Abstract
Diabetes-induced cognitive deficit (DICD) is a prevalent disease with substantial morbidity and mortality and as a global health problem with serious economic burdens. Astaxanthin (AST) has a good prospect in production of nutritional, medical, and particularly functional health drug. The present study was aimed to study the effect of AST on DICD in diabetes mellitus (DM) rat through suppression of oxidative stress, nitric oxide synthase (NOS) pathway, inflammatory reaction and upregulation of PI3K/Akt. In the study, Morris water maze teat was used to detect the cognitive function of DM rat. Afterwards, we measured the body weight and blood glucose levels of DM rats. Then, oxidative stress, the activities of eNOS and iNOS, and inflammatory factors were analyzed using a commercial kit in cerebral cortex and hippocampus. Finally, the caspase-3/9 and phosphoinositide 3-kinase (PI3K)/Akt expressions were also checkout with Real Time PCR and immunoblotting, respectively. In this experiment, AST could availably enhance the body weight and reduce blood glucose levels of DM rats. Moreover, AST could observably perfect cognitive function of DM rat. Next, the activities of oxidative stress, nitric oxide synthase and inflammation were distinctly diminution in DM rat, after the treatment of AST. Furthermore, our present results demonstrated that AST had the protective effect on the brain cell of DM rat, decreased the caspase-3/9 expression and promoted the expression of PI3K/Akt in cerebral cortex and hippocampus.
Keywords: Diabetes-induced cognitive deficit, astaxanthin, oxidative stress, nitric oxide synthase, inflammatory, PI3K/Akt
Introduction
With the change in lifestyles and the aging of population, the incidence rate of diabetes mellitus (DM) shows an increasing trend [1]. As a systemic disease, DM can cause a variety of structural and functional changes in tissues and organs, and the lesions can affect the whole body. The studies on DM combined with lesions of heart, brain, kidney and retina have been very extensive, and the studies of the impact on cognitive function gain more and more attention. Early recognition and treatment of diabetes-induced cognitive deficits (DICD) can delay and reduce the incidence of dementia and improve the quality of life of patients with DM [2].
Studies have shown that DICD is closely related to the oxidative stress of the body [3]. The fluctuation state of high blood sugar inside the body is found through in-vitro experiments, which is high blood sugar fluctuation of the body of causes some unusual biochemical pathways so that the production of inflammatory cytokines is increased and gene expression and regulation is altered, resulting in over-transported mitochondrial electrons and the excessive oxide produced, thus catalyzing oxidative stress and then the endothelial cells are damaged, resulting in vascular lesions. Studies have shown that high blood sugar fluctuation in the body will increase oxidative stress, resulting in enlarged oxidative stress, and promote the apoptosis of endothelial cells, thereby affecting cognitive function [4].
More recently, the role of nitric oxide (NO) in the effects of DM on the central nervous system is attracting adding attention, which is considered to be a connection between diabetes neuropathy vascular doctrine and the theory of metabolize. The regulations of the body on the different functions of NO are mainly realized by the fine regulation of different nitric oxide synthase (NOS), in order to make NO achieves different accumulation degrees in different media inside and outside of cells [5]. Next, endothelial nitric oxide synthase (eNOS) present in fewer neurons is primarily implicated in the regulation of vascular function, however inducible nitric oxide synthase (iNOS) is involved in pathological conditions and unspecific immune response of nervous tissue [6].
DM is an autoimmune and chronic inflammatory disease. The levels NF-κB p65, tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) are closely associated with abnormally elevated insulin resistance (IR), which is one of the most important reasons for DM microangiopathy and neuropathy [7]. Nervous system and immune system constitute inflammation reflex loop via the vagus nerve, regulating systemic or local inflammatory reactions: activating the vagus nerve and promoting the increased secretion of the neurotransmitter acetylcholine so that can antagonize or inhibit the synthesis and the release of proinflammatory cytokinein tissue [8]. Mao et al. concluded that HupA ameliorated diabetes-associated cognitive decline through oxidative stress and inflammation (NF-κB p65 unit, TNF-α, IL-1β, IL-6) [9]. Meanwhile, Wang et al. reported that chronic treatment with Oxymatrine alleviated diabetes-associated cognitive decline through the NF-κB p65 unit, TNF-α, IL-1β levels in rats [10].
Phosphoinositide 3-kinase (PI3K)/Akt is an important signal transduction molecule in cell. The activated Akt is further activated by phosphorylation or inhibits its downstream target protein, thus further playing a role in regulating cell proliferation, differentiation, glucose metabolism and migration. There has been part of the trail about the apoptosis inhibition effect and mechanism of PI3K/Akt. Li et al. reported that chitosan coating markedly ameliorates diabetes-induced impaired bio-performance of titanium alloy implant through reactivation of PI3K/AKT pathway [11]. Francis et al. showed intranasal insulin prevents cognitive decline through upregulation of PI3K/Akt in murine type I diabetic encephalopathy [12].
