Abstract
Purpose: This study aimed to review the clinicopathological, histochemical, and prognostic features of Alpha-Fetoprotein (AFP) positive gastric cancer. Patients and methods: Six hundred and fifty one patients with gastric cancer who underwent gastrectomy between January 2009 and December 2012 at The First Hospital of Jilin University were enrolled in the study. Among them, 45 patients were identified as AFP positive gastric cancer. The clinicopathologic characteristics and prognosis of the AFP positive gastric cancer patients were analyzed. Results: Among the 45 AFP positive patients, serum levels of AFP were < 100 µg/L in nine patients. The histological classification of 45 patients was as follows: hepatoid type, 25 (55.6%) cases; fetal gastrointestinal type, 12 (26.7%) cases; yolk sac tumor type, 2 (4.4%) cases; and mixed type, 6 (13.3%) cases. Twenty nine (64.4%) cases were AFP positive by immunohistochemical analysis; we found no significant difference in AFP positivity and histologic type. However, the differences in the number of metastasis lymph nodes, the maximum tumor diameter, pathological stage, vascular invasion and liver metastasis between the AFP positive group and the negative group were significant. At the same T stage, the liver metastasis status of the AFP positive group was higher than that of the negative group. The AFP positive group had a much poorer prognosis than the negative group. Conclusion: AFP positive gastric cancer is associated with aggressive behavior and poorer prognosis compared to that of AFP negative gastric cancer.
Keywords: AFP, gastric cancer, prognosis
Introduction
Alpha-Fetoprotein (AFP) is produced mainly by babies’ liver and yolk sac, which has been generally used as a specific tumor marker for screening primary liver cancer and yolk sac tumor [1]. Other tumors in the human body also produce AFP, and the most common case is gastric cancer [2-8]. In 1970, Bourreille first reported one gastric cancer case with synchronous liver metastasis and increased serological AFP [9], which led to the concept of AFP positive gastric cancer. Further studies demonstrated that AFP positive gastric cancer has the characteristics of high stage, easy occurrence of liver metastasis, and poor prognosis [10-14]. Moreover, AFP positive gastric cancer has the characteristics of stronger proliferation, lower cell apoptosis and more neovascularization compared with AFP negative gastric cancer [15]. Motoyama et al. proposed a concept of histological type of AFP positive gastric cancer, and divided AFP positive gastric cancer into hepatoid type, fetal gastrointestinal type and yolk sac tumor type [16]. However, Li et al. proposed that AFP positive gastric cancer should be divided into four types: hepatoid type, fetal gastrointestinal type, yolk sac tumor type and mixed type [17]. The pathogenesis of AFP positive gastric cancer remains elusive; chemotherapy, biotherapy or interventional therapy cannot cure AFP positive gastric cancer and radical surgery is required.
Although the incidence of gastric cancer is high in northeast China, the data on AFP positive gastric cancer in this area are very limited. This study enrolled pathologically diagnosed primary gastric cancer patients who underwent surgery at the Department of Gastric and Colorectal Surgery at The First Hospital of Jilin University during the period of January 2009 to December 2012. We compared preoperative serological AFP examination, analyzed clinicopathologic characteristics and prognosis factors of AFP positive gastric cancer and investigated the relationship between histological type and clinical prognosis.
Patients and methods
Patients
Six hundred and fifty one patients were enrolled who were pathologically diagnosed as primary gastric cancer and underwent preoperative serological AFP examination at the Department of Gastric and Colorectal Surgery at The First Hospital of Jilin University from January 2009 to December 2012. The selection criteria were as follows: (1) pathologically diagnosed as primary gastric cancer; (2) underwent radical surgery for gastric cancer or palliative gastric cancer resection; (3) underwent preoperative serological AFP examination; and (4) had detailed contact information and complete clinical data. The exclusion criteria were as follows: (1) had liver disease, such as acute or chronic hepatitis, cirrhosis, fatty liver or alcoholic liver, or had medical history of these diseases; and (2) had yolk sac tumor, teratoma or primary liver cancer. 17 patients with hepatitis or cirrhosis were excluded according to the exclusion criteria, and the 634 remaining patients were selected. 45 out of the 634 patients with increased serum AFP levels were included in the AFP positive group and 589 patients with normal AFP levels were included in the AFP negative group. The clinicopathologic data of 634 patients were collected, including age, gender, preoperative serum AFP level, surgery, resection method, lymph node dissection, position of tumor, size of tumor, invasion depth, histological type, differentiation degree, vessel cancer embolus, nerve invasion, lymph mode metastasis, liver metastasis and tumor stage.
Immunohistochemical staining
Hematoxylin and eosin (HE) stained sections retrieved from the AFP positive group were rechecked by the pathologists. The paraffin blocks of the tissue specimen were selected and cut into serial section of 5 um for HE and immunohistochemical staining. For HE staining, the sections were bathed in water after being dewaxed, and then stained in hematoxylin at ambient temperature for 1 min, differentiated in 1% hydrochloric acid alcohol for three seconds, stained again in eosin at ambient temperature for five seconds, dehydrated gradually to be transparent and sealed by neutral gum. Based on HE staining, the samples were classified into four histological types: hepatoid type, fetal gastrointestinal type, yolk sac tumor type or mixed type.
