Abstract
The aim of this study was to investigate signaling pathways for reversal of microRNA-127-mediated multi-drug resistance (MDR) in gliomas cells. Adriamycin-resistant glioma cell lines U251/adr and U87-MG/adr were established and we found that anti-microRNA-127 markedly reduced microRNA-127 expression levels in a time-dependent manner, leading to distinct inhibition of cell proliferation and increased apoptosis and the content of intracellular Rh123. Silencing of microRNA-127 significantly increased the sensitivity of U251/ADR and U87-MG/adr cells to adriamycin, compared to cells transfected with negative control siRNA. Silencing of microRNA-127 also significantly reduced the mRNA and protein expression levels of MDR1 and MRP1, which are major ATP-binding cassette (ABC) transporter linked to multi-drug resistance in cancer cells. And Runx2, p53, bcl-2 and survivin, which are important role in cell apoptosis, also markedly changed after microRNA-127 silencing. In addition, down-regulating microRNA-127 decreased the level of phosphorylated-Akt. Our data indicate that down-regulation of micorRNA-127 can trigger apoptosis and overcome drug resistance of gliomas cells. Therefore, this resistance of adriamycin in gliomas can be cancelled by silencing expression of microRNA-127.
Keywords: microRNA-127, gliomas, adriamycin, drug resistance reversal
Introduction
Glioma, as a common neurological malignancy, has become a major public health issue in the contemporary society. The treatment of malignant glioma is mainly relying on surgical excision at present, supported by postoperative radiotherapy and chemotherapy [1,2]. However, the treatment of tumor is a complex process. Resistance of cancer cells to chemotherapy continues to be a major clinical obstacle to the successful treatment of cancer, including gliomas. Cause of tumor multidrug resistance (MDR) is very complexed and reduced survival of the patients [3,4], resolving the issue of tumor MDR and searching for the target to overcome drug resistance has important significance in the treatment of many tumors, including gliomas.
MicroRNAs, a group of short non-coding RNAs, which play important roles in a variety of biologic processes. They have been shown to have both diagnostic and prognostic significance and to constitute a novel target for cancer treatment [5]. Recently, miRNAs have been associated with cell chemosensitivity or chemotherapy resistance in a variety of cancer cell types, including gliomas [6,7]. microRNA-127 was differentially expressed in a number of metabolic pathways, including cell proliferation, cycle arrest, angiogenesis and apoptosis, and acted as a tumor suppressor gene in several tumor development processes [8-12]. Zhou J et al found that miR-127 was proposed to be a tumor suppressor in human hepatocellular carcinoma cells [13]. These results indicated that microRNA-127 might be linked to gliomas chemotherapy resistance. However, the role of microRNA-127 in chemotherapy resistance in gliomas remains elusive.
The objective of this study was to investigate whether the down-regulation of microRNA-127 could sensitize gliomas cells to adriamycin. We down-regulated the expression of microRNA-127 by specific miRNA inhibitor, and conducted cellular proliferation, cell cycle, apoptosis, Rh123 content and drug sensibility assays to elucidate the role of microRNA-127 in adriamycin-resistance human glioma cell lines.
Materials and methods
Main reagents and instruments
Human glioma cell lines U251 and U87-MG were purchased from Cell Bank (Shanghai) of Type Culture Collection Committee of the Chinese Academy of Sciences; viral vectors and related reagents for shRNAs-mediated silencing were purchased from GenePharma (Shanghai); MTS and RT-PCR kit were purchased from Promega; flow cytometry kit was purchased from BD Biosciences; various monoclonal antibodies were purchased from Santa Cruz; ECL immunoblotting substrate kit was purchased from Millipore; microplate reader: Thermo; PCR instrument: Thermo; flow cytometer: BD Biosciences.
