Abstract
Polygonatum sibiricum polysaccharide (PSP) is a traditional Chinese medicine and is widely used to treat many diseases for hundreds of years conventionally. This study was to access the effects of PSP on the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) in the mice. Cells collected from BALB/C mice in the bone marrow were isolated and cultured with osteogenic medium (OM) with different concentrations of PSP. The proliferation and morphological changes of BMSCs were observed using an inverted microscope. Flow cytometric analysis was used to identify the BMSCs. MTT test was performed to analyze the proliferation and viability of the cells. ELISA was used to determine the expression levels of alkaline phosphatase (ALP), osteocalcin (OC), N-terminal propeptide of type I procollagen (PINP) and bone morphogenetic protein-2 (BMP-2). Immunocytochemistry and western blot were respectively used to determine the expressions of bone sialoprotein (BSP) and SPARC/osteonectin (OSN). The growth curves of the proliferation and differentiation of the Control, OM, 17β-E2 and PSP groups were increased. Compared to the Control and OM groups, the expression levels of ALP, OC, PINP and BMP-2 were significantly increased in the PSP induced group (P<0.05). Immunocytochemistry and western blot showed that BSP and SPARC were increased after induction of PSP compared to the OM group (P<0.05). The study demonstrates that PSP promotes the proliferation and enhances the viability of BMSCs during osteogenic differentiation. Therefore, PSP may be a potential treatment of osteoporosis in the clinic.
Keywords: Polygonatum sibiricum polysaccharide, bone marrow mesenchymal stem cells, osteoblasts, alkaline phosphatase, osteocalcin
Introduction
Polygonatum sibiricum grows wild and is cultivated as a traditional medicinal herb and foodstuffs in China; as a common Chinese medicine, P. Sibiricum is considered to have the functions of replenishing vital essence, removing dryness, promoting secretion of fluid, and quenching thirst [1]. In recent years, many compounds such as polysaccharides [2] have been isolated from P. Sibiricum. Polygonatum Sibiricum polysaccharide (PSP) belongs to Polygonatum species, and can be derived from mayflower solomonseal rhizome commonly known as ‘Huang Jing’. PSP is water-soluble extract of P. Sibiricum Rhizome and was desiccated to be brownish-yellow powder (Polysaccharides ≥98%). As an important component of Polygonatum sibiricum, Polygonatum sibiricum polysaccharide (PSP) is known to have low toxicity and is considered to be suitable for long-term administration. Our previous research has shown that PSP reduces the expressions of interleukin 1 (IL-1) and interleukin 6 (IL-6) in rats with osteoporotic fractures [3]. But no studies thus far have investigated the effects of PSP on the recovery of bone loss; we performed this study to assess the effects of PSP on osteogenic differentiation using BMSCs.
Osteoporosis is commonly age-related and a widespread metabolic bone disease, involving a reduction in bone mass and micro-architectural weakening of bone tissues with a resultant increase in bone fragility and vulnerability to fractures [4,5]. Hormone replacement therapy (HRT) is the current foundation for the prevention of postmenopausal osteoporosis; however, some patients refuse HRT because they fear the possible side effects [6,7]. PSP is known to have low toxicity and is considered suitable for long-term administration. Thus, in this study, we investigated the effects of PSP on the osteogenic differentiation of mouse bone marrow mesenchymal stem cells to test whether it could be a potential therapeutic approach to the clinical treatment of osteoporosis.
Materials and methods
Ethics statement
The protocol of this study was approved by the Institutional Animal Experiment Committee of Guangxi Medical University (Nanning, China; Permission numbers: NO. 2013, KY-E-006). All animal experiments were performed in accordance with the Guidelines for the Ethical Care and Treatment of Rats from the European Community Guidelines (EEC Directive of 1986; 86/609/EEC).
Materials
Fetal bovine serum (FBS), IMDM medium and trypsin were obtained from Hyclone (Logan, UT, USA). PSP was purchased from Chengdu institute of biology, Chinese academy of sciences (the extent of PSP purity is 98%). 1% penicillin and streptomycin, 17β-ethinylestradiol (17β-E2), β-glycerin sodium and 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dexamethasone was obtained from the Guangdong Succhi Pharmaceutical Company (China). Vitamin C was obtained from the Xuzhou Lai’en Pharmaceutical Company (China). The alkaline phosphatase staining kit was obtained from Sigma (USA). ALP, PINP, BMP-2 and OC ELISA kits were purchased from the Wuhan Cusabio Biotech Company (China). Rabbit polyclonal antibody anti-BSP, rabbit polyclonal antibody anti-SPARC, anti-beta actin and ECL detection kit were purchased from Bioss Inc (China). The osteogenic media (OM) was composed of IMDM medium with 10% BSA, 1% penicillin and streptomycin, 0.1 μmol/L dexamethasone, 10 mmol/L β-glycerine sodium and 50 μmol/L vitamin C.
