Abstract
Curcumin is a polyphenol compound extracted from ginger plant, turmeric, commonly used in a variety of food coloring and flavoring additives. Curcumin has many effects such as anti-inflammatory, anti-tumor, antioxidant and anti-microbial effects. However, the mechanism underlying the anti-cancer effect of curcumin on human osteoclastoma (Giant cell tumor, GCT) cells remains unclear. The objectives of this study were to determine the efficacy of curcumin on proliferation and apoptosis of GCT cells and its related mechanisms. In our study, cell viability, cellular apoptosis and caspase-3 activity of GCT cells were analyzed using 3.3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, flow cytometry (FCM) assay and commercial kits, respectively. Next, MMP-9 gene expression quantity, NF-κB activity and JNK protein expression of GCT cells were tested with real-time polymerase chain reaction (RT-PCR), commercial kits and western blotting assay, respectively. Firstly, MMP-9, NF-κB and JNK inhibitors were added into GCT cells and which was researched the mechanism of curcumin on human GCT cells. In this study, the efficacy of curcumin reduced cell viability, induced cellular apoptosis and increased caspase-3 activity of GCT cells. Furthermore, curcumin inhibited the MMP-9 gene expression quantity and NF-κB activity, and activated JNK protein expression in GCT cells. Meanwhile, down-regulation of MMP-9 gene expression quantity and NF-κB activity could promote the anti-cancer effect of curcumin on cell viability of GCT cells. Interesting, down-regulation of JNK protein expression could also reversed the anti-cancer effect of curcumin on cell viability of GCT cells. Taken together, our results suggest that curcumin inhibits cell proliferation and promotes apoptosis in osteoclastoma cell through suppression of MMP-9 and NF-κB, and activation JNK signaling pathways.
Keywords: Curcumin, osteoclastoma, MMP-9, NF-κB, JNK
Introduction
Osteoclastoma (giant cell tumor, GCT) is an invasive primary benign bone tumor with local recurrence tendency composed by the proliferative mononuclear cells and osteoclast-like multinucleated giant cells, and as it can have distant (lung) metastasis, it is also considered to be as middle or low-grade malignant bone tumor [1,2].
MMPs are a class of proteolytic enzymes, which can degrade all the components of the extracellular matrix [3]. Studies have shown that solid tumor growth, invasion and metastasis depend on the tiny blood vessels within the tumor. One of MMPs family members, MMP-9, can degrade the main component collagen of vascular basement membrane, thus considered to have close relationship with invasion and metastasis of GCT [4].
NF-κB is closely related to cell apoptosis and involved in the transcriptional regulations of various apoptosis-related genes, with two-way effect-inhibiting cell apoptosis and promoting cell apoptosis [5]. No matter what induces NF-κB activation, its main role is to inhibit cell death, and therefore NF-κB is considered to be anti-apoptotic factor. Meanwhile NF-κB is an important indicator of cell proliferation, of which the expression is significantly enhanced in malignant tumors. NF-κB plays important roles in the anti-cancer effect of paclitaxel on GCT [6].
c-Jun N-terminal kinase is protein kinase across the nuclear membrane, belonging to one of the mitotic activator protein kinase family members, which is an important signal transduction pathway for regulating the cell proliferation and apoptosis [7]. Research identified that Increased of c-JUN was also found in stromal cells of human giant cell tumor of bone[8].
Curcumin from is a natural active ingredient extracted from the dry rhizome of Curcuma Genus plants, turmeric, curcuma aromatic and curcuma zedoary, with extensive pharmacological effects, low toxicity, and good tolerance [9]. With increasing depth of research on curcumin, it has been found to have a wide range of pharmacological effects such as anti-inflammatory [10], antioxidant [11], lipid regulation [12], anti-viral [13], anti-infective [14], anti-tumor [15], anticoagulant [16], anti-liver fibrosis [17] and atherosclerosis [18], with low toxicity, and few side effects. However, whether curcumin has anti-cancer effects on GCT cells, as well as the underlying mechanism remain unknown to clinic treatment. In this study, we investigated the anti-cancer effects of curcumin on proliferation and apoptosis of GCT cells. We further explored whether curcumin could inhibit MMP-9 and NF-κB and activated JNK signaling pathways in GCT cells.
Materials and methods
Ethics statement
Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS) and TRIzol reagent were obtained from Hyclone (Invitrogen Company, USA). The chemical structure of curcumin (Sigma, with a purity ≥ 80%) was revealed in Figure 1. 3.3-(4,5-dimethylthiazol-2-yl)-2,5 -diphenyltetrazolium bromide (MTT) and dimethyl-sulfoxide (DMSO) were obtained from Sigma (USA). Annexin V-FITC/PI Apoptosis Detection kit and Apo-ONE Homogeneous Caspase-3 assay were obtained from BeastBio (Shanghai, China). SuperScript-II RT system was obtained from Life Technologies (Carlsbad, CA, USA). luciferase assay kit SYBR Green PCR master mix and Bicinchoninic Acid protein assay (BCA) were obtained from Beyotime (Nanjing, China).
