Abstract
INTRODUCTION
The importance of internal mammary nodes (IMNs) in the staging and treatment of breast cancer patients is controversial.
METHODS
A retrospective cohort of patients diagnosed with primary breast cancer over a 4-year period (January 2009 to December 2012) was assessed. The number and size of any IMNs visible on spiral computed tomography (CT) were recorded.
RESULTS
A total of 830 patients were diagnosed with primary breast cancer within the identified time frame, of which 150 patients met the inclusion criteria. Of these 42% (63) had IMNs present, although the majority were small (<5 mm). However, 16% (25) had larger nodes, greater than 5 mm in short axis, present on CT. Significantly more patients with the presence of large (>5 mm) IMNs had more advanced disease with CT evidence of other distant spread.
CONCLUSION
We have demonstrated that IMNs are present in a substantial number of our primary breast cancer patients. We suggest that further histological research is required to establish reliable CT size criterion for pathological IMNs. In addition, routine imaging of the IM chain, as well as axilla, should be considered in the staging of breast cancer.
Keywords: internal mammary nodes (IMN), breast carcinoma, breast cancer, imaging, computer tomography (CT), staging
Introduction
The importance of internal mammary lymph nodes (IMNs) in the staging and treatment of breast cancer patients has long been a controversial issue.1 Lymphoscintigraphic studies have demonstrated that a significant proportion of breast cancers’ have primary IMN drainage, including 30% of medial and 15% of lateral tumors.2 In addition, up to 45% of tumors have partial IMN drainage.3 However, historical studies demonstrated that extended mastectomy to include dissection of IMNs conferred no significant benefit over a mastectomy alone.4 Further historical studies suggest that irradiation of the IMN chain may increase cardiac morbidity, possibly as a result of cardiac irradiation.5 Recent papers,6–8 however, suggest that targeted radiotherapy of the IMN chain is now possible. Additional reports suggest that IMN metastases can be safely removed surgically. 9,10 Therefore, if it were possible to demonstrate the IMN involved with noninvasive imaging techniques, there are new treatment techniques that could be used with relatively low morbidity.
To our knowledge and on review of the literature, there is no recognized size criterion for abnormal IMN on computed tomography (CT). However Noushi et al11 states that normal IMN are very small (3–4 mm) and Kinoshita et al12 reports a 90.7% accuracy for magnetic resonance imaging (MRI) using the size-based criterion of ≥5 mm in the long axis.
Methods
We performed an audit of all CT thorax examinations performed on patients within 12 months following the diagnosis of primary breast cancer from January 2009 to December 2012 (48 months). This was approved and registered with the local NHS trust. As the data were collected retrospectively from existing databases, specific ethical approval and patient consent was not required. All CT scans were performed on a Toshiba Aquilion 64-slice CT scanner in the County Durham and Darlington NHS Foundation Trust (CDDFT). Patients were identified from existing cancer services databases within the NHS trust. Using the picture archiving and communications system and radiology information system, those patients who had a spiral CT thorax performed after the diagnosis of primary breast cancer, but within a 12-month window of this diagnosis, were identified. High-resolution CT was excluded.
The CT imaging was then reviewed by a radiology registrar and supervised by a consultant radiologist. Axial, coronal, and sagittal image sets were reviewed. The presence or absence of IMNs was documented. Thus, where lymph nodes were present, it was recorded whether the nodes were 5 mm or greater in the short-axis diameter as it was felt that these were more likely to represent pathological nodes. The clinical indication for the CT thorax examination and whether the breast cancer was a primary or recurrent malignancy were also noted. The site of the breast cancer was recorded (laterality and position within the breast) as documented in the prior diagnostic imaging reports: mammography and ultrasound (US). Note was made of any other evidence of disease spread (axillary adenopathy or distant metastases) on the CT imaging.
