Abstract
Oxidative stress and inflammation are the important pathological basis of atherogenesis. So, attenuating oxidative stress and inflammation has a very important significance in the prevention and treatment of atherosclerosis. The aim of present study was to investigate whether anti-atherosclerotic effect of Tongxinluo (TXL), a compound traditional Chinese medicine, is related to its anti-oxidation and anti-inflammation in human cardiac microvascular endothelial cells (HCMEC). We found that TXL treatment significantly reduced serum lipid levels and atherosclerotic plaque formation of apoE-deficient mice, and improved endothelial cell function as evidenced by increased expression of CD31 and eNOS. TXL pretreatment could abrogate the up-regulation of ROS and MDA induced by C16. Further experiments showed that the anti-oxidative effect of TXL may be related to inhibiting the expression of p22phox, p47phox and HO-1 in HCMECs. We also found that TXL could inhibit the release of IL-1β and TNFα induced by C16, which is mediated by inhibiting the expression and activation of NF-κB. In conclusion, TXL decreases atherosclerotic plaque formation and improves endothelial cell function by inhibiting oxidative stress and inflammation in HCMECs. This finding provides a new molecular mechanism for the anti-atherosclerotic effect of TXL.
Keywords: TXL, human cardiac microvascular endothelial cells, atherosclerosis, oxidative stress, inflammation
Introduction
Atherosclerosis (AS) is one of the most important pathological bases of cardiovascular and cerebrovascular diseases. Oxidative stress and inflammation play the central role in the development and progression of atherosclerosis. Reactive oxygen species(ROS) such as superoxide mediates a wide range of pathological processes including lipid peroxidation [1], reduction of NO bioactivity [2], and induction of inflammatory genes [3]. Recently, several lines of data have indicated that NADPH oxidase complex, which is composed of the membrane-associated proteins nox1, nox4, and p22phox, and cytosolic components p47phox and p67phox, is the major sources of ROS production in several cardiovascular diseases [4]. Our study has demonstrated that overexpression of p47phox protein occurs in the neointima formation induced by balloon injury and this increased p47phox protein level is consistent with the increased superoxide production [5]. An important mechanism for ROS-mediated atherosclerosis appears to be through stimulation of proinflammatory events. Previous study showed the critical role of inflammation in the atherosclerotic lesion formation [6]. It has been reported that lack of apolipoprotein E (apoE) results in a marked increase in cholesterol-rich materials in blood circulation and subsequent atherosclerotic lesion formation [7]. Therefore, apoE-deficient mice can rapidly develop atherosclerotic lesions that are very similar to the advanced lesions in humans. Besides, high fat diet (HFD) can accelerate the pathological process of atherosclerosis in the apoE-deficient mice. Hence, this model has been widely used to analyze the pathogenesis of the atherosclerotic lesion formation.
Tongxinluo (TXL) was registered in the State Food and Drug Administration of China for treatment of angina pectoris in 1996. TXL is extracted, concentrated, and freeze-dried from a group of herbal medicines, such as ginseng, radix paeoniae rubra, borneol, and spiny jujuba seed, which contains multiple active components that may be responsible for its antianginal effects [8,9]. It is widely used as the clinical emergency medicine without obvious discomfort, side effects, and drug resistance. A previous study showed that TXL enhances the stability of vulnerable plaques and reduces plaque area by lowering expression of oxidized LDL-RI, matrix metalloproteinase(MMP)-1, MMP-3, NF-kB, and systemic and local inflammatory factors [9]. Although anti-atherosclerotic effect of TXL has been well known, it remains unclear whether TXL decreases atherosclerotic lesion formation through attenuating oxidative stress and inflammation.
This study was carried out to test the hypothesis that TXL inhibits atherosclerotic lesion formation via its anti-oxidation and anti-inflammation in human cardiac microvascular endothelial cells (HCMEC) and apoE-deficient mice.
Materials and methods
Animals
Eight-week-old male C57BL/6 mice and apoE-deficient mice on the C57BL/6J background were obtained from Peking University Animal Technology Co., Ltd. (Beijing, China). They were fed on a high-fat diet which Contained 21% fat and 0.15% cholesterol. All animal procedures were performed in accordance with the Animal Ethics Committee of Hebei Medical University of Traditional Chinese Medicine. Animal housing rooms were maintained at a constant room temperature (25°C) and with 12-h light/dark cycle (light on from 8:00 AM to 8:00 PM).
Components and preparation of TXL
TXL powder was provided by Shijiazhuang Yiling Pharmaceutic (Hebei, China). The herbal drugs were authenticated and standardized on marker compounds according to the Chinese Pharmacopoeia 2005. TXL contains 12 medicinal components, which were ground to superfine powder with the diameter ≤ 10 μm by a micronizer. To reduce the dose variability of TXL among different batches, the species, origin, harvest time, medicinal parts, and concocted methods for each component were strictly standardized. Moreover, high performance liquid chromatography (HPLC), high performance capillary electrophoresis, and gas chromatography were applied to quantity the components of the TXL.
Drug treatment
The apoE-deficient mice with similar body weight were randomly divided to model and treatment groups respectively (5 mice in each group). In addition, C57BL/6J mice were fed with a control diet. And then all mice were treated by intragastric administration with normal saline or TXL (0.75 g/kg/day) or atorvastatin (5 mg/kg/day). The drugs were grinded into a powder and dissolved in normal saline.
