Abstract
Mannose has been reported to prevent acute lung injury (ALI), and mannose receptor (MR) has been demonstrated to have a role. The rationale for this study is to characterize the mechanism by which mannose and MR prevent lipopolysaccharide (LPS)-induced ALI. Male ICR mice were pretreated mannose by intravenous injection 5 min before and 3 h after intratracheal instillation of LPS. Pathological changes, proinflammatory mediator, peroxisome proliferator activated receptor gamma (PPARγ), MR, and transforming growth factor β1 (TGF-β1) levels were determined. The RAW264.7 cells were pretreated with mannose and stimulated with LPS for 3 h. Proinflammatory mediator and TGF-β1 in the culture media, PPARγ, MR, and TGF-β1 expression in RAW 264.7 cells were measured. Mannose markedly attenuated the LPS-induced histological alterations and inhibited the production of proinflammatory mediator in mice and in RAW 264.7 cells. Mannose increased PPARγ and MR expression, and inhibited TGF-β1 stimulated by LPS. Interestingly, competitive inhibition of MR with mannan was associated with elimination of the anti-inflammatory effects of mannose, and reversed effects of mannose of regulation to PPARγ and TGF-β1. MR is important in increasing PPARγ and decreasing TGF-β1 expression and plays a critical role in mannose’s protection against ALI.
Keywords: Acute lung injury, anti-inflammation, mannose, mannose receptor, PPARγ, TGF-β1
Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are still life-threatening diseases in critically ill patients, despite significant improvement in the understanding of their pathophysiology, the mortality rate remains high at 35-40%. Several promising therapies, include exogenous surfactant therapy, beta(2)-adrenergic receptor agonists, antioxidants, immunomodulating agents and HMG-CoA reductase inhibitors (statins), are currently being investigated in preclinical and clinical trials for treatment of ALI, but to date, no single pharmacotherapy has proven effective in decreasing mortality in adult patients with ALI [1]. Therefore, there is still a need for novel effective pharmacotherapies.
Mannose, a simple hexose sugar with a molecular weight of 180.2, has been shown to inhibit the neutrophil oxidative burst, which plays an important role in inflammation [2]. Our previous studies have demonstrated that mannose was effective in reducing lipopolysaccharide (LPS)-induced ALI [3]. Further study have also demonstrated a role for mannose receptor (MR), a member of the C-type lectin family, and impaired nuclear transcription factor (NF-κB) activation in mannose-mediated prevention of ALI [4].
Peroxisome proliferator activated receptors (PPARs) are ligand-activated transcription factors related to thyroid hormone, steroid and retinoid receptors [5]. Recently however, PPARα and PPARγ have been shown to exert a potent anti-inflammatory activity, mainly through their ability to down regulate pro-inflammatory gene expression and inflammatory cell functions. Pretreatment with rosiglitazone (a ligand of PPARγ) protects ALI by activating PPARγ, inhibiting NF-κB activation, and inhibiting the transforming growth factor beta 1 (TGF-β1) signaling [6,7]. And the regulation of MR activity is related to PPARγ activity. A recent report has stated that ligands to PPARγ promote MR expression and that the interleukin 13 (IL-13)- induced up-regulation of MR is, in fact, mediated via generation of PPARγ ligands [8].
This study was, therefore, designed to investigate the protective effect of mannose in LPS-induced ALI in both mice and in vitro macrophages, and elucidated its underlying mechanisms involves MR, PPARγ, and TGF-β1 signaling pathway.
Materials and methods
All experiments in this study were approved by the Animal Experiments Committee of Zhejiang University, and were performed in accordance with the Chinese National Regulations for Animal Care.
Reagents
LPS (isolated from Escherichia coli), 0127:B8), D-mannose, and mannan (isolated from Saccharomyces cerevisiae) were purchased from Sigma (St. Louis, MO, USA). The protein assay kit was purchased from Thermo (Waltham, MA, USA). IL-6, IL-10, TNF-α, and TGF-β1 enzyme-linked immunoassay (ELISA) kits were from eBioscience (San Diego, CA, USA). Cell culture media was from Cellgro (Herndon, VA, USA), and fetal bovine serum (FBS) was from PAA Laboratories (Colbe, Germany). Mouse monoclonal antibodies against TGF-β1 and MR were from Abcam (Cambridge, MA, USA), and the rabbit monoclonal antibody against PPARγ was from Cell Signaling Technology (Beverly, MA, USA). HRP-conjugated anti-mouse and anti-rabbit secondary antibodies were from ZSGB-BIO (Beijing, China).
