Abstract
Hydrogen sulfide (H2S) has been reported to be interwined in multiple systems, specifically in the cardiovascular system. However, the mechanisms underlying remain controversial. In the present study, we assessed the cardio-protective effects of H2S in the rat hemorrhagic shock model. Hemorrhagic shock was induced in adult male Sprague-Dawley rats by drawing blood from the femoral artery to maintain the mean arterial pressure at 35-40 mmHg for 1.5 h. The rats were assigned to four groups and the H2S donor, NaHS (28 μmol/kg, i.p.), was injected before the resuscitation in certain groups. After resuscitation the animals were observed and then killed to harvest the hearts. The morphological investigation and ultrastructural analyses were done and apoptotic cells were detected. The levels of relevant proteins were examined using Western blotting and immunohistochemical analyses. Resuscitated hemorrhagic shock induced heart injury and significantly increased the levels of serum myocardial enzymes, creatine kinase (CK) and lactate dehydrogenase (LDH) levels. Furthermore, it caused marked increase of apoptotic cells in heart tissue. Moreover, the expression of death receptor Fas and Fas-ligand, as well as the expression of apoptosis-relevant proteins active-caspase 3 and active-caspase 8 were markedly increased. Administration of NaHS significantly ameliorated hemorrhagic shock caused hemodynamic deterioration, decreased myocardial enzymes elevation, protected myocardial ultrastructure, and inhibited the expression of apoptosis-relevant proteins. It suggested that H2S might exert its cardio-protective roles via both the extrinsic Fas/FasL/caspase-8/caspase-3 pathway and the intrinsic mitochondria-involved pathways.
Keywords: Hydrogen sulphide, hemorrhagic shock, cardio-protective effect, apoptosis
Introduction
With the disrepute of “rotten-egg-smell gas”, hydrogen sulfide (H2S) was once received attention primarily as a toxic gas and an environmental pollution. More recently, however, increasing evidence has revealed H2S as a newly accepted gaseous signaling molecule, which possesses a variety of bio-effects on multiple systems [1,2]. Since 1996 Ade and Kimura began the investigation of H2S [3], various physiological and pathophysiological roles of H2S have been gradually explored, specifically on the cardiovascular system [4-8]. Bian JS and colleagues have found that endogenous H2S contributes to cardioprotection induced by ischemia, which effect may involve both protein kinase C and sarcolemmal KATP channels [9]. H2S may also be of value in cytoprotection during the evolution of myocardial infarction and administration of H2S may be of clinical benefit in ischemic disorders [10]. Latest study has demonstrated that H2S upregulates the expression of endothelial nitric oxide synthase and increases nitric oxide bioavailability, consequently prevents the transition from compensated to decompensate heart failure [11]. Studies mentioned above have confirmed the cardio-protective effects of H2S, however, the mechanisms underlying remain controversial.
Hemorrhagic shock (HS) is the leading cause of death in civilian and military trauma. The pathological process of hemorrhagic shock is very complex, including hypovolemia, hypoxemia, microcirculatory disturbances, oxidative stress, the release of significant amounts of toxic chemical mediators, and apoptosis [12]. The mechanisms of myocardial injury induced by hemorrhagic shock have not been fully elucidated, nonetheless, apoptosis is considered to be one of the most important factors. Up to date, the research on the apoptosis-inhibiting roles of H2S during hemorrhagic shock is limited. It has been reported that H2S protects cardiomyocytes from myocardial ischemia-reperfursion injury by enhancing phosphorylation of apoptosis repressor with caspase recruitment domain [13]. In the previous study, our team has also discovered that, by decreasing the expression of Fas/Fas-Ligand (FasL) and increasing the expression of apoptosis-related proteins, administration of NaHS, the H2S donor, can protect lung injury against hemorrhagic shock [14]. Therefore, we hypothesized that the similar mechanisms may also exist in the cardioprotective roles of H2S.
In the present research, we observed the pathological changes of myocardial cells during hemorrhagic shock, and explored the mechanisms of how H2S exerts protective effects in a rat model.
Materials and methods
Materials
NaHS and pentobarbital sodium were purchased from Sigma (Sigma-Aldrich CO LLC, MO, USA). Serum creatine kinase (CK) and lactate dehydrogenase (LDH) detection kits were purchased from Jiancheng Bioengineering Institute (Nanjing, P. R. China). The primary antibodies against Fas, FasL, active-caspase 8, active-caspase 3 were all purchased from Abcam (Abcam, Cambridge, UK). The commercial in situ cell death detection kit was from Roche Ltd (Basel, Switzerland). The commercial immunohistochemistry staining kit was from ZSGB-BIO CO Ltd (Beijing, China).
