Abstract
SGEF (SH3-containing Guanine Nucleotide Exchange Factor) is a RhoGEF of unknown function. We found the SGEF protein to be expressed in many established cell lines and highly expressed in human liver tissue. SGEF stimulated the formation of large interconnected membrane ruffles across dorsal surfaces when expressed in fibroblasts. SGEF required its proline-rich amino-terminus to generate dorsal, but not lateral, membrane ruffles and a functional SH3 domain to colocalize with filamentous actin at sites of membrane protrusion. Full-length SGEF activated RhoG, but not Rac, when expressed in fibroblasts. Further, recombinant SGEF DH/PH protein exchanged nucleotide on RhoG, but not on Rac1 or Rac3, in vitro. Scanning electron microscopy of fibroblasts demonstrated that SGEF induced dorsal ruffles that were morphologically similar to those generated by constitutively active RhoG, but not constitutively active Rac1. Transient expression of SGEF stimulated fibroblast uptake of 10-kDa dextran, a marker of macropinocytosis. This required the full-length protein and a catalytically active DH domain. Finally, activated RhoG was found to be more effective than activated Rac, and comparable to SGEF, in its ability to trigger dextran uptake. Together, this work establishes SGEF as a RhoG exchange factor and provides evidence that both SGEF and RhoG regulate membrane dynamics in promotion of macropinocytosis.
INTRODUCTION
The Rho proteins are low-molecular-weight GTP-binding proteins that cycle between GDP and GTP bound states. Binding of GTP “activates” Rho GTPases by inducing structural shifts that support association of effector molecules that transmit downstream signals. Rho GTPases are negatively regulated primarily by the sequestering activity of Rho guanine dissociation inhibitors (RhoGDIs; Olofsson, 1999) and by the Rho GTPase-activating proteins (RhoGAPs), which trigger GTP hydrolysis upon binding (Moon and Zheng, 2003). Conversely, Rho GTPases are positively regulated by Rho guanine nucleotide exchange factors (RhoGEFs) that catalyze the exchange of bound GDP for GTP (Schmidt and Hall, 2002). Rho GTPases are modified by the addition of a prenyl moiety to their carboxyl-terminal cysteine residue. This posttranslational event is necessary for GTPase function (Ando et al., 1992, Ohya et al., 1993), presumably by allowing interactions with phospholipid membranes and other proteins.
There are currently 22 identified Rho family members that synergistically govern a number of cell functions, such as gene expression, cell adhesion, and cytoskeletal dynamics (Burridge and Wennerberg, 2004). Emerging goals of this field are to assign temporal and spatial activation patterns of the GTPases and understand how these enzymes engage in “cross-talk” with each other to effectively regulate cell function. For example, Gauthier-Rouviere et al. (1998) discovered that GTP-bound RhoG was an upstream activator of both Rac1, a major regulator of membrane protrusion, and Cdc42, a protein linked with membrane protrusion, cell polarity, and vesicular trafficking.
RhoG, an ubiquitously expressed GTPase, was isolated from a screen for mRNA molecules upregulated in G1 phase (Vincent et al., 1992). RhoG shares significant homology with Rac and binds to a number of the same effector proteins, with the notable exception of p21-activated kinase (PAK; Gauthier-Rouviere et al., 1998; Wennerberg et al., 2002). Functionally, RhoG activity is sufficient to drive neurite outgrowth in PC12 cells and is required for NGF stimulation of outgrowth (Katoh et al., 2000). Rac and Cdc42 signaling is also necessary; however, neither GTPase is sufficient to drive outgrowth when overexpressed alone (Katoh et al., 2000), indicating that additional RhoG signaling and/or RhoG's ability to coordinate Rac and Cdc42 activation is needed in the formation of neurite extensions. Katoh and Negishi (2003) discovered that GTP-RhoG binds to ELMO and forms a ternary complex with ELMO and DOCK180. Formation of this ternary complex is postulated to advance DOCK180 exchange factor activity against Rac, thereby providing a molecular connection between RhoG and downstream Rac activation.
SGEF (SH3-containing Guanine Nucleotide Exchange Factor) was initially found in a screen for androgen-responsive genes in human prostate (Qi et al., 2003). Two alternatively transcribed forms of SGEF gene were isolated, one which encodes the full-length molecule of 871 amino acids and the other an androgen-inducible splice-variant of 139 amino acids that contains only a small segment of the pleckstrin homology (PH) domain and the entire carboxyl Src homology 3 (SH3) domain. Northern blot analysis of SGEF mRNA revealed an ubiquitous low-level expression in a variety of tissues, with pronounced expression in liver. The 139-amino acid splice variant was restricted to liver and prostate. SGEF has been classified as a putative exchange factor for Rho GTPases based on the presence of a Dbl homology (DH) domain, the conserved catalytic core of most RhoGEF proteins. However, the expression, specificity, and function of the SGEF protein are unknown. We report here that SGEF is a RhoG guanine nucleotide exchange factor that stimulates macropinocytosis.
MATERIALS AND METHODS
Reagents
Bovine serum albumin (BSA) and buffer reagents were acquired from Sigma (St. Louis, MO). Polyvinylidene fluoride (PVDF) membranes were purchased from Millipore (Billerica, MA). Rac1 and Cdc42 monoclonal antibodies were purchased from BD Transduction Laboratories (Lexington, KY).
SGEF Cloning
Full-length SGEF cDNA was cloned from a Marathon Brain cDNA library (BD Clontech, Palo Alto, CA) using end primers designed from the published SGEF cDNA sequence (GenBank Accession NM015595). Isolated clones were identical to the published sequence with the exception of an A→C nucleotide substitution in position 2343 in the coding sequence, resulting in a serine instead of an arginine at residue 781. Several clones were isolated from the cDNA library and they were all of the C2343 variant. A BLAST search for alternative deposited sequences for SGEF revealed that all GenBank entries except the NM015595 and its derivates contained a C in the 2343 position. We have deposited our version of the SGEF coding sequence as GenBank accession number AY552599.
Expression Vectors
SGEF cDNA was subcloned using BamHI/EcoRI restriction sites into pCMV6M, an amino-terminal Myc epitope-tagged eukaryotic expression vector (gift of Dr. Jonathan Chernoff, Fox Chase Cancer Center). SGEF truncations were created using restriction primers, all of which utilized BamHI/EcoRI restriction sites, and subcloned into pCMV6M. The cDNA fragment encoding the DH/PH domains was additionally subcloned into the prokaryotic expression vector pET28a (Novagen, Madison, WI). Mutations of SGEF cDNA were created through PCR mutagenesis using the Quickchange mutagenesis kit (Stratagene, La Jolla, CA) and confirmed by DNA sequencing. Generation of eukaryotic expression vectors pCMV-Myc-Rac(Q61L), pCMVMyc-RhoG(Q61L), pCMV-Myc-RhoG, pCMV-Myc-POSH (RBD), and pEGFP-N1-oncoVAV2 (amino acids 184-878) was described previously by our laboratories (Liu and Burridge, 2000; Wennerberg et al., 2002). pCMY-122 (Myc-tagged RhoGIP122) was provided by Dr. Anne Blangy, Centre de Recherches en Biochimie Macromoleculaire, France (Blangy et al., 2000). pGEX-4T-1-TC10 was engineered by subcloning the TC10 cDNA into pGEX-4T-1 using EcoRI/XhoI restriction sites. Generation of the prokaryotic expression constructs pGEX 2T-PBD, pGEX 4T-1-RhoA, pGEX 4T-1-Rac1, pGEX 4T-1-Cdc42, pGEX 4T-1-RhoG, pGEX4T-1-RhoG(15A), pGEX4T-1-Rac(15A), pGEX4T-1-Cdc42(15A), and pGEX4T-1-RhoA(17A) was described previously by our laboratories (Wennerberg et al., 2002; Arthur et al., 2002; Ellerbroek et al., 2003). pGEX 4T-2-ELMO was a kind gift of Dr. Kodimangalam Ravichandran, University of Virginia. pGEX 2T-Rac3 was a gift of Dr. Adrienne Cox, University of North Carolina at Chapel Hill (Joyce and Cox, 2003).
