Abstract
Nestin, a member of type VI intermediate filament protein family, is widely expressed in mammalian nervous tissue and stem/precursor cells of non-neuronal normal tissues. Nestin has also been investigated to determine possible tumor-promoting functions. However, whether Nestin is involved in colorectal cancer (CRC) cells remains unclear. In this report, Nestin expression was upregulated in stromal cells of human CRC tissues. Endogenous Nestin expression in CRC cell lines SW480 and HCT116 was knocked down by a lentivirus. MTT and colony formation assays revealed that Nestin deletion significantly inhibits the proliferation of CRC cell lines; flow cytometer analysis showed that Nestin deletion causes cell cycle arrest at S phase. Transwell chamber and wound healing scratch assays also revealed that Nestin deletion suppresses cell migration. Our findings indicated that Nestin plays an essential role in CRC progression; thus, Nestin can be applied as a therapeutic target of CRC.
Keywords: Nestin, colorectal cancer, metastasis
Introduction
Colorectal cancer (CRC) is regarded as the third most common type of cancer and the fourth leading cause of common cancer mortality worldwide [1,2]. CRC mortality rate has shown a decreasing trend in the United States, and disease-specific mortality rate is approximately 33% in developed countries [3,4]. Although advances in therapeutic strategies have been applied in patients, treatment breakthroughs remain insufficient to improve the survival rate of patients with advanced disease or metastasis [1]. Therefore, the underlying molecular mechanisms of CRC initiation and progression should be further investigated to determine new biomarkers for diagnosis or development of effective and side effect-free treatments.
Nestin is considered as a type VI intermediate filament protein, which was originally described as a neuronal stem cell marker during central nervous system development [5]. Nestin is also widely expressed in non-neuronal immature or progenitor cells of normal tissues; Nestin expression levels are downregulated with cellular differentiation [6-9]. Nestin has also been reported in various types of cell lines established from human solid tumors, such as gastrointestinal stromal tumors [10], pancreatic cancer [11], malignant melanoma [12,13], breast cancer [14], thyroid tumors [15], and nasopharyngeal carcinoma [16]. Nestin is required for cell migration and invasion of prostate cancer cells [17]. Nestin is also necessary to promote cell proliferation of nasopharyngeal carcinoma [16] and astrocytoma cells [18]. Furthermore, Nestin is associated with increased aggressiveness in several tumors in the nervous system [19]. Therefore, Nestin is closely related to tumorigenesis. However, the precise role of Nestin and the relationship between Nestin and CRC progression remain unknown.
In this study, lentivirus-mediated stable transfection was introduced to CRC cell line SW480 and HCT116 to downregulate the expression of Nestin and to elucidate the role of Nestin in CRC. Our results showed that the knockdown of Nestin arrested cell cycle at S phase and inhibited cell proliferation and migration of CRC cells. We also detected Nestin upregulation in CRC samples. Nestin expression was located in stromal cells and small-sized tumor vessel endothelial cells of human CRC tissues. These findings elucidated that Nestin can be applied as a therapeutic marker of CRC because this protein elicits inhibitory effects on cell proliferation and migration.
Materials and methods
Specimens
CRC tissues and adjacent normal tissues were collected from the Department of General Surgery of the Shanghai 10th People’s Hospital. Tissue fragments were immediately frozen in liquid nitrogen at the time of surgery and stored at -80°C. The pathological type of each colorectal tissue was identified as adenocarcinoma. All of the included patients or their guardians provided written informed consent to use their tissue specimens for medical study. No patient received chemotherapy or radiotherapy prior to surgery.
Immunohistochemistry
Primary antibodies against Nestin (1:300 dilution; Merck, Germany) were used in our study, as described in a previous study [16]. In brief, tissue sections were treated with xylene and graded alcohol and then equilibrated in PBS. Antigen was retrieved by microwaving the slides for 15-20 min in a buffer containing 10 mM sodium citrate (pH 6.0). Endogenous peroxidase was blocked by incubating the slides in methanol containing 3% H2O2 at room temperature for 10 min. Nonspecific binding was blocked by incubating with normal goat serum (Invitrogen, USA) for 30 min at room temperature; sections were incubated with anti-Nestin antibody (1:300 dilution) for 1 h at room temperature. Slides were washed several times in PBS; afterward, the slides were incubated with biotinylated anti-mouse IgG (1:100 dilution) for 30 min at room temperature and with peroxidase-labeled streptavidin (1:200 dilution; Sigma-Aldrich, USA) for 30 min at room temperature. The antigen was visualized through incubation for 10-20 min at room temperature with diaminobenzidine tetrahydrochloride hydrade according to the manufacturer’s instructions.
