Abstract
Aims
Prior studies have reported that elevated concentrations of several plasma amino acids (AA) in plasma, particularly branched chain (BCAA) and aromatic AA predict the onset of type 2 diabetes. We sought to test the hypothesis that circulating BCAA, aromatic AA and related AA metabolites decline in response to the use of insulin sensitizing agents in overweight/obese adults with impaired fasting glucose or untreated diabetes.
Methods
We performed a secondary analysis of a randomized, double-blind, placebo, controlled study conducted in twenty five overweight/obese (BMI~30 kg/m2) adults with impaired fasting glucose or untreated diabetes. Participants were randomized to three months of pioglitazone (45 mg per day) plus metformin (1000 mg twice per day, N = 12 participants) or placebo (N = 13). We measured insulin sensitivity by the euglycemic-hyperinsulinemic clamp and fasting concentrations of AA and AA metabolites using ultra-pressure liquid chromatography tandem mass spectrometry before and after the three-month intervention.
Results
Insulin sensitizer therapy that significantly enhanced insulin sensitivity reduced 9 out of 33 AA and AA metabolites measured compared to placebo treatment. Moreover, insulin sensitizer therapy significantly reduced three functionally clustered AA and metabolite pairs: i) phenylalanine/tyrosine, ii) citrulline/arginine, and iii) lysine/α-aminoadipic acid.
Conclusions
Reductions in plasma concentrations of several AA and AA metabolites in response to three months of insulin sensitizer therapy support the concept that reduced insulin sensitivity alters AA and AA metabolites.
Key Terms: Insulin Resistance, Biomarkers, Metabolomics, Obesity, Diabetes
1. INTRODUCTION
In people with insulin resistance plasma concentrations of branched chain amino acids (BCAA; leucine, isoleucine, and valine), aromatic amino acids (AAA; phenylalanine and tyrosine), and amino acid (AA) metabolites are elevated [1–4]. Moreover, BCAA were recently reported to be elevated in metabolically well compared to metabolically unwell adults, independent of obesity [5,6]. Importantly, elevations in BCAA and AAA occur approximately a decade prior to the development of type 2 diabetes (T2D), which has led to the proposal that these AA are useful predictors for future T2D [7]. In vitro, supraphysiolgical doses of AA impair insulin signaling at several steps critical for glucose uptake [8] and glycogen synthesis [9]. In vivo, the infusion of AA, especially BCAA, reduces insulin sensitivity [10–12]. BCAA deprivation in rodents also improves insulin sensitivity in insulin resistant mice [13]. On the other hand, the rapid increase in insulin sensitivity and improvement in glucose homeostasis following weight loss surgery coincides with reductions in circulating BCAA, AAA, and several AA metabolites [14–16]. In addition, three months of metformin monotherapy has been reported to reduce AAA in patients with T2D [17]. Therefore, monitoring changes in BCAA, AAA, and AA metabolites may provide insight into the early responses to T2D therapies [18]. The close association between elevations AA, especially BCAA and AAA, and insulin resistance has led to the hypothesis that elevations in BCAA and AAA cause insulin resistance [1]. However, another equally plausible hypothesis is that reductions in the activity of branched chain ketoacid dehydrogenase (BCKD) and tyrosine aminotransferase (TAT) in states of insulin resistance lead to increased tissue and circulating BCAA and AAA concentrations, respectively [19].
We proposed that pharmacologically improving insulin sensitivity would result in reductions in plasma concentrations of BCAA, AAA, or other AA metabolites in insulin-resistant adults. Insulin is a potent antiproteolytic hormone that results in hypoaminoacidemia when systemically administered [20–22] due to a concomitant reduction in skeletal muscle protein breakdown [23] and increased AA transport into the muscle [24]. Previous studies have also shown that insulin deprivation in c-peptide negative adults with type 1 diabetes increases protein turnover (i.e., synthesis and breakdown) [25], AA oxidation [25], and transamination rates of leucine [26], and is accompanied by large increases in circulating AA concentrations, especially BCAA [26]. Moreover, protein degradation in multiple tissues is increased in people with T2D compared to non-diabetic controls under hyperglycemic conditions [27]. As stated previously, reductions in BCKD activity, TAT activity, and amino acid oxidation in states of insulin resistance lead to increased tissue and circulating BCAA and AAA concentrations [19]. Reductions in the degradation of BCAA also could lead to reductions in branched chain α-ketoacids concentrations and the synthesis of monomethyl branched-chain fatty acids with adipose tissue and perhaps muscle [28]. Monomethyl branched-chain fatty acids have recently been demonstrated to positively correlate with insulin sensitivity [28]. Together these data support the premise that a reduction in insulin action contributes to elevations in tissue and circulating BCAA, AAA, and AA metabolite concentrations. Since it has been reported that BCAA, especially leucine, can inhibit insulin action [1] there may be a type of feed-forward relationship between insulin resistance and the concentration of circulating AA.
The current study was designed to determine whether chronically enhancing insulin sensitivity reduces plasma BCAA, AAA, and AA metabolites in insulin resistant adults. Specifically, we investigated the impact of three months of dual insulin sensitizer therapy (45 mg pioglitazone per day plus 1000 mg metformin twice per day) in overweight/obese adults who had impaired fasting glucose or untreated diabetes.
2. METHODS
The Mayo Clinic’s Intuitional Review Board approved the study protocol in accordance to the principles of the Declaration of Helsinki. All participants provided written and informed consent prior to participation.
2.1 Study Design and Participants
We previously reported the overall study design for the parent study [29]. The current report primarily examines the effect of three months of insulin sensitizer therapy on plasma concentrations of BCAA, AAA, and AA metabolites in overweight/obese adults with fasting hyperglycemia, defined as either impaired fasting glucose or untreated diabetes [29]. Briefly, 25 drug naïve, Northern European American participants with fasting blood glucose concentrations of 108–180 mg/dL were randomized to receive either 45 mg of pioglitazone per day plus 1 g of metformin twice per day (n=12) or placebo (n=13) for 12 weeks. We chose metformin based on its proven effect on hepatic insulin sensitivity and pioglitazone based on its effect on peripheral insulin sensitivity. Current use of hypoglycemic medications excluded participants from the present study.