Animals
Adult age-matched male Sprague-Dawley (SD) rats (average weight 280 g, range 250-350 g) were used in this study. All the animals were received human care and in compliance with the Declaration of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1996). Animal experimental protocols and surgical procedures adopted in this study were reviewed and approved by the Animal Care and Use Committee of the Third Affiliated Hospital of Soochow University. All animals were fed a standard rodent diet (purchased from the Animal Center of the Third Affiliated Hospital of Soochow University).
Induction of hemorrhagic shock and protocol design
In the present study, a total of 20 rats were used randomly assigned to 4 groups (5 rats for each group): (1) Sham-operated animals (sham group); (2) Sham-operated animals treated with sodium sulphide (NaHS) (sham+NaHS group); (3) hemorrhagic shock animals (HS group); and (4) hemorrhagic shock animals treated with NaHS (HS+NaHS group). After 1 week of acclimatization, the rats were fasted overnight prior to the experiments but allowed free access to water. The rat hemorrhagic shock model was performed as has previously been described [15]. Briefly, two PE-50 catheters were inserted in both femoral arteries and the right femoral vein. The catheter was linked with a transducer which the other end linked to a PoweLab system (AD Instruments, Colorado Springs, CO, USA), and mean arterial pressure (MAP) was recorded. Blood was drawn from femoral artery within 10 min to a MAP of 35-40 mmHg. At this point, the volume of drawn-out blood, defined as maximal drawn out (MDO), was up to 60% of total blood volume. The MAP was maintained at 35-40 mmHg for 1.5 h by slowly injecting Ringer’s lactate solution till about 40% volume of the MDO. The rats were treated with NaHS (28 μmol/kg, i.p.) 10 min before resuscitation. Then the rats were resuscitated by evenly injecting Ringer’s lactate solution over 60 min with a volume of triple MDOs. Rats were observed for a total of 6 hours. During this time, blood samples were collected through femoral vein at 0, 1.5, 2, 3, 4 and 6 h. The sham group animals underwent the same procedures, but hemorrhage was not induced and resuscitation was not performed. The sham+NaHS group underwent the same procedures as the sham group, except that NaHS was administered at the same time as resuscitation. The HS group underwent the same procedures except the rats were not treating with NaHS before resuscitation. All animals were killed by injecting an overdose of pentobarbital sodium after the procedures. The hearts were harvested immediately, and the heart-apexes were sectioned into 3 mm-wide slices and immersed in neutral buffer (pH 7.4) containing 10% formalin for the following experiments. Smaller heart tissues (width and height < 1 mm, length < 5 mm) were obtained and fixed in 2% glutaraldehyde for transmission electron microscopy analysis. The remaining heart tissues were kept in a -70°C freezer for subsequent experiments.
Myocardial enzymes assays
Serum CK and LDH levels were measured by commercial chemical colorimetry kits according to the manufacturer’s instructions. In brief, the collected blood samples were stored in a 4°C freezer for 15 min. After that the samples were centrifuged at 4°C, then collected and regents were added. The optical density values were detected by a spectrophotometer (PowerWave XS, BioTek Inc, Vermont, USA) at 460nm, and the relative CK and LDH levels were then calculated.
Morphological investigation and ultrastructural analyses
To investigate morphological changes of rat hearts after hemorrhagic shock, hematoxylin and eosin (HE) staining was performed according to the standard protocol. The morphological changes in HE staining were analyzed under light microscope (LM) by an experienced pathologist. Ultrastructure damage was detected by transmission electron microscopy (TEM, JEM 2000 EX electron microscope; JEOL Ltd., Tokyo, Japan), and the pathological changes were also examined by a pathologist. All the morphological investigations and ultrastructural analysis were conducted in a double-blind manner.
Terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) staining
A commercial in situ cell death detection kit was used in this study according to the manufacturer’s instructions as has previously been described [14]. In brief, the tissue sections were dewaxed according to standard protocols and then were microwave heat treated. The slides were immersed in Tris-HCl (0.1 mol/L, pH 7.5) containing 3% BSA and 20% normal bovine serum for 30 min. To get a better look of the apoptotic cells, we used the DAPI (ready-to-use, Sigma-Aldrich CO LLC, MO, USA), which could preferentially stain dsDNA. The corresponding secondary antibody was incubated for 60 min at 37°C. After being washed twice in PBS, the slides were covered with the TUNEL reaction mixture. The slides were then incubated for 60 min at 37°C in a humidified chamber in the dark. Then, the slides were washed three times with PBS for 5 min each. The apoptotic cells were counted under a fluorescence microscope by an experienced pathologist. The apoptotic cells in 10 consecutive visual fields were counted in each histological section using a ×40 objective.
