Abstract
Objective: To establish a canine model of crush syndrome (CS). Methods: A total of 16 healthy adult female Beagle dogs were randomly divided into the control group (n=8) and the experimental group (n=8). The crush injury was created in the left hind leg of each dog in the experimental group. Results: The biochemical indexes in the experimental group changed significantly compared to the values before extrusion. And they were also significantly different from the values of the control group. The glomerular capillary dilation, renal tubular epithelial cell degeneration, and renal interstitial lymphocytic infiltration were found in the kidneys. Conclusion: The canine CS model established by the digital crush injury device platform was successful according with the diagnosis of CS. It is good for the investigation of the CS mechanism and treatment using this model.
Keywords: Crush syndrome, crush injury device platform, dogs
Introduction
Crush syndrome (CS) also known as traumatic rhabdomyolysis syndrome is defined as traumatic compression of muscle tissue with resulting limb swelling, muscular necrosis, hyperkalemia, myoglobinuria and acute kidney injury (AKI). The main symptom of CS is rhabdomyolysis, leading to the muscle cell contents exuding into the extracellular fluid and blood circulation and causing hypovolemia, electrolyte imbalance and complications like AKI and multiple organ dysfunctions. In 1941, CS was first described in Bywaters’s report of patients who were trapped beneath rubble after the air strike in London with symptoms of swollen limbs, circulatory disturbance, dark urine (caused by myohemoglobin in urine) and acute renal failure [1]. Besides the swollen limbs and muscular necrolysis, severe secondary systemic pathological changes always appear in CS patients, like serious damages of remote organs including kidney, heart, lung and liver [2]. CS patients without an effective first aid may be multiple organ failure to death. CS may present at patients who were buried or extruded in explosive terrorist attacks, natural disasters (earthquakes and landslides), traffic accidents and other severe disasters (mine accidents and house collapses). Since the incidence rate of CS after an earthquake is about 10% and the mortality rate is up to 40%, CS is the second leading cause of death for patients in earthquakes [3-6].
Some studies indicated that continuous renal replacement therapy (CRRT) is an effective treatment for CS patients [7,8]. However doctors always use CRRT on the basis of their own experiences for the lack of laboratory experiments for the optimal timing, dose and duration of treatment. Currently animal models of CS are established using small animals like mice, rats or rabbits [9,10]. But these animals are not suitable for the treatment of CRRT because of their thin cardiac vein and small blood volume. Dogs have rich muscles in their limbs, large blood volume, thick arteries and veins, so it is easy to build access of blood purification. In our study, a canine model of CS was established using the self-made digital crush injury device platform (patent No. 201220049194.0) (Figures 1, 2) for the further investigation of continuous blood purification to treat CS.
Figure 1.
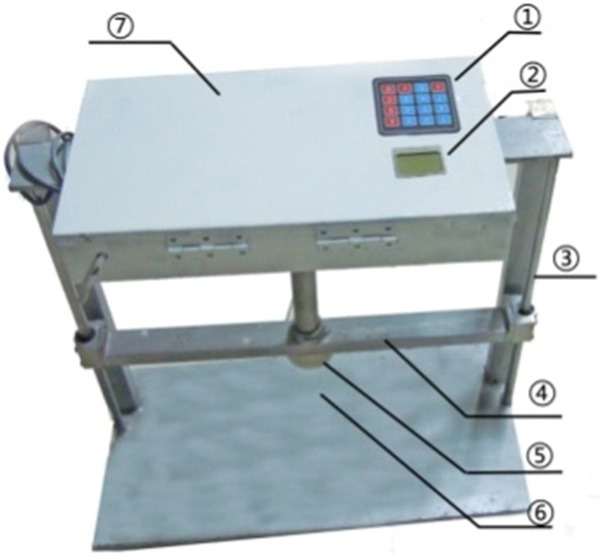
The digital crush injury device platform, picture of the real product. ① controling keyboard; ② display screen; ③ guiding shaft; ④ lifting lever; ⑤ extrusion sliding block; ⑥ substrate (with pressure sensor); ⑦ control chamber.
Figure 2.