Animals
Ten eight-week-old BALB/C mice were used for this experiment. All the animals were purchased from the Guangxi Medical College Animal Experimentation Center. And the protocol of this study was approved by the Institutional Animal Experiment Committee of Guangxi Medical University in institutional guidelines for the care and use of laboratory animals (Nanning, China; Permission numbers: NO. 2013, KY-E-006).
Isolation and culture of mouse BMSCs
The suffering of these animals was minimized. The mice were sacrificed by cervical dislocation and were soaked in 75% alcohol for 5 min. Then, the femur and tibia of the mice were collected with sterile operating procedures. The bone marrow was flushed with a syringe into a tube with Percoll cell separation fluid at a density of 1.082 and was made into a single cell suspension. Then, the tube was centrifuged at 1000 rpm for 5 min, and the supernatant was discarded. The middle layer of cells in the tube was washed three times with PBS and inoculated into culture medium (density of 1.5×105 pcm2) with 10% fetal bovine serum and 1% penicillin and streptomycin. The cells were then placed into an incubator at 37°C (5% CO2). Every three days, the media was replaced, and the non-adherent cells were discarded. The cells were split at a ratio of 1:2, and the third passage of the cells was used for this experiment. These cells were randomly assigned to additional groups as follows: blank control group (Control), blank control-induced group (OM), 17β-E2-induced group (E2+OM) and PSP induced group. The blank control group was cultured in IMDM with 10% fetal bovine serum and 1% penicillin and streptomycin. The blank control-induced group was cultured in osteogenic medium (OM) [8]. The 17β-E2-induced group was cultured in OM with 10-8 mol/L 17β-E2 [9]. The PSP200-, PSP300-, PSP400- and PSP500-induced groups were cultured in OM with 200, 300, 400 and 500 mg/ml PSP, respectively.
BMSCs Identification
BMSCs were identified with fluorescein isothiocyanate-conjugated anti-rat CD34 antibody (FITC-CD34, EB Biosciences) and phycoerythrin cyanine-5-conjugated anti-rat CD44 antibody (PE-Cy5-CD44, EB Biosciences). After 10 days culturing, the third generation of the cells was digested with 0.25% trypsin. The cells were then centrifuged in a test tube at 1000 rpm for 5 min at 4°C. The liquid was removed, and the cells were washed three times with PBS. Then, the cells were transferred to 24-well plates, fixed with 4% paraformaldehyde, permeabilized with 0.1% saponin and stained with fluorescent antibodies against CD34 and CD44. Finally, the cells were analyzed using a flow cytometer (BD Biosciences), and the data were collected by Cell Quest software (BD Biosciences).
MTT cell viability assay
The viability of BMSCs in this experiment was measured with an MTT assay. The cells of each group were placed into 96-well microtiter plates at a density of 1×104 per well with 200 µl of medium. On the 3rd, 5th, 7th, 9th, 11th and 13th day, the MTT assay was performed as follows. Firstly, the cells of each group were washed with PBS, and 20 µl of a 5 mg/ml MTT solution was added per well. After 4 h, the culture medium with dye was removed, and 150 μL of DMSO was added to each well for formazan solubilization. To dissolve the crystals in the well adequately, the culture plates were vortexed for 10 min. Finally, the absorbance value of each well was measured using an enzyme-labeled instrument (Guangxi Medical University). The optical density (OD) was read at 490 nm, and the cell viability was measured. Each experiment was performed in triplicate.
Osteoblast identification by ALP staining
Osteoblasts were identified using an alkaline phosphatase (ALP) staining kit according to the manufacturer’s instructions to confirm the osteogenic differentiation of BMSCs. Firstly, cell smears of each group were prepared and then fixed using stationary liquid for 3 min. Secondly, the substrate reaction liquid was added onto each cell smear. These samples were then placed in an incubator for 15 min to activate alkaline phosphatase. Finally, the cells were stained with hematoxylin for 3 min. The stained cells were washed with PBS to remove the remaining stain solution and were photographed with a camera.
ALP, OC, PINP and BMP-2 assay
The level of ALP, OC, PINP and BMP-2 secreted into the culture medium was determined using an ELISA kit according to the manufacturer’s instructions. In order to normalize these markers expressions for quantification, medium supernatant from each subgroup was collected to evaluate the ALP, PINP, BMP-2 and OC levels at a same density of 1×107 cells per well. After 7 days culturing, the medium supernatant from each subgroup was collected and used to evaluate the ALP level. After 15 days culturing, the medium supernatant of each subgroup was collected and used to evaluate the PINP, BMP-2 and OC levels. Firstly, 100 µl of the cell media for each group was placed in a 96-well microtiter plate at 37°C. The plates were then covered with plastic film and incubated at room temperature for 2 h. The liquid was removed from each well without washing, 100 µl of biotin antibody was added to each well, and the plates were then incubated for an additional 1 h at 37°C. After washing, the unbound antibodies were washed off, followed by the addition of avidin-HRP (1:1000 dilutions). The plates were incubated for 1 h at 37°C. Finally, the color reaction was developed by incubation with a TMB substrate, and the enzyme reaction was terminated with 50 μl of Stop Solution. The OD was read at 450 nm using an ELISA plate reader (Bio-Rad). All samples were assayed in duplicate.