Immunohistochemistry (IHC) assays
After being dewaxed, the paraffin sections (4 μm thick) were incubated at 4°C overnight in the presence of primary antibodies raised against Fas (1:150), and FasL (1:150). Then, the sections were incubated with the appropriate secondary antibodies at 37°C for 1 h. The final antibody complex was visualized using a commercial immunohistochemistry staining kit. The stained sections were analyzed using Image-Pro Plus software (version 6.0, Media Cybernetics, Inc, MD, USA) by a pathologist.
Western blotting assays
The heart tissue samples were homogenized in RIPA lysis buffer (Beyotime Inc, Haimen, China). The homogenates were then centrifuged at 800× g to remove the cellular debris. The supernatant was again centrifuged at 12 000× g for 10 min at 4°C. The resulting supernatant (cytosolic fraction) was collected. The resulting pellet was resuspended in mitochondrial lysis buffer to obtain the mitochondrial fraction. The nuclear protein was extracted using a commercial kit (BestBio Inc, Shanghai, China) according to the manufacturer’s instructions. Equivalent amounts of protein (30 μg) from each sample were separated on 12% SDS-polyacrylamide gels and were then transferred onto 0.22 μmol/L nitrocellulose filter membranes (Millipore, Bedford, USA). The anti-active-caspase 3 (1:2000) and anti-active-caspase 8 (1:2000) primary antibodies were incubated at 4°C overnight. The secondary antibodies were incubated, and the signals were detected using an ECL kit (BestBio Inc, Shanghai, China).
Statistical analysis
All values were expressed as mean ± SD. Data were analyzed using one-way ANOVA, followed by a Fisher’s LSD post hoc multiple comparisons (SPSS for Windows version 16.0, Chicago, USA). A P-value < 0.05 was considered statistically significant.
Results
Exogenous H2S prevented hemodynamic deterioration in hemorrhagic shock rats and decreased CK and LDH levels
Hemorrhagic shock caused a remarkably decrease in MAPs. Compared with sham and sham+NaHS groups, MAP was lower in HS and HS+NAHS groups at all time points (Figure 1; P < 0.01). Administration of NaHS effectively elevated the MAP after resuscitation, and the pressure remained constantly higher than that without the administration of NaHS (Figure 1).
Figure 1.
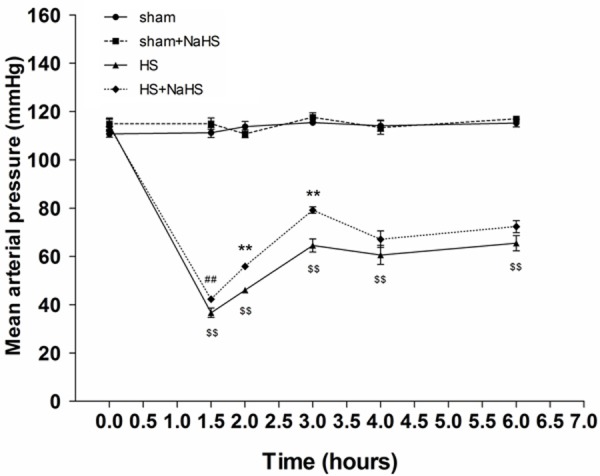
Effects of NaHS on MAP. HS caused a remarkably decrease in MAPs, which were elevated by NaHS administration (n=5). ##P < 0.05 vs. time-matched value in the HS group. **P < 0.01 vs. time-matched value in the HS group. $$P < 0.01 vs. time-matched value in the sham and sham+NaHS groups.
CK and LDH represented the damage severity of hearts. Hemorrhagic shock resulted in a significant elevation of serum LDH and CK levels, which were notably attenuated by NaHS treatment (Figure 2A, 2B).
Figure 2.
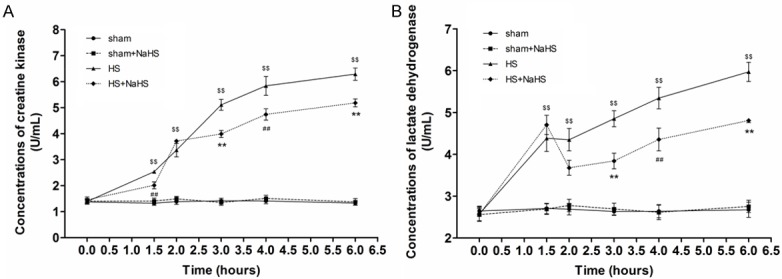
Effects of NaHS on Serum CK and LDH Levels. HS resulted in a significant elevation of serum LDH and CK levels, which were attenuated by NaHS treatment (n=5). ##P < 0.05 vs. time-matched value in the HS group. **P < 0.01 vs. time-matched value in the HS group. $$P < 0.01 vs. time-matched value in the sham and sham+NaHS groups.