Statistics
Comparison of all the variables described above was made between the cohort of patients with no IMNs and the cohort with IMNs present using Fisher’s exact and chi-squared tests. A subsequent comparison of the cohort without IMNs and the cohort with IMNs ≥5 mm was made. Patient age was analyzed using a t-test. The test used for each variable is indicated in the footnote. A P-value of ≤0.05 was considered significant. MedCalc version 14.8.1.0 was used for all calculations except Fisher’s exact test for greater than two variables. In these instances, VassarStats: Website for Statistical Computation © Rich-ard Lowry 1998–2015 (http://vassarstats.net) was used for statistical analysis.
Results
A total of 830 patients were diagnosed within CDDFT with primary breast cancer within the identified 48-month time frame. Of these, 196 had CT imaging within 12 months of their diagnosis and met the eligibility criteria for this audit. Sixteen patients were excluded because they had an additional malignancy; therefore, it would be impossible to be certain whether enlarged IMNs were a result of metastatic spread from the breast cancer, as opposed to the other underlying malignancy. A further 31 patients were excluded because either the IMN chain was incompletely imaged (22 patients) or the CT images could not be retrieved in full (9 patients). The remaining 149 patients were included in the data analysis.
The patient age within each group was similar, with means ranging from 60.77 to 62.63 years. The vast majority of CTs were performed as part of staging work-up, with a minority being CT pulmonary angiograms performed to exclude pulmonary emboli. Of the 149 patients included, 42% (62) had IMNs present, although the majority were small (<5 mm). However, 16% (25) had larger nodes greater than 5 mm present on CT. Of these, the IMNs were only commented on in the CT report in four cases. In one of these instances, the node present was greater than 10 mm in the short axis (Fig. 1). Comparison between patients with no IMNs present and IMNs of any size is displayed in Table 1. Table 2 demonstrates the comparison between patients with no IMNs and those with IMNs greater than 5 mm in the short axis.
Figure 1.
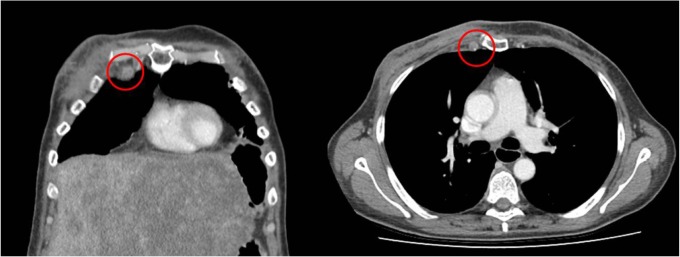
Right IMN >10 mm in short axis in a patient with florid liver metastases.
Table 1.
Patients without IMNs present compared to patients with IMNs of any size present.
| NO IMN | IMN (ANY SIZE) | P VALUE | |
|---|---|---|---|
| Age | 60.77 +/− 15.07 | 62.63 +/− 15.52 | P = 0.46* |
| Primary/Recurrent | |||
| Primary | 63 (0.72) | 53 (0.85) | |
| Recurrent | 19 (0.22) | 6 (0.10) | |
| Unknown | 5 (0.06) | 3 (0.05) | P = 0.12¥ |
| Indication | |||
| ?PE | 10 (0.16) | 3 (0.05) | |
| Staging | 77 (0.86) | 59 (0.95) | P = 0.24¥ |
| Laterality | |||
| Right | 43 (0.49) | 26 (0.42) | |
| Left | 41 (0.47) | 32 (0.52) | |
| Bilateral | 3 (0.03) | 4 (0.06) | P = 0.54¥ |
| Position | |||
| Medial | 12 (0.14) | 7 (0.11) | |
| Central | 18 (0.21) | 14 (0.23) | |
| Lateral | 33 (0.32) | 29 (0.47) | |
| Diffuse/multicentric | 6 (0.07) | 5 (0.08) | |
| Other/unknown | 18 (0.02) | 7 (0.02) | P = 0.58# |
| Distant disease | |||
| Present (incl. axillary cl) | 54 (0.62) | 46 (0.74) | |
| Absent | 33 (0.38) | 16 (0.26) | P = 0.15¥ |
| Distant disease | |||
| Present | 34 (0.39) | 29 (0.47) | |
| Axillary clearance only | 20 (0.23) | 17 (0.27) | |
| Absent | 33 (0.38) | 16 (0.26) | P = 0.30¥ |
| Metastases | |||
| Liver | |||
| Present | 6 (0.07) | 9 (0.15) | |
| Absent | 73 (0.84) | 51 (0.82) | |
| Unknown | 8 (0.09) | 2 (0.03) | P = 0.15¥ |
| Lung | |||
| Present | 9 (0.10) | 12 (0.19) | |
| Absent | 75 (0.86) | 50 (0.81) | |
| Unknown | 3 (0.03) | 0 (0.00) | P = 0.12¥ |
| Bone | |||
| Present | 18 (0.21) | 11 (0.18) | |
| Absent | 69 (0.68) | 51 (0.82) | P = 0.68¥ |
| Other | |||
| Present | 11 (0.13) | 11 (0.18) | |
| Absent | 76 (0.87) | 51 (0.82) | P = 0.48¥ |
Notes:
T-test,
Fisher’s exact test,
Chi-squared test.
