Abstract
Downregulation of p57Kip2 is involved in tumor progression, and S-phase kinase-associated protein 2 (Skp2) is an E3 ligase that regulates a variety of cell cycle proteins. However, the prognostic value of p57Kip2 and its correlation with Skp2 in breast cancer have not been fully elucidated. Here we report our study on the expression of p57Kip2 and Skp2 in 102 breast cancer patients by immunohistochemistry, and analysis of clinicopathologic parameters in relation to patient prognosis. The expression of p57Kip2 was negatively associated with Skp2 expression in breast cancer (r = −0.26, P = 0.009). Kaplan–Meier analysis indicated that both high Skp2 and low p57Kip2 correlated with poor disease-free survival (DFS) (P = 0.05), and a group with the combination of high Skp2/low p57Kip2 demonstrated even worse DFS (log-rank = 21.118, P < 0.001). In addition, univariate analysis showed that Skp2, p57Kip2, histological grade, lymph node metastasis, and estrogen and progesterone receptors (ER and PR) were all associated with DFS, and multivariate analysis revealed that lymph node metastasis and Skp2 were independent prognostic biomarkers. The correlation between p57 and Skp2 was further demonstrated in multiple breast cancer cell lines and cell cycle phases. Half-life and immunoprecipitation (IP) experiments indicated that Skp2 directly interacts with p57Kip2 and promotes its degradation, rather than its mutant p57Kip2 (T310A). Overall, our findings demonstrate that Skp2 directly degrades p57Kip2, and an inverse correlation between these proteins (high skp2/low p57Kip2) is associated with poor prognosis in breast cancer. Thus, our results indicate a combined prognostic value of these markers in breast cancer diagnosis and treatment.
Keywords: Skp2, p57Kip2, breast cancer, degradation, prognosis
Introduction
Breast cancer is a leading cause of death from malignant cancer among women worldwide. According to estimates, 235,030 new breast cancer cases were diagnosed and 40,430 patients died in 2014.1,2 Although many generally accepted biomarkers and therapeutic targets [such as estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER-2), and BRCA1] have already been employed in clinical evaluation, variations in patient outcomes are difficult to explain. Therefore, identifying reliable biomarkers for diagnosis, prognosis, and treatment is an urgent goal in breast cancer research.
Cyclin-dependent kinase inhibitors (CKIs) are a family of enzymes that govern cell cycle progression by negatively regulating cyclin-dependent kinases (CDKs). CKI deregulation leads to uncontrolled proliferation and tumorigenesis.3 The newest member of the Cip/Kip family, p57Kip2 (whose function has not been fully elucidated), is considered to be an important putative tumor suppressor gene. A number of studies, including our recent one,4 have shown that p57Kip2 protein expression is markedly reduced in a variety of cancers, including hepatocellular carcinoma,4–6 colorectal carcinoma,7 ovarian cancer,8–10 and breast cancer.11–13 Moreover, several studies have shown that p57Kip2 is implicated in breast tumorigenesis. p57Kip2 is expressed at low levels during human mammary epithelial cell immortalization,14 which is regulated by estradiol in mammary carcinoma cells.15,16 Pamela et al have assessed the p57Kip2 allele imbalance/loss of heterozygosity (AI/LOH) and the p57Kip2 protein level in breast cancer samples and found that this gene does not undergo AI/LOH, but p57Kip2 protein levels are reduced compared to those in normal breast tissue. While these studies suggest that p57Kip2 may be a prognostic marker in breast cancer, the prognostic value of p57Kip2 is not yet clear.
The S-phase kinase-associated protein 2 (Skp2), an indispensable member of the SKP1/CUL1/F-box protein (SCF) ubiquitin ligase complex, was identified as a protein that interacts with cyclin A and CDK2 and controls G1-Sprogression.17 Skp2 has been reported to be overexpressed in breast cancer and is related to poor prognosis.18–20 Recent studies have shown that Skp2 is also involved in the ubiquitin-mediated degradation of p27Kip1,21 p57Kip2,22 E2F-123 and cyclin E.24 Kamura et al have shown that p57Kip2 accumulates in Skp2−/− mouse embryonic fibroblast (MEF) cells during S phase and can bind to Skp2 in the HeLa cervical cancer cell line.22 In non-small-cell lung cancer (NSCLC), both p27Kip1 and p57Kip2 are expressed at much lower levels in cancer tissues and are associated with increased Skp2 expression.25 These studies demonstrate that Skp2 plays a vital role in regulating p57Kip2. However, the correlation between p57Kip2 and Skp2 in breast cancer has not been fully elucidated.
In this study, we examined the expression of p57Kip2 and Skp2 in breast cancer tissues and analyzed the clinicopathologic features in relation to patient prognosis. Furthermore, an inverse correlation between p57Kip2 and Skp2 and their functional relationship was found in breast cancer cell lines, indicating the combined prognostic value of p57Kip2 and Skp2 in breast cancer.