Cell culture
Human CRC cell lines HCT116 and SW480 cells were purchased from the Chinese Academy of Sciences (Shanghai, China). HCT116 cells were maintained in DMEM/high glucose (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) at 37°C and 5% CO2. SW480 cells were cultured in RPMI-1640 (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) in a humidified incubator at 37°C with 5% CO2.
Stable infection
HCT116 or SW480 cells were infected with lentivirus expressing shRNA targeting Nestin (Genchem, China) or its negative control at 30%-50% confluency to perform stable knockdown of Nestin. The monoclonal population of stably infected cells was selected through limiting dilution assay and positive clone was detected through qRT-PCR and Western blot analysis.
Quantitative RT-PCR analysis
Total RNA was extracted from tissues and cultured cells by using Trizol Reagent (Invitrogen) according to the manufacturer’s instructions. Reverse transcription reaction was performed using PrimeScript™ RT-PCR kit (TaKaRa, Japan) in accordance with the manufacturer’s instructions. Qualitative RT-PCR (qRT-PCR) was performed using SYBR premix ExTaq (TaKaRa) and 7900 real-time PCR system (Applied Biosystems, USA) equipped with analytical software. The following forward and reverse primer pairs were designed for Nestin and 18S rRNA: Nestin (forward, 5’-GGAAGAGTCTGACCCTGT-3’; reverse, 5’-AGACTAGCGGCATTCCTT-3’) and 18SrRNA (forward, 5’-CCTGGATACCGCAGCTAGGA-3’; reverse, 5’-GCGGCGCAATACGAATGCCCC-3’). 2-∆∆Ct method for relative quantization was used to determine gene expression.
Western blot analysis
Infected cells were subjected to Western blot analysis with anti-Nestin (1:500 MERCK) and actin (1:1500 Santa Cruz, USA) antibodies. Cells were harvested and lysed in RIPA buffer (Beyotime, China) with protease inhibitor mixture tablet (Roche, USA). Protein (20 μg) was processed for 8% SDS-PAGE. Proteins were electrophoretically transferred to PVDF membranes (Millipore, USA) and incubated with primary antibody and then with an HRP-conjugated secondary antibody (Santa Cruz). After the membranes were washed with TBS, bound antibodies were visualized through enhanced chemiluminescence (Pierce, USA) and recorded on X-ray films.
Cell proliferation assay
Cell proliferation was determined using an MTT assay kit (Sigma) in accordance with the manufacturer’s instructions. In brief, treated cells (2 × 103 cells) were plated into 96-well plates. MTT assays were performed at 1 d to 7 d. The absorbance of samples was determined using a spectrophotometer at 490 nm. Each assay was performed in triplicate.
Colony formation assay
Treated cells were seeded in 6-well plates (100 cells per well) and incubated for 10 d to allow colony growth. Colonies were stained with crystal violet; colonies containing 50 or more cells were counted. To calculate plating efficiency, we divided the average number of colonies per dish by the number of plated cells. We also calculated survival fractions by normalizing to the plating efficiency of appropriate control groups.
Transwell chamber assay
In accordance with Transwell chamber instructions, 5 × 104 shCON or shNES cells were seeded in a Matrigel-coated chamber with 8.0 μM pores (Corning Costar, USA). The cells were seeded in serum-free medium in the upper chamber and translocated to complete growth medium for 18 h. The lower compartment of the chamber was fixed in 1.5% glutaraldehyde for 1 h, stained with 0.1% violet crystal dye for 15 min, and washed twice with distilled water. The stained cells were viewed under a light microscope (Olympus, USA). Membrane-binding crystal violet was dissolved in 300 μl of 33% glacial acetic acid; absorbance at 573 nm was determined using a microplate reader.