Table 2.
Patients without IMNs present compared to patients with IMNs greater than 5 mm present.
| NO IMN | IMN (>5mm) | P VALUE | |
|---|---|---|---|
| Age | 60.77 +/− 15.07 | 62.49 +/− 14.82 | P = 0.49* |
| Primary/Recurrent | |||
| Primary | 63 (0.72) | 20 (0.80) | |
| Recurrent | 19 (0.22) | 3 (0.12) | |
| Unknown | 5 (0.06) | 2 (0.08) | P = 0.60¥ |
| Indication | |||
| ?PE | 10 (0.16) | 0 (0.00) | |
| Staging | 77 (0.86) | 25 (1.00) | P = 0.11¥ |
| Laterality | |||
| Right | 43 (0.49) | 12 (0.48) | |
| Left | 41 (0.47) | 12 (0.48) | |
| Bilateral | 3 (0.03) | 1 (0.04) | P = 0.99¥ |
| Position | |||
| Medial | 12 (0.14) | 3 (0.12) | |
| Central | 18 (0.21) | 6 (0.24) | |
| Lateral | 33 (0.38) | 9 (0.36) | |
| Diffuse/multicentric | 6 (0.07) | 3 (0.12) | |
| Other/unknown | 18 (0.21) | 4 (0.16) | P = 0.91# |
| Distant disease | |||
| Present (incl. axillary cl) | 54 (0.62) | 19 (0.88) | |
| Absent | 33 (0.38) | 3 (0.12) | P = 0.04¥ |
| Distant disease | |||
| Present | 34 (0.39) | 16 (0.64) | |
| Axillary clearance only | 20 (0.23) | 6 (0.24) | |
| Absent | 33 (0.38) | 3 (0.12) | P = 0.03¥ |
| Metastases | |||
| Liver | |||
| Present | 6 (0.07) | 4 (0.16) | |
| Absent | 73 (0.84) | 21 (0.84) | |
| Unknown | 8 (0.09) | 0 (0.00) | P = 0.13¥ |
| Lung | |||
| Present | 9 (0.10) | 6 (0.24) | |
| Absent | 75 (0.86) | 19 (0.76) | |
| Unknown | 3 (0.03) | 0 (0.00) | P = 0.18¥ |
| Bone | |||
| Present | 18 (0.21) | 3 (0.12) | |
| Absent | 69 (0.79) | 22 (0.88) | P = 0.39¥ |
| Other | |||
| Present | 11 (0.13) | 5 (0.20) | |
| Absent | 76 (0.87) | 20 (0.80) | P = 0.34¥ |
Notes:
T-test,
Fisher’s exact test,
Chi squared test.
The cohorts were similar in all variables recorded, except for the presence of distant disease spread and axillary adenopathy, which was significantly more common in patients with IMNs greater than 5 mm compared to those without IMN adenopathy (Table 2). When sites of distant metastases were analyzed separately; liver, lung, and bone, no significant difference was identified between the groups. This may be in part due to the small numbers involved, especially in the cohort with IMNs greater than 5 mm.