Cell culture and induction of adriamycin-resistant cell lines
The cells were cultured in an incubator under 37°C, 5% CO2 and saturated humidity condition. The culture medium was DMEM supplemented with 10% FBS. The cells were digested with 0.25% trypsin-EDTA for passaging. Cells in logarithmic growth phase were used in all experiments. To obtain adriamycin-resistant cell lines, U251 and U87-MG were cultured in medium containing 10 nM adriamycin. One day later, the medium was changed to adriamycin-free medium to resume the culture for 6 d. Using 7 d as a cycle, adriamycin concentration was doubled each time; the above procedures were repeated until the cell clones were able to tolerate 2 μM or higher concentration of adriamycin. The adriamycin-resistant cell lines were named U251/Adr and U87-MG/Adr, respectively.
microRNA-127 inhibitor preparation
Two miArrest miRNA inhibitors, which are vector-based microRNA inhibitors, block microRNA-127 regulation of target gene expression were offered by GeneCopoeia company (Guangzhou, China). And the two miArrest miRNA inhibitors were named sh1 and sh2.Transfection was carried out and then the successfully transfected cells were screened out in accordance with the reagent instructions.
MTS assay
The cells in logarithmic growth phase, 1×105 cells/ml, were seeded in 96-well microplates, 100 μl/well, and cultured overnight to allow cell adherence. The medium was removed after continuous culture for 72 h; MTS was added in accordance with the reagent instructions to continue the culture for 4 h. Finally, the OD value was measured at 490 nm wavelength with a microplate reader to represent the cell counts. The inhibition rate of this drug on cells was calculated as follows: inhibition rate = (1-experimental group OD/control group OD) ×100%.
Flow cytometry
The cells in logarithmic growth phase were digested with trypsin and the density was adjusted to 106 cells per ml with PBS. In cell cycle assay, PI staining technique was used; the cells were first fixed with 70% ethanol for 2 h; after washing off the ethanol, the cells were stained with the staining solution containing 50 μg/mL RNaseA, 1% Triton X-100 and 40 μg/mL PI (BD) for 30 min to be analyzed with a flow cytometer. In apoptosis assay, the collected cells were directly stained with Annexin V-FITC/PI in accordance with the kit instructions. In Rh-123 uptake assay, the cells were stained with 10 μM Rh-123 for 1 h prior to analysis.
Western blot
The cells were lysed to extract proteins from the cell lysate. The proteins were separated in 12% SDS-PAGE and then transferred onto a PVDF membrane; the target proteins were detected with different antibodies (4°C overnight). After washing off the primary antibodies, the membrane was incubated with HRP-conjugated secondary antibody for 1 h. After several washes, ECL kit was used to develop the immunoreactive bands. Then β-actin was used as an internal control to determine not only the expression levels of MDR1, MPR1, Runx2, p53, bcl-2, survivin, and ErbB4, but also the phosphorylation levels of AKT in these cells.
qRT-PCR
Total RNA was extracted from each group using Trizol method. Real-Time PCR Kit was used to carry out reverse transcription to obtain the cDNA; then, MDR1, MPR1, p53, Runx2, bcl-2, survivin, and ErbB4 mRNA levels were detected; The PCR primers used were as follows: 5’-CCACATCGCTCAGACACCAT-3’ (sense) and 5’-ACCAGGCGCCCAATACG-3’ (antisense) for GAPDH; 5’-CGGATTGACTGAATGCTGATT-3’ (sense) and 5’-ACTCACTTCAGGAAGCAACCA-3’ (antisense) for MDR1; 5’-GTGATCCTCGACAGGAAGGA-3’ (sense) and 5’-ATGCTCACTTTCTGGCTGGT-3’ (antisense) for MRP1; 5’-GAGGTTGGCTCTGACTGTACC-3’ (sense) and 5’-TCCGTCCCAGTAGATTACCAC-3’ (antisense) for p53; 5’-CCTAAATCACTGAGGCGGTC-3’ (sense) and 5’-CAGTAGATGGACCTCGGGAA-3’ (antisense) for Runx2; 5’-CGGGAAGCAACAACTCTGAT-3’ (sense) and 5’-GGTGCATCTGGTGATGTGAG-3’ (antisense) for Bcl-2; 5’-CTTTCTCCGCAGTTTCCTCA-3’ (sense) and 5’-TTGGTGAATTTTTGAAACTGGA-3’ (antisense) for survivin; 5’-GCAGATGCTACGGACCTTACG-3’ (sense) and 5’-GACACTGAGTAACACATGCTCC-3’ (antisense) for ErbB4. The real-time PCR reaction was conducted under the following conditions: 95°C for 30 s, 40 cycles of 95°C for 5 s and 60°C for 60 s.