Wound healing scratch assay
For the wound healing scratch assay, SW480 cells were seeded in 6-well plates. After 24 h, wound was made by scratching a line across the bottom of the dish on the monolayer of the confluent cells with a sterile p-200 pipette tip. The cells were rinsed very gently with PBS and then cultivated in the corresponding serum-deprived medium supplemented with 0.5% FBS. The same area of the gap was imaged at 50 × magnifications by using a microscope equipped with a digital camera (Olympus) at 0, 24, and 48 h after scratching was performed and then quantified using Image J software (http://rsbweb.nih.gov/ij/). The difference between initial and final areas was calculated.
Cell cycle analysis
Parental and transfected cells in the log phase of growth were stained with propidium iodide (PI) and examined using a fluorescence-activated cell sorting (FACS) flow cytometer. DNA histograms were analyzed with modified software. shCON and shNES cells were synchronized in serum-free medium for 24 h and cultured in complete medium with 10 nM of doxorubicin for 10 h to examine the effect of Nestin suppression on cell cycle. The cells were harvested at 0, 24, and 48 h and analyzed through FACS (FACS Caliber II Sorter, BD Biosciences, USA) to determine DNA content.
Statistical analysis
Data were presented as mean ± standard deviation (SD) from at least three independent experiments. Two-tailed t-test was performed to evaluate differences between groups. P<0.05 indicated statistically significant results.
Results
Nestin was upregulated in human CRC clinical specimens
We compared Nestin expression profiles between 34 pairs of CRC tissues and matched adjacent normal colon tissues. qRT-PCR results revealed that the mRNA expression of Nestin was higher in human CRC tissues than in normal tissues (Figure 1A). Nestin was also observed in vascular endothelial cells of small-sized tumor vessels and stromal cells in human CRC tissues but not in adjacent normal colon tissues (Figure 1B). These results indicated that Nestin was associated with tumorigenesis of CRC.
Figure 1.
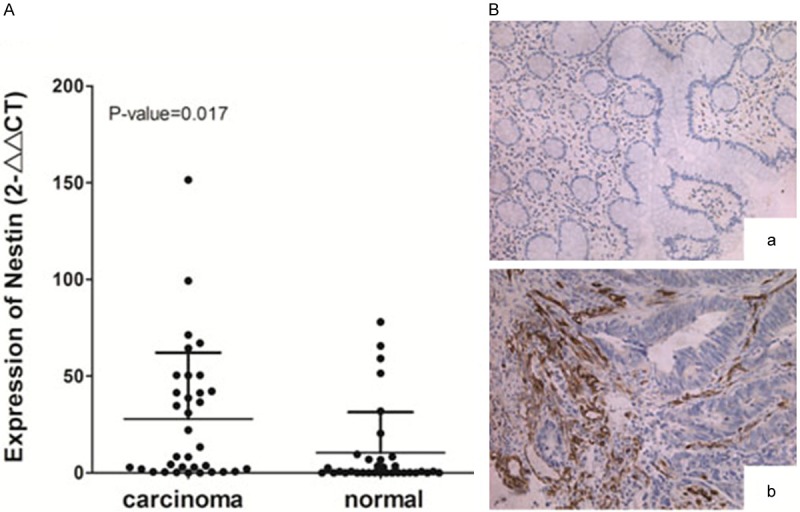
Nestin is upregulated in human CRC clinical specimens. (A) Nestin expression was determined through qRT-PCR in tissues of patients with CRC compared with adjacent normal tissues. Data are shown as 2-∆∆Ct values relative to 18S rRNA expression in box plots. P=0.017 compared with adjacent normal tissues. (B) Representative immunohistochemistry images showing Nestin expression in (A) adjacent normal tissues and (B) CRC tissues. Magnification =200 ×.