Astaxanthin (AST), oxygen-containing derivative of carotenoids, can effectively quench reactive oxygen, having high nutritional and medicinal value. AST has been separated from the shells of shrimp and crab since 1930s, of which the physiological function gained widespread attention till 1980s [13]. Since then many scholars have proved through animal and clinical trials that AST can inhibit tumorigenesis, enhance immune function and prevent cardiovascular disease with a wide range of physiological functions, having broad application prospects [14]. Therefore, the current study aims to investigate the effect of AST to ameliorate DICD in rats. Meanwhile, we designed the present study to explore the potential effect of AST on DICD rats and also to elucidate the underline mechanism.
Materials and methods
Drugs and chemicals
AST (purity ≥ 97%) were purchased from Sigma (St. Louis, MO, USA) and the chemical structure of it was represented in Figure 1. Malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione (GSH) ELISA kits were provided from KeyGen Biotech (Nanjing, China). A commercial NOS radioimmunoassay (RIA) kit was provided from Jiancheng Bioengineering Institute (Nanjing, China). NF-κB p65, TNF-α, IL-1β and IL-6 ELISA immunoassay kits were provided from Sangon Biotech (Shanghai, China). RNeasy Plus Mini kit and cDNA was transcribed using the RevertAid H Minus Reverse Transcriptase were provided from Beyotime (Nanjing, China). Power SYBR Green was provided from (TAKARA, Japan). Bicinchoninic Acid (BCA) protein assay kit was provided from Bio-Rad, (Hercules, CA, USA).
Figure 1.
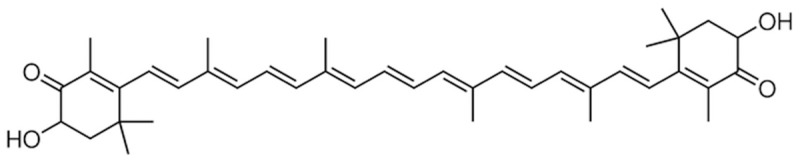
The chemical structure of AST.
Animals
Male Wistar rats (300 ± 10 g) were acquired from the Shanghai Experimental Animal Center (Shanghai, China). The present study was conducted according to the National Institutes of Health guide for the care and use of laboratory animals. All rats were housed in a laboratory animal room for at least 1 week before the start of the experiments and maintained at 25°C ± 1°C with 65% ± 5% humidity on a 12-h light/dark cycle (lights on: 07:30-19:30). The experiment rats were freely given food and water ad libitum.
Induction and measurement of diabetes
After 1 week of acclimatization, a single dose of 65 mg/kg streptozotocin was injected intraperitoneally (i.p.) to induce diabetes except in normal healthy controls [15]. Fasting blood glucose levels was confirmed using an enzymatic glucose oxidase peroxidase diagnostic kit by the elevated glucose level in plasma, determined at 48 h after injection. Fasting blood glucose levels > 250 mg/dL were considered diabetic and used for further.
Experimental design
Animal were randomly divided into 5 groups of eight rats each: (1) control group (Con) (n = 8): normal rats were injected with physiological saline (0.1 mL/100 g, i.p.); DM group (n = 8): the diabetic rats were received with physiological saline (0.1 mL/100 g, i.p.); (3-5) DM + AST group (DM + AST (10), DM + AST (20) and DM + AST (40)) (n = 8): the diabetic rats were treated with AST at doses of 10, 20 and 40 mg/kg, respectively [16]. All rats were anesthesia using chloral hydrate (300 mg/kg, i.p.) in the study. Immediately, the rats were sacrificed, the brains were removed and blood samples were collected under anesthesia. Meanwhile, the samples were stored at -80°C until used for further experimentation measurements.
Morris water maze teat
After the treatment of AST for 5 days, Morris water maze was evaluated by slightly modifying the protocol adopted in previous studies [17,18]. All rats were received the non-visible platform trial twice per day (every morning and afternoon) for the first 5 days, a probe trial on the 6th day, and a visible platform trial on the 7th day. The apparatus consisted of a circular water tank (diameter, 120 cm; height, 50 cm), filled with water (25 ± 5°C) made opaque with milk power. Each time, the mouse was put into the pool from different quadrants for training for 120 seconds. The latency and swimming distance in finding the platform were recorded. If the mouse did not find the platform within 120 seconds, the latency was recorded as 120 seconds. Then, the mouse was replaced on the platform for 20 seconds, and the next training was performed after 120 seconds of rest. On day 5 of training, the platform was removed, and the number of crossings of the platform location within 120 seconds (crossing number) was recorded.
Measurement of oxidative stress
After the treatment of AST for 5 days, a portion of the brain tissue was homogenized in physiological saline (0.1 mL/100 g) and then centrifuged at 15,000 g for 15 minutes. The clear upper supernatants were collected for analysis of oxidative stress (MDA, SOD and GSH). In according to the manufacturer’s instructions (KeyGen Biotech, Nanjing, China), the MDA, SOD and GSH concentrations were measured by MDA, SOD and GSH ELISA kits, respectively.
Measurement of eNOS and iNOS activities
After the treatment of AST for 5 days, a portion of the brain tissue was homogenized in physiological saline (0.1 mL/100 g) and then centrifuged at 15,000 g for 15 minutes. The clear upper supernatants were collected for analysis of NOS (eNOS and iNOS) activity assay. In according to the manufacturer’s instructions (Jiancheng Bioengineering Institute), the activities of eNOS and iNOS were detected using a commercial NOS radioimmunoassay (RIA) kit.