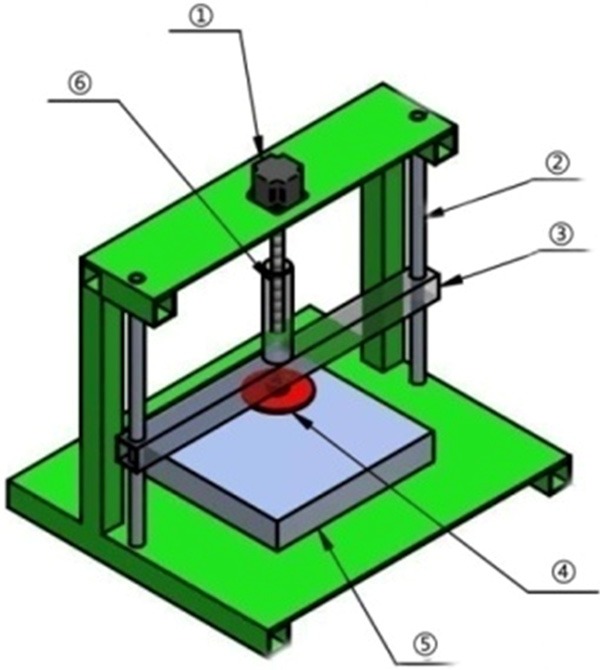
The digital crush injury device platform, diagrammatic drawing of the machinery. ① stepper motor; ② guiding shaft; ③ horizontal axis of the lifting lever; ④ extrusion sliding block; ⑤ pressure sensor; ⑥ vertical axis of the lifting lever.
Materials and methods
Animals
A total of 16 healthy adult Beagle dogs between 10 and 12 kg body-weight were provided by the Animal Center of Academy of Military Medical Sciences (experimental animal production license: SCXK (Army) 2012-0002). Room temperature was maintained at 20-28°C with relative humidity of 40-70%. Experimental procedures were performed in accordance with the Helsinki Convention and the Guide for the Care and Use of Laboratory Animals [11] and were approved by the Animal Ethics Committee of Affiliated Hospital of Logistics University of Chinese People’s Armed Police Forces.
Crush injury
Dogs were randomly divided into the control group (n=8) and the experimental group (n=8). Fasting time before anesthesia was 12 hours. Dogs were narcotized using Sumianxin II injection (size: 1.5 ml/L, Veterinary Institute of Changchun Quartermaster University, China). The first intramuscular injection of 1.5 ml was followed by an additional 0.5-1 ml every two hours. Dogs were anesthetized until decompression. Dogs were placed on the crush injury device platform (Figure 3) in the supine position with retention catheterization and restraint straps to fix limbs and mouth. A venous indwelling needle in right femoral vein was used for blood collection and transfusion. Prepare the left hind leg by shaving and continuously extrude the point 1 cm below the left groin by the self-made digital crush injury device platform with the extrusion area of 10 cm×10 cm and the oppressive weight of 50 kg (about 5 times the dog’s weight). Decompress dogs following 8 h extrusion. Blood samples were collected before extrusion, at 4 h and 8 h extrusion and at 1 h, 4 h, 8 h, 12 h and 24 h after decompression. Following 24 h decompression, all the dogs were sacrificed by exsanguination under anesthesia. And the kidney, crush-injured muscle and myocardial tissue samples were harvested. Dogs in control group were fixed in the supine position but not extruded.
Figure 3.
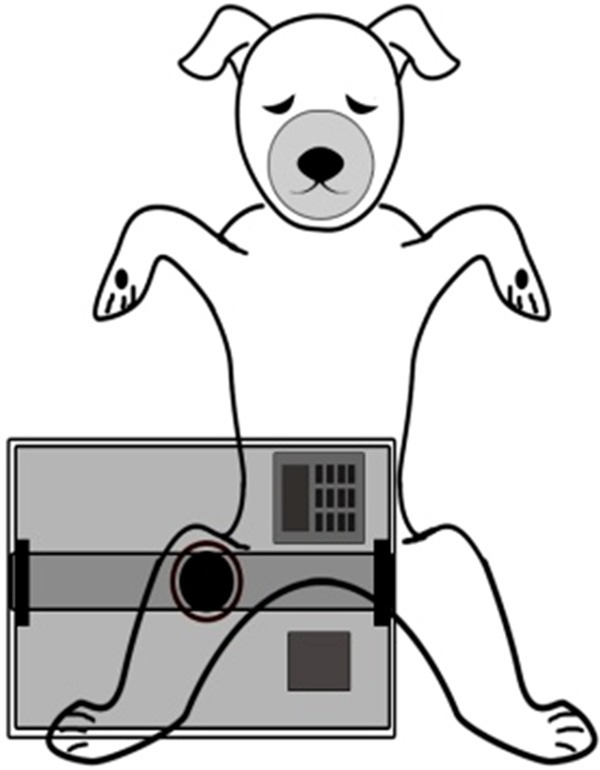
The diagrammatic drawing of the extrusion process.