Figure 1.
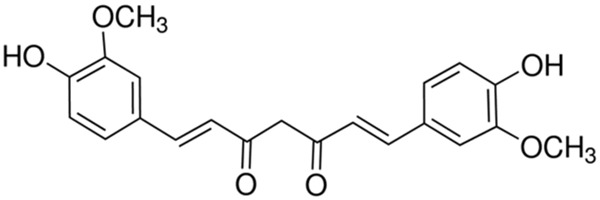
Chemical structure ofcurcumin.
GCT sample collection
The study was approved by the regional ethics committee of General Hospital of Tianjin Medical University and written informed consent was obtained from the patients. The diagnosis of GCT was established by biopsy prior to surgical excision. In this study, GCT samples were collected from five cases of GCT patients in General Hospital of Tianjin Medical University.
Primary cell lines and cultures
Primary cell of GCT were isolated, characterized and established from fresh GCT tissue samples as described in the previous study [19]. The primary GCT cells were freshly minced in DMEM (Hyclone, Invitrogen Company, USA) producing a cell suspension with small fragments of tissue. The resultant cell suspension were transferred to 25-cm2 flasks and incubated in DMEM (Hyclone, Invitrogen Company, USA) supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37°C in a water-saturated atmosphere of 95% air and 5% CO2. 50% the culture medium was replaced with fresh complete medium every 2 days.
Cell viability assay
GCT cells (2.0 × 104 cells/well) were incubated in 96-well plates and cultured with curcumin (0, 10, 20, 40 and 80 μM) at 37°C in a water-saturated atmosphere of 95% air and 5% CO2 for 0, 1 and 2 days . MTT dye (5 mg/mL, Sigma, USA) was added to each well and incubated for at least 4 hours of treatment. Afterwards, 150 µl DMSO (Sigma, USA) was added into each well and incubated for 20 min whilst being shaken. Optical density of GCT cells was measured at 570 nm wavelength on a multiwell plate reader.
Detection of cellular apoptosis by flow cytometry (FCM)
GCT cells (1 × 106 cells/well) were incubated in 6-well plates and cultured with curcumin (0, 10, 20 and 40 μM) for 1 day. Then, GCT cells were washed twice with ice-PBS and stained with 5 µL annexin V-FITC for 30 min at darkness. Next, 10 µL PI was added into each well cells and cellular apoptosis was immediately detected using FCM (Beckman Coulter Canada, ON).
Caspase-3 activity assay
GCT cells (2 × 104 cells/well) were incubated in 96-well plates and cultured with curcumin (0, 10, 20 and 40 μM) for 1 day. Then, GCT cells were performed to quantitate caspase-3 activity levels with The Apo-ONE Homogeneous Caspase-3 assay according to the manufacturer’s protocol (BeastBio, Shanghai, China). GCT cells were incubated for four hours at ambient temperature prior to recording fluorescence (E = 450).
Real-time polymerase chain reaction (RT-PCR) for MMP-9 gene expression quantity
GCT cells (2 × 104 cells/well) were incubated in 96-well plates and cultured with curcumin (0, 10, 20 and 40 μM) for 1 day. The total RNA from the GCT cells was isolated using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, Company, USA). Total RNA was converted to cDNA using SuperScript-II RT system according to the manufacturer’s instructions (Life Technologies, Carlsbad, CA, USA). RT-PCR was performed with 20 µl in the presence of SYBR Green PCR master mix (Beyotime, Nanjing, China). The primers used to amplify MMP-9: forward, 5’-CACCGCCAACTACGACCGGG-3’ and reverse, 5’-GGTGGTAGCGCACCAGA GGC-3’; and GAPDH forward, 5’-GAACGGGAAGCTCACTGGCATGGC-3’ and reverse, 5’-TGAGGTCCACCACCCTGTTGCTG-3’. Cycling consisted of 40 cycles of 15 s at 95°C, 30 s at 60°C, and 30 s at 72°C.
NF-κB activity
GCT cells (2 × 104 cells/well) were incubated in 96-well plates and cultured with curcumin (0, 10, 20 and 40 μM) for 1 day. NF-κB activity was measured with a luciferase assay kit according to the manufacturer’s instructions (Promega, Madison, WI, USA).