Measurement of inflammation
The p65 subunit has a positive correlation with activated NF-κB signaling. After the treatment of AST for 5 days, a portion of the brain tissue was homogenized in physiological saline (0.1 mL/100 g) and then centrifuged at 15,000 g for 15 minutes. The clear upper supernatants were collected for analysis of inflammatory cytokines (NF-κB p65, TNF-α, IL-1β and IL-6). In according to the manufacturer’s instructions (Sangon Biotech, Shanghai, China), the activities of NF-κB p65, TNF-α, IL-1β and IL-6 were measured using ELISA immunoassay kits.
Real Time PCR
After the treatment of AST for 5 days, total RNA was isolated from the brain tissue using an RNeasy Plus Minikit (Beyotime, Nanjing, China). cDNA was transcribed using the RevertAid H Minus Reverse Transcriptase (Beyotime, Nanjing, China). Real time PCR reactions were performed using Power SYBR Green (TAKARA, Japan) and Applied Biosystems 7500 Instrument. The typical thermal profile: 95°C for 5 min, followed by 35 cycles of 95°C for 30 s and 58°C for 30 s. The primer sets used were rat caspase-3: 5’-GCATGATCCGCGACGTGGAA-3’, 5’-AGATCCATGCCGTTGGCCAG-3’, respectively; rat caspase-9: 5’-ATGCAGGTCCCTGTCATG-3’, 5’-GCTTGAGGTGGTTGTGGA-3’; and β-actin: 5’-AGAGGGAAATCGTGCGTGAC-3’, 5’-CAATAGTGATGACCTGGCCGT-3’. All primers were compound and purchased form (Sangon Biotech, Shanghai, China).
Immunoblotting
The brain tissues were homogenized with lysis buffer and centrifuged at 1000 × g for 3000 min at 4°C. Protein content was measured using with the Bicinchoninic Acid (BCA) protein assay kit (Bio-Rad, Hercules, CA, USA). An equal amount of protein were separated by SDS-PAGE, loaded onto 12% polyacrylamide gels and then transferred to PVDF membrane (Millipore, Bedford, MA). Membranes were blocked at room temperature for 2 h in blocking buffer containing 5% (v/v) nonfat milk for 2 h in TBST buffer. The membranes were then incubated with anti-PI3K (1:2000, Santa Cruz Biotechnology, Inc, Calif, USA), anti-Akt (1:1500, Santa Cruz Biotechnology, Inc, Calif, USA) and anti-β-actin (Boster Biological, Wuhan, China). The membranes were washed 3 times with TBST for 30 min. Then membranes were detected by incubating with anti-mouse IgG (1:1000, Santa Cruz Biotechnology, Inc, Calif, USA) conjugated with horseradish peroxidase for 2 h. The relative band intensity was visualized using the ECL Western Blotting Detection kit (Bio-Rad, USA) and X-ray films (Bio-Rad, USA).
Statistical analysis
Statistical analyses were done using SPSS 19.0. Data were presented as means ± standard deviation (S.D). One-way analysis of variance was used for the determination of differences in measurements between the groups. A p-value of less than 0.05 was considered the statistically significant.
Results
Effect of AST on body weight and blood glucose levels
To verify the establishment of the diabetic model, we first evaluated the body weight and blood glucose levels of all rats at the onset and at the end of the experiment. Our results indicated that the body weight of the DM group was observably decrease form 302.30 ± 5.31 to 241.70 ± 4.29 g, compared with the control group (Table 1). However, after the treatment of AST (10, 20 and 40 mg/kg) for 5 days, the body weight of diabetic rats were significantly reversed from 299.80 ± 5.68 to 268.60 ±6.12 g, 301.80 ± 6.68 to 273.70 ± 6.28 g, and 304.20 ± 5.26 to 286.40 ± 6.88 g, respectively, compared with the DM group (Table 1). Meanwhile, our results reveal that the blood glucose of the DM group was distinctly increased from 116.40 ± 1.36 to 612.70 ± 3.73 g, compared with the control group (Table 1). However, after the treatment of AST (10, 20 and 40 mg/kg) for 5 days, the body weight of diabetic rats were significantly reversed from 115.80 ± 1.27 to 321.10 ± 3.21 g, 111.20 ± 2.31 to 304.70 ± 3.18 g, and 109.80 ± 2.41 to 298.50 ± 3.82 g, respectively, compared with the DM group (Table 1).
Table 1.
The body weight and blood glucose levels of rats at the onset and at the end of the experiment (n = 8, mean ± S.D.)
| Treatment | Body weight (g) | Plasma glucose (mg/dl) | ||
|---|---|---|---|---|
|
|
|
|||
| Onset of study | End of study | Onset of study | End of study | |
| Con | 303.10 ± 4.51 | 308.40 ± 6.82 | 118.40 ± 1.38 | 120.20 ± 1.28 |
| DM | 302.30 ± 5.31 | 241.70 ± 4.29** | 116.40 ± 1.36 | 612.70 ± 3.73** |
| DM + AST (10) | 299.80 ± 5.68 | 268.60 ±6.12## | 115.80 ± 1.27 | 321.10 ± 3.21## |
| DM + AST (20) | 301.80 ± 6.68 | 273.70 ± 6.28## | 111.20 ± 2.31 | 304.70 ± 3.18## |
| DM + AST (40) | 304.20 ± 5.26 | 286.40 ± 6.88## | 109.80 ± 2.41 | 298.50 ± 3.82## |
P<0.01 compared with Con group;
P<0.01 compared with DM group.