Insulin sensitivity was measured using a hyperinsulinemic-euglycemic clamp as previously described [29]. In brief, participants received a continuous infusion of insulin (1.5 mU/kg-FFM/min) for 8 hours. Participants also received a continuous infusion of AA (5.4% NephrAmine, B. Braun Medical Inc.) to prevent insulin-induced hypoaminoacidemia. The AA analyses in the current report were performed on fasting blood samples that were acquired before any infusions began. Arterialized-venous blood was used to measure plasma glucose concentrations every 10 min using an automated glucose analyzer (GM9, Analox Instruments, London, UK). The glucose infusion rate (GIR) (40% dextrose) was adjusted to maintain euglycemia [~5.0 mmol/L (90 mg/dL)] during the 8h insulin infusion. Insulin sensitivity was defined as the steady-state glucose infusion rate (GIR, μmol/kg-FFM/min) achieved during the last 2h of the 8h hyperinsulinemic-euglycemic clamp [30]. Fasting glucose and insulin concentrations were used to calculate the Quantitative Insulin Sensitivity Check Index (QUICKI = 1/log[glucose]+log[insulin]) [31]. Fasting plasma AA and AA metabolite concentrations were measured using ultra-pressure liquid chromatography tandem mass spectrometry (UPLC-MS/MS) in the Mayo Clinic Metabolomics Core as previously described [32]. In brief, we drew arterialized-venous blood samples into plasma EDTA tubes, processed, and stored them at −80°C until analysis. Storage of plasma/serum samples at −80°C for qualitative and quantitative metabolomics using mass spectrometry are routinely done, demonstrating little to no evidence of storage related decay including samples stored greater than 20 years [7,33]. The frozen samples were thawed on ice and spiked with C13-labeled internal standards and processed for analysis as previously described [32]. Amino acids in particular are relatively stable for several years, with the exception of their keto-acids (e.g., KIC), which we have not included in the present analysis. Subsequently, we separated the samples using an Acquity UPLC system, followed by mass detection using a TSQ Ultra Quantum from Thermo Finnigan running in ESI positive mode as previously described [32]. We calculated the AA and AA metabolites concentrations using 10 point standard curves as previously described [32]. We handled the pre- and post-intervention samples similarly and analyzed them in the same run. The reproducibility of the UPLC/MS/MS method in the MCRMC is excellent (average CV~9.5%) [32].
Additionally, we measured plasma AA and AA metabolites in samples collected during a second study in which an acute (7-hours) infusion of insulin (1.5 mU/kg FFM/min) with glucose replacement and without AA replacement compared to saline alone (n = 9 each, both groups 5 M/4 F) [34]. The original goal of that protocol was to assess the effect of insulin on muscle protein metabolism in young, healthy, normal weight men and women. We previously reported that the plasma concentrations of 16 AA (out of 17 measured) declined during the 7 hour insulin infusion [34]. For the current report, we expanded the panel of measured AA and AA metabolites. The rationale for including those analyses in the current report is to help determine whether changes in AA and AA metabolites following insulin sensitizer therapy were attributable to the improvement of insulin action or to the direct effect of the pharmacological agents.
2.2 Metabolic Assessment
All measurements for both studies were performed in the Mayo Clinic’s Center for Translational Science Activities’ Clinical Research Unit (CRU). Body composition was measured using dual-energy x-ray absorptiometry (Lunar DPX-L; Lunar Radiation, Madison, WI).
2.3 Statistical Methods
All statistical analyses were performed using SAS software (Version 9.3, Cary, NC). Results are presented as medians with interquartile ranges. Spearman rank correlations (ρ) were used to examine univariate associations between AA/AA metabolites and the GIR at baseline. Wilcoxon Rank Sum tests were used to determine significant differences between treatment groups at baseline and between treatment groups with respect to their pre- to post-intervention change scores (Δ). The sample size of insulin sensitizer study (n=25) was not conducive to principal components analysis, but we wanted to test for systematic changes over the entire panel of AA/AA metabolites between groups. To do this, O’Brien’s nonparametric global test statistic was used [35]. This test is an overall (omnibus) test for differences in a composite score based on the ranked order of Δ for each AA/AA metabolite in the panel. Briefly, the Δ observed with treatment for each AA/AA metabolite was ranked either ascending or descending order based on the correlation with the Δ in GIR. The sum of the ranks for each AA/AA metabolite by person was used as the dependent variable for the global test statistic. The SAS MACRO %GlobTest was used for implementation of the global test [36]. Statistical significance was set at α=0.05 for all comparisons.
3. RESULTS
We previously reported the clinical and demographic characteristics of participants [29]. In brief, the participants had a mean age of ~52 y, BMI of ~31 kg/m2, body fat of ~45%, fasting glucose of ~126 mg/dL, fasting insulin of ~13 μU/mL and steady-state glucose infusion rate during the hyperinsulinemic clamp of ~25 μmol/kgFFM/min [29]. All of these outcomes are consistent with a phenotype of overweight/obesity and T2D or high risk for T2D.
Spearman rank correlations (ρ) revealed inverse correlations between the glucose infusion rate and several AA/AA metabolites at baseline. As expected, the glucose infusion rate was inversely correlated with the BCAA: leucine (ρ=−0.52, p=0.007), isoleucine (ρ=−0.41, p=0.043), and valine (ρ=−0.51, p=0.010); and AAA: phenylalanine (ρ=−0.70, p=0.007), tyrosine (ρ=−0.55, p=0.005). In addition, the glucose infusion rate was also inversely correlated with methionine (ρ=−0.56, p=0.004), tryptophan (ρ=−0.47, p=0.018), lysine (ρ=−0.41, p=0.044), and α-aminoadipic acid (ρ=−0.49, p=0.012).
Table 1 presents the baseline fasting plasma AA/AA metabolite concentrations by treatment group. At baseline, there were no significant differences between treatment groups for any of the fasting plasma AA/AA metabolites concentrations (p>0.05).
Table 1.