Statistical analysis
Experimental data were expressed as mean ± standard deviation; SPSS13.0 software was used for analysis. One-way ANOVA was carried out for comparison; P<0.05 indicated statistically significant differences.
Results
Generation of adriamycin-resistant glioma cell lines
After the in vitro screening, an adriamycin-resistant cell lines was obtained, and named U251/Adr and U87-MG/Adr, respectively. MTS assay showed that the IC50 of adriamycin of U251/Adr and U87-MG/Adr were 3.5±0.04 and 2.3±0.5 μM, respectively, compared to their parental cells 1.05±0.13 μM and 0.7±0.12 μM, the IC50 of adriamycin increased markedly (P<0.05). qRT-PCR assay showed that U251/Adr and U87-MG/Adr over-expressed microRNA-127, as shown in Figure 1.
Figure 1.
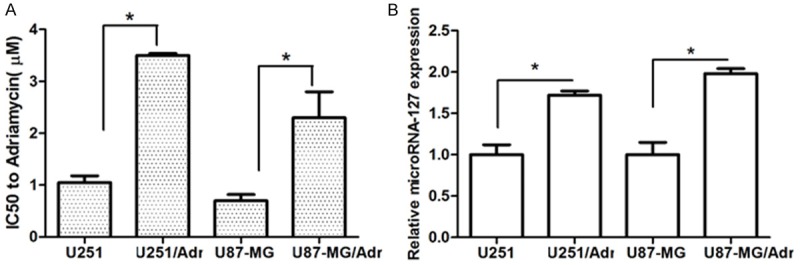
The expression of microRNA-127 was up-regulated in U251/Adr and U87-MG/Adr cell lines. A. The IC50 of adriamycin in adriamycin-resistant glioma cell lines and their parental cell lines were detected by MTS assay (*P<0.05). B. The expression of microRNA-127 in these cell lines were detected by qRT-PCR (*P<0.05).
miRNA inhibitor significantly inhibit microRNA-127 expression level
The shRNA-transfected cell clones were obtained successfully and qRT-PCR results showed that, after microRNA-127 silencing, microRNA-127 expression level in U251/Adr and U87-MG/Adr decreased significantly when compared to shControl group (P<0.05 and P<0.05, respectively Figure 2).
Figure 2.
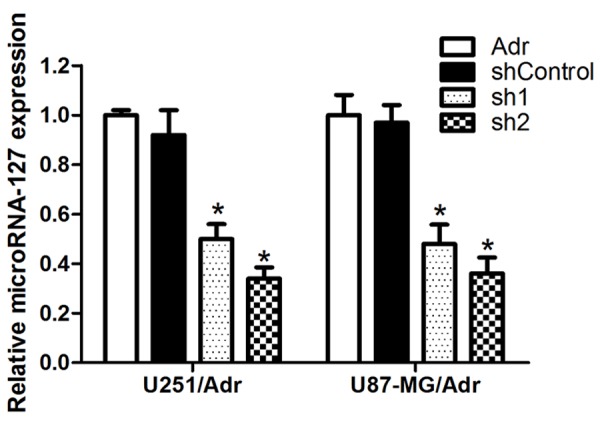
Effect of miRNA inhibitor on microRNA-127 expression. After transfected with miRNA inhibitor of microRNA-127, the expression of microRNA-127 was measured by qRT-PCR. Results are expressed as mean ± SD for three replicate determinations (*P<0.05).
microRNA-127 silencing significantly affects cell growth and increases the sensitivity to adriamycin
MTS cell proliferation assay showed that, when microRNA-127 knocked down, cell growth of U251/Adr and U87-MG/Adr were significantly inhibited (P<0.05), as shown in Figure 3. In the meantime, the sensitivity to adriamycin in U251/Adr and U87-MG/Adr increased significantly. The results revealed that down-regulating microRNA-127 could restrain the proliferation of U251/Adr and U87-MG/Adr cells; microRNA-127 might participate in drug-resistant to adriamycin in U251/Adr and U87-MG/Adr cells, and down-regulating microRNA-127 expression might improve the drug sensibility.