Exogenous H2S alleviates pathological changes of myocardial cells in hemorrhagic shock
HE staining showed well-defined and neatly-arranged cardiomyocytes in sham and sham+NaHS groups (Figure 3A, 3B). Hemorrhagic shock resulted in myocardial injury characterized by edema, unevenly-dyed cardiac muscle fibre, and abnormally-distributed cardiac muscle gaps (Figure 3C). Administration of NaHS notably attenuated these changes of cardiomyocytes induced by hemorrhagic shock (Figure 3D).
Figure 3.
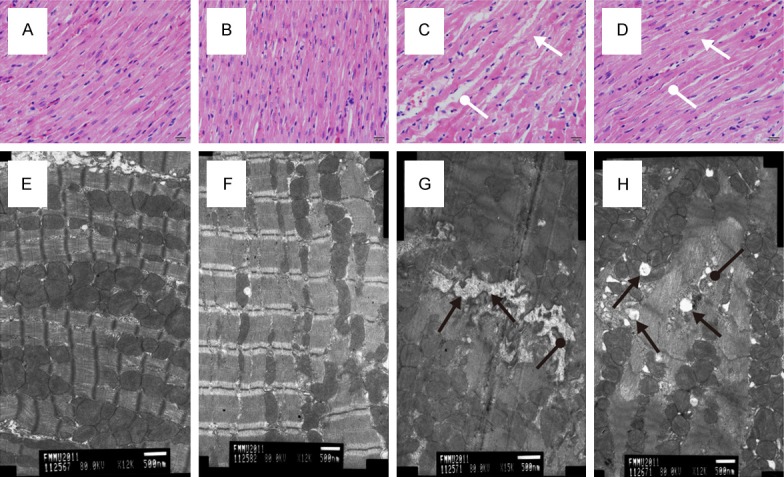
Effects of NaHS on Rat Cardiac Tissue. HE staining showed well-defined and neatly-arranged cardiomyocytes in sham (A) and sham+NaHS (B) groups. HS resulted in myocardial injury characterized by edema, unevenly-dyed cardiac muscle fibre (white arrows), and abnormally-distributed cardiac muscle gaps (white round arrows, (C). Administration of NaHS notably attenuated these changes (D). Transmission electron microscopy showed that, compared with sham (E) and sham+NaHS (F) groups, poorly-arranged cardiac sarcomeres, ill-defined cardiac stripes, swollen mitochondria with disorganized fractured cristae (black round arrows), and dilated sarcoplasmic reticula (black arrows) were observed in the cardiac tissue of HS group (G). In the HS+NaHS group, the sarcoplasmic reticula were not significantly dilated, and the structure of mitochondria was relatively clear and complete compared with HS group (H).
Transmission electron microscopy showed that, compared with sham and sham+NaHS groups, poorly-arranged cardiac sarcomeres, ill-defined cardiac stripes, swollen mitochondria with disorganized fractured cristae, and dilated sarcoplasmic reticula were observed in the cardiac tissue of HS group (Figure 3E-G). On the other hand, in the HS+NaHS group, the sarcoplasmic reticula were not significantly dilated, and the structure of mitochondria was relatively clear and complete compared with HS group (Figure 3H).
NaHS attenuates apoptosis induced by hemorrhagic shock
TUNEL staining showed that few apoptotic cells were detected in the sham and sham+NaHS groups (Figure 4A, 4B). However, we observed an obvious increase in the number of apoptotic cells in the rat hearts after hemorrhagic shock (Figure 4C; P < 0.01 vs. the sham and sham+NaHS groups). In contrast, NaHS administration evidently alleviated the apoptotic response (Figure 4D; P < 0.05 compared with HS group).
Figure 4.