Amino acid and amino acid metabolite concentrations (μmol/L) at baseline stratified by treatment group. Data presented as median (interquartile range).
| Placebo | Pioglitizone plus Metformin | Wilcoxon-Rank Sum* P-Value | |
|---|---|---|---|
| N | 13 | 12 | - |
| Leucine | 141.4 (123.3, 189.4) | 142.2 (122.4, 169.9) | 0.683 |
| Isoleucine | 70.6 (59.3, 93.4) | 66.4 (56.9, 87.2) | 0.497 |
| Valine | 276.7 (254.4, 324.9) | 260.2 (241.5, 334.1) | 0.532 |
| Phenylalanine | 59.4 (57.4, 64.9) | 58.0 (52.3, 63.9) | 0.765 |
| Tyrosine | 74.3 (56.8, 88.1) | 74.6 (67.0, 91.8) | 0.369 |
| Lysine | 193.3 (170.8, 228.6) | 207.1 (196.2, 238.1) | 0.201 |
| Arginine | 75.6 (68.3, 94.1) | 88.8 (75.0, 99.1) | 0.462 |
| Methionine | 20.8 (16.8, 26.4) | 22.0 (19.6, 26.2) | 0.314 |
| Glutamine | 590.4 (268.5, 769.4) | 616.7 (249.2, 740.8) | 0.807 |
| Threonine | 112.6 (108.0, 151.1) | 115. 8 (101.6, 151.9) | >0.999 |
| Alanine | 384.9 (319.5, 409.0) | 359.7 (298.4, 393.9) | 0.644 |
| Aspartic Acid | 2.1 (1.8, 2.8) | 2.1 (1.8, 2.4) | 0.765 |
| Glutamic Acid | 63.5 (40.1, 71.4) | 59.5 (47.1, 68.1) | 0.849 |
| Serine | 84.7 (76.4, 106.7) | 78.5 (72.0, 94.9) | 0.644 |
| Glycine | 153.9 (137.3, 191.6) | 157.2 (134.7, 218.8) | 0.892 |
| Histidine | 76.1 (72.7, 83.7) | 69.3 (58.9, 77.8) | 0.121 |
| 1-Methylhistidine | 16.2 (10.9, 18.3) | 8.1 (6.9, 10.9) | 0.077 |
| 3-Methylhistidine | 5.7 (5.1, 6.7) | 4.7 (4.3, 6.1) | 0.221 |
| β-Alanine | 2.7 (2.0, 3.2) | 2.5 (2.1, 2.8) | 0.683 |
| α-Aminoadipic-acid | 1.2 (0.7, 1.3) | 1.0 (0.7, 1.3) | 0.369 |
| β-Aminoisobutyric-acid | 0.9 (0.8, 1.0) | 0.8 (0.6, 1.3) | 0.573 |
| α-Amino-N-butyric-acid | 24.7 (19.9, 29.6) | 26.4 (22.2, 31.0) | 0.605 |
| Asparagine | 59.8 (52.5, 66.2) | 63.3 (54.3, 66.3) | 0.683 |
| Citrulline | 31.4 (28.6, 35.8) | 32.2 (25.5, 34.9) | 0.724 |
| Ornithine | 51.5 (37.9, 67.4) | 53.2 (44.7, 60.3) | 0.978 |
| Taurine | 46.0 (40.5, 49.6) | 40.6 (35.7, 47.3) | 0.242 |
| Ethanolamine | 6.0 (5.0, 7.2) | 6.2 (5.1, 6.9) | 0.978 |
| Sarcosine | 1.2 (1.1, 1.4) | 1.1 (1.0, 1.4) | 0.765 |
| Proline | 192.0 (152.8, 240.0) | 199.7 (176.6, 221.3) | 0.849 |
| Hydroxyproline | 7.3 (7.0, 9.4) | 7.3 (6.2, 8.2) | 0.463 |
| Hydroxylysine 2 | 0.4 (0.3, 0.5) | 0.3 (0.2, 0.4) | 0.849 |
| Cystine | 76.1 (54.0, 82.5 | 90.8 (32.6, 106.6) | 0.369 |
| Tryptophan | 57.6 (53.2, 59.5) | 60.9 (50.0, 65.5) | 0.605 |
Wilcoxon Rank Sum, normal approximation
As previously reported [29] three months of insulin sensitizer therapy reduced fasting plasma glucose and insulin concentrations (all p < 0.001). Insulin sensitizer therapy also increased insulin sensitivity estimated the steady-state glucose infusion rate during the hyperinsulinemic-euglycemic clamp (p < 0.001) as previously reported [29].
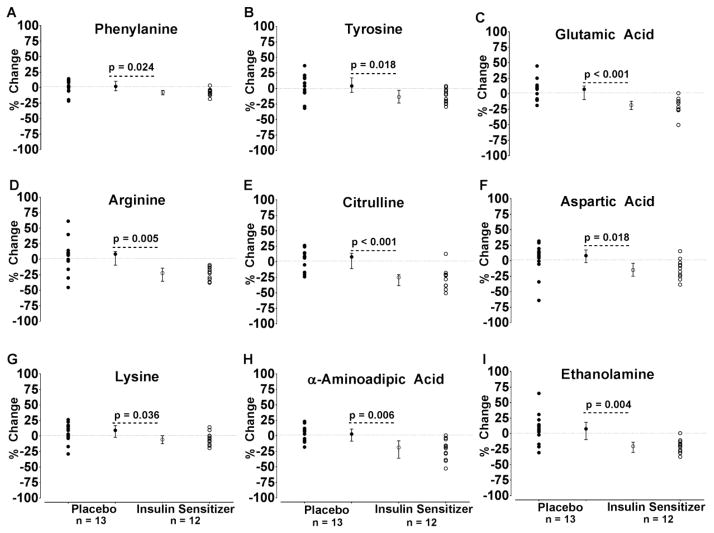
In response to three months of insulin sensitizer therapy plasma BCAA concentrations were not significantly reduced as hypothesized (Table 2). However, the plasma phenylalanine and tyrosine concentrations were reduced (Figure 1A–B and Table 2) as were the plasma concentrations of glutamic acid, arginine, citrulline, aspartic acid, lysine, α-aminoadipic acid, and ethanolamine (Figure 1C–I and Table 2). In contrast, insulin sensitizer therapy increased the plasma serine and glycine concentrations (Table 2). When the O’Brien’s global test statistic was applied to the entire AA/AA metabolite panel to determine the overall effect of treatment, there was a highly significant difference between groups (p<0.001) (Table 2). Moreover, the global rank score was inversely associated with change in glucose infusion rate (ρ =−0.74, p<0.001).
Table 2.