Cell Culture and Transfections
NIH3T3 cells were maintained in growth medium (DMEM supplemented with 10% bovine calf serum; Sigma). Cells were transiently transfected with the expression vectors indicated in each experiment according to the manufacturer's protocol using LipofectAMINE PLUS (Life Technologies, Rockville, MD). For immunofluorescence assays, cells were grown on coverslips and transfected in 24-well cluster plates. After introduction of the expression vectors for 3 h, transfection medium was supplemented with an equal volume of serum-containing medium and incubated for 16 h before the processing of cells.
Detection of SGEF Protein
Rabbit immunizations and serum production were performed by Covance (Denver, PA). The SGEF immunogen corresponded to amino acids 850-870 of the full-length molecule with an additional carboxyl cysteine residue for conjugation purposes (QATIDKNVERMGRLLGLETNC). Rabbits were immunized with the immunogen thiol bonded to keyhole limpet hemocyanin. Affinity purifications were performed by conjugating the immunogen via the carboxyl cysteine to SulfoLink Coupling Gel (Pierce, Rockford, IL), incubation with SGEF antisera, washing, and low pH elution. Eluted antibodies were concentrated by ammonium sulfate precipitation, dialyzed, and stored in phosphate-buffered saline (PBS) with NaN3 at -20°C. Protein analysis with the SGEF antibody was performed with human tissue membranes from IMGENEX (San Diego, CA) or with 15 μg of whole cell lysates from the indicated cells. Membranes were also stained with Ponceau S (Sigma) before immunoblot analysis to ensure that comparable amounts of total protein in each extract sample were used.
Immunofluorescence
Transfected NIH3T3 fibroblasts on glass coverslips were fixed for 7 min in 3.7% formaldehyde/PBS and then permeabilized for 2 min with 0.5% Triton X-100/PBS. Filamentous actin was labeled with Alexa Fluor 594 - conjugated phalloidin (Molecular Probes, Eugene, OR). Exogenously expressed proteins were stained for the c-Myc antigen (clone 9E10, Sigma) and visualized with FITC-conjugated donkey anti-mouse secondary antibody (Covance, Richmond, CA). Images were obtained on an Axiophot microscope (Zeiss, Thornwood, NY) using a MicroMAX 5 MHz cooled CCD camera (Princeton Instrument, Trenton, NJ) and Metamorph Image software (Universal Imaging Corp., West Chester, PA). For confocal microscopy, cells were processed as above and z-series images collected on a Leica SP2 laser scanning confocal microscope using the Leica Confocal Software program.
Scanning Electron Microscopy
Transfected NIH3T3 were grown on glass coverslips and then fixed in 2.5% glutaraldehyde/PBS for 30 min at room temperature and processed for scanning electron microscopy (SEM) similar to as previously described (Brunk et al., 1981). Briefly, samples were incubated with 2% aqueous osmium tetroxide for 45 min, dehydrated in a graded ethanol series, and then critical point dried in liquid CO2 using the Balzers CPD 010 (Balzers Instruments, Balzers, Liechtenstein). Samples were mounted on aluminum stubs (Ted Pella, Inc., Redding, CA) and sputter coated with gold/palladium using the Polaron SEM. Coating Unit E5100 (Thermo VG Scientific, Beverly, MA). Fibroblasts were examined on a JEOL 820 scanning electron microscope (JEOL USA, Peabody, MA) at 15 kV and at magnifications ranging from 400 to 3500×.
Rho GTPase Activation Assays
The amount of activated, GTP-bound Rho proteins was measured using a technique similar to the method described by Ren et al. (1999). For Rac and Cdc42 activation assays, transfected cells were lysed in 350 μl of 50 mM Tris, pH 7.4, 10 mM MgCl2, 500 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% deoxycholate, and protease inhibitors. Lysates (500-750 μg) were cleared at 16,000 × g for 5 min. Supernatants were rotated for 20 min with 35 μg GST-PBD (GST fusion protein containing the Rac/Cdc42 binding domain of PAK1) bound to glutathione-Sepharose beads (Amersham Pharmacia Biotech, Uppsala, Sweden). Samples were washed in 50 mM Tris, pH 7.4, 10 mM MgCl2, 150 mM NaCl, 1% Triton X-100, and protease inhibitors. Pull downs and lysates were then Western blotted for either GTPase. For RhoG activation assays, a low-level transient coexpression of Myc epitope-tagged RhoG was utilized because of a lack of a specific antibody that detects endogenous RhoG protein. Cells were lysed and clarified as above and the supernatant rotated for 20 min with 75 μg GST-ELMO (GST fusion protein containing the full-length RhoG effector, ELMO) conjugated to glutathione-Sepharose beads. Samples were washed as above and then Western blotted for the c-Myc epitope tag.
Fusion Proteins
GST-PBD, GST-ELMO, and GST-Rho fusion proteins (GST-RhoG, GST-Cdc42, GST-Rac1, GST-Rac3, GST-RhoA) were purified from BL21 Escherichia coli cells (Stratagene) using glutathione-Sepharose 4B (Amersham Pharmacia Biotech). GST-Rho proteins were eluted with free and reduced glutathione in TBSM (50 mM Tris, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT) and stored in 30% glycerol. SGEF DH/PH-His6 was purified from BL21 E. coli cells using Ni NTA-sepharose (Qiagen, Valencia, CA). SGEF DH/PH-His6 sepharose was incubated with GEF elution buffer (50 mM Tris, 150 mM NaCl, 100 mM EDTA, 1 mM DTT), equilibrated with 50 mM MgCl2, and then stored in 30% glycerol at -70°C. Purified Vav2 DH/PH/CRD-His6 fusion protein was kindly provided by Dr. Michelle Booden, University of North Carolina at Chapel Hill (Booden et al., 2002).
In Vitro Guanine Nucleotide Exchange Factor Assays
Fluorescence spectroscopic analysis of N-methylanthraniloyl (mant)-GTP (Biomol, Plymouth Meeting, PA) incorporation into GDP-preloaded GST-Rho proteins was carried out using a FLUOstar fluorescence microplate reader at 25°C similar to as described previously (Leonard et al., 1994). GST-Rho GTPase, 2 μM, was prepared and allowed to equilibrate in exchange buffer (20 mM Tris, pH 7.5, 50 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol, 50 μg/ml BSA, 1% glycerol) for 5 min before addition of 500 nM mant-GTP. At the indicated time, 400 nM of SGEF DH/PH-His6, 150 nM Vav2 DH/PH/CRD-His6, or an equal volume of GEF elution buffer was added and the change of mant-GTP fluorescence (excitation = 360 nm, emission = 460 nm) was monitored. The final condition for GEF exchange reactions were 20 mM Tris, pH 7.5, 50 mM NaCl, 11 mM MgCl2, 2 mM EDTA, 1 mM dithiothreitol, 50 μg/ml BSA, and 1% glycerol. Experiments were performed in duplicate for every reaction.
