One group of CIN85-interacting endocytic effectors, inositol 5′ phosphatases (synaptojanin 2B1 and SHIP-1), is implicated in membrane dynamics by controlling concentration of phosphoinositides (De Camilli et al., 1996). Synaptojanins have been found in complexes with numerous endocytic proteins, including endophilin or amphiphysins (Micheva et al., 1997). Synaptojanin 1, due to its ability to dephosphorylate phosphatidylinositol 4,5-bisphosphate to phosphatidylinositol 3-phosphate, has been proposed to function as a negative regulator of interactions between coat proteins and membrane phospholipids (McPherson et al., 1996; Cremona et al., 1999; Guo et al., 1999). On the other hand, siRNA-mediated decrease in synaptojanin 2 levels had an inhibitory effect on EGF receptor endocytosis, suggesting that synaptojanin 1 and 2 do not have a completely overlapping function (Rusk et al., 2003). The PxxxPR motif is present in ubiquitously expressed synaptojanins 2, as well as in brain-specific synaptojanin 1; however, we have not detected CIN85/synaptojanin 1 complex formation (our unpublished observations). It is therefore likely that different functions of synaptojanins 1 and 2, which share the same catalytic activity, are dependent on their specific binding partners. Overexpression of synaptojanin 2B1, but not its R1249A mutant, increased the amount of CIN85 found in the complex with activated EGF receptor (our unpublished data), and therefore synaptojanin 2, similarly to Cbl, provides an inducible link between CIN85 and receptor (Figure 3D), possibly controlling early stages of receptor trafficking. This additional interaction could provide an explanation why carboxyl terminal truncations of Cbl have only a partial inhibitory effect on the EGFR internalization (Soubeyran et al., 2002). It will be of interest to further investigate the function of CIN85/synaptojanins complexes in EGFR endocytosis.
Another role CIN85 may play in regulation of EGF receptor trafficking could be to bridge endocytic events with cytoskeletal changes via its interactions with Hip1R, ARAP3, and ASAP1. CIN85-mediated clustering of Hip1R (Figure 2) could have a dual function both in assembly of clathrin-coated vesicles and also linking receptor internalization with locally regulated actin rearrangements (Engqvist-Goldstein et al., 1999, 2001). ARAP3 contains a Rho GAP domain that is able to inactivate Rho family proteins (Krugmann et al., 2002). On the other hand, CIN85 binds directly to p115 Rho GEF, an activator of RhoGTPases (Hart et al., 1998). CIN85 may thus regulate positively and negatively the activity of Rho GTPases and thereby coordinate changes in membranes and actin that are necessary for proper maturation and movement of endosomes and transport vesicles
CIN85 has a potential to elicit similar functions by affecting Arf family proteins, established regulators of membrane traffic and cytoskeletal dynamics (Randazzo et al., 2000b). Arfs are regulated by nucleotide exchange factors that activate Arf, and GAPs that inactivate Arf (Randazzo et al., 2000b). Two phosphoinositide-dependent Arf GAPs, ASAP1 and ARAP3, sharing structural motifs such as a PH domain and ankyrin repeats in addition to the ArfGAP domain, are constitutively associated with CIN85 (Figures 1 and 5A) and importantly can be found in one complex due to interactions mediated by multiple SH3 domains of CIN85 (Figure 4). Both ASAP1 and ARAP subfamily Arf GAPs have been found to regulate actin cytoskeletal structures (Randazzo et al., 2000a; Krugmann et al., 2002; Miura et al., 2002) and membrane trafficking in the cell periphery (Nie, Hirsch, and Randazzo, unpublished observations). Interestingly, CIN85 overexpression resulted in the recruitment of ASAP1 to the vesicular structures in cells (Figure 6D). Although we could not demonstrate any effect of ASAP1 on EGFR internalization, we have observed that overexpression of wild-type ASAP1 led to an increase in the rate of EGF receptor recycling (Figure 6A). Importantly, overexpression of ASAP1 R1041A impaired in its ability to bind to CIN85 and decreased the rate of receptor recycling below the control levels (Figure 6, A and B), implying that it exerts a dominant interfering effect on receptor recycling. The described effects were specific for the ligand induced recycling of the EGF receptors to the plasma membrane, as the constitutive recycling of transferrin receptors was not influenced by the presence of ASAP1 (Figure 6C). Thus, our data suggest an important role for the CIN85/ASAP1 complex in directing receptors toward recycling pathways. Remarkably, the focal contacts formation at a leading edge of a moving cell involves, in addition to actin rearrangements, recycling of the membrane internalized from the cell surface (de Curtis, 2001; Turner and Brown, 2001). ASAP1 could therefore, similarly to the Arf GAPs of the GIT family (Matafora et al., 2001), be involved both in focal contact formation as well as in membrane recycling via its interaction with CIN85.