Induction of ALI in mice
Male Imprinting Control Region mice weighing 25 to 35 g were purchased from Shanghai Slac Laboratory Animal Co. Ltd (Shanghai, China). Mice were anesthetized by intraperitoneal injection with pentobarbital (40 mg/kg) prior to administration of saline solution, dexamethasone (DXM; 0.5 mg/kg), or mannose (50, 150, and 450 mg/kg). All treatments were delivered by intravenous injection in the tail vein 5 min before and again 3 h after intratracheal instillation of LPS (4 mg/kg). In addition to intravenous injection of saline, the control group received intratracheal instillation of saline solution. Samples were collected 6 h after administration of LPS.
Histopathologic examination of the lung tissue
The right lower lobe was fixed with 10% formalin, embedded in paraffin, sectioned into 5 μm slices, and stained with hematoxylin-eosin or Masson. Pathologic examination was performed under the light microscope. The lung pathology of LPS-induced acute lung injury shows hemorrhage and edema in the interstitial and alveolar spaces and infiltration of inflammatory cells [9].
Cell culture and treatment
The RAW264.7 murine macrophage cell line was obtained from BIOK&KM (Jiangsu, China). RAW264.7 macrophages were plated into six-well plates at a concentration of 1×106 cells/well and cultured in Dulbecco’s modified Eagle’s medium (DEME) containing 10% FBS. Cells were cultured at 37°C in 5% CO2 in a humidified incubator. Macrophages were divided into the following groups: control group, incubated in the culture medium alone; LPS group, stimulated with LPS 1 μg/ml; mannose groups, received mannose (0.1, 1, and 10 mM) 5 min before stimulation with LPS 1 μg/ml; mannan group, pre-incubated with mannan (2 mg/ml) 30 min before and received mannose 1 mM 5 min before stimulation with LPS 1 μg/ml. Samples were collected 3 h after administration of LPS.
Cytokine ELISA
Levels of TNF-α, IL-6, IL-10 and TGF-β1 in bronchoalveolar lavage (BAL) fluid, serum and cell culture supernatants were measured by sandwich ELISA specific for mice, following the manufacturer’s instructions.
Real-time RT-PCR analysis
Lung samples containing the middle right lobe were collected and ground into powder in liquid nitrogen containing 1 ml Trizol reagent (Takara Bio, Japan). Each sample was then homogenized on ice for 30 s with a handheld homogenizer. The homogenized samples were incubated on ice for 5 min prior to addition of 0.2 ml chloroform. Samples were incubated on ice for 3-4 min and then centrifuged at 12,000× g at 4°C for 15 min to remove any insoluble material. Supernatants were transferred to sterile tubes and 0.5 ml of isopropanol was added. The samples were incubated at room temperature for 15 min and then centrifuged at 12,000× g at 4°C for 15 min to separate the isopropanol layers. The lower layer containing the RNA was washed three times using an equal volume of 75% anhydrous ethanol, then was diluted in nuclease-free DEPC-treated water. RNA quantity and purity was assessed using an ultraviolet spectrophotometer. For real-time reverse transcriptase polymerase chain reaction (RT-PCT) analysis, the following components were added to a 10 μl reaction: 1 μl of cDNA, 0.4 μl of each forward and reverse primer (10 μM initial concentration), 0.2 μl of 50× ROX Reference Dye II (Takara Bio, Japan), 3 μl of PCR grade water, and 5 μl of 2× SYBR Premix Ex TaqTM II (Takara Bio, Japan). The sequences of the primer pairs used are as follows: MR, forward: 5’ GCTCTAGAATGGAACACACACTCTGGGCCATG, reverse: 5’ GCTCTAGAATGGAACACTCTGGGCCATG; TGF-β1, forward: 5’ CCACCTGCAAGACCATCGAC, reverse: 5’ CTGGCGAGCCTTAGTTTGGAC; PPARγ, forward: 5’ GAGATCATCTACACGATGCTGGC, reverse: 5’ CGCAGGCTTTTGAGGAACTC. PCR conditions consisted of one 30 s cycle at 95°C, followed by 40 cycles of 95°C for 5 s and 60°C for 34 s, followed by a melting curve analysis. PCR reactions and analysis were performed using a 7500 Real Time PCR system (Applied Biosystems; Life Technologies, Grand Island, NY, USA). Expression of PPARγ, TGF-β1, and MR were calculated by the ΔΔCT method, using the GAPDH to normalize values.
RNA was isolated from RAW264.7 cells using Trizol after 3 h of incubation with mannose and/or LPS. The experimental setup for real-time RT-PCR was as same as above.
