Abstract
The Na,K-ATPase consists of an α- and β-subunit. Moloney sarcoma virus-transformed MDCK cells (MSV-MDCK) express low levels of Na,K-ATPase β1-subunit. Ectopic expression of Na,K-ATPase β1-subunit in these cells increased the protein levels of the α1-subunit of Na,K-ATPase. This increase was not due to altered transcription of the α1-subunit gene or half-life of the α1-subunit protein because both α1-subunit mRNA levels and half-life of the α1-subunit protein were comparable in MSV-MDCK and β1-subunit expressing MSV-MDCK cells. However, short pulse labeling revealed that the initial translation rate of the α1-subunit in β1-subunit expressing MSV-MDCK cells was six- to sevenfold higher compared with MSV-MDCK cells. The increased translation was specific to α1-subunit because translation rates of occludin and β-catenin, membrane and cytosolic proteins, respectively, were not altered. In vitro cotranslation/translocation experiments using rabbit reticulocyte lysate and rough microsomes revealed that the α1-subunit mRNA is more efficiently translated in the presence of β1-subunit. Furthermore, sucrose density gradient analysis revealed significantly more α1-subunit transcript associated with the polysomal fraction in β1-subunit expressing MSV-MDCK cells compared with MSV-MDCK cells, indicating that in mammalian cells the Na,K-ATPase β1-subunit is involved in facilitating the translation of the α1-subunit mRNA in the endoplasmic reticulum.
INTRODUCTION
Na,K-ATPase, also known as sodium pump, is a key enzyme that regulates intracellular Na+ and K+ homeostasis in animal cells. It catalyzes an ATP-dependent transport of three sodium ions out and two potassium ions into the cell per pump cycle, thereby generating a transmembrane sodium gradient. The sodium gradient generated by the enzyme provides the primary energy for uptake and extrusion of a wide variety of solutes by epithelial cells and is crucial for efficient functioning of other Na+-coupled transport systems (Katz, 1988; Lingrel and Kuntzweiler, 1994).
The Na,K-ATPase is an oligomeric transmembrane protein consisting of a noncovalently linked α- and β-subunit. Recently, a third subunit, the γ-subunit has been described, but in contrast to α- and β-subunit, which are ubiquitously expressed, γ-subunit expression is restricted to certain tissues (Therien et al., 1997; Arystarkhova et al., 1999). In mammals at least four α-isoforms (Shamraj and Lingrel, 1994; Blanco et al., 1999; Woo et al., 2000), and three isoforms of the β-subunit (Mercer, 1993; Lingrel and Kuntzweiler, 1994) have been described which exhibit tissue-specific distribution and differences in functional properties. The isoforms predominantly expressed in kidney are α1 and β1 (Mercer, 1993). The α1-subunit (∼112 kDa; Shull et al., 1985) has 10 membrane-spanning segments and contains the catalytic and ligand-binding sites of the enzyme. The β1-subunit (∼55 kDa; Shull et al., 1986) is a glycosylated single transmembrane protein with a short cytoplasmic tail and a larger extracellular domain. Although the precise function of the β-subunit is not known, it is required for normal activity of the enzyme (Noguchi et al., 1987; Horowitz et al., 1990; McDonough et al., 1990; Eakle et al., 1994; Hasler et al., 1998).
Several lines of evidence indicate that the α-subunit and the β-subunit of Na,K-ATPase cotranslationally associate in the endoplasmic reticulum (ER) and are transported to the cell surface as a heterodimer (Geering, 1990; McDonough et al., 1990; Chow and Forte, 1995). Noguchi et al. (1990a) demonstrated that when increasing amounts of β-subunit mRNA were coinjected with a fixed amount of α-subunit mRNA into Xenopus oocytes, the plasma membrane expression of the α-subunit as well as the Na,K-ATPase activity increased indicating that the β-subunit facilitates the correct assembly of the α-subunit and its transport to the cell surface. Ackermann and Geering (1990) have shown in Xenopus oocytes that the β-subunit is necessary for the stability of the newly synthesized α-subunit. A recent study has demonstrated that the β-subunit of Na,K-ATPase may shield a degradation signal in the M7/M8 loop of the α-subunit and might protect the α-subunit from ER degradation (Béguin et al., 2000). When the avian α-subunit alone was overexpressed in a mouse cell line, it was predominantly located intracellularly in the ER (Takeyasu et al., 1988). These studies collectively suggest that the β-subunit plays a role in the synthesis, stability, and the transport of α-subunit of Na,K-ATPase. Because most of the mammalian cells express high endogenous levels of α- and β-subunits of Na,K-ATPase, most of the above-mentioned studies used heterologous systems to understand the role of β-subunit in regulating the α-subunit of Na,K-ATPase.
We have shown previously that Na,K-ATPase β1-subunit protein levels are reduced in human renal clear-cell carcinoma (Rajasekaran et al., 1999). Subsequently, we showed that Moloney sarcoma virus-transformed Madin-Darby canine kidney (MSV-MDCK) cells also express reduced protein levels of β1-subunit of Na,K-ATPase (Rajasekaran et al., 2001). In this study, we utilized MSV-MDCK cells as a model to study the role of β-subunit in regulating the α-subunit levels. We show that ectopic expression of β1-subunit increases the α1-subunit protein at the translational level on the endoplasmic reticulum and demonstrate that the β1-subunit is involved in increasing the translation rate of the α1-subunit in MSV-MDCK cells.
MATERIALS AND METHODS
Cell Lines and Antibodies
Canine Na,K-ATPase α1-subunit (kindly provided by Dr. Askari Amir, Medical College of Ohio, Toledo, OH) and canine Na,K-ATPase β1-subunit (gift from Dr. Robert Farley, University of Southern California, Los Angeles, CA) were subcloned into pCDNA3 (Invitrogen, Carlsbad, CA) as described (Rajasekaran et al., 2001). MSV-transformed MDCK cells expressing Na,K-ATPase β1-subunit (MSV-NaKβ-cl 1 and MSV-NaKβ-cl 2) and vector-control cells (MSV-pCDNA3) were described earlier (Rajasekaran et al., 2001).
Mouse monoclonal antibodies raised against Na,K-ATPase α1- (M8-P1-A3 and M7-PB-E9) and β1- (M17-P5-F11) subunit and rabbit polyclonal antisera (833, 754) against the α1-subunit have been described earlier (Ball and Lane, 1986; Abbott and Ball, 1993; Sun and Ball, 1994). Rabbit polyclonal occludin antibody was obtained from Zymed Laboratories (South San Francisco, CA), mouse monoclonal β-catenin and horseradish peroxidase (HRP)-conjugated anti-mouse antibody from Transduction Laboratories (Lexington, KY) and FITC-labeled, affinity-purified secondary antibody from Jackson ImmunoResearch Laboratories (West Grove, PA).