Immunocytochemistry
The glass coverslips used for immunocytochemistry were sterilized and then were put into the 24 well plates. Cells in each groups were plated and grown the glass coverslips at a density of 2×104. After 7 days, cells were washed 3 times×2 min by PBS and fixed using 4% paraformaldehyde at a room temperature (RT) for 15 min. After drying in the air for 5 min, cells were washed 3 times×2 min by PBS. Then, cells were incubated with 0.5% Triton X-100 for 20 min. Wash the cells 3 times×2 min by PBS and used 3% H2O2 to block endogenous peroxidase for 15 min at RT. Next, cells were washed 3 times×2 min by PBS and were blocked with 5% bovine serum albumin (BSA, Sigma) at RT for 20 min. Then cells were incubated with primary antibodies BSP (rabbit anti-mouse bone sialoprotein IgG, 1:200 dilutions) and SPARC (rabbit anti-mouse SPARC IgG, 1:200 dilutions) for 1 h at 37°C in a humid chamber. After that, cells were incubated with peroxidase-conjugated goat anti-rabbit IgG (dilution 1:1000) for 30 min at RT. Washed the coverslips 3 times×5 min with PBS. Finally, using 1, 3-diaminobenzidine (DAB) for 10 min in a dark room to visualize the peroxidase activity and hematoxylin to counterstain the cells for 5 min. 5 visual fields were randomly chosen per coverslip. Positive immunostaining were viewed using a light microscope (Eclipse E800, Nikon, Japan).
Western Blot analysis
After culturing for 14 days, cells of each groups were washed with PBS for 3 times and lysed at 4°C with lysis buffer (RIPA lysate containing 1% PMSF) for 30 min. The total protein of the cell was extracted following the manufacturer’s protocols (Beyotime Technology Inc, Jiangsu, China). Then, the Protein concentration was determined in the supernatant using a BCA Protein Assay Reagent Kit (Beyotime Technology Inc). An equivalent amount of 40 µg total protein samples were dissolved in 20 µl of 1×sodium dodecyl sulfate loading dye and boiled for 5 minutes. After an equivalent amount of the sample was added, the proteins were separated by 8 and 12% SDS-PAGE gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were blocked for 2 h at room temperature with a blocking solution (5% nonfat milk in Tris buffered saline with Tween 20 (TBST) and incubated overnight at 4°C with rabbit polyclonal antibody anti-BSP (1:1000), rabbit polyclonal antibody anti-SPARC (1:1000) and anti-beta actin (1:2500). After washing 3 times×10 min TBST, the membranes were incubated with secondary antibodies (1:2500, Beyotime Technology Inc) for 1 h at room temperature. Then, wash the membranes with TBST (3 times×10 min). Finally, the immunoreactive proteins were visualized with the use of ECL detection kit according to the manufacturer’s instructions and exposed to a radiograph film. The results of the graphs were analyzed by the Digital Gel Imaging Analyst (Nikon 990-Doc 1000, USA). β-actin was used as a loading control for comparison between samples.
Statistical analyses
All data are presented as the means ± SEM. Statistical analyses were performed using SPSS16.0 (SPSS Company, USA). One-way ANOVA followed by the LSD multiple-range test was used to analyze the differences between groups. P<0.05 (two-tailed) indicates statistical significance.
Results
The morphological characteristics of BMSCs
The morphology of the cells was observed using an inverted microscope. Few spindle shaped cells were observed after two days culturing. After three days in culture, multilateral form fiber cells were observed (Figure 1A).
Figure 1.

A. The morphological characteristics of BMSCs. The morphology of the cells was observed using an inverted microscope (100×). After three days in culture, multilateral form fiber cells were observed. B. BMSCs identification using FCM. The expressions of the BMSCs marker CD44 and the hemopoietic stem cell (HTC) marker CD34 were evaluated in the cells collected from the bone marrow. Numbers in upper right quadrants and the lower right quadrants indicate the separate percentages of CD44+ cells and CD34+ cells, respectively. The dot-plots from a representative experiment are shown. The percentages within gated populations represent the means of two biological replicates. Lineage marker expressions were analyzed based on least 105 gated cells. Gates were set in accordance with the non-stained sample (not shown).
Identification of BMSCs using flow cytometry
Flow cytometry was used to identify BMSCs in this experiment. BMSCs are positive for the surface antigens CD44 but negative for CD14 and CD34 [10,11]. Therefore, cells that fit these criteria were identified as BMSCs (Figure 1B).