Tissue preparation
After the end of administration, blood was collected and then the mice were sacrificed immediately. Dissection of the whole aorta from the aortic arch to the iliac artery bifurcation was performed under aseptic conditions. The aortas were carefully cleaned of adherent connective tissue under a dissecting microscope. A part of the aortas were embedded in OCT compound (Tissue-Tek), frozen on dry ice, and stored at -80°C for morphometry or immunohistochemistry. Others were wrapped with aluminum foil for detecting expression of gene related to plaque stability by reverse transcription-polymerase chain reaction.
Serum lipid levels
After the administration of TXL, mice were fasted for 12 hours and blood was collected from the canthus, centrifuged for 15 minutes at 3000 rpm/min, the serum was separated. The serum total cholesterol (TC), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), and triglyceride (TG) levels were quantified by semi-automatic biochemical analyzer.
Morphometric analysis of the aortas
For macroscopic analysis of plaque extension, the adventitia was removed from the vessels and fixed overnight in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The aortas were stained with Oil-red O, and then photographed. The results were quantified with Image-Pro-Plus 6.0.
Immunohistochemistry
For Immunohistochemical staining, sections were deparaffinized and rehydrated in graduated alcohol. Then they were treated in a 0.1 mol/L sodium citrate buffer and heated for 30 min for antigen retrieval, and then the sections were incubated with CD31 (Abcam, 1:50), HO-1 (Epitomics, 1:100), p22 (Abcam, 1:500), p47 (Bioworld, 1:50) antibodies. After overnight incubation, the sections were incubated with the secondary antibodies (Abcam), and visualized with 3,3-diaminobenzidine (DAB) and counterstained using hematoxylin. Brown and yellow colors indicated positive results.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
Aortic tissues were harvested and total RNA was isolated with Trizol reagent (Roche, Mannheim, Germany). Reverse Transcription-Polymerase Chain Reaction assay was performed according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). The sequences of the PCR primer are as follows: mouse p47, 5’-ACCTGTCGGAGAAGGTGGT-3’(forward); 5’-TAGGTCTGAAGGATGATGGG-3’ (reverse); mouse HO-1, 5’-TCCAGACACCGCTCCTCCAG-3’ (forward), 5’-GGATTTGGGGCTGCTGGTTTC-3’ (reverse); mouse p22, 5’-TGCGGGACGCTTCACGCAGTGG-3’ (forward), 5’-GGTTGGTAGGTGGCTGCTTGATGG-3’ (reverse); mouse NF-κB, 5’-TGTCTGCTGCTGCTGGTGGC-3’ (forward); 5’-AAGCAGGCAGCCAGCCAAGG-3’ (reverse); human p47, 5’-CCCTGAGCCCAACTATGCA-3’ (forward); 5’-CCCCCTCCACAGCAGTGTA3-3’ (reverse); human p22, 5’-TGGGCGGCTGCTTGATGGT-3’ (forward), 5’-GTTTGTGTGCCTGCTGGAGT-3’ (reverse); human HO-1, 5’-CAGGCAGAGAATGCTGAGTTC-3’ (forward), 5’-GCTTCACATAGCGCTGCA-3’ (reverse); human NF-κB, 5’-CATGGTGGTTGGCTTTGCA-3’ (forward); 5’-AGCCCCTAATACACGCCTCTGT-3’ (reverse); human eNOS, 5’-TGGTAACCAGCACATTTGGGA-3’ (forward); 5’-CCTGGGCAACATGGCGA-3’ (reverse). The relative quantitative expressions of tissue-specific markers were calculated after normalization with GAPDH as an endogenous reference gene.
Measurement of ROS levels
Superoxide in human cardiac microvascular endothelial cells (HCMECs) on chamber slides were detected using 2 µmol/L dihydroethidium (DHE) for 30 minutes at 37°C, then visualized using laser scanning confocal microscope.
Determination of SOD, nitric oxide (NO) and malondialdehyde (MDA)
Human cardiac microvascular endothelial cells (HCMECs) were cultured in Endothelial Cell Medium with 10% fetal bovine serum (FBS) and maintained in 5% CO2 at 37°C in a humidified atmosphere. Cells were pretreated with various concentrations of TXL before stimulation with C16. The supernatant was used to determine SOD, NO, MDA levels, which were measured spectrophotometrically using diagnostic kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute).
ELISA
The concentration of TNF-α and IL-1β was determined in Human cardiac microvascular endothelial cells (HCMECs) culture medium using a human TNF-α Elisa kit or a IL-1β Elisa kit (Ray Biotech, Norcross, Georgia, USA), respectively. The absorbance was measured with a microplate reader (SPECTRAFluor Plus, Tecan).