General measurement
The color and amount of urine, the hemoglobinuria and death were recorded. Physical activities and the perimeter of the crush-injured limbs were observed at 1 h, 4 h, 8 h, 12 h and 24 h after decompression.
Biochemical analysis
Creatine kinase (CK), creatine kinase-MB (CK-MB), serum creatinine (Scr), blood urea nitrogen (BUN), alanine transaminase (ALT) and aspartate aminotransferase (AST) were analyzed using the Beckman-Coulter LX-20 (Beckmann Coulter Inc. Fullerton, CA). Serum potassium (K+) and carbon dioxide combining power (CO2CP) were analyzed using the i-STAT 1 Wireless (Abbott Point of Care Inc., Illinois, USA).
Histological examinations
Tissue samples were fixed in 4% paraformaldehyde, dehydrated in graded ethanol, deparaffinized in xylene, routine paraffin-embedded, sectioned into 5 μm thick, HE stained and examined for pathological changes.
Diagnostic criteria
According to the human diagnostic criteria of CS [12-14], dogs with muscles rich areas extruded for a long time, continue oliguria or anuria, hemoglobinuria, serum CK 5 times higher than baseline, AKI and one or more complications like hyperkalemia, metabolic acidosis and etc. were diagnosed with CS.
Statistics analysis
SPSS 16.0 was used for statistical analysis. All data were expressed as mean ± standard deviation (SD) (x̅±s). The statistical differences among different groups were assessed by repeated measures ANOVA. Multiple comparisons within groups were performed using LSD test. P<0.05 indicated a significant difference.
Results
General conditions of dogs
Dogs in control group were in good condition with normal breathing and heart rate. Experimental dogs were listless with rapid shallow breathing, increased heart rate and clammy extremities. Dogs in control group had normal physical activities and sensory function with unchanged skin and perimeter of limbs. While experimental dogs were hypoesthesia, and some of them had subcutaneous bleeding, tension blisters or even necrosis. The crush-injured limbs of experimental dogs couldn’t act normally and were swelling. The peak of crush-injured limb perimeter appeared at 8 h after decompression and was significantly greater than time before decompression (P<0.05, data are not shown). 3 experimental dogs died in 24 hours after decompression however there was no death in the control group.
Myoglobinuria and hypourocrinia occurred in experimental dogs
There was no myoglobinuria in the control group. In the experimental group, there was no myoglobinuria before or at 4 h extrusion, in 1 dog (12.5%) at 8 h extrusion and in the other 7 dogs (87.5%) at 1 h after decompression. The urinary output in the experimental group was significantly poorer than the control group after decompression (P<0.05, data are not shown) and got poorer with the time passing. The urinary output of experimental dogs was <0.5 ml/Kg/h at 8-24 h after decompression which met the diagnostic criteria of AKI [15].
Biochemical indexes changed significantly in experimental dogs
As shown in Table 1, the levels of CK and CK-MB in experimental dogs at 4 h and 8 h after extrusion and 1 h, 4 h, 8 h, 12 h and 24 h after decompression were significantly higher than the time before extrusion (P<0.01) and higher than the values in the control group (P<0.01) (Figure 4). The levels of CK and CK-MB in the experiment group increased to the highest values at 8 h after decompression and then declined but were still significantly higher than the control group. However the levels of CK and CK-MB in the control group didn’t change significantly with the time.
Table 1.