Materials and Methods
Clinical samples
Fresh surgical tissue samples were randomly obtained from 102 breast cancer patients in the Oncology Department of the First Affiliated Hospital, Xi’an Jiaotong University, between 2006 and 2008. All patients were diagnosed for breast cancer, which was histologically confirmed. Treatment after surgery was strictly administered according to the NCCN (National Comprehensive Cancer Network) guidelines. Clinicopathological data, including age, menopause status, ER, PR, HER-2, tumor size, differentiation, lymph nodes metastasis, TNM stage, disease-free survival (DFS), and overall survival, were collected for all cases. The follow-up time for all patients was over 5 years, and 42 of the 102 patients relapsed or died.
Ethics
This study was approved by the Ethics Committee of the Medical College, Xi’an Jiaotong University. Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Immunohistochemistry
Resected specimens were fixed with 10% formaldehyde and embedded in paraffin blocks. Five micrometer sections were deparaffinized with xylene and rehydrated in a series of ethanol concentrations. Endogenous peroxidase activity was blocked by immersion in 0.3% methanolic peroxide for 15 minutes. Immunoreactivity of the target antigens was enhanced by microwaving the sections for 10 minutes in 0.1 M citrate buffer, pH 6.0. Test sections were incubated with rabbit anti-Skp2 monoclonal antibody (1:200; Cell Signaling Technology) and rabbit anti-p57 antibody (C-20, 1:300; Santa Cruz Biotechnology) in the blocking solution at 4°C overnight and then with biotinylated secondary antibodies at a dilution of 1:250. The antibody binding sites were finally visualized by an avidin–biotin peroxidase complex solution and 3,3′-diaminobenzidine. For negative controls, the anti-Skp2 and anti-p57Kip2 antibodies were replaced with 1% bovine serum albumin in phosphate buffered saline (PBS). Skp2 and p57Kip2 immunoreactivity was observed in all samples, which were evaluated by three pathologists in a coded manner. Each section was observed in 10 random 20× power fields, including a minimum of 1000 cells, and scored for the degree of expression. The p57Kip2 sections were graded for the percentage of positive nuclear/cytoplasmic staining as follows: 1, <25%; 2, 26%–50%; 3, 51%–75%; and 4, >76%. The staining intensity was scored as follows: 0, no staining; 1, weak; 2, moderate; 3, strong. These two scores were added together and reanalyzed. Based on the sum, all p57Kip2 samples were categorized into two groups: 0–3, low expression; 4–7, high expression. To define high and low protein expression, we used a cutoff of 10% for Skp2 (which was used in previous studies)19,26,27 because it correlated well with quantitative immunoblot data.
Cell culture and transfection
MCF-7, SKBR3, MDA-MB-231, MDA-MB-468, and BT474 were cultured at 37°C under an atmosphere of 5% CO2 in DMEM or RPMI 1640 media (HyClone) supplemented with 10% fetal bovine serum (FBS; HyClone), 100 units/mL penicillin, and 100 g/mL streptomycin. T47D cells were transfected in the logarithmic growth phase with Lipofectamine™ 2000 (Invitrogen) following the manufacturer’s instructions, and subsequent experiments were performed 48 hours after transfection. Complementary DNAs (cDNAs) encoding human p57Kip2 (WT) and a non-phosphorylatable mutant p57Kip2 (T310A) were inserted into the pIRES2-EGFP vector. A cDNA encoding human Skp2 with an HA tag was subcloned into pcDNA3. A small interfering RNA (siRNA) and the control fragment for p57 RNAi were designed and synthesized by Shanghai GenePharma Co. Ltd (Shanghai). Cell synchronization was performed as previously described.28
Immunoblot analysis
Forty-eight hours after transfection, cells were scraped into the lysis buffer (150 mM NaCl, 25 mM Tris-HCl, pH 7.6, 1% NP-40, 0.1% SDS, and 1% sodium deoxycholate) and incubated for 30 minutes on ice. Normalized protein amounts were separated on 4%–12% SDS-PAGE gels and transferred to PVDF membranes (Millipore) by wet blotting. After blocking with 5% BSA at room temperature for 1 hour, the membranes were incubated with anti-p57Kip2 antibody (1:1000, Cell Signaling Technology), Skp2 antibody (1:1000, Cell Signaling Technology), or β-actin antibody (Sigma Chemicals) overnight at 4°C. The membranes were then incubated with horseradish peroxidase-labeled goat anti-rabbit IgG antibody (1:5000; Santa Cruz Biotechnology) for one hour at 25°C and developed using ECL (enhanced chemiluminescent) detection (Millipore).
Immunoprecipitation
MDA-MB-231 cells were transfected with Flag-p57 (WT), Flag-p57 (T310A), or a control vector for 48 hours, collected in modified lysis buffer (50 mM Tris-HCl, pH 7.5, 200 mM NaCl, 0.5% NP-40, 0.5 mM phenylmethysulfonyl fluoride, 1 mM NaF, and 0.1 mM sodium orthovanadate), and incubated for 30 minutes on ice. After centrifuging, the supernatants from the cell lysate were incubated with prepared Anti-Flag M2 Affinity Gel (Sigma Chemicals) for 2.5 hours at 4°C. The collected beads were washed three times with the lysis buffer and then eluted with SDS-PAGE sample buffer. The immunoblotting was performed as previously described. For immunodetection, primary anti-HA or anti-Flag rabbit antibodies (1:1000; Cell Signaling Technology) were used.