Western blot analysis
Human cardiac microvascular endothelial cells (HCMECs) were cultured in Endothelial Cell Medium with 10% fetal bovine serum (FBS) and maintained in 5% CO2 at 37°C in a humidified atmosphere. Cells were harvested and lysed with lysis buffer containing 1% NP-40, 150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 10% glycerin, 1 mM Na3VO4, 1 mM PMSF and 1 mM DTT. Proteins were isolated from HCMECs then separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto polyvinylidene difluoride membrane (Millipore). Membranes were blocked with 5% milk in Tris-HCl Tween buffer solution for 2 h at 37°C and incubated overnight at 4°C with specific. HO-1 (Epitomics, 1:1000), p22 (Abcam, 1:1000), p47 (Bioworld, 1:500) and p-p22 (Sigma, 1:1000), p-p47 (Bioworld, 1:500), p-NF-κB (Cell Signaling, 1:500), NF-κB (Abcam, 1:500), eNOS (Novus, 1:1000), IκB (Bioworld, 1:500) antibodies. After incubation with appropriate secondary antibody, the membranes were developed with the Chemiluminescence Plus Western blot analysis kit (Millipore).
Statistical analysis
All data were analyzed using SPSS version 16 software (SPSS Inc) and expressed as means ± SD. Statistical comparison between different treatments was done by one-way ANOVA. Differences were considered statistically significant for P < 0.05.
Results
TXL and AT decreased atherosclerotic plaque formation in the apoE-deficient mice
To generate a mouse model of atherosclerosis, apoE-deficient mice were fed with HFD for 12 weeks with or without TXL and atorvastatin (AT) treatment. Then aortas were dissected out and sectioned. The sections were stained with Hematoxylin eosin (H&E) and Oil-red O. As shown in Figure 1A, atherosclerotic plaques were observed clearly in the aortic sections of apoE-deficient mice fed HFD. TXL and AT treatment significantly reduced plaque area. B-mode ultrasonic examination got the same results (Figure 1B). During the treatment, body weights of the mice were measured and there was no difference in different groups (data not shown). Serum lipid levels were detected by semi-automatic biochemical analyzer. Treatment with TXL and AT significantly reduced serum TG, TC and LDL levels, compared with model group (Figure 1C). However, there were no statistical differences in the amounts of HDL. These results suggested that TXL and AT decrease the development of atherosclerotic plaque through reducing serum lipid levels. To further investigate whether decreased serum lipid level by TXL and AT improved endothelial function in apoE-deficient mice fed HFD, the expression of CD31 (endothelial-specific marker) was detected by immunohistochemisty. As shown in Figure 1D, TXL and AT treatment significantly increased CD31 expression in atherosclerotic plaque of apoE-deficient mice. There was no significant difference between TXL and AT. These results prompt us to assume that TXL inhibited atherosclerotic plaque formation through regulating the function of endothelial cells.
Figure 1.
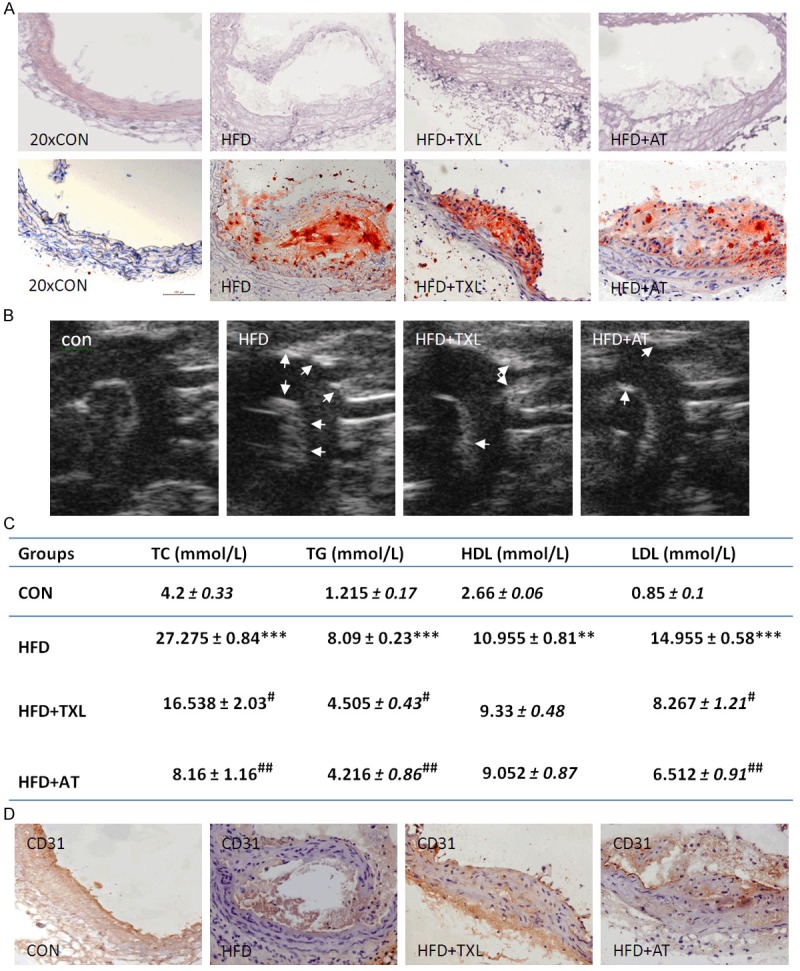
TXL decreased atherosclerotic plaque formation. A. Representative photographs of HE-and oil red O-stained thoracic aortic sections (upper panel). Quantitative analysis of lipid deposition. The mean percentages of plaque areas of total vessel areas in all mice of 1 group are shown with SEM (n = 3) (lower panel). B. Images of the ascending aorta captured with UBM with the atherosclerotic plaques delineated. C. Effect of TXL and AT on serum lipid levels. **P < 0.01 and ***P < 0.001 vs. control group; #P < 0.05 and ##P < 0.01 vs. HFD group (n = 5 in each group). The bars represent mean ± SEM. D. Immunohistochemical staining on sections of the thoracic artery with antibody against CD31 (× 20).