Nestin expression was downregulated by lentivirus-mediated shRNA
HCT116 and SW480 cells were infected with lentivirus shRNA specifically targeting Nestin to knockdown Nestin expression. As a marker of lentivirus infection efficiency, a high GFP expression was observed in HCT116 and SW480 cell lines after these cells were infected for 48 h (Figure 2A and 2B). mRNA (Figure 2C and 2D) and protein (Figure 2E) levels of Nestin also significantly decreased 4 d after infection. These results showed that endogenous Nestin expression in CRC was effectively downregulated by lentivirus.
Figure 2.

Relative Nestin expression in SW480 and HCT116 cells infected by shRNA-targeting Nestin. Representative images of (A) SW480 and (B) HCT116 cells infected with lentiviral-EGFP. mRNA expression of Nestin in (C) SW480 and (D) HCT116 cells determined through qRT-PCR. Data are shown as 2-∆∆Ct values standardized to 18SrRNA expression and normalized to 1.0 in shCON-transduced cells. *P<0.05, **P<0.01 compared with shCON. (E) Protein levels of Nestin in SW480 and HCT116 cells revealed through Western blot analysis. Data represent at least three independent experiments with similar results.
Knockdown of Nestin suppressed the proliferation of CRC cells
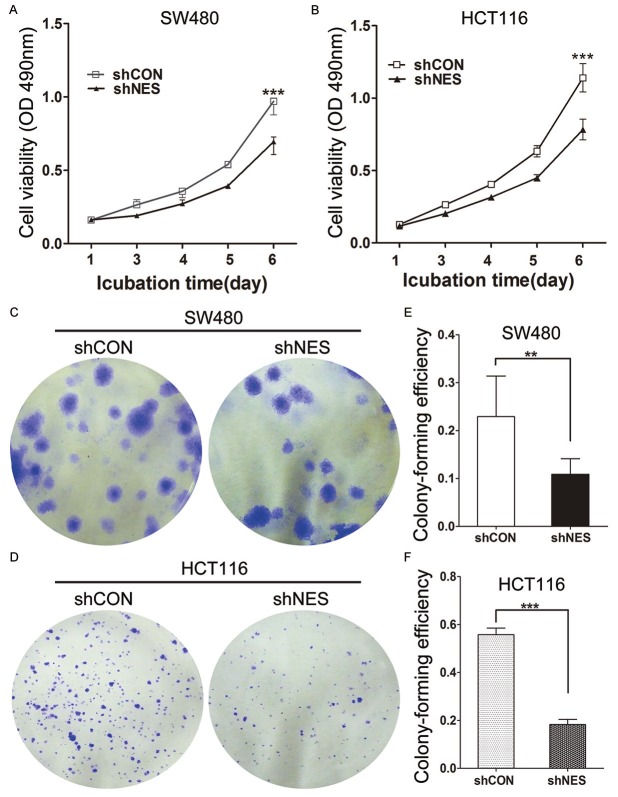
MTT and colony formation assays were performed in Nestin-depleted cells to investigate the effects of Nestin on CRC cell proliferation. The optical density (OD) value (0.695 ± 0.045) of Nestin-depleted SW480 cells at 6 d decreased by 28.4% compared with the OD (0.970 ± 0.046) of control cells (Figure 3A). Similar results were observed in HCT116 cells (Figure 3B); the OD (0.783 ± 0.071) of Nestin-depleted cells was significantly lower than the OD (1.14 ± 0.098) of the control cells. We also found that Nestin depletion significantly suppressed colony-forming efficiency of SW480 cells (Figure 3C and 3D) and HCT116 cells (Figure 3E and 3F). These data indicated that Nestin promoted CRC cell proliferation.
Figure 3.
Knockdown of Nestin suppressed the proliferation and colony-formation ability of CRC cells. MTT analysis performed in (A) SW480 and (B) HCT116 cells after a time course of transfection. Data represent mean ± SD from three independent experiments. (C-F) Colony-forming efficiency of transfected cells. Representative images of the plates used for enumeration of colonies are shown in (C and D) Values of colony-forming efficiency (E and F) represent the means ± SD of three independent experiments. **P<0.01, ***P<0.001 compared with shCON.