Western blotting assay
GCT cells (2 × 104 cells/well) were incubated in 96-well plates and cultured with curcumin (0, 10, 20 and 40 μM) for 1 day. GCT cells were incubated with ice-cold RIPA lysis buffer including protease and phosphatase inhibitors for 30 min on ice. Then, cells liquid were centrifuged at 10,000 g for 10 minutes at 4°C. Supernatant was collected to measure the protein concentration using Bicinchoninic Acid protein assay (BCA, Beyotime, Nanjing, China). Equal protein were resuspended in a loading buffer and electrophoresed on 12% SDS-polyacrylamide gels followed by transferring to polyvinylidene fluoride membranes (Invitrogen, 0.22 mm). After the membranes were blocked with Tris-buffered saline (TBS) containing 5% milk for 2 hour, the membranes were incubated with anti-JNK (1:1000; Santa Cruz, USA) and anti-β-actin (1:500, Sangon Biotech, Shanghai, China) overnight at 4°C. Washing 3 times with Tris Buffered Saline Tween (TBST) after primary antibody and followed by secondary antibodies (1:1000, Santa Cruz Biotechnology, Inc, Calif, USA) added on the membranes and incubated for 2 hour at room temperature. The proteins were detected using enhanced chemiluminescence (Thermo Scientific, USA).
Statistical analysis
The SPSS 17.0 software was applied to complete data processing. All quantitative values are performed at least three times and given as mean ± S.D. Statistical significance was analyzed by Student’s t-test. Values of P < 0.05 were considered statistically significant.
Results
Effect of curcumin on cell viability of GCT cells
Firstly, we examined the anti-cancer effects of curcumin on the cell viability of GCT cells. The cells were exposed to curcumin at different concentrations (0, 10, 20, 40 and 80 μM) for 0, 1 and 2 day, and cell viability of GCT cells was inspected by MTT assay. The dose- and time-dependent the anti-cancer effects of curcumin on cells proliferation were decreased (Figure 2).
Figure 2.
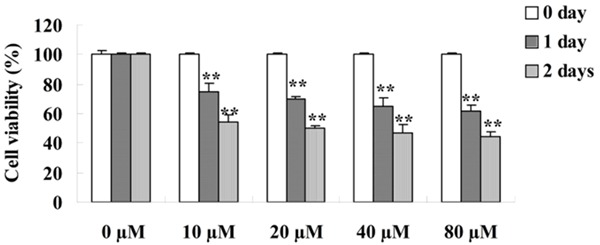
Effect of curcumin on cell viability of GCT cells. **P < 0.01 compared with 0 μM curcumin-treated group.
Effect of curcumin on cellular apoptosis of GCT cells
To detect the anti-cancer effects of curcumin on the cellular apoptosis of GCT cells, the cellular apoptosis were performed by FCM. After treatment with different concentrations curcumin (0, 10, 20 and 40 μM) for 1 day, the cellular apoptosis of GCT cells were dramatically increased and showed a dose-dependent (Figure 3).
Figure 3.
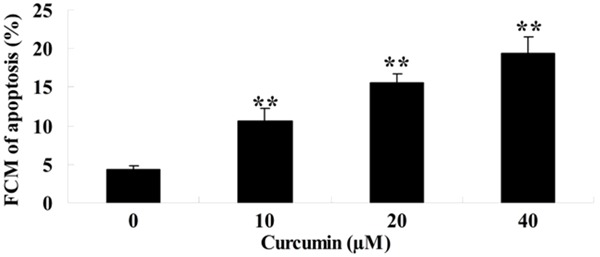
Effect of curcumin on cellular apoptosis of GCT cells. **P < 0.01 compared with 0 μM curcumin-treated group.
Effect of curcumin on caspase-3 activity of GCT cells
To further analysis the anti-cancer effects of curcumin on caspase-3 activity of GCT cells, the caspase-3 activity was measured by commercial kits. GCT cells were treated with curcumin (0, 10, 20 and 40 μM) for 1 day. The caspase-3 activity was boosted by treatment with curcumin and showed a dose-dependent (Figure 4).
Figure 4.
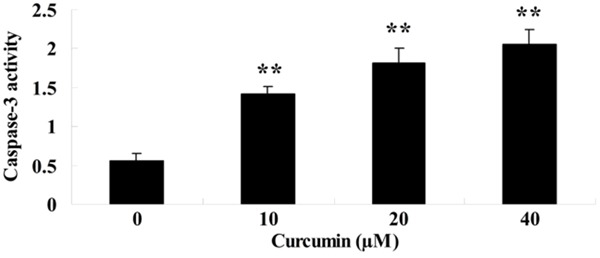
Effect of curcumin on caspase-3 activity of GCT cells. **P < 0.01 compared with 0 μM curcumin-treated group.