TXL exerted anti-oxidative effect in endothelial cells
To further explore whether the role of TXL in improving endothelial cell function was related to its anti-oxidative properties, anti-oxidative effect of TXL was analyzed using C16 as an oxidative stress inducer. As shown in Figure 2, the ROS and MDA levels in the endothelial cells treated with 600 μmol/L of C16 were significantly increased (Figure 2A and 2B), while the NO and SOD level was significantly decreased (Figure 2C and 2D) when compared with the cells treated without C16. The TXL treatment significantly reduced levels of ROS and MDA (Figure 2A and 2B). By contrast, the levels of NO and SOD were increased in dose-dependent manner (Figure 2C and 2D). These results suggest that TXL had markedly antioxidative effect in endothelial cells.
Figure 2.
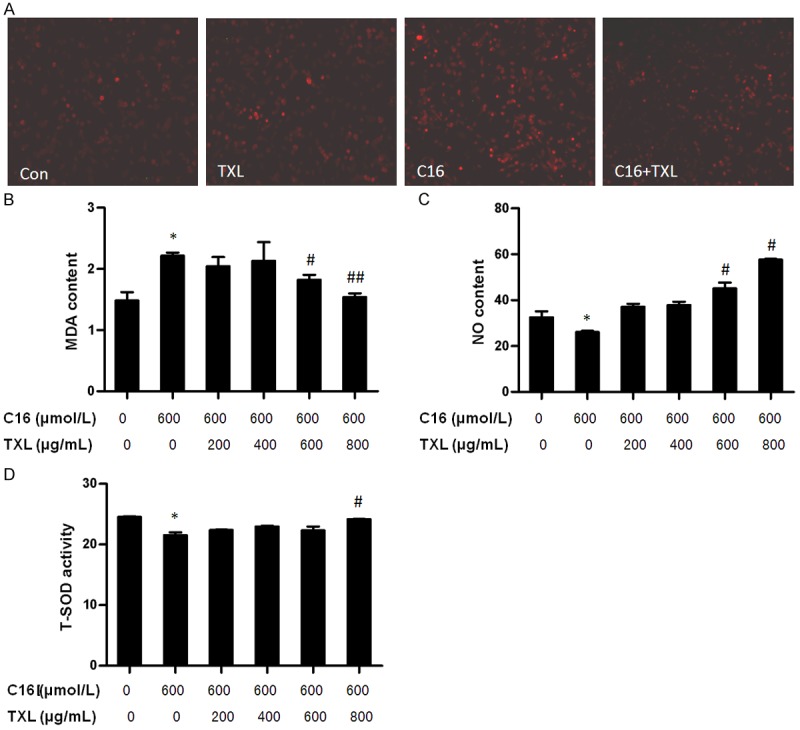
TXL exerted anti-oxidative effect. (A) HCMECs were grown in six-well plates for 24 h, and then were pre-incubated with different doses of TXL before stimulating with C16 (600 μmol/L) for 12 h. ROS was detected by DHE staining. MDA content (B), NO content (C) and T-SOD activity (D) were detected by TBA, nitrate reductase and hydroxylamine method. *P < 0.05 vs. C16-untreated group; #P < 0.05 and ##P < 0.01 vs. C16-treated group without TXL treatment. The bars represent the mean ± SEM. from 3 independent experiments.
TXL inhibited expression of NADPH oxidase and HO-1 in endothelial cells
To examine whether antioxidative effect of TXL is mediated by NADPH oxidase and HO-1, HCMECs were pre-incubated with the different doses of TXL for 24 h and then treated with 600 μmol/L of C16. As shown in Figure 3A, C16-treated endothelial cells showed significantly higher p22phox, p47phox and HO-1 mRNA expression levels than the cells treated without C16. Treatment with different doses (200 to 800 μg/ml) of TXL significantly decreased p22phox, p47phox and HO-1 mRNA expression in dose-dependent manner (Figure 3A). Accordingly, the expression of p22phox, p47phox and HO-1 proteins was detected by Western blotting. The results showed that oxidative stress inducer C16 dose-dependently induced the expression of p22phox, p47phox and HO-1 proteins, while TXL treatment markedly decreased their expression in dose-dependent manner, especially when 600 μg/ml and 800 μg/ml TXL were used (lanes 4 and 5) (Figure 3B). To further investigate the effect of TXL on the expression of NADPH oxidase and HO-1 proteins in vivo, apoE-deficient mice were used to examine p22phox, p47phox and HO-1 expression by immunohistochemical staining. As shown in Figure 3C, atherosclerotic mice showed significantly higher p22phox, p47phox and HO-1 expression levels than the control groups. Treatment with TXL (0.75 g/kg/day) markedly decreased p22phox, p47phox and HO-1 expression as evidenced by immunohistochemisty (Figure 3C) and RT-PCR (Figure 3D), without significant difference between TXL and atorvastatin (AT). These results indicated that TXL exerts its anti-oxidative effect through regulating the expression of HO-1 and NADPH oxidase subunits.