Statistics
All statistical analyses were performed using the SPSS version 16.0 (SPSS/IBM). Chi-square and Fisher’s exact tests were used to compare frequencies. The Kaplan–Meier method was used to assess prognosis after surgery, and the log-rank test was used to compare survival curves. Univariate and multivariate analyses were performed using the Cox regression model. The pathological variables were as follows: age (<50 years vs ≥50 years), menopausal status, histological grade (G1, G2, or G3), tumor size (<2 cm vs ≥2 cm), lymph nodes metastasis (negative or positive), stage (I, II, III), and ER, PR, and Her-2 levels. A P-value <0.05 was considered statistically significant.
Results
The correlation between Skp2 or p57Kip2 expression and clinicopathological parameters in breast cancer
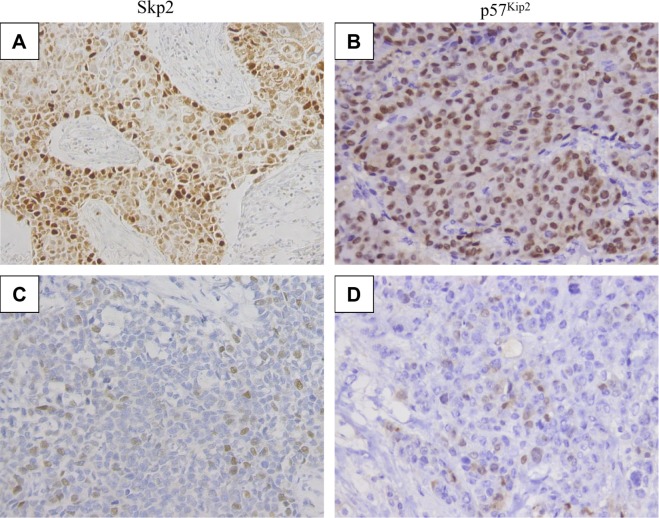
To assess the expression of Skp2 and p57Kip2, immunohistochemistry was performed in a series of 102 primary human breast cancers. Typical Skp2 and p57Kip2 staining is shown in Figure 1. Forty-one (40.2%) patients expressed high levels of Skp2 and low levels of p57Kip2, 24 (23.5%) patients expressed low levels of Skp2 and high levels of p57Kip2, and 15 (14.7%) patients expressed high levels of both Skp2 and p57Kip2, while 22 (21.6%) patients expressed low levels of both proteins. An inverse correlation was found between Skp2 and p57Kip2 expression in breast cancer (r = −0.26, P = 0.009) (Table 1).
Figure 1.
Representative slides demonstrating expression of p57Kip2 and Skp2 in breast cancer tissues. Tumor cells exhibit (A) high (×200) and (C) low (×200) Skp2 nuclear/cytoplasmic staining and (B) high (×200) and (D) low (×200) p57Kip2 stains in breast cancer tissues.
Table 1.
Correlation of Skp2 and p57 expression in breast cancer.
| Skp2 | r | P-VALUE | ||
|---|---|---|---|---|
| LOW | HIGH | |||
| p57 | ||||
| Low | 22 | 41 | −0.26 | 0.009 |
| High | 24 | 15 | ||
To examine the relationship between the expression levels of Skp2 and p57Kip2 and the clinicopathological patient data, the Skp2 and p57Kip2 levels were compared with the age at diagnosis, menopausal status, tumor differentiation, tumor size, lymph node metastasis, TNM stage, as well as ER, PR, and HER-2 expression (Table 2). The results suggest that the data represent an unselected group of breast cancers, and expression of both Skp2 and p57Kip2 was significantly associated with histological grade (P = 0.01). High expression of Skp2 was more frequently observed in higher grade (poorly differentiated) tumors than in lower grade tumors. In contrast, high expression of p57Kip2 was more frequently observed in lower grade (well or moderately differentiated) tumors. However, the Skp2 and p57Kip2 levels did not significantly correlate with the age at diagnosis, menopausal status, tumor size, lymph node metastasis, TNM stage, or ER, PR, or HER-2 levels (P = 0.05). Furthermore, Spearman correlation analysis revealed that the inverse correlation between Skp2 and p57Kip2 expression is much stronger in patients with poor tumor differentiation (G3). (r = −0.398, P = 0.006). However, no significant correlation between Skp2 and p57Kip2 was observed among the patients with well to moderately differentiated tumors (G1 or G2) (P > 0.05) (Table 3).
Table 2.
Relationship between the expression of Skp2 and p57kip2 and clinicopathological parameters.