Figure 2 illustrates a patient with prominent axillary adenopathy and a concurrent IMN measuring greater than 5 mm the in short axis. By comparison, Figure 3 illustrates a patient with no other evidence of distant spread on CT.
Figure 2.
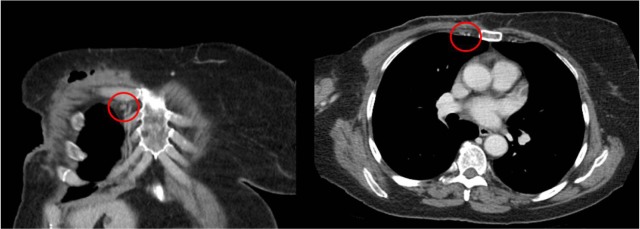
Right IMN >5 mm in short axis in a patient with prominent axillary adenopathy.
Figure 3.
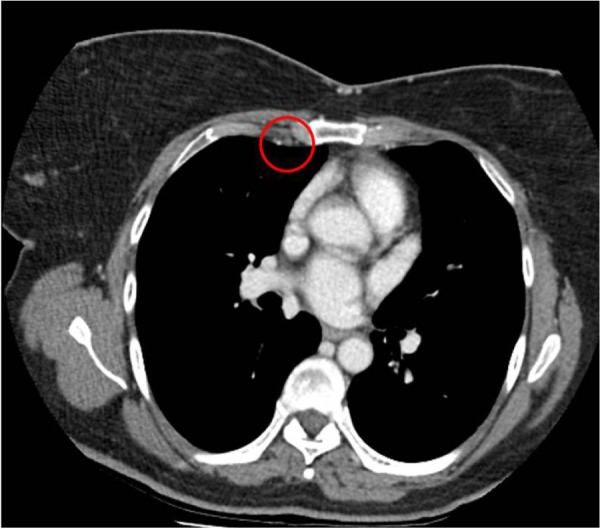
Right IMN with no other evidence of distant spread.
Discussion
In this retrospective review, we have demonstrated an incidence of 42% of IMNs present on CT in our population of patients within a year of diagnosis of a primary breast cancer. Although the majority were small (<5 mm), 16% (25) had larger nodes (>5 mm). Our results are similar to those of Zhang et al,13 who looked at the presence of IMN spread diagnosed on imaging studies. IMNs were considered positive on review of the imaging reports. They did not specify the size nor morphology criteria that were used for identifying suspicious nodes. We have identified no universally recognized size criteria for pathological internal mammary lymph nodes on CT, although Noushi et al11 describes normal IMNs as very small (3–4 mm). A small retrospective historadiological study of dissected nodes of 16 women with primary breast cancer and preoperative MRI studies concluded that 5 mm in long axis was an accurate size discriminator for pathological IMNs. They found that MRI had a 90.7% accuracy, 93.3% sensitivity, and 89.3% specificity using size-based criterion (≥5 mm as positive).10 These findings support our suggestion of using the size-criteria ≥5 mm in short axis; in fact, they suggest that we may be underestimating the incidence of pathological IMN in our population. By contrast, Eubank et al14 only classified nodes greater than 11 mm in short axis on CT as pathological, referring to the nodal map of Naruke et al.15 This map was designed for staging lung cancer and may not be appropriate for breast cancer staging. Interestingly, they identified abnormal fluorodeoxyglucose uptake on positron emission tomography (PET) in the mediastinum or IM chain approximately twice as frequently as abnormal nodes were identified on CT. We suggest that this discrepancy in PET and CT findings may be related to the lack of suspicion of smaller IMNs. From our own data, while the one case with an IMN >10 mm was commented on in the report, only in three of the remaining 24 cases with IMNs >5 mm were these documented in the original imaging report. This is certainly not a criticism of missed pathology on the part of the imaging radiologists who originally reported the CT imaging. As we have discussed above, the size criteria for what precisely constitutes an abnormal IMN is uncertain.
