Abstract
Background: In this study, we will establish a stable and optimized rat model that can meet strictly diagnosed criteria and serve as a tool to investigate the potential of novel therapeutics in this preclinical model through comparative analysis of systemic alterations, levels of pro-inflammatory cytokines in serum and infiltrated numbers of inflammatory cells in distant organ between 30% and 50% TBSA with a full-thickness burn. Materials and methods: The adult male Wistar rats were randomly divided into the following groups: control group, 30% TBSA with a full-thickness burn group, and 50% TBSA with a full-thickness burn group. The blood and serum samples in the 3 groups were collected and detected by blood routine examination and biochemical detection at 6 h, 12 h, 24 h and 48 h post burn. The levels of TNF-α, IL-1β and IL-6 in serum were detected by ELISA. The sections of lung, renal, liver and heart were analyzed by H&E and immunohistochemical staining detection. Results: Our results showed that temperature in 50% TBSA with a full-thickness burn group was always hypothermia, and lower than 36°C at defined timepoints post burn, that was in 30% TBSA with a full-thickness burn group was lower than 36°C only at 48 h post burn. The levels of TNF-α, IL-1β and IL-6 were significantly increased in 30% and 50% groups at 6 h, 12 h, 24 h and 48 h post burn. The apoptosis in distant organs and the biochemical parameters such as ALT, AST, troponin, CK, CK-MB, LDH, urea and creatinine in 30% and 50% groups were also increased at different degrees at defined timepoints after burn, but changes in 50% group were more obvious than that in 30% group. Conclusion: We choose 50% TBSA with a full-thickness burn to establish a stable and optimized rat model that can meet strictly diagnosed criteria and serve as a tool to investigate the potential of novel therapeutics in this preclinical model.
Keywords: Animal model, excessively inflammatory response, systemic changes, severe burn
Introduction
Severe burns are one of dangerous surgical emergency. Despite improved prognosis, increased morbidity and mortality still remain major concerns in burns. Not only do they cause accidental death, they also result in considerable morbidity and disfigurement leading to significant functional and social [1-3].
The severe burn injury triggers an excessive inflammatory response and serious metabolic disturbances at early stage post burn [4]. Meanwhile, other systemic disorders are also accompanied by excessive inflammatory response such as cardiac dysfunction, acute respiratory distress syndrome, acute renal failure, increased intestinal permeability resulting in bacterial translocation, hypermetabolism, hypercatabolism and sepsis [5]. These intense disruptions in body’s homeostatic balance may result in multiple organ failure and death [6]. The excessive inflammatory reaction caused by severe burn of the different total body surface area (BSA) is also different. Total body surface area (BSA) burned is bigger, these patients are more likely to die from excessive inflammatory response due to the massive release of inflammatory mediators from the burn body. Each one percent increase in total body surface area burned was associated with a six percent increase in mortality risk [7-12]. Furthermore, majority of what is known about that animal models play a pivotal role in the discovery of potential therapeutic targets for this condition thereby furthering biomedical advances [13,14]. Therefore, comparing systemic inflammation response and vital organ damage induced by different area severe burns is needed for establishing a stable animal model to research the pathogenesis and treatment of the excessive inflammation after severe burn injury.
Materials and methods
Animal care
All studies adhered to procedures consistent with the International Guiding Principles for Biomedical Research Involving Animals issued by the Council for the International Organizations of Medical Sciences (CIOMS) and were approved by the Institutional Animal Care and Use Committee at the First Affiliated Hospital to PLA General Hospital. Wistar rats were obtained from the local animal facility, housed at the Institute of Animal Experiments of the First Affiliated Hospital to PLA General Hospital in stables with a temperature of 22°C, a relative humidity of 55% and a day/night cycle of 12/12 hours, with food and water ad libitum.
Six-week-old male Wistar rats (180-220 g) we-ighing were anesthetized by intraperitoneal injection of 300 mg/kg Avertin (20 mg/ml) (2,2,2-tribromoethanol, Sigma, USA) [15]. Animals in control group were subjected to identical procedure and resuscitation, but immersed in water at 37°C for 12 s. At the end of the experiment, all rats were sacrificed with an overdose of 10% chloral hydrate.