The viability of BMSCs
MTT assay was used to confirm the viability of BMSCs. Compared with the blank control group, cell viability in the induced groups was significantly increased (Figure 2). In addition, cell viability in the PSP-induced groups increased in a dose- and time-dependent manner on the 3rd, 5th, 7th, 9th, 11th and 13th day; particularly, the PSP500-induced group showed a greater increase in cell viability than other groups (Figure 2).
Figure 2.
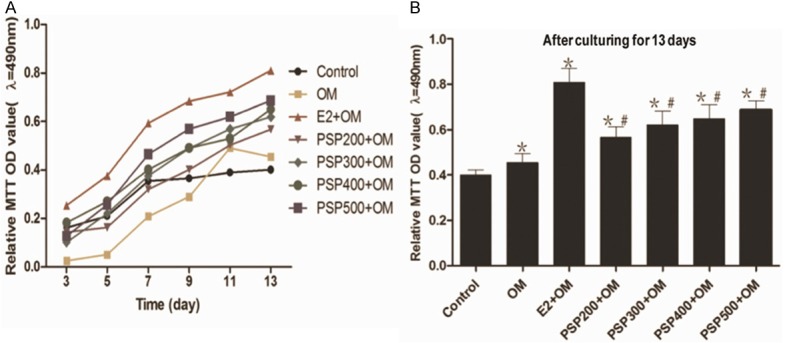
The viability of BMSCs using a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Cells cultured with different concentrations of PSP or 17β-E2 exhibited an increase in viability in a dose-dependent manner, which became apparent with a 500 mg/ml concentration of PSP. The data are presented as the means ± the SD. *Significant difference vs. the control (P<0.05), #Significant difference vs. OM (P<0.05), ∆Significant difference vs. E2+OM (P<0.05).
Osteoblast identification
In this experiment, osteoblasts, formed from the osteogenic differentiation of BMSCs, were identified using alkaline phosphatase (ALP) staining. Cells with red particles filling in the cell cytoplasm were identified as osteoblasts (Figure 3).
Figure 3.
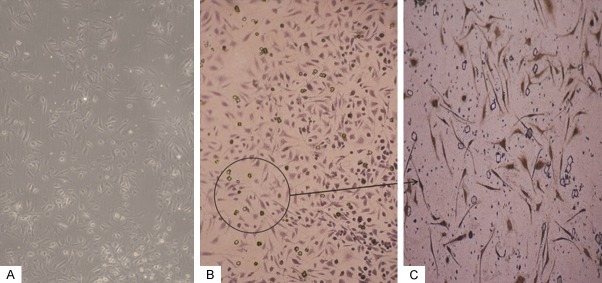
BMSCs alkaline phosphatase (ALP) staining (magnification 200×). The BMSCs were cultured with different medium conditions. (A) The morphological changes of BMSCs before ALP staining. (B) The cell cytoplasms of osteoblasts are filled with red particles. (C) Insert from (B), magnification 400×.
The expression of ALP, OC, PINP and BMP-2
The expression levels of ALP, OC, PINP and BMP-2 were quantified using the ELISA kit. Compared with the blank control group, the expression of ALP, OC, PINP and BMP-2 in induced groups showed a significant increase. In addition, the expression levels of ALP, PINP, BMP-2 and OC in PSP-induced groups increased in a dose-dependent manner; particularly, the PSP500-induced group showed a significant increase in the expression levels of these proteins over the other groups (Figure 4).
Figure 4.

The expressions of ALP, PINP, BMP-2 and OC of BMSCs cultured under different conditions. A. The ALP expressions of BMSCs cultured under different medium conditions. B. The PINP expressions of BMSCs cultured under different medium conditions. C. The BMP-2 expressions of BMSCs cultured under different medium conditions. D. The OC expressions of BMSCs cultured under different medium conditions. The data are presented as the mean ± the SD. #P<0.05 versus control, #P<0.05 versus OM, ΔP<0.05 versus E2+OM.