Figure 3.
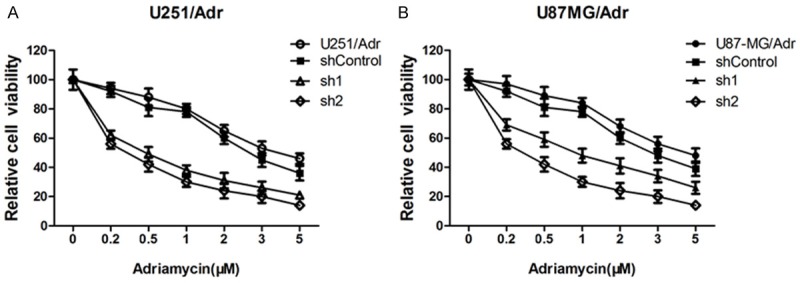
Effect of microRNA-127 Silenced on cell growth and the sensitivity to adriamycin. After transfected with miRNA inhibitor, cell viability of U251/Adr and U87-MG/Adr were measured by MTS assay.
microRNA-127 silencing arrests the cell cycle, potentiates adriamycin-induced apoptosis, and increases cellular Rh-123 uptake
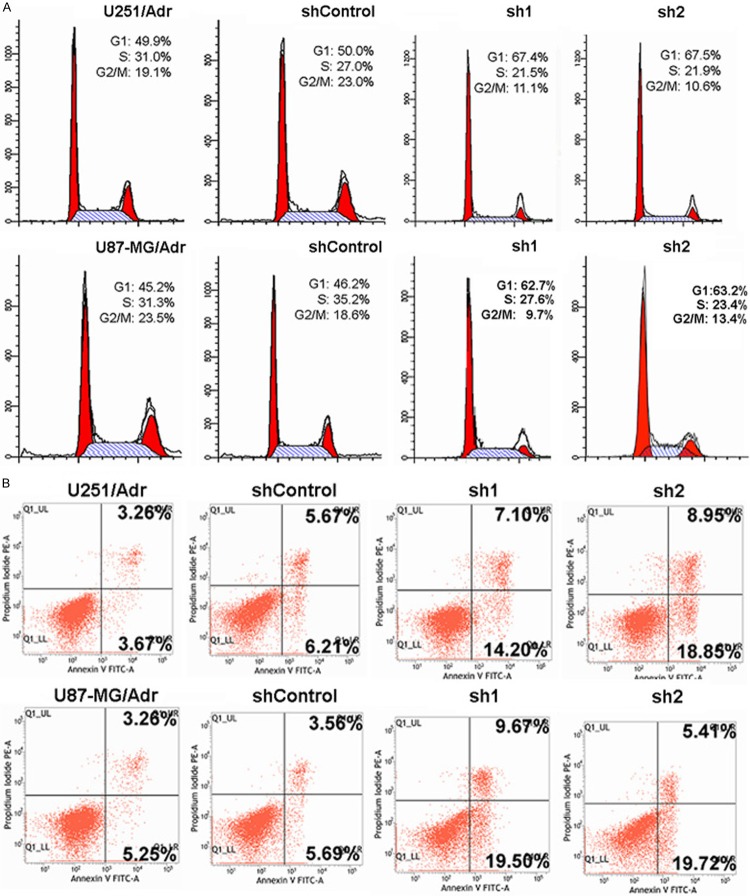
When compared with the control group after microRNA-127 silencing, the proportion of cells in G0/G1 phase increased, as shown in Figure 4. When compared with the control group after the cells have been treated with 0.5 μM adriamycin for 24 h, the proportion of apoptosis in sh1 and sh2 groups increased significantly, as shown in Figure 4. Meanwhile, the intracellular Rh-123 fluorescence intensity in sh1 and sh2 group increased significantly, suggesting that the cellular Rh-123 uptake increased. There was a dose-effect relationship between all these effects and microRNA-127 silencing (Figure 5).
Figure 4.