For AFP immunohistochemical staining, serial sections were dewaxed and washed by PBS three times, then incubated in deionized water containing 3% H2O2 for 10 min to eliminate endogenous peroxidase activity, washed by distilled water and immersed in PBS for 5 min. Next, the sections were subjected to antigen retrieval and incubated in goat serum at ambient temperature for 15 min to block heterogenetic antigen. The sections were incubated with primary antigen overnight at 4°C, washed again by PBS three times and incubated with biotin secondary antigen at 37°C for 15 min, then washed by PBS three times. Afterwards, the sections were stained by diaminobenzidine (DAB) and washed sufficiently by tap-water, stained again by hematoxylin, dehydrated and sealed. PBS buffer was substituted for primary antibody as a negative control.
Evaluation of immunohistochemical staining
The staining was evaluated by two pathologists, based on the percentage of the stained cells and staining density. The grades for the percentage of stained cells were marked from 0 to 4 (0 for unstained cells, 1 for 1-10% stained cells, 2 for 11-50% stained cells, 3 for 51-80% stained cells, and 4 for 81-100% stained cells). The grades for staining density were marked from 0 to 3 (0 for unstained cells, 1 for slightly stained cells, 2 for medially stained cells, and 3 for highly stained cells). The two sets of data were multiplied, resulting in the results of immunohistochemical staining: 0 points: -; 1-4 points: +; 5-8 points: ++; and 9-12 points: +++.
Statistical analysis
SPSS 20.0 software was used for statistical analysis. The Chi-square Test and Fisher Probabilistic Methods were used for correlation tests, Kaplan-Meier was employed for survival curve analysis, log-rank was used for significance testing, and the COX method was employed for the analysis of independent risk factors associated with survival. P<0.05 was considered statistically significant.
Results
Serum AFP level of gastric cancer patients
Among 634 patients who underwent a preoperative serological AFP test, 45 patients had increased serum AFP levels, while 589 patients had a normal range of serum AFP level. The average AFP level was 2.758 µg/L (range: 0.61 to 6.98, standard error: 0.053) in the AFP negative group, but was 118.263 µg/L (range: 7 to 1210, standard error: 40.367) in the AFP positive group. Serological AFP positive patients were divided into two groups according to their AFP values: < 100 µg/L (n=36) and ≥ 100 µg/L (n=9). 80% of patients had a serum AFP level < 100 µg/L.
Histological types of serological AFP positive gastric cancer
According to the histological classification standards of AFP positive gastric cancer, serological AFP positive patients were divided as follows: hepatoid type, 25 cases (55.6%); fetal gastrointestinal type, 12 cases (26.7%); yolk sac tumor type, 2 cases (4.4%); and mixed type, 6 cases (13.3%) (Figure 1).
Figure 1.
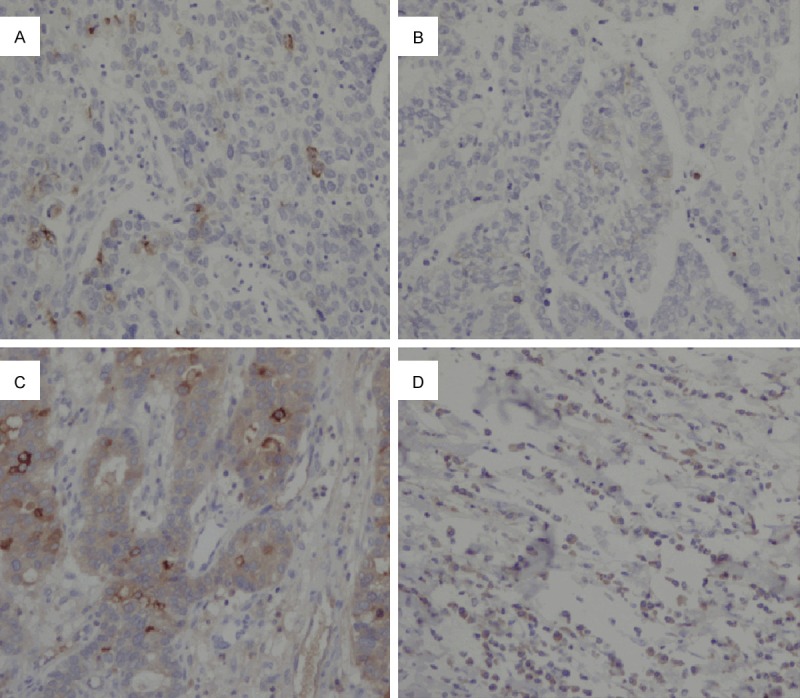
Histological types of AFP positive gastric cancer: A. Hepatoid type; B. Fetal gastrointestinal type; C. Yolk sac tumor-like type; D. Mixed type.