Con, control group; DM, diabetes group; DM + AST (10), astaxanthin (10 mg/kg)-treated group; DM + AST (20), astaxanthin (20 mg/kg)-treated group; DM + AST (40), astaxanthin (40 mg/kg)-treated group.
Effect of AST on diabetes-induced cognitive deficit
To test whether AST would affect cognitive function of DM rat, we performed Morris water maze tests. The effect of AST on DICD, there was a significant reduction of the escape latency in the DM rats from second day to 4th day training. After treatment with AST (10, 20 and 40 mg/kg) for 5 days, shorter escape latency was markedly reduce from second day to 4th day training (Figure 2A). Meanwhile, the mean path length of DM rats was observably reduced at 5th day training, compared with that of control rats (Figure 2B). The mean path length of DM/ AST rats was significantly lower than that of DM rats at 5th day training (Figure 2B). In the probe trials, when compared to that of control rats, DM rats spent less time in the target quadrant, and these differences were statistically significant (Figure 2C). Furthermore, the number of times the animals crossed the former platform location of DM rats was also markedly reduced, compared with that of control group (Figure 2D). After treatment of AST (10, 20 and 40 mg/kg), spent more time of DM/AST rats could significantly mitigated these changes, compared to those of DM rats (Figure 2D). However, no difference in the swimming speed was observed in the each group (Figure 2E).
Figure 2.
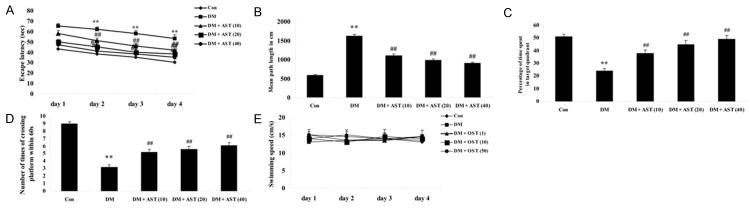
Effect of AST on diabetes-induced cognitive deficit. Effect of AST on the escape latency (A) mean path length (B) mean percentage of time spent in the target quadrant (C) the number of times of crossing platform (D) and swimming speed (E). **P<0.01 compared with Con group; ##P<0.01 compared with DM group. Con, control group; DM, diabetes group; DM + AST (10), AST (10 mg/kg)-treated group; DM + AST (20), AST (20 mg/kg)-treated group; DM + AST (40), AST (40 mg/kg)-treated group.
Effect of AST on diabetes-induced changes in oxidative stress
To explore the mechanism underlying the effect of AST (10, 20 and 40 mg/kg) on restraining oxidative stress of DM rat brains, we detected the MDA, SOD and GSH concentrations after the treatment of AST for 5 days. This result indicates that the MDA level in cerebral cortex and hippocampus of DM rats were markedly increased compared with the control group (Figure 3A). However, the expression of MDA level in cerebral cortex and hippocampus of DM/AST rats were effectually reduce compared with that in the DM rat group (Figure 3A). Oppositely, the SOD and GSH concentrations in cerebral cortex and hippocampus of DM rats were dramatically decreased compared with the control group (Figure 3B, 3C). Certainly, the SOD and GSH concentrations in cerebral cortex and hippocampus of DM/AST rats were augmented compared with that in the DM rat group (Figure 3B, 3C).
Figure 3.
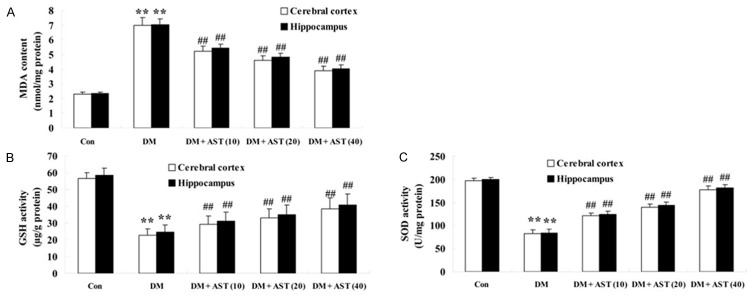
Effect of AST on diabetes-induced changes in oxidative stress. Effect of AST on the expression of the MDA content (A), GSH (B), and SOD (C). **P<0.01 compared with Con group; ##P<0.01 compared with DM group. Con, control group; DM, diabetes group; DM + AST (10), AST (10 mg/kg)-treated group; DM + AST (20), AST (20 mg/kg)-treated group; DM + AST (40), AST (40 mg/kg)-treated group.