Figure 3.
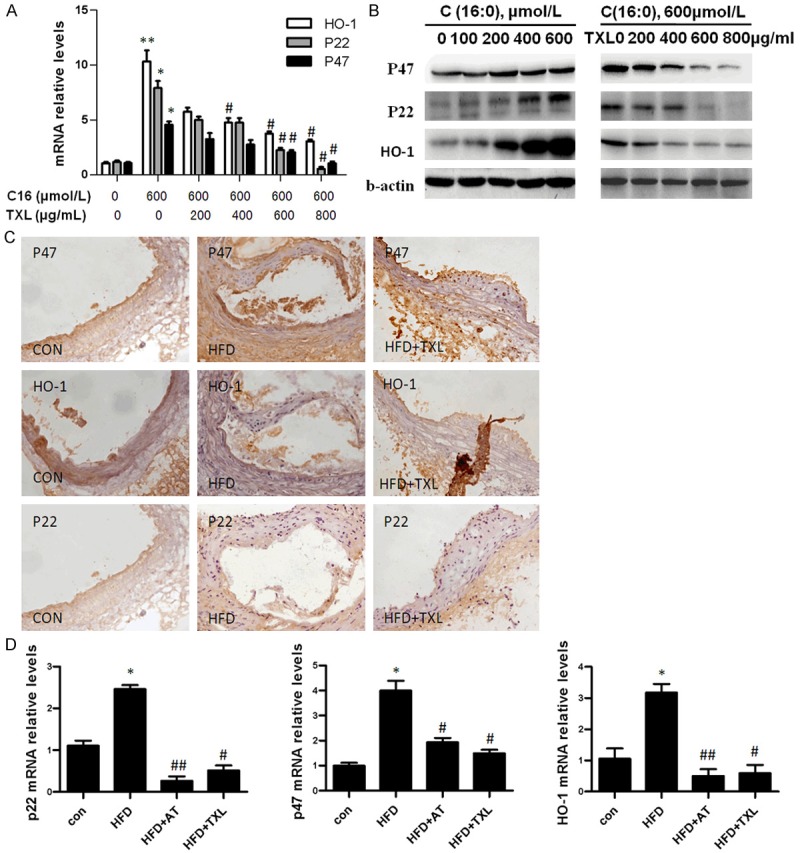
TXL inhibited expression of NADPH oxidase and HO-1. A. HCMECs were grown in six-well plates for 24 h, and then were pre-incubated with different doses of TXL before stimulating with 600 μmol/L of C16 for 24 h. The expression of p22phox, p47phox and HO-1 was detected by real-time PCR. *P < 0.05 and **P < 0.01 vs. C16-untreated group; #P < 0.05 vs. C16-treated group without TXL treatment. The bars represent the mean ± SEM. from 3 independent experiments. B. HCMECs were grown in six-well plates for 24 h, and then were stimulated with different doses of C16 for 12 h (left panel), or were pre-incubated with different doses of TXL before stimulating with 600 μmol/L of C16 for 24 h (right panel). The expression of p22phox, p47phox and HO-1 was detected by Western blotting. C. Immunohistochemical staining on sections of the thoracic artery with antibodies against p22phox, p47phox and HO-1. (× 20). D. ApoE-/- mice fed high-fat diet were orally administrated with TXL (0.75 g/kg/day). The expression of p22phox, p47phox and HO-1 in the thoracic artery was detected by real-time PCR. *P < 0.05 vs. control group; #P < 0.05 and ##P < 0.01 vs. HFD group (n = 5 in each group). The bars represent mean ± SEM.
TXL inhibited inflammation in C16-stimulated HCMECs
Except for oxidative stress, the inflammation is also involved in the atherogenesis. To detect the effect of TXL on inflammation, HCMECs were treated with C16 and the expression of IL-1â, TNFα and NF-κB was detected. As shown in Figure 4A, C16 treatment increased the level of proinflammatory cytokines IL-1β and TNFα in the cultured medium of HCMECs in a dose-dependent manner. Treatment with different doses of TXL dose-dependently decreased the release of IL-1β and TNFα to the medium, suggesting that TXL decreases the inflammation induced by C16 in HCMECs. Further, to gain mechanistic insight into how TXL modulates the expression of proinflammatory cytokines, NF-κB expression in C16-treated HCMECs was examined. We found that C16 increased, but TXL decreased NF-κB mRNA expression (Figure 4B) and protein expression (Figure 4C) in dose-dependent manner. Conversely, TXL increased the expression of eNOS in C16-treated HCMECs (Figure 4B), suggesting that TXL can improve HCMECs function. Atherosclerotic mice also showed that treatment with TXL and AT could decrease NF-κB mRNA expression (Figure 4D). These results make us believe that inhibitory effect of TXL on atherogenesis may be related to its anti-inflammation by decreasing the expression of NF-κB and proinflammatory cytokines in HCMECs.
Figure 4.