Levels of biochemical indexes during the experiment in each group
| Before extrusion | 4 h extrusion | 8 h extrusion | 1 h after decompression | 4 h after decompression | 8 h after decompression | 12 h after decompression | 24 h after decompression | |
|---|---|---|---|---|---|---|---|---|
| CK (U/L) | ||||||||
| Control group | 349.25±21.42 | 340.01±22.19 | 324.50±24.68 | 316.50±15.67 | 325.25±16.38 | 331.50±19.36 | 333.45±19.81 | 340.00±13.63 |
| Experimental group | 345.88±36.39 | 662.88±197.56*,† | 2470.88±327.03*,† | 17740.88±1393.92*,† | 45456.13±3312.7*,† | 58436.01±3744.87*,† | 50319.75±3195.04*,† | 28319.50±2529.29*,† |
| CK-MB (U/L) | ||||||||
| Control group | 121.25±25.73 | 125.12±27.03 | 122.01±20.90 | 119.79±22.38 | 124.67±19.65 | 121.79±26.12 | 128.05±27.11 | 128.91±28.09 |
| Experimental group | 124.25±27.73 | 197.75±42.11*,† | 215.01±39.02*,† | 711.03±106.48*,† | 2162.87±222.61*,† | 3206.38±266.88*,† | 2652.13±238.25*,† | 828.01±137.39*,† |
| BUN (mmol/L) | ||||||||
| Control group | 4.38±0.62 | 4.55±0.78 | 5.52±0.91 | 5.50±0.81 | 5.55±1.12 | 5.60±0.56 | 5.45±0.69 | 5.83±1.10 |
| Experimental group | 4.26±0.79 | 5.04±0.99 | 6.91±0.76 | 8.54±1.43*,† | 12.01±1.86*,† | 16.45±2.83*,† | 19.05±1.92*,† | 24.50±1.37*,† |
| Scr (μmol/L) | ||||||||
| Control group | 72.75±12.09 | 75.75±13.96 | 72.95±12.18 | 79.75±14.07 | 85.25±15.12 | 82.07±16.27 | 85.75±16.30 | 86.50±15.19 |
| Experimental group | 78±17.68 | 77.5±13.52 | 92.88±19.32 | 103.38±22.36*,† | 160±52.57*,† | 196.5±61.16*,† | 234.13±50.74*,† | 404.63±53.51*,† |
| ALT (U/L) | ||||||||
| Control group | 24.50±2.52 | 28.25±2.63 | 31.25±3.30 | 35.75±7.22 | 34.25±5.38 | 36.50±7.85 | 35.75±5.68 | 35.00±6.68 |
| Experimental group | 23.25±2.25 | 25.50±4.72 | 31.50±4.04 | 52.50±19.73*,† | 131.00±27.17*,† | 290.38±92.66*,† | 358.50±51.60*,† | 532.00±32.67*,† |
| AST (U/L) | ||||||||
| Control group | 32.00±3.16 | 43.00±3.56 | 41.75±2.99 | 44.50±6.02 | 42.50±3.87 | 40.50±7.14 | 47.00±2.44 | 46.25±1.25 |
| Experimental group | 26.88±4.42 | 53.38±16.00 | 64.88±24.86 | 214.50±46.74*,† | 670.25±246.77*,† | 1292.38±238.86*,† | 1629.13±180.11*,† | 2091.50±237.65*,† |
| K+ (mmol/L) | ||||||||
| Control group | 4.17±0.49 | 4.72±0.36 | 4.88±0.49 | 4.68±0.37 | 4.92±0.43 | 4.65±0.59 | 4.22±0.10 | 4.05±0.44 |
| Experimental group | 4.56±0.42 | 4.88±0.65 | 5.43±0.59 | 6.39±0.51*,† | 6.51±0.46*,† | 6.63±0.57*,† | 7.15±0.53*,† | 7.18±0.55*,† |
| CO2CP (mmol/L) | ||||||||
| Control group | 24.48±3.30 | 23.25±3.25 | 25.38±3.21 | 24.43±2.77 | 25.10±3.26 | 24.01±3.52 | 24.15±3.22 | 24.57±3.49 |
| Experimental group | 23.59±3.18 | 22.46±3.33 | 20.66±3.52*,† | 18.11±3.15*,† | 16.86±3.27*,† | 15.91±3.32*,† | 14.41±3.67*,† | 11.21±3.32*,† |
P<0.01 vs. the time before extrusion;
P<0.01 vs. control group.
CK, creatine kinase; CK-MB, creatine kinase-MB; Scr, serum creatinine; BUN, blood urea nitrogen; ALT, alanine transaminase; AST, aspartate aminotransferase; K+, serum potassium; CO2CP, carbon dioxide combining power.
Figure 4.
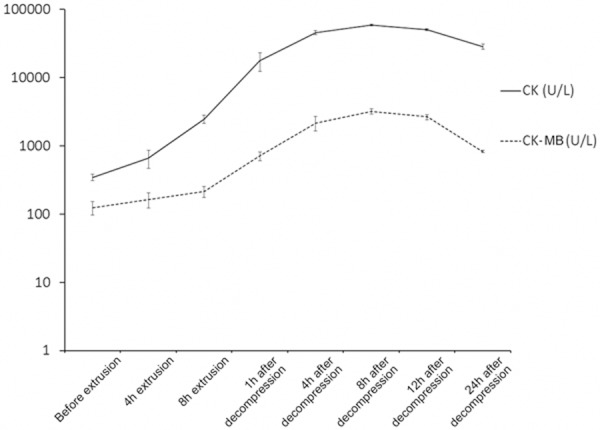
The levels of CK and CK-MB in the experiment.