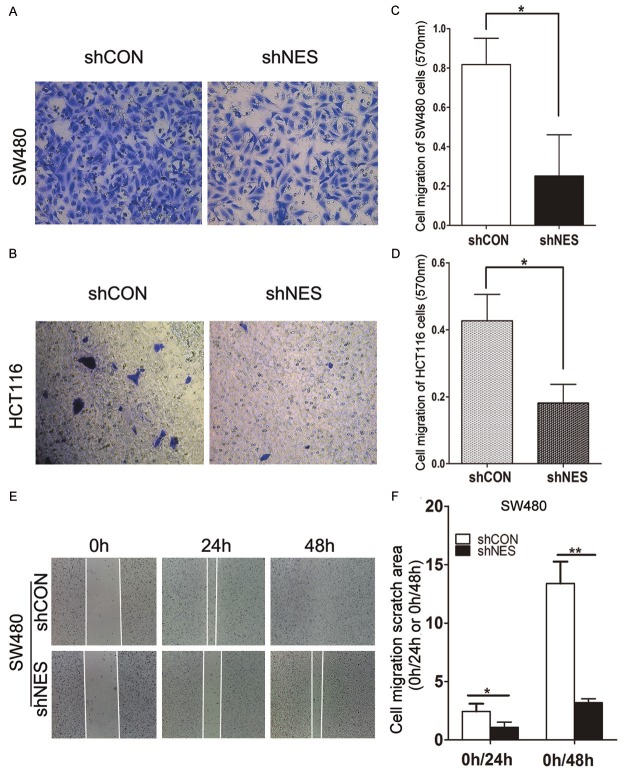
Knockdown of Nestin inhibited the migration of CRC cells
To investigate the effects of Nestin on the migration of CRC cells, we performed a Transwell chamber assay and assessed cell migration in SW480 and HCT116 cells. We found that Nestin knockdown notably reduced the migration ability of CRC cells (Figure 4A-D). HCT116 cells showed lower migration ability than SW480 cells; thus, only SW480 cells were subsequently subjected to wound-healing scratch assay to determine the effects of Nestin on cell migration. Control cells or Nestin-depleted cells were cultured in a medium with 10% FBS for 24 h; confluent monolayer of these cells was scratched and cultured in a medium with 0.5% FBS for 48 h. The same wounded area/well was examined through phase-contrast microscopy after scratching was performed at 0, 24, and 48 h. The knockdown of Nestin significantly inhibited scratch healing in SW480 cells (Figure 4E and 4F). These data suggested that Nestin could promote CRC cell migration.
Figure 4.
Knockdown of Nestin inhibited CRC cell migration. (A, B) Treated cells were re-plated in serum-free medium layered onto the top chambers and invaded the bottom chamber containing serum-supplemented medium for 18 h at 37°C. (C, D) Quantification of cell invasion of (A) and (B) by detecting absorbance at 570 nm. Data represent at least three independent experiments with similar results.*P<0.05 compared with shCON. (E) Phase-contrast microscopy images of the same wounded area at 0, 24, and 48 h after scratching. (F) Cell migration quantification of (E) was assessed on the basis of the scratch area at 0 h normalized to 24 h or 48 h. Data represent at least three independent experiments with similar results.*P<0.05, **P<0.01 compared with shCON.
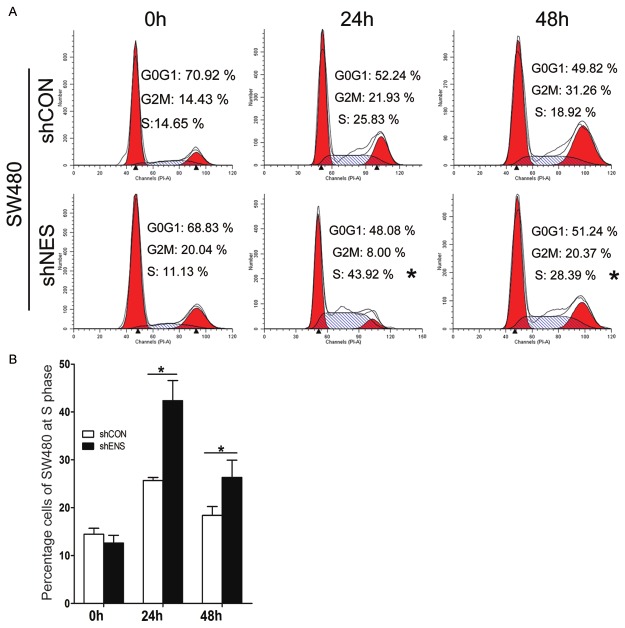
Knockdown of Nestin arrested the cell cycle of CRC cells
Control and Nestin-depleted cells were harvested at 0, 24, and 48 h after pure cells were cultured again in a complete medium cell cycle. Cell cycle distribution in SW480 cells was then analyzed through FACS. Our results showed that the population of Nestin-depleted cells and control cells at S phase significantly increased (43.92% and 25.83% at 24 h and 28.39% and 18.92% at 48 h) (Figure 5A and 5B). Therefore, Nestin knockdown arrested SW480 cells at S phase, thereby contributing to the suppression of cell proliferation.