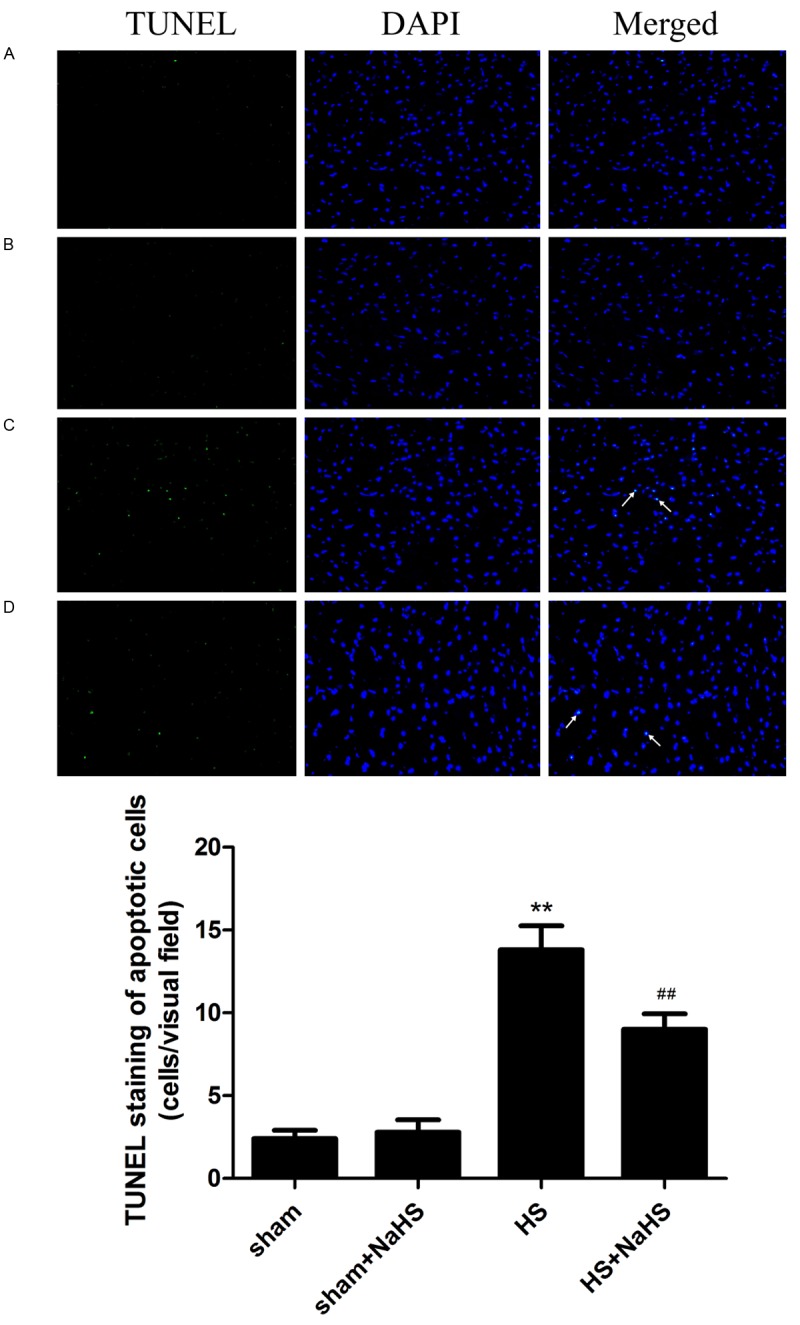
Effects on NaHS on apoptosis. The green stained cells indicated by white arrows are the apoptotic cells induced by HS. TUNEL staining showed that few apoptotic cells were detected in the sham (A) and sham+NaHS (B) groups. The number of apoptotic cells in the rat hearts remarkably increased after HS (**P < 0.01 vs. the sham and sham+NaHS groups, (C), and NaHS administration evidently alleviated the apoptotic response (##P < 0.05 vs. the HS group, D).
NaHS inhibits the expression of Fas and FasL during hemorrhagic shock
Hemorrhagic shock elevated the expression of death receptor Fas (Figure 5C). Whereas, there was no difference between the Sham and Sham+NaHS groups (Figure 5A, 5B). Administration of NaHS reduced the expression of Fas (Figure 5D). Similarly, the expression of FasL also increased in the HS group, and NaHS treatment significantly reduced the expression of FasL (Figure 5E-H).
Figure 5.
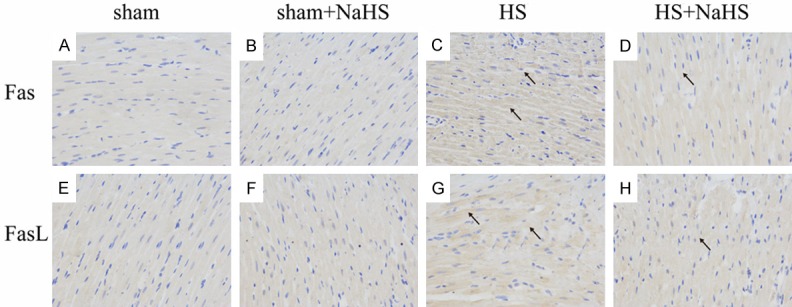
Effects on NaHS on the expression of Fas and FasL. The black arrows indicate cells positively stained for Fas and FasL. There was no difference between the sham (A) and sham+NaHS (B) groups and HS elevated the expression of Fas (C). Administration of NaHS reduced the expression of Fas (D). Similarly, the expression of FasL also was low in the sham (E) and sham+NaHS (F), and increased in the HS group (G), and NaHS treatment significantly reduced the expression of FasL (H).