Effect of curcumin on MMP-9 of GCT cells
To further explore the potential mechanism of curcumin on GCT cells, RT-PCR was used to test MMP-9 gene expression quantity during treatment with curcumin (0, 10, 20 and 40 μM) on GCT cells for 1 day. MMP-9 gene expression quantity was effectively reduced by treatment with curcumin and which showed a dose-dependent (Figure 5).
Figure 5.
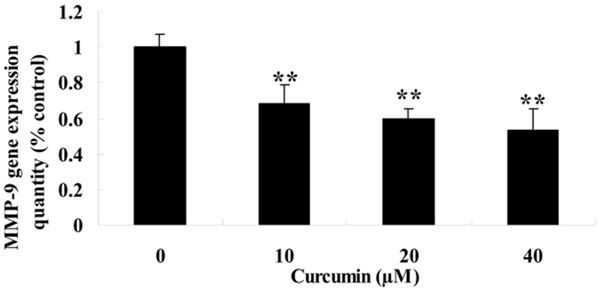
Effect of curcumin on MMP-9 of GCT cells. **P < 0.01 compared with 0 μM curcumin-treated group.
MMP-9 inhibitor influences the anti-effect of curcumin on GCT cells
To further analyze the anti-effect of curcumin on MMP-9 of GCT cells, GCT cells were treated with curcumin (10 μM) or curcumin (10 μM) combination with MMP-9 inhibitor (GM6001 180 nM) for 1 day. Firstly, MMP-9 gene expression quantity of GCT cells was decreased in curcumin (10 μM) combination with MMP-9 inhibitor group compared to the curcumin group (Figure 6A). Next, the cell viability of GCT cells was restrained in curcumin (10 μM) combination with MMP-9 inhibitor group compared to the curcumin group (Figure 6B).
Figure 6.
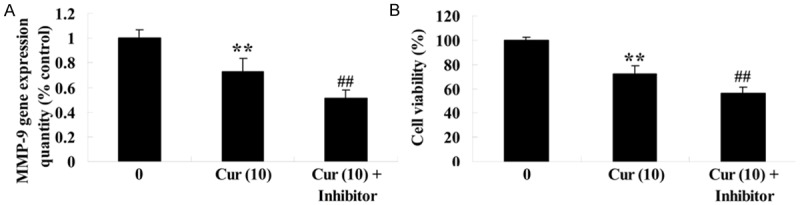
MMP-9 inhibitor influences the anti-effect of curcumin on GCT cells. MMP-9 inhibitor influences MMP-9 gene expression quantity (A) and the anti-effect of curcumin on GCT cells (B). **P < 0.01 compared with 0 μM curcumin-treated group, ##P < 0.01 compared with 10 μM curcumin-treated group.
Effect of curcumin on NF-κB activity of GCT cells
To probe the potential mechanism of curcumin on GCT cells, NF-κB activity was measure by commercial kits. NF-κB activity was also availably attenuated by treatment with curcumin (0, 10, 20 and 40 μM) for 1 day and which showed a dose-dependent (Figure 7).
Figure 7.
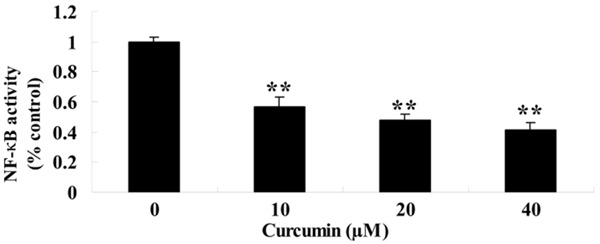
Effect of curcumin on NF-κB activity of GCT cells. **P < 0.01 compared with 0 μM curcumin-treated group.
NF-κB inhibitor influences the anti-effect of curcumin on GCT cells
To further research the anti-effect of curcumin on NF-κB activity of GCT cells, GCT cells were treated with curcumin (10 μM) or curcumin (10 μM) combination with NF-κB inhibitor (PDTC 100 μM) for 1 day. Firstly, curcumin (10 μM) combination with NF-κB inhibitor lessened NF-κB activity of GCT cells compared to the curcumin group (Figure 8A). Meanwhile, the cell viability of GCT cells was ulteriorly suppressed by curcumin (10 μM) combination with NF-κB inhibitor compared to the curcumin group (Figure 8B).
Figure 8.