The effect of BMSCs on osteoblastic markers
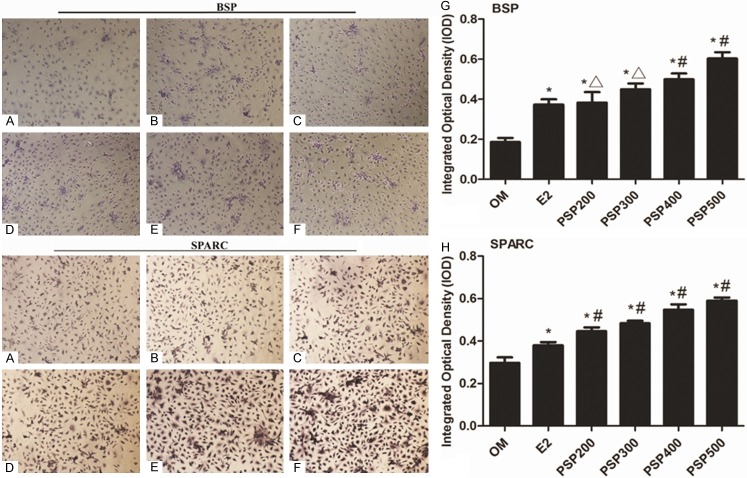
Immunocytochemistry staining of BSP and SPARC were both shown in pictures. Cells in the PSP induced groups were both BSP and SPARC positively increased compared with the OM group (P<0.05) (Figure 5). Western blotting of BSP and SPARC was performed to detect the protein expression of osteoblastic markers. Increased osteoblastic protein expression was observed in each of the PSP induced groups compared with the OM group (P<0.05) (Figure 6).
Figure 5.
Immunocytochemistry staining for BSP and SPARC. A. OM group. B. E2 group. C. PSP200 group. D. PSP300 group. E. PSP400 group. F. PSP500 group. G. Bar graph demonstrating BSP positively expressed cells. H. Bar graph demonstrating SPARC positively expressed cells. Cells in the PSP induced groups were both BSP and SPARC positively increased compared with the OM group (P<0.05 vs. OM). *P<0.05 vs. OM, #P<0.05 vs. E2 and ΔP>0.05 vs. E2.
Figure 6.
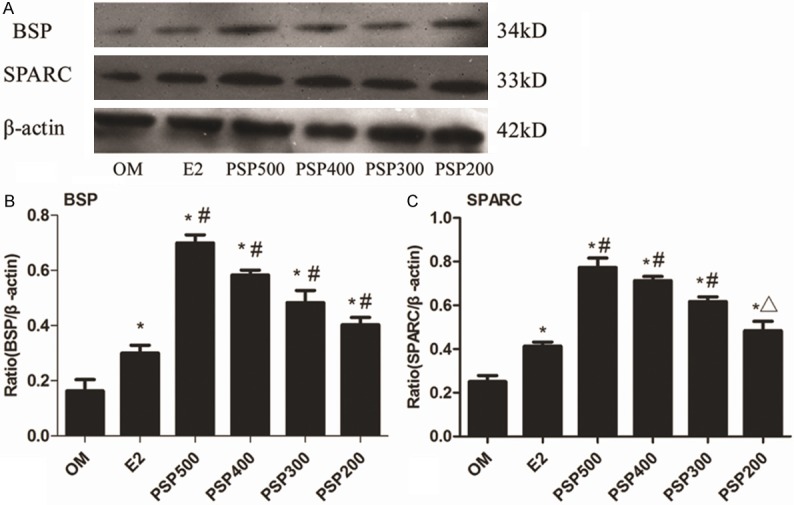
Western blot analysis of BSP and SPARC. A. Representative photographs of BSP and SPARC protein levels. B. Bar graph quantifying the BSP protein level. BSP protein levels increased after induction of PSP compared to the OM group. C. Bar graph quantifying the SPARC protein level. SPARC protein levels increased after induction of PSP compared to the OM group. The results are expressed as means ± SD. *P<0.05 vs. OM, #P<0.05 vs. E2 and ΔP>0.05 vs. E2. β-actin was used as the internal control.
Discussion
In traditional Chinese medicine, Polygonatum sibiricum is widely used for thousands of years as a tonic herb. As an important component of Polygonatum siibiricum which was included in the Chinese Pharmacopoeia (ChP) [12], Polygonatum siibiricum polysaccharide (PSP) is used for the treatment of bone fractures in promoting the fracture healing. From our previous descriptions, we knew that PSP was considered to be effective in the treatment of bone fractures and could prevent ovariectomy-induced bone loss in rats [3]. Therefore, PSP, similar to estrogen, could be used to prevent and treat bone loss as a treatment of osteoporosis. To analyze the effects of PSP on the improvement of osteogenic differentiation of BMSCs, we selected different concentrations of PSP in this study.
Osteoporosis, a systemic skeletal disease that is characterized by low bone mass and micro-architectural deterioration of the bone tissue, is a debilitating disease that affects millions of people worldwide [13]. The incidence of osteoporosis is most common in postmenopausal women because the rate of bone turnover dramatically increases with estrogen deficiency and results in continuous bone loss [14]. Thus, in our experiment, we selected 17β-E2 with osteogenic medium (E2+OM) as a positive control. The concentration of estrogen (10-8 mol/L) we selected was determined by preliminary studies as an appropriate concentration.