Exogenous H2S attenuates the increase of apoptosis-related protein expression induced by hemorrhagic shock
There was no difference in caspase-3 and caspase-8 expression between sham and sham+NaHS groups (Figure 6A-C; P > 0.05). A remarkably increase in caspase-3 and caspase-8 expression were observed in hemorrhagic shock, and exogenous H2S decreased these changes in HS+NaHS group (Figure 6A-C; P < 0.01).
Figure 6.
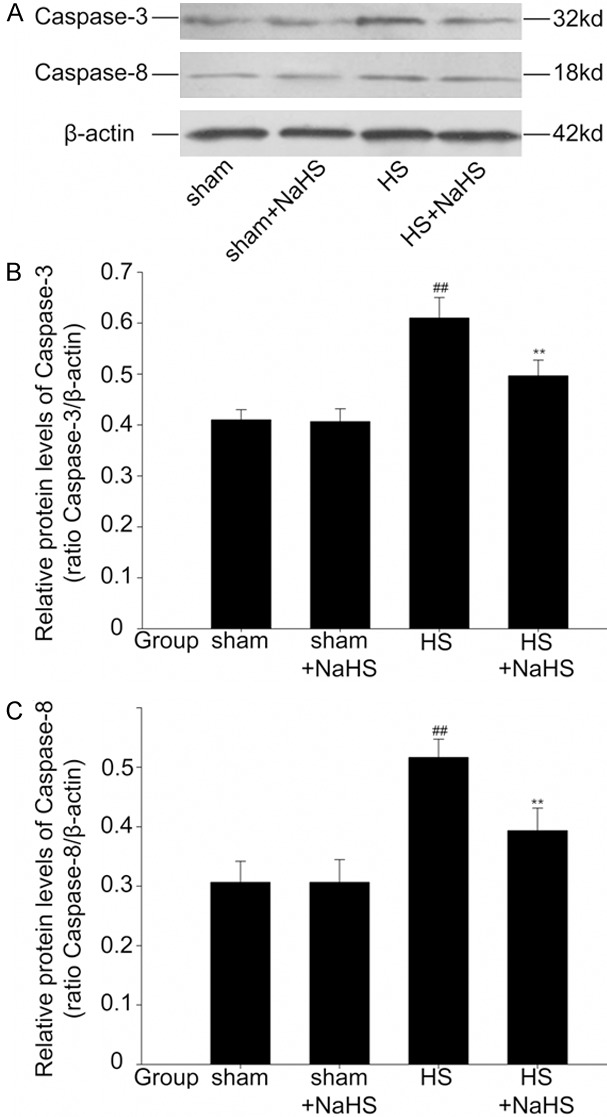
Effects of NaHS on the expression of caspase-3 and caspase-8. Western blotting assays showed that there was no difference in caspase-3 and caspase-8 expression between sham and sham+NaHS groups. A remarkably increase in caspase-3 and caspase-8 expression were observed in HS, and NaHS decreased these changes in HS+NaHS group. ##P < 0.01 vs. The sham and sham+NaHS groups. **P < 0.01 vs. The HS group.
Discussion
NaHS, the exogenous H2S donor, has been shown to play important roles in varieties of disease. In the present study, we revealed that, administration of NaHS (28 μmol/kg, i.p.) showed cardioprotective effects characterized by ameliorating hemodynamic deterioration, attenuating the increase of CK and LDH, and alleviating the pathological injuries of cardiomyocytes in a rat hemorrhagic shock model. This result is in accordance with our previous finding which has been published in 2012 [15]. However, some research reported conflicting results. The study addressed by Drabek and colleagues demonstrated that H2S did not induce hypothermia or improve survival from hemorrhagic shock in pigs [16]. It is considered that discrepancies may exist in different animal models. Therefore, caution is needed in interpreting the results because it provides limitations of the H2S effect among different species and difficulties in translating the H2S effect to large animals, even to human beings, in a clinically relevant post-insult paradigm.