Immunoblotting and Cell Surface Biotinylation
Experiments were carried out as described earlier (Rajasekaran et al., 2001). Briefly, confluent monolayers were lysed in 95 mM NaCl, 25 mM Tris, pH 7.4, 0.5 mM EDTA, 2% SDS, 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 μg/ml each of antipain, leupeptin, and pepstatin), briefly sonicated and centrifuged. Protein, 100 μg, was separated by 10% SDS-PAGE, transferred, and immunoblotted with either α1-subunit (M7-PB-E9) or β1-subunit (M17-P5-F11) mAb. The protein bands were detected with HRP-conjugated anti-mouse secondary antibody and ECL (NEN Life Science Products, Boston, MA). Densitometric analysis was carried out with an ImageQuant software package (Molecular Dynamics, Sunnyvale, CA).
For cell surface biotinylation cell surface proteins were labeled with membrane-impermeable EZ-Link Sulfo-NHS-Biotin (Pierce, Rockford, IL) in TEA (150 mM NaCl, 10 mM triethanolamine, pH 9, 1 mM CaCl2, 1 mM MgCl2). The cells were quenched with ammonium chloride and lysed in 150 mM NaCl, 20 mM Tris, pH 8, 5 mM EDTA, 1% Triton X-100, 0.1% BSA, 1 mM PMSF, 5 μg/ml each of antipain, leupeptin, and pepstatin. Biotinylated proteins were precipitated with Ultralink streptavidin beads (Pierce) as reported earlier (Rajasekaran et al., 2001) and immunoblotted for α1- and β1-subunits as described above.
Immunofluorescence
Confluent monolayers were fixed in ice-cold methanol and processed for α1-subunit (M7-PB-E9) immunofluorescence as described earlier (Rajasekaran et al., 2001). Epifluorescence analysis was performed using an Olympus AX70 Provis microscope.
Ouabain-sensitive Rubidium Uptake and Sodium Measurements
The ouabain-sensitive transport of 86Rb+ was essentially determined as described (Rajasekaran et al., 2001). Briefly, cells washed in ice-cold wash solution (144 mM NaCl, 0.5 mM CaCl2, 10 mM HEPES, pH 7.4) were incubated for 10 min at 37°C with buffer containing 144 mM NaCl, 10 mM HEPES, pH 7.4, 0.5 mM MgCl2, 0.5 mM CaCl2, 1 mM RbCl, 1 mg/ml glucose, 1 μCi 86Rb+. The cells were washed and lysed, and the amount of solubilized, radioactive 86Rb+ was determined. To determine the ouabain-sensitive transport cells were preincubated with 50 μM ouabain for 30 min. All samples were normalized for protein content.
Intracellular Na+ levels were determined by atomic emission spectrometry as described (Rajasekaran et al., 2001). The intracellular Na+ concentrations were measured at 588.995 nm and normalized to the total Mg2+ content of the cells (internal control).
Northern Blot Analysis
Total RNA was isolated using the acid/phenol/guanidinium thiocyanate procedure described by Chomczynski and Sacchi (1987). RNA, 10 μg, was denatured, subjected to electrophoresis on a 1.5% MOPS/formaldehyde/agarose, and transferred onto Nytran Plus nylon membranes (Schleicher & Schuell, Keene, NH). Membranes were hybridized with a random primed full-length Na,K-ATPase α1-subunit cDNA probe according to standard protocols.
Pulse Chase Analysis
For metabolic labeling and pulse-chase experiments equal numbers of cells were plated on 60-mm dishes and allowed to grow for 24 h. Before metabolic labeling the cells were incubated for 2 h at 37°C with methionine- and cysteine-free DMEM containing 1% of FBS that had been dialyzed against PBS. The cells were pulsed for 20 min with 0.5 mCi/ml trans-35S-label (ICN Biochemicals, Irvine, CA), washed, and chased in regular DMEM culture medium at 37°C. For short pulse-labeling experiments with 2.5-min pulses the amount of tran-35S-label was increased to 2 mCi/ml. At the indicated time points the cells were lysed in 10 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 40 mM N-octylglucoside, 0.2 mM sodium vanadate, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, and 5 μg/ml each of antipain, leupeptin, and pepstatin on ice for 30 min. The cell lysates were briefly sonicated and centrifuged at 4°C for 10 min at 14,000 rpm in a microfuge. The supernatants were immunoprecipitated at 4°C for 16-18 h with 40 μl of protein A-agarose beads coated with rabbit anti-mouse IgG (for monoclonal antibodies) and 1 μg/ml primary antibody (anti-Na,K-ATPase α1-subunit, anti-β-catenin, or anti-occludin). The immunoprecipitates were separated by 10% SDS-PAGE, detected by fluorography, and quantitated by densitometric analysis.
In Vitro Translation and Translocation Analysis
The cDNAs of canine Na,K-ATPase α1- and β1-subunit, both cloned into plasmid pCDNA3, were digested with ApaI and XhoI, respectively, and blunt-ended with T4 DNA polymerase. The linearized fragments were purified with a QIAEX II gel extraction kit (Qiagen, Valencia, CA). Linearized, purified cDNA, 2 μg, was used for in vitro transcription reactions using the RiboMAX large scale RNA production system (T7, Promega, Madison, WI) according to manufacturer's instructions. The transcripts were diluted with water (1:15 for α1-subunit and 1:60 for β1-subunit) and used for in vitro translation reactions. The translation reactions in a rabbit reticulocyte lysate system were carried out as described in the Promega technical manual. Briefly, a 25-μl translation reaction mixture contained 17.5 μl of reticulocyte lysate, 0.5 μl methionine-free amino acid mixture, 1 μl of 10 mCi/ml [35S]methionine (in vitro translation grade, ICN Biomedicals, Inc., Irvine, CA), 0.1 μl RNasin (Promega), 1 μl canine pancreatic microsomal membranes (Promega), and the indicated amounts of in vitro-transcribed RNA. α-factor RNA provided with the microsomal membranes was used as nonspecific control RNA. The reaction mixture was incubated at 30°C for 90 min and chilled immediately after the incubation. In vitro-translated α1-subunit was immunoprecipitated in a buffer containing 150 mM NaCl, 20 mM Tris, pH 8.0, 5 mM EDTA, 1% Triton X-100, 0.1% bovine serum albumin, 1 mM PMSF, and 5 μg/ml each of antipain, leupeptin, and pepstatin as described above. The immunoprecipitates were incubated in Laemmli sample buffer at 50°C for 10 min, separated by 10% SDS-PAGE, detected by fluorography, and quantitated by densitometric analysis.