Macropinocytosis Assays
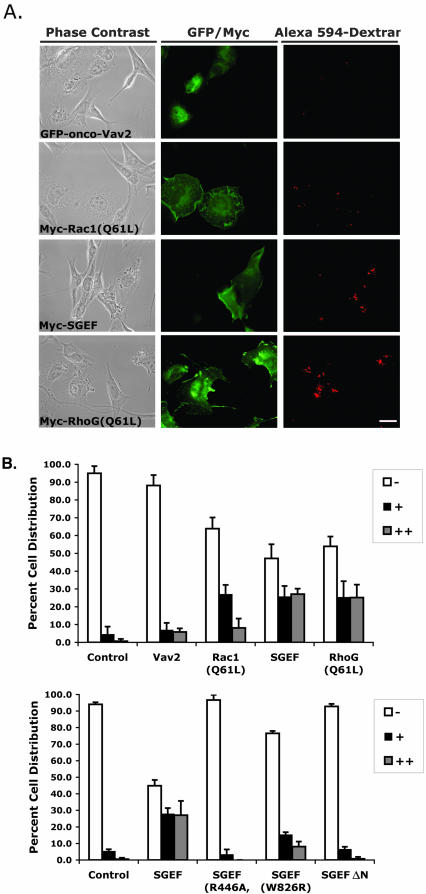
Transfected NIH3T3 cells were cultured to 70% confluency on glass coverslips in a 24-well plate. Cells were washed twice in DMEM and then incubated with warm (37°C) internalization medium (DMEM plus 0.15% BSA) for 5 min. Medium was removed and cells incubated with 250 μl of 50 μg/ml Alexa Fluor 594 - conjugated 10-kDa dextran (Molecular Probes) in internalization medium at 37°C for 5 min. Dextran uptake was stopped by adding ice-cold PBS. After washing twice with 1-ml volumes of ice-cold PBS, cells were immediately fixed with 3.7% formaldehyde at 25°C for 7 min and processed for immunofluorescence. Internalization of dextran was scored by fluorescence microscopy as described in the figure legend (Figure 11).
Figure 11.
Myc-SGEF and RhoG stimulate macropinocytosis in NIH3T3 cells. Cells were transiently transfected overnight with expression plasmids for the indicated protein before incubation with Alexa Fluor 594-conjugated 10-kDa dextran in uptake buffer (DMEM w/0.15% BSA) for 5 min. When appropriate, cells were immunostained for the Myc epitope tag to identify expressing cells. Cells were scored as a (-) if dextran uptake was the same as untransfected cells, and as (+) or (++) as macropinocytosis increased. (A) GFP-onco-Vav2, although a strong activator of Rho GTPases, does not significantly promote dextran uptake (-), while Myc-Rac1(Q61L) stimulated uptake (+). Both Myc-SGEF and Myc-RhoG(Q61L) consistently promoted the greatest uptake of dextran (++). (B) Dextran uptake was scored for each condition from three independent coverslips according to the (-), (+), and (++) grading system. Note that stimulation of dextran uptake by SGEF required a full-length catalytically active enzyme with a functional SH3 domain.
RESULTS
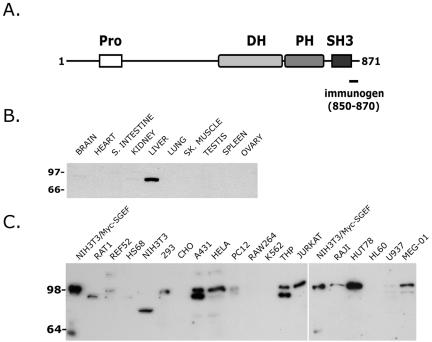
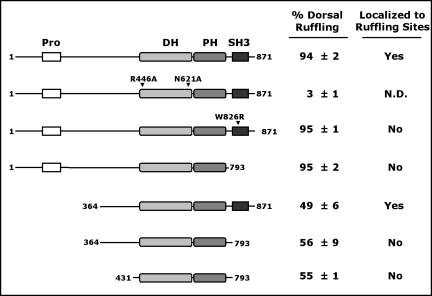
SGEF is an 871-amino acid protein consisting of an amino-terminal proline-rich region, followed closely by a tandem DH/PH domain characteristic of many RhoGEFs, and a carboxyl-terminal SH3 domain (Figure 1A). Sequence comparison of the SGEF DH domain with that of other RhoGEFs showed strongest sequence identity with a subfamily that includes VSM-RhoGEF, TIM, Ephexin, and Neuroblastoma (Schmidt and Hall, 2002). All of these GEFs, except VSM-RhoGEF, contain an SH3 domain immediately after their DH/PH domains, furthering the classification of this group as a structurally related subfamily of enzymes.
Figure 1.
(A) Full-length SGEF is an 871 amino acid protein containing an amino-terminal proline-rich region (Pro), a Dblhomology (DH) domain followed by a Pleckstrin homology (PH) domain, and a carboxyl-terminal Src-homology 3 domain (SH3). A 21-mer peptide corresponding to residues 850-870 was used to generate rabbit antiserum against SGEF (bar). (B) A highly expressed 80-kDa form of SGEF was detected in liver using affinity-purified SGEF antibodies. (C) Lysates (15 μg) collected from a variety of cell lines contain the full-length 97-kDa SGEF protein. Lane 1 in either blot contains 2 μg of NIH3T3 fibroblast lysate containing exogenous Myc epitope-tagged SGEF to demonstrate migration of the full-length molecule. A subset of cell lines contained a 90-kDa (Rat1, A431, THP, U937, MEG-01) or a 75-kDa (NIH3T3) immunoreactive protein.
To examine SGEF protein expression, a polyclonal antiserum was raised against a peptide corresponding to residues 850-870 of the molecule. Affinity-purified antibodies revealed that SGEF was predominately expressed in human liver tissues, with an apparent molecular weight of 80 kDa (Figure 1B). Longer exposures revealed low-level expression of SGEF in brain and kidney tissues (unpublished data). Analysis of established cell lines (Figure 1C) revealed that SGEF was expressed as two major forms, a 97-kDa protein that comigrated with exogenously expressed Myc epitope-tagged enzyme and a smaller 90-kDa species, neither of which were mutually exclusive. SGEF was expressed at very low levels in fibroblasts, with the exception of NIH3T3 cells, which contained a potentially unique 75-kDa form of the protein. SGEF was also detected at low levels in the human embryonic kidney cell line 293T and the rat neuronal cell line PC12. The enzyme was expressed at high levels in the human epithelial cell lines A431 and HeLa, but was undetectable in CHO hamster cells. Lastly, SGEF was expressed in a number of hematopoietic cell lines (THP, Jurkat T-cells, RAJI, HUT78, U937, and Meg-01), but undetectable in others (RAW264, K562, and HL-60). Together, we conclude SGEF is an ubiquitously expressed protein.
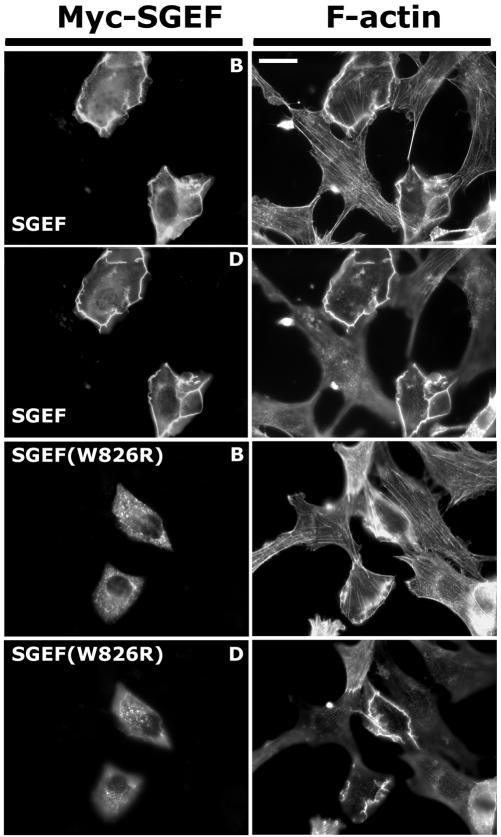
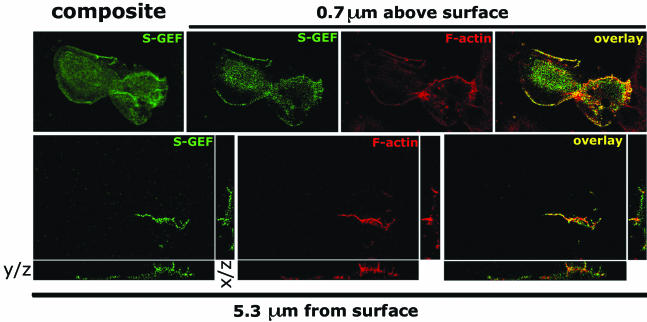
As a putative RhoGEF, we predicted that SGEF will influence cell morphology through its regulation of Rho GTPase(s). NIH3T3 fibroblasts transiently overexpressing full-length Myc epitope-tagged SGEF lost stress fibers and displayed enhanced ruffling activity around their periphery (Figure 2). Adjustments in focal plane revealed that peripheral ruffles extended up and continued across the dorsal surface of the cells (Figure 2). Immunostaining for the Myc epitope tag indicated that Myc-SGEF localized to sites of membrane ruffling. To extend this observation, cells were processed for z-series analysis by confocal microscopy (Figure 3). Myc-SGEF colocalized with filamentous actin (F-actin) at sites of lateral ruffling. On the dorsal surface, Myc-SGEF was evident at the tips of F-actin rich dorsal ruffles, indicating that Myc-SGEF is targeted to sites of membrane protrusion.