The C-terminal tail of CIN85 contains a coiled-coil region that seems to be critical for oligomerization of CIN85 in mammalian cells (Watanabe et al., 2000). Interestingly, this motif is present in numerous endocytic proteins, including Eps-15, amphiphysin, EEA1, Hrs, or SNARE (Stenmark et al., 1996; Wigge et al., 1997; Burkhard et al., 2001; Raiborg et al., 2001). The coiled-coil region of CIN85 also is engaged in heterologous interactions with several endocytic effectors, such as Hip1R and amphiphysins 1 and 2 (our unpublished data). Additionally, the presence of an intact coiled-coil is critical for formation of large CIN85-bound protein complexes (Figure 3E). Therefore, the coiled-coil-mediated homo- and heterooligomerization of CIN85 might further promote receptor clustering in the vesicular compartments and ensure its proper trafficking.
It is becoming obvious that endocytosis of transmembrane receptors is governed by specific scaffold proteins able to coordinate extensive networks of protein-protein interactions. For example, β-arrestins and SARA proteins control down-regulation of their respective receptors, G protein-coupled receptors or transforming growth factor-β receptors (Tsukazaki et al., 1998; Hall and Lefkowitz, 2002). Our results indicate that multiple steps in EGF receptor down-regulation are controlled by a protein network assembled through the endocytic scaffold CIN85. Analogous function has been ascribed to intersectin, an adaptor protein implicated in the regulation of clathrin-mediated endocytosis (Yamabhai et al., 1998). Interestingly, the two carboxyl-terminal SH3 domains of intersectin share >50% homology with the SH3 domains of CIN85, which places them among the most closely related SH3 domain sequences found in the public databases. Structural and functional similarities between CIN85 and intersectin open intriguing questions regarding their potential cooperation in orchestrating dynamic assembly of endocytic proteins around receptor complexes.
Acknowledgments
We thank David Drubin, Akihiko Yoshimura, Peter T. Hawkins, and Christophe Erneux for providing reagents used in this study. This work was funded in part by grants from the Swedish Strategic Funds and Boehringer Ingelheim Foundation (to I.D.). K.K. is supported by a scholarship from the Boehringer Ingelheim Fonds.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-09-0683. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-09-0683.