Immunohistochemical staining of AFP in serological AFP positive patients
For the 45 preoperative serological AFP positive patients, immunohistochemical staining showed that 29 cases were immunohistochemically positive; the immunohistochemical positive ratio was 64.4%. AFP had different expression levels in the primary lesion and metastatic lymph nodes of AFP positive gastric cancer patients. The cases could be divided into four groups according to AFP staining: - (16 cases), + (14 cases), ++ (7 cases), and +++ (8 cases) (Figure 2). No significant difference in AFP positivity or histologic type was observed (Table 1).
Figure 2.
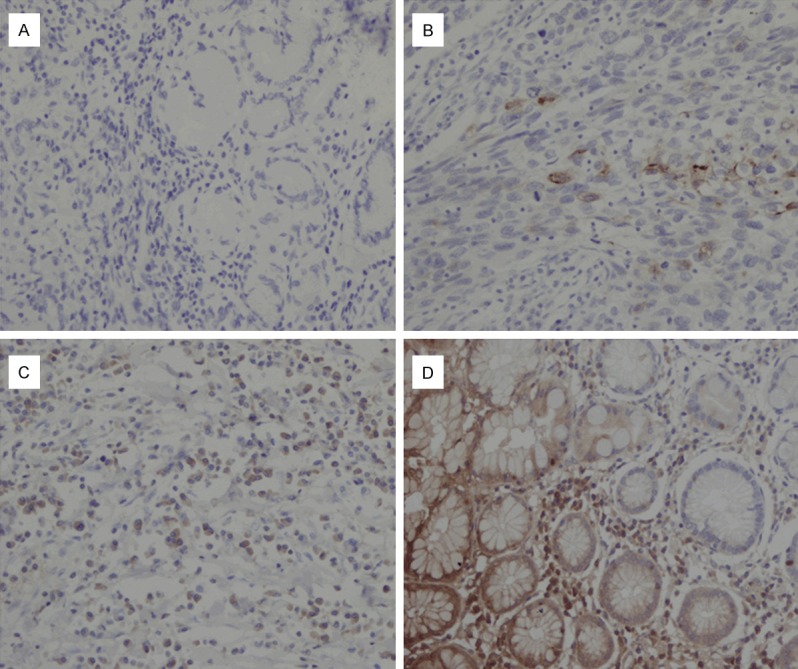
AFP positive cases were divided into four different groups according to the immune reactivity for AFP staining. A. -; B. +; C. ++; D. +++.
Table 1.
Correlation between AFP immunoreactivity and histologic type of gastric cancer
| Immunoreactivity | Histologic types (n=45) | p-value | |||
|---|---|---|---|---|---|
|
| |||||
| Hepatoid type (n=25) | Fetal gastrointestinal type (n=12) | Yolk sac tumor-like type (n=2) | Mixed type (n=6) | ||
| - | 8 | 7 | 0 | 1 | 0.386 |
| + | 10 | 2 | 0 | 2 | |
| ++ | 4 | 1 | 1 | 1 | |
| +++ | 3 | 2 | 1 | 2 | |
Comparison of clinicopathologic characteristics between AFP positive and negative groups
Comparison of clinicopathologic characteristics between AFP positive and negative groups showed that there were no significant differences in gender, age distribution, tumor location, degree of differentiation, T stage, N stage, perineural invasion, P53 or Ki-67 expression between the two groups. However, there were significant differences in the number of metastasis lymph nodes, the maximum tumor diameter, pathological stage, vascular invasion and liver metastasis between the AFP positive and negative groups (Table 2).
Table 2.
Comparison of clinicopathologic characteristics between AFP positive and negative groups
| Variables | AFP positive (n=45) | AFP negative (n=589) | P-value |
|---|---|---|---|
| Gender | 0.767 | ||
| Male | 31 | 418 | |
| Female | 14 | 171 | |
| Age (years) | 0.845 | ||
| ≥60 | 21 | 266 | |
| <60 | 24 | 323 | |
| Tumor location | 0.384 | ||
| Upper third | 4 | 86 | |
| Middle third | 12 | 150 | |
| Lower third | 27 | 344 | |
| Two-thirds or more | 2 | 9 | |
| Histologic differentiation | 0.486 | ||
| Moderate | 9 | 145 | |
| Poor | 36 | 444 | |
| Depth of tumor invasion | 0.168 | ||
| T1 | 3 | 119 | |
| T2 | 5 | 63 | |
| T3 | 28 | 303 | |
| T4 | 9 | 104 | |
| N status | 0.126 | ||
| N0 | 11 | 214 | |
| N1 | 12 | 183 | |
| N2 | 11 | 109 | |
| N3 | 11 | 83 | |
| No. of lymph node metastases | 10.62±1.937 | 6.49±0.389 | 0.042 |
| TNM stage | 0.030 | ||
| I | 5 | 149 | |
| II | 7 | 92 | |
| III | 13 | 195 | |
| IV | 20 | 153 | |
| Tumor size (cm) | 5.68±0.40 | 4.38±0.11 | 0.001 |
| Vascular invasion | 0.032 | ||
| No | 8 | 196 | |
| Yes | 37 | 393 | |
| Nerve invasion | 0.295 | ||
| No | 17 | 270 | |
| Yes | 28 | 319 | |
| P53 (%) | 46.22±5.46 | 43.15±1.48 | 0.581 |
| Ki-67 (%) | 66.44±3.41 | 67.07±0.91 | 0.855 |
| Liver metastasis | 0.000 | ||
| No | 19 | 567 | |
| Yes | 26 | 22 |
+/-Represents standard deviation.