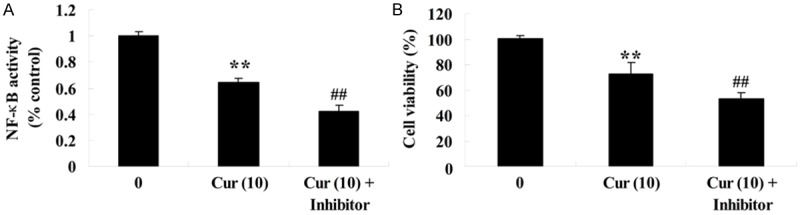
NF-κB inhibitor influences the anti-effect of curcumin on GCT cells.NF-κB inhibitor influences NF-κB activity (A) and the anti-effect of curcumin on GCT cells (B). **P < 0.01 compared with 0 μM curcumin-treated group, ##P < 0.01 compared with 10 μM curcumin-treated group.
Effect of curcumin onJNK protein expression of GCT cells
To determine the potential mechanism of curcumin on GCT cells, JNK protein expression of GCT cells was measure by western blotting assay. After GCT cells were treated with curcumin (0, 10, 20 and 40 μM) for 1 day, JNK protein expression of GCT cells was efficaciously activated and which showed a dose-dependent (Figure 9A, 9B).
Figure 9.
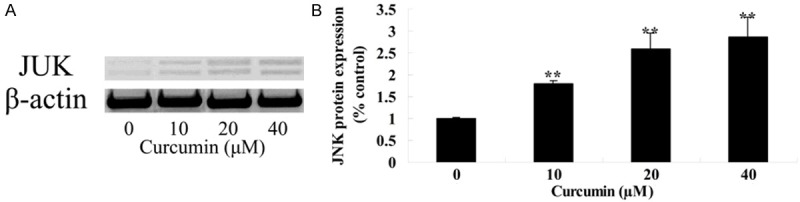
Effect of curcumin on JNK protein expression of GCT cells. **P < 0.01 compared with 0 μM curcumin-treated group.
JNK inhibitor influences the anti-effect of curcumin on GCT cells
To further detect the anti-effect of curcumin on JNK protein expression of GCT cells, GCT cells were treated with curcumin (10 μM) or curcumin (10 μM) combination with JNK inhibitor (SP600125 20 μM) for 1 day. Firstly, curcumin (10 μM) combination with JNK inhibitor augmented JNK protein expression of GCT cells compared to the curcumin group (Figure 10A, 10B). Meanwhile, the cell viability of GCT cells was ulteriorly reduced by curcumin (10 μM) combination with JNK inhibitor compared to the curcumin group (Figure 10C).
Figure 10.
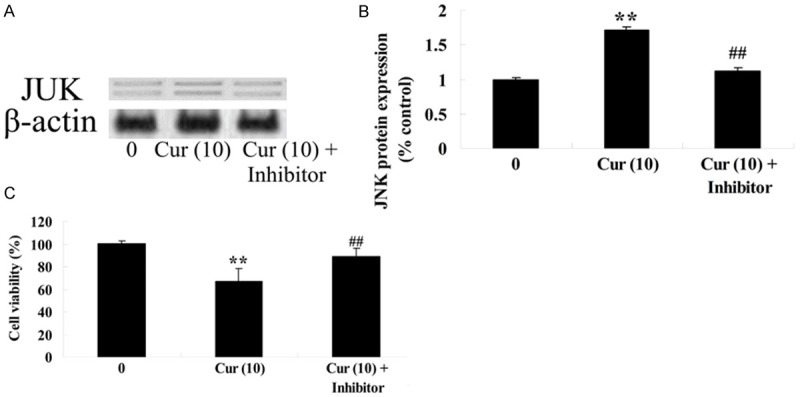
JNK inhibitor influences the anti-effect of curcumin on GCT cells. JNK inhibitor influences JNK protein expression by western blotting assays (A), statistical analysis of JNK protein expression (B) and the anti-effect of curcumin on GCT cells (C). **P < 0.01 compared with 0 μM curcumin-treated group, ##P < 0.01 compared with 10 μM curcumin-treated group.
Discussion
Osteoclastoma is a locally invasive bone tumor, with the characteristics of postoperative recurrence, of which the recurrence rate is up to 46% [20]. The fibroblast-like spindle cells in cellular components of osteoclastoma are tumor stromal cells, and multinucleated giant cells have bone-absorption activity like osteoclast. Our study suggested that curcumin could suppress cell viability, activated cellular apoptosis and induced caspase-3 activity of GCT cells. In recent years, several studies have indicated that curcumin inhibited cell viability and promoted cellular apoptosis of pancreatic cancer though suppression of HSP90, NF-κB, and HIF-1α [21]. Ye et al. reported that curcumin promoted apoptosis of non-small cell lung cancer by activating the p53-miR-192-5p/215-XIAP pathway [22].