The levels of BUN, Scr, ALT, AST and K+ in experimental dogs at 1 h, 4 h, 8 h, 12 h and 24 h after decompression were significantly higher than the time before extrusion (P<0.01) and higher than the values in the control group (P<0.01). However those levels in the control group didn’t change much.
Blood CO2CP in the experimental group was weaker at 8 h extrusion and at 1 h, 4 h, 8 h, 12 h and 24 h after decompression than the time before extrusion (P<0.01) and weaker than that in control group (P<0.01) which didn’t change significantly.
Pathological changes in kidney, crush-injured muscle and myocardial tissues of experimental dogs
As show in renal pathological sections, in the control group, the structure of glomerulus was normal, the renal tubular epithelial cells were integral and there were no casts in lumen (Figure 5A). In the experimental group, part of the renal capsule thickened, part of the glomerular capillary dilated or congested, part of the renal tubular dilated, epithelial cells swelled and some were vacuolar degeneration or necrosis, necrotic cell debris and exudates were found in part of the renal tubular lumen, myoglobin casts were seen and there was an infiltration of renal interstitial lymphocytes (Figure 5B, 5C).
Figure 5.
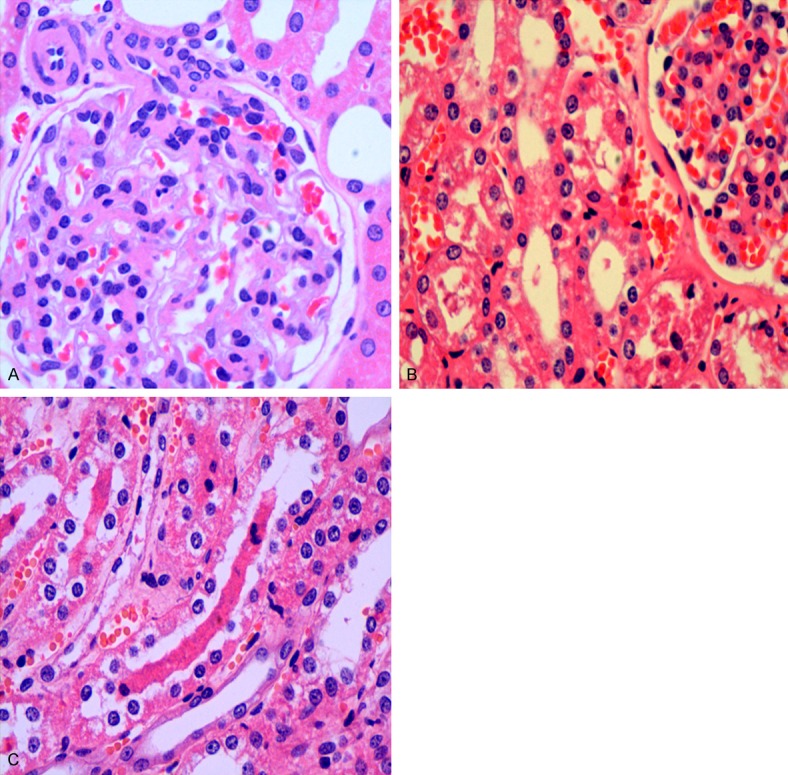
The pathological analysis of renal tissues. A: Control group HE staining ×400; B, C: Experimental group HE staining ×400.
As shown in muscle pathological sections, in the control group, the structure of muscle fibers was normal, horizontal grains were clear and cells were completed (Figure 6A). In the experimental group, bleeding was in muscle tissues, degeneration, swelling and necrosis appeared in part of the muscle fibers and lymphocytic infiltration and fat cell infiltration were seen (Figure 6B, 6C).
Figure 6.