References
- Aaronson, S.A. (1991). Growth factors and cancer. Science 254, 1146-1153. [DOI] [PubMed] [Google Scholar]
- Berry, D.M., Nash, P., Liu, S.K., Pawson, T., and McGlade, C.J. (2002). A high-affinity Arg-X-X-Lys SH3 binding motif confers specificity for the interaction between Gads and SLP-76 in T cell signaling. Curr. Biol. 12, 1336-1341. [DOI] [PubMed] [Google Scholar]
- Bogler, O., Furnari, F.B., Kindler-Roehrborn, A., Sykes, V.W., Yung, R., Huang, H.J., and Cavenee, W.K. (2000). SETA: a novel SH3 domain-containing adapter molecule associated with malignancy in astrocytes. Neurooncology 2, 6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borinstein, S.C., Hyatt, M.A., Sykes, V.W., Straub, R.E., Lipkowitz, S., Boulter, J., and Bogler, O. (2000). SETA is a multifunctional adapter protein with three SH3 domains that binds Grb2, Cbl, and the novel SB1 proteins. Cell Signal. 12, 769-779. [DOI] [PubMed] [Google Scholar]
- Brown, M.T., Andrade, J., Radhakrishna, H., Donaldson, J.G., Cooper, J.A., and Randazzo, P.A. (1998). ASAP1, a phospholipid-dependent arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol. Cell. Biol. 18, 7038-7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhard, P., Stetefeld, J., and Strelkov, S.V. (2001). Coiled coils: a highly versatile protein folding motif. Trends Cell Biol. 11, 82-88. [DOI] [PubMed] [Google Scholar]
- Cormont, M., Meton, I., Mari, M., Monzo, P., Keslair, F., Gaskin, C., McGraw, T.E., and Le Marchand-Brustel, Y. (2003). CD2AP/CMS Regulates Endosome Morphology and Traffic to the Degradative Pathway Through its Interaction with Rab4 and c-Cbl. Traffic 4, 97-112. [DOI] [PubMed] [Google Scholar]
- Cremona, O., et al. (1999). Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 99, 179-188. [DOI] [PubMed] [Google Scholar]
- De Camilli, P., Emr, S.D., McPherson, P.S., and Novick, P. (1996). Phosphoinositides as regulators in membrane traffic. Science 271, 1533-1539. [DOI] [PubMed] [Google Scholar]
- de Curtis, I. (2001). Cell migration: GAPs between membrane traffic and the cytoskeleton. EMBO Rep. 4, 277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic, I. (2002). CIN85/CMS family of adaptor molecules. FEBS Lett. 529, 110-115. [DOI] [PubMed] [Google Scholar]
- Dikic, I. (2003). Mechanisms controlling EGF receptor endocytosis and degradation. Biochem. Soc. Trans. 31, 1178-1181. [DOI] [PubMed] [Google Scholar]
- Dikic, I., and Giordano, S. (2003). Negative receptor signalling. Curr. Opin. Cell Biol. 15, 128-135. [DOI] [PubMed] [Google Scholar]
- Dikic, I., Szymkiewicz, I., and Soubeyran, P. (2003). Cbl signaling networks in the regulation of cell function. Cell Mol. Life Sci. 60, 1805-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin, M.L., et al. (1998). A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 94, 667-677. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein, A.E., Kessels, M.M., Chopra, V.S., Hayden, M.R., and Drubin, D.G. (1999). An actin-binding protein of the Sla2/Huntingtin interacting protein 1 family is a novel component of clathrin-coated pits and vesicles. J. Cell Biol. 147, 1503-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engqvist-Goldstein, A.E., Warren, R.A., Kessels, M.M., Keen, J.H., Heuser, J., and Drubin, D.G. (2001). The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro. J. Cell Biol. 154, 1209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout, I., Middleton, G., Adu, J., Ninkina, N.N., Drobot, L.B., Filonenko, V., Matsuka, G., Davies, A.M., Waterfield, M., and Buchman, V.L. (2000). Negative regulation of PI 3-kinase by Ruk, a novel adaptor protein. EMBO J. 19, 4015-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, S., Stolz, L.E., Lemrow, S.M., and York, J.D. (1999). SAC1-like domains of yeast SAC1, INP52, and INP53 and of human synaptojanin encode polyphosphoinositide phosphatases. J. Biol. Chem. 274, 12990-12995. [DOI] [PubMed] [Google Scholar]