Rat model of burn injury
The 96 adult male Wistar rats were randomly divided into 3 groups (n=32): control group, 30% TBSA with a full-thickness burn group and 50% TBSA with a full-thickness burn group. Each group was divided equally into four subgroups of eight rats according to the period of euthanasia at 6, 12, 24, 48 hours post burn. The models of 30% and 50% TBSA with full-thickness burn were prepared. After the rats were anesthetized by intraperitoneal injection of Avertin (300 mg/kg), the dorsal hair was removed completely, first with clippers and then through the application of Veet depilatory cream. The whole backside of these rats was then placed in hot water (94°C) for 12 s, which caused 30% TBSA with a full-thickness burn. Both whole backside and abdomen of those rats in 50% TBSA groups were also placed in hot water (94°C) for 12 s and 6 s, respectively. According to different area, balanced salt solution (40 mg/kg) of the different doses was injected into enterocoelia for anti-shock. The wound was then treated with 1% tincture of iodine and kept dry to prevent infection. Wounds were left open and animals were sacrificed at defined time points post burn.
Specimen collection and detection
The blood samples of aortaventralis were taken at 6 h, 12 h, 24 h, and 48 h post-burn. They were transferred immediately to heparin-cotaining tubes for blood routine examination and collected serum after centrifuging for biochemical detection and ELISA assay. Meanwhile, vital organs including lung, renal, heart and liver were also collected. These organs samples was carefully removed, rinsed in PBS. Then they were fixed in 4% paraformaldehyde for histological examination.
Blood routine examination and biochemical detection
The blood and serum samples were detected by the Central Institute of Clinical Chemistry and Laboratory Medicine of the First Affiliated Hospital to PLA General Hospital. A 4 ml aliquot of blood was taken for full blood count (FBC) analysis to determine all the whole blood parameters. FBC was analyzed using a Sysmex XE 2100 (Sysmex UK, Milton Keynes, UK) automated hematology analyzer within 2 hrs of collection. These parameters, including alanine transaminase (ALT), aspartate transaminase (AST), troponin, creatine kinase (CK), MB isoform of CK (CK-MB), lactate dehydrogenase (LDH), urea and creatinine (CREA) in serum samples were measured spectrophotometrically using commercially available standard Roche- Hitachi methodology (Roche Diagnostics GmbH, Mannheim, Germany).
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of TNF-α, IL-1β and IL-6 were normalized to the total protein content. The levels of TNF-α, IL-1β and IL-6 in serum samples were determined using ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Histological analyses
After fixation with 4% paraformaldehyde for 24 h at room temperature, the specimens were embedded in paraffin and sectioned in a plane perpendicular to the incision. Five-micrometer-thick sections were prepared, deparaffinized in dimethylbenzene, and rehydrated. Preparative sections were stained with H&E in accordance with standard procedures. Some sections were incubated with specific antibodies (monoclonal mouse antibodies against rat MPO and CD68; Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with the corresponding secondary antibody and the PAP (peroxidase-anti-peroxidase) complex, and exposure using DAB (3,3’-diaminobenzidine). Other sections were stained with In Situ Cell Death Detection Kit (Roche Applied Science, Germany) following the directions of the manufacturer. MPO+, CD68+ cells and apoptotic nuclei were shown to be brown. The OD value of neutrophil, positive number of macrophage and cell apoptosis rate in the lung, liver, heart and renal tissues were counted in 5 randomly selected fields of the each slide by an experienced and independent cell scientist in a blinded manner.
Statistical analysis
All data are expressed as the mean ± SD (x̅ ± s) and were analyzed using SPSS 16.0 (SPSS Inc, Chicago, IL, USA). The data were analyzed using the ANOVA test (factorial design) was applied using Prism software (GraphPad Soft-ware, La Jolla, CA, USA). The differences were considered to be statistically significant at *P < 0.05, **P < 0.01, @P < 0.05, @@P < 0.01.
Results
Systemic change
Compare with normal temperature of rats in control group, temperature changes in 30% and 50% TBSA with a full-thickness burn groups were significantly different at 6 h, 12 h, 24 h, and 48 h after burn. The temperature change in 30% TBSA with a full-thickness burn group was very slightly, while temperature in 50% TBSA with a full-thickness burn group was decreased significantly and its value was (34.60±0.36)°C at 6 h post burn. At other time points post burn, temperatures in both groups firstly increased then decreased markedly, and their peak values were (37.67±0.15)°C and (35.73±0.22)°C, respectively. In general, temperature in 50% TBSA with a full-thickness burn group was always hypothermia, and lower than 36°C at defined time points post burn (Figure 1A).
Figure 1.