Effect of AST on diabetes-induced changes in the activities of eNOS and iNOS
To test whether AST would affect the activity NO of DM rat brains, we performed the activities of eNOS and iNOS using NOS radioimmunoassay (RIA) kit after the treatment of AST (10, 20 and 40 mg/kg) for 5 days. We detected the activities of eNOS and iNOS in cerebral cortex and hippocampus of DM rats were promoted compared with the control group (Figure 4A, 4B). Interestingly, we found that AST could significantly reversed the tendency and decrease the activities of eNOS and iNOS in cerebral cortex and hippocampus of DM/AST rats compared with that in the DM rat group (Figure 4A, 4B).
Figure 4.
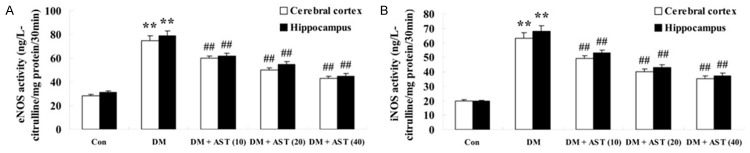
Effect of AST on diabetes-induced changes in the activities of eNOS and iNOS. Effect of AST on diabetes-induced changes in the activities of eNOS and iNOS (A and B). **P<0.01 compared with Con group; ##P<0.01 compared with DM group. Con, control group; DM, diabetes group; DM + AST (10), AST (10 mg/kg)-treated group; DM + AST (20), AST (20 mg/kg)-treated group; DM + AST (40), AST (40 mg/kg)-treated group.
Effect of AST on diabetes-induced changes in the activities of inflammation
To check the inflammation activities of DM rat brains, the activities of NF-κB p65, TNF-α, IL-1β and IL-6 were analyzed using ELISA immunoassay kits. The result showed that the activities of NF-κB p65, TNF-α, IL-1β and IL-6 were significantly increased in cerebral cortex and hippocampus of DM rats compared with the control group (Figure 5A, 5D). However, these inflammatory factors of cerebral cortex and hippocampus of DM/AST rats were markedly reduced compared with that in the DM rat group (Figure 5A, 5D).
Figure 5.
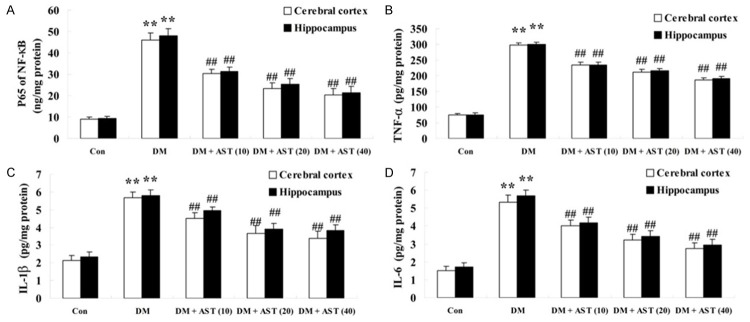
Effect of AST on diabetes-induced changes in the activities of inflammation. Effect of AST on diabetes-induced changes in the activities of NF-κB p65 (A), TNF-α (B), IL-1β (C) and IL-6 (D). **P<0.01 compared with Con group; ##P<0.01 compared with DM group. Con, control; DM, diabetes; DM + AST (10), AST (10 mg/kg)-treated group; DM + AST (20), AST (20 mg/kg)-treated group; DM + AST (40), AST (40 mg/kg)-treated group.
Effect of AST on the expression of caspase-3 and caspase-9
To test if AST prevented apoptosis of DM rat brains, the activities of caspase-3 and caspase-9 were detected using real time PCR after the treatment of AST (10, 20 and 40 mg/kg) for 5 days. In this study we therefore found the activities of caspase-3 and caspase-9 in cerebral cortex and hippocampus of DM rats were increased compared with the control group (Figure 6A, 6B). Furthermore, the activities of caspase-3 and caspase-9 in cerebral cortex and hippocampus of the DM/AST rats were significantly reduced compared with that in the DM rat group (Figure 6A, 6B).
Figure 6.
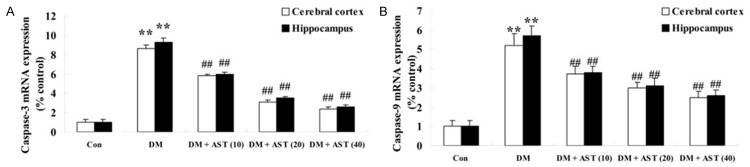
Effect of AST on the activities of caspase-3 and caspase-9. Effect of AST on the activities of caspase-3 and caspase-9 (A and B). **P<0.01 compared with Con group; ##P<0.01 compared with DM group. Con, control; DM, diabetes; DM + AST (10), AST (10 mg/kg)-treated group; DM + AST (20), AST (20 mg/kg)-treated group; DM + AST (40), AST (40 mg/kg)-treated group.
Effect of AST on diabetes-induced changes in the expression of PI3K/Akt
We next sought to study that the effect of AST on diabetes-induced changes in the expression of PI3K/Akt. We found that AST remarkably promoted the expressions of PI3K and Akt protein in cerebral cortex and hippocampus of the DM/AST rats compared with that in the DM rat group (Figure 7A-D).
Figure 7.