Studies show in MMPs family, MMP-9 expression is the strongest in osteoclastoma, related to tumor invasion and metastasis [23]. Studies have indicated that, positive expression of MMP-9 is common in multinucleated giant cells of osteoclastoma, while in mononuclear stromal cells only a small number of expressions are positive, suggesting that MMP-9 is mainly secreted by multinucleated giant cells [24]. As MMP-9 has gelatin and collagen degradation effect, the over-expression of MMP-9 may be helpful to vascular invasion and bone absorption of osteoclastoma, which is closely related to the invasion behaviors of osteoclastoma such as cortical bone destruction, surrounding soft tissue invasion and distant metastasis [25]. In this study, our results revealed that treatment with curcumin reduced MMP-9 gene expression quantity of GCT. Meanwhile, down-regulation of MMP-9 gene expression suppressed the cell viability of GCT cells. Cao et al. suggested that curcumin inhibited MMP-9 expression in phorbol 12-myristate 13-acetate induced macrophages [26]. Chiablaem et al. showed that curcumin reduced cell migration and MMP-9 production in human hepatocellular carcinoma cells [27].
The results show the positive expression of NF-κB protein in osteoclastoma is significantly higher that of osteochondroma, indicating NF-κB expression intensity is related to benign or malignant degree of tumors [28]. At the same time there has been reported that osteoclastoma may be a kind of malignant tumor with high proliferation and apoptosis. In this study, our data showed that curcumin could attenuate NF-κB activity of GCT cells. Interesting, suppression of NF-κB activity decreased the cell viability of GCT cells. Jiang et al. suggested that curcumin induced apoptosis through suppression of NF-κB, p38 and p53 [29]. Meiyanto et al. showed that curcumin enhance sensitivity of resistant MCF-7 cells to doxorubicin through suppression of HER2 and NF-κB activation [30].
c-Jun N-terminal kinase family is a member of the mitogen-activated protein kinase superfamily, and c-Jun can mediate the apoptosis induced by extracellular stimuli of various cells (such as stress, Fas, TN F-α, etc.), involved in a number of cell apoptosis, which plays an important role in the occurrence and development of many diseases and pathological injuries such as neurodegenerative diseases, tumor, I diabetes mellitus, chronic hepatitis, ischemia-reperfusion injury and so on [31]. In this study, our results showed curcumin activated JNK protein expression of GCT cells. Down-regulation of JNK expression increased the cell viability of GCT cells. Zikaki’s study also showed curcumin induced the apoptotic intrinsic pathway via up-regulation of JNKs [32]. Cao et al. suggested that curcumin induced apoptosis of AGS and HT-29 cells up-regulation of JNK protein [33].
In conclusion, the present study identified that the anti-cancer effect of curcumin inhibited cell proliferation and promoted apoptosis of GCT cell through suppression of MMP-9 and NF-κB, and activation of JNK signaling pathways. Further studies are needed to determine the in vivo anti-cancer effects of curcumin in animal models in order to better evaluate the therapeutic potential of curcumin on GCT cells and the potential benefits of curcumin for clinical practice in the future.
Acknowledgements
This work was also supported by Special Program for Sino-Russian Joint Research which was Sponsored by the Ministry of Science and Technology, China (2014DFR31210) and Key Program which was Sponsored by the Tianjin Science and Technology Committee, China (13RCGFSY19000, 14ZCZDSY00044).
Disclosure of conflict of interest
None.
References
- 1.Haque AU, Moatasim A. Giant cell tumor of bone: a neoplasm or a reactive condition? Int J Clin Exp Pathol. 2008;1:489–501. [PMC free article] [PubMed] [Google Scholar]
- 2.Akahane T, Mori N, Yoshida K. Giant cell tumor of the tendon sheath extending around the patellar tendon and invading the knee joint and tibia: A case report. Oncol Lett. 2014;8:2800–2802. doi: 10.3892/ol.2014.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Lu H, Liu R, Chen B, Wang S, Ma J, Fu J. The dineolignan from Saururus chinensis, manassantin B, inhibits tumor-induced angiogenesis via downregulation of matrix metalloproteinases 9 in human endothelial cells. Oncol Rep. 2014;32:659–667. doi: 10.3892/or.2014.3244. [DOI] [PubMed] [Google Scholar]