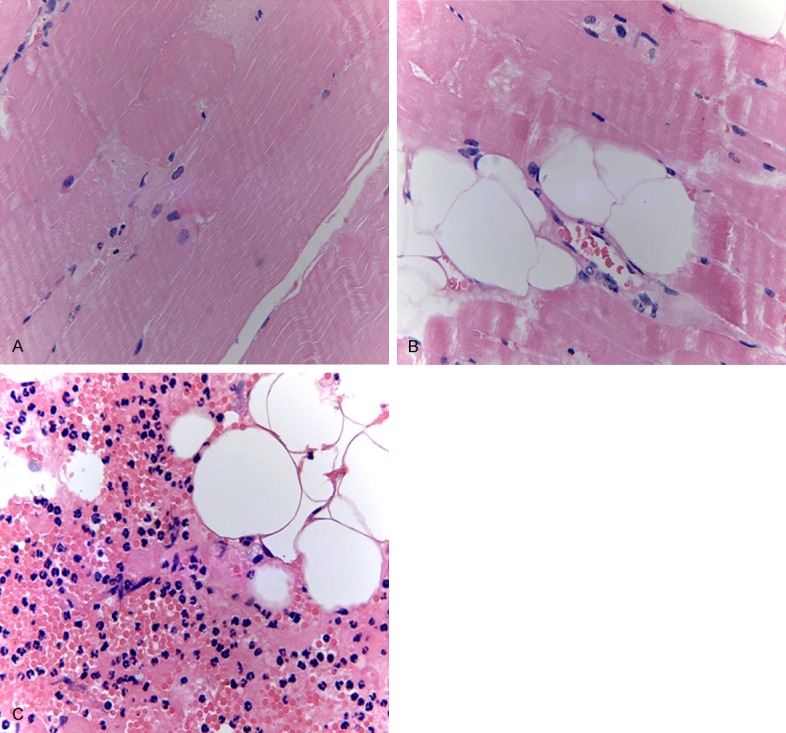
The pathological analysis of left hind muscle tissues. A: Control group HE staining ×400; B, C: Injured parts of experimental group HE staining ×400.
As shown in Figure 7, part of the heart capsule thickened, fat cell infiltration and inflammatory cell infiltration were found and some myocardial cells were necrosis.
Figure 7.
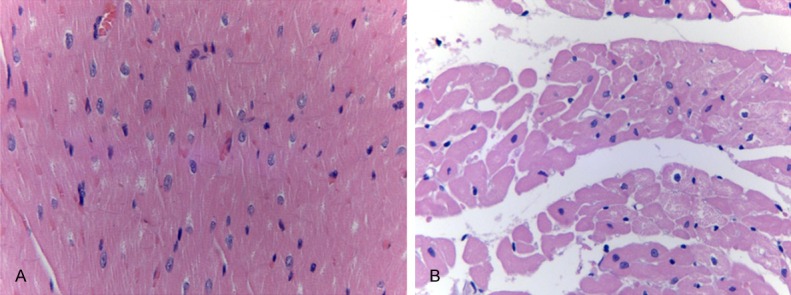
The pathological analysis of myocardium tissues. A: Control group HE staining ×400; B: Experimental group HE staining ×400.
Discussion
Establishing animal model of CS is the basic method to investigate the mechanism and treatments of CS. Nowadays there are two main methods to build an animal model of CS: the first is tying up the certain part of animals by ropes or springs to block the blood flows [16,17]; the second is extruding the certain part of animals by heavy things to cause necrosis of muscle tissue. The second method is similar to the way causing CS in earthquakes, landslides and other disasters or accidents. In 2005, Akimau [18] designed a rat model of CS by putting heavy things on some parts of the rat. But the area of thrust surface in the model couldn’t be controlled accurately. In 2013, a new crush injury device was made by Chen, et al. [19] so that rats could drink and eat in the extrusion. However the device could not show the area of thrust surface and the real time pressure. In our study, we used a single chip microcomputer Intel 89C52 (Intel Corporation, USA) as the control unit of our crush injury device platform, used a pressure sensor to measure the pressure and used different extrusion modules to control the area of thrust surface. The pressure and time for extrusion could be set in our self-made digital crush injury device and the temperature, pressure and the remaining time for extrusion could be shown.
The lower limbs have rich muscles and they are easily injured in earthquakes, landslides and traffic accidents resulting in a CS. In our research, the left hind legs of Beagle dogs were extruded by a 50 kg weight for 8 h using the self-made digital crush injury device platform. And then they were found to be congestion, swelling and perimeter increasing. Myoglobinuria was found in 1 h after decompression in the experimental group. In some studies, myohemoglobin in blood and urine were used as the important diagnostic criteria [20]. CK and CK-MB as sarcoplasmic proteins in myocytes will enter into the blood when myocytes are damaged. They are closely related to the injured range and degree of the skeletal muscle necrotic. In our study, the levels of CK and CK-MB increased and reached to the peak level beginning with the extrusion until 8 h after decompression. The peak value of CK in the experimental group was >50000 U/L significantly higher than the control group at the same time reaching the diagnostic criteria of CS. So CK and CK-MB were the sensitive indicators of CS. The levels of CK and CK-MB declined after the peak in the experimental group but still higher than the control group. That was the same as the previous reports, and that might be related to the amount and the injury degree of striated muscle cells damaged by the extrusion [21].