Western blot analysis
Lung samples containing the upper right lobe were grounded into powder in liquid nitrogen, and lysed in ice-cold lysis buffer (2% Triton X-100, 10 mM Tris-HCl, pH 8, 150 mM NaCl, 2 mM NaN3, 2 mM EDTA) containing protease inhibitors for 2 h at 4°C. Lysates were harvested and centrifuged at 2000 rpm in a tabletop centrifuge to remove nuclei and were stored at -20°C until further use. Protein concentration was determined using the protein assay kit (Thermo Fisher Scientific co., MA, USA). Cell lysates and supernatants were electrophoresed in a 6% or 8% sodium dodecyl sulfate polyacrylamide gel under non-reducing conditions and were transferred to nitrocellulose. Western blotting for MR, TGF-β1, or PPARγ was performed using anti-MR, anti-TGF-β1, or anti-PPARγ primary antibodies and an appropriate HRP-conjugated secondary antibody. Bands were detected using an enhanced chemiluminescence reagent.
RAW264.7 cells were pretreated with mannose and/or LPS for 3 h. After removing the media, cells were washed in phosphate buffered saline and lysed in ice-cold lysis buffer containing protease inhibitors for 2 h at 4°C. The samples were then harvested using the same methods as above.
Statistical analysis
All continuous data were presented as mean ± standard deviation (SD). Statistical significance was determined by one-way analysis of variance, a Dunnett’s multiple comparison was used to compare the effect among groups. Values with P<0.05 were considered significant. Analysis was carried out using Statistical Product and Service Solutions (SPSS; version 11.5) (SPSS Inc, Chicago, IL, USA).
Results
In vivo study
Mannose improved the histological changes induced by LPS
To confirm the ability of mannose to prevent lung injury at a histological level, we examined lungs from mice after 6 h of intratracheal instillation of LPS. Hematoxylin-eosin staining of pulmonary sections from the LPS alone group demonstrated obvious hemorrhage, edema, a thickened alveolar septum, formation of hyaline membranes, and infiltration of inflammatory cells in alveolar spaces (Figure 1B). In contrast, a control group receiving saline showed none of these effects (Figure 1A). In mice treated with 50, 150, or 450 mg/kg of mannose, a dose dependent decrease in interstitial edema and inflammatory cell infiltration was observed (Figure 1C-E, respectively). Treatment with DXM also resulted in a reduction in these changes (Figure 1F). However Masson’s staining did not demonstrate quantitative differences in collagen deposition between groups (data not shown).
Figure 1.
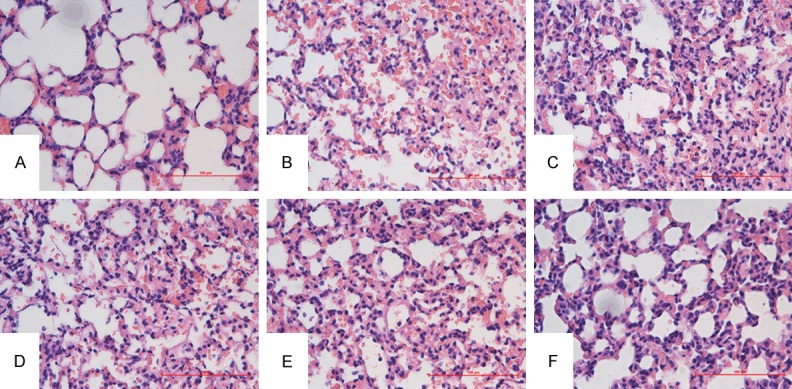
Effects of mannose on histological changes of LPS-induced ALI in mice. Mice randomly received a saline solution or mannose (50, 150, 450 mg/kg) by intravenous injection in the tail vein 5 min before and 3 h after intratracheal instillation of LPS (4 mg/kg). Samples were collected 6 h after administration of LPS. Pathological changes in lung tissues were observed by HE staining (light microscopy, ×400). A. Saline-treated control mice. B. LPS only treated mice. C-E. Mice treated with LPS and 50, 150, or 450 mg/kg mannose, respectively. F. Mice treated with LPS and 0.5 mg/kg DXM. The images presented are representative of three independent experiments.
Effects of mannose on the BAL fluid and serum levels of TNF-α, IL-6, IL-10, and TGF-β1
To further characterize the protective role of mannose during ALI, we sought to determine the extent to which LPS-induced proinflammatory cytokines, could be reduced by mannose. The BAL fluid and serum levels of TNF-α, IL-6, IL-10, and TGF-β1 were all significantly elevated following LPS treatment. In contrast, intravenous administration of mannose resulted in a dose-dependent decrease in the levels of all tested cytokines. Treatment with the positive control DXM also attenuated the effects of LPS on cytokine production (Figures 2, 3). These results demonstrated that mannose treatment had a marked inhibitory effect on LPS-induced cytokines production and suggested an important mechanism for mannose in reduction of ALI.
