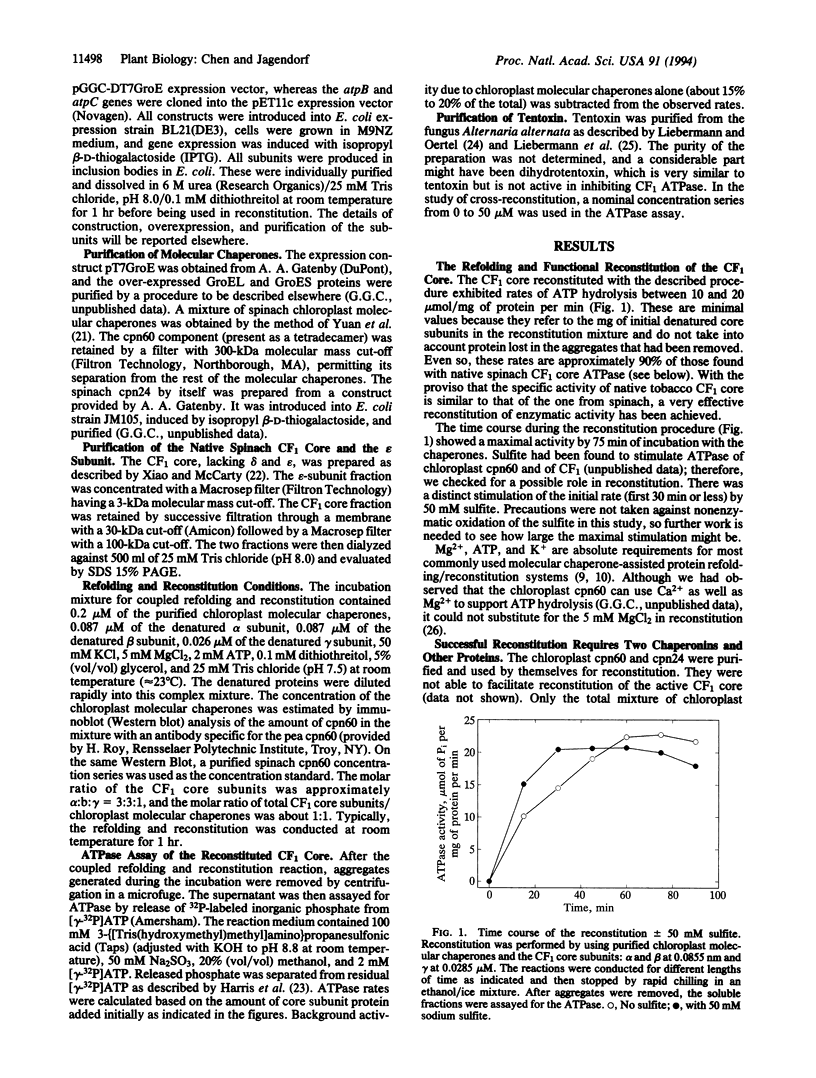
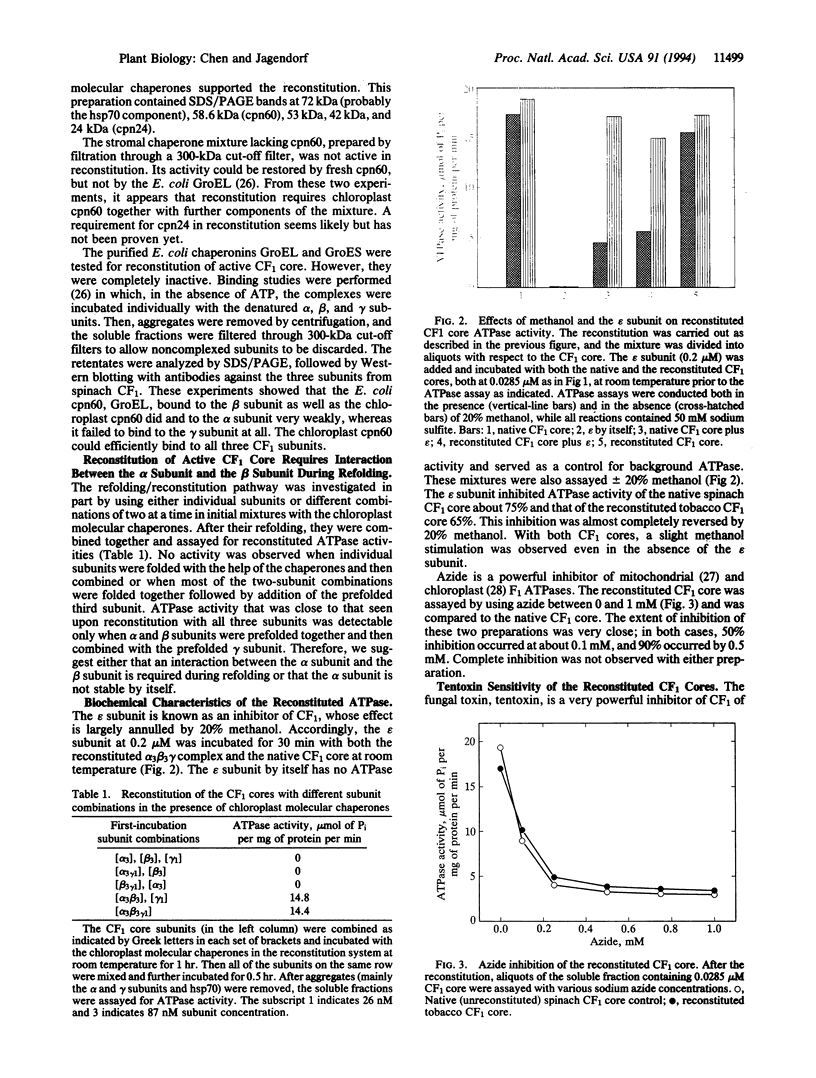
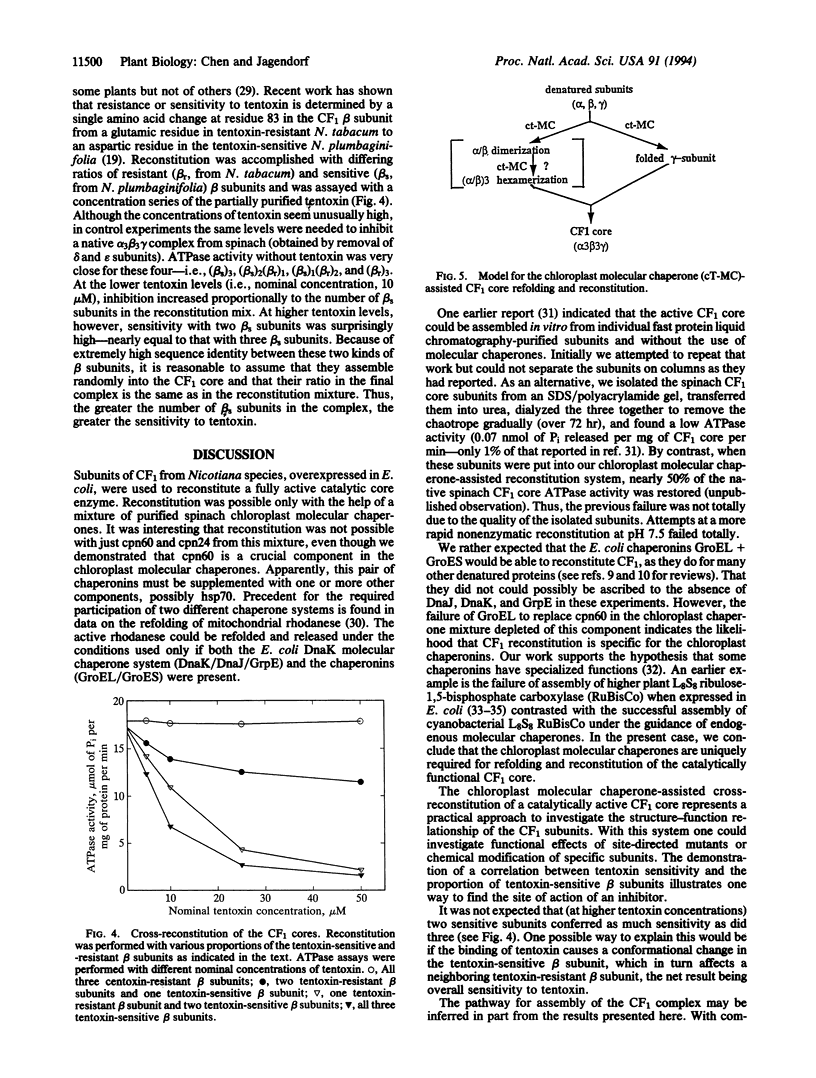
Abstract
The chloroplast coupling factor 1 (CF1) is composed of five kinds of subunits with a stoichiometry of alpha 3 beta 3 gamma delta epsilon. Reconstitution of a catalytically active alpha 3 beta 3 gamma core from urea-denatured subunits at a physiological pH is reported here. A restoration of approximately 90% of the CF1 ATPase activity has been observed. The reconstitution was achieved by using subunits overexpressed in Escherichia coli, purified, and combined in the presence of MgATP, K+, and a mixture of several chloroplast molecular chaperones at pH 7.5. The combination of chaperonin 60 and chaperonin 24 failed to reconstitute the active CF1 core, as did the GroEL/GroES pair (E. coli chaperonin 60/10 homologues). Characteristics of the reconstituted ATPase were very close to those of the native complex, including methanol-reversible inhibition by the purified epsilon subunit of CF1 and sensitivity to inhibition by azide and by tentoxin. In reconstitution with a mixture of tentoxin-resistant and -sensitive beta subunits, the extent of inhibition by tentoxin depended on the proportion of sensitive subunits in the reconstitution mixture. Finally, a model for the assembly of the CF1 core alpha 3 beta 3 gamma structure is proposed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avital S., Gromet-Elhanan Z. Extraction and purification of the beta subunit and an active alpha beta-core complex from the spinach chloroplast CFoF1-ATP synthase. J Biol Chem. 1991 Apr 15;266(11):7067–7072. [PubMed] [Google Scholar]