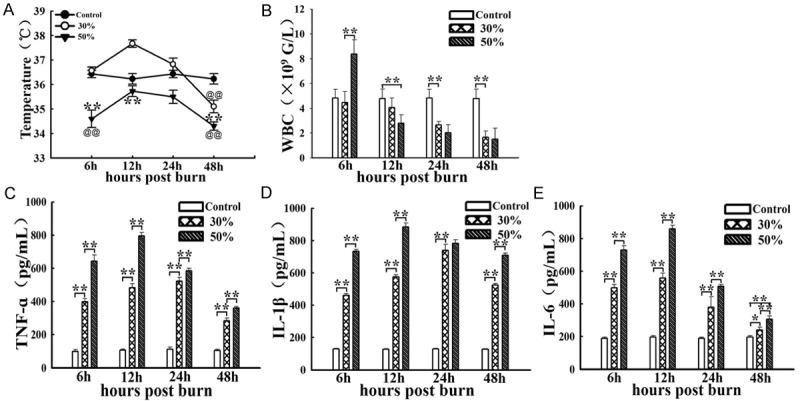
Comparison of systemic changes at defined timepoints post burn in 30%, 50% TBSA with a full-thickness burn groups and control group. A. The temperature changes of rats in the 3 groups at 6 h, 12 h, 24 h, and 48 h after burn. B. The leukocyte changes of rats in the 3 groups at 6 h, 12 h, 24 h, and 48 h after burn. C-E. The levels changes of TNF-α, IL-1β and IL-6 in the 3 groups at 6 h, 12 h, 24 h and 48 h post burn. Values are represented as mean ± SD (n=8), asterisk (*) and double asterisk (**) stand for P < 0.05 and P < 0.01 compared with 30% TBSA with a full-thickness burn, respectively. single @ and double @@ stand for P < 0.05 and P < 0.01 compared with control group, respectively.
Furthermore, full blood count (FBC) analysis shows that leukocyte number in 50% TBSA with a full-thickness burn group increased significantly at 6 h post burn, then decreased rapidly and significantly, even lower than 4×109/L at 12 h, 24 h and 48 h post burn. However, leukocyte number in 30% TBSA with a full-thickness burn group were not obvious at 6 h and 12 h post burn, decreased markedly at 24 h and 48 h post burn. leukocyte count was significant differences in both groups at 6 h and 12 h post burn. In general, leukocyte count in 50% TBSA with a full-thickness burn group were always lower than 4×109/L at defined time points post burn other at 6 h post burn (Figure 1B).
Inflammatory cells infiltration and structure of the vital organ change
To assess whether excessive inflammatory reaction at early stage after 30% and 50% TBSA with a full-thickness burn, we still need to detect the numbers of infiltrating neutrophils and macrophages, apoptosis rates of cells in the remote organs such as lung, liver, renal and heart by immunohistochemical and TUNEL staining detection.
Lung
Our results showed that the numbers of infiltrating neutrophils (MPO+) and macrophages (CD68+) in lung tissue in 50% group significantly higher than that in 30% group at 24 h post burn. In addition, both of the two groups were higher than control group (Figure 2A). These results of the quantitative analysis are presented in the corresponding histogram (Figure 2B, 2C).
Figure 2.
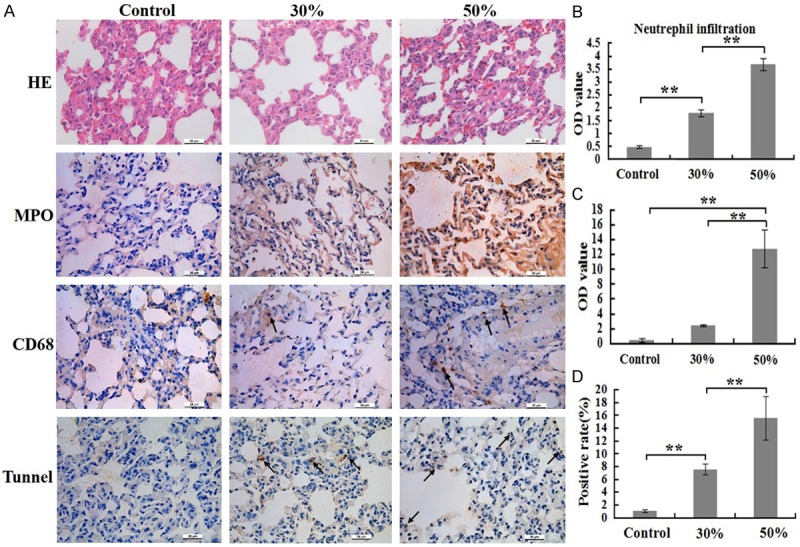
Comparison of inflammatory cells numbers and cells apoptosis rates of lung at 24 h post burn in 30%, 50% TBSA with a full-thickness burn groups and control group. A. The inflammatory cells infiltrations and cells apoptosis rates in lung were assessed by H&E staining, immunohistochemistry and Tunnel detection. B. A quantitative analysis of positive staining for neutrophils (MPO) is shown in the corresponding histogram. C. A quantitative analysis of positive staining for macrophages (CD68) is shown in the corresponding histogram. D. A quantitative analysis of positive staining for apoptosis cells is shown in the corresponding histogram. Values are represented as mean ± SD (n=8), asterisk (*) stands for P < 0.05 and double asterisk (**) stands for P < 0.01.