Incidence of IMN involvement
Zhang et al13 reported an incidence of 13.8% (112 of 809) using all modalities (CT, PET/CT, and US) with solitary nodal involvement in only 10 cases. In their study, of the 218 patients who had a CT thorax, 39 (17.9%) had IMN spread identified. This is very similar to the incidence of 16% we found of the lymph nodes greater than 5 mm in diameter. The MD Anderson Cancer Center routinely stage the IM chain using US and perform US-guided fine-needle aspirates (FNAs) of suspicious nodes when a positive result would significantly alter patient management.16 Over a 10-year period from 1996, they identified IMN involvement in 11% of patients.15 While this figure is lower that the incidence we identified, this may be accounted for by the different imaging modalities. In addition, it is likely that not all the nodes we identified as suspicious were truly sites of metastatic spread.
Effect of IMN involvement on treatment and survival
Breast cancer patients with IMN drainage identified on lymphoscintigraphy have previously been found to have a significantly worse distant disease-free survival (DDFS)18 and overall survival (OS).19 Kong et al18 found that DDFS was poorer in patients with IMN drainage if there was no difference in the rates of IMN irradiation in comparison to patients without IMN drainage. By comparison, Heuts et al20 demonstrated no significant difference in OS or DDFS between IMN-negative and IMN-positive patients. Importantly, they routinely perform internal mammary sentinel node biopsy following lymphoscintigraphy and tailor adjuvant therapy accordingly. This suggests that tailored therapy improves patient OS and DDFS to levels comparable to IMN-negative patients. Further studies indicate change to both chemotherapy regimens and radiation fields following histological confirmation of IMN involvement.13,21,22 There is meta-analysis evidence to support additional regional radiotherapy to the IMN, demonstrating a significant improvement in DDFS, distant metastasis-free survival and OS in stage I–III.23 These findings emphasize the importance of identifying pathological IMN to allow appropriate, modified treatment regimens to be instituted. Modifications may include instigating systemic therapy such as chemotherapy or hormonal therapy, which may not otherwise be used, for example, in instances where the sentinel node in the axilla was negative or increasing the radiotherapy field to include the IMN chain. Selection algorithms for identifying patients most at risk of IMN involvement have been proposed.13,21 However, they require lymphoscintigraphy data, not routinely available at many centers, including our own. As such, their application is limited.
IMN and distant disease
Within our data, significantly more patients with large (>5 mm) IMN adenopathy have distant spread elsewhere, either distant metastases or additional adenopathy. This is supported by previous literature, which finds the histopathological axillary nodal status to be important in assessing the risk of IMN involvement.1 From our data, it is not possible to assess whether there is a causal relationship. It is possible that IMN involvement increases the risk of developing metastatic disease, perhaps due to undertreatment or as an indicator of micrometastatic disease. However, it is equally possible that IMN adenopathy tends to occur late in disease progression, after the disease has already spread elsewhere.
There is limited evidence of the incidence of proven IMN involved in the literature. Both Choi24 and Wang et al25 sampled IMN identified as suspicious on PET/CT. Of sampled nodes, 72.2% and 80%, respectively, proved positive. Interestingly, Choi24 found no significant difference in the standardized uptake values (SUVs) of metastatic and nonmetastatic nodes. While this suggests that the SUV cannot be used as a substitute for tissue diagnosis, this may be slightly misleading, as it seems unlikely that nodes with low SUVs would have been considered suspicious and sampled in the first place. The mean size of the IM lymph nodes identified on PET/CT by Wang et al25 was 7.7 mm (standard deviation, 6.8 mm; range, 1.4–61 mm). This is comparable with our cohort of patients with larger IMNs. FNAs were performed in 25 cases, and of these, 20 (80%) were cytologically proven metastases.
Limitations
Our review may underestimate the true number of patients with IMN involvement. As this is a retrospective review, the majority of patients included had been scanned as part of staging work-up; this is only routinely performed for those with positive sentinel axillary nodes. Thus, a patient with the sentinel node in the IMN chain and no additional axillary involvement would not routinely have had a staging CT and would not, therefore, be included in our review. A further limitation to our study is the lack of histological confirmation of lymph node involvement.