Figure 5.
Knockdown of Nestin arrested cell cycle progression. (A) Transduced cells harvested at 0, 24, and 48 h after pure cells were cultured in a complete medium; cell cycle distribution in SW480 cells was analyzed through FACS. (B) Percentage of transduced cells at S phase. *P<0.05 compared with shCON.
Discussion
Surgery, radiotherapy, and chemotherapy are the main strategies applied to treat CRC. The five-year relative survival of patients with CRC has reached approximately 65% in high-income countries, but remained >50% in low-income countries [20-22]. Hence, molecular mechanisms underlying CRC development should be investigated for accurate prognosis and treatment response of patients with CRC [23].
In this study, we found average mRNA levels of Nestin were higher in CRC tissues than in adjacent normal tissues. Increased Nestin expression was also detected in endothelial cells of small-sized tumor vessels and stromal cells. This result is consistent with that of Teranishi [24]. However, Nestin is not detected in cancer cells of human CRC tissues. Studies have also demonstrated that Nestin is expressed in newly formed small-sized blood vessels in various tumor tissues, including CRC [24], gastric adenocarcinoma [25], glioblastoma [26], pancreatic cancer [27], and prostate cancer [28]. This finding indicates that Nestin is associated with tumor angiogenesis and prognosis of multiple human cancers [29,30]. Therefore, Nestin was not detected in cancer cells of CRC tissues. Nevertheless, Nestin can be used as a marker of CRC diagnosis and prognosis.
Nestin is involved in various tumor progression stages, such as cell proliferation, apoptosis, migration, and invasion. The knockdown of Nestin remarkably decreases migration of prostate cancer cells [17,31], inhibits proliferation of nasopharyngeal carcinoma [16] and lung cancer [32] cells, and arrests G2/M cell cycle of nasopharyngeal carcinoma [16]. In the present study, Nestin could be detected in CRC cell lines SW480 and HCT116. This study is the first to demonstrate that lentivirus-mediated knockdown of Nestin not only inhibits CRC cell proliferation and migration, but also promotes CRC cell cycle arrest at S phase. However, further studies should be conducted to characterize the functions of Nestin in promoting cell proliferation and migration.
In conclusion, Nestin can promote cell proliferation and migration of CRC cells. Nestin exhibits abnormally high expression in small-sized tumor vessels of endothelial cells and stromal cells of CRC tissues. This finding confirms the significant role of Nestin in diagnosis and prognosis of CRC.
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program, 2012CB966904), the National Natural Science Foundation of China (81171778, 81371913, 81301704 and 31171086), the New Hundred Talents Program at municipal and ministerial level of Shanghai (Climbing training project, 04.01.13017), the programs of Shanghai Tenth People’s Hospital (The Reserve Doctoral Tutor Training Project, 12HBBD118), and the programs of Shanghai Tenth People’s Hospital (Excellent Young Talents Training Project, 12RQ101).
Disclosure of conflict of interest
None.