Furthermore, H&E staining of the lung sections revealed that the structure damage of alveolar, bleeding, edema, and neutrophil accumulation in 50% group was more serious than that in other two groups (Figure 2A). TUNEL staining displayed that apoptotic rate of cells in 50% group was markedly higher than that in other two groups, as well as apoptosis rates of cells in 30% group higher than that in control group (Figure 2A). The result of the quantitative analysis are presented in the corresponding histogram (Figure 2D).
Liver
The numbers of infiltrating neutrophils and macrophages in liver in 50% group significantly higher than that in 30% group as well as numbers of infiltrating neutrophils and macrophages in 30% group higher than that in control group at 24 h post burn (Figure 3A). These results of the quantitative analysis are presented in the corresponding histogram (Figure 3B, 3C). However, the numbers of infiltrating macrophages in liver were less than that in lung at same time point post burn.
Figure 3.
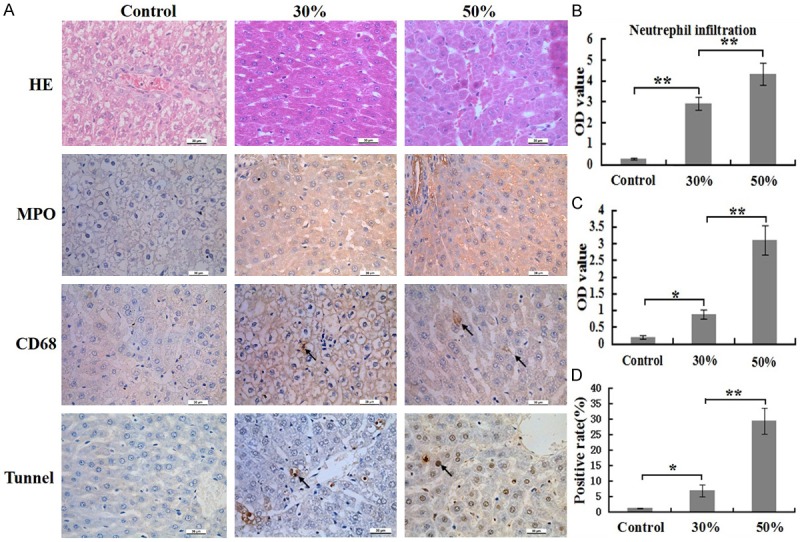
Comparison of inflammatory cells numbers and cells apoptosis rates of liver at 24 h post burn in 30%, 50% TBSA with a full-thickness burn groups and control group. A. The inflammatory cells infiltrations and cells apoptosis rates in liver were assessed by H&E staining, immunohistochemistry and Tunnel detection. B. A quantitative analysis of positive staining for neutrophils (MPO) is shown in the corresponding histogram. C. A quantitative analysis of positive staining for macrophages (CD68) is shown in the corresponding histogram. D. A quantitative analysis of positive staining for apoptosis cells is shown in the corresponding histogram. Values are represented as mean ± SD (n=8), asterisk (*) stands for P < 0.05 and double asterisk (**) stands for P < 0.01.
H&E staining revealed that cell swelling, interstitial hemorrhage of liver in 50% group, while these changes not found in other two groups (Figure 3A). TUNEL staining displayed that cell apoptosis rate in 50% group was markedly higher than that in other two groups, as well as apoptosis rates of cells in 30% group higher than that in control group (Figure 3A). The result of the quantitative analysis are presented in the corresponding histogram (Figure 3D).
Kidney
The numbers of infiltrating neutrophils and macrophages in renal tubules in 30% and 50% group significantly higher than that in control group at 24 h post burn, but the numbers of infiltrating macrophages in 30% and 50% group were so little (Figure 4A). These results of the quantitative analysis are presented in the corresponding histogram (Figure 4B, 4C).
Figure 4.