- Haglund, K., Shimokawa, N., Szymkiewicz, I., and Dikic, I. (2002). Cbl-directed monoubiquitination of CIN85 is involved in regulation of ligand-induced degradation of EGF receptors. Proc. Natl. Acad. Sci. USA 99, 12191-12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund, K., Sigismund, S., Polo, S., Szymkiewicz, I., Di Fiore, P.P., and Dikic, I. (2003). Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 5, 461-466. [DOI] [PubMed] [Google Scholar]
- Hall, R.A., and Lefkowitz, R.J. (2002). Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ. Res. 91, 672-680. [DOI] [PubMed] [Google Scholar]
- Hart, M.J., Jiang, X., Kozasa, T., Roscoe, W., Singer, W.D., Gilman, A.G., Sternweis, P.C., and Bollag, G. (1998). Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science 280, 2112-2114. [DOI] [PubMed] [Google Scholar]
- Kam, J.L., Miura, K., Jackson, T.R., Gruschus, J., Roller, P., Stauffer, S., Clark, J., Aneja, R., and Randazzo, P.A. (2000). Phosphoinositide-dependent activation of the ADP-ribosylation factor GTPase-activating protein ASAP1. Evidence for the pleckstrin homology domain functioning as an allosteric site. J. Biol. Chem. 275, 9653-9663. [DOI] [PubMed] [Google Scholar]
- Kirsch, K.H., Georgescu, M.M., Ishimaru, S., and Hanafusa, H. (1999). CMS: an adapter molecule involved in cytoskeletal rearrangements. Proc. Natl. Acad. Sci. USA 96, 6211-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowanetz, K., Szymkiewicz, I., Haglund, K., Kowanetz, M., Husnjak, K., Taylor, J.D., Soubeyran, P., Engstrom, U., Ladbury, J.E., and Dikic, I. (2003a). Identification of a novel proline-arginine motif involved in CIN85-dependent clustering of Cbl and down-regulation of epidermal growth factor receptors. J. Biol. Chem. 278, 39735-39746. [DOI] [PubMed] [Google Scholar]
- Kowanetz, K., Terzic, J., and Dikic, I. (2003b). Dab2 links CIN85 with clathrin-mediated receptor internalization. FEBS Lett. 554, 81-87. [DOI] [PubMed] [Google Scholar]
- Krugmann, S., et al. (2002). Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol Cell 9, 95-108. [DOI] [PubMed] [Google Scholar]
- Kurakin, A.V., Wu, S., and Bredesen, D.E. (2003). Atypical recognition consensus of CIN85/SETA/Ruk SH3 domains revealed by target-assisted iterative screening. J. Biol. Chem. 278, 34102-34109. [DOI] [PubMed] [Google Scholar]
- Liu, Q., Berry, D., Nash, P., Pawson, T., McGlade, C.J., and Li, S.S. (2003). Structural basis for specific binding of the Gads SH3 domain to an RxxK motif-containing SLP-76 peptide: a novel mode of peptide recognition. Mol. Cell 11, 471-481. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Loijens, J.C., Martin, K.H., Karginov, A.V., and Parsons, J.T. (2002). The association of ASAP1, an ADP ribosylation factor-GTPase activating protein, with focal adhesion kinase contributes to the process of focal adhesion assembly. Mol. Biol. Cell 13, 2147-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, D.K., Winata, S., Lyons, R.J., Hughes, W.E., Lehrbach, G.M., Wasinger, V., Corthals, G., Cordwell, S., and Daly, R.J. (2003). A cortactin/CD2-associated protein (CD2AP) complex provides a novel link between epidermal growth factor receptor endocytosis and the actin cytoskeleton. J. Biol. Chem. 278, 21805-21813. [DOI] [PubMed] [Google Scholar]