- 4.Kumta SM, Huang L, Cheng YY, Chow LT, Lee KM, Zheng MH. Expression of VEGF and MMP-9 in giant cell tumor of bone and other osteolytic lesions. Life Sci. 2003;73:1427–1436. doi: 10.1016/s0024-3205(03)00434-x. [DOI] [PubMed] [Google Scholar]
- 5.Shan RF, Zhou YF, Peng AF, Jie ZG. Inhibition of Aurora-B suppresses HepG2 cell invasion and migration via the PI3K/Akt/NF-kappaB signaling pathway. Exp Ther Med. 2014;8:1005–1009. doi: 10.3892/etm.2014.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang ES, Pavlos NJ, Chim SM, Feng HT, Scaife RM, Steer JH, Zheng MH, Xu J. Paclitaxel inhibits osteoclast formation and bone resorption via influencing mitotic cell cycle arrest and RANKL-induced activation of NF-kappaB and ERK. J Cell Biochem. 2012;113:946–955. doi: 10.1002/jcb.23423. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Chen H, Wu Y, Liu B, Li Z, Wang Z. Adiponectin exerts antiproliferative effect on human placenta via modulation of the JNK/c-Jun pathway. Int J Clin Exp Pathol. 2014;7:2894–2904. [PMC free article] [PubMed] [Google Scholar]
- 8.Mak IW, Turcotte RE, Popovic S, Singh G, Ghert M. AP-1 as a Regulator of MMP-13 in the Stromal Cell of Giant Cell Tumor of Bone. Biochem Res Int. 2011;2011:164197. doi: 10.1155/2011/164197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Y, Shu L, Zhang C, Su ZY, Kong AN. Curcumin inhibits anchorage-independent growth of HT29 human colon cancer cells by targeting epigenetic restoration of the tumor suppressor gene DLEC1. Biochem Pharmacol. 2015;94:69–78. doi: 10.1016/j.bcp.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esatbeyoglu T, Ulbrich K, Rehberg C, Rohn S, Rimbach G. Thermal stability, antioxidant, and anti-inflammatory activity of curcumin and its degradation product 4-vinyl guaiacol. Food Funct. 2015;6:887–93. doi: 10.1039/c4fo00790e. [DOI] [PubMed] [Google Scholar]
- 11.El-Bahr SM. Effect of curcumin on hepatic antioxidant enzymes activities and gene expressions in rats intoxicated with aflatoxin b1. Phytother Res. 2015;29:134–140. doi: 10.1002/ptr.5239. [DOI] [PubMed] [Google Scholar]
- 12.Zhao X, Chen Q, Liu W, Li Y, Tang H, Liu X, Yang X. Codelivery of doxorubicin and curcumin with lipid nanoparticles results in improved efficacy of chemotherapy in liver cancer. Int J Nanomedicine. 2015;10:257–270. doi: 10.2147/IJN.S73322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chauhan G, Rath G, Goyal AK. In-vitro anti-viral screening and cytotoxicity evaluation of copper-curcumin complex. Artif Cells Nanomed Biotechnol. 2013;41:276–281. doi: 10.3109/21691401.2012.742096. [DOI] [PubMed] [Google Scholar]
- 14.Dutta K, Ghosh D, Basu A. Curcumin protects neuronal cells from Japanese encephalitis virus-mediated cell death and also inhibits infective viral particle formation by dysregulation of ubiquitin-proteasome system. J Neuroimmune Pharmacol. 2009;4:328–337. doi: 10.1007/s11481-009-9158-2. [DOI] [PubMed] [Google Scholar]
- 15.Gong G, Pan Q, Wang K, Wu R, Sun Y, Lu Y. Curcumin-incorporated albumin nanoparticles and its tumor image. Nanotechnology. 2015;26:045603. doi: 10.1088/0957-4484/26/4/045603. [DOI] [PubMed] [Google Scholar]
- 16.Leong PK, Chiu PY, Ko KM. Prooxidant-induced glutathione antioxidant response in vitro and in vivo: a comparative study between schisandrin B and curcumin. Biol Pharm Bull. 2012;35:464–472. doi: 10.1248/bpb.35.464. [DOI] [PubMed] [Google Scholar]
- 17.Gao D, Chen X, Yang X, Wu Q, Jin F, Wen H, Jiang Y, Liu H. Stable isotope labeling strategy for curcumin metabolite study in human liver microsomes by liquid chromatography-tandem mass spectrometry. J Am Soc Mass Spectrom. 2015;26:686–94. doi: 10.1007/s13361-014-1064-z. [DOI] [PubMed] [Google Scholar]
- 18.Sahebkar A. Dual effect of curcumin in preventing atherosclerosis: the potential role of pro-oxidant-antioxidant mechanisms. Nat Prod Res. 2015;29:491–492. doi: 10.1080/14786419.2014.956212. [DOI] [PubMed] [Google Scholar]