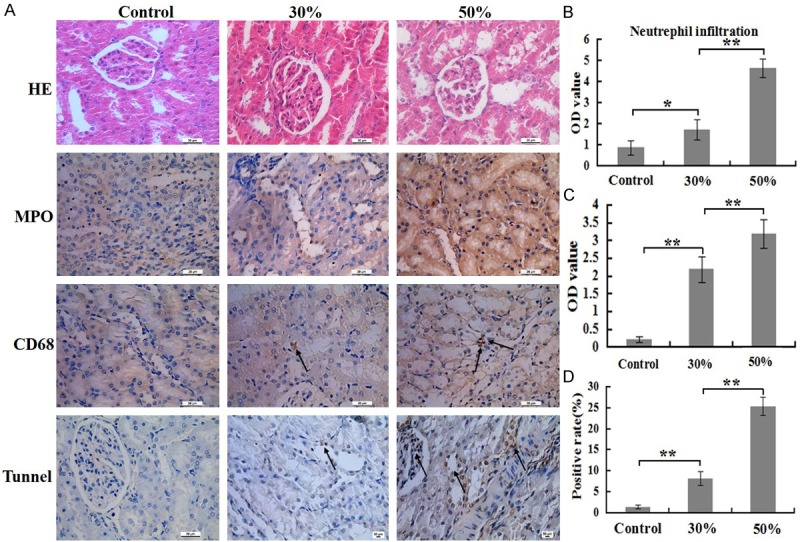
Comparison of inflammatory cells numbers and cells apoptosis rates of renal at 24 h post burn in 30%, 50% TBSA with a full-thickness burn groups and control group. A. The inflammatory cell infiltrations and cells apoptosis rates in renal was assessed by H&E staining, immunohistochemistry and Tunnel detection. B. A quantitative analysis of positive staining for neutrophils (MPO) is shown in the corresponding histogram. C. A quantitative analysis of positive staining for macrophages (CD68) is shown in the corresponding histogram. D. A quantitative analysis of positive staining for apoptosis cells is shown in the corresponding histogram. Values are represented as mean ± SD (n=8), asterisk (*) stands for P < 0.05 and double asterisk (**) stands for P < 0.01.
H&E staining revealed that structure of glomerular and renal tubular were relatively complete, but brush border of renal tubular was detachment and part of the epithelial cells of renal tubular fell off in 50% group, while these changes not found in other two groups (Figure 4A). TUNEL staining displayed that cell apoptosis rate in 50% group was significantly higher than that in other two groups, as well as cell apoptosis rate in 30% group higher than that in control group (Figure 4A). The result of the quantitative analysis is presented in the corresponding histogram (Figure 4D).
Heart
The degree of infiltrating neutrophils and macrophages in heart in 50% group significantly higher than that in 30% group at 24 h post burn. In addition, both of the two groups were higher than control group (Figure 5A). These results of the quantitative analysis are presented in the corresponding histogram (Figure 5B, 5C).
Figure 5.
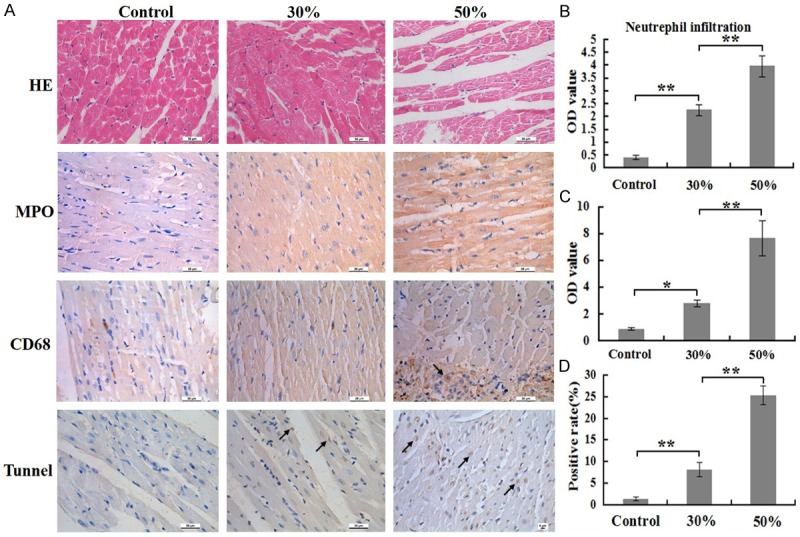
Comparison of inflammatory cells numbers and cells apoptosis rates of heart at 24 h post burn in 30%, 50% TBSA with a full-thickness burn groups and control group. A. The inflammatory cells infiltrations and cells apoptosis rates in heart were assessed by H&E staining, immunohistochemistry and Tunnel detection. B. A quantitative analysis of positive staining for neutrophils (MPO) is shown in the corresponding histogram. C. A quantitative analysis of positive staining for macrophages (CD68) is shown in the corresponding histogram. D. A quantitative analysis of positive staining for apoptosis cells is shown in the corresponding histogram. Values are represented as mean ± SD (n=8), asterisk (*) stands for P < 0.05 and double asterisk (**) stands for P < 0.01.