| CHARACTERISTIC | Skp2 | p57 | ||||
|---|---|---|---|---|---|---|
| LOW | HIGH | P-VALUE | LOW | HIGH | P-VALUE | |
| Age | ||||||
| ≤50 y | 25 | 30 | 0.938 | 33 | 22 | 0.692 |
| >50 y | 21 | 26 | 30 | 17 | ||
| Menopausal status | ||||||
| Pre- | 19 | 27 | 0.485 | 30 | 16 | 0.515 |
| Post- | 27 | 29 | 33 | 23 | ||
| Histological grade | ||||||
| G1 | 13 | 7 | 0.02* | 6 | 14 | 0.003** |
| G2 | 18 | 16 | 22 | 12 | ||
| G3 | 15 | 33 | 35 | 13 | ||
| Tumor size | ||||||
| ≤2 cm | 18 | 15 | 0.185 | 17 | 16 | 0.141 |
| >2 cm | 28 | 41 | 46 | 23 | ||
| Lymph node status | ||||||
| Negative | 23 | 19 | 0.101 | 22 | 20 | 0.103 |
| Positive | 23 | 37 | 41 | 19 | ||
| AJCC stage | ||||||
| I | 8 | 3 | 0.121 | 5 | 6 | 0.507 |
| II | 26 | 33 | 37 | 22 | ||
| III | 12 | 20 | 21 | 11 | ||
| ER | ||||||
| Negative | 17 | 23 | 0.672 | 26 | 14 | 0.589 |
| Positive | 29 | 33 | 37 | 25 | ||
| PR | ||||||
| Negative | 17 | 26 | 0.335 | 29 | 14 | 0.314 |
| Positive | 29 | 30 | 34 | 25 | ||
| HER2 | ||||||
| Negative | 27 | 23 | 0.076 | 30 | 20 | 0.719 |
| Positive | 19 | 33 | 33 | 19 | ||
| Total | 46 | 56 | 63 | 39 | ||
Notes:
P < 0.05;
P < 0.01.
Table 3.
Correlation of Skp2 and p57 expression in different histological grades.
| p57 | Skp2 | r | P-VALUE | ||
|---|---|---|---|---|---|
| LOW | HIGH | ||||
| G1 | Low | 5 | 1 | 0.252 | 0.260 |
| High | 8 | 6 | |||
| G2 | Low | 10 | 12 | −0.203 | 0.236 |
| High | 8 | 4 | |||
| G3 | Low | 7 | 28 | −0.398 | 0.006** |
| High | 8 | 5 | |||
Note:
P < 0.01.
Prognostic significance of Skp2 and p57Kip2 expression
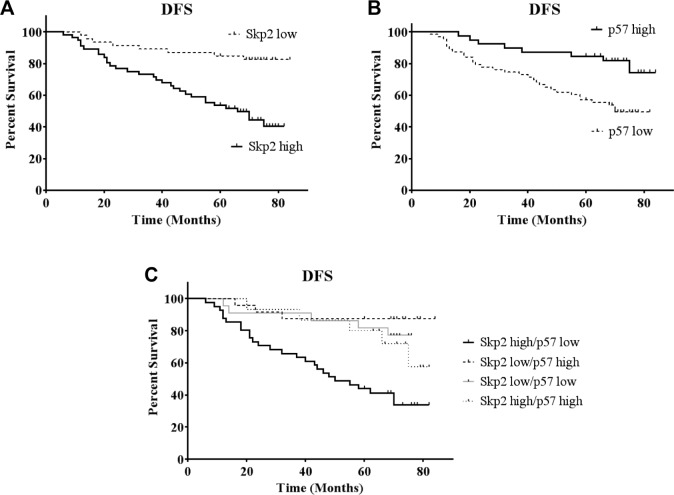
The patients were divided into two groups based on Skp2 expression levels. In terms of DFS, 33 of 56 (58.9%) patients with high Skp2 expression died or experienced relapse, whereas only 9 of 46 (19.6%) patients with low Skp2 expression died or experienced relapse. Kaplan–Meier analysis revealed that Skp2 expression was significantly associated with DFS (log-rank = 14.836, P < 0.001) in breast cancer patients. Specifically, increased Skp2 expression was associated with worse prognosis in these patients (Fig. 2A). If the patients were divided into two groups based on p57Kip2 expression, the DFS of patients with high p57Kip2 expression was significantly longer than those with low p57Kip2 expression (log-rank = 8.485, P = 0.004) (Fig. 2B). Therefore, in breast cancer patients, high Skp2 expression correlates with poor prognosis, whereas low p57Kip2 expression correlates with worse prognosis. To better evaluate the prognostic value of Skp2 and p57Kip2, the DFS of patients with the combination of high Skp2 and low p57Kip2 expression was evaluated (Fig. 2C). This group demonstrated a significantly shorter DFS than the other groups, which was much more obvious compared with Skp2 or p57Kip2 alone (log-rank = 21.118, P = 0.001).
Figure 2.
Association between Skp2 or p57Kip2 expression with overall survival. Disease-free survival was plotted as a function of (A) Skp2 expression, (B) p57Kip2 expression, and (C) Skp2 high/p57 low group. Analysis was done by Kaplan–Meier method.
To evaluate the prognostic value for breast cancer and further confirm the Kaplan–Meier analysis results, we used the univariate Cox proportional hazards regression model to analyze Skp2 and p57Kip2 expression, clinical pathological factors and the prognosis of breast cancer patients. Skp2, p57Kip2, histological grade, lymph node metastasis, and ER and PR expression were all associated with DFS. Furthermore, the difference in DFS between patients with low Skp2 expression and those with high Skp2 expression was significant (4.104-fold, 95% CI 1.881–8.954, P < 0.001) (Table 4).
Table 4.