Figure 2.
SGEF generates dorsal-ruffling in NIH3T3 fibroblasts. NIH3T3 fibroblasts transiently expressing the indicated Myc-SGEF protein were immunostained for the Myc-antigen (Myc-SGEF) and incubated with Alexa Fluor 594-Phalloidin to identify filamentous actin (F-actin) as indicated. On the basal plane of focus (marked as B), Myc-SGEF expressing cells displayed a loss of actin stress fibers. On the dorsal plane of focus (marked as D), Myc-SGEF expressing fibroblasts contained actin-rich ruffles around their edges and across their dorsal surfaces. Myc-SGEF colocalized with F-actin on either planes of focus. When the SH3 domain was inactivated by a point mutation of an invariant trytophan, Myc-SGEF (W826R) adopted a punctate localization throughout the cell body and no longer colocalized with F-actin on either plane of focus. However, there was little difference in the quantity or quality of dorsal-ruffles stimulated by Myc-SGEF (W826R) compared with the wild-type enzyme. Bar, 20 μm.
Figure 3.
SGEF colocalizes with F-actin at the tips of dorsal ruffles. Fibroblasts transiently expressing Myc-SGEF were immunostained for the Myc epitope tag (green) and incubated with Alexa Fluor 594-Phalloidin to identify F-actin (red). Z-series images were captured by confocal microscopy. S-GEF and F-actin colocalized along the lateral edges of fibroblasts (overlay, 0.7 μm above surface) and at the tips of the dorsal ruffles (overlay, 5.3 μm above surface). The y/z and x/z planes are provided for protein localization 5.3 μm above surface.
To examine if the SH3 domain is required for the ruffling phenotype, we expressed full-length Myc-SGEF molecules containing a single amino acid substitution of an invariant tryptophan (W826R) within the SH3 domain. Substitution of this key residue has been used as a SH3 domain inactivation mutation in other studies (Hing et al., 1999), including work involving the RhoGEF Trio (Estrach et al., 2002). Myc-SGEF(W826R) elicited a dorsal ruffling phenotype with similar potency as the wild-type protein; however, it failed to localize to sites of membrane ruffling (Figure 2). Identical results were observed when the entire SH3 was deleted from the molecule (unpublished data). Thus, although the SH3 domain may influence subcellular localization, it was dispensable for actin reorganization activity.
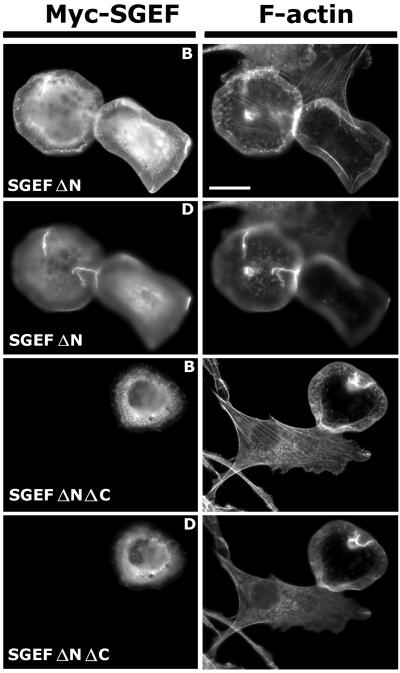
Removal of amino-terminal sequences upstream of the DH domain has been found to be an activating event for many RhoGEFs (Katzav et al., 1991; van Leeuwen et al., 1995). Surprisingly, Myc-SGEFΔN (aa 364-871) was significantly less effective than the full-length protein in generating dorsal ruffles. Instead, cells adopted a lateral lamellipodial phenotype. Additional removal of the SH3 domain excluded Myc-SGEFΔNΔC (aa 364-793) from actin-rich lamellipodial extensions (Figure 4). The ability of different Myc-SGEF missense and truncation mutants to stimulate and localize to dorsal ruffles is summarized in Figure 5.
Figure 4.
SGEFΔN (aa 364-871) promotes lamellipodia instead of dorsal-ruffles. NIH3T3 fibroblasts transiently expressing the indicated Myc-SGEF protein were immunostained for the Myc-epitope tag (Myc-SGEF) and incubated with Alexa Fluor 594-Phalloidin to identify F-actin as indicated. On the basal surface (marked as B), Myc-SGEF ΔN stimulated lateral lamellipodia formation and displayed diffuse staining with limited colocalization with F-actin. Unlike the full-length protein, Myc-SGEFΔN either failed to generate or weakly stimulated actin-rich ruffles on the dorsal surface (marked as D). Myc-SGEF ΔNΔC (aa 364-793), which lacks the SH3 domain, also promoted lateral lamellipodia, but was restricted to the cell body and failed to localize to small ruffles on the dorsal surface in contrast to Myc-SGEFΔN. Bar, 20 μm.
Figure 5.
Summation of Myc-SGEF-induced dorsal ruffling and Myc-SGEF localization to dorsal ruffles in NIH3T3 fibroblasts. Cells were scored for the presence or absence of Myc-SGEF-induced dorsal ruffles and presented as % expressing ± SD of three scored coverslips. Catalytically dead Myc-SGEF (E446A, N621A) did not affect cell morphology (Figure 10B). Myc-SGEFΔN proteins produced significantly weaker and less numerous dorsal ruffles (compare Figures 2 and 4). Myc-SGEF lacking the SH3 domain or carrying an SH3-inactivating mutation (W826R) failed to localize with F-actin at sites of dorsal ruffling. ND, not determined.
Membrane ruffling is a phenomenon that has been connected to the activation of Rho GTPases of the Rac subfamily, including Rac1 and RhoG (Ridley et al., 1992; Gauthier-Rouviere et al., 1998). To extend our characterization of the dorsal ruffling phenotype and to compare the morphological effects of Myc-SGEF with that of constitutively active Myc-Rac1(Q61L) and Myc-RhoG(Q61L), fibroblasts expressing the respective proteins were processed for SEM (Figure 6). Myc-SGEF-expressing fibroblasts displayed large interconnected membrane ruffles across their dorsal surfaces, with a number of circular dorsal ruffles evident at higher magnification. Expression of Myc-Rac1(Q61L) universally promoted a flattened cell phenotype with shorter and more numerous dorsal ruffles than Myc-SGEF expressing cells. Conversely, a population of fibroblasts expressing Myc-RhoG(Q61L) possessed interconnected dorsal ruffles of similar appearance and magnitude as Myc-SGEF-expressing cells. In contrast to the high percentage of ruffling cells caused by full-length Myc-SGEF (Figure 5), it was estimated by F-actin staining that only 30-35% of Myc-RhoG(Q61L) fibroblasts contained the large interconnected dorsal ruffles, with the remainder of cells expressing a range of lateral ruffling phenotypes.