- 19.Pichiorri F, Suh SS, Ladetto M, Kuehl M, Palumbo T, Drandi D, Taccioli C, Zanesi N, Alder H, Hagan JP, Munker R, Volinia S, Boccadoro M, Garzon R, Palumbo A, Aqeilan RI, Croce CM. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc Natl Acad Sci U S A. 2008;105:12885–12890. doi: 10.1073/pnas.0806202105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajpai J, Saini S, Bajpai A, Khera R. Rare presentation of giant cell tumor of bone in the lateral end of the clavicle. Am J Case Rep. 2013;14:235–237. doi: 10.12659/AJCR.889121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagaraju GP, Zhu S, Ko JE, Ashritha N, Kandimalla R, Snyder JP, Shoji M, El-Rayes BF. Antiangiogenic effects of a novel synthetic curcumin analogue in pancreatic cancer. Cancer Lett. 2015;357:557–565. doi: 10.1016/j.canlet.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Ye M, Zhang J, Zhang J, Miao Q, Yao L, Zhang J. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015;357:196–205. doi: 10.1016/j.canlet.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Bolkun L, Lemancewicz D, Sobolewski K, Mantur M, Semeniuk J, Kulczynska A, Kloczko J, Dzieciol J. The evaluation of angiogenesis and matrix metalloproteinase-2 secretion in bone marrow of multiple myeloma patients before and after the treatment. Adv Med Sci. 2013;58:118–125. doi: 10.2478/v10039-012-0048-0. [DOI] [PubMed] [Google Scholar]
- 24.Chen YF, Wu KJ, Wood WG. Paeonia lactiflora Extract Attenuating Cerebral Ischemia and Arterial Intimal Hyperplasia Is Mediated by Paeoniflorin via Modulation of VSMC Migration and Ras/MEK/ERK Signaling Pathway. Evid Based Complement Alternat Med. 2013;2013:482428. doi: 10.1155/2013/482428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang YK, Wang H, Leng Y, Li ZL, Yang YF, Xiao FJ, Li QF, Chen XQ, Wang LS. Overexpression of microRNA-29b induces apoptosis of multiple myeloma cells through down regulating Mcl-1. Biochem Biophys Res Commun. 2011;414:233–239. doi: 10.1016/j.bbrc.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 26.Cao J, Han Z, Tian L, Chen K, Fan Y, Ye B, Wang C, Huang Z. Curcumin inhibits EMMPRIN and MMP-9 expression through AMPK-MAPK and PKC signaling in PMA induced macrophages. J Transl Med. 2014;12:266. doi: 10.1186/s12967-014-0266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiablaem K, Lirdprapamongkol K, Keeratichamroen S, Surarit R, Svasti J. Curcumin suppresses vasculogenic mimicry capacity of hepatocellular carcinoma cells through STAT3 and PI3K/AKT inhibition. Anticancer Res. 2014;34:1857–1864. [PubMed] [Google Scholar]
- 28.Park SL, Won SY, Song JH, Kim WJ, Moon SK. EPO gene expression induces the proliferation, migration and invasion of bladder cancer cells through the p21WAF1mediated ERK1/2/NF-kappaB/MMP-9 pathway. Oncol Rep. 2014;32:2207–2214. doi: 10.3892/or.2014.3428. [DOI] [PubMed] [Google Scholar]
- 29.Jiang AJ, Jiang G, Li LT, Zheng JN. Curcumin induces apoptosis through mitochondrial pathway and caspases activation in human melanoma cells. Mol Biol Rep. 2015;42:267–275. doi: 10.1007/s11033-014-3769-2. [DOI] [PubMed] [Google Scholar]
- 30.Meiyanto E, Putri DD, Susidarti RA, Murwanti R, Sardjiman , Fitriasari A, Husnaa U, Purnomo H, Kawaichi M. Curcumin and its analogues (PGV-0 and PGV-1) enhance sensitivity of resistant MCF-7 cells to doxorubicin through inhibition of HER2 and NF-κB activation. Asian Pac J Cancer Prev. 2014;15:179–184. doi: 10.7314/apjcp.2014.15.1.179. [DOI] [PubMed] [Google Scholar]
- 31.Wang TB, Hu BG, Liu DW, Shi HP, Dong WG. The influence of lentivirus-mediated CXCR4 RNA interference on hepatic metastasis of colorectal cancer. Int J Oncol. 2014;44:1861–1869. doi: 10.3892/ijo.2014.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zikaki K, Aggeli IK, Gaitanaki C, Beis I. Curcumin induces the apoptotic intrinsic pathway via upregulation of reactive oxygen species and JNKs in H9c2 cardiac myoblasts. Apoptosis. 2014;19:958–974. doi: 10.1007/s10495-014-0979-y. [DOI] [PubMed] [Google Scholar]
- 33.Cao A, Li Q, Yin P, Dong Y, Shi H, Wang L, Ji G, Xie J, Wu D. Curcumin induces apoptosis in human gastric carcinoma AGS cells and colon carcinoma HT-29 cells through mitochondrial dysfunction and endoplasmic reticulum stress. Apoptosis. 2013;18:1391–1402. doi: 10.1007/s10495-013-0871-1. [DOI] [PubMed] [Google Scholar]