References
- 1.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 4.Enewold L, Horner MJ, Shriver CD, Zhu K. Socioeconomic disparities in colorectal cancer mortality in the United States, 1990-2007. J Community Health. 2014;39:760–766. doi: 10.1007/s10900-014-9824-z. [DOI] [PubMed] [Google Scholar]
- 5.Andressen C, Stocker E, Klinz FJ, Lenka N, Hescheler J, Fleischmann B, Arnhold S, Addicks K. Nestin-specific green fluorescent protein expression in embryonic stem cell-derived neural precursor cells used for transplantation. Stem Cells. 2001;19:419–424. doi: 10.1634/stemcells.19-5-419. [DOI] [PubMed] [Google Scholar]
- 6.Terling C, Rass A, Mitsiadis TA, Fried K, Lendahl U, Wroblewski J. Expression of the intermediate filament nestin during rodent tooth development. Int J Dev Biol. 1995;39:947–956. [PubMed] [Google Scholar]
- 7.Frojdman K, Pelliniemi LJ, Lendahl U, Virtanen I, Eriksson JE. The intermediate filament protein nestin occurs transiently in differentiating testis of rat and mouse. Differentiation. 1997;61:243–249. doi: 10.1046/j.1432-0436.1997.6140243.x. [DOI] [PubMed] [Google Scholar]
- 8.Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci. 1993;106:1291–1300. doi: 10.1242/jcs.106.4.1291. [DOI] [PubMed] [Google Scholar]
- 9.Kachinsky AM, Dominov JA, Miller JB. Myogenesis and the intermediate filament protein, nestin. Dev Biol. 1994;165:216–228. doi: 10.1006/dbio.1994.1248. [DOI] [PubMed] [Google Scholar]
- 10.Sarlomo-Rikala M, Tsujimura T, Lendahl U, Miettinen M. Patterns of nestin and other intermediate filament expression distinguish between gastrointestinal stromal tumors, leiomyomas and schwannomas. APMIS. 2002;110:499–507. doi: 10.1034/j.1600-0463.2002.100608.x. [DOI] [PubMed] [Google Scholar]
- 11.Hunziker E, Stein M. Nestin-expressing cells in the pancreatic islets of Langerhans. Biochem Biophys Res Commun. 2000;271:116–119. doi: 10.1006/bbrc.2000.2611. [DOI] [PubMed] [Google Scholar]
- 12.Brychtova S, Fiuraskova M, Hlobilkova A, Brychta T, Hirnak J. Nestin expression in cutaneous melanomas and melanocytic nevi. J Cutan Pathol. 2007;34:370–375. doi: 10.1111/j.1600-0560.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- 13.Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007;20:102–107. doi: 10.1038/modpathol.3800720. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Chen B, Zhu J, Zhang R, Yao F, Jin F, Xu H, Lu P. Clinical implications for nestin protein expression in breast cancer. Cancer Sci. 2010;101:815–819. doi: 10.1111/j.1349-7006.2009.01422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada H, Takano T, Ito Y, Matsuzuka F, Miya A, Kobayashi K, Yoshida H, Watanabe M, Iwatani Y, Miyauchi A. Expression of nestin mRNA is a differentiation marker in thyroid tumors. Cancer Lett. 2009;280:61–64. doi: 10.1016/j.canlet.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Sun F, Li C, Zhang Y, Xiao W, Li Z, Pan Q, Zeng H, Xiao G, Yao K, Hong A, An J. Depletion of intermediate filament protein Nestin, a target of microRNA-940, suppresses tumorigenesis by inducing spontaneous DNA damage accumulation in human nasopharyngeal carcinoma. Cell Death Dis. 2014;5:e1377. doi: 10.1038/cddis.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleeberger W, Bova GS, Nielsen ME, Herawi M, Chuang AY, Epstein JI, Berman DM. Roles for the stem cell associated intermediate filament Nestin in prostate cancer migration and metastasis. Cancer Res. 2007;67:9199–9206. doi: 10.1158/0008-5472.CAN-07-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei LC, Shi M, Cao R, Chen LW, Chan YS. Nestin small interfering RNA (siRNA) reduces cell growth in cultured astrocytoma cells. Brain Res. 2008;1196:103–112. doi: 10.1016/j.brainres.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Thomas SK, Messam CA, Spengler BA, Biedler JL, Ross RA. Nestin is a potential mediator of malignancy in human neuroblastoma cells. J Biol Chem. 2004;279:27994–27999. doi: 10.1074/jbc.M312663200. [DOI] [PubMed] [Google Scholar]