Figure 6.
Myc-SGEF expressing fibroblasts are morphologically similar to cells expressing activated RhoG. NIH3T3 cells were transiently transfected with expression plasmids for the indicated Myc-tagged protein, with mock cells receiving empty vector. Fibroblasts were then processed for scanning electron microscopy as described in MATERIALS AND METHODS. Myc-SGEF-induced dorsal ruffles were evident around cellular edges and across the dorsal surface of the fibroblast. Higher magnification revealed that the dorsal ruffles were highly connected with some degree of circular ruffling. Myc-Rac1(Q61L) expressing cells appeared flat with numerous dorsal ruffles that were shorter in stature than SGEF-induced ruffles. Cells expressing Myc-RhoG(Q61L) exhibited dorsal ruffles that were reminiscent of Myc-SGEF cells in both their connectivity and stature. Like Myc-SGEF cells, Myc-RhoG(Q61L) cells were less spread than Mock or Myc-Rac1(Q61L)-expressing cells. Bar, 10 μm.
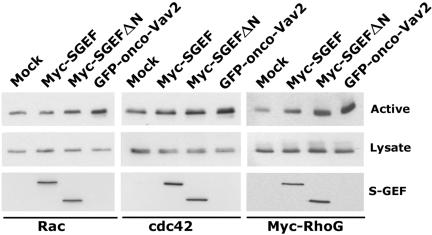
Fibroblasts expressing Myc-SGEF appear morphologically more similar to cells expressing constitutively active RhoG than fibroblasts expressing activated Rac1. To examine if Myc-SGEF activates Rac, Cdc42, or RhoG, we performed effector pull-down assays to measure changes in the intracellular GTP loading of these Rho proteins (Figure 7). To detect changes in the activation state for RhoG, a low level of Myc-RhoG was expressed in the absence or presence of the indicated GEF and then activated Myc-RhoG pulled down with GST-ELMO. Full-length Myc-SGEF efficiently activated Myc-RhoG, but not Rac or Cdc42. Myc-SGEFΔN strongly activated Myc-RhoG and weakly promoted GTP-loading of Rac and Cdc42. As a positive control, GFP-onco-Vav2 stimulated increased GTP-loading of all three GTPases. Together, these data indicate that amino-terminal truncation of SGEF is an activating event that results in GTP-loading of Rac, Cdc42, and RhoG, whereas only RhoG is activated by the intact full-length molecule.
Figure 7.
Myc-SGEF stimulates RhoG activation in NIH3T3 cells. GST-PBD was used to pull down GTP-loaded Rac1 or Cdc42. GST-ELMO was used to pull down GTP-loaded Myc-RhoG. Low-level expression of Myc-RhoG was used in place of the endogenous protein for lack of a specific RhoG antibody. Myc-SGEF, Myc-SGEFΔN, or GFP-onco-Vav2 (positive control) was transiently expressed and the resulting change in GTPase activation (labeled active) compared with empty vector-transfected fibroblasts (Mock).
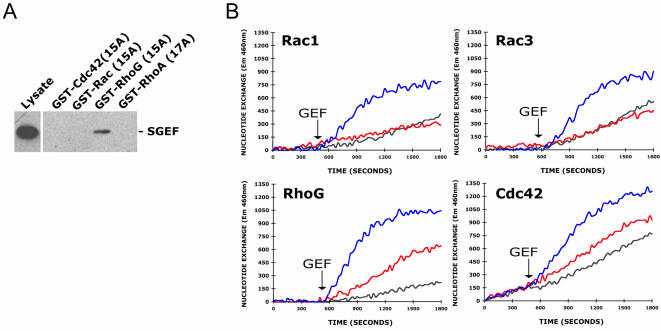
To identify which GTPases are substrates for SGEF, we examined the ability of different Rho proteins to bind Myc-SGEF DH/PH/SH3 (aa 431-871). Myc-SGEF DH/PH/SH3 was expressed in fibroblasts and then pulled down using GST-Rho fusion proteins containing a point mutation that disrupts binding of guanine nucleotides. Myc-SGEF DH/PH/SH3 was specifically pulled down with nucleotide-free RhoG, but not Rac, Cdc42, or RhoA (Figure 8A). To confirm that SGEF is an exchange factor, purified DH/PH domain of SGEF (amino acids 431-793) was incubated with recombinant GST-tagged Rho GTPases in vitro. Exchange for nucleotide was measured using a fluorescence assay (Figure 8B), with the DH/PH/CRD of Vav2 used as a positive control. Although Myc-SGEFΔN generated a Rac-like phenotype (Figure 4) and weakly promoted activation of Rac in fibroblasts (Figure 7), SGEF DH/PH did not exchange nucleotide on Rac1 or Rac3. On the other hand, SGEF DH/PH weakly exchanged Cdc42 and, consistent with nucleotide-free Rho GTPase pull-down results, strongly exchanged nucleotide on RhoG. SGEF did not exchange nucleotide on RhoA or TC10 (unpublished data).
Figure 8.
SGEF preferentially binds and exchanges RhoG in vitro. (A) Myc-SGEF DH/PH/SH3 (aa 431-871) was expressed in fibroblasts and then pulled down with the indicated GST-Rho fusion protein containing a point mutation that disrupts binding of guanine nucleotides. Myc-SGEF DH/PH/SH3 bound nucleotide-free RhoG. (B). GST-GTPase fusion proteins (Rac1, Rac3, RhoG, or Cdc42) were incubated with Mant-GTP and the rate of nucleotide incorporation assessed over time (Δ460 nm). SGEF DH/PH (red), Vav2 DH/PH/CRD (blue), or GEF elution buffer (black) were added to the reactions at the indicated time (GEF with arrow) and the resulting change in the rate of Mant-GTP incorporation monitored. SGEF exchanged nucleotide on RhoG and, to a lesser extent, Cdc42 in vitro.
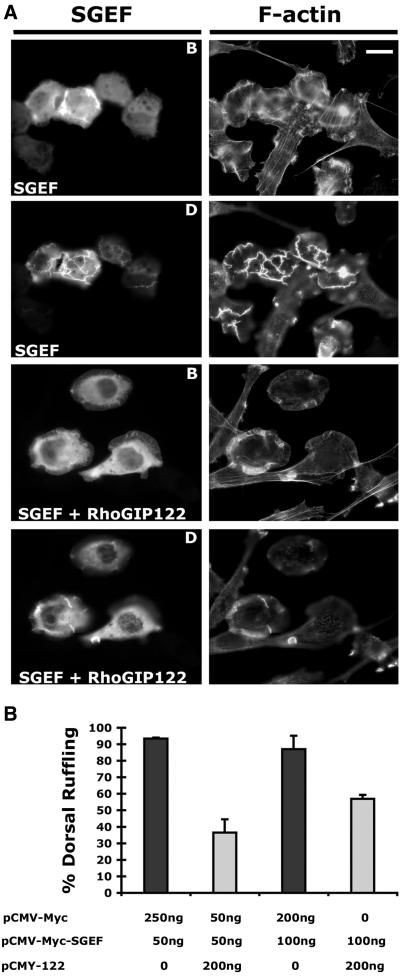
The ability of SGEF to activate RhoG in vivo and in vitro, along with the morphological similarities between SGEF and a subset of constitutively active RhoG expressing fibroblasts, indicates that obstructing RhoG signaling should block dorsal ruffling of SGEF-expressing cells. To test this possibility, fibroblasts were cotransfected with expression plasmids for Myc-SGEF and the dominant negative RhoG effector fragment Myc-RhoGIP122 (Blangy et al., 2000). Coexpression of Myc-RhoGIP122 effectively blocked SGEF-induced dorsal ruffling (Figure 9, A and B), demonstrating that SGEF requires RhoG signaling for this activity. The RhoGIP122 fragment was either not sufficient or suitably expressed to eliminate SGEF-induced lamellipodium formation.