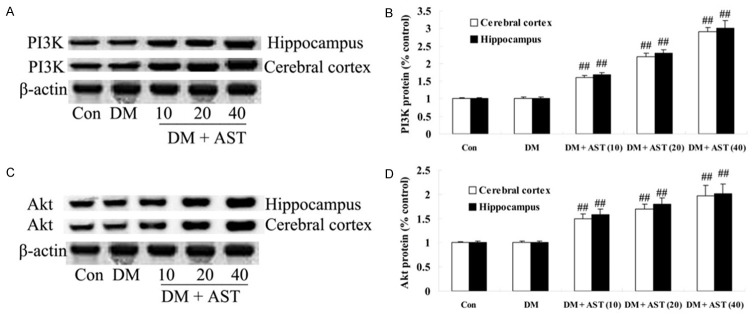
Effect of AST on diabetes-induced changes in the expression of PI3K/Akt. Indicated representative western blotting analysis of PI3K and Akt protein levels (A and C) and statistical analysis of PI3K and Akt protein level (B and D). ##P<0.01 compared with DM group. Con, control; DM, diabetes; DM + AST (10), AST (10 mg/kg)-treated group; DM + AST (20), AST (20 mg/kg)-treated group; DM + AST (40), AST (40 mg/kg)-treated group.
Discussion
DICD is the result of multiple elements and multiple factors, of which the pathogenesis is related to hyperglycemia and hypoglycemia, as well as the lack of insulin action, insulin resistance, vascular factor, etc. [19]. Early DICD may be mainly due to the direct damage on the central nervous cells from high blood sugar; with the further development of the disease, if the blood sugar cannot be effectively controlled, more and more pathogenic factors are involved, including high blood pressure, high cholesterol and DM complications, which can increase cognitive impairment, even lead to dementia [20]. Therefore, early detection and treatment is especially important. Our most significant finding was that AST could observably augment the body weight and decrease blood glucose levels of DM rats. Zhao et al. reported AST could ameliorate diabetic endothelial dysfunction through down-regulation the ox-LDLLOX-1-eNOS pathway [21]. Moreover, our data revealed, AST could effectively improve cognitive function of DM rat. Previous studies suggested that AST improves cognitive function in the healthy aged individuals [22].
SOD and GSH are important antioxidant enzymes in vivo, and play an important role in protecting the body from oxidative stress, removing superoxide anion radicals and protecting cells from damage. Experimental results show that the SOD and GSH activities decrease in rat brain model, and MDA value is significantly increased, indicating oxidative damage of free radicals has been occurred in rat brain to some extent. Researchers have found that increasing SOD and GSH activity and decreasing MDA content can protect the brain nerve from oxidative stress damage. In the present study, the effect of AST could availably changes oxidative stress of DM rat. In cerebral cortex and hippocampus, the MDA level was markedly increased and the SOD and GSH concentrations were dramatically decreased in DM/AST –treatment rats. Previous studies also indicated that AST could significantly increased plasma and kidney MDA levels for the rats [23]. Leite et al. showed that ASTA caused a reduction in SOD and GSH activities in dental pulp tissue [24]. Meanwhile, AST could significantly decrease the activities of eNOS and iNOS in cerebral cortex and hippocampus of diabetic rats of DM rats. Ohgami et al. reported that AST also decreased production of NO, activity of iNOS in RAW264.7 cells [25].
TNF-α and IL-6 levels in the serum of DICD patient are high, correlated to the degree of cognitive impairment. For the patient of TNF-α, both the Wechsler Adult Intelligence Scale and Mini-Mental State Examination scores are low. Overexpression of IL-6 can accelerate neural apoptosis, and decrease learning ability [26]. Moreover, AST also reduced NF-κB p65 unit, TNF-α, IL-1β and IL-6 levels in the cerebral cortex and hippocampus of DM rats. Park et al. reported that AST decreased oxidative stress and inflammation (TNF-α and IL-6) of humans [27]. Kim et al. suggest that AST regulated IL-6 production through NF-κB p65-dependent pathway in activated microglial cells. Nagendraprabhu et al. indicated that AST inhibited tumor invasion via modulating the expressions of NF-κB-p65 [28].
PI3K-Akt signaling pathway is necessary for the regulation of cell proliferation and apoptosis. PI3K/Akt signaling pathway is involved in the differentiation process from endothelial progenitor cells into endothelial cells. High-density lipoprotein can activate PI3K/Akt signaling pathway to promote progenitor cells differentiation into endothelial-like cells; HMG-CoA reductase inhibitors and vascular endothelial growth factor can promote the differentiation of progenitor cells via PI3K/Akt pathway. These studies indicate that PI3K/Akt an important way to promote progenitor cell proliferation and differentiation. Our present results demonstrated that AST could effectually reduce the activities of caspase-3 and caspase-9 in cerebral cortex and hippocampus. Taken together, these results explained that AST have protected and reduced apoptosis of the heart cells. Song et al. suggested that AST inhibits apoptosis, the activation of caspase-9, caspase-3 in alveolar epithelial cells and SH-SY5Y cells [29,30]. Meanwhile, we found AST remarkably activated the expressions of PI3K and Akt protein in cerebral cortex and hippocampus of the DM rats. Li et al. reported that AST protected ARPE-19 cells from oxidative stress through upregulation of activation of PI3K/Akt [31]. Zhang et al. provided the evidence that AST alleviated early brain injury following subarachnoid hemorrhage through involvement of Akt/bad signaling in rats [32].