Effect of microRNA-127 silenced on cell cycle and apoptosis in U251/Adr and U87-MG/Adr. A. The effects of microRNA-127 silence on cell cycle. Down-regulating microRNA-127 expression led to the percentage of cells in G1/G0 phase increased obviously (P<0.05) and the microRNA-127 silencing increased the sensitivity to ADR. B. microRNA-127 silencing induced cell apoptosis in U251/Adr and U87-MG/Adr cell lines (*P<0.05, **P<0.01).
Figure 5.
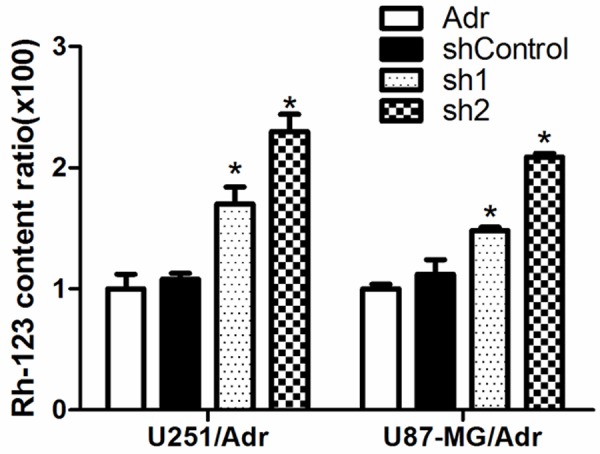
microRNA-127 silencing increased the intracellular Rh-123 in U251/Adr and U87-MG/Adr. After transfected with miRNA inhibitor, intracellular Rh-123 content was measured by flow cytometry.
microRNA-127 silencing down-regulates MDR1, MRP1, Runx2, Bcl-2, Survivin and ErbB4 expression while up-regulates p53 expression
Western blot results showed that, in sh1 and sh2 group versus the control group, the intracellular expression level of drug transport-related proteins MDR1 and MRP1 were down-regulated; the expression level of cell growth-promoting and anti-apoptosis proteins Runx2, Bcl-2, Survivin and ErbB4 were also down-regulated; the expression level of tumor suppressor gene p53 was up-regulated; as shown in Figure 6. qRT-PCR results showed that regulation of the expression of all these proteins occurred at transcriptional level, as shown in Figure 6.
Figure 6.
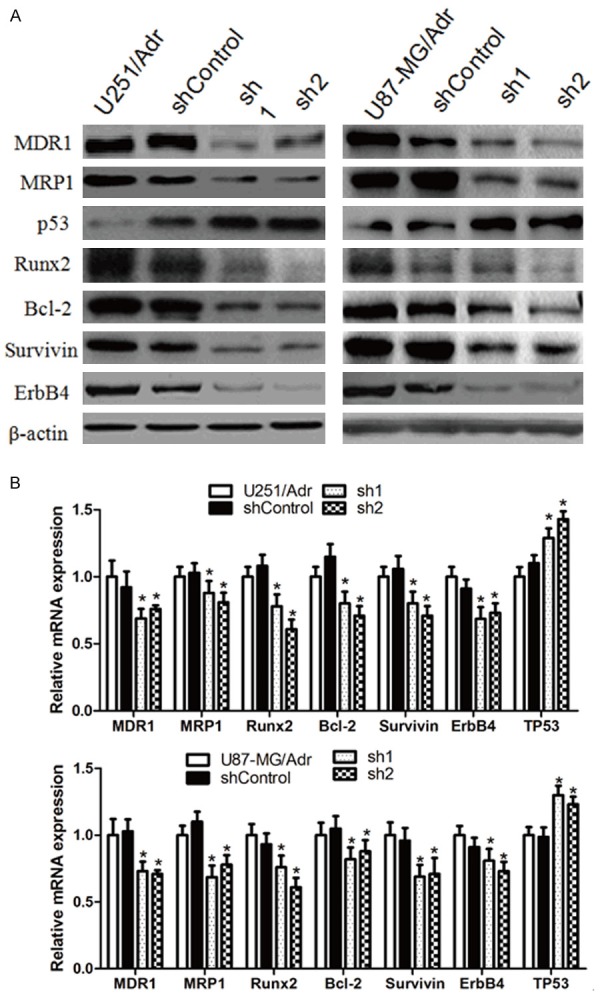
Effect of microRNA-127 silenced on drug transport-related proteins, cell cycle and apoptosis related genes in U251/Adr and U87-MG/Adr. The protein and mRNA expression levels of MDR1, MRP1, Runx2, Bcl-2, Survivin and ErbB4 were detected by Western blot (A) and qRT-PCR (B). (*P<0.05).
microRNA-127 silencing inhibits AKT phosphorylation
WB results showed that AKT expression was not affected significantly after microRNA-127 silencing, but its phosphorylation level decreased significantly, suggesting that the activity of AKT signaling pathway was inhibited (Figure 7).