Figure 9.
Myc-SGEF requires RhoG signaling to stimulate dorsal ruffling. (A) NIH3T3 fibroblasts were transiently cotransfected with 100 ng of Myc-SGEF expression plasmid and either 200 ng of empty vector (marked SGEF) or 200 ng of CMY-122, a Myc-RhoGIP122 expression plasmid (marked SGEF + RhoGIP122). Myc-RhoGIP122 is a RhoG effector fragment that obstructs RhoG signaling (Blangy et al., 2000). Fibroblasts were immunostained with the SGEF polyclonal antibody to ensure only SGEF-expressing cells were visualized. Myc-RhoGIP122 expression effectively blocked ruffling on the dorsal surface (marked as D), but was not sufficient to eliminate lamellipodia formation on the basal surface (marked as B). Bar, 10 μm. (B) Quantification of dorsal ruffling. NIH3T3 fibroblasts were transiently cotransfected with the indicated amounts of expression plasmids. SGEF-expressing cells were scored for the presence or absence of dorsal ruffling. Error bars represent the SD between two independent coverslips for every condition.
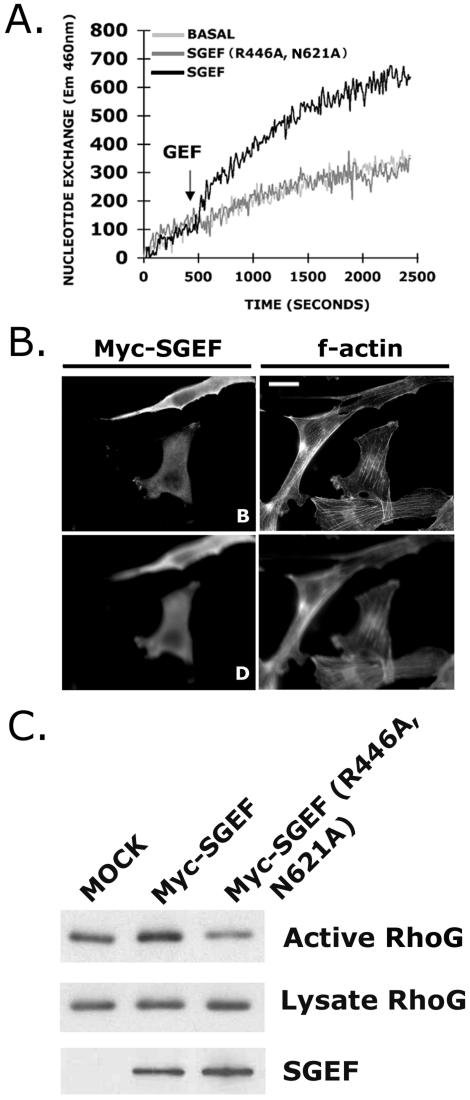
To confirm whether the effects of Myc-SGEF on cell morphology are due to its exchange activity, we inactivated the DH domain of SGEF through a pair of mutations, E446A and N621A. These residues were chosen based on a separate study demonstrating the requirement of analogous residues for Rho protein binding and exchange activity of the RacGEF TIAM1 (C. Der, J. Lambert, M. Pham, and J. Sondek, unpublished results). Recombinant SGEF DH/PH(E446A, N621A) failed to exchange nucleotide on RhoG in vitro (Figure 10A). Moreover, expression of comparable amounts of full-length Myc-SGEF(E446A, N621A) did not affect cell morphology (Figure 10B) or activate Myc-RhoG in cells (Figure 10C), establishing that Myc-SGEF exchange activity is required for generation of a ruffling phenotype.
Figure 10.
Myc-SGEF (E446, N621A) is catalytically dead and does not affect NIH3T3 cell morphology. To examine whether Myc-SGEF requires exchange activity to promote ruffling, catalytic function was knocked-out through mutation of two key residues of the DH domain, E446 and N621. Purified SGEF DH/PH-6xHis (E446A, N621A) does not promote exchange of RhoG (A), nor does expression of Myc-SGEF (E446, N621A) affect cell morphology (B) or stimulate RhoG activation in cells (C). The basal (B) and dorsal (D) planes of focus are provided in panel B.
Macropinocytosis is a cellular mechanism for nonselective uptake of solute macromolecules (Johannes and Lamaze, 2002). This event can proceed constitutively or be stimulated shortly after growth factor receptor activation (e.g., epidermal or platelet-derived growth factor receptors). The process of macropinocytosis involves production of actin-rich circular ruffles that eventually fold back and fuse with the plasma membrane, thereby forming fluid vesicles called macropinosomes. Macropinosomes are then internalized and ultimately release their soluble cargo. The appearance of circular dorsal ruffles on the surface of Myc-SGEF-expressing cells led us to hypothesize that SGEF positively regulates macropinocytic events. To examine this possibility, NIH3T3 fibroblasts were transiently transfected with either Myc-SGEF, Myc-Rac1(Q61L), Myc-RhoG(Q61L), or GFP-oncoVav2 as a control for the effects of general and aberrant Rho GTPase activation in these fibroblasts, and then uptake of Alexa Fluor 594-conjugated 10-kDa dextran was imaged and then scored as described in the figure legend (Figure 11). Even though GFP-oncoVav2 strongly activates Rac, Cdc42, and RhoG (Figure 7), it does not influence uptake of 10-kDa dextran over the time course of this study. As previously reported (Ridley et al., 1992; Dharmawardhane et al., 2000), expression of constitutively active Rac stimulated macropinocytosis. Strikingly, Myc-SGEF and constitutively active RhoG were more effective than Myc-Rac1(Q61L) in stimulating macropinocytosis in fibroblasts (Figure 11). Removal of Myc-SGEF DH domain function abrogated the ability of the GEF to stimulate dextran uptake, demonstrating that downstream GTPase activation is required. Moreover, although Myc-SGEFΔN catalyzed RhoG activation in fibroblasts (Figure 7) and Myc-SGEF (W826R) generated dorsal ruffles (Figure 3), their ability to stimulate dextran uptake was strongly attenuated, indicating that a full-length SGEF molecule with an intact SH3 domain is required for macropinocytosis.
DISCUSSION
SGEF was found to activate RhoG, and to a lesser extent Cdc42, in vitro and in vivo. Other known exchange factors for RhoG are Trio, Kalirin, Dbs, and Vav1/2 (Schuebel et al., 1998; Blangy et al., 2000; May et al., 2002, Wennerberg et al., 2002). With the exception of Dbs, these GEFs also exchange Rac, making SGEF unusual in its ability to discern between these two highly related potential substrates. Functionally, both Trio and Kalirin are implicated as a catalyst for RhoG activation during neurite outgrowth (Blangy et al., 2000; May et al., 2002). SGEF mRNA was detected in brain extracts (Qi et al., 2003), providing the possibility that a similar function(s) exists for SGEF. At the same time, the expression of SGEF in hematopoietic cell lines is noteworthy when considering that RhoG regulates gene transcription in B and T cells and homozygous disruption of the RhoG gene in mice results in elevated levels of immunoglobulins in serum and a mild increase in lymphocyte response to antigen (Vigorito et al., 2004).
Using an affinity-purified antibody, we readily detected in a number of cell lines a 97-kDa protein that comigrated with exogenously expressed full-length SGEF. We also observed a variable presence of smaller immunoreactive proteins (90, 80, and 75 kDa). These low-molecular-weight bands probably reflect that SGEF RNA is alternatively spliced to produce protein isoforms of varying functionality, as was suggested in the description of the SGEF gene (Qi et al., 2003) and recently described for the closely related exchange factor ARHGEF5/TIM (Debily et al., 2004). In support of this possibility, prostate and liver tissues have been reported to produce an alternate 139-amino acid truncated form of SGEF upon androgen stimulation that contains 50 amino acids of the PH domain and the entire SH3 domain (CSGEF, aa 732-871). The function of this fragment remains unclear, as we found that coexpression of a GFP-fusion protein containing the SH3 domain of SGEF (aa 793-871) did not influence the morphological effects or cellular localization of full-length SGEF proteins (unpublished data).