BMSCs are hierarchical postnatal stem cells/progenitor cells having the capability of self-renewal and differentiation into osteoblasts, chondrocytes, adipocytes and neural cells [15,16] and are thought to be derived from the bone marrow stromal compartment [17]. It is widely accepted that BMSCs express SH2 (CD105), SH3/SH4 (CD73), integrin β1 (CD29), CD44, Thy-1 (CD90), CD71 and vascular cell adhesion molecule-1 (CD106) [10] but lack expression of hematopoietic surface molecules, including CD34, integrin αM (CD11b) and CD14 [11]. In this study, BMSCs were isolated and cultured in vitro with different culture medium conditions and were identified using flow cytometry (FCM). This experiment induced BMSCs differentiation with osteogenic medium (OM) and confirmed the osteoblasts, which were differentiated from BMSCs using ALP staining (Figure 3).
The viability of BMSCs in this study reflects the cells’ proliferation. Our MTT results showed that, compared to the control group, the viability of BMSCs was increased in the OM, E2+OM and PSP+OM groups. When compared to the OM group, the E2+OM and PSP+OM groups showed a greater increase in viability, especially in the PSP500+OM group, which exhibited a greater increase than the E2+OM group. It indicates that PSP may play a role in promoting the proliferation of BMSCs, in a similar manner as estrogen, particularly at high concentrations.
Osteoblasts are derived from MSCs; and once differentiated, osteoblasts begin to synthesize alkaline phosphatase, type I collagen, osteocalcin, and bone sialoprotein and deposit them to bone extracellular matrix (ECM) [18]. We therefore know that the process of bone formation is determined by osteoblasts. Osteogenesis involves the differentiation of mesenchymal cells into pre-osteoblasts, which in turn differentiate into mature osteoblasts, which finally induce the synthesis and deposition of bone matrix proteins leading to bone formation [19]. In addition, a large number of paracrine, autocrine and endocrine factors affect osteoblast development and maturation, including bone morphogenetic proteins (BMPs) [20]. BMPs belong to the TGF-β super family and have been implicated in tissue growth and remodeling, which may contribute to the formation of bone and connective tissues [21,22]. What’s more, BMP-2 is a well-described stimulator of osteoblastic cell differentiation characterized by increasing expressions of ALP, type I collagen, and OC [23-25]. For these biochemical markers of bone are direct or indirect products or enzymes of active osteoblasts [26], we focused on the expression levels of ALP, BMP-2, PINP and OC to study the effects of PSP on the osteogenic differentiation of BMSCs.
ALP is widely recognized as an early marker of osteoblast differentiation, which shoots up during differentiation [27]. Some studies indicated that ALP activity was increased during osteoblast differentiation in monolayer and collagen scaffold [28]. Moreover, mature osteoblasts also produce regulators of matrix mineralization, such as osteocalcin (OC) [29], which has also been referred to as Bone Gla-Protein (BGP). Osteoblasts secret ALP and collagen type-1 proteins at early stage of differentiation and osteocalcin at later stage, and eventually induce hydroxyapatite nodule formation in the culture medium [19]. In addition, the fully differentiated osteoblast is characterized by the co-expression of ALP and N-terminal propeptide of type I procollagen (PINP), which could reflect the activity of osteoblasts [30]. Previous studies have shown that PINP is more specific and sensitive in the process of bone formation than osteocalcin [31], a non-collagenous protein, which is secreted in the later stages of osteoblast differentiation and is known to have high affinity with hydroxyapatite crystals and regulates nucleation for bone mineralization [32]. According to our results, the expression levels of ALP, PINP, BMP-2 and OC in the OM, E2+OM and PSP+OM groups were increased compared to the control group, especially in the PSP500+OM group. It indicated that PSP might affect various levels of the cell differentiation process and differentiation of osteoblast cells from early stages to terminal stages.
Some researchers found that bone sialoprotein (BSP), a non-collagenous glycosylated phosphoprotein, was produced primarily by osteoblasts and was considered to be a marker of the osteoblastic phenotype which may serve as a matrix-associated signal that directly promotes osteoblast differentiation [33,34]. SPARC (secreted protein, acidic and rich in cysteine), also termed osteonectin (OSN) as a major non-collagenous protein of bone matrix [35] is a positive regulator of bone formation, having vital functions in the regulation of calcium turnover and initiation of mineralization [36]. Thus, we used western bolt to detect the protein expression levels of BSP and SPARC in OM, E2 and PSP induced groups. Our results showed that both BSP and SPARC were increased in PSP induced groups, especially in PSP500 group in a dose-dependent manner.
In the clinical treatment of osteoporosis, some studies have shown that estrogen replacement therapy (ERT) might lead to some side effects, such as breast cancer and venous thromboembolism [22,37]. Therefore, as an antioxidant, PSP may benefit for osteoblast differentiation and osteoporosis’ treatment. In recent years, the characterization of phytoestrogens from herbal medicines has raised the possibility of developing alternative forms of treatment to hormone replacement therapy (HRT) [38]. Thus, it is necessary to find a new drug in the treatment of osteoporosis that has fewer side effects than those caused by ERT. Furthermore, combined with previous research, our results demonstrate that PSP, similar to estrogen but with fewer side effects, may be a potential therapeutic approach to the clinical treatment of osteoporosis. However, whether PSP directly activates the expression of ALP, PINP, BMP-2, OC, BSP and SPARC during osteogenic differentiation at the genetic level or these responses are mediated through protein signaling pathways is not clear, and these mechanisms remain to be investigated.