Figure 7.
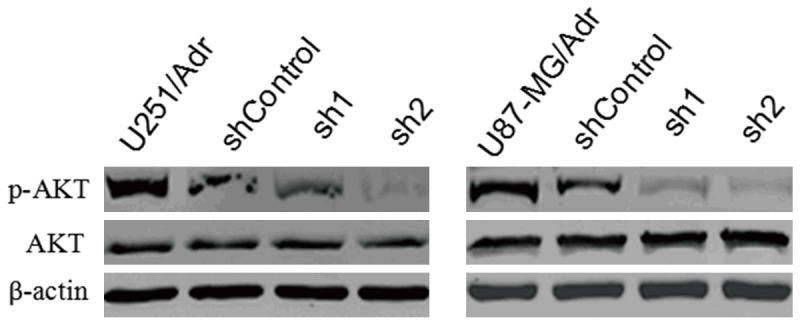
Effect of microRNA-127 silenced on phosphorylated-Akt in U251/Adr and U87-MG/Adr. The expression levels of total AKT and p-AKT were detected by Western blot analysis.
Discussion
In this study, we first obtained adriamycin-resistant gliomas U251/Adr and U87-MG/Adr cell lines over-expressing microRNA-127 and then, obtained microRNA-127-silencing cell clones using vector-based microRNA inhibitors. MTS assay showed that microRNA-127 silencing slowed down the growth of these cells and increased their sensitivity to adriamycin. These results suggest that microRNA-127 plays an important role in adriamycin resistance of gliomas U251/Adr and U87-MG/Adr cell lines. Therefore, we further investigated and verified the mechanism responsible for reversing U251/Adr and U87-MG/Adr cell resistance by microRNA-127 silencing.
Since microRNA-127 silencing itself can inhibit cell growth, we first analyzed cell cycle distribution after microRNA-127 silencing. The flow cytometry results showed that the proportion of G0/G1 phase increased after microRNA-127 silencing. Because the cells cease to grow in G0 phase and G1 phase is the starting point of a cell cycle, the increase in this proportion of cells corresponds with cell growth arrest. Western blot assay showed that p53 protein expression level was up-regulated after microRNA-127 silencing. p53, a tumor suppressor gene, can arrest the cell cycle in G1 phase and induce apoptosis simultaneously [14,15]. However, arresting the cell cycle in G0/G1 phase may not be the direct reason that silencing microRNA-127 reverses drug resistance. Under normal circumstances, a considerable portion of the tumor cells are damaged as a result of exposure to chemotherapeutic drugs, leading to apoptosis, while the drug-resistant tumor cells can often resist apoptosis to repair the damages and survive [16]. We further analyzed whether microRNA-127 silencing increased apoptosis when the cells were exposed to adriamycin. The flow cytometry analysis of apoptosis confirmed our hypothesis. Another common mechanism responsible for tumor cell drug resistance is to over-express drug transport proteins on the membrane, such as MDR1 and MRP1, in order to pump drugs out of the cells to reduce the intracellular drug concentration [17]. We first used flow cytometry to determine cellular Rh-123 uptake; the results showed that Rh-123 uptake declined in drug-resistant cells and, after microRNA-127 silencing, significantly resumed. Because Rh-123 is the transport substrate of both MDR1 and MRP1, the cellular Rh-123 uptake can indirectly reflect drug absorption by the cells [18,19]. We used Western blot and qRT-PCR to determine intracellular MDR1 and MRP1 expression. The results confirmed that their expression was regulated by microRNA-127. RUNX2, a key transcription factor associated with osteoblast differentiation, was reported to play important role in cell proliferation and apoptosis [20]. In gliomas cells, Runx2 expression pattern in these cells correlated completely with that of galectin-3 [21]. Clinically, RUNX2 over-expression was closely related to the poor prognosis of patients [22], while silencing or inhibiting RUXN2 activity was able to suppress tumor growth [23,24]. p53, bcl-2, survivin and ErbB4 are all also key molecules regulating the transition of cell cycle and apoptosis [25]. This study showed that silencing microRNA-127 caused down-regulation of their expression, except p53, implying that they may play a role in U251/Adr and U87-MG/Adr drug resistance. AKT signaling pathway that plays an important role in tumorigenesis and drug resistance [26]. It was found in breast cancer cells that microRNA-200c could increase the sensitivity of breast cancer cells to doxorubicin through the suppression of E-cadherin-mediated PTEN/Akt signaling [27]. This study showed that microRNA-127 silencing inhibited AKT phosphorylation that reflects the activity of AKT signaling pathway, suggesting that AKT also plays a role in microRNA-127-mediated adriamycin resistance.