H&E staining revealed that myocardial fiber trophy and fibers gap widened in 50% group, while these changes not found in other two groups (Figure 5A). TUNEL staining displayed those apoptosis rates of cells in 30% and 50% group was markedly higher than that in control group, while apoptosis rates of cells in 50% group markedly higher than that in 30% group (Figure 5A). The result of the quantitative analysis is presented in the corresponding histogram (Figure 5D).
All results suggest that infiltrated of inflammatory cells, destruction of the structure and cells apoptosis occurred at different degree in the distant organs, including lung, liver, renal and heart of rats in 30% and 50% group, but the infiltrated numbers of inflammatory cells and apoptosis rates of cells in 50% group was significantly higher than that in 30% group.
Change in the biochemical parameters
The serum samples of the rats in the 3 groups at 6 h, 12 h, 24 h and 48 h post burn were analyzed for the crucial and sensitive biochemistry parameters as summarized in Table 1. Serum ALT activity were significantly firstly increased from 6 h to 12 h then decreased after 12 h in both of the severely burned groups, but ALT activity in 50% group was markedly higher than that in 30% group at 6 h, 12 h, 24 h and 48 h post burn. AST activity in serum showed similar tendency but the time point of peak value in 50% group was 12 h post burn and that in 30% group was 24 h post burn. AST and activity in 50% group was significantly higher than that in 30% group at defined timepoints post burn. Levels of high sensitive troponin activity were explicitly increased post severe burn as compared to normal value in control group. In addition, a strong increase in serum CK and CK-MB activities in 50% group was observed at 6 h post burn and peak values arrived at (10973.75±4239.34) U/L and (4516.40±173.13) U/L, which [16] resulted in a 8.3-fold and 3.2-fold increase compared to control group. The levels of LDH, urea and creatinine was also increased at 6 h, 12 h, 24 h and 48 h post burn, but they exceeded range only slightly.
Table 1.
Values changes of the biochemical parameters at defined timepoints in 30%, 50% TBSA with a full-thickness burn groups and control group
| Control | 30% | 50% | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 6 h | 12 h | 24 h | 48 h | 6 h | 12 h | 24 h | 48 h | ||
| ALT (U/L) | 53.67±2.89 | 70.20±19.10 | 103.75±1.89@@ | 86.33±6.66@ | 78.33±18.48 | 121.00±21.49@@,** | 164.00±14.07@@,** | 139.00±12.38@@,** | 114.00±25.50@@,* |
| AST (U/L) | 138±25.36 | 212.80±83.19@@ | 315.00±15.68@@ | 332.00±1.47@@ | 182.00±10.15@ | 442.40±121.12@@,* | 505.00±53.75@@,** | 492.67±10.38@@,** | 380.50±33.49@@,** |
| Troponin (pg/mL) | 206.33±2.36 | 248.11±3.08@@ | 263.31±9.28@@ | 218.77±4.03@@ | 210.13±4.16 | 287.34±3.19@@,** | 262.15±3.21@@ | 234.25±4.11@@,** | 215.22±2.08@ |
| CK (U/L) | 1335.33±176.38 | 4235.80±422.64 | 4106.75±361.39@@ | 4011.33±538.83@@ | 2583.33±164.51@@ | 10973.75±4239.34@@,* | 8626.75±211.56@@,** | 5580.75±262.95@@,** | 3835.50±20.09@@,** |
| CK-MB (U/L) | 1420.80±61.56 | 3310.67±72.00@@ | 2841.33±58.96@@ | 2928.25±62.24@@ | 1962.00±27.03@@ | 4516.40±173.13@@,* | 3210.67±61.56@@,** | 2218.75±89.22@@,** | 2034.00±262.31@@,** |
| LDH (U/L) | 1198.00±162.14 | 1659.80±395.80@@ | 1710.25±60.40@@ | 1438.00±58.89@@ | 1865.50±174.03@@ | 2193.25±278.03@@,** | 2297.25±68.21@@,** | 1867.75±49.92@@,** | 1336.33±82.25@,** |
| Urea (mmol/L) | 4.68±0.10 | 9.54±0.53@@ | 8.83±1.22@@ | 5.01±0.82@@ | 7.00±0.84@@ | 11.08±0.26@@,** | 14.43±1.02@@,** | 8.38±0.71@@,** | 8.25±0.61@@ |
| Crea (μmol/L) | 17.33±0.50 | 24.40±0.36@@ | 22.75±0.75@@ | 21.67±0. 26@@ | 21.75±0. 56@@ | 29.50±0.11@@,** | 24.33±0.48@@ | 22.07±0.38@@ | 20.67±0.31@@ |
ALT = alanine transaminase; AST = aspartate transaminase; CK = Creatine kinase; CK-MB = MB isoform of CK; LDH = lactate dehydrogenase; Crea = Creatinine. Values are represented as mean ± SD (n=8);
stand for P < 0.05 compared with 30% TBSA with a full-thickness burn, respectively;
stand for P < 0.01 compared with 30% TBSA with a full-thickness burn, respectively.
stand for P < 0.05 compared with control group, respectively;
stand for P < 0.01 compared with control group, respectively.