Conclusion
This study demonstrates that PSP could not only promote the proliferation of BMSCs during osteogenic differentiation but also improve the expression of ALP, PINP, BMP-2, OC, BSP and SPARC in osteoblasts. Therefore, PSP may be a potential clinical treatment of osteoporosis as an alternative to estrogen, which has been confirmed in the clinical treatment of osteoporosis.
Acknowledgements
In this study, we would like to thank the National Natural Science Foundation of China (81360279) for its support.
Disclosure of conflict of interest
None.
References
- 1.Xu DP, Hu CY, Zhang Y. Two new steroidal saponins from the rhizome of Polygonatum sibiricum. J Asian Nat Prod Res. 2009;11:1–6. doi: 10.1080/10286020802513681. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Dong Q, Dong XT, Fang JN, Ding K. Structural investigation of two neutral polysaccharides isolated from rhizome of Polygonatum sibiricum. Carbohyd Polym. 2007;70:304–309. [Google Scholar]
- 3.Zeng GF, Zhang ZY, Lu L, Xiao DQ, Zong SH, Xiong CX, Zhao YX. Effects of polygonatum polysaccharide on the expression of interleukin-1 and 6 in rats with osteoporotic fracture. Chinese Journal of Tissue Engineering Research. 2012;16:220–222. [Google Scholar]
- 4.Cheung AM, Feig DS, Kapral M, Diaz-Granados N, Dodin S Canadian Task Force on Preventive Health Care. Prevention of osteoporosis and osteoporotic fractures in postmenopausal women: recommendation statement from the Canadian Task Force on Preventive Health Care. CMAJ. 2004;170:1665–1667. doi: 10.1503/cmaj.1030757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng GF, Zhang ZY, Lu L, Xiao DQ, Xiong CX, Zhao YX, Zong SH. Protective effects of Polygonatum sibiricum polysaccharide on ovariectomy-induced bone loss in rats. J Ethnopharmacol. 2011;136:224–229. doi: 10.1016/j.jep.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 6.Mori-Okamoto J, Otawara-Hamamoto Y, Yamato H, Yoshimura H. Pomegranate extract improves a depressive state and bone properties in menopausal syndrome model ovariectomized mice. J Ethnopharmacol. 2004;92:93–101. doi: 10.1016/j.jep.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Baber RJ, O’Hara JL, Boyle FM. Hormone replacement therapy: to use or not to use? Med J Aust. 2003;178:630–633. [PubMed] [Google Scholar]
- 8.Sakamoto F, Hashimoto Y, Kishimoto N, Honda Y, Matsumoto N. The utility of human dedifferentiated fat cells in bone tissue engineering in vitro. Cytotechnology. 2015;67:75–84. doi: 10.1007/s10616-013-9659-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Sun YP, Zhu GY. Effects of estrogen on rat osteoblasts in vitro. Journal of Clinical Rehabilitative Tissue Engineering Research. 2008;12:2061–2064. [Google Scholar]
- 10.Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama K, You YO, Yamaza T, Chen C, Tang L, Jin Y, Chen XD, Gronthos S, Shi S. Characterization of bone marrow derived mesenchymal stem cells in suspension. Stem Cell Res Ther. 2012;3:40. doi: 10.1186/scrt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinese pharmacopoeia. Beijing: China Medical Science Press; 2010. National Pharmacopoeia Committee. [Google Scholar]
- 13.Antebi B, Pelled G, Gazit D. Stem cell therapy for osteoporosis. Curr Osteoporos Rep. 2014;12:41–47. doi: 10.1007/s11914-013-0184-x. [DOI] [PubMed] [Google Scholar]
- 14.Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194:S3–11. doi: 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 15.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 17.Friedenstein AJ. Stromal mechanisms of bone marrow: cloning in vitro and retransplantation in vivo. Haematol Blood Transfus. 1980;25:19–29. doi: 10.1007/978-3-642-67319-1_3. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita M, Ying SX, Zhang GM, Li C, Cheng SY, Deng CX, Zhang YE. Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell. 2005;121:101–113. doi: 10.1016/j.cell.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaturvedi R, Singha PK, Dey S. Water soluble bioactives of nacre mediate antioxidant activity and osteoblast differentiation. PLoS One. 2013;8:e84584. doi: 10.1371/journal.pone.0084584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin L, Qiu P, Wang L, Li X, Swarthout JT, Soteropoulos P, Tolias P, Partridge NC. Gene expression profiles and transcription factors involved in parathyroid hormone signaling in osteoblasts revealed by microarray and bioinformatics. J Biol Chem. 2003;278:19723–19731. doi: 10.1074/jbc.M212226200. [DOI] [PubMed] [Google Scholar]