Relationship between AFP level and liver and lymph node metastasis
In the same T stage, the frequency of liver metastasis in the AFP positive group was significantly higher than that of the AFP negative group. In the T1 stage, the frequency of lymph node metastasis in the AFP positive group was significantly higher than that of the AFP negative group; while in the T2-T4 stages, the frequency of lymph node metastasis showed no obvious difference between the AFP positive and negative groups (Tables 3 and 4).
Table 3.
Relationship between AFP levels and liver metastasis status
| Liver metastasis | AFP positive (n=45) | AFP negative (n=589) | P-value |
|---|---|---|---|
| T1 | 0.000 | ||
| Yes | 1 | 1 | |
| No | 2 | 118 | |
| T2 | 0.000 | ||
| Yes | 2 | 0 | |
| No | 3 | 63 | |
| T3 | 0.000 | ||
| Yes | 14 | 18 | |
| No | 13 | 285 | |
| T4 | 0.000 | ||
| Yes | 9 | 3 | |
| No | 1 | 101 |
Table 4.
Relationship between AFP levels and lymph node metastasis status
| Lymph node metastasis | AFP positive (n=45) | AFP negative (n=589) | P-value |
|---|---|---|---|
| T1 | 0.010 | ||
| Yes | 3 | 24 | |
| No | 0 | 95 | |
| T2 | 0.180 | ||
| Yes | 4 | 28 | |
| No | 1 | 35 | |
| T3 | 0.817 | ||
| Yes | 21 | 233 | |
| No | 7 | 70 | |
| T4 | 0.169 | ||
| Yes | 7 | 90 | |
| No | 3 | 14 |
Survival analysis
Compared with the AFP negative group, the cases in the AFP positive group were more liable to develop liver metastasis, but both groups showed no significant differences in peritoneal metastasis and local recurrence (Table 5).
Table 5.
Comparison of the recurrence between AFP positive group and negative group
| AFP positive (n=45) | AFP negative (n=589) | P value | |
|---|---|---|---|
| Liver metastasis | 0.000 | ||
| Yes | 26 | 22 | |
| No | 19 | 567 | |
| Peritoneal metastasis | 0.719 | ||
| Yes | 3 | 29 | |
| No | 42 | 560 | |
| LR recurrence | 0.234 | ||
| Yes | 2 | 11 | |
| No | 43 | 578 |
Univariate analysis showed that the size of tumor, TNM (T: tumor; N: node; M: metastasis) stage, vascular invasion, and liver metastasis were the risk factors for poor prognosis. Kaplan-Meier survival curves indicated that the prognosis of the AFP positive group was much poorer than that of the negative group (Figure 3). Multivariate Cox regression analysis showed that age, AFP level and TNM stage were independent risk factors for prognosis (Table 6).
Figure 3.
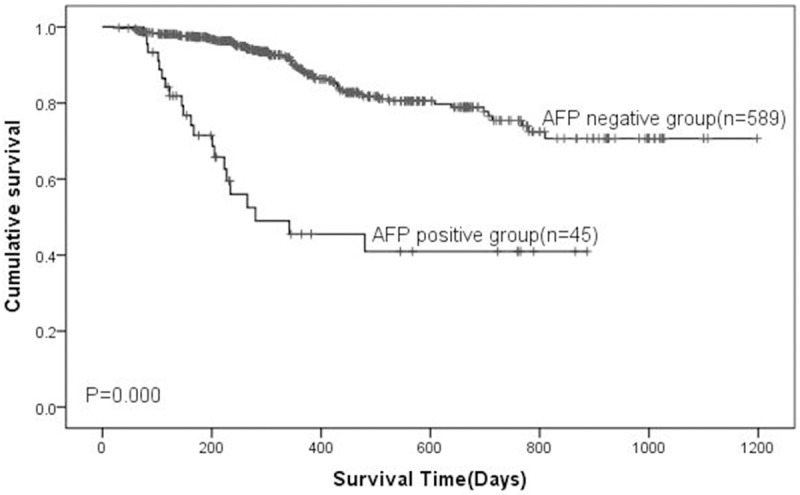
Kaplan-Miere survival analysis of patients with gastric cancer. The overall survival of the patients was determined based on preoperative serum AFP levels. P-values were determined by log-rank test.
Table 6.