Pre-to-post intervention change in amino acid and amino acid metabolite concentrations (μmol/L) in response to three months of insulin sensitizer therapy or placebo. Data presented as median (interquartile range).
| Placebo | Pioglitizone plus Metformin | Wilcoxon-Rank Sum* P-Value | |
|---|---|---|---|
| n | 13 | 12 | - |
| Leucine | 13.3 (8.5, 22.8) | 6.3 (−3.9, 16.8) | 0.369 |
| Isoleucine | 8.1 (0.7, 16.1) | 8.6 (−4.2, 12.3) | 0.644 |
| Valine | 22.7 (−9.9, 39.3) | 1.5 (−5, 38.6) | 0.683 |
| Phenylalanine | 1.2 (−2.8, 5.4) | −4.5 (−6.5, −2.6) | 0.024 |
| Tyrosine | 3.2 (−4, 9.1) | −9.7 (−15.1, −4) | 0.018 |
| Lysine | 17.5 (−1.5, 24.3) | −11.5 (−31.4, 0.5) | 0.036 |
| Arginine | 6.1 (−2.2, 10.1) | −21.0 (−31.8, −10.9) | 0.005 |
| Methionine | 1.7 (−0.4, 3.1) | 2.3 (−0.1, 3.1) | 0.765 |
| Glutamine | 0.7 (−25.0, 44.1) | 24.3 (−74.5, 87.1) | 0.644 |
| Threonine | 2.4 (−11, 13.8) | 3.0 (0.1, 21.7) | 0.289 |
| Alanine | −2.7 (−35.9, 42.9) | 9.5 (−26.9, 35.4) | 0.724 |
| Aspartic Acid | 0.2 (0.0, 0.4) | −0.3 (−0.6, −0.1) | 0.018 |
| Glutamic Acid | 4.3 (−5, 6.5) | −10.1 (−15.9, −5.6) | <0.001 |
| Serine # | −3.1 (−8.1, 7.1) | 14.6 (9.5, 22.3) | 0.002 |
| Glycine # | −0.3 (−14.7, 14.3) | 26.2 (10.6, 42.8) | 0.013 |
| Histidine # | 0.6 (−2, 6) | 6.4 (−0.7, 9.5) | 0.265 |
| 1-Methylhistidine | 0.3 (−2.6, 5.3) | 0.0 (−3, 2.3) | 0.644 |
| 3-Methylhistidine | −0.1 (−0.3, 0.7) | −0.1 (−0.7, 0.2) | 0.765 |
| β-Alanine | 0.2 (−0.1, 0.3) | −0.1 (−0.3, 0.3) | 0.724 |
| α-Aminoadipic-acid | 0.0 (−0.1, 0.1) | −0.2 (−0.3, −0.1) | 0.006 |
| β-Aminoisobutyric-acid # | 0.0 (−0.1, 0.1) | 0.2 (0, 0.2) | 0.061 |
| α-Amino-N-butyric-acid # | 0.1 (−2.5, 1.3) | 1.7 (−0.6, 6.9) | 0.135 |
| Asparagine # | 3.5 (−2.5, 9.7) | 10.0 (2.1, 13.3) | 0.201 |
| Citrulline | 2.9 (−1.3, 3.9) | −8.9 (−12.2, −7.1) | <0.001 |
| Ornithine # | 2.0 (−3, 7.4) | −0.1 (−4, 6.6) | 0.849 |
| Taurine | −1.9 (−6.6, 4.9) | −2.9 (−5.2, 2.1) | 0.978 |
| Ethanolamine | 0.4 (−0.1, 0.9) | −1.3 (−1.6, −0.9) | <0.001 |
| Sarcosine | 0.1 (0.0, 0.3) | 0.0 (0.0, 0.1) | 0.369 |
| Proline # | 10.2 (−13.2, 29.6) | 37.7 (8.6, 63) | 0.097 |
| Hydroxyproline | 0.2 (−0.2, 0.4) | 0.6 (−0.5, 2) | 0.683 |
| Hydroxylysine 2 # | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.1) | 0.497 |
| Cystine # | 2.2 (−11.1, 4.9) | −2.3 (−6.9, 5.3) | >0.999 |
| Tryptophan | −0.9 (−2, 3.4) | −3.9 (−6.8, −1.4) | 0.053 |
| Global Test Statistic, sum of rankings across panel of 33 AA/AA metabolites# | 487 (456, 533) | 356 (323, 387) | <0.001 |
Wilcoxon Rank Sum, normal approximation
These AA/AA metabolites were sorted in descending order when computing the rankings for the global test statistics. The remaining AA/AA metabolites were sorted in ascending order when computing the rankings for the global test statistics. A lower mean value for the global test statistic in the Pioglitizone plus Metformin group is interpretable as this group having larger decreases in the panel AA/AA metabolites over the study period (note: the largest decrease in an AA would results in a ranking of 1).
Fig. 1.
Effects of three months of insulin sensitizer therapy on amino acids and amino acid metabolites. Twenty five overweight/obese (BMI~30 kg/m2) adults with impaired fasting glucose or untreated diabetes were randomized to three months of pioglitazone (45 mg per day) plus metformin (1000 mg twice per day, N = 12 participants) or placebo (n=13). Compared to placebo, insulin sensitizer therapy increased fasting plasma concentrations of phenylalanine (A), tyrosine (B), glutamic acid (C), arginine (D), citrulline (E), aspartic acid (F), lysine (G), α-aminoadipic acid (H), and ethanolamine (I). The values represent the individual percent changes for the insulin sensitizer (open circles) and placebo (closed circles) groups. In addition, the median percent change and interquartile ranges are also provided. P-Values are from the Wilcoxon Rank Sum Test for the difference between the absolute change scores using the normal approximation.
Spearman rank correlations (ρ) between the absolute pre-to-post intervention change in the glucose infusion rate and the absolute pre-to-post intervention changes in the plasma BCAA and AAA in the insulin sensitizer treated group revealed no statistically significant associations. Likewise, the Spearman rank correlations (ρ) between the relative pre-to-post intervention percent change in the glucose infusion rate and the relative pre-to-post intervention percent changes in the plasma BCAA and AAA in the insulin sensitizer treated group revealed also no statistically significant associations. These data indicate that improvements in insulin sensitivity in response to dual insulin sensitizer therapy can be dissociated from changes in BCAA and AAA.
As described in the reference study on the effects of acute insulin infusion [34],. plasma concentrations of leucine, isoleucine, valine, phenylalanine, tyrosine, lysine, arginine, methionine, glutamine, alanine, aspartic acid, glutamic acid, serine, glycine, histidine, and 3-methylhistidine declined (all p<0.05) in young adults during the 7 hour insulin infusion compared to a control group receiving saline. For the current report we expanded the panel of metabolites measured in that study and found that compared the saline infusion, the insulin infusion reduced plasma concentrations of citrulline [−56% (−45%, −37%) vs. −8% (−11%, −6%)], proline [−37% (−39%, −36%) vs. −15% (−16%, −7%)], ornithine [−41% (−44%, −37%) vs. −4% (−7%, −4%)], hydroxyproline [−30% (−35%, −28%) vs. −8% (−18%, −4%)], α-aminoadipic acid [−34% (−41%, −33%) vs. 2% (−13%, 8%)], taurine [−13% (−23%, −6%) vs. 3% (−2%, 5%)], hydroxlysine-2 [−6% (−11%, −3%) vs. 6% (3%, 9%)], β-aminoisobutyric acid [−49% (−52%, −46%) vs. 21% (7%, 60%)], asparagine [−36% (−43%, −35%) vs. −11% (−15%, −7%)], and α-amino-n-butyric acid [−60% (−62%, −58%) vs. 13% (2%, 27%)] (all p<0.05).