Figure 2.
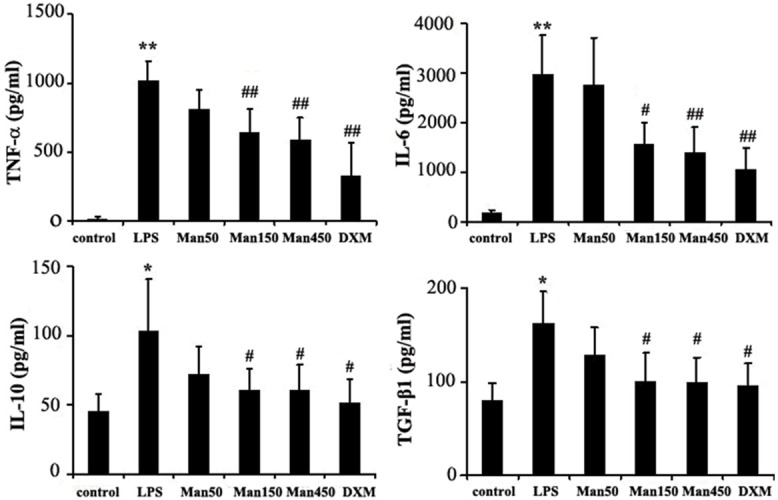
Effects of mannose on the BAL fluid levels of cytokines of LPS-induced ALI in mice. Mice randomly received a saline solution or mannose (50, 150, 450 mg/kg) by intravenous injection in the tail vein 5 min before and 3 h after intratracheal instillation of LPS (4 mg/kg). Samples were collected 6 h after administration of LPS. The TNF-α, IL-6, IL-10 and TGF-β1 levels in the BAL fluid were evaluated by ELISA. Results are presented as mean ± SD (n=8). *P<0.05 and **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. LPS group.
Figure 3.

Effects of mannose on the serum levels of cytokines of LPS-induced ALI in mice. Mice randomly received a saline solution or mannose (50, 150, 450 mg/kg) by intravenous injection in the tail vein 5 min before and 3 h after intratracheal instillation of LPS (4 mg/kg). Samples were collected 6 h after administration of LPS. The TNF-α, IL-6, IL-10 and TGF-β1 levels in the BAL fluid were evaluated by ELISA. Results are presented as mean ± SD (n=8). *P<0.05 and **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. LPS group.
Mannose treatment upregulates the expression of PPARγ and MR, but downregulates TGF-β1
To further investigate the role by which mannose treatment protects from ALI, the expression of the PPARγ, MR, and TGF-β1 in the murine lung was examined by real-time RT-PCR and western blot analysis. Following LPS treatment, a decrease of 18.5% in PPARγ and 29.9% in MR mRNA expression was observed by real-time RT-PCR (Figure 4A, 4B). LPS exposure also resulted in a 485% increase in TGF-β1 mRNA expression (Figure 4C). Mannose treatment reversed the effects of LPS and resulted a dose-dependent increase in PPARγ and MR mRNA expression (Figure 4A, 4B). Moreover, the LPS-induced upregulation of TGF-β1 mRNA expression was reduced by treatment with 150 or 450 mg/kg of mannose, as well as with 0.5 mg/kg DXM (Figure 4C).
Figure 4.

Effect of mannose on PPARγ, MR, and TGF-β1 expression in lungs of ALI mice. Mice randomly received a saline solution or mannose by intravenous injection in the tail vein 5 min before and 3 h after intratracheal instillation of LPS (4 mg/kg). Samples were collected 6 h after administration of LPS and detected by real-time RT-PCR and western blot. A. Measurement of PPARγ mRNA levels. B. Measurement of MR mRNA levels. C. Measurement of TGF-β1 mRNA levels. D. Measurement of PPARγ, MR, and TGF-β1 protein production. Lane 1 lung tissues from control group, lane 2 from LPS group, lanes 3, 4, 5 and 6 from treatment group with mannose (50, 150, 450 mg/kg) and DXM, β-actin was sued as a control. The data represent the mean ± SD of three independent experiments. *P<0.05 and **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. LPS group.
Similarly, Western blotting analysis showed that the PPARγ and MR protein expression in the lung tissues was decreased, but TGF-β1 protein increased significantly by treatment with LPS. Mannose treatment reversed the LPS-induced changes in PPARγ, MR, and TGF-β1 protein levels (Figure 4D). Moreover, we show that DXM treatment exhibits similar and a little stronger effects than mannose treatment (Figure 4).