For Endoglycosidase H (Endo H) treatment, in vitro-translated β1-subunit was immunoprecipitated under same conditions as the α1-subunit. The immunoprecipitates were dissolved in 30 μl 1% SDS solution containing 50 mM DTT and boiled for 3 min. An equal volume of 0.2 M sodium citrate buffer (pH 5.5) containing 0.2 μg/ml Trasylol was added. Both, a sample containing 2 μl of 5 mU/μl Endo H (Roche Diagnostics, Indianapolis, IN) and one without Endo H were incubated overnight at 37°C. After addition of Laemmli sample buffer, the samples were boiled, separated by 10% SDS-PAGE, and detected by fluorography.
Polysomal RNA Fractionation and RT-PCR
Polysomal and subpolysomal RNAs were isolated by centrifugation on discontinuous sucrose gradients as previously described (Baum and Wormington, 1985; Twiss et al., 2000). Briefly, cultures were rinsed in PBS containing 0.1 mM cycloheximide and then incubated in polysome lysis buffer (300 mM KCl, 2 mM MgCl2, 20 mM TrisCl, pH 7.4, 2 mM DTT, 0.05% deoxycholic acid, 100 U/ml RNasin, and 0.1 mM cycloheximide) for 20 min at 4°C. Lysates were cleared by centrifiguation at 13,000 × g for 15 min. Cleared lysates were layered onto 1 ml polysome lysis buffer containing 20% sucrose. As a negative control for fractionation of polysomes, cleared lysates were brought to 50 mM EDTA before centrifugation to dissociate ribosome subunits; EDTA was also included in the sucrose solution used for centrifugation for these control lysates (Zheng et al., 2001). Gradients were subjected to ultracentrifugation at 105,000 × g, 4°C in RP55S swinging bucket rotor with a microultracentrifuge (Kendro Laboratory Products, Asheville, NC). After 2 h centrifugation, the bottom 0.4 ml of each gradient was collected as the polysomal fraction, with the remainder of the gradient serving as the subpolysomal fraction. RNA was extracted from these fractions by phenol-chloroform and then precipitated overnight at -80°C using isopropanol with glycogen as a carrier. After washing with 70% ethanol, RNA was resuspended in 30 μl DEPC-treated water (Ambion, Austin, TX) and equivalent proportions from each fraction were used for reverse transcription with MMLV-RT and oligo-dT primer (Ambion). Na,K-ATPase α1- and β1-subunit were amplified with an annealing temperature of 55°C and using the following primers: α1-subunit (sense) 5′-CAGAGGAGGTTGTAATCGG-3′; α1-subunit (antisense) 5′-CTTTCACGCAGTTGGTTGAG; β1-subunit (sense) 5′-ACTGAAATTTCCTTTCGTCCTAA-3′; β1-subunit (antisense) 5′-ATCACTGGGTAAGTCTCCA-3′.
RESULTS
Increased Na,K-ATPase α1-subunit Protein Levels in Na,K-ATPase β1-subunit Expressing MSV-MDCK Cells
We recently showed that MSV-MDCK cells express reduced protein levels of α1- and β1-subunit of Na,K-ATPase compared with wild-type MDCK cells (Rajasekaran et al., 2001). To study the role of Na,K-ATPase β1-subunit in Na,K-ATPase α1-subunit regulation we used two clones of MSV-MDCK cells ectopically expressing canine β1-subunit of Na,K-ATPase (MSV-NaKβ-cl 1, MSV-NaKβ-cl 2). MSV-NaKβ-cl 1 and MSV-NaKβ-cl 2 cells express three- and fivefold more β1-subunit, respectively, than MSV-MDCK cells transfected with the vector alone (MSV-pCDNA3; Figure 1, A and B). Interestingly, MSV-NaKβ-cl 1 and MSV-NaKβ-cl 2 cells express three- and sixfold more Na,K-ATPase α1-subunit, respectively, than MSV-pCDNA3 cells (Figure 1, C and D). Although, MSV-pCDNA3 cells contained low levels of β1-subunit, the α1-subunit was clearly localized on the plasma membrane as revealed by immunofluorescence (Figure 1E) and cell surface biotinylation assays (Figure 1, J and K). Increased expression of β1-subunit enhanced the cell surface localization of the α1-subunit in both MSV-NaKβ-cl 1 and MSV-NaKβ-cl 2 cells (Figure 1, F and G). Cell surface biotinylation showed about a three- and eightfold increase in the α1-subunit levels expressed on the cell surface in MSV-NaKβ-cl 1 and MSV-NaKβ-cl 2 cells, respectively (Figure 1, J and K), which correlated with the increased β1-subunit expression on the cell surface (Figure 1, H and I). Ectopic expression of β1-subunit also increased the Na,K-ATPase activity as determined by ouabain-sensitive rubidium flux (Figure 1L), which was accompanied by a decrease in the intracellular sodium levels in these cell lines (Figure 1M). These results demonstrate that repletion of the β1-subunit increases the α1-subunit protein level, cell surface expression, and activity of the Na,K-ATPase in MSV-MDCK cells.
Figure 1.
Expression of Na,K-ATPase β-subunit in MSV-MDCK cells. (A-D) Expression levels of Na,K-ATPase α1- and β1-subunit. Total cell lysates of MSV-pCDNA3, MSV-NaKβ-cl 1, and MSV-NaKβ-cl 2 cells were separated on a 10% SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-Na,K-ATPase β-subunit (A) or α-subunit (C) antibody. The blots from two gels each for β-subunit (B) and α-subunit (D) were quantified by densitometric analysis. The α- and β-subunit expression levels in MSV-NaKβ cells were compared with the α- and β-subunit levels in MSV-pCDNA3 cells. Bars, means ± SE. (E-K) Cell surface expression of Na,K-ATPase α- and β-subunit. (E-G) Immunofluorescence of Na,K-ATPase α-subunit. Confluent monolayers of MSV-pCDNA3 (E), MSV-NaKβ-cl 1 (F), and MSV-NaKβ-cl 2 (G) cells were washed, fixed, and stained with anti-Na,K-ATPase α-subunit and FITC-conjugated anti-mouse secondary antibody. Bar, 20 μm. (H-K) Cell surface biotinylation. Cell surface proteins of MSV-pCDNA3, MSV-NaKβ-cl 1, and MSV-NaKβ-cl 2 cells were labeled with membrane-impermeable Sulfo-NHS-biotin and biotinylated proteins were precipitated with streptavidin-conjugated beads. The precipitates were separated by SDS-PAGE and immunoblotted for Na,K-ATPase α-subunit (J) or β-subunit (H). The quantitative data of α-subunit (K) and β-subunit (I) cell surface expression of two independent experiments are shown. Bars, means ± SE. (L-M) Na,K-ATPase function in β-subunit expressing MSV-MDCK cells. Ouabain-sensitive 86Rb+ flux measurements (L) and atomic emission spectrometry to determine intracellular Na+ levels (M) were done as described in MATERIALS AND METHODS. The results were expressed relative to the levels in MSV-pCDNA3 cells. Error bars, SE of the mean of two independent determinations done in triplicates (L) and for two single independent determinations (M), respectively.