Univariate and multivariate COX risk model.
| VARIABLES | UNIVARIATE | MULTIVARIATE | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-VALUE | HR | 95% CI | P-VALUE | |
| Age | 0.600 | 0.312–1.154 | 0.126 | |||
| Grade | 1.723 | 1.082–2.744 | 0.022* | 1.495 | 0.879–2.544 | 0.138 |
| Size | 1.588 | 0.774–3.260 | 0.207 | |||
| Node | 3.681 | 1.689–8.025 | 0.001** | 2.837 | 1.246–6.458 | 0.013* |
| ER | 0.400 | 0.213–0.754 | 0.005** | 0.528 | 0.244–1.143 | 0.105 |
| PR | 0.420 | 0.222–0.794 | 0.008** | 0.630 | 0.286–1.386 | 0.251 |
| HER2 | 1.306 | 0.693–2.461 | 0.408 | |||
| p57 | 0.333 | 0.153–0.726 | 0.006** | 0.700 | 0.286–1.713 | 0.435 |
| Skp2 | 4.104 | 1.881–8.954 | <0.001** | 2.552 | 1.098–5.931 | 0.029* |
Notes:
P < 0.05;
P < 0.01.
The multivariate Cox proportional hazards regression model was used to evaluate the independent factors that affect DFS. The results demonstrated that lymph node metastasis (HR = 2.837, 95% CI 1.246–6.458, P = 0.013) and Skp2 expression (HR = 2.552, 95% CI 1.098–5.931, P = 0.029) affected the DFS. Taken together, Skp2 expression (but not p57Kip2 expression) is an independent factor that can be used to evaluate prognosis in breast cancer patients.
The inverse correlation between Skp2 and p57Kip2 expression in breast cancer cell lines
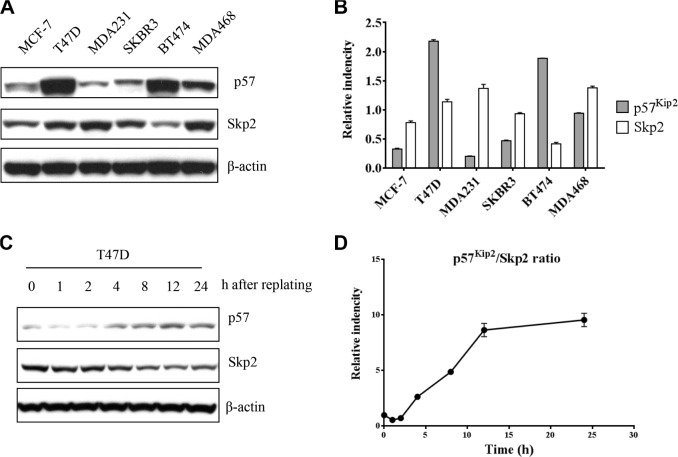
Because Skp2 and p57Kip2 expression is inversely correlated in clinical samples, we also assessed the protein expression of Skp2 and p57Kip2 in six breast cancer cell line models. Although all these cell lines were derived from human breast cancer, western blotting revealed that the levels of Skp2 and p57Kip2 expression differed between cell lines. However, all six cell lines demonstrated an inverse relationship between the levels of Skp2 and p57Kip2 (Fig. 3A, B). Among these cell lines, p57Kip2 expression was lowest in MDA-MB-231 and highest in T47D. Therefore, these two cell lines were selected for further study. Skp2 and p57 protein expression was then examined after synchronization induced by nocodazole in T47D cells. An inverse correlation between Skp2 and p57 was also observed in different cell cycle phases after release from prometaphase (Fig. 3C, D).
Figure 3.
Reverse expression of Skp2 and p57Kip2 in breast cancer cell lines. (A) Expression of p57Kip2 and Skp2 in six breast cancer cell lines. β-actin was used as a loading control. (B) The results were quantified in the graphs in the right. (C) T47D cells were released from 18 h nocodazole (0.4 μM/mL)-induced prometaphase arrest and collected at the indicated times. Skp2 and p57Kip2 expression showed reverse expression in different time points. The results were quantified to Skp2/p57Kip2 ratio in the graph as (D).
Promotion of p57Kip2 degradation by Skp2
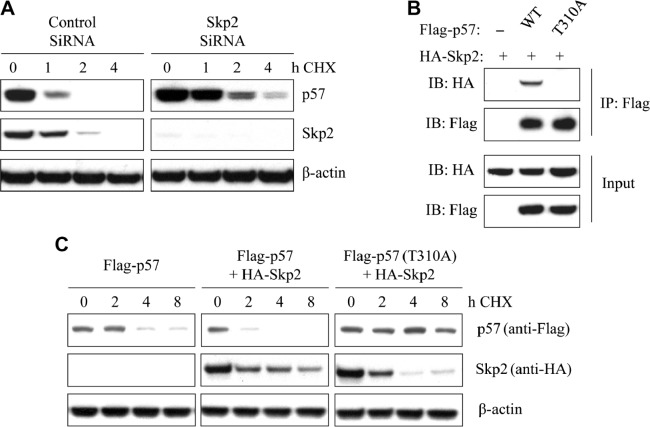
We hypothesized that Skp2 regulates the stability of p57Kip2 in breast cancer. T47D breast cancer cells were transfected with siRNA-Skp2 or control, and then incubated with the protein synthesis inhibitor CHX (cycloheximide, 20 μg/mL) for 0, 1, 2, and 4 hours. After exposure to CHX, T47D cells exhibited an almost complete loss of p57Kip2 within two hours. The degradation of endogenous p57Kip2 was much slower in Skp2-knockdown T47D cells compared with the control cells (Fig. 4A). These results indicate that knockdown of Skp2 inhibits the degradation of p57Kip2 in T47D cells.