- 21.Cui L, Liu YY, Wu T, Ai CM, Chen HQ. Osteogenic effects of D+beta-3,4-dihydroxyphenyl lactic acid (salvianic acid A, SAA) on osteoblasts and bone marrow stromal cells of intact and prednisone-treated rats. Acta Pharmacol Sin. 2009;30:321–332. doi: 10.1038/aps.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beral V, Banks E, Reeves G. Evidence from randomised trials on the long-term effects of hormone replacement therapy. Lancet. 2002;360:942–4. doi: 10.1016/S0140-6736(02)11032-4. [DOI] [PubMed] [Google Scholar]
- 23.Gazzerro E, Gangji V, Canalis E. Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J Clin Invest. 1998;102:2106–2114. doi: 10.1172/JCI3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hay E, Hott M, Graulet AM, Lomri A, Marie PJ. Effects of bone morphogenetic protein-2 on human neonatal calvaria cell differentiation. J Cell Biochem. 1999;72:81–93. doi: 10.1002/(sici)1097-4644(19990101)72:1<81::aid-jcb9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 25.Hughes FJ, Collyer J, Stanfield M, Goodman SA. The effects of bone morphogenetic protein-2, -4, and -6 on differentiation of rat osteoblast cells in vitro. Endocrinology. 1995;136:2671–2677. doi: 10.1210/endo.136.6.7750491. [DOI] [PubMed] [Google Scholar]
- 26.Risteli L, Risteli J. Biochemical markers of bone metabolism. Ann Med. 1993;25:385–393. doi: 10.3109/07853899309147301. [DOI] [PubMed] [Google Scholar]
- 27.Lecoeur L, Ouhayoun JP. In vitro induction of osteogenic differentiation from non-osteogenic mesenchymal cells. Biomaterials. 1997;18:989–993. doi: 10.1016/s0142-9612(97)00025-2. [DOI] [PubMed] [Google Scholar]
- 28.George J, Kuboki Y, Miyata T. Differentiation of mesenchymal stem cells into osteoblasts on honeycomb collagen scaffolds. Biotechnol Bioeng. 2006;95:404–411. doi: 10.1002/bit.20939. [DOI] [PubMed] [Google Scholar]
- 29.Eriksen EF. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord. 2010;11:219–227. doi: 10.1007/s11154-010-9153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005;19:1093–1104. doi: 10.1101/gad.1276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernández MV, Guañabens N, Alvarez L, Monegal A, Peris P, Riba J, Ercilla G, Martínez de Osaba MJ, Muñoz-Gómez J. Immunocytochemical evidence on the effects of glucocorticoids on type I collagen synthesis in human osteoblastic cells. Calcif Tissue Int. 2004;74:284–293. doi: 10.1007/s00223-002-1095-5. [DOI] [PubMed] [Google Scholar]
- 32.Hunter GK, Hauschka PV, Poole AR, Rosenberg LC, Goldberg HA. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. The Biochem J. 1996;317:59–64. doi: 10.1042/bj3170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuno M, Imai T, Fujisawa R, Tani H, Kuboki Y. Bone sialoprotein (BSP) is a crucial factor for the expression of osteoblastic phenotypes of bone marrow cells cultured on type I collagen matrix. Calcif Tissue Int. 2000;66:388–396. doi: 10.1007/s002230010078. [DOI] [PubMed] [Google Scholar]
- 34.Gordon JA, Tye CE, Sampaio AV, Underhill TM, Hunter GK, Goldberg HA. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone. 2007;41:462–473. doi: 10.1016/j.bone.2007.04.191. [DOI] [PubMed] [Google Scholar]
- 35.Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 36.Hiltunen A, Aro HT, Vuorio E. Regulation of extracellular matrix genes during fracture healing in mice. Clin Orthop Relat Res. 1993:23–27. [PubMed] [Google Scholar]
- 37.Miller J, Chan BK, Nelson HD. Postmenopausal estrogen replacement and risk for venous thromboembolism: a systematic review and meta-analysis for the U. S. Preventive Services Task Force. Ann Intern Med. 2002;136:680–690. doi: 10.7326/0003-4819-136-9-200205070-00011. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Zhang ZJ, Geng F, Su SB, White KN, Bligh SW, Branford-White CJ, Wang ZT. Treatment with Qing’E, a kidney-invigorating Chinese herbal formula, antagonizes the estrogen decline in ovariectomized mice. Rejuvenation Res. 2010;13:479–488. doi: 10.1089/rej.2009.1000. [DOI] [PubMed] [Google Scholar]