Discussion
Thermal burn represents a pathophysiological condition in which hyperactive macrophages are primed to stimulate the downregulation or upregulation of certain inflammatory cytokines [17]. Abnormal levels of proinflammatory mediators, such as tumor necrosis factor alpha (TNF-β), interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-8 (IL-8) contributed to the progress of tissue necrosis and possible serious complications following the initial trauma, such as excessive inflammatory response and severe metabolic imbalance [18,19]. Fur-thermore, excessive inflammatory response is an essential process during the occurrence of multiorgan failure which contributes to deaths in severely burned patients [7,20].
It was worth noting that the diagnosis about excessive inflammatory reaction met at least two points in the following four points: (1) T > 38°C or T < 36°C; (2) heart rate > 90 times/min; (3) breathing > 20 times/min or PaCO2 < 32 mmHg; (4) leukocyte count > 12×109/L or < 4×109/L [21]. However, these parameters were chosen to be quite sensitive, but they clearly lacked specificity for a clinically meaningful di-agnosis [22]. Therefore, researchers thought that excessively inflammatory mediators in plasma, infiltrated inflammatory cells and damage and dysfunction of distant organ were thought to play a key role in the diagnosis of the excessively inflammatory response [4,23,24]. In this study, we will establish a stable and optimized rat model through comparative analysis of systemic alterations, levels of pro-inflammatory cytokines in serum and infiltrated numbers of inflammatory cells and apoptosis rates of cells in distant organ between 30% and 50% TBSA with a full-thickness burn.
Our results showed that hypothermia, low numbers of leukocyte count, high levels of TNF-α, IL-1β and IL-6 in 50% TBSA with a full-thickness burn group comprehensively met diagnosed criteria of excessive inflammatory response at defined timepoints after burn, while that in 30% TBSA with a full-thickness burn groups met in part. Meanwhile, our research also displayed that the infiltrating numbers of neutrophils and macrophages in the lung, liver, renal and heart in 50% TBSA with a full-thickness burn group were significantly higher than that in 30% TBSA with a full-thickness burn group. The biochemical parameters such as ALT, AST, troponin, CK, CK-MB, LDH, urea and creatinine in 30% and 50% groups were also increased at different degrees at defined timepoints after burn, but the changes in 50% group were more obvious than that in 30% group. In addition, destruction of the structure and cells apoptosis in the distant organs in 30% group were so slightly, while that in 50% group were so severely. Therefore, using 50% TBSA with a full-thickness burn to establish a rat model of excessive inflammation response post burn was a stable and optimized model that can meet strictly diagnosed criteria and serve as a tool to investigate the potential of novel therapeutics in this preclinical model.
Acknowledgements
The authors gratefully acknowledge Drs. Yonghui Yu, Hongjie Duan, Huinan Yin and Shaoxia Wang for their expert technical assistance and fruitful discussion. This work was supported by two research projects of the National Natural Science Foundation of China (81372052) and (02010210) and project of the National Natural Science Foundation of Beijing (7144250).
Disclosure of conflict of interest
None.