Figure 4.
Association of Skp2 with p57Kip2 degradation in breast cancer cell lines. (A) After transfection with Skp2 siRNA or control for 48 hours, T47D cells were incubated for the indicated times with CHX (20 μg/mL). Cell lysates were then subjected to immunoblot analysis with antibodys to p57Kip2 or Skp2. (B) Cells were transfected with HA-Skp2 and Flag-p57 (WT) or Flag-p57 (T310A) for 48 hours and then incubated with 10 μM MG132 for 4 hours. After that, cell extracts were immunoprecipitated with an antibody against Flag and analyzed by immunoblotting. (C) After transfection with HA-skp2 and Flag-p57 (WT) or Flag-p57 (T310A) for 48 hours, cells were incubated for the indicated times with CHX (20 μg/mL). Cell lysates were then used for immunoblot analysis with antibodies to Flag or HA. β-Actin was used as a loading control.
To further investigate the association between Skp2 and p57Kip2, MDA-MB-231 cells were transfected with HA-Skp2 together with either wild-type (WT) Flag-p57Kip2 or a T310A mutant. At 48 hours after transfection, the cells were treated with 10 μM MG132 for an additional four hours. IP was performed to assess interactions between Skp2 and the WT or mutant forms of p57Kip2. The IP results showed that Skp2 could bind to the WT p57Kip2, but not the T310A mutant, in MDA-MB-231 cells (Fig. 4B).
MDA-MB-231 cells were then transfected with HA-Skp2, Flag-p57Kip2 (WT), or Flag-p57Kip2 (T310A) and incubated with CHX (20 μg/mL) for various periods. Overexpression of Skp2 promoted the degradation of p57Kip2. However, mutant p57Kip2 (T310A), which could not be phosphorylated, was degraded at a much slower rate than WT p57Kip2. This result suggests that phosphorylation of p57Kip2 at Thr310 is involved in p57Kip2 degradation (Fig. 4C).
Discussion
Skp2 is frequently expressed at high levels in breast cancer tissues18–20,29 and is closely related to the cell cycle.30 Previous studies have also shown that Skp2 is involved in the ubiquitin-mediated degradation of Cip/Kip family members.24,31 Here we have demonstrated that the expression of Skp2 and p57Kip2 is inversely correlated in breast cancer patient samples. Moreover, this correlation is stronger in poorly differentiated tumors compared with well and moderately differentiated tumors. This negative correlation was observed not only in clinical samples but also in multiple cell lines and cell cycle phases. These data suggest that Skp2 and p57Kip2 have underlying biological connections in breast cancer. IP and half-life experiments showed that Skp2 could directly bind to WT p57Kip2 but not mutant p57Kip2 (T310A). In addition, phosphorylation at this site is known to accelerate p57Kip2 degradation through Skp2. Previous studies in HeLa cervical cancer cells have demonstrated that Skp2 can ubiquitinate p57Kip2 but not the p57Kip2 (T310A) mutant in the context of Cks1 and cyclin E-CDK2 involvement.22 Our results are consistent with the data on HeLa cells and, therefore, suggest that high Skp2 expression may lead to an acceleration of p57Kip2 degradation, resulting in worse clinical outcome. As far as we know, one small-molecule inhibitor of Skp2 has been developed; this inhibitor can cause an increase in p27Kip1 levels without affecting its transcription.32,33 Perhaps this drug will be effective in patients with high Skp2/low p57 levels. Alternatively, the future development of a new inhibitor targeting both p57Kip and Skp2 might lead to promising therapeutic effects.
p57Kip2 is generally considered to be an important tumor suppressor gene in many cancers.34 As an imprinted gene located at chromosome 11p15.5, p57Kip2 has potent functions in regulating cyclins and CDKs, controlling DNA replication, and participating in tumor cell proliferation during G1-S and G2-M phases.35 Most p57Kip2-null mice die after birth and display severe developmental defects as a result of increased apoptosis and delayed differentiation.36 Therefore, we hypothesize that p57Kip2 may serve as a prognostic biomarker for breast cancer. Previous studies, including our own,4–6 have shown that p57 is frequently downregulated and associated with clinical outcome and tumor invasiveness in multiple types of cancers.7–13 In the present study, high p57Kip2 expression indeed correlated with a better tumor differentiation grade and longer DFS in breast cancer (P < 0.01). Moreover, we also found that cases with the combination high Skp2/low p57 expression had a significantly worse overall survival (P < 0.01). Univariate analysis showed that p57 expression was associated with DFS, but not an independent factor for prognosis. In contrast, Skp2 expression was elevated in cancer tissue and was considered to be an independent factor for the overall survival according to the multivariate Cox proportional analysis (P < 0.05).