- Malecz, N., McCabe, P.C., Spaargaren, C., Qiu, R., Chuang, Y., and Symons, M. (2000). Synaptojanin 2, a novel Rac1 effector that regulates clathrin-mediated endocytosis. Curr. Biol. 10, 1383-1386. [DOI] [PubMed] [Google Scholar]
- March, M.E., and Ravichandran, K. (2002). Regulation of the immune response by SHIP. Semin. Immunol. 14, 37-47. [DOI] [PubMed] [Google Scholar]
- Masuhara, M., Nagao, K., Nishikawa, M., Sasaki, M., Yoshimura, A., and Osawa, M. (2000). Molecular cloning of murine STAP-1, the stem-cell-specific adaptor protein containing PH and SH2 domains. Biochem. Biophys. Res. Commun. 268, 697-703. [DOI] [PubMed] [Google Scholar]
- Matafora, V., Paris, S., Dariozzi, S., and de Curtis, I. (2001). Molecular mechanisms regulating the subcellular localization of p95-APP1 between the endosomal recycling compartment and sites of actin organization at the cell surface. J. Cell Sci. 114, 4509-4520. [DOI] [PubMed] [Google Scholar]
- McPherson, P.S., Garcia, E.P., Slepnev, V.I., David, C., Zhang, X., Grabs, D., Sossin, W.S., Bauerfeind, R., Nemoto, Y., and De Camilli, P. (1996). A presynaptic inositol-5-phosphatase. Nature 379, 353-357. [DOI] [PubMed] [Google Scholar]
- Micheva, K.D., Kay, B.K., and McPherson, P.S. (1997). Synaptojanin forms two separate complexes in the nerve terminal. Interactions with endophilin and amphiphysin. J. Biol. Chem. 272, 27239-27245. [DOI] [PubMed] [Google Scholar]
- Miura, K., Jacques, K.M., Stauffer, S., Kubosaki, A., Zhu, K., Hirsch, D.S., Resau, J., Zheng, Y., and Randazzo, P.A. (2002). ARAP 1, a point of convergence for Arf and Rho signaling. Mol. Cell 9, 109-119. [DOI] [PubMed] [Google Scholar]
- Nemoto, Y., Wenk, M.R., Watanabe, M., Daniell, L., Murakami, T., Ringstad, N., Yamada, H., Takei, K., and De Camilli, P. (2001). Identification and characterization of a synaptojanin 2 splice isoform predominantly expressed in nerve terminals. J. Biol. Chem. 276, 41133-41142. [DOI] [PubMed] [Google Scholar]
- Oshiro, T., Koyama, S., Sugiyama, S., Kondo, A., Onodera, Y., Asahara, T., Sabe, H., and Kikuchi, A. (2002). Interaction of POB1, a downstream molecule of small G protein Ral, with PAG2, a paxillin-binding protein, is involved in cell migration. J. Biol. Chem. 277, 38618-38626. [DOI] [PubMed] [Google Scholar]
- Pawson, T., and Scott, J.D. (1997). Signaling through scaffold, anchoring, and adaptor proteins. Science 278, 2075-2080. [DOI] [PubMed] [Google Scholar]
- Petrelli, A., Gilestro, G.F., Lanzardo, S., Comoglio, P.M., Migone, N., and Giordano, S. (2002). The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature 416, 187-190. [DOI] [PubMed] [Google Scholar]
- Raiborg, C., Bremnes, B., Mehlum, A., Gillooly, D.J., D'Arrigo, A., Stang, E., and Stenmark, H. (2001). FYVE and coiled-coil domains determine the specific localisation of Hrs to early endosomes. J. Cell Sci. 114, 2255-2263. [DOI] [PubMed] [Google Scholar]
- Randazzo, P.A., Andrade, J., Miura, K., Brown, M.T., Long, Y.Q., Stauffer, S., Roller, P., and Cooper, J.A. (2000a). The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 97, 4011-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo, P.A., Nie, Z., Miura, K., and Hsu, V.W. (2000b). Molecular aspects of the cellular activities of ADP-ribosylation factors. Sci STKE 2000, RE1. [DOI] [PubMed] [Google Scholar]