Na,K-ATPase α1-subunit mRNA levels Are Not Altered in MSV-NaKβ Cells
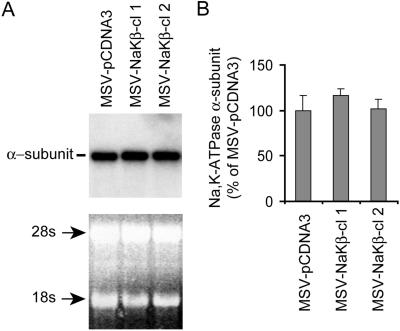
The increased α1-subunit protein levels in MSV-MDCK cells could be explained by increased transcription of the α1-subunit gene. To address this possibility, we compared the levels of α1-subunit mRNA in MSV-pCDNA3, MSV-NaKβ-cl 1, and MSV-NaKβ-cl 2 cells by Northern blot analysis. Similar α1-subunit mRNA levels in all the three cell lines (Figure 2, A and B) indicated that upregulation of the α1-subunit protein levels in β1-subunit-expressing MSV-MDCK cells is posttranscriptional rather than transcriptional upregulation of the α1-subunit gene.
Figure 2.
Northern blot analysis of Na,K-ATPase α-subunit. (A) Total RNA from MSV-pCDNA3, MSV-NaKβ-cl 1, and MSV-NaKβ-cl 2 cells was separated by electrophoresis, transferred to nylon membrane, and hybridized with a 32P-labeled full-length Na,K-ATPase α-subunit cDNA probe. 28S and 18S rRNA are shown to confirm equal loading. (B) Intensity of the α-subunit mRNA bands was quantitated by PhosphorImager analysis and expressed relative to MSV-pCDNA3 cells. Bars, SD of the mean of two independent determinations.
The Protein Half-life of the Na,K-ATPase α1-subunit Is Comparable in MSV-pCDNA3 and MSV-NaKβ Cells
Earlier studies indicated that in the absence of Na,K-ATPase β1-subunit expression the α1-subunit is retained in the ER and subsequently degraded (Ackermann and Geering, 1990; Geering, 1990). Therefore, we sought to test whether expression of the β1-subunit increases the stability of the α1-subunit protein accounting for the increased α1-subunit levels observed in MSV-NaKβ cells. Pulse-chase analysis revealed a half-life of 35 h for the α1-subunit protein in MSV-pCDNA3 cells and of 28 and 27 h for MSV-NaKβ-cl 1 and MSV-NaKβ-cl 2 cells, respectively (Figure 3, A and B), suggesting that repletion of β1-subunit in MSV-MDCK cells did not significantly affect the half-life of the α1-subunit. Therefore, an increased stability of the α1-subunit protein may not account for the increased levels of this protein observed in MSV-NaKβ cells. Why MSV-MDCK cells with low β1-subunit levels have an increased half-life of the α1-subunit compared with MSV-NaKβ cells is not known. It is possible that factors other than the β1-subunit might be involved in the stabilization of the α1-subunit in transformed cells which express less β-subunit.
Figure 3.
Pulse-chase analysis of the Na,K-ATPase α-subunit. (A) MSV-pCDNA3, MSV-NaKβ-cl 1, and MSV-NaKβ-cl 2 cells were pulsed for 20 min with [35S]methionine, washed, and chased for the indicated times. Na,K-ATPase α-subunit was immunoprecipitated, separated by SDS-PAGE, and detected by fluorography. The arrow indicates the α-subunit band. (B) The intensity of the bands was quantitated by densitometric analysis. Bars, SE of the mean of three independent experiments done in duplicates.
Increase in Newly Synthesized α1-subunit Protein in MSV-NaKβ Cells
It has been shown that α- and β-subunit cotranslationally associate in the ER and are transported to the cell surface as a complex (Hiatt et al., 1984; Tamkun and Fambrough, 1986). It is possible that cotranslational association of the β-subunit with the α-subunit in the ER might positively influence the synthesis of the α-subunit. To test this possibility we pulsed MSV-pCDNA3 and MSV-NaKβ cells with [35S]methionine for 20 min, immunoprecipitated the α1-subunit, and determined the rate of new synthesis of α1-subunit among these cell lines. The identity of the α1-subunit band was confirmed by immunoblot analysis (unpublished data). In MSV-NaKβ cells the label incorporated into the α1-subunit was five- to sixfold more compared with MSV-pCDNA3 cells (Figure 4, A and B), indicating that the initial synthesis of the α-subunit on the endoplasmic reticulum is dramatically increased in MSV-NaKβ cells. Incorporation of [35S]methionine into occludin, a transmembrane protein localized to tight junctions in epithelial cells (Furuse et al., 1993) was comparable in MSV-pCDNA3 and MSV-NaKβ cells. Furthermore, the incorporation of [35S]methionine into β-catenin, a cytosolic protein was also similar in MSV-pCDNA3 cells and MSV-NaKβ cells, indicating that the increased synthesis rate of the α1-subunit in MSV-NaKβ cells is specific to the α1-subunit.
Figure 4.
Metabolic labeling of β-subunit expressing MSV-MDCK cells. (A and B) Twenty-minute pulse labeling. MSV-pCDNA3, MSV-NaKβ-cl 1, and MSV-NaKβ-cl 2 cells were pulsed for 20 min with [35S]methionine, washed, and lysed and the Na,K-ATPase α-subunit was immunoprecipitated. Subsequently, β-catenin and occludin were immunoprecipitated from the same lysates. The immunoprecipitates were separated by SDS-PAGE and detected by fluorography. The intensity of the bands was quantitated by densitometric analysis. Bars, the SE of the mean of three independent experiments done in duplicates. (C and D) 2.5-min pulse labeling. MSV-pCDNA3 and MSV-NaKβ-cl 2 cells were pulsed for 2.5 min with [35S]methionine, washed, and lysed. Na,K-ATPase α-subunit was immunoprecipitated either with mAb M8 (as for the 20-min pulse) or with polyclonal antibodies 833 and 754 recognizing different epitopes. The immunoprecipitates were separated by SDS-PAGE, detected by fluorography, and quantitated by densitometric analysis. Bars, the SE of the mean of three independent experiments.