- 20.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 21.Brenner H, Bouvier AM, Foschi R, Hackl M, Larsen IK, Lemmens V, Mangone L, Francisci S. Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: the EUROCARE study. Int J Cancer. 2012;131:1649–1658. doi: 10.1002/ijc.26192. [DOI] [PubMed] [Google Scholar]
- 22.Sankaranarayanan R, Swaminathan R, Brenner H, Chen K, Chia KS, Chen JG, Law SC, Ahn YO, Xiang YB, Yeole BB, Shin HR, Shanta V, Woo ZH, Martin N, Sumitsawan Y, Sriplung H, Barboza AO, Eser S, Nene BM, Suwanrungruang K, Jayalekshmi P, Dikshit R, Wabinga H, Esteban DB, Laudico A, Bhurgri Y, Bah E, Al-Hamdan N. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol. 2010;11:165–173. doi: 10.1016/S1470-2045(09)70335-3. [DOI] [PubMed] [Google Scholar]
- 23.De Sousa EMF, Wang X, Jansen M, Fessler E, Trinh A, de Rooij LP, de Jong JH, de Boer OJ, van Leersum R, Bijlsma MF, Rodermond H, van der Heijden M, van Noesel CJ, Tuynman JB, Dekker E, Markowetz F, Medema JP, Vermeulen L. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. 2013;19:614–618. doi: 10.1038/nm.3174. [DOI] [PubMed] [Google Scholar]
- 24.Teranishi N, Naito Z, Ishiwata T, Tanaka N, Furukawa K, Seya T, Shinji S, Tajiri T. Identification of neovasculature using nestin in colorectal cancer. Int J Oncol. 2007;30:593–603. [PubMed] [Google Scholar]
- 25.Kim HS, Kang HS, Messam CA, Min KW, Park CS. Comparative evaluation of angiogenesis in gastric adenocarcinoma by nestin and CD34. Appl Immunohistochem Mol Morphol. 2002;10:121–127. doi: 10.1097/00129039-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Hlobilkova A, Ehrmann J, Knizetova P, Krejci V, Kalita O, Kolar Z. Analysis of VEGF, Flt-1, Flk-1, nestin and MMP-9 in relation to astrocytoma pathogenesis and progression. Neoplasma. 2009;56:284–290. doi: 10.4149/neo_2009_04_284. [DOI] [PubMed] [Google Scholar]
- 27.Yamahatsu K, Matsuda Y, Ishiwata T, Uchida E, Naito Z. Nestin as a novel therapeutic target for pancreatic cancer via tumor angiogenesis. Int J Oncol. 2012;40:1345–1357. doi: 10.3892/ijo.2012.1333. [DOI] [PubMed] [Google Scholar]
- 28.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. Proliferation of immature tumor vessels is a novel marker of clinical progression in prostate cancer. Cancer Res. 2009;69:4708–4715. doi: 10.1158/0008-5472.CAN-08-4417. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi Y, Tucker SL, Kitadai Y, Koura AN, Bucana CD, Cleary KR, Ellis LM. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch Surg. 1997;132:541–546. doi: 10.1001/archsurg.1997.01430290087018. [DOI] [PubMed] [Google Scholar]
- 30.Ishiwata T, Matsuda Y, Naito Z. Nestin in gastrointestinal and other cancers: effects on cells and tumor angiogenesis. World J Gastroenterol. 2011;17:409–418. doi: 10.3748/wjg.v17.i4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu T, Xu F, Du X, Lai D, Zhao Y, Huang Q, Jiang L, Huang W, Cheng W, Liu Z. Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol Cell Biochem. 2010;340:265–273. doi: 10.1007/s11010-010-0426-5. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, Wang J, Cai L, Zhong B, Luo H, Hao Y, Yu W, Wang B, Su C, Lei Y, Bella AE, Xiang AP, Wang T. Role of the stem cell-associated intermediate filament nestin in malignant proliferation of non-small cell lung cancer. PLoS One. 2014;9:e85584. doi: 10.1371/journal.pone.0085584. [DOI] [PMC free article] [PubMed] [Google Scholar]