The necrotic cell lysates of injured muscle tissues were absorbed into blood leading to the hyperkalemia, metabolic acidosis and AKI [22]. In our study, dogs in experimental group suffered from hyperkalemia after the decompression and the level of serum potassium was significantly higher than the control group (P<0.01). The reason seems to be that a large number of intracellular potassium ions outflow into the blood when muscle cells injured by the extrusion. Blood gas analysis for the experimental dogs showed that the value of CO2CP at 8 h extrusion and after decompression was significantly lower than the time before extrusion (P<0.01). It indicated that metabolic acidosis aggravated with the process of the extrusion. AKI is one of the outstanding features of CS and is an important prognostic factor of CS [23]. The mechanism of AKI induced by CS is still not fully clear. There were reports suggesting that myoglobin entering into kidney with the blood flow after the rhabdomyolysis was filtered in glomerulus and then resolved in proximal tubule. It became into jelly in an acid environment and then formed casts with shedding tubular epithelial cells to block the renal distal convoluted tubule and the collecting duct leading to AKI. In an internal environment of metabolic acidosis, the iron ligand valence of myoglobin changed and ferrous myoglobin was generated to induce a dose-dependent apoptosis of renal tubular epithelial cells [24,25]. In our study, the urine color of experimental dogs changed from light red to dark brown after decompression. Experimental dogs got urinary output significantly reduced at 5 to 8 h after decompression and even anuria at 8 to 24 h after decompression. All the experimental dogs met the TIFLE diagnosis of AKI after decompression. And the pathological analysis further confirmed the diagnosis of AKI.
As was reported that liver dysfunction could be found in CS patients [7]. The levels of ALT and AST of experimental dogs in this study increased significantly suggesting a liver dysfunction. Myocardial damage was rarely reported so far. In our study, edema, necrosis and inflammatory cell infiltration were observed in part of the myocardial cells of experimental dogs. It indicated that a myocardial damage was induced by CS. That should be confirmed by further studies with more tests of myocardial injury markers.
In conclusion, dogs in the experimental group suffering from 8 h extrusion got obvious swelling and muscle trauma in injured limbs, significant changes of blood biochemical indexes and pathological changes in kidney, crush-injured muscle and myocardial tissues, meeting the diagnostic criteria of CS. The further studies about the mechanism and treatment of CS could be performed easily using this canine CS model established by the digital crush injury device platform.
Disclosure of conflict of interest
This research was supported by grants from the Fundation of Key Laboratory of Emergency and Disaster Medicine in Chinese People’s Liberation Army (PLA) (JY1404).
References
- 1.Bywaters EG, Beall D. Crush Injuries with Impairment of Renal Function. Br Med J. 1941;1:427–432. doi: 10.1136/bmj.1.4185.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malinoski DJ, Slater MS, Mullins RJ. Crush injury and rhabdomyolysis. Crit Care Clin. 2004;20:171–192. doi: 10.1016/s0749-0704(03)00091-5. [DOI] [PubMed] [Google Scholar]
- 3.Sever MS, Lameire N, Vanholder R. Renal disaster relief: from theory to practice. Nephrol Dial Transplant. 2009;24:1730–1735. doi: 10.1093/ndt/gfp094. [DOI] [PubMed] [Google Scholar]
- 4.Sever MS, Vanholder R. Management of crush victims in mass disasters: highlights from recently published recommendations. Clin J Am Soc Nephrol. 2013;8:328–335. doi: 10.2215/CJN.07340712. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Sun X, Zhang L, Cai G, Ding X, Ma Z, Wang R, Wang Y, Wang L, Wang L, Fu P, Liu B, Liu Z, Xiang J, Bi W, Tang L, Xu Q, He Y, Zhang L, Zhang J, Li Y, Li W, Li H, Xiao J, Qiu Q, Chen J, Chen F, Lin H, Miao L, Duan Z, Dang S, Xi X, Guo D, Guo L, Mei C, Huang X. Expert consensus of the crush syndrome diagnosis and treatment of acute kidney injury. Natl Med J China. 2013;93:1297–1300. [Google Scholar]