It is possible that partially synthesized α1-subunit is rapidly degraded in the absence of β1-subunit and is not detected after a 20-min pulse. Further, the anti-α1-subunit antibody M8-P1-A3 used for immunoprecipitation has been shown to bind to a synthetic peptide sequence encompassing amino acids 496-506 (Ball and Loftice, 1987) and it can be argued that this antibody will not detect partially synthesized N-terminal fragments of the α1-subunit. To address these issues, we first pulse-labeled MSV-NaKβ-cl 2 and MSV-pCDNA3 cells for 2.5 min and compared the incorporation of [35S]methionine into the α1-subunit. Using the M8 antibody for immunoprecipitation a 2.5-min pulse, similar to the 20-min pulse, showed a 5.4 ± 1.4-fold increase in [35S]methionine incorporation into the α1-subunit in MSV-NaKβ-cl 2 cells compared with MSV-pCDNA3 cells (Figure 4, C and D), indicating that the increased synthesis of the α1-subunit is evident even in shorter pulse experiments. To rule out the possibility that M8 antibody was not detecting partially synthesized N-terminal fragments, we used two additional peptide antisera (833 and 754) raised against the N-terminus of the α1-subunit for immunoprecipitations (Ball and Lane, 1986). A seven- to eight-fold increase in [35S]methionine incorporation into the α1-subunit was observed after a 2.5-min pulse (Figure 4, C and D). Together, these experiments confirmed that the increased [35S]methionine incorporation into the α1-subunit observed after a 20-min pulse was neither due to a prolonged pulse time nor to a failed detection of partially synthesized α1-subunit.
Increased Translation of the α1-subunit mRNA in the Presence of β1-subunit in an In Vitro Translation Translocation System
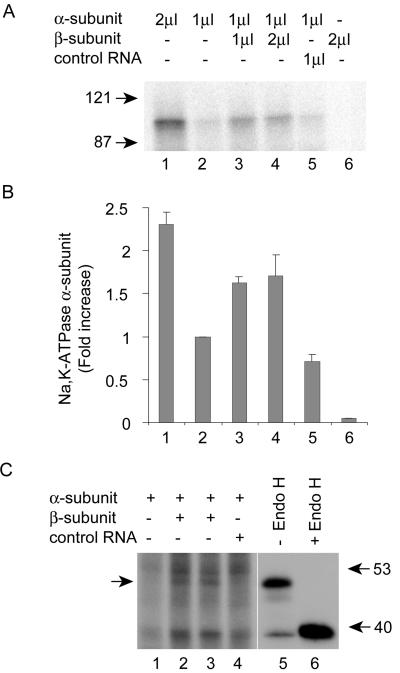
To further confirm that the presence of the β1-subunit positively influences the translation of the α1-subunit, we utilized an in vitro cotranslational translocation system, which we have extensively characterized for studying translocation of the α-subunit of Na,K-ATPase into rough microsomes (Xie et al., 1996). Na,K-ATPase α1- and β1-subunit cDNAs were transcribed in vitro, and the mRNAs were then translated in a rabbit reticulocyte lysate containing [35S]methionine and purified rough microsomes. Including rough microsomes in the in vitro translation system allows for monitoring cotranslational translocation of the newly synthesized, 35S-labeled proteins into the ER membrane. After the translation-translocation reaction, the α1- or β1-subunit protein was immunoprecipitated, separated on a SDS-PAGE, and autoradiographed and the densities of respective bands were quantified. We first confirmed that the α1-subunit mRNA was fully translated in the presence of microsomes using two different concentrations of α1-subunit mRNA (1 and 2 μl). A 2.3-fold increase in the translated α1-subunit (100 kDa) was observed when 2 μl of α1-subunit RNA were added as compared with reactions with 1 μl of α1-subunit RNA (Figure 5, A and B, compare lanes 2 and 1). This band was not present in control immunoprecipitates of reactions that contained no α1-subunit but only β1-subunit mRNA (Figure 5, lane 6). Presence of the β1-subunit mRNA in the translation-translocation system consistently showed an increased translation of the α1-subunit. Translation of 1 μl α1-subunit mRNA in the presence of 1 μl β1-subunit mRNA resulted in a 1.6-fold increase in the translation of the α1-subunit (Figure 5, A and B, compare lanes 2 and 3). Doubling the amount of β1-subunit mRNA produced only a slight increase in the α1-subunit level compared with the reaction containing 1 μl of β1-subunit mRNA (Figure 5, A and B, compare lanes 3 and 4). Synthesis of the α1-subunit did not increase in the presence of an irrelevant control mRNA (Figure 5, A and B, compare lanes 2 and 5), which was clearly translated under these conditions (unpublished data). These results confirmed that the increased translation of the α1-subunit mRNA was specific to the presence of the β1-subunit mRNA.
Figure 5.
In vitro translation-translocation of Na,K-ATPase α- and β-subunit mRNAs. (A) The indicated amounts of in vitro-transcribed Na,K-ATPase α- and β-subunit mRNAs were used for translation-translocation reactions in a rabbit reticulocyte lysate system containing microsomal membranes as described in MATERIALS AND METHODS. Na,K-ATPase α-subunit was immunoprecipitated, separated on a SDS-PAGE, and detected by fluorography. (B) The intensity of the bands was quantitated by densitometric analysis and expressed relative to the intensity of the α-subunit band in the reaction containing 1 μl of α-subunit mRNA. Bars, SE of four independent experiments. (C) α-subunit mRNA was translated and translocated in the presence or absence of β-subunit mRNA. The α-subunit was immunoprecipitated and separated on a SDS-PAGE. The arrow indicates the coimmunoprecipitated β-subunit. Presence of the high mannose-form of the β-subunit was confirmed by digesting β-subunit immunoprecipitates with Endo H (lanes 5 and 6).
As reported earlier, the β-subunit associates with the α-subunit cotranslationally (Geering, 1990; McDonough et al., 1990; Chow and Forte, 1995). Consistent with these observations the α1-subunit immunoprecipitates contained a band of molecular mass ∼45 kDa, the molecular mass of the high mannose form of β1-subunit (Figure 5C, arrow). Immunoprecipitation with β1-subunit specific antibody clearly pulled down a 45-kDa band, which was completely sensitive to Endo H digestion (Figure 5C, compare lanes 5 and 6), confirming that the 45-kDa band was the ER high mannose form of the β1-subunit. Furthermore, a similar 45-kDa band was not detected in α1-subunit immunoprecipitates of samples that did not contain β1-subunit mRNA (Figure 5C, lanes 1 and 4). The small increase in the translation of the α1-subunit in vitro compared with in vivo could be due to the limitation of the microsomal system to translate these messages. We consistently observed that it was necessary to translate both the messages simultaneously to obtain efficient translation of the α1-subunit. Taken together, these results strongly indicated that the Na,K-ATPase β1-subunit plays a role in increasing the translation of the α1-subunit mRNA in MSV-MDCK cells.