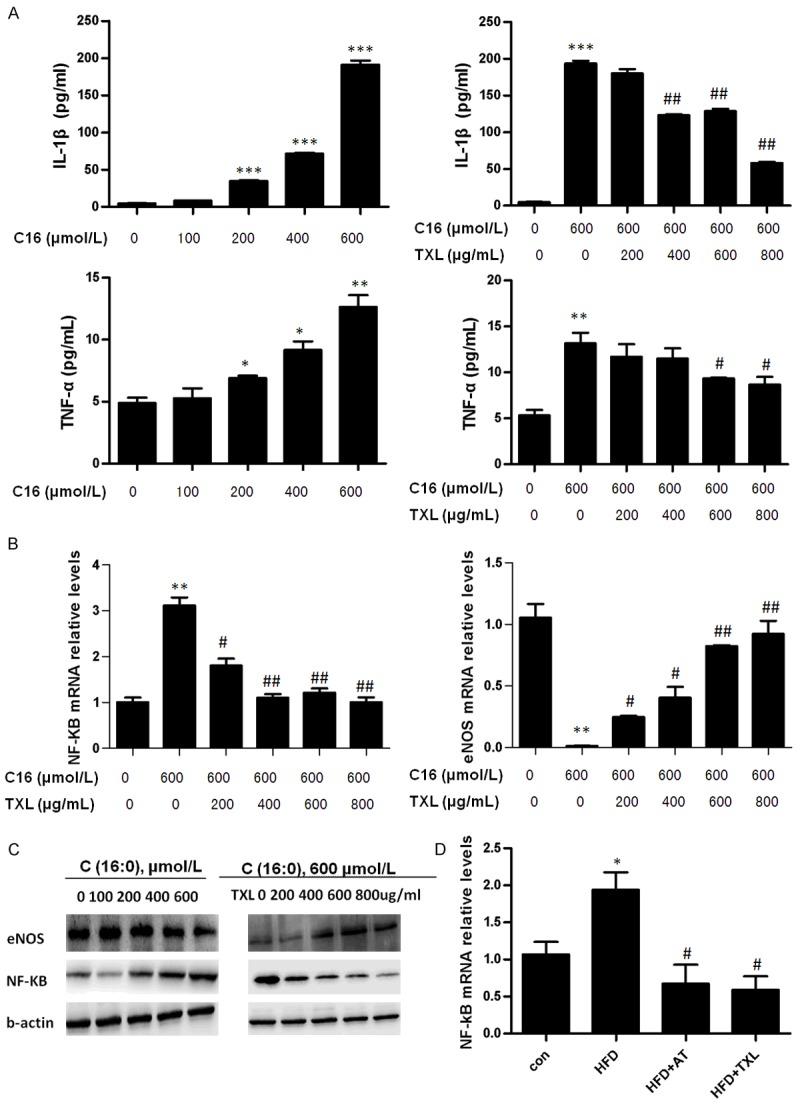
TXL inhibited inflammation in C16-treated HCMECs. A. HCMECs were grown in six-well plates for 24 h, and then were stimulated with different doses of C16 for 12 h, or were pre-incubated with different doses of TXL before treating with 600 μmol/L of C16. The cultured medium of cells was harvested and the content of IL-1β and TNFαwas examined by ELISA. B. HCMECs were grown in six-well plates for 24 h, and then were pre-incubated with different doses of TXL before stimulating with 600 μmol/L of C16 for 24 h. the expression of eNOS and NF-κB was detected by real-time PCR. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. C16-untreated group; #P < 0.05 and ##P < 0.01 vs. C16-treated group without TXL treatment. The bars represent the mean ± SEM. from 3 independent experiments. C. HCMECs were grown in six-well plates for 24 h, and then were stimulated with different doses of C16 for 12 h, or were pre-incubated with different doses of TXL before stimulating with 600 μmol/L of C16 for 24 h. The expression of eNOS and NF-κB was detected by Western blotting. D. ApoE-/- mice fed high-fat diet were orally administrated with TXL (0.75 g/kg/day). The expression of NF-κB in the thoracic artery was detected by real-time PCR. *P < 0.01 vs. control group; #P < 0.05 vs. HFD group (n = 5 in each group). The bars represent mean ± SEM.
TXL decreased inflammation oxidative stress by inhibiting the activation of NF-κB and p47 induced by C16
We and others have shown that NF-κB plays an important role in the regulation of the expression of multiple genes involved in the inflammatory responses [10,11]. To gain mechanistic insight into how TXL modulates inflammation in endothelial cells, HCMECs were pre-incubated with TXL and then treated with C16 for 1 h. As shown in Figure 5, the phosphorylation of p22phox, p47phox and NF-κB was significantly increased in HCMECs, following stimulation with 600 μmol/L of C16 for 1 h. TXL pre-treatment decreased C16-stimulated p47phox and NF-κB phosphorylation, but phosphorylation of p22phox was little changed by the TXL treatment, suggesting that TXL treatment leads to the suppression of p47phox and NF-κB activation by C16, subsequently reducing the inflammation and oxidative stress generation.
Figure 5.