Independent predictors of survival based on the multivariate analysis
| Variables | HR | 95% CI | P value | |
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| AFP | 19.186 | 1.950 | 5.751 | 0.000 |
| TNM | 13.315 | 1.406 | 3.101 | 0.000 |
| Age | 5.366 | 1.084 | 2.631 | 0.021 |
| Gender | 1.765 | 0.412 | 1.186 | 0.184 |
| Differentiation | 3.191 | 0.941 | 3.710 | 0.074 |
| Location | 0.053 | 0.777 | 1.375 | 0.818 |
| Tumor size | 3.044 | 0.992 | 1.154 | 0.081 |
| Vascular invasion | 0.666 | 0.642 | 3.142 | 0.414 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
The median survival time of all postoperative AFP positive cases was 484 days. Kaplan-Meier survival curves indicated that there was no obvious difference in survival time among the subtypes after subtypes were separated (Figure 4), but survival time showed a significant difference between the AFP positive and negative groups according to AFP immunohistochemical staining (Figure 5).
Figure 4.
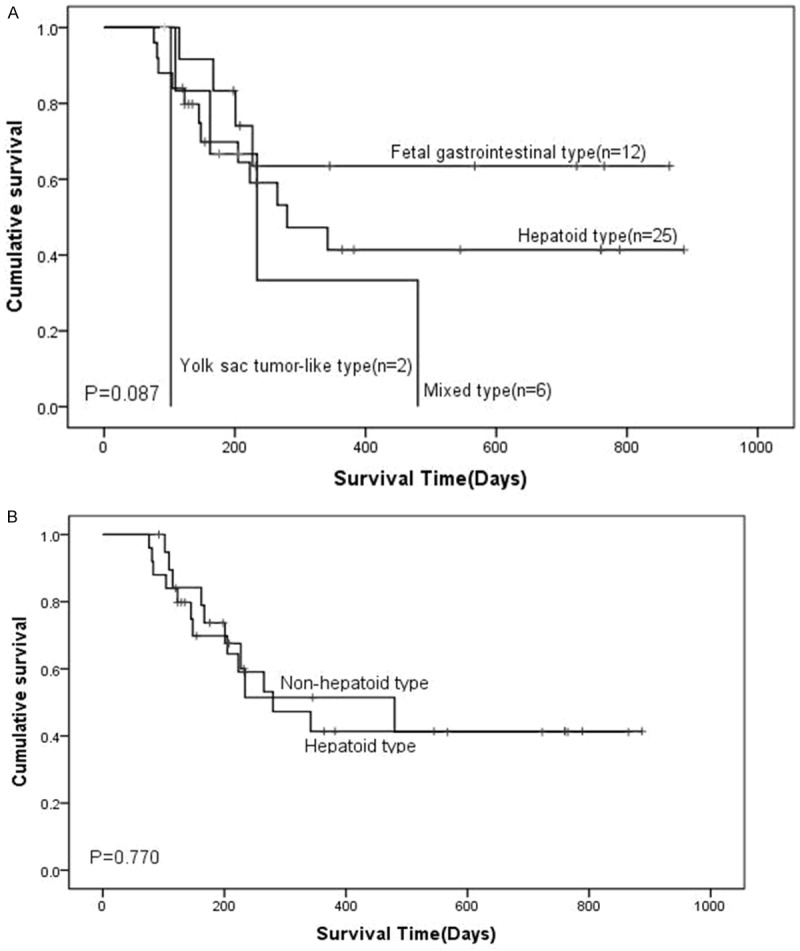
Kaplan-Miere survival analysis of patients with gastric cancer. A. The overall survival of the patients was determined based on four different histological types. B. The overall survival of gastric cancer patients with hepatoid type and non-hepatoid type. P-values were determined by log-rank test.
Figure 5.
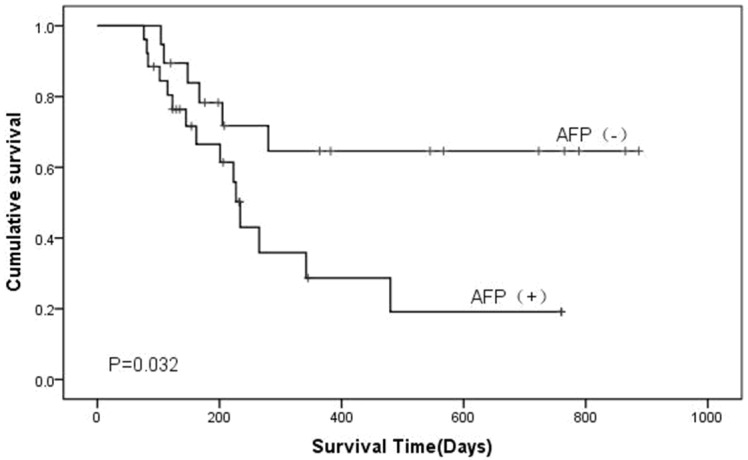
Kaplan-Miere survival analysis of patients with gastric cancer. The overall survival of the patients was determined based on AFP staining. P-values were determined by log-rank test.
Discussion
AFP has been proposed as a tumor marker for screening liver tumor and yolk sac tumor in the clinic [18,19]. 70-95% of primary liver cancer cases may be associated with increased serum AFP level. The incidence of AFP positive gastric cancer is 1.3-15% worldwide [20]. In this study, there were 45 serological positive gastric cancer cases in the 634 cases studied; the incidence of AFP positive gastric cancer was 7.1%. However, only 29 cases were AFP immunohistochemically positive, and the incidence of AFP positive gastric cancer was 4.6% according to immunohistochemical analysis. The incidence we reported was higher than the 2.3% incidence reported by Fudan University Shanghai Cancer Center [21]. This difference may be due to regional distribution. For the diagnosis of AFP positive gastric cancer, immunohistochemical staining is necessary. Although there were AFP positive gastric cancer cases with negative serological and positive immunohistochemistry staining [22], the cases were very few, indicating the importance of histological analysis in the diagnosis of gastric cancer.