Furthermore, the consequences of H2S treatment differ on different drug concentrations and, to some extent, different drug types and administration routes. Dombkowski and colleagues reported that H2S played an either vasodilation or vasoconstriction role in a dose-dependent manner, and seemed to suit both organ-specific and species-specific homeostatic requirements [17]. In mice model, Simon F. and colleagues observed that the sulfide infusion rate (initial bolus 0.2 mg/kg followed by continuous i.v. 2 mg/kg/h during the 2 h before aortic occlusion, 0.5 mg/kg/h during the 90 min of aortic occlusion, and 1 mg/kg/h during the 8 h reperfusion period) avoided the adverse effects, while a higher sulfide infusion rate (2 mg/kg/h over 10 h) markedly reduced energy expenditure but caused a deterioration of pulmonary gas exchange [18]. In the present study, administration of NaHS (28 μmol/kg, i.p.) showed cardio-protective effects in rat hemorrhagic shock model. Understanding these discrepancies may be useful in developing new drugs which target H2S pathways under different conditions.
Using the light microscopy and transmission electron microscopy, we observed that H2S alleviated the morphological changes of myocardial tissue induced by hemorrhagic shock. It provided direct evidence for cardio-protective effects of H2S. Further investigations found the decrease of apoptosis cells and amendment of mitochondria injury after H2S administration, which suggested that H2S might exert the protective roles via apoptosis signal pathways.
It is well-established that there are two fundamental signaling pathways mediating apoptosis, the extrinsic and the intrinsic pathways [19]. The extrinsic pathways are activated by external death ligands, such as Fas and FasL. Ligand binding results in recruiting adapter proteins and initiating caspases (caspase-8/-10), which subsequently activates the downstream caspases (caspase-3/-6/-7) or intrinsic pathways. The intrinsic pathways are triggered by internal apoptotic signals and involved in mitochondria, which play vital roles in executing apoptosis. The anti-apoptotic effects of H2S have been widely reported, however, few studies have been conducted with regard to the cardio-protection in hemorrhagic shock. In the present study, we detected a significant increase in Fas and FasL expression, as well as the caspase-8 and caspase-3 expression, while H2S remarkably attenuate the increasing trend. It suggests that the apoptosis of myocardiocytes induced by hemorrhagic shock may be mediated via extrinsic pathways, and administration of H2S suspends the apoptosis by interrupting the Fas/FasL/caspase-8/caspase-3 signal pathway. It is partially consistent with the previous research performed by Kang K. and colleagues [20]. Complementary, the ultrastructure observation of myocardiocytes revealed the morphological changes of mitochondria during hemorrhagic shock which can be alleviated by H2S. Possibility exists that H2S may inhibit apoptosis by preserving the mitochondria functions and interfering the intrinsic pathways. It was consistent with a new report stating that H2S reduces regional myocardial ischemia injury through protection of mitochondria function [21]. Further experiments to study the mechanisms of mitochondria-protective roles of H2S can be taken in the following researches.
Some limitations of our study should be addressed. The sample size was not large enough for comprehensive analysis. The data generated was largely phenomenological and the findings are associative. Further studies using knockout animals or giving pharmacologic agents to test the internal mechanisms will be performed in the near future. Besides, we could not take all possible factors and it is uncertain to draw a direct line between the anti-apoptosis effects of H2S and its protective effects. Nevertheless, strengths of our study should be acknowledged. It is one of the few researches to investigate the cardio-protective roles of H2S in hemorrhagic shock, and to our knowledge, is the first research to reveal that H2S may exert it via the possible extrinsic Fas/FasL/caspase-8/caspase-3 and intrinsic mitochondria-involved apoptosis signal pathways.
Conclusions
Our in vivo study showed that administration of NaHS, the H2S donor, ameliorated hemorrhagic shock caused hemodynamic deterioration, decreased myocardial enzymes elevation, and protected myocardial ultrastructure. It suggested that H2S might exert its cardio-protective roles via both the extrinsic Fas/FasL/caspase-8/caspase-3 pathway and the intrinsic mitochondria-involved pathways.
Acknowledgements
This study was supported by national natural science foundation of China (81171653, 30972703, 81201741, 81301960 and 81302047); Natural science foundation of Jiangsu province (BK2011246, BK2011247 and BK20130243); The project of Six batch of major talent summit (BRA2010037); Society development plans, department of science and technology Changzhou (CJ20112020, CZ20110024, CS20102020); the Guiding Project of Changzhou Health Bureau (WZ200602), and the Innovative Talents Training Project of Changzhou Health Bureau.
Disclosure of conflict of interest
None.