In vitro study
Effect of mannose treatment on cytokine production in LPS-stimulated RAW264.7 cells
The RAW264.7 macrophage cell line was treated with LPS or LPS and mannose, and the effect of mannose on cytokine levels was determined by ELISA. As compared to the control, a 19.11-fold, 6.05-fold, 2.18-fold, and 3.70-fold increase of TNF-α, IL-6, IL-10, and TGF-β1 production was seen in RAW264.7 cell culture supernatants 3 h after LPS exposure (1 μg/ml), respectively (Figure 5). As expected, mannose treatment (0.1, 1, and 10 mM) reduced the effects of LPS-induced cytokine production in a dose-dependent manner, while the MR inhibitor mannan suppressed the inhibitory effect of mannose (Figure 5).
Figure 5.
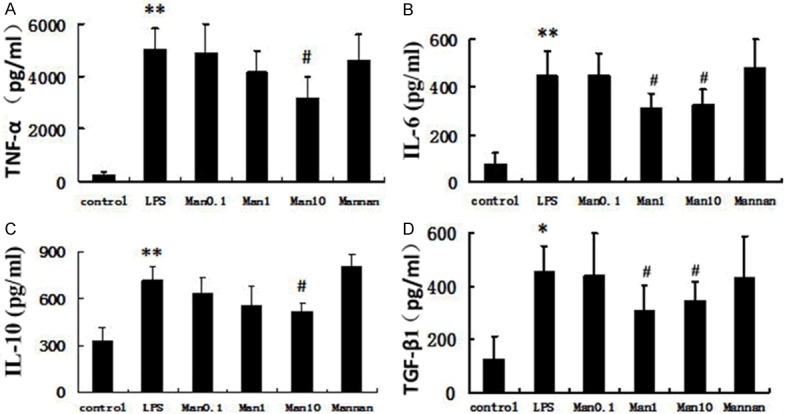
Inhibition of cytokine protein by mannose in RAW 264.7 cells. The murine macrophage RAW264.7 cells were pretreated with the indicated concentration of mannose (0.1, 1, and 10 mM) and stimulated with 1 μg/ml LPS for 3 h. Mannan group, pre-incubated with mannan (2 mg/ml) 30 min before and received mannose 1 mM 5 min before stimulation with LPS 1 μg/ml. Results are presented as mean ± SD (n=4). *P<0.05 and **P<0.01 vs. control group; #P<0.05 vs. LPS group.
Effect of mannose and mannan on expression of PPARγ, MR, and TGF-β1 in LPS-stimulated RAW264.7 cells
To explore the mechanism of mannose-mediated inhibition of pro-inflammatory cytokines, real-time PCR and Western blotting were performed to determine whether mannose could regulate expression of PPARγ, MR, and TGF-β1 in RAW264.7 cells. LPS treatment reduced PPARγ and MR mRNA levels, but markedly increased TGF-β1 mRNA levels. Similarly, Western blotting analysis showed that LPS treatment reduced PPARγ and MR protein expression, but significantly increased TGF-β1 protein expression (Figure 6).
Figure 6.
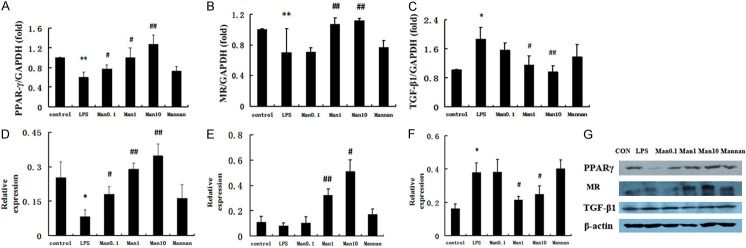
Effect of mannose on PPARγ, MR, and TGF-β1 expression in RAW 264.7 cells. The murine macrophage RAW264.7 cells were pretreated with the indicated concentration of mannose and stimulated with 1 μg/ml LPS for 3 h (real-time PCR) or 6 h (Western blot). Mannan group, pre-incubated with mannan (2 mg/ml) 30 min before and received mannose 1 mM 5 min before stimulation with LPS 1 μg/ml. A. Measurement of PPARγ mRNA levels. B. Measurement of MR mRNA levels. C. Measurement of TGF-β1 mRNA levels. D. Measurement of PPARγ protein production. E. Measurement of MR protein production. F. Measurement of TGF-β1 protein production. G. Measurement of PPARγ, MR, and TGF-β1 protein production. Lane 1 cells from control group, lane 2 from LPS group, lanes 3, 4, 5 and 6 from treatment group with mannose (0.1, 1, and 10 mM) and mannan, β-actin was sued as a control. The data represent the mean ± SD of three independent experiments. *P<0.05 and **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. LPS group.