By screening preoperative serum AFP levels from 634 cases, we found 45 cases with increased serum AFP levels. The average serum AFP level was 118.263 µg/L for the AFP positive cases, which was significantly higher than the average 2.758 µg/L for the AFP negative cases. Moreover, among 45 gastric cancer cases with increased AFP serum levels, 29 cases presented positive immunohistochemistry staining of AFP. This immunohistochemistry positive rate of 64.4% is lower than reported previously [21]. The AFP positive group was divided into four groups: -, 16 cases; +, 14 cases; ++, 7 cases; and +++, 8 cases. Statistical analysis indicated that serum AFP level was not associated with AFP immumohistochemical staining, in agreement with the results obtained from Fudan University [21].
Motoyama et al. [16] reported that AFP positive gastric cancer could be divided into hepatoid type, yolk sac tumor type and fetal gastrointestinal type, of which hepatoid type was the most common histological type in AFP positive gastric cancer. Li et al. [17] added another histological type of AFP positive gastric cancer, mixed type. In this study, we classified AFP positive gastric cancer as follows: hepatoid type, 25 cases (55.6%); fetal gastrointestinal type, 12 cases (26.7%); yolk sac tumor type, 2 cases (4.4%); and mixed type, 6 cases 1 (3.3%). Our results are similar to those reported by Li et al [17]. In the 45 cases of AFP positive gastric cancer, 25 cases were hepatoid type, which was the most common type and is consistent with the findings of Motoyama et al [16]. We also analyzed the correlation between AFP immunohistochemical staining and histological subtype. Seventeen cases of positive immunohistochemical staining were of the hepatoid type, with a positive rate was 68%; five cases of positive immunohistochemical staining were of the fetal gastrointestinal type, with a positive rate was 41.7%; two cases of positive immunohistochemical staining were of the yolk sac tumor type, with a positive rate was 100%; and five cases of positive immunohistochemical staining were of the mixed type, with a positive rate was 83.3%. Based on these data, the yolk sac tumor type had the highest AFP immunohistochemical positive rate, followed by mixed type, hepatoid type and fetal gastrointestinal type. Comparison of the hepatoid type and fetal gastrointestinal type showed that the hepatoid type had a higher AFP immunohistochemical positive rate, which indicates that the hepatoid type of AFP positive gastric cancer has a higher tendency of producing AFP.
Next, we analyzed the correlation of histological subtype with liver metastasis. The results showed that the hepatoid type was prone to liver metastasis and had a higher rate of preoperative liver metastasis. Survival analysis of gastric cancer with histological subtypes showed that the kinds of histological subtype had no significant effect on the survival of AFP positive gastric cancer, suggesting that histological subtype is not a risk factor for postoperative survival time of AFP positive gastric cancer patients.
For the clinicopathologic characteristics of the AFP positive and negative groups, the male to female ratio was 2.2 in the positive group and 2.4 in the negative group, and the ratio of patients over 60 years old to those under 60 years old was 46.7% in the positive group and 45.2% in the negative group. 60% of tumors were located in gastric antrum in the positive group and 58% tumors in the negative group, which demonstrated that the gastric antrum was the predilection site of gastric cancer. The two groups had no significant differences in the degree of differentiation or T stage. The AFP positive group exhibited much higher lymph node metastasis than the negative group, and the difference was statistically significant.
AFP positive gastric cancer had the characteristics of later staging and high malignant degree, and the prognosis of AFP positive gastric cancer was worse than ordinary gastric cancer. Adachi et al. [23] found that the T3 stage accounted for 20% and the T4 stage accounted for 55% of the 270 patients, similar to the results in this study. The average maximum tumor diameter was 5.68 cm in the AFP positive group, which was much larger than that in the AFP negative group (4.38 cm). These data are in contrast to an early report that the difference in maximum tumor diameter between AFP positive and negative groups exhibited no statistical difference [24]. The AFP positive group also had much higher vascular invasion than the negative group. Consistent with our results, Chun et al. [24] showed that AFP positive gastric cancer cases had the characteristics of high occurrence of liver metastasis, poor five-year survival and short median survival time. Chang et al. [25] reported that in early AFP positive gastric cancer, the occurrence of liver metastasis as well as vessel and lymphatic invasion was much higher than that of early AFP negative gastric cancer. Liu et al. [21] showed that AFP positive gastric cancer was prone to lymph node metastasis and vascular invasion. Kamei et al. showed that the expression of vascular endothelial growth factor (VEGF) was much higher in AFP positive gastric cancer than in AFP negative gastric cancer [26]. This may explain why AFP positive gastric cancer is prone to vascular and lymphatic invasion.