References
- 1.Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 2.Lowicka E, Beltowski J. Hydrogen sulfide (H2S)-the third gas of interest for pharmacologists. Pharmacol Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- 3.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 5.Chan MV, Wallace JL. Hydrogen sulfide-based therapeutics and gastrointestinal diseases: translating physiology to treatments. Am J Physiol Gastrointest Liver Physiol. 2013;305:G467–G473. doi: 10.1152/ajpgi.00169.2013. [DOI] [PubMed] [Google Scholar]
- 6.Polhemus DJ, Calvert JW, Butler J, Lefer DJ. The cardioprotective actions of hydrogen sulfide in acute myocardial infarction and heart failure. Scientifica (Cairo) 2014;2014:768607. doi: 10.1155/2014/768607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Di Sante M, Morelli A, Cirino G, Wallace JL. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 8.Muniraj N, Stamp LK, Badiei A, Hegde A, Cameron V, Bhatia M. Hydrogen sulfide acts as a pro-inflammatory mediator in rheumatic disease. Int J Rheum Dis. 2014 doi: 10.1111/1756-185X.12472. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther. 2006;316:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- 10.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G Sr, Gojon G Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. H(2)S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127:1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angele MK, Schneider CP, Chaudry IH. Bench-to-bedside review: latest results in hemorrhagic shock. Critical Care. 2008;12:218. doi: 10.1186/cc6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao X, Tan G, He C, Gao Y, Pan S, Jiang H, Zhang Y, Sun X. Hydrogen sulfide protects cardiomyocytes from myocardial ischemia-reperfusion injury by enhancing phosphorylation of apoptosis repressor with caspase recruitment domain. Tohoku J Exp Med. 2012;226:275–285. doi: 10.1620/tjem.226.275. [DOI] [PubMed] [Google Scholar]
- 14.Xu DQ, Gao C, Niu W, Li Y, Wang YX, Gao CJ, Ding Q, Yao LN, Chai W, Li ZC. Sodium hydrosulfide alleviates lung inflammation and cell apoptosis following resuscitated hemorrhagic shock in rats. Acta Pharmacol Sin. 2013;34:1515–1525. doi: 10.1038/aps.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao C, Xu DQ, Gao CJ, Ding Q, Yao LN, Li ZC, Chai W. An exogenous hydrogen sulphide donor, NaHS, inhibits the nuclear factor kappaB inhibitor kinase/nuclear factor kappab inhibitor/nuclear factor-kappaB signaling pathway and exerts cardioprotective effects in a rat hemorrhagic shock model. Biol Pharm Bull. 2012;35:1029–1034. doi: 10.1248/bpb.b110679. [DOI] [PubMed] [Google Scholar]
- 16.Drabek T, Kochanek PM, Stezoski J, Wu X, Bayir H, Morhard RC, Stezoski SW, Tisherman SA. Intravenous hydrogen sulfide does not induce hypothermia or improve survival from hemorrhagic shock in pigs. Shock. 2011;35:67–73. doi: 10.1097/SHK.0b013e3181e86f49. [DOI] [PubMed] [Google Scholar]
- 17.Dombkowski RA, Russell MJ, Schulman AA, Doellman MM, Olson KR. Vertebrate phylogeny of hydrogen sulfide vasoactivity. Am J Physiol Regul Integr Comp Physiol. 2005;288:R243–252. doi: 10.1152/ajpregu.00324.2004. [DOI] [PubMed] [Google Scholar]
- 18.Simon F, Giudici R, Duy CN, Schelzig H, Oter S, Gröger M, Wachter U, Vogt J, Speit G, Szabó C, Radermacher P, Calzia E. Hemodynamic and metabolic effects of hydrogen sulfide during porcine ischemia/reperfusion injury. Shock. 2008;30:359–364. doi: 10.1097/SHK.0b013e3181674185. [DOI] [PubMed] [Google Scholar]
- 19.Lu Q, Harrington EO, Rounds S. Apoptosis and lung injury. Keio J Med. 2005;54:184–189. doi: 10.2302/kjm.54.184. [DOI] [PubMed] [Google Scholar]
- 20.Kang K, Zhao M, Jiang H, Tan G, Pan S, Sun X. Role of hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in rats. Liver Transpl. 2009;15:1306–1314. doi: 10.1002/lt.21810. [DOI] [PubMed] [Google Scholar]
- 21.Xie YH, Zhang N, Li LF, Zhang QZ, Xie LJ, Jiang H, Li LP, Hao N, Zhang JX. Hydrogen sulfide reduces regional myocardial ischemia injury through protection of mitochondrial function. Mol Med Rep. 2014;10:1907–1914. doi: 10.3892/mmr.2014.2391. [DOI] [PubMed] [Google Scholar]