DISCUSSION
The present investigation demonstrates that three months of dual insulin sensitizer therapy (metformin plus pioglitazone) on average reduces fasting plasma AA and AA metabolite concentrations in overweight/obese adults with fasting hyperglycemia or previously untreated T2D. The major new finding was that insulin sensitizer therapy resulted in reduced concentrations of three functional pairs of AA/AA metabolites: 1) phenylalanine and tyrosine, 2) lysine and α-aminoadipic acid, and 3) arginine and citrulline. The same AA/AA metabolites also declined in response to a single 7-hour insulin infusion. Together, these findings support the premise that enhanced insulin action per se is responsible for the changes in these functional pairs of AA/AA metabolites, rather than being attributable to a more direct effect of the pharmacological agents, pioglitazone and/or metformin.
An important finding of the present investigation is that dual insulin sensitizer therapy in overweight/obese adults with fasting hyperglycemia or previously untreated T2D reduces plasma concentrations of both phenylalanine and tyrosine (Figure 1A–B and Table 2). The combination of these two insulin sensitizers is commonly used in T2D. Of interest, increase in insulin sensitivity was not significantly correlated with the decline in fasting plasma phenylalanine or tyrosine. This may indicate that the reduction in these amino acids in response to improvements in insulin sensitivity has a floor effect and not a dose response. A previous report demonstrated that three months of metformin monotherapy reduces fasting AAA in people with T2D [17]. Moreover, three months of pioglitazone has also been shown to reduce fasting AAA concentrations in people with NASH [37].
We hypothesized that insulin sensitizer-induced improvement in insulin sensitivity would translate into reduced fasting concentrations of plasma BCAA. Although insulin sensitivity increased, plasma BCAA concentrations were unchanged (Table 2). Skeletal muscle protein breakdown, adipose tissue degradation of BCAA by BCKD, and subsequent oxidation of branched chain ketoacids (BCKA) by mitochondria play important roles in regulating fasting plasma BCAA concentrations [19,38]. Plasma BCAA concentrations may not have changed due to opposing effects of metformin and pioglitazone. It was shown, for example that two days of metformin therapy increased plasma BCAA in insulin resistant adults [18]. In contrast, pioglitazone, a peroxisome proliferator-activated receptor-γ (PPARγ) agonist, promotes the degradation of BCAA by increasing the activity of BCKD in adipose tissue [39] and has also been shown to reduce fasting plasma BCAA in obese patients with non-alcoholic fatty liver disease [37]. Six months of rosiglitazone, another PPARγ agonist, also reduces BCAA in patients with T2D [40]. It is also feasible that the decline in fasting insulin offsets the increase in insulin sensitivity and insulin-mediated suppression skeletal muscle protein breakdown. In support of this possibility, the insulin sensitizer treatment had no effect on plasma 3-methylhistidine, a byproduct of myofibrillar protein breakdown. The decline in fasting insulin could also reduce the uptake of amino acids into peripheral tissues. Indeed, elevations in insulin secretion associated with sitagliptin therapy lead to reduced circulating BCAA concentrations following a mixed meal in patients with T2D [41]. Our findings are also consistent with a previous report in which it was shown that an alteration in insulin action on glucose metabolism following insulin treatment may not be accompanied by a change in the insulin effect on protein metabolism [42]. Other plausible explanations for the lack of effect of insulin sensitizer therapy on BCAA concentrations include an adaptation of BCAA metabolism to chronically high circulating insulin in people with insulin resistance, or a blunted effect of insulin sensitizers on BCAA transamination, a process that is typically increased in people with diabetes [26,43]. A change in dietary protein intake could also be impact circulating BCAA [44] but participants in the current study consumed a standard diet for three days prior to both the baseline and post-intervention studies to minimize the effect of diet as a confounding variable.
Although some previous studies showed that pharmacological doses of AA, especially BCAA, reduced in vitro and in vivo insulin action, BCAA in the physiological range appear to play a critical role in maintaining or enhancing insulin sensitivity. For example, dietary leucine supplementation enhanced insulin action in high-fat fed rodents [45,46]. Leucine, and its metabolite β-hydroxy-methylbutyrate (HMB), can act synergistically with pharmacological agents to increase their potency [47–49]. When combined with metformin, leucine and HMB improved insulin sensitivity by activating AMPK and SIRT1 [47]. Leucine content is high in dairy products and has been identified as a key component for lowering the risk for T2D [50].
In the present investigation insulin sensitizer therapy reduced the concentration of lysine, and its metabolite α-aminoadipic acid (Figures 1G and 1H and Table 2). In the presence of hyperglycemia, lysine can be converted to allysine through a non-enzymatic Strecker-type reaction [51]. Oxidative stress and/or low concentrations of the anti-oxidant glutathione lead to further oxidation of allysine to α-aminoadipic acid [52]. As a result, α-aminoadipic acid has been proposed as a potential biomarker of protein oxidation in T2D [53], is elevated in people with diabetes [53], and is a predictor for the future development of T2D [33]. We previously demonstrated that insulin withdrawal for 8 hours increased oxidative stress, which was reflected by an accelerated oxidation of lysine to allysine in de novo synthesized apoA1 [54] and a concomitant elevation in α-aminoadipic acid in people with type 1 diabetes [32]. Therefore, we speculate that the insulin sensitizer induced reductions in α-aminoadipic acid reflect reductions in overall oxidative stress mediating further improvements in insulin sensitivity.
Arginine and citrulline concentrations declined in response to insulin sensitizer therapy (Figure 1D–E and Table 2), but the underlying mechanism(s) are not yet clear. Arginine and citrulline are key components of nitric oxide biosynthesis and the urea. Reductions in citrulline likely reflect insulin sensitizer-induced reductions in urea biosynthesis, secondary to reductions in gluconeogenesis [4,55]. Insulin acutely reduces ornithine level affecting the catabolic aspect of arginine metabolism consistent with the anti-catabolic effect of insulin on AA metabolism.