Interestingly, PPARγ and MR expression were upregulated following exposure to a combination of mannose and LPS as compared to the LPS only group, but this effect was almost completely abolished by pretreatment with mannan (Figure 6). Meanwhile, pretreatment of RAW264.7 cells with mannose significantly reduced the LPS-induced expression of TGF-β1 in a dose-dependent manner. The MR inhibitor mannan (2 mg/ml) significantly impaired the effect of mannose treatment on reduction of TGF-β1 mRNA and protein (Figure 6). These data indicate that mannose up-regulates PPARγ and MR expression, and down-regulates TGF-β1 expression, and this effect may rely on the macrophage surface MR.
Discussion
In this study, we used a mouse endotoxemic model induced by intratracheal instillation of LPS, which may mimic sepsis-associated ALI in humans, and the RAW264.7 murine macrophage cell line, to study the effect and the mechanisms of mannose treatment on ALI. Our results showed that pretreatment with mannose attenuated lung damage and inflammatory cell migration into the lung induced by LPS, decreased proinflammatory cytokine production, upregulated PPARγ and MR expression, whereas downregulated TGF-β1 expression. Meanwhile, competitive inhibition of MR with mannan in RAW264.7 macrophage cells, was associated with elimination of the anti-inflammatory effects of mannose, and reversed effects of mannose of regulation to PPARγ and TGF-β1.
In this endotoxin model, LPS induced obvious hemorrhage, edema, a thickened alveolar septum, formation of hyaline membranes, and the infiltration of inflammatory cells in alveolar spaces, as observed by HE staining. The inflammatory state observed in this study is consistent with our previous work [3]. We used DXM, a glucocorticoid, as a positive control, as the effects of corticosteroids in ALI models are well documented [10,11]. In the present study, administration of mannose or DXM partially reversed these inflammatory histopathologic changes.
Proinflammatory cytokines are known to play a critical role in ALI and ARDS, and persistently elevated levels of proinflammatory cytokines, are associated with worse outcome in patients with ALI or sepsis [12]. Our study found that mannose or DXM, reversed the LPS-induced increase of TNF-α, IL-6, and IL-10 both in BAL fluid and in serum. During our in vitro RAW264.7 macrophage study, the production of cytokines was markedly stimulated by LPS, and were inhibited by mannose. This indicated that mannose may attenuate lung inflammation by suppressing the activation of alveolar macrophages and subsequent production of inflammatory cytokines. The anti-inflammatory cytokine IL-10, which was increased by LPS and decreased by mannose, changed its concentration levels similar to TNF-α and IL-6. This indicated that IL-10 may act to promote rather than dampen the amplification of early inflammatory signals.
The cytokine TGF-β1 plays a critical role in tissue repair after injury in multiple organs, including the lung. In ALI, the role of TGF-β1 has been most thoroughly evaluated during the late phase of tissue repair, where it plays a critical role in the development of the fibroproliferative response [13]. Moreover, recent studies have shown that expression of several TGF-β1 inducible genes were dramatically increased early following injury, and could contribute to the pulmonary edema early in the course of ALI [14,15]. We found expression of TGF-β1 following LPS-induced lung injury was increased dramatically as early as 6 h after intratracheal instillation of LPS, and mannose treatment resulted in a dose-dependent downregulation of TGF-β1 in the BALF, serum, and lung tissue. Similar results were seen in the RAW 264.7 macrophage cell line. These results indicate that the protective effects of mannose may be attributed to the inhibition of TGF-β1 during the early phase of ALI. Though TGF-β1 also participates in the lung fibrosis phase of ALI, our Masson staining study showed no significant improvement in lung fibrosis following mannose treatment (data not shown), perhaps 6 h was too short a timespan to find obvious changes in lung fibrosis.
The macrophage MR, is considered to be a pattern recognition receptor involved in host defense, innate immunity, triggering cytokine production, and modulating cell surface receptors [16]. The macrophage MR plays an important role in the anti-inflammatory response, and cross-linking of the MR activates an anti-inflammatory immunosuppressive program that results in down-regulation of Th1-polarized immune responses [17]. We found the LPS treatment resulted in decreases in macrophage MR mRNA and protein expression in vivo and in vitro, and this effect reversed by mannose or DXM. Our results are consistent with previously published results which demonstrate that for nervous system macrophages, macrophage MR could be downregulated by LPS or upregulated by DXM, by increasing mRNA levels [18]. Mannose, as the natural ligand of MR, binds to the C-type lectin-like domains of MR [19]. Mannose dose-dependently increased levels of macrophage MR, even more effective (when the dose 450 mg/kg) than DXM. This indicates that the up-regulation of MR is involved in protective effect of mannose.