References
- 1.Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-beta family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- 2.Botan A. Epilepsy and full-thickness burns. Ann Burns Fire Disasters. 2010;23:67–71. [PMC free article] [PubMed] [Google Scholar]
- 3.Ferraris VA, Ferraris SP, Saha SP. The relationship between mortality and preexisting cardiac disease in 5,971 trauma patients. J Trauma. 2010;69:645–652. doi: 10.1097/TA.0b013e3181d8941d. [DOI] [PubMed] [Google Scholar]
- 4.Dahiya P. Burns as a model of SIRS. Front Biosci (Landmark Ed) 2009;14:4962–4967. doi: 10.2741/3580. [DOI] [PubMed] [Google Scholar]
- 5.Kott M, Elke G, Reinicke M, Winoto-Morbach S, Schadler D, Zick G, Frerichs I, Weiler N, Schutze S. Acid sphingomyelinase serum activity predicts mortality in intensive care unit patients after systemic inflammation: a prospective cohort study. PLoS One. 2014;9:e112323. doi: 10.1371/journal.pone.0112323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatakeyama N, Matsuda N. Alert cell strategy: mechanisms of inflammatory response and organ protection. Curr Pharm Des. 2014;20:5766–5778. doi: 10.2174/138161282036140912122809. [DOI] [PubMed] [Google Scholar]
- 7.Farina JA Jr, Rosique MJ, Rosique RG. Curbing inflammation in burn patients. Int J Inflam. 2013;2013:715645. doi: 10.1155/2013/715645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarma BP. Prevention of burns: 13 years’ experience in Northeastern India. Burns. 2011;37:265–272. doi: 10.1016/j.burns.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Chen XL, Xia ZF, Wei HF. Escharectomy and allografting during shock stage reduces insulin resistance induced by major burn. J Burn Care Res. 2011;32:e59–66. doi: 10.1097/BCR.0b013e31820aaf96. [DOI] [PubMed] [Google Scholar]
- 10.Ipaktchi K, Arbabi S. Advances in burn critical care. Crit Care Med. 2006;34:S239–244. doi: 10.1097/01.CCM.0000232625.63460.D4. [DOI] [PubMed] [Google Scholar]
- 11.Meshulam-Derazon S, Nachumovsky S, Ad-El D, Sulkes J, Hauben DJ. Prediction of morbidity and mortality on admission to a burn unit. Plast Reconstr Surg. 2006;118:116–120. doi: 10.1097/01.prs.0000221111.89812.ad. [DOI] [PubMed] [Google Scholar]
- 12.Sakallioglu AE, Basaran O, Karakayali H, Ozdemir BH, Yucel M, Arat Z, Haberal M. Interactions of systemic immune response and local wound healing in different burn depths: an experimental study on rats. J Burn Care Res. 2006;27:357–366. doi: 10.1097/01.BCR.0000216330.93056.06. [DOI] [PubMed] [Google Scholar]
- 13.Domergue S, Jorgensen C, Noel D. Advances in Research in Animal Models of Burn-Related Hypertrophic Scarring. J Burn Care Res. 2014 doi: 10.1097/BCR.0000000000000167. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Abdullahi A, Amini-Nik S, Jeschke MG. Animal models in burn research. Cell Mol Life Sci. 2014;71:3241–3255. doi: 10.1007/s00018-014-1612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Yu Y, Hou Y, Chai J, Duan H, Chu W, Zhang H, Hu Q, Du J. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS One. 2014;9:e88348. doi: 10.1371/journal.pone.0088348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheeler AP. Recent developments in the diagnosis and management of severe sepsis. Chest. 2007;132:1967–1976. doi: 10.1378/chest.06-2535. [DOI] [PubMed] [Google Scholar]
- 17.Hur J, Yang HT, Chun W, Kim JH, Shin SH, Kang HJ, Kim HS. Inflammatory cytokines and their prognostic ability in cases of major burn injury. Ann Lab Med. 2015;35:105–110. doi: 10.3343/alm.2015.35.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Q, Berthiaume F, Androulakis IP. A quantitative model of thermal injury-induced acute inflammation. Math Biosci. 2011;229:135–148. doi: 10.1016/j.mbs.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatakeyama N, Matsuda N. Mechanisms of Inflammatory Response and Organ Dysfunction: Organ-Protective Strategy by Anesthetics. Curr Pharm Des. 2014 doi: 10.2174/138161282036140912122809. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Huang G, Liang B, Liu G, Liu K, Ding Z. Low dose of glucocorticoid decreases the incidence of complications in severely burned patients by attenuating systemic inflammation. J Crit Care. 2015;30:436, e7–11. doi: 10.1016/j.jcrc.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000;16:179–192. doi: 10.1016/s0749-0704(05)70106-8. [DOI] [PubMed] [Google Scholar]
- 22.Balk RA. Systemic inflammatory response syndrome (SIRS): where did it come from and is it still relevant today? Virulence. 2014;5:20–26. doi: 10.4161/viru.27135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orman MA, Nguyen TT, Ierapetritou MG, Berthiaume F, Androulakis IP. Comparison of the cytokine and chemokine dynamics of the early inflammatory response in models of burn injury and infection. Cytokine. 2011;55:362–371. doi: 10.1016/j.cyto.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis TA, Stojadinovic A, Anam K, Amare M, Naik S, Peoples GE, Tadaki D, Elster EA. Extracorporeal shock wave therapy suppresses the early proinflammatory immune response to a severe cutaneous burn injury. Int Wound J. 2009;6:11–21. doi: 10.1111/j.1742-481X.2008.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