In summary, this study shows that microRNA-127 plays a role in adriamycin resistance of U251/Adr and U87-MG/Adr, and silencing microRNA-127 can reverse its resistance. Its mechanisms of action may be related to regulating resistance-related gene expression and promoting cell cycle arrest and apoptosis.
Disclosure of conflict of interest
None.
References
- 1.Hochberg FH, Atai NA, Gonda D, Hughes MS, Mawejje B, Balaj L, Carter RS. Glioma diagnostics and biomarkers: an ongoing challenge in the field of medicine and science. Expert Rev Mol Diagn. 2014;14:439–452. doi: 10.1586/14737159.2014.905202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desjardins A, Rich JN, Quinn JA, Vredenburgh J, Gururangan S, Sathornsumetee S, Reardon DA, Friedman AH, Bigner DD, Friedman HS. Chemotherapy and novel therapeutic approaches in malignant glioma. Front Biosci. 2005;10:2645–2668. doi: 10.2741/1727. [DOI] [PubMed] [Google Scholar]
- 3.Tsuruo T, Naito M, Tomida A, Fujita N, Mashima T, Sakamoto H, Haga N. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003;94:15–21. doi: 10.1111/j.1349-7006.2003.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haar CP, Hebbar P, Wallace GC 4th, Das A, Vandergrift WA 3rd, Smith JA, Giglio P, Patel SJ, Ray SK, Banik NL. Drug resistance in glioblastoma: a mini review. Neurochem Res. 2012;37:1192–1200. doi: 10.1007/s11064-011-0701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang JC, Kwok WK, Chen Z, Ng HK. Oncogenic role of microRNAs in brain tumors. Acta Neuropathol. 2009;117:599–611. doi: 10.1007/s00401-009-0525-0. [DOI] [PubMed] [Google Scholar]
- 6.Ma J, Dong C, Ji C. MicroRNA and drug resistance. Cancer Gene Therapy. 2010;17:523–535. doi: 10.1038/cgt.2010.18. [DOI] [PubMed] [Google Scholar]
- 7.Song B, Wang Y, Xi Y, Kudo K, Bruheim S, Botchkina GI, Gavin E, Wan Y, Formentini A, Kornmann M, Fodstad O, Ju J. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene. 2009;28:4065–4074. doi: 10.1038/onc.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA, Jones PA. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9:435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Xie T, Liang J, Liu N, Wang Q, Li Y, Noble PW, Jiang D. MicroRNA-127 inhibits lung inflammation by targeting IgG Fcγ receptor I. J Immunol. 2012;188:2437–2344. doi: 10.4049/jimmunol.1101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo LH, Li H, Wang F, Yu J, He JS. The tumor suppressor roles of miR-433 and miR-127 in gastric cancer. Int J Mol Sci. 2013;14:14171–14184. doi: 10.3390/ijms140714171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao X, Duan Z, Liu X, Wang B, Wang X, He J, Yao Z, Yang J. MicroRNA-127 is downregulated by Tudor-SN protein and contributes to metastasis and proliferation in breast cancer cell line MDA-MB-231. Anat Rec (Hoboken) 2013;296:1842–1849. doi: 10.1002/ar.22823. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Wang M, Guo M, Xie Y, Cong YS. miR-127 regulates cell proliferation and senescence by targeting BCL6. PLoS One. 2013;8:e80266. doi: 10.1371/journal.pone.0080266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J, Lu S, Yang S, Chen H, Shi H, Miao M, Jiao B. MicroRNA-127 post-transcriptionally downregulates Sept7 and suppresses cell growth in hepatocellular carcinoma cells. Cell Physiol Biochem. 2014;33:1537–1546. doi: 10.1159/000358717. [DOI] [PubMed] [Google Scholar]