- Avni A., Anderson J. D., Holland N., Rochaix J. D., Gromet-Elhanan Z., Edelman M. Tentoxin sensitivity of chloroplasts determined by codon 83 of beta subunit of proton-ATPase. Science. 1992 Aug 28;257(5074):1245–1247. doi: 10.1126/science.1387730. [DOI] [PubMed] [Google Scholar]
- Avni A., Avital S., Gromet-Elhanan Z. Reactivation of the chloroplast CF1-ATPase beta subunit by trace amounts of the CF1 alpha subunit suggests a chaperonin-like activity for CF1 alpha. J Biol Chem. 1991 Apr 25;266(12):7317–7320. [PubMed] [Google Scholar]
- Bertsch U., Soll J., Seetharam R., Viitanen P. V. Identification, characterization, and DNA sequence of a functional "double" groES-like chaperonin from chloroplasts of higher plants. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8696–8700. doi: 10.1073/pnas.89.18.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daggett S. G., Tomaszek T. A., Jr, Schuster S. M. Interaction of azide with beef heart mitochondrial ATPase. Arch Biochem Biophys. 1985 Feb 1;236(2):815–824. doi: 10.1016/0003-9861(85)90688-5. [DOI] [PubMed] [Google Scholar]
- Dunn S. D., Futai M. Reconstitution of a functional coupling factor from the isolated subunits of Escherichia coli F1 ATPase. J Biol Chem. 1980 Jan 10;255(1):113–118. [PubMed] [Google Scholar]