Interestingly, contrary to previous lymphoscintigraphic studies, which demonstrated that a greater proportion of medial breast cancers have primary IMN drainage,2,3 we demonstrated no significant difference in the site of the breast cancer between the cohort of patients without any IMNs and those with IMNs present. This suggests that it is not possible to target screening or treatment of the IMN chain purely toward those with medial cancers and they should be considered a potential source of disease spread for all patients.
Conclusion
We have demonstrated that IMNs are present in a substantial number of our primary breast cancer patients. There was no significant difference in the incidence of IMN according to the site of breast cancer. Published data suggest that DDFS and OS are improved in IMN-positive patients when they receive modified treatment.20,23 Further research, including histological analysis, is required to establish reliable size criteria for identifying pathological IMNs on CT in breast cancer patients. This will allow subsequent appropriate modification of treatment regimens. We suggest that routine imaging of the IMN chain, as well as axilla, should be considered in the staging of all breast cancer patients.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: SS, JC. Analyzed the data: SS. Wrote the first draft of the manuscript: SS. Contributed to the writing of the manuscript: JC. Agree with manuscript results and conclusions: SS, JC, JS. Jointly developed the structure and arguments for the paper: SS, JC, JS. Made critical revisions and approved final version: SS, JC, JS. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Delaney G, Stebbing J, Thompson A. Breast Cancer (non-metastatic) BMJ Clin Evid. 2011;2011:0102. [PMC free article] [PubMed] [Google Scholar]
- 2.Manca G, Volterrani D, Mazzarri S, et al. Sentinel lymph node mapping in breast cancer: a critical reappraisal of the internal mammary chain issue. Q J Nucl Med Mol Imaging. 2014;58(2):114–126. [PubMed] [Google Scholar]
- 3.Cranenbroek S, van der Sangen MJ, Kuijt GP, Voogd AC. Diagnosis, treatment and prognosis of internal mammary lymph node recurrence in breast cancer patients. Breast Cancer Res Treat. 2005;89(3):271–275. doi: 10.1007/s10549-005-2469-y. [DOI] [PubMed] [Google Scholar]
- 4.Lacour J, Bucalossi P, Cacers E, et al. Radical mastectomy versus radical mastectomy plus internal mammary dissection. Five-year results of an international cooperative study. Cancer. 1976;37:206–214. doi: 10.1002/1097-0142(197601)37:1<206::aid-cncr2820370130>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Chen RC, Lin NU, Golshan M, Harris JR, Bellon JR. Internal mammary nodes in breast cancer: diagnosis and implications for patient management—a systematic review. J Clin Oncol. 2008;26(30):4981–4989. doi: 10.1200/JCO.2008.17.4862. [DOI] [PubMed] [Google Scholar]
- 6.Mansur DB, El Naqa I, Kong F, et al. Localization of internal mammary lymph nodes by CT simulation: implications for breast therapy planning. Radiother Oncol. 2004;73(3):355–357. doi: 10.1016/j.radonc.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Kirova YM, Servois V, Campana F, et al. CT-scan based localization of the internal mammary chain and supra clavicular nodes for breast cancer radiation therapy planning. Radiother Oncol. 2006;79(3):310–315. doi: 10.1016/j.radonc.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Caudrelier JM, Meng J, Esche B, Grimard L, Ruddy T, Amjadi K. IMRT sparing normal tissues in locoregional treatment of breast cancer. Radiat Oncol. 2014;22(9):161. doi: 10.1186/1748-717X-9-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrado-Abrão F, Das-Neves-Pereira JC, Fernandes A, Jatene FB. Thoracoscopic approach in the treatment of breast cancer relapse in the internal mammary lymph nodes. Interact Cardiovasc Thorac Surg. 2010;11(3):328–330. doi: 10.1510/icvts.2010.240606. [DOI] [PubMed] [Google Scholar]