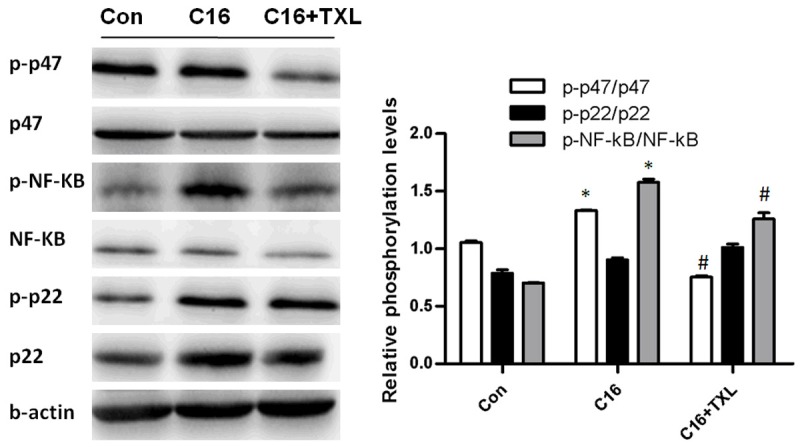
TXL inhibited inflammation and oxidative stress by inhibiting the activation of NF-κB and p47 by C16. HCMECs were grown in six-well plates for 24 h, and then were pre-incubated with TXL before stimulating with C16 for 1 h. The expression of p47, p-p47, p22, p-p22, NF-κB, and p-NF-κB was detected by Western blotting. *P < 0.05 vs. C16-untreated group; #P < 0.05 vs. C16-treated group without TXL treatment. The bars represent the mean ± SEM. from 3 independent experiments.
Discussion
TXL is a Chinese herbal compound formulated according to the meridian theory of a traditional Chinese medicine. A number of clinical and animal studies have showed that TXL has a variety of effects that are potentially of therapeutic value, such as improving endothelial cell function, lowering lipids, reducing inflammation, preventing apoptosis, and enhancing angiogenesis [9,12-15]. In this study, we evaluated the role of TXL in anti-oxidation and anti-inflammation in C16-induced endothelial cells and apoE-deficient mice. We found that the oxidative stress inducer C16 markedly up-regulated expression of NADPH oxidase subunits p22phox, p47phox and inflammatory factors TNFα, IL-1β and NF-κB, whereas the these protein expression levels were obviously decreased following pretreatment with TXL, indicating that TXL may play an important role in improving vascular endothelial function damaged by C16.
The present result showed that apoE-deficient mice treated with TXL decreased the markers of oxidative stress and inflammation, including MDA, T-SOD, IL-1β and TNF-α, suggesting that TXL may improve endothelium-dependent function and reduce oxidative stress and inflammation in the whole animal. Previous evidence indicates that TXL exerts its cellular effects by inhibiting phosphorylation of NF-κB and p47phox [16,17], thus preventing the activation of NF-κB and p47phox. In agreement, here we present in vivo evidence that TXL could suppress the activation of NF-κB and p47phox by C16 in endothelial cells. The results of the present study also provide insight into the mechanisms by which NF-κB inhibition by TXL improves vascular endothelial function and decreases formation of atherosclerotic lesion. In turn, NF-κB stimulates the production of reactive oxygen species at least in part via activation of NADPH oxidase [18]. As such, we reasoned that inhibition of NF-κB by TXL might improve endothelium-dependent function by reducing oxidative stress. In support of this, we found that ROS was reduced in C16-treated endothelial cells after TXL treatment, providing molecular evidence for reduced vascular endothelial oxidative stress after treating with TXL. It has been reported that p47phox is required for atherosclerotic lesion progression in apoE-deficient mice [19], suggesting that ROS production through NADPH oxidase activation plays an important role in the development and progression of atherosclerosis. In the present study, we demonstrated that TXL also reduced the p47phox expression and activation induced by C16, indicating that TXL exerts its anti-oxidation through inhibiting the p47phox. Together, the present functional and molecular data are consistent with the idea that oxidative stress induced by C16 is related to an upregulation of NF-κB activation, p47phox expression and oxidative stress generation in vivo and in vitro, and they, in turn, contribute to the impaired endothelial function. In addition, reactive oxygen species also can activate NF-κB. However, the present data do not provide insight into the direct relationship between these events. In addition to modulating redox signaling in the cell, NF-κB facilitates transcription of a large number of proinflammatory genes [20]. As a result, NF-κB also may suppress endothelial function via increased proinflammatory signaling. In the present study, TXL treatment decreased the expression of the proinflammatory cytokines TNFα and IL-1β via inhibiting NF-κB activation. Thus, although it is possible, we have no direct evidence that TXL improved endothelial function and reduced formation of atherosclerotic lesion through modulation of inflammatory signaling independent of oxidative stress signaling.
We believe that the present findings have important clinical relevance. Vascular endothelial dysfunction induced by oxidative stress is considered a central feature of clinical vascular disorders. Despite this, the cellular and molecular mechanisms that contribute to impaired endothelial function in humans are incompletely understood. The present results provide unique and direct in vivo evidence that supports a key role for the NF-κB and p47phox in mediating vascular endothelial dysfunction in humans and provide insight into the underlying mechanisms.
In summary, TXL decreases atherosclerotic plaque formation and improves endothelial cell function by inhibiting oxidative stress and inflammation in HCMECs. This finding provides a new molecular mechanism for the anti-atherosclerotic effect of TXL.
Acknowledgements
This study was supported by the National Basic Research Program of China (No. 2012CB518601), the National Natural Science Foundation of China (No. 31271396, No. 31271224).
Disclosure of conflict of interest
None.