- 14.Yuan L, Zhang Y, Xia J, Liu B, Zhang Q, Liu J, Luo L, Peng Z, Song Z, Zhu R. Resveratrol induces cell cycle arrest via a p53-independent pathway in A549 cells. Mol Med Rep. 2015;11:2459–2464. doi: 10.3892/mmr.2014.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao ZM, Dawson MI, Li XS, Rishi AK, Sheikh MS, Han QX, Ordonez JV, Shroot B, Fontana JA. p53 independent G0/G1 arrest and apoptosis induced by a novel retinoid in human breast cancer cells. Oncogene. 1995;11:493–504. [PubMed] [Google Scholar]
- 16.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 17.Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract. 2005;14(Suppl 1):35–48. doi: 10.1159/000086183. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann T, Kohler C, Weidauer E, Taege C, Foth H. Expression of MRP1 and related transporters in human lung cells in culture. Toxicology. 2001;167:59–72. doi: 10.1016/s0300-483x(01)00458-9. [DOI] [PubMed] [Google Scholar]
- 19.Al-Mohizea AM, Al-Jenoobi FI, Alam MA. Rhodamine-123: A p-glycoprotein marker complex with sodium lauryl sulfate. Pak J Pharm Sci. 2015;28:617–622. [PubMed] [Google Scholar]
- 20.Eliseev RA, Dong YF, Sampson E, Zuscik MJ, Schwarz EM, O’Keefe RJ, Rosier RN, Drissi MH. Runx2-mediated activation of the Bax gene increases osteosarcoma cell sensitivity to apoptosis. Oncogene. 2008;27:3605–3614. doi: 10.1038/sj.onc.1211020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vladimirova V, Waha A, Lückerath K, Pesheva P, Probstmeier R. Runx2 is expressed in human glioma cells and mediates the expression of galectin-3. J Neurosci Res. 2008;86:2450–2461. doi: 10.1002/jnr.21686. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Xu S, Lin S, Zhao W. Overexpression of runt-related transcription factor-2 is associated with advanced tumor progression and poor prognosis in epithelial ovarian cancer. J Biomed Biotechnol. 2012;2012:456534. doi: 10.1155/2012/456534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra S, Vaughn AD, Devore DI, Roth CM. Delivery of siRNA silencing Runx2 using a multifunctional polymer-lipid nanoparticle inhibits osteogenesis in a cell culture model of heterotopic ossification. Integr Biol (Camb) 2012;4:1498–1507. doi: 10.1039/c2ib20200j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma J, Lu H, Wang S, Chen B, Liu Z, Ke X, Liu T, Fu J. The anthraquinone derivative Emodin inhibits angiogenesis and metastasis through downregulating Runx2 activity in breast cancer. Int J Oncol. 2015;46:1619–1628. doi: 10.3892/ijo.2015.2888. [DOI] [PubMed] [Google Scholar]
- 25.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 26.Fu X, Tian J, Zhang L, Chen Y, Hao Q. Involvement of microRNA-93, a new regulator of PTEN/Akt signaling pathway, in regulation of chemotherapeutic drug cisplatin chemosensitivity in ovarian cancer cells. FEBS Lett. 2012;586:1279–1286. doi: 10.1016/j.febslet.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Sun Y, Chen L, Xu X, Zhang X, Wang B, Min L, Liu W. miRNA-200c increases the sensitivity of breast cancer cells to doxorubicin through the suppression of E-cadherin-mediated PTEN/Akt signaling. Mol Med Rep. 2013;7:1579–1584. doi: 10.3892/mmr.2013.1403. [DOI] [PubMed] [Google Scholar]