Amino-terminal truncation of SGEF enhanced its exchange activity in cells, but unexpectedly promoted a phenotypic shift from dorsal ruffling to lateral lamellipodia formation. RhoG(Q61L) also stimulated dorsal ruffles (Figure 6), demonstrating that a failure to restrict RhoG activation through aberrant GEF activity is not a sufficient explanation for the loss of dorsal ruffling. Rather, a more likely alternative is that the amino-terminus of SGEF facilitates dorsal ruffling through as yet unidentified interaction(s).
The RhoG effector fragment RhoGIP122 blocked SGEF-induced dorsal ruffling, confirming that SGEF signals through RhoG to control dorsal membrane dynamics (Figure 9). The RhoGIP122 fragment was not sufficient to block lamellipodial formation. However, the efficacy of Rho-GIP122 under our assay conditions is likely a limiting factor as this fragment did not block constitutively active RhoG-induced ruffling (unpublished data) and was sensitive to the level of SGEF expression (Figure 9B). SGEF does not activate Rac in cells as measured by effector-pull-down assays, nor does the SGEF catalytic fragment exchange Rac1 or Rac3 in vitro. At the same time, we observed that coexpression of the Rho-binding fragment of the Rac-specific effector POSH eliminated both constitutively active RhoG- and SGEF-induced lamellipodia (unpublished data), indicating that some level of basal Rac activity is required for SGEF and RhoG-mediated lamellipodial formation.
A third of known RhoGEFs contain at least one putative SH3 domain, many of which reside after a PH domain (Schmidt and Hall, 2002). Previous work has demonstrated that inactivation of SH3 domain function through point mutation eliminates the ability of Trio to stimulate neurite outgrowth (Estrach et al., 2002) and Vav1 to promote cellular transformation (Groysman et al., 1998). In the case of the two SH3 domains of Vav1, the carboxyl SH3 domain has been found to bind a number of proteins, including zyxin (Hobert et al., 1996), whereas both SH3 domains work in unison to bind the adapter protein Grb2 (Ye and Baltimore, 1994). We found that inactivation of the SGEF SH3 domain through point mutation disrupts SGEF subcellular localization, but does not affect the ability of the protein to reorganize actin. This finding suggests that the SGEF amino-terminus and SH3 carboxyl domain work in concert to initiate dorsal ruffling and then stabilize SGEF localization at sites of actin-rich protrusion to advance dorsal ruffling events, such as macropinocytosis (Figure 11B).
Macropinocytosis is an efficient cellular process for the uptake of nutrients and macromolecules from the extracellular environment (Johannes and Lamaze, 2002). In most cells, macropinocytosis is transient and downstream of growth factor receptor activation. Conversely, immature dendritic cells use constitutive macropinocytosis as a mechanism for sampling of antigens during maturation (Sallusto et al., 1995). Macropinocytosis also contributes to infections, as many bacteria, such as Salmonella and Legionella (Chen et al., 1996; Watarai et al., 2001), and even viruses, such as HIV (Marechal et al., 2001), utilize macropinocytosis as a mechanism for entry into macrophages and other cells.
Previous studies have identified the GTPases Rac and Cdc42 as positive regulators of macropinocytic events. Constitutively active Rac and the downstream effector PAK1 localize to pinocytic vesicles (Schlunck et al., 2004; Dharmawardhane et al., 1997) and transient expression of constitutively active forms of either protein in fibroblasts is sufficient to promote macropinocytosis (Ridley et al., 1992; Dharmawardhane et al., 2000). In other work, Cdc42, and to a lesser extent Rac1, are required for Salmonella internalization into COS-1 cells by macropinocytosis (Chen et al., 1996). Moreover, expression of dominant-negative Rac(T17N) or Cdc42(T17N) inhibit constitutive macropinocytosis of immature dendritic cells (West et al., 2000; Garrett et al., 2000). Interestingly, neither Rac nor Cdc42 are required for dorsal ruffling of dendritic cells (West et al., 2000), leading to the theory that another GTPase is involved. We found that catalytically active SGEF and constitutively active RhoG stimulate 10-kDa dextran uptake in fibroblasts. This is the first identification of a mammalian RhoGEF that promotes macropinocytosis, and it is noteworthy that its downstream substrate, RhoG, is more effective than Rac at stimulating dextran uptake. It will be important in future studies to address the exciting possibility that RhoG, whose expression accumulates in response to growth factor signaling (Vincent et al., 1992), functions downstream of growth factor receptor activation to specifically control dorsal ruffling and generation of macropinosomes. This hypothesis is especially attractive considering that RhoG can function upstream of the established regulators of macropinoncytosis, Rac and Cdc42 (Gauthier-Rouviere et al., 1998).
Acknowledgments
We thank Dr. Bill Dunty for his help with the scanning electron microscopy and Dr. Erika Wittchen and The Michael Hooker Microscopy Facility at the University of North Carolina, Chapel Hill for assistance with the confocal microscopy. The authors are grateful to Drs. John Lambert and John Sondek for comments and valuable discussions. This work was supported by Postdoctoral Grant PF-02-019-01-TBE from the American Cancer Society (S.M.E.) and by National Institutes of Health Grants GM29860 and HL45100 and a Kenan Distinguished Professorship (K.B.)
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-02-0146. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-02-0146.
Abbreviations used: GDI, guanine dissociation inhibitor; GEF, guanine nucleotide exchange factor; GAP, GTPase-activating protein; SGEF, SH3 domain-containing GEF; DH, Dbl-homology; PH, pleckstrin homology; CRD, cysteine-rich domain; GST, glutathione S-transferase; PBD, the Rac/Cdc42-binding domain of PAK1; RhoGIP, RhoG-interacting protein; SEM, scanning electron microscopy; F-actin, filamentous actin.