Insulin sensitizer therapy also reduced the concentration of glutamic acid/glutamate (Figure 1C and Table 2). Glutamic acid/glutamate is an essential precursor for gluconeogenesis so the elevation in glutamic acid/glutamate may contribute to the development of fasting hyperglycemia in insulin-resistant states. Likewise, the reduced fasting glutamic acid/glutamate following pioglitazone and metformin treatment could contribute to a concomitant reduction in gluconeogenesis. Metformin has previously been shown to reduce gluconeogenesis and hepatic glucose production [56] and this effect may be at least partially mediated via reduction in glutamic acid/glutamate concentration, although gluconeogenesis is highly regulated by the rate-limiting enzyme, phosphoenolpyruvate carboxykinase. There is also recent evidence demonstrating that metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerol phosphate dehydrogenase [57]. Taken together, glutamic acid/glutamate may play a permissive role in the regulation of gluconeogenesis.
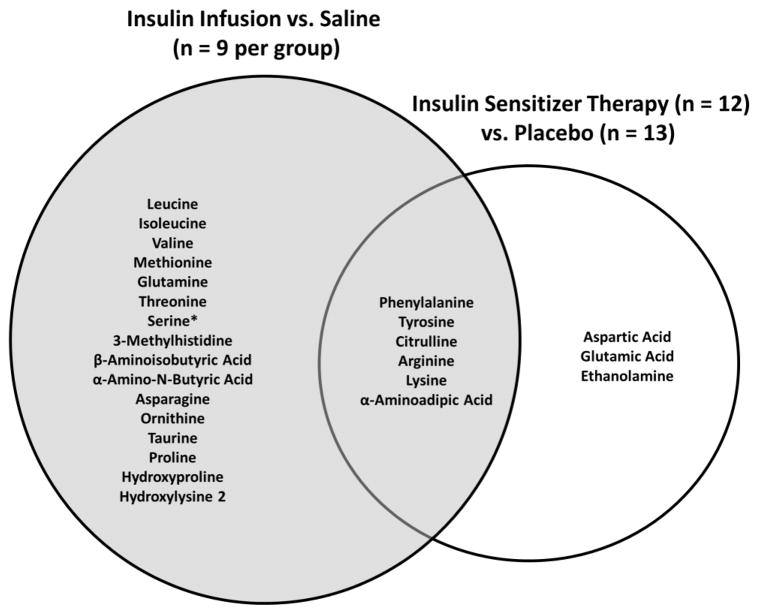
We found that nine AA or AA metabolites were reduced in response to three months of insulin sensitizer therapy, whereas in the reference study 22 AA or AA metabolites declined in response to an acute insulin infusion (Figure 2). The higher number of changes in the reference study may be because the participants were all young, healthy adults with high insulin sensitivity who were studied during 7 hours of insulin infusion without a meal or AA supplement. In contrast, the participants undergoing insulin sensitizer treatment were older, had higher body fat, lower insulin sensitivity and were studied in the overnight fasted state. Nevertheless, six AA and AA metabolites were reduced in both studies, and represent three functional pairs: i) phenylalanine and tyrosine, ii) lysine and α-aminoadipic acid, and iii) arginine and citrulline.
Fig. 2.
Venn diagram for plasma amino acid and amino acid metabolites that were reduced in response to an acute or chronic increase in insulin action. Compared to saline, 22 amino acids and amino acid metabolites were reduced in response to an acute infusion of insulin in healthy young adults (n = 9 per group) (grey circle). Compared to placebo, nine amino acids and amino acid metabolites were reduced in response to three months of insulin sensitizer therapy in overweight/obese (BMI~30 kg/m2) adults with impaired fasting glucose or untreated diabetes (n = 13, placebo; n = 12 insulin sensitizer) (white circle). Three functional pairs of amino acids and amino acid metabolites (phenylalanine/tyrosine, lysine/α-aminoadipic acid, and arginine/citrulline) were reduced in response to both the acute infusion of insulin as well as three months of insulin sensitizer therapy. Finally, serine concentrations were reduced in response to the acute infusion of insulin, while it was increased in response to three months of insulin sensitizer therapy.
Among the strengths of the present study was that it was placebo controlled and double blinded [29]. Secondly, the insulin sensitizer therapy substantially improved insulin sensitivity in all treated participants. A potential limitation is that insulin sensitizer therapy could have altered the plasma AA and AA metabolite concentrations both directly and indirectly. However, the analysis of samples from the reference insulin infusion study [34] clearly demonstrated that changes in AA metabolites can occur if insulin action is acutely increased. Additionally, since we used both pioglitazone and metformin together their separate effects on the study outcomes have yet to be determined.
In summary, three months of insulin sensitizer therapy with metformin and pioglitazone in a double-blind trial results in a global reduction in plasma AA and AA metabolite concentrations. Elevations in BCAA, AAA, and AA metabolites have the potential to be used as a biomarker for both diabetes risk and monitoring the effects of strategies designed to lower that risk. Importantly, insulin sensitizer therapy significantly reduced three functional pairs of AA/AA metabolites (phenylalanine/tyrosine, lysine/α-aminoadipic acid, and arginine/citrulline). The long-term clinical impact of those changes has not yet been determined. It remains to be determined whether these metabolites could predict individuals who are or are not benefiting from the insulin sensitizing therapy and therefore more or less likely to develop T2D.
Acknowledgments
We acknowledge the Clinical Research Unit staff, Maureen Bigelow, RN, Jill Schimke, Kate Klaus, Dawn Morse, Roberta Soderberg, Deborah Sheldon, and Melissa Aakree for their skilled support. The project described was supported by National Institutes of Health R01-AG09531 and R01-DK41973 the Dole-Murdock Professorship (to K.S.N.). The project described was also supported by the National Center for Research Resources, UL1-RR024150 and KL2-RR024151, which is now the National Center for Advancing Translational Sciences, UL1-RR024150 and KL2-RR024151. The project described was also supported by U24DK100469 from the National Institute of Diabetes and Digestive and Kidney Diseases and originates from the National Institutes of Health Director’s Common Fund also supported the study.
Footnotes
Trial Registration: clinicaltrials.gov identifier: NCT00443755
Author Contributions
BAI collected data, interpreted data and wrote the manuscript. REC performed biostatistical analyses, interpreted data and revised manuscript. MS, LT, KRS, RB designed study, collected data, and revised manuscript. AW, HS, HK collected data. KSN designed study, supervised collection and analyses of data, interpreted data, and revised manuscript. All authors provided critical feedback to the manuscript.