- Gatenby A. A., Ellis R. J. Chaperone function: the assembly of ribulose bisphosphate carboxylase-oxygenase. Annu Rev Cell Biol. 1990;6:125–149. doi: 10.1146/annurev.cb.06.110190.001013. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Ellis R. J. Purification and properties of ribulosebisphosphate carboxylase large subunit binding protein. Plant Physiol. 1986 Jan;80(1):269–276. doi: 10.1104/pp.80.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick J. P., Hartl F. U. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Larsson K. H., Napier J. A., Gray J. C. Import and processing of the precursor form of the gamma subunit of the chloroplast ATP synthase from tobacco. Plant Mol Biol. 1992 May;19(2):343–349. doi: 10.1007/BF00027359. [DOI] [PubMed] [Google Scholar]
- Madueno F., Napier J. A., Gray J. C. Newly Imported Rieske Iron-Sulfur Protein Associates with Both Cpn60 and Hsp70 in the Chloroplast Stroma. Plant Cell. 1993 Dec;5(12):1865–1876. doi: 10.1105/tpc.5.12.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel R., Cloney L. P., Pelcher L. E., Hemmingsen S. M. Unique composition of plastid chaperonin-60: alpha and beta polypeptide-encoding genes are highly divergent. Gene. 1990 Oct 15;94(2):181–187. doi: 10.1016/0378-1119(90)90385-5. [DOI] [PubMed] [Google Scholar]
- Patrie W. J., McCarty R. E. Specific binding of coupling factor 1 lacking the delta and epsilon subunits to thylakoids. J Biol Chem. 1984 Sep 10;259(17):11121–11128. [PubMed] [Google Scholar]
- Richter M. L., Gromet-Elhanan Z., McCarty R. E. Reconstitution of the H+-ATPase complex of Rhodospirillum rubrum by the beta subunit of the chloroplast coupling factor 1. J Biol Chem. 1986 Sep 15;261(26):12109–12113. [PubMed] [Google Scholar]