References
- Ando, S. et al. (1992). Post-translational processing of rac p21s is important both for their interaction with the GDP/GTP exchange proteins and for their activation of NADPH oxidase. J. Biol. Chem. 267, 25709-25713. [PubMed] [Google Scholar]
- Arthur, W.T., Ellerbroek, S.M., Der, C.J., Burridge, K., and Wennerberg, K. (2002). XPLN, a guanine nucleotide exchange factor for RhoA and RhoB, but not RhoC. J. Biol. Chem. 277, 42964-42972. [DOI] [PubMed] [Google Scholar]
- Blangy, A., Vignal, E., Schmidt, S., Debant, A., Gauthier-Rouviere, C., and Fort, P. (2000). TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J. Cell Sci. 113, 729-739. [DOI] [PubMed] [Google Scholar]
- Booden, M.A., Campbell, S.L., and Der, C.J. (2002). Critical but distinct roles for the pleckstrin homology and cysteine-rich domains as positive modulators of Vav2 signaling and transformation. Mol. Cell. Biol. 22, 2487-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk, U., Collins, V.P., and Arro, E. (1981). The fixation, dehydration, drying and coating of cultured cells of SEM. J. Microsc. 123, 121-131. [DOI] [PubMed] [Google Scholar]
- Burridge, K., and Wennerberg, K. (2004). Rho and Rac take center stage. Cell 116, 167-179. [DOI] [PubMed] [Google Scholar]
- Chen, L.M., Hobbie, S., and Galan, J.E. (1996). Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science 274, 2115-2118. [DOI] [PubMed] [Google Scholar]
- Debily, M.A. et al. (2004). Expression and molecular characterization of alternative transcripts of the ARHGEF5/TIM oncogene specific for human breast cancer. Hum. Mol. Genet. 13, 323-334. [DOI] [PubMed] [Google Scholar]
- Dharmawardhane, S., Sanders, L.C., Martin, S.S., Daniels, R.H., and Bokoch, G.M. (1997). Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J. Cell Biol. 138, 1265-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmawardhane, S., Schurmann, A., Sells, M.A., Chernoff, J., Schmid, S.L., and Bokoch, G.M. (2000). Regulation of macropinocytosis by p21-activated kinase-1. Mol. Biol. Cell 11, 3341-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbroek, S.M., Wennerberg, K., and Burridge, K. (2003). Serine phosphorylation negatively regulates RhoA in vivo. J. Biol. Chem. 278, 19023-19031. [DOI] [PubMed] [Google Scholar]
- Estrach, S., Schmidt, S., Diriong, S., Penna, A., Blangy, A., Fort, P., and Debant, A. (2002). The Human Rho-GEF trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Curr. Biol. 12, 307-312. [DOI] [PubMed] [Google Scholar]
- Garrett, W.S., Chen, L.M., Kroschewski, R., Ebersold, M., Turley, S., Trombetta, S., Galan, J.E., and Mellman, I. (2000). Developmental control of endocytosis in dendritic cells by Cdc42. Cell 102, 325-334. [DOI] [PubMed] [Google Scholar]
- Gauthier-Rouviere, C., Vignal, E., Meriane, M., Roux, P., Montcourier, P., and Fort, P. (1998). RhoG GTPase controls a pathway that independently activates Rac1 and Cdc42Hs. Mol. Biol. Cell 9, 1379-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groysman, M., Nagano, M., Shaanan, B., and Katzav, S. (1998). Mutagenic analysis of Vav reveals that an intact SH3 domain is required for transformation. Oncogene 17, 1597-1606. [DOI] [PubMed] [Google Scholar]
- Hing, H., Xiao, J., Harden, N., Lim, L., and Zipursky, S.L. (1999). Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell 97, 853-863. [DOI] [PubMed] [Google Scholar]
- Hobert, O., Schilling, J.W., Beckerle, M.C., Ullrich, A., and Jallal, B. (1996). SH3 domain-dependent interaction of the proto-oncogene product Vav with the focal contact protein zyxin. Oncogene 12, 1577-1581. [PubMed] [Google Scholar]
- Johannes, L., and Lamaze, C. (2002). Clathrin-dependent or not: is it still the question? Traffic 3, 443-451. [DOI] [PubMed] [Google Scholar]
- Joyce, P.L., and Cox, A.D. (2003). Rac1 and Rac3 are targets for geranylgeranyltransferase I inhibitor-mediated inhibition of signaling, transformation, and membrane ruffling. Cancer Res. 63, 7959-7967. [PubMed] [Google Scholar]
- Katoh, H., Yasui, H., Yamaguchi, Y., Aoki, J., Fujita, H., Mori, K., and Negishi, M. (2000). Small GTPase RhoG is a key regulator for neurite outgrowth in PC12 cells. Mol. Cell. Biol. 20, 7378-7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, H., and Negishi, M. (2003). RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature 424, 461-464. [DOI] [PubMed] [Google Scholar]
- Katzav, S., Cleveland, J.L., Heslop, H.E., and Pulido, D. (1991). Loss of the amino-terminal helix-loop-helix domain of the vav proto-oncogene activates its transforming potential. Mol. Cell. Biol. 11, 1912-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard, D.A., Evans, T., Hart, M., Cerione, R.A., and Manor, D. (1994). Investigation of the GTP-binding/GTPase cycle of Cdc42Hs using fluorescence spectroscopy. Biochemistry 33, 12323-12328. [DOI] [PubMed] [Google Scholar]
- Liu, B.P., and Burridge, K. (2000). Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not beta1 integrins. Mol. Cell. Biol. 20, 7160-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marechal, V., Prevost, M.C., Petit, C., Perret, E., Heard, J.M., and Schwartz, O. (2001). Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 75, 11166-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, V., Schiller, M.R., Eipper, B.A., and Mains, R.E. (2002). Kalirin Dblhomology guanine nucleotide exchange factor 1 domain initiates new axon outgrowths via RhoG-mediated mechanisms. J. Neurosci. 22, 6980-6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, S.Y., and Zheng, Y. (2003). Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 13, 13-22. [DOI] [PubMed] [Google Scholar]
- Ohya, Y., Qadota, H., Anraku, Y., Pringle, J.R., and Botstein, D. (1993). Suppression of yeast geranylgeranyl transferase I defect by alternative prenylation of two target GTPases, Rho1p and Cdc42p. Mol. Biol. Cell 4, 1017-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson, B. (1999). Rho guanine dissociation inhibitors: pivotal molecules in cellular signaling. Cell Signal. 11, 545-554. [DOI] [PubMed] [Google Scholar]
- Qi, H., Fournier, A., Grenier, J., Fillion, C., Labrie, Y., and Labrie, C. (2003). Isolation of the novel human guanine nucleotide exchange factor Src homology 3 domain-containing guanine nucleotide exchange factor (SGEF) and of C-terminal SGEF, an N-terminally truncated form of SGEF, the expression of which is regulated by androgen in prostate cancer cells. Endocrinology 144, 1742-1752. [DOI] [PubMed] [Google Scholar]
- Ren, X.D., Kiosses, W.B., and Schwartz, M.A. (1999). Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A.J., Paterson, H.F., Johnston, C.L., Diekmann, D., and Hall, A. (1992). The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401-410. [DOI] [PubMed] [Google Scholar]
- Sallusto, F., Cella, M., Danieli, C., and Lanzavecchia, A. (1995). Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182, 389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlunck, G., Damke, H., Kiosses, W.B., Rusk, N., Symons, M.H., Waterman-Storer, C.M., Schmid, S.L., and Schwartz, M.A. (2004). Modulation of Rac localization and function by dynamin. Mol. Biol. Cell 15, 256-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, A., and Hall, A. (2002). Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 16, 1587-1609. [DOI] [PubMed] [Google Scholar]
- Schuebel, K.E., Movilla, N., Rosa, J.L., and Bustelo, X.R. (1998). Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J. 17, 6608-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen, F.N., van der Kammen, R.A., Habets, G.G., and Collard, J.G. (1995). Oncogenic activity of Tiam1 and Rac1 in NIH3T3 cells. Oncogene 11, 2215-2221. [PubMed] [Google Scholar]
- Vigorito, E., Bell, S., Hebeis, B.J., Reynolds, H., McAdam, S., Emson, P.C., McKenzie, A., and Turner, M. (2004). Immunological function in mice lacking the Rac-related GTPase RhoG. Mol. Cell. Biol. 24, 719-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent, S., Jeanteur, P., and Fort, P. (1992). Growth-regulated expression of rhoG, a new member of the ras homolog gene family. Mol. Cell. Biol. 12, 3138-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai, M., Derre, I., Kirby, J., Growney, J.D., Dietrich, W.F., and Isberg, R.R. (2001). Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus. J. Exp. Med. 194, 1081-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg, K., Ellerbroek, S.M., Liu, R.Y., Karnoub, A.E., Burridge, K., and Der, C.J. (2002). RhoG signals in parallel with Rac1 and Cdc42. J. Biol. Chem. 277, 47810-47817. [DOI] [PubMed] [Google Scholar]
- West, M.A., Prescott, A.R., Eskelinen, E.L., Ridley, A.J., and Watts, C. (2000). Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr. Biol. 10, 839-848. [DOI] [PubMed] [Google Scholar]
- Ye, Z.S., and Baltimore, D. (1994). Binding of Vav to Grb2 through dimerization of Src homology 3 domains. Proc. Natl. Acad. Sci. USA 91, 12629-12633. [DOI] [PMC free article] [PubMed] [Google Scholar]