These results prompted us to hypothesize that the relationship between p57Kip2 expression and prognosis is complicated. As discussed above, high levels of Skp2 can directly affect p57Kip2 degradation in breast cancer cells. The association between p57Kip2 and DFS might result from regulation by Skp2, which would affect p57Kip2 in the multivariate analysis calculations. Moreover, if we consider only the node, histological grade, and p57Kip2 expression in the multivariate analysis, p57 can be considered to be an independent prognostic marker (Supplementary Table 1). This phenomenon further suggests that p57Kip2 alone is not a reliable marker for prognosis, but a Skp2/p57 index could serve as a more promising marker for predicting clinical outcome.
Conclusion
In conclusion, we found that the expression of Skp2 and p57Kip2 negatively correlates in breast cancer tumor samples and cell lines. Both p57Kip2 and Skp2 were associated with breast cancer patient prognosis, and a high Skp2/low p57 index can predict an even worse prognosis. In addition, we have demonstrated that Skp2 can directly regulate p57Kip2 degradation in vivo in breast cancer cells. These findings demonstrate that Skp2 plays an important role in regulating p57Kip2, thus suggesting that the combination of these markers has prognostic value in breast cancer diagnosis and treatment.
Supplementary materials
Supplementary table 1. Multivariate COX risk model.
Acknowledgments
The authors wish to thank Kishina Gala, Zhiqiang Li, and Weiyi Toy for useful suggestions.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,241 words, excluding any confidential comments to the academic editor.
FUNDING: This study was supported in part by grants from the National Natural Science Foundation of China (Nos. 81172361, 81402506) and the Xi’an Social Development Plan. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: HG. Wrote the first draft of the manuscript and conducted most experiments: CY. Carried out immunostaining and data analysis: HN, YZ, and JM. Evaluated pathology of slices: LH, QG, JM. Developed the structure and arguments for the paper: LJ. Made critical revisions and approved final version: KN. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 3.Cicenas J, Valius M. The CDK inhibitors in cancer research and therapy. J Cancer Res Clin Oncol. 2011;137(10):1409–1418. doi: 10.1007/s00432-011-1039-4. [DOI] [PubMed] [Google Scholar]
- 4.Hu T, Guo H, Wang W, et al. Loss of p57 expression and RhoA overexpression are associated with poor survival of patients with hepatocellular carcinoma. Oncol Rep. 2013;30(4):1707–1714. doi: 10.3892/or.2013.2608. [DOI] [PubMed] [Google Scholar]
- 5.Nan K-J, Guo H, Ruan Z-P, Jing Z, Liu SX. Expression of p57 (kip2) and its relationship with clinicopathology, PCNA and p53 in primary hepatocellular carcinoma. World J Gastroenterol. 2005;11(8):1237–1240. doi: 10.3748/wjg.v11.i8.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo H, Lv Y, Tian T, et al. Downregulation of p57 accelerates the growth and invasion of hepatocellular carcinoma. Carcinogenesis. 2011;32(12):1897–1904. doi: 10.1093/carcin/bgr220. [DOI] [PubMed] [Google Scholar]
- 7.Li JQ, Wu F, Usuki H, et al. Loss of p57KIP2 is associated with colorectal carcinogenesis. Int J Oncol. 2003;23(6):1537–1543. [PubMed] [Google Scholar]
- 8.Guo J, Cai J, Yu L, Tang H, Chen C, Wang Z. EZH2 regulates expression of p57 and contributes to progression of ovarian cancer in vitro and in vivo. Cancer Sci. 2011;102(3):530–539. doi: 10.1111/j.1349-7006.2010.01836.x. [DOI] [PubMed] [Google Scholar]
- 9.Lu M, Zhao Y, Xu F, Wang Y, Xiang J, Chen D. The expression and prognosis of FOXO3a and Skp2 in human ovarian cancer. Med Oncol. 2012;29(5):3409–3415. doi: 10.1007/s12032-012-0275-z. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg E, Demopoulos RI, Zeleniuch-Jacquotte A, et al. Expression of cell cycle regulators p57(KIP2), cyclin D1, and cyclin E in epithelial ovarian tumors and survival. Hum Pathol. 2001;32(8):808–813. doi: 10.1053/hupa.2001.26462. [DOI] [PubMed] [Google Scholar]
- 11.Larson PS, Schlechter BL, King CL, et al. CDKN1C/p57kip2 is a candidate tumor suppressor gene in human breast cancer. BMC Cancer. 2008;8(1):68. doi: 10.1186/1471-2407-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Karuturi RK, Sun F, et al. CDKN1C (p57) is a direct target of EZH2 and suppressed by multiple epigenetic mechanisms in breast cancer cells. PLoS One. 2009;4(4):e5011. doi: 10.1371/journal.pone.0005011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu XY, Wang WQ, Zhang L, et al. Clinical implications of p57KIP2 expression in breast cancer. Asian Pac J Cancer Prev. 2012;13(10):5033–5036. doi: 10.7314/apjcp.2012.13.10.5033. [DOI] [PubMed] [Google Scholar]
- 14.Nijjar T, Wigington D, Garbe JC, Waha A, Stampfer MR, Yaswen P. p57KIP2 expression and loss of heterozygosity during immortal conversion of cultured human mammary epithelial cells. Cancer Res. 1999;59(20):5112–5118. [PubMed] [Google Scholar]