Comparison of liver metastasis and lymph node metastasis in the same T stage of AFP positive and negative groups proved that, in the same T stage, the occurrence of liver metastasis in the AFP positive group was much higher than that in the negative group. For AFP positive gastric cancer in the T1 stage, the liver metastasis rate was 33.3%, which showed that there is a great risk of liver metastasis even for early AFP positive gastric cancer. In the T1 stage, the occurrence of lymph node metastasis was 100% in the AFP positive group, much higher than the rate of 20% in the negative group. In the T2-T4 stages, the occurrence of lymph node metastasis showed no difference between the AFP positive and negative groups, which may indicate that along with the increase in T stage, the chance of lymph node metastasis increases.
Adachi et al. [23] found that in 270 AFP positive gastric cancer cases, the 5-year survival rate was 22% and the median survival was 14 months. Nagai et al. [27] reported that the 5-year survival rate of AFP positive gastric cancer was only 11.9%. Liu et al. [21] showed that 1-year, 3-year, and 5-year survival rates were 53%, 35% and 28%, respectively. In this study we found that the 1-year, 2-year and 3-year survival rates in the AFP positive group were lower than those in the AFP negative group. Survival curve analysis indicated that the prognosis of the AFP positive group was poorer than that of the AFP negative group. COX regression analysis showed that serological AFP, the size of tumor, TNM stage, vascular invasion and liver metastasis were the risk factors for poor prognosis. Furthermore, multivariate COX multiple-factor analysis showed that AFP level and TNM stage were independent risk factors of poor prognosis.
In conclusion, AFP positive gastric cancer is associated with aggressive behavior and poorer prognosis compared to AFP negative gastric cancer. AFP positive gastric cancer has the characteristics of stronger aggressive biological activity, later staging, more vascular invasion, lymph node metastasis and liver metastasis, short survival time and poor prognosis. This information may provide important guidance on strategies for the therapy and prognosis of AFP positive gastric cancer.
Acknowledgements
This study was supported and funded by the Departments of Gastrointestinal Surgery and Departments of Pathology of the First Hospital of Jilin University.
Disclosure of conflict of interest
None.
References
- 1.Bergstrand CG, Czar D. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest. 1956;8:174. doi: 10.3109/00365515609049266. [DOI] [PubMed] [Google Scholar]
- 2.Isonishi S, Ogura A, Kiyokawa T, Suzuki M, Kunito S, Hirama M, Tachibana T, Ochiai K, Tanaka T. Alpha-fetoprotein (AFP)-producing ovarian tumor in an elderly woman. Int J Clin Oncol. 2009;14:70–73. doi: 10.1007/s10147-008-0800-4. [DOI] [PubMed] [Google Scholar]
- 3.Hamanaka W, Yoneda S, Shirakusa T, Shirahama H, Tashiro Y, Iwasaki A, Shiraishi T, Tsuru H. Alpha-fetoprotein (AFP)-producing adrenocortical carcinoma-long survival with various therapeutic strategies including a lung resection: Report of a case. Surg Today. 2008;38:275–278. doi: 10.1007/s00595-007-3610-9. [DOI] [PubMed] [Google Scholar]
- 4.Yamagata T, Yamagata Y, Nakanishi M, Shirahama H, Tashiro Y, Iwasaki A, Shiraishi T, Tsuru H. A case of primary lung cancer producing alpha-fetoprotein. Can Respir J. 2004;11:504–506. doi: 10.1155/2004/510350. [DOI] [PubMed] [Google Scholar]
- 5.Ueno M, Nakashima J, Ohigashi T, Deguchi N, Ban S, Akita M, Murai M. Establishment of a testicular carcinoma cell line producing alpha-fetoprotein. BJU Int. 2001;88:611–621. doi: 10.1046/j.1464-410x.2001.02357.x. [DOI] [PubMed] [Google Scholar]
- 6.Saito S, Hatano T, Hayakawa M, Koyama Y, Ohsawa A, Iwamasa T. Studies on alphafetoprotein produced by renal cell carcinoma. Cancer. 1989;63:544–549. doi: 10.1002/1097-0142(19890201)63:3<544::aid-cncr2820630324>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Hocking GR, Shembrey M, Hay D, Ostör AG. Alpha-fetoproteinproducing adenocarcinoma of the sigmoid colon with possible hepatoid differentiation. Pathology. 1995;27:277–279. doi: 10.1080/00313029500169113. [DOI] [PubMed] [Google Scholar]
- 8.Kumar Dhar D, Kubota H, Tabara H, Kotoh T, Monden N, Igarashi M, Kohno H, Nagasue N. nm23 in the primary and metastatic sites of gastric carcinoma. Relation to AFPproducing carcinoma. Oncology. 1999;56:122–128. doi: 10.1159/000011952. [DOI] [PubMed] [Google Scholar]