Numerous studies have demonstrated natural AST has the potential effect of physiological regulation in the human body, so that it has a good prospect in production of nutritional, medical, and particularly functional health drug [33]. According to the strong antioxidant effect and the potential role in the human body of AST, it can be inferred that supplementing AST is expected to be effective in regulating body functions and maintaining human health [34]. In summary, the present study provides an evidence of the potential protective effect of AST on DICD through suppression of oxidative stress, activity of NOS, inflammatory reaction and up-regulation of PI3K/Akt in diabetes rat. Future studies are needed to complete the picture of the cellular mechanisms of effect on neurological function damage.
Disclosure of conflict of interest
None.
References
- 1.Wang YB, Wang S, Bai R, Du JL, Xing Q, Ba Y, Yang Y, Zhang XY, Shi CH, Yao JJ. Efficacy of switching from premixed insulin to insulin glargine regimen in Type 2 diabetes mellitus patients with different islet functions. Mol Med Rep. 2014;10:1096–1102. doi: 10.3892/mmr.2014.2263. [DOI] [PubMed] [Google Scholar]
- 2.Datusalia AK, Sharma SS. Amelioration of Diabetes-induced Cognitive Deficits by GSK-3beta Inhibition is Attributed to Modulation of Neurotransmitters and Neuroinflammation. Mol Neurobiol. 2014;50:390–405. doi: 10.1007/s12035-014-8632-x. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffari-Khosravi H, Ahadi Z, Fallah Tafti M. The Effect of Green Tea versus Sour Tea on Insulin Resistance, Lipids Profiles and Oxidative Stress in Patients with Type 2 Diabetes Mellitus: A Randomized Clinical Trial. Iran J Med Sci. 2014;39:424–432. [PMC free article] [PubMed] [Google Scholar]
- 4.Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Li Q, Tomiyama H, Kobayashi Y, Noda A, Sasamata M, Shibasaki M. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715:246–255. doi: 10.1016/j.ejphar.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Savu O, Iosif L, Bradescu OM, Serafinceanu C, Papacocea R, Stoian I. L-arginine catabolism is driven mainly towards nitric oxide synthesis in the erythrocytes of patients with type 2 diabetes at first clinical onset. Ann Clin Biochem. 2015;52:135–43. doi: 10.1177/0004563214531739. [DOI] [PubMed] [Google Scholar]
- 6.Murphy S. Production of nitric oxide by glial cells: regulation and potential roles in the CNS. Glia. 2000;29:1–13. doi: 10.1002/(sici)1098-1136(20000101)29:1<1::aid-glia1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki S, Shibata M, Gonda K, Kanke Y, Ashizawa M, Ujiie D, Suzushino S, Nakano K, Fukushima T, Sakurai K, Tomita R, Kumamoto K, Takenoshita S. Immunosuppression involving increased myeloid-derived suppressor cell levels, systemic inflammation and hypoalbuminemia are present in patients with anaplastic thyroid cancer. Mol Clin Oncol. 2013;1:959–964. doi: 10.3892/mco.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppieters KT, von Herrath MG. The type 1 diabetes signature: hardwired to trigger inflammation? Diabetes. 2014;63:3581–3583. doi: 10.2337/db14-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao XY, Cao DF, Li X, Yin JY, Wang ZB, Zhang Y, Mao CX, Zhou HH, Liu ZQ. Huperzine A ameliorates cognitive deficits in streptozotocin-induced diabetic rats. Int J Mol Sci. 2014;15:7667–7683. doi: 10.3390/ijms15057667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang SB, Jia JP. Oxymatrine attenuates diabetes-associated cognitive deficits in rats. Acta Pharmacol Sin. 2014;35:331–338. doi: 10.1038/aps.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Ma XY, Feng YF, Ma ZS, Wang J, Ma TC, Qi W, Lei W, Wang L. Osseointegration of chitosan coated porous titanium alloy implant by reactive oxygen species-mediated activation of the PI3K/AKT pathway under diabetic conditions. Biomaterials. 2015;36:44–54. doi: 10.1016/j.biomaterials.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Francis GJ, Martinez JA, Liu WQ, Xu K, Ayer A, Fine J, Tuor UI, Glazner G, Hanson LR, Frey WH 2nd, Toth C. Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathy. Brain. 2008;131:3311–3334. doi: 10.1093/brain/awn288. [DOI] [PubMed] [Google Scholar]