Disclosure Summary
We have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell metabolism. 2009 Apr;9(4):311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tai ES, Tan ML, Stevens RD, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010 Apr;53(4):757–767. doi: 10.1007/s00125-009-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huffman KM, Shah SH, Stevens RD, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009 Sep;32(9):1678–1683. doi: 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaham O, Wei R, Wang TJ, et al. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214. doi: 10.1038/msb.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batch BC, Shah SH, Newgard CB, et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism. 2013 Jul;62(7):961–969. doi: 10.1016/j.metabol.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badoud F, Lam KP, DiBattista A, et al. Serum and adipose tissue amino acid homeostasis in the metabolically healthy obese. J Proteome Res. 2014 Jul 3;13(7):3455–3466. doi: 10.1021/pr500416v. [DOI] [PubMed] [Google Scholar]
- 7.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011 Apr;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. The Journal of clinical investigation. 1998 Apr 1;101(7):1519–1529. doi: 10.1172/JCI1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krebs M, Krssak M, Bernroider E, et al. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes. 2002 Mar;51(3):599–605. doi: 10.2337/diabetes.51.3.599. [DOI] [PubMed] [Google Scholar]
- 10.Flakoll PJ, Kulaylat M, Frexes-Steed M, Hill JO, Abumrad NN. Amino acids enhance insulin resistance to exogenous glucose infusion in overnight-fasted humans. JPEN J Parenter Enteral Nutr. 1991 Mar-Apr;15(2):123–127. doi: 10.1177/0148607191015002123. [DOI] [PubMed] [Google Scholar]
- 11.Schwenk WF, Haymond MW. Decreased uptake of glucose by human forearm during infusion of leucine, isoleucine, or threonine. Diabetes. 1987 Feb;36(2):199–204. doi: 10.2337/diab.36.2.199. [DOI] [PubMed] [Google Scholar]
- 12.Robinson MM, Soop M, Sohn TS, et al. High insulin combined with essential amino acids stimulates skeletal muscle mitochondrial protein synthesis while decreasing insulin sensitivity in healthy humans. The Journal of clinical endocrinology and metabolism. 2014 Sep 15;:jc20142736. doi: 10.1210/jc.2014-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao F, Yu J, Guo Y, et al. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism. 2014 Jun;63(6):841–850. doi: 10.1016/j.metabol.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Laferrere B, Reilly D, Arias S, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011 Apr 27;3(80):80re82. doi: 10.1126/scitranslmed.3002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magkos F, Bradley D, Schweitzer GG, et al. Effect of Roux-en-Y Gastric Bypass and Laparoscopic Adjustable Gastric Banding on Branched-Chain Amino Acid Metabolism. Diabetes. 2013 Aug;62(8):2757–2761. doi: 10.2337/db13-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lips MA, Van Klinken JB, van Harmelen V, et al. Roux-en-Y Gastric Bypass Surgery, but Not Calorie Restriction, Reduces Plasma Branched-Chain Amino Acids in Obese Women Independent of Weight Loss or the Presence of Type 2 Diabetes. Diabetes Care. 2014 Dec;37(12):3150–3156. doi: 10.2337/dc14-0195. [DOI] [PubMed] [Google Scholar]
- 17.Huo T, Cai S, Lu X, Sha Y, Yu M, Li F. Metabonomic study of biochemical changes in the serum of type 2 diabetes mellitus patients after the treatment of metformin hydrochloride. Journal of pharmaceutical and biomedical analysis. 2009 May 1;49(4):976–982. doi: 10.1016/j.jpba.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Walford GA, Davis J, Warner AS, et al. Branched chain and aromatic amino acids change acutely following two medical therapies for type 2 diabetes mellitus. Metabolism. 2013 Dec;62(12):1772–1778. doi: 10.1016/j.metabol.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Advances in nutrition. 2011 Nov;2(6):445–456. doi: 10.3945/an.111.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukagawa NK, Minaker KL, Rowe JW, et al. Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. The Journal of clinical investigation. 1985 Dec;76(6):2306–2311. doi: 10.1172/JCI112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meek SE, Persson M, Ford GC, Nair KS. Differential regulation of amino acid exchange and protein dynamics across splanchnic and skeletal muscle beds by insulin in healthy human subjects. Diabetes. 1998;47:1824–1835. doi: 10.2337/diabetes.47.12.1824. [DOI] [PubMed] [Google Scholar]
- 22.Chow LS, Albright RC, Bigelow ML, Toffolo G, Cobelli C, Nair KS. Mechanism of insulin’s anabolic effect on muscle: measurements of muscle protein synthesis and breakdown using aminoacyl-tRNA and other surrogate measures. American journal of physiology. 2006 Oct;291(4):E729–736. doi: 10.1152/ajpendo.00003.2006. [DOI] [PubMed] [Google Scholar]
- 23.Fryburg DA, Jahn LA, Hill SA, Oliveras DM, Barrett EJ. Insulin and insulin-like growth factor-I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. The Journal of clinical investigation. 1995 Oct;96(4):1722–1729. doi: 10.1172/JCI118217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. The Journal of clinical investigation. 1995 Feb;95(2):811–819. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair KS, Garrow JS, Ford C, Mahler RF, Halliday D. Effect of poor diabetic control and obesity on whole body protein metabolism in man. Diabetologia. 1983 Nov;25(5):400–403. doi: 10.1007/BF00282518. [DOI] [PubMed] [Google Scholar]
- 26.Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J. Protein dynamics in whole body and in splanchnic and leg tissues in type I diabetic patients. The Journal of clinical investigation. 1995 Jun;95(6):2926–2937. doi: 10.1172/JCI118000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Short KR, Irving BA, Basu A, Johnson CM, Nair KS, Basu R. Effects of type 2 diabetes and insulin on whole-body, splanchnic, and leg protein metabolism. The Journal of clinical endocrinology and metabolism. 2012 Dec;97(12):4733–4741. doi: 10.1210/jc.2012-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su X, Magkos F, Zhou D, et al. Adipose tissue monomethyl branched-chain fatty acids and insulin sensitivity: Effects of obesity and weight loss. Obesity (Silver Spring, Md. 2014 Oct 18; doi: 10.1002/oby.20923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCoy RG, Irving BA, Soop M, et al. Effect of insulin sensitizer therapy on atherothrombotic and inflammatory profiles associated with insulin resistance. Mayo Clin Proc. 2012 Jun;87(6):561–570. doi: 10.1016/j.mayocp.