- 10.van Geel AN, Wouters MW, van der Pol C, Schmitz PIM, Lans T. Chest wall resection for internal mammary lymph node metastases of breast cancer. Breast. 2009;18(2):94–99. doi: 10.1016/j.breast.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Noushi F, Spillane AJ, Uren RF, Gebski V. Internal mammary lymph node metastases in breast cancer: predictive models to assist with prognostic influence. Breast. 2011;20(3):278–283. doi: 10.1016/j.breast.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita T, Odagiri K, Andoh K, et al. Evaluation of small internal mammary lymph node metastases in breast cancer by MRI. Radiat Med. 1999;17(3):189–193. [PubMed] [Google Scholar]
- 13.Zhang YJ, Oh JL, Whitman GJ, et al. Clinically apparent internal mammary nodal metastasis in patients with advanced breast cancer: incidence and local control. Int J Radiat Oncol Biol Phys. 2010;77(4):1113–1119. doi: 10.1016/j.ijrobp.2009.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eubank WB, Mankoff DA, Takasugi J, et al. 18Fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J Clin Oncol. 2001;19(15):3516–3523. doi: 10.1200/JCO.2001.19.15.3516. [DOI] [PubMed] [Google Scholar]
- 15.Naruke T, Suemasu K, Ishikawa S. Lymph node mapping and curability at various levels of metastases in resected lung cancer. J Thorac Cardiovasc Surg. 1978;76:832–839. [PubMed] [Google Scholar]
- 16.Fornage BD. Local and regional staging of invasive breast cancer with sonography: 25 years of practice at MD Anderson Cancer Centre. Oncologist. 2014;19(1):5–15. doi: 10.1634/theoncologist.2013-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyengar P, Strom EA, Zhang YJ, et al. The value of ultrasound in detecting extra-axillary regional node involvement in patients with advanced breast cancer. Oncologist. 2012;17:1401–1408. doi: 10.1634/theoncologist.2012-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong AL, Tereffe W, Hunt KK, et al. Impact of internal mammary lymph node drainage identified by preoperative lymphoscintigraphy on outcomes in patients with stage I to stage III breast cancer. Cancer. 2012;118(24):6287–6296. doi: 10.1002/cncr.27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao MS, Kurland BF, Smith AH, et al. Internal mammary nodal chain drainage is a prognostic indicator in axillary node-positive breast cancer. Ann Surg Oncol. 2007;14(10):2985–2993. doi: 10.1245/s10434-007-9473-x. [DOI] [PubMed] [Google Scholar]
- 20.Heuts EM, van der Ent FWC, Hulsewe KWE, von Meyenfeldt MF, Voogd AC. Results of tailored treatment for breast cancer patients with internal mammary lymph node metastases. Breast. 2009;18(4):254–258. doi: 10.1016/j.breast.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Bevilacqua JL, Gucciardo G, Cody HS, et al. A selection algorithm for internal mammary sentinel lymph node biopsy in breast cancer. Eur J Surg Oncol. 2002;28:603–614. doi: 10.1053/ejso.2002.1269. [DOI] [PubMed] [Google Scholar]
- 22.Caudle AS, Yi M, Hoffman KE, Mittendorf EA. Impact of identification of internal mammary sentinel lymph node metastases in breast cancer patients. Ann Surg Oncol. 2014;21:60–65. doi: 10.1245/s10434-013-3276-z. [DOI] [PubMed] [Google Scholar]
- 23.Budach W, Kammers K, Boelke E, Matuschek C. Adjuvant radiotherapy of regional nodes in breast cancer—a meta-analysis of randomised trials. Radiat Oncol. 2013;8:267. doi: 10.1186/1748-717X-8-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi JE. The metastatic rate of internal mammary lymph nodes when metastasis of internal mammary lymph node is suspected on PET/CT. J Breast Cancer. 2013;16(2):202–207. doi: 10.4048/jbc.2013.16.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang CL, Eissa MJ, Rogers JV, Aravkin AY, Porter BA, Beatty JD. (18)F-FDG PET/CT-positive internal mammary lymph nodes: pathologic correlation by ultrasound-guided fine-needle aspiration and assessment of associated risk factors. AJR Am J Roentgenol. 2013;200(5):1138–1144. doi: 10.2214/AJR.12.8754. [DOI] [PubMed] [Google Scholar]