- 13.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jyonouchi H, Sun S, Iijima K, Gross MD. Antitumor activity of astaxanthin and its mode of action. Nutr Cancer. 2000;36:59–65. doi: 10.1207/S15327914NC3601_9. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui O, Sun Y, Liu JC, Chien YW. Facilitated transdermal transport of insulin. J Pharm Sci. 1987;76:341–345. doi: 10.1002/jps.2600760416. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Zhao H, Shao Y, Wang P, Wei Y, Zhang W, Jiang J, Chen Y, Zhang Z. Nephroprotective effect of astaxanthin against trivalent inorganic arsenic-induced renal injury in wistar rats. Nutr Res Pract. 2014;8:46–53. doi: 10.4162/nrp.2014.8.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murakami Y, Zhao Q, Harada K, Tohda M, Watanabe H, Matsumoto K. Choto-san, a Kampo formula, improves chronic cerebral hypoperfusion-induced spatial learning deficit via stimulation of muscarinic M1 receptor. Pharmacol Biochem Behav. 2005;81:616–625. doi: 10.1016/j.pbb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Q, Murakami Y, Tohda M, Watanabe H, Matsumoto K. Preventive effect of chotosan, a Kampo medicine, on transient ischemia-induced learning deficit is mediated by stimulation of muscarinic M1 but not nicotinic receptor. Biol Pharm Bull. 2005;28:1873–1878. doi: 10.1248/bpb.28.1873. [DOI] [PubMed] [Google Scholar]
- 19.Zhao B, Pan BS, Shen SW, Sun X, Hou ZZ, Yan R, Sun FY. Diabetes-induced central neuritic dystrophy and cognitive deficits are associated with the formation of oligomeric reticulon-3 via oxidative stress. J Biol Chem. 2013;288:15590–15599. doi: 10.1074/jbc.M112.440784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu F, Jin Z, Jin J. Hypoglycemic effects of glabridin, a polyphenolic flavonoid from licorice, in an animal model of diabetes mellitus. Mol Med Rep. 2013;7:1278–1282. doi: 10.3892/mmr.2013.1330. [DOI] [PubMed] [Google Scholar]
- 21.Zhao ZW, Cai W, Lin YL, Lin QF, Jiang Q, Lin Z, Chen LL. Ameliorative effect of astaxanthin on endothelial dysfunction in streptozotocin-induced diabetes in male rats. Arzneimittelforschung. 2011;61:239–246. doi: 10.1055/s-0031-1296194. [DOI] [PubMed] [Google Scholar]
- 22.Katagiri M, Satoh A, Tsuji S, Shirasawa T. Effects of astaxanthin-rich Haematococcus pluvialis extract on cognitive function: a randomised, double-blind, placebo-controlled study. J Clin Biochem Nutr. 2012;51:102–107. doi: 10.3164/jcbn.D-11-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sila A, Ghlissi Z, Kamoun Z, Makni M, Nasri M, Bougatef A, Sahnoun Z. Astaxanthin from shrimp by-products ameliorates nephropathy in diabetic rats. Eur J Nutr. 2014;54:301–7. doi: 10.1007/s00394-014-0711-2. [DOI] [PubMed] [Google Scholar]
- 24.Leite MF, Lima AM, Otton R. Combination of astaxanthin and fish oil supplementation alters antioxidant enzyme profile of dental pulp tissue. Int Endod J. 2012;45:1109–1115. doi: 10.1111/j.1365-2591.2012.02080.x. [DOI] [PubMed] [Google Scholar]
- 25.Ohgami K, Shiratori K, Kotake S, Nishida T, Mizuki N, Yazawa K, Ohno S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Invest Ophthalmol Vis Sci. 2003;44:2694–2701. doi: 10.1167/iovs.02-0822. [DOI] [PubMed] [Google Scholar]
- 26.Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 27.Park JS, Chyun JH, Kim YK, Line LL, Chew BP. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (Lond) 2010;7:18. doi: 10.1186/1743-7075-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagendraprabhu P, Sudhandiran G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NFkB and COX-2. Invest New Drugs. 2011;29:207–224. doi: 10.1007/s10637-009-9342-5. [DOI] [PubMed] [Google Scholar]
- 29.Song X, Wang B, Lin S, Jing L, Mao C, Xu P, Lv C, Liu W, Zuo J. Astaxanthin inhibits apoptosis in alveolar epithelial cells type II in vivo and in vitro through the ROS-dependent mitochondrial signalling pathway. J Cell Mol Med. 2014;18:2198–212. doi: 10.1111/jcmm.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeda Y, Tsuji S, Satoh A, Ishikura M, Shirasawa T, Shimizu T. Protective effects of astaxanthin on 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J Neurochem. 2008;107:1730–1740. doi: 10.1111/j.1471-4159.2008.05743.x. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y, Hou D, Zhang X. Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Mol Vis. 2013;19:1656–1666. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang XS, Zhang X, Wu Q, Li W, Zhang QR, Wang CX, Zhou XM, Li H, Shi JX, Zhou ML. Astaxanthin alleviates early brain injury following subarachnoid hemorrhage in rats: possible involvement of Akt/bad signaling. Mar Drugs. 2014;12:4291–4310. doi: 10.3390/md12084291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 2003;21:210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 34.Abadie-Guedes R, Guedes RC, Bezerra RS. The impairing effect of acute ethanol on spreading depression is antagonized by astaxanthin in rats of 2 young-adult ages. Alcohol Clin Exp Res. 2012;36:1563–1567. doi: 10.1111/j.1530-0277.2012.01766.x. [DOI] [PubMed] [Google Scholar]