- Rusk, N., Le, P.U., Mariggio, S., Guay, G., Lurisci, C., Nabi, I.R., Corda, D., and Symons, M. (2003). Synaptojanin 2 functions at an early step of clathrin-mediated endocytosis. Curr. Biol. 13, 659-663. [DOI] [PubMed] [Google Scholar]
- Schlessinger, J. (2000). Cell signaling by receptor tyrosine kinases. Cell 103, 211-225. [DOI] [PubMed] [Google Scholar]
- Sorkin, A.D., and Waters, C.M. (1993). Endocytosis of growth factor receptors. Bioessays 6, 375-382. [DOI] [PubMed] [Google Scholar]
- Soubeyran, P., Barac, A., Szymkiewicz, I., and Dikic, I. (2003). Cbl-ArgBP2 complex mediates ubiquitination and degradation of c-Abl. Biochem. J. 370, 29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubeyran, P., Kowanetz, K., Szymkiewicz, I., Langdon, W.Y., and Dikic, I. (2002). Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature 416, 183-187. [DOI] [PubMed] [Google Scholar]
- Stenmark, H., Aasland, R., Toh, B.H., and D'Arrigo, A. (1996). Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J. Biol. Chem. 271, 24048-24054. [DOI] [PubMed] [Google Scholar]
- Szymkiewicz, I., Kowanetz, K., Soubeyran, P., Dinarina, A., Lipkowitz, S., and Dikic, I. (2002). CIN85 participates in Cbl-b-mediated down-regulation of receptor tyrosine kinases. J. Biol. Chem. 277, 39666-39672. [DOI] [PubMed] [Google Scholar]
- Take, H., Watanabe, S., Takeda, K., Yu, Z.X., Iwata, N., and Kajigaya, S. (2000). Cloning and characterization of a novel adaptor protein, CIN85, that interacts with c-Cbl. Biochem. Biophys. Res. Commun. 268, 321-328. [DOI] [PubMed] [Google Scholar]
- Thien, C.B., and Langdon, W.Y. (2001). Cbl: many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell. Biol. 2, 294-307. [DOI] [PubMed] [Google Scholar]
- Tsukazaki, T., Chiang, T.A., Davison, A.F., Attisano, L., and Wrana, J.L. (1998). SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell 95, 779-791. [DOI] [PubMed] [Google Scholar]
- Turner, C.E., and Brown, M.C. (2001). Cell motility: ARNO and ARF6 at the cutting edge. Curr. Biol. 11, R875-R877. [DOI] [PubMed] [Google Scholar]
- Watanabe, S., Take, H., Takeda, K., Yu, Z.X., Iwata, N., and Kajigaya, S. (2000). Characterization of the CIN85 adaptor protein and identification of components involved in CIN85 complexes. Biochem. Biophys. Res. Commun. 278, 167-174. [DOI] [PubMed] [Google Scholar]
- Waterman, H., and Yarden, Y. (2001). Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS. Lett. 490, 142-152. [DOI] [PubMed] [Google Scholar]
- Whitmarsh, A.J., and Davis, R.J. (1998). Structural organization of MAP-kinase signaling modules by scaffold proteins in yeast and mammals. Trends. Biochem. Sci. 12, 481-485. [DOI] [PubMed] [Google Scholar]
- Wigge, P., Kohler, K., Vallis, Y., Doyle, C.A., Owen, D., Hunt, S.P., and McMahon, H.T. (1997). Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis. Mol. Biol. Cell 8, 2003-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich, L., Goher, A., Farago, A., Downward, J., and Buday, L. (1999). Requirement of multiple SH3 domains of Nck for ligand binding. Cell Signal. 11, 253-262. [DOI] [PubMed] [Google Scholar]
- Yamabhai, M., Hoffman, N.G., Hardison, N.L., McPherson, P.S., Castagnoli, L., Cesareni, G., and Kay, B.K. (1998). Intersectin, a novel adaptor protein with two Eps15 homology and five Src homology 3 domains. J. Biol. Chem. 273, 31401-31407. [DOI] [PubMed] [Google Scholar]