- Richter M. L., Patrie W. J., McCarty R. E. Preparation of the epsilon subunit and epsilon subunit-deficient chloroplast coupling factor 1 in reconstitutively active forms. J Biol Chem. 1984 Jun 25;259(12):7371–7373. [PubMed] [Google Scholar]
- Tsugeki R., Nishimura M. Interaction of homologues of Hsp70 and Cpn60 with ferredoxin-NADP+ reductase upon its import into chloroplasts. FEBS Lett. 1993 Apr 12;320(3):198–202. doi: 10.1016/0014-5793(93)80585-i. [DOI] [PubMed] [Google Scholar]
- Wang H., Goffreda M., Leustek T. Characteristics of an Hsp70 homolog localized in higher plant chloroplasts that is similar to DnaK, the Hsp70 of prokaryotes. Plant Physiol. 1993 Jul;102(3):843–850. doi: 10.1104/pp.102.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. B., Feist G. L., Hemmingsen S. M. A modified Escherichia coli chaperonin (groEL) polypeptide synthesized in tobacco and targeted to the chloroplasts. Plant Mol Biol. 1993 Sep;22(6):1087–1100. doi: 10.1007/BF00028979. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Sone N., Hirata H., Kagawa Y. A highly stable adenosine triphosphatase from a thermophillie bacterium. Purification, properties, and reconstitution. J Biol Chem. 1975 Oct 10;250(19):7910–7916. [PubMed] [Google Scholar]
- Yuan J., Henry R., Cline K. Stromal factor plays an essential role in protein integration into thylakoids that cannot be replaced by unfolding or by heat shock protein Hsp70. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8552–8556. doi: 10.1073/pnas.90.18.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]