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. The American journal of physiology. 1979 Sep;237(3):E214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 31.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. The Journal of clinical endocrinology and metabolism. 2000 Jul;85(7):2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 32.Lanza IR, Zhang S, Ward LE, Karakelides H, Raftery D, Nair KS. Quantitative metabolomics by H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PloS one. 2010;5(5):e10538. doi: 10.1371/journal.pone.0010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang TJ, Ngo D, Psychogios N, et al. 2-Aminoadipic acid is a biomarker for diabetes risk. The Journal of clinical investigation. 2013 Oct 1;123(10):4309–4317. doi: 10.1172/JCI64801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barazzoni R, Short KR, Asmann Y, Coenen-Schimke JM, Robinson MM, Nair KS. Insulin fails to enhance mTOR phosphorylation, mitochondrial protein synthesis, and ATP production in human skeletal muscle without amino acid replacement. American journal of physiology. 2012 Nov 1;303(9):E1117–1125. doi: 10.1152/ajpendo.00067.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984 Dec;40(4):1079–1087. [PubMed] [Google Scholar]
- 36.Dmitrienko A, SAS Institute. Analysis of clinical trials using SAS: a practical guide. Cary, NC: SAS Institute; 2005. [Google Scholar]
- 37.Kakazu E, Kondo Y, Ninomiya M, et al. The influence of pioglitazone on the plasma amino acid profile in patients with nonalcoholic steatohepatitis (NASH) Hepatol Int. 2013 Jun 01;7(2):577–585. doi: 10.1007/s12072-012-9395-y. [DOI] [PubMed] [Google Scholar]
- 38.Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. The Journal of biological chemistry. 2010 Apr 9;285(15):11348–11356. doi: 10.1074/jbc.M109.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sears DD, Hsiao G, Hsiao A, et al. Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization. Proceedings of the National Academy of Sciences of the United States of America. 2009 Nov 3;106(44):18745–18750. doi: 10.1073/pnas.0903032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bao Y, Zhao T, Wang X, et al. Metabonomic variations in the drug-treated type 2 diabetes mellitus patients and healthy volunteers. J Proteome Res. 2009 Apr;8(4):1623–1630. doi: 10.1021/pr800643w. [DOI] [PubMed] [Google Scholar]
- 41.Muscelli E, Frascerra S, Casolaro A, et al. The amino acid response to a mixed meal in patients with type 2 diabetes: effect of sitagliptin treatment. Diabetes, obesity & metabolism. 2014 Nov;16(11):1140–1147. doi: 10.1111/dom.12350. [DOI] [PubMed] [Google Scholar]
- 42.Copeland KC, Nair KS, Kaplowitz PB, Robbins DC, Calles-Escandon J. Discordant metabolic actions of insulin in extreme lipodystrophy of childhood. The Journal of clinical endocrinology and metabolism. 1993 Nov;77(5):1240–1245. doi: 10.1210/jcem.77.5.8077317. [DOI] [PubMed] [Google Scholar]
- 43.Halvatsiotis P, Short KR, Bigelow M, Nair KS. Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes. 2002 Aug;51(8):2395–2404. doi: 10.2337/diabetes.51.8.2395. [DOI] [PubMed] [Google Scholar]
- 44.Walrand S, Short KR, Bigelow ML, Sweatt AJ, Hutson SM, Nair KS. Functional impact of high protein intake on healthy elderly people. American journal of physiology. 2008 Oct;295(4):E921–928. doi: 10.1152/ajpendo.90536.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macotela Y, Emanuelli B, Bang AM, et al. Dietary leucine--an environmental modifier of insulin resistance acting on multiple levels of metabolism. PloS one. 2011;6(6):e21187. doi: 10.1371/journal.pone.0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H, Xu M, Lee J, He C, Xie Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. American journal of physiology. 2012 Nov 15;303(10):E1234–1244. doi: 10.1152/ajpendo.00198.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruckbauer A, Zemel MB. Synergistic effects of metformin, resveratrol, and hydroxymethylbutyrate on insulin sensitivity. Diabetes, metabolic syndrome and obesity: targets and therapy. 2013;6:93–102. doi: 10.2147/DMSO.S40840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruckbauer A, Zemel MB. Synergistic effects of polyphenols and methylxanthines with Leucine on AMPK/Sirtuin-mediated metabolism in muscle cells and adipocytes. PloS one. 2014;9(2):e89166. doi: 10.1371/journal.pone.0089166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruckbauer A, Zemel MB, Thorpe T, et al. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutrition & metabolism. 2012;9(1):77. doi: 10.1186/1743-7075-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirahatake KM, Slavin JL, Maki KC, Adams SH. Associations between dairy foods, diabetes, and metabolic health: potential mechanisms and future directions. Metabolism. 2014 May;63(5):618–627. doi: 10.1016/j.metabol.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akagawa M, Sasaki T, Suyama K. Oxidative deamination of lysine residue in plasma protein of diabetic rats. Novel mechanism via the Maillard reaction. Eur J Biochem. 2002 Nov;269(22):5451–5458. doi: 10.1046/j.1432-1033.2002.03243.x. [DOI] [PubMed] [Google Scholar]
- 52.Fan X, Zhang J, Theves M, et al. Mechanism of lysine oxidation in human lens crystallins during aging and in diabetes. The Journal of biological chemistry. 2009 Dec 11;284(50):34618–34627. doi: 10.1074/jbc.M109.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sell DR, Strauch CM, Shen W, Monnier VM. 2-aminoadipic acid is a marker of protein carbonyl oxidation in the aging human skin: effects of diabetes, renal failure and sepsis. The Biochemical journal. 2007 Jun 1;404(2):269–277. doi: 10.1042/BJ20061645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaleel A, Henderson GC, Madden BJ, et al. Identification of de novo synthesized and relatively older proteins: accelerated oxidative damage to de novo synthesized apolipoprotein A-1 in type 1 diabetes. Diabetes. 2010 Oct;59(10):2366–2374. doi: 10.2337/db10-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Felig P. Amino acid metabolism in man. Annu Rev Biochem. 1975;44:933–955. doi: 10.1146/annurev.bi.44.070175.004441. [DOI] [PubMed] [Google Scholar]
- 56.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995 Aug 31;333(9):550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 57.Madiraju AK, Erion DM, Rahimi Y, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014 Jun 26;510(7506):542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]