References
- 1.Wood LG, Gibson PG, Garg ML. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur Respir J. 2003;21:177–186. doi: 10.1183/09031936.03.00017003a. [DOI] [PubMed] [Google Scholar]
- 2.Xia N, Daiber A, Habermeier A, Closs EI, Thum T, Spanier G, Lu Q, Oelze M, Torzewski M, Lackner KJ, Munzel T, Forstermann U, Li H. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J Pharmacol Exp Ther. 2010;335:149–154. doi: 10.1124/jpet.110.168724. [DOI] [PubMed] [Google Scholar]
- 3.Clempus RE, Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res. 2006;71:216–225. doi: 10.1016/j.cardiores.2006.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Touyz RM, Yao G, Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:981–987. doi: 10.1161/01.ATV.0000069236.27911.68. [DOI] [PubMed] [Google Scholar]
- 5.Zhang HB, Wen JK, Zhang J, Miao SB, Ma GY, Wang YY, Zheng B, Han M. Flavonoids from Inula britannica reduces oxidative stress through inhibiting expression and phosphorylation of p47(phox) in VSMCs. Pharm Biol. 2011;49:815–820. doi: 10.3109/13880209.2010.550055. [DOI] [PubMed] [Google Scholar]
- 6.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler Thromb Vasc Biol. 2000;20:2587–2592. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- 8.Li XD, Yang YJ, Geng YJ, Jin C, Hu FH, Zhao JL, Zhang HT, Cheng YT, Qian HY, Wang LL, Zhang BJ, Wu YL. Tongxinluo reduces myocardial no-reflow and ischemia-reperfusion injury by stimulating the phosphorylation of eNOS via the PKA pathway. Am J Physiol Heart Circ Physiol. 2010;299:H1255–1261. doi: 10.1152/ajpheart.00459.2010. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Liu Y, Lu XT, Wu YL, Zhang C, Ji XP, Wang R, Liu CX, Feng JB, Jiang H, Xu XS, Zhao YX, Zhang Y. Traditional Chinese medication Tongxinluo dose-dependently enhances stability of vulnerable plaques: a comparison with a high-dose simvastatin therapy. Am J Physiol Heart Circ Physiol. 2009;297:H2004–2014. doi: 10.1152/ajpheart.00208.2009. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, Han M, Wen JK. Acetylbritannilactone Inhibits Neointimal Hyperplasia after Balloon Injury of Rat Artery by Suppressing Nuclear Factor-{kappa}B Activation. Journal Pharmacol Exp Ther. 2008;324:292–298. doi: 10.1124/jpet.107.127407. [DOI] [PubMed] [Google Scholar]
- 11.Fan J, Frey RS, Rahman A, Malik AB. Role of neutrophil NADPH oxidase in the mechanism of tumor necrosis factor-alpha -induced NF-kappa B activation and intercellular adhesion molecule-1 expression in endothelial cells. J Biol Chem. 2002;277:3404–3411. doi: 10.1074/jbc.M110054200. [DOI] [PubMed] [Google Scholar]
- 12.Chang LP, Wei C, Jia ZH. [Effects of tongxinluo on angiogenesis and the volume of blood perfusion in ischemic stroke rats] . Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:1667–1670. [PubMed] [Google Scholar]
- 13.Li N, Yang YJ, Cui HH, Zhang Q, Jin C, Qian HY, Dong QT, Zhang H. Tongxinluo decreases apoptosis of mesenchymal stem cells concentration-dependently under hypoxia and serum deprivation conditions through the AMPK/eNOS pathway. J Cardiovasc Pharmacol. 2014;63:265–273. doi: 10.1097/FJC.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Wu Y, Jia Z, Zhang Y, Shen HY, Wang XL. Protective effects of a compound herbal extract (Tong Xin Luo) on free fatty acid induced endothelial injury: implications of antioxidant system. BMC Complement Altern Med. 2008;8:39. doi: 10.1186/1472-6882-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang RN, Zheng B, Li LM, Zhang J, Zhang XH, Wen JK. Tongxinluo inhibits vascular inflammation and neointimal hyperplasia through blocking positive feedback loop between miR-155 and TNF-alpha. Am J Physiol Heart Circ Physiol. 2014;307:H552–62. doi: 10.1152/ajpheart.00936.2013. [DOI] [PubMed] [Google Scholar]
- 16.Wu YL, You JH, Yuan GQ, Liang JQ, Jia ZH, Liu KJ, Wei C. [The effects of Tongxinluo Supermicro Powder on nuclear factor-kappaB, intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 expression in aorta of rabbits fed with high-lipid diet] . Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:271–274. [PubMed] [Google Scholar]
- 17.Bu PL, Zhao XQ, Wang LL, Zhao YX, Li CB, Zhang Y. Tong-xin-luo capsule inhibits left ventricular remodeling in spontaneously hypertensive rats by enhancing PPAR-gamma expression and suppressing NF-kappaB activity. Chin Med J (Engl) 2008;121:147–154. [PubMed] [Google Scholar]
- 18.Han W, Li H, Cai J, Gleaves LA, Polosukhin VV, Segal BH, Yull FE, Blackwell TS. NADPH oxidase limits lipopolysaccharide-induced lung inflammation and injury in mice through reduction-oxidation regulation of NF-kappaB activity. J Immunol. 2013;190:4786–4794. doi: 10.4049/jimmunol.1201809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(-/-) mice. J Clin Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]