Increased α1-subunit mRNA Levels in Polysomal Fractions upon Expression of the Na,K-ATPase β1-subunit
An increased translation rate is defined as an increased number of translation products being produced by ribosomes from a single transcript during a specific period of time (Mazumder et al., 2003). During translation several ribosomes associate with one mRNA to produce a protein. These mRNA-associated ribosomes, often termed as polyribosomes, can be fractionated by sucrose density gradients as “polysomal” and “subpolysomal” fractions (Baum and Wormington, 1985; Rajasekaran et al., 1995; Twiss et al., 2000). To provide direct evidence for a role of β1-subunit in increasing the translation rate of the α1-subunit, polysomal and subpolysomal RNAs were isolated from MSV-pCDNA3 and MSV-NaKβ cells using discontinuous sucrose gradient ultracentrifugation. The relative levels of α1-subunit mRNA in the polysomal and subpolysomal fractions were determined by RT-PCR. To control for appropriate isolation of polysomal mRNAs, we used EDTA to dissociate ribosomal subunits, therefore shifting the polyribosomal mRNAs into the subpolysomal fractions.
Figure 6 shows the distribution of β1-subunit (Figure 6A) and α1-subunit mRNAs (Figure 6B) in polysomal and subpolysomal fractions from MSV-pCDNA3 and MSV-NaKβ-cl 2 cells. As expected very little β1-subunit mRNA was detected in the MSV-pCDNA3 cells (Figure 6A, lane 1 and 2). In the MSV-NaKβ-cl 2 cells the majority of the β1-subunit mRNA was detected in the polysomal fraction (Figure 6A, lanes 5 and 6). Addition of EDTA shifted the β1-subunit mRNA to the subpolysomal fraction (Figure 6A, lanes 7 and 8), showing appropriate isolation of polysomal mRNA in lane 5.
Figure 6.
Relative distribution of α- and β-subunit mRNAs in polysomal fractions. Polysomal and subpolysomal fractions from MSV-pCDNA3 and MSV-NaKβ-cl 2 cells were obtained after discontinuous sucrose gradient ultracentrifugation as described in MATERIALS AND METHODS. The relative levels of β-subunit mRNA (A) and α-subunit mRNA (B) in polysomal and subpolysomal fractions were determined by RT-PCR using subunit-specific primers. As a control for appropriate isolation of polysomal mRNAs, EDTA was used to dissociate the ribosomal subunits. Addition of EDTA resulted in a shift of polysomal mRNAs to subpolysomal fractions. (C) Relative distributions of α-subunit mRNA in polysomal and subpolysomal fractions. The intensities of the α-subunit cDNA bands were determined using an AlphaImager 2200 (Alpha Innotech, San Leandro, CA). Bars, SD of the mean of two independent determinations.
We then determined the levels of α1-subunit mRNA by RT-PCR using α1-subunit specific primers in the polysomal and subpolysomal fractions of MSV-pCDNA3 and MSV-NaKβ cells. As shown in Figure 6B, the α1-subunit mRNA was near equally distributed between the polysomal and subpolysomal fractions in MSV-pCDNA3 cells that have low levels of β1-subunit expression (Figure 6B, lanes 1 and 2). Addition of EDTA appropriately shifted the α1-subunit mRNA from polysomal to the subpolysomal fraction (Figure 6B, lanes 3 and 4). Strikingly, in MSV-NaKβ cells, the vast majority of α1-subunit mRNA resided in the polysomal fraction (Figure 6B, lanes 5 and 6). Addition of EDTA confirmed that this distribution represents ribosome-bound α1-subunit mRNA since the transcript shifted this message to the subpolysomal fraction in the EDTA-treated lysates (Figure 6, lanes 7 and 8). The ratio of the α1-subunit mRNA in polysomal and subpolysomal fractions in the absence of EDTA in MSV-NaKb-cl 2 cells was 3.8-fold higher than in MSV-pCDNA3 cells (Figure 6C). Increased levels of α1-subunit transcript in the polysomal fraction in MSV-NaKβ cells compared with MSV-pCDNA3 cells provide conclusive evidence that the β-subunit of Na,K-ATPase facilitates the translation rate of the α-subunit.
DISCUSSION
Our investigations on reduced β1-subunit levels in human renal clear-cell carcinoma (Rajasekaran et al., 1999) enabled us to identify MSV-MDCK cells that express low levels of endogenous β1-subunit (Rajasekaran et al., 2001). Using this cell line we were able to demonstrate novel functions of Na,K-ATPase β1-subunit in epithelial polarization, suppression of invasion, and cell motility (Rajasekaran et al., 2001). In this report we utilized MSV-MDCK cells as model to further study the role of the Na,K-ATPase β1-subunit in the regulation of Na,K-ATPase α1-subunit synthesis and uncovered a role for β1-subunit in facilitating the translation of α1-subunit mRNA in mammalian cells. We showed that ectopic expression of β1-subunit in MSV-MDCK cells results in a more than threefold increase in the α1-subunit protein level. This increase in the α1-subunit level is not due to an increased transcription of the α1-subunit gene or increased stability of the α1-subunit protein, but is rather due to increased synthesis of the α1-subunit in the endoplasmic reticulum. The increase in α1-subunit synthesis was demonstrated in vivo in two independent clones (MSV-NaKβ-cl 1 and MSV-NaKβ-cl 2), using short radioactive pulse experiments as well as in vitro cotranslation by translocation assays using rabbit reticulocyte lysate and microsomes. Finally, we show that in MSV-NaKβ cells significantly more α1-subunit transcript associated with the polysomes compared with MSV-MDCK cells, demonstrating that β1-subunit expression increases the translation efficiency of the α1-subunit in MSV-MDCK cells. To our knowledge this is the first study describing that in a mammalian cell line and under physiological conditions Na,K-ATPase β1-subunit is involved in the regulation of α1-subunit protein levels by increasing the translation rate of the α1-subunit.
Ackermann and Geering (1990) showed that the β-subunit confers a stable configuration to the newly synthesized α-subunit and that α-subunit synthesized in the absence of the β-subunit is degraded. Noguchi et al. (1990b) reported that when a fixed amount of α-subunit was synthesized in the presence of increasing amounts of β-subunit the amounts of newly synthesized αβ-complexes increased. A recent study from Geering and coworkers elegantly demonstrated that the β-subunit association with the M7/M8 loop of the α-subunit might protect the α-subunit from degradation in the ER (Béguin et al., 2000). Lescale-Matys et al. (1990) have shown that in LLC-PK1 cells, a pig kidney proximal tubule cell line, increased β-subunit synthesis under K+ depletion (a condition known to inhibit Na,K-ATPase) also increased the protein levels of α-subunit and the Na,K-ATPase enzyme activity. Collectively, these studies strongly suggest that the β-subunit is necessary for the stability of the α-subunit during its synthesis in the ER.