PPARγ is a prime candidate for an intracellular molecular switch based on its central role in controlling macrophage inflammatory responses, and it has been implicated in the down-regulation of proinflammatory responses [20]. In this study, we found that LPS administration significantly decreased PPARγ expression in the lungs and in the RAW 264.7 macrophages. Decreased expression of PPARγ could contribute to the ongoing pulmonary inflammation and tissue injury in endotoxemia [21]. Pretreatment with mannose reversed the LPS-induced decrease in PPARγ mRNA and protein expression in a dose-dependent manner, indicating that the mechanisms by which mannose treatment upregulates PPARγ are associated with activation of mRNA transcription. These results suggest that mannose protects ALI through a pathway at least partially dependent on PPARγ activation.
Activation of PPARγ could result in increased MR expression, leading to enhanced MR-mediated endocytosis, elevated cross-presentation of soluble antigens, and the induction of T cell tolerance [22]. Alternatively, the engagement of the MR, could induce PPARγ expression, which regulates the macrophage inflammatory response [23]. To further demonstrate the effect of mannose and the pathway between PPARγ and MR, we used the RAW 264.7 macrophage cell line and mannan, a high-affinity natural ligand of MR [24]. We found that anti-inflammatory effects and increases in PPARγ expression by mannose were reversed by mannan treatment, suggesting that the effects of mannose on macrophages are MR dependent, and blocking MR downregulates PPARγ activity to mannose. Negative regulation of PPARγ has been described to altering the expression of many inflammatory genes, modulating macrophage differentiation and activation, and attenuating the respiratory burst [25]. Further studies are required to determine the exact signaling pathway between PPARγ and MR.
Activation of PPARγ also has been shown to attenuate TGF-β1-induced epithelial-to-mesenchymal transition, exert anti-inflammatory, anti-fibrotic, and vaculo-protective effects on different diseases [26]. Simultaneously, TGF-β1 can influence PPARγ expression, which is mediated through β-catenin pathway, and subsequently contribute to fibrosis [27]. However, the specific signaling pathway that links inhibition of inflammation and expression of PPARγ and TGF-β1 has not completely established. Our study showed that the regulation of PPARγ and TGF-β1 was partially MR-dependent, as evidenced by administering mannan partially reversed the mannose-induced up-regulation of PPARγ and down-regulation of TGF-β1.
In summary, the present results support our previous work showing that mannose has a protective effect against LPS-induced ALI, attenuates LPS-induced histological changes, and suppresses proinflammatory cytokine release. Moreover, our data describe a novel regulatory feedback loop between MR, PPARγ and TGF-β1 signaling in macrophages. In this loop, mannose up-regulates MR expression, an increased MR expression drives up PPARγ expression, induction of PPARγ represses TGF-β1 expression, and inhibits the inflammatory process. Thus, mannose administration may represent a promising therapeutic option in the future for the reduction of ALI.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (No. 81100051, No. 81170038).
Disclosure of conflict of interest
None.
References
- 1.Bosma KJ, Taneja R, Lewis JF. Pharmacotherapy for prevention and treatment of acute respiratory distress syndrome. Drugs. 2010;70:1255–1282. doi: 10.2165/10898570-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kössi J, Peltonen J, Ekfors T, Niinikoski J, Laato M. Effects of hexose sugars: glucose, fructose, galactose and mannose on wound healing in the rat. Eur Surg Res. 1998;31:74–82. doi: 10.1159/000008623. [DOI] [PubMed] [Google Scholar]
- 3.Xu XL, Xie QM, Shen YH, Jiang JJ, Chen YY, Yao HY, Zhou JY. Mannose prevents lipopolysaccharide-induced acute lung injury in rats. Inflamm Res. 2008;57:104–110. doi: 10.1007/s00011-007-7037-y. [DOI] [PubMed] [Google Scholar]