- 15.Moggs JG, Murphy TC, Lim FL, et al. Anti-proliferative effect of estrogen in breast cancer cells that re-express ERα is mediated by aberrant regulation of cell cycle genes. J Mol Endocrinol. 2005;34(2):535–551. doi: 10.1677/jme.1.01677. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez BA, Weng YI, Liu TM, et al. Estrogen-mediated epigenetic repression of the imprinted gene cyclin-dependent kinase inhibitor 1C in breast cancer cells. Carcinogenesis. 2011;32(6):812–821. doi: 10.1093/carcin/bgr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Kobayashi R, Galaktionov K, Beach D. pl9skp1 and p45skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell. 1995;82(6):915–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 18.Sonoda H, Inoue H, Ogawa K, Utsunomiya T, Masuda TA, Mori M. Significance of skp2 expression in primary breast cancer. Clin Cancer Res. 2006;12(4):1215–1220. doi: 10.1158/1078-0432.CCR-05-1709. [DOI] [PubMed] [Google Scholar]
- 19.Signoretti S, Di Marcotullio L, Richardson A, et al. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J Clin Invest. 2002;110(5):633–641. doi: 10.1172/JCI15795. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Traub F, Mengel M, Luck HJ, Kreipe HH, von Wasielewski R. Prognostic impact of Skp2 and p27 in human breast cancer. Breast Cancer Res Treat. 2006;99(2):185–191. doi: 10.1007/s10549-006-9202-3. [DOI] [PubMed] [Google Scholar]
- 21.Bloom J, Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin Cancer Biol. 2003;13(1):41–47. doi: 10.1016/s1044-579x(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 22.Kamura T, Hara T, Kotoshiba S, et al. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci U S A. 2003;100(18):10231–10236. doi: 10.1073/pnas.1831009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutterlüty H, Chatelain E, Marti A, et al. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1(4):207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama K, Nagahama H, Minamishima YA, et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. EMBO J. 2000;19(9):2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pateras IS, Apostolopoulou K, Koutsami M, et al. Downregulation of the KIP family members p27KIP1 and p57KIP2 by SKP2 and the role of methylation in p57KIP2 inactivation in nonsmall cell lung cancer. Int J Cancer. 2006;119(11):2546–2556. doi: 10.1002/ijc.22214. [DOI] [PubMed] [Google Scholar]
- 26.Davidovich S, Ben-Izhak O, Shapira M, Futerman B, Hershko DD. Overexpression of Skp2 is associated with resistance to preoperative doxorubicin-based chemotherapy in primary breast cancer. Breast Cancer Res. 2008;10(4):R63. doi: 10.1186/bcr2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravaioli A, Monti F, Regan MM, et al. International Breast Cancer Study Group p27 and Skp2 immunoreactivity and its clinical significance with endocrine and chemo-endocrine treatments in node-negative early breast cancer. Ann Oncol. 2008;19(4):660–668. doi: 10.1093/annonc/mdm547. [DOI] [PubMed] [Google Scholar]
- 28.Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCFSkp2-Cks1 ubiquitin ligase by the APC/CCdh1 ubiquitin ligase. Nature. 2004;428(6979):190–193. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- 29.Shapira M, Ben-Izhak O, Linn S, Futerman B, Minkov I, Hershko DD. The prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer. 2005;103(7):1336–1346. doi: 10.1002/cncr.20917. [DOI] [PubMed] [Google Scholar]
- 30.Susaki E, Nakayama K, Yamasaki L, Nakayama KI. Common and specific roles of the related CDK inhibitors p27 and p57 revealed by a knock-in mouse model. Proc Natl Acad Sci U S A. 2009;106(13):5192–5197. doi: 10.1073/pnas.0811712106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Z, Hunter T. Ubiquitylation and proteasomal degradation of the p21Cip1, p27Kip1and p57Kip2CDK inhibitors. Cell Cycle. 2014;9(12):2342–2352. doi: 10.4161/cc.9.12.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan CH, Morrow JK, Li CF, et al. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell. 2013;154(3):556–568. doi: 10.1016/j.cell.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu L, Grigoryan AV, Li Y, Hao B, Pagano M, Cardozo TJ. Specific small molecule inhibitors of Skp2-mediated p27 degradation. Chem Biol. 2012;19(12):1515–1524. doi: 10.1016/j.chembiol.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo H, Tian T, Nan K, Wang W. p57: a multifunctional protein in cancer (review) Int J Oncol. 2010;36(6):1321–1329. doi: 10.3892/ijo_00000617. [DOI] [PubMed] [Google Scholar]
- 35.Pateras IS, Apostolopoulou K, Niforou K, Kotsinas A, Gorgoulis VG. p57KIP2: “Kip”ing the cell under control. Mol Cancer Res. 2009;7(12):1902–1919. doi: 10.1158/1541-7786.MCR-09-0317. [DOI] [PubMed] [Google Scholar]
- 36.Yan Y, Frisen J, Lee M-H, Massague J, Barbacid M. Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev. 1997;11(8):973–983. doi: 10.1101/gad.11.8.973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1. Multivariate COX risk model.