- 6.Reingardiene D, Jodziuniene L, Lazauskas R. [Muscle crush injury and crush syndrome] . Medicina (Kaunas) 2010;46:435–441. [PubMed] [Google Scholar]
- 7.Wei Q, Baihai S, Ping F, Xiaolei C, Jing L, Rong Z. Successful treatment of crush syndrome complicated with multiple organ dysfunction syndrome using hybrid continuous renal replacement therapy. Blood Purif. 2009;28:175–180. doi: 10.1159/000227786. [DOI] [PubMed] [Google Scholar]
- 8.Li CY, Gu JW, Li YM, Peng T, Gan XY. Continuous renal replacement therapy and blood transfusions in treating patients with crush syndrome: 8 Case studies from the Wenchuan earthquake. Transfus Apher Sci. 2011;45:257–260. doi: 10.1016/j.transci.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Dobek GL, Fulkerson ND, Nicholas J, Schneider BS. Mouse model of muscle crush injury of the legs. Comp Med. 2013;63:227–232. [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama T, Fujita M, Ishihara M, Ishihara M, Ogata S, Yamamoto Y, Shimizu M, Maehara T, Kanatani Y, Tachibana S. Improved survival rate by temperature control at compression sites in rat model of crush syndrome. J Surg Res. 2014;188:250–259. doi: 10.1016/j.jss.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Guide for the care and use of laboratory animals. National Academies Press; 1996. Institute of Laboratory Animal Resources. [PubMed] [Google Scholar]
- 12.Wang WZ, Fang XH, Stephenson LL, Khiabani KT, Zamboni WA. Ischemia/reperfusion-induced necrosis and apoptosis in the cells isolated from rat skeletal muscle. J Orthop Res. 2008;26:351–356. doi: 10.1002/jor.20493. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z. The investigation of the timing, pattern and dose of blood purification for crush syndrome. Chin J Blood Purif. 2008;7:498–499. [Google Scholar]
- 14.Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore) 1982;61:141–152. doi: 10.1097/00005792-198205000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–84. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 16.Richards NT, Tattersall J, McCann M, Samson A, Mathias T, Johnson A. Dialysis for acute renal failure due to crush injuries after the Armenian earthquake. BMJ. 1989;298:443–445. doi: 10.1136/bmj.298.6671.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaoyang Z, Chunhua Z, Mingmin W, Yue W, Weisi S, Yali X, Jiyou L, Shasha Y, Xiaoyuan Z. A canine model of crush syndrome. China Journal of Emergency Resuscitation and Disaster Medicine. 2013;8:811–814. [Google Scholar]
- 18.Akimau P, Yoshiya K, Hosotsubo H, Takakuwa T, Tanaka H, Sugimoto H. New experimental model of crush injury of the hindlimbs in rats. J Trauma. 2005;58:51–58. doi: 10.1097/01.ta.0000154066.11748.38. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Liu Y, Xu W, Qin T, Zhao L, Liu S, Zhang Y, Tan H, Zhou Y. Experimental study on establishment of a simple model of rats crush injury-crush syndrome. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013;27:77–82. [PubMed] [Google Scholar]
- 20.Hong D, Zhang Y, Zhang P, Pu L, Wu S, Gao H, Chen J, He Q, Wang F, Chen X, Wang L. Urine markers of acute kidney injury in patients with quake-associated crush syndrome. Chin J Nephrol Dial Transpl. 2009;18:334–337. [Google Scholar]
- 21.Huerta-Alardin AL, Varon J, Marik PE. Bench-to-bedside review: Rhabdomyolysis: an overview for clinicians. Crit Care. 2005;9:158–169. doi: 10.1186/cc2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sever MS, Erek E, Vanholder R, Ozener C, Yavuz M, Ergin H, Kiper H, Korular D, Canbakan B, Arinsoy T, VanBiesen W, Lameire N Marmara Earthquake Study Group. The Marmara earthquake: admission laboratory features of patients with nephrological problems. Nephrol Dial Transplant. 2002;17:1025–1031. doi: 10.1093/ndt/17.6.1025. [DOI] [PubMed] [Google Scholar]
- 23.Sever MS, Kellum J, Hoste E, Vanholder R. Application of the RIFLE criteria in patients with crush-related acute kidney injury after mass disasters. Nephrol Dial Transplant. 2011;26:515–524. doi: 10.1093/ndt/gfq426. [DOI] [PubMed] [Google Scholar]
- 24.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62–72. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- 25.Valentovic MA, Minigh J. Pyruvate attenuates myoglobin in vitro toxicity. Toxicol Sci. 2003;74:345–351. doi: 10.1093/toxsci/kfg135. [DOI] [PubMed] [Google Scholar]