- 4.Xu XL, Xie QM, Shen YH, Lu GH, Yao HY, Chen YY, Zhou JY. Involvement of mannose receptor in the preventive effects of mannose in lipopolysaccharide-induced acute lung injury. Eur J Pharmacol. 2010;641:229–237. doi: 10.1016/j.ejphar.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Xu S, Wang WX, Ding YM, Yu KH, Wang B, Chen XY. Rosiglitazone attenuates the severity of sodium taurocholate-induced acute pancreatitis and pancreatitis-associated lung injury. Arch Med Res. 2009;40:79–88. doi: 10.1016/j.arcmed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Liu ZN, Zhao M, Zheng Q, Zhao HY, Hou WJ, Bai SL. Inhibitory effects of rosiglitazone on paraquat-induced acute lung injury in rats. Acta Pharmacol Sin. 2013;34:1317–1324. doi: 10.1038/aps.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu MH, Melichian DS, Chang E, Matthew WB, Ghosh AK, Varga J. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-γ. Am J Pathol. 2009;174:519–533. doi: 10.2353/ajpath.2009.080574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coste A, Lagane C, Filipe C, Authier H, Amandine G, Bernad J, Victorine DE, Lepert JC, Patricia B, Linas MD, Arnal JF, Johan A, Bernard P. IL-13 attenuates gastrointestinal candidiasis in normal and immunodeficient RAG-2-/-mice via peroxisome proliferator-activated receptor-γ activation. J Immunol. 2008;180:4939–4947. doi: 10.4049/jimmunol.180.7.4939. [DOI] [PubMed] [Google Scholar]
- 9.Kao SJ, Wang D, Yeh DY W, Hsu K, Hsu YH, Chen HI. Static inflation attenuates ischemia/reperfusion injury in an isolated rat lung in situ. Chest. 2004;126:552–558. doi: 10.1378/chest.126.2.552. [DOI] [PubMed] [Google Scholar]
- 10.Tang Y, Chen Y, Chu Z, Yan B, Xu L. Protective effect of cryptotanshinone on lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol. 2014;723:494–500. doi: 10.1016/j.ejphar.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Yang W, Qiang D, Zhang M, Ma L, Zhang Y, Qing C, Xu Y, Zhen C, Liu J, Chen YH. Isoforskolin pretreatment attenuates lipopolysaccharide-induced acute lung injury in animal models. Int Immunopharmacol. 2011;11:683–692. doi: 10.1016/j.intimp.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Minamino T, Komuro I. Regeneration of the endothelium as a novel therapeutic strategy for acute lung injury. J Clin Invest. 2006;116:2316–2319. doi: 10.1172/JCI29637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giri SN, Hyde DM, Hollinger MA. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993;48:959–966. doi: 10.1136/thx.48.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJD, Morris D, Huang X, Sheppard D, Heller RA. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci. 2000;97:1778–1783. doi: 10.1073/pnas.97.4.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pittet JF, Griffiths MJD, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LAS, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-β is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–1544. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92:1177–1186. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- 17.Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, Monti P, Piemonti L, Biondi A, Mantovani A, Introna M, Allavena P. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 18.Marzolo MP, von Bernhardi R, Inestrosa NC. Mannose receptor is present in a functional state in rat microglial cells. J Neurosci Res. 1999;58:387–395. [PubMed] [Google Scholar]
- 19.Wileman TE, Lennartz MR, Stahl PD. Identification of the macrophage mannose receptor as a 175-kDa membrane protein. Proc Natl Acad Sci. 1986;83:2501–2505. doi: 10.1073/pnas.83.8.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Liu D, Zeng BX, Shang Y. Decreased expression of peroxisome proliferator-activated receptor gamma in endotoxin-induced acute lung injury. Physiol Res. 2006;55:291–299. doi: 10.33549/physiolres.930822. [DOI] [PubMed] [Google Scholar]
- 22.Klotz L, Hucke S, Thimm D, Classen S, Gaarz A, Schultze J, Edenhofer F, Kurts C, Klockgether T, Limmer A, Knolle P, Burgdorf S. Increased antigen cross-presentation but impaired cross-priming after activation of peroxisome proliferator-activated receptor γ is mediated by up-regulation of B7H1. J Immunol. 2009;183:129–136. doi: 10.4049/jimmunol.0804260. [DOI] [PubMed] [Google Scholar]
- 23.Rajaram MVS, Brooks MN, Morris JD, Torrelles JB, Azad AK, Schlesinger LS. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor γ linking mannose receptor recognition to regulation of immune responses. J Immunol. 2010;185:929–942. doi: 10.4049/jimmunol.1000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeeprae W, Kawakami S, Yamashita F, Hashida M. Effect of mannose density on mannose receptor-mediated cellular uptake of mannosylated O/W emulsions by macrophages. J Control Release. 2006;114:193–201. doi: 10.1016/j.jconrel.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Luo Q, Xiong Z, Liang W, Chen L, Xiong X. Telmisartan counteracts TGF-β1 induced epithelial-to-mesenchymal transition via PPAR-γ in human proximal tubule epithelial cells. Int J Clin Exp Pathol. 2012;5:522–529. [PMC free article] [PubMed] [Google Scholar]
- 27.Qian J, Niu M, Zhai X, Zhou Q, Zhou Y. β-Catenin pathway is required for TGF-β1 inhibition of PPARγ expression in cultured hepatic stellate cells. Pharmacol Res. 2012;66:219–225. doi: 10.1016/j.phrs.2012.06.003. [DOI] [PubMed] [Google Scholar]