Although, α1-subunit mRNA levels were comparable in MSV-pCDNA3 and MSV-NaKβ cells, the α1-subunit protein levels were low in MSV-pCDNA3 cells, suggesting that the α1-subunit mRNA is not efficiently translated in MSV-MDCK cells expressing low levels of β1-subunit. Repletion of the β1-subunit in these cells revealed a six- to sevenfold increase in the synthesis of the α1-subunit, an increase that was specific to the α1-subunit of Na,K-ATPase because the synthesis of occludin and β-catenin was not increased in MSV-NaKβ cells. Furthermore, a substantially increased α1-subunit transcript level in the polysome fraction in β1-subunit expressing MSV-MDCK cells indicates that the β-subunit facilitates the translation of the α-subunit. From our results we suggest that the β1-subunit might be a limiting factor for the translation rate of the α1-subunit on the polysomes.
Previous studies using the Xenopus oocyte system have shown that in the absence of β-subunit expression the α-subunit is retained in the endoplasmic reticulum and degraded (Ackermann and Geering, 1990). Although MSV-MDCK cells express reduced levels of β1-subunit the α1-subunit is clearly localized on the plasma membrane in these cells. Immunofluorescence analysis did not reveal a localization of the α1-subunit in any other distinct intracellular compartment, suggesting that the low level of β1-subunit expression in MSV-MDCK cells is sufficient for the surface expression of the α1-subunit in these cells. When avian Na,K-ATPase α1-subunit was overexpressed in mouse L-cells it predominantly localized intracellularly (Takeyasu et al., 1988), leading the authors to conclude that the β-subunit might become a limiting factor in the transport of the α-subunit to the plasma membrane. Our studies are consistent with this conclusion. Overexpression of the α1-subunit in MSV-MDCK cells resulted in a similar observation. Most of the α1-subunit was localized intracellularly with reduced levels on the cell surface (Rajasekaran et al., 2001). However, immunofluorescence and cell surface biotinylation of MSV-NaKβ cells revealed that expression of the β1-subunit in MSV-MDCK cells not only increased total α1-subunit protein levels but also the amount of α1-subunit expressed at the cell surface. Therefore, the β1-subunit may not only be a limiting factor during the synthesis of the α1-subunit on the ER but also in the transport of the α1-subunit to the plasma membrane.
How can the β-subunit of Na,K-ATPase increase the translation of the α-subunit? The exact mechanism by which β1-subunit increases the translation of the α1-subunit is currently not known. However, it is well known that the β-subunit associates with the α-subunit in the endoplasmic reticulum (Geering, 1990; McDonough et al., 1990; Chow and Forte, 1995). We propose that αβ-subunit association in the ER might facilitate proper anchoring of the α-subunit transcript at translation-translocation sites during its synthesis. This might expedite either recruitment of more ribosomes or facilitate retaining of the already bound ribosomes on the α-subunit transcript during its synthesis on the ER membrane. Either or both these possibilities should result in increased levels of α-subunit transcript in the polysome fraction thus facilitating the efficient synthesis of this polytopic protein.
Acknowledgments
We thank Dr. William James Ball, Jr. for generous gift of Na,K-ATPase antibodies and Dr. Lawrence Palmer for critical reading of the manuscript. This work is supported by a National Institutes of Health Grant DK56216 (A.K.R.) and NS415096 (J.L.T.). S.A.R. is supported by USHHS Institutional National Research Service Award T32CA09056. S.A.R. and A.K.R. dedicate this paper to Dr. Takashi Morimoto.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-03-0222. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-03-0222.
References
- Abbott, A., and Ball, W.J., Jr. (1993). The epitope for the inhibitory antibody M7-PB-E9 contains Ser-646 and Asp-652 of the sheep Na+,K(+)-ATPase alpha-subunit. Biochemistry 32, 3511-3518. [DOI] [PubMed] [Google Scholar]
- Ackermann, U., and Geering, K. (1990). Mutual dependence of Na,K-ATPase alpha- and beta-subunits for correct posttranslational processing and intracellular transport. FEBS Lett. 269, 105-108. [DOI] [PubMed] [Google Scholar]
- Arystarkhova, E., Wetzel, R.K., Asinovski, N.K., and Sweadner, K.J. (1999). The gamma subunit modulates Na(+) and K(+) affinity of the renal Na,K-ATPase. J. Biol. Chem. 274, 33183-33185. [DOI] [PubMed] [Google Scholar]
- Ball, W.J., Jr., and Lane, L.K. (1986). Immunochemical comparison of cardiac glycoside-sensitive (lamb) and -insensitive (rat) kidney (Na+ + K+)-ATPase. Biochim. Biophys. Acta 873, 79-87. [DOI] [PubMed] [Google Scholar]
- Ball, W.J., Jr., and Loftice, C.D. (1987). Immunochemical studies of (Na+ + K+)-ATPase using site-specific, synthetic peptide directed antibodies. Biochim. Biophys. Acta 916, 100-111. [DOI] [PubMed] [Google Scholar]
- Baum, E.Z., and Wormington, W.M. (1985). Coordinate expression of ribosomal protein genes during Xenopus development. Dev. Biol. 111, 488-498. [DOI] [PubMed] [Google Scholar]
- Béguin, P., Hasler, U., Staub, O., and Geering, K. (2000). Endoplasmic reticulum quality control of oligomeric membrane proteins: topogenic determinants involved in the degradation of the unassembled Na,K-ATPase alpha subunit and in its stabilization by beta subunit assembly. Mol. Biol. Cell 11, 1657-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco, G., Melton, R.J., Sanchez, G., and Mercer, R.W. (1999). Functional characterization of a testes-specific alpha-subunit isoform of the sodium/potassium adenosinetriphosphatase. Biochemistry 38, 13661-13669. [DOI] [PubMed] [Google Scholar]
- Chomczynski, P., and Sacchi, N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156-159. [DOI] [PubMed] [Google Scholar]
- Chow, D.C., and Forte, J.G. (1995). Functional significance of the beta-subunit for heterodimeric P-type ATPases. J Exp. Biol. 198, 1-17. [DOI] [PubMed] [Google Scholar]