- 9.Bourreille J, Metayer P, Sauger F, Matray F, Fondimare A. Existence d’alphafoetoproteine au cours d’un cancer secondaire du foie d’origine gastrique. Presse Med. 1970;78:1277–1278. [PubMed] [Google Scholar]
- 10.Kono K, Amemiya H, Sekikawa T, Iizuka H, Takahashi A, Fujii H, Matsumoto Y. Clinicopahtologic features of gastric cancers producing alpha-fetoprotein. Dig Surg. 2002;19:359–365. doi: 10.1159/000065838. [DOI] [PubMed] [Google Scholar]
- 11.Chang YC, Nagasue N, Kohno H, Taniura H, Uchida M, Yamanoi A, Kimoto T, Nakamura T. Clinicopathologic features and long term results of alpha-fetoprotein-producing gastric cancer. Am J Gastroenterol. 1990;85:1480–1485. [PubMed] [Google Scholar]
- 12.Chang YC, Nagasue N, Abe S, Kohno H, Kumar DD, Nakamura T. Alpha fetoprotein producing early gastric cancer with liver metastasis: Report of three cases. Gut. 1991;32:542–545. doi: 10.1136/gut.32.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsurumachi T, Yamamoto H, Watanae K, Honda I, Watanabe S, Yamada S, Jingu K, Satomi D, Fujita M. Resection of liver metastasis from alpha-fetoprotein-producing early gastric cancer: Report a case. Surg Today. 1997;27:563–566. doi: 10.1007/BF02385813. [DOI] [PubMed] [Google Scholar]
- 14.Shibata Y, Sato K, Kodama M, Nanjyo H. Alpha-fetoproteinproducing early gastric cancer of the remnant stomach: Report of a case. Surg Today. 2007;37:995–999. doi: 10.1007/s00595-007-3501-0. [DOI] [PubMed] [Google Scholar]
- 15.Koide N, Nishio A, Igarashi J, Kajikawa S, Adachi W, Amano J. Alpha-fetoprotein-producing gastric cancer: Histochemical analysis of cell proliferation, apoptosis and angiogenesis. Am J Gastroenterol. 1999;94:1658–1663. doi: 10.1111/j.1572-0241.1999.01158.x. [DOI] [PubMed] [Google Scholar]
- 16.Motoyama T, Aizawa K, Watanabe H, Fukase M, Saito K. α-Fetoprotein-producing gastric carcinomas: a comparative study of three different subtypes. Acta Pathol Jpn. 1993;43:654–61. doi: 10.1111/j.1440-1827.1993.tb02549.x. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Shi F, Le M, Zhang T, Wang C, Lai R, Yang H. A study of histopathology and classification on AFP-positive gastric carcinoma. Chin J Clin Exp Pathol. 1999;15:293–296. [Google Scholar]
- 18.O’Conor GT, Tatarinov YS, Abelev Gl, Uriel J. A collaborative study for the evaluation of a serologic test for Primary liver cancer. Cancer. 1970;25:1091–1098. doi: 10.1002/1097-0142(197005)25:5<1091::aid-cncr2820250514>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 19.Motoyama T, Watanabe H, Yamamoto T, Sekiguchi M. Production of alpha-fetoprotein by human germ cell tumors in vivo and in vitro. Acta Pathol JPn. 1987;37:1263–1277. doi: 10.1111/j.1440-1827.1987.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 20.McIntiere KR, Waldman TA, Moertel CG, Go VL. Serum alpha-fetoprotein in patients with neoplasms of the gastrointestinal tract. Cancer Res. 1975;35:991–996. [PubMed] [Google Scholar]
- 21.Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long Z, Zhu H, Wang Y. Clinico-pathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol. 2010;102:249–255. doi: 10.1002/jso.21624. [DOI] [PubMed] [Google Scholar]
- 22.Hirasaki S, Tanimizu M, Tsuzuki T, Tsubouchi E, Hidaka S, Hyodo I, Tajiri H. Seronegative alpha-fetoprotein-producing early gastric cancer treated with endoscopic mucosal resection and additional surgery. Intern Med. 2004;4:926–30. doi: 10.2169/internalmedicine.43.926. [DOI] [PubMed] [Google Scholar]
- 23.Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S. AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology. 2003;65:95–101. doi: 10.1159/000072332. [DOI] [PubMed] [Google Scholar]
- 24.Chun H, Kwon SJ. Clinicopathological characteristics of Alpha-Fetoprotein-producing gastric cancer. J Gastric Cancer. 2011;11:23–30. doi: 10.5230/jgc.2011.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang YC, Nagasue N, Abe S, Kohno H, Yamanoi A, Uchida M, Nakamura T. The characters of AFP-producing early gastric cancer. Nihon Geka Gakkai Zasshi. 1990;91:1574–80. [PubMed] [Google Scholar]
- 26.Kamei S, Kono K, Amemiya H, Takahashi A, Sugai H, Ichihara F, Fujii H, Matsumoto Y. Evaluation of VEGF and VEGF-C expression in gastric cancer cells producing alpha-fetoprotein. J Gastroenterol. 2003;38:540–7. doi: 10.1007/s00535-002-1099-y. [DOI] [PubMed] [Google Scholar]
- 27.Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. Cancer. 1993;72:1827–35. doi: 10.1002/1097-0142(19930915)72:6<1827::aid-cncr2820720606>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]