- Eakle, K.A., Kabalin, M.A., Wang, S.G., and Farley, R.A. (1994). The influence of beta subunit structure on the stability of Na+/K(+)-ATPase complexes and interaction with K+. J. Biol. Chem. 269, 6550-6557. [PubMed] [Google Scholar]
- Furuse, M., Hirase, T., Itoh, M., Nagafuchi, A., Yonemura, S., and Tsukita, S. (1993). Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 123, 1777-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering, K. (1990). Subunit assembly and functional maturation of Na,K-ATPase. J. Membr. Biol. 115, 109-121. [DOI] [PubMed] [Google Scholar]
- Hasler, U., Wang, X., Crambert, G., Beguin, P., Jaisser, F., Horisberger, J.D., and Geering, K. (1998). Role of beta-subunit domains in the assembly, stable expression, intracellular routing, and functional properties of Na,K-ATPase. J. Biol. Chem. 273, 30826-30835. [DOI] [PubMed] [Google Scholar]
- Hiatt, A., McDonough, A.A., and Edelman, I.S. (1984). Assembly of the (Na+ + K+)-adenosine triphosphatase. Post-translational membrane integration of the alpha subunit. J. Biol. Chem. 259, 2629-2635. [PubMed] [Google Scholar]
- Horowitz, B., Eakle, K.A., Scheiner-Bobis, G., Randolph, G.R., Chen, C.Y., Hitzeman, R.A., and Farley, R.A. (1990). Synthesis and assembly of functional mammalian Na,K-ATPase in yeast. J. Biol. Chem. 265, 4189-4192. [PubMed] [Google Scholar]
- Katz, A.I. (1988). Role of Na-K-ATPase in kidney function. Prog. Clin. Biol. Res. 268, 207-232. [PubMed] [Google Scholar]
- Lescale-Matys, L., Hensley, C.B., Crnkovic-Markovic, R., Putnam, D.S., and McDonough, A.A. (1990). Low K+ increases Na,K-ATPase abundance in LLC-PK1/Cl4 cells by differentially increasing beta, and not alpha, subunit mRNA. J. Biol. Chem. 265, 17935-17940. [PubMed] [Google Scholar]
- Lingrel, J.B., and Kuntzweiler, T. (1994). Na+,K(+)-ATPase. J. Biol. Chem. 269, 19659-19662. [PubMed] [Google Scholar]
- Mazumder, B., Seshadri, V., and Fox, P.L. (2003). Translational control by the 3′-UTR: the ends specify the means. Trends. Biochem. Sci. 28, 91-98. [DOI] [PubMed] [Google Scholar]
- McDonough, A.A., Geering, K., and Farley, R.A. (1990). The sodium pump needs its beta subunit. FASEB J. 4, 1598-1605. [DOI] [PubMed] [Google Scholar]
- Mercer, R.W. (1993). Structure of the Na,K-ATPase. Int. Rev. Cytol. 139-168. [PubMed]
- Noguchi, S., Higashi, K., and Kawamura, M. (1990a). Assembly of the alpha-subunit of Torpedo californica Na+/K(+)-ATPase with its pre-existing beta-subunit in Xenopus oocytes. Biochim. Biophys. Acta 1023, 247-253. [DOI] [PubMed] [Google Scholar]
- Noguchi, S., Higashi, K., and Kawamura, M. (1990b). A possible role of the beta-subunit of (Na,K)-ATPase in facilitating correct assembly of the alpha-subunit into the membrane. J. Biol. Chem. 265, 15991-15995. [PubMed] [Google Scholar]
- Noguchi, S., Mishina, M., Kawamura, M., and Numa, S. (1987). Expression of functional (Na+ + K+)-ATPase from cloned cDNAs. FEBS Lett. 225, 27-32. [DOI] [PubMed] [Google Scholar]
- Rajasekaran, A.K., Langhans-Rajasekaran, S.A., Gould, R.M., Rodriguez-Boulan, E., and Morimoto, T. (1995). A simple biochemical approach to quantitate rough endoplasmic reticulum. Am. J. Physiol. 268, C308-C316. [DOI] [PubMed] [Google Scholar]
- Rajasekaran, S.A., Ball, W.J., Jr., Bander, N.H., Liu, H., Pardee, J.D., and Rajasekaran, A.K. (1999). Reduced expression of beta-subunit of Na,K-ATPase in human clear-cell renal cell carcinoma. J. Urol. 162, 574-580. [PubMed] [Google Scholar]
- Rajasekaran, S.A., Palmer, L.G., Quan, K., Harper, J.F., Ball, W.J., Jr., Bander, N.H., Peralta Soler, A., and Rajasekaran, A.K. (2001). Na,K-ATPase beta-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol. Biol. Cell 12, 279-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamraj, O.I., and Lingrel, J.B. (1994). A putative fourth Na+,K(+)-ATPase alpha-subunit gene is expressed in testis. Proc. Natl. Acad. Sci. USA 91, 12952-12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull, G.E., Lane, L.K., and Lingrel, J.B. (1986). Amino-acid sequence of the beta-subunit of the (Na+ + K+)ATPase deduced from a cDNA. Nature 321, 429-431. [DOI] [PubMed] [Google Scholar]
- Shull, G.E., Schwartz, A., and Lingrel, J.B. (1985). Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature 316, 691-695. [DOI] [PubMed] [Google Scholar]
- Sun, Y., and Ball, W.J., Jr. (1994). Identification of antigenic sites on the Na+/K(+)-ATPase beta-subunit: their sequences and the effects of thiol reduction upon their structure. Biochim. Biophys. Acta 1207, 236-248. [DOI] [PubMed] [Google Scholar]
- Takeyasu, K., Tamkun, M.M., Renaud, K.J., and Fambrough, D.M. (1988). Ouabain-sensitive (Na+ + K+)-ATPase activity expressed in mouse L cells by transfection with DNA encoding the alpha-subunit of an avian sodium pump. J. Biol. Chem. 263, 4347-4354. [PubMed] [Google Scholar]
- Tamkun, M.M., and Fambrough, D.M. (1986). The (Na+ + K+)-ATPase of chick sensory neurons. Studies on biosynthesis and intracellular transport. J. Biol. Chem. 261, 1009-1019. [PubMed] [Google Scholar]
- Therien, A.G., Goldshleger, R., Karlish, S.J., and Blostein, R. (1997). Tissue-specific distribution and modulatory role of the gamma subunit of the Na,K-ATPase. J. Biol. Chem. 272, 32628-32634. [DOI] [PubMed] [Google Scholar]
- Twiss, J.L., Smith, D.S., Chang, B., and Shooter, E.M. (2000). Translational control of ribosomal protein L4 mRNA is required for rapid neurite regeneration. Neurobiol. Dis. 7, 416-428. [DOI] [PubMed] [Google Scholar]
- Woo, A.L., James, P.F., and Lingrel, J.B. (2000). Sperm motility is dependent on a unique isoform of the Na,K-ATPase. J. Biol. Chem. 275, 20693-20699. [DOI] [PubMed] [Google Scholar]
- Xie, Y., Langhans-Rajasekaran, S.A., Bellovino, D., and Morimoto, T. (1996). Only the first and the last hydrophobic segments in the COOH-terminal third of Na,K-ATPase alpha subunit initiate and halt, respectively, membrane translocation of the newly synthesized polypeptide. Implications for the membrane topology. J. Biol. Chem. 271, 2563-2573. [DOI] [PubMed] [Google Scholar]
- Zheng, J.Q., Kelly, T.K., Chang, B., Ryazantsev, S., Rajasekaran, A.K., Martin, K.C., and Twiss, J.L. (2001). A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J. Neurosci. 21, 9291-9303. [DOI] [PMC free article] [PubMed] [Google Scholar]